Abstract
An array of neuromodulators, including monoamines and neuropeptides, regulate most behavioural and physiological traits. In the past decade, dramatic progress has been made in mapping neuromodulatory circuits, in analysing circuit dynamics, and interrogating circuit function using pharmacogenetic, optogenetic and imaging methods. This review will focus on several distinct neural networks (acetylcholine/GABA/glutamate; histamine/GABA; orexin/glutamate; and relaxin-3/GABA) that originate from neural hubs that regulate wakefulness and related attentional and cognitive processes, and highlight approaches that have identified dual transmitter roles in these behavioural functions. Modulation of these different neural networks might be effective treatments of diseases related to arousal/sleep dysfunction and of cognitive dysfunction in psychiatric and neurodegenerative disorders.
Keywords: Acetylcholine, Arousal and attention, GABA, Glutamate, Histamine, Learning and memory, Orexin, Hypocretin, Relaxin-3
1. INTRODUCTION
Neuromodulators, including acetylcholine, monoamines and neuropeptides regulate most if not all behavioural and physiological traits, including arousal, sleep, motivation, emotion and memory. We define arousal as a response to counteract actual or potential dangers, such as potentially threatening sensory stimuli, or increased attention demand, such as during learning processes or reward seeking, in addition to waking from sleep. A critical but understudied feature of these systems is that they contain more than a single transmitter or modulator. Neuromodulation by, for example amines and neuropeptides, occurs simultaneously with classical neurotransmission of small molecule neurotransmitters, which is thought to provide neural network flexibility and enhanced complexity in the synaptic integration of relayed information (Nusbaum et al., 2001). This is perhaps not surprising since most, if not all neurons, appear to release one or more classical transmitters and one or more neuropeptides (Hnasko and Edwards, 2012; Vaaga et al., 2014) and this has been reported for the vast majority of neuropeptide systems (see Table 1 for several key examples).
Table 1.
General overview of dual neurotransmitter systems.
While co-transmitters have long been recognised to expand the spatial and temporal signalling repertoires of neurons across phyla (Nusbaum et al., 2001), approaches to understand the functional significance and interactions of co-transmission/release in systems regulating arousal, learning and memory have only recently been developed. This review will focus on four dual transmitter systems that uniquely originate from centralised ‘hubs’ of neurons with long-range, highly connected projections to diverse brain regions regulating arousal and memory, where some of these systems have clearly identified functions in these processes (e.g. cholinergic/GABA, histaminergic/GABA and orexinergic/glutamate) while the functions of others, like the relaxin-3/GABA system, are emerging (Figure 1). In addition, these systems display a substantial degree of interaction, including between orexin/glutamate and monoamine neurons (Kohlmeier et al., 2013), between orexin/glutamate and histamine/GABA neurons in driving wakefulness (Mieda et al., 2011; Yu et al., 2015), and between multiple peptide systems and cholinergic/GABA networks (Ishibashi et al., 2015; Damborsky et al., 2016), essential for attention and learning processes (Hasselmo and Sarter, 2011; Hangya et al., 2015). There are also less familiar interactions between relaxin-3/GABA and orexin/glutamate (Blasiak et al., 2015), corticotropin-releasing factor (CRF) (Ma et al., 2013) and serotonin systems (Lawther et al., 2015). We will highlight new data on the function of these systems and the known or putative roles of their co-transmitters.
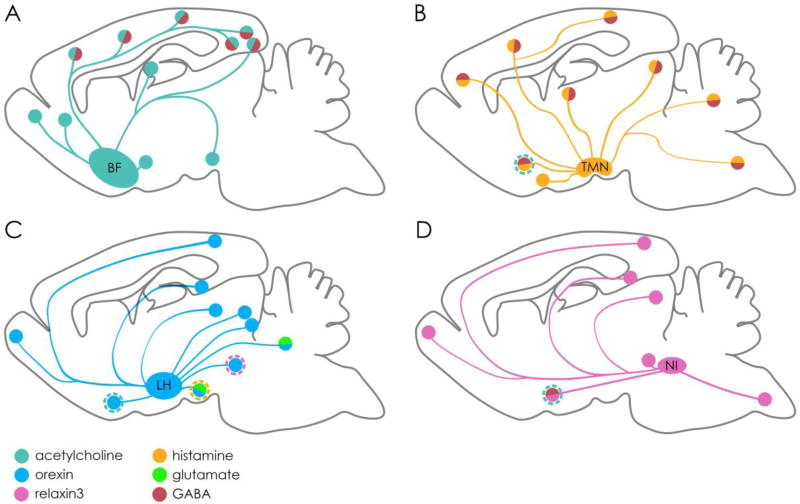
Figure 1. Dual neurotransmitter neural hubs regulating arousal, attention, learning and memory.
(A) Basal forebrain (BF) cholinergic projections. Known sites of co-transmission are labelled by dual colour. (B) Histaminergic projections from the tuberomammillary nucleus (TMN). (C) Orexin projections from the lateral hypothalamus (LH). (D) Relaxin-3 projections from the nucleus incertus (NI). Known interactions between these systems are marked by dashed outlines.
2. Basal forebrain neurons that release acetylcholine and GABA may control cortical plasticity and learning
The basal forebrain (BF) gives rise to one of the best characterised neuromodulatory hubs, the cholinergic projection system. The unusually long axon trees of cholinergic neurons (Wu et al., 2014) innervate virtually all cortical areas and many subcortical centres (Figure 1A; Saper, 1984; Zaborszky et al., 2015). This wide-ranging network has been associated with many cognitive functions, most notably sustained attention, and learning and memory (Everitt and Robbins, 1997; Hasselmo and Sarter, 2011). Recently, evidence supporting the co-release of the neurotransmitter GABA from cholinergic terminals has been reported (Saunders et al., 2015a,b; Tritsch et al., 2016) and here we discuss possible implications of this finding in light of recent advances regarding cholinergic function.
2.1. Acetylcholine co-transmitters
Recent studies have demonstrated that, in addition to acetylcholine (ACh), forebrain cholinergic neurons can release other neurotransmitters. It has been shown that two separate cholinergic systems, the tonically-active interneurons of the striatum (Gras et al., 2002; Guzman et al., 2011; Higley et al., 2011) and the cholinergic cells of the medial habenula (Ren et al., 2011) can release both ACh and glutamate. Co-localisation of the vesicular glutamate transporter VGLUT3 with cholinergic markers was also demonstrated in the basal forebrain (Manns et al., 2001; Gritti et al., 2006; Nickerson Poulin et al., 2006) and cultured BF neurons were shown to release both acetylcholine and glutamate (Allen et al., 2006; Huh et al., 2008). However, basal forebrain neurons expressing both choline acetyltransferase (ChAT) and VGLUT3 appear to, more or less, specifically project to the amygdala, and co-release of glutamate in the cortex by cholinergic neurons has not been detected (Nickerson Poulin et al., 2006).
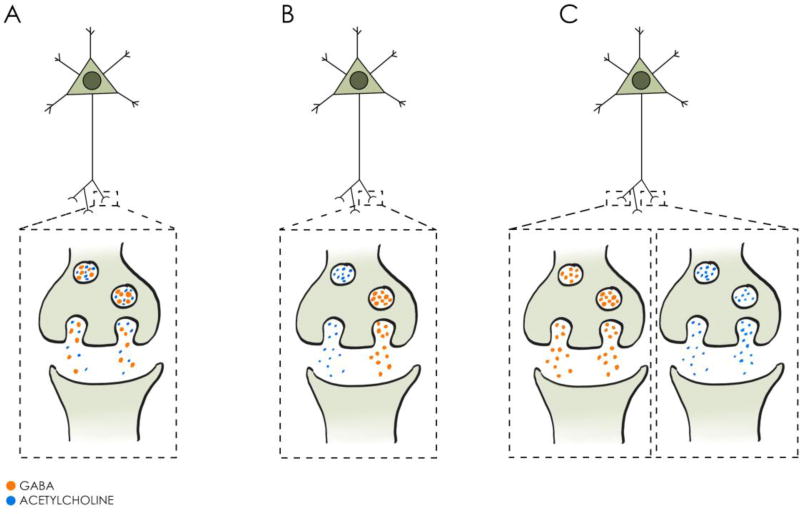
While cortical co-release of ACh and glutamate hitherto lacks solid support, a recent study demonstrated that cholinergic cells can also release the inhibitory transmitter, GABA (Saunders et al., 2015b). This is in line with previous reports of the co-localisation of ChAT and the vesicular GABA transporter (VGAT) (Kosaka et al., 1988; Fisher and Levine, 1989; Beaulieu and Somogyi, 1991). Saunders and colleagues (2015b) employed optogenetic stimulation of cholinergic axons and applied pharmacological blockade of postsynaptic currents to demonstrate the dual nature of transmitter release onto layer 1 interneurons in the cortex. Therefore, the question remains whether ACh and GABA are released from the same vesicles (‘co-release’), different vesicles of the same terminals (‘co-transmission’) or even different terminals (Figure 2). Saunders et al. (2015b) performed array tomography to quantify co-localisation at the terminal level and found that both single-transmitter and double-transmitter terminals can be identified in the cortex. It is worth noting that BF cholinergic neurons may not be unique regarding a capability of ACh-GABA co-transmission, as cortical (GABA) interneurons immunopositive for vasoactive intestinal peptide (VIP) co-express ChAT. Whether this co-expression is a reflection of functional co-transmission is not known. Nevertheless, this should be taken into account when investigating co-release from BF cholinergic neurons, since global ChAT-ChR2 transgenic lines as well as staining at terminal levels do not distinguish local cortical and BF cholinergic/GABAergic terminals.
Figure 2. Co-release vs co-transmission of acetylcholine and GABA.
ACh and GABA may be released from: (A) the same vesicles, (B) separate vesicles in the same terminals, or (C) separate terminals of cholinergic neurons.
2.2. Inputs that stimulate basal forebrain acetylcholine/GABA neurons and downstream effects
There is a wealth of anatomical evidence for diverse inputs to BF cholinergic neurons, including from the striatum, frontal cortex, brainstem, lateral hypothalamus and other neuromodulatory centres (Zaborszky et al., 2012). Recently, two elegant studies using rabies viruses for monosynaptic retrograde tract tracing reached similar conclusions regarding the proportional inputs to cholinergic neurons (Do et al., 2016; Hu et al., 2016). However, little is known about the firing of the input neurons to cholinergic cells during behaviour and how these inputs are integrated by cholinergic dendrites and cell bodies to drive activity.
In contrast, many studies have probed the downstream effects of the cholinergic system on the activity of target neurons, cortical and subcortical networks and behaviour. At the cellular level, both principal neurons and interneurons express a diverse array of muscarinic (metabotropic) and nicotinic (ionotropic) cholinergic receptors, both pre- and postsynaptically. ACh is capable of depolarising and/or hyperpolarising specific target neurons through these receptors on multiple time scales, and can exert an intricate influence on the release of multiple neurotransmitters (Gu, 2002; Demeter et al., 2013; Lin et al., 2015). At the circuit level, ACh has been shown to desynchronise cortical networks, decrease pairwise correlations among neurons, increase the processing of bottom-up versus top-down inputs, and increase cortical activation in general (Buzsaki et al., 1988; Metherate et al., 1992; Pinto et al., 2013; Avery et al., 2014; Sugihara et al., 2016). Importantly, ACh has a strong influence on synaptic plasticity both in terms of controlling sensory receptive fields and tuning curves, and controlling spike timing-dependent plasticity on a fine temporal scale (Kilgard and Merzenich, 1998a; Disney et al., 2007; Froemke et al., 2007; Seol et al., 2007; Berg, 2011; Gu and Yakel, 2011). Finally, at the behavioural level, the ability of central cholinergic neurons to increase cortical activation and fine-tune synaptic plasticity may underlie their widely observed effects on attention, and learning and memory (Everitt and Robbins, 1997; Shuler and Bear, 2006; Hasselmo and Sarter, 2011; Letzkus et al., 2011; Pinto et al., 2013).
