Abstract
Background
Traffic-related air pollution (TRAP) exposure has been linked to type 2 diabetes and metabolic dysfunction in humans. Animal studies suggest that air pollutants may alter the composition of the gut microbiota, which may negatively impact metabolic health through changes in the composition and/or function of the gut microbiome.
Objectives
The primary aim of this study was to determine whether elevated TRAP exposure was correlated with gut bacterial taxa in overweight and obese adolescents from the Meta-AIR (Metabolic and Asthma Incidence Research) study. The secondary aim was to examine whether gut microbial taxa correlated with TRAP was also correlated with risk factors for type 2 diabetes (e.g., fasting glucose levels). We additionally explored whether correlations between TRAP and these metabolic risk factors could be explained by the relative abundance of these taxa.
Methods
Participants (17–19 years; n=43) were enrolled between 2014–2016 from Southern California. The CALINE4 line dispersion model was used to model prior year residential concentrations of nitrogen oxides (NOx) as a marker of traffic emissions. The relative abundance of fecal microbiota was characterized by 16S rRNA sequencing and spearman partial correlations were examined after adjusting for body fat percent.
Results
Freeway TRAP was correlated with decreased Bacteroidaceae (r=−0.48; p=0.001) and increased Coriobacteriaceae (r=0.48; p<0.001). These same taxa were correlated with fasting glucose levels, including Bacteroidaceae (r=−0.34; p=0.04) and Coriobacteriaceae (r=0.41; p<0.01). Further, freeway TRAP was positively correlated fasting glucose (r=0.45; p=0.004) and Bacteroidaceae and Coriobacteriaceae explained 24% and 29% of the correlation between TRAP and fasting glucose levels.
Conclusions
Increased TRAP exposure was correlated with gut microbial taxa and fasting glucose levels. Gut microbial taxa that were correlated with TRAP partially explained the correlation between TRAP and fasting glucose levels. These results suggest that exposure to air pollutants may negatively impact metabolic health via alterations in the gut microbiota.
Keywords: Traffic-related air pollution exposure, gut microbiome
Introduction
While type 2 diabetes is strongly linked with traditional risk factors such as poor diet and low physical activity and socio-economic status, recent reports suggest that exposures to traffic-related air pollution (TRAP) may also play an important and independent role in disease development beginning in childhood (Jerrett et al., 2014; Thiering et al., 2016; Toledo-Corral et al., 2016). The mechanisms underlying these correlations remain uncertain, yet one hypothesis involves exposure-induced effects of TRAP on the gut microbiota (Beamish et al., 2011). Studies have shown that the composition of the gut microbiota is altered during obesity and type 2 diabetes (Ley et al., 2006; Ross et al., 2015; Turnbaugh et al., 2006) and that each disease is transmissible through fecal transplants (Ridaura et al., 2013; Vrieze et al., 2012). Despite this emerging evidence, no published studies in humans have examined whether air pollution exposure alters the composition and/or function of the gut microbiome as a possible mechanism by which increased exposure to TRAP increases type 2 diabetes risk.
Increased long-term exposure to air pollutants, including nitrogen oxides (NOx), nitrogen dioxide (NO2) and particulate matter (PM10 and PM2.5) have been shown to be associated with greater risk for type 2 diabetes (Alderete et al., 2017; Thiering et al., 2013; Weinmayr et al., 2015). Recent studies support a link between air pollution exposure and the gut microbiota, where ultrafine particles may reach the intestine through inhalation and diffusion from the lungs into systemic circulation or ingestion of inhaled particles following mucociliary clearance from the airways (Riva et al., 2017). Similar to TRAP, cigarette smoke represents a complex mixture of inhaled combustion products that may alter the composition and/or function of the gut microbiome. In humans, smoking cessation has been shown to increase gut microbial diversity (Biedermann et al., 2013) and mice exposed to chronic cigarette smoke had significant shifts in gut bacteria and altered intestinal mucins that support gut barrier integrity (Allais et al., 2016). Studies examining correlations between air pollution exposure and intestinal disease further support effects of air pollutants on the gut (Beamish et al., 2011; Kaplan et al., 2010; 2012). Rodent studies also indicate that ingestion of airborne sources of PM alter the gut microbiome and increase intestinal inflammation (Dybdahl, 2003; Kish et al., 2013; Mutlu et al., 2011). Lastly, recent work found that increased concentrations of airborne PM altered bacterial biofilms as well as bacterial colonization in mice (Hussey et al., 2017). Collectively, this work suggests several possible mechanisms in which increased environmental concentrations of air pollutants may impact the human microbiome.
While emerging evidence indicates that air pollutants may impact the gut, it remains unknown whether these environmental stressors are associated with the composition of the human gut microbiota, which has the potential to impact metabolic health. The primary objective of this study was to determine whether residential based estimates of TRAP exposure were associated with the relative abundance of gut bacterial taxa in a subset of adolescents from the Meta-AIR (Metabolic and Asthma Incidence Research) study. We hypothesized that higher TRAP exposure would be associated with the relative abundance of bacterial lineages using 16S rRNA sequencing of stool. As a secondary aim, we investigated whether specific gut microbial taxa associated with air pollutants were also associated with risk factors for type 2 diabetes (i.e., fasting glucose, fasting insulin, insulin resistance, and 1- and 2-hour glucose). We additionally explored whether correlations between TRAP and these metabolic risk factors could be partially explained by the relative abundance of these gut bacterial taxa.
