Abstract
Melanoma represents an ever-increasing problem in the western world as incidence rates continue to climb. Though manageable during early stages, late stage metastatic disease is highly resistant to current intervention. We have previously shown that gamma-interferon-inducible lysosomal thiol-reductase (GILT) enhances HLA class II antigen processing and immune detection of human melanoma cells. Here we report that GILT expression inhibits a potential target, paired box-3 (PAX-3) protein, in late stage human metastatic melanoma. We also show that GILT transfection or induction by IFN-γ, decreases PAX-3 protein expression while upregulating the expression of Daxx, which is also a repressor of PAX-3. Confocal microscopic analysis demonstrated that GILT co-localizes with PAX-3 protein, but not with Daxx within melanoma cells. Immunoprecipitation and immunoblotting studies suggest that GILT expression negatively regulates PAX-3 through the autophagy pathway, potentially resulting in increased susceptibility to conventional treatment in the form of chemotherapy or radiotherapy. While high-dose radiation is a common treatment for melanoma patients, our data suggest that GILT expression significantly increased the susceptibility of melanoma cells to low-dose radiation therapy via upregulation of tumor suppressor protein p53. Overall, these data suggest that GILT has multiple roles in inducing human melanoma cells as better targets for radiation and immunotherapy.
Keywords: MELANOMA, PAX, GILT, DAXX, AUTOPHAGY, RADIATION
INTRODUCTION
Melanoma is an aggressive skin cancer and is the 5th most commonly found malignancy in the Western Hemisphere [Siegel et al., 2015]. When melanoma arises, early diagnosis is crucial to survival with 80% of cases treated successfully with excision of the tumor [Siegel et al., 2015; Zhu et al., 2009]. However, metastatic tumors are highly resistant to common treatments, resulting in the development of widespread tumor metastases to the lymph nodes, lungs, and brain [Alexandrescu et al., 2010; Constantinou, 2015; Zbytek et al., 2008]. Melanoma is also associated with a high rate of cellular transformation and mutations that lead to both neovasculature involvement and in tumor migration through both the lymphatic and circulatory system resulting in metastasis [Constantinou, 2015].
One factor influencing this malignant transformation may be the melanocyte transcriptional regulator paired box-3 (PAX-3), which is expressed in early development, but is suppressed in adult melanocytes [Bailey et al., 2012]. PAX-3 forms a complex with SRY-homeobox 10 (SOX10) protein to bind to the promoter of microphthalmia transcription factor (MITF) to determine the fate of melanocyte differentiation, regulate the maintenance of melanoblasts and melanocyte stem cells, retain the ability to enter the cell cycle, or initiate apoptosis [Hornyak et al., 2001; Lang et al., 2005; Medic and Ziman, 2009; Widlund and Fisher, 2003]. PAX-3 expression has been found in stages I and IV malignancies, but not in intermediate stages of melanoma [Fang et al., 2013; Scholl et al., 2001]. In some cases, PAX-3 has been used as a staging marker and in the detection of circulating melanoma cells [Kiyohara et al., 2014; Koyanagi et al., 2005]. This indicates that the regulation of PAX-3 could be a key factor in melanoma initiation, progression, and metastasis [Cao et al., 2015; Iyengar et al., 2014; Jin et al., 2015; Kubic et al., 2015].
PAX-3 regulation may be due to several proteins, one of which is believed to be the death domain associated protein (Daxx) [Li et al., 2000; Salomoni et al., 2006]. Daxx has been identified through its interaction with the FAS/CD95 transmembrane death receptor, and is also regulated by a STAT1-mediated pathway [Salomoni and Khelifi, 2006; Zimmerer et al., 2007]. It has been reported that Daxx binds to the homeodomain and the octapeptide epitopes, thus blocking the ability of PAX-3 to activate downstream promoters [Kubic et al., 2008]. However, the role of PAX-3 in differentiated melanocytes and in metastatic melanoma is not well defined.
Our laboratory has shown multiple roles for gamma-interferon-inducible lysosomal thiol reductase (GILT) when present in melanoma cells [Goldstein et al., 2008; Haque et al., 2002; O’Donnell et al., 2004]. GILT is a lysosomal thiol reductase that is expressed in professional antigen presenting cells (APCs), but is absent or expressed at low levels in melanoma [Haque et al., 2002; Singh and Cresswell, 2010]. We have previously shown that GILT is involved in many roles in enzymatic processing, including the reduction of oxidized and cysteinylated proteins and peptides, the enhancement of acidic cathepsin expression and activity, upregulating HLA-DM protein expression, and its regulation by STAT1 [Arunachalam et al., 2000; Goldstein et al., 2008; Haque et al., 2002; Phan et al., 2000]. It remains unknown if GILT has a secondary role in the alteration of tumorigenic molecules involved in cellular differentiation and/or transformation of late stage melanoma. Because PAX-3 expression is believed to be suppressed by Daxx, and Daxx/GILT proteins are known to be regulated by STAT1, we wanted to explore whether GILT expression influences PAX-3 in metastatic melanoma.
Therefore in this study, we generated several human melanoma cell lines expressing variable levels of GILT proteins and examined the role GILT may have on the expression of PAX-3 in these human melanoma cells. Results obtained show for the first time that GILT expression downregulates PAX-3 in melanoma cells and may act through the induction of Daxx protein expression in melanoma cells. We also demonstrate an interaction of cytoplasmic PAX-3 with lysosomal GILT proteins via induction of autophagy and a possible degradation of PAX-3 in the lysosomal pathway, implicating GILT’s ability to affect PAX-3 molecules. Interestingly, GILT expression decreased PAX-3 protein in melanoma cells, and induced increased cell death when exposed to low-dose radiation. These findings propose a novel function of GILT in suppressing the highly aggressive, metastatic nature of late stage melanoma and could lead to increased susceptibility of melanoma tumors to current standards of treatment namely chemotherapy and radiotherapy.