Since ACh/GABA co-release has only been demonstrated recently, we can only speculate on the interactions between these co-transmitters. Given the widespread involvement of the cholinergic system in plasticity, one candidate function of the GABA co-transmission may be the fine control of cortical plasticity (Seol et al., 2007; Gu and Yakel, 2011; Hasselmo and Sarter, 2011; Lin et al., 2015; Tritsch et al., 2016). Thus, GABA signalling at postsynaptic sites may influence how the cholinergic system tunes synaptic weights to modify receptive fields, tuning curves, and its contribution to reinforcement learning processes (Kilgard and Merzenich, 1998b; Yu and Dayan, 2005; Disney et al., 2007; Froemke et al., 2007, 2013). Alternatively, GABA release may also be a source of plasticity in the sense that differential control of presynaptic GABA release, both temporally and spatially, including during bursts of cholinergic neurons, may influence the strength of cholinergic modulation in a spatiotemporally and cell type specific manner. Therefore pre- and postsynaptic GABA signalling may provide rapid regulation of cholinergic control of cortical plasticity. Additionally, by inhibiting interneurons that target principal cells, GABA released from cholinergic terminals may add to the disinhibitory effects mediated by cholinergic activation, including disinhibition via cortical layer 1 and VIP interneurons (Letzkus et al., 2011; Lee et al., 2013; Pi et al., 2013; Fu et al., 2014; Granger et al., 2016; Tritsch et al., 2016). This may lead to enhancement of the signal to noise ratio in processing sensory signals and contribute to the cholinergic enhancement of ‘bottom-up’ information transfer (Sarter et al., 2001).
2.3. Temporal release of acetylcholine and its co-transmitters
Understanding the behavioural contingencies that determine the precise timing of acetylcholine release would inform how cholinergic neurons mediate different cognitive processes. By measuring ACh release with choline sensitive voltammetry probes, it has been revealed that cholinergic transients in the medial prefrontal cortex are related to successful detection of visual cues in a sustained attention task and are considerably faster than previously appreciated (Parikh et al., 2007; Sarter et al., 2009). Moreover, these cholinergic transients were absent after consecutive detections, and were therefore associated with attentional shifts (Howe et al., 2013).
Nevertheless, there is little direct data on the activity of cholinergic neurons in behaving animals. However, a recent study probed the activity of optogenetically identified cholinergic neurons of the mouse nucleus basalis and horizontal nucleus of the diagonal band of Broca, two distinct parts of the BF cholinergic system with non-overlapping efferent projections (Hangya et al., 2015). Mice were trained on an auditory detection task that required sustained attention and incorporated reinforcement learning. Surprisingly, while some non-cholinergic neurons exhibited strong attentional correlates, BF cholinergic neurons were instead activated with short latency and high precision after punishment and reward. This is in agreement with two calcium imaging studies in which cholinergic fibres in the hippocampus (Lovett-Barron et al., 2014) and cholinergic cell bodies in the BF (Harrison et al., 2016) were found to report airpuff punishments. Moreover, cholinergic responses scaled with the unexpectedness of the reinforcer, i.e., ‘reinforcement surprise’ (Hangya et al., 2015), or more simply, cholinergic neurons responded when reward was unexpected, but displayed no or very little response to expected reward (Hangya et al., 2015). This is the likely reason for a failure to detect strong reward responses in a related study using imaging techniques, where reward delivery was predictable and thus behaviourally expected (Harrison et al., 2016).
These results suggest cholinergic neurons may be strongly involved in learning through reinforcement (Everitt and Robbins, 1997; Yu and Dayan, 2005; Hasselmo and Sarter, 2011; Letzkus et al., 2011), and are capable of precisely timing cortical activation with respect to reinforcement delivery (Buzsaki et al., 1988; Disney et al., 2007; Pinto et al., 2013). Whether there is a one-to-one coupling between acetylcholine release and firing of cholinergic neurons (Sarter et al., 2009, 2014), and whether ACh and GABA are released with similar temporal dynamics after reward and punishment, are important open questions.
3. Histaminergic neurons that promote wakefulness co-transmit GABA
Histamine is well known as a neurotransmitter involved in peripheral immune system signalling, as upon noxious stimulation, mast cells co-release large quantities of histamine and other chemicals which cause vasodilation. A common side-effect of antihistamines (specifically histamine H1 receptor antagonists) is drowsiness, providing direct support for the wake-promoting role for histamine (Monnier et al., 1967; Nicholson et al., 1991). Its presence in brain was discovered in 1943 (Kwiatkowski, 1943), and histamine neurons are exclusively located in the posterior hypothalamic tuberomammillary nucleus (TMN) (Watanabe et al., 1983; Panula et al., 1984; Takeda et al., 1984), as these neurons express histidine decarboxylase (HDC), the enzyme which synthesises histamine by decarboxylating the amino acid, histidine (Haas et al., 2008). Although TMN neurons are the only neuronal source of brain histamine, microglia have been reported to also express hdc mRNA (Katoh et al., 2001). In this regard, central histamine also regulates microglial migration and cytokine release underlying brain immune responses (Rocha et al., 2014).
3.1. Histamine co-transmitters
It has been known for many years that histamine neurons express glutamic acid decarboxylase (GAD) enzymes and GABA (Takeda et al., 1984; Airaksinen et al., 1992; Trottier et al., 2002; Kukko-Lukjanov and Panula, 2003; Sundvik and Panula, 2012), and in fact, TMN neurons were identified as GABAergic before they were identified to be histaminergic (Vincent et al., 1983). TMN histamine neurons also express the vesicular GABA transporter, VGAT (Yu et al., 2015), which is consistent with the mRNA distributions of GABAergic markers gad1, gad2 and vgat mRNA in the mouse TMN (www.brain-map.org; Lein et al., 2007). In addition to GABA, a subpopulation of histaminergic TMN neurons in the rat co-express thyrotropin releasing hormone and galanin (Airaksinen et al., 1992; Chotard et al., 2002), but the latter expression was not observed in human (Airaksinen et al., 1992; Trottier et al., 2002).
Histamine acts as a classical neuromodulator in a similar manner to noradrenaline, dopamine or ACh (Bolam and Ellender, 2016). It regulates, enables and adjusts the activity of various neural circuits by binding to metabotropic H1, H2 or H3 receptors (Haas et al., 2008; Panula et al., 2015). For example, histamine inhibits ACh, glutamate or GABA release by binding to presynaptic hetero-H3 receptors, it can depolarise neurons by binding to H1 or H2 receptors, or generate changes in phosphorylation of ion channels which influence the rate at which neurons fire action potentials (Atzori et al., 2000).
3.2. Inputs that stimulate TMN histamine/GABA neurons and downstream effects to promote wakefulness
TMN histaminergic neurons are inhibited by GABAergic and galaninergic inputs from the ventrolateral preoptic area, which promote sleep (Sherin et al., 1998). Other neural inputs include hypothalamic orexin/glutamate neurons that provide some excitatory input to histamine neurons (Schone et al., 2014). Similar to the other neural projection hubs discussed in this review (ACh, orexin, relaxin-3), TMN histamine neurons send long-range projections throughout the brain, for example, to the neocortex, striatum and other hypothalamic areas (Figure 1B; Panula et al., 1989; Wada et al., 1991) and vesicular histamine is transported via the monoamine transporter, VMAT2. Notably, histaminergic axons do not form synapses, as assessed by electron microscopy (Takagi et al., 1986), consistent with volume or paracrine, rather than synaptic transmission (Bolam and Ellender, 2016).
In vivo unit recordings in mice have demonstrated a correlation between a population of TMN neurons and wakefulness, whereby ~5 Hz action potentials were observed after transition from slow-wave sleep to wake, continued firing occurred during wakefulness, and a cessation of firing occurred before the onset of electroencephalogram (EEG) synchronisation and sleep (Sakai et al., 2010). Interestingly, these firing characteristics were unaltered in TMN neurons of hdc-knockout mice (Sakai et al., 2010), suggesting that endogenous histamine is not necessary for the expression of the firing properties of these neurons. Consistent with a wealth of earlier findings (Monnier et al., 1967; Lin et al., 1988, 1989; Nicholson et al., 1991; Parmentier et al., 2002; Haas et al., 2008; Schone et al., 2014), direct and selective pharmacogenetic activation of histamine neurons causes hyperactivity and wakefulness (Yu et al., 2015). Histamine neuron activation also promotes feeding, sexual appetite and locomotion, and motivated behaviour in general, all diverse aspects of wakefulness (Torrealba et al., 2012; Riveros et al., 2015).
3.3. Dissecting the paracrine contributions of histamine and GABA functions co-release
Despite the long-standing suspicion that histamine and GABA were putative co-transmitters, the specific function of GABA was unclear, even though the presence of GABA in histaminergic neurons of all vertebrates examined, suggested an essential function. Considering the paracrine fashion of histamine release (Takagi et al., 1986), GABA likely functions via similar volume transmission. In this regard, GABA is an electroneutral zwitterion at physiological pH, with reduced binding to the extracellular matrix (Roberts and Sherman, 1993). Thus, it can diffuse widely, and would only be limited by GABA reuptake transporters that limit long-range diffusion. Ambient, non-synaptic GABA has been demonstrated to produce sustained inhibitory currents (known as Gtonic) by activating high-affinity extrasynaptic ionotropic GABAA receptors that contain delta subunits (Brickley and Mody, 2012; Yu et al., 2015).
Prior to current genetic manipulation technology, there was no clear way to selectively test the GABAergic role of histamine neurons. More recently, their expression of the hdc gene allowed a way to selectively manipulate these neurons through generation of HDC-Cre recombinase mice (Zecharia et al., 2012). This mouse line was used to ‘knock down’ the vgat gene, and thus GABA release, selectively in histamine neurons (Yu et al., 2015). The resultant mice exhibited increased locomotion, and significantly enhanced wakefulness during the dark (active) phase (Yu et al., 2015). Selective optogenetic stimulation of histaminergic projections to the neocortex and caudate-putamen increased Gtonic onto pyramidal neurons and medium spiny neurons, respectively, and vgat knock-down abolished these evoked tonic inhibitory currents, but they still occurred in the presence of H1 and H2 receptor antagonists (Yu et al., 2015). Thus, during wakefulness, GABA is delivered widely in neocortical and striatal circuitry by volume transmission and is co-transmitted with histamine to regulate and constrain promotion of wakefulness by histamine. However, there appears to be heterogeneity in TMN histamine neurons, whereby not all of them co-transmit GABA. Selective optogenetic stimulation of histamine neuron projections to the preoptic hypothalamus induces only histamine release which, however, stimulates local GABAergic neurons to produce an overall net inhibitory effect (Williams et al., 2014). Thus, histamine/GABA co-transmission is circuit specific.
