Abstract
Sudden unexpected death in epilepsy (SUDEP) is a devastating epilepsy complication. Seizure-induced respiratory arrest (S-IRA) occurs in many witnessed SUDEP patients and animal models as an initiating event leading to death. Thus, understanding the mechanisms underlying S-IRA will advance the development of preventive strategies against SUDEP. Serotonin (5-HT) is an important modulator for many vital functions, including respiration and arousal, and a deficiency of 5-HT signaling is strongly implicated in S-IRA in animal models, including the DBA/1 mouse. However, the brain structures that contribute to S-IRA remain elusive. We hypothesized that the dorsal raphe (DR), which sends 5-HT projections to the forebrain, is implicated in S-IRA. The present study used optogenetics in the DBA/1 mouse model of SUDEP to selectively activate 5-HT neurons in the DR. Photostimulation of DR 5-HT neurons significantly and reversibly reduced the incidence of S-IRA evoked by acoustic stimulation. Activation of 5-HT neurons in the DR suppressed tonic seizures in most DBA/1 mice without altering the seizure latency and duration of wild running and clonic seizures evoked by acoustic stimulation. This suppressant effect of photostimulation on S-IRA is independent of seizure models, as optogenetic stimulation of DR also reduced S-IRA induced by pentylenetetrazole, a proconvulsant widely used to model human generalized seizures. The S-IRA-suppressing effect of photostimulation was increased by 5-hydroxytryptophan, a chemical precursor for 5-HT synthesis, and was reversed by ondansetron, a specific 5-HT3 receptor antagonist, indicating that reduction of S-IRA by photostimulation of the DR is specifically mediated by enhanced 5-HT neurotransmission. Our findings suggest that deficits in 5-HT neurotransmission in the DR are implicated in S-IRA in DBA/1 mice, and that targeted intervention in the DR is potentially useful for prevention of SUDEP.
Keywords: Photostimulation, serotonin, 5-hydroxytryptophan, ondansetron, generalized seizures, pentylenetetrazole
Introduction
Sudden unexpected death in epilepsy (SUDEP) is a devastating complication of epilepsy, accounting for up to 17% of deaths in patients with epilepsy and ranking second in public health burden among common neurological disorders (Devinsky et al., 2016; Feng and Faingold, 2017; Hughes, 2009; Thurman et al., 2014; Tomson et al., 2008). Among several proposed mechanisms underlying SUDEP (Devinsky et al., 2016; Goldman et al., 2016; Hirsch, 2010; Klassen et al., 2014; Sarkis et al., 2015), respiratory and cardiac dysfunctions have received considerable attention (Feng and Faingold, 2017; Lhatoo et al., 2015). Clinical studies observed that many witnessed SUDEP or near-SUDEP patients exhibit breathing difficulties after generalized tonic–clonic seizures (Bateman et al., 2008; Blum, 2009; Langan et al., 2000; Pezzella et al., 2009; Ryvlin et al., 2013; So et al., 2000), leading to seizure-induced respiratory arrest (S-IRA), followed by terminal asystole (Ryvlin et al., 2013). These changes in respiratory and cardiac systems observed in humans have been consistently recapitulated in several animal models relevant to SUDEP. For example, S-IRA occurs prior to terminal asystole in DBA/1 mice following generalized audiogenic seizures (AGSz) evoked by acoustic stimulation (Faingold et al., 2010). The occurrence of S-IRA and subsequent terminal asystole was also observed during generalized seizures evoked by maximal electroshock in serotonin (5-HT)-deficient Lmx1b(f/f) mice (Buchanan et al., 2014). Our recent study showed that S-IRA and subsequent terminal asystole occur in most DBA/1 and some C57BL/6J mice that incurred sudden death after generalized tonic-clonic seizures evoked by pentylenetetrazole (PTZ) (Zhang et al., 2016), a proconvulsant widely used to model human generalized seizures (White, 1997). These studies of SUDEP in humans and animal models strongly support the idea that S-IRA is an important initiating event that leads to seizure-related mortality in many cases. Thus, investigation of the mechanisms underlying S-IRA is important for better understanding the pathophysiology of SUDEP and for development of therapeutics to prevent this fatal epileptic event.
Studies show that serotonergic neurotransmission is implicated in S-IRA (Feng and Faingold, 2017). Several selective 5-HT reuptake inhibitors (SSRIs) suppress S-IRA in DBA and 5-HT-deficient Lmx1b(f/f) mice (Buchanan et al., 2014; Faingold et al., 2011; Tupal and Faingold, 2006; Zeng et al., 2015). Our recent data demonstrated that direct enhancement of 5-HT synthesis reduces the incidence of S-IRA evoked by acoustic stimulation or PTZ in DBA/1 mice (Zhang et al., 2016). Antagonism of 5-HT3 receptors abolishes the S-IRA-blocking effect of the SSRI, fluoxetine, in DBA/1 mice (Faingold et al., 2016b), suggesting that 5-HT3 receptors are involved in pathogenesis of S-IRA in this animal model. However, it is still unknown which brain structures are involved in S-IRA. 5-HT neurons are mainly located in the medullary raphe nuclei and midbrain dorsal raphe (DR) and modulate many vital functions including increased respiration and arousal induced by elevated levels of CO2 (Feldman et al., 2003; Sowers et al., 2013). These functions are often impaired by seizures (Sowers et al., 2013; Zhan et al., 2016), contributing to S-IRA. The SSRI, fluoxetine, prevents S-IRA but does not augment basal ventilation in the absence of seizures. On the other hand, drugs that directly stimulate respiration exert no effect on S-IRA evoked by acoustic stimulation in DBA/1 mice (Zeng et al., 2015). These findings suggest that SSRIs may not reduce S-IRA by a direct action at the medullary respiratory center, which is mainly innervated by projections from medullary raphe nuclei (Brust et al., 2014; Depuy et al., 2011). Thus, we hypothesized that a deficiency of 5-HT neurotransmission mediated by the DR may contribute to S-IRA in DBA/1 mice.
