Summary
Diffuse malignant mesothelioma of the pleura is a highly aggressive tumor typically associated with short survival. ALCAM (CD166), a type I transmembrane protein, is a member of the immunoglobulin superfamily. In normal cells, ALCAM regulates physiological processes such as angiogenesis and immune response. In cancer, it is associated with neoplastic progression, including invasion, migration, and metastasis. Furthermore, ALCAM is considered one of the cancer stem cell markers such as ALDH1 (ALDH1A1) and SALL4. The PD-L1 (CD274)/PD-1 (PDCD1, CD279) pathway is crucial for the modulation of immune responses in normal cells. Nevertheless, pathologic activation of the PD-L1/PD-1 pathway participates in immune evasion by tumor cells. Many PD-L1–expressing tumor cells have been identified in different types of cancer, including malignant mesothelioma. In this study, 175 well-characterized primary diffuse pleural mesotheliomas, including the epithelioid (n = 148), biphasic (n = 15), and sarcomatoid (n = 12) histotypes, were evaluated immunohistochemically for cancer stem cell markers (ALCAM, ALDH1, and SALL4) and PD-L1 expression. Twenty-five percent of the mesotheliomas (43/175) expressed ALCAM, whereas ALDH1 and SALL4 positivity was seen in 1% to 2% of cases. Thirty-three percent of the analyzed tumors (57/175) contained PD-L1–positive cells. Overall survival was significantly decreased in the cohort of patients with ALCAM- or PD-L1–positive tumors (both P < .01). Furthermore, the multivariate Cox hazards regression analysis identified ALCAM and PD-L1 (both P < 0.01) as potential independent risk factors. Thus, a combination of these two markers might be useful for prognostication and planning the treatment of patients with malignant pleural mesothelioma.
Keywords: ALCAM, activated leukocyte cell adhesion molecule CD166; ALDH1A1, aldehyde dehydrogenase, 1A1; MPM, malignant pleural mesothelioma; PD-L1 (CD274), programmed cell death ligand 1; SALL4, spalt-like transcription factor 4
1. Introduction
Malignant mesothelioma (MPM) is an aggressive neoplasm arising from mesothelial cells of the pleura, peritoneum, and pericardium [1]. As many as 80% of cases are of pleural origin and are defined as MPM. Despite advances in the chemotherapy modalities, the prognosis of MPM patients is still poor. [2]
ALCAM (activated leukocyte cell adhesion molecule, CD166) is a type I transmembrane protein and a member of the immunoglobulin superfamily. It consists of five extracellular immunoglobulin-like domains, a transmembrane domain, and a short COOH-terminal cytoplasmic tail [3,4]. ALCAM was first identified on activated leukocytes as a ligand of CD6, and ALCAM–CD6 association was proved to accelerate T-cell activation or proliferation [5,6]. Under physiologic conditions, ALCAM also regulates angiogenesis and the immune response [7,8]. In neoplastic conditions, ALCAM accelerates tumor cell migration, invasion, and metastasis [9–11]. Furthermore, ALCAM is considered a cancer stem cell marker along with ALDH1A1 and SALL4 [12–14]. Expression of cancer stem cell markers has been reported in malignant mesotheliomas, and such expression seemed to be associated with a more malignant phenotype in vitro [9,15]. Among the cancer stem cell markers, only Nestin, a type VI intermediate filament protein, was immunohistochemically analyzed for its prognostic impact on mesothelioma patients [16].
CD274 has been identified as a cell-surface glycoprotein belonging to the B7 family [17]. CD279, a physiological receptor for PD-L1, belongs to the immunoglobulin superfamily and is expressed mainly on activated T cells, as well as on non–T lymphocytes such as B cells and natural killer (NK) cells upon induction [18,19]. In physiologic conditions, the PD-L1/PD-1 axis is crucial for the modulation of the immune system to reduce collateral tissue damage from the inflammatory response in peripheral tissues. This signaling also is important for T-cell responses and the maintenance of self-tolerance to avoid autoimmune diseases [20,21]. When T cells are exposed to chronic antigen stimulation, such as by viral infection or cancer, high expression of PD-1 is induced and leads to T-cell exhaustion or anergy [22]. Expression of PD-L1 has been reported in various tumors, including mesothelioma, in some cases associated with greater tumor aggressiveness and poor clinical outcome [23–25].
