Abstract
Exposure to ambient particulate matter (PM) has been linked to adverse pulmonary and cardiovascular health effects. Activation of both inflammatory and oxidative stress pathways has been observed and may be a probable cause of these outcomes. We tested the hypothesis that in human monocytes, PM-induced oxidative and inflammatory responses are interrelated. A human monocytic cell line (THP-1) was used to determine if dose and differentiation state plays a role in the cellular response after a 24hr exposure to particles. Primary human monocytes derived from eight female, nonsmoker donors (aged: 21, 24, 27, 28, 48, 49, 54 & 60yo) were used to determine if the age of donors modulates the response. Cells were treated with aqueous suspensions of ambient ultrafine particles (UFP, defined as smaller than 0.2 μm in size) or a media control for 24hr. After exposure, reactive oxygen species (ROS) formation was increased irrespective of dose or differentiation state of THP-1 cells. In the primary human monocytes, ROS formation was not significantly changed. The release of the proinflammatory cytokine, tumor necrosis factor alpha (TNF-α), was dose-dependent and greatest in differentiated compared to undifferentiated THP-1 cells exposed to UFP. In the Primary human monocytes, TNF-α secretion was increased irrespective of the age of the donor. Our results suggest that after a 24hr exposure to particles, general reactive oxygen species formation was nonspecific and uncorrelated to cytokine secretion which was consistently enhanced. Cytokines play an important role in orchestrating many immune responses and thus the ability of ambient particles to enhance robust secretion of a proinflammatory cytokine from primary human monocytes, and how this may influence the response to pathogens and alter disease states, needs to be further evaluated.
Keywords: Ultrafine Particles (UFP), Monocytes, Reactive Oxygen Species (ROS), Oxidative Stress, Tumor necrosis factor alpha (TNF-α), Inflammation
Introduction
Particle formation from vehicle exhaust and industrial emissions is a prominent cause of air pollution in the modern urban environment. Epidemiological studies have found a correlation between exposure to ambient particles and increased risk of morbidity and premature mortality (Dockery et al., 1993; Pope et al., 2002; Brook et al., 2004; Chen et al., 2013; Chen et al., 2017; Li et al., 2017). PM associated mortality was mainly contributed to cardiopulmonary causes and lung cancer (Pope et al., 1995). According to the World Health Organization (WHO), in 2012, exposure to ambient air pollution was estimated to cause approximately three million deaths. Of these mortalities, 8% was associated with chronic obstructive pulmonary disease; 15% connected with ischemic heart disease; 17% linked to respiratory infection and 25% related to lung cancer (WHO, 2017).
Ambient particulate matter (PM) comprises a wide range of metallic, inorganic and organic compounds, which determine its toxic potential. Many studies show that particles have the capability of enhancing cellular oxidative and inflammatory responses in vitro (Schwarze et al., 2007; Araujo and Nel, 2009; Steenhof et al., 2011; Ghio et al., 2012; Thomson et al., 2016). Particle induced oxidative stress and inflammation also play an important causative role in many diseases including cardiovascular disease (Lawal et al., 2017). The objective of this study was to test the hypothesis that PM-induced oxidative and inflammatory changes in human monocytes are interconnected and influenced by dose, differentiation state, and age of donors.
Inhaled ultrafine particles (UFP, defined as particles smaller than approximately 0.2 μm) have been shown to translocate from the lung to the blood (Nemmar et al., 2001; Nemmar et al., 2002). Once particles are in the peripheral circulation, monocytes are one of the first cellular defense responders. To test how dose (2 or 20μg/ml) and differentiated state of monocytes influence response, THP-1 cells were used. This inexpensive and well-characterized in vitro model has been well established to study the potential of ambient particles to activate immune-competent cells (Qin et al., 2012; Wu et al., 2014). The concentration chosen is based on other in vitro studies which use a wide range (1–500 μg/mL) for toxicity testing (Bhavaraju et al., 2014; Wu et al., 2014; Thomson et al., 2016). Although reactive oxygen species (ROS) formation was enhanced, this was independent of dose or differentiation state of the cells. On the other hand, differentiated THP-1 cells were more capable compared to undifferentiated cells of releasing the proinflammatory cytokine, tumor necrosis factor alpha (TNF-α), in response to particle exposure. This response was dose-dependent. Primary human monocytes from eight different donors (ages: 21, 24, 27, 28, 48, 49, 54, and 60 years old) were exposed to the lower and more biologically relevant concentration of UFP (2μg/mL) for 24hr. Although particles did not induce ROS formation in primary monocytes, they caused a robust secretion of TNF-α independent of the age of the donor. Our results indicate that contrary to our hypothesis, the oxidative and proinflammatory response of human monocytes to UFP do not appear to be correlated.
