Abstract
Poor decision-making is a central feature of all substance use disorders (SUD), but substances vary in the legal and health consequences associated with their use. For example, while the negative health consequences associated with cigarette smoking are often years away, the consequences of heroin abuse can be fatal in mere hours. It remains unclear if users of these substances show decision-making patterns that differ with the relative riskiness of their drug of choice. To address this question, we reviewed studies that compared decision-making of individuals using different substances. We focused on studies assessing two of the most commonly investigated decision-making processes—delay discounting and risk taking—and specifically focused on decision-making that involved selection between options for hypothetical monetary rewards. For delay discounting, we reviewed studies that assessed decisions regarding delayed or immediate monetary rewards, and for risk-taking we reviewed studies using the Iowa Gambling Task. Studies directly comparing different SUD groups were limited in number and tended to compare alcohol or cocaine users to other substance users. Overall, these studies do not support the hypothesis that decision-making differed by drug of choice. Major limitations in the literature include failing to account for comorbid substance use and a lack of prospective longitudinal studies. Due to these limitations, conclusions should be considered provisional. Nonetheless, current findings suggest that these two facets of decision-making are similar across drugs of abuse.
Introduction
We all make decisions every day and, while some have trivial consequences, others can profoundly alter the course of our lives. Poor decision-making is a central feature of substance use disorder (SUD). Risky choices may lead to trying an addictive substance for the first time or continuing to use a drug despite devastating consequences. Decision-making involves many factors and can be affected by a range of internal and external influences, such as stress, mood, and social pressures (Giordano et al, 2002). A fundamental question is whether an individual’s drug of choice can inform us about their decision-making. Does an individual who uses a substance with a risk of fatal overdose such as heroin rather than a more benign substance such as cannabis have especially poor judgment? Neurobiological evidence on the etiology of SUD offers mixed evidence. On the one hand, individuals may have pre-existing neurobiological characteristics which may affect their choices and lead to abuse of a particular drug (Ersche et al, 2012). Furthermore, different drugs alter specific neurotransmitter systems and may therefore differentially affect decision-making. On the other hand, there may be vulnerability factors (Tsuang et al, 1998), neuroanatomical pathways (Nestler, 2005) and, potentially, decision-making patterns common to all addictive substances.
To determine whether decision-making differences exist between individuals primarily dependent upon one substance relative to another, we conducted a review of the literature. We defined decision-making as the selection of an action from among available alternatives, resulting in an outcome that engenders a specified neural, cognitive, or emotional state (Paulus, 2007). We limited the scope of our review to two primary decision-making processes: delay discounting and risk-taking. To assess delay discounting, we reviewed studies which employed variants of the monetary delay discounting tasks developed by Rachlin and colleagues (1991), Kirby and colleagues (1999), Bickel and colleagues (1999) and Green and colleagues (1994). To assess risk-taking, we reviewed studies which employed the Iowa Gambling Task (IGT) developed by Bechara and colleagues (1994). These tasks present participants with repeated opportunities to make decisions that only vary on a few parameters, and thereby reduce variation in task design, which can significantly impact decision-making and study results. For example, framing of decisions (Kahneman and Tversky, 1984), type of reward (Chapman and Elstein, 1995), and even the presence of irrelevant information (Tversky and Kahneman, 1974) can alter decision-making. Thus, by using these tasks, we sought to examine the measures of decision-making that are most frequently employed in SUD populations while limiting task variability.
While a large literature exists comparing IGT and delay discounting in individuals with a specific SUD to controls (Bechara, 2005; MacKillop et al, 2011), only one meta-analysis has assessed whether delay discounting differs between substance users of one type relative to another (Amlung et al, 2017). Importantly, this meta-analysis assessed the relationship between continuous measures of addiction severity and delay discounting measures, but did not review studies which directly compared substance using groups. While comparing across studies in this fashion provides important information, different samples may harbor idiosyncrasies related to recruitment strategies, task modifications, inclusion and exclusion criteria, and regional differences in populations. Thus, there is reason to believe that head-to-head comparisons of different substance using groups in a single study may fill a void in the understanding of the literature. No prior attempts have been made to synthesize the literature investigating such comparisons for either delay discounting or risk-taking. Our goal is to review the findings from head-to-head comparisons of groups who used different substances and discuss what remains to be done to resolve whether differences truly exist across users of different substances in their patterns of decision-making.
Methods
We conducted a literature search on April 14, 2017 on PubMed using the following terms “Delay Discounting” OR “Iowa Gambling Task” AND “Substance-related Disorders”. We examined each of these studies to determine if they met the following inclusion criteria: 1) an original data paper, 2) use of either a monetary delay discounting task or the Iowa Gambling Task, 3) examination of participants who were diagnosed with a substance use disorder for either alcohol, nicotine, cocaine, opioids, amphetamines, or marijuana (or who reported heavy use of these substances as defined by the study authors), 4) direct comparison of at least two distinct substance using groups (e.g. marijuana users versus cocaine users). The search yielded 113 results. Of these, 20 met the inclusion criteria (10 delay discounting, 10 IGT) and are described in the sections below.
