Abstract
Background
Exposure to particulate matter (PM) is increasing worldwide as a result of increased human activity, the rapid industrialization of developing countries, and effects of climate change. Adverse effects of PM on human health are well documented, and because PM exposure occurs mostly through the airways, PM has especially deleterious impact on the lungs.
Objective
We investigated whether surrogate PM particles like carbon black (CB), diesel exhaust particle (DEP), coal fly ash (CFA) can recapitulate the allergic airway inflammatory response induced by urban particulate matter.
Methods
We compared the pro-inflammatory potential of urban PM collected from New York (NYC) and Baltimore (BM) with CB, DEP and CFA surrogate PM particles. Eight to ten weeks old BALB/cJ mice were exposed through the airways to particulate material, and markers of airway inflammation were determined. Specifically, we assessed cellular influx, mucus production, lung function, cytokine levels as well as immune cell profiling of the lungs.
Results
Herein, we demonstrate that exposure to equivalent mass of stand-alone surrogate PM particles like CB, DEP and CFA, fails to induce significant airway inflammatory response seen after similar exposure to urban PMs. Specifically, we observe that PM collected from New York (NYC) and Baltimore city (Balt) triggers a mixed Th2/Th17 response accompanied by eosinophilic and neutrophilic influx, mucus production and airway hyperresponsiveness (AHR). Although the immune profile of NYC and Baltimore PMs are similar, they demonstrate considerable differences in their potency. Baltimore PM induced more robust airway inflammation, AHR, and Th2 cytokine production, possibly due to the greater metal content in Baltimore PM.
Conclusions
Urban particulate matter with its unique physiochemical properties and heterogeneous composition elicits a mixed Th2/Th17 allergic airway response that is not seen after similar exposures to surrogate PM particles.
Keywords: particulate matter, fly ash, carbon black, diesel exhaust, inflammation, pulmonary, metals
1. INTRODUCTION
A growing number of epidemiologic studies have shown that exposure to urban particulate matter (PM) can seriously affect lung health. PM is monitored worldwide as a metric of air quality, and recent environmental data suggest an increase in global air pollution burden and exposure to PM content that is highest in low-income urban centers (World Health Organization 2016). Exposure to elevated PM has been linked to both upper and lower airways afflictions such as sore throat, rhinitis, sinusitis, and wheezing, as well as exacerbating asthma and emphysema (Andersen et al. 2008; Nachman and Parker 2012; Paulin and Hansel 2016; Renner et al. 2012; World Health Organization 2016).
Many sources of PM have been used as surrogates for the study of ambient urban particulate matter. As carbon is the primary component of various forms of particulate matter, numerous studies have used fine (0.1–1 μm) and ultrafine (<0.1 μm) carbon black to study the effect of PM on biological functions (Bennett et al. 2012; Gilmour et al. 2004; Oberdorster et al. 1992; Ohtsuka et al. 2000; Saputra et al. 2014). While carbon particles can impair macrophage function (Renwick et al. 2004), facilitate cytokine secretion and airway inflammation (Gilmour et al. 2004; Shwe et al. 2005), carbon black is devoid of the organic and inorganic components of typical urban PM.
Recognizing these limitations, several investigators have used more complex chemical mixtures, such as diesel exhaust particles (DEP) or coal fly ash (CFA), which contribute to the complex make-up of urban-collected PM (Karimi et al. 2015; Laden et al. 2000; Veronesi et al. 2002). Indeed, several investigators report significant lung inflammation in response to airway exposure to DEP and fly ash (Acciani et al. 2013; Brandt et al. 2015; Smith et al. 2006). Although DEP and fly ash contain metals, sulfates, nitrates, and polyaromatic hydrocarbons in addition to carbon (Cassee et al. 2013; Laden et al. 2000), DEP and CFA do not completely recapitulate the complex mixture of urban PM and do not trigger the entirety of allergic airway responses seen with urban PM, including eosinophilic influx, and the development of airway hyperresponsiveness (AHR) (Walters et al. 2001; Walters et al. 2002).
Apart from chemical constituents, biological contaminants (aeroallergens, endotoxin, mold) are another important and dominant constituent of PM collected from urban centers (Frohlich-Nowoisky et al. 2009; Morakinyo et al. 2016). While the identity of the essential components of urban PM that mediate the potent inflammatory properties remain unclear, studies have identified several immune processes as mediators of PM-induced inflammation. For example, numerous reports demonstrate that exposure to ambient urban PM potentiates innate immune activation, and downstream recruitment of various types of leukocytes, which results in a mixed immune response. In addition, innate sensing of PM by epithelial cells and dendritic cells has been shown to produce a battery of immune modulating mediators that shape the downstream inflammatory and pathologic responses.
A number of different types of airborne particles, including Carbon Black (CB), coal fly ash (CFA) and diesel exhaust particles (DEP), are common constituents of urban PM and are often used in studies to investigate health effects of PM. It is unknown, however, whether such types of particles can effectively mimic the pathobiological effects mediated by urban PM and thus act as surrogates to urban PM in laboratory studies. To address this, we have used PM collected from New York city and Baltimore city as well as CB, DEP and CFA. Here, we demonstrate that repeated exposure to urban PM drives lung inflammation and impairs lung function, and that these effects are not mediated by the sole action of major constituents of PM like CB, DEP and CFA.
2. Materials and methods
2.1 Particulate matter
Carbon black was obtained from Cabot Corp (Hamade et al. 2008). Baltimore ambient particulate matter (Balt) was collected in urban Baltimore during the fall of 2012, using a modified high-volume cyclone system that collects particles between 0.3 and 10 μm aerodynamic diameter when operated at a flow rate of 1 m3/min (London et al. 2016). NYC PM was collected from Queens, New York between December 2009 to January 2010 using the fine fraction (0.3 to 3 μm) bulk PM collected with a high volume sequential cyclone system operated at 1 m3/min(Han et al. 2012; Rule et al. 2010). Diesel Exhaust Particles (DEP) (Standard Reference Material 1650b) was purchased from the National Institute for Standards and Technology (NIST). SRM 1650b particulate material was collected from the heat exchangers of a dilution tube facility following 200 engine hours of particle accumulation and represents diesel exhaust from heavy duty vehicles. Coal fly ash was purchased from Brandon Shores Unit power plant, Baltimore. Particles were resuspended in PBS at 10 mg/ml. The physical characteristics of PM are summarized in Table 1 and metal content of urban PMs are outlined in Supplementary Table 1.
Table 1.
Characteristics of carbon black, diesel exhaust particle, coal fly ash, NYC and Baltimore PM
| PM type (source) | Collection system | Collection date(s) | Size range | Physical/chemical Characterization |
|---|---|---|---|---|
| Carbon black (Regal 660) | Purchased in bulk | Purchased circa 1990 | Bulk PM Mean Diameter = 0.7 um Size between 0.1– 1.0 μm |
density= 1.95 g/cm3; specific surface area= 112 m2/g; composition= 96.90% carbon, 1.42% oxygen, 0.30% hydrogen |
| SRM 1650b (DEP) | Collected from four cycle heavy duty diesel engines. Obtained from Coordinating research council, Atlanta, GA. |
collected in 1983 | Bulk PM Mean diameter = 0.18um Size between 0.12–0.33 μm |
Derived from SRM1650a. |
| Coal Fly Ash (CFA) | Brandon Shores Unit, Baltimore | Collected in 1998 | Size between 10– 100 μm | The principal components of bituminous coal fly ash are silica, alumina, iron oxide with varying amounts of carbon. |
| NYC PM | Collected in Queens, NY using a high volume sequential cyclone | collected Nov 2009 to Jan 2010 | 0.3<d<2.5 Size between 0.3 and 2.5 μm |
Characterized for metals and ions. |
| Mean concentration = 8.21+/−2.5 μg/m3 (Han et al., JA & WMA 2012) | ||||
| Baltimore PM10 | Collected in Baltimore, MD with a modified high volume sequential cyclone # (Rule et al., JEM 2010) | Collected Fall 2012 | 0.3<d<10 Size between 0.3 and 10 μm |
Characterized for metals and ions |
| $ Mean concentration = 14.3+/− 7.5 μg/m3 |
Modified by eliminating the middle cyclone that would have collected PM between 2.5 μm and 10 μm
d: diameter
2.2. Mice and PM exposures
Male BALB/cJ and C57BL/6 mice (8–10 weeks) were purchased from Jackson and housed in the Johns Hopkins School of Public Health animal facility. Mice were provided autoclaved food (Lab diet 5010) and water ad libitum. Mice were anesthetized with isoflurane and given 400 μg PM (40 μl) intranasally on days 0, 3, 6, 9 and 12 (van Voorhis et al. 2013). All procedures were approved by the Animal Care and Use Committee of Johns Hopkins University.
