Abstract
This review critically examines neurobehavioral theoretical developments in decision making in addiction in the 21st century. We specifically compare each theory reviewed to seven benchmarks of theoretical robustness, based on their ability to address: why some commodities are addictive; developmental trends in addiction; addiction-related anhedonia; self-defeating patterns of behavior in addiction; why addiction co-occurs with other unhealthy behaviors; and, finally, means for the repair of addiction. We have included only self-contained theories or hypotheses which have been developed or extended in the 21st century to address decision making in addiction. We thus review seven distinct theories of decision making in addiction: learning theories, incentive-sensitization theory, dopamine imbalance and systems models, opponent process theory, strength models of self-control failure, the competing neurobehavioral decision systems theory, and the triadic systems theory of addiction. Finally, we have directly compared the performance of each of these theories based on the aforementioned benchmarks, and highlighted key points at which several theories have coalesced.
Keywords: addiction, decision making, theory, human, neurobehavioral
1. Introduction
Addiction is a serious public health problem leading to excess morbidity and mortality and considerable economic burden (National Institute on Drug Abuse, 2015). For example, the opioid epidemic has resulted in a greater number of deaths than` automobile accidents (Warner, Chen, Makuc, Anderson, & Miniño, 2011). Recognizing that addiction results from the choices made by those with that disorder, the field of addiction science, beginning in the 21st century, has increasingly focused its attention on decision making processes. Indeed, a hallmark of addiction is the fact that the individual suffering from it continues to use despite punishing and negative consequences. Thus, the response of this field has, as an important part of its agenda, the goal of understanding the decisional processes that result in the continued use of one or more substances.
Progress in identifying the neurobehavioral decision processes underlying disadvantageous decision making evident in addiction could facilitate the transition from a symptom-focused approach to a mechanism-focused approach, and this may further the development of treatments for these conditions. Indeed, this growing emphasis on decision making renders the field of addiction closer to the Research Domain Criteria effort (Insel et al., 2010). The Research Domain Criteria unit within the National Institutes on Mental Health seeks to identify constructs and domains of functioning which can be examined on multiple units of analysis, from genomics to self-report, to understand how basic dimensions of functioning undergird both normal and abnormal human behavior. Although basic science of decision making has made and continues to make advances, using these decisional processes to mechanistically understand disorders remains a significant scientific gap (Goschke, 2014). However, this has not prevented the formulation of theories to understand the dysfunctional decision making evident in addiction. Such theoretical undertakings are important and useful for the field because theories typically serve three roles; that is, theories (1) explain the extant data collected, (2) predict what will be observed in novel circumstances and (3) generate new ideas about the range of events to which the theory may apply. Many of these theories are derived inductively and are refined over time as new data are obtained. Interestingly for the addiction science field, several contemporary theories of decision making in addiction are competing for scientific attention. From a Kuhnian perspective (Kuhn, 1961), these diverse theories would be considered in a pre-paradigmatic stage. However, some evidence is suggestive of coalescing around common elements across several theories.
In this paper, we seek to briefly evaluate the extant theories of addiction that have been developed since the beginning of the 21st century. We limit our paper to this timeframe because, as we noted above, the decision making emphasis within the field has gained momentum since the early 2000s. Thus, we will only review theories from the last century to the extent they are actively used and/or updated. We will use the word addiction as opposed to other terms (such as alcohol or drug use disorder or dependence) because we recognize that addictive-like behaviors can occur with a variety of commodities and events in addition to alcohol and drugs. In this paper, we first review the phenomena that could be used as a benchmark by which to evaluate any comprehensive theory of addiction. These benchmarks are a further elaboration of earlier phenomena we have identified (Bickel, Mueller, & Jarmolowicz, 2013). This will be followed by a brief introduction to extant theories and evaluation against these benchmarks. Finally, we will conclude with our views of the future direction of decision making theories of addiction and remaining questions emerging from these theoretical developments.
2. Benchmarks for a Theory of Decision Making in Addiction
A theory of decision making in addiction ideally should satisfy several functions. In order to (1) explain extant collected data, (2) predict observations from novel circumstances, and (3) generate new ideas about theoretical applications, a theory must address or account for multiple components of addiction. The task that follows is identification of the components of addiction (see also Bickel et al., 2013) that are meaningful to meeting these three criteria. Specific phenomena widely acknowledged in addiction that can be used as criteria by which to evaluate these theories have been specified in prior work (Edwards & Lader, 1990; Peele, 1985). Included in these phenomena is the development of interventions that flow from the theory which speaks to, in part, generating new ideas from theoretical applications. Specifically, theories of addiction should thus be able to answer the following questions. (1) Why are some commodities addictive when others are not? (2) Why does addiction follow common developmental trends? (3) Why do some individuals, as their valuation of drugs increases, also exhibit a decrease in valuation of non-addictive commodities? (4) Why do individuals with addiction engage in consistent self-defeating patterns of behavior? (5) Why are individuals with addiction also likely to engage in other unhealthy behaviors? And finally (6) what interventions have been or are derivable from the tenets of these theories? Together, these six questions comprise benchmarks by which a theory of decision making in addiction can be assessed.
First, why are some commodities addictive when others are not? When referring to this, we are not addressing the sociological status of a commodity, which may vary over time (e.g., opioids and cocaine at the turn of the 19th century may not have been considered addictive). Rather, we are speaking to potential for addictive consumption, which can be assessed through the study of abuse liability of commodities, as is standard practice in application for approval of new pharmacologic agents (Balster & Bigelow, 2003; L. P. Carter & Griffiths, 2009; Collins, Weeks, Cooper, Good, & Russell, 1984). A continuum exists from some drugs having no addictive potential (e.g., chlorpromazine, Griffiths, Bigelow, & Liebson, 1979) to those which have substantial potential for abuse (e.g., heroin, Comer, Sullivan, Whittington, Vosburg, & Kowalczyk, 2008). Moreover, among abused substances, the physical dependence and incidence of addictive consumption can vary. For example, a 2009 panel of addiction experts (including researchers and clinicians) identified “degree to which a drug can lead to dependence” as a distinct component of the overall harm profile of drugs, and rated some drugs (such as tobacco or crack cocaine) as significantly more likely to lead to dependence than others (such as LSD or psilocybin; Nutt, King, Phillips, & Independent Scientific Committee on Drugs, 2010).
Second, why does addiction follow common developmental trends? Exposure to addictive commodities most often begins in early adolescence (Kosterman, Hawkins, Guo, Catalano, & Abbott, 2000), and, in some susceptible individuals, addictive consumption follows in adolescence and emerging adulthood. Notably, many individuals who are exposed to these addictive commodities never become addicted, and identifying underlying risk factor(s) for this progression is a key component of understanding developmental trends. Furthermore, this addictive consumption tends to diminish as individuals age into early and middle adulthood; this is known as maturing out (see Moffitt, 1993; O Malley, 2004). Maturing out may not be constant across all addictive commodities, but is especially pronounced in some, see Figure 1. Indeed, spontaneous remission (recovery from addiction without traditional treatment methods) may be the norm, in alcohol (Cunningham, Sobell, Sobell, & Kapur, 1995) and opiate addiction (Price, Risk, & Spitznagel, 2001).
Figure 1.
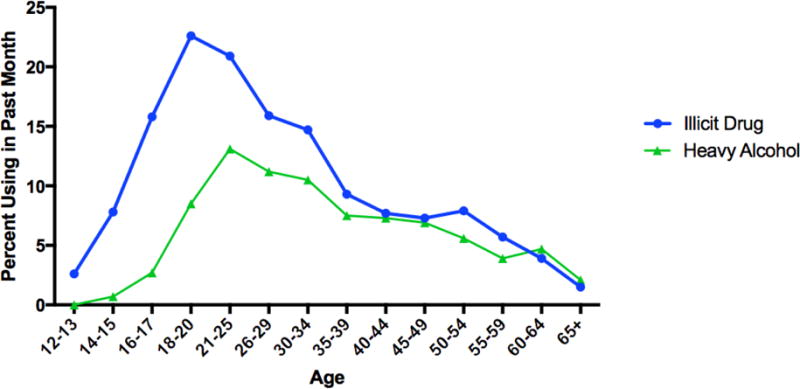
Alcohol and Illicit Drug Use among Persons Age 12 and Over
CAPTION: Figure 1 depicts past month use of alcohol to binge levels (defined as consumption of five or more drinks per occasion), heavy levels (defined as five or more binges in the past month), or any past month use of illicit substances (including marijuana/hashish, cocaine (including crack), heroin, hallucinogens, inhalants, or prescription type psychotherapeutics used nonmedically). Data taken from the 2013 National Survey of Drug Use and Health (SAMHSA, 2014).
Third, why do some individuals as their valuation of drugs increase also exhibit a decrease in valuation of non-addictive commodities? Individuals in the most advanced and severe stages of addiction may demonstrate concurrent patterns of overvaluation of addictive commodities, and undervaluation of alternative reinforcers, such as social and family engagement (Currie, 1994). Indeed, anhedonia, or the lack of ability to experience pleasure from commonly-enjoyable activities, may be widespread in individuals with addiction (Lubman et al., 2009; Sussman & Leventhal, 2014) and critical to the understanding of addictive behavior and motivation (Wise, 2008). Specifically, the relative insensitivity to otherwise rewarding events may have physiological underpinnings among individuals who are addicted (Koob & Volkow, 2010), and may be paired with relatively heightened sensitivity to the rewards of drugs and other addictive commodities (Leventhal et al., 2014).
Fourth, why do individuals with addiction engage in consistent self-defeating patterns of behavior? Although many consequences of addiction are delayed and uncertain (such as the probability of lung cancer after tobacco smoking), others are more concrete (such as the relatively higher likelihood of jail time after a failed drug test during parole), and yet an individual who is addicted may still choose to consume. This insensitivity to consequences leads to a general pattern of self-defeating behavior (Leshner, 1997), where individuals choose to consume even when they are aware of possible consequences. Indeed, most individuals who use substances report awareness of at least some of their general negative effects (Siahpush, McNeill, Hammond, & Fong, 2006), but may also be unrealistically optimistic about the consequences of their personal use (Mcgregor, Darke, Ali, & Christie, 1998). Overall, addictive consumption seems insensitive to these consequences. These self-defeating patterns of foreseeing harm and still engaging in the harmful activity are not commonly observed in healthy controls (Baumeister & Scher, 1988).
Fifth, why are individuals with addiction also likely to engage in other unhealthy behaviors? In this context, we are not referring in any way to psychiatric diagnoses. Instead, we are focused on a variety of health behaviors. For example, alcohol and nicotine use commonly co-occur with poor dietary choices (Padrão, Lunet, Santos, & Barros, 2007; Ryle & Thomson, 1984) and alcohol use disorder co-occurs with risky sexual behaviors (Cooper, 2002). Drug use, including alcohol, and illicit drug use are also associated with engagement in risky driving practices (Kelly, Darke, & Ross, 2004; Lonczak, Neighbors, & Donovan, 2007), and increased criminality (Haggård-Grann, Hallqvist, Långström, & Möller, 2006). Addiction is also common among pathological gamblers (Petry, Stinson, & Grant, 2005). Furthermore, addiction to one substance commonly co-occurs with addiction to (or heavy use of) other substances, a condition known as polydrug use or polydrug dependence. Polydrug users may account for as much as half of the population seeking treatment for addiction (Kedia, Sell, & Relyea, 2007). Although capturing population-level associations between addiction and other health conditions is challenging, these data support that overall, unhealthy behaviors, including addiction cluster together. Of note, the prevalence of addiction, but not necessarily prevalence of misuse, may vary inversely with socioeconomic status (Gilman, Abrams, & Buka, 2003; e.g., Keyes & Hasin, 2008; van Oers, Bongers, van de Goor, & Garretsen, 1999). This is similar to the prevalence of other challenging health behaviors/conditions, such as obesity (Baum & Ruhm, 2009), and both stressful life events (Lantz, House, Mero, & Williams, 2016) and physiologic markers of stress (Beckie, 2012).
Sixth, what interventions have been or are derivable from the tenets of these theories? Of course, a theory could generate new ideas and predict what will be observed in novel circumstances in addition to treatment. However, given the negative health consequences associated with addiction, we have chosen to evaluate therapeutic interventions as the most translationally relevant. These interventions must be derivable from the theory. If the theory is robust then the proposed treatment should be efficacious. This benchmark is especially critical given the efficacy of existing treatments. Although these interventions are efficacious and replicable, considerable opportunity for improvement remains, and a large gap persists between needs in the population for intervention and the small percentage who avail themselves of it (SAMHSA, 2015). If the interventions which are derived from a given theory of addiction prove effective, then the theory not only serves to explain one or more of the diverse set of phenomena associated with addiction but also provides a means for its repair.
As we review theories of decision making in addiction, we will explore the extent to which they address each of these six benchmarks. Presumably, those theories that can explain a greater proportion of these benchmarks will have greater theoretical robustness and practical utility. In the following sections, we review theories that address decision making and are active and/or modified in this century.
3. An Overview of Theories of Decision Making in Addiction
Addiction, as a disorder of excessive consumption, develops from many individual instances of choice to consume or not consume substances. Each of these choices is the product of a decision making process, in which an individual selects a course of action from among several possible alternatives. In the following sections, we will provide a brief overview of theories and hypotheses that speak to this decision making process in addiction, and evaluate these theories and hypotheses against the six benchmarks described above. We have not included all explanations of addiction, but only those that meet three criteria. First, the explanation of addiction must be designated as a theory or hypothesis. Second, the theory or hypothesis must have been developed or extended in the 21st century, generating considerable experimental research to understand addiction in human subjects. Third, the theory or hypothesis must address decision making in addiction. For example, we have excluded many explanations of addiction, including failures modes (Redish, 2015) and implicit cognition (Stacy & Wiers, 2010), the self-medication theory of addiction (Khantzian, 1987),and the trans-theoretical model of behavior change (Prochaska & Velicer, 1997), for each of these three reasons, respectively. For discussion of some of these other theories, see West and Brown (2013). Furthermore, we have not included within this review a discussion of the fundamental nature of addiction, particularly the question of whether addiction is a brain disease or a disorder of choice (Heyman, 2009; e.g., Volkow, Koob, & McLellan, 2016).
We have included seven main theories in this review. All of the theories which we have included based on the three criteria above also involved neurobehavioral components at their inception or have since been developed to include them in the 21st century. We have ordered theories based on common features or histories where logical. Thus, we will present three dopamine-related theories, one opponent process theory, two types of self-regulation theory, and two dual-decision system or dual-decision system-derived theories. The reviews of these theories presented below are not intended to be complete statements of the totality of each theory; rather, these introductions are meant to orient the reader to the fundamental components of the theory. Each brief description will thus contain: a statement of the earliest form of the theory; definitions of the key concepts or structures invoked by the theory; summary of exemplary empirical work in support of the theory, and finally a restatement of the most recent version of the theory, if the theory has been updated since its earliest form. Then, we compare each of these theories to the benchmarks.
3.1 Dopamine-Related Theories
During the 1960s, several seminal research reports influenced by Skinner’s (Skinner, 1938, 1953) concept of reinforcement provided a new paradigm for the field of addiction by demonstrating that non-human animals would self-administer many common drugs of abuse, thus establishing that drugs could act as reinforcers in a manner similar to food and water (Pickens & Thompson, 1968; Schuster & Thompson, 1969). This research spawned considerable work which later identified the role of dopamine- (DA) dependent, neuronal communication in these reinforcing actions (Crow, 1972). Indeed, many drugs of abuse, including amphetamine, cocaine, heroin, nicotine, cannabis, and alcohol, while having very different primary molecular targets, all have the common action of increasing DA transmission in the nucleus accumbens (Wise, 1987, 1988). We now know that the mesolimbic DA pathway (DA cells in the ventral tegmental area (VTA) projecting into ventral striatum, in particular the nucleus accumbens (NAc)), is critical for the rewarding effects of drugs (Wise, 2009; Wise & Rompre, 1989), while other DA pathways such as the mesostriatal (DA cells in the substantia nigra projecting into dorsal striatum) and mesocortical (DA cells in the VTA projecting to the frontal cortex) also contribute to drug reward and addiction (Wise, 2009). The initial work was extended by Schultz and colleagues (Schultz, 1997; Schultz, Dayan, & Montague, 1997), who showed that over time, the DA response to reinforcer receipt shifts to the predictive stimuli preceding reinforcer delivery (Schultz, 2007). Finally, an improved understanding of associative learning mechanisms that conceive behavioral output as an interaction between Pavlovian and operant learning processes have helped to understand the mechanisms underlying the transition from drug abuse to addiction (Dickinson & Balleine, 1994; White & McDonald, 2002).
