Abstract
The zebrafish is a social vertebrate and an excellent translational model for a variety of human disorders. Abnormal social behavior is a hallmark of several human brain disorders. Social behavioral problems can arise as a result of adverse early social environment. Little is known about the effects of early social isolation in adult zebrafish. We compared zebrafish that were isolated for either short (7 days) or long duration (180 days) to socially housed zebrafish, testing their behavior across ontogenesis (ages 10, 30, 60, 90, 120, 180 days), and shoal cohesion and whole-brain monoamines and their metabolites in adulthood. Long social isolation increased locomotion and decreased shoal cohesion and anxiety in the open-field in adult. Additionally, both short and long social isolation reduced dopamine metabolite levels in response to social stimuli. Thus, early social isolation has lasting effects in zebrafish, and may be employed to generate zebrafish models of human neuropsychiatric conditions.
Keywords: Zebrafish, social behavior, locomotor activity, anxiety, social isolation, dopamine
Introduction
Early social environment has remarkable and long-lasting effects on the behavior and physiology of an animal. Developmental social isolation has been used extensively to study the functional importance of early social stimuli in a variety of group-living species. For example, early social isolation models exist in rodents (Fone & Porkess, 2008; Shams et al, 2012; Lomanowska et al, 2017), birds (Leitner & Catchpole, 2007; Town, 2011), and non-human primates (Harlow et al, 1965; Higley et al, 1991), where early deprivation from social stimuli or social contact has been found to lead to an array of deficits in adulthood, associated with altered behavioral, physiological, and neural development. However, as all above cited species are altricial (i.e. their young require maternal care), developmental isolation in these species does not allow investigation of the effects of social isolation without the confounding effects of maternal deprivation.
In contrast, zebrafish is a precocial species where young zebrafish require no maternal care, and developmental isolation can be induced immediately post-fertilization. The zebrafish is also easy and economical to keep in the laboratory, and provides a suitable model system due to evolutionary conservation of key features, including their genes, whose nucleotide sequence is often found highly similar to that of mammalian homologs (Howe et al, 2013; Rinkwitz et al, 2011). Social interaction is an important and highly developed zebrafish behavior, and its study has been suggested for the modeling and analysis of human disorders associated with social abnormalities (Morris, 2009; Tropepe & Sive, 2003). Accordingly, there has been a rise in efforts to characterize zebrafish social behavior (Miller & Gerlai, 2007; Saverino & Gerlai, 2008; Buske & Gerlai, 2011; Green et al, 2012; Maaswinkel et al, 2013b; Qin et al, 2014). However, the effect of isolation on zebrafish has rarely been analyzed. Similarly, while the importance of studying underlying functional mechanisms has been highlighted (Bailey et al, 2013; Gerlai, 2014; Fonseka et al, 2016), specific brain areas or neurotransmitter systems involved in zebrafish social behavior remain poorly understood.
A typical characteristic of zebrafish social behavior is shoaling, i.e., forming and remaining in a tight group called a shoal (Miller & Gerlai, 2007). Shoaling is not observed in fish younger than 7 days post-fertilization (dpf), and starts to develop after this age becoming progressively stronger as the fish mature (Buske & Gerlai, 2011; Dreosti et al, 2015; Hinz & de Polaveija, 2017). Furthermore, the development of shoaling has been found to be associated with rising whole brain dopamine levels (Buske & Gerlai, 2012). Like in mammals, early social environment of zebrafish can affect later expression of social behavior, for example, early social experience has been found to determine excursions away from the shoal and aggression towards shoal-mates in adulthood (Moretz et al, 2007).
The presence of visual and olfactory social cues in the environment during early development (first week of life) may be critical for appropriate ontogenesis of social behavior (Mann et al, 2003; Engeszer et al, 2004; Gerlach et al, 2008; Dreosti et al, 2015). Gerlach et al (2008) found 21 dpf to 30 dpf zebrafish fry to exhibit olfactory preference for kin vs. non-kin when raised with siblings during the first 7 days of life, while fry isolated from all conspecifics during the first 7 days did not differentiate between odor cues from kin and non-kin. It is unknown if these effects persist in adulthood. Engeszer et al (2004) also demonstrated that early social experience, i.e. visual cues, such as pigment pattern of the stimulus shoal fish, determine adult shoaling preference, and also found that the preference for shoal-mates with the particular pigment pattern is diminished in 90-days old zebrafish raised in isolation (Engeszer et al, 2004).
Thus, it appears that socially-deprived zebrafish fail to show social preference due to absence of social cues during development. Specific mechanistic processes responsible for the development of preference for particular social cues or for the impairment of such preference remain unknown in zebrafish. It is possible that isolation-reared zebrafish are unable to associate conspecifics as a rewarding experience due to changes in reward pathways that engage the dopamingeric system. Several direct and indirect pieces of evidence demonstrate the involvement of the dopaminergic system in shoaling. First, in a developmental analysis, Buske & Gerlai (2012) found strong correlation between age-dependent strengthening of shoaling behavior and increasing levels of dopamine and DOPAC, relative to total brain protein weight, as the fish matured. Second, Saif et al (2013) demonstrated that in response to social stimuli, dopamine and DOPAC levels rapidly rise in the brain of adult zebrafish. Third, in a psychopharmacology study, Scerbina et al (2012) showed that dopamine D1-receptor antagonism dose dependently impaired shoaling in adult zebrafish. Last, Al-Imari & Gerlai (2008) found that visual access to conspecifics could serve as a positive reinforcer and motivate zebrafish to learn in an associative learning task.
Early social environment may influence not only social but also non-social behaviors. For example, Zellner et al (2011) isolated young zebrafish from 1 dpf to 5 dpf, and found decreased locomotor activity in the isolated fish on 6 dpf compared to fish kept in social groups. However, these authors did not observe the fish past the age of 6 dpf. Social deprivation in adulthood has also been shown to alter anxiety-related behaviors and serotonin levels in brains of adult zebrafish (Collymore et al, 2015; Shams et al, 2015). It is possible that the manipulation of early social environment of zebrafish may also affect anxiety-related behavioral responses and not only the dopaminergic but also the serotoninergic neurotransmitter system. It is also probable that complete, as opposed to short-term, social deprivation starting from early development would have a stronger effect on subsequent adult behaviors, however, this question has not been experimentally answered yet.
To address some of the above questions, we manipulated the early social environment of zebrafish. We generated experimental zebrafish that were reared in social isolation from post-fertilization day (pfd) 0. In order to test the effects of short, as opposed to long, developmental social isolation, we also isolated zebrafish from pfd 0 only to pfd 7, the period that was previously thought to be critical for the development of social responses (Gerlach et al, 2008; Zellner et al, 2011), e.g. for the development of preference for certain social cues. We compared fish in long or short isolation conditions to control fish that were reared in social groups, and monitored the changes of locomotor activity as well as open-field behavioral responses related to anxiety across development (testing behavior on pfd 10, 30, 60, 90, 120, 150, 180). Furthermore, we quantified shoaling behavior and whole-brain levels of neurotransmitters dopamine, serotonin, and their metabolites of adult fish in these three groups.
Methods & Materials
Fish Husbandry & Housing
All zebrafish were bred and housed in the vivarium at the University of Toronto Mississauga as directed by the Canadian Council on Animal Care and the Local Animal Care Committee. Adult 6th generation descendants of AB strain of zebrafish (Danio rerio), originally obtained from the Zebrafish International Research Center (ZIRC, Eugene, OR), were used to breed experimental fish. Parent pairs were bred to collect fertilized eggs, which were bleached (0.5% bleach solution, 2 minutes) within 2hrs of fertilization as per standard protocol (Westerfield, 2007). Developing fry and adult zebrafish were maintained on a high-density recirculating aquatic housing system (Aquaneering Inc., San Diego, CA, USA) equipped with a multi-stage filtration that included activated carbon filtration, fluidized bed biological filtration, and UV sterilization. Housing rack water temperature, salinity and pH were maintained in the ranges of 28–30 °C, 150–300 μS, and 6.5–8.5, respectively. Fish were kept in a 12hr light:12hr dark cycle (lights on at 0900 h). All fish were fed at least twice daily with food appropriate for the age, size, and number of fish in a given tank, to support sufficient growth. Between post-fertilization day (pfd) 5 and30, fish were fed Larval Artificial Plankton 100 (microparticle size below 100μm, from ZeiglerBros, Inc., Gardners, PA, USA), and after pfd 30, with alternating diets that included nauplii of brine shrimp (Artemia salina) and dried flake food, a 1:1 mixture of Tertramin Tropical fish flake food (Tetra Co, Melle, Germany) and spirulina (Jehmco Inc., Lambertville, NJ, USA). A total of 80 zebrafish were used. We recorded behavior from 45 fish across development. Additional 35 fish were habituated to the developmental testing procedure, but were not recorded or analyzed during development. This was done to assure sufficiently large sample sizes for social interaction testing in the adult.