2.3. Airway measurements
Briefly, 48h after the last PM exposure, mice were anesthetized by i.p. administration of ketamine/xylazine and tracheotomized before insertion of an 18-gauge cannula into the trachea. Mice were paralyzed with suxamethonium chloride (3 mg/kg), intubated and respirated at a rate of 120 breaths per minute with a constant tidal volume (0.2 ml). After a stable baseline was achieved, mice were exposed to 30 mg/ml nebulized methacholine (Sigma). After 10 seconds, dynamic airway pressure (cm H20×s) was recorded for 3 min. Following airway reactivity measurements, BAL fluid, and lungs were collected, and processed as previously described (Lajoie et al. 2010; Lewkowich et al. 2008).
2.4. Bronchoalveolar lavage fluid (BAL) collection and cell count
The lungs were lavaged using 1 ml of sterile HBBS to collect all the inflammatory cells in the BAL fluid. Total cells were stained with AccuStain and counted via the ADAM-MC automated cell counter (Digital Bio)(Sussan et al. 2015). Differential cell counts were performed on cytospin preps stained with Diff-Quik stain and determined based on standard cytological criteria.
2.5. Cytokine ELISAs
Mouse lung cells were obtained by digestion of lung tissue with 0.05 mg/ml Liberase TL (Roche) and 0.5 mg/ml DNaseI (Sigma) for 45 min at 37°C in 5% CO2. Digested tissue was filtered through a 70-μm nylon mesh (BD Biosciences) and centrifuged. Pellet was resuspended in red blood cell lysis buffer (ACK lysis buffer). Recovered cells were counted (trypan blue exclusion) and plated in a 96-well flat bottom tissue culture plate. Cells were re-stimulated with 4 μg/ml concanavalin A (Sigma) for 3 days. IL-5, IL-13, IL-17A, and IFNγ levels were measured using ELISA DuoSets (R&D Systems)(Lajoie et al. 2010).
2.6. Histological analysis of the lung sections
To assess the effects of particulate matter on overall inflammation in the lungs and mucus production from the airway epithelial cells, lungs were excised and fixed in 10% neutral buffered formalin, processed, paraffin embedded and sectioned. Sections were stained with Hematoxylin and Eosin (H&E) or Periodic Acid Schiff (PAS). Slides were read in a blinded fashion and scored according to the following scale:
0, no inflammation;
1+, peri-bronchial lymphocytic inflammation, size <20μm;
2+, peri-bronchial lymphocytic inflammation, size <50μm;
3+, peri-bronchial and alveolar inflammation, size <100μm;
4+, peri-bronchial and alveolar inflammation, size >100μm.
For quantifying mucus producing airway epithelial cells, % of PAS-positive cells in the left lobe of the lung were determined using a light microscope. Results are presented as mean+SE for 3–4 mice per group.
2.6. Real time RT-PCR
Total RNA was extracted from lung tissue using TRIzol RNA isolation reagent (Invitrogen). The reverse transcription reaction was performed using a high capacity cDNA synthesis kit (Applied Biosystems). Quantitative PCR analyses of mouse genes were performed by using assay-on-demand primers and probe sets (Applied Biosystems)(Singh et al. 2006). Actb (β-actin) was used for normalization.
2.8. Flow cytometry analysis
Mouse lung cells were prepared as above. Cells were plated at 4×106 cells/ml and either stained directly (myeloid) or stimulated (lymphoid) with phorbol 12-myristate 13-acetate (PMA, 50 ng/ml) and ionomycin (1 μg/ml) for 16h, then brefeldin A and monensin (eBioscience) were added for the last 3–4h (Lajoie et al. 2010). Cells were filtered using a 40- μm nylon mesh (BD Biosciences), washed with PBS and labeled with live/dead dye (Zombie Aqua, Biolegend) for 10 min at RT, and blocked with anti-CD16/32 (TrueStain FcX Anti-mouse, BioLegend) for an additional 20 min at RT. For the detection of myeloid populations, cells were stained with Brilliant violet 786-conjugated anti-CD11b (M1/70, BioLegend), Alexa Fluor-700-conjugated CD11c (N418, BioLegend), APC-Cy7-conjugated anti-Ly-6G (1A8, BioLegend), Brilliant Violet 605-conjugated anti-Ly-6C (HK1.4, BioLegend), Alexa Fluor 488-conjugated I-A/I-E (clone, BioLegend), Brilliant Violet 421-conjugated anti-Siglec-F (E50-2440, BD Biosciences), PerCP-Cy5.5-conjugated anti-CD103 (2E7, BioLegend). For the identification of cytokine-producing lymphoid subsets, PMA/ionomycin-stimulated cells were stained with APC-Fire750-conjugated anti-CD4 (RM4–5, eBioscience), PE-Cy7-conjugated anti-CD8 (53-6.7, BioLegend), Brilliant Violet 786-conjugated CD3ε (145-2C11, BD Biosciences), PerCP-Cy5.5-conjugated CD49b (DX5, BD Biosciences). For intracellular cytokine staining, cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) for 10 min at RT and permeabilized in 0.1% saponin (Sigma) for 20 min at RT. Cells were then stained with Brilliant Violet 421-conjugated anti-IL-17A (clone TC11-18H10, BD Biosciences), and Alexa Fluor 700-conjugated anti-IFNγ (clone XMG1.2, BioLegend). Data was acquired on an LSRII flow cytometer (BD Biosciences), and gated to exclude debris and to select single cells (FSC-W/FSC-A+SSC-W/SSC-A). Data was analyzed using FACSDiVa (BD Biosciences). Positive gates were based on Fluorescence Minus One (FMO) controls.
2.9. Statistical analysis
We determined differences between multiple groups using ANOVA, followed by Dunnett’s multiple comparison test or Kruskall-Wallis followed by Dunnett’s multiple comparison test. Significance was assumed at p < 0.05.
3. RESULTS
3.1. Exposure to urban PM induces a mixed eosinophilic/neutrophilic response
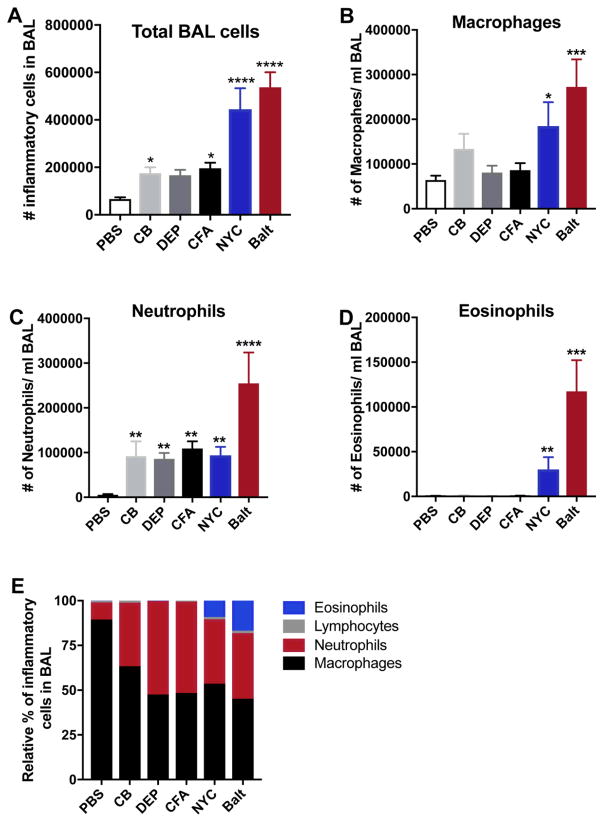
To determine the patterns and magnitudes of inflammatory pulmonary response after exposure of mouse lungs to urban PM collected from sites in New York (NYC) and Baltimore city (Balt), and to surrogate PMs (CB, FA and DEP), mice were exposed intranasally to various PM preparations as described above. All PM exposure induced accumulation of inflammatory cells in the BAL fluid, as compared to PBS-treated mice, with urban PM-exposed mice displaying markedly higher levels of inflammatory cells compared to mice exposed to surrogate PMs (Fig. 1A). This result is consistent with other studies showing that various types of PM can induce variable levels of airway inflammation. Also, consistent with other reports (Saunders et al. 2010), we noted that PM is a strong inducer of BAL macrophages and neutrophilia (Fig. 1B–C). Remarkably, urban PM as well as surrogate PM particles caused similar inductions of neutrophils.
