Abstract
β-cell autoantibodies against insulin (IAA), GAD65 (GADA) and IA-2 (IA-2A) precede onset of childhood type 1 diabetes (T1D). Incidence of the first appearing β-cell autoantibodies peaks at a young age and is patterned by T1D-associated genes, suggesting an early environmental influence. Here, we tested if gestational infections and interactions with child’s human leukocyte antigen (HLA) and non-HLA genes affected the appearance of the first β-cell autoantibody. Singletons of mothers without diabetes (n=7472) with T1D-associated HLA-DR-DQ genotypes were prospectively followed quarterly through the first 4 years of life, then semiannually until age 6 years, using standardized autoantibody analyses. Maternal infections during pregnancy were assessed via questionnaire 3–4.5 months post-delivery. Polymorphisms in twelve non-HLA genes associated with the first appearing β-cell autoantibodies were included in a Cox regression analysis. IAA predominated as the first appearing β-cell autoantibody in younger children (n=226, median age at seroconversion 1.8 years) and GADA (n=212; 3.2 years) in children aged ≥2 years. Gestational infections were not associated with the first appearing β-cell autoantibodies overall. However, gestational respiratory infections (G-RI) showed a consistent protective influence on IAA (HR 0.64, 95% CI 0.45–0.91) among CTLA4-(AG, GG) children (G-RI*CTLA4 interaction, p=0.002). The predominant associations of HLA-DR-DQ 4-8/8-4 with IAA and HLA-DR-DQ 3-2/3-2 with GADA were not observed if a G-RI was reported (G-RI*HLA-DR-DQ interaction, p=0.03). The role of G-RI may depend on offspring HLA and CTLA-4 alleles and supports a bidirectional trigger for IAA or GADA as a first appearing β-cell autoantibody in early life.
Keywords: β-cell autoantibodies, glutamic acid decarboxylase, IA-2, insulin, HLA, autoimmunity, type 1 diabetes, autoimmune diabetes
1. Introduction
The etiology of type 1 diabetes (T1D) remains unresolved. For children with genetic predisposition to T1D, recent studies suggest the pathogenesis is triggered early in life resulting in the appearance of autoantibodies against pancreatic islet β-cell autoantigens, specifically against insulin (IAA), glutamic acid decarboxylase 65 (GADA), insulinoma-associated antigen-2 (IA-2A) or zinc transporter 8 (ZnT8A)(1–3). The genetic etiology of T1D is closely associated with human leukocyte antigen (HLA) class II genes; in particular the DR3-DQ2 and DR4-DQ8 haplotypes (4, 5). These well-known associations were used in The Environmental Determinants of Diabetes in the Young (TEDDY) study to genetically screen and select newborns from the general population (GP) (90%) and in first degree relatives (FDR) (10%) at increased risk for T1D. In this prospective study, the first appearing β-cell autoantibody is the first primary endpoint and children are being followed for the appearance of additional autoantibodies until the clinical onset of diabetes (second primary endpoint) (5–10).
Prior epidemiological studies, with T1D as the end-point, suggest the gestational environment may play a role as exposures such as older maternal age (11), preeclampsia of mothers in some (12) but not all (13) studies, and jaundice caused by blood group incompatibility (12, 14), low birth weight (14–16), or a short birth length (17) affected the risk for T1D. A meta-analysis of 20 studies suggested that caesarean section increased the risk for T1D (18). In addition, smoking known to be associated with low birth weight (19) was reported to either decrease (15, 20) or increase (21) risk for T1D in the offspring.
Conflicting findings on the association between gestational infections and T1D risk have been reported. Maternal coxsackie B virus infection appears to increase (22, 23) or have no effect (24) on the risk for T1D in the offspring. Other studies suggested that the prevalence of newborn β-cell autoantibodies consistently varied with season and reported gestational infections (25). Changing epidemiological trends in T1D incidence also suggest an improved hygienic environment and lower exposure to infections in early life may be leading to a dysfunctional immune response to pathogens later in life (26, 27).
Interestingly, humoral immune responses to enterovirus infections were increased in mothers with T1D-associated HLA DR-DQ genotypes (28, 29). Furthermore, offspring of mothers, who reported gestational infections, were more likely to have higher relative birth weight and the association was dependent on HLA-DR-DQ genotypes of the of the offspring (30). Interactions between gestational infections with HLA and non-HLA (31) genes on risk for the first β-cell autoantibody (1, 3) have not previously been analyzed yet a dependency on risk genes may help explain conflicting associations between gestational infections and T1D.
The TEDDY study revealed that the age at appearance and order of the first appearing β-cell autoantibodies were distinctly characterized by HLA genotypes as well as by non-HLA effects of PTPN22, INS and CTLA4 gene polymorphisms, indicating that the influence of triggers may be age dependent (1). Furthermore, using non-HLA single nucleotide polymorphisms (SNP) that reached significance in genome-wide association studies carried out by the Type 1 Diabetes Genetics Consortium (32), it was found that six additional SNPs were associated with the first appearing β-cell autoantibody rather than T1D (31). When the TEDDY children were analysed for about 186,000 SNP using the Illumina ImmunoChip custom array, three additional polymorphisms in the complement region within the ITGAM gene were also found nominally associated with the first appearing β-cell autoantibody (33). These non-HLA genetic factors need to be considered when investigating the trigger of the first β-cell autoantibody (1).
Using the TEDDY study, we set out to examine if gestational infections were associated with risk of either IAA or GADA as the first appearing β-cell autoantibody. We hypothesize that gestational infections show interaction with T1D genes of the child in their association with the age-related first appearing β-cell autoantibodies. Such evidence would create stronger support for the role of intrauterine environment and prenatal exposures on the risk for β-cell autoimmunity and T1D.
2. Material and methods
2.1 Study population
The Environmental Determinants of Diabetes in the Young (TEDDY) is a prospective cohort study funded by the National Institutes of Health with the primary goal to identify environmental causes of T1D (10). It includes six clinical research centers - three in the US: Colorado, Georgia/Florida, Washington and three in Europe: Finland, Germany, and Sweden. Detailed study design and methods have been previously published (1, 10). Infants with T1D-associated high-risk HLA-DR-DQ genotypes younger than 4.5 months were eligible for the follow-up (10). After screening, TEDDY enrolled 8,676 infants. Of these infants 7472 singleton babies included (Twins/Triplets, n=241) were confirmed at 9 months to be carrying one of the TEDDY eligible HLA genotypes (5) (ineligible HLA, n=118), the mothers did not experience pre-gestational type 1, type 2 or gestational diabetes (n=790), and they were analyzed for autoantibodies during follow-up (indeterminate autoantibodies; n=55). Written informed consents were obtained for all study participants from a parent or primary caretaker, separately, for genetic screening and participation in prospective follow-up. The study was approved by local Institutional Review Boards and is monitored by an External Advisory Board formed by the National Institutes of Health.
2.2 Non-HLA genotyping
As previously described (1) when the child was 9 to 12 months of age, the HLA-DR-DQ genotypes were confirmed by reverse blot hybridization at the central HLA Reference Laboratory at Roche Molecular Systems, Oakland, CA (5), along with the INS-23Hph1 (rs689), CTLA4 T17A (rs231775) and PTPN22 R620W (rs2476601) SNP primer pairs. Further SNP analysis was performed by the Center for Public Health Genomics at University of Virginia using the Illumina ImmunoChip. The final selection of SNPs containing 186,000 SNPs in 186 regions for 12 autoimmune diseases, was decided by the ImmunoChip Consortium. TEDDY previously examined whether any non-HLA SNPs previously shown to be associated with T1D conferred risk for IA (31, 33). Six additional SNPs were identified: rs2816316 in RGS1, rs10517086 in a region on chromosome 4p15.2 without any known genes, rs2292239 in ERBB3, rs3184504 in SH2B3, rs4948088 in COBL and rs12708716 in CLEC16A. Also, TEDDY previously examined 15 SNPs within the complement gene and identified three within ITGAM associated with risk of IA, rs1143678, rs1143683 and rs4597342 (33). These twelve SNPs were included in this report.
2.3 Gestational Infections
Maternal and gestational factors were obtained through questionnaires when the child was 3–4.5 months old and included infections during any stage of pregnancy. The maternal infections, from the list in the questionnaire, were categorized as upper respiratory tract infection (flu or bad cold, sore throat, sinusitis, ear infection), lower respiratory tract infection (bronchitis or pneumonia); gastroenteritis (or diarrhea); and other infections that included skin infection or rash; kidney, bladder or urinary tract infection; cold sores; genital herpes or unknown infection with fever.
2.4 Other Environmental Exposures
Our previous publication showed that introduction of probiotics before the age 28 day was associated with lower risk of β-cell autoantibodies and may be a confounder (34). Probiotic exposure was defined as timing of first introduction to probiotics from either dietary supplementation or infant formula that were monitored from birth using questionnaire and diaries. Early infectious related conditions were recorded at the 3-month clinic visit. The most commonly reported infectious conditions were diarrhea and respiratory tract-related illnesses. Upper respiratory infections for the infant was defined as having a cold or runny nose in addition to having either an ear infection or eye discharge. A lower respiratory infection was defined as having either pneumonia, difficulty of breathing or respiratory problems.
