Abstract
Placebo treatments are pharmacologically inert, but are known to alleviate symptoms across a variety of clinical conditions. Associative learning and cognitive expectations both play important roles in placebo responses, however we are just beginning to understand how interactions between these processes lead to powerful effects. Here, we review the psychological principles underlying placebo effects and our current understanding of their brain bases, focusing on studies demonstrating both the importance of cognitive expectations and those that demonstrate expectancy-independent associative learning. To account for both forms of placebo analgesia, we propose a dual-process model in which flexible, contextually driven cognitive schemas and attributions guide associative learning processes that produce stable, long-term placebo effects. According to this model, the placebo-induction paradigms with the most powerful effects are those that combine reinforcement (e.g., the experience of reduced pain after placebo treatment) with suggestions and context cues that disambiguate learning by attributing perceived benefit to the placebo. Using this model as a conceptual scaffold, we review and compare neurobiological systems identified in both human studies of placebo analgesia and behavioral pain modulation in rodents. We identify substantial overlap between the circuits involved in human placebo analgesia and those that mediate multiple forms of context-based modulation of pain behavior in rodents, including forebrain-brainstem pathways and opioid and cannabinoid systems in particular. This overlap suggests that placebo effects are part of a set of adaptive mechanisms for shaping nociceptive signaling based on its information value and anticipated optimal response in a given behavioral context.
1. Introduction
Drug treatments can reduce negative symptoms associated with a wide array of clinical disorders. However, placebo treatments—which have no direct pharmacological effect—can also alleviate symptoms across a wide array of disorders. These include, but are not limited to, chronic pain (Kaptchuk et al., 2010; Kaptchuk et al., 2008; Vase et al., 2002), depression (Fields et al., 1991; Franklin, 1989; Kirsch et al., 2008; Leuchter et al., 2014), Parkinson’s disease (Benedetti et al., 2016; de la Fuente-Fernández et al., 2001; Lidstone et al., 2010; Pollo et al., 2002), and asthma (Kemeny et al., 2007; Luparello et al., 1968).
A growing literature focuses on the brain and psychological mechanisms underlying placebo effects (Benedetti, 2014; Buchel et al., 2014; Finniss et al., 2010; Geuter et al., 2017b; Wager and Atlas, 2015). Placebos mitigate disease signs or symptoms by virtue of a patient’s perceptions and beliefs about the treatment and its context. The mechanisms of placebo effects are likely not specific to placebos alone, and also contribute to symptom relief following standard drug treatment (Atlas et al., 2012; Bingel et al., 2011; Colloca et al., 2004; Schenk et al., 2014). Thus, standard ‘open-label’ drug treatment can be thought of as working in two ways: via specific drug actions, on one hand, and treatment context effects on the other. In some cases, the effect of treatment context may be as great or greater than the simple drug effect (Khan et al., 2012; Kirsch and Sapirstein, 1998; Tuttle et al., 2015). Placebo studies isolate those context effects, and so provide a way of studying the mechanisms of endogenous self-regulation and healing.
Understanding the psychological and brain mechanisms underlying placebo effects is thus important for understanding all kinds of treatments, and is the focus of this review. In particular, we focus here on inferring mechanisms of placebo analgesia, the best-studied area in placebo research, by comparing findings from human neuroimaging with those of invasive studies in non-human animal models. A large literature on the mechanisms of analgesia in animal models, particularly rodents, reveal much about the neural pathways and neuropharmacology of pain control that is likely conserved in humans and provides a neurobiological substrate for human placebo effects.
In this review, we first briefly explore differences between placebo and drug effects, establishing definitions and clarifying the different levels at which placebos can operate. Then, we present evidence supporting the standard “expectation model” of placebo analgesia, which focuses on patients’ cognitive beliefs, as well as evidence that is incompatible with this explanation. We propose a dual-process model of placebo analgesia to account for both cognitively mediated (i.e., expectation-dependent) and cognition-independent placebo effects.
Using the dual-process model as a framework, we draw connections between psychological processes and neural mechanisms underlying placebo analgesia, focusing on both neuroanatomical and neurochemical systems. While the neurobiological systems underlying expectation-dependent placebo analgesia have been outlined across many studies (Bingel et al., 2006; Eippert et al., 2009a; Petrovic et al., 2002; Wager et al., 2004; Wager et al., 2007; Zubieta et al., 2005), few studies have directly examined the systems underlying expectation-independent analgesia (Benedetti et al., 2011). We will review neurobiological studies of conditioned analgesia in rodents to infer which mechanisms may underlie expectation-independent analgesia in humans. To conclude, we explore potential implications of this model, discussing how it could generalize to placebo effects outside of pain and how the processes identified in the model could impact clinical treatment outcomes.
2. Psychological principles underlying placebo effects
2.1 Placebo and drug effects
When comparing placebos and drugs, drug effects refer to symptom changes directly caused by pharmacological properties of a drug. Similarly, placebo effects describe symptom improvement caused by a placebo treatment, although the placebo itself is inert. Placebo effects are directly caused by the participant or patient undergoing a treatment procedure, and cannot be explained by regression to the mean or other statistical artifacts (Wager and Fields, 2013). By contrast, the term placebo response, as used in clinical trials, typically refers to overall improvement after placebo treatment, without attempting to estimate the causal effects of the treatment. Many placebo-controlled clinical studies examine drug effects, and include placebo conditions in order to estimate drug effects over and above a placebo response. However, these paradigms do not isolate placebo effects; for that, it is necessary to compare the placebo group to a natural history group that receives no treatment, to account for regression to the mean and other biases (Kirsch, 2003).
Standard clinical treatments are usually delivered ‘open-label,’ with the patient’s knowledge that they are receiving the treatment. Thus, their therapeutic benefits are caused jointly by a person’s response to the specific treatment (e.g., a drug) and their response to the act of treatment and related context effects (Bialosky et al., 2011; Kemeny et al., 2007; Kirsch and Sapirstein, 1998; Meissner et al., 2011). Thus, symptom improvement resulting from treatment across a variety of disorders is partly attributable to placebo effects. Varying changes to the environmental context can enhance or diminish the level of symptom relief following these treatments.
As placebo effects are an inescapable part of open drug administration, they are an important component of the treatment process that can be deliberately manipulated to achieve desired effects. Rather than simply being used as a control for drug effects, placebo effects should be emphasized as useful enhancements to standard treatment regimens. For example, ethical manipulations of the context surrounding drug treatment could enhance symptom relief without explicitly giving the patients a placebo. Even when using open administration of placebos instead of drugs (Kaptchuk et al., 2010), educating patients about placebos and how they work can enhance treatment efficacy when compared to non-informed patients (Kisaalita et al., 2014). Understanding how and why placebo effects occur could allow clinicians and researchers to capitalize on these effects to improve patient response to treatment across a wide array of disorders and symptomologies.
2.2 The adaptive value of placebo and related context effects
The context surrounding a treatment is rich, and includes a variety of factors that can influence placebo effects. Some examples of these factors includes expectations for symptom relief (Kirsch et al., 2014; Kotsis et al., 2012; Price et al., 1999), physician communication (Dutt-Gupta et al., 2007; Kaptchuk et al., 2008), hidden versus open administration of a treatment (Atlas et al., 2012; Bingel et al., 2011; Colloca et al., 2004), and prior experience with both specific treatments (Colloca et al., 2010; Schafer et al., 2015; Voudouris et al., 1990) and treatments as a whole (Leuchter et al., 2014). Collectively, these and other contextual factors comprise the ‘active’ ingredients that elicit placebo effects. Any manipulation of these factors, whether purposeful or accidental, could affect therapeutic outcomes.
In order to successfully harness these contextual factors in clinical treatment, we must understand why, how, and under what conditions they operate. What are the internal mechanisms that drive placebo effects, and what is their purpose? The central nervous system, from the spinal cord and retina to the prefrontal cortex, is adapted to make accurate inferences about which behaviors are optimal in a given environment. Part of this adaptation is the use of contextual information to constrain perception, which confers potential advantages to both speed and accuracy in noisy sensory environments. For example, ganglion cells in the retina do not simply respond to stimulation; they anticipate the position of a moving stimulus (Berry et al., 1999) which permits the perception of moving objects. Similar predictive behavior is observed in neuronal activation across multiple brain regions, and theories of ‘predictive coding’ suggest that it represents a general principle nervous system function (Barrett and Simmons, 2015; Buchel et al., 2014; Friston, 2005; Geuter et al., 2017a; Muckli et al., 2015; Summerfield and de Lange, 2014).
Consistent with the notion of predictive coding, the behavioral response to potentially threatening stimuli can vary based on the surrounding environmental context. The threat value associated with the jagged, white outlines of a dog’s teeth or an indistinct shadow lurking outside your home lies not in the percept itself, but in its projected effects. The anticipated threat of a territorial dog defending its home is markedly different from a dog getting its teeth cleaned, and a shadow is much less eerie if one is expecting company. The appropriate behavioral response in these and other cases depends on the context of an event, and each affords different responses depending on the particulars of the scenario. It is plausible then, that placebo effects are specific instantiations of adaptation processes that prepare organisms to respond to anticipated stimuli and events based on contextual cues (Buchel et al., 2014; Eikelboom and Stewart, 1982).
2.3 Pain as a target system for studying placebo effects
Pain provides critical information that is used to identify appropriate behavioral responses in the environment. In most cases, pain is adaptive, as it encourages rest, helps to prevent further injury, and increases the survival rate of injured animals in life-threatening situations (Crook et al., 2014). However, pain experience is not a veridical representation of tissue damage, or even a direct mapping of ascending nociceptive input to the brain (Baliki and Apkarian, 2015; Buchel et al., 2014; Melzack and Katz, 2013). Rather, pain is a fluid representation of the body that, like other adaptive responses, is shaped by anticipated outcomes based on contextual information (Geuter et al., 2017a). This context can include external environmental factors (Lester and Fanselow, 1985; Walf and Frye, 2003), internal state (Foo and Mason, 2011; Onen et al., 2001; Tomim et al., 2015), and cognitively directed goals (Buhle et al., 2012; Ford et al., 2015; Sprenger et al., 2012). The flexibility of the relationship between pain experience and body state means that it can be adaptively modulated up or down to provide the appropriate amount of information for an organism to balance pursuit of current motivational goals with the potential worsening of an injury (Fields, 2004, 2006).
Pain serves as a good model for studying placebo effects for several reasons. First, the neural systems that modulate and induce pain experience are well-defined (Millan, 2002; Ossipov et al., 2010), and activation of these pain control mechanisms are often implicated in placebo analgesia and other forms of behaviorally induced pain relief (Butler and Finn, 2009; Eippert et al., 2009a; Geuter et al., 2013; Wager et al., 2004). Furthermore, placebo effects on pain have been studied extensively within both healthy and clinical populations (Pollo et al., 2001; Vase et al., 2011; Vase et al., 2002), with significant overlap between the biological systems underlying these effects. Thus, models of placebo analgesia in healthy populations are likely to apply to pain management within clinical populations. Finally, numerous studies have explored the neurobiological mechanisms underlying other forms of pain modulation in rodents, including fear-conditioned analgesia (Butler and Finn, 2009; Fanselow and Baackes, 1982), social threat (Rodgers and Hendrie, 1983; Rodgers and Randall, 1986), predator odor effects (Kavaliers et al., 1997; Vendruscolo et al., 2006; Walf and Frye, 2003), and distraction (Ford et al., 2015). There is extensive overlap between the neurobiological mechanisms related to pain relief under these non-placebo manipulations and the systems modulated by placebo administration (De Felice et al., 2011; Heinricher et al., 2009; Meng et al., 1998). Examining how these regions interact during induced analgesia in rodents may provide insight into how placebo effects work in humans.
2.4 Placebo effects are not simple response biases
One explanation of placebo analgesia is that it represents a reporting bias rather than true reductions in pain experience (Allan and Siegel, 2002). If placebo effects are limited to decisions about how to report pain, then they are not relevant for ‘organic’ disease, and their mechanisms cannot be harnessed in therapeutic treatments. This view has been used to argue that significant placebo effects in chronic pain are not meaningful (Hróbjartsson and Gøtzsche, 2001), and that pain patients who respond to placebo sympathetic blockade must not be experiencing genuine pain (Ochoa, 1997).
While placebo treatments are almost certain to influence decision processes, their effects are not limited to subjective reports. Placebos can influence health-relevant physiological outcomes that cannot be ascribed to decision biases, including autonomic (Geuter et al., 2013; Jepma and Wager, 2015; Koban and Wager, 2016; Nakamura et al., 2012), endocrine (Meissner, 2009, 2011), and immune effects (Albring et al., 2014; Vits et al., 2011). Placebo treatment can influence pupillary, electrodermal, and cardiovascular responses to painful events (Nakamura et al., 2012), in a manner that is also influenced by subject expectations (Geuter et al., 2013). Placebo treatment reduces pain-related activity in the spinal cord (Eippert et al., 2009b), while a “nocebo” treatment—where subjects believe a treatment will enhance their pain—increases pain-related spinal activity (Geuter and Buchel, 2013; Tinnermann et al., 2017). Additionally, many placebo effects on pain are reversed by systemic administration of naloxone, an opioid antagonist (Amanzio and Benedetti, 1999; Eippert et al., 2009a; Levine et al., 1978). This indicates that at least some forms of placebo analgesia are directly mediated by the release of endogenous opioids, and thus are not reducible to changes in response bias.
2.5 Expectations as a driver of placebo analgesia
This section will first present and summarize evidence that supports the expectation model of placebo analgesia, focusing on how many manipulations used to induce placebo analgesia also enhance expectations for pain relief. Afterward, we review studies that counter this theoretical understanding and require analgesic processes that operate independent of explicit, reportable expectations for pain relief. The discrepancy between these two sets of results sets the stage for our proposed dual-process model of placebo analgesia.
Placebo analgesia is typically induced in the laboratory using two different manipulations. The first is an expectation manipulation, where subjects are encouraged to believe in the effectiveness of a treatment. The other is a conditioning manipulation, where a placebo treatment is paired with surreptitious reductions in pain intensity (Voudouris et al., 1985). Some theories of placebo analgesia suggest that conditioning and expectation manipulations induce placebo analgesia via a final common pathway that critically depends on expectation for pain relief (Kirsch et al., 2014; Meissner et al., 2011; Stewart-Williams and Podd, 2004). This is supported by the finding that conditioning manipulations fail to induce placebo analgesia when expectations are lowered (Montgomery and Kirsch, 1997). This conceptualization will be referred to as the expectation model of placebo analgesia.
2.5.1 Evidence supporting the expectation model of placebo analgesia
Expectation for pain relief following treatment is often measured by self-report, and is associated with subsequent placebo analgesia across multiple studies (de Jong et al., 1996; Kirsch et al., 2014; Price et al., 1999; Watson et al., 2006). Procedures that diminish expectations for pain relief from a placebo also reduce pain relief following treatment with that placebo (Montgomery and Kirsch, 1997; Price et al., 2008). Expectation manipulations performed without a conditioning manipulation can be used to induce placebo analgesia (Geers et al., 2010; Pollo et al., 2001), and this is particularly effective among subjects who score higher on specific personality traits such as openness (Yu et al., 2014), dispositional optimism (Geers et al., 2010; Morton et al., 2009) or ego resiliency and other factors related to agreeableness (Pecina et al., 2013).
If expectations for pain relief are reduced, either through explicit information or the omission of instructions regarding associations with pain relief, subsequent analgesia is reduced. When using a conditioning manipulation to associate a cue with pain relief, subjects who are explicitly informed that the cues are associated with different stimulation intensities report greater cue-dependent differences in pain levels at test (Carlino et al., 2015). Even when subjects are told the placebo will reduce their pain, revealing that the intensity of the painful stimulus is reduced following placebo administration can prevent the attribution of pain relief to the placebo. In this case, subjects have lower expectations for pain relief, and fail to report reductions in pain for a placebo treatment during test (Montgomery and Kirsch, 1997; Watson et al., 2006).
When combined, conditioning and expectation manipulations induce stronger analgesia as compared to expectation manipulations alone (Carlino et al., 2015; Vase et al., 2002). However, this combination also generates greater expectations for pain relief (Colloca et al., 2008; Colloca et al., 2009; Kirsch et al., 2014; Klinger et al., 2007; Voudouris et al., 1990). While including a conditioning manipulation enhances analgesia, this effect could be fully mediated by an expectation-dependent process. Prior to conditioning, an initial pairing of a placebo with very painful stimulation reduces the magnitude of a subsequent analgesic response (Colloca and Benedetti, 2006; Kessner et al., 2013). The pairing of high pain with a placebo treatment is likely to reduce expectations for pain relief, which inhibits the subsequent acquisition of a placebo response. Even after a conditioning manipulation, verbal suggestions of hyperalgesia can attenuate an analgesic response and sometimes abolish it entirely (Benedetti et al., 2003; Goffaux et al., 2007).
Expectations are important for modulating pain experience other ways beyond standard placebo paradigms. In one study, conditioned cues (shapes) were paired with visual representations of stimulus intensity (pictures of thermometers) rather than actual changes in pain experience (Jepma and Wager, 2015). In this ‘symbolic conditioning’ paradigm, participants learned associations between visual cues and pain intensity without experiencing any actual physical pain—thus, in the absence of any primary reinforcement. In a later test phase, participants reported more pain at equivalent stimulation intensities for cues that were previously paired with higher symbolic representations of temperature. Furthermore, these changes in pain experience were mediated by changes in expected pain. In another example, visual cues that ostensibly signaled other participants’ experiences of pain were presented immediately before participants experienced painful stimuli (Koban and Wager, 2016). High- and low-pain cues were presented, but they were never systematically reinforced, meaning that equal numbers of high-pain, medium-pain, and low-pain stimuli were delivered following each cue type. Nonetheless, pain and pain-related autonomic responses were strongly influenced by the false social information. This paradigm influences pain-related brain responses as well (Yoshida et al., 2013). Critically, the pain associations in both of these examples were generated via changes in expectations and did not rely on conditioned associations with physical experience, highlighting the importance of expectations in the modulation of pain. They parallel learning procedures used mainly in animal models, such as sensory preconditioning, that also suggest that the information value of cues is a critical ingredient of conditioning in animal models (Rescorla, 1988; Schoenbaum et al., 2009).