The precise role for co-release of GABA and histamine, and its apparent stop-go nature, with GABA being inhibitory and histamine being excitatory, is not known. The GABA component may be neuroprotective, as excessive stimulation of circuits for wakefulness could be excitotoxic. In this regard, mice with vgat knock down selectively in histaminergic neurons are extremely hyperactive during their circadian wake period (Yu et al., 2015). Alternatively, tonic GABA inhibition could mediate precision of, for example, pyramidal cell firing (Wlodarczyk et al., 2013), whereby the Gtonic component of histamine signalling could serve to strengthen and complement the enhancement of cognitive and attentional processes produced by histamine. Studies on GABA co-transmission or co-release, with peptides or glutamate, observed fast monosynaptic GABAA receptor responses, and not a slow Gtonic response (Tong et al., 2008; Tritsch et al., 2012; Jego et al., 2013; Root et al., 2014; Shabel et al., 2014; Tritsch et al., 2014, 2016). An intriguing example is GABA co-release with dopamine, where GABA is not synthesised by dopamine neurons, but is instead transported into axons by mGAT1 and mGAT4 for packaging into dopamine vesicles by VMAT2 (Tritsch et al., 2012, 2014, 2016). For histamine neurons, no compensatory function of VMAT2 was observed (Yu et al., 2015). Furthermore, GABA containing vesicles are distinct from those containing histamine in TMN neurons, suggestive that these neurotransmitters are co-transmitted and not co-released (Kukko-Lukjanov and Panula, 2003). Future studies would be needed to determine if different firing patterns or frequencies differentially promote GABA and/or histamine release.
4. Orexin and glutamate co-transmission in promoting arousal
In 1998, two laboratories independently discovered the neuropeptides orexin-A/hypocretin-1 and orexin-B/hypocretin-2 peptides (de Lecea et al., 1998; Sakurai et al., 1998). These peptides were shown to have excitatory activity in cultured hypothalamic neurons (van den Pol et al., 1998) and to be synthesised exclusively in the CNS by a neural hub in the perifornical lateral hypothalamus with widespread projections (Figure 1C; Peyron et al., 1998). Among many target areas, these projections conspicuously terminated within regions associated with feeding, metabolism, arousal and reward (Kilduff and Peyron, 2000), including the histaminergic TMN and other hypothalamic structures, the noradrenergic locus coeruleus (LC), the serotonergic dorsal raphe (DR), the dopaminergic ventral tegmental area (VTA), and the cholinergic BF and mesopontine laterodorsal tegmental nucleus (LDT) and pedunculopontine nucleus (PPT). Through a convergence of compelling genetic studies, a key function of these neurons was revealed to be the stabilisation of waking and sleep states. Indeed genetic deletion of orexin peptides or their two known receptors in mice resulted in a phenotype strikingly similar to the human sleep-disorder, narcolepsy (Chemelli et al., 1999; Lin et al., 1999; Kinsanuki et al., 2001; Mochizuki et al., 2004; Kalogiannis et al., 2011). Moreover, the canarc-1 gene, which confers heritable narcolepsy in dogs was discovered to be a null mutation of the orexin-2 receptor gene (Lin et al., 1999). These animal models all displayed key features of narcolepsy in humans, including fragmentation of wake and sleep periods and epochs of sudden postural atonia during waking, termed cataplexy. Shortly thereafter, it was shown that human narcoleptics with cataplexy were orexin-peptide deficient (Peyron et al., 2000) and that the orexin neurons themselves are largely absent in brains from adult narcoleptic patients (Thannickal et al., 2000). Thus, loss of orexin peptides or receptors in animals produces a phenotype very similar to human narcolepsy, which itself results from a loss of orexin neurons.
4.1. Orexin co-transmitters
Orexin neurons express a number of other neuropeptides, including galanin (Håkansson et al., 1999), dynorphin (Chou et al., 2001) and neuronal activity-regulated pentraxin (Reti et al., 2002). There is also strong evidence for co-expression of vesicular glutamate transporters, VGLUT1 and VGLUT2 (VGLUT2>VGLUT1) (Rosin et al., 2003; Henny et al., 2010), but not GAD67, suggesting that glutamate and orexin and perhaps other peptides, are co-transmitters. Indeed, electron microscopy studies in the TMN and other hypothalamic areas, and in VTA, LC, LDT and DR, indicate orexin-immunoreactive neurons form asymmetric and some symmetric synapses, having a typical synaptic ultrastructure with small synaptic vesicles clustered near active zones, and larger, dense-core orexin-immunoreactive vesicles further away (Horvath et al., 1999; Diano et al., 2003; Torrealba et al., 2003; Balcita-Pedicino and Sesack, 2007; Cid-Pellitero and Garzon, 2011; Del Cid-Pellitero and Garzón, 2011).
Physiological evidence for glutamate co-transmission in orexin projections has been obtained in the TMN and LC, using optogenetic stimulation of orexin axons (Figure 1C) (Schöne et al., 2012, 2014; Sears et al., 2013). In the TMN, brief light pulses that evoked presynaptic action potentials, also evoked short latency EPSPs that were abolished by AMPA receptor antagonism with CNQX (Schöne et al., 2012, 2014). Moreover, these EPSPs rapidly increased the firing of TMN neurons with trains of light pulses. These light-evoked fast EPSPs also displayed paired-pulse depression, which caused the evoked EPSPs to substantially diminish in size across pulse trains of 10 s. Interestingly, it was only during this time course of synaptic depression that orexin was released to act on TMN neurons and promote firing by means of a slow onset and long lasting depolarising current (Schone et al., 2014). Thus, as observed in other peptide-releasing terminals, orexin release requires sustained high-frequency firing (> 10 Hz) (for review, see van den Pol, 2012).
One putative function of glutamate and orexin co-transmission is to provide complementary signals related to orexin neuron firing, with the fast EPSPs conveying a signal proportional to the time derivative of input firing, and orexin conveying a signal proportional to the time integral of input firing (Schöne et al., 2014). This type of control, if deployed in a feedback circuit can effectively promote circuit stability and may help maintain consolidated states of waking, depending on circuit structure (Schöne et al., 2014).
4.2. Inputs that stimulate hypothalamic orexin/glutamate neurons and downstream effects to promote wakefulness
Neural inputs regulating orexin neurons arise from BF cholinergic neurons, preoptic area GABAergic neurons, serotonergic raphe neurons, lateral septum, bed nucleus of the stria terminalis, dorsomedial nucleus, periaqueductal grey, amygdala, and many hypothalamic regions (Sakurai et al., 2005; Yoshida et al., 2006). The orexin/glutamate system has been proposed to receive negative feedback from wake-promoting modules in order to provide dynamic behavioural control of arousal and wakefulness (Schöne and Burdakov, 2016). Evidence for this negative feedback schema is strongest for the serotonergic system since orexin neurons are inhibited by serotonergic inputs from the dorsal and median raphe. Optogenetic activation of serotonergic terminals in the lateral hypothalamus directly inhibited orexin neurons via 5HT1A receptors, and also indirectly inhibited orexin neurons by facilitating GABAergic inhibitory inputs, without affecting glutamatergic inputs (Chowdhury and Yamanaka, 2016). Moreover, orexin neurons receive dense glutamatergic and GABAergic inputs from the substantia innominata and magnocellular preoptic area, but no innervation from BF cholinergic neurons (Agostinelli et al., 2017).
In addition to discovering orexin peptides, Sakurai et al. (1998) identified two (previously orphan) G-protein-coupled receptors that bind and are activated by orexins, which they termed the orexin-1 and -2 receptors (OX1, OX2). These receptors have distinct but partially overlapping expression patterns throughout the CNS (Marcus et al., 2001). Many studies have investigated the excitatory/depolarising effects of orexin/glutamate neurons on target brain nodes regulating sleep/wakefulness (via BF, LDT/PPT, preoptic area, raphe nucleus, TMN and LC), feeding (amygdala, nucleus accumbens, hypothalamic paraventricular and arcuate nuclei, and nucleus of the solitary tract) and reward/emotion (lateral septum, amygdala, nucleus accumbens, hypothalamic paraventricular nucleus, VTA, and nucleus incertus) (Kohlmeier et al., 2013; Schöne et al., 2014; Kastman et al., 2016; for reviews see Leonard and Kukkonen, 2014; Schöne and Burdakov, 2016). Given its relatively slow time course, orexin signalling is well suited to modulate the postsynaptic integration of fast synaptic inputs, including glutamatergic EPSPs arising from co-transmission, as suggested by two recent studies. The first examined whether the unusually large membrane current noise associated with orexin depolarisation in cholinergic LDT and PPT neurons and serotonergic DR neurons could provide high frequency input, in addition to a slow depolarisation (Ishibashi et al., 2015). The ‘noisy’ orexin input doubled the spectral amplitude of membrane current at gamma band frequencies in cholinergic neurons and more than tripled it in serotonergic neurons. Using a dynamic clamp to add a virtual orexin current further revealed that this noisy input activates an intrinsic, Ca2+-dependent resonance centred on theta and alpha frequencies in these neurons. These findings suggest the noisy orexin input can function to boost the effectiveness of EPSPs in the theta and alpha frequency range by stochastic resonance and by engaging intrinsic postsynaptic resonances within these target neurons. The second study revealed that activation of OX1 and OX2 strongly enhanced the post-spike afterhyperpolarisation in serotonergic DR neurons (Ishibashi et al., 2016). This orexin-enhanced AHP strongly altered the firing properties of DR neurons by increasing spike-frequency adaptation. In signal-processing terms, enhanced spike-frequency adaptation would increase the high-pass filter characteristics of these neurons by attenuating firing to steady inputs, without attenuating firing to rapidly varying inputs like those encoding behavioural events.
In the DR, this may be particularly important for reward signal processing, since orexin neurons increase firing during reward (Hassani et al., 2016) and the firing of most serotonergic DR neurons transition from tonic to burst firing during reward acquisition (Li et al., 2016). Thus, orexin receptor signalling during active waking, when orexin neurons are firing, would enhance encoding of rapidly varying signals from glutamate inputs, including those mediated by co-transmission, and promote phasic outflow from raphe neurons. In the absence of orexin, this signal-processing would be impaired and the effectiveness of fast synaptic inputs including those mediated by co-transmission would be attenuated. Indeed, perhaps the loss of these postsynaptic tuning actions in the absence of orexin, limit the behavioural impact of signals conveyed by glutamate co-transmission (see next section). Further experiments will be necessary to directly test this idea.
4.3. Implications of orexin and glutamate co-transmission for behaviour
To date, the possible behavioural implications of glutamate/orexin co-transmission have been explored indirectly, via three lines of experimentation. First, optogenetic stimulation of orexin neurons in mice produced sleep-to-wake transitions at an average latency of ~30 s, which was shorter than the average latency following stimulation in control mice (~60 s) (Adamantidis et al., 2007). If glutamate co-transmission was an important contributor to this latency shortening, waking latencies would be expected to remain shorter than control following disrupted orexin signalling. However, optical stimulation of orexin neurons did not produce significantly shorter latency waking when OX1 was blocked by an antagonist or in orexin peptide null mice. This indicates orexin signalling is necessary and suggests that glutamate co-transmission is insufficient to drive short latency waking.
Secondly, while genetic ablation of orexin neurons would be expected to result in more severe phenotypes than just the knockout of the orexin peptides, total ablation of orexin neurons, produced by expressing a toxic ataxin-3 gene product in them, produced a narcolepsy phenotype with only subtle differences to that produced by orexin peptide deletion (Hara et al., 2001). These findings suggest orexin signalling is required for stabilising behavioural states and suppressing cataplexy, rather than potential co-transmitters. However, these experiments need to be interpreted with caution since phenotype severity is often sensitive to genetic background and environmental factors, which was clearly illustrated by the late-onset obesity which is much more severe in orexin/ataxin-3 mice than orexin knockout mice with mixed genetic backgrounds than when both transgenes are on a pure C57BL6 genetic background. Despite this, mice lacking orexin neurons still become more obese than orexin null mice when they are both on a C57BL6 background and fed high-fat chow, suggesting that one or more co-transmitter plays a discernible role in regulating metabolism (Hara et al., 2005).