Figure 1. Exposure to PM induces airway inflammation.
Male BALB/cJ mice were intranasally exposed to saline, CB, DEP, CFA or urban PM, 3 days a week for 2 weeks. 48 hours after the last exposure, mice were sacrificed, and total BAL cells (A) were counted. Macrophages (B), Neutrophils (C) and Eosinophils (D) in the BAL fluid were enumerated by differential cell counting. (E) Bar graph showing relative percentage of macrophages, neutrophils and eosinophils in the control and treatment groups. Data represents means+SEM and representative of 2 independent experiments (n=5 mice/group) or pooled from 2–3 independent experiments (8–15 mice/group). *compared to PBS. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Next, we assessed the effect of PM exposure on eosinophil accumulation in the lungs, a distinctive feature of type 2 immune responses. In contrast to the similar induction of neutrophils seen after exposures to all types of PM tested, we noted that urban PMs cause substantial eosinophil accumulations in lungs of BALB/c mice (Fig. 1D), as measured by cell counts in the BAL. On the other hand, exposures to CB, CFA or DEP did not induce eosinophils in either BALB/c (Fig. 1D). Our findings, suggest that exposures to different types of PM lead to both conserved physiological responses, such as neutrophilia, as well as different cellular responses, such as eosinophil accumulations seen after exposure to urban PM (Fig. 1E). Moreover, our data demonstrates that exposure to CB, FA and DEP surrogate PMs cannot completely recapitulate the complex immune response seen after exposure to urban PM.
3.2 Urban PM exposure induces a mixed effector cytokine response
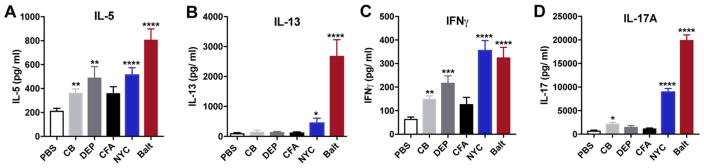
We next evaluated the nature of effector cytokines that are induced in lungs by exposure to urban PM and tested whether surrogate PMs are equally potent in modulating the expression of these effector cytokines. First, we measured IL-5, a cytokine central in driving eosinophilic airway inflammation. Consistent with our findings of increased eosinophils in urban PM exposed BAL fluid, we observed elevated IL-5 secretion in the lungs of NYC and Baltimore PM-exposed animals (Fig. 2A). Remarkably, exposure to CB, FA and DEP also induced IL-5, although we failed to observe eosinophil recruitment in these mice.
Figure 2. PM-driven effector cytokines.
Single cell suspension from PM-exposed male BALB/cJ mice were stimulated with concanavalin A (con A) for 3 days and levels of IL-5 (A), IL-13 (B), IFNγ (C) and IL-17A (D) levels in the supernatants were determined by ELISA. Data represents means+SEM, and is pooled from 2–3 independent experiments (10 mice/group). *compared to PBS. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
We also measured IL-13, a central mediator of asthma pathogenesis (Wills-Karp et al. 1998), and found that this key Th2 cytokine is significantly and selectively induced by urban PM in lungs (Fig. 2B), with our sample of Baltimore PM inducing substantially more IL-13 than NYC PM. Notably, CB, FA and DEP exposures had no effect on IL-13 production in our experiments, suggesting that unlike urban PMs, these particles have limited potential to drive aberrant type 2 responses and subsequent allergic airway inflammation. By driving the secretion of the Th1 cytokine IFNγ (Fig. 2C), urban PM responses also contrast to those seen after exposures to allergens such as dust mite, molds, cockroach, pollen or dander, where Th1 responses are either repressed or un-induced. CB and DEP also induce increases in IFNγ levels although of lower magnitude than that seen after exposure to urban PM.
Recent publications have reported the importance of PM-induced IL-17A in exacerbating allergen-induced asthma (Brandt et al. 2013; van Voorhis et al. 2013). We therefore sought to determine whether various sources of PM or surrogate particles have differential IL-17A-inducing properties. Interestingly, we observed that NYC and Baltimore PM are potent inducers of IL-17A expression (Fig. 2D), suggesting that this cytokine might play a role in driving airway inflammation to these urban PMs. In contrast to previous reports that found small but statistically significant induction of IL-17A by DEP (Brandt et al. 2013; Brandt et al. 2015), exposures to CFA and DEP in our experiments failed to induce IL-17A and CB induced only marginal secretion of IL-17A in the lungs. Consistent with the cytokine protein data, we also detected similar Il5 and Il13 expression patterns in the whole lung, whereas expression of Il4 did not change across the different treatment groups (Supplementary Fig. 1). Thus, urban PM leads to a mixed Th1/Th2/Th17 immune response, consistent with previous reports, and while different urban PMs show similar patterns of immune activation, variation in their composition also leads to different potencies in driving Th2 and Th17 cytokines. Moreover, we demonstrate that the IL-13- and IL-17A-driving constituents of urban PM are not found in CB, FA or DEP surrogate particles. Similar induction of Il5 and Il13 mRNA was observed in urban PM exposed lungs from C57BL/6 mice (Supplementary Fig. 2).
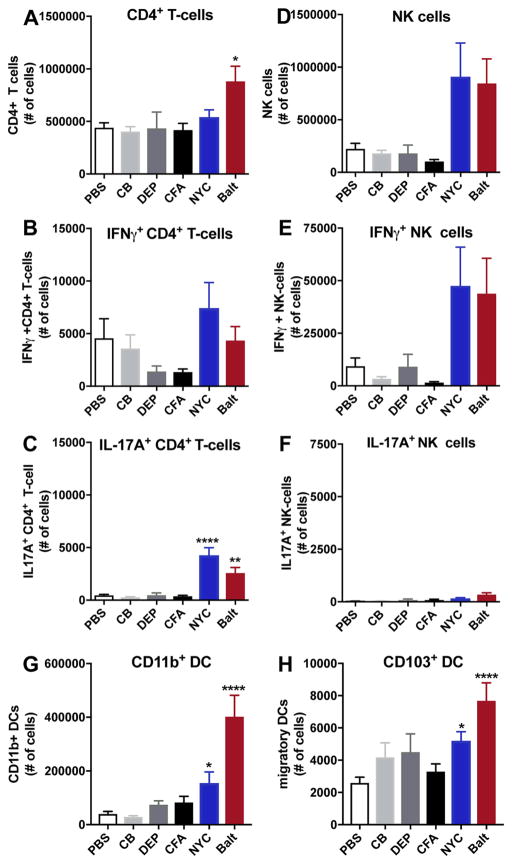
3.3. CD4+ T and NK cells are the primary source of PM-induced cytokines in the lungs
As we observe that PM induces cytokines in the lungs, we next sought to determine the nature of cytokine-secreting cells. The numbers of total CD4+ T cells remained largely unchanged after exposure to PM, with only the Baltimore PM-exposed group showing an increase in numbers of CD4+ T cells over PBS-treated mice (Fig. 3A). While PM exposure had no effect on Th1 (IFNγ+CD4+) cells (Fig. 3B), NYC and Baltimore PM exposures led to significant accumulation of Th17 cells (Fig. 3C). However, consistent with our IL-17A data, we observed that CB, DEP and CFA do not promote Th17 (IL-17A+CD4+) recruitment. Also, we do not observe the presence of double positive IFNg+IL-17A CD4+ T cells after PM exposure (Supplementary Fig. 3). Urban PM from NYC and Baltimore similarly induced an IL-17A-dominant response in C57BL/6 lungs whereas CB, DEP, CFA exposure induced minimal changes (Supplementary Fig. 4)
Figure 3. Identification of cytokine-producing cells and antigen-presenting cell profile in the lungs of PM-exposed mice.