2.5 β-cell autoimmunity and clinical type 1 diabetes
β-cell autoimmunity was defined as the presence of one or more persistent confirmed β-cell autoantibodies against GAD65 (GADA) IA-2 (IA-2A), or insulin (IAA) on two or more consecutive samples (1). The autoantibodies were measured in two laboratories (Barbara Davis Center, Aurora, Colorado, and the University of Bristol Laboratory, Bristol, UK) depending upon the location of the clinical site. All samples identified as positive in one laboratory were sent to the other laboratory for confirmation (35). Blood sample was collected from the 3 month study visit and continued at a 3 month interval up to 4 years of age. If a child developed β-cell autoantibodies, then they continued on the 3 month interval schedule up to age 15 years; otherwise, they switched to a 6 month interval schedule after the age of 4 years. This study examined the child’s first appearing β-cell autoantibodies against one of GAD65, IA-2 or insulin referred to as GADA first, IA-2A first or IAA first.
2.6 Statistics
Proportional hazard models were used to test whether gestational infections alone or together with the T1D genetic factors were associated with GADA or IAA as the first appearing β-cell autoantibody. Children negative for the specific first appearing β-cell autoantibodies were right censored after the date of the last negative sample, the child’s seven year birthday, or the date of seroconversion for any competing first appearing autoantibody, whichever came first. Hazard ratios (HR) and 95% Confidence Intervals (CI) estimated the autoantigen-specific, “cause-specific”, risk of β-cell autoimmunity. The associations between gestational infections and the first appearing β-cell autoantibodies were tested for effect modification by the genetic factors by including an interaction term in the model. Differences in association with IAA first and GADA first, two mutually exclusive events, were also examined by comparing parameter estimates from the respective models using a Wald test. A family-wise Bonferroni correction was made to correct for number of separate SNP interactions (n=12) with a p-value <0.004 considered significant. Significant associations were adjusted for other related β-cell autoantibody risk factors (HLA-DR-DQ, SNPs, probiotics, birth weight, early infections) and examined for consistency across countries. Consistency by age was also examined graphically by plotting the general trend in the age-specific incidence of autoantibodies within groups. The observed incidence was calculated within each visit window (3-month window up to 4 years and 6-month thereafter) and a coarse LOWESS curve was fitted through the incidences using GraphPad PRISM 5.03 (GraphPad Software Inc., San Diego, CA). For each SNP the minor allele was examined for associations with outcomes. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
3. Results
3.1 Gestational infections
Among the mothers of children residing within the TEDDY countries (US, n=3201; Finland, n=1507; Germany; n=434; and Sweden, n=2330), 45% (3359/7472) reported a gestational upper respiratory tract infection; 4% (298/7472) a lower respiratory tract infection, 21.4% (1601/7472) gastroenteritis, and 30.2% (2259/7472) another specified infection, (Supplementary Figure 1). Maternal gastroenteritis (Supplementary Table 1) or other non-respiratory infections were not associated with the appearance of β-cell autoantibodies in the child and were not further examined. Of the maternally reported upper respiratory tract infections (n=3359), 73% reported an influenza “flu” or bad cold, 43% sore throat, 25% sinusitis, 6% ear infection. Of the lower respiratory tract (n=298), 92% reported bronchitis, 14% pneumonia and only 18% (52/298) of the mothers reporting lower tract infection did not report an upper tract infection. Of the mothers not reporting a respiratory infection, 3647/4016 (91%) answered no to every symptom while the remaining 414/4016 (9%) did not answer or answered unknown at least once.
3.2 First appearing β-cell autoantibodies
The first appearing β-cell autoantibody was determined in 447 of the 536 singleton children who seroconverted to β-cell autoantibodies up to the age of six years (IAA first, n=226; IA-2A first, n=9, GADA first, n=212). The median (IQR) age of seroconversion for children developing IAA as the first β-cell autoantibody (1.75, (0.99 – 3.01) years) was lower (p<0.001) compared to children who developed GADA as the first β-cell autoantibody (median (IQR) age (3.18, (1.78 – 4.71) years). In comparison, children seroconverting for IA-2A first were older (4.78 (3.99 – 5.58) years. The remaining 89/536 β-cell autoantibody positive children (IA-2A and GADA, n=1; GADA and IAA, n=69, IAA and IA-2A, n=4; GADA, IAA and IA-2A, n=15) developed two or more β-cell autoantibodies since their last sample and thus the first appearing β-cell autoantibody could not be determined (median age of seroconversion was 2.27 (1.27 – 3.26) years). Gestational Respiratory Infection (G-RI) information was available on 211/226 (93%) of the children developing IAA first and 206/212 (97%) of the children developing GADA first. Of the 69 children with GADA and IAA appearing together as the first β-cell autoantibodies at seroconversion, a majority 64% (44/69) had the highest T1D risk HLA-DR-DQ3-2/4-8 genotype of which 42/44 had available G-RI information.