Finally, the narcolepsy phenotype in orexin neuron-ablated mice was effectively rescued by either ICV orexin or non-specific central re-expression of the prepro-orexin peptide (Mieda et al., 2004), suggesting that orexin is also sufficient to stabilise waking and suppress cataplexy in the absence of orexin neurons and their co-transmitters. In this regard, it is noteworthy that in addition to making morphological synapses, orexin-immunoreactive processes containing dense-core vesicles also make membrane appositions and free varicosities lacking synaptic structure, suggesting the non-synaptic release of orexin without glutamate release may also occur (Balcita-Pedicino and Sesack, 2007; Cid-Pellitero and Garzon, 2011; Del Cid-Pellitero and Garzón, 2011). These studies do not rule out a behavioural role for glutamate co-transmission, but suggest that a role in these behaviours depends on the integrity of orexin signalling.
5. Relaxin-3/GABA and nucleus incertus networks in promoting arousal
In comparison to the ACh/GABA, histamine/GABA and orexin/glutamate systems, the relaxin-3/GABA system is currently not as well characterised. Relaxin-3 is still considered a relatively ‘novel’ neuropeptide, although it was discovered quite soon after the orexins (Bathgate et al., 2002). It is synthesised in neuronal populations of the midbrain and pons, and the distribution of these neurons and relaxin-3-immunoreactive projections have been mapped in rat (Burazin et al., 2002; Tanaka et al., 2005; Ma et al., 2007), mouse (Smith et al., 2010), and Cynomolgus monkey (Ma et al., 2007). Although it the most recently discovered member of the relaxin peptide family, bioinformatics analysis revealed that the relaxin-3 gene is highly conserved and relaxin-3 is the ancestral peptide of the relaxin and insulin-like peptide superfamily (Wilkinson et al., 2005). Similarly, analysis of relaxin-3 immunoreactivity in neural projections of rodent and primate brain reveals the neuroanatomical distribution of relaxin-3 and its projection targets are highly conserved (Ma et al., 2007, 2009b; Smith et al., 2010).
Relaxin-3 is synthesised in four pontine/midbrain regions: nucleus incertus (NI), pontine raphe (PnR), periaqueductal grey (PAG) and an area dorsal to the substantia nigra (dSN) (Figure 1D). The receptor for relaxin-3 is known as RXFP3 (relaxin family peptide 3 receptor) (Liu et al., 2003; Ma et al., 2017b), which is similarly distributed to relaxin-3-containing nerve fibres. RXFP3 is generally associated with inhibitory actions on intracellular cAMP signalling via Gi/o-protein coupling (Liu et al., 2003), but in brain, RXFP3 activation is capable of both hyperpolarisation and depolarisation of neurons (Blasiak et al., 2013).
5.1. Relaxin-3 co-transmitters
The NI contains the major population of relaxin-3 neurons (Ma et al., 2017b), and is a compact GABAergic nucleus located caudal to DR, below the fourth ventricle and dorsal tegmental nucleus of Gudden, midline of the LC and LDT, and anterior to the prepositus hypoglossal nucleus (Goto et al., 2001; Olucha-Bordonau et al., 2003). It is positioned among other well characterised brainstem neural hubs important for arousal and sleep (Brown and McKenna, 2015; Weber and Dan, 2016). While largely GABAergic (Ford et al., 1995), NI neurons are neurochemically and functionally heterogeneous. In this regard, all relaxin-3 neurons are GABAergic, reflected by colocalisation with GAD65/7 and the calcium-binding protein, calbindin (Ma et al., 2007). However, the nature of, and interaction between, relaxin-3 and GABA transmission in these neurons remains to be examined, using similar electrophysiological and genetic technology to that applied to the histamine/GABA and orexin/glutamate systems.
5.2. Inputs that stimulate brainstem relaxin-3/GABA neurons and downstream effects on behaviour and arousal
In the rat brain, the NI receives primary neural inputs from pyramidal neurons of the prelimbic and anterior cingulate cortices, lateral habenula, interpeduncular nucleus, median raphe, medial septum, preoptic area and lateral hypothalamus (Goto et al., 2001; Olucha-Bordonau et al., 2003). These inputs suggest the NI integrates information largely related to behavioural planning and hippocampal function, however the excitatory or inhibitory nature of these inputs, and inputs specific to relaxin-3 neurons, remain to be characterised. Approximately half of all NI neurons express CRF receptor-1 (CRF1), which includes all relaxin-3 neurons; and in urethane-anesthetised rats, all relaxin-3 neurons (and some non-relaxin-3 neurons) exhibited increased spontaneous firing in response to CRF, whereas a population of non-relaxin-3 neurons exhibited decreased firing (Ma et al., 2013). This CRF input appears to originate from the preoptic area (Ma et al., 2013). More recent studies demonstrated that intra-NI infusion of an CRF1 antagonist blocked yohimbine-induced reinstatement of alcohol seeking in alcohol-preferring (iP) rats (Walker et al., 2016), whereas intra-NI infusion of a dopamine D2/3 agonist, resulted in hypolocomotion via inhibitory D2 receptors expressed by CRF1/relaxin-3 neurons (Kumar et al., 2015).
Orexins also regulate the activity of NI and relaxin-3 neurons via OX2 (Blasiak et al., 2015; Kastman et al., 2016). NI relaxin-3 neurons receive an orexinergic innervation from neurons in the lateral hypothalamus and perifornical area, and orexin-A produced depolarisation and action potential firing of NI neurons in vitro via OX2 (Blasiak et al., 2015). Furthermore, intra-NI injection of an OX2 antagonist attenuated yohimbine-induced reinstatement of alcohol seeking in iP rats (Kastman et al., 2016) and intra-NI injections of orexin-A enhanced locomotor activity and food intake in Sprague-Dawley rats.
Similar to the aforementioned systems, neural tract-tracing of NI neurons revealed broad, long-range projections to cortical and sub-cortical regions (Figure 1D). These connections suggest that the NI is positioned to modulate prefrontal and hippocampal cortical activity, locomotor behaviour, attentive states, and learning processes, in part via dense connections with components of the septohippocampal system and cortex (Goto et al., 2001; Olucha-Bordonau et al., 2003), and there is accumulating experimental evidence that supports these functions (for review see Ma and Gundlach, 2015). The projection pattern of NI neurons corresponds well with the distribution of relaxin-3-immunoreactive axons/terminals and RXFP3 mRNA (Ma et al., 2007), suggesting that relaxin-3 is largely produced and transported by NI neurons.
Early functional studies demonstrated that electrical stimulation or lesion of the NI induced or attenuated hippocampal theta rhythm (4–12 Hz oscillations in neural activity), respectively (Nunez et al., 2006); and hippocampal theta rhythm is important for memory processes (Vertes, 2005) and mood (Gray and McNaughton, 2000). Inactivation of the NI also impaired spatial learning and memory in rats trained in the Morris water maze task (Nategh et al., 2015). A recent study aimed at better understanding the function of NI neurons used a chemogenetic approach to stimulate the NI network in awake rats and assessed the effects on behaviour and cortical activity, via electroencephalogram (EEG) recordings. Clozapine-N-oxide (CNO)-induced activation of the hM3Dq DREADD (i.e. designer receptor activated by designer drug), expressed in NI neurons and throughout their projections resulted in increased wakefulness and locomotor activity in the homecage, detected in EEG and telemetry activity recordings, during the normal inactive phase (Ma et al., 2017a). Detailed spectral analyses identified enhanced cortical desynchronisation, particularly during periods of rest, such that cortical activity was similar that observed during active movement (Ma et al., 2017a). Furthermore, in rats conditioned to associate an audible tone with footshock, NI stimulation was associated with increased ‘active’, vigilant behaviours, such as head-scanning and darting, as opposed to passive behaviours, such as freezing, in response to impending threat (Ma et al., 2017a). Increased locomotor activity and enhanced emotional reactivity is consistent with the ‘operational definition’ of general arousal (Quinkert et al., 2011).
Further studies are required to determine the sites of action and neuronal target populations that underlie the different effects of NI stimulation, as well as the contribution of relaxin-3 vs GABA transmission or relaxin-3/GABA co-transmission to these effects, perhaps using the approach adopted to characterise the histamine/GABA system (Yu et al., 2015). Nonetheless, data consistent with these findings was reported, with conventional electrical stimulation of the NI producing forward locomotion (Farooq et al., 2016). Taken together, these findings strongly suggest the NI receives multiple neurochemical inputs that are important for behavioural arousal and motivated behaviours, actions that are likely mediated by NI GABA transmission and, in some part, by relaxin-3/RXFP3 signalling.
5.3. Putative target nodes underlying relaxin-3/GABA signalling effects on arousal and behaviour
Likely downstream targets of the NI that mediate these behavioural effects include the medial septum, which is densely innervated by NI neurons (Goto et al., 2001; Olucha-Bordonau et al., 2003) and which, via pacemaker cell connections with the hippocampus, contributes to spatial navigation and learning and memory (Hangya et al., 2009; Pang et al., 2011; Roland et al., 2014). Moreover, recent studies have demonstrated that glutamatergic neurons in the medial septum control initiation and velocity of locomotion and associated entrainment of hippocampal theta oscillations (Bender et al., 2015; Fuhrmann et al., 2015; Robinson et al., 2016). In this regard, the medial septum expresses high levels of RXFP3 and infusion of an RXFP3-specific agonist into the region enhanced hippocampal theta oscillations, whereas an antagonist attenuated hippocampal theta oscillations and resulted in dose-dependent impairment of spatial working memory, assessed by the spontaneous alternation task in rats (Ma et al., 2009a).
Any interaction between the cholinergic and relaxin-3/NI systems is currently unclear, although high levels of acetylcholinesterase have been detected in the NI (Olucha-Bordonau et al., 2003), suggesting that cholinergic regulation of NI neurons may occur. Furthermore, NI neurons innervate the broad lateral preoptic area and BF (Goto et al., 2001; Olucha-Bordonau et al., 2003), which receives a dense relaxin-3 innervation and expresses high levels of RXFP3 (Ma et al., 2007). Therefore, it will be of interest to examine the possible interaction of NI/relaxin-3 afferents with cortically-projecting cholinergic and GABAergic neurons in the basal forebrain and the impact on cortical activity, attention and arousal.
It has been established that NI relaxin-3 neurons interact with neighbouring serotonergic neurons in the DR, whereby relaxin-3 neurons express 5-HT1A receptors and central serotonin depletion by p-chlorophenylalanine administration resulted in elevated relaxin-3 mRNA expression (Miyamoto et al., 2008). A more recent study demonstrated that rats treated with the anxiogenic benzodiazepine, FG-7142, displayed enhanced anxiety-like behaviour that was associated with activated populations of relaxin-3 NI neurons and serotonergic DR neurons (Lawther et al., 2015), suggesting a functional association between these signalling systems in anxiety, associated with co-activation of serotonergic and relaxin-3 systems. Conversely, relaxin-3 fibres from the NI course through the DR, which contains high levels of RXFP3 mRNA (Ma et al., 2007), although the effects of relaxin-3/RXFP3 signalling on serotonin neuron activity remains to be investigated.