Methods
Particle Collection & Characterization
UFP (particles with aerodynamic diameter less than 0.2 μm) were collected continuously for approximately 40 days by means of a high-volume ultrafine particle (HVUP) sampler, operating at 400L/min (Misra et al., 2002). Sampling was conducted in downtown Los Angeles. The sampling site represents a typical urban background site impacted by mostly traffic sources and located about 150m east of the I-110 freeway (Ning et al., 2007). For the purpose of chemical speciation, the sampler was loaded with zefluor filters (supported PTFE, 3.0μm pore, 8” × 10”, Pall Life Sciences). The collected particles were then transferred into an aqueous suspension by soaking of the particle-loaded filter in ultrapure water, followed by 5min vortexing and 15min sonication. Aliquots of the UFP slurry samples were analyzed for total organic carbon (TOC) as well as total metals and trace elements. TOC content was determined using a Sievers 900 Total Organic Carbon Analyzer (Stone et al., 2009). Metals and elements were quantified by means of high- resolution magnetic sector inductively coupled plasma mass spectrometry (SF-ICP-MS, Thermo-Finnigan Element 2), following acidification (16N HNO3) of the slurry sample (Zhang et al., 2008).
Exposure
THP-1 cells
THP-1 human leukemic monocytes (ATCC) were maintained at 37°C in 5% CO2. The cells were grown in RPMI-1640 medium (ATCC) containing 0.05mM 2-mercaptoethanol, 10% fetal bovine serum, and 1% Pen/Strep. THP-1 cells were seeded (10,000 cells/well) in a 96-well plate. For differentiation, cells were incubated with 12-O-tetradecanoylphorbol-13-acetate (TPA) for 48hr (Tsuchiya et al., 1982). Cells were exposed to 0 (control), 2 or 20μg/mL of UFP for 24hr. Before and after exposure, the cells were visualized and changes were captured using an EVOS FL Cell Imaging System (Life Technologies). After the 24hr exposure, supernatant was collected. The cells were immediately used for ROS assay. Cell cytotoxicity was measured in THP-1 cells by evaluating lactate dehydrogenase (LDH) in the supernatant using the LDH-cytotoxicity colorimetric assay kit from Biovision (Milpitas, CA). The cellTiter-Glo luminescent cell viability assay by Promega (Madison, WI) was used to evaluate cell viability in primary human monocytes at the conclusion of the last kinetic reading for ROS formation.
Primary monocytes
CD14+ primary monocytes, isolated from peripheral blood, were purchased from StemCell Technologies (Cambridge, MA). Eight different donors were selected as biologically independent samples and each of the samples were run in quadruplicates. The donors were all female Caucasian nonsmokers. The ages were (21, 24, 27, 28, 48, 49, 54 and 60 years old). To better delineate the association of age to particle response, the data is presented per individual as well as divided into two age groups (21, 24, 27, 28 = younger donors and (48, 49, 54, 60 = older donors). Cells were thawed and grown in RPMI 1640 medium supplemented with 10% FBS following the manufacturer’s protocol. Cells were seeded at a density of 10,000/well in 96 well plates for 24hr before exposure with 2μg/mL of UFP. Supernatant was collected and ROS formation was determined followed by CellTiter-Glo luminescent cell viability assay (Promega, Wisconsin). Secreted LDH levels were also quantitated as another measure of cytotoxicity.
Reactive Oxygen Species Formation
2′,7′-dichlorofluorescein diacetate (DCFH-DA) was used to measure non-specific ROS formation after PM exposure. This dye rapidly diffuses into cells and is hydrolyzed by intracellular esterases to dichlorofluorescein (DCFH). DCFH is oxidized to the fluorescent 2′,7′-dichloro-fluorescein (DCF) by generated reactive oxygen species. DCF standard curve was used to quantify ROS formation. BioTek Synergy HT Microplate Reader was used to measure the fluorescence generated kinetically for 1hr at 10min intervals at an excitation of 485nm and emission of 528nm. Results shown are for amount of DCF generated in 30 minutes and normalized based on viable cells in corresponding well.
Viability assays
CellTiterGlo-Luminescent cell viability assay was used to measure the number of viable primary human monocytes following the manufacturer’s protocol. This assay measures the amount of ATP present in each sample and is based on the assumption that the number of viable cells are directly proportional to the ATP content. Briefly, 100μl of the CellTiter-Glo reagent is added to each well and the samples are incubated for 10min at room temperature. The luminescence was recorded and the data was calculated as percent control. Levels of LDH in the supernatant was evaluated for THP-1 cells using the LDH-cytotoxicity colorimetric assay kit provided by Biovision. This assay measures the activity of the enzyme as a marker for membrane damage. Substrate is added to the samples and after a 30min incubation, the absorption was measured at 450nm using a BioTek Synergy HT Microplate Reader.
Cytokine Levels
ELISA kits purchased from Invitrogen (Carlsbad, CA) were used to measure Human TNF-α in 96 well plates coated with antibodies for the cytokine in accordance with the manufacturer’s protocol. The absorbance was recorded at 450nm using a BioTek Synergy HT Microplate Reader.
Statistical Analysis
Statistical analysis was conducted using GraphPad Prism 6.0. Statistical significance was determined using either a One-Way or Two-Way ANOVA where appropriate followed by a Bonferroni post-hoc test. Significant values had a p of ≤ 0.05. In all figures, the error bars represent SEM.