In clinical populations, expectation manipulations are particularly effective at reducing pain associated with Irritable Bowel Syndrome (IBS) (Vase et al., 2003). Among these patients, placebo treatment can be as effective as treatment with lidocaine, a topical analgesic, and can remain effective for extended periods of time (Vase et al., 2005). Verbal reassurances that the placebo is effective at reducing pain can dramatically reduce pain intensity in IBS (Craggs et al., 2014; Price et al., 2007). Furthermore, encouraging patients to believe in the effectiveness of the placebo can elicit pain relief in ‘open-label’ treatments where patients are informed that the treatment is chemically inert (Kam-Hansen et al., 2014; Kaptchuk et al., 2010).
Despite the strong association between expectations and pain relief, expectation manipulations sometimes fail to reliably induce analgesia (Colloca et al., 2008; Colloca et al., 2009; de Jong et al., 1996; Reicherts et al., 2016; Voudouris et al., 1990). One potential explanation for these results is that the expectation manipulation failed to produce sufficiently strong expectations for pain relief. The increased effectiveness of expectation manipulations on optimists and individuals high on openness (Geers et al., 2010; Morton et al., 2009; Yu et al., 2014), agreeableness (Pecina et al., 2013), and suggestibility (De Pascalis et al., 2001) may simply be because those subjects are predisposed towards belief in the treatment and less likely to require confirming evidence as compared to their more pessimistic counterparts. Alternatively, greater expectation-induced analgesic effects within subjects high on ego resiliency may instead reflect that expectation manipulations tend to fail in those who are more susceptible to stress, effectively blocking any placebo response from being induced by the manipulation (Pecina et al., 2013). Either of these cases may explain the increased difficulty in reliably inducing placebo analgesia in healthy populations without a conditioning manipulation, as the effectiveness of an expectation manipulation alone may partially depend on the specific distribution of personality traits within a given sample.
2.5.2 Expectation-independent processes in placebo analgesia
While conditioning procedures enhance both expectations and subsequent pain relief, placebo effects induced via conditioning do not always act via expectation-mediated processes. Informing subjects that the intensity of a painful stimulus is reduced during placebo conditioning trials prevents acquisition of a placebo response (Montgomery and Kirsch, 1997), however this effect is weakened if conditioning and testing sessions are performed on different days. Under these procedures, placebo analgesia induced by standard expectation and conditioning manipulations in one group was not significantly different from a second group who were informed about the temperature manipulation during conditioning (de Jong et al., 1996). This finding suggests that when conditioning occurs across multiple days, memory consolidation processes may make placebo effects expectation-independent.
When subjects complete a single session of conditioning, the subsequent analgesia is correlated with expectations and can be attenuated or abolished by reducing expectations for pain relief (Benedetti et al., 2003; Schafer et al., 2015). However, this manipulation fails to abolish placebo analgesia if subjects have participated in multiple conditioning sessions. Increasing the number of conditioning sessions can lead to stronger analgesia without enhancing expectations (Colloca et al., 2010; Schafer et al., 2015). Furthermore, in one recent study (Schafer et al., 2015), subjects who completed four sessions of conditioning across multiple days continued to experience placebo analgesia after a subsequent reversal of expectations, despite a nearly complete lack of expectations for analgesia after the reversal. This provides evidence that placebo analgesia is not always dependent on reportable beliefs and expectations.
In addition to variations in the common paradigms used to induce placebo analgesia, other manipulations can also induce expectation-independent analgesic effects. Following repeated sessions of pharmacological conditioning, where pain is reduced via active medication and the drug is replaced by a placebo during the test phase, the placebo effect is fully dependent on expectations if an opiate is used, but only partially dependent when conditioned with ketorolac, a non-steroidal anti-inflammatory drug (NSAID) (Amanzio and Benedetti, 1999). Furthermore, analgesic responses can be induced without manipulating conscious expectations at all. After pairing a set of face images with either high or low pain, subliminal presentation of those faces modulates subsequent pain responses up or down accordingly (Jensen et al., 2012). Even when faces are displayed subliminally during the conditioning phase, subliminal presentation of conditioned face cues at test continues to modulate pain response (Jensen et al., 2015). Together, these results show that analgesia induced across a variety of different paradigms cannot be explained using the standard expectation model.
Expectation-independent analgesic effects cannot be explained by relying on a single expectation-dependent process to induce pain relief. Instead, at least two learning processes underlie placebo analgesia: one that can flexibly adjust the placebo response following changes in expectations and beliefs about the treatment, and another that influences the placebo response based on evidence slowly accumulated over multiple pairings of the treatment context with pain relief. Together, these processes can explain and account for discrepancies in placebo effects reported in the literature. The following section will explore this dual-process model of placebo analgesia, using it to explain findings that cannot be interpreted within the standard expectation model of placebo analgesia.
3. A dual-process model of placebo analgesia
In our proposed dual-process model of placebo analgesia, the response to contextual information is acquired through two different process categories (Table 1). These categories are analogous to the two systems described in psychological theories of reasoning (termed ‘System 1’ and ‘System 2’) that are used to capture differences in the way we reason, learn, and respond to stimuli in our environment (Kahneman and Frederick, 2002; Stanovich and West, 2000). In general, ‘System 1’ processes are more automatic and ingrained, requiring little to no conscious effort to activate. In contrast, ‘System 2’ includes reasoning and decision making processes that rely on conscious awareness and effortful cognition. These two reasoning processes operate simultaneously to enable one to navigate through the environment. Note that these systems parallel ideas from reinforcement learning theory, which distinguishes between model-free and model-based learning (Dayan and Berridge, 2014).
Table 1.
Dual Learning Processes1
| Dynamic | Accumulative | |
|---|---|---|
| Learning Speed | Fast | Slow |
| Modality Specific | No | Yes |
| Automatic | No | Yes |
| Flexible | Yes | No |
This table summarizes key differences between the dynamic and accumulative learning processes. Under the dynamic process, information is learned rapidly, and can generalize to other modalities. Activation of this process requires explicit awareness and is flexible, as it can be rapidly changed with new information. In comparison, learning under the accumulative processes is slower and may not generalize to situations that are too different from the learned context. However, placebo effects induced via this process do not require explicit cognitive control, nor are they be immediately affected by new information.
The dual-process model of placebo analgesia is comprised of a dynamic and an accumulative process. The dynamic process is comparable to ‘System 2’ reasoning processes and its function is largely analogous to the expectation model of placebo analgesia. This process acts by generating a mental schema within which to understand the surrounding context. In this instance, a schema is a mental pattern used to represent the situation as a whole that can be used to guide behavioral outcomes (DiMaggio, 1997; Wager and Atlas, 2015). This schema is flexible and can be rapidly changed after learning new information, which in turn affects the intensity of the placebo effect. The accumulative process relates to the ‘System 1’ form of reasoning and involves the slow accumulative of information over time to form pre-cognitive associations that do not require conscious thought to activate. Within this process, specific contextual elements are associated with experienced outcomes—e.g., via conditioning procedures—which after learning trigger adaptive associative responses. For example, changes in pain experience can be initially paired with visual cues. Afterward, subliminal presentation of those cues can increase or decrease pain experience without influencing conscious awareness (Jensen et al., 2015; Jensen et al., 2012). Placebo effects that arise from an accumulative learning process are not dependent upon reportable beliefs and expectations, but rather on the strength of conditioned associations.
Under the dynamic learning process, new information is evaluated purposefully and rapidly, leading to placebo effects that can be reversed with new information. This form of learning has the advantage of being able to encode a single experience into a mental schema, and use that schema to extrapolate to non-experienced potential outcomes. This form of learning is amodal, in that information acquired in one sensory modality (e.g. verbal suggestions of pain relief absent painful stimulation) can directly influence response in another (e.g. pain response). This is in contrast to a conditioning procedure where a treatment context must be first paired with changes in pain before subsequent presentations of that context can induce pain relief.
Many manipulations used to induce placebo analgesia focus on enhancing expectations for pain relief, and thus rely primarily on this dynamic learning process to induce effects. Both expectation and conditioning manipulations can be used to strengthen a mental schema of pain relief, and the subsequent analgesia is generally correlated with reported expectations for pain relief regardless of the manipulation used (de Jong et al., 1996; Jepma and Wager, 2015; Koban and Wager, 2016; Montgomery and Kirsch, 1997). Verbal and other manipulations of expectations (e.g. physician cues, professional environment) can enhance the ‘pain relief schema’ associated with the placebo and lead to greater placebo effects. Similarly, experience with a placebo treatment reducing pain during a conditioning manipulation can reinforce the expectation that a treatment will elicit pain relief. A recent study demonstrated the importance of mental schemas in relationship to placebo treatments. Locher et al. (2017) tested two groups with open-label placebos. Both groups were told that they received a placebo, but the second group was also told that the placebo could still reduce their pain via endogenous mechanisms. Interestingly, placebo analgesia was only evident in the second group, highlighting the role of activated mental schemas.
The effect of personality on verbal induction of placebo analgesia can also be understood within the dual-process model. Optimistic and open individuals may simply be more inclined to believe assurances that a treatment will reduce their pain, and consequently are more likely to generate a mental schema where a placebo leads to pain relief (Geers et al., 2010; Morton et al., 2009; Yu et al., 2014). In contrast, more pessimistic individuals may require evidence that the treatment works before they will create a similar schema (Pecina et al., 2013; Watson et al., 2006). However, the dynamic process alone cannot account for instances when placebo analgesia occurs in the absence of participant expectations.
Under an accumulative learning process, associations between contextual elements in the environment, subsequent actions, and expected outcomes are learned slowly over time. As the association between the placebo and pain relief becomes stronger, preparatory responses become more ‘stamped in’ and are less flexible compared to responses dependent on mental schemas. Furthermore, the accumulative responses are specific to a given treatment context, and may not generalize to novel treatments. However, placebo responses learned this way have the benefit of creating a rapid response to a treatment cue without the need for explicit cognitive control, and this response can occur much more rapidly than the more flexible responses governed by mental schemas (Kahneman and Frederick, 2002).
The combination of these two processes can be used to explain inconsistencies among the results of various placebo studies. Verbal manipulations of expectations induce placebo effects via a dynamic process without engaging the slower accumulative learning process. In contrast, conditioning procedures that pair a placebo with experienced pain relief induce placebo effects via both dynamic and accumulative learning processes by simultaneously enhancing expectations and creating a low-level association between the placebo and pain relief. Furthermore, the separate action of the accumulative process explains why conditioning over longer periods of time can lead to stronger placebo effects without measurable increases in expected analgesia that are only reduced, rather than eliminated, by a reversal of expectations (Colloca et al., 2010; Schafer et al., 2015).
The function of these two processes on placebo analgesia is at least partially additive, as it is possible to separate the effects of these processes via experimental manipulation. For example, following two sessions of conditioning with a NSAID, subjects report analgesia in response to a placebo administered within the same context. This occurs even when subjects are told that the placebo is not an analgesic but is instead an antibiotic with no effect on pain (Amanzio and Benedetti, 1999). This placebo response is reduced, though still significant, compared to the response of subjects who believe the placebo to be an active analgesic, consistent with additive effects of dynamic and accumulative responses.
In another study, subjects experienced either one or four sessions of conditioning, and placebo analgesia was assessed both before and after they were made aware that the placebo did not possess any pharmacologically active ingredients. After this information was revealed, reported expectations for pain relief were no different between the two conditioning groups. However, only those subjects who had received multiple sessions of conditioning continued to experience pain relief despite being aware that the treatment was an inert placebo (Schafer et al., 2015). As in the pharmacological conditioning study, analgesia within this group was diminished following the reversal of expectations, but remained significant. The subjects in this “long” conditioning group were the only ones who gained sufficient experience with the treatment context to engage the accumulative process to induce placebo analgesia, and thus were the only group to continue to report pain relief following a reversal of expectations.
Both of these cases demonstrate placebo effects that are partially dependent on both expectations and previous experiences. This demonstrates the behavioral separability and additive effects of dynamic and accumulative processes on placebo analgesia. However, the extent to which these processes are subsumed by different neural mechanisms is unknown. Both processes likely influence analgesia by attenuating ascending pain signals at the level of the spinal cord (Basbaum and Fields, 1984; Goffaux et al., 2007; Hohmann and Suplita, 2006; Lichtman and Fanselow, 1991; Matre et al., 2006), but it is unclear at which level these processes diverge. The following section will aim to address this by reviewing how neuroanatomical and neurochemical systems involved in endogenous pain control could separately influence placebo analgesia mediated by these different processes.
4. Neurobiological Mechanisms of Placebo Analgesia
While behavioral manipulations can reveal how dynamic and accumulative processes can separately contribute to placebo analgesia, it is unknown how these processes separately map onto neurobiological systems. The brain is well suited to make predictions and adapt to changes in the environment using both accumulative and dynamic processes (Buchel et al., 2014; Friston, 2005), and placebo effects represent one aspect of this adaptability. Though a number of studies and reviews have clarified the neurobiological systems involved in placebo analgesia (Benedetti and Amanzio, 2013; Geuter et al., 2017b; Wager and Atlas, 2015; Wager and Fields, 2013), it is unclear which mechanisms, if any, are specific to dynamic or accumulative processes, and which are shared between the two.
Although previous work has found that accumulative and dynamic processes can be partially separated at a behavioral and pharmacological level (Amanzio and Benedetti, 1999; de Jong et al., 1996; Schafer et al., 2015), to date no neuroimaging study has been performed that can assess the similarities and differences in the neural mechanisms that underlie placebo analgesia learned through these different processes. To address this gap, we first review different neuroanatomical and neurochemical systems involved in endogenous pain control. We then examine how activity within these systems changes during placebo analgesia in humans. To assess how separate neurobiological mechanisms may govern the dynamic and accumulative processes involved in pain control, we compare systems activated during placebo analgesia in humans to those involved in studies of conditioned pain relief in rodents. Using this comparison, we infer distinctions between neural systems that elicit either dynamic or accumulative placebo analgesia, and generate a full dual-process model that explains placebo effects at both a neurobiological and psychological level.
4.1 The architecture of pain control
Modulation of nociceptive signals occurs at multiple stages along pain processing pathways. For example, pain modulation can be achieved by a reduction of receptor potentials in the periphery (Janson and Stein, 2003; Labuz et al., 2007; Obara et al., 2004; Richardson et al., 1998), within the spinal cord (Eippert et al., 2009b; Goffaux et al., 2007; Matre et al., 2006; Sprenger et al., 2012), or in cortical regions that evaluate the meaning and context surrounding nociceptive signals (Krummenacher et al., 2010). Today, several descending pain-modulating networks have been identified. These networks involve multiple pathways and neurochemical systems, including opioids, cannabinoids, serotonin, dopamine, norepinephrine, oxytocin, cholecystokinin, galanin, and NK-1 (Altier and Stewart, 1999; Millan, 2002; Ossipov et al., 2010; Watkins and Mayer, 1982). Much of the work identifying these pathways has been accomplished in rodent research, but similar systems have been identified in humans (Eippert et al., 2009a; Matre et al., 2006; Vogt et al., 1995; Yelle et al., 2009; Zubieta et al., 2005).
The descending pain modulation system includes the periaqueductal gray (PAG), rostral ventromedial medulla (RVM), and projections to the spinal cord (SC) (Basbaum and Fields, 1984; Heinricher and Fields, 2013; Heinricher et al., 2009; McNally, 1999). The descending pain modulation system receives direct and indirect input from multiple cortical and subcortical brain regions, including the dorsolateral prefrontal cortex (dlPFC), ventromedial prefrontal cortex (vmPFC), rostral anterior cingulate cortex (rACC), anterior insula (aIns), amygdala, nucleus accumbens (NAc), and hypothalamus (Millan, 2002). Direct electrical stimulation of PAG elicits strong analgesia (Fardin et al., 1984; Reynolds, 1969); however PAG neurons themselves do not directly synapse onto nociceptive neurons in the spinal cord. Instead, PAG neurons affect neurotransmission in the dorsal horn via projections to both the RVM and the dorsolateral pontine tegmentum (Benarroch, 2008; Moreau and Fields, 1986; Roychowdhury and Fields, 1996). The RVM constitutes a major target of PAG projections, and RVM efferents contact nociceptive neurons in laminae I, II and V of the dorsal horn where they can either inhibit or facilitate nociceptive signals (Antal et al., 1996; Fields, 2004; Fields et al., 1995; Finnegan et al., 2004; Vanegas et al., 1984).
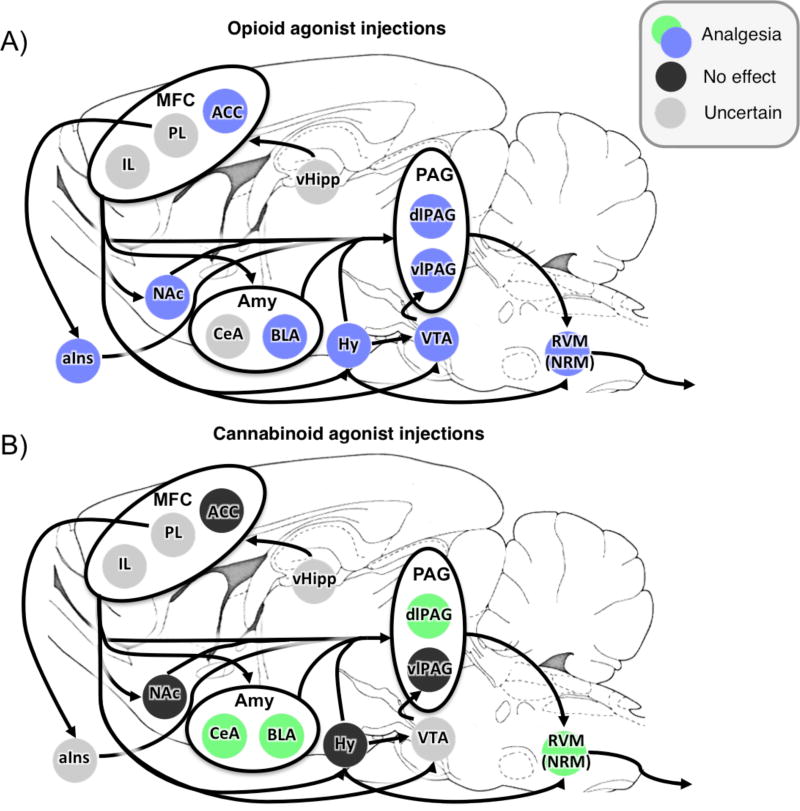
Within the PAG, GABAergic interneurons synapse onto output neurons that project to other neuroanatomical structures (Park et al., 2010). Opioids and cannabinoids inhibit these GABAergic interneurons (Chieng and Christie, 1994; Chiou and Huang, 1999; Vaughan and Christie, 1997), resulting in a net activation of the PAG output neurons via disinhibition (Drew et al., 2009; Park et al., 2010). Injection of opioids or μ-opioid agonists into PAG induces analgesia (Lewis and Gebhart, 1977; Sharpe et al., 1974) (Table 2), whereas opioid-antagonists in the PAG can attenuate the analgesic effects of systemic morphine (Heinricher and Fields, 2013). In addition to opioidergic actions in the PAG, the cannabinoidergic CB-1 receptor is densely expressed in the PAG (Tsou et al., 1998) and CB-1 activation within the dorsolateral PAG also elicits analgesia (Martin et al., 1999; Walker et al., 1999) (Table 3).