The function of the smaller relaxin-3 neuron populations located in the PnR and dSN has not been investigated. The relaxin-3 neurons in the PAG have been shown to strongly innervate the thalamic intergeniculate leaflet (IGL) (Blasiak et al., 2013), which contains neuropeptide-Y (NPY) neurons that are known to modulate suprachiasmatic nucleus function and associated circadian rhythms (Muscat and Morin, 2006). The IGL is also a site of orexin action and modulation of sleep-wake balance (Nixon and Smale, 2005; Palus et al., 2015), but interactions between orexin and relaxin-3 signalling within the IGL have not yet been examined (Ma et al., 2007).
6. Conclusions
Although it was established several decades ago that exocytosis of one or more neuropeptides accompanies the release of classical neurotransmitters in most neurons (Burnstock, 2004), recent evidence is identifying the physiological importance of co-transmission of multiple neuromodulators with classical small-molecule neurotransmitters. The precise functions of neuromodulator/neurotransmitter co-release are still unclear, but a recent review on GABA co-transmission by Tritsch et al. (2016) speculated that it may contribute to (i) the fine-tuning of the magnitude and duration of synaptic effects, as is the case for histamine-GABA neurons, whereby GABA serves to provide a brake on overactivation by histamine (Yu et al., 2015), (ii) fine-tuning of the membrane potential of target neurons to optimise the actions of cotransmitters, and/or (iii) complementary modulation of the plasticity of synapses and neuronal circuits. Here we reviewed four co-transmitter systems that originate from hubs of neurons with long-range, highly connected projections - ACh/GABA, histamine/GABA, orexin/glutamate, and relaxin-3/GABA, all of which have been demonstrated to regulate arousal, wakefulness, and attention, as well as associated cognitive functions including learning and memory. Therefore, it is not surprising that these networks have identified anatomical and functional interactions. However, understanding how these multiple dual transmission systems are coordinated to cooperate or compete in facilitating similar cognitive functions, and how co-transmission shapes these network level interactions and behavioural functions, requires further research.
Highlights.
Acetylcholine and GABA co-transmission may control cortical plasticity and learning.
Histamine and GABA co-transmission are necessary for appropriate wakefulness.
Orexin and glutamate co-transmission may function to stabilize wakefulness.
Relaxin-3 and GABA co-transmission influence stress-related arousal and behaviours.
Acknowledgments
We thank Katalin Sviatkó for help with the figures. SM was supported by a National Health and Medical Research Council of Australia project grant (1067522). BH was supported by the ‘Lendület’ Program of the Hungarian Academy of Sciences (LP2015-2/2015) and he is a member of the FENS-Kavli Network of Excellence. CSL was funded by PHS grant NS027881. WW holds a joint Wellcome Trust Investigator Award (together with Nicholas P Franks; 107841/Z/15/Z). ALG was supported by a National Health and Medical Research Council of Australia Research Fellowship (1005985) and project grant (1067522), and a Brain & Behavior Research Foundation (USA) NARSAD Independent Investigator Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors claim no conflict of interest.
References
- Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostinelli LJ, Ferrari LL, Mahoney CE, Mochizuki T, Lowell BB, Arrigoni E, Scammell TE. Descending projections from the basal forebrain to the orexin neurons in mice. J Comp Neurol. 2017;525:1668–1684. doi: 10.1002/cne.24158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen MS, Alanen S, Szabat E, Visser TJ, Panula P. Multiple neurotransmitters in the tuberomammillary nucleus: comparison of rat, mouse, and guinea pig. J Comp Neurol. 1992;323:103–116. doi: 10.1002/cne.903230109. [DOI] [PubMed] [Google Scholar]
- Allen TG, Abogadie FC, Brown DA. Simultaneous release of glutamate and acetylcholine from single magnocellular "cholinergic" basal forebrain neurons. J Neurosci. 2006;26:1588–1595. doi: 10.1523/JNEUROSCI.3979-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori M, Lau D, Tansey EP, Chow A, Ozaita A, Rudy B, McBain CJ. H2 histamine receptor-phosphorylation of Kv3.2 modulates interneuron fast spiking. Nat Neurosci. 2000;3:791–798. doi: 10.1038/77693. [DOI] [PubMed] [Google Scholar]
- Avery MC, Dutt N, Krichmar JL. Mechanisms underlying the basal forebrain enhancement of top-down and bottom-up attention. Eur J Neurosci. 2014;39:852–865. doi: 10.1111/ejn.12433. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- Bathgate RAD, Samuel CS, Burazin TCD, Layfield S, Claasz AA, Reytomas IG, Dawson NF, Zhao C, Bond C, Summers RJ, Parry LJ, Wade JD, Tregear GW. Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. Novel members of the relaxin peptide family. J Biol Chem. 2002;277:1148–1157. doi: 10.1074/jbc.M107882200. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Somogyi P. Enrichment of cholinergic synaptic terminals on GABAergic neurons and coexistence of immunoreactive GABA and choline acetyltransferase in the same synaptic terminals in the striate cortex of the cat. J Comp Neurol. 1991;304:666–680. doi: 10.1002/cne.903040412. [DOI] [PubMed] [Google Scholar]
- Bender F, Gorbati M, Cadavieco MC, Denisova N, Gao X, Holman C, Korotkova T, Ponomarenko A. Theta oscillations regulate the speed of locomotion via a hippocampus to lateral septum pathway. Nat Commun. 2015;6:8521. doi: 10.1038/ncomms9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DK. Timing is everything, even for cholinergic control. Neuron. 2011;71:6–8. doi: 10.1016/j.neuron.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak A, Blasiak T, Lewandowski MH, Hossain MA, Wade JD, Gundlach AL. Relaxin-3 innervation of the intergeniculate leaflet of the rat thalamus - neuronal tract-tracing and in vitro electrophysiological studies. Eur J Neurosci. 2013;37:1284–1294. doi: 10.1111/ejn.12155. [DOI] [PubMed] [Google Scholar]
- Blasiak A, Siwiec M, Grabowiecka A, Blasiak T, Czerw A, Blasiak E, Kania A, Rajfur Z, Lewandowski MH, Gundlach AL. Excitatory orexinergic innervation of rat nucleus incertus - Implications for ascending arousal, motivation and feeding control. Neuropharmacology. 2015;99:432–447. doi: 10.1016/j.neuropharm.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Ellender TJ. Histamine and the striatum. Neuropharmacology. 2016;106:74–84. doi: 10.1016/j.neuropharm.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Somogyi P, Takagi H, Fodor I, Smith AD. Localization of substance P-like immunoreactivity in neurons and nerve terminals in the neostriatum of the rat: a correlated light and electron microscopic study. J Neurocytol. 1983;12:325–344. doi: 10.1007/BF01148468. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, McKenna JT. Turning a negative into a positive: ascending GABAergic control of cortical activation and arousal. Front Neurol. 2015;6:135. doi: 10.3389/fneur.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burazin TCD, Bathgate RAD, Macris M, Layfield S, Gundlach AL, Tregear GW. Restricted, but abundant, expression of the novel rat gene-3 (R3) relaxin in the dorsal tegmental region of brain. J Neurochem. 2002;82:1553–1557. doi: 10.1046/j.1471-4159.2002.01114.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MJ, Arrigoni E, Maratos-Flier E. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci. 2015;35:3644–3651. doi: 10.1523/JNEUROSCI.4187-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chotard C, Ouimet T, Morisset S, Sahm U, Schwartz JC, Trottier S. Effects of histamine H3 receptor agonist and antagonist on histamine co-transmitter expression in rat brain. J Neural Transm (Vienna) 2002;109:293–306. doi: 10.1007/s007020200024. [DOI] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Yamanaka A. Optogenetic activation of serotonergic terminals facilitates GABAergic inhibitory input to orexin/hypocretin neurons. Sci Rep. 2016;6:36039. doi: 10.1038/srep36039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid-Pellitero E, Garzon M. Hypocretin1/OrexinA axon targeting of laterodorsal tegmental nucleus neurons projecting to the rat medial prefrontal cortex. Cereb Cortex. 2011;21:2762–2773. doi: 10.1093/cercor/bhr070. [DOI] [PubMed] [Google Scholar]
- Damborsky JC, Smith KG, Jensen P, Yakel JL. Local cholinergic-GABAergic circuitry within the basal forebrain is modulated by galanin. Brain Struct Funct. 2016;222:1385–1400. doi: 10.1007/s00429-016-1283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJ, Jr, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol. 1999;413:113–128. [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cid-Pellitero E, Garzón M. Medial prefrontal cortex receives input from dorsal raphe nucleus neurons targeted by hypocretin1/orexinA-containing axons. Neuroscience. 2011;172:30–43. doi: 10.1016/j.neuroscience.2010.10.058. [DOI] [PubMed] [Google Scholar]
- Demeter E, Guthrie SK, Taylor SF, Sarter M, Lustig C. Increased distractor vulnerability but preserved vigilance in patients with schizophrenia: evidence from a translational Sustained Attention Task. Schizophr Res. 2013;144:136–141. doi: 10.1016/j.schres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Diano S, Horvath B, Urbanski HF, Sotonyi P, Horvath TL. Fasting activates the nonhuman primate hypocretin (orexin) system and its postsynaptic targets. Endocrinology. 2003;144:3774–3778. doi: 10.1210/en.2003-0274. [DOI] [PubMed] [Google Scholar]
- Dicken MS, Hughes AR, Hentges ST. Gad1 mRNA as a reliable indicator of altered GABA release from orexigenic neurons in the hypothalamus. Eur J Neurosci. 2015;42:2644–2653. doi: 10.1111/ejn.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicken MS, Tooker RE, Hentges ST. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J Neurosci. 2012;32:4042–4048. doi: 10.1523/JNEUROSCI.6032-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do JP, Xu M, Lee SH, Chang WC, Zhang S, Chung S, Yung TJ, Fan JL, Miyamichi K, Luo L, Dan Y. Cell type-specific long-range connections of basal forebrain circuit. Elife. 2016;5 doi: 10.7554/eLife.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KJ, Sawchuk MA, Hochman S. Phenotypic diversity and expression of GABAergic inhibitory interneurons during postnatal development in lumbar spinal cord of glutamic acid decarboxylase 67-green fluorescent protein mice. Neuroscience. 2009;163:909–919. doi: 10.1016/j.neuroscience.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, Saper CB, Elmquist JK. Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol. 2001;432:1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fan J, Zeng H, Olson DP, Huber KM, Gibson JR, Takahashi JS. Vasoactive intestinal polypeptide (VIP)-expressing neurons in the suprachiasmatic nucleus provide sparse GABAergic outputs to local neurons with circadian regulation occurring distal to the opening of postsynaptic GABAA ionotropic receptors. J Neurosci. 2015;35:1905–1920. doi: 10.1523/JNEUROSCI.2661-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq U, Kumar JR, Rajkumar R, Dawe GS. Electrical microstimulation of the nucleus incertus induces forward locomotion and rotation in rats. Physiol Behav. 2016;160:50–58. doi: 10.1016/j.physbeh.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Faust TW, Assous M, Tepper JM, Koos T. Neostriatal GABAergic interneurons mediate cholinergic inhibition of spiny projection neurons. J Neurosci. 2016;36:9505–9511. doi: 10.1523/JNEUROSCI.0466-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Levine MS. Transmitter cosynthesis by corticopetal basal forebrain neurons. Brain Res. 1989;491:163–168. doi: 10.1016/0006-8993(89)90099-1. [DOI] [PubMed] [Google Scholar]