Results
PM Characterization
Table 1 shows the mass fraction of Total Organic Carbon (TOC), as well as trace elements and metals to the overall mass of the ultrafine particles. TOC, accounting for about 56.4% of the total ultrafine mass, typically consists of organic compounds directly emitted from combustion sources (e.g. Polycyclic Aromatic Hydrocarbons (PAHs), Hopanes, Steranes and Alkanes), in addition to water soluble organic compounds formed through photochemical reactions in the atmosphere (Hasheminassab et al., 2013). The most abundant elements in the UFP sample were sulfur (S: 6.2%), sodium (Na: 6.1%), Calcium (Ca: 5.4%), magnesium (Mg: 1.7%), aluminum (Al: 1.5%) and potassium (K: 1.1%). Transition metals: iron (Fe), manganese (Mn), vanadium (V), chromium (Cr), copper (Cu), nickel (Ni), zinc (Zn), and lead (Pb) each comprised less than 1% of the total UFP mass. Although the contribution of these metals to the total UFP mass is trivial, they are capable of inducing oxidative stress upon interaction of PM with cells, and therefore are important from a toxicological perspective (Shi et al., 2003; Valavanidis et al., 2005). These metals originate from a variety of sources in the Los Angeles area, most notably vehicular emissions, ship emissions and industrial activities (Saffari et al., 2013).
TABLE 1.
PM Characterization: mass fraction of total organic carbon (TOC), as well as select metals and elements in the UFP slurry.
| Percent (%) Species Fraction |
Ultrafine PM |
|---|---|
| Total Organic Carbon | 56.7 |
| Na | 6.1 |
| Al | 1.5 |
| K | 1.1 |
| Mg | 1.7 |
| Ca | 5.4 |
| Fe | 0.5 |
| Mn | 0.05 |
| S | 6.2 |
| Ti | 0.02 |
| V | 0.006 |
| Cr | 0.014 |
| Cu | 0.1 |
| Ni | 0.04 |
| Zn | 0.5 |
| Ba | 0.12 |
| Pb | 0.02 |
Response of THP-1 cells to UFP
THP-1 cells treated with 2 or 20μg/mL of UFP showed enhanced ROS formation independent of dose (Figure 1A). However, these cells displayed a dose-dependent increase in TNF-α secretion consequent to UFP exposure (Figure 1B). Morphologically, untreated THP-1 cells were spherical in appearance and spread throughout the wells with negligible clumping (Figure 2A). After 24hr of exposure to UFP, cells appeared to aggregate substantially towards visible particle agglomerates (Figure 2B). After differentiation, THP-1 cells became more irregular in appearance (Figure 2C). These cells did not appear to aggregate around visible particles (Figure 2D). There was no change in cell viability after exposure to UFP (data not shown). ROS formation was increased after exposure to ambient particles but there was no difference based on the differentiation state of the cells (Figure 3A). The levels of TNF-α were increased after exposure and the response was greater in differentiated cells. The differentiated monocytes also displayed a heightened baseline cytokine secretion profile compared to the non-differentiated cells (Figure 3B).
FIGURE 1.
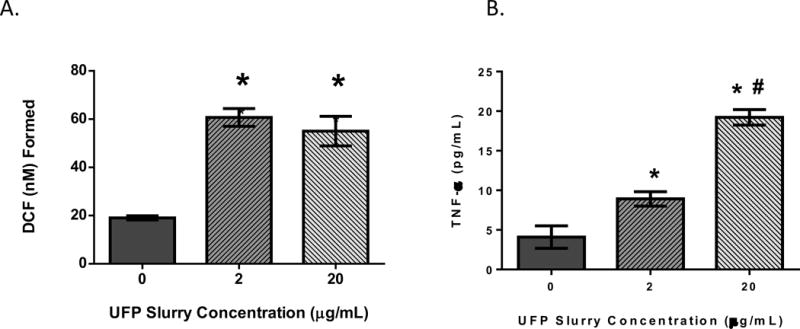
THP-1 cells were treated with 0 (control), 2 or 20μg/mL of UFP. Rate of ROS formation (A) and TNF-α secretion (B) is shown. Data is representative of three experiments conducted on separate days; n=8 biological replicates. * denotes significance p < 0.05 from control group. #: denotes significance p < 0.05 between the 2 & 20μg/mL exposure groups.
FIGURE 2.
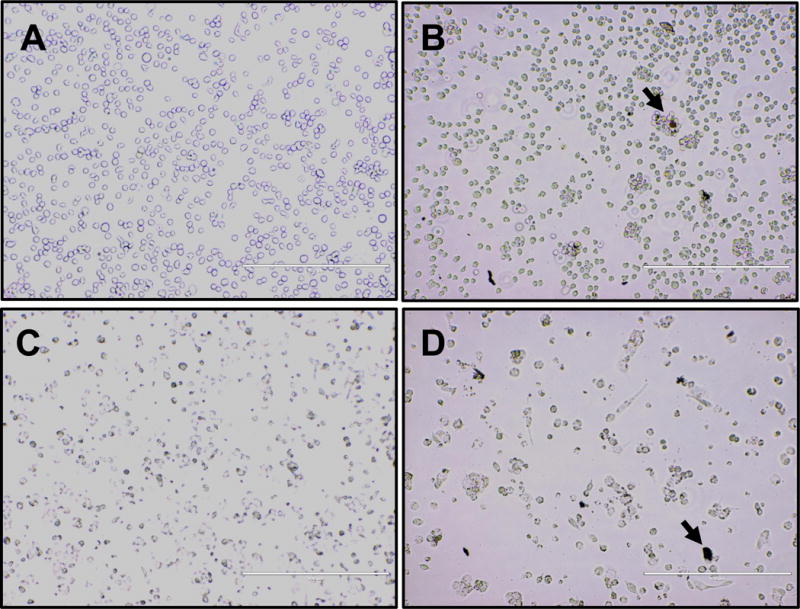
Representative images showing the morphology of undifferentiated (A & B) or differentiated (C & D) THP-1 cells after exposure to control media (A & C) or 2μg/mL UFP (B & D). Arrow indicates an example of a visible particle agglomerate.