Developmental Isolation & Experimental Groups
Following fertilization, embryos from the same clutch were randomly assigned to one of the experimental groups: long isolation (no social experience from pfd 0 to pfd 180), short isolation (isolated from pfd 0 to pfd 7 and social experience from pfd 8 to pfd 180), or no isolation, control/social fish (always kept in groups of five from pfd 0 to pfd 180). Isolation was achieved by placing the fish singly in 1.4 liter trapezoid Plexiglas isolation tanks (23 × 5 × 24 cm, length × width × depth). In these single tanks, the isolated fish had no access to visual, olfactory, or tactile cues of other fish. Social housing was achieved by placing the fish in 2.8 liter trapezoid Plexiglas tanks (23 × 15 × 24 cm, length × width × depth) in groups of five per tank. To block any visual cues from neighboring non-experimental fish for both social and isolation conditions, gray polycarbonate dividers were placed between tanks throughout development. The internal filtration system of the system rack could not be used because the filtered water would have provided olfactory cues from other fish on the rack. Thus, to maintain good water quality, 50% of the water in all experimental tanks was changed manually every day between pfd 1 and pfd 10. Subsequently, standard recirculation water flow was delivered automatically by the aquatic housing system to all fish tanks. Except for the experimental manipulation of social environment of the home tank, all other procedures were the same for the three groups. As experimental group assignment and testing took place before sexual maturity, we could not determine or control the sex ratio of the experimental fish populations across development. Once fish reached adulthood, the distribution was determined to be approximately 50% males and 50% females.
Behavioral Apparatus and Testing
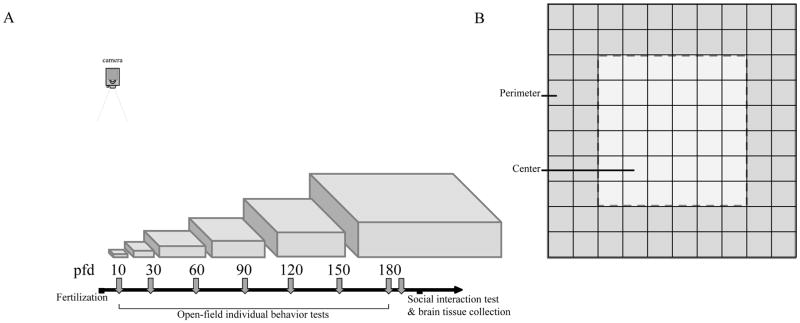
All experimental testing took place between 1000 and 1800 h. For open-field testing, fish was tested repeatedly across development at pfd 10, 30, 60, 90, 120, 150, and 180 age (Figure 1A). At each developmental age, fish from the three groups were tested singly in an open field proportional to their body size, with linear dimensions of the open field tank being approximately 20× the body length of the experimental fish. For example, at pfd 90, most fish were 2.5–3 cm in body length. We used the 50×50 cm arena for the smaller fish (body length ~ 2.5cm) and 60×60 cm arena for the larger fish (body length ~ 3cm). Open-field tanks were filled with water from the home system-rack to 50% of the depth of the tank (to allow better 2-d tracking), and walls were covered with thin wax-paper to make them translucent. Each fish was netted from their home tank, put in the center of the testing arena alone, and was allowed to explore freely. Afterwards, each tested fish was taken from the open-field and returned to a tank similar to their previous home tank (with size-matched conspecifics of the same experimental group for social housing conditions). Testing sessions lasted 30 minutes and were recorded with an over-head (bird’s eye view) digital video-camera (Sony HDR-XR550, Sony Corporation, Japan). These recordings were transferred to a hard drive, and later tracked with our in-house tracking software ‘TheRealFishTracker’, which previously has been used successfully by our lab and others (Mahabir et al, 2013; Saif et al, 2013; Buske & Gerlai, 2014; Felix et al, 2017). The X-Y coordinates generated by the tracking software were used to extract path parameters using the R-language (Mahabir et al, 2013; Buske & Gerlai, 2014). Three parameters were extracted: distance traveled (cm), percent of time spent in the perimeter, and number of entries made to the center. Distance traveled is a measure of locomotor activity, time spent in perimeter measure thigmotaxis or wall-hugging, and center entries quantify the transitions between thigmotaxis and center exploration. Thigmotaxis has been established as an expression of anxiety in several animal species, including zebrafish (Maximino et al, 2010; Schnorr et al, 2012; Shams et al, 2015; Baiamonte et al, 2016). Anxious animals spend more time in the perimeter and make less entries in the center of the open-field. To quantify thigmotaxis, each side of the square open-field was divided into ten equal sections, giving a 10 × 10 grid (Figure 1B). The perimeter was defined as the area within two linear-units from the walls, while the center was defined as the area further than two linear-units.
Figure 1.
(A) Experimental timeline showing ages (in post-fertilization days; pfd) when open-field behavior of experimental fish was recorded across development. The arena sizes (proportional to the length of experimental fish) used for the open-field testing are also shown. (B) Representation of the open-field perimeter and center used to quantify thigmotaxis (wall-hugging) with a grid pattern shown. Perimeter is defined as two liner unit area close to the wall. Center is the 6 × 6 liner unit square in the middle of the open-tank. Greater percentage of time spent in the perimeter indicate increased thigmotaxis, while higher number of entries in the center square indicate center exploration, i.e., reduced thigmotaxis.
Lastly, fish were tested in a shoaling task at age pfd 185, in a large open-field (70 × 70 × 25 cm, length × width × depth). Fish were netted from their home tank and released simultaneously in groups of five in the center of the testing tank. Social fish were tested with social fish from different home tanks (to prevent potential effects of established hierarchy), while isolated fish were tested with isolated fish (long with long, and short with short). The behavior of fish was video-recorded using an over-head camera, their swim paths were tracked as described above and as developed by Buske & Gerlai (2014). Using X-Y coordinates of individual fish, we extracted inter-individual distances (IID) between every pair of fish in all combination in the 5 member shoals we recorded. Inter-individual distance is often used as a measure of shoal cohesion (Miller & Gerlai, 2008; Ladu et al, 2014). This measure quantifies how far a given shoal member is, on average, from all of its shoal-mates. The advantage of this measure is that it incorporates all distances between that focal fish and its shoal-mates, and thus it comes with no loss of information. The disadvantage of the measure is that its value is influenced by the number of shoal members, which may vary among different studies. Another popular measure is the nearest neighbor distance (NND). The advantage of this measure is that it does not depend on the number of shoal members as it only quantifies the distance between the focal fish and the shoal member closest to it. For this same reason, however, this measure loses information, as it does not incorporate the other distances, i.e. the distances between the focal fish and shoal members that are further away from it than its nearest neighbor. For these reasons, we calculated both measures, and compared our adult fish of the three isolation treatment groups. In addition to the 45 fish observed throughout development, additional 20 social and 15 long-isolated fish were habituated and observed during social interaction. In total, 7 shoals of social/control fish, 3 shoals of short-term isolated fish, and 6 shoals of long-term isolated fish were recorded and analyzed. Note that the unit of statistical analysis here was the shoal and thus the above specified shoal numbers represent the sample sizes.
Neurochemical Quantification
Immediately following the adult shoaling task, all fish were euthanized by decapitation. The subjects’ bodies were weighed and their brains were dissected on dry ice and stored in a −80 °C freezer. Brains were processed as previously described (Chatterjee & Gerlai, 2009) with slight modifications. Briefly, each zebrafish brain sample was homogenized in artificial cerebral spinal fluid (ACSF, Harvard Apparatus, UK) and stabilizers. Each brain sample was centrifuged and the supernatant was analyzed for levels of dopamine and serotonin and their metabolites in a single run. Samples (5 μL) were processed using BAS 460 MICRO-Bore-HPLC system chemical detection (Bio-analytical Systems Inc., West Lafayette, IN) with a Uniget C-18 reverse phase microbore column (BASi, Catalogue # 8949) as the stationary phase, and an acetonitrile and tetrahydrofuran buffer mobile phase (Chatterjee et al, 2014). Neurochemicals in the experimental samples were quantified using known standard concentrations of chemically pure serotonin, dopamine, 3,4-Dihydroxyphenylacetic acid (DOPAC), serotonin, and 5-hydroxyindoleacetic acid (5HIAA) (Sigma-Aldrich, Oakville, ON, CA) and normalized to individual weight and brain protein for each fish (stated as ng/mg of brain protein).
Statistical Analyses
Data from 45 fish were observed across development in the control, short-term isolation and long-term isolation conditions (n=15 for each group). Due to technical error, videos were lost for 15 fish for age pfd 10 and 30. Thus, analysis was done for 30 fish at ages pfd 10 and pfd 30, and for all 45 fish the rest of the time points. Outliers were identified and removed using visual box plot analysis (Williamson, Parker & Kendrick, 1989). Final sample sizes were between 10–15 for each group for this analysis. One-way repeated measures ANOVAs were conducted to analyze the effect of isolation (the between-subject factor) and age (the within-subject factor) on the behavior of experimental zebrafish. One way ANOVA was also performed for each age separately as appropriate, followed by Post-hoc Tukey HSD analysis to investigate differences among and between treatment groups. In addition, to analyze potential time-dependent changes within each testing session, at each developmental age, repeated measures ANOVA was performed with isolation as between-subject factor and time bins (2-min intervals) as repeated within-subject factor.
For social interaction, the unit of analysis was a shoal, and group differences were analyzed by repeated measures ANOVA with isolation as a between-subject factor and time bins (four 5-min intervals) as a within-subject factor. Final sample sizes were 3–7 shoals for each group.
Finally, data for neurochemicals were analyzed by one-way ANOVA with isolation as the between-subject factor for individual fish. If there was a significant main effect of isolation, post-hoc multiple comparison Tukey HSD tests were carried out. Final sample size was 15 for each group for this analysis. All statistical analyses were conducted using IBM SPSS Statistics (version 23.0). The null hypothesis was rejected when p < 0.05.