MATERIALS AND METHODS
CELL LINES
The human melanoma cell lines HT-144 and DM-331 were obtained from Dr. Craig L. Slingluff Jr. (University of Virginia, Virginia, USA)[Hossain et al., 2012; Zhao et al., 2011]. The melanoma line J3 was obtained from Dr. Janice Blum (Indiana University, Indianapolis, USA). Cells were cultured in complete RPMI containing 10% fetal bovine serum (FBS) (HyClone), 50 U/ml penicillin, 50 μg/ml streptomycin, and enriched with L-glutamate (Mediatech Inc., Herndon, VA)[God et al., 2015; Zhao et al., 2011]. J3.GILT cysteine mutants (J3.GILT.C46S and J3.GILT.C49S) originally created by Dr. P. Creswell lab (Yale University) [Hastings et al., 2006] were a gift from Dr. Janice Blum. J3.GILT.C46S and J3.GILT.C49S cells express cysteine mutants of the reductase active site of GILT (C46S, C49S), and were cultured in IMDM media as described [Hastings et al., 2006].
DNA TRANSFECTION ASSAYS
The human melanoma J3 cells were transduced using retroviral vectors for constitutive expression of HLA-DR4 (DRB*0401) with linked drug selection markers for puromycin and histidinol resistance [Haque et al., 2002]. Expression of surface HLA-DR4 complexes on cells was confirmed by flow cytometric analysis using the DR4-specific mAb, 359-F10 [Hiraiwa et al., 1990]. The J3.DR4 cell line was further transfected with an empty vector or GILT cDNA to generate J3.DR4.GILT [Haque et al., 2002; O’Donnell et al., 2004]. The human melanoma cell lines HT-144 and DM-331, which constitutively express HLA-DR4 molecules on the cell surface, were also transfected with empty vectors or GILT as described [O’Donnell et al., 2004]. The expression of GILT was confirmed by western blot analysis.
REAGENTS AND ANTIBODIES
IFN-γ was purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against GILT (T18, sc-2187), PAX-3 (6288D4a, sc-81351), Daxx (M112, sc-7152), STAT1 (C-111, sc-417), p53 (sc-6243), and Beclin-1 (G-11) proteins were purchased from Santa Cruz Biotechnology, Inc. Antibodies against LC3 (N-20) was purchased from Bioscience. The secondary antibodies used for western blotting were horseradish peroxidase conjugated anti-mouse (Pierce), anti-rabbit (Santa Cruz), or anti-goat IgG (Sigma-Aldrich). Monoclonal antibody for β-actin (Santa Cruz) was used as a protein loading control for western blot analysis. Bafilomycin A1 (Invitrogen, Grand Island, NY) was diluted to 20nM in culture medium and used to block autophagy in melanoma cells overnight.
WESTERN BLOT ANALYSIS
J3.DR4, J3.DR4.GILT, HT-144.vec, HT-144.GILT, DM-331.vec, and DM-331.GILT were cultured in complete RPMI, washed, and cell lysates were obtained using a standard lysis buffer (10mM Trizma base, 150 mm NaCl, 1% Triton-X 100) [Haque et al., 2002; O’Donnell et al., 2004; Phan et al., 2000]. Equal protein concentrations from designated cell lysates were separated on a 4–12% Bis/Tris NuPage gel (Invitrogen). Proteins were transferred onto a nitrocellulose membrane (Pierce), and probed with antibodies for the expression of GILT, PAX-3, Daxx, Beclin-1, p53, and LC3. The secondary antibodies used for western blotting were horseradish peroxidase conjugated anti-mouse (Pierce), anti-rabbit (Santa Cruz), or anti-goat IgG (Sigma-Aldrich). Monoclonal antibody for β-actin was used as a protein loading control. Relative protein expression was also assessed using a Bio-Rad scanning densitometer and further stated as a ratio of specific protein expressed/β-actin for each sample [Goldstein et al., 2008].
CONFOCAL MICROSCOPY
HT-144.GILT cells were cultured on glass coverslips (cat# 12-545-80, Fisher Scientific). Cells were washed, fixed with (1:1) acetone/methanol mixture for 10 minutes at room temperature. Cells were then permeabilized with 0.1% Triton-X 100 for 15 minutes, blocked with 5% normal serum for 10 minutes, and incubated with GILT, PAX-3, Daxx, and STAT1 antibodies (1:50 dilution) at 37°C for 1 hour. Following incubation, cells were washed twice with 1% BSA (cat. No. 2930, OmniPur, EMD, Cincinnati, OH) in PBS, and incubated with Alexa 488-FITC conjugated donkey anti-goat Ig (Santa Cruz) and Alexa 543-rhodamine conjugated goat anti-mouse Ig (Santa Cruz) at a 1:100 dilution for 1 hour. Cells were also counter-stained with DAPI (4′-6-Diamidino-2-phenylindole) for nuclear localization. The slides were mounted in fluorescent mounting medium G (South Biotechnology, Inc.), observed with a 63x N.A.1.4 oil immersion objective lens, and analyzed by a Leica TCS SP5 confocal laser scanning microscope using Las-AF software (Leica Lasertechnik) [Goldstein et al., 2008].
IMMUNOPRECIPITATION
HT-144.GILT and J3.GILT melanoma cells were also subjected to immunoprecipitation (IP) using protein G beads (Genscript) on cell lysate acquired as previously described [Yang et al., 2007]. GILT antibody (Santa Cruz) and a matched isotype control (NN4) were used to immunoprecipitate GILT proteins followed by western blot analysis for PAX-3, Daxx, and GILT proteins as described above.
FLOW CYTOMETRY
HT-144 +/− GILT cells were reconstituted to 1×106 cells/ml. Cells were washed with staining buffer (PBS + 1 % heat-inactivated BGS) (HyClone), and resuspended in a permeabilization buffer (Staining buffer + 0.5% Saponin) [God et al., 2015]. Cells were incubated with rabbit anti-p53 antibody (Clone: FL-393, cat. no. sc-6243, Santa Cruz), washed and stained with anti-rabbit-FITC (Santa Cruz, sc-2365). Samples were then analyzed on a FACScan using CellQuest software (BD Bioscience, Mountain View, CA) for p53 expression and displayed as cell counts. Experiments were repeated at least three times.