Recently, we reported that colorectal carcinomas defined by PD-L1 expression displayed features associated with tumors arising via the serrated neoplasia pathway such as an involvement of the right or transverse colon, poorly differentiated and solid/medullary histology, BRAF V600E mutation, and MMR deficiency [26]. Moreover, close association between PD-L1 expression and the “stem-like” immunophenotype (lack of intestinal differentiation marker CDX2 with prominent expression of ALCAM) was noted in these tumors [26].
This study examined the expression of cancer stem cell markers (ALCAM, ALDH1, and SALL4) as well as PD-L1 in malignant pleural mesothelioma. Also, their association with clinicopathologic features and impacts on prognosis were analyzed to assess their possibility for clinical use.
2. Materials and methods
2.1. Tumors
Thirty tumors were included in a clinical trial, and informed consent was obtained from all patients at the National Institutes of Health (NIH). One hundred forty-five additional anonymous malignant pleural mesotheliomas were collected. This project was completed under the Office of Human Subject Research Exemption with specimens freed of all links to the patient. The tumors selected had been extensively characterized clinically, histologically, and immunohistochemically. All tumors were positive for cytokeratin cocktail AE1/AE3, and all were positive for calretinin and/or WT1. Both TTF-1 and CEA were absent from all tumors. All tumors were multiple (diffuse mesothelioma), and none of them was localized.
2.2. Immunohistochemistry staining
One rectangular tumor sample derived from each surgical specimen was assembled into multitumor blocks containing as many as 40 tissue samples as previously described [27]. The size of the tumor sample was estimated to exceed the size of a single 0.6-mm2 core by a factor of 10 to 15.
Immunohistochemistry staining with ALCAM, ALDH1, SALL4 and PD-L1 antibodies was performed using the Leica Bond-Max automation and Leica Refine detection kit (Leica Biosystems, Bannockburn, IL). The protocols are detailed in Table 1 and include previously published (SALL4 and PD-L1) immunohistochemical procedures [25–27]. For the sequential double staining, additional antibody was depicted by Fast Red chromogen. According to the past report, the immunoreactivity of ALCAM (cell membranous), PD-L1 (cell membranous and/or cytoplasmic), ALDH1 (cytoplasmic), and SALL4 (nuclear) was evaluated with a detection cut-off of 5% [25,26]. Expression of PD-L1 in tumor-associated immune cells (TAIs, mostly macrophages) was also evaluated.
Table 1.
Antibodies and conditions for immunohistochemistry staining
| Gene | Retrieval | Dilution | Antibody |
|---|---|---|---|
| ALDH1 | H1 | 64,000 | Clone 44, BD Biosciences (San Jose CA) |
| ALCAM | H2 | 1000 | Leica Biosystems (San Diego CA) |
| PD-L1 | H2 | 200 | Clone E1L3N, Cell Signaling Technology (Danvers MA) |
| SALL4 | H2 | 200 | Clone 6E3, Biocare Medical (Concord CA) |
NOTE. Antigen retrieval was performed with heat activation in Bond low (H1) or high (H2) pH buffer.
2.3. Statistical analysis
All statistical analyses were performed with EZR version 1.32 software [28]. The χ2 or Fisher exact test was performed to investigate the statistical correlation between categorical data. To analyze the overall survival of mesothelioma patients, the log-rank test was used. For multiple comparisons, the Holm method was used. Moreover, Cox proportional hazards regression analysis was performed to analyze the association of survival and other factors including age (<65 vs ≥65 years old), sex (male vs female), and tumor histology (epithelial vs biphasic vs sarcomatoid). The data from the immunohistochemical analyses (positive vs negative), such as ALDH1, ALCAM, and SALL4, as well as PD-L1 expression in tumors or TAIs, were included in the initial model. A backward elimination with a threshold of P = .05 was used to select variables in the final model. Cases with missing information were eliminated from the statistical analysis of that feature.