Delay Discounting
Delay discounting refers to a trait observed across many species (Mazur, 1987) to weigh immediate outcomes more heavily than future ones. For example, most children would rather have a cookie now than wait until after dinner. In human research, the majority of studies assess monetary rewards as the outcome of interest (Fishburn and Rubinstein, 1982), although discounting rates correlate across reinforcers, such that an individual who rapidly discounts delayed money is also likely to rapidly discount delayed food or drugs (Odum, 2011). The two primary factors that affect such decisions are the length of the delay and the amounts of money. If $1000 and $2000 are both offered immediately, $2000 is clearly preferable. If someone must wait to receive $2000, however, at some point the delay becomes too long. Would you prefer $1000 today or $2000 in ten years? The future time when the subjective value of the large reward diminishes to the equivalent of the small immediate reward is called the indifference point. Studies of delay discounting typically establish a person’s indifference point for a variety of monetary values and delay periods. From this, it is possible to fit an individual’s indifference points to a hyperbolic function defined by the following equation (Mazur, 1987): V = A/(1 + kD), where V is the subjective value, A is the actual monetary value, and D is the time delay. Higher levels of delay discounting are represented by higher values of k, which can be referred to as the delay discounting constant. Since k is not normally distributed, there are a variety of ways to compare groups, such as logarithmic transformation of k or nonparametric analyses. Alternatively, as the hyperbolic function does not always fit a person’s decisions, some studies use the area under the curve of the indifference point plot to index delay discounting.
Meta-analyses of studies comparing substance using groups to controls have found small to medium effect sizes for delay discounting differences (MacKillop et al, 2011), but no evidence of differences in effect sizes between users of different substances (Amlung et al, 2017). Importantly, these meta-analyses found little evidence of publication bias, indicating that these effects are reliable. These studies provide strong evidence that individuals with an SUD prefer immediate relative to delayed rewards compared to controls. In the following section, we will examine the studies that met our search criteria to determine if delay discounting tendencies differ as a function of substance of abuse.
Discounting of Delayed Monetary Rewards Across SUD Groups
The most common comparisons in monetary delay discounting studies have been between cocaine, alcohol, and nicotine users. As seen in Table 1, existing studies have not found any differences in delay discounting between cocaine and alcohol users (Kirby and Petry, 2004; Moody et al, 2016b), alcohol and nicotine users (Moallem and Ray, 2012; Moody et al, 2016b), and alcohol and polysubstance users (Taylor et al, 2016). In contrast, two studies have found that cocaine users discount at higher rates than nicotine users (García-Rodríguez et al, 2013; Moody et al, 2016b). Another study compared smoking and nonsmoking polysubstance users to a smoking only group and found no difference across groups (Businelle et al, 2010). Thus, cocaine users may have steeper rates of delay discounting than nicotine users, but smokers discount money similarly to other substance using groups.
Table 1.
Studies Assessing Discounting of Delayed Monetary Rewards
| Study | Substances | Group 1 | N | Group 2 | N | Estimated Effect Sizea | Significant Group Difference |
|---|---|---|---|---|---|---|---|
| Alcohol | |||||||
|
| |||||||
| Kirby and Petry 2004 | Opioids, Cocaine, Alcohol | Alcohol | 33 | Heroin | 27 | 0.88 | Yes |
| Alcohol | 33 | Cocaine | 41 | 0.66 | No | ||
| Moallem and Ray, 2012 | Alcohol, Nicotine | Alcohol + Nicotine | 213 | Nicotine | 67 | 0.16 | No |
| Alcohol + Nicotine | 213 | Alcohol | 107 | 0.31 | Yes | ||
| Alcohol | 107 | Nicotine | 67 | — | No | ||
| Taylor et al. 2016 | Alcohol, Polysubstance | Alcohol | 27 | Polysubstance | 59 | 0.00 | No |
| Moody et al. 2016b | Cocaine, Alcohol, Nicotine | Alcohol | 47 | Nicotine | 137 | 0.12 | No |
| Alcohol | 47 | Cocaine | 28 | 0.19 | No | ||
|
| |||||||
| Cocaine | |||||||
|
| |||||||
| Bornovalova et al. 2005 | Cocaine, Opioids | Cocaine | 16 | Heroin | 11 | 0.93 | Yes |
| García- Rodríguez et al. 2013 | Cocaine, Nicotine | Cocaine | 17 | Nicotine | 30 | 1.18 | Yes |
| Cocaine + Nicotine | 30 | Nicotine | 30 | 1.44 | Yes | ||
| Cocaine + Nicotine | 30 | Cocaine | 17 | 0.35 | No | ||
| Mejía-Cruz et al. 2016 | Cocaine, Marijuana | Cocaine | 77 | Marijuana | 44 | —b | No |
| Moody et al. 2016b | Cocaine, Alcohol, Nicotine | Cocaine | 28 | Nicotine | 137 | 0.63 | Yes |
|
| |||||||
| Opioids | |||||||
|
| |||||||
| Kirby and Petry 2004 | Opioids, Cocaine, Alcohol | Heroin | 27 | Cocaine | 41 | 0.20 | No |
| Robles et al. 2011 | Opioids | Methadone only | 30 | Methadone + illicit opioids | 30 | <0.30 | No |
| Karakula et al. 2016 | Opioids | Heroin | 106 | Prescription- opioids | 33 | 0.49 | Yes |
|
| |||||||
| Polysubstance | |||||||
|
| |||||||
| Polysubstance | Polysubstance | 25 | Nicotine | 20 | <0.18 | No | |
| Businelle et al. 2010 | Polysubstance + Nicotine | 36 | Nicotine | 20 | <0.18 | No | |
| Polysubstance + Nicotine | 36 | Polysubstance | 25 | <0.18 | No | ||
Effect size is reported as Cohen's d. If this was not reported, it was calculated by a) means and standard deviations, b) test-statistics and degrees of freedom, or c) conversion from another measure of effect size.