Table 2.
Opioid agonists and analgesia2
| Site | Type | Effect | References |
|---|---|---|---|
| Systemic | μ Agonist | Analgesia | (Fanselow et al., 1989b) |
| κ Agonist | Analgesia | (Fanselow et al., 1989b; Helmstetter et al., 1995) | |
| δ Agonist | Analgesia | (Fanselow et al., 1989b; Helmstetter et al., 1995) | |
| Agonist | Analgesia | (Azami et al., 1982; Deakin and Dostrovsky, 1978; Dostrovsky and Deakin, 1977; Gilbert and Franklin, 2002; Hart et al., 1983; Young et al., 1984) | |
|
| |||
| ACC | Agonist | Analgesia | (Pavlovic et al., 1996) |
|
| |||
| aIns | Agonist | Analgesia | (Burkey et al., 1996) |
|
| |||
| CeA | Agonist | Analgesia | (Pavlovic et al., 1996) |
|
| |||
| NAc | δ Agonist | None | (Schmidt et al., 2002) |
| μ Agonist | None | (Schmidt et al., 2002) | |
| δ + μ Agonist | Analgesia | (Schmidt et al., 2002) | |
|
| |||
| Hy | Agonist | Analgesia | (Fuchs and Melzack, 1995; Manning et al., 1994) |
|
| |||
| VTA | Agonist | Analgesia | (Altier and Stewart, 1997, 1998; Franklin, 1989; Manning et al., 1994) |
|
| |||
| dPAG | Agonist | Analgesia | (Jensen and Yaksh, 1986; Levy and Proudfit, 1979; Manning et al., 1994; Miczek et al., 1985; Pert and Walter, 1976) |
|
| |||
| vPAG/DRN | Agonist | Analgesia | (Jensen and Yaksh, 1986; Levy and Proudfit, 1979; Lewis and Gebhart, 1977; Manning et al., 1994;Sharpe et al., 1974; Young et al., 1984) |
|
| |||
| RVM | μ Agonist | Analgesia | (Heinricher et al., 1994) |
| Agonist | Analgesia | (Dickenson et al., 1979; Jensen and Yaksh, 1986; Levy and Proudfit, 1979) | |
This table summarizes the effect of local and systemic injections of opioid agonists on pain.
Table 3.
Cannabinoid agonists and analgesia3
| Site | Type | Effect | References |
|---|---|---|---|
| Systemic | Agonist | Analgesia | (Martin et al., 1999; Meng et al., 1998) |
| Antagonist | Hyperalgesia | (Meng et al., 1998) | |
|
| |||
| Cingulate | Agonist | None | (Martin et al., 1999) |
|
| |||
| BLA | Agonist | Analgesia | (Martin et al., 1999) |
|
| |||
| CeA | Agonist | Analgesia | (Martin et al., 1999) |
|
| |||
| NAc | Agonist | None | (Martin et al., 1999) |
|
| |||
| dlPAG | Agonist | Analgesia | (Martin et al., 1999) |
|
| |||
| RVM | Agonist | Analgesia | (Martin et al., 1999; Martin et al., 1998; Meng and Johansen, 2004) |
| Agonist | Analgesia | (Martin et al., 1998) | |
This table summarizes the effect of local and systemic injections of opioid agonists on pain.
There is some evidence that anatomical regions involved in opioid and cannabinoid dependent analgesia in the PAG may be partially distinct. For example, electrical stimulation of dorsal PAG elicits strong analgesia which can be attenuated by cannabinoid antagonists, whereas ventral PAG stimulation elicits opioid-dependent analgesia (Cannon et al., 1982; Walker et al., 1999). There is a large proportion of opioid-sensitive GABAergic interneurons in the ventrolateral PAG (Chiou and Huang, 1999; Park et al., 2010), and injection of morphine into ventral PAG is more effective at eliciting analgesia than injections into dorsal PAG (Sharpe et al., 1974). This spatial distinction can also be found in studies of conditioned analgesia in rodents, where analgesia can be attenuated by injections of cannabinoid antagonists in dorsolateral PAG (Hohmann et al., 2005; Olango et al., 2012) or opioid antagonists in ventrolateral PAG (Helmstetter and Landeira-Fernandez, 1990).
Projections from the PAG activate descending neurons governing pain response in the RVM (Behbehani and Fields, 1979; Drew et al., 2009; Lau and Vaughan, 2014; Morgan et al., 2008). Within the RVM, ‘OFF’ cells pause in firing immediately following a painful stimulus, and activation of these cells inhibits ascending pain signals at the dorsal spinal cord (Fields, 2004; Fields et al., 1995). As in the PAG, projection neurons in the RVM are modulated by both cannabinoid and opioid release through similar disinhibition processes (Drew et al., 2009; Katona et al., 2001; Lau and Vaughan, 2014; Millan, 2002; Pan et al., 1990; Vaughan et al., 2000). Injections of cannabinoid (Martin et al., 1999; Martin et al., 1998; Meng and Johansen, 2004; Meng et al., 1998) or opioid (Heinricher et al., 1994) agonists into the RVM is sufficient to induce analgesia. In addition to generating analgesia, inactivating the RVM through either lesions or reversible chemical processes can prevent various forms of analgesia. These include systemic administration of either morphine (Azami et al., 1982; Young et al., 1984) or cannabinoid (Meng et al., 1998) agonists, microinjections of morphine into the PAG (Young et al., 1984), and microinjections of opioid agonists into the amygdala (Helmstetter et al., 1998). These studies demonstrate that RVM function is central to descending pain control, and is a common point of integration for antinociceptive pathways in an opioid- and cannabinoid-dependent manner.
Placebo analgesia has been associated with the action of endogenous opioids and cannabinoids (Amanzio and Benedetti, 1999; Benedetti et al., 2011; Eippert et al., 2009a; Levine et al., 1978), and both neurochemical systems play a large role on pain modulation within the PAG-RVM-SC system (Basbaum and Fields, 1984; Drew et al., 2009; Hohmann and Suplita, 2006; Park et al., 2010). Moreover, placebo effects have been associated with changes in both the primary descending pain control network and a variety of higher-order input systems. The next few sections will review how these systems are activated during placebo treatment, before exploring the function of these systems during conditioned analgesia in rodents.
4.2. Neuroanatomical systems underlying placebo analgesia
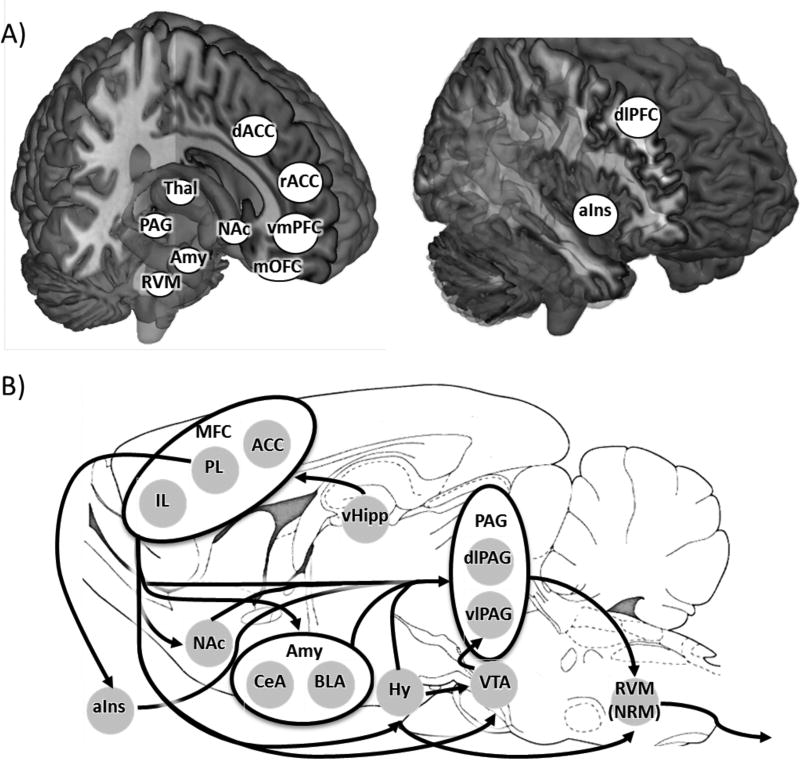
Human neuroimaging studies have identified placebo induced activations and deactivations within the PAG-RVM-SC system (Eippert et al., 2009a; Eippert et al., 2009b; Wager et al., 2004) as well as in cortical input areas to this pain modulatory network. Many forebrain regions show increased activity during placebo analgesia and are thought to inhibit pain processing. These regions include dlPFC (Eippert et al., 2009a; Lui et al., 2010), rACC (Bingel et al., 2006; Eippert et al., 2009a; Kong et al., 2006; Petrovic et al., 2002; Wager et al., 2004), vmPFC (Petrovic et al., 2002), medial orbitofrontal cortex (mOFC), and NAc (Lee et al., 2012). In contrast, activity within regions that are activated during painful stimulation is reduced following placebo treatment (Bingel et al., 2006). These regions include aIns (Eippert et al., 2009a; Lee et al., 2012; Lu et al., 2010; Wager et al., 2004), thalamus (Elsenbruch et al., 2012; Wager et al., 2004), dorsal anterior cingulate cortex (dACC) (Eippert et al., 2009a; Lee et al., 2012; Lu et al., 2010; Wager et al., 2004), amygdala (Eippert et al., 2009a), and somatosensory cortex (Lu et al., 2010) (Figure 1A).
Figure 1. Endogenous pain network in the human and rat.
A) In humans, pain-induced activity in PAG, RVM, and other pain-related brain areas is affected by placebo administration. Placebo administration reduces activity in regions associated with pain experience such as aIns, dACC, Thal, and Amy. In contrast, activity in regions thought to be involved in pain modulation such as dlPFC, rACC, vmPFC, mOFC, and NAc increases with placebo treatment. B) This figure shows a selection of projections descending from MFC to regions involved in pain nociception and regulation in the rat. Black arrows represent connections between brain regions that were identified via either anterograde or retrograde labeling. These connections do not represent an exhaustive list. Of the pathways shown, only a few have been explicitly tested and shown to be relevant to endogenous modulation of pain experience.
Reductions in pain following placebo treatment are often correlated with changes in brain activation within pain-responsive regions. Greater pain relief is associated with reduction in pain-related activity within insular cortex, thalamus, dACC, and somatosensotry cortex (Geuter et al., 2013; Lu et al., 2010; Wager et al., 2004). Similarly, stronger pain relief is associated with greater increases in activity within rACC (Bingel et al., 2006; Ellingsen et al., 2013; Geuter et al., 2013; Kong et al., 2006), vmPFC (Kong et al., 2006; Wager et al., 2004), and dlPFC (Geuter et al., 2013; Lui et al., 2010; Wager et al., 2004). Activation of dlPFC in particular is required to induce placebo analgesia, as inhibition of this region via transcranial magnetic stimulation is sufficient to abolish a conditioned placebo response (Krummenacher et al., 2010).
The pain-modulatory function of many of these brain regions operates via direct and indirect connections to the PAG (Millan, 2002). White matter connectivity between PAG and both rACC and dlPFC is correlated with individual variation in placebo analgesia (Stein et al., 2012), and functional coupling between dlPFC and PAG predicts future placebo analgesia (Sevel et al., 2015b). Furthermore, functional connectivity between the PAG and each of rACC (Bingel et al., 2006; Eippert et al., 2009a; Petrovic et al., 2002; Valet et al., 2004), dlPFC (Wager et al., 2004), and vmPFC (Wager et al., 2004) is enhanced during behaviorally induced analgesia, though in some cases the connectivity between dlPFC and PAG is actually reduced (Sevel et al., 2015a).
In particular, rACC plays a strong modulatory role during placebo analgesia. Functional connectivity between rACC and a brainstem region containing RVM increases during placebo treatment (Petrovic et al., 2002). Furthermore, greater rACC-PAG coupling predicted greater RVM activity (Eippert et al., 2009a) and reduced activation of somatosensory cortex (Ellingsen et al., 2013) during placebo analgesia. Rostral ACC serves as a general hub for top-down pain control, and functional coupling of rACC with other pain-responsive regions including vmPFC, amygdala, and NAc is increased following placebo treatment (Bingel et al., 2006; Ellingsen et al., 2013). These functional pathways are mirrored in recent work demonstrating PFC-induced analgesia can be attenuated by inactivation of NAc (Lee et al., 2015).
Placebo-related changes in brain activation are often correlated with expectations, such that greater expectations for pain relief are associated with greater activation within rACC (Geuter et al., 2013) and reduced activation within thalamus (Craggs et al., 2014; Geuter et al., 2013), insula (Geuter et al., 2013; Schmid et al., 2013), amygdala (Schmid et al., 2013), dACC (Craggs et al., 2014) and somatosensory cortex (Schmid et al., 2013) during placebo treatment. These expectation-related changes are not specific to placebos alone. Greater expectation for analgesia from opioid treatment leads to diminished insula and thalamus and increased dlPFC, rACC, vmPFC activation (Bingel et al., 2011). Furthermore, open vs. hidden administration of an analgesic is correlated with reduced activity in aIns, secondary somatosensory cortex (S2), thalamus, dACC, and amygdala and increased activation within dlPFC and vmPFC (Atlas et al., 2012; Schenk et al., 2014). Inducing expectations for hyperalgesia attenuates pain relief from these analgesics and leads to increased activation of the thalamus, dACC and aIns (Bingel et al., 2011), further highlighting the importance of expectation in determining analgesic response.
The regions identified above all play a role in eliciting pain relief following placebo treatment. However, it is unclear whether the neuroanatomical systems identified here play a role in placebo analgesia in general, or whether they are specifically involved in either dynamic or accumulative processes. Each of these studies used either a verbal manipulation of expectations or a combination of an expectation manipulation with a brief conditioning manipulation to generate placebo analgesia. This form of placebo analgesia has been shown to rely strongly on expectations and is reversible by verbal information (Benedetti et al., 2003; Montgomery and Kirsch, 1997). Thus, it is difficult to use these studies to dissociate between brain regions specifically involved with the different learning processes. The neurobiological mechanisms identified in these studies primarily inform about dynamic and general mechanisms underlying placebo analgesia, and do not address separable accumulative mechanisms.
4.3 Neurochemical systems underlying placebo analgesia
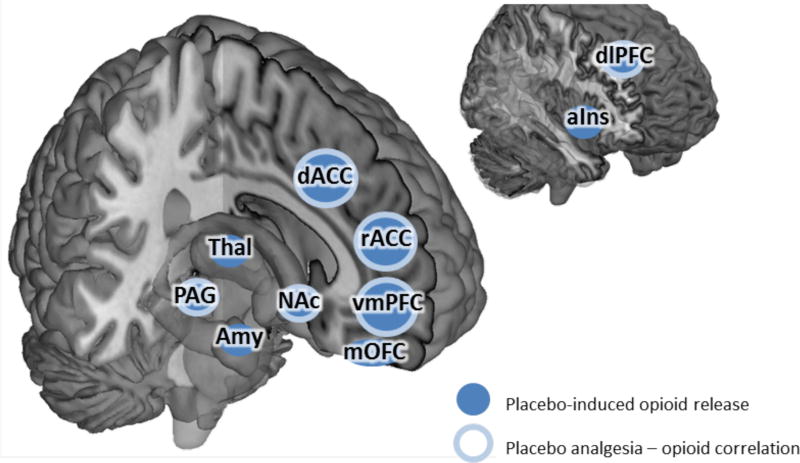
In humans, manipulating expectation for pain relief has a strong effect on how well placebo and drug treatments modulate pain experience (Atlas et al., 2012; Bingel et al., 2011; Montgomery and Kirsch, 1997; Price et al., 2008), and the strength of these changes is often correlated with opioid activity in the brain (Pecina et al., 2014b; Scott et al., 2008). Given these results, it is plausible that the net effect of expectations on placebo analgesia is mediated at the neurobiological level by endogenous opioid release (Pecina and Zubieta, 2014; Zubieta et al., 2005). It is thus reasonable to assert that when placebo analgesia is governed by mental schemas, it is also mediated by endogenous opioid release. However, it is less clear whether the release of opioids, cannabinoids, or some combination of the two generates placebo analgesia via an accumulative learning process. For example, opioid activity in ACC is correlated with error signals analogous to accumulative learning rather than to explicit expectations (Pecina et al., 2014b).
While expectation-independent placebo analgesia has been shown to be mediated by endogenous cannabinoid release in specific cases (Benedetti et al., 2011), it is unknown whether cannabinoid release is a simple conditioned response to the analgesics used during conditioning in this instance, or instead represents a critical mechanism of expectation-independent placebo analgesia in general.