- Foley P, Hughes PD, Bradford HF, Ghatei MA, Khandanian N, Bloom SR. The presence of neuropeptides in GABAergic and cholinergic rat cerebrocortical synaptosome sub-populations. Neuropeptides. 1992;23:67–72. doi: 10.1016/0143-4179(92)90080-g. [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, Martins AR, Zaika N, Bernstein H, Wachs M, Levis PA, Polley DB, Merzenich MM, Schreiner CE. Long-term modification of cortical synapses improves sensory perception. Nat Neurosci. 2013;16:79–88. doi: 10.1038/nn.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Fu LY, van den Pol AN. GABA excitation in mouse hilar neuropeptide Y neurons. J Physiol. 2007;579:445–464. doi: 10.1113/jphysiol.2002.019356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T, Jefferson SJ, Hooper A, Yee PH, Maguire J, Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann F, Justus D, Sosulina L, Kaneko H, Beutel T, Friedrichs D, Schoch S, Schwarz MK, Fuhrmann M, Remy S. Locomotion, theta oscillations, and the speed-correlated firing of hippocampal neurons are controlled by a medial septal glutamatergic circuit. Neuron. 2015;86:1253–1264. doi: 10.1016/j.neuron.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Goto M, Swanson LW, Canteras NS. Connections of the nucleus incertus. J Comp Neurol. 2001;438:86–122. doi: 10.1002/cne.1303. [DOI] [PubMed] [Google Scholar]
- Gracia-Llanes FJ, Crespo C, Blasco-Ibanez JM, Marques-Mari AI, Martinez-Guijarro FJ. VIP-containing deep short-axon cells of the olfactory bulb innervate interneurons different from granule cells. Eur J Neurosci. 2003;18:1751–1763. doi: 10.1046/j.1460-9568.2003.02895.x. [DOI] [PubMed] [Google Scholar]
- Granger AJ, Mulder N, Saunders A, Sabatini BL. Cotransmission of acetylcholine and GABA. Neuropharmacology. 2016;100:40–46. doi: 10.1016/j.neuropharm.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety. Oxford University Press; Oxford: 2000. [Google Scholar]
- Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience. 2006;143:1051–1064. doi: 10.1016/j.neuroscience.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71:155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Crespo C, Blasco-Ibanez JM, Gracia-Llanes FJ, Marques-Mari AI, Martinez-Guijarro FJ. Characterization of somatostatin- and cholecystokinin-immunoreactive periglomerular cells in the rat olfactory bulb. J Comp Neurol. 2005;489:467–479. doi: 10.1002/cne.20649. [DOI] [PubMed] [Google Scholar]
- Guzman MS, De Jaeger X, Raulic S, Souza IA, Li AX, Schmid S, Menon RS, Gainetdinov RR, Caron MG, Bartha R, Prado VF, Prado MA. Elimination of the vesicular acetylcholine transporter in the striatum reveals regulation of behaviour by cholinergic-glutamatergic co-transmission. PLoS Biol. 2011;9:e1001194. doi: 10.1371/journal.pbio.1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Hajos N, Acsady L, Freund TF. Target selectivity and neurochemical characteristics of VIP-immunoreactive interneurons in the rat dentate gyrus. Eur J Neurosci. 1996;8:1415–1431. doi: 10.1111/j.1460-9568.1996.tb01604.x. [DOI] [PubMed] [Google Scholar]
- Håkansson M, de Lecea L, Sutcliffe JG, Yanagisawa M, Meister B. Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol. 1999;11:653–663. doi: 10.1046/j.1365-2826.1999.00378.x. [DOI] [PubMed] [Google Scholar]
- Hangya B, Borhegyi Z, Szilágyi N, Freund TF, Varga V. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. J Neurosci. 2009;29:8094–8102. doi: 10.1523/JNEUROSCI.5665-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangya B, Ranade SP, Lorenc M, Kepecs A. Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell. 2015;162:1155–1168. doi: 10.1016/j.cell.2015.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Hara J, Yanagisawa M, Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci Lett. 2005;380:239–242. doi: 10.1016/j.neulet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Harrison TC, Pinto L, Brock JR, Dan Y. Calcium imaging of basal forebrain activity during innate and learned behaviors. Front Neural Circuits. 2016;10:36. doi: 10.3389/fncir.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harthoorn LF, Sane A, Nethe M, Van Heerikhuize JJ. Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell Mol Neurobiol. 2005;25:1209–1223. doi: 10.1007/s10571-005-8184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Krause MR, Mainville L, Cordova CA, Jones BE. Orexin neurons respond differentially to auditory cues associated with appetitive versus aversive outcomes. J Neurosci. 2016;36:1747–1757. doi: 10.1523/JNEUROSCI.3903-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Brischoux F, Mainville L, Stroh T, Jones BE. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience. 2010;169:1150–1157. doi: 10.1016/j.neuroscience.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges ST, Nishiyama M, Overstreet LS, Stenzel-Poore M, Williams JT, Low MJ. GABA release from proopiomelanocortin neurons. J Neurosci. 2004;24:1578–1583. doi: 10.1523/JNEUROSCI.3952-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A, Maguire J. Characterization of a novel subtype of hippocampal interneurons that express corticotropin-releasing hormone. Hippocampus. 2016;26:41–53. doi: 10.1002/hipo.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van Den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Lustig C, Sarter M. Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: converging electrochemical and fMRI evidence from rats and humans. J Neurosci. 2013;33:8742–8752. doi: 10.1523/JNEUROSCI.5809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Jin S, He X, Xu F, Hu J. Whole-brain monosynaptic afferent inputs to basal forebrain cholinergic system. Front Neuroanat. 2016;10:98. doi: 10.3389/fnana.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh CY, Danik M, Manseau F, Trudeau LE, Williams S. Chronic exposure to nerve growth factor increases acetylcholine and glutamate release from cholinergic neurons of the rat medial septum and diagonal band of Broca via mechanisms mediated by p75NTR. J Neurosci. 2008;28:1404–1409. doi: 10.1523/JNEUROSCI.4851-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Gumenchuk I, Kang B, Steger C, Lynn E, Molina NE, Eisenberg LM, Leonard CS. Orexin receptor activation generates gamma band input to cholinergic and serotonergic arousal system neurons and drives an intrinsic Ca2+-dependent resonance in LDT and PPT cholinergic neurons. Front. Neurol. 2015;6:120. doi: 10.3389/fneur.2015.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Gumenchuk I, Miyazaki K, Inoue T, Ross WN, Leonard CS. Hypocretin/orexin peptides alter spike encoding by serotonergic dorsal raphe neurons through two distinct mechanisms that increase the late afterhyperpolarization. J Neurosci. 2016;36:10097–10115. doi: 10.1523/JNEUROSCI.0635-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Ayzenshtat I, Karnani MM, Yuste R. VIP+ interneurons control neocortical activity across brain states. J Neurophysiol. 2016;115:3008–3017. doi: 10.1152/jn.01124.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic P, Leranth C. Dual role of substance P/GABA axons in cortical neurotransmission: synaptic triads on pyramidal cell spines and basket-like innervation of layer II–III calbindin interneurons in primate prefrontal cortex. Cereb Cortex. 1997;7:359–373. doi: 10.1093/cercor/7.4.359. [DOI] [PubMed] [Google Scholar]
- Janzso G, Valcz G, Thuma A, Szoke B, Lendvai Z, Abraham H, Kozicz T, Halasy K. Cocaine- and amphetamine-regulated transcript (CART) peptide-immunopositive neuronal elements in the lateral septum: rostrocaudal distribution in the male rat. Brain Res. 2010;1362:40–47. doi: 10.1016/j.brainres.2010.09.079. [DOI] [PubMed] [Google Scholar]
- Jarvie BC, Hentges ST. Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. J Comp Neurol. 2012;520:3863–3876. doi: 10.1002/cne.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, Friedman J, Burdakov D, Adamantidis AR. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Patterns of expression of neuropeptides in GABAergic nonprincipal neurons in the mouse hippocampus: Quantitative analysis with optical disector. J Comp Neurol. 2003;461:333–349. doi: 10.1002/cne.10700. [DOI] [PubMed] [Google Scholar]
- Kalogiannis M, Hsu E, Willie JT, Chemelli RM, Kisanuki YY, Yanagisawa M, Leonard CS. Cholinergic modulation of narcoleptic attacks in double orexin receptor knockout mice. PLoS One. 2011;6:e18697. doi: 10.1371/journal.pone.0018697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastman HE, Blasiak A, Walker L, Siwiec M, Krstew EV, Gundlach AL, Lawrence AJ. Nucleus incertus Orexin2 receptors mediate alcohol seeking in rats. Neuropharmacology. 2016;110:82–91. doi: 10.1016/j.neuropharm.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Niimi M, Yamamoto Y, Kawamura T, Morimoto-Ishizuka T, Sawada M, Takemori H, Yamatodani A. Histamine production by cultured microglial cells of the mouse. Neurosci Lett. 2001;305:181–184. doi: 10.1016/s0304-3940(01)01835-3. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998a;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998b;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsanuki YY, Chemelli RM, Tokita S, Willie JT, Sinton CM, Yanagisawa M. Behavioral and polysomnographic characterization of orexin-1 receptor and orexin-2 receptor double knockout mice. Sleep. 2001;24:A22. [Google Scholar]
- Kohlmeier KA, Tyler CJ, Kalogiannis M, Ishibashi M, Kristensen MP, Gumenchuk I, Chemelli RM, Kisanuki YY, Yanagisawa M, Leonard CS. Differential actions of orexin receptors in brainstem cholinergic and monoaminergic neurons revealed by receptor knockouts: implications for orexinergic signaling in arousal and narcolepsy. Front Neurosci. 2013;7:246. doi: 10.3389/fnins.2013.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono J, Konno K, Talukder AH, Fuse T, Abe M, Uchida K, Horio S, Sakimura K, Watanabe M, Itoi K. Distribution of corticotropin-releasing factor neurons in the mouse brain: a study using corticotropin-releasing factor-modified yellow fluorescent protein knock-in mouse. Brain Struct Funct. 2017;222:1705–1732. doi: 10.1007/s00429-016-1303-0. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Tauchi M, Dahl JL. Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res. 1988;70:605–617. doi: 10.1007/BF00247609. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, Hirai Y, Morishima M, Kawaguchi Y. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex. 2011;21:1803–1817. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- Kukko-Lukjanov TK, Panula P. Subcellular distribution of histamine, GABA and galanin in tuberomamillary neurons in vitro. J Chem Neuroanat. 2003;25:279–292. doi: 10.1016/s0891-0618(03)00043-7. [DOI] [PubMed] [Google Scholar]