— Indicates lack of information
This study compared nicotine, alcohol, and alcohol + nicotine groups using three different monetary reward magnitudes: small, medium, and large. There was no effect of group on medium or large rewards, but there was an effect of group on the small reward values, such that the alcohol + nicotine group showed steeper delay discounting relative to both the alcohol and nicotine group.
There is mixed evidence as to whether opioid users have higher rates of delay discounting compared to other substance users (see Table 1 sections “Opioids” and “Alcohol” and “Cocaine”). Heroin users have been found to have greater delay discounting than alcohol users but not cocaine users (Kirby and Petry, 2004). However, this study employed unconventional substance use thresholds to define each group (daily use of heroin, drinking alcohol to the point of intoxication 3x/week) rather than using clinical interviews to determine formal diagnoses. A small study set in a residential treatment program found that crack cocaine users had higher discounting rates relative to heroin users (Bornovalova et al, 2005). The study had a potential confound, however, as the cocaine users were also more likely to use alcohol, marijuana and PCP, implying that polysubstance abuse, and SUD severity, is associated with steeper delay discounting (MacKillop et al, 2011). A third study compared two groups of opioid users that used either heroin or prescription opioids and found that heroin users showed greater rates of discounting than prescription opioid users (Karakula et al, 2016). As heroin users tends to have more severe use disorders than prescription opioid users, despite similar pharmacology of their drug of choice, this finding supports the idea that decision-making differences may be a product of severity of substance use disorder rather than reflecting differences across substances of abuse. However, another study compared two groups of patients who had been receiving methadone maintenance therapy for two years. One group continued using heroin, cocaine, and alcohol during treatment, while the other adhered strictly to methadone, but the groups showed no differences in delay discounting (Robles et al, 2011).
There are a limited number of studies comparing individuals with other substance use disorders. We did not find any specific studies comparing amphetamine users to other substance users. Only one study recruited marijuana users and found that they did not differ from a cocaine using group (Mejía-Cruz et al, 2016). More effort should be made to study individuals with these disorders.
In summary, most studies showed no delay discounting differences between users of different substances, although two studies showed that cocaine users discounted delayed monetary rewards more rapidly than smokers. There was mixed evidence that more severe drug use behaviors, such as injecting heroin rather than ingesting prescription opioids, were associated with higher rates of discounting. The meta-analysis by Amlung and colleagues (2017) suggests no difference in effect size between users of different drugs and controls, implying that substance using groups have similar rates of discounting, and the studies reviewed here concur with this conclusion. In the studies reviewed here, all but one of the comparisons between substance users and controls showed a statistically significant difference (Supplemental Table 1). Although a formal meta-analysis was not conducted, the median effect size for comparisons between two SUD groups was Cohen’s d = 0.31 (interquartile range = 0.70; see Table 1). In contrast, the median effect size for case-control comparisons was Cohen’s d = 0.61 (interquartile range = 0.57, see Supplemental Table 1), indicating that there is a larger difference in delay discounting between cases and controls than between substance using groups. However, decisions regarding delayed monetary rewards represent only one aspect of the broader domain of decision-making.
Measuring Risk Taking Using the Iowa Gambling Task
The Iowa Gambling Task (IGT) was developed to probe for neuropsychological abnormalities following lesions to the ventromedial prefrontal cortex (Bechara et al, 1994). Patients with these lesions performed normally on standardized neurocognitive tests, but they made troubling decisions in their real lives. For example, following bilateral ablation of the ventral medial prefrontal cortex, one patient divorced his wife, was fired from his job, and lost his savings by investing in a risky business plan, yet he maintained a high IQ (Eslinger and Damasio, 1985). The IGT probes decision-making by asking participants to choose a card from one of four decks to receive money. Two of the decks are associated with large short-term gains, but even larger eventual losses, leading to a net loss. The other two decks are associated with smaller payouts but an overall net gain. Participants are not informed of these contingencies, so decision-making on this task involves an element of risk-taking (Gowin et al, 2013) and an element of learning from the outcomes of each choice. IGT behavior is typically summarized with a numeric score equal to the number of selections from the net-gain decks minus selections from the net-loss decks, where a higher score indicates more advantageous decision-making. While healthy individuals tend to learn which decks are associated with a net gain and shift toward selecting from those decks across trials, patients with ventromedial prefrontal cortex lesions continue to choose from the disadvantageous decks across the task, showing a slower rate of learning contingencies (Bechara et al, 1994).
Since its development, many studies have examined the behavior of substance users on the IGT. Interestingly, individuals with SUD show deficits on the IGT that parallel those of the frontal lesion patients, in that they show a slower rate of learning to choose the advantageous decks and they continue to select more often from the disadvantageous decks throughout the task (Bechara et al, 2001). Studies have shown that alcohol, nicotine, methamphetamine, cocaine, marijuana, heroin, and polysubstance users all choose from the disadvantageous decks at higher rates than controls (Barry and Petry, 2008; Bolla et al, 2003; Gonzalez et al, 2007; Kjome et al, 2010; Petry et al, 1998; Stephan et al, 2016; Stout et al, 2004; Whitlow et al, 2004; Xiao et al, 2008). In the following section, we will examine the studies that met our search criteria to determine if performance on the IGT differ as a function of substance of abuse.