3.3 Gestational Respiratory infection (G-RI) on IAA-first
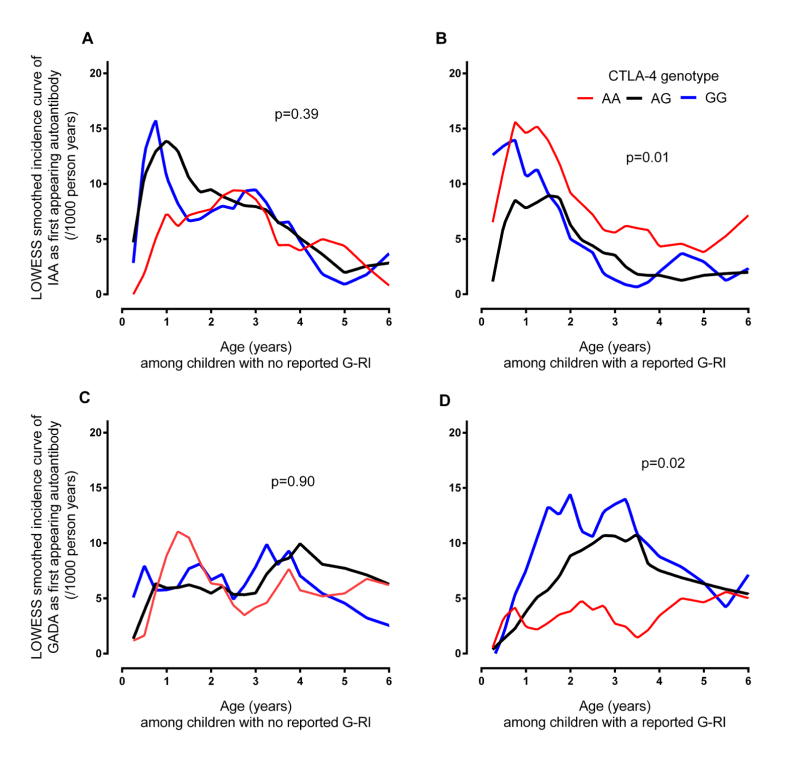
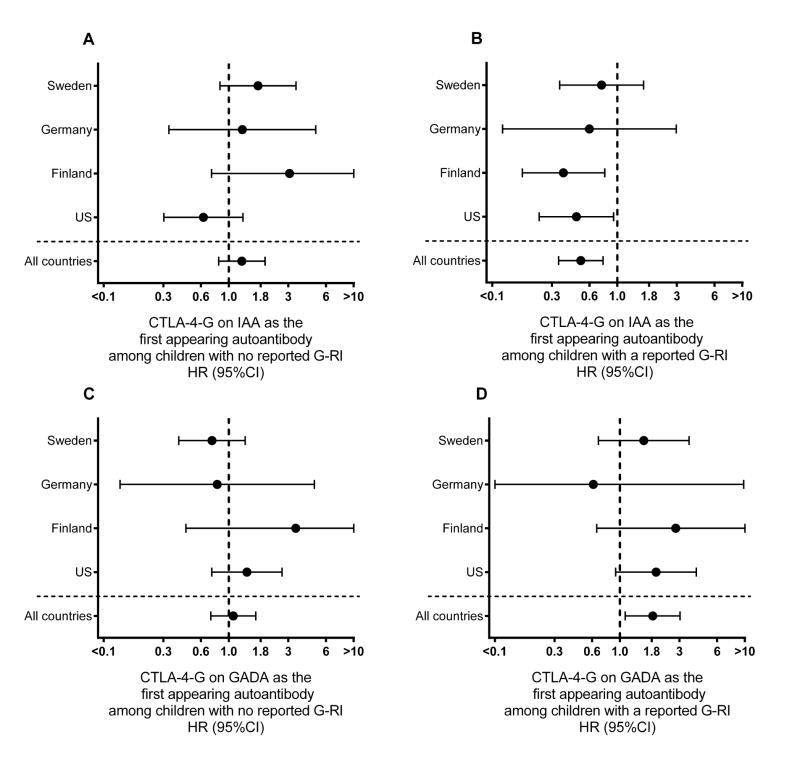
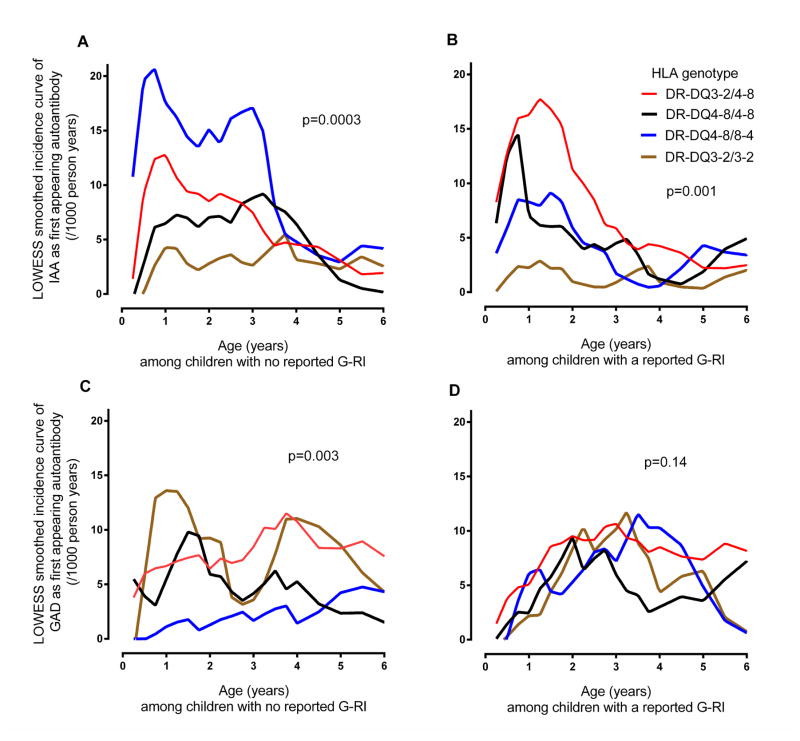
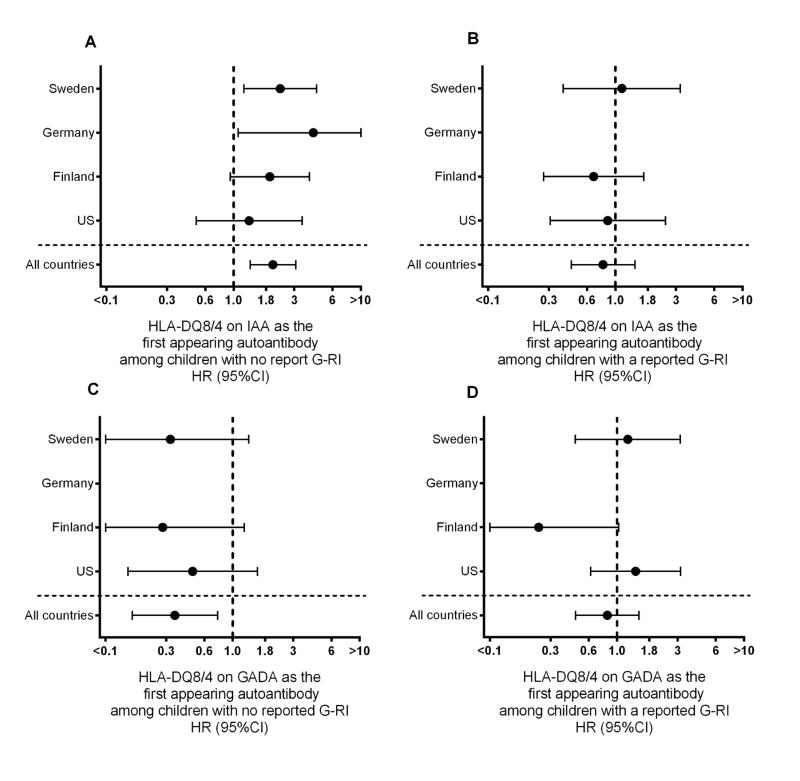
Overall, G-RI did not influence the incidence of IAA as the first appearing β-cell autoantibody (HR=0.88, 95%CI = 0.67–1.15). However, a significant interaction was observed with the HLA-DQ genotypes (p=0.03) and CTLA-4 alleles (p=0.002). G-RI was associated with a reduced risk of IAA among HLA-DR-DQ4-8/8-4 (HR = 0.45, 95%CI = 0.24 – 0.84) and CTLA-4-(AG, GG) children (HR = 0.64, 95%CI = 0.45 – 0.91) but no significant association was seen among the CTLA-4-AA children and other HLA-DR-DQ genotypes (Table 1). While the protective association of G-RI on IAA was similar for HLA-DR-DQ4-8/8-4 and CTLA-4-(AG, GG), the impact of G-RI on the variation in IAA incidence across HLA-DR-DQ and CTLA-4 genotypes was different. Compared to CTLA-4-AA, the incidence of IAA first among CTLA-4-(AG, GG) children was consistently lower by age of the child (Figure 1B) and country (Figure 2B), when a G-RI was reported, however it was close to average incidence or inconsistent when a G-RI was not reported (Figure 1A, Figure 2A). In contrast, when a G-RI was not reported, HLA-DR-DQ4-8/8-4 children had the highest incidence of IAA first across the HLA-DR-DQ genotypes (Figure 3A), an association that was consistent by country (Figure 4A), but IAA incidence was close to average if a G-RI was reported (Figure 3B, Figure 4B). The increased risk of IAA first among HLA-DR-DQ4-8/8-4 children in the absence of a G-RI and reduced risk of IAA first by CTLA-4-(AG, GG) following a G-RI remained when adjusting for other risk factors (Table 2).
Table 1.
Gestational Respiratory Infection (G-RI) in relation to IAA and GADA as the first appearing β-cell autoantibodies by genetic factors
| Genetic factor | G-RI in relation to IAA event as first appearing beta cell autoantibodies | G-RI in relation to GADA event as first appearing beta cell autoantibodies | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Total | Events | G-RI on IAA-first | Events | G-RI on GADA-first | ||||
|
| ||||||||
| N | n | HR (95%CI) | p-valueA | n | HR (95%CI) | p-valueA | ||
|
| ||||||||
| All children | 7058 | 211 | 0.88 (0.67, 1.15) | 0.35 | 206 | 0.95 (0.73, 1.25) | 0.73 | |
|
| ||||||||
| General Population (GP) | 6530 | 171 | 0.90 (0.67, 1.22) | 0.59 | 175 | 1.06 (0.79, 1.43) | 0.05 | |
| First Degree Relative (FDR) | 528 | 44 | 0.75 (0.40, 1.39) | 31 | 0.48 (0.22, 1.01) | |||
|
| ||||||||
| HLA-DQ | DQ8/8 | 1396 | 36 | 1.16 (0.60, 2.23) | 0.03 | 31 | 1.09 (0.55, 2.20) | 0.06 |
| DQ2/8 | 2778 | 101 | 1.19 (0.80, 1.77) | 103 | 1.02 (0.69, 1.49) | |||
| DQ8/4 | 1221 | 49 | 0.45 (0.24, 0.84)B | 21 | 2.26 (0.91, 5.60)B | |||
| DQ2/2 | 1510 | 16 | 0.48 (0.17, 1.38) | 50 | 0.54 (0.30, 0.97) | |||
|
| ||||||||
| SNPs | ||||||||
|
| ||||||||
| rs2476601 (PTPN22-A) | No | 4991 | 144 | 0.90 (0.65, 1.25) | 0.86 | 146 | 1.12 (0.81, 1.56) | 0.05 |
| Yes | 1265 | 64 | 0.85 (0.52, 1.40) | 57 | 0.59 (0.35, 1.02) | |||
|
| ||||||||
| rs689 (INS-A) | No | 3404 | 153 | 0.94 (0.68, 1.29) | 0.43 | 106 | 0.78 (0.53, 1.14) | 0.13 |
| Yes | 2853 | 55 | 0.73 (0.43, 1.25) | 98 | 1.18 (0.79, 1.76) | |||
|
| ||||||||
| rs231775 (CTLA4-G) | No | 1971 | 72 | 1.59 (0.99, 2.54)B | 0.002 | 52 | 0.62 (0.35, 1.08)B | 0.08 |
| Yes | 4286 | 136 | 0.64 (0.45, 0.91)B | 152 | 1.10 (0.80, 1.51)B | |||
|
| ||||||||
| rs2292239 (ERBB3-T) | No | 1657 | 70 | 0.94 (0.59, 1.50) | 0.55 | 74 | 0.94 (0.60, 1.48) | 0.80 |
| Yes | 3104 | 124 | 0.78 (0.55, 1.12) | 118 | 1.01 (0.71, 1.45) | |||
|
| ||||||||
| rs3184504 (SH2B3-T) | No | 1813 | 50 | 1.07 (0.62, 1.87) | 0.31 | 40 | 1.21 (0.65, 2.26) | 0.47 |
| Yes | 3948 | 144 | 0.77 (0.55, 1.07) | 152 | 0.94 (0.68, 1.29) | |||
|
| ||||||||
| rs12708716 (CLEC16A_G) | No | 2528 | 91 | 1.10 (0.73, 1.65) | 0.08 | 96 | 0.95 (0.64, 1.42) | 0.78 |
| yes | 3218 | 103 | 0.65 (0.44, 0.98) | 96 | 1.03 (0.69, 1.54) | |||
|
| ||||||||
| rs10517086-A | No | 2951 | 93 | 0.70 (0.46, 1.06) | 0.26 | 105 | 0.90 (0.61, 1.32) | 0.50 |
| yes | 2810 | 101 | 0.97 (0.66, 1.44) | 87 | 1.10 (0.72, 1.67) | |||
|
| ||||||||
| rs4948088 (COBL-A) | No | 5240 | 185 | 0.82 (0.61, 1.09) | 0.76 | 178 | 0.98 (0.73, 1.32) | 0.94 |
| yes | 521 | 9 | 1.00 (0.27, 3.73) | 14 | 0.95 (0.33, 2.73) | |||
|
| ||||||||
| rs2816316 (RGS1-C) | No | 3845 | 129 | 0.81 (0.57, 1.15) | 0.81 | 130 | 1.09 (0.77, 1.54) | 0.29 |
| yes | 1916 | 65 | 0.87 (0.54, 1.43) | 62 | 0.78 (0.47, 1.30) | |||
p-value is test of association between G-RI and first appearing autoantibody for all children and, when stratifying by genetic factor, p-value is test of multiplicative interaction between genetic factor and G-RI on first appearing autoantibody.