Many variations on DA-related theories of addiction now exist. All propose that the actions of DA on receptors in the NAc, related limbic regions, and projections to the prefrontal cortex (PFC) play a critical role in the decision making process in addiction. Here, we discuss three prominent contemporary DA-related theories: learning and habit theories (Everitt et al., 2008; Hogarth, Balleine, Corbit, & Killcross, 2013; Hyman, Malenka, & Nestler, 2006), incentive-sensitization theory (Robinson & Berridge, 1993, 2008), and the dopamine imbalance model of addiction (Volkow & Baler, 2014; Volkow, Wang, Fowler, Tomasi, & Telang, 2011). All of these theories recognize the crucial importance of DA, neuronal adaptations, and associative learning processes in decision-making throughout the addiction process, but differ in specific mechanisms underlying and maintaining addiction.
3.1.1 Learning or Habit Theories
The commonality of action of drugs of abuse on DA has led to the widely held view that the mesolimbic DA system has a general role in the reinforcing effects of drugs, perhaps stemming from a more general role in mediating natural aspects of reward. From the late 1980s throughout the 1990s, following research that linked the striatum’s role in associative learning and memory with addictive behavior, Norman White began developing perhaps the earliest form of contemporary learning and habit theories with the hypothesis that addiction was a disorder of normal learning and mnemonic processes (White, 1989, 1996). The importance of associative learning mechanisms for human addiction was further gleaned from the observation that much drug-taking, including relapses after periods of abstinence, follows exposure to cues previously associated with drug use (O’Brien, Childress, Ehrman, & Robbins, 1998; Tiffany, 1990; Wikler & Pescor, 1967). Anthony Dickinson then developed the formal associative learning account based on research with non-drug rewards (Dickinson, 1985; Dickinson & Balleine, 1994) that was later translated into behavioral neuroscience research with addictive drugs with Trevor Robbins and Barry Everitt (Everitt, Dickinson, & Robbins, 2001; cf. Everitt & Robbins, 2005). Hyman, Malenka, and Nestler (2006), Everitt et al. (2008), and most recently Hogarth et al. (2013) have extended and built upon these ideas, forming overlapping learning and habit theories of addiction. Due to their many similarities, these theories will be discussed together below; differences will be explicitly noted as applies.
These learning and habit theories argue that drug addiction is a synthesis of multiple controllers. These theories hypothesize the following key concepts. First, drug addiction can be viewed as the endpoint of a series of transitions from initial voluntary drug use to loss of control over drug-seeking and -taking. Second, addiction can be understood in terms of the processes and mechanisms of normal learning and memory brain systems. As discussed in detail by Dickinson and Balleine (1994), these theories distinguish between Pavlovian and instrumental learning processes underlying drug addiction. Pavlovian learning processes are sensitive to the contingency between stimuli and reinforcers or outcomes whereas instrumental processes are sensitive to the contingencies between actions or responses and outcomes. Goal-directed learning involves learning the contingency between the drug-seeking response and the drug’s outcome or value. These processes can then be contrasted with a second instrumental process in which drug-seeking is a simple response habit elicited by environmental and drug-associated stimuli; this is known as habit learning. These learning and habit theories then argue that drug-seeking is initiated under the control of goal-directed processes, but with the onset of addiction become compulsive under the control of habit processes, even while the subjective effects of the drug are reduced compared to the initial drug effects. Hogarth et al. (2013) elaborated on this notion, arguing that continuing drug exposure impairs retrieval or utilization of knowledge of specific outcomes and, thus, control of action by specific outcomes or contingencies, resulting in the more general control of behavior by antecedent stimuli. These learning and habit theories also acknowledge the important role of incentive-sensitization in drug addiction and are, at a fundamental level, consistent with Robinson and Berridge’s (1993) incentive-sensitization theory (discussed below) including similar principles but different underlying mechanisms.
Each of these theories also acknowledges the importance of DA brain systems to learning and memory processes and addiction, in particular the PFC and the striatum. When drugs are chronically self-administered, these DA-heavy, learning and memory systems are hypothesized to be pathologically subverted (Everitt et al., 2008, 2001; Everitt & Robbins, 2005), or usurped (Hyman, 2005; Hyman et al., 2006), thereby leading to the establishment of compulsive drug-seeking habits through the associative mechanisms discussed above, strengthened by the motivational capacity of drug-associated stimuli, and occurring at the expense of other sources of reinforcement. Hyman (2005; cf. Hyman et al., 2006) and Everitt and Robbins (cf. Everitt et al., 2008; 2005) both hypothesize that the change from voluntary drug use (goal-directed processes) to more habitual and compulsive drug use (habit learning processes) represents a transition at the neural level from prefrontal cortical to striatal control over drug-seeking and drug-taking as well as a progression from ventral DA action to more dorsal domains of the striatum. Further, these neurobehavioral transitions themselves are said to depend on the neuroplasticity in both cortical and striatal structures that is induced by chronic self-administration of drugs.
In support of the learning and habit theories, drug-associated stimuli can induce subjective states of craving (Childress, McElgin, Mozley, & O’Brien, 1999), as well as drug-seeking and increase susceptibility to relapse after abstinence (Sinha & Li, 2007). The outcome-devaluation procedure (see Dickinson, 1985) has demonstrated goal-directed control of drug-seeking in both humans responding for tobacco (Hogarth, 2012; Hogarth & Chase, 2011) and non-humans responding for cocaine (Olmstead, Lafond, Everitt, & Dickinson, 2001) and heroin (Hutcheson, Everitt, Robbins, & Dickinson, 2001). The outcome-devaluation procedure has also demonstrated habit-controlled drug-seeking by measuring the insensitivity of responding to drug devaluation during extinction in rats responding for alcohol (Dickinson, Wood, & Smith, 2002) and cocaine (Miles, Everitt, & Dickinson, 2003).
At the neurobiological level, the hypothesis that the progressive engagement of dorsal striatal mechanisms underlies the transition from drug use to addiction is supported by in vivo microdialysis studies illustrating increased DA release selectively in the nucleus accumbens core (ventral striatum) during early periods of drug-seeking, but during prolonged periods of drug-seeking, DA release is elevated only in the dorsal striatum (Ito, Dalley, Howes, Robbins, & Everitt, 2000; Ito, Dalley, Robbins, & Everitt, 2002). Further, DA release in the dorsal striatum has also been shown to aid in the maintenance of drug seeking, as cocaine seeking was greatly reduced by DA receptor blockade at this site, but this treatment had no effect when infused into the nucleus accumbens core (Vanderschuren, Di Ciano, & Everitt, 2005). At the cellular level, the downregulation of striatal DA D2 receptors is a reliable marker of chronic cocaine (Goldstein & Volkow, 2002) and alcohol (Heinz et al., 2004) abuse in humans, with the latter study also demonstrating a negative correlation between D2-like receptor availability and alcohol craving severity and cue-induced activation of frontal brain regions. Moreover, the presentation of drug cues to human cocaine addicts induces drug craving which has been shown to be correlated with activation of the dorsal striatum (Garavan et al., 2000; Volkow et al., 2006).
In summary, the most recent learning and habit theories of addiction posit that drug addiction can be understood in terms of normal learning and memory neuronal systems which, through chronically self-administered drug actions, are pathologically subverted, thereby leading to the establishment of compulsive drug-seeking habits. These habits are strengthened, through Pavlovian and operant mechanisms, by the motivational capacity of drug-associated stimuli, but, importantly, these drug-seeking habits occur at the expense of other sources of reinforcement.
3.1.2 Incentive-Sensitization Theory
The central theme of incentive-sensitization theory, introduced in its earliest form by Robinson and Berridge (Robinson & Berridge, 1993) and refined in the years since (Berridge & Robinson, 2016; Robinson & Berridge, 2008), was that repeated administration of potentially addictive drugs can persistently change brain circuits that normally regulate the attribution of incentive salience to stimuli, a neurobehavioral process that imbues perception of stimuli with salience, making them attractive and “wanted,” “incentive” stimuli. The resulting neuroadaptations caused by repeated drug use render the brain circuits hypersensitive, resulting in a pathological enhancement in the incentive salience the nervous system attributes to the act of drug taking. The co-activation of associative learning mechanisms directs the focus of this neurobehavioral system to specific targets that are associated with drugs and pathological focus of incentive salience on drug-related stimuli.
Thus, the key concepts of the theory are that stimuli acquire incentive properties by being associatively paired with a drug-related reward, and these conditioned stimuli that have been imbued with incentive salience have three fundamental characteristics: (1) They can elicit approach towards them (become ‘wanted’), acting as a motivational magnets (Harmer & Phillips, 1998); (2) they energize ongoing actions (e.g., drug-related responding) by eliciting cue-triggered wanting for their associated unconditional drug rewards (Wyvell & Berridge, 2001); and (3) they can act as reinforcers on their own, such as strengthening the acquisition of a new instrumental response (Di Ciano, 2008). Although these characteristics of conditioned stimuli are entirely consistent with and in fact a result of the associative learning mechanisms described in the learning and habit theories above, in this perspective of incentive-sensitization theory, while these associative learning mechanisms specify the object of desire (e.g., drug), drug-related learning per se is not enough for pathological motivation to take drugs. Whereas the learning and habit theories posit that associative learning mechanisms underlie and drive the transition from drug abuse to maintained addiction, incentive-sensitization theory hypothesizes that the repeated use of addictive drugs hypersensitizes DA and related systems to drugs and drug-associated stimuli. While the sensitization is gated and guided by associative learning mechanisms, these are considered secondary in the addiction process to the motivational component of elevated incentive-sensitization; that is, stimulus-response habits are considered solely a symptom of addiction, but not the origin. An additional motivational component must also be involved because stimulus-response learning habits are not modulated by motivational factors (but see Hogarth et al., 2013; Robinson & Berridge, 2008). However, incentive sensitization theory suggests that such habits do contribute to the automatized behavior and rituals involved in consuming drugs once obtained (Robinson & Berridge, 2004).
In recent years, incentive sensitization theory has expanded its key concepts to include neurobiological mechanisms, positing the following: addictive drugs (1) enhance mesotelencephalic DA signaling, which, in turn, (2) affects decision making by increasing the incentive salience of, and preference for, events correlated with drug-taking, (3) when repeatedly used incrementally sensitizes the mesotelencephalic DA system to drugs and drug-related stimuli in susceptible individuals. Finally, the processes associated with drug “wanting” (i.e., incentive salience) and drug “liking” (i.e., subjective euphoric drug effects) can be independent. Therefore, sensitization of incentive salience to drug-related stimuli may support continued drug taking even if the subjective pleasurable effects of the drug are diminished (see also Everitt & Robbins, 2005).
In support of this theory, prior exposure to drugs of abuse enhances rewarding drug-effects (Robinson & Berridge, 2000, 2003). In non-humans, sensitization facilitates drug self-administration, drug-conditioned place preferences, and motivation to exert effort or work for drugs (e.g., Vezina, 2004; Ward, Läck, Morgan, & Roberts, 2006). Behavioral sensitization increases neural activations in brain systems that mediate the incentive value of the reinforcing stimulus. For example, amphetamine sensitization in rats increases specific firing patterns of DA mesolimbic structures (e.g., striatum) that code the incentive salience of reward-conditioned stimuli (Tindell, Berridge, Zhang, Peciña, & Aldridge, 2005). Similarly, in humans, repeated amphetamine administration increases DA release with subsequent doses, even after a year since last dose (Boileau et al., 2006), and drug-related stimuli elicit robust DA responses in the same brain reward systems discussed above (Boileau et al., 2007; Childress et al., 2008). These results support the theory’s most recent, fundamental premise that addiction is caused primarily by drug-induced sensitization in brain mesocorticolimbic DA systems responsible for mediating Pavlovian conditioned incentive motivational processes (i.e., attributing salience), thereby invigorating drug-related motivation (i.e., wanting) and drug-reinforced responding (Berridge & Robinson, 2016).
3.1.3 Dopamine Imbalance Theory
Based largely on imaging evidence, Volkow and Baler (see also Koob & Volkow, 2016; Volkow & Baler, 2014; Volkow et al., 2016; Volkow, Wang, Fowler, & Tomasi, 2012; 2011) recently posited a DA-related theory that, in its earliest form, was based on the premise that the capacity to inhibit drug use during craving and withdrawal requires interaction of six neuronal circuits. The key concepts of this theory are that the six neuronal circuits exert top-down control to contest conditioned responses preceding drug taking. The DA imbalance model of addiction suggests that addiction affects (1) the DA reward/salience circuitry, as well as circuits directing (2) motivation, (3) memory, learning-conditioning, and habits, (4) inhibitory control and executive function, (5) interoception, and (6) aversion avoidance and stress reactivity (Volkow & Baler, 2014; Volkow et al., 2016). These circuits are directly innervated by DA neurons, and are also connected with one another through direct and indirect excitatory glutamatergic, inhibitory GABAergic, and modulatory monoaminergic inputs. Their concerted actions broadly impact behavior, which aids in explaining the multidimensional nature and robustness of addiction. We note that this theory is primarily neurobiological with decision making discussed more aptly as a secondary, downstream process linked heavily to DA.
As mentioned above, DA is clearly linked to the prediction of drug reward and assessing saliency. All drugs of abuse increase DA levels in the NAc, through various molecular targets depending on drug class (Koob, 1992). In support of this theory, Positron Emission Tomography (PET) studies in humans have illustrated that stimulants (Drevets et al., 2001; Volkow et al., 1994, 1999), nicotine (Brody et al., 2009), alcohol (Boileau et al., 2003), and marijuana (Bossong et al., 2009) stimulate DA release in the dorsal and ventral striatum. Furthermore, drug-induced DA increases in the striatum are proportional to the intensity of self-reported euphoria (Volkow, Fowler, Wang, Baler, & Telang, 2009). The finding that even reward-predicting stimuli induce quick and large DA increases illustrates that through the conditioning process, innocuous stimuli correlated with drug reinforcers acquire the ability to increase striatal DA on their own (Schultz et al., 1997).
In the striatum, DA D2 receptors mediate neurotransmission in the “indirect pathway” that moderate PFC regions; therefore, dysregulation of DA D2 receptor functioning and signaling could alter PFC activity and impair inhibitory control (for a review, see Volkow & Fowler, 2000). Indeed, further support for this theory lies in the significant reductions in DA D2 receptor availability observed in the striatum of individuals with addiction, and these reductions persist for months following abstinence in humans (Volkow et al., 2009) and non-humans (Nader et al., 2006; Thanos, Michaelides, Benveniste, Wang, & Volkow, 2007; Volkow et al., 2001). This reduction in striatal DA D2 receptors is associated with decreased baseline glucose metabolism in the dorsolateral PFC (dlPFC), orbitofrontal cortex (OFC), and the anterior cingulate cortex (ACC), indicating decreased PFC activity overall (Volkow et al., 1993, 2001, 2007). This research suggests that D2 receptor-mediated DA signaling produces dysregulation in these areas and may underlie the elevated incentive value of drugs and loss of control over drug intake in those with addiction (Volkow & Fowler, 2000). Moreover, DA acts in circuits modulating motivational control over goal directed behavior within the NAc, dlPFC, OFC, ACC, amygdala, dorsal striatum, and ventral pallidum (Salamone, Correa, Farrar, & Mingote, 2007), leading to consequent changes in decision making processes.
The DA imbalance theory also recognizes the role of the insular cortex in addiction, however, the insula’s role in mediating interoceptive drug signals will be discussed below in detail in the triadic neurocognitive theory of addiction (Section 3.4.2). Similarly, the DA imbalance theory notes that avoidance and stress reactivity are integral to addiction, but because these processes are primary components of Koob and Le Moal’s opponent processes theory (Section 3.2.1) they will not be further discussed here.