Iowa Gambling Task Performance Across SUD Groups
Several studies directly compared individuals who abused different substances (see Table 2 for a list of studies and estimated effect sizes). Most studies that have compared SUD groups found no evidence of differences in decision-making, including cocaine-dependent and heroin-dependent individuals (Verdejo-García et al, 2007) cocaine, alcohol and methamphetamine users (van der Plas et al, 2009), and cocaine- and marijuana-dependent individuals (Verdejo-Garcia et al, 2007). Further, cocaine-only dependent males did not differ from cocaine- and heroin-dependent males (Vassileva et al, 2007). One study assessed substance use disorders in combination with comorbid bipolar disorder (type I or II) and similarly found no difference in total score between cocaine and methamphetamine dependent individuals (Nejtek et al, 2013). However, bipolar disorder has been associated with impulsivity and risky decision-making (Sloan et al, 2014; Adida et al, 2011) and this may have obscured the effects of drug use in this sample. Collectively, these studies argue against substance-specific effects.
Table 2.
Risk-Taking Studies Using the Iowa Gambling Task
| Study | Substances | Group 1 | N | Group 2 | N | Estimated Effect Sizea | Significant Group Difference |
|---|---|---|---|---|---|---|---|
| Alcohol | |||||||
|
| |||||||
| Gonzalez et al. 2007 | Alcohol, Amphetamine | Alcohol | 17 | Methamphetamine | 16 | 0.63 | Yes |
| Barry and | Alcohol, Cocaine, | Alcohol | 34 | Cocaine | 42 | 0.01 | No |
| Petry 2008 | Opioids | Alcohol | 34 | Heroin | 28 | 0.11 | No |
| Alcohol | 34 | Polysubstance | 27 | 0.46 | No | ||
| Cantrell et al. 2008c | Alcohol, Marijuana, Nicotine | Alcohol | No | ||||
| van der Plas et al. 2009 | Alcohol, Cocaine, Amphetamine | Alcohol | 33 | Cocaine | 27 | — | No |
| Alcohol | 33 | Methamphetamine | 38 | — | No | ||
| Kornreich et al. 2013 | Polysubstance, Alcohol | Alcohol | 25 | Polysubstance | 25 | — | No |
|
| |||||||
| Cocaine | |||||||
|
| |||||||
| Vassileva et al. 2007 | Cocaine, Opioids | Cocaine | 47 | Cocaine + Heroin | 53 | No | |
| Verdejo- Garcia et al. 2007b | Cocaine, Marijuana | Cocaine | 11 | Marijuana | 10 | — | No |
| Barry and Petry 2008 | Alcohol, Cocaine, Opioids | Cocaine | 42 | Polysubstance | 27 | 0.46 | No |
| van der Plas et al. 2009 | Alcohol, Cocaine, Amphetamine | Cocaine | 27 | Methamphetamine | 38 | — | No |
| Nejtek et al. 2013 | Methamphetamine, Cocaine | Cocaine | 41 | Methamphetamine | 22 | 0.33 | No |
|
| |||||||
| Opioids | |||||||
|
| |||||||
| Verdejo-Garcia et al. 2007a | Cocaine, Opioids | Heroin | 25 | Cocaine | 39 | — | No |
| Barry and Petry 2008 | Alcohol, Cocaine, Opioids | Heroin | 28 | Cocaine | 42 | 0.13 | No |
| Heroin | 28 | Polysubstance | 27 | 0.45 | No | ||
|
| |||||||
| Polysubstance | |||||||
|
| |||||||
| Businelle et al 2008 | Polysubstance | Polysubstance | 19 | Nicotine | 26 | —b | No |
| Polysubstance + Nicotine | 40 | Nicotine | 26 | — | No | ||
| Polysubstance + Nicotine | 40 | Polysubstance | 19 | — | No | ||
| Hanson et al. 2008 | MDMA, Polysubstance | MDMA + polysubstance | 22 | Polysubstance | 30 | 0.00 | No |
Effect size is reported as Cohen's d. If this was not reported, it was calculated by a) means and standard deviations, b) test-statistics and degrees of freedom, or c) conversion from another measure of effect size.
— Indicates lack of information
While this study did not include separate groups for marijuana or nicotine, it compared two analyses that either adjusted for, or did not adjust for marijuana and nicotine use.
Several other studies have compared polysubstance abusers to individuals dependent on specific substances or attempted to parse out the effects of specific drugs in individuals with polysubstance abuse (see Table 2 subsections “Polysubstance” and “Alcohol”). One study comparing non-smokers with an SUD, smokers with an SUD, and smokers without an SUD found no difference in net score on the IGT by smoking status, but individuals with an SUD had lower net scores than individuals with no SUD (Businelle et al, 2008). Interestingly, this study contained many overlapping participants with a delay discounting study with the same lead author, and the lack of differences in IGT performance parallel the lack of delay discounting differences across groups. Studies have found no difference between individuals dependent on alcohol, cocaine or heroin and polysubstance users (Barry and Petry, 2008; Kornreich et al, 2013). In a large study comparing alcohol dependent individuals to controls, dependent individuals had lower scores than the controls, but covarying for marijuana, nicotine, and other substance use did not explain any additional variance in IGT score (Cantrell et al, 2008), indicating common covariance among substance use variables. Lastly, a study comparing MDMA polysubstance users to a polysubstance group that never used MDMA showed no difference in IGT score between the groups, although both groups showed lower scores relative to controls, and less evidence of shifting toward the advantageous decks across blocks (Hanson et al, 2008). Overall, these studies provide little evidence of an effect of polysubstance abuse on IGT scores relative to other substance abusing groups.