Among stratified group, HR of G-RI on IAA-first differs from HR of G-RI on GADA-first; Wald test: p-value <0.05
Fig 1. Smoothed incidence curve of IAA and GADA as first appearing β-cell autoantibodies from birth to age six years by report of a Gestational Respiratory Infection (G-RI) and offsprings CTLA-4 genotype.
A LOWESS curve was plotted through the observed incidences of first appearing β-cell autoantibodies calculated at 3 month intervals during the first 4 years of life and at 6 month intervals thereafter. Incidence curves for IAA-first (panels A and B) and GADA-first (panels C and D) were drawn for children with the CTLA-4 AA (red), AG (black) and GG (blue) genotypes and separately for those offspring of mothers who did (panels B and D) or did not report a G-RI (panels A and C). P-values describing the differences in the risk of the first appearing β-cell autoantibody across the CTLA-4 genotypes were calculated from Proportional Hazard models of CTLA-4 genotypes regressed on the autoantigen-specific hazard of β-cell autoimmunity. The overall Wald test p-value is shown in each panel. In panel (A) IAA-first appeared in 29/1016 with CTLA-4 AA genotype, 61/1576 with CTLA-4 AG and 24/651 with CTLA-4 GG among children with no reported G-RI. In panel (B) IAA-first appeared in 43/955 with CTLA-4 AA, 35/1498 with CTLA-4 AG and 16/561 with CTLA-4 GG among children with a reported G-RI. In panel (C) GADA-first appeared in 33/1016 with CTLA-4 AA, 54/1576 with CTLA-4 AG and 21/651 with CTLA-4 GG among children with no reported G-RI. In panel (D) GADA-first appeared in 19/955 with CTLA-4 AA, 51/1498 with CTLA-4 AG and 26/561 with CTLA-4 GG among children with a reported G-RI.
Fig 2. Country specific assocaiation between CTLA-4 G allele and first appearing β-cell autoantibodies by whether or not a gestational respiratory infection (G-RI) was reported.
The association between a child having the CTLA-4 G allele and risk of IAA (panels A and B) or GADA (panels C and D) as the first appearing β-cell autoantibodies are evaluated by Proportional Hazard models. The autoantigen-specific hazard ratios (HR) and 95% CI are provided overall and by country both for offspring of mothers who did (panels B and D) and did not (panels A and C) a reported G-RI. HR<1 indicated children with CTLA-4 G allele had a lower autoantigen-specific risk of the first appearing autoantibody. In panels A and C, CTLA-4 G was shown in relation to IAA-first (A) and GADA-first (C) for 3243 offspring (1273 US, 658 Finland, 155 Germany, 1157 Sweden) of mothers not reporting a G-RI. In panels B and D, CTLA-4 G was shown in relation to IAA-first (B) and GADA-first (D) for 3014 offspring (1307 US, 646 Finland, 198 Germany, 863 Sweden) of mothers who did report a G-RI.
Fig 3. Smoothed incidence curve of IAA and GADA as first appearing β-cell autoantibodies from birth to age six years by report of a gestational respiratory Infection (G-RI) and offsprings HLA-DR-DQ genotype.
A LOWESS curve was plotted through the observed incidences of first appearing β-cell autoantibodies calculated at 3 month intervals during the first 4 years of life and at 6 month intervals thereafter. Incidence curves for IAA-first (panels A and B) and GADA-first (panels C and D) were drawn for children with the HLA-DR-DQ3-2/4-8 (red), HLA-DR-DQ4-8/4-8 (black), HLA-DR-DQ4-8/8-4 (blue) and HLA-DR-DQ3-2/3-2 (brown) genotypes and separately for those offspring of mothers who did (panels B and D) or did not report a G-RI (panels A and C). P-values describing the differences in the risk of the first appearing β-cell autoantibody across the HLA genotypes were calculated from Proportional Hazard models that regressed HLA on the autoantigen-specific hazard of β-cell autoimmunity. The overall Wald test p-value is shown in each panel. In panel (A) IAA-first appeared in 47/1415 with HLA-DR-DQ3-2/4-8 genotype, 18/741 with HLA-DR-DQ4-8/4-8, 35/645 with HLA-DR-DQ4-8/8-4 and 11/755 with HLA-DR-DQ3-2/3-2 for children with no reported G-RI. In panel (B) IAA-first appeared in 54/1363 with HLA-DR-DQ3-2/4-8, 18/655 with HLA-DR-DQ4-8/4-8, 14/576 with HLA-DR-DQ4-8/8-4 and 5/735 with HLA-DR-DQ3-2/3-2 for children with a reported G-RI. In panel (C) GADA-first appeared in 52/1415 with HLA-DR-DQ3-2/4-8, 16/741 with HLA-DR-DQ4-8/4-8, 7/645 with HLA-DR-DQ4-8/8-4 and 33/775 with HLA-DR-DQ3-2/3-2 for children with no reported G-RI. In panel (D) GADA-first appeared in 51/1363 with HLA-DR-DQ3-2/4-8, 15/655 with HLA-DR-DQ4-8/4-8, 14/576 with HLA-DR-DQ4-8/8-4 and 17/735 with HLA-DR-DQ3-2/3-2 for children with no reported G-RI.
Fig 4. Country specific association between HLA-DR-DQ4-8/8-4 (DQ8/4) genotype and first appearing β-cell autoantibodies by whether or not a gestational respiratory infection (G-RI) was reported.
The association between a child having the HLA-DQ8/4 genotype and risk of IAA (panels A and B) or GADA (panels C and D) as the first appearing β-cell autoantibodies are evaluated by Proportional Hazard models. The autoantigen-specific hazard ratios (HR) and 95% CI are provided overall and by country, both for offspring of mothers who did (panels B and D) and did not (panels A and C) a reported G-RI. HR<1 indicated children with the HLA-DQ8/4 genotype had a lower autoantigen specific risk of the first appearing autoantibody. In panels A and C, HLA-DQ8/4 was shown in relation to IAA-first (A) and GADA-first (C) for 3647 offspring (1491 US, 712 Finland, 188 Germany, 1256 Sweden) of mothers not reporting a G-RI. In panels B and D, HLA-DQ8/4 was shown in relation to IAA-first (B) and GADA-first (D) for 3411 offspring (1563 US, 688 Finland, 235 Germany, 925 Sweden) of mothers who did report a G-RI.
Table 2.