3. Results
3.1. Expression of cancer stem cell markers and PD-L1 in mesotheliomas
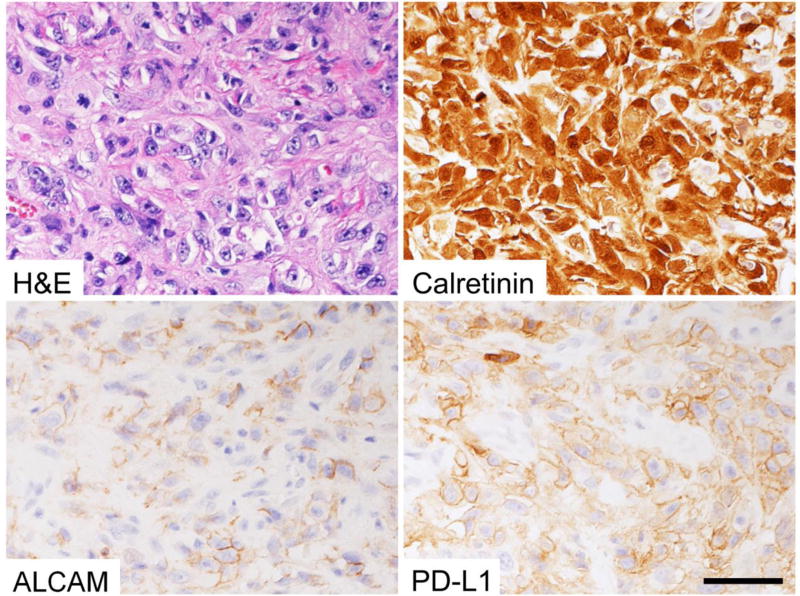
Forty-three of the 175 mesotheliomas (25%) showed variable ALCAM expression on the cell membrane with 5% to 100% (median 20%) of all tumor cells positive (representative image in Fig. 1). Clinical, pathologic, and immunohistochemical features of the tumors are summarized in Table 2 according to ALCAM expression.
Fig. 1.
Representative images of ALCAM and PD-L1 expression in a malignant pleural mesothelioma. Calretinin also was positive. Bar = 50 µm.
Table 2.
Clinical, pathologic, and immunohistochemical features of tumors according to ALCAM expression
| Total, n (%) | ALCAM expression, n (%) | P | ||
|---|---|---|---|---|
|
| ||||
| Positive | Negative | |||
| Total | 175 (100%) | 43 (25%) | 132 (75%) | |
| Sex | 1a | |||
| Male | 101 (69) | 24 (69) | 77 (69) | |
| Female | 45 (31) | 11 (31) | 34 (31) | |
| Mean age, y (mean ± SD) | 59.2 ± 11.8 | 60.1 ± 9.38 | 58.9 ± 12.5 | .60b |
| Histology | .94c | |||
| Epithelial | 148 (85) | 36 (84) | 112 (85) | |
| Biphasic | 15 (9) | 4 (9) | 11 (8) | |
| Sarcomatoid | 12 (7) | 3 (7) | 9 (7) | |
| PD-L1 in tumor cells | .04a | |||
| Positive | 57 (33) | 20 (47) | 37 (28) | |
| Negative | 118 (67) | 23 (53) | 95 (72) | |
| PA-L1 in TAIs | <.01a | |||
| Positive | 35 (20) | 15 (35) | 20 (15) | |
| Negative | 140 (80) | 28 (65) | 112 (85) | |
Calculated by the χ2 test for ALCAM expression.
The t test was used to compare the means of age.
Calculated by Fisher’s exact test for ALCAM expression.
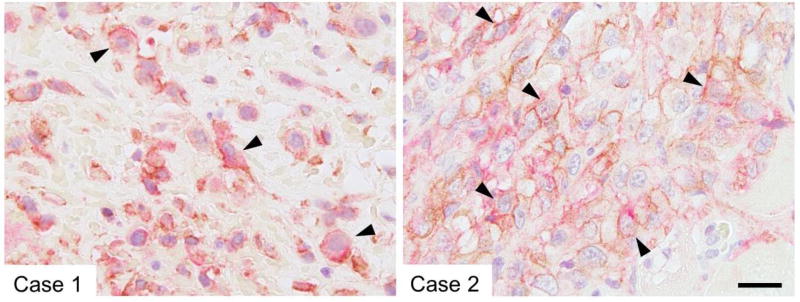
The PD-L1 protein was expressed in 57 tumors (33%, with 5% to 100% [median 60%] of cells being positive; Fig. 1). Statistical analysis showed a mild but significant association between ALCAM and PD-L1 expression (P = .04; Table 2). Among the cases positive for ALCAM and PD-L1, several contained cell populations co-expressing ALCAM and PD-L1 (Fig. 2).