Research Design and Methods
Participants
The 43 participants included in this pilot study were recruited from the ongoing Meta-AIR study at the University of Southern California (USC) between 2014–2016. The Meta-AIR Study is recruiting 200 participants from Cohort E of the Children’s Health Study (CHS), which was a large school-based cohort study that began in 2002–2003. The primary aim of the Meta-AIR study is to examine the effects of TRAP exposures on metabolic health and adiposity during adolescence. As such, Meta-AIR participants are being recruited to represent the extremes of TRAP exposures in Southern California CHS communities using probability weighted sampling from their last visit (October 2011–June 2012). Based on this recruitment strategy, participants represent a wide range of modeled exposures to air pollutants. For example, mean freeway NOx exposure was 6.4 parts per billion (ppb) (range: 0.01 to 26.5 ppb) in this pilot study, which was comparable to that currently observed in the ongoing Meta-Air study (mean: 6.1 ppb and range: 0.001 to 28.6 ppb). Inclusion criteria for Meta-AIR included participation in Cohort E of the CHS, age- and sex-specific body mass index (BMI) of ≥ 85th percentile at the date of their last visit, and the absence type 1 or type 2 diabetes. Smokers were not excluded in this study but smoking was not highly prevalent and only four participants (9%) reported ever smoking in the previous week. Participants were excluded if they were using a medication or diagnosed with a condition known to influence insulin and/or glucose metabolism or body composition. For the current pilot study, participants were further excluded if they reported using antibiotics in the two weeks prior to their clinical visit. Before testing, informed written consent/assent was obtained from the parents/participant. The USC Institutional Review Board approved these studies.
Clinical Assessments
Participants from the Meta-AIR study are being extensively phenotyped for adiposity and metabolic outcomes during a clinical visit at the USC Diabetes and Obesity Research Institute. Briefly, clinical measures include height, weight, waist circumference, blood pressure, heart rate, body composition via dual-energy X-ray absorptiometry (DEXA) scans, and an oral glucose tolerance test (OGTT) after a 12-hour fast. For the current analysis, metabolic outcomes of interest included fasting glucose, fasting insulin, and the homeostatic model assessment of insulin resistance (HOMA-IR). Fasting insulin was assayed by Human Insulin ELISA Kit (EMD Millipore) and glucose was assayed by hexokinase-mediated reaction assay with Roche Covas C501. Insulin resistance was calculated by HOMA-IR [fasting glucose (mg/dL) * fasting insulin (μU/mL)/405]. Dietary information was available in 39 participants and was assessed using 24-hour diet recalls. Nutrition data were analyzed using the Nutrition Data System for Research (version 2014) developed at the University of Minnesota and provided average total energy and macronutrient intake.
Assessment of Exposure to Traffic-Related Pollutants
Residential exposure to traffic-modeled pollutants from local freeway and non-freeway sources was estimated using street-level residential addresses of participants. Street-level residential addresses of participants were standardized and geocoded at the parcel centroid (the likely center of a residential building). These parcel level and match quality codes were obtained using the Texas A&M Geocoder (http://geoservices.tamu.edu/Services/Geocode/). Addresses that did not match a parcel centroid were corrected based on the best available knowledge of the participant’s residence location. When participants moved (n=9) or had more than one address (n=13) in the year prior to their visit, exposures estimates were weighted by time spent at each residence (e.g., home, college).
The California Line Source Dispersion Model (CALINE4) was used to model annual-average residential TRAP exposure by estimating levels of nitrogen oxides (NOx) from motor vehicle activity on roads within 5 km of each residence in the year prior to each clinical visit. CALINE4 takes into account local meteorology (e.g., wind speed, direction, mixing heights), traffic counts, road geometry, and emission factors (Benson, 1992). The CALINE4 model provided estimates of the annual-average traffic impact from each road class, including freeways or highways, major roadways, and minor roadways based on the Streetmap Premium database (ArcGIS 10.1, Environmental Systems Research Institute Inc., Redlands, CA). Total TRAP was defined as the sum of freeway and non-freeway TRAP, and non-freeway TRAP was defined as the sum of major and minor roadways. These TRAP measures modeled NOx exposures as a marker for the mixture of traffic pollutants from each of these roadways since traffic pollutants are a complex mixture of particles and gases, including NOx, carbon monoxide, particulate matter, organic compounds, elemental carbon, and polycyclic aromatic hydrocarbons (Fujita et al., 2007) that vary by roadways and traffic volumes (Clements et al., 2009; Zhu et al., 2009).
Gut Microbiota
One fecal sample per participant was collected using commercial collection kits (Second Genome, San Francisco, CA) and stored at −80°C within an average of 2 days after collection until analysis. The relative abundance of bacterial taxa was determined using 16S rRNA amplicon sequencing (Second Genome, San Francisco, CA). Briefly, nucleic acid isolation was performed with the MoBio PowerMag® Microbiome kit (Carlsbad, CA) according to manufacturer’s guidelines and optimized for high-throughput processing. All samples were quantified via the Qubit® Quant-iT dsDNA High Sensitivity Kit (Invitrogen, Life Technologies, Grand Island, NY) to ensure that they met minimum concentration and mass of DNA. To enrich the sample for bacterial 16S V4 rDNA region, DNA was amplified utilizing V4 fusion primers as described by Caporaso et al. (Caporaso et al., 2011). Samples that met the post-PCR quantification minimum and were advanced for pooling and sequencing on the Illumina Miseq v3 sequencer platform. The 16S rDNA sequence reads were quality filtered, clustered into operational taxonomic units (OTUs) with a shared 97% identity by UPARSE (de novo OTU clustering), and a representative consensus sequence per de novo OTU was aligned against the Greengenes reference database (DeSantis et al., 2006) and assigned taxonomy to determine community profiles. The UPARSE clustering algorithm comprises a chimera filtering and discards likely chimeric OTUs. All non-strain sequences that passed the quality filtering were mapped to the representative consensus sequences to generate an abundance table for de novo OTUs. Representative OTU sequences were assigned taxonomic classification via Mothur’s Bayesian classifier, trained against the Greengenes reference database of 16S rRNA gene sequences clustered at 99%. The reprehensive sequences for these OTUs can be found in Supplemental File 1.