Single cell suspension from PM-exposed mice were analyzed by flow cytometry for total CD3+CD4+ T cells (A), IFNγ+CD4+ T cells (B), and IL-17A+CD4+ T cells (C), total NK cells (CD3–NK1.1+) (D), (IFNγ+ NK (E) and IL-17A+NK (F). Numbers of CD11b+ DCs (G), and CD103+ migratory DCs (H). Data represents means +SEM, pooled from 2 independent experiments (10 mice/group). *compared to PBS. *p<0.05, ****p<0.0001.
While some investigators have observed an influx of CD8+ T cells in the lungs after PM exposure (Deiuliis et al), we fail to see increased numbers of either total CD8+, IFNg+CD8+ or IL-17A+CD8+ T cells after PM exposure (Supplementary Fig. 5). In contrast to CD4+ or CD8+ T cells, the recruitment of NK cells into the lungs induced by NYC and Baltimore PM was greater than that seen by PBS, CB, DEP and CFA (Fig. 3D). Similarly, the numbers IFNγ+NK cells in the lungs of animals exposed to urban PM were greater than lungs of animals exposed to PBS, DEP or CFA (Fig. 3E). Interestingly, the increases in levels of IFNγ in the lungs after PM exposure are not accompanied by increases in IFNγ+CD4+ cells or in IFNγ+CD8+ cells (Fig. 3B and Supplementary Fig. 5), suggesting NK cells as the main source of IFNγ in lungs exposed to urban PM.
In addition to T cells, NK cells can also secrete IL-17A, however, only Baltimore urban PM significantly induced IL-17A+NK cells in the BALB/cJ lungs as compared to PBS (Fig. 3F). Still, the numbers of urban PM-recruited IL-17A+NK cells are several fold lower than that of IL-17A+CD4+ cells, suggesting that CD4+ T cells are likely the primary source of PM-induced IL-17A. Similar to our observation with BALB/cJ strain, we observed increased IL-17A+NK cells in Baltimore PM exposed lung samples from C57BL/6 strain, however, the difference did not reach statistical significance (Supplementary Fig. 4).
3.4. Urban PM triggers a unique pattern of monocytic and dendritic cell influx into the lungs
PM is well known to activate macrophages and dendritic cells after phagocytosis (Brugha et al. 2014; Matthews et al. 2016; Mukae et al. 2000; Porter et al. 2007; Provoost et al. 2010; Williams et al. 2007). For this reason, we wanted to test whether PM could alter antigen presenting cell (APC) influx into the lungs (see Supplementary Fig. 6 for gating scheme). Notably, we did not detect increases in lung resident alveolar macrophages (CD11c+Siglec-F+) after any PM exposure (Supplementary Fig. 7). We next looked at CD11b+ DCs (CD11b+CD11c+MHCIIhi), a strongly immunogenic DC subset. Exposure to CB, DEP and CFA had no effect on CD11b+ DC recruitment, while NYC and Baltimore PM induced a significant accumulation of these DCs in the lungs from BALB/cJ (Fig. 3G) and C57BL/6 strains of mice (Supplementary Fig. 4). Likewise, we observed a significant increase in migratory CD103+ DCs (CD11b−/loCD11c+CD103+) after exposure to NYC and Baltimore PM as compared to CB, DEP and CFA (Fig. 3H). These findings indicate that urban PMs are distinctive in their ability to recruit these important DC subsets. These findings further suggest that both CD11b+ and CD103+ DCs are critical for recruiting innate IL-17A-producing cells and Th17 cells (Scott et al. 2015; Zelante et al. 2015), and that both NYC and Baltimore PM can lead to specific downstream effector responses because of their ability to recruit and activate these DC populations.
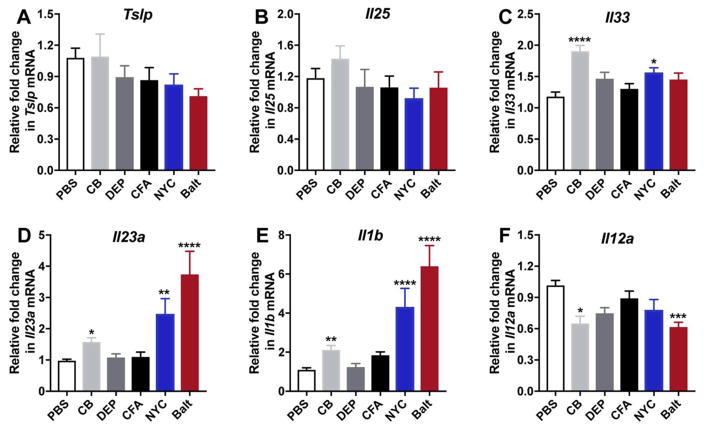
3.5. PM exposures drive differential epithelial- and APC-derived innate cytokines
Epithelial- and APC-derived innate cytokines are central in driving downstream Th1, Th2 and Th17 responses. As we observed that different urban PM can preferentially induce adaptive T cell responses, we wanted to investigate whether these PMs trigger downstream polarized responses by altering innate cytokine production. Because epithelial-derived IL-25, TSLP and IL-33 are central in driving type 2 responses, we evaluated whether PM modulated their expression. Our data demonstrates that while any particulate matter has little effect on Tslp (Fig. 4A) and Il25 (Fig. 4B), CB and NYC PM only marginally increased Il33 mRNA as compared to PBS (Fig. 4C). However, both DEP, CFA and Baltimore PM had no impact on Il33 expression (Fig. 4C). These data suggest that PM may drive type 2 responses through the action of other innate mediators.
Figure 4. Levels of innate mediators in lung exposed to PM.
Transcript levels of Tslp (A), Il25 (B), Il33 (C), Il23a (D) Il1b (E) and Il12a (F) were determined in whole lung by RT-PCR. Data represents means+SEM, pooled from 2 independent experiments (10 mice/group). *compared to PBS. *p<0.05, **p<0.01, ****p<0.0001.
IL-17A is strongly induced by Baltimore PM and to a lesser extent by NYC PM, and accordingly, mice exposed to Baltimore PM have the highest levels of PM-induced Il23a (Fig. 4D) and Il1b (Fig. 4E), two DC-derived cytokines required for the production of IL-17A-producing lymphocytes. CB induces minimal IL-17A secretion, and we find that only CB upregulates Il23a or Il1b transcription, but FA and DEP have no apparent effect on Il23a or Il1b mRNA in the lungs (Fig. 4D, E). This observation suggests the presence of components within NYC and Baltimore PM that directly activate dendritic cells to produce both IL-23 and IL-1β. Although our data shows that PM can induce IFNγ we did not detect PM-mediated induction of Il12a (Fig. 4F), the prototypical DC-produced cytokine that drives Th1 responses, suggesting that either, a) timing of IL-12 production occurs earlier and was not captured by our data, or b) that other IL-12-independent pathways lead to Th1 differentiation in PM-exposed mice (Skokos and Nussenzweig 2007; Xing et al. 2000).