RADIATION THERAPY
Cells were grown to 80% confluency and resuspended to 1×106 cells/ml. Cells were then subjected to 5, 10, or 20Gy radiation by a Varian 2100C linear irradiator (MUSC Hollings Cancer Center). Cells were plated on 96 well plates and incubated for 5 days. After incubation, cells were counted using the trypan blue dye exclusion method for number of live cells. Assays were repeated 3 times, and data are expressed as percent cytotoxicity ± SD of triplicate wells.
STATISTICAL ANALYSIS
Data from each experimental group were subjected to statistical analysis. ANOVA with post-hoc tests, Repeated Measures ANOVA with post-hoc tests, or Student’s t-tests were performed using GraphPad Prism 7.0, as appropriate. Two-sided tests were used in all cases and p values < 0.05 were considered statistically significant.
RESULTS
GILT EXPRESSION DOWNREGULATES PAX-3 AND INCREASES DAXX PROTEINS IN HUMAN MELANOMA CELLS
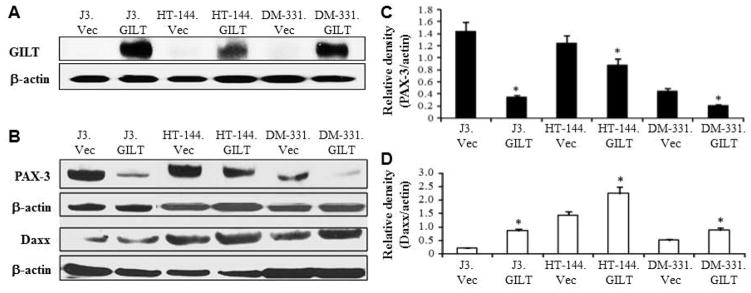
To examine GILT’s effect on tumorigenic PAX-3, it was first necessary to express GILT in multiple melanoma cell lines as melanoma tumors generally express low to no detectable GILT. Using vector/GILT cDNA and a Lipofectamine Transfection Reagent [Haque et al., 2002; O’Donnell et al., 2004], we successfully generated melanoma cell lines J3.Vec, J3.GILT, HT-144.Vec, HT-144.GILT, DM-331.Vec, DM-331.GILT, and steady state GILT expression was confirmed by western blot analysis (Fig. 1A). Following successful transfection, we next examined GILT’s effect on PAX-3 protein expression (Fig. 1B). In all three melanoma cell lines tested, GILT expression significantly downregulated PAX-3 protein levels (Fig. 1C). Patient melanoma samples obtained from the Tumor Bank of Hollings Cancer Center at the Medical University of South Carolina were also subjected to western blot analysis for PAX-3 protein expression. Late stage metastatic melanoma displayed high levels of PAX-3 proteins while early stage melanoma expressed little to no PAX-3 molecules (data not shown). Previous studies have shown that GILT is regulated by the STAT1 pathway and not by the CIITA master class II regulator [O’Donnell et al., 2004]. Studies have also shown that Daxx is involved in the regulation of PAX-3, and is also governed by the STAT1 regulatory pathway [Zimmerer et al., 2007]. Therefore, we next looked at potential intermediary proteins which could result in decreased PAX-3 expression. Western blotting as well as densitometric analysis indicated that GILT expression significantly increased Daxx protein expression in all melanoma cell lines tested, most notably in the HT-144.GILT cell line (Fig. 1D). Taken together, these data suggest that GILT expression decreases the protein expression of PAX-3 via upregulation of Daxx proteins in melanoma cells.
Fig. 1. GILT expression alters PAX-3 and Daxx proteins in human melanoma cells.
(A) Western blot analysis shows successful GILT transfection in melanoma cells with β-actin as a loading control. (B) Western blot analysis for PAX-3 and Daxx protein expression in melanoma cell lines +/− GILT. β-actin was used as a loading control. Data are representative of at least three separate experiments. (C&D) Densitometric analyses of the protein bands detected in Figure 1B. Data suggest that GILT expression significantly reduces PAX-3 proteins while upregulating Daxx proteins in human melanoma cells. * = p<0.05. Statistical analyses included the Student t-test with standard error.
FUNCTIONAL GILT IS REQUIRED FOR DOWNREGULATION OF PAX-3 PROTEINS IN HUMAN MELANOMA CELLS
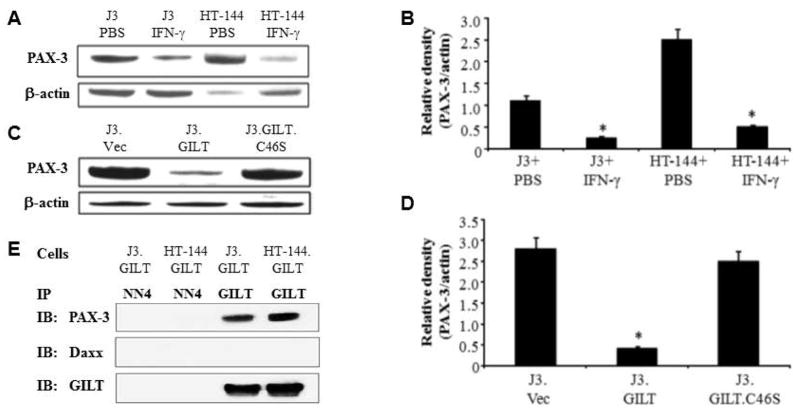
To examine whether IFN-γ-induced GILT can modulate PAX-3 protein expression, we treated J3 and HT-144 melanoma cells with 200 U/ml of IFN-γ for 48 h, and tested for PAX-3 molecules (Fig. 2A). Western blotting and densitometric analysis showed that PAX-3 protein levels were significantly decreased by IFN-γ treatment (Fig. 2B). The blots were re-probed with GILT antibody to confirm GILT expression (data not shown). Dr. Cresswell’s laboratory has generated multiple GILT mutants for mature GILT expression but devoid of reductase activity [Hastings et al., 2006]. J3 cells stably transfected with either wild-type human GILT or a cysteine mutant of the reductase active site (C46S) were also tested for PAX-3 proteins. Western blot analysis showed that the PAX-3 protein expression was not significantly altered in mutant J3.GILT.C46S cells (Fig. 2, C&D), suggesting the requirement of functional reductase (GILT) to alter PAX-3 in melanoma cells.