Results
Effect of isolation and age on open-field behaviors across development
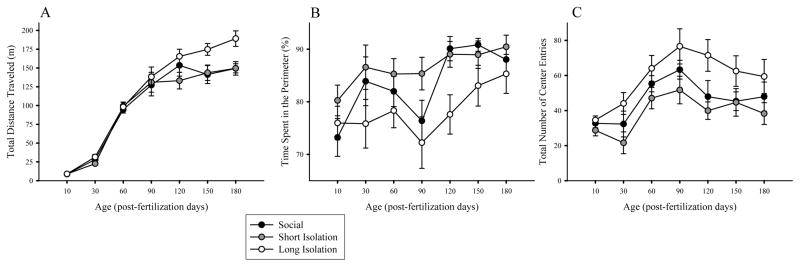
Figure 2 depicts the performance of fish as they mature. Figure 2A shows the ontogenetic changes in distance traveled for fish of the three isolation treatment groups. The results suggest that fish traveled longer distances as they matured, and also that fish of the three treatment groups behaved differently at certain ages. These observations were confirmed by ANOVA of distance traveled, which detected a significant main effect of isolation (F(2,25) = 6.804, p = 0.004), and of age (F(6,150) = 75.627, p < 0.001), without a significant isolation × age interaction (F(12, 150) = 0.76, p = 0.61). It is notable, however, that ANOVA is known to be underpowered to detect significance of interaction between main effects (Wahlsten, 1990), and thus to further investigate potential treatment effects, we conducted one-way ANOVA for each age followed by Post-hoc Tukey HSD analysis. These analyses showed no group differences between ages pfd 10–120 (F(2, 29–44) < 2.492, p > 0.10, for all age points). For age pfd 150, one-way ANOVA showed that the isolation treatment effect approached significance (F(2,43) = 3.194, p = 0.051) and by age pfd 180 it has become highly significant (F(2,44) = 7.041, p = 0.002). Tukey HSD analysis confirmed that fish of the long isolation group traveled significantly longer distance than fish of the short isolation or control groups (p < 0.05).
Figure 2.
Average (A) total distance traveled, (B) time spent in the perimeter and (C) number of entries in the center during 30 minutes of open-field testing across the various age points for social control group, and short-term and long-term developmental isolation groups. Mean ± S.E.M. are shown. n = 10–15 fish per treatment group. Each fish was allowed to swim singly and the x-y coordinates were recorded and used to calculate distances, time spent in perimeter, and center entries. Note that compared to social/control fish, long-term isolation led to hyperactivity in adulthood, and decreased anxiety-related behavior across development. However, short-term isolation (pfd 0–7) did not.
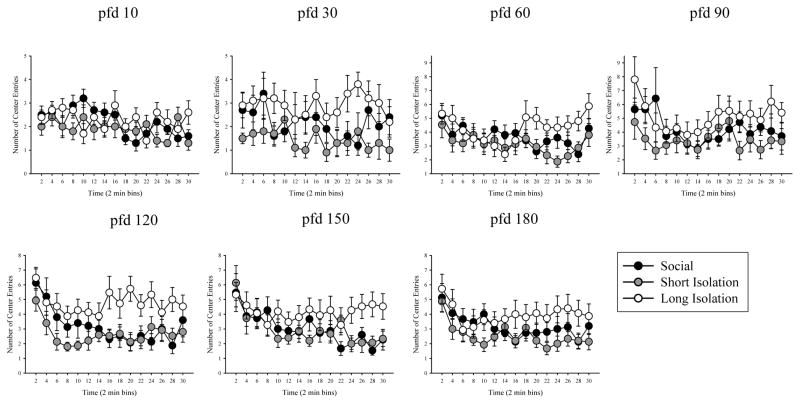
Figure 2B depicts the percent of time fish of the three groups spent in the perimeter of the open-field across the different age points. Again, the results suggest isolation treatment induced differences as well as age-related changes in behavior. ANOVA detected significant main effects of isolation (F(2,27) = 4.998, p = 0.014), and of age (F(6,162) = 3.989, p = 0.004), but found no significant interaction between isolation and age (F(12,162) = 0.477, p = 0.876). Subsequent ANOVAs performed for each age point separately confirmed no isolation treatment induced differences at age pfd 10, 30, 60, 150, and 180, (F(2, 29–44) < 2.103, p > 0.135, for all). At age pfd 90, the difference among treatment groups approached but did not reach significance (F(2,42) = 2.768, p = 0.074), but at age pfd 120, it did (F(2,44) = 5.708, p = 0.006). Tukey’s HSD analysis conducted for this age point (pfd 120) showed that fish of the long isolation group spent significantly (p < 0.05) less time in the perimeter, compared to fish of the control and short isolation groups. Likewise, Figure 2C suggests that the number of entries to the center of the open field was changing as the fish matured, and also that fish of the three treatment groups may not have behaved the same manner. ANOVA confirmed these observations and found the effect of isolation (F(2,26) = 4.927, p = 0.015) and of age (F(6,150) = 7.128, p < 0.001) both significant. However, isolation × age interaction was found non-significant (F(12, 156) = 0.308, p = 0.97). ANOVA conducted separately for each age point revealed no group differences on pfd 10, 60, 90, 150, and 180 (F(2,44) < 2.422, p > 0.10). At pfd 30 the difference among isolation treatment groups approached but did not reach significance (F(2,29) = 2.859, p = 0.075). But at pfd 120, the treatment groups were found to significantly differ (F(2,44) = 4.227, p = 0.021), such that fish of the long isolation group made significantly more center entries compared to fish of the short isolation group (p < 0.05).
Effect of social isolation on temporal changes in locomotor activity within testing sessions
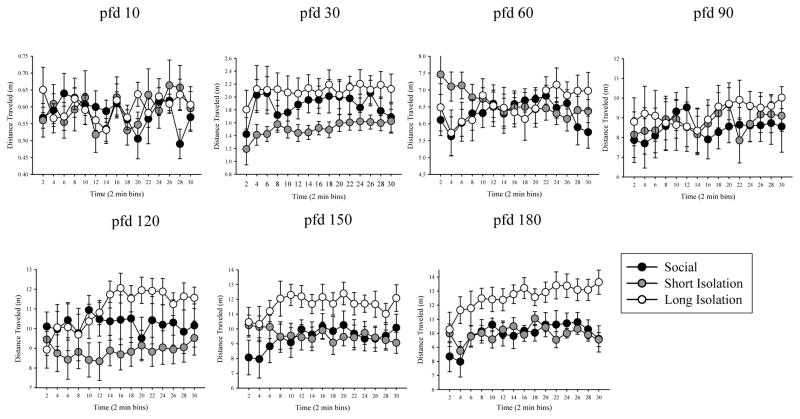
Next, we examined potential temporal changes in distance traveled within the 30 minutes testing session. We conducted this analysis for each age point separately, and compared the three isolation treatment groups of the same age. Short-term, i.e. within session, temporal changes in the quantified behavioral measures are expected due to habituation, e.g., as a result of changes in the level of anxiety and exploratory drive (Brenes et al, 2009; Maaswinkel et al 2013a). We found significant main effect of time at pfd 30 and 180 but not for other age groups (see Table 1). Isolation did not affect activity levels for any ages between pfd 10–150, as no main effects were found for these ages (Figure 3, Table 1). For pfd 150, the main effect of isolation approached statistical significance, and by pfd 180 it became highly significant. Isolation × time interaction was found non-significant for any age point tested (Table 1).
Table 1.
Results from the various separate repeated-measures ANOVAs are presented for distance traveled, percentage of time spent in perimeter and center entries. Statistics for the main effect of isolation, temporal effects across the testing period, and the isolation × time interactions are shown. Bold font indicates significant results (p ≤ 0.05).