Hazard ratios (HR) from a multivariate proportional hazard model for IAA as first appearing β-cell autoantibodies in the offspring of mothers who did and did not report gestational respiratory infection
| Factor | Comparison | Gestational respiratory infection | |||
|---|---|---|---|---|---|
| No (Total, n=2879) (IAA-first, n = 104) | Yes (Total, n=2652) (IAA-first, n= 80) | ||||
| Factors on IAA-first | |||||
|
| |||||
| HR (95%CI) | p-value | HR (95%CI) | p-value | ||
|
| |||||
| Sex | Male vs. Female | 1.23 (0.83, 1.82) | 0.30 | 1.38 (0.88, 2.16) | 0.17 |
|
| |||||
| Family History | FDR Dad/Sibling vs. GP | 2.74 (1.56, 4.81) | 0.0004 | 3.85 (1.95, 7.59) | 0.0001 |
|
| |||||
| HLA | DQ2/8 vs. DQ8/8 | 1.44 (0.80, 2.57) | 0.22 | 1.31 (0.74, 2.32) | 0.35 |
| DQ8/4 vs. DQ8/8 | 2.63 (1.41, 4.91) | 0.002 | 0.83 (0.40, 1.72) | 0.61 | |
| DQ2/2 vs. DQ8/8 | 0.66 (0.30, 1.46) | 0.30 | 0.17 (0.05, 0.60) | 0.005 | |
| DQ2,8/xA vs. DQ8/8 | 1.10 (0.36, 3.38) | 0.86 | 0.49 (0.14, 1.66) | 0.25 | |
|
| |||||
| SNPs | |||||
|
| |||||
| rs689 (INS-A) | Yes vs. No | 0.48 (0.31, 0.73) | 0.0007 | 0.36 (0.21, 0.61) | 0.0002 |
|
| |||||
| rs231775 (CTLA4-G) | Yes vs. No | 1.43 (0.91, 2.26) | 0.13 | 0.50 (0.32, 0.79) | 0.003 |
|
| |||||
| rs2476601 (PTPN22-A) | Yes vs. No | 1.91 (1.25, 2.91) | 0.003 | 1.81 (1.13, 2.91) | 0.01 |
|
| |||||
| rs2292239 (ERBB3-T) | Yes vs. No | 1.72 (1.14, 2.58) | 0.009 | 1.44 (0.91, 2.27) | 0.12 |
|
| |||||
| rs3184504 (SH2B3-T) | Yes vs. No | 1.67 (1.04, 2.68) | 0.03 | 1.06 (0.65, 1.73) | 0.81 |
|
| |||||
| rs12708716 (CLEC16A-G) | Yes vs. No | 0.97 (0.65, 1.43) | 0.86 | 0.65 (0.42, 1.02) | 0.06 |
|
| |||||
| rs10517086-(A) | Yes vs. No | 0.89 (0.60, 1.32) | 0.56 | 1.36 (0.87, 2.12) | 0.18 |
|
| |||||
| rs4948088 (COBL-A) | Yes vs. No | 0.44 (0.18, 1.08) | 0.07 | 0.62 (0.22, 1.70) | 0.35 |
|
| |||||
| rs2816316 (RGS1-C) | Yes vs. No | 0.98 (0.64, 1.48) | 0.90 | 1.11 (0.69, 1.78) | 0.67 |
|
| |||||
| Birth weight | /Kg | 1.33 (0.91, 1.94) | 0.14 | 0.83 (0.53, 1,32) | 0.43 |
|
| |||||
| Probiotics at 28 days | Yes vs. No or ≥28 days | 0.38 (0.14, 1.05) | 0.06 | 0.67 (0.28, 1.61) | 0.37 |
|
| |||||
| Child conditions before first clinical visit (age 3 months) | Upper Resp. (Yes vs. No) | 1.76 (1.14, 2.70) | 0.01 | 1.10 (0.67, 1.82) | 0.71 |
|
|
|||||
| Lower Resp. (Yes vs. No) | 0.71 (0.34, 1.48) | 0.36 | 1.20 (0.61, 2.37) | 0.59 | |
|
|
|||||
| Diarrhea (Yes vs. No) | 1.00 (0.46, 2.19) | 0.99 | 2.25 (1.26, 4.01) | 0.006 | |
HLA-DQ8/X or HLA-DQ2/X children with a dad or sibling having T1D (~3.4% of total)
3.4 Gestational Respiratory infection (G-RI) on GADA first
G-RI did not influence the incidence of GADA as the first appearing autoantibody (HR=0.95, 95%CI = 0.73–1.25) and there was no significant interactions by the T1D genetic factors (Table 1). Nevertheless, among HLA-DR-DQ3-2/3-2 children, G-RI was associated with a moderately lower the risk of GADA (HR=0.54, 95%CI = 0.30 – 0.97). Also, while the incidence of GADA among the HLA-DR-DQ4-8/8-4 children was low, the correlation between G-RI and GADA (HR=2.26) was significantly different compared to G-RI and IAA (HR=0.45, p=0.004)(Table 1). As a result a significant variation in risk of GADA across the four HLA-DR-DQ genotypes was observed only if a G-RI was not reported (Figures 3C, 4C, Table 3). There was also evidence that CTLA-4-G increased the risk of GADA first if a G-RI was reported, (Figures 1D, 2D) but the association was reduced after adjusting for other factors (Table 3).
Table 3.
Hazard ratios (HR) from a multivariate proportional hazard model for GADA as first appearing β-cell autoantibodies in the offspring of mothers who did and did not report gestational respiratory infection
| Factor | Comparison | Gestational respiratory infection | |||
|---|---|---|---|---|---|
| No (total, n=2879) (GADA-first, n= 95) | Yes (total, n=2652) (GADA-first, n= 86) | ||||
| Factors on GADA-first | |||||
|
| |||||
| HR (95%CI) | p-value | HR (95%CI) | p-value | ||
|
| |||||
| Sex | Male vs. Female | 1.17 (0.78, 1.76) | 0.46 | 0.96 (0.63, 1.49) | 0.87 |
|
| |||||
| Family History | FDR Dad/Sibling vs. GP | 3.69 (2.20, 6.21) | <0.0001 | 2.62 (1.28, 5.39) | 0.009 |
|
| |||||
| HLA | DQ2/8 vs. DQ8/8 | 2.10 (1.11, 3.97) | 0.02 | 1.71 (0.94, 3.11) | 0.08 |
| DQ8/4 vs. DQ8/8 | 0.77 (0.30, 1.99) | 0.59 | 0.99 (0.46, 2.16) | 0.98 | |
| DQ2/2 vs. DQ8/8 | 2.29 (1.16, 4.49) | 0.02 | 0.81 (0.38, 1.73) | 0.58 | |
| DQ2,8/xA vs. DQ8/8 | 0.30 (0.04, 2.44) | 0.26 | - | - | |
|
| |||||
| SNPs | |||||
|
| |||||
| rs689 (INS-A) | Yes vs. No | 0.85 (0.56, 1.28) | 0.43 | 1.43 (0.94, 2.20) | 0.10 |
|
| |||||
| rs231775 (CTLA4-G) | Yes vs. No | 1.17(0.75, 1.82) | 0.50 | 1.65 (0.98, 2.79) | 0.06 |
|
| |||||
| rs2476601 (PTPN22-A) | Yes vs. No | 2.15 (1.40, 3.31) | 0.0005 | 1.20 (0.71, 2.00) | 0.50 |
|
| |||||
| rs2292239 (ERBB3-T) | Yes vs. No | 1.42 (0.94, 2.15) | 0.10 | 1.39 (0.90, 2.15) | 0.14 |
|
| |||||
| rs3184504 (SH2B3-T) | Yes vs. No | 1.77 (1.06, 2.97) | 0.03 | 1.51 (0.92, 2.47) | 0.11 |
|
| |||||
| rs12708716 (CLEC16A-G) | Yes vs. No | 0.68 (0.45, 1.02) | 0.06 | 0.78 (0.51, 1.19) | 0.25 |
|
| |||||
| rs10517086-(A) | Yes vs. No | 0.80 (0.53, 1.20) | 0.28 | 0.96 (0.62, 1.46) | 0.84 |
|
| |||||
| rs4948088 (COBL-A) | Yes vs. No | 0.81 (0.39, 1.69) | 0.58 | 0.77 (0.33, 1.77) | 0.54 |
|
| |||||
| rs2816316 (RGS1-C) | Yes vs. No | 0.99 (0.65, 1.52) | 0.97 | 0.71 (0.44, 1.16) | 0.17 |
|
| |||||
| Birth weight | /Kg | 1.17 (0.78, 1.74) | 0.45 | 1.18 (0.77, 1.80) | 0.46 |
|
| |||||
| Probiotics at 28 days | Yes vs. No or ≥28 days | 1.03 (0.43, 2.46) | 0.95 | 0.43 (0.13, 1.41) | 0.16 |
|
| |||||
| Child conditions before first clinical visit (age 3 months) | Upper Resp. (Yes vs. No) | 0.84 (0.49, 1.42) | 0.51 | 1.19 (0.74, 1.91) | 0.48 |
|
|
|||||
| Lower Resp. (Yes vs. No) | 1.33 (0.72, 2.46) | 0.36 | 1.21 (0.65, 2.25) | 0.54 | |
|
|
|||||
| Diarrhea (Yes vs. No) | 0.79 (0.32, 1.97) | 0.61 | 1.25 (0.64, 2.45) | 0.51 | |
HLA-DQ8/X or HLA-DQ2/X children with a dad or sibling having T1D (~3.4% of total)
No interactions were observed between G-RI and the other eleven polymorphisms (Table 1), including the three in the ITGAM gene (data not shown).
Among the highest risk HLA-DR-DQ3-2/4-8 children, G-RI did not influence the risk of IAA, GADA as first appearing, or the appearance of both GADA and IAA at the same time.