FIGURE 3.
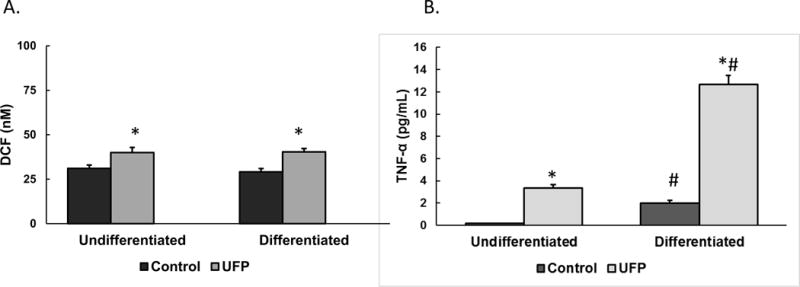
ROS generation (A) and TNF-α secretion (B) from undifferentiated or differentiated THP-1 cells treated with 0 (control) or 2μg/mL of UFP. Data was normalized from 3 different experimental days conducted with different technical replicates per exposure condition (n=22–23 for the ROS assay and n=10 for the TNF-α levels). * denotes significance p < 0.05. #: denotes significance p < 0.05 between undifferentiated and differentiated cells.
Response of Primary Monocytes to UFP
After exposure to particles, there did not appear to be significant changes in the appearance of the monocytes based on exposure to UFP or age (Figure 4). There appeared to be some aggregation (Figure 4B) and engulfment (Figure 4D) of particles by the cells. ROS formation was not altered in response to UFP exposure although there appeared to be donor-specific differences in baseline ROS levels (Figure 5). Cell viability data did not correspond to the levels of ROS generation, although the number of cells were increased after exposure in monocytes derived from the 21yo and 48yo samples (Figure 6). These cells did have a tendency to clump which may have contributed to this effect. In contrast to the ROS response, UFP-induced TNF-α release was consistent and significantly increased in monocytes derived from all donors (Figure 7). To better determine if the age of the donor influenced the response to UFP, donors were evaluated as younger (21, 24, 27, 28yo) and older (48, 49, 54, 60yo) groups. Although the baseline ROS formation seemed to be lower in the monocytes derived from older donors, this did not reach statistical significance (Figure 8A). The TNF-α release in response to particles also appeared lower in the older group but again, this did not reach statistical significance (Figure 8B).
FIGURE 4.
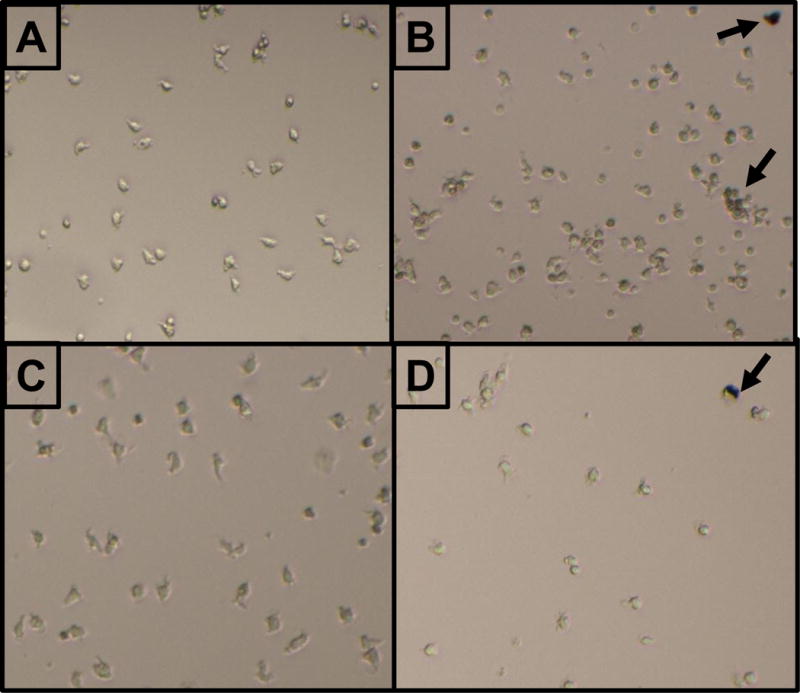
Representative images showing the morphology of primary monocytes derived from the 21yo donor (A & B) or 54yo donor (C & D). Monocytes were exposed to control media (A & C) or 2μg/mL UFP (B & D). Arrow indicates an example of a visible particle agglomerate.
FIGURE 5.