Fig. 2. GILT induction regulates PAX-3 protein expression in melanoma cells.
(A) J3 and HT-144 cells were treated with either PBS or IFN-γ (200 U/ml) for 48 h, and subjected to western blotting for PAX-3 proteins. (B) Densitometric analysis of the PAX-3 protein bands detected in Figure 2A in IFN-γ-treated J3 and HT-144 cells. * = p<0.05. (C) J3.Vec, J3.GILT and the cysteine mutant J3.GILT.C46S were analyzed by western blotting for steady state PAX-3 protein expression. β-actin was used as a loading control. (D) Densitometric analysis of the PAX-3 protein bands detected in Figure 2C. * = p<0.05. (E) GILT interacts with PAX-3 proteins through a direct protein-protein interaction in HT-144.GILT melanoma cells. Co-immunoprecipitation studies in J3.DR4.GILT and HT-144.GILT cells for GILT and PAX-3 proteins. The NN4 antibody was used as an isotype control, and GILT was used as a loading control. Immunoblotting was performed by using antibodies against PAX-3, Daxx, and GILT molecules. Data are representative of three separate experiments.
GILT CO-LOCALIZES WITH PAX-3 PROTEINS IN HUMAN MELANOMA CELLS
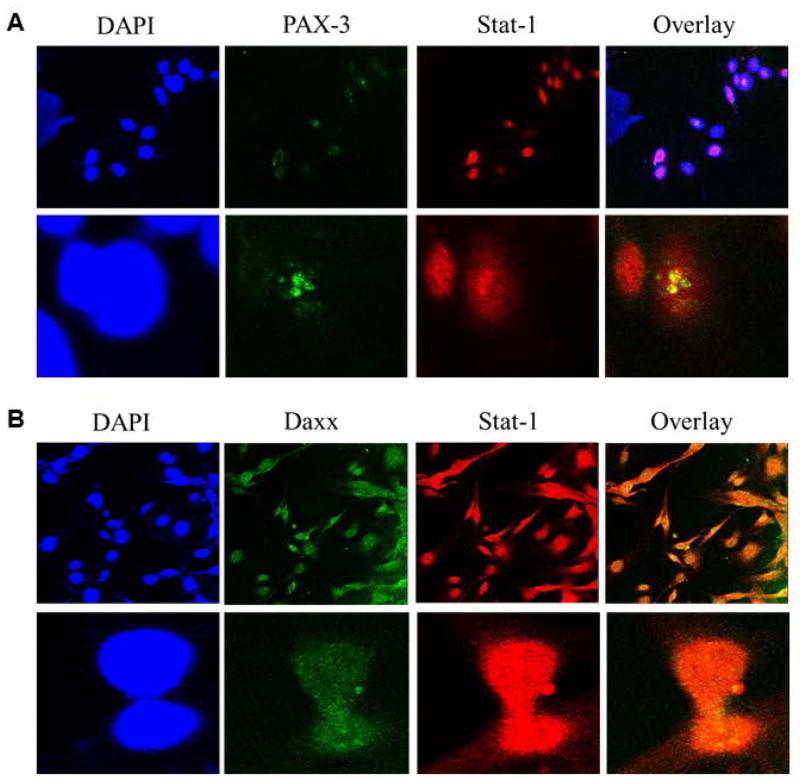
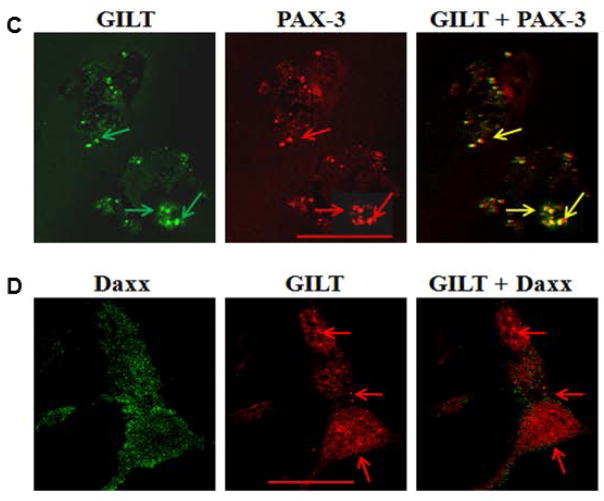
The interaction of GILT and PAX-3 molecules were further confirmed through immunoprecipitation studies of J3.GILT and HT-144.GILT melanoma cells. Cellular lysate was extracted and co-incubated with primary antibodies for GILT or NN4 (isotype control), followed by western blotting for PAX-3/Daxx protein expression (Fig. 2E). Data obtained suggest that PAX-3 and GILT undergo a direct protein-protein interaction within melanoma cells potentially resulting in the reduction of PAX-3 in tumor cells. It is possible that GILT and Daxx could be regulated through a STAT1-mediated pathway. Thus, we first tested interactions of STAT1 and PAX-3 (Fig. 3A), and STAT1 and Daxx proteins (Fig. 3B) in HT-144.GILT cells by confocal microscopy. Results indicated a possible protein-protein interaction between STAT1 and PAX-3 as well as STAT1 with Daxx in GILT-transfected melanoma cells. These data also suggest that GILT expression may influence multiple cytoplasmic proteins in melanoma via unknown pathway(s). Confocal microscopic analysis of GILT and PAX-3 confirmed colocalization of these two proteins within HT-144.GILT cells (Fig. 3C, yellow arrows). Yet confocal microscopy of GILT and Daxx molecules in the melanoma cell line HT-144.GILT showed no cellular colocalization between the two proteins (Fig. 3D). These are interesting findings as these proteins are normally localized in two different cellular compartments. The question then arises how GILT and PAX-3 proteins from two different cellular compartments interact together in GILT-positive melanoma cells.