Fig. 2.
Sequential double staining shows coexpression of ALCAM (brown; membranous) and PD-L1 (red; membranous, cytoplasmic, or both) in malignant mesothelioma (arrowheads). Bar = 20 µm.
Expression of PD-L1 on TAIs was detected in 35 cases (20%). Significant association was detected between PD-L1 positivity in TAIs and ALCAM expression (P < .01) or PD-L1 expression (P < .01) on tumor cells (Table 2 and Supplementary Table S1).
The ALDH1 and SALL4 proteins were expressed in only 2 (1%) and 3 (2%) cases, respectively (Supplementary Table S1; Supplementary Fig. S1).
3.2. Survival analyses
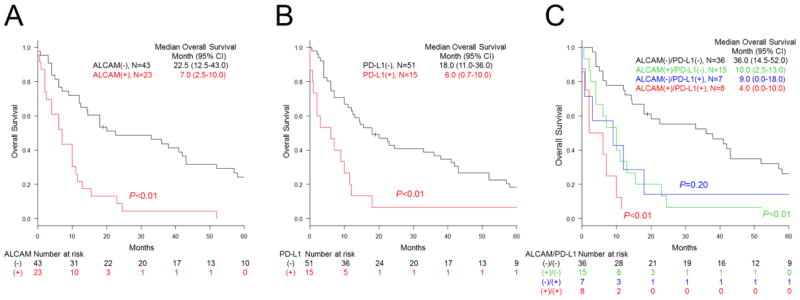
The characteristics of the 66 mesothelioma patients analyzed for survival are summarized in Table 3. Patients were followed for as long as 120 months. Survival was significantly shorter for patients with mesothelioma expressing ALCAM on the cell membrane (7.0 months median vs 22.5 months without ALCAM expression or cytoplasmic labeling only; P < .01; Fig. 3A and Supplementary Fig. S2A). The patients with mesothelioma expressing PD-L1 also had a shorter survival (6.0 months median vs 18.0 months without PD-L1; P < .01; Fig. 3B and Supplementary Fig. S2B). The patients with mesothelioma co-expressing ALCAM and PD-L1 showed the shortest survival (median 4 months vs 36.0 months without ALCAM or PD-L1; P < .01; Fig. 3C). However, no statistical significance was detected in the survival of the patients with ALCAM and PD-L1 double-positive tumors and those with ALCAM-positive/PD-L1–negative (P = .20) or ALCAM-negative/PD-L1–positive tumors (P = .31). Multivariate Cox hazards regression analysis revealed ALCAM (hazard ratio [HR] 3.26; 95% confidence interval [CI] 1.80, 5.92; P < .01) and PD-L1 (HR 2.34; 95% CI 1.26, 4.33; P < .01) to be potential independent risk factors (Table 4). In the present study, no significant difference in clinical outcome was detected among the groups classified according to tumor histology (Supplementary Fig. S2C).
Table 3.
Characteristics of 66 mesothelioma patients according to survival
| Characteristic | n (%) |
|---|---|
| Age (y) | |
| Mean | 60.9 ± 10.5 |
| Median (range) | 61 (27–87) |
| Sex (%) | 50 (76)/16 (24) |
| Histology | |
| Epithelial | 56 (85) |
| Biphasic | 7 (11) |
| Sarcomatoid | 3 (5) |
| Double positive for ALCAM and PDL-1 (%) | 8 (12) |
| ALCAM positive | 23 (35) |
| PD-L1 positive in tumor cells | 15 (23) |
| PD-L1 positive in TAIs | 16 (24) |
Fig. 3.