Statistical Analysis
Abundance of microbial sequence read counts were normalized for each taxonomic group in order to account for varying sequencing depths across samples using the following equation: ([samplei counts/total counts in samplei] * average number of counts across samples). Evenness, richness (number of unique taxa), and Shannon diversity (measures of richness and evenness) were calculated using the vegan package in R statistical program. Taxa were retained if they were present in at least 40% of samples. This approach was used to reduce multiple testing but may have limited our ability to examine rare taxa. One outlier was removed due an estimated total TRAP exposure that was greater than five standard deviations above the mean. Additionally, one outlier was removed when examining metabolic outcomes due to a fasting insulin level that was greater than four standard deviations above the mean. For the primary aim, non-parametric Spearman partial correlations were used to assess correlations between TRAP exposures and the normalized abundance of gut taxa after adjusting for body fat percent. In a secondary analysis, partial correlations were further examined after adjusting for diet, including energy and macronutrient intake. Exact p-values were used for the Spearman partial correlation with 2,000 permutations. Correction for multiple testing was performed using the false discovery rate (FDR) with the Benjamini-Hochberg procedure, with values of ≤0.10 considered statistically significant. However, due to the limited sample size in this pilot study, FDR values of ≤0.15 were further explored and reported. For the secondary aim, Spearman partial correlations were used to assess relationships between metabolic outcomes and 1) TRAP exposures and 2) gut bacterial taxa found to be associated with TRAP, adjusting for body fat percent. In addition, we determined the amount of the correlation between TRAP and metabolic outcomes that could be explained by the relative abundance of gut bacterial taxa. This was accomplished by assessing the proportional reduction in the correlation between TRAP and metabolic outcomes after adjusting for body fat percent and gut bacterial taxa that was found to be correlated with TRAP. All analyses were conducted in R statistical package version 3.3.3 and figures were produced using RStudio or Prism 7 (GraphPad Software, Inc.).
Results
This study included 43 participants from the Meta-AIR Study. General characteristics and the average levels of TRAP exposures are reported in Table 1. The mean age of participants was 18.8 years (range 17.7 – 20.2), 53% were male, and approximately 65% of the adolescents self-identified as Hispanic. On average, participants in this study were overweight or obese with an average BMI of 30.5 kg/m2 (range 20.7 – 47.4). Overall, the gut bacterial community composition was dominated by the phylum Firmicutes, Bacteroidetes and Actinobacteria. The most abundant families from these phyla were Bacteroidaceae, Lachnospiraceae and Ruminococcaceae (Supplemental Figure 1).
Table 1.
Baseline Characteristics and Prior Year Residential Traffic-Related Air Pollutant (TRAP) Concentrations in Adolescents from the Meta-AIR Study
| Mean ± SD | |
|---|---|
| General Characteristics | n=43 |
|
| |
| Age (years) | 18.8 ± 0.5 |
| Sex (Males/Females, %) | 25/18, 58% |
| Ethnicity (Hispanic/Non-Hispanic, %) | 28/15, 65% |
|
| |
| Metabolic Indices | |
|
| |
| Fasting Glucose (mg/dL)* | 90.5 ± 7.7 |
| 1-Hour Glucose (mg/dL)* | 145.9 ± 28.6 |
| 2-Hour Glucose (mg/dL)* | 119.3 ± 21.7 |
| Fasting Insulin (μU/mL)* | 6.0 ± 3.2 |
| HOMA-IR* | 1.4 ± 0.8 |
|
| |
| Adiposity | |
|
| |
| Body Mass Index (kg/m2) | 30.5 ± 5.9 |
| Normal Weight/Overweight and Obese, % | 8/35, 18.6% |
| Total Fat Mass (kg) | 29.2 ± 11.9 |
| Total Lean Mass (kg) | 53.6 ± 14.9 |
| Body Fat Percent (%) | 33.1 ± 9.3 |
|
| |
| Energy Intake and Macronutrients | |
|
| |
| Energy Intake (Kcal)† | 1,987.6 ± 606.3 |
| Carbohydrates (g)† | 247.6 ± 72.8 |
| Fat (g)† | 79.4 ± 35.9 |
| Protein (g)† | 76.6 ± 27.6 |
| Fiber (g)† | 16.5 ± 7.2 |
|
| |
| Prior Year Average Exposure to TRAP** | |
|
| |
| Total TRAP (ppb) | 8.4 ± 6.5 |
| Freeway TRAP (ppb) | 6.4 ± 6.1 |
| Non-Freeway TRAP (ppb) | 1.9 ± 1.4 |
Baseline characteristics and prior one-year average exposure to TRAP (i.e., total, freeway, and non-freeway) from the Meta-AIR study visit.
Average prior year TRAP exposure was based on residential addresses where nitrogen oxides (NOx; ppb) were used as a marker of traffic emissions. Data are reported as mean with standard deviation (SD). Sample size is indicated as
40 and
39.