Fig. 3. GILT colocalizes with PAX-3 molecules in HT-144.GILT melanoma cells.
(A) Melanoma cell line HT-144.GILT was tested for STAT1 and PAX-3 protein expression through confocal microscopy. The overlay reveals a colocalization between these two proteins. (B) HT-144.GILT cells were also tested for STAT1 and Daxx expression. Results indicate that, like PAX-3, Daxx colocalizes with STAT1 which signifies a possible protein-protein interaction. (C) HT-144.GILT melanoma cells were subjected to confocal microscopy using antibodies against GILT (left panel, green arrows) and PAX-3 (middle panel, red arrows) proteins. Overlay shows colocalization of PAX-3 and GILT proteins (right panel, yellow arrows) in HT-144.GILT cells. Bar, 26.1 μM. (D) Confocal microscopy of HT-144.GILT cells for Daxx (left panel, green) and GILT (middle panel, red) molecules. Overlay shows no colocalization of Daxx and GILT proteins (right panel, red arrows) in HT-144.GILT cells. Bar, 17.6 μM. Data are representative of three separate experiments. Data are representative of at least three separate experiments.
GILT EXPRESSION REGULATES PAX-3 THROUGH THE AUTOPHAGY AND LYSOSOMAL DEGRADATION PATHWAY IN HUMAN MELANOMA CELLS
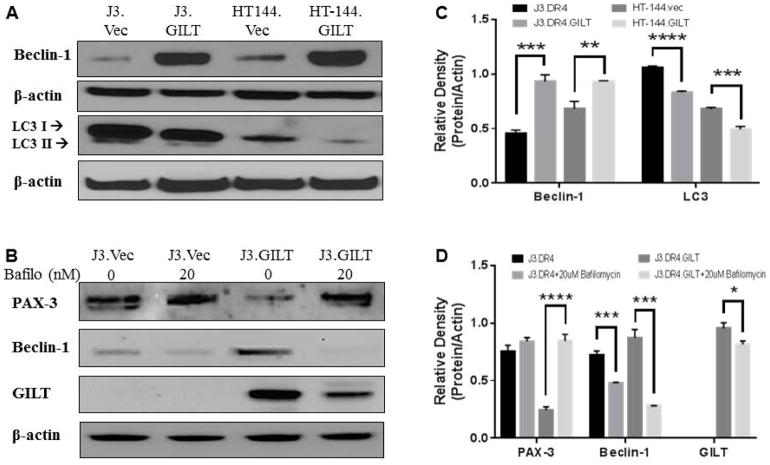
GILT resides with the endosomal/lysosomal compartments of cells, while PAX-3 mainly is expressed in the cytoplasm [Kubic et al., 2008; Phan et al., 2000]. Our current study suggests that GILT expression downregulates PAX-3 through a protein-protein interaction, and functional GILT is necessary for this decrease. Possible interaction between these two proteins may be a result of autophagy, a lysosome-dependent degradation process designed to maintain cellular homeostasis by clearing damaged cytosolic organelles and long-lived proteins [Chiang and Maric, 2011]. Once autophagy is activated, this process helps reduce oxidative damage and maintains redox homeostasis [Scherz-Shouval and Elazar, 2007]. Therefore, autophagic proteins were tested in melanoma cell lines +/− GILT expression through western blot analysis (Fig. 4). Results indicate that GILT expression significantly increased Beclin-1 proteins while decreasing whole LC3 protein (Fig. 4A), thus affecting multiple proteins involved in autophagy. Because GILT is able to downregulate PAX-3, it is possible that GILT is leading PAX-3 through the lysosomal degradation pathway.
Fig. 4. GILT regulates PAX-3 through the autophagy and lysosomal degradation pathway.
(A) Western blot analysis of whole cell lysates from J3. DR4.vc, J3.DR4.GILT, HT-144.vec, HT-144.GILT cells showing protein expression of Beclin-1 and LC3. (B) Melanoma cell lines J3.DR4 and J3.DR4.GILT were treated with or without 20nM bafilomycin overnight, which blocks proteins from processing via the lysosomal degradation pathway. Western blot analysis from whole cell lysates of these cell lines were tested for PAX-3, Beclin-1, and GILT expression. Data are representative of at least three independent experiments. (C&D) Densitometric analysis of autophagic proteins detected in Figure 4 in J3.DR4 and HT-144.vec cells +/− GILT expression. (C) Densitometric analysis for Beclin-1 and LC3 in all cell lines tested showed a significant increase in GILT-expressing cells compared to the vector counterpart. (D) Densitometric analysis for PAX-3 proteins displayed a significant increase in J3.DR4.GILT cells when treated with bafilomycin A1, while Beclin-1 and GILT showed a significant decrease in the same cells treated with bafilomycin A1. Figure 4C, Beclin-1 (***p<0.001, ** = p<0.05); LC3 (**** p = <0.04, ***p<0.05). Figure 4D, PAX-3 (****p<0.001): Beclin-1 (***p<0.01); GILT (***p<0.05). Statistical analyses included the Student t-test with standard error.
To test whether GILT regulates PAX-3 through this pathway, cells were treated with 20nM bafilomycin A1 overnight. Bafilomycin has been shown to inhibit the late phase of autophagy by preventing maturation of autophagic vacuoles, thus inhibiting fusion between autophagosomes and lysosomes. Therefore, J3.DR4 +/− GILT cells were treated with bafilomycin A1 overnight, whole cell lysates were obtained, and subjected to western blot analysis. As shown in Fig. 4B, J3.DR4 cell lysates were analyzed for PAX-3 as well as Beclin-1 and GILT. When cells are treated with bafilomycin, there is a significant increase in PAX-3 expression, specifically in GILT positive cells (Fig. 4, C&D). Beclin-1 and GILT were used as controls to determine the effectiveness of bafilomycin treatment. This data suggests that when autophagy/lysosome is blocked, PAX-3 expression persists. When GILT is present in melanoma cells, GILT is able to regulate PAX-3 through the autophagy and lysosomal degradation pathway.