Opioids play a major role in placebo analgesia. Placebo related changes in activation within vmPFC, dlPFC, rACC, dACC, PAG, thalamus, hypothalamus, and RVM are all reversible by the μ-opioid antagonist naloxone, as is the rACC-PAG coupling and subsequent enhancement of RVM activity following placebo treatment (Eippert et al., 2009a). Changes in endogenous opioid activity are also associated with placebo analgesia. Placebo treatment elicits increased μ-opioid activity in the rACC (Petrovic et al., 2002; Wager et al., 2007; Zubieta et al., 2005), and there is a positive correlation between dlPFC μ-opioid activity and expectations for reduced pain (Pecina et al., 2014b; Zubieta et al., 2005). The strength of placebo analgesia is associated with enhanced μ-opioid activity within rACC, vmPFC, insula, amygdala, NAc and PAG (Pecina et al., 2013; Petrovic et al., 2002; Scott et al., 2008; Wager et al., 2007) (Table 4). Furthermore, stronger expectations for pain relief are associated with greater release of opioids as well as greater placebo analgesia (Scott et al., 2008).
Table 4.
Opioid activity and placebo analgesia4
| Brain Region | Contrast | References |
|---|---|---|
| dlPFC | Placebo | (Wager et al., 2007; Zubieta et al., 2005) |
| Placebo Analgesia | (Wager et al., 2007) | |
|
| ||
| vmPFC | Placebo | (Pecina et al., 2013; Scott et al., 2008; Wager et al., 2007) |
|
| ||
| rACC | Placebo | (Scott et al., 2008; Wager et al., 2007; Zubieta et al., 2005) |
| Placebo Analgesia | (Pecina et al., 2013; Pecina et al., 2014a) | |
|
| ||
| dACC | Placebo | (Pecina et al., 2013) |
| Placebo Analgesia | (Pecina et al., 2013; Pecina et al., 2014a) | |
|
| ||
| aIns | Placebo | (Pecina et al., 2013; Scott et al., 2008; Wager et al., 2007; Zubieta et al., 2005) |
|
| ||
| Amygdala | Placebo | (Pecina et al., 2013; Scott et al., 2008; Wager et al., 2007) |
|
| ||
| NAc | Placebo | (Pecina et al., 2013; Scott et al., 2008; Zubieta et al., 2005) |
| Placebo Analgesia | (Scott et al., 2008) | |
|
| ||
| Thalamus | Placebo | (Wager et al., 2007) |
|
| ||
| PAG | Placebo | (Pecina et al., 2013; Scott et al., 2008; Wager et al., 2007) |
| Placebo Analgesia | (Pecina et al., 2013) | |
This table summarizes studies that found enhanced opioid activity following placebo treatment, or found opioid activity to correlate with behavioral analgesia.
Conditioning with an opiate analgesic such as morphine induces placebo analgesia that is dependent on expectations and blocked by opioid antagonists. However, conditioning with a NSAID such as ketorolac elicits opioid-independent analgesia that is only partially mediated by expectations (Amanzio and Benedetti, 1999). When ketorolac conditioning is used, the expectation-dependent component of the analgesic response is still opioid-dependent and reversible by naloxone. However the expectation-independent component is specifically mediated by cannabinoid release and blocked by the CB-1 receptor antagonist rimonabant (Benedetti et al., 2011). Given the link between expectation-independent analgesia and cannabinoid release in this case, it is possible that endogenous cannabinoid release underlies expectation-independent placebo effects identified in other paradigms (Jensen et al., 2015; Jensen et al., 2012; Schafer et al., 2015).
Placebo analgesia is related to reductions in brain activity within regions associated with pain experience, however the connection between the release of opioids and cannabinoids to different components of placebo analgesia remains unclear. Previous studies have consistently found analgesia to be correlated with opioid release (Pecina et al., 2013; Pecina and Zubieta, 2014; Wager et al., 2007), however each of these studies used procedures that induce expectation-dependent placebo analgesia. While opioid release is related to expectation-mediated placebo analgesia that is rapidly learned and schema-dependent, the relationship between opioid release and expectation-independent placebo analgesia is unclear. To date, no study has systematically compared the differences in neural mechanisms underlying expectation-dependent and expectation-independent placebo analgesia. In the following section, we examine the neural mechanisms underlying context dependent analgesia within other paradigms to infer whether separate mechanisms may underlie these different types of placebo analgesia.
4.4. Neural mechanisms underlying conditioned analgesia in rodents
Neurobiological studies within rodents have explored both opioid and non-opioid mechanisms governing pain (Butler et al., 2012; Helmstetter and Fanselow, 1987; Helmstetter and Landeira-Fernandez, 1990; Helmstetter et al., 1998; Hohmann et al., 2005; Martin et al., 1998; Olango et al., 2012) (Figure 1B), and demonstrated how different manipulations of context learning can elicit one or both of these modulatory processes (Lichtman and Fanselow, 1991). It is possible that differences in neurochemical mechanisms governing analgesia under different behavioral paradigms in rodents similarly explain the variation in expectation dependence within placebo analgesia. To explore this idea, we review contextual learning processes and neural structures involved in conditioned analgesia within rodents and infer how these systems may be separately activated during expectation-dependent and expectation-independent placebo analgesia.
Placebo analgesia-like responses can be conditioned in rodents using morphine (Guo et al., 2011; Miller et al., 1990; Valone et al., 1998) and other opioid agonists (Bryant et al., 2009), though conditioned analgesia effects can be inconsistent across studies (McNabb et al., 2014; Nolan et al., 2012). Similar to examples in humans, opiate conditioning in rodents elicits placebo analgesia-like reductions in pain that can be reversed by opioid antagonists (Guo et al., 2010; Zhang et al., 2013). Moreover, conditioning with NSAIDs (e.g., aspirin) induces opioid-independent placebo analgesia (Guo et al., 2010). However, it is unknown whether this analgesia is mediated by endogenous cannabinoids as it is in humans (Benedetti et al., 2011).
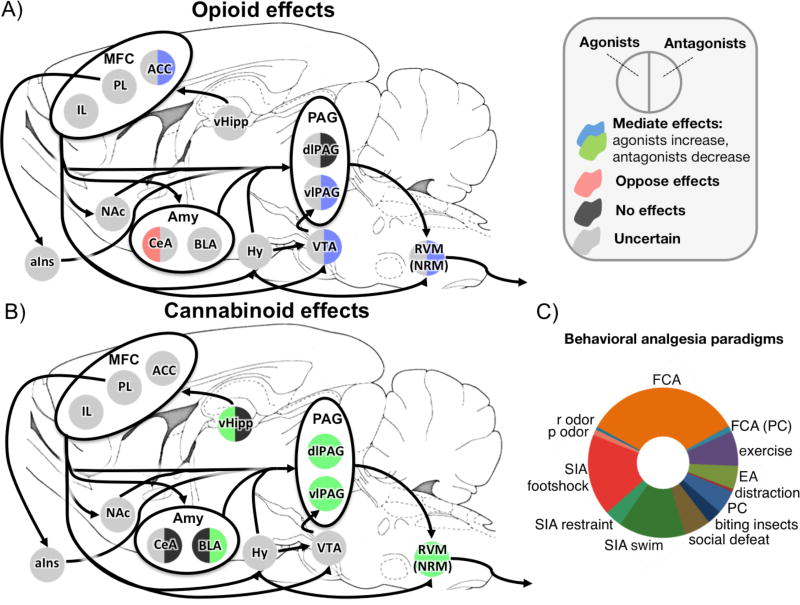
Stress-induced analgesia (SIA) refers to analgesia expressed following a stressful experience. As a paradigm, SIA experiments in rodents are far more numerous when compared to the pharmacologically conditioned analgesia studies described above, and the neurobiological mechanisms needed to induce these analgesic responses are better identified. Various stressors can be used to induce SIA, including restraint stress, swim stress, conditioned fear, and mild shock (Butler and Finn, 2009). Among these varied manipulations, conditioned fear is unique in that, rather than testing pain response immediately following the offset of a painful stimulus or stressor, a threatening context previously associated with pain is used to induce analgesia. When conditioned fear is used as the stressor, the resulting analgesia is often termed fear-conditioned analgesia (FCA). A common method for inducing FCA involves first placing rats in a novel context and administering uncontrollable painful stimuli such as foot shocks. When later returned to the shock context and subjected to a novel painful stimulus (often a formalin injection), fear conditioned rats exhibit diminished recuperative pain-related behaviors (e.g. raising paw, licking paw) compared to non-conditioned controls (Hayes et al., 1978; Helmstetter and Fanselow, 1987; Watkins et al., 1982). This behavior is related to anticipated threat, as it can be extinguished by additional presentations of the threat context without a corresponding shock prior to testing (Fanselow, 1984).
SIA and FCA are not specific to rodents, as humans can also demonstrate analgesic responses to stress (Willer et al., 1981). In an exemplary study of FCA in humans, an experimental group was conditioned to a visual CS with loud white noise plus a mental arithmetic task over several days. During the subsequent test phase, this group showed increased pain thresholds and tolerance when compared to a control group. The final tests were conducted in a different room than the conditioning, so that potential confounds due to environmental context were mitigated. In this case, the CS alone was sufficient to induce analgesia for the experimental group (Flor and Grüsser, 1999). This form of conditioned analgesia can also be evoked by auditory stimuli (Flor et al., 2002) or faces (Williams and Rhudy, 2007). While the specific anatomical structures underlying FCA in humans are unknown, these effects do rely on opioidergic neurotransmission (Flor et al., 2002; Willer et al., 1981).
Within rodents, FCA is mediated by both opioid (Table 5) and cannabinoid (Table 6) release, as it can be attenuated by central injections of either opioid (Butler et al., 2008; Fanselow et al., 1989; Helmstetter and Fanselow, 1987) or cannabinoid (Finn et al., 2004) antagonists. However, enhancement of standard FCA by cannabinoid agonists (Butler et al., 2012) is completely blocked by injection of an opioid antagonist (Butler et al., 2008), implying that opioid activity can “gate” cannabinoid-mediated analgesia. However, this gating effect may specifically apply to changes in FCA behavior, as injection of an opioid antagonist fails to reduce analgesia following treatment with a cannabinoid agonist (Meng et al., 1998).
Table 5.
Opioidergic modulation of behavioral analgesiai
| Site | Type | Manipulation | Effect | References |
|---|---|---|---|---|
|
| ||||
| Systemic | δ antagonist | FCA, SIA | Less analgesia | (Fanselow et al., 1989a; Hart et al., 1983) |
| κ antagonist | FCA, SIA, Social Defeat, Biting Insects | Less analgesia, None | (Fanselow et al., 1989b; Kavaliers et al., 1998; McLaughlin et al., 2006; McLaughlin et al., 2003) | |
| μ antagonist | FCA | Less analgesia | (Fanselow et al., 1989b) | |
| naloxone | FCA, SIA, Social Defeat, Morphine conditioning, Biting Insects, Predator Odor | Less analgesia, (None) | (Bodnar et al., 1978; Bragin, 1986; Butler et al., 2008; Colwell and Kavaliers, 1990; Fanselow, 1984; Galdino et al., 2014b; Galdino et al., 2010; Good and Westbrook, 1995; Guo et al., 2010; Hart et al., 1983, 1985; Hayes et al., 1978; Helmstetter and Fanselow, 1987b; Kavaliers et al., 1998; Kavaliers et al., 1997; Kurrikoff et al., 2008; Lee et al., 2016; Lewis et al., 1981; Lewis et al., 1980; Marek et al., 1992; Miczek et al., 1982; Miller et al., 1990; Przewlocka et al., 1990; Rodgers and Hendrie, 1983; Rodgers and Randall, 1986; Teskey et al., 1984; Watkins et al., 1982; Zhang et al., 2013) | |
| naltrexone | FCA, SIA, Social Defeat, Morphine conditioning, Odor of different shocked rat | Less analgesia, (None) | (Fanselow, 1985; Fanselow and Baackes, 1982; Girardot and Holloway, 1984, 1985; Grisel et al., 1993; Helmstetter and Fanselow, 1987a, b; Kelly and Franklin, 1987; Lee et al., 2016; Lichtman and Fanselow, 1991; Meagher et al., 1989; Miczek et al., 1982; Terman et al., 1986) | |
|
| ||||
| rACC | δ antagonist | Morphine conditioning | None | (Zhang et al., 2013) |
| κ antagonist | Morphine conditioning | None | (Zhang et al., 2013) | |
| μ antagonist | Morphine conditioning | Less analgesia | (Zhang et al., 2013) | |
| naloxone | Morphine conditioning | Less analgesia | (Zhang et al., 2013) | |
|
| ||||
| CeA | morphine | FCA | Less analgesia | (Good and Westbrook, 1995) |
|
| ||||
| VTA | naltrexone | SIA | Less analgesia | (Altier and Stewart, 1996) |
|
| ||||
| dlPAG | κ antagonist | FCA | None | (Bellgowan and Helmstetter, 1998) |
| μ antagonist | FCA | None | (Bellgowan and Helmstetter, 1998) | |
| naltrexone | SIA | None | (Hohmann et al., 2005) | |
|
| ||||
| vlPAG | κ antagonist | FCA | None | (Bellgowan and Helmstetter, 1998) |
| μ antagonist | FCA | Less analgesia | (Bellgowan and Helmstetter, 1998) | |
| naltrexone | FCA | Less analgesia | (Helmstetter and Landeira-Fernandez, 1990) | |
|
| ||||
| RVM | κ agonist | FCA | Less analgesia | (Foo and Helmstetter, 2000a) |
| δ antagonist | FCA | None | (Foo and Helmstetter, 1999) | |
| κ antagonist | FCA | None | (Foo and Helmstetter, 1999) | |
| μ antagonist | FCA | Less analgesia | (Foo and Helmstetter, 1999, 2000b) | |
This table summarizes the effect of local and systemic injections of opioid agonists and antagonists on various types of behavioral analgesia.
Table 6.
Cannabinoidergic modulation of behavioral analgesia5
| Site | Type | Manipulation | Effect | References |
|---|---|---|---|---|
|
| ||||
| Systemic | Agonist | FCA, Exercise, SIA | More analgesia | (Butler et al., 2012; Butler et al., 2008; Galdino et al., 2014a; Galdino et al., 2014b; Hohmann et al., 2005; Suplita et al., 2005) |
| CB-1 Antagonist | FCA, SIA, Exercise, Distraction | Less analgesia | (Finn et al., 2004; Ford et al., 2015; Galdino et al., 2014a; Galdino et al., 2014b; Kurrikoff et al., 2008; Lee et al., 2016; Olango et al., 2014; Rea et al., 2013; Suplita et al., 2005) | |
| CB-2 Antagonist | Exercise | Less analgesia | (Galdino et al., 2014a; Galdino et al., 2014b) | |
|
| ||||
| BLA | Agonist | SIA | None | (Connell et al., 2006) |
| CB-1 Antagonist | FCA, SIA | Less analgesia, None | (Connell et al., 2006; Rea et al., 2013; Roche et al., 2010; Roche et al., 2007) | |
|
| ||||
| CeA | CB-1 Antagonist | FCA, SIA | None | (Connell et al., 2006; Rea et al., 2013) |
|
| ||||
| vHipp | Agonist | FCA | More analgesia | (Ford et al., 2011) |
| CB-1 Antagonist | FCA | None | (Ford et al., 2011) | |
|
| ||||
| dlPAG | Agonist | SIA | More analgesia | (Hohmann et al., 2005; Suplita et al., 2005) |
| CB-1 Antagonist | FCA, SIA | Less analgesia | (Hohmann et al., 2005; Olango et al., 2012; Suplita et al., 2005) | |
| CB-2 Antagonist | SIA | None | (Hohmann et al., 2005) | |
|
| ||||
| vlPAG | Agonist | SIA | More analgesia | (Hohmann et al., 2005) |
| CB-1 Antagonist | SIA | Less analgesia | (Lee et al., 2016) | |
|
| ||||
| RVM | Agonist | SIA | More analgesia | (Suplita et al., 2005) |
| CB-1 Antagonist | SIA | Less analgesia | (Suplita et al., 2005) | |
This table summarizes the effect of local and systemic injections of cannabinoid agonists and antagonists on various types of behavioral analgesia.
The neural structures most commonly investigated within FCA are the amygdala, PAG and RVM (Helmstetter and Tershner, 1994; Helmstetter et al., 1998). The amygdala coordinates behavioral responses to painful stimuli (Herry et al., 2007), and projections from amygdala to PAG are directly involved in descending pain control (Davis, 1994; Helmstetter et al., 1998; Hopkins and Holstege, 1978; Oka et al., 2008). Like many prefrontal regions involved in pain modulation, opioid release in the amygdala is associated with pain relief (Finnegan et al., 2005; Helmstetter et al., 1998). In contrast to these cortical regions, however, the amygdala can also induce pain relief in a cannabinoid dependent manner. Direct injections of CB-1 agonists into cingulate cortex fail to elicit analgesic responses, while microinjections of cannabinoid agonists into the amygdala induce analgesia (Martin et al., 1999). There is a large concentration of cannabinoid receptors within basolateral amygdala (BLA), and activation of these receptors presynaptically modulates GABAergic neurons that may be involved in descending pain control (Katona et al., 2001).
Amygdala activation is required for inducing fear conditioned analgesia in rodents (Helmstetter, 1992; Helmstetter and Bellgowan, 1993) in an opioid (Butler et al., 2008) and cannabinoid (Connell et al., 2006) dependent manner. Specifically, FCA is mediated by increased cannabinoid activity (Rea et al., 2013) and decreased concentration of GABA (Rea et al., 2009) within BLA, consistent with both the high concentration of CB-1 receptors (Katona et al., 2001) and the inhibition of GABAergic neurons by cannabinoids. However, other researchers have reported that cannabinoid antagonists within BLA have no effect on FCA per se, but rather work in the short term to briefly attenuate expression of pain independent of fear conditioning (Roche et al., 2007). While the specific role of endogenous cannabinoids within the amygdala in FCA is unclear, the amygdala is an important hub for generating FCA (Helmstetter, 1992; Helmstetter and Bellgowan, 1993) and relays signals to the PAG that serve to initiate descending pain control mechanisms (Finnegan et al., 2005; Millan, 2002).