The Composition of the Gut Microbiota was Associated with TRAP Exposures and Glucose Levels
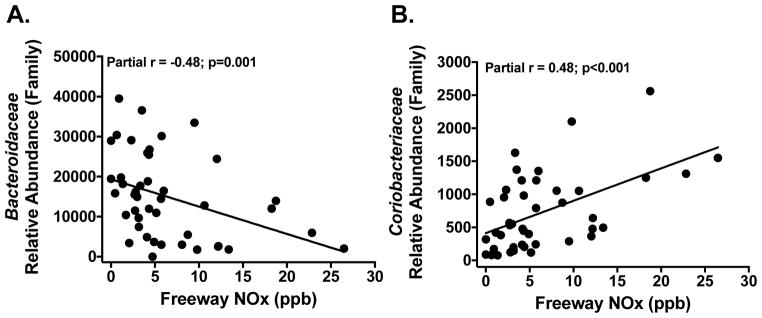
Higher total and freeway TRAP exposures were significantly correlated with the relative abundance of gut bacterial taxa after adjusting for body fat percent (Table 2 and Figure 1) and these correlations were consistently stronger for freeway compared to total TRAP (Table 2). Further, the strength of these correlations remained consistent after adjusting for diet, including energy and macronutrient intake (Supplement Table 1). At the phylum level, freeway TRAP was positively associated with the relative abundance of Actinobacteria (r=0.30; p=0.02; FDR=0.14) and negatively correlated with Proteobacteria (r=−0.29; p=0.04; FDR=0.14). Actinobacteria was primarily composed of the class Coriobacteriia that was positively correlated with freeway TRAP (r=0.48; p=0.001; FDR=0.006). Higher freeway TRAP was also associated with the relative abundance of other bacterial families, including Bacteroidaceae (r=−0.48; p=0.001; FDR=0.02), Tissierellaceae (r=−0.45; p<0.001; FDR=0.01), Corynebacteriaceae (r=−0.37; p=0.007; FDR=0.08), and Coriobacteriaceae (r=0.48; p<0.001; FDR=0.01). Bacteroidaceae was only moderately correlated with Tissierellaceae (r=0.4; p=0.01) and Coriobacteriaceae (r=−0.33; p=0.03), suggesting that the observed correlations between TRAP and these bacterial families represented independent findings (Supplemental Figure 2). Additionally, within these bacterial families, there were similar correlations at the level of the genus with freeway TRAP, including Bacteroides (r=−0.48; p=0.002; FDR=0.08), Peptoniphilus (r=−0.45; p=0.001; FDR=0.05), Corynebacterium (r=−0.37; p=0.007; FDR=0.23), and Collinsella (r=0.51; p=0.001; FDR=0.05), respectively.
Table 2.
Correlations Between Prior Year Traffic-Related Air Pollutant (TRAP) Exposure with the Gut Microbiota at the Level of the Family in Adolescents from the Meta-AIR Study
| Total TRAP | Freeway TRAP | Non-Freeway TRAP | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Partial r | p-value | FDR | Partial r | p-value | FDR | Partial r | p-value | FDR | |
| Bacteroidaceae | −0.49 | <0.0001 | <0.0001* | −0.48 | 0.001 | 0.02* | −0.33 | 0.02 | 0.34 |
| Tissierellaceae | −0.41 | 0.002 | 0.06* | −0.45 | <0.001 | 0.01* | −0.09 | 0.28 | 0.44 |
| Coriobacteriaceae | 0.41 | 0.005 | 0.09* | 0.48 | <0.001 | 0.01* | 0.27 | 0.04 | 0.34 |
| Corynebacteriaceae | −0.35 | 0.01 | 0.17 | −0.37 | 0.007 | 0.08* | −0.09 | 0.29 | 0.44 |
Partial Spearman correlations are shown after adjusting for body fat percent. P-values in bold denote significant for exact p-values at <0.05.
FDR p-values significant and a ≤0.10 false discover rate.
Figure 1. Prior Year Exposure to Traffic-Related Air Pollutants (TRAP) was Associated with the Relative Abundance of Bacteriodaceae and Coriobacteriaceae in Adolescents from the Meta-AIR Study.
A–B: Unadjusted plots between prior year freeway TRAP exposure and gut microbial taxa using partial Spearman correlations and exact p-values after adjustment for body fat percent. Prior year TRAP exposure was based on residential addresses where nitrogen oxides (NOx; ppb) were used as a marker of traffic emissions.
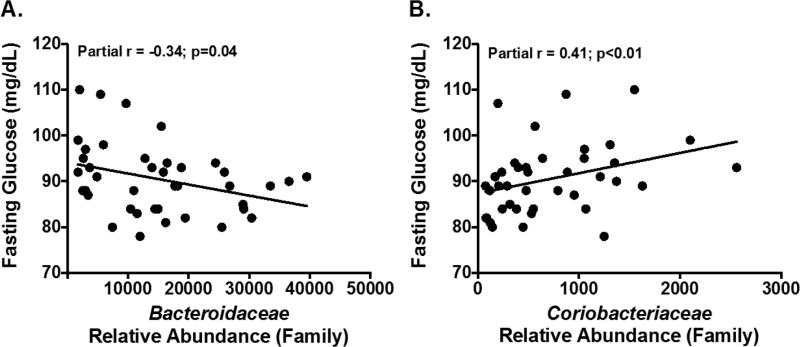
Among the adolescents included in this study, TRAP exposure was associated with impaired glucose homeostasis after adjusting for body fat percent. For example, fasting glucose levels were positively associated with freeway TRAP (r=0.45; p=0.004), which remained significant after additionally adjusting for energy intake (r=0.52; p=0.001). Fasting glucose levels were also significantly associated with two bacterial families that were associated with TRAP exposure, including Bacteroidaceae (r=−0.34; p=0.04) and Coriobacteriaceae (r=0.41; p<0.01) (Figure 2). The strength of the correlation between freeway TRAP and fasting glucose levels was attenuated after adjustment for Bacteroidaceae or Coriobacteriaceae (Table 3). Specifically, after adjusting for body fat percent, the relative abundance of Bacteroidaceae and Coriobacteriaceae explained 24% and 29% of the correlation between TRAP and fasting glucose levels. In regard to impaired glucose homeostasis, 2-hour glucose levels were also positively correlated with Coriobacteriaceae (r=0.32; p=0.047).