GILT EXPRESSION UPREGULATES P53 AND SENSITIZES HUMAN MELANOMA CELLS TO RADIATION THERAPY
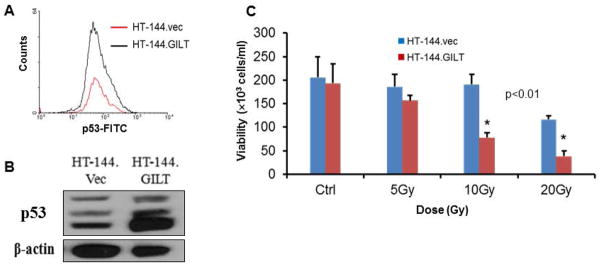
PAX-3 plays a critical role in cell survival during melanocytogenesis. However, it is unknown whether this is true in the development of melanoma. Interestingly, with GILT downregulating PAX-3 expression in melanoma cells, the next step was to determine whether this reduction would allow melanoma cells to be susceptible to cellular death. One protein that is essential for cell regulation under these conditions is p53. Oftentimes, melanoma cells do not express p53 through loss-of-function, or p53 is mutated. Seminal studies looking at neural crest development have shown that PAX-3 inactivates p53 function by stimulating its ubiquitination and degradation [Morgan et al., 2008; Pani et al., 2002; Wang et al., 2011]. Thus, we tested and compared p53 expression in melanoma cells +/−GILT through intracellular flow cytometry and western blot analysis (Fig. 5A–B). Results indicate a higher p53 expression in HT-144.GILT cells compared to its vector counterpart.
Fig 5. GILT expression upregulates p53 and sensitizes human melanoma cells to radiation therapy.
(A) HT-144.Vec and HT-144.GILT cells were tested by intracellular flow cytometry for p53 expression. Data is expressed as cell counts. (B) HT-144.Vec and HT-144.GILT cells were tested by western blot analysis to show 3 isoforms of p53 protein expression. (C) Cells were subjected to 5, 10, or 20 Gy radiation and incubated for 5 days. Cells were tested for viability using trypan blue dye exclusion method. Data are expressed as percent cytotoxicity ± SD of triplicate wells. (*p<0.01).
Interestingly, because of the elevated p53 expression displayed in GILT-expressing cells, we wanted to determine whether these cells would be sensitive to common treatments used for melanoma patients, such as high-dose radiation. However, using lower doses of radiation would be less toxic to patients. Therefore, we tested human melanoma cell lines HT-144.vec and HT-144.GILT in a radiation assay using various doses and incubated for 5 days to determine the role of GILT in cell viability (Fig. 5C). Results show that the presence of functional GILT allowed more cells to die when compared to vector alone, specifically utilizing lower radiation doses. This data suggests that GILT regulation of PAX sensitizes melanoma cells to radiation.
DISCUSSION
Standard metastatic melanoma treatments such as chemotherapy, radiation therapy, and targeted inhibitor therapy have not had much success in combating this aggressive disease. Background on the mechanisms for these treatment failures has not been well defined. Thus, we set to determine underlying molecules and pathways to delineate why treatments such as radiation therapy fail in late stage melanoma. Our focus was on the transcription factor PAX-3, which has been implicated in the transition to aggressive-metastatic disease. This transition of initial cellular irregularity to aggressive-metastatic disease is a complex process involving multiple intra and extra-cellular signaling pathways and molecules. Although PAX-3 has been associated with this shift to metastatic potential, it is unclear whether targeting PAX-3 would lead to a less aggressive form of disease.
Our study highlights that GILT regulates PAX-3 through the autophagy-lysosomal degradation pathway, resulting in a p53-dependent cellular susceptibility to radiation. Given the localization of GILT and PAX-3 within melanoma cells, lysosomal and cytosolic/nuclear respectively, it was thought that GILT may not be acting on PAX-3 directly, rather through a protein intermediary. As previously stated, both GILT and PAX-3-inhibitory protein Daxx are governed by the STAT1 pathway, which suggested a possible link between GILT and PAX-3. Our investigation supported this notion by showing GILT enhancing Daxx protein expression in melanoma cells. However, when confocal microscopy displayed no direct interaction between GILT and Daxx, the upregulation of Daxx in GILT-positive cells may be caused via secondary effects. Surprisingly, confocal microscopy showed that that GILT directly interacts with PAX-3 proteins within melanoma cells potentially leading to PAX-3 downregulation, confirmed by co-immunoprecipitation, suggesting a direct protein-protein interaction is taking place. Interestingly, GILT induction by IFN-γ significantly decreased PAX-3 protein levels, while elevating Daxx protein expression in melanoma cells. While GILT expression decreased PAX-3, disruption of the reductase active site of GILT failed to decrease PAX-3 protein levels, suggesting that reductive processing and degradation in the endolysosomal compartments may play a significant role in this process.
Autophagy is critical for recycling cellular components to enable survival during cellular stress. Within the endosomal/lysosomal compartments, proteins may be trafficked through and tagged for autophagy through the lysosomal degradation pathway. Higher levels of autophagic events in tumor cells may also promote lysosomal degradation where GILT can influence protein processing by other acidic proteases such as cathepsins [Goldstein et al., 2008]. Because of our findings that GILT downregulates PAX-3, we hypothesized that GILT may be regulating PAX-3 expression through the autophagic degradation pathway. Our study further supports our hypothesis, showing that Beclin-1 is increased in GILT-expressing cells while GILT promotes degradation of LC3I and LC3II and lysosomal degradation. Because autophagy is a dynamic, ongoing process, it is difficult to measure proteins and assemblies to test autophagy activity. Thus, inhibiting autophagy activity by increasing lysosomal pH or blocking autophagosome fusion is best to determine if cells are undergoing this process. Cells treated with 20nM bafilomycin displayed decreased Beclin-1 and GILT proteins while PAX-3 protein was increased (Fig. 4B). This further validates that GILT regulates PAX-3 through the autophagic degradation pathway. Along with its primary role in enhancing antigen processing and T cell recognition, these findings suggest an important secondary role of GILT in melanoma cells by altering tumorigenic proteins, further stressing GILT’s inclusion in future melanoma vaccine design.