| Isolation | Time | Isolation × Time | |
|---|---|---|---|
|
Locomotor Activity – Total Distance traveled by individual fish
| |||
| pfd 10 | F(2,27) = 0.046, p = 0.956 | F(14,378) = 1.398, p = 0.195 | F(28,378) = 1.319, p = 0.183 |
|
|
|||
| 30 | F(2,27) = 2.492, p = 0.102 | F(14,378) = 2.860, p = 0.025 | F(28,378) = 0.802, p = 0.607 |
|
|
|||
| 60 | F(2,42) = 0.169, p = 0.845 | F(14,588) = 0.729, p = 0.565 | F(28,588) = 1.964, p = 0.059 |
|
|
|||
| 90 | F(2,41) = 0.164, p = 0.849 | F(14,574) = 1.287, p = 0.278 | F(28,574) = 0.762, p = 0.634 |
|
|
|||
| 120 | F(2,42) = 1.915, p = 0.160 | F(14,588) = 1.847, p = 0.132 | F(28,588) = 1.988, p = 0.061 |
|
|
|||
| 150 | F(2,41) = 3.194, p = 0.051 | F(14,574) = 1.285, p = 0.283 | F(28,574) = 1.161, p = 0.332 |
|
|
|||
| 180 | F(2,42) = 7.041, p = 0.002 | F(14,588) = 4.198, p = 0.002 | F(28,588) = 1.216, p = 0.285 |
|
|
|||
|
Anxiety behavior – Time Spent in the Perimeter by individual fish
| |||
| pfd 10 | F(2,27) = 1.192, p = 0.319 | F(14,378) = 2.71, p = 0.010 | F(28,378) = 1.233, p = 0.253 |
|
|
|||
| 30 | F(2,27) = 1.544, p = 0.232 | F(14,378) = 0.632, p = 0.805 | F(28,378) = 0.632, p = 0.805 |
|
|
|||
| 60 | F(2,42) = 1.321, p = 0.278 | F(14,588) = 3.109, p = 0.012 | F(28,588) = 0.782, p = 0.639 |
|
|
|||
| 90 | F(2,41) = 2.768, p = 0.074 | F(14,588) = 2.881, p = 0.019 | F(28,588) = 1.670, p = 0.098 |
|
|
|||
| 120 | F(2,42) = 5.708, p = 0.006 | F(14,588) = 4.639, p < 0.001 | F(28,588) = 1.153, p = 0.327 |
|
|
|||
| 150 | F(2,42) = 2.103, p = 0.135 | F(14,588) = 3.896, p = 0.003 | F(28,588) = 1.030, p = 0.418 |
|
|
|||
| 180 | F(2,42) = 0.805, p = 0.454 | F(14,588) = 5.211, p = 0.001 | F(28,588) = 0.903, p = 0.508 |
|
|
|||
|
Anxiety behavior – Entries made in the center by individual fish
| |||
| pfd 10 | F(2,27) = 1.267, p = 0.298 | F(14,378) = 2.048, p = 0.038 | F(28,378) = 1.639, p = 0.055 |
|
|
|||
| 30 | F(2,27) = 2.859, p = 0.075 | F(14,378) = 1.231, p = 0.278 | F(28,378) = 1.001, p = 0.454 |
|
|
|||
| 60 | F(2,42) = 1.924, p = 0.159 | F(14,588) = 2.469, p = 0.015 | F(28,588) = 1.312, p = 0.191 |
|
|
|||
| 90 | F(2,41) = 2.422, p = 0.101 | F(14,574) = 2.147, p = 0.047 | F(28,574) = 0.753, p = 0.702 |
|
|
|||
| 120 | F(2,42) = 4.227, p = 0.021 | F(14,588) = 5.087, p < 0.001 | F(28,588) = 1.439, p = 0.141 |
|
|
|||
| 150 | F(2,42) = 1.848, p = 0.170 | F(14,588) = 4.658, p < 0.001 | F(28,588) = 1.307, p = 0.165 |
|
|
|||
| 180 | F(2,42) = 1.655, p = 0.203 | F(14,588) = 4.693, p < 0.001 | F(28,588) = 1.302, p = 0.433 |
|
|
|||
Figure 3.
Distance traveled during 30 minutes (averaged in 2-min bins) of open-field testing for social/control, short isolation, and long isolation groups on pfd 10, 30, 60, 90, 120, 150, and 180. Mean ± S.E.M. are shown. n = 10–15 fish per treatment group. Each fish was allowed to swim alone in an arena (whose linear dimensions were 20 times of the body length of the experimental subject). The x-y coordinates were used to quantify distances traveled. Fish subjected to long isolation traveled more distances in adulthood than fish in short isolation and social control group.
Effect of social isolation on temporal changes in percent of time in the perimeter and the number of entries to the center recorded within testing sessions
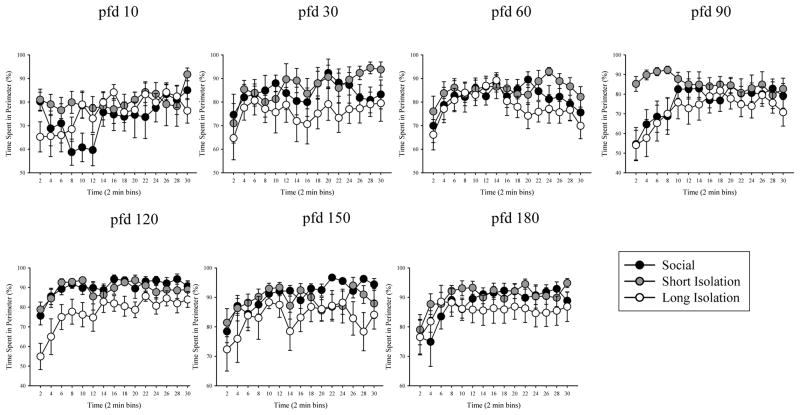
Table 1 summarizes the results of statistical analysis of open-tank behaviors, time spent in the perimeter and number of entries made to the center of the open-field. Figure 4 depicts the percentage of time fish spent in the perimeter while Figure 5 shows the number of center entries for the different age points across development. For both time spent in perimeter and center entries, significant effects of time were revealed on all days, except pfd 30 (see Table 1). We found no effects of isolation at any age point, except at pfd 120. Furthermore, isolation × time interaction was also found non-significant for percent of time in the perimeter and for the number of center entries recorded at all age points.
Figure 4.
Average percentage of time spent in the perimeter of the arena during 30 minutes (in 2-min bins) of open-field testing for social/control, short isolation, and long isolation groups on pfd 10, 30, 60, 90, 120, 150, and 180. Mean ± S.E.M. are shown. n = 10–15 fish per treatment group. Compared to social/control fish, fish in long-term isolation group spent less time in the perimeter but fish in short isolation did not.
Figure 5.
Average number of entries made in the center of the arena during 30 minutes (in 2-min bins) of open-field testing for social/control, short isolation, and long isolation groups on pfd 10, 30, 60, 90, 120, 150, and 180. Mean ± S.E.M. are shown. n = 10–15 fish per treatment group. Compared to social/control fish, fish in long isolation group made more center entries but fish in short isolation group did not.
Effect of developmental isolation on social interaction in adulthood
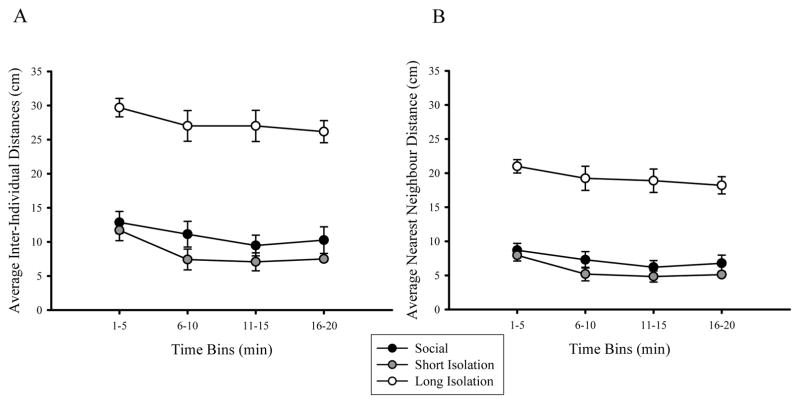
Inter-individual distances and distances to nearest neighbors of adult zebrafish measured during the shoal task are depicted in Figure 6. Repeated measures ANOVA of inter-individual distances revealed a significant main effect of social isolation (F(2,13) = 39.740, p < 0.001) and of time (F(3,39) = 6.026, p = 0.002), but no significant interaction of social isolation × time was found (F(6,39) = 0.309, p = 0.889). Post-hoc analysis showed that shoal cohesion in fish of the long isolation group was significantly reduced as these fish were significantly further from each other compared to control fish or to fish of the short isolation group (p < 0.05, figure 6A), a difference that remained consistent across all time bins analyzed. Similarly, repeated measures ANOVA of nearest-neighbor distances showed a significant main effect of social isolation (F(2,13) = 43.301, p < 0.001) and of time (F(3,39) = 6.484, p = 0.004), but no significant interaction of isolation × time (F(6,39) = 0.758, p = 0.960, figure 6B). Post-hoc analysis showed that long isolation fish were significantly further from their nearest neighbors, compared to control and short isolation fish (p < 0.05, figure 6B) for each time bin.
Figure 6.
Average (A) inter-individual distances and (B) nearest neighbor distances during 20 minutes of social interaction between shoals of adult fish in social/control, short-term isolation, and long-term isolation group. Mean ± S.E.M. are shown calculated for 5 min time bins. n = 3–7 shoals per treatment group. Fish were allowed to interact freely and the x-y coordinates of individual fish were used to calculate distances between shoal mates. Compared to control group, long-term social isolation (pfd 0–180) significantly increased inter-individual distances and nearest neighbor distances between shoal mates, but short-term early social isolation (pfd 0–7) did not.