In summary, the most recent iteration of the DA imbalance theory is primarily a neurobiological theory of addiction that depends on the activation of and interaction between six DA-innervated neuronal circuits. When the circuits are in balance, normal decision-making and proper inhibitory control result; during addiction, the value of the drug in reward, motivational, and mnemonic systems overcomes the inhibitory control circuit, resulting in a positive feedback-loop beginning with the consumption of the drug and perpetuated by enhanced activation of the motivational/drive and memory circuits.
3.1.3 Benchmark Evaluation
The DA-related decision theories described above have contributed to a number of domains related to decision making and addiction. Further, these DA-related theories have laid a solid foundation for future research aimed at understanding the mechanisms underlying decision making in addiction. Here, we evaluate the learning and habit theories, incentive-sensitization theory, and the dopamine imbalance theory according to their ability to answer the questions posed in Section 2 of this manuscript (see Table 1). We organize this section to examine the benchmarks explained by (1) all theories, (2) mixed evidence/explanations or two or fewer theories, and (3) none of the theories.
All DA-related theories provided comprehensive explanations of Benchmark 1, “why are certain commodities addictive when others are not?”, and account for Benchmark 3, “why do some individuals, as their valuation of drugs increases, also exhibit a decrease in valuation of non-addictive commodities?”. Moreover, each of these theories generally account for these phenomena by similar mechanisms. In all of these theories, commodities are posited to be addictive because of their reinforcing properties, which depend on the stimulation and activation of the mesolimbic DA system, in particular the nucleus accumbens and striatum. The learning and habit theories, incentive-sensitization theory, and the DA imbalance theory acknowledge that drug-associated stimuli can become valued in their own right through mesolimbic DA signaling. Further, in relation to Benchmark 3, these theories posit that drug abuse causes overstimulation of DAergic signalling which renders DA circuits insensitive to non-drug reinforcers; these theories also recognize that the excessive positive reinforcement and incentive value that comes to be attributed to drugs and drug-related stimuli causes a greater proportion of behavior to be allocated to drugs, leaving less opportunity for non-addictive commodities to be attributed reinforcing value.
All of the DA-related theories of addiction also provide similar explanations of Benchmark 2, “why does addiction follow common developmental trends?”. These theories suggest that during certain developmental stages, especially the transition from adolescence to early adulthood, structural and functional imbalances in DAergic systems in brain regions critical for decision making (e.g., PFC & NAc) leave adolescents and young adults vulnerable to maladaptive behavior (e.g., Doremus-Fitzwater, Varlinskaya, & Spear, 2010; Everitt et al., 2008; Spear, 2013). In addition, these theories offer hypotheses concerning why only certain individuals become and remain addicted when so many have used drugs at some point in their life. In particular, they suggest that certain individuals are susceptible because of either baseline differences in DA reward system function, drug-induced neurobehavioral adaptations, and/or environmental influences over associative drug learning although the precise nature of these differences are not fully explicated. That is, development of addiction could be related to abnormal baseline functioning of DA-related neurobehavioral signaling that is exacerbated by drug exposure in a conducive environment.
All DA theories provide mixed evidence or less than complete explanations (see Table 1 for details) for Benchmark 4, “why do individuals with addiction engage in consistent self-defeating patterns of behavior?”. Incentive-sensitization theory and the DA imbalance theory each hypothesize that dysregulated DAergic signaling in motivational circuits (e.g., NAc) enhances drug-seeking motivation, which is mediated by sensitization of incentive salience. Indeed, the DA imbalance theory invokes incentive-sensitization as the mechanism for enhanced motivation to obtain drugs (see Volkow et al., 2012, pp. 330–331). Thus, in each of these DA-related theories such dysregulation is inferred to explain why individuals addicted to drugs decide to procure drugs even in the face of known adverse consequences. The learning and habit theories explain the consistent self-defeating patterns of behavior seen in addiction by appealing to the changes in associative learning mechanisms purported to underlie the drug abuse and dependence. That is, the transition from goal-directed behavior to habitual responding means that unhealthy or self-defeating patterns of behavior become entrenched and semi-automated due to the repeated and extended patterns of reinforcement.
An important point of departure between the DA-related theories is in relation to Benchmark 6, “what interventions have been or are derivable from the tenets of these theories?”. Incentive-sensitization theory does not explicitly offer any potential treatments (i.e., Benchmark 6), although a treatment such as cue exposure is a consequence of the basic tenets of this theory. However, the learning and habit theories have discussed several implications for treatment strategies. Hogarth et al. (2013) argue that if addiction reflects a progressive weakening of the role of drug outcome retrieval/utilization (i.e., goal-directed learning) in the execution of behavior, allowing habitual drug-related action to dominate, treatments such as expectancy challenge (B. T. Jones & Young, 2011) and extinction training (Bouton, 2002) may not prove adequate. Instead, the authors argue that treatments that enhance the capacity to engage in prospective thinking, such as working memory training (Bickel, Yi, Landes, Hill, & Baxter, 2011) combined with facilitation of alternative reinforcement contingencies (Iguchi, Belding, Morral, Lamb, & Husband, 1997), may be more efficacious in redirecting addicts from their entrenched habitual responding. Additionally, because goal-directed control has been shown to be reinstated by manipulations of brain function (Corbit, Nie, & Janak, 2012; Killcross & Coutureau, 2003; Zapata, Minney, & Shippenberg, 2010) and by uncertainty, which has definable neural substrates (Daw, Niv, & Dayan, 2005), neuropsychopharmacological methods could complement such associative learning approaches to provoke new action patterns. Similarly, the DA imbalance model of addiction suggests that potential treatments lie in the identification of neurotransmitter receptors and transporters involved in the processes of drug reward and neuroplasticity in addiction. This expands the list of molecular targets that have potential for the development of medications to combat addiction (Volkow & Skolnick, 2012). This theory also points to the expanding field of pharmacogenomics to provide an understanding of individual differences in response to existing treatments. Polymorphisms of certain genes that regulate DA functioning have been identified in a number of reward- and withdrawal-related functions, such as OPRM1 and CYP2A6, which have been shown to predict clinical responses to existing treatments, such as naltrexone in alcoholism (Ooteman et al., 2009) and nicotine replacement therapy in smoking (Malaiyandi et al., 2006). These genes, or others, could be potential, independent, therapeutic targets in addiction.
Finally, the benchmark that all DA-related theories fail to explain is Benchmark 5, “why are individuals with addiction also likely to engage in other unhealthy behaviors?”. These theories are certainly well-developed enough to offer hypotheses related to this benchmark, but they do not presently explain the co-occurence of addiction with other behavioral maladies.
3.2 The Dark Side of Addiction: Withdrawal, Negative Reinforcement, and Opponent Process Theories
Drug addiction has been conceptualized as a disorder involving elements of both impulsivity and compulsivity. This yields a cycle of addiction with three stages: preoccupation/anticipation, binge/intoxication, and withdrawal/negative affect (Koob & Le Moal, 2001, 2005, 2008a). Within this cycle, multiple sources of positive and negative reinforcement contribute to impulsive and compulsive drug use (Everitt & Robbins, 2005). The above DA-related theories emphasized the positive reinforcing effects of drugs. Indeed, impulsivity often dominates recursively during the earlier stages of this cycle (i.e., binge/intoxication) and, more broadly, in the course of addiction. However, compulsivity dominates in terminal stages (i.e., withdrawal/negative affect). As behavior shifts from impulsivity to compulsivity, the motivation for drug-related behavior also shifts from positive to negative reinforcement. The present section focuses on the most prominent theory of addiction often referred to as the “dark side”; that is the development of the withdrawal and aversive states that drives drug use to decrease those aversive states (i.e., negative reinforcement as stated in Koob & Le Moal, 2001, 2005).
3.2.1 Opponent Process Theories
The earliest form of theories concerning the dark side of addiction postulated that this state results from two competing motivational processes, first identified by Solomon and Corbit (Solomon & Corbit, 1974) and extended by Koob and Le Moal (2001, 2005, 2008a). This original opponent process theory stated that the a-process or positive hedonic response occurs during the initial acute effects of a drug and is opposed by the b-process, or homeostatic changes in brain circuits that function to dampen this positive response. The a-process occurs rapidly after the presentation of the drug, shows tolerance, and is closely aligned with its quality, intensity, and duration. As the a-process terminates, the b-process begins and is characterized by slow onset and decay with increases in magnitude following repeated exposure. Dependence and tolerance are inextricably linked in this theory. Indeed, following chronic exposure, hedonic effects of the drug decrease (a-process) and aversive states increase (b-process), producing the negative reinforcing processes in addiction (i.e., removal of b-process or aversive state) that drive compulsive drug consumption.
In more recent years, the opponent process theory has been expanded to explain the neurobehavioral decision processes of drug addiction from a neurocircuitry perspective. Based on their prior work, Koob and Le Moal (2008a) described an allostatic model of brain motivational systems to explain the persistent, drug-dependent motivational changes seen in addiction. Moreover, Koob and Mason (2016) recently extended this model to account for existing drug treatments and also proposed future drugs for the treatment of the dark side of addiction. In this model, influenced heavily by McEwen (2000), the key concepts begin with allostasis, the process of achieving homeostasis (e.g., stability) through change; allostatic state is the relatively permanent state of deviation of brain regulatory systems from their homeostatic (normal) operating levels brought about by chronic drug abuse; and allostatic load is the accumulating damage to particular brain systems over time that results from this chronic deviation. Thus, Koob and Le Moal argue that viewing addiction as simple homeostatic imbalance is insufficient. Specifically, because drug addiction is heavily influenced by environmental circumstances and leaves an abiding neuroadaptive vestige, it creates conditions that support relapse even after long periods of drug abstinence. Such characteristics of addiction imply an aggressive and chronic departure from homeostasis of brain systems (i.e., an allostatic state) that causes accumulating dysregulation over time, thus reflecting states of pathology and accumulation of damage (i.e., allostatic load; see also McEwen, 2000).
In this framework, addiction is a cycle of increasingly dysregulated brain reward and antireward systems driving compulsive drug use. The brain reward system is exemplified by activation of networks (including DA and opioid peptide neurons located in the VTA and NAc) mediating positive reinforcement with a veneer of positive hedonic valence (i.e., the a-process). In the antireward system, neuroadaptations counter this excessive activation (i.e., the b-process). These counteradaptive, allostatic b-processes can be mediated by within-system and/or between-system neuroadaptations. Within-system neuroadaptations comprise molecular or cellular changes within a singular reward system that abet over-activation of the a-process, causing decreased reward functioning with an overlay of negative hedonic valence. For example, mesolimbic DA and opioid peptide function decreases during withdrawal after chronic drug exposure, providing support for these concepts and dark side theories (Koob & Le Moal, 2008a).
In contrast, a between-system neuroadaptation is an opposing change in circuitry defined when one brain system is activated by excessive recruitment of another. Notably, much evidence supports that brain stress and emotional circuits are recruited following chronic activation of the brain reward system, therefore providing an additional variety of b-processes and source of negative hedonic valence (Koob & Le Moal, 2008a). Accordingly, this additional source of negative hedonic valence results from a major between-system neuroadaptation that defines the theory’s antireward system: during chronic drug use, brain stress or antireward circuits (e.g., the hypothalamic-pituitary-adrenal (HPA) axis) are recruited to oppose the inordinate activation of a-processes in the brain reward system. For instance, as dependence and withdrawal develop, corticotropin-releasing factor (CRF), noradrenaline (NA) and NAergic neurons are recruited in the central nucleus of the amygdala, while dynorphin is recruited in the extended amygdala, each producing aversive states marked by chronic irritability, emotional pain, anhedonia, dysphoria, and loss of motivation for unconditional rewards (Koob, 2003; Nestler, 2001). Concurrently, decreases in DA and opioid peptide neurotransmission within the ventral striatum-extended amygdala result in decreased reward system function. Thus, the most recent research by Koob and colleagues regarding the dark side of addiction indicate that the combination of decreases in reward system function and recruitment of antireward systems drives the compulsive drug-related behavior to diminish the aversive states seen in the latter stages of addiction.
3.2.2 Benchmark Evaluation
Although many overlaps exist, the opponent process theory provides a different perspective of decision making processes in addiction than the DA-related theories discussed above, specifically by focusing on the dark side of addiction (e.g., negative reinforcement and withdrawal). Below, we evaluate this theory in relation to our benchmark criteria.
As with the DA-related theories of addiction, the opponent process theory thoroughly explains Benchmark 1, “why are some commodities addictive when others are not?” and Benchmark 3, “why do some individuals, as their valuation of drugs increases, also exhibit a decrease in valuation of non-addictive commodities?”. In contrast, the opponent process theory also provides detailed accounts for Benchmark 4, “why do individuals with addiction engage in consistent self-defeating patterns of behavior?”, and Benchmark 6, “what interventions have been or are derivable from the tenets of these theories?”.
First, the opponent process theory postulates that the addictiveness of certain commodities depends on both their properties of positive reinforcement (a-process) and negative reinforcement (b-process). As in the DA-related theories, the positive reinforcing properties of addictive commodities depend on the stimulation and activation of the mesolimbic DA system. In turn, the negative reinforcing properties of addictive commodities are hypothesized to be driven by these positive reinforcing effects. As discussed above, the combination of decreased brain reward system function and recruitment of antireward systems that progressively worsen with chronic drug abuse creates a powerful source of negative reinforcement that drives impulsive and compulsive drug-taking during the addiction cycle (Benchmark 1). Concurrently, because these within- and between-system neuroadaptations repeatedly drive the allostatic state, possibly resulting in allostatic load, non-drug rewards have a blunted rewarding effect on the organism, which may ultimately cause anhedonia (Benchmark 3). Consequently, the explanations regarding why dependent individuals consistently engage in patterns of self-defeating behavior are the same as those responsible for the cyclical nature of addiction discussed previously. That is, self-defeating behavior occurs to escape or avoid the negative emotional and affective states and withdrawal resulting from the allostatic, within- and between-systems neuroadaptations induced by chronic drug use (Benchmark 4).
Due to the heavy focus on within- and between-systems neuroadaptations and negative reinforcement as driving forces in addiction, Koob and Le Moal propose that the best treatment options for addiction lie in the neurobiological arena (i.e., Benchmark 6). Indeed, Koob and Mason (2016) provided a comprehensive overview of existing and future drug treatment options that focus on the negative reinforcing aspects of drug addiction and, importantly, these treatment options are still contained within the opponent process theory. First, they note the importance of managing withdrawal effects during acute and protracted abstinence, including the effects of stress signalling in the antireward system. Some antagonists of this system have reduced alcohol consumption in a double-blind, placebo-control trial (Mason, Goodman, Chabac, & Lehert, 2006). Second, they suggest the translation of non-human findings that CRF and dynorphin opioid receptor antagonists targeting the extended amygdala decrease self-administration of heroin (Greenwell et al., 2009), alcohol (Bruijnzeel, Small, Pasek, & Yamada, 2010), and nicotine (Bruijnzeel, Zislis, Wilson, & Gold, 2007). Koob and Mason (2016) describe several other drug classes and targeted brain regions that may be clinically relevant for treating addiction; for example, antagonizing glutamatergic receptors in brain stress systems to reduce alcohol consumption (e.g., Mason et al., 2006), inhibiting endocannabinoid clearance in brain stress systems to reduce the aversive states associated with dependence and withdrawal (e.g., Serrano & Parsons, 2011), and identifying genetic markers associated with drug-induced neuroadaptations to account for individual differences in drug treatment (e.g., Treutlein et al., 2006).