4. Discussion
The TEDDY study is the largest prospective investigation in children at the highest genetic risk for T1D to study infectious agents, dietary factors, and other environmental agents, as triggers of β-cell autoimmunity and/or T1D. Gestational events as a risk factor for T1D figure prominently in the literature. It is therefore of interest that the principal finding in the present study of first appearing β-cell autoantibodies demonstrates that children with the HLA-DR-DQ 4-8/8-4 genotype and, independently, children with CTLA-4 G allele, had a reduced risk for IAA as the first appearing β-cell autoantibody if born to a mother who reported a G-RI. The fact that the reduced risk was seen irrespective of country of birth, age of child (for CTLA-4 but not HLA-DQ) or other gestational factors underscore the significance of the observation. Aside from the protective association the HLA-DR-DQ 4-8/8-4 genotype and CTLA-4 G minor allele had with IAA as first appearing β-cell autoantibody, the same alleles, again in the presence of a G-RI, was associated with a small increased risk of GADA that appears first in older children. This suggests that while G-RI together with HLA and CTLA-4 did not influence cumulative incidence of the β-cell autoantibodies overall, they may delay the appearance of the second, third or fourth autoantibody and thereby also T1D as the larger the number of β-cell autoantibodies the greater the risk for progression to the clinical onset of T1D (2). TEDDY will follow these children exposed to a G-RI until 15 years of age to investigate this.
The peak incidence of IAA as the first appearing autoantibody was about 12 months of age in DQ2/8 TEDDY children (1). TEDDY (1) and other studies (3) have recently shown that HLA-DQ8 is strongly linked with the appearance of IAA as the first autoantibody in young children while HLA-DQ2 is predominately linked with GADA as the first autoantibody in older children (1). The present observations show that these associations are altered when G-RI are taken into account. Our findings allow for more complex gene-environmental interactions to be examined by testing associations directly with the autoantigen-specific risk of first appearing autoantibodies.
Previously, we showed that the INS and CTLA-4 gene polymorphisms affected the proportion of children having GADA over IAA as their first autoantibody (1). This would further support the heterogeneous role of environmental factors that may trigger a β-cell autoantibody and may help explain conflicting associations between environmental factors and T1D as reported in the literature. The order of the first appearing autoantibody may be of clinical and pathological importance as IAA first may trigger GADA or IA-2A as second or third autoantibody to signify a more aggressive pathogenesis including insulitis and a subsequent early age at onset. It is important to note that the incidence rate of IAA first was decreasing with increasing age while that of GADA first reached a plateau (1). GADA first may therefore signify a less aggressive pathogenesis as the second and third autoantibody may appear later to accelerate the pathogenesis.
Little is known about the mechanisms that are responsible for the appearance of the first β-cell autoantibody. It is assumed that the autoantigen is processed and presented on HLA class II heterodimers on an antigen-presenting cell be it a macrophage, dendritic cell or B cell. Insulin-peptides would be presented primarily on DR4-DQ8 and GAD65 peptides on DR3-DQ2. The resulting trimolecular complex would be seen by TCR on CD4+ T cells, which in turn are able to activate CD8+ T cells as well as B cells recognizing the autoantigen. This initiating immune response is yet to be studied in relation to a hypothetical triggering event. TEDDY was designed to provide sufficient power to ask questions related to the appearance of a first autoantibody (10). Unexpectedly, the analysis of the appearance of a first β-cell autoantibody up until the TEDDY children were 6 years of age revealed that it was primarily either IAA first in DR4-DQ8 children or GADA first in DR3-DQ2 children(1). This hypothetical series of events would apparently be influenced by G-RI. As the median age of appearance of IAA was about 12 months, a potential effect of gestational events could not be excluded. TEDDY was designed to obtain blood samples from 3–4 months of age and onwards every three months to detect a first appearing β-cell autoantibody. Since mothers may be β-cell autoantibody positive, whether with T1D (36, 37), or not (25, 37–39), we excluded FDR mothers and adjusted for maternal β-cell autoantibody exposure that was determined by analysing IAA, GADA and IA-2A in a blood sample from the mothers when the child was 6–9 months old (1). In our analysis, 51 children were found to be exposed to maternal autoantibodies but there was no association with risk of IAA or GADA in the child. Nevertheless, this was important to consider, as fetal exposure to maternal β-cell autoantibodies may be protective against future β-cell autoimmunity (36) and diabetes (40) or increased if the autoantibody was IA-2A(41). Information about gestational events was obtained via the mother’s self report at the very first visit to the TEDDY clinic which is a potential weakness in cases of inaccurate recall. The percentage of new mothers of children born between February and June reporting a G-RI (51%) was 10% higher than new mothers of children born October to December (41%) where the mother was not pregnant at any time during the winter (Nov – Feb). However, no interactions of genetic factors or G-RI were observed with season of birth on autoantibody outcomes. Unfortunately sampling during pregnancy was not possible in TEDDY and thus timing of the gestational infections during pregnancy was not available. Nevertheless with an estimated 45% of mother reporting a G-RI overall, further studies are warranted as first trimester enterovirus IgM increased the risk for β-cell autoantibodies in DQ2 mothers (29) without necessarily affecting the offspring (23, 24, 42, 43).
The major finding that the CTLA-4 G allele interacts with reported G-RI to reduce the risk for IAA, but not GADA, as the first β-cell autoantibody is a novel finding, which to our knowledge has not previously been reported. First, linkage and association between CTLA-4 and T1D was first reported in the Belgian Diabetes Registry (44) to be followed by numerous reports [for a review see (45, 46), also demonstrating that this T-cell regulating protein is important to a host of autoimmune diseases (47)]. Indeed, the CTLA-4 rs231775 SNP overall showed association with T1D, but when analyzed in patients with both T1D and autoimmune thyroid disease, the genetic effect of CTLA-4 was stronger (reviewed in (48)). Second, the initial indication of a gestational effect of CTLA-4 was the observation of an increased risk for T1D by a distorted segregation of CTLA-4 on maternal HLA-DR3 (49). Third, in women with the CTLA-4 G allele there was an increased risk for placental abruption and preeclampsia (50) or idiopathic recurrent miscarriage (51, 52) suggesting that G-RI in the mother may represent a gene-environment effect on the CTLA-4 G allele in the TEDDY child. Fourth, cord blood compared to postnatal T cells appear to show reduced CTLA-4 expression (53), which may have contributed to the observation that early activation of the fetal immune system as a consequence of maternal autoimmunity and transplacental passage of GADA may influence fetal regulatory T cells (54). Finally, the mechanisms that explain the linkage among G-RI, CTLA-4 type of the child and appearance of IAA first are yet to be dissected. Further studies of postnatal T cells in children born to mothers with documented G-RI should prove useful.
In Sweden it has been reported that the incidence of T1D has not changed among the 0–35 years old but rather the age of diagnosis has shifted to a younger age (55). Other studies show the incidence of T1D is increasing more rapidly in younger children (56). With fewer respiratory infections being reported, these results indicate the shift to a younger age may be related to an increasing incidence of IAA among DQ8 children. The relationship between G-RI, CTLA-4 and IAA as the first appearing autoantibody also explains difficulties in validating existing epidemiological frameworks, such as the hygiene hypothesis, as our data suggest that the same exposure may both increase and reduce risk for the first appearing autoantibody depending on genetic background.
The observed dichotomy of CTLA-4 G allele interacting with G-RI to yield the first appearing β-cell autoantibody either via reduced risk for IAA in DQ8 children or increased risk for GADA in DQ2 children has several implications for future investigations. First, in order to understand the mechanisms of susceptibility to a hypothetical trigger, the role of CTLA-4 in the development of the immune system needs to be explored. Why is the AA genotype reducing the early risk for IAA in the absence but increasing the risk of IAA in the presence of G-RI? Clearly, the G-RI infection would seem to contribute to the incidence of IAA as the first autoantibody at about one year of age (1). Similarly, why are the GA and GG genotypes increasing the risk for GADA in children born to mothers reporting G-RI? The AA genotype did not seem to contribute to the incidence rate of GADA first while the G allele had a seemingly dominant effect for GADA first in children born to mothers reporting G-RI. In either of the two scenarios, it would be of interest to separately determine the expression of CTLA-4 in cord blood T lymphocytes separately in DR4-DQ8 and DR3-DQ2 children, born to mothers with and without documented G-RI. It cannot be excluded that the susceptibility to a perinatal or postnatal trigger may differ dependent on maternal infectious exposures and the CTLA-4 genotype of the child.
In conclusion, reported gestational respiratory infection in mothers (G-RI) of TEDDY children showed distinct and different interactions with the HLA-DQ and CTLA-4 genotypes of the child and the type of first appearing β-cell autoantibody. First, in children with the HLA DR4-DQ8 haplotype, G-RI was associated with a reduced risk for IAA as the first β-cell autoantibody and, irrespective of HLA, CTLA-4 AG and GG children had a consistently lower risk of IAA in the presence of a G-RI. Second, in children with the DR3-DQ2 haplotype, G-RI, was associated with a reduced risk for GADA as first autoantibody however CTLA-4 AG and GG children had an elevated risk of GADA in the presence of a G-RI. These novel data suggest that gestational events such as reported respiratory infection interact with CTLA-4, a well-known T cell regulatory protein, to influence how either DR4-DQ8 or DR3-DQ2 children react to a hypothetical trigger occurring during the first years of life. The recognition of underlying immunogenetic propensities should prove useful to further analyze the apparent bisectional etiology of the first appearing β-cell autoimmunity be it marked by either IAA or GADA. The challenge remains as to whether it is one triggering event, or many, interacting with HLA-DR-DQ haplotype of the child resulting in either IAA or GADA as the first β-cell autoantibody.