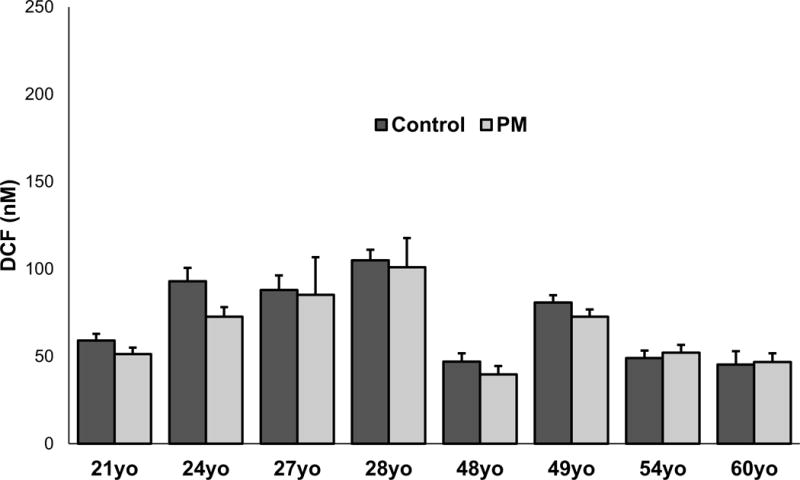
ROS formation in monocytes derived from eight biologically independent donors (ages 21, 24, 27, 28, 48, 49, 54 or 60). Monocytes were exposed to control media or 2μg/mL UFP. Data is representative of three separate experiments conducted on separate days. Each sample was ran in quadruplicates (n=4). * denotes significance p < 0.05.
FIGURE 6.
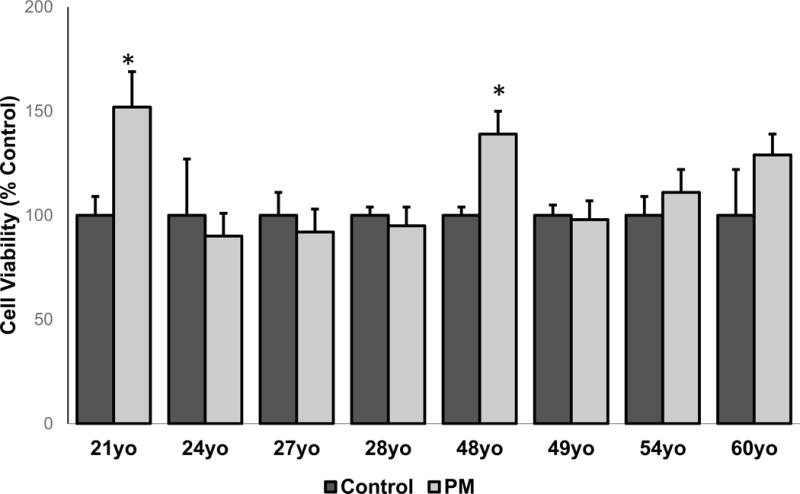
Cell viability in monocytes derived from eight biologically independent donors (ages 21, 24, 27, 28, 48, 49, 54 or 60). Monocytes were exposed to control media or 2μg/mL UFP. Data is representative of three separate experiments conducted on separate days. Each sample was ran in quadruplicates (n=4). * denotes significance p < 0.05.
FIGURE 7.
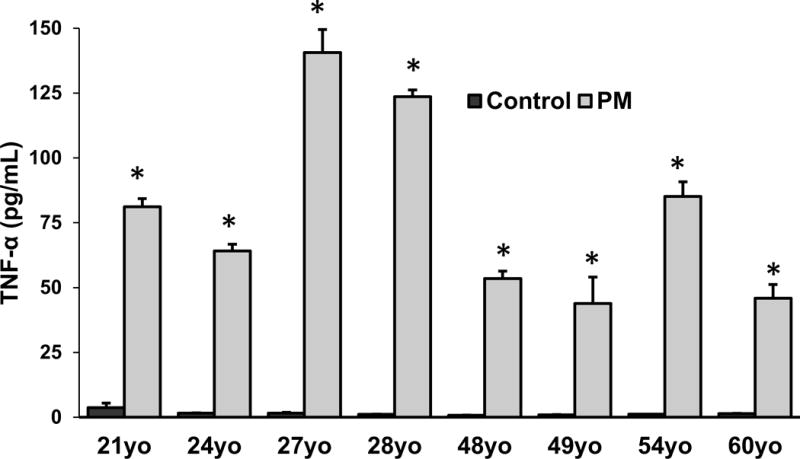
Secreted levels of TNF-α in monocytes derived from eight biologically independent donors ages 21, 24, 27, 28, 48, 49, 54 or 60. Monocytes were exposed to control media or 2μg/mL UFP. Data is representative of three separate experiments conducted on separate days. Each sample was ran in quadruplicates (n=4 technical replicates). * denotes significance p < 0.05.
FIGURE 8.
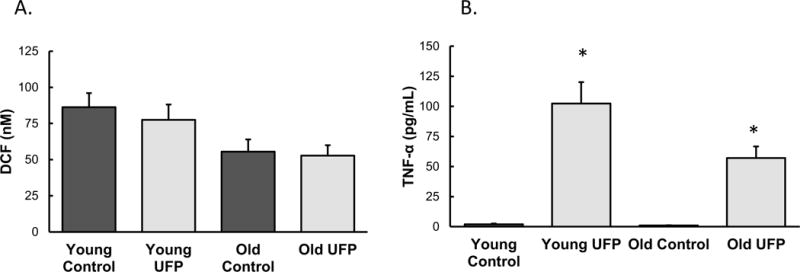
Age-dependent changes in ROS formation (A) and TNF-α secretion (B) assessed by grouping monocytes derived from younger donors (ages: 21, 24, 27, 28) and older donors (ages: 48, 49, 54, 60). (n=4 independent biological samples). * denotes significance p < 0.05.