Figure 2. Gut Bacteria Associated with Prior Year Exposure to Traffic-Related Air Pollutants was also Associated with Fasting Glucose Levels in Adolescents from the Meta-AIR Study.
A–B: Unadjusted plots between gut microbial taxa associated with air pollutants using partial Spearman correlations after adjustment for body fat percent.
Table 3.
Impact of Gut Microbial Taxa on the Relationship Between Prior Year Exposure to Freeway Traffic-Related Air Pollutants (TRAP) and Fasting Glucose Levels in Adolescents from the Meta-AIR Study
| Variable Adjusted | Partial r | p-value | % Reduction Compared with no Adjustment for Taxa† |
|---|---|---|---|
| Body Fat Percent | 0.45 | 0.004 | –– |
| Body Fat Percent and Bacteroidaceae | 0.34 | 0.03 | 24% |
| Body Fat Percent and Coriobacteriaceae | 0.32 | 0.049 | 29% |
Partial Spearman correlations between freeway TRAP exposure and fasting glucose levels adjusted for body fat percent or body fat percent in conjunction with the relative abundance of bacterial taxa (i.e., Bacteroidaceae and Coriobacteriaceae).
Calculated as 100 X (partial correlation coefficient adjusted for body fat percent – partial correlation coefficient adjusted for body fat percent and bacterial taxa)/partial correlation coefficient adjusted for body fat percent.
Freeway TRAP was also positively associated with two unidentified families that belong to the phylum Firmicutes, class Clostridia, and order Clostridiales (r=0.42; p=0.006; FDR=0.08 and r=0.32; p=0.02; FDR=0.15). At the level of the OTU, 13 taxa were significantly associated with freeway TRAP (Supplemental Table 2). Overall, the correlations between total TRAP and the gut microbiota were weaker than those observed for freeway TRAP, suggesting that freeway-related exposures largely contributed to the observed correlations. Furthermore, non-freeway TRAP was not significantly associated with gut microbiota taxa after adjustment for multiple testing. Measures of diversity and richness were not associated with TRAP exposure. Also, the relative abundance of gut bacterial taxa was not associated with fasting insulin levels, HOMA-IR, or 1-hour glucose levels (Supplemental Table 3).
Discussion
The human gut microbiota has been linked with obesity, type 2 diabetes, and intestinal bowel disease (Ley et al., 2006; Morgan et al., 2012; Ross et al., 2015; Turnbaugh et al., 2006). Emerging evidence suggests that environmental factors, including TRAP, are also associated with these health outcomes (Jerrett et al., 2014; Salim et al., 2014; Thiering et al., 2016; Toledo-Corral et al., 2016). The aim of the current investigation was to examine the correlations between air pollution exposures with the gut microbiota. Results from this initial study demonstrate significant correlations between elevated TRAP exposure with the gut microbiota that were independent of body fat percent. The current study also found that fasting glucose levels were associated with two bacterial families that were also associated with TRAP exposure, including Bacteroidaceae and Coriobacteriaceae. Additionally, fasting glucose levels were positively associated with freeway TRAP exposure, yet the strength of this correlation was attenuated after adjustment for Bacteroidaceae or Coriobacteriaceae. Collectively, results from this study suggest that exposure to air pollutants may impact metabolic health in part via alterations in the gut microbiota.
This study showed that TRAP exposures were associated with the relative abundance Bacteroidaceae and Corynebacteriaceae, which have been associated with obesity, altered metabolism, and intestinal inflammation. For example, Bacteroidaceae has been shown to be depleted in obese children (Riva et al., 2017) and was found to be lower in morbidly obese adults with high compared to low insulin resistance (Moreno-Indias et al., 2016), suggesting that depletion of this taxa may independently contribute to metabolic dysfunction. Coriobacteriaceae has been linked with cholesterol metabolism (Martínez et al., 2013) and insulin resistance among overweight and obese pregnant women (Gomez-Arango et al., 2016). In mice, oral exposure to benzo[a]pyrene (a polycyclic aromatic hydrocarbon that is emitted from combustion sources) resulted in an increase in Coriobacteriaceae and intestinal inflammation (Ribière et al., 2016). In the current study, there was also a positive correlation between TRAP exposure and the genus Collinsella (family Coriobacteriaceae, phylum Actinobacteria), which has been reported in type 2 diabetes (Lambeth et al., 2015; Zhang et al., 2013) and insulin resistance during pregnancy (Gomez-Arango et al., 2016). Additionally, Coriobacteriaceae, including Collinsella, has the potential to directly interact with the host through colonization of mucosal surfaces and metabolism of amino acids (Clavel et al., 2014; Gomez-Arango et al., 2016).
The gut microbiome is known to contribute to an array of metabolic and immune processes (Palau-Rodriguez et al., 2015) through microbial-host exchanges of genes and metabolites (Nicholson et al., 2012) and also helps to maintain the intestinal barrier between bacteria in the lumen and systemic circulation. Therefore, any exposure-induced alterations in the composition and/or function of gut microbiome may decrease gut barrier integrity (Bischoff et al., 2014) and result in increased bacterial translocation and systemic inflammation that contributes to obesity and insulin resistance (Pekkala et al., 2015; Prajapati et al., 2014). TRAP may have the potential to modify the composition and/or function of resident bacteria, generate harmful byproducts through bacterial biotransformation (Choi et al., 2013; Maurice et al., 2013), alter the innate immune system, and trigger local inflammation and breakdown of the intestinal barrier (Thaiss et al., 2016). As such, increased air pollution exposure may contribute to obesity and insulin resistance through known inflammatory pathways related to changes in the gut microbiome (Boulangé et al., 2016; Cani et al., 2009).