We tested this hypothesis using optogenetics to enhance 5-HT neurotransmission in the DR of transgenic DBA/1 mice with 5-HT neurons selectively expressing channelrhodopsin-2 (ChR2) guided by the promoter of tryptophan hydroxylase-2 (TPH2), the key enzyme for 5-HT synthesis in the brain (Cote et al., 2003). Our data show that photostimulation of 5-HT neurons in the DR significantly reduced the incidence of S-IRA evoked by either acoustic stimulation or PTZ in transgenic DBA/1 TPH2-ChR2 mice. Using an agent to enhance 5-HT synthesis and a specific 5-HT3 receptor antagonist, we demonstrate that the reduction of S-IRA elicited by photostimulation of the DR is specifically mediated by enhanced 5-HT neurotransmission.
Material and Methods
Ethical statement
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital, which are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize animal use and to reduce the stress of animals during experiments.
Creation of transgenic DBA/1 TPH2-ChR2 mice
Transgenic C57BL/6J TPH2-ChR2 mice are commercially available from The Jackson Laboratory (Stock # 014555, Bar Harbor, ME, USA). Wild type DBA/1 mice were purchased from Envigo (Indianapolis, IN, USA). Hemizygous transgenic DBA/1 TPH2-ChR2 mice (referred as transgenic DBA/1 mice below) were created by backcrossing hemizygous transgenic C57BL/6J TPH2-ChR2 mice with wild type DBA/1 mice using marker-assisted accelerated backcrossing (MAX-BAX, Charles River Laboratories, Troy, NY, USA). Congenic transgenic DBA/1 mice (~100% of DBA/1 genetic background) were generated in the offspring of the fifth generation of backcrossing. Hemizygous transgenic DBA/1 mice were mated with wild type DBA/1 mice to produce hemizygous transgenic DBA/1 mice for experiments. Transgenic DBA/1 mice of both sexes were used in the experiments, as previous study showed that sex is not a variable in S-IRA in DBA/1 mice (Faingold and Randall, 2013). All of these mice were housed and bred in the Massachusetts General Hospital Center for Comparative Medicine animal facility in a temperature- and humidity-controlled environment (12-h light/dark cycle) and provided with rodent food and water ad libitum. DBA/1 mice were “primed” starting from postnatal day 26–28 by once daily subjecting to acoustic stimulation for 3–4 days to establish consistent susceptibility to S-IRA.
Surgical implantation
Surgical implantation of a fiberoptic cannula was performed as described in our previous studies (Feng and Faingold, 2002; Taylor et al., 2016; Van Dort et al., 2015). Briefly, a mouse at 8–10 weeks of age (14–22 g) was anesthetized by intraperitoneal (i.p.) injection of ketamine/xylazine (100/10 mg/kg) and placed in a stereotaxic device (Model 940, David Kopf Instruments, Tujunga, CA, USA). A midline incision was made, and the skin was pulled aside to expose the skull. A small craniotomy was made above the DR. A fiberoptic cannula (200 μm in diameter, Doric Lenses, Quebec, Canada) was implanted through the calvarium into the DR (AP −4.47 mm, ML 0 mm, V −3.5 mm) (Paxinos and Franklin, 2013). The fiberoptic cannula and several anchor screws (BASi, West Lafayette, IN, USA) were fixed to the skull using dental cement (A-M Systems, Sequim, WA, USA). After implantation, the mouse was separately housed for recovery, and optogenetic experiments were performed 7 days after surgery.
Experimental models of generalized seizures and S-IRA
Generalized seizures and S-IRA were evoked in DBA/1 mice either by acoustic stimulation or by systemic administration of PTZ (Zhang et al., 2016). To induce AGSz by acoustic stimulation in DBA/1 mice, each mouse was placed in a cylindrical plexiglass chamber in a sound-isolated room. Broadband acoustic stimulation was generated using an electric bell (96 dB SPL, UC4-150, Zhejiang People’s Electronics, China). The acoustic stimulus was given for a maximum duration of 60 s or until the mouse exhibited tonic seizures and S-IRA in most cases (Feng, 2014). DBA/1 mice that exhibited S-IRA were resuscitated within 5 s after the final respiratory gasp using a polyethylene tube connected to a rodent respirator (Harvard Apparatus 680, Holliston, MA, USA), which pumped room air at 180 strokes/min with a volume of 1 ml. Seizure-related behaviors were videotaped for offline analysis. If photostimulation reduced S-IRA, the return of susceptibility to S-IRA was tested 24 hr after photostimulation and at 24 hr intervals thereafter until susceptibility to S-IRA returned. Some transgenic DBA/1 mice were reused in photostimulation experiments. The criteria for reuse of a mouse include: 1) the mouse rested in the animal facility for at least one week to ensure that the effect of last photostimulation is cleared; 2) the mouse was randomly assigned to an experimental group; and 3) the susceptibility of this mouse to S-IRA was always confirmed 24 hr prior to the next test.
S-IRA also occurred in transgenic DBA/1 mice after generalized tonic-clonic seizures evoked by PTZ (75 mg/kg, i.p.), as described previously (Zhang et al., 2016).