Overall survival of patients with pleural mesothelioma according to ALCAM and PD-L1 expression. Kaplan-Meier curves for the patients carrying mesothelioma with or without ALCAM (A) and PD-L1 (B) expression. C, Kaplan-Meier curves for patients grouped according to ALCAM or PD-L1 expression or both. P values were calculated in comparison with ALCAM-negative/PD-L1–negative group.
Table 4.
Multivariate Cox hazards analysis of mesothelioma patients
| Hazard ratio |
95% CI | P | ||
|---|---|---|---|---|
|
| ||||
| Min | Max | |||
| ALCAM positivity | 3.26 | 1.80 | 5.92 | <.01 |
| PD-L1 positivity | 2.34 | 1.26 | 4.33 | <.01 |
NOTE. The model initially included age, sex, tumor histology, ALCAM expression, PD-L1 expression in tumor cells, PD-L1 expression in TAIs, ALDH1 expression, and SALL4 expression. A backward elimination with a threshold of P = .05 was used to select the variables in the final model.
4. Discussion
Cancer stem cell markers (ALCAM, ALDH1, and SALL4) and PD-L1 are variably expressed in different tumors, including malignant mesothelioma [12–14,25,26,29]. Upregulation of ALCAM enhances the malignant phenotype of mesothelioma cells in vitro [9,15]. However, the clinical significance of marker expression has not been fully evaluated in patients with mesothelioma [16]. Also, the associations of cancer stem cell markers and PD-L1 expression have not been analyzed systematically in malignant mesothelioma. In this study, 175 diffuse malignant pleural mesotheliomas were evaluated immunohistochemically for the expression of ALCAM, ALDH1, SALL4, and PD-L1. Also, the associations of these proteins with clinicopathologic features and survival were analyzed.
The ALCAM protein was variably expressed in 25% of mesotheliomas (43/175). In addition, significantly shorter survival was found in the patients with mesotheliomas expressing ALCAM on the cell membrane (median 7.0 months; P < .01). In nude mice with mesothelioma cell transplants, disturbance of the homophilic ALCAM interaction on the cell membrane significantly prolongs survival [15]. This result indicates the biologic significance of its membranous expression. Also, tumor cells expressing ALCAM on the cell membrane showed cancer stem cell properties [14].
These lines of evidence suggest that ALCAM expressed on the cell membrane regulates malignant features of mesothelioma cells, such as migration, invasion, and anchorage-independent colony formation, whereas cytoplasmic expression does not seem to have similar significance. Actually, in our preliminary study, patients with mesotheliomas expressing ALCAM on the cell membrane showed significantly shorter survival than those carrying tumors expressing no ALCAM (P < .01) or cytoplasmic ALCAM (P = .01). Recently, in hepatocellular carcinoma cells, ALCAM was identified as an upstream regulator of Yes-associated protein (YAP), a member of the Hippo signaling pathway, which is involved in mesotheliomagenesis [30]. In the near future, the importance of ALCAM-YAP signaling should be confirmed in mesothelioma cells. Also, ALCAM-targeting therapy by monoclonal antibodies might be considered with attention to physiologic ALCAM expression on colonic stem cells [14].
PD-L1 was expressed in 33% of mesotheliomas (57/175). The patients with PD-L1 expression had shorter survival (median 6.0 months; P < .01). Furthermore, in our preliminary study, PD-L1 expression showed worse prognostic effects in a proportion of cases in a tumor-dependent manner. This result parallels two previous reports on the adverse prognostic significance of PD-L1 expression in malignant mesothelioma [23,24]. Many tumor types express PD-L1 on the cell membrane, and this contributes to their evasion of the host immune system. Thus, PD-L1/PD-1 immune checkpoint inhibitors were used in treatment trials and showed significant anti-cancer effects in some cases [31,32]. At the time of submission of this manuscript, some clinical trials (such as NCT02497508 and NCT02716272) were in progress to assess PD-L1/PD-1 immune checkpoint inhibitors in patients with mesothelioma.