3.6. Exposure to urban PM induces mucus production and airway hyperresponsiveness (AHR) in the mice
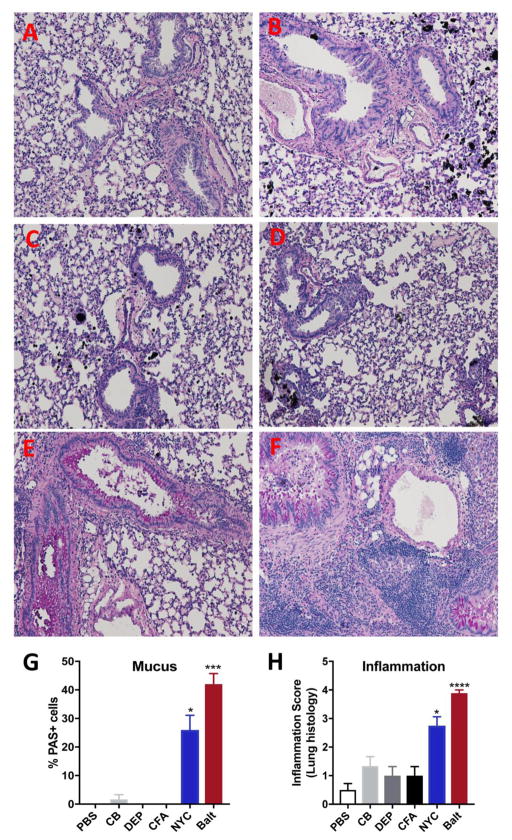
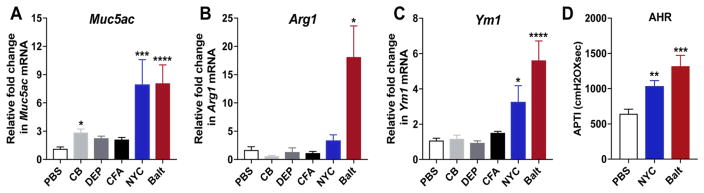
We next assessed PM-induced chronic inflammation and mucus production, both hallmark features of allergic airway inflammation. We find that consistent with increasing eosinophils and Th2 cytokines in lungs, exposure to Baltimore and NYC PM uniquely caused increases in the numbers of airways containing PAS+ mucus cells as well as numbers of PAS+ cells in each airway (Fig. 5A–G). Interestingly, exposure to CB, CFA or DEP did not induce mucus secretion in the lungs as indicated by absence of PAS+ cells. Assessment of pathologic changes in the lungs revealed acute peri-bronchial and alveolar inflammation with a size range of >50–100μm in the urban PM exposed group with Baltimore PM inducing more potent pathologic response and exhibiting maximum inflammation score of 4 compared to the NYC PM (Inflammation score 3) (Fig. 5H) and inflammation scores that were not significantly different from saline treated controls for animals exposed to CB, CFA or DEP (Fig. 5H). Consistent with this scoring of PAS staining, we observe that urban PM induces robust expression of Muc5ac mRNA (mRNA coding for a glycoprotein of mucus) as well as markers of alternatively activated macrophages (M2) like Arginase1 (Arg1) and Ym1 (Fig. 6A–C). Similar to our findings with BALB/cJ mice, urban PM also induced the expression of Muc5ac, Arg1 and Ym1 mRNAs in the lungs of C57BL/6 mice (Supplementary Fig. 2). Finally, we determined whether urban PM could also drive AHR and observed that both NYC and Baltimore PMs could induce AHR to cholinergic agonist stimulation (Fig. 6D). These data thus provide novel evidence that exposure to urban PM can specifically promote the development of Th2 and Th17 responses, mucus metaplasia and AHR whereas exposure to components of urban PM such as CB, FA and DEP are not able to drive allergic airway inflammation.
Figure 5. Exposure to urban PM induces pulmonary inflammation and mucus metaplasia.
PAS-stained sections of the lungs from saline (A), carbon black (B), Diesel exhaust particle (C), coal fly ash, New York PM (E) and Baltimore PM (F) exposed mice. (G) Quantification of PAS+ cells. (H) The same lung sections were scored for inflammation. Scoring was done using the following criteria: 0, no inflammation; 1+, peri-bronchial lymphocytic inflammation, size <20μm; 2+, peri-bronchial lymphocytic inflammation, size <50μm; 3+, peri-bronchial and alveolar inflammation, size <100μm; 4+, peri-bronchial and alveolar inflammation, size >100μm. Data represents means +SEM and representative of 2 independent experiments (n=5 mice/group) or pooled from 2–3 independent experiments (8–15 mice/group). *compared to PBS. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Figure 6. Exposure to urban PM induces Th2 cytokine driven gene expression and AHR.
Relative expression of Muc5AC (A) Arg1 (B) and Ym1 (C) mRNA in the whole lung. (D) AHR in mice receiving PBS or urban PM i.n. 3 days a week for 2 weeks. 24–36h after the last exposure, AHR was measured. Data represents means+SEM and representative of 2 independent experiments ( (8–15 mice/group). *compared to PBS. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
4. DISCUSSION
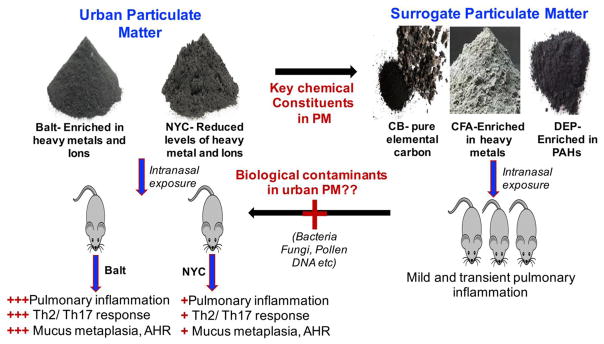
In this study, we sought to compare immunological responses in lungs exposed to equal mass of urban PM from various sources and to surrogate PM particles, including CB, DEP and CFA. Consistent with other studies (Saunders et al. 2010; Walters et al. 2001; Walters et al. 2002), our findings indicate that urban PM exposure is notable for causing substantial airway inflammation and impaired lung function (Fig. 7). Specifically, we found that short term exposure to PMs collected from New York and Baltimore cities trigger a mixed Th2/Th17 response accompanied by eosinophilic and neutrophilic influx, mucus production and airway hyperresponsiveness. Although the immune profiles in lungs exposed to PMs from the different urban sources are similar, we noted compelling differences in the potencies of PMs from these sources, with Baltimore PM inducing more robust airway inflammation, AHR, and Th2 cytokine production.
Figure 7. Schematic diagram of research hypothesis, design and observations.
CB, carbon black; DEP, diesel exhaust particle; CFA, coal fly ash; PAH, polyaromatic hydrocarbon; AHR, airway hyperressponsiveness.
Previous reports have focused on the immunobiological activity of single sources of PM, and found that they drive mostly mixed immune responses. A recent report shows that industrial PM collected at various sites display significantly different toxicities (Thomson et al. 2016), suggesting that various PM may drive pathology through different pathways. Nevertheless, it remains unclear whether exposure to different sources of PM, like carbon black, FA, DEP and urban-collected PMs, will drive distinct immunological outcomes. Our findings demonstrate that while urban PMs promote manifestations of allergic asthma characterized by mixed immune responses, major constituents of urban PM like CB, FA and DEP, on their own, cannot recapitulate these pathological effects.
Carbon is one of the unifying components of all PM, and for this reason carbon black is often used to study the biological effect of particles. We investigated the role of carbon particles in driving manifestations of airway inflammation, and found that consistent with others (Gilmour et al. 2004; Renwick et al. 2004; Saputra et al. 2014; Shwe et al. 2005; Zhang et al. 2014), it can drive inflammatory cell influx, primarily neutrophils, and induce some cytokines like IL-5 and IFNγ however these effects were minimal compared to urban PM. This suggest that the biological effects of city-collected PM are directly related to its complex composition. It is thought that particulate matter can adsorb various biologically active components like metals, organic compounds, hydrocarbons, but it remains unclear the identity of the chemical or biological entities within urban PM that drive lung inflammation.
Among the various components of PM, it is thought that metals are particularly toxic and likely to drive some of the inflammatory and deleterious effects of PM exposure (Chen and Lippmann 2009). Initial assessment of the metal composition of PM reveals some profound differences between NYC and Baltimore PM for the majority of the metals. In fact, several metals were in higher concentrations in Baltimore versus NYC PM, and may help explain the enhanced potency of Baltimore PM in driving some manifestations of airway inflammation. The higher zinc content of Baltimore PM, compared to that of NYC PM, could help explain why Baltimore PM can induces more eosinophils than NYC PM. Chronic airway exposure of zinc nanoparticles into rats and mice, as opposed to nickel or copper nanoparticles, lead to a significant accumulation of eosinophils in the BAL (Cho et al. 2012; Roy et al. 2014). However, in contrast to zinc or copper, chronic nickel exposure resulted in neutrophilia (Cho et al. 2012), and consistent with this, all PM (NYC and Baltimore) have comparable nickel content and thus drive neutrophilic influx. Interestingly, nickel strongly activates TLR4 in humans, resulting in IL-8 production, a potent neutrophil chemoattractant (Schmidt et al. 2010). In addition, metals like cadmium and aluminum, which are elevated in Baltimore PM as compared to NYC PM, are known to drive significant lung inflammation associated with greater cellular influx and the production if pro-inflammatory cytokines like IFNγ and TNFα, (Blum et al. 2014; Kirschvink et al. 2006) and decrements in lung function(Mazzoli-Rocha et al. 2010). On its own, airway exposure to arsenic in mice does not lead to significant accumulation of neutrophils or pro-inflammatory cytokines and only marginal macrophage accumulation, however, it significantly exacerbates the recruitment of inflammatory cells and the production of inflammatory mediators (Kozul et al. 2009; Ramsey et al. 2013) in mice infected with influenza. Conversely, in the context of allergic airway inflammation to ovalbumin, exposure to arsenic trioxide significantly reduces airway inflammation (Chu et al. 2010; Zhou et al. 2006). These seemingly conflicting findings suggest that the role of arsenic in the lungs may depend on the pre-existing immune microenvironment.