The opponent process theory provides some explanations for the developmental trends observed in addiction, Benchmark 2, “why does addiction follow common developmental trends?”, and co-occurrence of other unhealthy behaviors, Benchmark 5, “why are individuals with addiction also likely to engage in other unhealthy behaviors?”. First, concerning Benchmark 2, the authors appeal to the adolescent neuroadaptations, in particular those related to stress axis dysregulation, that result from exposure to drugs or other environmental stressors. Specifically, Koob and Le Moal (2008a) note that because drugs of abuse activate the hypothalamus response to stress and engage brain stress systems, brain and brain pituitary stress systems have a role in the initial vulnerability to drug abuse, the development of dependence, and the vulnerability to stress-induced relapse during the fragile period of adolescence. Further, the stress-induced propensity towards drug-taking in adolescence is linked to increased activation of mesolimbic DAergic transmission mediated by stress hormone release (e.g., glucocorticoids). Thus, these authors specify that adolescent drug use is, in part, a result of antireward system sensitization, but acknowledge research suggesting that vulnerability to addiction also involves other elements, including comorbidity with psychopathological conditions, genetic and epigenetic mechanisms, and developmental factors that may predispose some individuals, but not others, to proceed from drug-taking to addiction (e.g., Miller-Day, Alberts, Hecht, Trost, & Krizek, 2014).
Regarding Benchmark 5, Koob and Le Moal (2008b) hypothesize that concurrent unhealthy behavioral patterns are prominent factors in vulnerability and overlap with the ‘dark side’ neuroadaptations. More specifically, they invoke the self-medication hypothesis in which individuals take drugs as a means to cope with painful and threatening emotions in attempt to alleviate or medicate the dysregulation of certain affective states, painful affect, or inability to express personal feeling (Koob & Le Moal, 2008a). This explanation alone, however, does not completely meet our criteria for Benchmark 5 (see Table 1).
3.3: Strength models of self control failure
The self control failure model posits that our capacity for self control is a limited resource that is exhaustible (Baumeister, Heatherton, & Tice, 1994), leading to short-sighted decisions and impulsive actions. Moreover, when self control breaks down, it is related to criminal and impulsive behaviors such as illegal activity, violence, risky sex, and overuse of alcohol, cigarettes, and drugs (Baumeister & Vonasch, 2015; Gottfredson & Hirschi, 1990).
Here we describe two related sub-types of self control failure: the depletion model and emotion regulation. Although the theory of self control failure acts as an umbrella for two subtypes, their differences are worth comparing. Therefore, we treat the two sub-types as separate entities against each benchmark. Note, the literature has suggested that other executive dysfunctions (e.g., response disinhibition, attentional deficits, poor planning, and devaluing the future) are strongly associated with impulsive behaviors and, indeed, drug addiction (see Bickel, Jarmolowicz, Mueller, Gatchalian, & McClure, 2012; Bickel, Koffarnus, Moody, & Wilson, 2014; MacKillop et al., 2011 for reviews and meta-analysis). Although these patterns of executive function deficits create distinct hypotheses, they are not independent theories, and we do not review them as such. However, we acknowledge their contributions to the field, including their suggestions for targeting the executive system to treat drug addiction (Bickel, Koffarnus, et al., 2014).
3.3.1 Depletion model
In the depletion model’s earliest form, it proposed that individual self control is a limited resource that acts like a muscle (Baumeister, Bratslavsky, Muraven, & Tice, 1998). Given that some level of self control is necessary to resist drugs and alcohol, if an individual has adequate self control “muscle” strength they should exhibit more adaptable behaviors and be less tempted by drug and alcohol use (Baumeister & Vohs, 2012; Baumeister & Vonasch, 2015). However, an individual’s self control strength is not constant over time, or even over the course of a day. Consistent with the muscle analogy, and the key concept of this theory, using self-control in certain conditions has been demonstrated to cause temporary impairments (i.e., depletion) in the performance of future tasks (Baumeister, 2002; Baumeister, Vohs, & Tice, 2007). For example, individuals who expended self-control by forcing themselves to eat radishes, or suppress an attitude or emotion, demonstrated abbreviated persistence and/or diminished performance on a second unrelated task such as a puzzle or solving an unsolvable algorithm (Baumeister et al., 1998). Moreover, the depletion effect has been generalized to drug administration. Male social drinkers who engaged in a depletion task that required them to suppress a particular thought (i.e., thinking of a white bear) were then invited to drink beer. Participants who were asked to suppress their thoughts of the white bear (depleted group) consumed more beer and achieved higher blood alcohol content levels compared to the non-depleted participants (Muraven, Collins, & Nienhaus, 2002). Additionally, daily smokers who completed a depletion task exhibited 1) decreased latency to smoke a cigarette during a smoking cessation task compared to non-depleted smokers, and 2) increased craving for a cigarette over and above the effects of 12-hour deprivation on craving (Heckman et al., 2017)
The conditions that deplete self control are wide ranging. In fact, a meta-analysis found significant effects of depletion on task performance irrespective of task type or depleting condition (Hagger, Wood, Stiff, & Chatzisarantis, 2010). Interestingly, this analysis also demonstrated that depleting task duration, task complexity, and interim period between tasks all moderated the effect of depletion, all in support of the strength model of self control. These findings also support the idea that outside of the laboratory, depletion can be any form of stress. For example, and in support of the theory, Baumeister (1996) reviewed that during stressful conditions individuals are more likely to break diets, overeat, abuse drugs and alcohol, and smoke cigarettes. Moreover, risky sex, violent crimes, heavy drinking, and broken diets often occur late at night (Baumeister & Heatherton, 1996), indicative of depletion over the course of the day.
From the original model of this theory, combatting self control failure and depletion can be achieved a few ways. In addition to resting to replenish self control (Baumeister, 2002), the meta-analysis described above found that the use of glucose supplementation and self control strength training promoted better self control in depleted samples (Hagger et al., 2010). Depletion was demonstrated to decrease glucose levels, but replenishing glucose (as with a lemonade drink sweetened with sugar) reduced impairments (Gailliot et al., 2007).
Second, exercising self control as if it were a muscle is theorized to combat depletion. From this perspective self control may be trained, and over time, become more tolerant and enduring against small depletions. For example, 122 smokers were asked to practice self control by doing small tasks such as avoiding sweets or practicing with a handgrip tool for two weeks before quitting smoking. Those who practiced self control tasks were abstinent longer than a control group who completed tasks unrelated to self control, suggesting that practicing was a product of building self control strength (Muraven, 2010).
Functional MRI analyses support both the depletion model and emotion regulation (also described below) sub-types of the self control failure theory similarly. That is, an inverse relationship exists between brain regions associated with self control and immediate reward. While more evidence of this neurobiological mechanism is present for emotion regulation, a few studies have demonstrated similar patterns following tasks of depletion (Berkman & Miller-Ziegler, 2013). The first example of this effect was demonstrated when individuals, depleted from engaging in an attentional inhibition task, exhibited increased left amygdalar activation (i.e., greater emotion) and concurrent decreased activation between the amygdala and the ventro-medial prefrontal cortex (i.e., self control strength) while viewing negatively emotional scenes, compared to a non-depleted group (Wagner & Heatherton, 2013). A second study by the same group demonstrated similar effects in depleted chronic dieters, wherein viewing images of food during a cue-reactivity task increased activation of the orbitofrontal cortex (a region associated with brain reward value), and decreased activation in the inferior frontal gyrus (a region associated with self control; Wagner, Altman, Boswell, Kelley, & Heatherton, 2013). The results from both of these studies, although not performed in addicted populations, suggest that depletion may be associated with increased activation in brain reward and decreased “top down” self control activation, both consistent with the observable behavior of self control failure.
Although self control failure was originally widely adopted, the depletion model has recently been challenged by others failing to replicate its effect. For example, a 23 laboratory multi-site study that conducted the standard depletion task to examine the replicability of the depletion effect, concluded that the depletion effect is near zero (Hagger & Chatzisarantis, 2016). These findings support several other recent meta-analyses which reported that the original meta-analysis (Hagger et al., 2010) over-estimated the depletion effect. For example, Carter and McCullough (2014) found that the depletion effect was largely due to a publication bias of large effects from small sample sizes (i.e., small study bias). Moreover, in a follow up meta-analysis, these authors indicated that when controlling for small study bias, a small depletion effect was reduced to nonsignificance (E. C. Carter, Kofler, Forster, & McCullough, 2015). In addition, specific challenges to the model were raised when Job et. al. (2010) demonstrated that the way in which an individual interprets “willpower” (i.e., as exhaustible or not) influences whether they exhibit depletion in tasks such as eating behavior, procrastination, and self-regulated goal-striving. The skepticism continued with failures to replicate the depletion of glucose stores in replication studies (see Inzlicht, Schmeichel, & Macrae, 2014).
In response to these many challenges, (Baumeister & Vohs, 2016) adapted their theory in their most recent work to highlight the allocation of effort. Specifically, instead of proposing that self control is completely exhaustible, the authors suggest that self control is indeed rarely in danger of being completely exhausted (or their glucose stores completely depleted). In fact, the authors extend their muscle metaphor to saying that muscles are rarely, if ever, completely exhausted. Alternatively, they posit that individuals are aware of the energy they spend and self control may be conserved and/or allocated where needed (i.e., anticipation, motivation, etc.). Therefore, regardless of resources, the adapted theory argues that motivation is the driving force behind the allocation of self control towards a particular task. This theory has generated a considerable amount of research and the recent evidence has raised significant challenges. Only additional research will ascertain whether this theory has future utility.
3.3.2 Emotion regulation failure
Emotion regulation, by Heatherton and Baumeister (1991), in its earliest form was identical to the depletion model of self control failure, with the exception that it describes an individual’s attempt to cope with a particular situation by redirecting their emotions. That is, identical to depletion, individuals deficient in emotion coping strategies who attempt to effortfully suppress their emotions demonstrate impaired performance on secondary tasks (Baumeister et al., 1998; Muraven, Tice, & Baumeister, 1998). However, this perspective also describes the key concepts in which individuals engage in short term reinforcers such as eating fattening food, taking drugs, or procrastinating in an attempt to distract them from (Baumeister & Heatherton, 1996) or restabilize (Tice, Bratslavsky, & Baumeister, 2001) their emotions. For example, Cooper et al. (1995) provided support that both adolescents and adults consumed alcohol to regulate their emotions (both positive and negative), and drinking was significantly mediated by affect coping (see also Fox, Hong, & Sinha, 2008). Moreover, when individuals with cocaine dependence were presented with emotionally stressful negative scenarios during inpatient treatment, they reported more cocaine craving with earlier rates of relapse during the post-treatment period (Sinha, Garcia, Paliwal, Kreek, & Rounsaville, 2006).
The most recent form of the emotion regulation theory has been derived from a neuro-mechanistic perspective. Functional MRI research has demonstrated that emotion regulation occurs due to the inverse relationship between the brain regions associated with self control (e.g., dlPFC) and regions associated with emotions (e.g., amygdala; see Heatherton & Wagner, 2011; Salzman & Fusi, 2010; Zilverstand, Parvaz, Moeller, & Goldstein, 2016). For example, when individuals with cocaine dependence were asked to recall stressful past experiences, concurrent BOLD-signal decreases in brain regions associated with self control, emotion regulation, or both (e.g, anterior cingulate, left hippocampal/parahippocampal region, right fusiform gyrus, and the right postcentral gyrus), and BOLD- signal increases in brain regions associated with reward (e.g., caudate and dorsal striatum) were correlated with reported cocaine craving (Sinha et al., 2005). In contrast, when participants employed “top down” strategies for regulating negative emotions, such as reappraisal, researchers found increased lateral and medial prefrontal cortex activation, and concurrent decreased activation in the amygdala and orbitofrontal cortex (Ochsner, Bunge, Gross, & Gabrieli, 2002; Ray & Zald, 2012). These results suggest that emotion regulation and dysregulation is the concurrent activation/deactivation of self control prefrontal brain regions and the activation/deactivation of emotion-associated brain regions (Zilverstand et al., 2016) reminiscent of the dual decision systems and the somatic marker hypothesis discussed in detail in Section 3.4.
3.3.3 Benchmark Evaluation
We now examine whether the statements of each of these theories thus far satisfy the benchmarks required for a well-rounded theory of addiction. Here we review each theory sub-type based only on explicit and published explanations and/or supporting evidence. However we acknowledge that the updated depletion model (Baumeister & Vohs, 2016) is a very recent revision which may not yet explicate all of the nuances of the updated theory.
First, both sub-types, depletion and emotion regulation, seem to account for Benchmark 5, “why are individuals with addiction also likely to engage in other unhealthy behaviors?”. As described above, depletion and emotion dysregulation are demonstrated to lead to a variety of impulsive behaviors including criminal behavior, risky sexual behavior, and drug use (Baumeister & Heatherton, 1996; Baumeister & Vonasch, 2015; Gottfredson & Hirschi, 1990), irrespective of addiction. Moreover, comorbid drug addiction in low socioeconomic environments or poverty are explained by these two theories. That is, poverty and/or the scarcity of resources is stressful in nature and can lead to depletion and emotional distress and subsequent drug use (Baumeister & Heatherton, 1996; Sinha et al., 2006; Spears, 2010).
Evidence for Benchmark 4, “why do individuals with addiction continue to engage in self-defeating patterns of behavior?” may be gleaned from both theory sub-types, however neither theory explicitly expounds upon it. For example, depletion was demonstrated to decrease glucose levels (Gailliot et al., 2007) which may exacerbate reductions in self control and lead to engaging in continued use (Southwick & Steele, 1987), however the mechanism by which this occurs is not detailed. Also, significantly higher emotional dysregulation was reported by recently-abstinent cocaine users, compared to controls (Fox, Axelrod, Paliwal, Sleeper, & Sinha, 2007). While this dysregulation improved slightly over time, continued emotional dysregulation after acute abstinence leaves the potential for relapse vulnerability (Fox et al., 2007).
Second, only the depletion sub-type offers several potentially therapeutic treatments and treatment components (i.e., Benchmark 6). Baumeister and Heatherton (1996) suggested that self control failure occurs because of underregulation (lack of strength or no standards) and/or misregulation (attempted regulation of things that cannot be controlled or too much emphasis on emotions). Therefore, Baumeister and Heatherton (1996) suggest that the standard for recovery from alcohol, complete abstinence as prescribed by Alcoholics Anonymous, allows individuals to allocate their self control to meet this known standard, and thus are hypothesized to be more successful in abstaining. We note here, however, that while individuals may have the self control to engage in a 12-step program such as Alcoholics Anonymous, results from NIAAA’s project MATCH indicated that outcomes following these treatments were not much better than those observed following zero treatment sessions (Cutler & Fishbain, 2005). Moreover, as described above, programs that exercise one’s self control to strengthen it over time may be potentially therapeutic. Emotion regulation offers suggestions for regulating and redirecting emotions at five distinct points of the emotion generation process: (1) selection of the situation, (2) modification of the situation, (3) direction of attention, (4) change of cognitions (i.e., reappraisal), and (5) modulation of responses (Baumeister & Vohs, 2016; Gross, 1998). However, these suggestions are conjecture and the theory does not offer specific treatment approaches directed at addiction in particular.
In contrast, emotion regulation may offer a plausible explanation for why adolescents initiate use of addictive substances (i.e., Benchmark 2; “why does addiction follow common developmental trends?”). Although the theory does not formally address adolescent initiation of use, the following could be implied from the theory sub-type’s theme. Given that drug use is theorized to be motivated by attempts to obscure negative emotions and adolescence brings with it a substantial increase in negative emotions (Larson & Asmussen, 1991), the findings that adolescents and young adults use drugs to cope with their emotions due to poor emotion regulation are tenable (Fischer, Forthun, Pidcock, & Dowd, 2007; Wong et al., 2013). Similarly, if adults are unable to develop emotion regulation, negative affect drives drug use (Brandon, 1994; Carmody, 1989; Gross & Muñoz, 1995; Khantzian, 1985) making these individuals potentially vulnerable to overuse and addiction. From the perspective of the depletion sub-type, Muraven et al. (2002) found that an individual’s trait temptation for alcohol interacted with a depletion task producing more alcohol consumption in a laboratory study. The authors suggested that this interaction offers a mechanism by which some individuals are more likely to consume alcohol when depleted. In contrast, an individual’s strength of self control in the face of depletion might also explain initiation of drug use, however in reverse sense. That is, in a more recent version of the model (Baumeister & Vonasch, 2015), the authors propose that adolescents exhibit self-control upon first use to avoid feeling “uncool” among their peers. A young teen may feel sick the first time they smoke a cigarette, but they exert self-control and try again in order to be socially accepted (Baumeister & Vonasch, 2015). This explanation also appears plausible, however, it is a departure from the original sentiment of the model.