Optogenetic stimulation and S-IRA
To perform optogenetic stimulation, a primed transgenic DBA/1 mouse or a sibling of transgenic DBA/1 mouse without transgene expression was placed in a cylindrical plexiglass chamber. A patch cord (Doric Lenses) was connected to a diode blue light laser (473 nm, Power Technology, Alexander, AR, USA) and the implanted fiberoptic cannula via a rotary joint so that the free movement of the mouse was not constrained. Pulsed laser light stimulus was controlled using a 4-channel stimulus generator (MCSSTG4004, ALA Scientific Instruments, Farmingdale, NY, USA). The energy output (mW) at the tip of the fiberoptic cannula was measured prior to implantation using a digital optic power meter (Thorlabs, Newton, NJ, USA). The susceptibility of primed DBA/1 mice to S-IRA evoked by acoustic stimulation was always re-confirmed 24 hr prior to optogenetic stimulation. Photostimulation (20 ms pulse duration, 20 Hz) with a duty cycle of 40% at either of two different energy levels (9 mW or 15 mW) was used to stimulate 5-HT neurons in the DR. Similar optogenetic stimulus parameters were used to effectively activate 5-HT neurons in previous studies (Cai et al., 2014; Zhao et al., 2011). For each energy level, a series of stimulation durations (1, 5, 10 and 15 min for 9 mW, and 3 and 5 min for 15 mW) were applied. Induction of AGSz was initiated immediately after optogenetic stimulation. The effect of each photostimulation on the incidence of S-IRA, the latency to AGSz and the duration of wild running and clonic seizures evoked by acoustic stimulation was examined. In the PTZ model, two groups of transgenic DBA/1 mice were used. One group was injected with PTZ (75 mg/kg, i.p.) without photostimulation, and the other group received photostimulation (9 mW, 20 ms pulse duration, 20 Hz) for 15 min, followed by the PTZ injection. The incidence of S-IRA and the latency to seizures evoked by PTZ in two groups of transgenic DBA/1 mice were examined.
To further examine the role of 5-HT in S-IRA susceptibility, 5-hydroxytryptophan (5-HTP), a precursor for 5-HT synthesis, or ondansetron (OND), a 5-HT3 receptor antagonist, was administered prior to photostimulation, and the action of these agents on the effect of photostimulation on S-IRA and characteristics of seizures evoked by acoustic stimulation was investigated. Vehicle or 5-HTP was administered in transgenic DBA/1 mice once daily for two days with 5-HTP at a dose (50 mg/kg, i.p.) that is known to produce no effect on S-IRA in wild type DBA/1 mice (Zhang et al., 2016). One hr following the second 5-HTP treatment, photostimulation (9 mW, 20 ms pulse duration, 20 Hz) was applied in these transgenic DBA/1 mice with a stimulation duration that had been previously determined to have no effect on S-IRA. The effect of coupling 5-HTP (or vehicle) administration with photostimulation on S-IRA, the latency to AGSz and the duration of wild running and clonic seizures evoked by acoustic stimulation in transgenic DBA/1 mice was examined. Vehicle or OND (2 mg/kg, i.p.) (Faingold et al., 2016b) was administered in transgenic DBA/1 mice 30 min prior to photostimulation (9 mW, 20 ms pulse duration, 20 Hz) with a stimulation duration that had been previously determined to reduce S-IRA in these mice. The effect of coupling OND (or vehicle) administration with photostimulation on S-IRA, the latency to AGSz and the duration of wild running and clonic seizures evoked by acoustic stimulation in transgenic DBA/1 mice was examined.
PTZ (P6500), 5-HTP (107751) and OND (1478571) were purchased from Sigma-Aldrich (St. Louis, MO, USA). PTZ and 5-HTP were dissolved in saline, and OND was dissolved in distilled water for i.p. administration.
Immunohistochemistry and histology
At the end of the experiment, the placement of the fiberoptic cannula tip in each implanted mouse was verified by histology. For c-fos expression study, transgenic DBA/1 mice without or with photostimulation (9 mW for 15 min) were sacrificed about 1 hr after induction of AGSz. Each DBA/1 mouse was deeply anesthetized with an overdose of ketamine/xylazine and transcardially perfused with 10 ml PBS (pH 7.4), followed by 10 ml 4% paraformaldehyde. The brain was removed and post-fixed in 4% paraformaldehyde at 4°C. Each brain was sectioned into 60-μm thickness of coronal slices with a vibratome (VT1000 S, Leica Biosystems, Buffalo Grove, IL, USA) at room temperature. Brain slices were blocked with 5% bovine serum albumin (BSA, Life Technologies, Grand Island, NY, USA) for 2 hr and incubated overnight with primary antibody for GFP (GFP Tag Alexa Fluor 488 conjugate, 1:500 dilution, A-21311, Thermo Fisher Scientific, Waltham, MA, USA) and TPH2 (1:500 dilution, T0678, Sigma-Aldrich) for examining the expression of ChR2 and TPH2 shown in Figure 1 and Figure 5, and with primary antibody for GFP and c-fos (1:500 dilution, sc-8047, Santa Cruz Biotechnology, Dallas, TX, USA) for examining the expression of ChR2 and c-fos presented in Figure 6. After being incubated for 10 hr, slices were washed for 3 times at the interval of 15 min and then rinsed and incubated with goat anti-mouse Alexa Fluor-conjugated secondary antibody (Alexa Fluor 555 conjugate, 1:300 dilution, A-21424, Thermo Fisher Scientific) for 2 hr. As the primary antibody for GFP is conjugated with Alexa, a secondary antibody was not needed for detection of GFP. Slices were mounted on slides with Vectashield (Vector Laboratories, Burlingame, CA, USA), dried and sealed with coverslip using nail polish.