One plausible explanation for greater metal content and robust immunotoxicological effects of Baltimore PM relative to NYC PM is that Baltimore PM collection site is located close to an industrial area whereas NYC PM was collected in a residential area of the city. Furthermore, NYC PM was collected over a period that included the winter holidays and traffic and industrial emissions could have been lower in that time. More importantly, although Baltimore PM is enriched in metals, its overall metal content is comparable to urban PM NIST 1648a (Mitkus et al. 2013). To conclude, similar to urban PM, CFA and DEP also contain metals although mostly in the oxidized form, induced early neutrophil accumulation but failed to recruit eosinophils into the lungs suggesting that metal composition and content of CFA and DEP may not be enough to drive eosinophilic influx. (Huggins et al. 2000; Miyabara et al. 1998; Takano et al. 1998).
Besides metals, PMs are rich in polyaromatic hydrocarbons (PAH), and traffic emissions are the major contributor to PAH exposure (Dubowsky et al. 1999). PAH are recognized by the aryl hydrocarbon receptor (AhR) and this drives Th17 responses (Quintana et al. 2010; Veldhoen et al. 2008). Responsiveness to PAHs has been shown to be important for PM-induced Th17 responses (Brandt et al. 2013; van Voorhis et al. 2013). Surprisingly, CFA and DEP which contain various PAHs (Bergvall and Westerholm 2008; Rohr et al. 2015), induced little to no IL-17A in the lungs, suggesting that these PAH varieties may not be sufficient to drive Th17 responses.
In addition to carrying chemical toxins, urban PM is known to contain biological contaminants of fungal and bacterial origin (Dong et al. 1996; Frohlich-Nowoisky et al. 2009; Gilmour et al. 2007; Morakinyo et al. 2016; Mueller-Anneling et al. 2004; Soukup and Becker 2001; Yan et al. 2016). These microbial products are strong activators of dendritic cells, and result in the production of IL-23 and IL-1β that drive IL-17A-dominated downstream responses (Mann et al. 2017; Matthews et al. 2016). Recognition of these microbial moieties in urban PM could explain its potent Th17-driving effects. Interestingly, the dose of exposure to these biological contaminants can skew immune activation. While larger doses of endotoxins predominantly drives Th1 and Th17 responses (Chapman et al. 2013; Eisenbarth et al. 2002), contact with low doses of endotoxins favor type 2 inflammation (Eisenbarth et al. 2002). Indeed, some sources of urban PM contain small quantities of endotoxins and this could drive Th2 responses, nonetheless it remains unclear what constituent of urban PM may favor Th2 inflammation. Our data, and that of others (Saunders et al. 2010; Walters et al. 2001) show that the pro-Th2 effect of urban PM is clear, however, analysis of the Th2-skewing innate cytokines, IL-33, IL-25 and TSLP, revealed PMs had little to no effect on their expression. These data suggest that other PM-driven innate mediators, which have been shown to be induced by PM, like C3 (Walters et al. 2002), IL-1α (Watterson et al. 2012) (Campbell et al. 2005) or GM-CSF (Ohta et al. 1999), could contribute to the development of PM-dependent Th2 responses.
5. Conclusions
In summary, our data establishes that the airway inflammatory effects of urban PM cannot be truly recapitulated by components of PM like CB, CFA and DEP alone and suggests the possibility that unidentified chemical or biological components of urban PM drive pathological immune responses in the lungs. Specifically, exposure to urban PM leads to a unique Th2/Th17 responses, not seen with either CB, CFA or DEP, possibly explaining the unique ability of urban PM to drive major manifestations of allergic airway disease.
Supplementary Material
Highlights.
CB, DEP and CFA fail to recapitulate the immunotoxicological effects of urban PM.
Urban PM induces allergic airway inflammation characterized by a mixed Th2/Th17 response.
Baltimore PM stimulates a more robust and sustained inflammatory response as compared to NYC PM.
The immunotoxicological determinants of urban PM remain to be identified.
Acknowledgments
This work was supported by the FHB33CRF- Maryland State Cigarette Restitution Fund (A.S and S.L), the Flight Attendant Medical Research Institute (A.S), NIH R56AI118791 (S.L), NIH R01AI127644 (S.L) and NCI R01 CA190610 (J.D.G). NG was supported by NIEHS T32ES007141. We thank Drs. Marsha Wills-Karp and Shyam Biswal at JHU for generously sharing facilities and equipment. Animal related procedures were approved by the Animal Care and Use Committee of Johns Hopkins University.
Footnotes
Financial Disclosure:
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acciani TH, Brandt EB, Khurana Hershey GK, Le Cras TD. Diesel exhaust particle exposure increases severity of allergic asthma in young mice. Clin Exp Allergy. 2013;43:1406–1418. doi: 10.1111/cea.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ZJ, Loft S, Ketzel M, Stage M, Scheike T, Hermansen MN, et al. Ambient air pollution triggers wheezing symptoms in infants. Thorax. 2008;63:710–716. doi: 10.1136/thx.2007.085480. [DOI] [PubMed] [Google Scholar]
- Bennett BA, Mitzner W, Tankersley CG. The effects of age and carbon black on airway resistance in mice. Inhal Toxicol. 2012;24:931–938. doi: 10.3109/08958378.2012.731436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergvall C, Westerholm R. Determination of 252–302 da and tentative identification of 316–376 da polycyclic aromatic hydrocarbons in standard reference materials 1649a urban dust and 1650b and 2975 diesel particulate matter by accelerated solvent extraction-hplc-gc-ms. Anal Bioanal Chem. 2008;391:2235–2248. doi: 10.1007/s00216-008-2182-x. [DOI] [PubMed] [Google Scholar]
- Blum JL, Rosenblum LK, Grunig G, Beasley MB, Xiong JQ, Zelikoff JT. Short-term inhalation of cadmium oxide nanoparticles alters pulmonary dynamics associated with lung injury, inflammation, and repair in a mouse model. Inhal Toxicol. 2014;26:48–58. doi: 10.3109/08958378.2013.851746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of il-17a contributes to severe asthma. J Allergy Clin Immunol. 2013;132:1194–1204. e1192. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt EB, Biagini Myers JM, Acciani TH, Ryan PH, Sivaprasad U, Ruff B, et al. Exposure to allergen and diesel exhaust particles potentiates secondary allergen-specific memory responses, promoting asthma susceptibility. J Allergy Clin Immunol. 2015;136:295–303. e297. doi: 10.1016/j.jaci.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugha RE, Mushtaq N, Round T, Gadhvi DH, Dundas I, Gaillard E, et al. Carbon in airway macrophages from children with asthma. Thorax. 2014;69:654–659. doi: 10.1136/thoraxjnl-2013-204734. [DOI] [PubMed] [Google Scholar]
- Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26:133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Cassee FR, Heroux ME, Gerlofs-Nijland ME, Kelly FJ. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal Toxicol. 2013;25:802–812. doi: 10.3109/08958378.2013.850127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TJ, Emo JA, Knowlden SA, Rezaee F, Georas SN. Pre-existing tolerance shapes the outcome of mucosal allergen sensitization in a murine model of asthma. J Immunol. 2013;191:4423–4430. doi: 10.4049/jimmunol.1300042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LC, Lippmann M. Effects of metals within ambient air particulate matter (pm) on human health. Inhal Toxicol. 2009;21:1–31. doi: 10.1080/08958370802105405. [DOI] [PubMed] [Google Scholar]
- Cho WS, Duffin R, Poland CA, Duschl A, Oostingh GJ, Macnee W, et al. Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology. 2012;6:22–35. doi: 10.3109/17435390.2011.552810. [DOI] [PubMed] [Google Scholar]