Only one study has shown differences between substance using groups, finding that methamphetamine users had lower net scores than alcohol users with a medium effect size (Gonzalez et al, 2007). However, the alcohol group did not differ significantly from controls despite having lower scores on average. Notably, the sample size of this study was relatively small, and the difference between alcohol and methamphetamine users contradicts a larger study showing no differences between users of these substances (van der Plas et al, 2009). Further, the lack of a difference between the alcohol group and controls contrasts the meta-analytic findings suggesting that alcohol dependence is associated with poorer performance than controls (Stephan et al, 2016). Thus, conclusions regarding differences between methamphetamine and alcohol users will require additional studies with larger sample sizes. Collectively, the IGT literature provides a much clearer picture than the delay discounting literature regarding differences between substance using groups. Only one study reviewed here showed evidence of a difference between SUD groups, but the small sample size of the study raises the possibility that this is a false positive effect. The head-to-head comparisons using the IGT indicate that performance is similar across substance using groups. While formal meta-analyses were not conducted, the median effect size across the included studies was Cohen’s d = 0.33 (interquartile range 0.35; see Table 2). In contrast, the median effect size for case-control comparisons was Cohen’s d = 0.55 (interquartile range = 0.25, see Supplemental Table 2), suggesting that the substance using groups are more similar to each other than they are to non-substance abusing groups. However, with regards to differences between substance users and healthy controls, the IGT studies reviewed here were less consistent than their delay discounting counterparts, with multiple studies showing no statistically significant differences between cases and controls. Future studies should systematically review the broader IGT literature comparing cases to controls to address these heterogeneous findings.
These results expand on the delay discounting findings and suggests that similarities in decision-making across substance of abuse extends beyond choices regarding immediate versus delayed rewards into choices involving risk-taking preferences and the ability to learn contingencies. They may also suggest that the type of decision-making probed by the IGT, relative to delay discounting, has less variance with regard to substance use effects. For example, whereas six of the ten delay discounting studies showed at least some evidence of a difference between substance using groups, only one of the ten IGT studies found a difference. The mixed findings in the delay discounting literature may be a product of variable task design, as there is substantial heterogeneity across and within studies as to the duration of delay and the magnitude of rewards. The IGT, in contrast, employs the same contingencies and levels of riskiness across most studies. Alternatively, the discrepancy between the IGT and delay discounting literature may suggest that decision-making involving delayed rewards shows some sensitivity to drug of choice or SUD status, whereas decision-making on the IGT does not.
Discussion
In summary, and consistent with other recent reviews, we conclude that there is insufficient evidence to support the hypothesis that there are differences in decision-making patterns across substances of abuse. The reviewed literature shows that substance users have consistent differences relative to controls (see Supplemental Tables 1 and 2), but the differences between users of different substances were either substantially smaller or undetectable. Confidence in this conclusion should be tempered by the small number of comparisons and the paucity of studies designed to assess the contribution of psychiatric and substance use comorbidities. While many individuals with a SUD are polysubstance users (Yoon et al, 2015), most studies reviewed assumed that decision-making behavior resulted from the influence of a primary drug. In reality the confluence of a primary SUD, concurrent drug use, and other psychopathologies (Moody et al, 2016a; Petry, 2002) may jointly contribute to these effects. These factors should be more carefully addressed in future studies.
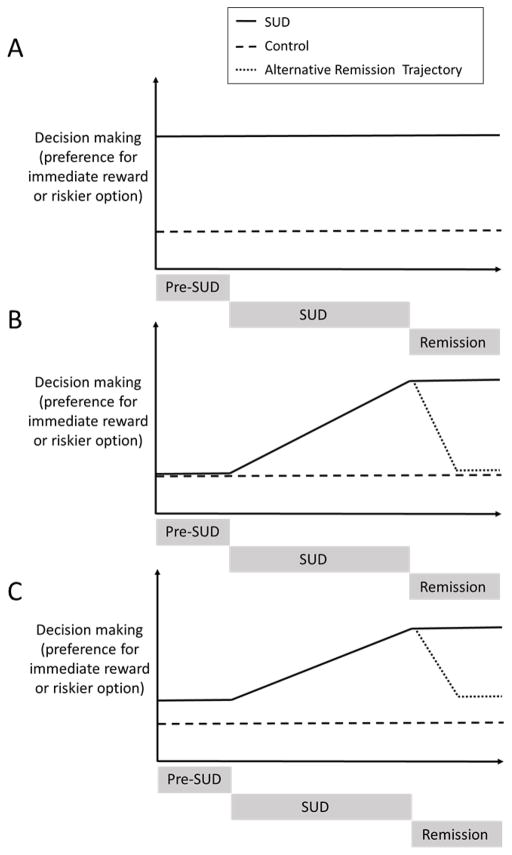
It remains unclear whether decision-making deficits precede or are caused by substance use. To address these possibilities, prospective studies are needed, such as the recently initiated Adolescent Brain Cognitive Development (ABCD) study. Such studies should follow a cohort of adolescents to identify pre-existing decision-making deficits and track how these decision-making deficits progress across the lifespan in both healthy individuals and substance users. Longitudinal studies are essential to determine whether decision-making differs across drug of abuse, and could help address three possible relationships between substance use and decision-making that are depicted in Figure 1. Individuals with any SUD may possess common inherent differences in decision-making that are entirely present prior to substance use (Panel A). Here, decision-making patterns should not vary by drug of choice. Second, repeated substance use may be solely responsible for decision-making deficits (Panel B). In this case, individuals with SUD would only display deficits following the neuroadaptive sequelae of chronic substance use (Koob and Volkow, 2016) that would likely vary by drug of choice. Third, there could be an interaction between pre-existing decision-making deficits and substance use consequences (Panel C). Small differences in decision-making patterns that exist prior to substance use may be exacerbated by either the drug’s chronic pharmacological action or by psychopathology associated with SUD, and exacerbation may vary by drug of choice.