Supplementary Material
Highlights.
The first β-cell autoantibody to appear in children depends on T1D-associated genes
IAA as first appears in younger children, GADA-first in children older than 2 years
Gestational respiratory infections (G-RI) protective of IAA-first among CTLA4-G children
Strong HLA association with IAA-first and GADA-first not observed if a G-RI reported
G-RI role in early life depends on offspring HLA and CTLA-4 alleles
Acknowledgments
Funding Sources
Funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Juvenile Diabetes Research Foundation (JDRF), and Centers for Disease Control and Prevention (CDC). This work supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
See Supplemental Acknowledgments for TEDDY study group details. The authors also give a special acknowledgement to the TEDDY families for their continued participation in this study.
Footnotes
Author Contributions
Å.L. and K.F.L proposed the analysis and wrote the manuscript. K.F.L performed and H.S., K.V, J.P.K and E.B. contributed to the statistical analysis. J.P.K, Å.L., W.H., M.R., J-X.S., O.G.S, J.T., A.Z., and B.A designed the study and and along with C.T., H.E.L and M.J.H acquired the data. All authors helped with interpretion of the results and critically reviewed the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krischer JP, Lynch KF, Schatz DA, Ilonen J, Lernmark A, Hagopian WA, Rewers MJ, She JX, Simell OG, Toppari J, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58(5):980–7. doi: 10.1007/s00125-015-3514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. Jama. 2013;309(23):2473–9. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilonen J, Hammais A, Laine AP, Lempainen J, Vaarala O, Veijola R, Simell O, Knip M. Patterns of beta-cell autoantibody appearance and genetic associations during the first years of life. Diabetes. 2013;62(10):3636–40. doi: 10.2337/db13-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57(4):1084–92. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagopian WA, Erlich H, Lernmark A, Rewers M, Ziegler AG, Simell O, Akolkar B, Vogt R, Jr, Blair A, Ilonen J, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatric Diabetes. 2011;12(8):733–43. doi: 10.1111/j.1399-5448.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nejentsev S, Sjoroos M, Soukka T, Knip M, Simell O, Lovgren T, Ilonen J. Population-based genetic screening for the estimation of Type 1 diabetes mellitus risk in Finland: selective genotyping of markers in the HLA-DQB1, HLA-DQA1 and HLA-DRB1 loci. Diabet Med. 1999;16(12):985–92. doi: 10.1046/j.1464-5491.1999.00186.x. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48(3):460–8. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 8.Rewers M, Bugawan TL, Norris JM, Blair A, Beaty B, Hoffman M, McDuffie RS, Jr, Hamman RF, Klingensmith G, Eisenbarth GS, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY) Diabetologia. 1996;39(7):807–12. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 9.Larsson K, Elding-Larsson H, Cederwall E, Kockum K, Neiderud J, Sjoblad S, Lindberg B, Lernmark B, Cilio C, Ivarsson SA, et al. Genetic and perinatal factors as risk for childhood type 1 diabetes. Diabetes Metab Res Rev. 2004;20(6):429–37. doi: 10.1002/dmrr.506. [DOI] [PubMed] [Google Scholar]
- 10.Group TS. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8(5):286–98. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 11.Dahlquist G, Kallen B. Maternal-child blood group incompatibility and other perinatal events increase the risk for early-onset type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1992;35(7):671–5. doi: 10.1007/BF00400261. [DOI] [PubMed] [Google Scholar]
- 12.Dahlquist GG, Patterson C, Soltesz G. Perinatal risk factors for childhood type 1 diabetes in Europe. The EURODIAB Substudy 2 Study Group. Diabetes Care. 1999;22(10):1698–702. doi: 10.2337/diacare.22.10.1698. [DOI] [PubMed] [Google Scholar]
- 13.Stene LC, Magnus P, Lie RT, Sovik O, Joner G. No association between preeclampsia or cesarean section and incidence of type 1 diabetes among children: a large, population-based cohort study. Pediatr Res. 2003;54(4):487–90. doi: 10.1203/01.PDR.0000081301.25600.5D. [DOI] [PubMed] [Google Scholar]
- 14.Dahlquist GG, Pundziute-Lycka A, Nystrom L. Birthweight and risk of type 1 diabetes in children and young adults: a population-based register study. Diabetologia. 2005;48(6):1114–7. doi: 10.1007/s00125-005-1759-6. [DOI] [PubMed] [Google Scholar]
- 15.Dahlquist G, Bennich SS, Kallen B. Intrauterine growth pattern and risk of childhood onset insulin dependent (type I) diabetes: population based case-control study. Bmj. 1996;313(7066):1174–7. doi: 10.1136/bmj.313.7066.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardwell CR, Stene LC, Joner G, Davis EA, Cinek O, Rosenbauer J, Ludvigsson J, Castell C, Svensson J, Goldacre MJ, et al. Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data. Diabetologia. 2010;53(4):641–51. doi: 10.1007/s00125-009-1648-5. [DOI] [PubMed] [Google Scholar]
- 17.Larsson HE, Hansson G, Carlsson A, Cederwall E, Jonsson B, Larsson K, Lynch K, Neiderud J, Lernmark A, Ivarsson SA. Children developing type 1 diabetes before 6 years of age have increased linear growth independent of HLA genotypes. Diabetologia. 2008;51(9):1623–30. doi: 10.1007/s00125-008-1074-0. [DOI] [PubMed] [Google Scholar]
- 18.Cardwell CR, Stene LC, Joner G, Cinek O, Svensson J, Goldacre MJ, Parslow RC, Pozzilli P, Brigis G, Stoyanov D, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2008;51(5):726–35. doi: 10.1007/s00125-008-0941-z. [DOI] [PubMed] [Google Scholar]
- 19.Ramakrishnan U. Nutrition and low birth weight: from research to practice. Am J Clin Nutr. 2004;79(1):17–21. doi: 10.1093/ajcn/79.1.17. [DOI] [PubMed] [Google Scholar]
- 20.Robertson L, Harrild K. Maternal and neonatal risk factors for childhood type 1 diabetes: a matched case-control study. BMC Public Health. 2010;10:281. doi: 10.1186/1471-2458-10-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattsson K, Jonsson I, Malmqvist E, Larsson HE, Rylander L. Maternal smoking during pregnancy and offspring type 1 diabetes mellitus risk: accounting for HLA haplotype. Eur J Epidemiol. 2015;30(3):231–8. doi: 10.1007/s10654-014-9985-1. [DOI] [PubMed] [Google Scholar]
- 22.Dahlquist GG, Ivarsson S, Lindberg B, Forsgren M. Maternal enteroviral infection during pregnancy as a risk factor for childhood IDDM. A population-based case-control study. Diabetes. 1995;44(4):408–13. doi: 10.2337/diab.44.4.408. [DOI] [PubMed] [Google Scholar]
- 23.Hyoty H, Hiltunen M, Knip M, Laakkonen M, Vahasalo P, Karjalainen J, Koskela P, Roivainen M, Leinikki P, Hovi T, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995;44(6):652–7. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- 24.Viskari HR, Roivainen M, Reunanen A, Pitkaniemi J, Sadeharju K, Koskela P, Hovi T, Leinikki P, Vilja P, Tuomilehto J, et al. Maternal first-trimester enterovirus infection and future risk of type 1 diabetes in the exposed fetus. Diabetes. 2002;51(8):2568–71. doi: 10.2337/diabetes.51.8.2568. [DOI] [PubMed] [Google Scholar]
- 25.Lynch KF, Lernmark B, Merlo J, Cilio CM, Ivarsson SA, Lernmark A. Cord blood islet autoantibodies and seasonal association with the type 1 diabetes high-risk genotype. Journal of perinatology: official journal of the California Perinatal Association. 2008;28(3):211–7. doi: 10.1038/sj.jp.7211912. [DOI] [PubMed] [Google Scholar]
- 26.Vehik K, Ajami NJ, Hadley D, Petrosino JF, Burkhardt BR. The changing landscape of type 1 diabetes: recent developments and future frontiers. Curr Diab Rep. 2013;13(5):642–50. doi: 10.1007/s11892-013-0406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knip M, Veijola R, Virtanen SM, Hyoty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S125–36. doi: 10.2337/diabetes.54.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- 28.Sadeharju K, Knip M, Hiltunen M, Akerblom HK, Hyoty H. The HLA-DR phenotype modulates the humoral immune response to enterovirus antigens. Diabetologia. 2003;46(8):1100–5. doi: 10.1007/s00125-003-1157-x. [DOI] [PubMed] [Google Scholar]