Rodent studies suggest that TRAP may impact the gut microbiome as well as intestinal inflammation. In mice, ingestion of airborne particulate matter altered the gut microbiome and induced intestinal inflammation (Kish et al., 2013; Mutlu et al., 2011), which appeared to be through increased intestinal cell responsiveness to lipopolysaccharide (Powell et al., 2000). Further, rats exposed to diesel exhaust particles through the diet showed increased oxidative stress, resulting in apoptosis and protein oxidation in colon mucosa (Dybdahl, 2003). In humans, few studies have examined correlations between air pollutants and the gut microbiota, yet ambient NO2 and PM (pollutants highly correlated with traffic emissions) have been shown to be associated with intestinal disease. For example, adolescents who lived in regions with greater NO2 concentrations were more likely to be diagnosed with Crohn’s disease (Kaplan et al., 2010). Also, when air pollutants (e.g., NO2, PM with aerodynamic diameter less than 2.5 micrometers) were elevated in ambient air, adolescents and young adults visited emergency rooms more often for non-specific abdominal pain (Kaplan et al., 2012). Although the precise mechanisms underlying the correlations between the gut microbiota with exposure to air pollutants are not fully understood, ultrafine particles are known to rapidly cross into systemic circulation (Nemmar et al., 2002) and particulate matter can enter into the intestinal epithelia via M cells on Peyer’s patches and through enterocytes (Lai et al., 2009; Lavelle et al., 1995). Studies also suggest that particles may reach the intestine through ingestion of inhaled particles following mucociliary clearance from the airways (Beamish et al., 2011; Möller et al., 2004; Nemmar et al., 2002; Salim et al., 2014). Lastly, another study found that increased concentrations of PM, specifically black carbon, altered key aspects of bacterial colonization and survival as well as bacterial colonization in mice (Hussey et al., 2017), presenting another potential mechanism through which air pollutants may alter the human microbiome.
Results from this study provide some of the first evidence that elevated exposure to air pollutants may impact the human gut microbiota. These findings were independent of body fat percent and were unchanged after adjusting for dietary factors known to influence the composition of the gut microbiota. Despite the strengths of this study, limited statistical power due to a relatively sample size may have reduced the number of correlations that remained significant after correction for multiple comparisons and limited our ability to investigate any potential gut bacterial differences by sex or race/ethnicity. To address these issues, we are currently working on validating our findings in a larger cohort of adolescents using untargeted metagenomics. Exposure misclassification may have occurred with residential based estimates of TRAP exposures, but any exposure misclassification would likely be non-differential and would diminish any observed effects (Nerriere et al., 2005). Finally, residual confounding may have affected the study findings since poor diet and lack of physical activity may be associated with increased risk of metabolic dysfunction, the composition of the gut microbiota, and could also be correlated with residential proximity to sources of air pollution (Fram et al., 2015; Hajat et al., 2013). Yet, this seems unlikely given that the reported findings were independent of body fat percent and remained unchanged after adjustment for dietary intake. This study was also limited in that vegetation was not assessed since vegetation has been shown to influence the microbial composition of nearby air (Lymperopoulou et al., 2016) and may have contributed to some of the observed correlations.
To our knowledge, this is the first study to show that increased TRAP exposure is associated with the gut microbiota. This study supports the hypothesis that environmental exposures, such as TRAP, have the potential to alter the relative abundance of gut bacteria. Results from this study also suggest that increased TRAP may negatively impact metabolic health through alterations in the composition and/or function of the gut microbiome, but larger studies are needed to confirm these initial findings.
Supplementary Material
Highlights.
Traffic-related air pollution (TRAP) was positively correlated with fasting glucose
TRAP exposure was correlated with specific gut microbial taxa
These taxa partially explained the correlation between TRAP and fasting glucose
TRAP exposure may negatively impact metabolic health via the gut microbiota
Acknowledgments
T.L. Alderete conceived the research question, study design, collected and reviewed data, ran analysis, and wrote the manuscript. R. Jones and Z. Chen assisted with data analysis, provided statistical consultation, and carefully reviewed the results, and contributed to writing of the manuscript. J. Kim performed clinical visits, collected data, designed and maintained the database, and contributed to writing of the manuscript. R. Habre guided geocoding efforts and contributed to writing of the manuscript. F.D. Gilliland conceived the primary study design and F.D. Gilliland and M.I. Goran contributed to writing of the manuscript. F. Lurmann provided air pollution exposure data and contributed to manuscript writing. All authors reviewed the article. T.L. Alderete, F.D. Gilliland, and M.I. Goran are the guarantors of this work, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by NIH NIEHS K99ES027853 (TLA), the Southern California Environmental Health Sciences Center Pilot Projects Program (TLA and MIG) funded by the NIH NIEHS P30ES007048 (FDG), and the NIH NIEHS P01ES022845 (FDG).
Footnotes
Human Subjects: Informed written consent/assent was obtained from the parents/participant. The University of Southern California Institutional Review Board approved these studies.