Figure 1.
This schematic depicts three possible relationships between SUD and decision-making. In panel A, an exaggerated preference for immediate rewards exists prior to the onset of SUD and likely contributes to SUD. In this possibility, it is unlikely that differences in decision-making exist across drug-type. In panel B, individuals who develop SUD do not differ from controls prior to developing a disorder. Decision-making differences coincide with the onset of their disorder, and are likely caused by substance use. The slope of the change in decision-making may differ by drug type. Remission of SUD may (dotted line) or may not (solid line) be associated with recovery of decision-making. In panel C, pre-existing differences relative to controls may contribute to the development of an SUD. Onset of SUD may exacerbate these differences. Slope of these differences may differ by drug type. Remission of SUD may (fine dotted line) or may not (solid line) be associated with recovery of decision-making.
There is evidence that decision-making differences are present prior to the development of substance use disorders and may reflect familial influences. A study of young adults stratified by family history of substance use problems showed that family history positive individuals had worse scores on the IGT, although some participants had developed a SUD so it was not possible to isolate the effect on IGT performance due to family history(O’Brien et al, 2014). However, not all studies using the IGT have found an effect of family history, as two studies of adults (Acheson et al, 2009; Lovallo et al, 2006) were both negative. As for delay discounting, there is consistent evidence that adolescents and young adults with a family history of substance abuse show greater discounting of delayed rewards, although the effect sizes of these differences have been small (Acheson et al, 2011; Dougherty et al, 2014, 2015; VanderBroek et al, 2016). Greater discounting has also been linked to earlier onset of alcohol, cigarette and marijuana use in college students (Kollins, 2003) and adolescents (Richardson and Edalati, 2016). Thus, altered patterns of decision-making appear to precede substance use. Since the magnitude of early differences are small, however, it is possible that disparities are less pronounced prior to the onset of SUD, which would support model C from Figure 1. However, the delay discounting meta-analyses (MacKillop et al, 2011; Amlung et al, 2017) also indicate small effect sizes associated with SUD in adults populations, which may be consistent with model A from Figure 1, where differences between SUD and control remain constant throughout the progression of SUD. Longitudinal studies are needed to clarify the trajectory of decision-making patterns across the course of SUDs.
Abstinence may affect decision-making. Two studies using the IGT found that decision-making deficits lingered in alcoholics following one year (Körner et al, 2015) and six years of abstinence (Fein et al, 2004), showing no evidence of recovery. In contrast, heroin users who had been abstinent longer performed better on the IGT (Zhang et al, 2011). Those who had been abstinent for a month had better scores than those abstinent for only three days and, remarkably, individuals abstinent for 2 years no longer differed from control subjects. Delay discounting studies have reported similar evidence of recovery, as current users of heroin, methamphetamine, nicotine and marijuana all showed greater rates of discounting relative to controls, but ex-users and controls did not differ (Bickel et al, 1999; Bretteville-Jensen, 1999; Johnson et al, 2010). These findings suggest that some portion of decision-making deficits can be attributed to active drug use, but these deficits may be partially reversible following abstinence, which would be consistent with the recovery trajectory from Figure 1, potentially supporting model C. It remains to be seen whether rate of recovery differs by drug of abuse.
Reducing decision-making deficits may be an important target for treatment. For example, in a study of abstinent heroin dependent individuals on methadone maintenance, weekly assessments showed that frequency of risky choices on a task increased in the weeks leading up to a lapse to heroin use, but decreased again after several weeks of abstinence (Konova et al, 2016). This suggests that decision-making patterns may track treatment progress. Many of the existing manualized therapies focus on teaching individuals to make better life decisions (Marlatt and Donovan, 2005). Although the impact of these interventions on standardized decision-making task performance has rarely been evaluated, there is some evidence that risk-taking decreases following 28-day residential treatment (Aklin et al, 2009). There is also evidence in abstinent users undergoing treatment that a working memory training program could reduce delay discounting rates (Bickel et al, 2011). It will be important to see if this finding can be replicated and whether it contributes to better treatment outcomes.
Conclusions
The literature does not support the idea of unique patterns of decision-making across substances of abuse. However, there are currently a limited number of studies with important confounding factors, thus this conclusion remains provisional. With regards to specific decision-making assessments, there is a consensus among studies using the IGT that there is no effect of drug of choice, and the delay discounting literature offers similar conclusions. There is, however, some contradictory evidence that certain drugs, such as cocaine, may be associated with greater discounting rates than nicotine (see Garcia-Rodriguez et al, 2013 and Moody et al, 2016b in Table 1, subsection “Cocaine”). Current data indicate that individuals with a family history of addiction show pre-existing differences in decision-making that may increase risk of developing an SUD. These differences may be amplified as an individual’s substance use progresses and structural and functional neuroadaptations manifest due to the neurochemical effects of the abused substance (Ungless et al, 2001). In some cases, as the disorder abates, so too do decision-making deficits, although the extent of the recovery remains undetermined. While these conclusions are supported by the literature, significant gaps in our knowledge remain, underscoring the need for well-designed longitudinal studies. This review corroborates that decision-making deficits are a prominent, well-established characteristic of individuals with SUD. Accordingly, continued work toward understanding decision-making deficits stands to advance basic knowledge of the etiology of SUDs. This knowledge may be translated into therapeutic interventions that will help individuals with SUDs learn to make better choices.
Supplementary Material
Highlights.
Substance abusers typically show impaired decision-making relative to healthy control groups.
Studies comparing decision-making between individuals who abused different substances were reviewed.