- Chu KH, Lee CC, Hsin SC, Cai BC, Wang JH, Chiang BL. Arsenic trioxide alleviates airway hyperresponsiveness and eosinophilia in a murine model of asthma. Cell Mol Immunol. 2010;7:375–380. doi: 10.1038/cmi.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Lewtas J, Luster MI. Role of endotoxin in tumor necrosis factor alpha expression from alveolar macrophages treated with urban air particles. Exp Lung Res. 1996;22:577–592. doi: 10.3109/01902149609046043. [DOI] [PubMed] [Google Scholar]
- Dubowsky SD, Wallace LA, Buckley TJ. The contribution of traffic to indoor concentrations of polycyclic aromatic hydrocarbons. J Expo Anal Environ Epidemiol. 1999;9:312–321. doi: 10.1038/sj.jea.7500034. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent t helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich-Nowoisky J, Pickersgill DA, Despres VR, Poschl U. High diversity of fungi in air particulate matter. Proc Natl Acad Sci U S A. 2009;106:12814–12819. doi: 10.1073/pnas.0811003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour MI, McGee J, Duvall RM, Dailey L, Daniels M, Boykin E, et al. Comparative toxicity of size-fractionated airborne particulate matter obtained from different cities in the united states. Inhal Toxicol. 2007;19(Suppl 1):7–16. doi: 10.1080/08958370701490379. [DOI] [PubMed] [Google Scholar]
- Gilmour PS, Ziesenis A, Morrison ER, Vickers MA, Drost EM, Ford I, et al. Pulmonary and systemic effects of short-term inhalation exposure to ultrafine carbon black particles. Toxicol Appl Pharmacol. 2004;195:35–44. doi: 10.1016/j.taap.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hamade AK, Rabold R, Tankersley CG. Adverse cardiovascular effects with acute particulate matter and ozone exposures: Interstrain variation in mice. Environ Health Perspect. 2008;116:1033–1039. doi: 10.1289/ehp.10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han I, Mihalic JN, Ramos-Bonilla JP, Rule AM, Polyak LM, Peng RD, et al. Assessment of heterogeneity of metal composition of fine particulate matter collected from eight u.S. Counties using principal component analysis. J Air Waste Manag Assoc. 2012;62:773–782. doi: 10.1080/10962247.2012.676593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins FE, Huffman GP, Robertson JD. Speciation of elements in nist particulate matter srms 1648 and 1650. J Hazard Mater. 2000;74:1–23. doi: 10.1016/s0304-3894(99)00195-8. [DOI] [PubMed] [Google Scholar]
- Karimi P, Peters KO, Bidad K, Strickland PT. Polycyclic aromatic hydrocarbons and childhood asthma. Eur J Epidemiol. 2015;30:91–101. doi: 10.1007/s10654-015-9988-6. [DOI] [PubMed] [Google Scholar]
- Kirschvink N, Martin N, Fievez L, Smith N, Marlin D, Gustin P. Airway inflammation in cadmium-exposed rats is associated with pulmonary oxidative stress and emphysema. Free Radic Res. 2006;40:241–250. doi: 10.1080/10715760500494657. [DOI] [PubMed] [Google Scholar]
- Kozul CD, Ely KH, Enelow RI, Hamilton JW. Low-dose arsenic compromises the immune response to influenza a infection in vivo. Environ Health Perspect. 2009;117:1441–1447. doi: 10.1289/ehp.0900911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six u.S. Cities. Environ Health Perspect. 2000;108:941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, et al. Complement-mediated regulation of the il-17a axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M. Allergen uptake, activation, and il-23 production by pulmonary myeloid dcs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS One. 2008;3:e3879. doi: 10.1371/journal.pone.0003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London NR, Jr, Tharakan A, Rule AM, Lane AP, Biswal S, Ramanathan M., Jr Air pollutant-mediated disruption of sinonasal epithelial cell barrier function is reversed by activation of the nrf2 pathway. J Allergy Clin Immunol. 2016;138:1736–1738. e1734. doi: 10.1016/j.jaci.2016.06.027. [DOI] [PubMed] [Google Scholar]
- Mann EH, Ho TR, Pfeffer PE, Matthews NC, Chevretton E, Mudway I, et al. Vitamin d counteracts an il-23-dependent il-17a+ifn-gamma+ response driven by urban particulate matter. Am J Respir Cell Mol Biol. 2017;57:355–366. doi: 10.1165/rcmb.2016-0409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews NC, Pfeffer PE, Mann EH, Kelly FJ, Corrigan CJ, Hawrylowicz CM, et al. Urban particulate matter-activated human dendritic cells induce the expansion of potent inflammatory th1, th2, and th17 effector cells. Am J Respir Cell Mol Biol. 2016;54:250–262. doi: 10.1165/rcmb.2015-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoli-Rocha F, Dos Santos AN, Fernandes S, Ferreira Normando VM, Malm O, Nascimento Saldiva PH, et al. Pulmonary function and histological impairment in mice after acute exposure to aluminum dust. Inhal Toxicol. 2010;22:861–867. doi: 10.3109/08958378.2010.489074. [DOI] [PubMed] [Google Scholar]
- Mitkus RJ, Powell JL, Zeisler R, Squibb KS. Comparative physicochemical and biological characterization of nist interim reference material pm2.5 and srm 1648 in human a549 and mouse raw264.7 cells. Toxicol In Vitro. 2013;27:2289–2298. doi: 10.1016/j.tiv.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Miyabara Y, Ichinose T, Takano H, Lim HB, Sagai M. Effects of diesel exhaust on allergic airway inflammation in mice. J Allergy Clin Immunol. 1998;102:805–812. doi: 10.1016/s0091-6749(98)70021-1. [DOI] [PubMed] [Google Scholar]
- Morakinyo OM, Mokgobu MI, Mukhola MS, Hunter RP. Health outcomes of exposure to biological and chemical components of inhalable and respirable particulate matter. Int J Environ Res Public Health. 2016:13. doi: 10.3390/ijerph13060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Anneling L, Avol E, Peters JM, Thorne PS. Ambient endotoxin concentrations in pm10 from southern california. Environ Health Perspect. 2004;112:583–588. doi: 10.1289/ehp.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukae H, Hogg JC, English D, Vincent R, van Eeden SF. Phagocytosis of particulate air pollutants by human alveolar macrophages stimulates the bone marrow. Am J Physiol Lung Cell Mol Physiol. 2000;279:L924–931. doi: 10.1152/ajplung.2000.279.5.L924. [DOI] [PubMed] [Google Scholar]
- Nachman KE, Parker JD. Exposures to fine particulate air pollution and respiratory outcomes in adults using two national datasets: A cross-sectional study. Environ Health. 2012;11:25. doi: 10.1186/1476-069X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Ferin J, Gelein R, Soderholm SC, Finkelstein J. Role of the alveolar macrophage in lung injury: Studies with ultrafine particles. Environ Health Perspect. 1992;97:193–199. doi: 10.1289/ehp.97-1519541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Yamashita N, Tajima M, Miyasaka T, Nakano J, Nakajima M, et al. Diesel exhaust particulate induces airway hyperresponsiveness in a murine model: Essential role of gm-csf. J Allergy Clin Immunol. 1999;104:1024–1030. doi: 10.1016/s0091-6749(99)70084-9. [DOI] [PubMed] [Google Scholar]
- Ohtsuka Y, Brunson KJ, Jedlicka AE, Mitzner W, Clarke RW, Zhang LY, et al. Genetic linkage analysis of susceptibility to particle exposure in mice. Am J Respir Cell Mol Biol. 2000;22:574–581. doi: 10.1165/ajrcmb.22.5.3895. [DOI] [PubMed] [Google Scholar]