Effect of developmental isolation on levels of monoamines and their metabolites
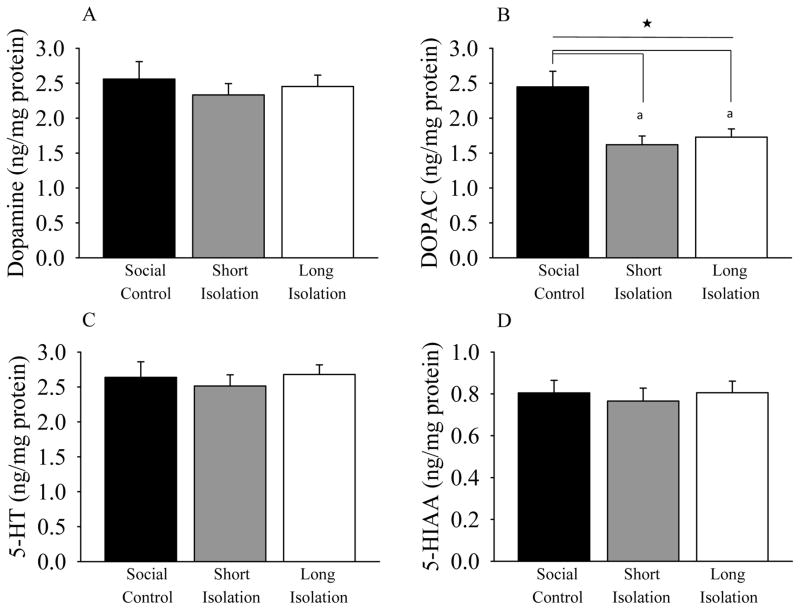
Fish body weights, taken after decapitation, were statistically indistinguishable across treatment groups (F(2,44) = 1.504, p = 0.234). Levels of whole-brain neurotransmitters dopamine and serotonin and their metabolites (DOPAC and 5HIAA respectively), were quantified in adult zebrafish (figure 6). We found no significant effect of isolation treatment on dopamine levels in the adult experimental zebrafish (ANOVA, F(2,44) = 0.336, p = 0.716; Figure 7A). However, analysis of levels of the dopamine metabolite, DOPAC, did reveal a significant main effect of social isolation (F(2,44) = 7.658, p = 0.001; Figure 7B). Post-hoc Tukey HSD test showed that compared to the social, i.e., control fish, both short-term isolated and long-term isolated fish had significantly reduced DOPAC levels (p < 0.05 for both). In contrast, ANOVA found no significant main effect of isolation on levels of serotonin (F(2,44) = 0.236, p = 0.791; Figure 7C) or on levels of serotonin’s metabolite, 5HIAA (F(2,44) = 0.145, p = 0.865; Figure 7D).
Figure 7.
Levels of whole-brain (A) dopamine, (B) DOPAC, (C) serotonin, (D) 5HIAA. Mean ± S.E.M. are shown. n = 15 fish per group. Compared to the control fish, early social isolation significantly reduced level of dopamine metabolite, DOPAC in both short-term (pfd 0–7) and long-term (pfd 0–180) isolated adult zebrafish. Levels of dopamine, serotonin, and its metabolite 5HIAA were not affected by social isolation. Samples were taken following 20-minutes of social interaction of groups fish. Neurochemical levels are expressed as weight of neurochemical per weight of total brain protein (ng/mg). Bars represent group mean (± S.E.M). Filled star (★) represent significant main effect of isolation and (a) denotes that long-term isolated and short-term isolated fish are significantly difference from social control fish at p < 0.05 as detected by a Tukey HSD post-hoc analysis.
Discussion
The social environment, especially during early stages of development can have lasting effects on brain function and behavior (Fone & Porkess, 2008; Levine et al, 2008; Lomanowska et al, 2017). These effects have been well established in humans (Kaler & Freeman, 1994; Chugani et al, 2001; Kumsta et al, 2010), and have been modelled most often using laboratory rodents, including mice and rats (Lapiz et al, 2000; Lukkes et al, 2009; Yasuda et al, 2016; Amiri et al, 2017). For example, deprived animals show locomotor hyperactivity, increased impulsivity, altered processing of reward and social deficits (Ferdman et al, 2007; Lovic et al, 2011; Lomanowska et al, 2017), while physiological changes include alterations in HPA axis response, sensitization to drugs of abuse, and altered synaptic transmission and neural plasticity (Fone et al, 1996; Lapiz et al, 2000; Weiss et al, 2001; Levine et al, 2008).
The zebrafish is a relative newcomer in behavioral neuroscience, but it has already been employed in a wide range of studies investigating human disorders from cancer (Berghmans et al, 2005; Liu & Leach, 2011) to neuropsychiatric disorders (Kalueff et al, 2014). Social behavior, i.e. shoaling, is a prominent feature of the zebrafish, and abnormalities associated with social behavior are part or defining symptoms of a number of human brain disorders (Tropepe & Sive, 2003; Guo et al, 2012; Norton, 2013; Meshalkina et al, 2017). Yet, only very few studies attempted to use the zebrafish as a model of human disorders with social abnormalities, or to investigate the effects of manipulation of the early and later social environment of zebrafish.
The current study represents a potential first step to address this hiatus. It investigated the effects of early short-term as well as of extended, long-term, social isolation on the ontogenesis of open field behavior (locomotor activity and thigmotaxis) as well as the lasting effects of this treatment on social behavior and on neurochemical features of adult fish. We report here for the first time that long-term social isolation, i.e. isolation from social cues throughout the life of zebrafish, leads to hyperactivity, reduction in anxiety-like responses, and significant disruption of shoaling in adult zebrafish, behavioral changes that are accompanied by a reduction of DOPAC levels without any changes in the levels of dopamine, serotonin, or 5HIAA. However, behavioral changes we observed in the adult zebrafish of the long-term social isolation group were not found in fish that were isolated only for the first week of their lives. For example, shoaling behavior and locomotor activity of the short-term isolated zebrafish were indistinguishable from those of control, non-isolated (social) fish. Nevertheless, significant reduction of DOPAC level was observed in the short-term isolated fish compared to control. These results thus suggest that, although our current behavioral tests did not reveal any changes in the short-term isolated fish, social isolation during the first week of the life of zebrafish does lead to lasting functional changes in the brain. They also suggest that the behavioral changes seen in the long-term isolated fish may not be fully, explained by the DOPAC level alteration seen in these fish.
Effects of Long-term Isolation on behavior
Long-term social isolation was found to result in significant hyperactivity in the adult fish. For example, we found hyperactivity, i.e. elevation of total distance travelled, manifesting as a significant change by pfd 120. This result is in line with reports showing isolation-induced hyperactivity in rodents (Varty et al, 1999; Lapiz et al, 2000; Levine et al, 2008), especially with those demonstrating that group differences in locomotor activity become more prominent with longer isolation (Garzon & Del Rio, 1981). The reasons for hyperactivity in animals isolated for extended periods remain unclear, but early isolation in rats has been shown to lead to behavioral impulsivity in adulthood (Lovic et al, 2011; Kirkpatrick et al, 2014).
For rodents, developmental isolation also elevates anxiety (Lomanowska et al, 2006; Lukkes et al, 2009; Yasuda et al, 2016; Amiri et al, 2017). In contrast, we found elevated number of entries to the center and reduction of time spent in the perimeter of the open-field in long-term isolated zebrafish. Both of these measures relate to thigmotaxis, and increased center exploration and reduction in wall-hugging are taken as evidence of reduced anxiety (Schnorr et al, 2012). The effects of developmental isolation on thigmotaxis have not been studied in zebrafish before, but 90-day-long isolation of adult zebrafish was found to decrease thigmotaxis (Shams et al, 2015). Reports of other anxiety behaviors after isolation have been inconsistent. For example, bottom dwelling is an anxiety-related behavior that increases in aversive environments. Compared to social control fish, Collymore et al (2015) reported increased bottom dwelling after 21-days of single housing, while Parker et al (2012) reported reduced bottom dwelling after 14 days of individual housing of adult zebrafish. Solitarily housing (for 2–3 days) was found to make adult three-spined sticklebacks bolder in risk-taking tasks, implying reduced anxiety (Jolles et al, 2016). None of these fish studies investigated the effect of developmental and/or of long-term isolation, but physiological measures of anxiety, e.g. quantification of cortisol (Lindsey & Tropepe, 2014), may aid our interpretation. For example, developmental isolation (lasting six months) in zebrafish did not affect cortisol levels (Lindsey & Tropepe, 2014) suggesting long-term isolation may not be stressful in zebrafish.
Analysis of shoaling responses may also allow us to evaluate anxiety or fear in zebrafish. One of the adaptive functions of shoaling is predator avoidance (Landeau & Terborgh, 1986; Hager & Helfman, 1991). Indeed, shoal cohesion increases in zebrafish exposed to aversive stimuli (Miller & Gerlai, 2007; 2011; Speedie & Gerlai, 2008). Thus, increased inter-individual distance and nearest neighbor distance, i.e. reduced shoal cohesion in the long-term isolated fish, may be interpreted as reduction of anxiety, a conclusion that is in line with the also detected reduction of thigmotaxis. Previously, Kerr (1962) stated that isolated zebrafish became compact and oriented in the same direction in a shoal sooner than socially-reared control fish. However, McCaan & Matthews (1974) found that isolation-reared fish spent significantly less time shoaling with social stimulus than control fish. Furthermore, Engeszer et al (2004) and Gerlach et al (2008) reported that socially-deprived zebrafish failed to show social preference. These latter developmental studies have shown that preference for specific conspecific stimuli is acquired via interactions with, or observation of, shoal mates at the young age of zebrafish (Engeszer et al, 2004; Moretz et al, 2007). Thus, lack of experience with conspecifics and absence of social cues during development likely underlie the social deficits we observed in our long-term isolated subjects. These results suggest that, unlike in rodents, long-term isolation in fish does not appear to be stressful, and in fact it may reduce anxiety-related behaviors such as thigmotaxis and shoaling. At this point, we are unable to decipher why long-term isolation leads to reduction in anxiety. Isolated fish did not get exposed to stress related to dominance hierarchies, competition for food, territorial behavior, or other types of aggression from conspecifics. Nonetheless, systematic and parametric analysis of numerous social and anxiety related responses along with physiological measures and pharmacological validation may be required before we can properly interpret our results and those published in the literature.