Within the brainstem, lesions of the RVM, dorsolateral PAG (dlPAG) or ventral/ventrolateral PAG (vPAG) can reduce FCA (Helmstetter and Tershner, 1994; Kinscheck et al., 1984; Watkins et al., 1983). Though all of these structures play a role in FCA, the neurochemical systems involved vary by region. Within the dlPAG, a pain modulatory response depends on endogenous cannabinoid, but not opioid, activity. FCA is unaffected by application of an opioid antagonist within dlPAG (Bellgowan and Helmstetter, 1998), but is attenuated by cannabinoid antagonists within the same region (Olango et al., 2012). Cannabinoid function within dlPAG is important within other pain modalities as well, as SIA induced from foot shocks is attenuated by cannabinoid antagonists and enhanced by cannabinoid agonists in dlPAG (Hohmann et al., 2005).
This dissociation between cannabinoid and opioid dependence is not as distinct within vPAG and RVM, however. Analgesia is influenced by opioid activity within these regions, as injection of opioid antagonists into either vPAG (Bellgowan and Helmstetter, 1998; Helmstetter and Landeira-Fernandez, 1990) or RVM (Foo and Helmstetter, 1999) attenuate FCA. The effect of cannabinoids within these regions is less clear, as no study has examined how local inhibition of cannabinoid function within vPAG or RVM affects FCA. However, cannabinoids in these regions do have an effect on endogenous pain modulation. Local injection of a cannabinoid agonist into RVM induces pain relief (Martin et al., 1999; Martin et al., 1998; Meng and Johansen, 2004), and systemic administration of a cannabinoid antagonist reduces the expression of pain-related genes in the RVM during FCA (Olango et al., 2014). Similarly, injections of cannabinoid agonists into vPAG enhances footshock-induced SIA (Hohmann et al., 2005), while intra-vPAG injections of cannabinoid antagonists attenuate restraint-induced SIA (Lee et al., 2016).
The neurochemical systems underlying FCA are not static, and can change with different behavioral manipulations. When animals are conditioned to a single extra session following criterion for fear conditioning, FCA can be blocked by naloxone and is thus opioid-dependent. However, if animals experience multiple conditioning sessions following criterion, FCA is no longer opioid-mediated and is not attenuated by naloxone administration (Lichtman and Fanselow, 1991). This has direct parallels to the shift of placebo analgesia from an expectation-dependent to expectation-independent state following multiple conditioning sessions (Schafer et al., 2015).
In summary, FCA is mediated by opioid and cannabinoid activity within the amygdala, RVM, and PAG. Expectation-dependent placebo analgesia has been shown to be dependent on opioid release in many cases, and conditioning with non-opioid analgesics induces expectation-independent analgesia that is mediated by cannabinoid release. However, these rodent studies demonstrate that with extended training, a conditioned analgesic response shifts from an opioid to a non-opioid system. It is possible, therefore, that when subjects in a placebo conditioning paradigm experience multiple conditioning sessions, the resultant placebo analgesia shifts from a largely opioid-mediated process that flexibly depends on expectations to incorporate a greater proportion of non-opioid elements via a slower accumulative process that function independent of expectations. Furthermore, given the importance of cannabinoid release in FCA (Butler et al., 2012; Olango et al., 2012) and previous associations of cannabinoid activity with some forms of expectation independent placebo analgesia (Benedetti et al., 2011), it is possible that non-opioid placebo analgesia following multiple conditioning sessions may specifically be mediated by cannabinoid release.
5. A dual process model of placebo analgesia
Placebo effects can be conceptualized as a learned adaptive response to contextual cues that prepares an organism to appropriately respond to external events. We argue that placebo analgesia is best understood as arising from two learning processes that are instantiated within separate, but connected, neurobiological systems. The first of these learning processes supports the acquisition of a placebo response by using information about the environment to update a mental schema of the placebo context. Expectations for pain relief operationalize the strength of this schema and are correlated with the magnitude of placebo analgesia. The activation of this schema depends on activity within frontal areas (Schuck et al., 2016), such as rACC and dlPFC, and culminates with inhibition of ascending pain signals in the spinal cord via disinhibition of projection neurons in PAG and RVM. This schema-dependent analgesia depends critically on the release of endogenous opioids.
The second learning system is activated by repeated pairing of a placebo with reduced pain resulting in the formation of pre-cognitive associations that can induce pain relief without relying on mental schemas (Jocham et al., 2016). We hypothesize that this analgesia is independent of prefrontal control, and instead utilizes connections between the basic associative learning systems (including the amygdala) and the PAG-RVM-SC system. These pre-cognitive associations can be used to induce analgesia dependent on either opioid or cannabinoid responses.
An interesting example of crosstalk between the two processes is the mitigation of morphine-conditioned opioid release by changes in the underlying schema—for example by informing subjects that they are not receiving an analgesic (Amanzio and Benedetti, 1999). One explanation for this phenomenon could be that opioid-dependent analgesia is always influenced by dynamic learning processes—even when enhanced via accumulative learning. Alternatively, it is also possible that conditioning with morphine induces a potential un-blinding effect during test, as participants may perceive different side effects following morphine vs. saline injection. Interestingly, when placebo conditioning was done via a non-opioid drug in this study, naloxone only partially blocked placebo analgesia (Amanzio and Benedetti, 1999). This suggests that the effect of mental schemas on analgesia learned via accumulative learning processes depends on the specific neurochemical system used to attenuate pain.
During fear conditioning in rodents, a single extra conditioning session following criterion for FCA elicits opioid-mediated analgesia, whereas adding further conditioning sessions renders FCA opioid-independent (Lichtman and Fanselow, 1991). In humans, a single session of conditioning tends to elicit placebo responses that are mediated by expectations (Montgomery and Kirsch, 1997), but multiple conditioning sessions can be used to generate placebo effects that are independent of expectations (Schafer et al., 2015). Given a) the involvement of cannabinoids in conditioned analgesia within rodents (Connell et al., 2006; Finn et al., 2004) and b) the importance of cannabinoids in conditioned expectation-independent analgesia within humans (Benedetti et al., 2011), it is reasonable to hypothesize that expectation-independent placebo analgesia is generally mediated by cannabinoid release. We would thus predict that placebo analgesia conditioned on masked, subliminal cues (Jensen et al., 2012) depends on the release of endogenous cannabinoids, rather than opioids.
The circumstances under which analgesia is mediated by opioidergic and cannabinoidergic mechanisms suggest the following explanatory hypotheses of conditioned analgesia: When conditioned analgesia is mediated by schema-dependent processes, it incorporates activity within a wide array of brain regions, including both cognitive and associative forebrain areas such as dlPFC, rACC, and NAc. Activation within these regions then activates descending pain control mechanisms within the PAG and RVM in an opioidergic manner (Figure 2). In contrast, when conditioned analgesia is independent of expectations, analgesia is mediated by a conditioned release of endogenous cannabinoids and relies on an intact pathway from amygdala to PAG and RVM to activate descending pain control.
Figure 2. Endogenous opioids and placebo analgesia in humans.
Placebo treatment is associated with enhanced opioid activity within dlPFC, aIns, dACC, rACC, vmPFC, mOFC, thalamus, NAc, amygdala, and PAG (dark blue fill). Opioid activity within PAG, NAc, rACC, vmPFC, and dlPFC is correlated with placebo analgesia (light blue outline).
5.1 Relationship between learning processes and modulatory mechanisms
Placebo effects are generated via changes within endogenous modulatory mechanisms. Critically, one can infer how learned contextual information affects those mechanisms by examining how different learning processes induce the related placebo effects. In cases where a placebo effect is independent of either dynamic or accumulative learning processes, the corresponding placebo mechanisms are also independent of those processes. This means that the reason placebo effects such as conditioned immunosuppression or growth hormone release are not mediated by expectations (Albring et al., 2012; Benedetti et al., 2003) is simply because the underlying mechanisms themselves are not affected by changes in mental schemas. The following sections will detail how the different learning processes that induce placebo analgesia can inform about the separability of the underlying neurobiological mechanisms for both placebo analgesia and endogenous pain modulation as a whole.
5.1.1. Neurochemical specificity of learning process in analgesia
As we have argued above, evidence suggests that the mechanisms associated with expectation-based and associative learning processes are partially distinct in placebo analgesia. However, this need not be the case across all classes of placebo effects, or all forms of pain modulation. For example, while placebo analgesia can be induced via both conditioning and expectation manipulations, conditioning does not seem induce appreciably greater hyperalgesia over and above manipulations of expectations (Colloca et al., 2008). This suggests that the neurobiological processes leading to hyperalgesia depend on mental schemas and may not be as affected by slowly learned pre-cognitive associations.
One explanation for this effect could be that the potential cost of an action determines the balance between these two processes. For example, there is a high cost to missing cues that predict enhanced pain, as such errors could result in significant injury or even death. In this case, a flexible learning system that can rapidly adapt to changes in the surrounding context is given preference over a slower accumulative process in order to minimize future harm experienced. This does not mean that associative processes cannot pair associations between cues and enhanced pain (Jensen et al., 2015), merely that these changes are more susceptible to changes in a mental schema as compared to conditioned analgesic associations.
The dual-process model hypothesizes that expectation-independent placebo analgesia is induced via pre-cognitive associations learned over time, and is mediated by the release of endogenous cannabinoids. The use of multiple conditioning sessions to shift placebo analgesia from an expectation and opioid dependent form to a partially expectation-independent form (Schafer et al., 2015) does so by recruiting cannabinoidergic modulatory processes (Benedetti et al., 2011). Studies of FCA in rats support this idea by demonstrating that multiple conditioning sessions can be used to elicit a non-opioid form of placebo analgesia, leaving open the possibility of mediation by endogenous cannabinoids (Lichtman and Fanselow, 1991).
This hypothesis raises some important questions. First, which neural regions would be involved in this cannabinoid release and how would they work to activate the descending pain control mechanisms that lead to placebo analgesia? Based on parallels drawn from FCA, the amygdala, PAG and RVM would be critical to this type of placebo analgesia (Helmstetter and Tershner, 1994; Kinscheck et al., 1984). Interestingly, a recent study in humans demonstrated that the amygdala supports non-contingent learning, in which humans associate stimuli with outcomes based on statistical patterns without being aware of those associations (Jocham et al., 2016). Activating this form of learning should require a procedure where pain relief is paired with a treatment context across multiple trials.
While a pain relief schema is not necessary to express expectation-independent analgesia, it is unclear whether such a schema is necessary during acquisition. Even if the conditioned analgesia is dependent on cannabinoid release, cannabinoid function in turn may depend on endogenous opioids. Studies in rodents find that increases in FCA from cannabinoid agonists can be completely abolished using opioid antagonists (Butler et al., 2008). This suggests that under certain circumstances opioid function is required to enable cannabinoid-induced analgesia, similar to a case where lack of a pain relief schema could suppress accumulative learning of an association between placebo treatment and pain relief. This view is supported by the observation that informing participants about reduced stimulation intensities during placebo conditioning prevents subsequent placebo analgesia—impairing the accumulative process while the dynamic process is set to a “no-analgesia” schema (Montgomery and Kirsch, 1997). Another study that induced placebo analgesia through a positive interpretation of the pain (Benedetti et al., 2013) reported that both cannabinoidergic and opioidergic antagonists blocked parts of the placebo analgesia. This observation and the fact that the combination of both antagonists completely abolished placebo analgesia, suggests parallel and independent neurochemical mechanisms are involved in some forms of placebo analgesia.
Even when associations are learned independent of the opioid system, a pain relief schema may still be necessary to condition associations between a placebo and pain relief. For example, both NSAID conditioning and subliminal conditioning can induce expectation-independent analgesia and should primarily rely on accumulative (and largely cannabinoidergic) processes to induce analgesia. If opioid activity is needed to “gate” the accumulative response in both of these cases, it is possible that an injection of naloxone or another opioid antagonist during conditioning could impair subsequent analgesia in both of these cases. From a psychological perspective, this would imply that explicit changes in the mental schemas prior to conditioning could also inhibit learning.
5.1.2. Context effects on learning and modulatory processes engaged
A major difference between FCA and placebo analgesia is that FCA occurs in response to a negatively valenced context associated with pain, whereas placebo analgesia is induced following a positively valenced context associated with pain relief. Fear conditioned analgesia can be interpreted as an adaptive response to prepare the organism to function under a threatening circumstance. This is completely at odds with the response to a placebo. In the case of placebo analgesia, an organism associates a context with pain relief, and then experiences relief, perhaps because of threat reduction or enhancement of a positive affective state or reward expectation (Scott et al., 2007).
In addition to FCA, conditioning with other forms of stress can be used to induce hyperalgesia rather than analgesia. For example, presentation of flavored water that has been previously associated with stomach pain leads to hyperalgesia (Wiertelak et al., 1994), as opposed to FCA induced by associating a novel context with footshocks (Hayes et al., 1978). Hence, the direction of pain modulation is susceptible to context valence – as in the differences between FCA and placebo analgesia – and also differences among the particular contexts paired with a given stimulus. Within placebo analgesia, disconfirming information that suggests a placebo does not induce pain relief, for example by initially pairing a placebo with high pain, prevents acquisition of an analgesia response (Colloca and Benedetti, 2006).
The exact reasons for why positively and negatively valenced contexts can lead to either analgesia or hyperalgesia remains an open question. One explanation may involve associations with the contexts themselves. For example, placebo effects are typically induced in comforting, familiar settings where subjects have at least some knowledge about what they are participating in, whereas FCA in rodents is induced within completely novel environments associated with uncertainty. A recent study in humans reported that uncertainty about the upcoming stimulus also enhances pain (Yoshida et al., 2013) and this uncertainty effect can interact with stimulus intensity (Jensen and Yaksh, 1986). An important contextual difference between FCA and placebo analgesia might be uncertainty about what to expect in this environment as uncertainty per se is aversive and associated with anxiety behavior as well as amygdala activation (Herry et al., 2007).
Another potential difference between these two paradigms are the actual stimuli associated with pain. Within FCA, pain is associated with a general environmental context, while in placebo analgesia, pain relief is associated with a specific treatment ritual (pill, injection, etc.) within an environmental context (social cues, setting, location, etc.). It is possible that this difference between conditioning the environmental context and the treatment ritual could underlie whether pain or pain relief could be used to elicit analgesia. Understanding why associations with both aversive and appetitive stimuli can be used to induce analgesic effects, and how those associations can sometimes reverse, could further enhance pain treatment outcomes in clinical settings.
5.2 Predictions of a dual-process model
One goal of the dual-process model is to account for differences in how expectation-dependent and expectation-independent placebo analgesia is formed across different paradigms. This framework was then extended to neurobiological systems to suggest how these two processes could operate in the brain. However, this model can also be used to explain individual differences in the systems underlying placebo analgesia, as well as make novel predictions about how pre-cognitive associations can be manipulated to change analgesic experience.
5.2.1. Individual differences in processes mirrored in neurochemical system
Personality traits such as openness and optimism have been shown to affect whether placebo analgesia can be induced in subjects using verbal manipulation of expectations alone (De Pascalis et al., 2002; Morton et al., 2009; Pecina et al., 2013). When interpreted within the dual-process framework, it is possible that “optimists” may simply have an easier time of forming, and believing in, a mental schema where the placebo elicits pain relief. Furthermore, it is possible that these differences are more accurately captured by individual differences in opioidergic and cannabinoidergic tone within the descending pain control system (King et al., 2013; Pecina et al., 2013).
These two neurochemical systems may combine differently within individual subjects based on idiosyncratic experiences and personal genetics. In one study, subjects who were told that a painful stimulus would strengthen their muscles demonstrated greater pain tolerance than controls. This tolerance was attenuated by either opioid or cannabinoid antagonists (naloxone and rimonabant, respectively) and fully blocked by a combination of the two (Benedetti et al., 2013). Critically, there was a strong negative correlation between the effectiveness of naloxone and rimonabant on attenuating pain tolerance, such that the stronger effect one antagonist had, the weaker effect the other had. While this suggests that these two systems may combine semi-independently to induce analgesia (Cichewicz and McCarthy, 2003; Wilson-Poe et al., 2013), the individual experiences and traits that lead to the preference of one system over the other remains unclear.
Another study found that a certain genotype in humans is associated with a less active form of FAAH, an enzyme in the body that metabolizes endogenous cannabinoids (Pecina et al., 2014a). Individuals with this genotype maintain higher levels of endogenous cannabinoids, and also show enhanced opioid release during placebo analgesia compared to individuals without this genotype. It is possible that these variations underlie individual differences in how placebo analgesia is induced in humans. Identifying whether personality traits, genotype, or opioidergic and cannabinoidergic tone affect different processes that in turn elicit placebo analgesia, is an important future goal and could explain why certain procedures are effective at inducing placebo effects in some individuals, but not others.
In addition to personality traits, person-by-situation interactions can explain additional variance in placebo responses (Atlas and Wager, 2012). Some people may be more susceptible to certain treatments compared to others (Geuter et al., 2013; Whalley et al., 2008) based on their neurochemical dispositions. Such interactions will make it harder to identify consistent placebo responders, which may be one of the reasons for the failure of so-called wash-in periods aiming at removing placebo responders from clinical trials.
5.2.2. Susceptibility of pre-cognitive associations to other conditioning manipulations
The dynamic learning process relies on the formation of a mental schema, and thus cannot generate expectation-independent analgesia. It is telling then, that all forms of expectation-independent placebo analgesia induced in the laboratory to date include some form of conditioning manipulation that could induce placebo effects via an accumulative learning process (Amanzio and Benedetti, 1999; de Jong et al., 1996; Jensen et al., 2012; Schafer et al., 2015). Although this mixture of processes may impede the isolation and study of relevant sub-processes, we can still make some predictions about the processes involved. If this accumulative process is comparable to the process by which conditioned and unconditioned stimuli are paired in classical conditioning, it is reasonable to hypothesize that many of the properties examined in studies of associative learning would also apply to these placebo effects. Thus, the dual-process model makes the prediction that expectation-independent analgesia should be extinguishable, be subject to spontaneous recovery, and be modality specific, such that responses conditioned to one form of treatment (e.g. oral ingestion of a placebo) fail to generalize to another (e.g. intravenous injection) (Delamater and Westbrook, 2014; Maren et al., 2013).