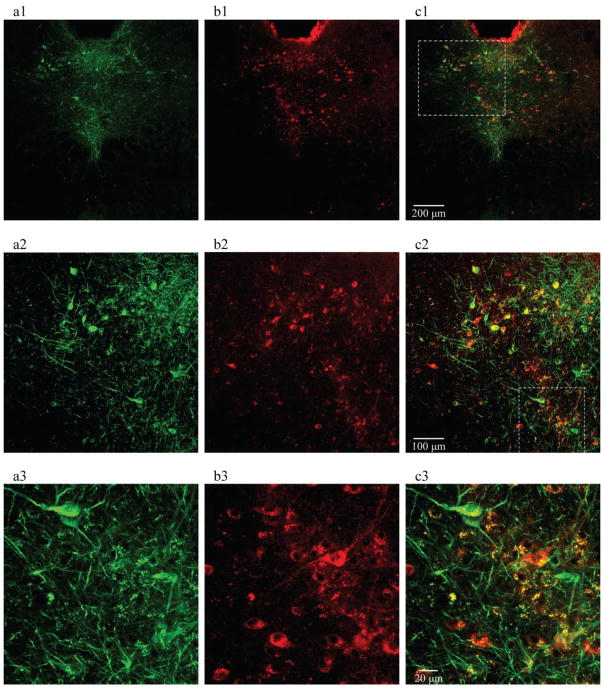
Figure 1. Selective expression of ChR2 on 5-HT neurons in the DR of transgenic DBA/1 mice.
a1, a2 and a3, neuronal immunostaining of GFP, a surrogate marker for ChR2, on 5-HT neurons in the DR of a coronal brain slice. b1, b2 and b3, immunostaining of TPH2, the key enzyme for 5-HT synthesis in the CNS. c1, c2 and c3, merged images, showing co-localization of TPH2 and GFP in 5-HT neurons. These data demonstrate that ChR2 is restrictively expressed on the surface of 5-HT neurons in the DR (n = 3 mice). The dashed-line frame in c1 indicates the region from which the magnified image was taken and presented in c2, and that in c2 indicates the region that was magnified in c3. Confocal image magnifications: a1–c1, 10x; a2–c2, 20x; a3–c3, 40x.
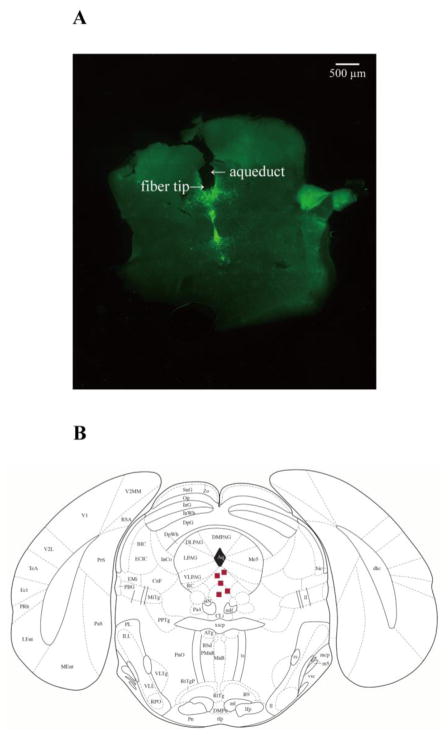
Figure 5. Placement of fiberoptic cannula tips in the DR.
A, an example of coronal brain slice, showing the location of a fiberoptic cannula tip in the DR of a transgenic DBA/1 mouse. B, distribution of representative locations of fiberoptic cannula tips (squares) in the DR, according to the mouse atlas of Paxinos and Franklin (Paxinos and Franklin, 2013).
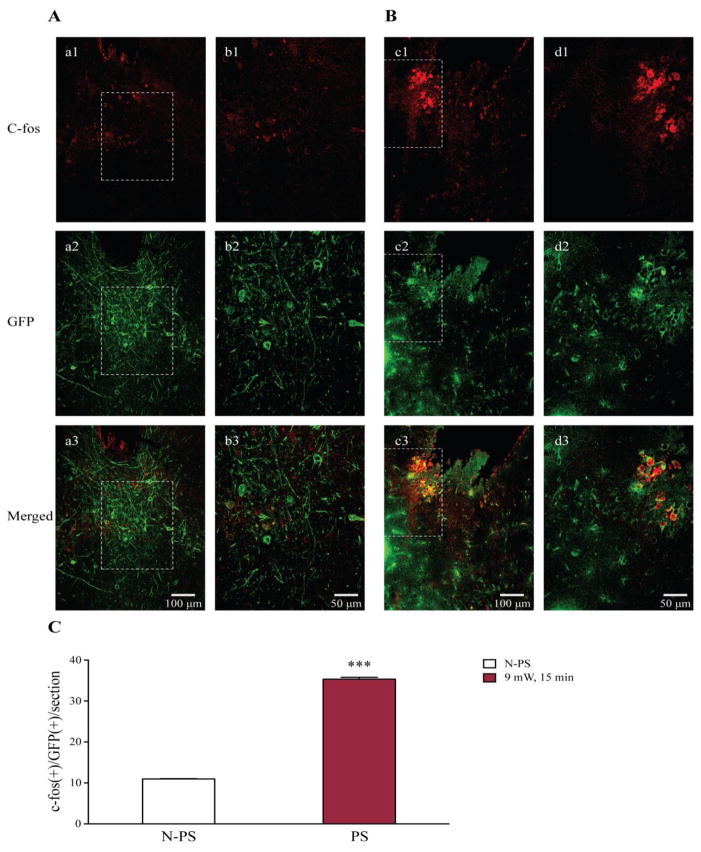
Figure 6. Photostimulation increases c-fos expression in 5-HT neurons in the DR of transgenic DBA/1 mice.