Effects of Short-term isolation on Behavior
Unlike in the long-term isolated zebrafish, we did not find significant behavioral changes in the short-term isolated zebrafish. For example, we found no changes in locomotor activity of short-term isolated fish. Our findings are inconsistent with hypoactivity reported in 6-day old zebrafish after isolation from pfd 0–5 (Zellner et al, 2011) and hyperactivity seen in juvenile angelfish after 4-day social isolation (Gómez-Laplaza & Morgan, 1991). The reasons for such discrepancies in locomotion following short isolation, are not known at this point, but could be due to numerous experimental factors, including the species or the genetic makeup of fish used, experimental conditions, e.g., lighting conditions, size of experimental tanks, and other procedural details. Many of these environmental factors can alter the balance between passive or active avoidance reactions to novelty, or influence the level of fear or anxiety vs. exploratory drive of the experimental subject.
The interpretation of our results for the short-term isolation is even more complex in the context of anxiety. Unlike the fish subjected to long-term isolation, we found short-term isolated fish to increase their time in the perimeter and to reduce the number of center entries. The complexity of the interpretation of these results becomes apparent, however, when one observes the high-resolution temporal changes in these two behaviors shown in figures 4 and 5. These figures suggest that the shortest amount of time experimental zebrafish spent in the perimeter of the open tank was right after they were placed in the tank, but as the session progressed, all fish gradually increased their time in the perimeter. Similarly, the number of entries to the center of the tank was highest at the beginning of the recording session, a response that gradually subsided within the first 10 min. This was surprising, since anxiety is expected to be highest at the start of the recording session and should habituate with time. At this point, we do not have an explanation for this finding. One working hypothesis is that our longitudinal design, i.e., the repeated testing using similar apparatus and procedure across development, led to habituation and thus reduced anxiety. However, it is also possible that these differences are related to the age of the fish, and not to the number of prior test exposures, possibilities that will be explored in the future.
It is also noteworthy that our short-term isolation group showed shoaling behavior in adulthood that was indistinguishable from that of control fish. There may be several possible explanations for this result. One, resocialization after the first week of social isolation may have reversed the effects of isolation induced changes, i.e. the mechanisms underlying the development of social isolation induced changes are plastic. Two, the reduced shoal cohesion seen in the long-term isolated zebrafish could have been induced by social isolation after the first week of development of these fish. Three, and related to the previous point, shoaling may not be sensitive to environmental manipulations in the first seven days of life, because such manipulations cannot interfere with social behavior related mechanisms that are not yet at work (Buske & Gerlai, 2011). Future studies that systemically manipulate the length of exposure to social stimuli after early isolation, along with biological assays revealing underlying mechanisms may allow a better understanding of this phenomenon.
Effect of isolation on Neurochemicals
Along with behavior, we also measured levels of neurochemicals from whole brain extracts including dopamine, serotonin, DOPAC and 5HIAA for the three groups. Interestingly, the level of DOPAC was reduced in short-term isolated zebrafish compared to control fish, and to the same degree as found in the long-term isolated zebrafish. Reduced DOPAC levels without changes in the amount of dopamine suggests that while production and storage of dopamine may not have been altered in the adult fish that were isolated, the metabolism of dopamine was significantly reduced. Upon dopamine release in the synapses, monoamine oxidase B located in the glia and astrocytes metabolizes dopamine to DOPAC (Nayak & Henchcliffe, 2008), and DOPAC levels have been used successfully as an indicator of dopamine release (Cheng & Wooten, 1981; Megaw et al, 2001). Thus, the reduced DOPAC levels found in fish of the two isolation treatment groups we interpret as a sign of blunted dopaminergic neurotransmission. Reduction of dopaminergic neurotransmission may be the result of numerous changes, including reduced number of dopaminergic neurons (altered cell differentiation during brain development), impaired connectivity of these neurons (circuitry level abnormalities resulting from abnormal cell migration and/or axonal growth behavior), and/or abnormal molecular machinery within these dopaminergic neurons (Varty et al, 1999; Schweitzer & Driever, 2009; Lindsey & Tropepe, 2014). Although at this point we cannot distinguish among these possibilities, we do note that the first hypothesis is less likely to be correct because reduction in the number of dopamine neurons would likely lead to reduced dopamine levels, which we did not find, and because dopaminergic neurons can be identified within 24 hours post-fertilization (Wullimann & Rink, 2001; Schweitzer & Driever, 2009; Yamamoto et al, 2010), at which point the developing embryo is still inside the egg, and is not yet possible to get influenced by social isolation. It is also notable that we found no changes in the serotoninergic system, at least at the level of neurochemicals analyzed, suggesting that the alteration of the dopaminergic system may not represent a pan-neuronal abnormality, but may be at least somewhat specific to this neurotransmitter system.
The dopaminergic system has been strongly implicated in two main functions, motivation (Koob, 1996; Phillips et al, 2008) and motor function (Beninger, 1983; Salamone, 1992), both of which could have direct relevance to our behavioral findings. For example, if the dopaminergic system malfunctions as a result of soci al isolation, the motivation to shoal, explore, or avoid aversive conditions may be altered, and thus altered shoaling responses to social stimuli, modified exploratory activity in a tank, and changed anxiety and fear responses may all manifest (Bergamini et al, 2016; Johnson et al, 2017; Kacprzak et al, 2017). Motor function, such as locomotory activity, may also change as a result of abnormal functioning of the dopaminergic system irrespective of motivational context (Lambert et al, 2012; Jay et al, 2015).
However, as noted before, a notable aspect of our findings is that although we did detect behavioral and DOPAC level changes in the long-term isolated fish, we found the DOPAC level change without behavioral alterations (open tank and shoaling) in the short-term isolated zebrafish. Thus, we conclude that the behavioral abnormalities we detected in the long-term isolated zebrafish are unlikely to be explained solely by changes in their dopaminergic system. Importantly, the HPLC method employed in the current study did not distinguish separate brain areas, and thus we could not map the potential neuroanatomical site-specific changes in neurotransmitter systems. For example, it is possible that site-specific changes in the serotoninergic system could have contributed to the behavioral abnormalities seen in the long-term isolated zebrafish. It is also possible that other neurotransmitter systems, we have not characterized, contributed to the behavioral alterations. In addition to a spatially refined HPLC analysis or higher resolution neuroanatomical studies (e.g. immunocytochemistry or in situ hybridization), one could also suggest functional characterization of circuitry using electrophysiological tools (McMahon, 1994; Lambert et al, 2012). Furthermore, transgenic reporter lines, e.g. dopaminergic neuron specific lines, may also be employed to illuminate how certain neurotransmitter systems may have been modified by social isolation. In summary, the question of what neurotransmitter systems contributed to the behavioral effects of long-term isolation must be systematically explored in the future. Conversely, the question of what behavioral changes the reduced DOPAC levels found in short-term isolated zebrafish may have led to, must also be systematically explored.
Clearly, our study raised more questions than it answered. Nevertheless, it did show that short term (7 day-long) early social isolation can have long lasting consequences in brain function. It also showed that the behavioral consequences of long-term social isolation are robust and different from short term developmental social isolation. These results now open a new line of research into how social behavior of zebrafish develops and what underlying biological mechanisms may support it.
Acknowledgments
Supported by NSERC (311637) and NIH/NIAAA (R01 AA14357-01A2). We are grateful to Diane Seguin for helping with experimental set-up and for reading an earlier draft of this manuscript, and to James McCrae for developing the RealFishTracker software. We are also thankful to the anonymous reviewers for their time and valuable feedback. Authors of this manuscript declare no conflict of interest.