Discussion
Exposure to ambient particles leads to enhanced markers of oxidative stress (Prahalad et al., 1999; Araujo et al., 2008; Weissenburg et al., 2010). Consistent with these reports, we found an overall increase in ROS formation in both differentiated and undifferentiated THP-1 cells after a 24hr exposure to UFP. Transition metals such as iron can directly produce ROS through the Fenton reaction, independent of cellular induction. However, in our experimental paradigm, the supernatant containing the particles was removed before measuring ROS formation in the cells. Notwithstanding, the particles or their constituents may have adhered to the cell surface and caused the general ROS formation observed. Alternatively, particles or soluble mediators may have entered the cells and changed cellular events in a manner that exacerbated ROS formation. One organelle that has been shown to be affected by PM is the mitochondrion (Xia et al., 2004; Chadhuri et al., 2012). Any change in mitochondria may disrupt oxidative potential and eventually lead to cellular dysfunction. The primary human monocytes did not show UFP-induced enhancement in ROS formation. It is important to note that there was significant variability between donors in the baseline levels of monocyte ROS generation. Although all donors were female, nonsmokers, genetic and lifestyle factors (such as diet and exercise) may have influenced the baseline oxidant properties of the monocytes. A clear age-related difference was not observed but this may be due to the limited number of samples used in this study. A more extensive age-range with a greater sample size may be necessary to discern if there are age-related differences in the monocyte response to UFP.
Primary human monocytes bind to and phagocytize diesel exhaust particles and this interaction leads to cytotoxicity attributed to mitochondrial and lysosomal dysfunction (Chadhuri et al., 2012). We observed that after a 24hr exposure, undifferentiated human THP-1 cells (and to a lesser extent the primary monocytes) began to associate with visible particle agglomerates. This observation indicates that the THP-1 cells may recognize components adhered to UFP in a manner consistent with the typical immune response. Our finding is in agreement with a study showing that a low concentration (1μg/mL) of biodiesel particles caused aggregation of rat alveolar macrophages (Bhavaraju et al., 2014). An extensive review of the response of alveolar macrophages to ambient PM described how both innate and adaptive immune systems are involved (Miyata and van Eeden, 2011) and our study is an agreement with the plethora of studies showing that particles induce inflammatory parameters in immune-competent cells. It is plausible that the composition of UFP determines the ability of monocytes to aggregate and interact with particles. Differentiated THP-1 cells were attached to the plate and as such did not appear to have the necessary mobility to move towards and surround the particles. However, they were more capable than the undifferentiated cells to secrete proinflammatory cytokines in response to UFP exposure. Thus, physical interaction with particles may not be a necessary factor for cytokine production and soluble mediators adhered to particles may be sufficient to activate monocytes. This in vitro observation is important since ambient particle exposure has been shown to mobilize monocytes and recruit them into tissues (Ishii et al., 2005) as well as atherosclerotic plaques (Yatera et al., 2008). Direct translocation of particles from lung to systemic circulation may not be a necessary factor for monocyte activation. Rather, the solubilization of the adhered constituents may be sufficient to cause a proinflammatory response.
Unlike the non-specific ROS generation, UFP exposure increased the levels of TNF-α in THP-1 cells in a dose dependent manner. The differentiated THP-1 cells had an enhanced baseline profile of TNF-α secretion which may be related to their activated state. Upon UFP exposure, these differentiated cells showed a greater response. Furthermore, an unequivocal increase in TNF-α secretion was observed in monocytes derived from all donors irrespective of age. Exposure to particulate air pollution has been correlated with progression of physical disability in the elderly (Weuve et al., 2016). A heightened proinflammatory state is observed in chronic age-associated diseases (Michaud et al., 2013). Thus the contribution of particles to disease pathology, by further exacerbating inflammatory processes, especially in susceptible individuals such as the elderly or immune-compromised individuals, needs to be further investigated.
Our results indicate that although particles can cause a general increase in ROS formation, this is not directly related to the degree of inflammatory effect which is consistent and dependent on both dose and differentiation state. The specificity of the inflammatory response is in agreement with another study demonstrating that particles collected from near-roadway environments activate inflammatory markers in THP-1 cells to a greater extent than particles derived from an urban background while ROS formation was not dependent to roadway proximity (Wu et al., 2014). A gene analysis study that directly correlated ROS generation with inflammation showed that 80% of altered inflammation-related genes did not correlate with ROS activity although there were four genes that were strongly interrelated between oxidative and inflammatory changes (Sijan et al., 2015). Furthermore, antioxidant treatment only partially blocked human bronchial epithelial cell secretion of the inflammatory cytokine IL-6 that was induced by diesel exhaust particles (Bach et al., 2015). Our results indicate that UFP enhance proinflammatory cytokine release from monocytes in a consistent manner unrelated to the degree of ROS formation.
Conclusions
Satellite-derived estimates of PM in Eastern North America show that exposure levels above the WHO air quality guidance (10 μg/m3) have declined from 62% (1998–2000) to 19% (2010–2012) in approximately a decade (van Donkelaar et al., 2015). In the U.S., the policy changes that improved air quality have been associated with an improvement in life expectancy (Pope et al., 2009). In the present political environment, there is a tendency to ignore the importance of environmental regulation. Our study indicates that ambient UFP may exacerbate mechanisms that are linked to the pathology of age-related diseases and thus continual air quality improvements are necessary to vouchsafe public health. Policies should continue to target the protection of human health so that detrimental long-term effects, such as reduction in life span, productivity, and quality of life, can be avoided.