Third, other parts of the development of addiction are not explained by either self control failure subtype. For example, maturing out of addictive behavior in later life may be a function of strengthening one’s self control and/or one’s emotion regulation to be strong enough to withstand the daily depletions and emotions present within one’s life, however this point, or its particular relevance for a given life stage, is not made in the presentation of either theory sub-type. Moreover, Benchmarks 1 “why are some commodities addictive when others are not?” and 3, “why do some individuals, as their valuation of drugs increases, also exhibit a decrease in valuation of non-addictive commodities?” are not accounted for at all in these theories. In fact, as described above, both perspectives suggest the notion that in the face of stressful or emotionally disruptive conditions, impulsive choices can and will be made towards any perceived high-value, immediately-available reward without prejudice.
3.4 Dual Decision System and Dual Decision System-Derived Theories
Dual-systems models and the dichotomy of systems into automatic and deliberative (or related terms) are not new to science or philosophy, but represent a seemingly timeless component of how humans think about their own behavior. In cognitive psychology, dual-systems models are especially prevalent, and the two systems are generally distinguished by inferred cognitive processes. Mischel and colleagues have referred to the two systems as “hot” and “cold” to highlight the common link between automatic control and “hot” emotional states and its antipode, the more “cool” rationally-based deliberation (Metcalfe & Mischel, 1999). Since that time, dual-systems theories have become more refined, both at the behavioral level and the neurobiological level. Importantly, dual-decision systems models have been developed explicitly to address addiction, and have been extended to include other systems found relevant to addictive processes (e.g., Bechara, 2005; see Figure 2). Indeed, even theories described elsewhere in the present review have sometimes included reference to dual-system control over behavior (e.g., Hogarth et al., 2013). Dual-system theories, to some extent, posit that addiction results from an imbalance or dysregulation between an automatic or impulsive system and a more deliberative or reflective system (e.g., executive functions). The functions of the impulsive-like system are generally consistent with those described above by incentive-sensitization theory, opponent-process and withdrawal theories, and the DA imbalance model, and, on the same note, the functions of the deliberative-like system are generally consistent with those mentioned above in strength models of self-control failure.
Figure 2.
Diagram of key brain structures in dual-systems models (Bechara, 2005)
CAPTION: A diagram of key brain structures comprising the impulsive system (red) and reflective/executive system (purple). Note that in this figure, the insula is identified with the executive/reflective system. However, in the competing neurobehavioral decision systems theory, the insula is identified with the impulsive system and in the triadic theory, the insula is considered a distinct component, the third system of the decisional triad. AC=anterior cingulate cortex; DA=dopamine; 5-HT=serotonin; Hip=hippocampus; A=amygdala; VMPC=ventromedial prefrontal cortex; DLPC = dorsolateral prefrontal cortex.
3.4.1 The Competing Neurobehavioral Decision Systems Theory
The competing neurobehavioral decision systems (CNDS) theory of addiction is a recent theory that, in its earliest form, was a dual-decision systems model developed within neuroeconomics that specifies the underlying neurobehavioral mechanisms and offers insights into the origin and treatment of addiction (e.g., Bickel et al., 2007; Bickel, Jarmolowicz, Mueller, Gatchalian, et al., 2012; Bickel, Snider, Quisenberry, Stein, & Hanlon, 2016; Bickel & Yi, 2008). The key concepts of the dual-decision systems approach of the CNDS begin with the notion that behavior emerges from relative control between two systems: (1) the reward-driven impulsive system, the functioning of which, as in the DA-related theories described in Section 3.1, is mediated by the limbic (e.g., midbrain, amygdala, habenular commissure, & striatum) and paralimbic (i.e., insula & NAc) regions, and (2) the evolutionarily newer executive system, which governs self-regulatory processes such as planning, complex decision making, and behavioral inhibition, and is mediated by prefrontal and parietal brain regions (e.g., dlPFC). These two systems compete for relative control during decision making. When the systems are in regulatory or homeostatic balance, individuals can flexibly modulate their behavior, and exhibit an extensive range of possible, appropriate decisions. However, when the regulatory balance between the two systems is disrupted, pathology (e.g., addiction) may result due to constriction of the range of behavioral options (Bickel, Koffarnus, et al., 2014).
In the CNDS, delay discounting, which is the devaluation of reward with delay, is viewed as a key behavioral measure of self-control that describes the relative strength of the two competing neurobehavioral systems (Bickel, Jarmolowicz, Mueller, Gatchalian, et al., 2012; S. M. McClure & Bickel, 2014). Delay discounting measures future valuation by asking participants to indicate preference between smaller, immediate amounts of a commodity and larger, but delayed amounts. Based on neuroimaging results (see S. M. McClure, Laibson, Loewenstein, & Cohen, 2004 as discussed below), the impulsive system drives preference towards immediate reward receipt (as well as maintaining sensitivity to any rewards), and the executive system allows for deliberation of the value of delaying gratification to receive the larger reward despite impulsive urges. Greater preference for smaller, immediate rewards may be described as a trans-disease process (Bickel, Jarmolowicz, Mueller, Koffarnus, & Gatchalian, 2012), wherein hyperactivation of the impulsive system and/or hypoactivation of the executive system act in concert to produce addictive-related patterns of behavior (Bickel, Mellis, Snider, Moody, Stein, et al., 2016). Indeed, strong support for these concepts include the observation that elevated discounting of future rewards is observed in nicotine- (Bickel, Odum, & Madden, 1999), alcohol- (Petry, 2001), cocaine- (Bickel, Landes, et al., 2011; Heil, Johnson, Higgins, & Bickel, 2006), and heroin-dependent (Madden, Petry, Badger, & Bickel, 1997) individuals relative to controls. In addition to predicting substance use, elevated discount rates are also associated with so-called behavioral addictions such as overeating and excessive gambling (Bickel, Jarmolowicz, Mueller, Koffarnus, et al., 2012).
Recent neuroeconomic studies have provided support and novel insights into neurobiological mechanisms associated with discounting of delayed reinforcers and confirmed neurobiological evidence for the actions of the CNDS. For example, McClure et al. (2004) had undergraduate students make choices between reinforcers that differed in both delay and magnitude. Examination of fMRI during decision making revealed that choices for immediate reinforcers were associated with relatively high levels of activation in limbic and paralimbic areas, whereas choices for delayed reinforcers were associated with relatively high levels of activation in the lateral prefrontal brain regions. Other fMRI studies have also demonstrated that choices for immediate reinforcers are associated with activation of impulsive system areas of the brain (S. M. McClure & Bickel, 2014). Thus, immediate reinforcement may preferentially and systematically engage areas and functions of the impulsive system. Additionally, some studies have also replicated the finding by McClure et al. (2004), demonstrating that choices for delayed reinforcers are associated with relatively high levels of activation in executive system areas of the brain (e.g., Bickel, Pitcock, Yi, & Angtuaco, 2009; Hare, Camerer, & Rangel, 2009; S. M. McClure, Ericson, Laibson, Loewenstein, & Cohen, 2007; Wesley & Bickel, 2014). Together, these imaging studies support the premises of the CNDS by mapping the brain regions correlated with choices for immediate and delayed reinforcers onto the decision systems described in the theory (for an alternative perspective, see Kable & Glimcher, 2010).
One additional theoretical innovation and key concept based on the CNDS is reinforcer pathology, which combines research in reinforcing value and impulsivity, as measured by delay discounting (Bickel, Johnson, Koffarnus, MacKillop, & Murphy, 2014; Carr, Daniel, Lin, & Epstein, 2011). Reinforcer pathology is a behavioral theory that is mainly concerned with observable and measurable features of human behaviors that may lead to addiction, and refers to the interaction of two decision making processes in addictive consumption: (1) excessive valuation of brief, intense reinforcers; and (2) excessive discounting of delayed compared to immediate reinforcers (Bickel, Johnson, et al., 2014; MacKillop et al., 2011). The interaction between valuation and delay discounting is presented with a two-by-two matrix, wherein individuals who exhibit both excessive valuation of brief, intense reinforcers and excessive discounting of delayed rewards are at highest risk of addiction (Bickel, Snider, Quisenberry, & Stein, 2016). This theory is supported by findings of significant association between estimates of valuation of drug commodities and real-world indices of drug use, dependence, and treatment outcomes (Bickel, Moody, Snider, Mellis, Stein, et al., 2016; Chase, Mackillop, & Hogarth, 2013; Gray & MacKillop, 2014; Liao et al., 2013; Wahlstrom, McChargue, & Mackillop, 2016); (Bernstein, Murphy, MacKillop, & Colby, 2014; E. A. McClure, Vandrey, Johnson, & Stitzer, 2013). Furthermore, cross-sectional findings have shown higher delay discounting rates among individuals with addiction to different substances such as cocaine, alcohol, cigarettes, and opioids (see Bickel, Kowal, & Gatchalian, 2006; MacKillop et al., 2011; Reynolds, 2006); obese women (Weller, Cook, Avsar, & Cox, 2008); pathological gamblers (Alessi & Petry, 2003), and others. Moreover, several studies have tested the relationship between reinforcing value of drugs and discounting and reported an association in which altering discounting rates alters valuation and/or self-administration of addictive commodities among individuals with addiction (Jarmolowicz, Reed, DiGennaro Reed, & Bickel, 2015). For example, findings from experimental research implicated that reducing rates of discounting (e.g., by using episodic future thinking) has the ability to reduce valuation for hypothetical drinks in alcohol misusers (Snider, LaConte, & Bickel, 2016), self-administration of cigarettes in smokers (Stein et al., 2016), and self-administration of highly palatable snacks in overweight/obese adults and children (Daniel, Said, Stanton, & Epstein, 2015; Daniel, Stanton, & Epstein, 2013; O’Neill, Daniel, & Epstein, 2016). As a theory that is still developing and, in part, an extension of the CNDS theory, we will not discuss reinforcer pathology theory independently of the more fully developed CNDS theory.
To summarize, the most recent update to the CNDS theory is built around the premise of a dual-decision system and the emergence of behavior resulting from competition for relative control between the reward-driven impulsive system and the regulatory executive system (Bickel et al., 2017; Bickel, Snider, Quisenberry, Stein, et al., 2016). The theory proposes that the relative control between the two systems can be measured and described by the interaction between valuation of reinforcers and the rate of delay discounting. In this view, addiction results from a reinforcer pathology, which involves excessive valuation of brief, intense reinforcers and elevated rates of discounting of delayed reinforcers.
3.4.2 Triadic, Neurocognitive Theory of Addiction
The triadic, neurocognitive theory of addiction, with the earliest form introduced by Noel, Brevars, and Bechara (2013) draws from and codifies prior work establishing the roles of somatic markers in addictive processes. Specifically, the somatic marker hypothesis (Damasio, Tranel, & Damasio, 1991) states that interoceptive processes (sensitivity to stimuli originating inside the body) and their associated somatic markers (visceral responses associated with various emotional states, such as rapid heart rate, skin conductance, or nausea) guide behavior by signalling consequences that these markers have been paired with in the past (Bechara & Damasio, 2005; Damasio, Everitt, & Bishop, 1996; Damasio et al., 1991). For example, somatic markers historically associated with undesirable outcomes signal that behavior producing those outcomes is likely to be punished. The somatic marker hypothesis, then, attributes deficits in decision making in part to a deficit in the ability to use emotion and its somatic markers to guide behavior.
The somatic marker hypothesis was first developed and applied to decision making in general, with support provided by early findings that patients with lesions to the vmPFC (a site implicated in processing of somatic states) experience not only deficits in learning from negative consequences, but also in emotional regulation. However, Verdejo-García and Bechara (2009) and others (e.g., Noël et al., 2013) later applied the hypothesis specifically to addiction, that the somatic markers associated with drug use are in conflict, signaling not only the immediate, hedonic gratification from drug use but also its delayed negative consequences. Addiction, then, arises from one of two non-mutually-exclusive possibilities: (1) hyperactivity in the amygdala and other impulsive areas (e.g., insula), which produces overly strong associations between somatic markers and immediate drug gratification, or (2) hypoactivity in vmPFC and other areas associated with executive functions, which produces weak associations between somatic markers and the negative consequences of drug use. The result in either case is the inability of somatic markers to promote future-oriented behavior.
Components of the somatic marker hypothesis were later integrated into a broader conceptual frame, including components of both dual-decision systems and the emotional and the self-regulatory systems noted in the somatic marker hypothesis. The key concepts of the triadic neurocognitive theory of addiction (Noël et al., 2013; see also Turel & Bechara, 2016) include that addiction is the product of abnormal functioning in the correspondence between three key neurocognitive systems: (1) an amygdala-striatum dependent neural system that promotes automatic cognitive processes and habits (similar to the impulsive system of the CNDS); (2) a PFC-dependent neural system that governs decision making, prospective thinking and planning, inhibitory control, and self-awareness (similar to the executive system of the CNDS, and here termed the “reflective” system); and (3) the insular cortex, which is key to modulating the dynamics and interactions between the impulsive and reflective systems (which, in the CNDS, is considered part of the impulsive decision system). More specifically, the anterior insula translates somatic markers into subjective experiences (e.g., craving), which, in turn, direct behavior.
Noel et al. (2013) argue that while dual-process models may explain certain components of addiction, these models are not sufficient to explain all aspects of addictive behaviors, such as homeostatic processes (e.g., craving). The major difference between the triadic neurocognitive theory of addiction and CNDS, then, is the role of the insula in mediating interoceptive processes: the CNDS views the insular cortex as a downstream element of the impulsive system, while the triadic theory views the insular cortex as a separate system from the impulsive system and reflective/executive system. That is, the triadic theory proposed a key role for the insular cortex (which is commonly activated during interoceptive processing) as a gate system involved in subjective, internal homeostatic response to perturbations (Craig, 2009). In relation to addiction, the insular cortex has been argued to contribute to the onset and maintenance of addiction by translating somatic states into subjective feelings of desire, anticipation, or inclination to approach-related behavioral patterns (Naqvi & Bechara, 2009; Paulus & Stewart, 2014; Verdejo-Garcia, Clark, & Dunn, 2012).
Support for this theory, in relation to the impulsive and executive system components, is identical to that mentioned above in relation to dual-decision systems more generally and in the CNDS. Evidence that the impulsive and executive system are influenced by interoceptive processes mediated by the insula include imaging studies illustrating activity within the insula correlating with subjects’ ratings of urge for cigarettes, cocaine, alcohol, and heroin (Craig, 2009; Naqvi & Bechara, 2009; Verdejo-Garcia et al., 2012). Insula activation has been shown to be closely associated with higher levels of nicotine dependence (Goudriaan, de Ruiter, van den Brink, Oosterlaan, & Veltman, 2010) and both attentional bias and the risk of relapse, with smokers at risk for relapse showing more insula activation while processing smoking-related words (Janes et al., 2010). Other evidence supporting the role of the insula in addiction processes arise from human brain lesion studies, with, for example, strokes that damage the insular cortex literally eliminating the urge to smoke in individuals previously addicted to cigarette smoking (Naqvi, Rudrauf, Damasio, & Bechara, 2007), indicating a role for the insula in maintaining addiction. Finally, some evidence exists supporting the notion that insular cortex activity increases the drive and motivation to seek and take drugs by altering the balance between the impulsive and executive systems (Wang et al., 2012).
Thus, the most recent iteration of this theory hypothesizes that insular cortex activation increases the motivation and drive to consume drugs by: (1) sensitizing or exacerbating the activity of the habit/impulsive system; and (2) subverting attention, reasoning, planning, and decision making processes and resources to devise plans of action to seek and procure drugs. That is, interoceptive signals processed by the insular cortex have the capacity to “hijack” processes responsible for inhibitory control (e.g., resisting temptation to use) or those to use drugs by redirecting activity of the PFC (e.g., Turel & Bechara, 2016).
3.4.3 Benchmark Evaluation
The dual decision and dual decision-derived theories outlined above each appeal to the interaction between two or more systems to account for decision making in addiction. Here, we evaluate both the CNDS and the triadic neurocognitive theories against the six benchmarks outlined in Section 2.