Sources of Support: Financial Support to RG by NSERC (311637) and NIH/NIAAA (R01 AA14357-01A2)
Footnotes
Authors Contribution:
SS – designed and ran the experiment, did the analysis, wrote the manuscript
SA – assisted with the tracking, analysis, and data-processing and revised the manuscript
CB – wrote the R code for quantification of behaviors and revised the manuscript
DC – helped with HPLC and revised the manuscript
RG – supervised the experiment and the analysis and revised the manuscript
References
- Al-Imari L, Gerlai R. Sight of conspecifics as reward in associative learning in zebrafish (Danio rerio) Behav Brain Res. 2008;189(1):216–219. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Amiri S, Haj-Mirzaian A, Amini-Khoei H, Razmi A, Shirzadian A, Rahimi-Balaei M, Ghazi-Khansari M. Protective effects of gabapentin against the seizure susceptibility and comorbid behavioral abnormalities in the early socially isolated mice. Eur J Pharmacol. 2017;797:106–114. doi: 10.1016/j.ejphar.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Baiamonte M, Parker MO, Vinson GP, Brennan CH. Sustained Effects of Developmental Exposure to Ethanol on Zebrafish Anxiety-Like Behaviour. PLoS One. 2016;11(2):e0148425. doi: 10.1371/journal.pone.0148425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J, Oliveri A, Levin ED. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res C Embryo Today. 2013;99(1):14–23. doi: 10.1002/bdrc.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Research Reviews. 1983;6(2):173–196. doi: 10.1016/0165-0173(83)90038-3. doi: https://doi.org/10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- Bergamini G, Sigrist H, Ferger B, Singewald N, Seifritz E, Pryce CR. Depletion of nucleus accumbens dopamine leads to impaired reward and aversion processing in mice: Relevance to motivation pathologies. Neuropharmacology. 2016;109:306–319. doi: 10.1016/j.neuropharm.2016.03.048. [DOI] [PubMed] [Google Scholar]
- Berghmans S, Jette C, Langenau D, Hsu K, Stewart R, Look T, Kanki JP. Making waves in cancer research: new models in the zebrafish. Biotechniques. 2005;39(2):227–237. doi: 10.2144/05392RV02. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Padilla M, Fornaguera J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav Brain Res. 2009;197(1):125–137. doi: 10.1016/j.bbr.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Buske C, Gerlai R. Shoaling develops with age in Zebrafish (Danio rerio) Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(6):1409–1415. doi: 10.1016/j.pnpbp.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske C, Gerlai R. Maturation of shoaling behavior is accompanied by changes in the dopaminergic and serotoninergic systems in zebrafish. Dev Psychobiol. 2012;54(1):28–35. doi: 10.1002/dev.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske C, Gerlai R. Diving deeper into Zebrafish development of social behavior: analyzing high resolution data. J Neurosci Methods. 2014;234:66–72. doi: 10.1016/j.jneumeth.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Gerlai R. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behav Brain Res. 2009;200(1):208–213. doi: 10.1016/j.bbr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D, Shams S, Gerlai R. Chronic and acute alcohol administration induced neurochemical changes in the brain: comparison of distinct zebrafish populations. Amino Acids. 2014;46(4):921–930. doi: 10.1007/s00726-013-1658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CH, Wooten GF. Simultaneous radioenzymatic assay of dopamine and dihydroxyphenylacetic acid: an index of in vivo dopamine release. J Pharmacol Methods. 1981;5(2):165–173. doi: 10.1016/0160-5402(81)90008-5. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14(6):1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Collymore C, Tolwani RJ, Rasmussen S. The Behavioral Effects of Single Housing and Environmental Enrichment on Adult Zebrafish (Danio rerio) J Am Assoc Lab Anim Sci. 2015;54(3):280–285. [PMC free article] [PubMed] [Google Scholar]
- Dreosti E, Lopes G, Kampff AR, Wilson SW. Development of social behavior in young zebrafish. Front Neural Circuits. 2015;9:39. doi: 10.3389/fncir.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeszer RE, Ryan MJ, Parichy DM. Learned social preference in zebrafish. Curr Biol. 2004;14(10):881–884. doi: 10.1016/j.cub.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Felix LM, Antunes LM, Coimbra AM, Valentim AM. Behavioral alterations of zebrafish larvae after early embryonic exposure to ketamine. Psychopharmacology (Berl) 2017;234(4):549–558. doi: 10.1007/s00213-016-4491-7. [DOI] [PubMed] [Google Scholar]
- Ferdman N, Murmu RP, Bock J, Braun K, Leshem M. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav Brain Res. 2007;180(2):174–182. doi: 10.1016/j.bbr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32(6):1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Fone KC, Shalders K, Fox ZD, Arthur R, Marsden CA. Increased 5-HT2C receptor responsiveness occurs on rearing rats in social isolation. Psychopharmacology (Berl) 1996;123(4):346–352. doi: 10.1007/BF02246645. [DOI] [PubMed] [Google Scholar]
- Fonseka TM, Wen XY, Foster JA, Kennedy SH. Zebrafish models of major depressive disorders. J Neurosci Res. 2016;94(1):3–14. doi: 10.1002/jnr.23639. [DOI] [PubMed] [Google Scholar]
- Garzon J, Del Rio J. Hyperactivity induced in rats by long-term isolation: further studies on a new animal model for the detection of antidepressants. Eur J Pharmacol. 1981;74(4):287–294. doi: 10.1016/0014-2999(81)90047-9. [DOI] [PubMed] [Google Scholar]
- Gerlach G, Hodgins-Davis A, Avolio C, Schunter C. Kin recognition in zebrafish: a 24-hour window for olfactory imprinting. Proc Biol Sci. 2008;275(1647):2165–2170. doi: 10.1098/rspb.2008.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Social behavior of zebrafish: from synthetic images to biological mechanisms of shoaling. J Neurosci Methods. 2014;234:59–65. doi: 10.1016/j.jneumeth.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Gomez-Laplaza LM, Morgan E. Effects of short-term isolation on the locomotor activity of the angelfish (Pterophyllum scalare) J Comp Psychol. 1991;105(4):366–375. doi: 10.1037/0735-7036.105.4.366. [DOI] [PubMed] [Google Scholar]
- Green J, Collins C, Kyzar EJ, Pham M, Roth A, Gaikwad S, … Kalueff AV. Automated high-throughput neurophenotyping of zebrafish social behavior. J Neurosci Methods. 2012;210(2):266–271. doi: 10.1016/j.jneumeth.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Guo S, Wagle M, Mathur P. Toward molecular genetic dissection of neural circuits for emotional and motivational behaviors. Dev Neurobiol. 2012;72(3):358–365. doi: 10.1002/dneu.20927. [DOI] [PubMed] [Google Scholar]
- Hager MC, Helfman GS. Safety in Numbers: Shoal Size Choice by Minnows under Predatory Threat. Behavioral Ecology and Sociobiology. 1991;29(4):271–276. [Google Scholar]
- Harlow HF, Dodsworth RO, Harlow MK. Total social isolation in monkeys. Proc Natl Acad Sci U S A. 1965;54(1):90–97. doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology (Berl) 1991;103(4):551–556. doi: 10.1007/BF02244258. [DOI] [PubMed] [Google Scholar]
- Hinz RC, de Polavieja GG. Ontogeny of collective behavior reveals a simple attraction rule. Proc Natl Acad Sci U S A. 2017;114(9):2295–2300. doi: 10.1073/pnas.1616926114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay M, De Faveri F, McDearmid JR. Firing dynamics and modulatory actions of supraspinal dopaminergic neurons during zebrafish locomotor behavior. Curr Biol. 2015;25(4):435–444. doi: 10.1016/j.cub.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Hamilton TJ. Modafinil decreases anxiety-like behaviour in zebrafish. PeerJ. 2017;5:e2994. doi: 10.7717/peerj.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles JW, Aaron Taylor B, Manica A. Recent social conditions affect boldness repeatability in individual sticklebacks. Anim Behav. 2016;112:139–145. doi: 10.1016/j.anbehav.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacprzak V, Patel NA, Riley E, Yu L, Yeh JJ, Zhdanova IV. Dopaminergic control of anxiety in young and aged zebrafish. Pharmacol Biochem Behav. 2017;157:1–8. doi: 10.1016/j.pbb.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SR, Freeman BJ. Analysis of environmental deprivation: cognitive and social development in Romanian orphans. J Child Psychol Psychiatry. 1994;35(4):769–781. doi: 10.1111/j.1469-7610.1994.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Gerlai R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci. 2014;35(2):63–75. doi: 10.1016/j.tips.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JP. Group behaviour of the zebrafish as influenced by social isolation. American Zool. 1962;2:532–533. [Google Scholar]
- Kirkpatrick K, Marshall AT, Smith AP, Koci J, Park Y. Individual differences in impulsive and risky choice: effects of environmental rearing conditions. Behav Brain Res. 2014;269:115–127. doi: 10.1016/j.bbr.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Hedonic valence, dopamine and motivation. Mol Psychiatry. 1996;1(3):186–189. [PubMed] [Google Scholar]
- Kumsta R, Stevens S, Brookes K, Schlotz W, Castle J, Beckett C, Sonuga-Barke E. 5HTT genotype moderates the influence of early institutional deprivation on emotional problems in adolescence: evidence from the English and Romanian Adoptee (ERA) study. J Child Psychol Psychiatry. 2010;51(7):755–762. doi: 10.1111/j.1469-7610.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- Ladu F, Butail S, Macri S, Porfiri M. Sociality modulates the effects of ethanol in zebra fish. Alcohol Clin Exp Res. 2014;38(7):2096–2104. doi: 10.1111/acer.12432. [DOI] [PubMed] [Google Scholar]
- Lambert AM, Bonkowsky JL, Masino MA. The conserved dopaminergic diencephalospinal tract mediates vertebrate locomotor development in zebrafish larvae. J Neurosci. 2012;32(39):13488–13500. doi: 10.1523/jneurosci.1638-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeau L, Terborgh J. Oddity and the ‘confusion effect’ in predation. Anim Behav. 1986;34(5):1372–1380. doi: https://doi.org/10.1016/S0003-3472(86)80208-1. [Google Scholar]
- Lapiz MD, Mateo Y, Parker T, Marsden C. Effects of noradrenaline depletion in the brain on response on novelty in isolation-reared rats. Psychopharmacology (Berl) 2000;152(3):312–320. doi: 10.1007/s002130000534. [DOI] [PubMed] [Google Scholar]