A, neuronal immunostaining of c-fos (a1, b1), GFP (a2, b2) and co-localization c-fos and GFP (a3, b3) in a transgenic DBA/1 mouse that was implanted with fiberoptic cannula, but no photostimulation was applied (n = 3 mice). B, immunostaining of c-fos (c1, d1), GFP (c2, d2) and co-localization c-fos and GFP (c3, d3) in an implanted transgenic DBA/1 mouse with photostimulation at 9 mW for 15 min (n = 3 mice). C, quantification of c-fos(+)/GFP(+) cells in implanted transgenic DBA/1 mice without photostimulation (blank bar) and those in implanted transgenic mice with photostimulation (red bar). N-PS, no photostimulation; PS, photostimulation. Confocal image magnifications: a1–a3 and c1–c3, 20x; b1–b3 and d1–d3, 40x. See figure 2 for photostimulation parameters.
*** p < 0.001: Significantly different from the number of c-fos(+)/GFP(+) cells per section in implanted transgenic DBA/1 mice with no photostimulation.
Co-localization of TPH2 and GFP (a surrogate marker for ChR2) as well as c-fos and GFP was visualized with the Nikon A1R confocal microscope (Nikon Instruments, Melville, NY, USA) at Microscopy Core Facility, Program in Membrane Biology, Massachusetts General Hospital. To compare the c-fos expression in DR 5-HT neurons in transgenic DBA/1 mice with or without photostimulation, cells with both c-fos and GFP expression were counted under a Nikon Eclipse 80i fluorescence microscope (Nikon Instruments) in 9 slices from 3 different mice in each group (with photostimulation vs. without photostimulation). For each slice, c-fos-positive and GFP-positive [c-fos(+)/GFP(+)] cells from 4 randomly selected sections (250 μm × 250 μm) within DR were manually counted (Cai et al., 2014).
Statistical analysis
Data are reported as mean ± SEM or median and 95% confidence interval. Statistical analyses were performed using Prism 6 software (GraphPad Software, La Jolla, CA, USA). The latency to AGSz and duration of wild running and clonic seizures in the absence and presence of photostimulation and/or drug treatment were compared using ANOVA with Tukey’s test as posthoc analysis or paired Student’s t test or using nonparametric tests (Wilcoxon matched-pairs signed rank test or Mann Whitney test) when the sample size of control and treatment group in an experiment was only 4–6 mice. Comparison of c-fos-positive cells in the DR of transgenic DBA/1 mice with and without photostimulation was carried out using ANCOVA with mouse as covariate. The incidence of S-IRA between two treatments was compared using Chi-square test. Statistical significance was inferred if p < 0.05.
Results
Selective expression of ChR2 on 5-HT neurons in the DR of transgenic DBA/1 mice
Transgenic DBA/1 mice were created by backcrossing the available hemizygous C57BL/6J TPH2-ChR2 mice (Zhao et al., 2011) with wild type DBA/1 mice. These transgenic DBA/1 mice have the same genetic background as wild type DBA/1 mice and display similar AGSz pattern and incidence of S-IRA to wild type DBA/1 mice (Feng, 2014). We first examined the expression of ChR2 and TPH2 in 5-HT neurons in the DR of transgenic DBA/1 mice using immunohistochemistry (n = 3 mice) (Figure 1). Consistent with previous immunohistochemistry studies in transgenic C57BL/6J TPH2-ChR2 mice (Zhao et al., 2011), the expression of GFP, a surrogate marker for ChR2, was predominantly localized on the membrane of cell body and axons (Figure 1, a1–a3), and that of TPH2 was confined to the cytosol of 5-HT neurons in the DR of transgenic DBA/1 mice (Figure 1, b1–b3). The merged images demonstrated the co-localization of TPH2 to the cell bodies and GFP to the cell membrane (Figure 1, c1–c3). Of all the cells counted, 99.6% (487/489) of cells were TPH2(+)/GFP(+) (percent/number of ChR2-GFP positive cells that were also TPH2 staining positive); 82.5% (462/560) of cells were GFP(+)/TPH2(+) (percent/number of TPH2 positive cells that were also ChR2-GFP staining positive), which is in general agreement with a previous study (Zhao et al., 2011). These data suggest that ChR2 is selectively expressed on 5-HT neurons in the DR of transgenic DBA/1 mice.