Highlights.
Exposure to ambient ultrafine particles (UFP) causes TNF-α secretion in primary human monocytes and THP-1 cells independently of reactive oxygen species formation.
Differentiated THP-1 cells are more responsive to UFP exposure.
Age of donor does not significantly contribute to the proinflammatory effect of UFP.
Acknowledgments
This work was supported by funds provided by BP/SCAQMD Public Benefits Programs Oversight Committee to AC; National Institutes of Health (R21AG040683, R21AG040753) and the South Coast Air Quality Management District (SCAQMD award #11527) to CS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest Statement:
The author(s) declare that they have no competing interests.
References
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo JA, Nel AE. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol. 2009;6:24. doi: 10.1186/1743-8977-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JG, Borm P, Cassee FRC, Castranova V, Donaldson K, Ghio A, Harrison RM, Hider R, Kelly F, Kooter IM, Marano F, Maynard RL, Mudway I, Nel A, Sioutas C, Smith S, Cho A, Duggan S, Froines J. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential—A workshop report and consensus statement. Inhalation toxicology. 2008;20:75–99. doi: 10.1080/08958370701665517. [DOI] [PubMed] [Google Scholar]
- Bach N, Bølling AK, Brinchmann BC, Totlandsdal AI, Skuland T, Holme JA, Låg M, Schwarze PE, Øvrevik J. Cytokine responses induced by diesel exhaust particles are suppressed by PAR-2 silencing and antioxidant treatment, and driven by polar and non-polar soluble constituents. Toxicol Lett. 2015;238:72–82. doi: 10.1016/j.toxlet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Bhavaraju L, Shannahan J, William A, McCormick R, McGee J, Kodavanti U, Madden M. Diesel and biodiesel exhaust particle effects on rat alveolar macrophages with in vitro exposure. Chemosphere. 2014;104:126–133. doi: 10.1016/j.chemosphere.2013.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Chadhuri N, Jary H, Lea S, Khan N, Piddock KC, Dockrell DH, Donaldson K, Duffin R, Singh D, Parker LC, Sabroe I. Diesel exhaust particle exposure in vitro alters monocyte differentiation and function. Plos one. 2012;7(12):e51107. doi: 10.1371/journal.pone.0051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ebenstein A, Greenstone M, Li H. Evidence on the impact of sustained exposure to air pollution on life expectancy from China’s Huai River policy. Proc Natl Acad Sci USA. 2013;110:12936–12941. doi: 10.1073/pnas.1300018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zhang Y, Zhang W, Li S, Williams G, Marks GB, Jalaludin B, Abramson MJ, Luo F, Yang D, Su X, Lin Q, Liu L, Lin J, Guo Y. Attributable risks of emergency hospital visits due to air pollutants in China: A multi-city study. Environ Pollut. 2017;228:43–49. doi: 10.1016/j.envpol.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Carraway MS, Madden M. Composition of Air Pollution Particles and Oxidative Stress in Cells, Tissues, and Living Systems. J Toxicol Env Health-Part B-Crit Rev. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Hasheminassab S, Daher N, Schauer JJ, Sioutas C. Source apportionment and organic compound characterization of ambient ultrafine particulate matter (PM) in the Los Angeles Basin. Atmos Environ. 2013;79:529–39. [Google Scholar]
- Ishii H, Hayashi S, Hogg JC, Fujii T, Goto Y, Sakamoto N, Mukae H, Vincent R, van Eeden SF. Alveolar macrophage-epithelial cell interaction following exposure to atmospheric particles induces the release of mediators involved in monocyte mobilization and recruitment. Respir Res. 2005;6:87. doi: 10.1186/1465-9921-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal AO. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol Lett. 2017;270:88–95. doi: 10.1016/j.toxlet.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Xue Li G, Zeng M, Cai Q, Pan Y, Meng XQ. Association between fine ambient particulate matter and daily total mortality: An analysis from 160 communities of China. Sci Total Environ. 2017;599–600:108–113. doi: 10.1016/j.scitotenv.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Misra C, Kim S, Shen S, Sioutas C. A high flow rate, very low pressure drop impactor for inertial separation of ultrafine from accumulation mode particles. Journal of Aerosol Sci. 2002;33:735–752. [Google Scholar]
- Miyata R, van Eeden SF. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol Applied Pharmacol. 2011;257:209–226. doi: 10.1016/j.taap.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PHM, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med. 2001;164:1665–1668. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PHM, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- Ning Z, Geller MD, Moore KF, Sheesley R, Schauer JJ, Sioutas C. Daily variation in chemical characteristics of urban ultrafine aerosols and inference of their sources. Env Sci Technol Lett. 2007;41:6000–6006. doi: 10.1021/es070653g. [DOI] [PubMed] [Google Scholar]