- Leitner S, Catchpole CK. Song and brain development in canaries raised under different conditions of acoustic and social isolation over two years. Dev Neurobiol. 2007;67(11):1478–1487. doi: 10.1002/dneu.20521. [DOI] [PubMed] [Google Scholar]
- Levine JB, Leeder AD, Parekkadan B, Berdichevsky Y, Rauch SL, Smoller JW, Yarmush ML. Isolation rearing impairs wound healing and is associated with increased locomotion and decreased immediate early gene expression in the medial prefrontal cortex of juvenile rats. Neuroscience. 2008;151(2):589–603. doi: 10.1016/j.neuroscience.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey BW, Tropepe V. Changes in the social environment induce neurogenic plasticity predominantly in niches residing in sensory structures of the zebrafish brain independently of cortisol levels. Dev Neurobiol. 2014;74(11):1053–1077. doi: 10.1002/dneu.22183. [DOI] [PubMed] [Google Scholar]
- Liu S, Leach SD. Zebrafish models for cancer. Annu Rev Pathol. 2011;6:71–93. doi: 10.1146/annurev-pathol-011110-130330. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Boivin M, Hertzman C, Fleming AS. Parenting begets parenting: A neurobiological perspective on early adversity and the transmission of parenting styles across generations. Neuroscience. 2017;342:120–139. doi: 10.1016/j.neuroscience.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Rana SA, McCutcheon D, Parker LA, Wainwright PE. Artificial rearing alters the response of rats to natural and drug-mediated rewards. Dev Psychobiol. 2006;48(4):301–314. doi: 10.1002/dev.20139. [DOI] [PubMed] [Google Scholar]
- Lovic V, Keen D, Fletcher PJ, Fleming AS. Early-life maternal separation and social isolation produce an increase in impulsive action but not impulsive choice. Behav Neurosci. 2011;125(4):481–491. doi: 10.1037/a0024367. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009;55(1):248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Le X, He L, Zhu L, Weng W. Dissociating the effects of habituation, black walls, buspirone and ethanol on anxiety-like behavioral responses in shoaling zebrafish. A 3D approach to social behavior. Pharmacol Biochem Behav. 2013;108:16–27. doi: 10.1016/j.pbb.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Zhu L, Weng W. Assessing social engagement in heterogeneous groups of zebrafish: a new paradigm for autism-like behavioral responses. PLoS One. 2013;8(10):e75955. doi: 10.1371/journal.pone.0075955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabir S, Chatterjee D, Buske C, Gerlai R. Maturation of shoaling in two zebrafish strains: a behavioral and neurochemical analysis. Behav Brain Res. 2013;247:1–8. doi: 10.1016/j.bbr.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KD, Turnell ER, Atema J, Gerlach G. Kin recognition in juvenile zebrafish (Danio rerio) based on olfactory cues. Biol Bull. 2003;205(2):224–225. doi: 10.2307/1543264. [DOI] [PubMed] [Google Scholar]
- Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouveia A., Jr Measuring anxiety in zebrafish: a critical review. Behav Brain Res. 2010;214(2):157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- McCann LI, Matthews JJ. The effects of lifelong isolation on species identification in Zebra fish (Brachydanio rerio) Dev Psychobiol. 1974;7(2):159–163. doi: 10.1002/dev.420070209. [DOI] [PubMed] [Google Scholar]
- McMahon DG. Modulation of electrical synaptic transmission in zebrafish retinal horizontal cells. J Neurosci. 1994;14(3 Pt 2):1722–1734. doi: 10.1523/JNEUROSCI.14-03-01722.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megaw P, Morgan I, Boelen M. Vitreal dihydroxyphenylacetic acid (DOPAC) as an index of retinal dopamine release. J Neurochem. 2001;76(6):1636–1644. doi: 10.1046/j.1471-4159.2001.00145.x. [DOI] [PubMed] [Google Scholar]
- Meshalkina DA, MNKEVK, Collier AD, Echevarria DJ, Abreu MS, Kalueff AV. Zebrafish models of autism spectrum disorder. Exp Neurol. 2017 doi: 10.1016/j.expneurol.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio) Behav Brain Res. 2007;184(2):157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Miller N, Gerlai R. Oscillations in shoal cohesion in zebrafish (Danio rerio) Behav Brain Res. 2008;193(1):148–151. doi: 10.1016/j.bbr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Gerlai R. Redefining membership in animal groups. Behav Res Methods. 2011;43(4):964–970. doi: 10.3758/s13428-011-0090-z. [DOI] [PubMed] [Google Scholar]
- Moretz JA, Martins EP, Robison BD. The effects of early and adult social environment on zebrafish (Danio rerio) behavior. [journal article] Environmental Biology of Fishes. 2007;80(1):91–101. doi: 10.1007/s10641-006-9122-4. [DOI] [Google Scholar]
- Morris JA. Zebrafish: a model system to examine the neurodevelopmental basis of schizophrenia. Prog Brain Res. 2009;179:97–106. doi: 10.1016/s0079-6123(09)17911-6. [DOI] [PubMed] [Google Scholar]
- Nayak L, Henchcliffe C. Rasagiline in treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2008;4(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- Norton W. Toward developmental models of psychiatric disorders in zebrafish. [Review] Front Neural Circuits. 2013;7(79) doi: 10.3389/fncir.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MO, Millington ME, Combe FJ, Brennan CH. Housing conditions differentially affect physiological and behavioural stress responses of zebrafish, as well as the response to anxiolytics. PLoS One. 2012;7(4):e34992. doi: 10.1371/journal.pone.0034992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacol Biochem Behav. 2008;90(2):236–249. doi: 10.1016/j.pbb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Qin M, Wong A, Seguin D, Gerlai R. Induction of social behavior in zebrafish: live versus computer animated fish as stimuli. Zebrafish. 2014;11(3):185–197. doi: 10.1089/zeb.2013.0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkwitz S, Mourrain P, Becker TS. Zebrafish: an integrative system for neurogenomics and neurosciences. Prog Neurobiol. 2011;93(2):231–243. doi: 10.1016/j.pneurobio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Saif M, Chatterjee D, Buske C, Gerlai R. Sight of conspecific images induces changes in neurochemistry in zebrafish. Behav Brain Res. 2013;243:294–299. doi: 10.1016/j.bbr.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD. Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology (Berl) 1992;107(2):160–174. doi: 10.1007/BF02245133. [DOI] [PubMed] [Google Scholar]
- Saverino C, Gerlai R. The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191(1):77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerbina T, Chatterjee D, Gerlai R. Dopamine receptor antagonism disrupts social preference in zebrafish: a strain comparison study. Amino Acids. 2012;43(5):2059–2072. doi: 10.1007/s00726-012-1284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorr SJ, Steenbergen PJ, Richardson MK, Champagne DL. Measuring thigmotaxis in larval zebrafish. Behav Brain Res. 2012;228(2):367–374. doi: 10.1016/j.bbr.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Schweitzer J, Driever W. Development of the dopamine systems in zebrafish. Adv Exp Med Biol. 2009;651:1–14. doi: 10.1007/978-1-4419-0322-8_1. [DOI] [PubMed] [Google Scholar]
- Shams S, Chatterjee D, Gerlai R. Chronic social isolation affects thigmotaxis and whole-brain serotonin levels in adult zebrafish. Behav Brain Res. 2015;292:283–287. doi: 10.1016/j.bbr.2015.05.061. [DOI] [PubMed] [Google Scholar]
- Shams S, Pawluski JL, Chatterjee-Chakraborty M, Oatley H, Mastroianni A, Fleming AS. Dendritic morphology in the striatum and hypothalamus differentially exhibits experience-dependent changes in response to maternal care and early social isolation. Behav Brain Res. 2012;233(1):79–89. doi: 10.1016/j.bbr.2012.04.048. [DOI] [PubMed] [Google Scholar]
- Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188(1):168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town SM. The effects of social rearing on preferences formed during filial imprinting and their neural correlates. Exp Brain Res. 2011;212(4):575–581. doi: 10.1007/s00221-011-2769-x. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2003;2(5):268–281. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Varty GB, Marsden CA, Higgins GA. Reduced synaptophysin immunoreactivity in the dentate gyrus of prepulse inhibition-impaired isolation-reared rats. Brain Res. 1999;824(2):197–203. doi: 10.1016/s0006-8993(99)01173-7. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. Insensitivity of the analysis of variance to heredity-environment interaction. Behavioral and Brain Sciences. 2011;13(1):109–120. doi: 10.1017/S0140525X00077797. [DOI] [Google Scholar]
- Weiss IC, Domeney AM, Heidbreder CA, Moreau JL, Feldon J. Early social isolation, but not maternal separation, affects behavioral sensitization to amphetamine in male and female adult rats. Pharmacol Biochem Behav. 2001;70(2–3):397–409. doi: 10.1016/s0091-3057(01)00626-8. [DOI] [PubMed] [Google Scholar]
- Westerfield M. A guide for the laboratory use of zebrafish (Danio rerio) 5. University of Oregon Press; Eugene: 2007. The Zebrafish Book. [Google Scholar]
- Williamson DF, Parker RA, Kendrick JS. The box plot: a simple visual method to interpret data. Ann Intern Med. 1989;110(11):916–921. doi: 10.7326/0003-4819-110-11-916. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Rink E. Detailed immunohistology of Pax6 protein and tyrosine hydroxylase in the early zebrafish brain suggests role of Pax6 gene in development of dopaminergic diencephalic neurons. Brain Res Dev Brain Res. 2001;131(1–2):173–191. doi: 10.1016/s0165-3806(01)00270-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ruuskanen JO, Wullimann MF, Vernier P. Two tyrosine hydroxylase genes in vertebrates New dopaminergic territories revealed in the zebrafish brain. Mol Cell Neurosci. 2010;43(4):394–402. doi: 10.1016/j.mcn.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Harauma A, Kato M, Ootomo Y, Hatanaka E, Moriguchi T. Artificially reared mice exhibit anxiety-like behavior in adulthood. Exp Anim. 2016;65(3):267–274. doi: 10.1538/expanim.15-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner D, Padnos B, Hunter DL, MacPhail RC, Padilla S. Rearing conditions differentially affect the locomotor behavior of larval zebrafish, but not their response to valproate-induced developmental neurotoxicity. Neurotoxicol Teratol. 2011;33(6):674–679. doi: 10.1016/j.ntt.2011.06.007. [DOI] [PubMed] [Google Scholar]