Studies have not consistently demonstrated differences in decision-making as a function of drug of choice.
Prospective studies are needed to determine the trajectory of decision-making deficits in individuals who go on to develop substance use disorders.
Footnotes
Disclosures: All authors report no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Drug Alcohol Depend. 2009;100:17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater discounting of delayed rewards in young adults with family histories of alcohol and drug use disorders: studies from the Oklahoma family health patterns project. Alcohol Clin Exp Res. 2011;35:1607–1613. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, et al. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–365. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Aklin WM, Tull MT, Kahler CW, Lejuez CW. Risk-Taking Propensity Changes Throughout the Course of Residential Substance Abuse Treatment. Pers Individ Dif. 2009;46:454–459. doi: 10.1016/j.paid.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt AG, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. Guilford Press; 2005. [Google Scholar]
- Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J. Steep delay discounting and addictive behavior: a meta-analysis of continuous associations. Addiction. 2017;112:51–62. doi: 10.1111/add.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry D, Petry NM. Predictors of decision-making on the Iowa Gambling Task: independent effects of lifetime history of substance use disorders and performance on the Trail Making Test. Brain Cogn. 2008;66:243–252. doi: 10.1016/j.bandc.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp Clin Psychopharmacol. 2005;13:311–318. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- Bretteville-Jensen AL. Addiction and discounting. J Health Econ. 1999;18:393–407. doi: 10.1016/s0167-6296(98)00057-5. [DOI] [PubMed] [Google Scholar]
- Businelle MS, Apperson MR, Kendzor DE, Terlecki MA, Copeland AL. The relative impact of nicotine dependence, other substance dependence, and gender on Bechara Gambling Task performance. Exp Clin Psychopharmacol. 2008;16:513–520. doi: 10.1037/a0013510. [DOI] [PubMed] [Google Scholar]
- Businelle MS, McVay MA, Kendzor D, Copeland A. A comparison of delay discounting among smokers, substance abusers, and non-dependent controls. Drug Alcohol Depend. 2010;112:247–250. doi: 10.1016/j.drugalcdep.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell H, Finn PR, Rickert ME, Lucas J. Decision making in alcohol dependence: insensitivity to future consequences and comorbid disinhibitory psychopathology. Alcohol Clin Exp Res. 2008;32:1398–1407. doi: 10.1111/j.1530-0277.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman GB, Elstein AS. Valuing the Future: Temporal Discounting of Health and Money. Medical decision making. 1995;15(4):373–386. doi: 10.1177/0272989X9501500408. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Charles NE, Mathias CW, Ryan SR, Olvera RL, Liang Y, et al. Delay discounting differentiates pre-adolescents at high and low risk for substance use disorders based on family history. Drug Alcohol Depend. 2014;143:105–111. doi: 10.1016/j.drugalcdep.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Lake SL, Mathias CW, Ryan SR, Bray BC, Charles NE, et al. Behavioral Impulsivity and Risk-Taking Trajectories Across Early Adolescence in Youths With and Without Family Histories of Alcohol and Other Drug Use Disorders. Alcohol Clin Exp Res. 2015;39:1501–1509. doi: 10.1111/acer.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2004;28:1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn PC, Rubinstein A. Time Preference. Int Econ Rev. 1982;23:677. [Google Scholar]
- García-Rodríguez O, Secades-Villa R, Weidberg S, Yoon JH. A systematic assessment of delay discounting in relation to cocaine and nicotine dependence. Behav Processes. 2013;99:100–105. doi: 10.1016/j.beproc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology. 2002;163:174–182. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: Preliminary observations. J Clin Exp Neuropsychol. 2007;29:155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Mackey S, Paulus MP. Altered risk-related processing in substance users: imbalance of pain and gain. Drug Alcohol Depend. 2013;132:13–21. doi: 10.1016/j.drugalcdep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. DISCOUNTING OF DELAYED REWARDS:. A Life-Span Comparison. Psychol Sci. 1994;5:33–36. [Google Scholar]
- Hanson KL, Luciana M, Sullwold K. Reward-related decision-making deficits and elevated impulsivity among MDMA and other drug users. Drug Alcohol Depend. 2008;96:99–110. doi: 10.1016/j.drugalcdep.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Exp Clin Psychopharmacol. 2010;18:99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Choices, values, and frames. Am Psychol. 1984;39:341–350. [Google Scholar]
- Karakula SL, Weiss RD, Griffin ML, Borges AM, Bailey AJ, McHugh RK. Delay discounting in opioid use disorder: Differences between heroin and prescription opioid users. Drug Alcohol Depend. 2016;169:68–72. doi: 10.1016/j.drugalcdep.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN. Bidding on the future: Evidence against normative discounting of delayed rewards. J Exp Psychol Gen. 1997;126:54–70. [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kjome KL, Lane SD, Schmitz JM, Green C, Ma L, Prasla I, et al. Relationship between impulsivity and decision making in cocaine dependence. Psychiatry Res. 2010;178:299–304. doi: 10.1016/j.psychres.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH. Delay discounting is associated with substance use in college students. Addict Behav. 2003;28:1167–1173. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Konova AB, Lopez-Guzman S, Urmanche A, Messinger J, Ross S, Louie K, Rotrosen J, Glimcher PW. Week-to-Week Fluctuations in Risky Decision Making Track Heroin Use in Treatment-Seeking. Neuropsychopharmacology. 2016;41:S53. [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner N, Schmidt P, Soyka M. Decision making and impulsiveness in abstinent alcohol-dependent people and healthy individuals: a neuropsychological examination. Subst Abuse Treat Prev Policy. 