Both the CNDS and triadic theories account for Benchmarks 1, “why are certain commodities addictive when others are not?”, and 4, “why do individuals with addiction engage in consistent self-defeating patterns of behavior?”, through the relative balance of impulsive and executive systems, although with different emphases. Regarding Benchmark 1, CNDS theory points to the observation that drugs of abuse are brief, intense, and reliable sources of reinforcement, which greatly appeal to the impulsive system. Thus, to an individual with a relatively stronger impulsive system, these reinforcers are hard to resist (see Bickel et al., 2013). The triadic theory would similarly point to the appeal of drug reinforcers to the impulsive system, and further emphasize the mediating role of the insular cortex in the translation of these drug craving signals into behavior (Noël et al., 2013; Turel & Bechara, 2016). Regarding Benchmark 4, both theories suggest that increased drug valuation is the product of relatively diminished control of the executive or reflective systems compared to the impulsive system (and hence, decreased likelihood of acting in one’s long-term self-interest). In CNDS theory, this imbalance manifests as reinforcer pathology (Bickel, Jarmolowicz, Mueller, & Gatchalian, 2011; Bickel, Johnson, et al., 2014; Carr et al., 2011). In the triadic theory, this manifests as pervasive, insula-mediated drug craving (Noël et al., 2013). In either case, diminished control of the executive or reflective systems compromise the ability of individuals suffering from addiction to pursue long-term goals, or avoid behavior in the present that produces delayed, negative consequences.
The CNDS and the triadic theory are also able to account for Benchmark 2, “why does addiction follow common developmental trends?”. As reviewed above, drug and alcohol use follows a predictable developmental time course, peaking in adolescence and receding through adulthood (see Figure 1). In both the CNDS theory and the triadic theory, differential development between the executive/reflective and impulsive systems accounts for this time course in that neural substrates in the impulsive system mature in early to late adolescence (Andersen, Thompson, Rutstein, Hostetter, & Teicher, 2000; Ernst & Paulus, 2005; Galvan et al., 2006; Sisk & Zehr, 2005), whereas maturation of the executive system follows later in early adulthood (Sisk & Zehr, 2005; Sowell et al., 2003). A central prediction of the CNDS theory is that increased relative control of the impulsive over the executive decision system (as seen in early adolescence) decreases self-control and hence the ability to avoid the temptations from drugs of abuse. Normal maturation of the executive system in later adulthood restores this balance in those who mature out of substance use (see Figure 1). For others, who do mature out of use or who are otherwise addicted to substances through adulthood, this balance may never tilt towards executive control (Bickel, Jarmolowicz, Mueller, Gatchalian, et al., 2012, see Figure 3).
The role of regulatory balance between decision systems in the CNDS and triadic theories points to novel treatment options for addiction, and hence accounts for Benchmark 6, “what interventions have been or are derivable from the tenets of these theories?”. If addiction is the product of diminished executive system control, then interventions that specifically engage and strengthen this system should restore balance and enhance self-control (Bickel, Mellis, Snider, Moody, Stein, et al., 2016; Bickel, Snider, Quisenberry, Stein, et al., 2016; Fraser & Rosen, 2012; Koffarnus, Jarmolowicz, Mueller, & Bickel, 2013). For example, excitatory stimulation of components of the executive/reflective systems (e.g., dlPFC) decreases cocaine use and cigarette smoking (Amiaz, Levy, Vainiger, Grunhaus, & Zangen, 2009; Terraneo et al., 2016) and reduces delay discounting (Sheffer et al., 2013). Moreover, in relation to the CNDS, individuals suffering from addiction show deficits in both planning for and imagining the future (Petry, Bickel, & Arnett, 1998; Wiehler, Bromberg, & Peters, 2015), which are important executive functions. These deficits can be remediated by targeted intervention in the form of episodic future thinking, which has consistently been shown to reduce both delay discounting and measures of drug consumption or valuation for cigarettes and alcohol (Bulley & Gullo, 2017; Snider et al., 2016; Stein et al., 2016). Importantly, these effects may be mediated by enhanced functional connectivity between the executive and impulsive systems (Peters & Büchel, 2010).
The triadic theory (Noël et al., 2013) suggested theoretically plausible strategies for reducing interoceptive signaling of homeostatic perturbations. Direct transcranial magnetic stimulation of the insula to decrease interoceptive “hijacking” of behavior would be a promising treatment option for addiction derived from this theory, but is not currently possible with existing delivery systems. Because a number of other brain regions could be altered indirectly by such stimulation, further studies are required to better characterize the brain regions altered by magnetic brain stimulation of the insula. Some alternative strategies which link the interoceptive and reflective systems have already been implemented, including the enhancement of the reflective system by modulating how individuals process and integrate interoceptive signals (e.g., through physical exercise and mindfulness; Paulus & Stewart, 2014), although not always in addicts (e.g., A. Jones et al., 2011; but see Kim et al., 2007; Verbruggen, Adams, & Chambers, 2012; Westbrook et al., 2013) and not always with clear, specific effects only on the referenced cognitive systems (see Gorelick, Zangen, & George, 2014).
In response to Benchmark 3, “why do some individuals, as their valuation of drugs increases, also exhibit a decrease in valuation of non-addictive commodities?”, the two theories differ. Although the CNDS theory accounts for the increase in valuation of drugs during the addiction cycle, it does not offer an explanation of the concurrent decrease in valuation of non-addictive commodities and the accompanying anhedonia. In contrast, the triadic theory notes the well-documented links between stressor exposure and addiction-related behavior (e.g., relapse), such as stressor modulation of reactivity to drug cues (e.g., Jasinska, Stein, Kaiser, Naumer, & Yalachkov, 2014), that may perilously impact the balance between the impulsive and reflective systems. Indeed, such imbalance is often seen in individuals struggling with their addiction, supporting blunted sensitivity to non-drug rewards, potentially causing anhedonia.
Regarding Benchmark 5, “why are individuals with addiction also likely to engage in other unhealthy behaviors?”, only CNDS theory offers an explicit answer. This theory would suggest that any unhealthy behavior which delivers immediate gratification and primarily long-term costs would be likely to emerge from the same errors of decision making common in addiction. Specifically, individuals with addiction commonly heavily discount delayed rewards. In addiction, the immediate gratification of consuming outweighs the delayed gratification of abstinence. In other unhealthy behaviors, texting while driving for example, the immediate gratification of sending or receiving a text message outweighs the possible long-term consequences of hazardous driving. Indeed, many other unhealthy behaviors are correlated with high delay discounting rates (Bickel et al., 2017), indicating that delay discounting may be a trans-disease process (Bickel, Jarmolowicz, Mueller, Koffarnus, et al., 2012).
4. Synthesis and Conclusion
In the preceding sections, we reviewed 21st century theories of decision making in addiction and evaluated their performance in addressing the following 6 benchmarks of theoretical utility:
why are some commodities addictive when others are not?
why does addiction follow common developmental trends?
why do some individuals, as their valuation of drugs increases, also exhibit a decrease in valuation of non-addictive commodities?
why do individuals with addiction engage in consistent self-defeating patterns of behavior?
why are individuals with addiction also likely to engage in other unhealthy behaviors? and finally
what interventions have been or are derivable from the tenets of these theories? (Table 1)
Table 1.
Evaluation of theories against the benchmarks
| Addictivepotential | Developmental trends | Anhedoniade | Self-defeating | Comorbidity | Treatment | Key citations | |
|---|---|---|---|---|---|---|---|
| Dopamine Based | |||||||
| Learning | Y | Y | Y | M | N | Y | (Hogarth, Balleine, Corbit, & Killcross, 2013; White, 1989) |
| Incentive-Sensitization | Y | Y | Y | M | N | N | (Berridge & Robinson, 2016; Robinson & Berridge, 1993) |
| Dopamine Imbalance | Y | Y | Y | M | N | Y | (Volkow, Koob, & McLellan, 2016; Volkow, Wang, Fowler, Tomasi, & Telang, 2011) |
| Dark Side of Addiction | |||||||
| Opponent Process | Y | M | Y | Y | M | Y | (Koob & Le Moal, 2001; Koob & Mason, 2016) |
| Self Control Failure | |||||||
| Depletion | N | M | N | M | Y | Y | (Baumeister, Bratslavsky, Muraven, & Tice, 1998; Baumeister & Vohs, 2016) |
| Emotion Regulation | N | N | N | M | Y | N | (Heatherton & Baumeister, 1991; Zilverstand, Parvaz, Moeller, & Goldstein, 2016) |
| Dual Decision and Derived Systems | |||||||
| CNDS | Y | Y | N | Y | Y | Y | (Bickel et al., 2007, 2017) |
| Triadic | Y | Y | Y | Y | N | Y | (Noël, Brevers, & Bechara, 2013) |
Comparing theories against common benchmarks of addiction. “Y” (yes) indicates that the theory provides an explicit theoretical explanation and empirical evidence for meeting the benchmark. “M” (mixed) indicates that the theory provides some evidence to support the benchmark, however the evidence is weakened for one of two reasons: (1) the theory implies support, however the theory never explicitly recognizes the benchmark itself, or (2) the theory answers part but not all of the phenomena described in the benchmark. “N” (no) indicates that the theory does not specifically recognize or empirically support the benchmark.
In Table 1, we summarized this evaluation, indicating whether individual theories satisfactorily answer the question posed by each benchmark. No one theory answers all of these questions, but in sum, all benchmarks are addressed by at least one theory. Not surprisingly, these theories vary between primarily molecular (e.g., the DA imbalance systems model of addiction) and primarily molar (e.g., strength models of self-control failure). An important future direction will thus be to determine whether these theories describe discrete phenomena of addiction or whether they are reducible to common elements across their differing levels of analysis (consistent with Research Domain Criteria efforts; Insel et al., 2010).
To this end, acknowledging where theories coalesce around common observations is important (see Table 1) and reveals common elements among theories. For example, where theories offer an explanation of Benchmark 1, “why are some commodities addictive when others are not?”, many point to the reinforcing properties of drugs, either at a molecular level (of DA activity) or a molar level (the subjective valuation of brief, intense reinforcers). Additionally, where theories offer an explanation of Benchmark 2, “why does addiction follow common developmental trends?”, they point to neurodevelopmental trends in the brain and how the time course of neurodevelopment may also map onto observations of life course variation in drug use (see Figure 1). Ultimately, these theories describe the same disease, but each selects different aspects to highlight and, in turn, to study. Thus, the exclusive validity of any one of these theories cannot be determined, because each refer to a set of real-world phenomena (addiction). Instead of the ability to describe the most facts (i.e., meet the most benchmarks), perhaps the most powerful basis for distinguishing between theories is on the pragmatic criterion of truth: that is, the theory’s practical utility (Zuriff, 1980). In this regard, treatment (Benchmark 6, “what interventions have been or are derivable from the tenets of these theories?”) remains both the largest hurdle and test of each of these theoretical perspectives.
Furthermore, other needs may exist in the development of current or novel theories of decision making in addiction. For example, those theories that have explicit principles can be tested and therefore their tenets proven or shown to be false. Importantly, some theories more explicitly state their core tenets than others, and are thus more susceptible to falsifiability criteria (Popper, 2005). Theories also vary in their description of the decision processes in addiction. Some describe decision making as a downstream consequence of other theoretical tenets, but the ideal theory of decision making in addiction will provide or help to generate evidence of the decisional processes contributing to the initiation, maintenance, relapse, and repair of this disease. And, as importantly, from an experimental medicine approach (Bernard, 1957), in which ability to treat disease in humans is directly used as a test of theoretical tenets, those decision processes that go awry in addiction should be modifiable, and provide a test of whether that decisional process modulates some aspect of addiction (again, see Benchmark 6). Only by application of such an experimental medicine approach will we be able to better understand the decision making process in addiction and be able to determine if these processes play not only a correlative role but a causal one in the stages of addiction.
Highlights.
The field of addiction science must understand the decisional processes that result in continued substance use.
We have reviewed six theories which have been developed or extended in the 21st century to the task of understanding decision making in addiction.
We have evaluated each theory on its ability to answer six key questions within the field of addiction.
No one theory provides complete and independent answers to all of these six key questions.
Acknowledgments
This work was supported by the National Institutes of Health [Grant numbers 5UH2DK109543, 5R01DA039456, 5R21DA040559, DP7OD018428, R01AA021529, R01DA034755, R01DA036017, U19CA157345, R01MD007054, 1P01CA200512, 1R01DA042535, 1R21AA023605, R01DA030241]. The funding sources were not involved in the development or preparation of this report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest:
The authors report no personal or financial relationships to influence this work.