In addition, multiple studies highlight PAX-3 regulation of p53 in neural crest and melanocyte development [Morgan et al., 2008; Pani et al., 2002; Wang et al., 2011], but no studies have examined these proteins in melanoma. One study suggests that PAX-3 reduction may lead to enhanced sensitivity to secondary treatment of metastatic melanoma [He et al., 2005]. Here, we discern a possible mechanism into enhanced targeting of PAX-3 can allow for standardized treatments (i.e. radiation) become more successful. Our study highlights that GILT regulation of PAX-3 leads to a p53-dependent cellular susceptibility to radiation. Our findings that GILT’s presence may increase radiation sensitivity of melanomas open more possibilities into exploring the role of GILT in melanoma cells. While radiotherapy can play a role in the management of many distinct melanomas, future studies may explore the combination of radiotherapy and immunotherapy to yield better results in advanced melanoma.
Acknowledgments
We thank Dr. Janice Blum (Indiana University School of Medicine, Indianapolis) and Craig L. Slingluff Jr. (University of Virginia School of Medicine, Charlottesville) for cell lines and reagents. We also thank Mr. Benjamin Johnson for technical help. This work was supported by grants from the National Institutes of Health (R01 CA129560) and the South Carolina Spinal Cord Injury Research Fund (SCIRF #2016 I-03) to A.H. The research presented in this article was also supported in part by the Cell and Molecular Imaging and the Flow cytometry Unit, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Alexandrescu DT, Ichim TE, Riordan NH, Marincola FM, Di Nardo A, Kabigting FD, Dasanu CA. Immunotherapy for melanoma: current status and perspectives. J Immunother. 2010;33:570–90. doi: 10.1097/CJI.0b013e3181e032e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam B, Phan UT, Geuze HJ, Cresswell P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT) Proc Natl Acad Sci U S A. 2000;97:745–50. doi: 10.1073/pnas.97.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CM, Morrison JA, Kulesa PM. Melanoma revives an embryonic migration program to promote plasticity and invasion. Pigment Cell Melanoma Res. 2012;25:573–83. doi: 10.1111/j.1755-148X.2012.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Dai X, Wan L, Wang H, Zhang J, Goff PS, Sviderskaya EV, Xuan Z, Xu Z, Xu X, Hinds P, Flaherty KT, Faller DV, Goding CR, Wang Y, Wei W, Cui R. The E3 ligase APC/C(Cdh1) promotes ubiquitylation-mediated proteolysis of PAX3 to suppress melanocyte proliferation and melanoma growth. Sci Signal. 2015;8:ra87. doi: 10.1126/scisignal.aab1995. [DOI] [PubMed] [Google Scholar]
- Chiang HS, Maric M. Lysosomal thiol reductase negatively regulates autophagy by altering glutathione synthesis and oxidation. Free Radic Biol Med. 2011;51:688–99. doi: 10.1016/j.freeradbiomed.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Constantinou M. Melanoma Genomics and Immunotherapy. R I Med J (2013) 2015;98:31–4. [PubMed] [Google Scholar]
- Fang WH, Ahmed M, Wang Q, Li HM, Kumar P, Kumar S. PAX3 promotes tumor progression via CD105 signaling. Microvasc Res. 2013;86:42–3. doi: 10.1016/j.mvr.2012.12.005. [DOI] [PubMed] [Google Scholar]
- God JM, Cameron C, Figueroa J, Amria S, Hossain A, Kempkes B, Bornkamm GW, Stuart RK, Blum JS, Haque A. Elevation of c-MYC disrupts HLA class II-mediated immune recognition of human B cell tumors. J Immunol. 2015;194:1434–45. doi: 10.4049/jimmunol.1402382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein OG, Hajiaghamohseni LM, Amria S, Sundaram K, Reddy SV, Haque A. Gamma-IFN-inducible-lysosomal thiol reductase modulates acidic proteases and HLA class II antigen processing in melanoma. Cancer Immunol Immunother. 2008;57:1461–70. doi: 10.1007/s00262-008-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque MA, Li P, Jackson SK, Zarour HM, Hawes JW, Phan UT, Maric M, Cresswell P, Blum JS. Absence of gamma-interferon-inducible lysosomal thiol reductase in melanomas disrupts T cell recognition of select immunodominant epitopes. J Exp Med. 2002;195:1267–77. doi: 10.1084/jem.20011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings KT, Lackman RL, Cresswell P. Functional requirements for the lysosomal thiol reductase GILT in MHC class II-restricted antigen processing. J Immunol. 2006;177:8569–77. doi: 10.4049/jimmunol.177.12.8569. [DOI] [PubMed] [Google Scholar]
- He SJ, Stevens G, Braithwaite AW, Eccles MR. Transfection of melanoma cells with antisense PAX3 oligonucleotides additively complements cisplatin-induced cytotoxicity. Mol Cancer Ther. 2005;4:996–1003. doi: 10.1158/1535-7163.MCT-04-0252. [DOI] [PubMed] [Google Scholar]
- Hiraiwa A, Yamanaka K, Kwok WW, Mickelson EM, Masewicz S, Hansen JA, Radka SF, Nepom GT. Structural requirements for recognition of the HLA-Dw14 class II epitope: a key HLA determinant associated with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1990;87:8051–5. doi: 10.1073/pnas.87.20.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornyak TJ, Hayes DJ, Chiu LY, Ziff EB. Transcription factors in melanocyte development: distinct roles for Pax-3 and Mitf. Mech Dev. 2001;101:47–59. doi: 10.1016/s0925-4773(00)00569-4. [DOI] [PubMed] [Google Scholar]