Immunohistochemical staining has been used as a potential biomarker to predict the response to such inhibitors [31,32]. However, PD-L1/PD-1 immunotherapy has shown anti-cancer effects identical to those of chemotherapy in tumors with low PD-L1 expression (1% or less of the population) [31]. These findings have led to the hypothesis that PD-L1 might be expressed on cancer cells harboring specific characteristics such as tumor-initiating cells or cancer stem cells. Actually, our recent study revealed the association between PD-L1 expression and a “stem-like” immunophenotype in colorectal cancer [26]. In the present study, statistical analysis revealed a significant association between ALCAM and PD-L1 expression (P = .04). Furthermore, in several cases, co-expression of ALCAM and PD-L1 was detected by sequential immunostaining. This suggests that a portion of PD-L1–positive mesothelioma cells might have stem cell–like properties. However, further experimental investigation is needed for confirmation of this idea.
Several reports have shown a significant association between PD-L1 expression and non-epithelioid histology in mesotheliomas [23,24]. Although biphasic (6/15; 40%) and sarcomatoid (5/12; 42%) mesotheliomas showed slightly higher PD-L1 positivity than epithelial mesothelioma (46/148; 31%), no clear association was detected between PD-L1 expression and mesothelioma histology. A larger study might clarify this point. Sarcomatoid mesothelioma often has been considered a product of epithelial–mesenchymal transition (EMT) [16]. This transition is associated with stem cell features. Therefore, in this study, the correlation of ALCAM expression and histologic variations were analyzed. However, no clear correlation was detected.
Expressions of ALCAM and PD-L1 were found to be independent risk factors for a shorter survival in patients with mesothelioma. Indeed, mesotheliomas co-expressing ALCAM and PD-L1 were associated with the shortest survival (median only 4 months), indicating additive negative effects of ALCAM and PD-L1 expression. Because of the limitations in patient clinical information such as complete tumor staging or treatment modality, as well as limited clinical samples (many of them being small biopsy specimens non-informative for staging purposes), it was difficult to include these features in the Cox proportional hazards regression model. Further study of a larger number of mesothelioma patients and their clinical treatments and outcomes might establish a better prognostication system using ALCAM and PD-L1 expression.
It is possible that PD-L1–expressing TAIs or follicular dendritic cells are able to help tumors evade immune action [33–35]. In the present study, PD-L1 expression on TAIs was detected in 20% of cases (35/175). In addition, a significant association between tumor ALCAM expression (P < .01) or tumor PD-L1 expression (P < .01) was detected. At present, the molecular mechanism(s) for the co-expression of PD-L1 in TAIs and mesothelioma cells is unclear; however, interferon-γ (IFNγ) from tumor-associated lymphocytes might induce PD-L1 expression on both types of cells. This mechanism(s) should be elucidated in the near future.
In conclusion, the present study demonstrated that expression of ALCAM and PD-L1 in malignant pleural mesothelioma is associated with shorter survival. These markers could be useful in determining the prognosis of patients with pleural mesothelioma and in planning treatment.
Supplementary Material
Highlights.
PD-L1 and stem cell marker expressions were analyzed in malignant mesotheliomas.
PD-L1 and ALCAM were expressed in 33% and 25% of mesothelioma cases, respectively.
The Cox proportional hazards model identified these proteins as independent risk factors for death.
Acknowledgments
Source of Funding: This work was supported as a part of the National Cancer Institute’s Intramural Research Program.