Following acquisition, there are several possibilities for how subsequent presentations of the placebo could affect extinction of the placebo response. For example, if pain relief is always experienced after placebo treatment, the placebo effect could be self-reinforcing as the association between the placebo and reduced pain is never broken (Vase et al., 2005; Watson et al., 2006). In the case of partial reinforcement, where the placebo mitigates but does not consistently reduce pain, repeated presentations lead to a slower extinction of placebo response than full reinforcement during acquisition (Au Yeung et al., 2014). Even if cases where placebos are fully self-reinforcing, however, placebo responses should still be extinguishable by surreptitious increases of pain intensity following placebo treatment.
While the accumulative and dynamic processes can separately induce analgesic responses, these two processes may interact when forming associative pairs. In this case, the time course for extinction of a placebo response would be influenced by subject expectations, such that lower expectations for pain relief would lead to more rapid extinction and vice versa. This would be consistent with models of conditioning where pairings are not simply low-level associations but instead incorporate value judgments and motivational state (Fanselow, 1984; Gallistel et al., 2004; Rescorla and Wagner, 1972). An activated schema could suppress prediction errors, which would otherwise drive the extinction as suggested by a recent study (Schenk et al., 2017).
5.3. Caveats to the dual-process model
There are several caveats and particulars about the dual-process model that must be clearly enumerated before concluding. It is important to note that PAG, RVM, and associated structures are not the only systems involved in contextual changes in pain experience. Rather, the purpose of this review is to clarify how the PAG, RVM, and related systems elicit context-dependent analgesia. Separate pathways for pain modulation that do not involve opioids, cannabinoids, PAG or RVM exist and these systems may also play a role in placebo and other forms of conditioned analgesia (Maire et al., 2016; Wager et al., 2011).
While the dual-process model predicts that schema-dependent analgesia is independent of cannabinoid release, that does not imply that this analgesia always depends on opioid function. For example, expectation-mediated analgesia in IBS is not always mediated by opioids (Vase et al., 2005), from which we can infer that the effect of mental schemas on pain experience cannot be purely governed by opioid function. While this review has focused primarily on the function of opioids and cannabinoids, other systems exist that can be used to modulate pain (e.g., dopaminergic and serotonergic systems), and these systems may underlie analgesia described in the study by Vase and colleagues (2005).
Some readers may note that the hippocampus, a critical neuroanatomical structure in the formation and retrieval of memories, has been excluded from discussion of learned placebo responses. The hippocampus plays a major role in regulating context dependent effects in a wide variety of situations (Maren et al., 2013). However, this review is primarily focused on how placebo effects induce analgesia so discussion of the importance of the hippocampus to context representation has been omitted. Several studies have found that hippocampus is needed to induce fear conditioned analgesia (Ford et al., 2011), and other conditioned associations (Kim and Fanselow, 1992; Selden et al., 1991), as well as nocebo hyperalgesia (Bingel et al., 2011; Dickenson et al., 1979; Kong et al., 2008; cf. Tinnermann et al., 2017). Thus, context associations mediated by hippocampal activation may also play a critical role in both associative and schema-dependent placebo effects, though it is not specific to conditioned analgesia in particular and instead plays a more general role in facilitating associative pairings.
6. Summary
The ubiquity of placebo effects across multiple illnesses and disorders represents an opportunity to enhance the effectiveness of drug treatments through relatively simple psychological manipulations with little to no adverse side effects. Placebo effects occur across such a wide variety of disorders and paradigms that the existence of separate and specific processes for each instance seems unlikely. Understanding how underlying neurobiological mechanisms and psychological processes induce placebo effects, and why some people respond to placebos and other do not, can help clinicians capitalize on these effects to enhance symptom relief from prescribed treatments without incurring additional costs and side effects.
Figure 3. Drug injection effects on pain.
A) Blue circles represent sites where local injection of opioid agonists reduces pain in rodents in at least one study, as measured by either reduction in pain-related behaviors or enhanced latency to escape from a painful stimulus. Gray circles represent regions that are involved in pain modulation where we failed to find studies testing the effect of local microinjections of opioid agonists on analgesia. B) Green circles represent sites where local injection of cannabinoid agonists reduces pain in rodents in at least one study, as measured by either reduction in pain-related behaviors or enhanced latency to escape from a painful stimulus. Gray circles represent regions that are involved in pain modulation where we failed to find studies testing the effect of local microinjections of cannabinoid agonists on analgesia. Black circles indicate that every reviewed study found no effect of local injection of cannabinoid agonists within this region on analgesia.
Figure 4. Drug effects on behaviorally mediated analgesia.
A) Local injection of opioid antagonists within ACC, vlPAG, and RVM reduce behavioral analgesia. In contrast, microinjections of opioid agonists into CeA reduce behavioral analgesia and microinjections of opioid antagonists in dlPAG have no effect on behavioral analgesia. Other regions involved in opioidergic pain control that have not been demonstrated to be involved in behavioral analgesia include aIns, NAc, BLA, Hy, and VTA. B) Local injection of cannabinoid antagonists within BLA, PAG, and RVM reduce behavioral analgesia. Similarly, microinjections of cannabinoid agonists within PAG, RVM, and vHipp enhance behavioral analgesia. Interestingly, while CeA injections of cannabinoid agonists are sufficient to induce analgesia, cannabinoid antagonists in CeA fail to reduce behavioral analgesia.
Figure 5. Distribution of drugs and pain measures across rodent studies.
A) Opioid interventions of behavioral analgesia paradigms including direct injections into brain regions (total: 171). B) Cannabinoid interventions of behavioral analgesia paradigms including direct injections into brain regions (total: 73). C) This chart shows the relative distribution of pain measures across the reviewed studies of pain modulation in rodents (total: 241).
Highlights.
We propose a dual-process model of placebo analgesia, in which strong placebo responses are created by appropriate flexible conceptual beliefs, reinforced by affective experiences (reward and punishment).
Cognitive schemas, mental representations of the self in context, are critical for many forms of placebo analgesia.
An extensive review of animal studies on behavioral analgesia is used to inform a neural systems implementation of the model
The model predicts that opioidergic neurotransmission underlies expectation-dependent placebo analgesia and cannabinoidergic neurotransmissions supports expectation-independent placebo analgesia.
Abbreviations
- ACC
anterior cingulate cortex
- aIns
anterior insula
- Amy
amygdala
- BLA
basolateral amygdala
- CeA
central amygdala
- dACC
dorsal anterior cingulate cortex
- dlPAG
dorsolateral periaqueductal gray
- dlPFC
dorsolateral prefrontal cortex
- DRN
dorsal raphe nucelus
- FCA
fear-conditioned analgesia
- Hy
hypothalamus
- IBS
Irritable Bowel Syndrome
- IL
infralimbic cortex
- mOFC
medial orbitofrontal cortex
- NAc
nucleus accumbens
- NRM
nucleus raphe magnus
- NSAID
non-steroidal anti-inflammatory drug
- PAG
periaqueductal gray
- PFC
prefrontal cortex
- PL
prelimbic cortex
- rACC
rostral anterior cingulate cortex
- RVM
rostral ventromedial medulla
- S2
secondary somatosensory cortex
- SC
spinal cord
- SIA
stress-induced analgesia
- Thal
thalamus
- vHipp
ventral hippocampus
- vlPAG
ventrolateral periaqueductal gray
- vPAG
ventral periaqueductal gray
- vmPFC
ventromedial prefrontal cortex
- VTA
ventral tegmental area.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albring A, Wendt L, Benson S, Nissen S, Yavuz Z, Engler H, Witzke O, Schedlowski M. Preserving learned immunosuppressive placebo response: perspectives for clinical application. Clinical pharmacology and therapeutics. 2014;96:247–255. doi: 10.1038/clpt.2014.75. [DOI] [PubMed] [Google Scholar]
- Albring A, Wendt L, Benson S, Witzke O, Kribben A, Engler H, Schedlowski M. Placebo effects on the immune response in humans: the role of learning and expectation. PloS one. 2012;7:e49477. doi: 10.1371/journal.pone.0049477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan LG, Siegel S. A Signal Detection Theory Analysis of the Placebo Effect. Evaluation & the Health Professions. 2002;25:410–420. doi: 10.1177/0163278702238054. [DOI] [PubMed] [Google Scholar]
- Altier N, Stewart J. The role of dopamine in the nucleus accumbens in analgesia. Life sciences. 1999;65:2269–2287. doi: 10.1016/s0024-3205(99)00298-2. [DOI] [PubMed] [Google Scholar]
- Amanzio M, Benedetti F. Neuropharmacological Dissection of Placebo Analgesia: Expectation-Activated Opioid Systems versus Conditioning-Activated Specific Subsystems. The Journal of Neuroscience. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal M, Petkó M, Polgár E, Heizmann CW, Storm-Mathisen J. Direct evidence of an extensive GABAergic innervation of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. Neuroscience. 1996;73:509–518. doi: 10.1016/0306-4522(96)00063-2. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Wager TD. How expectations shape pain. Neuroscience letters. 2012;520:140–148. doi: 10.1016/j.neulet.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:8053–8064. doi: 10.1523/JNEUROSCI.0383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au Yeung ST, Colagiuri B, Lovibond PF, Colloca L. Partial reinforcement, extinction, and placebo analgesia. Pain. 2014;155:1110–1117. doi: 10.1016/j.pain.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azami J, Llewelyn MB, Roberts MHT. The contribution of nucleus reticularis paragigantocellularis and nucleus raphe magnus to the analgesia produced by systemically administered morphine, investigated with the microinjection technique. Pain. 1982;12:229–246. doi: 10.1016/0304-3959(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Apkarian AV. Nociception, Pain, Negative Moods, and Behavior Selection. Neuron. 2015;87:474–491. doi: 10.1016/j.neuron.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nature reviews. Neuroscience. 2015;16:419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Enodgenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annual Reviews of Neuroscience. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Research. 1979;170:85–93. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- Bellgowan PS, Helmstetter FJ. The role of mu and kappa opioid receptors within the periaqueductal gray in the expression of conditional hypoalgesia. Brain Research. 1998;791:83–89. doi: 10.1016/s0006-8993(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Descending monoaminergic pain modulation. Neurology. 2008;71:217–221. doi: 10.1212/01.wnl.0000318225.51122.63. [DOI] [PubMed] [Google Scholar]
- Benedetti F. Placebo effects: from the neurobiological paradigm to translational implications. Neuron. 2014;84:623–637. doi: 10.1016/j.neuron.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M. Mechanisms of the placebo response. Pulmonary pharmacology & therapeutics. 2013;26:520–523. doi: 10.1016/j.pupt.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Rosato R, Blanchard C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nature medicine. 2011;17:1228–1230. doi: 10.1038/nm.2435. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Frisaldi E, Carlino E, Giudetti L, Pampallona A, Zibetti M, Lanotte M, Lopiano L. Teaching neurons to respond to placebos. The Journal of physiology. 2016 doi: 10.1113/JP271322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious Expectation and Unconscious Conditioning in Analgesic, Motor, and Hormonal Placebo/Nocebo Responses. The Journal of Neuroscience. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Thoen W, Blanchard C, Vighetti S, Arduino C. Pain as a reward: changing the meaning of pain from negative to positive co-activates opioid and cannabinoid systems. Pain. 2013;154:361–367. doi: 10.1016/j.pain.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Berry MJI, Brivanlou IH, Jordan TA, Meister M. Anticipation of moving stimuli by the retina. Nature. 1999;398:334–338. doi: 10.1038/18678. [DOI] [PubMed] [Google Scholar]
- Bialosky JE, Bishop MD, George SZ, Robinson ME. Placebo response to manual therapy: something out of nothing? The Journal of manual & manipulative therapy. 2011;19:11–19. doi: 10.1179/2042618610Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Science translational medicine. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Roberts KW, Culbertson CS, Le A, Evans CJ, Fanselow MS. Pavlovian conditioning of multiple opioid-like responses in mice. Drug and alcohol dependence. 2009;103:74–83. doi: 10.1016/j.drugalcdep.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Geuter S, Sprenger C, Eippert F. Placebo analgesia: a predictive coding perspective. Neuron. 2014;81:1223–1239. doi: 10.1016/j.neuron.2014.02.042. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Stevens BL, Friedman JJ, Wager TD. Distraction and placebo: two separate routes to pain control. Psychological science. 2012;23:246–253. doi: 10.1177/0956797611427919. [DOI] [PubMed] [Google Scholar]
- Butler RK, Finn DP. Stress-induced analgesia. Progress in neurobiology. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Butler RK, Ford GK, Hogan M, Roche M, Doyle KM, Kelly JP, Kendall DA, Chapman V, Finn DP. Fear-induced suppression of nociceptive behaviour and activation of Akt signalling in the rat periaqueductal grey: role of fatty acid amide hydrolase. J Psychopharmacol. 2012;26:83–91. doi: 10.1177/0269881111413823. [DOI] [PubMed] [Google Scholar]
- Butler RK, Rea K, Lang Y, Gavin AM, Finn DP. Endocannabinoid-mediated enhancement of fear-conditioned analgesia in rats: opioid receptor dependency and molecular correlates. Pain. 2008;140:491–500. doi: 10.1016/j.pain.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Cannon JT, Prieto GJ, Lee A, Liebeskind JC. Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain Research. 1982;243:315–321. doi: 10.1016/0006-8993(82)90255-4. [DOI] [PubMed] [Google Scholar]
- Carlino E, Torta DM, Piedimonte A, Frisaldi E, Vighetti S, Benedetti F. Role of explicit verbal information in conditioned analgesia. European journal of pain. 2015;19:546–553. doi: 10.1002/ejp.579. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Inhibition by opioids acting on μ-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994;113:303–309. doi: 10.1111/j.1476-5381.1994.tb16209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou LC, Huang L-YM. Mechanism underlying increased neuronal activity in the rat ventrolateral periaqueductal gray by a μ-opioid. Journal of Physiology. 1999;518:551–559. doi: 10.1111/j.1469-7793.1999.0551p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichewicz DL, McCarthy EA. Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. J Pharmacol Exp Ther. 2003;304:1010–1015. doi: 10.1124/jpet.102.045575. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124:126–133. doi: 10.1016/j.pain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. The Lancet Neurology. 2004;3:679–684. doi: 10.1016/S1474-4422(04)00908-1. [DOI] [PubMed] [Google Scholar]
- Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. Pain. 2010;151:430–439. doi: 10.1016/j.pain.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136:211–218. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Colloca L, Tinazzi M, Recchia S, Le Pera D, Fiaschi A, Benedetti F, Valeriani M. Learning potentiates neurophysiological and behavioral placebo analgesic responses. Pain. 2009;139:306–314. doi: 10.1016/j.pain.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Connell K, Bolton N, Olsen D, Piomelli D, Hohmann AG. Role of the basolateral nucleus of the amygdala in endocannabinoid-mediated stress-induced analgesia. Neuroscience letters. 2006;397:180–184. doi: 10.1016/j.neulet.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Craggs JG, Price DD, Robinson ME. Enhancing the placebo response: functional magnetic resonance imaging evidence of memory and semantic processing in placebo analgesia. The journal of pain : official journal of the American Pain Society. 2014;15:435–446. doi: 10.1016/j.jpain.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook RJ, Dickson K, Hanlon RT, Walters ET. Nociceptive sensitization reduces predation risk. Current biology : CB. 2014;24:1121–1125. doi: 10.1016/j.cub.2014.03.043. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in emotional learning. International Review of Neurobiology. 1994;36:225–266. doi: 10.1016/s0074-7742(08)60305-0. [DOI] [PubMed] [Google Scholar]
- Dayan P, Berridge KC. Model-based and model-free Pavlovian reward learning: revaluation, revision, and revelation. Cogn Affect Behav Neurosci. 2014;14:473–492. doi: 10.3758/s13415-014-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov MH, Vanderah TW, Lai J, Dussor GO, Fields HL, Price TJ, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain. 2011;152:2701–2709. doi: 10.1016/j.pain.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PJ, van Baast R, Arntz A, Merckelbach H. The Placebo Effect in Pain Reduction: The Influence of Conditioning Experiences and Response Expectancies. International Journal of Behavioral Medicine. 1996;3:14–29. doi: 10.1207/s15327558ijbm0301_2. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernández R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and Dopamine Release: Mechanism of the Placebo Effect in Parkinson's Disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Chiaradia C, Carotenuto E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain. 2002;96:393–402. doi: 10.1016/S0304-3959(01)00485-7. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Magurano MR, Bellusci A, Chen ACN. Somatosensory event-related potential and autonomic activity to varying pain reduction cognitive strategies in hypnosis. Clinical Neurophysiology. 2001;112:1475–1485. doi: 10.1016/s1388-2457(01)00586-7. [DOI] [PubMed] [Google Scholar]
- Delamater AR, Westbrook RF. Psychological and neural mechanisms of experimental extinction: a selective review. Neurobiology of learning and memory. 2014;108:38–51. doi: 10.1016/j.nlm.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson AH, Oliveras J-L, Besson J-M. Role of the nucleus raphe magnus in opiate analgesia as studied by the microinjection technique in the rat. Brain Research. 1979;170:95–111. doi: 10.1016/0006-8993(79)90943-0. [DOI] [PubMed] [Google Scholar]
- DiMaggio P. Culture and Cognition. Annu Rev Sociol. 1997;23:263–287. [Google Scholar]