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderete TL, Habre R, Toledo-Corral CM, Berhane K, Chen Z, Lurmann FW, Weigensberg MJ, Goran MI, Gilliland FD. Longitudinal Associations Between Ambient Air Pollution with Insulin Sensitivity, β-Cell Function, and Adiposity in Los Angeles Latino Children. Diabetes. 2017;66:1789–1796. doi: 10.2337/db16-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allais L, Kerckhof FM, Verschuere S, Bracke KR, De Smet R, Laukens D, Van den Abbeele P, De Vos M, Boon N, Brusselle GG, Cuvelier CA, Van de Wiele T. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ Microbiol. 2016;18:1352–1363. doi: 10.1111/1462-2920.12934. [DOI] [PubMed] [Google Scholar]
- Beamish LA, Osornio-Vargas AR, Wine E. Air pollution: An environmental factor contributing to intestinal disease. J Crohns Colitis. 2011;5:279–286. doi: 10.1016/j.crohns.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Benson PE. A review of the development and application of the CALINE3 and 4 models. Atmospheric Environment Part B Urban Atmosphere. 1992;26:379–390. doi: 10.1016/0957-1272(92)90013-I. [DOI] [Google Scholar]
- Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ, Steurer-Stey C, Frei A, Frei P, Scharl M. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS ONE. 2013;8:e59260. doi: 10.1371/journal.pone.0059260.s014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Eum SY, Rampersaud E, Daunert S, Abreu MT, Toborek M. Exercise Attenuates PCB-Induced Changes in the Mouse Gut Microbiome. Environ Health Perspect. 2013;121:725–730. doi: 10.1289/ehp.1306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel T, Desmarchelier C, Haller D, Gérard P, Rohn S, Lepage P, Daniel H. Intestinal microbiota in metabolic diseases. Gut Microbes. 2014;5:544–551. doi: 10.4161/gmic.29331. [DOI] [PubMed] [Google Scholar]
- Clements AL, Jia Y, DenBleyker A, McDonald-Buller E, Fraser MP, Allen DT, Collins DR, Michel E, Pudota J, Sullivan D, Zhu Y. Air pollutant concentrations near three Texas roadways, part II: Chemical characterization and transformation of pollutants. Atmospheric Environment. 2009;43:4523–4534. doi: 10.1016/j.atmosenv.2009.06.044. [DOI] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdahl M. DNA adduct formation and oxidative stress in colon and liver of Big Blue(R) rats after dietary exposure to diesel particles. Carcinogenesis. 2003;24:1759–1766. doi: 10.1093/carcin/bgg147. [DOI] [PubMed] [Google Scholar]
- Fram MS, Ritchie LD, Rosen N, Frongillo EA. Child experience of food insecurity is associated with child diet and physical activity. Journal of Nutrition. 2015;145:499–504. doi: 10.3945/jn.114.194365. [DOI] [PubMed] [Google Scholar]
- Fujita EM, Zielinska B, Campbell DE, Arnott WP, Sagebiel JC, Mazzoleni L, Chow JC, Gabele PA, Crews W, Snow R, Clark NN, Wayne WS, Lawson DR. Variations in speciated emissions from spark-ignition and compression-ignition motor vehicles in California’s south coast air basin. J Air Waste Manag Assoc. 2007;57:705–720. doi: 10.3155/1047-3289.57.6.705. [DOI] [PubMed] [Google Scholar]
- Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M SPRING Trial Group. Connections Between the Gut Microbiome and Metabolic Hormones in Early Pregnancy in Overweight and Obese Women. Diabetes. 2016;65:2214–2223. doi: 10.2337/db16-0278. [DOI] [PubMed] [Google Scholar]
- Hajat A, Diez Roux AV, Adar SD, Auchincloss AH, Lovasi GS, O’Neill MS, Sheppard L, Kaufman JD. Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2013;121:1325–1333. doi: 10.1289/ehp.1206337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey SJK, Purves J, Allcock N, Fernandes VE, Monks PS, Ketley JM, Andrew PW, Morrissey JA. Air pollution alters Staphylococcus aureus and Streptococcus pneumoniae biofilms, antibiotic tolerance and colonisation. Environ Microbiol. 2017;19:1868–1880. doi: 10.1111/1462-2920.13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, McConnell R, Wolch J, Chang R, Lam C, Dunton G, Gilliland F, Lurmann F, Islam T, Berhane K. Traffic-related air pollution and obesity formation in children: a longitudinal, multilevel analysis. Environ Health. 2014;13:49. doi: 10.1186/1476-069X-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GG, Hubbard J, Korzenik J, Sands BE, Panaccione R, Ghosh S, Wheeler AJ, Villeneuve PJ. The inflammatory bowel diseases and ambient air pollution: a novel association. Am J Gastroenterol. 2010;105:2412–2419. doi: 10.1038/ajg.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GG, Szyszkowicz M, Fichna J, Rowe BH, Porada E, Vincent R, Madsen K, Ghosh S, Storr M. Non-Specific Abdominal Pain and Air Pollution: A Novel Association. PLoS ONE. 2012;7:e47669. doi: 10.1371/journal.pone.0047669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish L, Hotte N, Kaplan GG, Vincent R, Tso R, Gänzle M, Rioux KP, Thiesen A, Barkema HW, Wine E, Madsen KL. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS ONE. 2013;8:e62220. doi: 10.1371/journal.pone.0062220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Advanced Drug Delivery Reviews. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth SM, Carson T, Lowe J, Ramaraj T, Leff JW, Luo L, Bell CJ, Shah VO. Composition, Diversity and Abundance of Gut Microbiome in Prediabetes and Type 2 Diabetes. J Diabetes Obes. 2015;2:1–7. doi: 10.15436/2376-0949.15.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle EC, Sharif S, Thomas NW, Holland J. The importance of gastrointestinal uptake of particles in the design of oral delivery systems. Advanced Drug Delivery Reviews. 1995;18:5–22. doi: https://doi.org/10.1016/0169-409X(95)00048-C. [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Lymperopoulou DS, Adams RI, Lindow SE. Contribution of Vegetation to the Microbial Composition of Nearby Outdoor Air. Appl Environ Microbiol. 2016;82:3822–3833. doi: 10.1128/AEM.00610-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I, Perdicaro DJ, Brown AW, Hammons S, Carden TJ, Carr TP, Eskridge KM, Walter J. Diet-induced alterations of host cholesterol metabolism are likely to affect the gut microbiota composition in hamsters. Appl Environ Microbiol. 2013;79:516–524. doi: 10.1128/AEM.03046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Indias I, Sánchez-Alcoholado L, García-Fuentes E, Cardona F, Queipo-Ortuño MI, Tinahones FJ. Insulin resistance is associated with specific gut microbiota in appendix samples from morbidly obese patients. Am J Transl Res. 2016;8:5672–5684. [PMC free article] [PubMed] [Google Scholar]
- Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller W, Häussinger K, Winkler-Heil R, Stahlhofen W, Meyer T, Hofmann W, Heyder J. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J Appl Physiol. 2004;97:2200–2206. doi: 10.1152/japplphysiol.00970.2003. [DOI] [PubMed] [Google Scholar]
- Mutlu EA, Engen PA, Soberanes S, Urich D, Forsyth CB, Nigdelioglu R, Chiarella SE, Radigan KA, Gonzalez A, Jakate S, Keshavarzian A, Budinger GRS, Mutlu GM. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part Fibre Toxicol. 2011;8:19. doi: 10.1186/1743-8977-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A, Hoet PM, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- Nerriere É, Zmirou-Navier D, Blanchard O, Momas I, Ladner J, Le Moullec Y, Personnaz MB, Lameloise P, Delmas V, Target A, Desqueyroux H. Can we use fixed ambient air monitors to estimate population long-term exposure to air pollutants? The case of spatial variability in the Genotox ER study. Environ Res. 2005;97:32–42. doi: 10.1016/j.envres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Palau-Rodriguez M, Tulipani S, Isabel Queipo-Ortuño M, Urpi-Sarda M, Tinahones FJ, Andres-Lacueva C. Metabolomic insights into the intricate gut microbial-host interaction in the development of obesity and type 2 diabetes. Front Microbiol. 2015;6:1151. doi: 10.3389/fmicb.2015.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkala S, Munukka E, Kong L, Pöllänen E, Autio R, Roos C, Wiklund P, Fischer-Posovszky P, Wabitsch M, Alen M, Huovinen P, Cheng S. Toll-like receptor 5 in obesity: the role of gut microbiota and adipose tissue inflammation. Obesity. 2015;23:581–590. doi: 10.1002/oby.20993. [DOI] [PubMed] [Google Scholar]
- Powell JJ, Harvey RS, Ashwood P, Wolstencroft R, Gershwin ME, Thompson RP. Immune potentiation of ultrafine dietary particles in normal subjects and patients with inflammatory bowel disease. J Autoimmun. 2000;14:99–105. doi: 10.1006/jaut.1999.0342. [DOI] [PubMed] [Google Scholar]
- Prajapati B, Jena PK, Rajput P, Purandhar K, Seshadri S. Understanding and modulating the Toll like Receptors (TLRs) and NOD like Receptors (NLRs) cross talk in type 2 diabetes. Curr Diabetes Rev. 2014;10:190–200. doi: 10.2174/1573399810666140515112609. [DOI] [PubMed] [Google Scholar]
- Ribière C, Peyret P, Parisot N, Darcha C, Déchelotte PJ, Barnich N, Peyretaillade E, Boucher D. Oral exposure to environmental pollutant benzo[a]pyrene impacts the intestinal epithelium and induces gut microbial shifts in murine model. Sci Rep. 2016;6:31027. doi: 10.1038/srep31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214–1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, Berry D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol. 2017;19:95–105. doi: 10.1111/1462-2920.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MC, Muzny DM, McCormick JB, Gibbs RA, Fisher-Hoch SP, Petrosino JF. 16S gut community of the Cameron County Hispanic Cohort. Microbiome. 2015;3:7. doi: 10.1186/s40168-015-0072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim SY, Kaplan GG, Madsen KL. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes. 2014;5:215–219. doi: 10.4161/gmic.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- Thiering E, Cyrys J, Kratzsch J, Meisinger C, Hoffmann B, Berdel D, von Berg A, Koletzko S, Bauer C-P, Heinrich J. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia. 2013;56:1696–1704. doi: 10.1007/s00125-013-2925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiering E, Markevych I, Brüske I, Fuertes E, Kratzsch J, Sugiri D, Hoffmann B, Berg von A, Bauer C-P, Koletzko S, Berdel D, Heinrich J. Associations of Residential Long-Term Air Pollution Exposures and Satellite-Derived Greenness with Insulin Resistance in German Adolescents. Environ Health Perspect. 2016 doi: 10.1289/ehp.1509967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Corral CM, Alderete TL, Habre R, Berhane K, Lurmann FW, Weigensberg MJ, Goran MI, Gilliland FD. Effects of air pollution exposure on glucose metabolism in Los Angeles minority children. Pediatr Obes. 2016;312:1218. doi: 10.1111/ijpo.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JET, Bloks VW, Groen AK, Heilig HGHJ, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JBL, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6. e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Weinmayr G, Hennig F, Fuks K, Nonnemacher M, Jakobs H, Möhlenkamp S, Erbel R, Jöckel KH, Hoffmann B, Moebus S, Nixdorf Heinz Recall Investigator Group. Long-term exposure to fine particulate matter and incidence of type 2 diabetes mellitus in a cohort study: effects of total and traffic-specific air pollution. Environ Health. 2015;14:53. doi: 10.1186/s12940-015-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE. 2013;8:e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Pudota J, Collins D, Allen D, Clements A, DenBleyker A, Fraser M, Jia Y, McDonald-Buller E, Michel E. Air pollutant concentrations near three Texas roadways, Part I: Ultrafine particles. Atmospheric Environment. 2009;43:4513–4522. doi: 10.1016/j.atmosenv.2009.04.018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.