- Pope CA, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;152:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- Pope CA, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360:376–386. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahalad AK, Soukup JM, Inmon J, Willis R, Ghio AJ, Becker S, Gallagher JE. Ambient air particles: effects on cellular oxidant radical generation in relation to particulate elemental chemistry. Toxicol Appl Pharmacol. 1999;158:81–91. doi: 10.1006/taap.1999.8701. [DOI] [PubMed] [Google Scholar]
- Qin Z. The use of thp-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis. 2012;221:2–11. doi: 10.1016/j.atherosclerosis.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Saffari A, Daher N, Shafer MM, Schauer JJ, Sioutas C. Seasonal and spatial variation of trace elements and metals in quasi-ultrafine (PM(0)(2)(5)) particles in the Los Angeles metropolitan area and characterization of their sources. Environmental pollution. 2013;181:14–23. doi: 10.1016/j.envpol.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Schwarze PE, Øvrevik J, Hetland RB, Becher R, Cassee FR, Låg M, Løvik M, Dybing E, Refsnes M. Importance of size and composition of particles for effects on cells in vitro. Inhal Toxicol. 2007;1:17–22. doi: 10.1080/08958370701490445. [DOI] [PubMed] [Google Scholar]
- Shi T, Schins RP, Knaapen AM, Kuhlbusch T, Pitz M, Heinrich J, et al. Hydroxyl radical generation by electron paramagnetic resonance as a new method to monitor ambient particulate matter composition. Journal of environmental monitoring. 2003;5:550–556. doi: 10.1039/b303928p. [DOI] [PubMed] [Google Scholar]
- Sijan Z, Antkiewicz DS, Heo J, Kado NY, Schauer JJ, Sioutas C, Shafer MM. An in vitro alveolar macrophage assay for the assessment of inflammatory cytokine expression induced by atmospheric particulate matter. Environ Toxicol. 2015;30:836–851. doi: 10.1002/tox.21961. [DOI] [PubMed] [Google Scholar]
- Steenhof M, Gosens I, Strak M, Godri KJ, Hoek G, Cassee FR, Mudway IS, Kelly FJ, Harrison RM, Lebret E, Brunekreef B, Janssen NA, Pieters RH. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential-the RAPTES project. Part Fibre Toxicol. 2011;8:26. doi: 10.1186/1743-8977-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Hedman CJ, Sheesley RJ, Shafer MM, Schauer JJ. Investigating the chemical nature of humic-like substances (HULIS) in North American atmospheric aerosols by liquid chromatography tandem mass spectrometry. Atmospheric Environment. 2009;43:4205–4213. [Google Scholar]
- Thomson EM, Breznan D, Karthikeyan S, MacKinnon-Roy C, Vuong NQ, Dabek-Zlotorzynska E, Celo V, Charland JP, Kumarathasan P, Brook JR, Vincent R. Contrasting biological potency of particulate matter collected at sites impacted by distinct industrial sources. Part Fibre Toxicol. 2016;13(1):65. doi: 10.1186/s12989-016-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42:1530–1536. [PubMed] [Google Scholar]
- Valavanidis A, Fiotakis K, Bakeas E, Vlahogianni T. Electron paramagnetic resonance study of the generation of reactive oxygen species catalysed by transition metals and quinoid redox cycling by inhalable ambient particulate matter. Redox report: communications in free radical research. 2005;10:37–51. doi: 10.1179/135100005X21606. [DOI] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Brauer M, Boys BL. Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environ Health Perspect. 2015;123:135–143. doi: 10.1289/ehp.1408646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenberg A, Sydlik U, Peuschel H, Schroeder P, Schneider M, Schins RP, Abel J, Unfried K. Reactive oxygen species as mediators of membrane-dependent signaling induced by ultrafine particles. Free Radic Biol Med. 2010;49:597–605. doi: 10.1016/j.freeradbiomed.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Weuve J, Kaufman JD, Szpiro AA, Curl C, Puett RC, Beck T, Evans DA, Mendes de Leon CF. Exposure to Traffic-Related Air Pollution in Relation to Progression in Physical Disability among Older Adults. Enviro Health Perspect. 2016;124:1000–1008. doi: 10.1289/ehp.1510089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, Global Health Observatory (GHO) data. http://www.who.int/gho/phe/outdoor_air_pollution/en/ Accessed May, 16, 2017.
- Wu W, Muller R, Berhane K, Fruin S, Liu F, Jaspers I, Diaz-Sanchez D, Peden DB, McConnell R. Inflammatory response of monocytes to ambient particles varies by highway proximity. Am J Respir Cell Mol Biol. 2014;51:802–809. doi: 10.1165/rcmb.2013-0265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Korge P, Weiss JN, Li N, Venkatesen MI, Sioutas C, Nel A. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: implications for ultrafine particle toxicity. Environ Health Perspect. 2004;112:1347–1358. doi: 10.1289/ehp.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatera K, Hsieh J, Hogg JC, Tranfield E, Suzuki H, Shih CH, Behzad AR, Vincent R, van Eeden SF. Particulate matter air pollution exposure promotes recruitment of monocytes into atherosclerotic plaques. Am J Physiol Heart Circ Physiol. 2008;294:944–953. doi: 10.1152/ajpheart.00406.2007. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Schauer JJ, Shafer MM, Hannigan MP, Cutton SJ. Source apportionment of in vitro reactive oxygen species bioassay activity from atmospheric particulate matter. Environmental Science & Technology. 2008;42:7502–7509. doi: 10.1021/es800126y. [DOI] [PubMed] [Google Scholar]