Activation of 5-HT neurons in the DR reduces S-IRA evoked by acoustic stimulation in transgenic DBA/1 mice
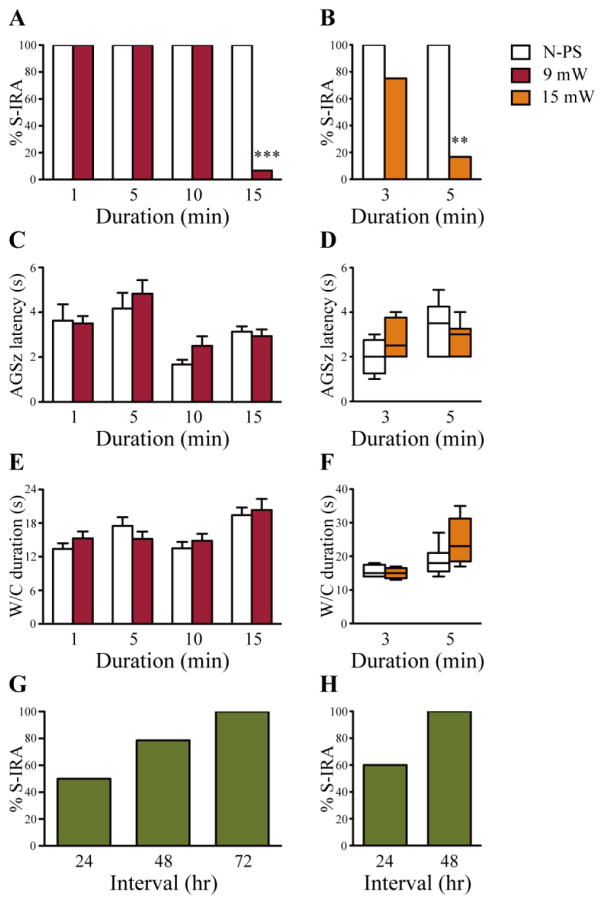
The effect of selective enhancement of 5-HT neurotransmission on S-IRA following generalized AGSz evoked by acoustic stimulation was examined by applying photostimulation (blue light, 20 ms pulse duration, 20 Hz) to 5-HT neurons in the DR of primed transgenic DBA/1 mice at an energy level of 9 mW with a series of stimulation durations (1, 5, 10 and 15 min). Photostimulation of DR for 15 min significantly reduced the incidence of S-IRA in transgenic DBA/1 mice (6.7%) (p < 0.001, n = 15) as compared with the incidence of S-IRA evoked by acoustic stimulation tested 24 hr prior to photostimulation (100%). However, photostimulation of the DR for 1 min (n = 8), 5 min (n = 6) or 10 min (n = 6) was without effect on the incidence of S-IRA evoked by acoustic stimulation as compared with that tested 24 hr prior to photostimulation (Figure 2A). Although tonic seizures were suppressed in the transgenic DBA/1 mice in which S-IRA was blocked by photostimulation at 9 mW for 15 min, the vast majority of the mice (92.9%) still exhibited wild running and/or clonic seizures. These data indicate that photostimulation of 5-HT neurons at 9 mW for 15 min in the DR did not significantly alter the susceptibility of these transgenic mice to AGSz, consistent with previous studies using 5-HT enhancing agents (Faingold et al., 2011; Zeng et al., 2015; Zhang et al., 2016). Photostimulation at 9 mW for 1–15 min did not alter the latency to AGSz (Figure 2C) and the duration of wild running and clonic seizures (Figure 2E) as compared with the corresponding seizure parameters tested 24 hr prior to photostimulation. The susceptibility to S-IRA in those transgenic DBA/1 mice in which S-IRA was suppressed by photostimulation at 9 mW for 15 min returned 24–72 hr after photostimulation (Figure 2G), indicating that the photostimulation-elicited reduction of S-IRA is reversible. These data indicate that, with a fixed energy level, the effect of photostimulation on S-IRA is dependent on the duration of stimulation.
Figure 2. Photostimulation of 5-HT neurons in the DR reduces S-IRA evoked by acoustic stimulation.
A, compared with the incidence of S-IRA evoked by acoustic stimulation 24 hr prior to photostimulation, S-IRA was significantly reduced by photostimulation at 9 mW for 15 min in transgenic DBA/1 mice (n = 15). However, photostimulation at 9 mW for 1 (n = 8), 5 (n = 6) or 10 min (n = 6) exerted no effect on S-IRA evoked by acoustic stimulation. B, increasing energy level to 15 mW significantly suppressed S-IRA evoked by acoustic stimulation for a shorter photostimulation duration of 5 min in transgenic DBA/1 mice (n = 6), although a further decrease in stimulation duration to 3 min produced no significant effect on S-IRA (n = 4) as compared to that observed 24 hr prior to photostimulation. C, D, for each stimulation duration, bar graphs or box plots show that photostimulation at either 9 mW or 15 mW did not alter the latency to AGSz in transgenic DBA/1 mice as compared with that tested 24 hr prior to photostimulation. E, F, for each stimulation duration, bar graphs or box plots show that photostimulation at either 9 mW or 15 mW did not affect the duration of wild running and clonic seizures in transgenic DBA/1 mice as compared with that tested 24 hr prior to photostimulation. G, H, for the transgenic DBA/1 mice in which S-IRA evoked by acoustic stimulation was suppressed by photostimulation, susceptibility of these mice to S-IRA evoked by acoustic stimulation returned 24–72 hr after photostimulation at 9 mW for 15 min and 24–48 hr after photostimulation at 15 mW for 5 min, respectively. Blank bars, the incidence of S-IRA, the latency to AGSz and the duration of wild running and clonic seizures evoked by acoustic stimulation 24 hr prior to photostimulation; red bars, the incidence of S-IRA, the latency to AGSz and the duration of wild running and clonic seizures following photostimulation at 9 mW; orange bars, the incidence of S-IRA, the latency to AGSz and the duration of wild running and clonic seizures following photostimulation at 15 mW; green bars, the return of the susceptibility to S-IRA after photostimulation. N-PS, no photostimulation. W/C, wild running and clonic seizures. Photostimulation parameters: blue light, 473 nm; pulse duration, 20 ms; frequency, 20 Hz.
** p < 0.01; *** p < 0.001: Significantly different from the incidence of S-IRA evoked by acoustic stimulation 24 hr prior to photostimulation.