- Kumar JR, Rajkumar R, Farooq U, Lee LC, Tan FC, Dawe GS. Evidence of D2 receptor expression in the nucleus incertus of the rat. Physiol Behav. 2015;151:525–534. doi: 10.1016/j.physbeh.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski H. Histamine in nervous tissue. J Physiol. 1943;102:32–41. doi: 10.1113/jphysiol.1943.sp004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther AJ, Clissold ML, Ma S, Kent S, Lowry CA, Gundlach AL, Hale MW. Anxiogenic drug administration and elevated plus-maze exposure in rats activate populations of relaxin-3 neurons in the nucleus incertus and serotonergic neurons in the dorsal raphe nucleus. Neuroscience. 2015;303:270–284. doi: 10.1016/j.neuroscience.2015.06.052. [DOI] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Kukkonen JP. Orexin/hypocretin receptor signalling: A functional perspective. Br J Pharmacol. 2014;171:294–313. doi: 10.1111/bph.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Lewin AE, Vicini S, Richardson J, Dretchen KL, Gillis RA, Sahibzada N. Optogenetic and pharmacological evidence that somatostatin-GABA neurons are important regulators of parasympathetic outflow to the stomach. J Physiol. 2016;594:2661–2679. doi: 10.1113/JP272069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, Jia C, Hu F, Zeng J, Guo Q, Fu L, Luo M. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun. 2016;7:10503. doi: 10.1038/ncomms10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Jouvet M. Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology. 1988;27:111–122. doi: 10.1016/0028-3908(88)90159-1. [DOI] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lin SC, Brown RE, Hussain Shuler MG, Petersen CC, Kepecs A. Optogenetic dissection of the basal forebrain neuromodulatory control of cortical activation, plasticity, and cognition. J Neurosci. 2015;35:13896–13903. doi: 10.1523/JNEUROSCI.2590-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Eriste E, Sutton S, Chen J, Roland B, Kuei C, Farmer N, Jornvall H, Sillard R, Lovenberg TW. Identification of relaxin-3/INSL7 as an endogenous ligand for the orphan G-protein coupled receptor GPCR135. J Biol Chem. 2003;278:50754–50764. doi: 10.1074/jbc.M308995200. [DOI] [PubMed] [Google Scholar]
- Lovett-Barron M, Kaifosh P, Kheirbek Ma, Danielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV, Losonczy A. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343:857–863. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubkemann R, Eberhardt J, Rohl FW, Janitzky K, Nullmeier S, Stork O, Schwegler H, Linke R. Identification and characterization of GABAergic projection neurons from ventral hippocampus to amygdala. Brain Sci. 2015;5:299–317. doi: 10.3390/brainsci5030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Allocca G, Ong-Palsson EK, Singleton CE, Hawkes D, McDougall SJ, Williams SJ, Bathgate RA, Gundlach AL. Nucleus incertus promotes cortical desynchronization and behavioral arousal. Brain Struct Funct. 2017a;222:515–537. doi: 10.1007/s00429-016-1230-0. [DOI] [PubMed] [Google Scholar]
- Ma S, Blasiak A, Olucha-Bordonau FE, Verberne AJ, Gundlach AL. Heterogeneous responses of nucleus incertus neurons to corticotrophin-releasing factor and coherent activity with hippocampal theta rhythm in the rat. J Physiol. 2013;591:3981–4001. doi: 10.1113/jphysiol.2013.254300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Bonaventure P, Ferraro T, Shen PJ, Burazin TCD, Bathgate RAD, Liu C, Tregear GW, Sutton SW, Gundlach AL. Relaxin-3 in GABA projection neurons of nucleus incertus suggests widespread influence on forebrain circuits via G-protein-coupled receptor-135 in the rat. Neuroscience. 2007;144:165–190. doi: 10.1016/j.neuroscience.2006.08.072. [DOI] [PubMed] [Google Scholar]
- Ma S, Gundlach AL. Ascending control of arousal and motivation: role of nucleus incertus and its peptide neuromodulators in behavioural responses to stress. J Neuroendocrinol. 2015;27:457–467. doi: 10.1111/jne.12259. [DOI] [PubMed] [Google Scholar]
- Ma S, Olucha-Bordonau FE, Hossain MA, Lin F, Kuei C, Liu C, Wade JD, Sutton SW, Nunez A, Gundlach AL. Modulation of hippocampal theta oscillations and spatial memory by relaxin-3 neurons of the nucleus incertus. Learn. Mem. 2009a;16:730–742. doi: 10.1101/lm.1438109. [DOI] [PubMed] [Google Scholar]
- Ma S, Sang Q, Lanciego JL, Gundlach AL. Localization of relaxin-3 in brain of Macaca fascicularis: identification of a nucleus incertus in primate. J Comp Neurol. 2009b;517:856–872. doi: 10.1002/cne.22197. [DOI] [PubMed] [Google Scholar]
- Ma S, Smith CM, Blasiak A, Gundlach AL. Distribution, physiology and pharmacology of relaxin-3/RXFP3 systems in brain. Br J Pharmacol. 2017b;174:1034–1048. doi: 10.1111/bph.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Mainville L, Jones BE. Evidence for glutamate, in addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain neurons projecting to the entorhinal cortex. Neuroscience. 2001;107:249–263. doi: 10.1016/s0306-4522(01)00302-5. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Zaric V. GABAergic somatostatin-immunoreactive neurons in the amygdala project to the entorhinal cortex. Neuroscience. 2015;290:227–242. doi: 10.1016/j.neuroscience.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander T, Hokfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A, Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci. 1986;6:3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and-2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31:6518–6526. doi: 10.1523/JNEUROSCI.6506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci U S A. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Watanabe Y, Tanaka M. Developmental expression and serotonergic regulation of relaxin 3/INSL7 in the nucleus incertus of rat brain. Regul Pept. 2008;145:54–59. doi: 10.1016/j.regpep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier M, Fallert M, Battacharya IC. The waking action of histamine. Experientia. 1967;23:21–22. doi: 10.1007/BF02142244. [DOI] [PubMed] [Google Scholar]
- Muscat L, Morin LP. Intergeniculate leaflet: contributions to photic and non-photic responsiveness of the hamster circadian system. Neuroscience. 2006;140:305–320. doi: 10.1016/j.neuroscience.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Nategh M, Nikseresht S, Khodagholi F, Motamedi F. Nucleus incertus inactivation impairs spatial learning and memory in rats. Physiol Behav. 2015;139:112–120. doi: 10.1016/j.physbeh.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Nicholson AN, Pascoe PA, Turner C, Ganellin CR, Greengrass PM, Casy AF, Mercer AD. Sedation and histamine H1-receptor antagonism: studies in man with the enantiomers of chlorpheniramine and dimethindene. Br J Pharmacol. 1991;104:270–276. doi: 10.1111/j.1476-5381.1991.tb12418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson Poulin A, Guerci A, El Mestikawy S, Semba K. Vesicular glutamate transporter 3 immunoreactivity is present in cholinergic basal forebrain neurons projecting to the basolateral amygdala in rat. J Comp Neurol. 2006;498:690–711. doi: 10.1002/cne.21081. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Smale L. Orexin fibers form appositions with Fos expressing neuropeptide-Y cells in the grass rat intergeniculate leaflet. Brain Res. 2005;1053:33–37. doi: 10.1016/j.brainres.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Nunez A, Cervera-Ferri A, Olucha-Bordonau F, Ruiz-Torner A, Teruel V. Nucleus incertus contribution to hippocampal theta rhythm generation. Eur J Neurosci. 2006;23:2731–2738. doi: 10.1111/j.1460-9568.2006.04797.x. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- Olucha-Bordonau FE, Teruel V, Barcia-Gonzalez J, Ruiz-Torner A, Valverde-Navarro AA, Martinez-Soriano F. Cytoarchitecture and efferent projections of the nucleus incertus of the rat. J Comp Neurol. 2003;464:62–97. doi: 10.1002/cne.10774. [DOI] [PubMed] [Google Scholar]
- Palus K, Chrobok L, Lewandowski MH. Orexins/hypocretins modulate the activity of NPY-positive and -negative neurons in the rat intergeniculate leaflet via OX1 and OX2 receptors. Neuroscience. 2015;300:370–380. doi: 10.1016/j.neuroscience.2015.05.039. [DOI] [PubMed] [Google Scholar]
- Pang KC, Jiao X, Sinha S, Beck KD, Servatius RJ. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: effects on proactive interference. Hippocampus. 2011;21:835–846. doi: 10.1002/hipo.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WL, Stark H, Thurmond RL, Haas HL. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol Rev. 2015;67:601–655. doi: 10.1124/pr.114.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci U S A. 1984;81:2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Forcelli PA, Luo R, Cashdan JM, Schulkin J, Valentino RJ, Vicini S. Stress increases GABAergic neurotransmission in CRF neurons of the central amygdala and bed nucleus stria terminalis. Neuropharmacology. 2016;107:239–250. doi: 10.1016/j.neuropharm.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrenoud Q, Rossier J, Geoffroy H, Vitalis T, Gallopin T. Diversity of GABAergic interneurons in layer VIa and VIb of mouse barrel cortex. Cereb Cortex. 2013;23:423–441. doi: 10.1093/cercor/bhs032. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi H-J, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci. 2013;16:1857–1863. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Sardella TC, Watanabe M, Todd AJ. Quantitative study of NPY-expressing GABAergic neurons and axons in rat spinal dorsal horn. J Comp Neurol. 2011;519:1007–1023. doi: 10.1002/cne.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Shehab SA, Watt C, Todd AJ. GABAergic neurons that contain neuropeptide Y selectively target cells with the neurokinin 1 receptor in laminae III and IV of the rat spinal cord. J Neurosci. 1999;19:2637–2646. doi: 10.1523/JNEUROSCI.19-07-02637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronneke A, Scheuer B, Wagener RJ, Mock M, Witte M, Staiger JF. Characterizing VIP neurons in the barrel cortex of VIPcre/tdTomato mice reveals layer-specific differences. Cereb Cortex. 2015;25:4854–4868. doi: 10.1093/cercor/bhv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinkert AW, Vimal V, Weil ZM, Reeke GN, Schiff ND, Banavar JR, Pfaff DW. Quantitative descriptions of generalized arousal, an elementary function of the vertebrate brain. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15617–15623. doi: 10.1073/pnas.1101894108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M. Habenula "cholinergic" neurons co-release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69:445–452. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Reti IM, Reddy R, Worley PF, Baraban JM. Selective expression of Narp, a secreted neuronal pentraxin, in orexin neurons. J Neurochem. 2002;82:1561–1565. doi: 10.1046/j.1471-4159.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- Riveros ME, Forray MI, Torrealba F. Infralimbic cortex activation and motivated arousal induce histamine release. Behav Pharmacol. 2015;26:338–344. doi: 10.1097/FBP.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E, Sherman MA. GABA-the quintessential neurotransmitter: electroneutrality, fidelity, specificity, and a model for the ligand binding site of GABAA receptors. Neurochem Res. 1993;18:365–376. doi: 10.1007/BF00967239. [DOI] [PubMed] [Google Scholar]
- Robinson J, Manseau F, Ducharme G, Amilhon B, Vigneault E, El Mestikawy S, Williams S. Optogenetic activation of septal glutamatergic neurons drive hippocampal theta rhythms. J Neurosci. 2016;36:3016–3023. doi: 10.1523/JNEUROSCI.2141-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha SM, Pires J, Esteves M, Graça B, Bernardino L. Histamine: a new immunomodulatory player in the neuron-glia crosstalk. Front Cell Neurosci. 2014;8:120. doi: 10.3389/fncel.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Stewart AL, Janke KL, Gielow MR, Kostek JA, Savage LM, Servatius RJ, Pang KC. Medial septum-diagonal band of Broca (MSDB) GABAergic regulation of hippocampal acetylcholine efflux is dependent on cognitive demands. J Neurosci. 2014;34:506–514. doi: 10.1523/JNEUROSCI.2352-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Alpar A, Hokfelt T, Harkany T. Molecular diversity of corticotropin-releasing hormone mRNA-containing neurons in the hypothalamus. J Endocrinol. 2017;232:R161–R172. doi: 10.1530/JOE-16-0256. [DOI] [PubMed] [Google Scholar]