- Paulin L, Hansel N. Particulate air pollution and impaired lung function. F1000Res. 2016:5. doi: 10.12688/f1000research.7108.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M, Karp M, Killedar S, Bauer SM, Guo J, Williams D, et al. Diesel-enriched particulate matter functionally activates human dendritic cells. Am J Respir Cell Mol Biol. 2007;37:706–719. doi: 10.1165/rcmb.2007-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provoost S, Maes T, Willart MA, Joos GF, Lambrecht BN, Tournoy KG. Diesel exhaust particles stimulate adaptive immunity by acting on pulmonary dendritic cells. J Immunol. 2010;184:426–432. doi: 10.4049/jimmunol.0902564. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and t cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KA, Foong RE, Sly PD, Larcombe AN, Zosky GR. Early life arsenic exposure and acute and long-term responses to influenza a infection in mice. Environ Health Perspect. 2013;121:1187–1193. doi: 10.1289/ehp.1306748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner B, Mueller CA, Shephard A. Environmental and non-infectious factors in the aetiology of pharyngitis (sore throat) Inflamm Res. 2012;61:1041–1052. doi: 10.1007/s00011-012-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick LC, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004;61:442–447. doi: 10.1136/oem.2003.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr AC, Campleman SL, Long CM, Peterson MK, Weatherstone S, Quick W, et al. Potential occupational exposures and health risks associated with biomass-based power generation. Int J Environ Res Public Health. 2015;12:8542–8605. doi: 10.3390/ijerph120708542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Kumar S, Verma AK, Sharma A, Chaudhari BP, Tripathi A, et al. Zinc oxide nanoparticles provide an adjuvant effect to ovalbumin via a th2 response in balb/c mice. Int Immunol. 2014;26:159–172. doi: 10.1093/intimm/dxt053. [DOI] [PubMed] [Google Scholar]
- Rule AM, Geyh AS, Ramos-Bonilla JP, Mihalic JN, Margulies JD, Polyak LM, et al. Design and characterization of a sequential cyclone system for the collection of bulk particulate matter. J Environ Monit. 2010;12:1807–1814. doi: 10.1039/c0em00034e. [DOI] [PubMed] [Google Scholar]
- Saputra D, Yoon JH, Park H, Heo Y, Yang H, Lee EJ, et al. Inhalation of carbon black nanoparticles aggravates pulmonary inflammation in mice. Toxicol Res. 2014;30:83–90. doi: 10.5487/TR.2014.30.2.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate matter-induced airway hyperresponsiveness is lymphocyte dependent. Environ Health Perspect. 2010;118:640–646. doi: 10.1289/ehp.0901461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Raghavan B, Muller V, Vogl T, Fejer G, Tchaptchet S, et al. Crucial role for human toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol. 2010;11:814–819. doi: 10.1038/ni.1919. [DOI] [PubMed] [Google Scholar]
- Scott CL, Bain CC, Wright PB, Sichien D, Kotarsky K, Persson EK, et al. Ccr2(+)cd103(−) intestinal dendritic cells develop from dc-committed precursors and induce interleukin-17 production by t cells. Mucosal Immunol. 2015;8:327–339. doi: 10.1038/mi.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwe TT, Yamamoto S, Kakeyama M, Kobayashi T, Fujimaki H. Effect of intratracheal instillation of ultrafine carbon black on proinflammatory cytokine and chemokine release and mrna expression in lung and lymph nodes of mice. Toxicol Appl Pharmacol. 2005;209:51–61. doi: 10.1016/j.taap.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, et al. Dysfunctional keap1-nrf2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokos D, Nussenzweig MC. Cd8- dcs induce il-12-independent th1 differentiation through delta 4 notch-like ligand in response to bacterial lps. J Exp Med. 2007;204:1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Veranth JM, Kodavanti UP, Aust AE, Pinkerton KE. Acute pulmonary and systemic effects of inhaled coal fly ash in rats: Comparison to ambient environmental particles. Toxicol Sci. 2006;93:390–399. doi: 10.1093/toxsci/kfl062. [DOI] [PubMed] [Google Scholar]
- Soukup JM, Becker S. Human alveolar macrophage responses to air pollution particulates are associated with insoluble components of coarse material, including particulate endotoxin. Toxicol Appl Pharmacol. 2001;171:20–26. doi: 10.1006/taap.2000.9096. [DOI] [PubMed] [Google Scholar]
- Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10:e0116861. doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano H, Ichinose T, Miyabara Y, Yoshikawa T, Sagai M. Diesel exhaust particles enhance airway responsiveness following allergen exposure in mice. Immunopharmacol Immunotoxicol. 1998;20:329–336. doi: 10.3109/08923979809038548. [DOI] [PubMed] [Google Scholar]
- Thomson EM, Breznan D, Karthikeyan S, MacKinnon-Roy C, Vuong NQ, Dabek-Zlotorzynska E, et al. Contrasting biological potency of particulate matter collected at sites impacted by distinct industrial sources. Part Fibre Toxicol. 2016;13:65. doi: 10.1186/s12989-016-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Voorhis M, Knopp S, Julliard W, Fechner JH, Zhang X, Schauer JJ, et al. Exposure to atmospheric particulate matter enhances th17 polarization through the aryl hydrocarbon receptor. PLoS One. 2013;8:e82545. doi: 10.1371/journal.pone.0082545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links th17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Veronesi B, de Haar C, Lee L, Oortgiesen M. The surface charge of visible particulate matter predicts biological activation in human bronchial epithelial cells. Toxicol Appl Pharmacol. 2002;178:144–154. doi: 10.1006/taap.2001.9341. [DOI] [PubMed] [Google Scholar]
- Walters DM, Breysse PN, Wills-Karp M. Ambient urban baltimore particulate-induced airway hyperresponsiveness and inflammation in mice. Am J Respir Crit Care Med. 2001;164:1438–1443. doi: 10.1164/ajrccm.164.8.2007121. [DOI] [PubMed] [Google Scholar]
- Walters DM, Breysse PN, Schofield B, Wills-Karp M. Complement factor 3 mediates particulate matter-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2002;27:413–418. doi: 10.1165/rcmb.4844. [DOI] [PubMed] [Google Scholar]
- Watterson TL, Hamilton B, Martin RS, Coulombe RA., Jr Urban particulate matter activates akt in human lung cells. Arch Toxicol. 2012;86:121–135. doi: 10.1007/s00204-011-0739-5. [DOI] [PubMed] [Google Scholar]
- Williams MA, Porter M, Horton M, Guo J, Roman J, Williams D, et al. Ambient particulate matter directs nonclassic dendritic cell activation and a mixed th1/th2-like cytokine response by naive cd4+ t cells. J Allergy Clin Immunol. 2007;119:488–497. doi: 10.1016/j.jaci.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: Central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- World Health Organization. [accessed 17 Jan 2016];Ambient (outdoor) air quality and health. 2016 Available: http://www.who.int/mediacentre/factsheets/fs313/en/
- Xing Z, Zganiacz A, Wang J, Divangahi M, Nawaz F. Il-12-independent th1-type immune responses to respiratory viral infection: Requirement of il-18 for ifn-gamma release in the lung but not for the differentiation of viral-reactive th1-type lymphocytes. J Immunol. 2000;164:2575–2584. doi: 10.4049/jimmunol.164.5.2575. [DOI] [PubMed] [Google Scholar]
- Yan D, Zhang T, Su J, Zhao LL, Wang H, Fang XM, et al. Diversity and composition of airborne fungal community associated with particulate matters in beijing during haze and non-haze days. Front Microbiol. 2016;7:487. doi: 10.3389/fmicb.2016.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T, Wong AY, Ping TJ, Chen J, Sumatoh HR, Vigano E, et al. Cd103(+) dendritic cells control th17 cell function in the lung. Cell Rep. 2015;12:1789–1801. doi: 10.1016/j.celrep.2015.08.030. [DOI] [PubMed] [Google Scholar]
- Zhang R, Dai Y, Zhang X, Niu Y, Meng T, Li Y, et al. Reduced pulmonary function and increased pro-inflammatory cytokines in nanoscale carbon black-exposed workers. Part Fibre Toxicol. 2014;11:73. doi: 10.1186/s12989-014-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LF, Zhu Y, Cui XF, Xie WP, Hu AH, Yin KS. Arsenic trioxide, a potent inhibitor of nf-kappab, abrogates allergen-induced airway hyperresponsiveness and inflammation. Respir Res. 2006;7:146. doi: 10.1186/1465-9921-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.