- Hossain A, Radwan FF, Doonan BP, God JM, Zhang L, Bell PD, Haque A. A possible cross-talk between autophagy and apoptosis in generating an immune response in melanoma. Apoptosis. 2012;17:1066–78. doi: 10.1007/s10495-012-0745-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar AS, Miller PJ, Loupe JM, Hollenbach AD. Phosphorylation of PAX3 contributes to melanoma phenotypes by affecting proliferation, invasion, and transformation. Pigment Cell Melanoma Res. 2014;27:846–8. doi: 10.1111/pcmr.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SG, Xiong W, Wu X, Yang L, Pfeifer GP. The DNA methylation landscape of human melanoma. Genomics. 2015;106:322–30. doi: 10.1016/j.ygeno.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara E, Hata K, Lam S, Hoon DS. Circulating tumor cells as prognostic biomarkers in cutaneous melanoma patients. Methods Mol Biol. 2014;1102:513–22. doi: 10.1007/978-1-62703-727-3_27. [DOI] [PubMed] [Google Scholar]
- Koyanagi K, Kuo C, Nakagawa T, Mori T, Ueno H, Lorico AR, Jr, Wang HJ, Hseuh E, O’Day SJ, Hoon DS. Multimarker quantitative real-time PCR detection of circulating melanoma cells in peripheral blood: relation to disease stage in melanoma patients. Clin Chem. 2005;51:981–8. doi: 10.1373/clinchem.2004.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubic JD, Little EC, Lui JW, Iizuka T, Lang D. PAX3 and ETS1 synergistically activate MET expression in melanoma cells. Oncogene. 2015;34:4964–74. doi: 10.1038/onc.2014.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubic JD, Young KP, Plummer RS, Ludvik AE, Lang D. Pigmentation PAX-ways: the role of Pax3 in melanogenesis, melanocyte stem cell maintenance, and disease. Pigment Cell Melanoma Res. 2008;21:627–45. doi: 10.1111/j.1755-148X.2008.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Lu MM, Huang L, Engleka KA, Zhang M, Chu EY, Lipner S, Skoultchi A, Millar SE, Epstein JA. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–7. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- Li R, Pei H, Watson DK, Papas TS. EAP1/Daxx interacts with ETS1 and represses transcriptional activation of ETS1 target genes. Oncogene. 2000;19:745–53. doi: 10.1038/sj.onc.1203385. [DOI] [PubMed] [Google Scholar]
- Medic S, Ziman M. PAX3 across the spectrum: from melanoblast to melanoma. Crit Rev Biochem Mol Biol. 2009;44:85–97. doi: 10.1080/10409230902755056. [DOI] [PubMed] [Google Scholar]
- Morgan SC, Lee HY, Relaix F, Sandell LL, Levorse JM, Loeken MR. Cardiac outflow tract septation failure in Pax3-deficient embryos is due to p53-dependent regulation of migrating cardiac neural crest. Mech Dev. 2008;125:757–67. doi: 10.1016/j.mod.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell PW, Haque A, Klemsz MJ, Kaplan MH, Blum JS. Cutting edge: induction of the antigen-processing enzyme IFN-gamma-inducible lysosomal thiol reductase in melanoma cells Is STAT1-dependent but CIITA-independent. J Immunol. 2004;173:731–5. doi: 10.4049/jimmunol.173.2.731. [DOI] [PubMed] [Google Scholar]
- Pani L, Horal M, Loeken MR. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: implications for Pax-3- dependent development and tumorigenesis. Genes Dev. 2002;16:676–80. doi: 10.1101/gad.969302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan UT, Arunachalam B, Cresswell P. Gamma-interferon-inducible lysosomal thiol reductase (GILT). Maturation, activity, and mechanism of action. J Biol Chem. 2000;275:25907–14. doi: 10.1074/jbc.M003459200. [DOI] [PubMed] [Google Scholar]
- Salomoni P, Guernah I, Pandolfi PP. The PML-nuclear body associated protein Daxx regulates the cellular response to CD40. Cell Death Differ. 2006;13:672–5. doi: 10.1038/sj.cdd.4401820. [DOI] [PubMed] [Google Scholar]
- Salomoni P, Khelifi AF. Daxx: death or survival protein? Trends Cell Biol. 2006;16:97–104. doi: 10.1016/j.tcb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–7. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Scholl FA, Kamarashev J, Murmann OV, Geertsen R, Dummer R, Schafer BW. PAX3 is expressed in human melanomas and contributes to tumor cell survival. Cancer Res. 2001;61:823–6. [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Singh R, Cresswell P. Defective cross-presentation of viral antigens in GILT-free mice. Science. 2010;328:1394–8. doi: 10.1126/science.1189176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Morgan SC, Loeken MR. Pax3 stimulates p53 ubiquitination and degradation independent of transcription. PLoS One. 2011;6:e29379. doi: 10.1371/journal.pone.0029379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlund HR, Fisher DE. Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene. 2003;22:3035–41. doi: 10.1038/sj.onc.1206443. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, Li Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–26. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Carlson JA, Granese J, Ross J, Mihm MC, Jr, Slominski A. Current concepts of metastasis in melanoma. Expert Rev Dermatol. 2008;3:569–585. doi: 10.1586/17469872.3.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Amria S, Hossain A, Sundaram K, Komlosi P, Nagarkatti M, Haque A. Enhancement of HLA class II-restricted CD4+ T cell recognition of human melanoma cells following treatment with bryostatin-1. Cell Immunol. 2011;271:392–400. doi: 10.1016/j.cellimm.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Wurdak H, Wang Y, Galkin A, Tao H, Li J, Lyssiotis CA, Yan F, Tu BP, Miraglia L, Walker J, Sun F, Orth A, Schultz PG, Wu X. A genomic screen identifies TYRO3 as a MITF regulator in melanoma. Proc Natl Acad Sci U S A. 2009;106:17025–30. doi: 10.1073/pnas.0909292106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerer JM, Lesinski GB, Radmacher MD, Ruppert A, Carson WE., 3rd STAT1-dependent and STAT1-independent gene expression in murine immune cells following stimulation with interferon-alpha. Cancer Immunol Immunother. 2007;56:1845–52. doi: 10.1007/s00262-007-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]