To further determine the relationship of stimulation durations vs. stimulation energy levels in photostimulation that modulates S-IRA in transgenic DBA/1 mice, we also examined the effect of photostimulation at a higher energy level (15 mW) on S-IRA. As compared with the incidence of S-IRA evoked by acoustic stimulation tested 24 hr prior to photostimulation (100%), photostimulation (20 ms pulse duration, 20 Hz) of DR at 15 mW significantly and reversibly reduced S-IRA in transgenic DBA/1 mice with a shorter stimulation duration of 5 min (16.7%) (p < 0.01, n = 6), although photostimulation of the DR at 15 mW for 3 min (n = 4) did not significantly suppress S-IRA (Figure 2B). In the transgenic DBA/1 mice in which S-IRA was suppressed by photostimulation at 15 mW for 5 min, tonic seizures were blocked in 60% of mice, but all of the mice continued to display wild running and/or clonic seizures. Photostimulation at 15 mW for 3–5 min did not change the latency to AGSz (Figure 2D) as well as the duration of wild running and clonic seizures (Figure 2F). The return of the susceptibility to S-IRA in those transgenic DBA/1 mice in which S-IRA was reduced by photostimulation occurred 24–48 hr after photostimulation (Figure 2H).
Photostimulation of the DR at either 9 mW for 15 min or 15 mW for 5 min produced no effect on S-IRA in siblings of transgenic DBA/1 mice that had no ChR2 expression on 5-HT neurons (n = 6). No obvious adverse effects were observed in DBA/1 mice with these photostimulation parameters.
The effect of photostimulation on S-IRA is dependent on 5-HT neurotransmission
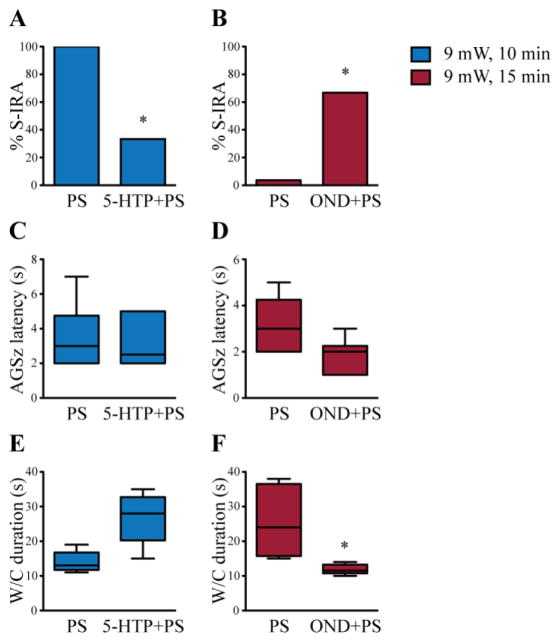
To determine if reduction of S-IRA by photostimulation is due to enhancement of 5-HT neurotransmission, a 5-HT enhancing agent or a 5-HT3 receptor antagonist was administered before photostimulation in transgenic DBA/1 mice. Consistent with a previous study in wild type DBA/1 mice (Zhang et al., 2016), administration of 5-HTP alone (50 mg/kg daily for two days), a chemical that augments 5-HT synthesis, exerted no effect on S-IRA and AGSz evoked by acoustic stimulation in transgenic DBA/1 mice in the current study (n = 8). However, photostimulation at 9 mW for 10 min following pre-treatment with the same doses of 5-HTP significantly reduced the incidence of S-IRA evoked by acoustic stimulation in transgenic DBA/1 mice (33.3%) as compared with that tested following vehicle treatment and photostimulation with the same parameters, which had no effect on S-IRA (100%) (p < 0.05, n = 6) (Figure 3A). 5-HTP treatment followed by photostimulation in transgenic DBA/1 mice did not alter the latency to AGSz (Figure 3C) and the duration of wild running and clonic seizures (p = 0.0625) (Figure 3E) as compared with the corresponding seizure parameters in those tested following vehicle treatment and photostimulation.
Figure 3. Alteration of 5-HT neurotransmission affects the inhibitory effect of photostimulation on S-IRA.
A, photostimulation of 5-HT neurons in the DR at 9 mW for 10 min had no effect on S-IRA evoked by acoustic stimulation in transgenic DBA/1 mice pre-treated with vehicle once daily for two days (n = 6). However, pre-treatment with 5-hydroxytryptophan (5-HTP, 50 mg/kg, i.p.), once daily for two days, significantly reduced S-IRA by photostimulation at 9 mW for 10 min (n = 6). B, photostimulation following pre-treatment with vehicle in the DR at 9 mW for 15 min blocked S-IRA evoked by acoustic stimulation in transgenic DBA/1 mice (n = 6), which exhibited 100% of S-IRA 24 hr prior to vehicle treatment without photostimulation. However, pre-treatment with ondansetron (OND, 2 mg/kg, i.p.) significantly reversed the blockade effect of photostimulation on S-IRA (n = 6). C, D, box plots show that as compared with corresponding photostimulation following vehicle treatment, pre-treatment with 5-HTP followed by photostimulation at 9 mW for 10 min did not result in a significant change in the latency to AGSz, nor did pre-treatment with OND followed by photostimulation at 9 mW for 15 min. E, F, box plots show that as compared with corresponding vehicle control, pre-treatment with 5-HTP followed by photostimulation did not significantly alter the duration of wild running and clonic seizures, however, pre-treatment with OND followed by photostimulation significantly reduced the duration of wild running and clonic seizures. Blue bars, photostimulation at 9 mW for 10 min; red bars, photostimulation at 9 mW for 15 min. PS, photostimulation; 5-HTP, 5-hydroxytryptophan; OND, ondansetron; W/C, wild running and clonic seizures. See figure 2 for photostimulation parameters.