We thank Dr. Yasuyuki Arai (National Institutes of Health) for advice on statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 3.Bowen MA, Patel DD, Li X, et al. Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med. 1995;181:2213–20. doi: 10.1084/jem.181.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelissen JM, Peters IM, de Grooth BG, et al. Dynamic regulation of activated leukocyte cell adhesion molecule-mediated homotypic cell adhesion through the actin cytoskeleton. Mol Biol Cell. 2000;11:2057–68. doi: 10.1091/mbc.11.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitney GS, Starling GC, Bowen MA, et al. The membrane-proximal scavenger receptor cysteine-rich domain of CD6 contains the activated leukocyte cell adhesion molecule binding site. J Biol Chem. 1995;270:18187–90. doi: 10.1074/jbc.270.31.18187. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman AW, Joosten B, Torensma R, et al. Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood. 2006;107:3212–20. doi: 10.1182/blood-2005-09-3881. [DOI] [PubMed] [Google Scholar]
- 7.Swart GW. Activated leukocyte cell adhesion molecule (CD166/ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol. 2002;81:313–21. doi: 10.1078/0171-9335-00256. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda K, Quertermous T. Molecular isolation and characterization of a soluble isoform of activated leukocyte cell adhesion molecule that modulates endothelial cell function. J Biol Chem. 2004;279:55315–23. doi: 10.1074/jbc.M407776200. [DOI] [PubMed] [Google Scholar]
- 9.Lunter PC, van Kilsdonk JW, van Beek H, et al. Activated leukocyte cell adhesion molecule (ALCAM/CD166/MEMD), a novel actor in invasive growth, controls matrix metalloproteinase activity. Cancer Res. 2005;65:8801–8. doi: 10.1158/0008-5472.CAN-05-0378. [DOI] [PubMed] [Google Scholar]
- 10.van Kempen LC, van den Oord JJ, van Muijen GN, et al. Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am J Pathol. 2000;156:769–74. doi: 10.1016/S0002-9440(10)64943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Kilsdonk JW, Wilting RH, Bergers M, et al. Attenuation of melanoma invasion by a secreted variant of activated leukocyte cell adhesion molecule. Cancer Res. 2008;68:3671–9. doi: 10.1158/0008-5472.CAN-07-5767. [DOI] [PubMed] [Google Scholar]
- 12.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng J, Deng R, Zhang P, et al. miR-219-5p plays a tumor suppressive role in colon cancer by targeting oncogene SALL4. Oncol Rep. 2015;34:1923–32. doi: 10.3892/or.2015.4168. [DOI] [PubMed] [Google Scholar]
- 14.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishiguro F, Murakami H, Mizuno T, et al. Activated leukocyte cell-adhesion molecule (ALCAM) promotes malignant phenotypes of malignant mesothelioma. J Thorac Oncol. 2012;7:890–9. doi: 10.1097/JTO.0b013e31824af2db. [DOI] [PubMed] [Google Scholar]
- 16.Thies S, Friess M, Frischknecht L, et al. Expression of the stem cell factor nestin in malignant pleural mesothelioma is associated with poor prognosis. PLoS One. 2015;10:e0139312. doi: 10.1371/journal.pone.0139312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 18.Fanoni D, Tavecchio S, Recalcati S, et al. New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett. 2011;134:157–60. doi: 10.1016/j.imlet.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Terme M, Ullrich E, Aymeric L, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71:5393–9. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22:265–8. doi: 10.1016/s1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 22.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 23.Cedres S, Ponce-Aix S, Zugazagoitia J, et al. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM) PLoS One. 2015;10:e0121071. doi: 10.1371/journal.pone.0121071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansfield AS, Roden AC, Peikert T, et al. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol. 2014;9:1036–40. doi: 10.1097/JTO.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaguma S, Wang Z, Lasota J, et al. Comprehensive immunohistochemical study of programmed cell death ligand 1 (PD-L1): analysis in 5536 cases revealed consistent expression in trophoblastic tumors. Am J Surg Pathol. 2016;40:1133–42. doi: 10.1097/PAS.0000000000000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaguma S, Lasota J, Wang Z, et al. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)–positive colorectal carcinomas. Mod Pathol. 2017;30:278–85. doi: 10.1038/modpathol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miettinen M. A simple method for generating multitissue blocks without special equipment. Appl Immunohistochem Mol Morphol. 2012;20:410–2. doi: 10.1097/PAI.0b013e318245c82f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miettinen M, Wang Z, McCue PA, et al. SALL4 expression in germ cell and non-germ cell tumors: a systematic immunohistochemical study of 3215 cases. Am J Surg Pathol. 2014;38:410–20. doi: 10.1097/PAS.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma L, Wang J, Lin J, et al. Cluster of differentiation 166 (CD166) regulated by phosphatidylinositide 3-kinase (PI3K)/AKT signaling to exert its anti-apoptotic role via yes-associated protein (YAP) in liver cancer. J Biol Chem. 2014;289:6921–33. doi: 10.1074/jbc.M113.524819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–37. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 35.Bloch O, Crane CA, Kaur R, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19:3165–75. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.