2015;10:24. doi: 10.1186/s13011-015-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich C, Brevers D, Ermer E, Hanak C, Verbanck P, Campanella S, et al. Polysubstance dependent patients display a more utilitarian profile in moral decision-making than alcohol-dependent patients, depressive patients and controls. Drug Alcohol Depend. 2013;132:434–440. doi: 10.1016/j.drugalcdep.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the Oklahoma family health patterns project. Alcohol Clin Exp Res. 2006;30:763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control patients: Drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An Adjusting Procedure for Studying Delayed Reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analyses of Behavior, v5. The effect of Delay and of Intervening Events on Reinforcement Value. 1987. pp. 55–73. [Google Scholar]
- Mejía-Cruz D, Green L, Myerson J, Morales-Chainé S, Nieto J. Delay and probability discounting by drug-dependent cocaine and marijuana users. Psychopharmacology. 2016;233:2705–2714. doi: 10.1007/s00213-016-4316-8. [DOI] [PubMed] [Google Scholar]
- Moallem NR, Ray LA. Dimensions of impulsivity among heavy drinkers, smokers, and heavy drinking smokers: singular and combined effects. Addict Behav. 2012;37:871–874. doi: 10.1016/j.addbeh.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody L, Franck C, Bickel WK. Comorbid depression, antisocial personality, and substance dependence: Relationship with delay discounting. Drug Alcohol Depend. 2016a;160:190–196. doi: 10.1016/j.drugalcdep.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody L, Franck C, Hatz L, Bickel WK. Impulsivity and polysubstance use: A systematic comparison of delay discounting in mono-, dual-, and trisubstance use. Exp Clin Psychopharmacol. 2016b;24(1):30–37. doi: 10.1037/pha0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejtek VA, Kaiser KA, Zhang B, Djokovic M. Iowa Gambling Task scores predict future drug use in bipolar disorder outpatients with stimulant dependence. Psychiatry Res. 2013;210:871–879. doi: 10.1016/j.psychres.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- O’Brien JW, Lichenstein SD, Hill SY. Maladaptive decision making and substance use outcomes in high-risk individuals: preliminary evidence for the role of 5-HTTLPR variation. J Stud Alcohol Drugs. 2014;75:643–652. doi: 10.15288/jsad.2014.75.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: trait variable? Behav Processes. 2011;87:1–9. doi: 10.1016/j.beproc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of delayed rewards in substance abusers: relationship to antisocial personality disorder. Psychopharmacology. 2002;162:425–432. doi: 10.1007/s00213-002-1115-1. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93:729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- van der Plas EAA, Crone EA, van den Wildenberg WPM, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2009;31:706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson CG, Edalati H. Application of a Brief Measure of Delay Discounting to Examine the Relationship Between Delay Discounting and the Initiation of Substance Use Among Adolescents. Subst Use Misuse. 2016;51:540–544. doi: 10.3109/10826084.2015.1126740. [DOI] [PubMed] [Google Scholar]
- Robles E, Huang BE, Simpson PM, McMillan DE. Delay discounting, impulsiveness, and addiction severity in opioid-dependent patients. J Subst Abuse Treat. 2011;41:354–362. doi: 10.1016/j.jsat.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan ME, Iskric A, Low NC. The treatment of bipolar patients with elevated impulsivity and suicide risk. J Psychiatry Neurosci. 2014;39:E34–5. doi: 10.1503/jpn.130274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan RA, Alhassoon OM, Allen KE, Wollman SC, Hall M, Thomas WJ, et al. Meta-analyses of clinical neuropsychological tests of executive dysfunction and impulsivity in alcohol use disorder. Am J Drug Alcohol Abuse. 2016:1–20. doi: 10.1080/00952990.2016.1206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon Bull Rev. 2004;11:742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- Taylor EM, Murphy A, Boyapati V, Ersche KD, Flechais R, Kuchibatla S, et al. Impulsivity in abstinent alcohol and polydrug dependence: a multidimensional approach. Psychopharmacology. 2016;233:1487–1499. doi: 10.1007/s00213-016-4245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Judgment under Uncertainty: Heuristics and Biases. Science. 1974;185:1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- VanderBroek L, Acker J, Palmer AA, de Wit H, MacKillop J. Interrelationships among parental family history of substance misuse, delay discounting, and personal substance use. Psychopharmacology. 2016;233:39–48. doi: 10.1007/s00213-015-4074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva J, Gonzalez R, Bechara A, Martin EM. Are all drug addicts impulsive? Effects of antisociality and extent of multidrug use on cognitive and motor impulsivity. Addict Behav. 2007;32:3071–3076. doi: 10.1016/j.addbeh.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet J-L, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García AJ, Perales JC, Pérez-García M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, et al. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Cen S, Grenard JL, Stacy AW, Gallaher P, et al. Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th-grade Chinese adolescent smokers. Nicotine Tob Res. 2008;10:1085–1097. doi: 10.1080/14622200802097530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Lane SD, Weaver MF. Opioid Analgesics and Nicotine: More Than Blowing Smoke. J Pain Palliat Care Pharmacother. 2015;29:281–289. doi: 10.3109/15360288.2015.1063559. [DOI] [PubMed] [Google Scholar]
- Zhang X-L, Shi J, Zhao L-Y, Sun L-L, Wang J, Wang G-B, et al. Effects of stress on decision-making deficits in formerly heroin-dependent patients after different durations of abstinence. Am J Psychiatry. 2011;168:610–616. doi: 10.1176/appi.ajp.2010.10040499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.