- 29.Resic Lindehammer S, Honkanen H, Nix WA, Oikarinen M, Lynch KF, Jonsson I, Marsal K, Oberste S, Hyoty H, Lernmark A. Seroconversion to islet autoantibodies after enterovirus infection in early pregnancy. Viral Immunol. 2012;25(4):254–61. doi: 10.1089/vim.2012.0022. [DOI] [PubMed] [Google Scholar]
- 30.Larsson HE, Lynch K, Lernmark B, Hansson G, Lernmark A, Ivarsson SA. Relationship between increased relative birthweight and infections during pregnancy in children with a high-risk diabetes HLA genotype. Diabetologia. 2007;50(6):1161–9. doi: 10.1007/s00125-007-0648-6. [DOI] [PubMed] [Google Scholar]
- 31.Torn C, Hadley D, Lee HS, Hagopian W, Lernmark A, Simell O, Rewers M, Ziegler A, Schatz D, Akolkar B, et al. Role of Type 1 Diabetes-Associated SNPs on Risk of Autoantibody Positivity in the TEDDY Study. Diabetes. 2015;64(5):1818–29. doi: 10.2337/db14-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rich SS, Akolkar B, Concannon P, Erlich H, Hilner JE, Julier C, Morahan G, Nerup J, Nierras C, Pociot F, et al. Overview of the Type I Diabetes Genetics Consortium. Genes and immunity. 2009;10(Suppl 1):S1–4. doi: 10.1038/gene.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torn C, Liu X, Hagopian W, Lernmark A, Simell O, Rewers M, Ziegler AG, Schatz D, Akolkar B, Onengut-Gumuscu S, et al. Complement gene variants in relation to autoantibodies to beta cell specific antigens and type 1 diabetes in the TEDDY Study. Sci Rep. 2016;6:27887. doi: 10.1038/srep27887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uusitalo U, Liu X, Yang J, Aronsson CA, Hummel S, Butterworth M, Lernmark A, Rewers M, Hagopian W, She JX, et al. Association of Early Exposure of Probiotics and Islet Autoimmunity in the TEDDY Study. JAMA Pediatr. 2016;170(1):20–8. doi: 10.1001/jamapediatrics.2015.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonifacio E, Yu L, Williams AK, Eisenbarth GS, Bingley PJ, Marcovina SM, Adler K, Ziegler AG, Mueller PW, Schatz DA, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95(7):3360–7. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koczwara K, Bonifacio E, Ziegler AG. Transmission of maternal islet antibodies and risk of autoimmune diabetes in offspring of mothers with type 1 diabetes. Diabetes. 2004;53(1):1–4. doi: 10.2337/diabetes.53.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Rewers M. Islet autoantibodies in cord blood: maternal, fetal, or neither? Diabetes Metab Res Rev. 2002;18(1):2–4. doi: 10.1002/dmrr.240. [DOI] [PubMed] [Google Scholar]
- 38.Hamalainen AM, Ilonen J, Simell O, Savola K, Kulmala P, Kupila A, Simell T, Erkkola R, Koskela P, Knip M. Prevalence and fate of type 1 diabetes-associated autoantibodies in cord blood samples from newborn infants of non-diabetic mothers. Diabetes Metab Res Rev. 2002;18(1):57–63. doi: 10.1002/dmrr.232. [DOI] [PubMed] [Google Scholar]
- 39.Stanley HM, Norris JM, Barriga K, Hoffman M, Yu L, Miao D, Erlich HA, Eisenbarth GS, Rewers M. Is presence of islet autoantibodies at birth associated with development of persistent islet autoimmunity? The Diabetes Autoimmunity Study in the Young (DAISY) Diabetes Care. 2004;27(2):497–502. doi: 10.2337/diacare.27.2.497. [DOI] [PubMed] [Google Scholar]
- 40.Yu L, Chase HP, Falorni A, Rewers M, Lernmark A, Eisenbarth GS. Sexual dimorphism in transmission of expression of islet autoantibodies to offspring. Diabetologia. 1995;38(11):1353–7. doi: 10.1007/BF00401769. [DOI] [PubMed] [Google Scholar]
- 41.Lundgren M, Lynch K, Larsson C, Elding Larsson H. Cord blood insulinoma-associated protein 2 autoantibodies are associated with increased risk of type 1 diabetes in the population-based Diabetes Prediction in Skane study. Diabetologia. 2015;58(1):75–8. doi: 10.1007/s00125-014-3394-6. [DOI] [PubMed] [Google Scholar]
- 42.Viskari H, Knip M, Tauriainen S, Huhtala H, Veijola R, Ilonen J, Simell O, Surcel HM, Hyoty H. Maternal enterovirus infection as a risk factor for type 1 diabetes in the exposed offspring. Diabetes Care. 2012;35(6):1328–32. doi: 10.2337/dc11-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahlquist G, Frisk G, Ivarsson SA, Svanberg L, Forsgren M, Diderholm H. Indications that maternal coxsackie B virus infection during pregnancy is a risk factor for childhood-onset IDDM. Diabetologia. 1995;38(11):1371–3. doi: 10.1007/BF00401772. [DOI] [PubMed] [Google Scholar]
- 44.Nistico L, Buzzetti R, Pritchard LE, Van der Auwera B, Giovannini C, Bosi E, Larrad MT, Rios MS, Chow CC, Cockram CS, et al. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Belgian Diabetes Registry. Hum Mol Genet. 1996;5(7):1075–80. doi: 10.1093/hmg/5.7.1075. [DOI] [PubMed] [Google Scholar]
- 45.Kristiansen OP, Larsen ZM, Pociot F. CTLA-4 in autoimmune diseases--a general susceptibility gene to autoimmunity? Genes Immun. 2000;1(3):170–84. doi: 10.1038/sj.gene.6363655. [DOI] [PubMed] [Google Scholar]
- 46.Qu HQ, Bradfield JP, Grant SF, Hakonarson H, Polychronakos C. Remapping the type I diabetes association of the CTLA4 locus. Genes and immunity. 2009;10(Suppl 1):S27–32. doi: 10.1038/gene.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev. 2011;241(1):180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huber A, Menconi F, Corathers S, Jacobson EM, Tomer Y. Joint genetic susceptibility to type 1 diabetes and autoimmune thyroiditis: from epidemiology to mechanisms. Endocr Rev. 2008;29(6):697–725. doi: 10.1210/er.2008-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fajardy I, Vambergue A, Stuckens C, Weill J, Danze PM, Fontaine P. CTLA-4 49 A/G dimorphism and type 1 diabetes susceptibility: a French case-control study and segregation analysis. Evidence of a maternal effect. Eur J Immunogenet. 2002;29(3):251–7. doi: 10.1046/j.1365-2370.2002.00309.x. [DOI] [PubMed] [Google Scholar]
- 50.Jaaskelainen E, Toivonen S, Keski-Nisula L, Paattiniemi EL, Helisalmi S, Punnonen K, Heinonen S. CTLA-4 polymorphism 49A-G is associated with placental abruption and preeclampsia in Finnish women. Clin Chem Lab Med. 2008;46(2):169–73. doi: 10.1515/CCLM.2008.034. [DOI] [PubMed] [Google Scholar]
- 51.Tsai AF, Kaufman KA, Walker MA, Karrison TG, Odem RR, Barnes RB, Scott JR, Schreiber JR, Stephenson MD, Ober C. Transmission disequilibrium of maternally-inherited CTLA-4 microsatellite alleles in idiopathic recurrent miscarriage. J Reprod Immunol. 1998;40(2):147–57. doi: 10.1016/s0165-0378(98)00073-4. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Lin Q, Ma Z, Hong Y, Zhao A, Di W, Lu P. Association of the A/G polymorphism at position 49 in exon 1 of CTLA-4 with the susceptibility to unexplained recurrent spontaneous abortion in the Chinese population. Am J Reprod Immunol. 2005;53(2):100–5. doi: 10.1111/j.1600-0897.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- 53.Miller RE, Fayen JD, Mohammad SF, Stein K, Kadereit S, Woods KD, Sramkoski RM, Jacobberger JW, Templeton D, Shurin SB, et al. Reduced CTLA-4 protein and messenger RNA expression in umbilical cord blood T lymphocytes. Exp Hematol. 2002;30(7):738–44. doi: 10.1016/s0301-472x(02)00831-7. [DOI] [PubMed] [Google Scholar]
- 54.Holm BC, Svensson J, Akesson C, Arvastsson J, Ljungberg J, Lynch K, Ivarsson SA, Lernmark A, Cilio CM. Evidence for immunological priming and increased frequency of CD4+ CD25+ cord blood T cells in children born to mothers with type 1 diabetes. Clin Exp Immunol. 2006;146(3):493–502. doi: 10.1111/j.1365-2249.2006.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rawshani A, Landin-Olsson M, Svensson AM, Nystrom L, Arnqvist HJ, Bolinder J, Gudbjornsdottir S. The incidence of diabetes among 0–34 year olds in Sweden: new data and better methods. Diabetologia. 2014;57(7):1375–81. doi: 10.1007/s00125-014-3225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–33. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.