- Drew GM, Lau BK, Vaughan CW. Substance P drives endocannabinoid-mediated disinhibition in a midbrain descending analgesic pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7220–7229. doi: 10.1523/JNEUROSCI.4362-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt-Gupta J, Bown T, Cyna AM. Effect of communication on pain during intravenous cannulation: a randomized controlled trial. British journal of anaesthesia. 2007;99:871–875. doi: 10.1093/bja/aem308. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Stewart J. Conditioning of Drug-Induced Physiological Responses. Psychological Review. 1982;89:507–528. [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009a;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Eippert F, Finsterbusch J, Bingel U, Buchel C. Direct Evidence for Spinal Cord Involvement in Placebo Analgesia. Science. 2009b;326:404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- Ellingsen DM, Wessberg J, Eikemo M, Liljencrantz J, Endestad T, Olausson H, Leknes S. Placebo improves pleasure and pain through opposite modulation of sensory processing. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17993–17998. doi: 10.1073/pnas.1305050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenbruch S, Kotsis V, Benson S, Rosenberger C, Reidick D, Schedlowski M, Bingel U, Theysohn N, Forsting M, Gizewski ER. Neural mechanisms mediating the effects of expectation in visceral placebo analgesia: an fMRI study in healthy placebo responders and nonresponders. Pain. 2012;153:382–390. doi: 10.1016/j.pain.2011.10.036. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Shock-induced analgesia on the formalin test: Effects of shock severity, naloxone, hypophysectomy, and associative variables. Behavioral Neuroscience. 1984;98:79–95. doi: 10.1037//0735-7044.98.1.79. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Baackes MP. Conditioned Fear-Induced Opiate Analgesia on the Formalin Test: Evidence for Two Aversive Motivational Systems. Learning and Motivation. 1982;13:200–221. [Google Scholar]
- Fanselow MS, Calcagnetti DJ, Helmstetter FJ. Delta opioid antagonist, 16-Me cyprenorphine, selectively attenuates conditional fear- and DPDPE-induced analgesia on the formalin test. Pharmacology Biochemistry & Behavior. 1989;32:469–473. doi: 10.1016/0091-3057(89)90181-0. [DOI] [PubMed] [Google Scholar]
- Fardin V, Oliveras J-L, Besson J-M. A Reinvestigation of the Analgesic Effects Induced by Stimulation of the Periaqueductal Gray Matter in the Rat. I. The Production of Behavioral Side Effects Together with Analgesia. Brain Research. 1984;306:105–123. doi: 10.1016/0006-8993(84)90360-3. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nature reviews. Neuroscience. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fields H. A Motivation-Decision Model of Pain: The Role of Opioids. In: Flor H, Kalso E, Dostrovsky JO, editors. Proceedings of the 11th World Congress on Pain. IASP Press; Seattle, USA: 2006. pp. 449–459. [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. Journal of Neurophysiology. 1995;74:1742–1759. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Richardson D, Kendall DA, Marsden CA, Chapman V. Evidence for differential modulation of conditioned aversion and fear-conditioned analgesia by CB1 receptors. The European journal of neuroscience. 2004;20:848–852. doi: 10.1111/j.1460-9568.2004.03509.x. [DOI] [PubMed] [Google Scholar]
- Finnegan TF, Chen SR, Pan HL. Effect of the μ opioid on excitatory and inhibitory synaptic inputs to periaqueductal gray-projecting neurons in the amygdala. J Pharmacol Exp Ther. 2005;312:441–448. doi: 10.1124/jpet.104.074633. [DOI] [PubMed] [Google Scholar]
- Finnegan TF, Li DP, Chen SR, Pan HL. Activation of μ-opioid receptors inhibits synaptic inputs to spinally projecting rostral ventromedial medulla neurons. J Pharmacol Exp Ther. 2004;309:476–483. doi: 10.1124/jpet.103.064808. [DOI] [PubMed] [Google Scholar]
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H, Birbaumer N, Schulz R, Grüsser SM, Mucha RF. Pavlovian conditioning of opioid and nonopioid pain inhibitory mechanisms in humans. European journal of pain. 2002;6:395–402. doi: 10.1016/s1090-3801(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Flor H, Grüsser SM. Conditioned stress-induced analgesia in humans. European journal of pain. 1999;3:317–324. doi: 10.1053/eujp.1999.0137. [DOI] [PubMed] [Google Scholar]
- Foo H, Helmstetter FJ. Hypoalgesia elicited by a conditioned stimulus is blocked by a, μ, but not a δ or a κ, opioid antagonist injected into the rostral ventromedial medulla. Pain. 1999;83:427–431. doi: 10.1016/S0304-3959(99)00125-6. [DOI] [PubMed] [Google Scholar]
- Foo H, Mason P. Ingestion analgesia occurs when a bad taste turns good. Behav Neurosci. 2011;125:956–961. doi: 10.1037/a0025542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford GK, Kieran S, Dolan K, Harhen B, Finn DP. A role for the ventral hippocampal endocannabinoid system in fear-conditioned analgesia and fear responding in the presence of nociceptive tone in rats. Pain. 2011;152:2495–2504. doi: 10.1016/j.pain.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Ford GK, Moriarty O, Okine BN, Tully E, Mulcahy A, Harhen B, Finn DP. Involvement of the endocannabinoid system in attentional modulation of nociceptive behaviour in rats. European journal of pain. 2015;19:1177–1185. doi: 10.1002/ejp.646. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ. Analgesia and the neural substrate of reward. Neuroscience and Biobehavioral Reviews. 1989;13:149–154. doi: 10.1016/s0149-7634(89)80024-7. [DOI] [PubMed] [Google Scholar]
- Friston K. A theory of cortical responses. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR, Fairhurst S, Balsam P. The learning curve: implications of a quantitative analysis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13124–13131. doi: 10.1073/pnas.0404965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers AL, Wellman JA, Fowler SL, Helfer SG, France CR. Dispositional optimism predicts placebo analgesia. The journal of pain : official journal of the American Pain Society. 2010;11:1165–1171. doi: 10.1016/j.jpain.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Boll S, Eippert F, Buchel C. Functional dissociation of stimulus intensity encoding and predictive coding of pain in the insula. Elife. 2017a;6 doi: 10.7554/eLife.24770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Buchel C. Facilitation of pain in the human spinal cord by nocebo treatment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:13784–13790. doi: 10.1523/JNEUROSCI.2191-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Eippert F, Hindi Attar C, Buchel C. Cortical and subcortical responses to high and low effective placebo treatments. NeuroImage. 2013;67:227–236. doi: 10.1016/j.neuroimage.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Koban L, Wager TD. The Cognitive Neuroscience of Placebo Effects: Concepts, Predictions, and Physiology. Annu Rev Neurosci. 2017b;40:167–188. doi: 10.1146/annurev-neuro-072116-031132. [DOI] [PubMed] [Google Scholar]
- Goffaux P, Redmond WJ, Rainville P, Marchand S. Descending analgesia--when the spine echoes what the brain expects. Pain. 2007;130:137–143. doi: 10.1016/j.pain.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Guo JY, Wang JY, Luo F. Dissection of placebo analgesia in mice: the conditions for activation of opioid and non-opioid systems. Journal of Psychopharmacology. 2010;24:1561–1567. doi: 10.1177/0269881109104848. [DOI] [PubMed] [Google Scholar]
- Guo JY, Yuan XY, Sui F, Zhang WC, Wang JY, Luo F, Luo J. Placebo analgesia affects the behavioral despair tests and hormonal secretions in mice. Psychopharmacology. 2011;217:83–90. doi: 10.1007/s00213-011-2259-7. [DOI] [PubMed] [Google Scholar]
- Hayes RL, Bennett GJ, Newlon PG, Mayer DJ. Behavioral and physiological studies of non-narcotic analgesia in the rat elicited by certain environmental stimuli. Brain Research. 1978;155:69–90. doi: 10.1016/0006-8993(78)90306-2. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Fields HL. Central Nervous System Mechanisms of Pain Modulation. In: McMahon SB, Koltzenburg M, Tracey I, Turk DC, editors. Wall & Melzack's Textbook of Pain. Saunders; 2013. pp. 129–142. [Google Scholar]
- Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain research reviews. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ. The amygdala is essential for the expression of conditional hypoalgesia. Behavioral Neuroscience. 1992;106:518–528. doi: 10.1037//0735-7044.106.3.518. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Lesions of the amygdala block conditional hypoalgesia on the tail flick test. Brain Research. 1993;612:253–257. doi: 10.1016/0006-8993(93)91669-j. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Fanselow MS. Effects of naltrexone on learning and performance of conditional fear-induced freezing and opioid analgesia. Physiology and Behavior. 1987;39:501–505. doi: 10.1016/0031-9384(87)90380-5. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Landeira-Fernandez J. Conditional hypoalgesia is attenuated by naltrexone applied to the periaqueductal gray. Brain Research. 1990;537:88–92. doi: 10.1016/0006-8993(90)90343-a. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Tershner SA. Lesions of the periaqueductal gray and rostral ventromedial medulla disrupt antinociceptive but not cardiovascular aversive conditional responses. The Journal of Neuroscience. 1994;14:7099–7106. doi: 10.1523/JNEUROSCI.14-11-07099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Tershner SA, Poore LH, Bellgowan PS. Antinociception following opioid stimulation of the basolateral amygdala is expressed through the periaqueductal gray and rostral ventromedial medulla. Brain Research. 1998;779:104–118. doi: 10.1016/s0006-8993(97)01104-9. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Luthi A, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL., 2nd Endocannabinoid mechanisms of pain modulation. The AAPS Journal. 2006;8:E693–E708. doi: 10.1208/aapsj080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Hróbjartsson A, Gøtzsche PC. Is the Placebo Powerless? An Analysis of Clinical Trials Comparing Placebo with No Treatment. The New England journal of medicine. 2001;344:1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- Janson W, Stein C. Peripheral opioid analgesia. Current pharmaceutical biotechnology. 2003;4:270–274. doi: 10.2174/1389201033489766. [DOI] [PubMed] [Google Scholar]
- Jensen K, Kirsch I, Odmalm S, Kaptchuk TJ, Ingvar M. Classical conditioning of analgesic and hyperalgesic pain responses without conscious awareness. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:7863–7867. doi: 10.1073/pnas.1504567112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15959–15964. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. I. Comparison of antinociceptive action of morphin in the periaqueductal gray, medial and paramedial medulla in rat. Brain Research. 1986;363:99–113. doi: 10.1016/0006-8993(86)90662-1. [DOI] [PubMed] [Google Scholar]
- Jepma M, Wager TD. Conceptual Conditioning: Mechanisms Mediating Conditioning Effects on Pain. Psychological science. 2015;26:1728–1739. doi: 10.1177/0956797615597658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham G, Brodersen KH, Constantinescu AO, Kahn MC, Ianni AM, Walton ME, Rushworth MF, Behrens TE. Reward-Guided Learning with and without Causal Attribution. Neuron. 2016;90:177–190. doi: 10.1016/j.neuron.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Frederick S. Representativeness revisited: Attribute substitution in intuitive judgment. In: Gilovich T, Griffin D, Kahneman D, editors. Heuristics and Biases: The Psychology of Intuitive Judgment. Cambridge University Press; 2002. [Google Scholar]
- Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, Kaptchuk TJ, Burstein R. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Science translational medicine. 2014;6:218ra215. doi: 10.1126/scitranslmed.3006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, Kowalczykowski M, Miller FG, Kirsch I, Lembo AJ. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PloS one. 2010;5:e15591. doi: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, Kirsch I, Schyner RN, Nam BH, Nguyen LT, Park M, Rivers AL, McManus C, Kokkotou E, Drossman DA, Goldman P, Lembo AJ. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. Bmj. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. The Journal of Neuroscience. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD, Perrot-Sinal TS. Opioid and non-opioid NMDA-mediated predator-induced analgesia in mice and the effects of parasitic infection. Brain Research. 1997;766:11–18. doi: 10.1016/s0006-8993(97)00521-0. [DOI] [PubMed] [Google Scholar]
- Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose RM, Berg-Smith SM, Kline JN. Placebo response in asthma: a robust and objective phenomenon. The Journal of allergy and clinical immunology. 2007;119:1375–1381. doi: 10.1016/j.jaci.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Kessner S, Wiech K, Forkmann K, Ploner M, Bingel U. The Effect of Treatment History on Therapeutic Outcome: An Experimental Approach. JAMA Internal Medicine. 2013;173:1468–1469. doi: 10.1001/jamainternmed.2013.6705. [DOI] [PubMed] [Google Scholar]
- Khan A, Faucett J, Lichtenberg P, Kirsch I, Brown WA. A systematic review of comparative efficacy of treatments and controls for depression. PloS one. 2012;7:e41778-e41778. doi: 10.1371/journal.pone.0041778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- King CD, Goodin B, Kindler LL, Caudle RM, Edwards RR, Gravenstein N, Riley JL, 3rd, Fillingim RB. Reduction of conditioned pain modulation in humans by naltrexone: an exploratory study of the effects of pain catastrophizing. Journal of behavioral medicine. 2013;36:315–327. doi: 10.1007/s10865-012-9424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinscheck IB, Watkins LR, Mayer DJ. Fear is not critical to classically conditioned analgesia: the effects of periaqueductal gray lesions and administration of chlordiazepoxide. Brain Research. 1984;298:33–44. doi: 10.1016/0006-8993(84)91144-2. [DOI] [PubMed] [Google Scholar]
- Kirsch I. Hidden administration as ethical alternatives to the balanced placebo design. Prevention & Treatment. 2003;6 [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration. PLoS Medicine. 2008;5:260–268. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I, Kong J, Sadler P, Spaeth R, Cook A, Kaptchuk TJ, Gollub R. Expectancy and conditioning in placebo analgesia: Separate or connected processes? Psychology of Consciousness: Theory, Research, and Practice. 2014;1:51–59. doi: 10.1037/cns0000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I, Sapirstein G. Listening to prozac but hearing placebo: A meta-analysis of antidepressant medication. Prevention & Treatment. 1998;1:2a. [Google Scholar]
- Kisaalita N, Staud R, Hurley R, Robinson M. Placebo use in pain management: The role of medical context, treatment efficacy, and deception in determining placebo acceptability. Pain. 2014;155:2638–2645. doi: 10.1016/j.pain.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger R, Soost S, Flor H, Worm M. Classical conditioning and expectancy in placebo hypoalgesia: a randomized controlled study in patients with atopic dermatitis and persons with healthy skin. Pain. 2007;128:31–39. doi: 10.1016/j.pain.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Koban L, Wager TD. Beyond conformity: Social influences on pain reports and physiology. Emotion. 2016;16:24–32. doi: 10.1037/emo0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, Rosen B, Kaptchuk TJ. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:13354–13362. doi: 10.1523/JNEUROSCI.2944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsis V, Benson S, Bingel U, Forsting M, Schedlowski M, Gizewski ER, Elsenbruch S. Perceived treatment group affects behavioral and neural responses to visceral pain in a deceptive placebo study. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24:935–e462. doi: 10.1111/j.1365-2982.2012.01968.x. [DOI] [PubMed] [Google Scholar]
- Krummenacher P, Candia V, Folkers G, Schedlowski M, Schonbachler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148:368–374. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Labuz D, Mousa SA, Schafer M, Stein C, Machelska H. Relative contribution of peripheral versus central opioid receptors to antinociception. Brain Res. 2007;1160:30–38. doi: 10.1016/j.brainres.2007.05.049. [DOI] [PubMed] [Google Scholar]
- Lau BK, Vaughan CW. Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Current opinion in neurobiology. 2014;29:159–164. doi: 10.1016/j.conb.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Lee HF, Hsieh JC, Lu CL, Yeh TC, Tu CH, Cheng CM, Niddam DM, Lin HC, Lee FY, Chang FY. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain. 2012;153:1301–1310. doi: 10.1016/j.pain.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Chang LY, Ho YC, Teng SF, Hwang LL, Mackie K, Chiou LC. Stress induces analgesia via orexin 1 receptor-initiated endocannabinoid/CB1 signaling in the mouse periaqueductal gray. Neuropharmacology. 2016;105:577–586. doi: 10.1016/j.neuropharm.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Manders TR, Eberle SE, Su C, D'Amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, Wang J. Activation of corticostriatal circuitry relieves chronic neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:5247–5259. doi: 10.1523/JNEUROSCI.3494-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester LS, Fanselow MS. Exposure to a cat produces opioid analgesia in rats. Behavioral Neuroscience. 1985;99:756–759. doi: 10.1037//0735-7044.99.4.756. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Hunter AM, Tartter M, Cook IA. Role of pill-taking, expectation and therapeutic alliance in the placebo response in clinical trials for major depression. The British journal of psychiatry : the journal of mental science. 2014;205:443–449. doi: 10.1192/bjp.bp.113.140343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Fields HL. The Mechanism of Placebo Analgesia. Lancet. 1978;2:654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- Lewis VA, Gebhart GF. Evaluations of the periaqueductal central gray (PAG) as a morphine-specific locus of action and examination of morphine-induced and stimulation-produced analgesia at coincident PAG loci. Brain Research. 1977;124:282–303. doi: 10.1016/0006-8993(77)90886-1. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Fanselow MS. Opioid and Nonopioid Conditional Analgesia: The Role of Spinal Opioid, Noradrenergic, and Serotonergic Systems. Behavioral Neuroscience. 1991;105:687–698. doi: 10.1037//0735-7044.105.5.687. [DOI] [PubMed] [Google Scholar]
- Lidstone SC, Schulzer M, Dinelle K, Mak E, Sossi V, Ruth TJ, de la Fuente-Fernández R, Phillips AG, Stoessl AJ. Effects of Expectation on Placebo-Induced Dopamine Release in Parkinson Disease. Arch Gen Psychiatry. 2010;67:857–865. doi: 10.1001/archgenpsychiatry.2010.88. [DOI] [PubMed] [Google Scholar]
- Locher C, Frey Nascimento A, Kirsch I, Kossowsky J, Meyer A, Gaab J. Is the rationale more important than deception? A randomized controlled trial of open-label placebo analgesia. Pain. 2017 doi: 10.1097/j.pain.0000000000001012. [DOI] [PubMed] [Google Scholar]
- Lu HC, Hsieh JC, Lu CL, Niddam DM, Wu YT, Yeh TC, Cheng CM, Chang FY, Lee SD. Neuronal correlates in the modulation of placebo analgesia in experimentally-induced esophageal pain: a 3T-fMRI study. Pain. 2010;148:75–83. doi: 10.1016/j.pain.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA. Neural bases of conditioned placebo analgesia. Pain. 2010;151:816–824. doi: 10.1016/j.pain.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Luparello T, Lyons HA, Bleecker ER, McFadden ER. Influences of Suggestion on Airway Reactivity in Asthmatic Subjects. Psychosomatic Medicine. 1968;30:819–825. doi: 10.1097/00006842-196811000-00002. [DOI] [PubMed] [Google Scholar]