References
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behavioural Processes. 2003;64(3):345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104(4):653–660. doi: 10.1111/j.1360-0443.2008.02448.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37(2):167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Balster RL, Bigelow GE. Guidelines and methodological reviews concerning drug abuse liability assessment. Drug and Alcohol Dependence. 2003;70(3 Suppl):S13–40. doi: 10.1016/s0376-8716(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Baum CL, Ruhm CJ. Age, socioeconomic status and obesity growth. Journal of Health Economics. 2009;28(3):635–648. doi: 10.1016/j.jhealeco.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Baumeister RF. Ego Depletion and Self-Control Failure: An Energy Model of the Self’s Executive Function. Self and Identity: The Journal of the International Society for Self and Identity. 2002;1(2):129–136. [Google Scholar]
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: Is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74(5):1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF. Self-regulation failure: An overview. Psychological Inquiry. 1996;7(1):1–15. [Google Scholar]
- Baumeister RF, Heatherton TF, Tice DM. Losing control: How and why people fail at self-regulation. Academic Press; 1994. [Google Scholar]
- Baumeister RF, Scher SJ. Self-defeating behavior patterns among normal individuals: Review and analysis of common self-destructive tendencies. Psychological Bulletin. 1988;104(1):3–22. doi: 10.1037/0033-2909.104.1.3. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD. Chapter 9: Self-Regulation and the Executive Function of the Self. In: Leary JPTMR, editor. Handbook of Self and Identity. 2nd. The Guilford Press; 2012. pp. 180–197. [Google Scholar]
- Baumeister RF, Vohs KD. Strength model of self-regulation as limited resource: Assessment, controversies, update. In: Olson James M, Zanna Mark P., editors. Advances in Experimental Social Psychology. Vol. 54. Academic Press; 2016. pp. 67–127. [Google Scholar]
- Baumeister RF, Vohs KD, Tice DM. The strength model of self-control. Current Directions in Psychological Science. 2007;16(6):351–355. [Google Scholar]
- Baumeister RF, Vonasch AJ. Uses of self-regulation to facilitate and restrain addictive behavior. Addictive Behaviors. 2015;44:3–8. doi: 10.1016/j.addbeh.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52:336–372. [Google Scholar]
- Beckie TM. A systematic review of allostatic load, health, and health disparities. Biological Research for Nursing. 2012;14(4):311–346. doi: 10.1177/1099800412455688. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Miller-Ziegler JS. Imaging depletion: fMRI provides new insights into the processes underlying ego depletion*. Social Cognitive and Affective Neuroscience. 2013;8(4):359–361. doi: 10.1093/scan/nss111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C. An Introduction to the Study of Experimental Medicine. Courier Corporation; 1957. [Google Scholar]
- Bernstein M, Murphy J, MacKillop J, Colby S. Alcohol demand indices predict outcomes among heavy-drinking young adults receiving a brief intervention. Drug and Alcohol Dependence. 2014;140:e13. [Google Scholar]
- Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. The American Psychologist. 2016;71(8):670–679. doi: 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM. The behavioral economics and neuroeconomics of reinforcer pathologies: Implications for etiology and treatment of addiction. Current Psychiatry Reports. 2011;13(5):406–415. doi: 10.1007/s11920-011-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, McClure SM. Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology. 2012;221(3):361–387. doi: 10.1007/s00213-012-2689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacology & Therapeutics. 2012;134(3):287–297. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Johnson MW, Koffarnus MN, MacKillop J, Murphy JG. The behavioral economics of substance use disorders: Reinforcement pathologies and their repair. Annual Review of Clinical Psychology. 2014;10:641–677. doi: 10.1146/annurev-clinpsy-032813-153724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014;76(Pt B):518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Kowal BP, Gatchalian KM. Understanding addiction as a pathology of temporal horizon. The Behavior Analyst Today. 2006;7(1):32–47. [Google Scholar]
- Bickel WK, Landes RD, Christensen DR, Jackson L, Jones BA, Kurth-Nelson Z, Redish AD. Single- and cross-commodity discounting among cocaine addicts: The commodity and its temporal location determine discounting rate. Psychopharmacology. 2011;217(2):177–187. doi: 10.1007/s00213-011-2272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Mellis AM, Snider SE, Moody L, Stein JS, Quisenberry AJ. Novel therapeutics for addiction: Behavioral and neuroeconomic approaches. Current Treatment Options in Psychiatry. 2016;3(3):277–292. doi: 10.1007/s40501-016-0088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug and Alcohol Dependence. 2007;90(Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Moody L, Snider SE, Mellis AM, Stein JS, Quisenberry AJ. The behavioral economics of tobacco products: Innovations in laboratory methods to inform regulatory science. In: Hanoch Y R, editor. Behavioral economics and health behaviors: Key concepts and current research. 2016. [Google Scholar]
- Bickel WK, Mueller ET, Jarmolowicz DP. What is addiction? Addictions: A Comprehensive Guidebook. 2013;2:3–16. [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146(4):447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Pitcock JA, Yi R, Angtuaco EJC. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(27):8839–8846. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Snider SE, Quisenberry AJ, Stein JS. Reinforcer pathology: The behavioral economics of abuse liability testing. Clinical Pharmacology and Therapeutics. 2016 doi: 10.1002/cpt.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Snider SE, Quisenberry AJ, Stein JS, Hanlon CA. Competing neurobehavioral decision systems theory of cocaine addiction: From mechanisms to therapeutic opportunities. Progress in Brain Research. 2016;223:269–293. doi: 10.1016/bs.pbr.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Stein JS, Moody L, Snider SE, Mellis AM, Quisenberry AM. Toward narrative theory: Interventions for reinforcer pathology in health behaviors. In: Stephens J, editor. Impulsivity: How time and risk influence decision making. Vol. 64. Springer; New York: 2017. [PubMed] [Google Scholar]
- Bickel WK, Yi R. Temporal discounting as a measure of executive function: insights from the competing neuro-behavioral decision system hypothesis of addiction. Advances in Health Economics and Health Services Research. 2008;20:289–309. [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49(4):226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Archives of General Psychiatry. 2006;63(12):1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C. Conditioned dopamine release in humans: a positron emission tomography [11C] raclopride study with amphetamine. Journal of Neuroscience. 2007;27(15):3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BNM, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, Kahn RS. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34(3):759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Brandon TH. Negative affect as motivation to smoke. Current Directions in Psychological Science. 1994;3(2):33–37. [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, London ED. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34(2):282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Small E, Pasek TM, Yamada H. Corticotropin-releasing factor mediates the dysphoria-like state associated with alcohol withdrawal in rats. Behavioural Brain Research. 2010;210(2):288–291. doi: 10.1016/j.bbr.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2007;32(4):955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Bulley A, Gullo MJ. The influence of episodic foresight on delay discounting and demand for alcohol. Addictive Behaviors. 2017;66:1–6. doi: 10.1016/j.addbeh.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Carmody TP. Affect Regulation, Tobacco Addiction, and Smoking Cessation. Journal of Psychoactive Drugs. 1989;21(3):331–342. doi: 10.1080/02791072.1989.10472175. [DOI] [PubMed] [Google Scholar]
- Carr KA, Daniel TO, Lin H, Epstein LH. Reinforcement Pathology and Obesity. Current Drug Abuse Reviews. 2011;4(3):190–196. doi: 10.2174/1874473711104030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter EC, Kofler LM, Forster DE, McCullough ME. A series of meta-analytic tests of the depletion effect: Self-control does not seem to rely on a limited resource. Journal of Experimental Psychology General. 2015;144(4):796–815. doi: 10.1037/xge0000083. [DOI] [PubMed] [Google Scholar]
- Carter EC, McCullough ME. Publication bias and the limited strength model of self-control: has the evidence for ego depletion been overestimated? Frontiers in Psychology. 2014;5:823. doi: 10.3389/fpsyg.2014.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug and Alcohol Dependence. 2009;105(Suppl 1):S14–25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Mackillop J, Hogarth L. Isolating behavioural economic indices of demand in relation to nicotine dependence. Psychopharmacology. 2013;226(2):371–380. doi: 10.1007/s00213-012-2911-x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, Wang Z, Li Y, Sciortino N, Hakun J, O’Brien CP. Prelude to passion: Limbic activation by “unseen” drug and sexual cues. PloS One. 2008;3(1):e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McElgin W, Mozley PD, O’Brien CP. BIOLOGICAL PSYCHIATRY. Vol. 45. ELSEVIER SCIENCE INC; 655 AVENUE OF THE AMERICAS, NEW YORK, NY 10010 USA: 1999. Limbic activation during cue-induced craving for cocaine and for natural rewards; pp. 53S–53S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology. 1984;82(1–2):6–13. doi: 10.1007/BF00426372. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2008;33(5):1179–1191. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML. Alcohol use and risky sexual behavior among college students and youth: evaluating the evidence. Journal of Studies on Alcohol Supplement. 2002;(14):101–117. doi: 10.15288/jsas.2002.s14.101. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69(5):990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biological Psychiatry. 2012;72(5):389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? the anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1) doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crow TJ. A map of the rat mesencephalon for electrical self-stimulation. Brain Research. 1972;36(2):265–273. doi: 10.1016/0006-8993(72)90734-2. [DOI] [PubMed] [Google Scholar]
- Cunningham JA, Sobell LC, Sobell MB, Kapur G. Resolution from alcohol problems with and without treatment: reasons for change. Journal of Substance Abuse. 1995;7(3):365–372. doi: 10.1016/0899-3289(95)90029-2. [DOI] [PubMed] [Google Scholar]
- Currie E. Reckoning: Drugs, the Cities, and the American Future. Macmillan; 1994. [Google Scholar]
- Cutler RB, Fishbain DA. Are alcoholism treatments effective? The Project MATCH data. BMC Public Health. 2005;5:75. doi: 10.1186/1471-2458-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Everitt BJ, Bishop D. The somatic marker hypothesis and the possible functions of the prefrontal cortex [and discussion] (Series B, Biological Sciences).Philosophical Transactions of the Royal Society of London. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio HC. Somatic markers and the guidance of behavior: Theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal Lobe Function and Dysfunction. Vol. 217. Oxford University Press; 1991. pp. 218–229. [Google Scholar]
- Daniel TO, Said M, Stanton CM, Epstein LH. Episodic future thinking reduces delay discounting and energy intake in children. Eating Behaviors. 2015;18:20–24. doi: 10.1016/j.eatbeh.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, Epstein LH. The future is now: reducing impulsivity and energy intake using episodic future thinking. Psychological Science. 2013;24(11):2339–2342. doi: 10.1177/0956797613488780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature Neuroscience. 2005;8(12):1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Di Ciano P. Facilitated acquisition but not persistence of responding for a cocaine-paired conditioned reinforcer following sensitization with cocaine. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2008;33(6):1426–1431. doi: 10.1038/sj.npp.1301542. [DOI] [PubMed] [Google Scholar]
- Dickinson A. Actions and Habits: The Development of Behavioural Autonomy. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1985;308(1135):67–78. [Google Scholar]
- Dickinson A, Balleine B. Motivational control of goal-directed action. Animal Learning & Behavior. 1994;22(1):1–18. [Google Scholar]
- Dickinson A, Wood N, Smith JW. Alcohol seeking by rats: action or habit? The Quarterly Journal of Experimental Psychology B, Comparative and Physiological Psychology. 2002;55(4):331–348. doi: 10.1080/0272499024400016. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition. 2010;72(1):114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry. 2001;49(2):81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Edwards G, Lader MH. The Nature of drug dependence. Oxford University Press; 1990. [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biological Psychiatry. 2005;58(8):597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. (Series B, Biological Sciences).Philosophical Transactions of the Royal Society of London. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Research. Brain Research Reviews. 2001;36(2–3):129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fischer JL, Forthun LF, Pidcock BW, Dowd DA. Parent relationships, emotion regulation, psychosocial maturity and college student alcohol use problems. Journal of Youth and Adolescence. 2007;36(7):912–926. [Google Scholar]
- Fox HC, Axelrod SR, Paliwal P, Sleeper J, Sinha R. Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug and Alcohol Dependence. 2007;89(2–3):298–301. doi: 10.1016/j.drugalcdep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Sinha R. Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Addictive Behaviors. 2008;33(2):388–394. doi: 10.1016/j.addbeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Fraser PE, Rosen AC. Transcranial direct current stimulation and behavioral models of smoking addiction. Frontiers in Psychiatry/Frontiers Research Foundation. 2012;3:79. doi: 10.3389/fpsyt.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF, DeWall CN, Maner JK, Plant EA, Tice DM, Schmeichel BJ. Self-control relies on glucose as a limited energy source: willpower is more than a metaphor. Journal of Personality and Social Psychology. 2007;92(2):325–336. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. The American Journal of Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Abrams DB, Buka SL. Socioeconomic status over the life course and stages of cigarette use: initiation, regular use, and cessation. Journal of Epidemiology and Community Health. 2003;57(10):802–808. doi: 10.1136/jech.57.10.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. The American Journal of Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Annals of the New York Academy of Sciences. 2014;1327:79–93. doi: 10.1111/nyas.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goschke T. Dysfunctions of decision-making and cognitive control as transdiagnostic mechanisms of mental disorders: advances, gaps, and needs in current research. International Journal of Methods in Psychiatric Research. 2014;23(S1):41–57. doi: 10.1002/mpr.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredson MR, Hirschi T. A general theory of crime. Stanford University Press; 1990. [Google Scholar]
- Goudriaan AE, de Ruiter MB, van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addiction Biology. 2010;15(4):491–503. doi: 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, MacKillop J. Interrelationships among individual differences in alcohol demand, impulsivity, and alcohol misuse. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2014;28(1):282–287. doi: 10.1037/a0032766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addiction Biology. 2009;14(2):130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow G, Liebson I. Human drug self-administration: Double-blind comparison of pentobarbital, diazepam, chlorpromazine and placebo. The Journal of Pharmacology and Experimental Therapeutics. 1979;210(2):301–310. [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Review of General Psychology: Journal of Division 1, of the American Psychological Association. 1998;2(3):271–299. [Google Scholar]
- Gross JJ, Muñoz RF. Emotion Regulation and Mental Health. Clinical Psychology: Science and Practice. 1995;2(2):151–164. [Google Scholar]
- Haggård-Grann U, Hallqvist J, Långström N, Möller J. The role of alcohol and drugs in triggering criminal violence: a case-crossover study*. Addiction. 2006;101(1):100–108. doi: 10.1111/j.1360-0443.2005.01293.x. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Chatzisarantis NLD. A multilab preregistered replication of the ego-depletion effect. Perspectives on Psychological Science: A Journal of the Association for Psychological Science. 2016;11(4):546–573. doi: 10.1177/1745691616652873. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NLD. Ego depletion and the strength model of self-control: a meta-analysis. Psychological Bulletin. 2010;136(4):495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behavioural Pharmacology. 1998;9(4):299–308. [PubMed] [Google Scholar]
- Heatherton TF, Baumeister RF. Binge eating as escape from self-awareness. Psychological Bulletin. 1991;110(1):86–108. doi: 10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences. 2011;15(3):132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman BW, MacQueen DA, Marquinez NS, MacKillop J, Bickel WK, Brandon TH. Self-control depletion and nicotine deprivation as precipitants of smoking cessation failure: A human laboratory model. Journal of Consulting and Clinical Psychology. 2017;85(4):381–396. doi: 10.1037/ccp0000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addictive Behaviors. 2006;31(7):1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser-Sinopoli SM, Bartenstein P. Correlation Between Dopamine D2 Receptors in the Ventral Striatum and Central Processing of Alcohol Cues and Craving. American Journal of Psychiatry. 2004;161(10):1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Heyman GM. Addiction: A Disorder of Choice. Harvard University Press; 2009. [Google Scholar]
- Hogarth L. Goal-directed and transfer-cue-elicited drug-seeking are dissociated by pharmacotherapy: evidence for independent additive controllers. Journal of Experimental Psychology. Animal Behavior Processes. 2012;38(3):266–278. doi: 10.1037/a0028914. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Balleine BW, Corbit LH, Killcross S. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Annals of the New York Academy of Sciences. 2013;1282:12–24. doi: 10.1111/j.1749-6632.2012.06768.x. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Chase HW. Parallel goal-directed and habitual control of human drug-seeking: implications for dependence vulnerability. Journal of Experimental Psychology. Animal Behavior Processes. 2011;37(3):261–276. doi: 10.1037/a0022913. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nature Neuroscience. 2001;4(9):943–947. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. The American Journal of Psychiatry. 2005;162(8):1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Iguchi MY, Belding MA, Morral AR, Lamb RJ, Husband SD. Reinforcing operants other than abstinence in drug abuse treatment: an effective alternative for reducing drug use. Journal of Consulting and Clinical Psychology. 1997;65(3):421–428. doi: 10.1037//0022-006x.65.3.421. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Schmeichel BJ, Macrae CN. Why self-control seems (but may not be) limited. Trends in Cognitive Sciences. 2014;18(3):127–133. doi: 10.1016/j.tics.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(19):7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22(14):6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmolowicz DP, Reed DD, DiGennaro Reed RD, Bickel WK. The behavioral and neuroeconomics of reinforcer pathologies: Implications for managerial and health decision making. Managerial and Decision Economics. 2015;37(4–5):274–293. [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job V, Dweck CS, Walton GM. Ego depletion–is it all in your head? implicit theories about willpower affect self-regulation. Psychological Science. 2010;21(11):1686–1693. doi: 10.1177/0956797610384745. [DOI] [PubMed] [Google Scholar]
- Jones A, Guerrieri R, Fernie G, Cole J, Goudie A, Field M. The effects of priming restrained versus disinhibited behaviour on alcohol-seeking in social drinkers. Drug and Alcohol Dependence. 2011;113(1):55–61. doi: 10.1016/j.drugalcdep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Jones BT, Young RM. Handbook of Motivational Counseling. John Wiley & Sons, Ltd; 2011. Changing Alcohol Expectancies and Self-Efficacy Expectations; pp. 489–504. [Google Scholar]
- Kable JW, Glimcher PW. An “as soon as possible” effect in human intertemporal decision making: Behavioral evidence and neural mechanisms. Journal of Neurophysiology. 2010;103(5):2513–2531. doi: 10.1152/jn.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedia S, Sell MA, Relyea G. Mono- versus polydrug abuse patterns among publicly funded clients. Substance Abuse Treatment, Prevention, and Policy. 2007;2:33. doi: 10.1186/1747-597X-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Darke S, Ross J. A review of drug use and driving: epidemiology, impairment, risk factors and risk perceptions. Drug and Alcohol Review. 2004;23(3):319–344. doi: 10.1080/09595230412331289482. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Hasin DS. Socio-economic status and problem alcohol use: the positive relationship between income and the DSM-IV alcohol abuse diagnosis. Addiction. 2008;103(7):1120–1130. doi: 10.1111/j.1360-0443.2008.02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. The American Journal of Psychiatry. 1985;142(11):1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence. In: Allen DF, editor. The Cocaine Crisis. Springer US; 1987. pp. 65–74. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebral Cortex. 2003;13(4):400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Kim JS, Park BK, Kim GJ, Kim SS, Jung JG, Oh MK, Oh JK. The role of alcoholics’ insight in abstinence from alcohol in male Korean alcohol dependents. Journal of Korean Medical Science. 2007;22(1):132–137. doi: 10.3346/jkms.2007.22.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. Journal of the Experimental Analysis of Behavior. 2013;99(1):32–57. doi: 10.1002/jeab.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Annals of the New York Academy of Sciences. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcoholism, Clinical and Experimental Research. 2003;27(2):232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nature Neuroscience. 2005;8(11):1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008a;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008b;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Mason BJ. Existing and future drugs for the treatment of the dark side of addiction. Annual Review of Pharmacology and Toxicology. 2016;56:299–322. doi: 10.1146/annurev-pharmtox-010715-103143. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3(8):760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterman R, Hawkins JD, Guo J, Catalano RF, Abbott RD. The dynamics of alcohol and marijuana initiation: patterns and predictors of first use in adolescence. American Journal of Public Health. 2000;90(3):360–366. doi: 10.2105/ajph.90.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn TS. The function of measurement in modern physical science. Isis; an International Review Devoted to the History of Science and Its Cultural Influences. 1961;52(2):161–193. [Google Scholar]
- Lantz PM, House JS, Mero RP, Williams DR. Stress, Life Events, and Socioeconomic Disparities in Health: Results from the Americans’ Changing Lives Study*. Journal of Health and Social Behavior. 2016 doi: 10.1177/002214650504600305. https://doi.org/10.1177/002214650504600305. [DOI] [PubMed]
- Larson R, Asmussen L. Anger, worry, and hurt in early adolescence: An enlarging world of negative emotions. Adolescent Stress: Causes and Consequences. 1991:21–41. [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278(5335):45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Trujillo M, Ameringer KJ, Tidey JW, Sussman S, Kahler CW. Anhedonia and the relative reward value of drug and nondrug reinforcers in cigarette smokers. Journal of Abnormal Psychology. 2014;123(2):375–386. doi: 10.1037/a0036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Luo X, Le CT, Chu H, Epstein LH, Yu J, Thomas JL. Analysis of cigarette purchase task instrument data with a left-censored mixed effects model. Experimental and Clinical Psychopharmacology. 2013;21(2):124–132. doi: 10.1037/a0031610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonczak HS, Neighbors C, Donovan DM. Predicting risky and angry driving as a function of gender. Accident; Analysis and Prevention. 2007;39(3):536–545. doi: 10.1016/j.aap.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yücel M, Kettle JWL, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Archives of General Psychiatry. 2009;66(2):205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216(3):305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control patients: Drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5(3):256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Molecular Psychiatry. 2006;11(4):400–409. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Goodman AM, Chabac S, Lehert P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: the role of patient motivation. Journal of Psychiatric Research. 2006;40(5):383–393. doi: 10.1016/j.jpsychires.2006.02.002. [DOI] [PubMed] [Google Scholar]
- McClure EA, Vandrey RG, Johnson MW, Stitzer ML. Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2013;15(1):139–148. doi: 10.1093/ntr/nts101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Bickel WK. A dual-systems perspective on addiction: contributions from neuroimaging and cognitive training. Annals of the New York Academy of Sciences. 2014;1327:62–78. doi: 10.1111/nyas.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27(21):5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2000;22(2):108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Mcgregor C, Darke S, Ali R, Christie P. Experience of non-fatal overdose among heroin users in Adelaide, Australia: circumstances and risk perceptions. Addiction. 1998;93(5):701–711. doi: 10.1046/j.1360-0443.1998.9357016.x. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological Review. 1999;106(1):3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Miles FJ, Everitt BJ, Dickinson A. Oral cocaine seeking by rats: action or habit? Behavioral Neuroscience. 2003;117(5):927–938. doi: 10.1037/0735-7044.117.5.927. [DOI] [PubMed] [Google Scholar]
- Miller-Day MA, Alberts J, Hecht ML, Trost MR, Krizek RL. Adolescent Relationships and Drug Use. Psychology Press; 2014. [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological Review. 1993;100(4):674–701. [PubMed] [Google Scholar]
- Muraven M. Building self-control strength: Practicing self-control leads to improved self-control performance. Journal of Experimental Social Psychology. 2010;46(2):465–468. doi: 10.1016/j.jesp.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M, Collins RL, Nienhaus K. Self-control and alcohol restraint: an initial application of the self-control strength model. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2002;16(2):113–120. doi: 10.1037//0893-164x.16.2.113. [DOI] [PubMed] [Google Scholar]
- Muraven M, Tice DM, Baumeister RF. Self-control as limited resource: regulatory depletion patterns. Journal of Personality and Social Psychology. 1998;74(3):774–789. doi: 10.1037//0022-3514.74.3.774. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nature Neuroscience. 2006;9(8):1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in Neurosciences. 2009;32(1):56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Trends & Statistics. 2015 Aug 20; Retrieved February 13, 2017, from https://www.drugabuse.gov/related-topics/trends-statistics.