- Rondini TA, Donato J, Jr, Rodrigues Bde C, Bittencourt JC, Elias CF. Chemical identity and connections of medial preoptic area neurons expressing melanin-concentrating hormone during lactation. J Chem Neuroanat. 2010;39:51–62. doi: 10.1016/j.jchemneu.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Zhang S, Wang HL, Hoffman AF, Lupica CR, Morales M. Single rodent mesohabenular axons release glutamate and GABA. Nat Neurosci. 2014;17:1543–1551. doi: 10.1038/nn.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- Sakai K, Takahashi K, Anaclet C, Lin JS. Sleep-waking discharge of ventral tuberomammillary neurons in wild-type and histidine decarboxylase knock-out mice. Front Behav Neurosci. 2010;4:53. doi: 10.3389/fnbeh.2010.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J Comp Neurol. 1984;222:313–342. doi: 10.1002/cne.902220302. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Howe WM, Gritton H, Berry AS. Deterministic functions of cortical acetylcholine. Eur J Neurosci. 2014;39:1912–1920. doi: 10.1111/ejn.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat Rev Neurosci. 2009;10:383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Granger AJ, Sabatini BL. Corelease of acetylcholine and GABA from cholinergic forebrain neurons. eLife. 2015a;4 doi: 10.7554/eLife.06412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Oldenburg Ia, Berezovskii VK, Johnson Ca, Kingery ND, Elliott HL, Xie T, Gerfen CR, Sabatini BL. A direct GABAergic output from the basal ganglia to frontal cortex. Nature. 2015b;521:85–89. doi: 10.1038/nature14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöne C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep. 2014;7:697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöne C, Burdakov D. Orexin/hypocretin and organizing principles for a diversity of wake-promoting neurons in the brain. Curr Top Behav Neurosci. 2016 doi: 10.1007/7854_2016_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöne C, Cao ZF, Apergis-Schoute J, Adamantidis A, Sakurai T, Burdakov D. Optogenetic probing of fast glutamatergic transmission from hypocretin/orexin to histamine neurons in situ. J Neurosci. 2012;32:12437–12443. doi: 10.1523/JNEUROSCI.0706-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears RM, Fink AE, Wigestrand MB, Farb CR, de Lecea L, Ledoux JE. Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc Natl Acad Sci U S A. 2013;110:20260–20265. doi: 10.1073/pnas.1320325110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Piriz J, Malinow R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. 2014;345:1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science (New York, N.Y.) 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- Smith CM, Shen PJ, Banerjee A, Bonaventure P, Ma S, Bathgate RA, Sutton SW, Gundlach AL. Distribution of relaxin-3 and RXFP3 within arousal, stress, affective, and cognitive circuits of mouse brain. J Comp Neurol. 2010;518:4016–4045. doi: 10.1002/cne.22442. [DOI] [PubMed] [Google Scholar]
- Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, Fukaya M, Shigemoto R, Watanabe M, Somogyi P. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19:552–569. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- Sosulina L, Graebenitz S, Pape HC. GABAergic interneurons in the mouse lateral amygdala: a classification study. J Neurophysiol. 2010;104:617–626. doi: 10.1152/jn.00207.2010. [DOI] [PubMed] [Google Scholar]
- Sugihara H, Chen N, Sur M. Cell-specific modulation of plasticity and cortical state by cholinergic inputs to the visual cortex. J Physiol Paris. 2016;110:37–43. doi: 10.1016/j.jphysparis.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Fukami T, Li T, Desai M, Ross MG. Preferential development of neuropeptide Y/GABA circuit in hypothalamic arcuate nucleus in postnatal rats. Brain Res. 2016;1635:27–40. doi: 10.1016/j.brainres.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Sundvik M, Panula P. Organization of the histaminergic system in adult zebrafish (Danio rerio) brain: neuron number, location, and cotransmitters. J Comp Neurol. 2012;520:3827–3845. doi: 10.1002/cne.23126. [DOI] [PubMed] [Google Scholar]
- Takagi H, Morishima Y, Matsuyama T, Hayashi H, Watanabe T, Wada H. Histaminergic axons in the neostriatum and cerebral cortex of the rat: a correlated light and electron microscopic immunocytochemical study using histidine decarboxylase as a marker. Brain Res. 1986;364:114–123. doi: 10.1016/0006-8993(86)90992-3. [DOI] [PubMed] [Google Scholar]
- Takatsuji K, Tohyama M. Geniculo-geniculate projection of enkephalin and neuropeptide Y containing neurons in the intergeniculate leaflet of the thalamus in the rat. J Chem Neuroanat. 1989;2:19–27. [PubMed] [Google Scholar]
- Takeda N, Inagaki S, Shiosaka S, Taguchi Y, Oertel WH, Tohyama M, Watanabe T, Wada H. Immunohistochemical evidence for the coexistence of histidine decarboxylase-like and glutamate decarboxylase-like immunoreactivities in nerve cells of the magnocellular nucleus of the posterior hypothalamus of rats. Proc Natl Acad Sci U S A. 1984;81:7647–7650. doi: 10.1073/pnas.81.23.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CO, Bullock D. Neuropeptide co-release with GABA may explain functional non-monotonic uncertainty responses in dopamine neurons. Neurosci Lett. 2008;430:218–223. doi: 10.1016/j.neulet.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Iijima N, Miyamoto Y, Fukusumi S, Itoh Y, Ozawa H, Ibata Y. Neurons expressing relaxin 3/INSL 7 in the nucleus incertus respond to stress. Eur J Neurosci. 2005;21:1659–1670. doi: 10.1111/j.1460-9568.2005.03980.x. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiong SY, Polgar E, van Kralingen JC, Watanabe M, Todd AJ. Galanin-immunoreactivity identifies a distinct population of inhibitory interneurons in laminae I–III of the rat spinal cord. Mol Pain. 2011;7:36. doi: 10.1186/1744-8069-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba F, Riveros ME, Contreras M, Valdes JL. Histamine and motivation. Front Syst Neurosci. 2012;6:51. doi: 10.3389/fnsys.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience. 2003;119:1033–1044. doi: 10.1016/s0306-4522(03)00238-0. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Granger AJ, Sabatini BL. Mechanisms and functions of GABA co-release. Nat Rev Neurosci. 2016;17:139–145. doi: 10.1038/nrn.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Oh WJ, Gu C, Sabatini BL. Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. Elife. 2014;3:e01936. doi: 10.7554/eLife.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier S, Chotard C, Traiffort E, Unmehopa U, Fisser B, Swaab DF, Schwartz JC. Co-localization of histamine with GABA but not with galanin in the human tuberomamillary nucleus. Brain Res. 2002;939:52–64. doi: 10.1016/s0006-8993(02)02546-5. [DOI] [PubMed] [Google Scholar]
- Urban-Ciecko J, Barth AL. Somatostatin-expressing neurons in cortical networks. Nat Rev Neurosci. 2016;17:401–409. doi: 10.1038/nrn.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaga CE, Borisovska M, Westbrook GL. Dual-transmitter neurons: functional implications of co-release and co-transmission. Curr Opin Neurobiol. 2014;29:25–32. doi: 10.1016/j.conb.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Hokfelt T, Skirboll LR, Wu JY. Hypothalamic gamma-aminobutyric acid neurons project to the neocortex. Science. 1983;220:1309–1311. doi: 10.1126/science.6857253. [DOI] [PubMed] [Google Scholar]
- Wada H, Inagaki N, Yamatodani A, Watanabe T. Is the histaminergic neuron system a regulatory center for whole-brain activity? Trends Neurosci. 1991;14:415–418. doi: 10.1016/0166-2236(91)90034-r. [DOI] [PubMed] [Google Scholar]
- Walker LC, Kastman HE, Koeleman JA, Smith CM, Perry CJ, Krstew EV, Gundlach AL, Lawrence AJ. Nucleus incertus corticotrophin-releasing factor 1 receptor signalling regulates alcohol seeking in rats. Addict Biol. 2016 doi: 10.1111/adb.12426. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Taguchi Y, Hayashi H, Tanaka J, Shiosaka S, Tohyama M, Kubota H, Terano Y, Wada H. Evidence for the presence of a histaminergic neuron system in the rat brain: an immunohistochemical analysis. Neurosci Lett. 1983;39:249–254. doi: 10.1016/0304-3940(83)90308-7. [DOI] [PubMed] [Google Scholar]
- Weber F, Dan Y. Circuit-based interrogation of sleep control. Nature. 2016;538:51–59. doi: 10.1038/nature19773. [DOI] [PubMed] [Google Scholar]
- Whissell PD, Cajanding JD, Fogel N, Kim JC. Comparative density of CCK-and PV-GABA cells within the cortex and hippocampus. Front Neuroanat. 2015;9:124. doi: 10.3389/fnana.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson TN, Speed TP, Tregear GW, Bathgate RA. Evolution of the relaxin-like peptide family. BMC Evol. Biol. 2005;5:14. doi: 10.1186/1471-2148-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RH, Chee MJ, Kroeger D, Ferrari LL, Maratos-Flier E, Scammell TE, Arrigoni E. Optogenetic-mediated release of histamine reveals distal and autoregulatory mechanisms for controlling arousal. J Neurosci. 2014;34:6023–6029. doi: 10.1523/JNEUROSCI.4838-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G, Hrabovszky E, Lechan RM. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J Comp Neurol. 2013;521:3287–3302. doi: 10.1002/cne.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk AI, Xu C, Song I, Doronin M, Wu YW, Walker MC, Semyanov A. Tonic GABAA conductance decreases membrane time constant and increases EPSP-spike precision in hippocampal pyramidal neurons. Front Neural Circuits. 2013;7:205. doi: 10.3389/fncir.2013.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J, Verma D, Lach G, Bonaventure P, Herzog H, Sperk G, Tasan RO. Structure and function of the amygdaloid NPY system: NPY Y2 receptors regulate excitatory and inhibitory synaptic transmission in the centromedial amygdala. Brain Struct Funct. 2016;221:3373–3391. doi: 10.1007/s00429-015-1107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Williams J, Nathans J. Complete morphologies of basal forebrain cholinergic neurons in the mouse. eLife. 2014;2014:1–17. doi: 10.7554/eLife.02444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur J Pharmacol. 2011;660:21–27. doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, España RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Sun QQ. GABAergic inhibitory interneurons in the posterior piriform cortex of the GAD67-GFP mouse. Cereb Cortex. 2009;19:3011–3029. doi: 10.1093/cercor/bhp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Yu X, Ye Z, Houston CM, Zecharia AY, Ma Y, Zhang Z, Uygun DS, Parker S, Vyssotski AL, Yustos R, Franks NP, Brickley SG, Wisden W. Wakefulness is governed by GABA and histamine cotransmission. Neuron. 2015;87:164–178. doi: 10.1016/j.neuron.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb Cortex. 2015;25:118–137. doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Pol AVD, Gyengesi E. The Basal Forebrain Cholinergic Projection System in Mice. In: Watson C, Paxinos G, Puelles L, editors. The mouse nervous system. 1. Elsevier; Amsterdam: 2012. pp. 684–718. [Google Scholar]
- Zecharia AY, Yu X, Gotz T, Ye Z, Carr DR, Wulff P, Bettler B, Vyssotski AL, Brickley SG, Franks NP, Wisden W. GABAergic inhibition of histaminergic neurons regulates active waking but not the sleep-wake switch or propofol-induced loss of consciousness. J Neurosci. 2012;32:13062–13075. doi: 10.1523/JNEUROSCI.2931-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]