- Maire JJ, Close LN, Heinricher MM, Selden NR. Distinct pathways for norepinephrine- and opioid-triggered antinociception from the amygdala. European journal of pain. 2016;20:206–214. doi: 10.1002/ejp.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature reviews. Neuroscience. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WJ, Coffin PO, Attias E, Balinsky M, Tsou K, Walker JM. Anatomical basis for cannabinoid-induced antinociception as revealed by intracerebral microinjections. Brain Research. 1999;822:237–242. doi: 10.1016/s0006-8993(98)01368-7. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Tsou K, Walker JM. Cannabinoid receptor-mediated inhibition of the rat tail-flick reflex after microinjection into the rostral ventromedial medulla. Neuroscience letters. 1998;242:33–36. doi: 10.1016/s0304-3940(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Matre D, Casey KL, Knardahl S. Placebo-induced changes in spinal cord pain processing. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:559–563. doi: 10.1523/JNEUROSCI.4218-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb CT, White MM, Harris AL, Fuchs PN. The elusive rat model of conditioned placebo analgesia. Pain. 2014;155:2022–2032. doi: 10.1016/j.pain.2014.07.004. [DOI] [PubMed] [Google Scholar]
- McNally GP. Pain facilitatory circuits in the mammalian central nervous system: their behavioral significance and role in morphine analgesic tolerance. Neuroscience and Biobehavioral Reviews. 1999;23:1059–1078. doi: 10.1016/s0149-7634(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Meissner K. Effects of placebo interventions on gastric motility and general autonomic activity. Journal of psychosomatic research. 2009;66:391–398. doi: 10.1016/j.jpsychores.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Meissner K. The placebo effect and the autonomic nervous system: evidence for an intimate relationship. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011;366:1808–1817. doi: 10.1098/rstb.2010.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner K, Bingel U, Colloca L, Wager TD, Watson A, Flaten MA. The placebo effect: advances from different methodological approaches. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:16117–16124. doi: 10.1523/JNEUROSCI.4099-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Katz J. Pain. Wiley interdisciplinary reviews. Cognitive science. 2013;4:1–15. doi: 10.1002/wcs.1201. [DOI] [PubMed] [Google Scholar]
- Meng ID, Johansen JP. Antinociception and modulation of rostral ventromedial medulla neuronal activity by local microinfusion of a cannabinoid receptor agonist. Neuroscience. 2004;124:685–693. doi: 10.1016/j.neuroscience.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Progress in neurobiology. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Miller JS, Kelly KS, Neisewander JL, McCoy DF, Bardo MT. Conditioning of morphine-induced taste aversion and analgesia. Psychopharmacology. 1990;101:472–480. doi: 10.1007/BF02244224. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Moreau J-L, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Research. 1986;397:37–46. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whittier KL, Hegarty DM, Aicher SA. Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain. 2008;140:376–386. doi: 10.1016/j.pain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DL, Watson A, El-Deredy W, Jones AK. Reproducibility of placebo analgesia: Effect of dispositional optimism. Pain. 2009;146:194–198. doi: 10.1016/j.pain.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Muckli L, De Martino F, Vizioli L, Petro LS, Smith FW, Ugurbil K, Goebel R, Yacoub E. Contextual Feedback to Superficial Layers of V1. Current biology : CB. 2015;25:2690–2695. doi: 10.1016/j.cub.2015.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Donaldson GW, Kuhn R, Bradshaw DH, Jacobson RC, Chapman CR. Investigating dose-dependent effects of placebo analgesia: A psychophysiological approach. Pain. 2012;153:227–237. doi: 10.1016/j.pain.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Nolan TA, Price DD, Caudle RM, Murphy NP, Neubert JK. Placebo-induced analgesia in an operant pain model in rats. Pain. 2012;153:2009–2016. doi: 10.1016/j.pain.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Przewlocki R, Przewlocka B. Local peripheral effects of mu-opioid receptor agonists in neuropathic pain in rats. Neuroscience letters. 2004;360:85–89. doi: 10.1016/j.neulet.2004.01.056. [DOI] [PubMed] [Google Scholar]
- Ochoa JL. Chronic Pains Associated with Positive and Negative Sensory, Motor, and Vasomotor Manifestations: CPSMV (RSD;CRPS?). Heterogeneous Somatic Versus Psychopathologic Origins. Journal of Contemporary Neurology. 1997;1997:2–20. [Google Scholar]
- Oka T, Tsumori T, Yokota S, Yasui Y. Neuroanatomical and neurochemical organization of projections from the central amygdaloid nucleus to the nucleus retroambiguus via the periaqueductal gray in the rat. Neuroscience research. 2008;62:286–298. doi: 10.1016/j.neures.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Olango WM, Géranton SM, Roche M, Hunt SP, Finn DP. Novel molecular correlates of endocannabinoid-mediated fear-conditioned analgesia in rats. European journal of pain. 2014;18:182–191. doi: 10.1002/j.1532-2149.2013.00369.x. [DOI] [PubMed] [Google Scholar]
- Olango WM, Roche M, Ford GK, Harhen B, Finn DP. The endocannabinoid system in the rat dorsolateral periaqueductal grey mediates fear-conditioned analgesia and controls fear expression in the presence of nociceptive tone. British Journal of Pharmacology. 2012;165:2549–2560. doi: 10.1111/j.1476-5381.2011.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. Journal of Sleep Research. 2001;10 doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. The Journal of clinical investigation. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT, Osborne PB. Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. Journal of Physiology. 1990;427:519–532. doi: 10.1113/jphysiol.1990.sp018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C, Kim JH, Yoon BE, Choi EJ, Lee CJ, Shin HS. T-type channels control the opioidergic descending analgesia at the low threshold-spiking GABAergic neurons in the periaqueductal gray. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14857–14862. doi: 10.1073/pnas.1009532107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Azhar H, Love TM, Lu T, Fredrickson BL, Stohler CS, Zubieta JK. Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:639–646. doi: 10.1038/npp.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Martinez-Jauand M, Hodgkinson C, Stohler CS, Goldman D, Zubieta JK. FAAH selectively influences placebo effects. Molecular psychiatry. 2014a;19:385–391. doi: 10.1038/mp.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Stohler CS, Zubieta JK. Neurobiology of placebo effects: expectations or learning? Social cognitive and affective neuroscience. 2014b;9:1013–1021. doi: 10.1093/scan/nst079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Zubieta JK. Molecular mechanisms of placebo responses in humans. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia - Imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Pollo A, Torre E, Lopiano L, Rizzone M, Lanotte M, Cavanna A, Bergamasco B, Benedetti F. Expectation modulates the response to subthalamic nucleus stimulation in Parkinsonian patients. Neuroreport. 2002;13:1382–1386. doi: 10.1097/00001756-200208070-00006. [DOI] [PubMed] [Google Scholar]
- Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annual review of psychology. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- Rea K, Lang Y, Finn DP. Alterations in extracellular levels of gamma-aminobutyric acid in the rat basolateral amygdala and periaqueductal gray during conditioned fear, persistent pain and fear-conditioned analgesia. The journal of pain : official journal of the American Pain Society. 2009;10:1088–1098. doi: 10.1016/j.jpain.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Rea K, Olango WM, Harhen B, Kerr DM, Galligan R, Fitzgerald S, Moore M, Roche M, Finn DP. Evidence for a role of GABAergic and glutamatergic signalling in the basolateral amygdala in endocannabinoid-mediated fear-conditioned analgesia in rats. Pain. 2013;154:576–585. doi: 10.1016/j.pain.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Reicherts P, Gerdes AB, Pauli P, Wieser MJ. Psychological Placebo and Nocebo Effects on Pain Rely on Expectation and Previous Experience. The journal of pain : official journal of the American Pain Society. 2016;17:203–214. doi: 10.1016/j.jpain.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning: It's not what you think it is. American Psychologist. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Roche M, O'Connor E, Diskin C, Finn DP. The effect of CB(1) receptor antagonism in the right basolateral amygdala on conditioned fear and associated analgesia in rats. The European journal of neuroscience. 2007;26:2643–2653. doi: 10.1111/j.1460-9568.2007.05861.x. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Hendrie CA. Social Conflict Activates Status-Dependent Endogenous Analgesic or Hyperalgesic Mechanisms in Male Mice: Effects of Naloxone on Nociception and Behaviour. Physiology and Behavior. 1983;30:775–780. doi: 10.1016/0031-9384(83)90177-4. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Randall JI. Acute Non-Opioid Analgesia in Defeated Male Mice. Physiology and Behavior. 1986;36:947–950. doi: 10.1016/0031-9384(86)90458-0. [DOI] [PubMed] [Google Scholar]
- Roychowdhury SM, Fields HL. Endogenous opioids acting at a medullary μ-opioid receptor contribute to the behavioral antinociception produced by GABA antagonism in the midbrain periaqueductal gray. Neuroscience. 1996;74:863–872. doi: 10.1016/0306-4522(96)00180-7. [DOI] [PubMed] [Google Scholar]
- Schafer SM, Colloca L, Wager TD. Conditioned placebo analgesia persists when subjects know they are receiving a placebo. The Journal of Pain. 2015;16:412–420. doi: 10.1016/j.jpain.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk LA, Sprenger C, Geuter S, Buchel C. Expectation requires treatment to boost pain relief: an fMRI study. Pain. 2014;155:150–157. doi: 10.1016/j.pain.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Schenk LA, Sprenger C, Onat S, Colloca L, Buchel C. Suppression of Striatal Prediction Errors by the Prefrontal Cortex in Placebo Hypoalgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2017;37:9715–9723. doi: 10.1523/JNEUROSCI.1101-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J, Theysohn N, Gass F, Benson S, Gramsch C, Forsting M, Gizewski ER, Elsenbruch S. Neural mechanisms mediating positive and negative treatment expectations in visceral pain: a functional magnetic resonance imaging study on placebo and nocebo effects in healthy volunteers. Pain. 2013;154:2372–2380. doi: 10.1016/j.pain.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature reviews. Neuroscience. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck NW, Cai MB, Wilson RC, Niv Y. Human Orbitofrontal Cortex Represents a Cognitive Map of State Space. Neuron. 2016;91:1402–1412. doi: 10.1016/j.neuron.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Selden NRW, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Sevel LS, Craggs JG, Price DD, Staud R, Robinson ME. Placebo analgesia enhances descending pain-related effective connectivity: a dynamic causal modeling study of endogenous pain modulation. The journal of pain : official journal of the American Pain Society. 2015a;16:760–768. doi: 10.1016/j.jpain.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevel LS, O'Shea AM, Letzen JE, Craggs JG, Price DD, Robinson ME. Effective connectivity predicts future placebo analgesic response: A dynamic causal modeling study of pain processing in healthy controls. NeuroImage. 2015b;110:87–94. doi: 10.1016/j.neuroimage.2015.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe LG, Garnet JE, Cicero TJ. Analgesia and hyperreactivity produced by intracranial microinjections of morphine into the periaqueductal gray matter of the rat. Behavioral Biology. 1974;11:303–313. doi: 10.1016/s0091-6773(74)90548-3. [DOI] [PubMed] [Google Scholar]
- Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Buchel C. Attention modulates spinal cord responses to pain. Current biology : CB. 2012;22:1019–1022. doi: 10.1016/j.cub.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Stanovich KE, West RF. Individual differences in reasoning: Implications for the rationality debate? Behavioral and Brain Sciences. 2000;23:645–726. doi: 10.1017/s0140525x00003435. [DOI] [PubMed] [Google Scholar]
- Stein N, Sprenger C, Scholz J, Wiech K, Bingel U. White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo analgesia. Pain. 2012;153:2210–2217. doi: 10.1016/j.pain.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychological bulletin. 2004;130:324–340. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- Summerfield C, de Lange FP. Expectation in perceptual decision making: neural and computational mechanisms. Nature reviews. Neuroscience. 2014;15:745–756. doi: 10.1038/nrn3838. [DOI] [PubMed] [Google Scholar]
- Tinnermann A, Geuter S, Sprenger C, Finsterbusch J, Buchel C. Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science. 2017;358:105–108. doi: 10.1126/science.aan1221. [DOI] [PubMed] [Google Scholar]
- Tomim DH, Pontarolla FM, Bertolini JF, Arase M, Tobaldini G, Lima MM, Fischer L. The Pronociceptive Effect of Paradoxical Sleep Deprivation in Rats: Evidence for a Role of Descending Pain Modulation Mechanisms. Molecular neurobiology. 2015 doi: 10.1007/s12035-014-9059-0. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tuttle AH, Tohyama S, Ramsay T, Kimmelman J, Schweinhardt P, Bennett GJ, Mogil JS. Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. Pain. 2015;156:2616–2626. doi: 10.1097/j.pain.0000000000000333. [DOI] [PubMed] [Google Scholar]
- Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Valone JM, Randall CK, Kraemer PJ, Bardo MT. Olfactory cues and morphine-induced conditioned analgesia in rats. Pharmacology Biochemistry & Behavior. 1998;60:115–118. doi: 10.1016/s0091-3057(97)00554-6. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Barbaro NM, Fields HL. Tail-flick related activity in medullospinal neurons. Brain Research. 1984;321:135–141. doi: 10.1016/0006-8993(84)90689-9. [DOI] [PubMed] [Google Scholar]
- Vase L, Norskov KN, Petersen GL, Price DD. Patients' direct experiences as central elements of placebo analgesia. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011;366:1913–1921. doi: 10.1098/rstb.2010.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vase L, Riley JL, 3rd, Price DD. Comparison of placebo effects in clincal analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–452. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- Vase L, Robinson ME, Verne GN, Price DD. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115:338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Vase L, Robinson ME, Verne NG, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal gray in vitro. Journal of Physiology. 1997;498:463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Connor M, Bagley EE, Christie MJ. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Molecular Pharmacology. 2000;57:288–295. [PubMed] [Google Scholar]
- Vendruscolo LF, Vendruscolo JC, Terenina-Rigaldie E, Raba F, Ramos A, Takahashi RN, Mormede P. Genetic influences on behavioral and neuroendocrine responses to predator-odor stress in rats. Neuroscience letters. 2006;409:89–94. doi: 10.1016/j.neulet.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Vits S, Cesko E, Enck P, Hillen U, Schadendorf D, Schedlowski M. Behavioural conditioning as the mediator of placebo responses in the immune system. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011;366:1799–1807. doi: 10.1098/rstb.2010.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Watanabe H, Grootoonk S, Jones AKP. Topography of diprenorphine binding in human cingulate gyrus and adjacent cortex derived from coregistered PET and MR images. Human brain mapping. 1995;3:1–12. [Google Scholar]
- Voudouris NJ, Peck CL, Coleman G. Conditioned Placebo Responses. Journal of Personality and Social Psychology. 1985;48:47–53. doi: 10.1037//0022-3514.48.1.47. [DOI] [PubMed] [Google Scholar]
- Voudouris NJ, Peck CL, Coleman G. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43:121–128. doi: 10.1016/0304-3959(90)90057-K. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nature reviews. Neuroscience. 2015;16:403–418. doi: 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Fields HL. Placebo Analgesia. In: Wall PD, Melzack R, editors. Textbook of Pain. 2013. pp. 362–373. [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Anti-nociception following exposure to trimethylthiazoline, peripheral or intra-amygdala estrogen and/or progesterone. Behavioural brain research. 2003;144:77–85. doi: 10.1016/s0166-4328(03)00067-6. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC. Pain modulation by release of the endogenous cannabinoid anandamide. PNAS. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Cobelli DA, Mayer DJ. Classical Conditioning of Front Paw and Hind Paw Footshock Induced Analgesia (FSIA): Naloxone Reversibility and Descending Pathways. Brain Research. 1982;243:119–132. doi: 10.1016/0006-8993(82)91125-8. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Mayer DJ. Organization of Endogenous Opiate and Nonopiate Pain Control Systems. Science. 1982;216:1185–1192. doi: 10.1126/science.6281891. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Young EG, Kinscheck IB, Mayer DJ. The neural basis of footshock analgesia: The role of specific ventral medullary nuclei. Brain Research. 1983;276:305–315. doi: 10.1016/0006-8993(83)90738-2. [DOI] [PubMed] [Google Scholar]
- Watson A, El-Deredy W, Bentley DE, Vogt BA, Jones AK. Categories of placebo response in the absence of site-specific expectation of analgesia. Pain. 2006;126:115–122. doi: 10.1016/j.pain.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Whalley B, Hyland ME, Kirsch I. Consistency of the placebo effect. Journal of psychosomatic research. 2008;64:537–541. doi: 10.1016/j.jpsychores.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Wiertelak EP, Smith KP, Furness L, Mooney-Heiberger K, Mayr T, Maier SF, Watkins LR. Acute and conditioned hyperalgesic response to illness. Pain. 1994;56:227–234. doi: 10.1016/0304-3959(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Willer JC, Dehen H, Cambier J. Stress-induced analgesia in humans: Endogenous opioids and naloxone-reversible depression of pain reflexes. Science. 1981;212:689–691. doi: 10.1126/science.6261330. [DOI] [PubMed] [Google Scholar]
- Williams AE, Rhudy JL. The influence of conditioned fear on human pain thresholds: does preparedness play a role? The journal of pain : official journal of the American Pain Society. 2007;8:598–606. doi: 10.1016/j.jpain.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Wilson-Poe AR, Pocius E, Herschbach M, Morgan MM. The periaqueductal gray contributes to bidirectional enhancement of antinociception between morphine and cannabinoids. Pharmacology, biochemistry, and behavior. 2013;103:444–449. doi: 10.1016/j.pbb.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:10264–10271. doi: 10.1523/JNEUROSCI.4648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W, Seymour B, Koltzenburg M, Dolan RJ. Uncertainty increases pain: evidence for a novel mechanism of pain modulation involving the periaqueductal gray. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5638–5646. doi: 10.1523/JNEUROSCI.4984-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EG, Watkins LR, Mayer DJ. Comparison of the effects of ventral medullary lesions on systemic and microinjection morphine analgesia. Brain Research. 1984;290:119–129. doi: 10.1016/0006-8993(84)90741-8. [DOI] [PubMed] [Google Scholar]
- Yu R, Gollub RL, Vangel M, Kaptchuk T, Smoller JW, Kong J. Placebo analgesia and reward processing: integrating genetics, personality, and intrinsic brain activity. Human brain mapping. 2014;35:4583–4593. doi: 10.1002/hbm.22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RR, Zhang WC, Wang JY, Guo JY. The opioid placebo analgesia is mediated exclusively through mu-opioid receptor in rat. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:849–856. doi: 10.1017/S1461145712000673. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]