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nature Reviews Neuroscience. 2001;2(2):119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Noël X, Brevers D, Bechara A. A triadic neurocognitive approach to addiction for clinical interventions. Frontiers in Psychiatry/Frontiers Research Foundation. 2013;4:179. doi: 10.3389/fpsyt.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD, Independent Scientific Committee on Drugs Drug harms in the UK: a multicriteria decision analysis. The Lancet. 2010;376(9752):1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? Journal of Psychopharmacology. 1998;12(1):15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Lafond MV, Everitt BJ, Dickinson A. Cocaine seeking by rats is a goal-directed action. Behavioral Neuroscience. 2001;115(2):394–402. [PubMed] [Google Scholar]
- Malley PMO. Maturing out of problematic alcohol use. Alcohol Research and Health. 2004;28(4):202. [Google Scholar]
- O’Neill J, Daniel TO, Epstein LH. Episodic future thinking reduces eating in a food court. Eating Behaviors. 2016;20:9–13. doi: 10.1016/j.eatbeh.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooteman W, Naassila M, Koeter MWJ, Verheul R, Schippers GM, Houchi H, van den Brink W. Predicting the effect of naltrexone and acamprosate in alcohol-dependent patients using genetic indicators. Addiction Biology. 2009;14(3):328–337. doi: 10.1111/j.1369-1600.2009.00159.x. [DOI] [PubMed] [Google Scholar]
- Padrão P, Lunet N, Santos AC, Barros H. Smoking, alcohol, and dietary choices: evidence from the Portuguese National Health Survey. BMC Public Health. 2007;7:138. doi: 10.1186/1471-2458-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76(Pt B):342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peele S. The meaning of addiction: Compulsive experience and its interpretation. Lexington Books/D. C. Heath and Com; 1985. [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66(1):138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154(3):243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93(5):729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Stinson FS, Grant BF. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of Clinical Psychiatry. 2005;66(5):564–574. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. The Journal of Pharmacology and Experimental Therapeutics. 1968;161(1):122–129. [PubMed] [Google Scholar]
- Popper K. The Logic of Scientific Discovery. Routledge; 2005. [Google Scholar]
- Price RK, Risk NK, Spitznagel EL. Remission from drug abuse over a 25-year period: patterns of remission and treatment use. American Journal of Public Health. 2001;91(7):1107–1113. doi: 10.2105/ajph.91.7.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. American Journal of Health Promotion: AJHP. 1997;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience and Biobehavioral Reviews. 2012;36(1):479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. The Wiley Handbook on the Cognitive Neuroscience of Addiction. John Wiley & Sons, Ltd; 2015. Addiction as a Symptom of Failure Modes in the Machineries of Decision Making; pp. 151–172. [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behavioural Pharmacology. 2006;17(8):651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive– sensitization view. Addiction. 2000;95(8s2):91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and drug “wanting. Psychopharmacology. 2004;171(3):352–353. [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. (Series B, Biological Sciences).Philosophical Transactions of the Royal Society of London. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryle PR, Thomson AD. Nutrition and vitamins in alcoholism. Contemporary Issues in Clinical Biochemistry. 1984;1:188–224. [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annual Review of Neuroscience. 2010;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. US Department of Health and Human Services; 2014. Retrieved from https://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf. [Google Scholar]
- SAMHSA. Mental Service and Administration (SAMHSA) Prevention of Substance Abuse and Mental Illness [Internet] 2015 [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Current Opinion in Neurobiology. 1997;7(2):191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annual Review of Neuroscience. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annual Review of Pharmacology. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacology & Therapeutics. 2011;132(3):215–241. doi: 10.1016/j.pharmthera.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffer CE, Mennemeier M, Landes RD, Bickel WK, Brackman S, Dornhoffer J, Brown G. Neuromodulation of delay discounting, the reflection effect, and cigarette consumption. Journal of Substance Abuse Treatment. 2013;45(2):206–214. doi: 10.1016/j.jsat.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siahpush M, McNeill A, Hammond D, Fong GT. Socioeconomic and country variations in knowledge of health risks of tobacco smoking and toxic constituents of smoke: results from the 2002 International Tobacco Control (ITC) Four Country Survey. Tobacco Control. 2006;15(Suppl 3):iii65–70. doi: 10.1136/tc.2005.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of General Psychiatry. 2006;63(3):324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183(2):171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CSR. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug and Alcohol Review. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Skinner BF. The Behavior of Organisms (Appleton-Century-Crofts, New York) Spaulding, WD; 1938. [Google Scholar]
- Storms L, Goodrich V, Sullivan M. Applications of Experimental Psychopathology in Psychiatric Rehabilitation. Schizophrenia Bulletin. 1986;12:560–577. doi: 10.1093/schbul/12.4.560. [DOI] [PubMed] [Google Scholar]
- Skinner BF. Science And Human Behavior. Simon and Schuster; 1953. [Google Scholar]
- Snider SE, LaConte SM, Bickel WK. Episodic Future Thinking: Expansion of the Temporal Window in Individuals with Alcohol Dependence. Alcoholism, Clinical and Experimental Research. 2016;40(7):1558–1566. doi: 10.1111/acer.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychological Review. 1974;81(2):119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Southwick L, Steele CM. Restrained Drinking: Personality Correlates of a Control Style. Journal of Drug Issues. 1987 https://doi.org/10.1177/002204268701700403.
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurodevelopment. The Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine. 2013;52(2 Suppl 2):S7–13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears DE. Economic decision-making in poverty depletes behavioral control. The BE Journal of Economic Analysis and Policy. 2010;11(1) https://doi.org/https://doi.org/10.2202/1935-1682.2973. [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annual Review of Clinical Psychology. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Wilson AG, Koffarnus MN, Daniel TO, Epstein LH, Bickel WK. Unstuck in time: episodic future thinking reduces delay discounting and cigarette smoking. Psychopharmacology. 2016;233(21–22):3771–3778. doi: 10.1007/s00213-016-4410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S, Leventhal A. Substance misuse prevention: addressing anhedonia. New Directions for Youth Development. 2014;2014(141):45–56. doi: 10.1002/yd.20085. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2016;26(1):37–44. doi: 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacology, Biochemistry, and Behavior. 2007;87(4):426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Tice DM, Bratslavsky E, Baumeister RF. Emotional distress regulation takes precedence over impulse control: if you feel bad, do it! Journal of Personality and Social Psychology. 2001;80(1):53–67. [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological Review. 1990;97(2):147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. The European Journal of Neuroscience. 2005;22(10):2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Molecular Psychiatry. 2006;11(6):594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Turel O, Bechara A. A Triadic Reflective-Impulsive-Interoceptive Awareness Model of General and Impulsive Information System Use: Behavioral Tests of Neuro-Cognitive Theory. Frontiers in Psychology. 2016;7:601. doi: 10.3389/fpsyg.2016.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005;25(38):8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers JA, Bongers IM, van de Goor LA, Garretsen HF. Alcohol consumption, alcohol- related problems, problem drinking, and socioeconomic status. Alcohol and Alcoholism. 1999;34(1):78–88. doi: 10.1093/alcalc/34.1.78. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Adams R, Chambers CD. Proactive motor control reduces monetary risk taking in gambling. Psychological Science. 2012;23(7):805–815. doi: 10.1177/0956797611434538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56(Suppl 1):48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neuroscience and Biobehavioral Reviews. 2012;36(8):1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neuroscience and Biobehavioral Reviews. 2004;27(8):827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD. Addiction science: Uncovering neurobiological complexity. Neuropharmacology. 2014;76(Pt B):235–249. doi: 10.1016/j.neuropharm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. The American Journal of Psychiatry. 2001;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14(2):169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. The New England Journal of Medicine. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Skolnick P. New medications for substance use disorders: challenges and opportunities. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2012;37(1):290–292. doi: 10.1038/npp.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, Pappas NR. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. The Journal of Pharmacology and Experimental Therapeutics. 1999;291(1):409–415. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, MacGregor R. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16(4):255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annual Review of Pharmacology and Toxicology. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27(46):12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Altman M, Boswell RG, Kelley WM, Heatherton TF. Self-regulatory depletion enhances neural responses to rewards and impairs top-down control. Psychological Science. 2013;24(11):2262–2271. doi: 10.1177/0956797613492985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Heatherton TF. Self-regulatory depletion increases emotional reactivity in the amygdala. Social Cognitive and Affective Neuroscience. 2013;8(4):410–417. doi: 10.1093/scan/nss082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom LC, McChargue DE, Mackillop J. Corrigendum to “DRD2/ANKK1 TaqI A genotype moderates the relationship between alexithymia and the relative value of alcohol among male college binge drinkers” [Pharmacol. Biochem. Behav. 102 (2012) 471–476] Pharmacology, Biochemistry, and Behavior. 2016;144:92. doi: 10.1016/j.pbb.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Wang GB, Zhang XL, Zhao LY, Sun LL, Wu P, Lu L, Shi J. Drug-related cues exacerbate decision making and increase craving in heroin addicts at different abstinence times. Psychopharmacology. 2012;221(4):701–708. doi: 10.1007/s00213-011-2617-5. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Läck C, Morgan D, Roberts DCS. Discrete-trials heroin self-administration produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology. 2006;185(2):150–159. doi: 10.1007/s00213-005-0288-9. [DOI] [PubMed] [Google Scholar]
- Warner M, Chen LH, Makuc DM, Anderson RN, Miniño AM. Drug poisoning deaths in the United States, 1980–2008. NCHS Data Brief. 2011;(81):1–8. [PubMed] [Google Scholar]
- Weller RE, Cook EW, 3rd, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51(3):563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Bickel WK. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biological Psychiatry. 2014;75(6):435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Social Cognitive and Affective Neuroscience. 2013;8(1):73–84. doi: 10.1093/scan/nsr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Brown J. Theory of Addiction. John Wiley & Sons; 2013. [Google Scholar]
- White NM. A functional hypothesis concerning the striatal matrix and patches: mediation of S-R memory and reward. Life Sciences. 1989;45(21):1943–1957. doi: 10.1016/0024-3205(89)90569-9. [DOI] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91(7):921–49. discussion 951–65. [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiology of Learning and Memory. 2002;77(2):125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Wiehler A, Bromberg U, Peters J. The Role of Prospection in Steep Temporal Reward Discounting in Gambling Addiction. Frontiers in Psychiatry/Frontiers Research Foundation. 2015;6:112. doi: 10.3389/fpsyt.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A, Pescor FT. Classical conditioning of a morphine abstinence phenomenon, reinforcement of opioid-drinking behavior and “relapse” in morphine-addicted rats. Psychopharmacologia. 1967;10(3):255–284. doi: 10.1007/BF00401386. [DOI] [PubMed] [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacology & Therapeutics. 1987;35(1–2):227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- Wise RA. The neurobiology of craving: implications for the understanding and treatment of addiction. Journal of Abnormal Psychology. 1988;97(2):118–132. doi: 10.1037//0021-843x.97.2.118. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotoxicity Research. 2008;14(2–3):169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal–not just mesocorticolimbic–dopamine in reward and addiction. Trends in Neurosciences. 2009;32(10):517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annual Review of Psychology. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wong CF, Silva K, Kecojevic A, Schrager SM, Bloom JJ, Iverson E, Lankenau SE. Coping and emotion regulation profiles as predictors of nonmedical prescription drug and illicit drug use among high-risk young adults. Drug and Alcohol Dependence. 2013;132(1–2):165–171. doi: 10.1016/j.drugalcdep.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive Sensitization by Previous Amphetamine Exposure: Increased Cue-Triggered “Wanting” for Sucrose Reward. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21(19):7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(46):15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MA, Moeller SJ, Goldstein RZ. Cognitive interventions for addiction medicine: Understanding the underlying neurobiological mechanisms. Progress in Brain Research. 2016;224:285–304. doi: 10.1016/bs.pbr.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuriff GE. Radical behaviorist epistemology. Psychological Bulletin. 1980;87(2):337. [Google Scholar]