* p < 0.05: Significantly different from vehicle control (PS at 9 mW for 10 min) in A or vehicle control (PS at 9 mW for 15 min) in B and F.
OND, a 5-HT3 receptor antagonist, had no effect on S-IRA when administered at 2 mg/kg (i.p.) alone in transgenic DBA/1 mice (n = 6), in line with a previous study (Faingold et al., 2016b). OND treatment did not alter the latency to AGSz and the duration of wild running and clonic seizures (p = 0.0579) as compared with that in vehicle treatment in transgenic DBA/1 mice. As compared with vehicle treatment followed by photostimulation at 9 mW for 15 min, which blocked S-IRA evoked by acoustic stimulation in all transgenic DBA/1 mice (0%), pre-treatment with OND (2 mg/kg, i.p.) significantly prevented the blockade of S-IRA elicited by photostimulation with the same parameters (66.7%) (p < 0.05, n = 6) (Figure 3B). Although pre-treatment with OND followed by photostimulation in transgenic DBA/1 mice did not significantly alter the latency to AGSz (p = 0.0545) (Figure 3D), it significantly decreased the duration of wild running and clonic seizures (p < 0.05) (Figure 3F) as compared with the corresponding seizure parameters in those tested following vehicle treatment and photostimulation.
Photostimulation in the DR suppresses S-IRA evoked by PTZ in transgenic DBA/1 mice
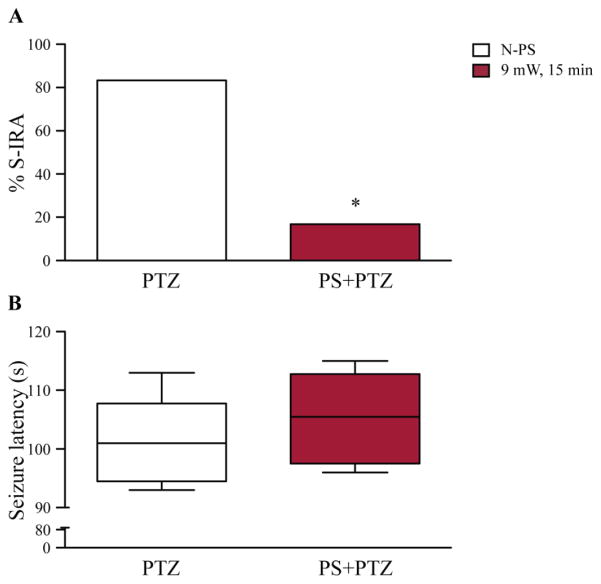
To determine if reduction of S-IRA by photostimulation in transgenic DBA/1 mice is dependent on seizure-induction methods, we also examined the effect of photostimulation on S-IRA evoked by PTZ in transgenic DBA/1 mice. Consistent with a previous study in wild type DBA/1 mice (Zhang et al., 2016), PTZ administration (75 mg/kg, i.p.) without photostimulation evoked a high incidence of S-IRA in transgenic DBA/1 mice (83.3%, n = 6). Photostimulation of the DR (9 mW for 15 min) significantly reduced S-IRA evoked by PTZ in transgenic DBA/1 mice (16.7%) (p < 0.05, n = 6) (Figure 4A). Photostimulation did not change the latency to seizures evoked by PTZ in transgenic DBA/1 mice (Figure 4B).
Figure 4. Photostimulation in the DR reduces S-IRA evoked by PTZ in transgenic DBA/1 mice.
A, S-IRA evoked by PTZ (75 mg/kg, i.p.) was significantly reduced in transgenic DBA/1 mice with photostimulation of 5-HT neurons in the DR at 9 mW for 15 min (n = 6) as compared with that in those transgenic DBA/1 mice without photostimulation (n = 6). B, box plots show that the latency to PTZ-evoked seizures was not affected by photostimulation as compared with that in the absence of photostimulation. Blank bars, the incidence of S-IRA or latency to seizures in the absence of photostimulation; red bars, the incidence of S-IRA or latency to seizures in the presence of photostimulation. N-PS, no photostimulation; PS, photostimulation; PTZ, pentylenetetrazole. See figure 2 for photostimulation parameters.
* p < 0.05: Significantly different from PTZ treatment alone.
Photostimulation increases c-fos expression in the 5-HT neurons in the DR
The placement of the fiberoptic cannula tip for each implanted transgenic DBA/1 mouse was verified by histology at the end of the experiment. Only those transgenic DBA/1 mice with the location of fiberoptic cannula tips confined to the DR were included in the analysis. An example of fiberoptic cannula tip placement in the DR of a transgenic DBA/1 mouse is shown in Figure 5A, and the representative distribution of the fiberoptic cannula tips in the DR is displayed in Figure 5B, based on the mouse atlas of Paxinos and Franklin (Paxinos and Franklin, 2013). No thermal injury due to photostimulation was observed in the area around the fiberoptic tips.
To determine if photostimulation increased the excitability of 5-HT neurons, we examined the neuronal expression of c-fos, an immediate early gene that is widely used as a marker for neuronal activity in optogenetics studies (Cai et al., 2014; Liu et al., 2012; Thanos et al., 2013), in the DR of transgenic DBA/1 mice with photostimulation and those without photostimulation (Figure 6). As compared with c-fos expression in 5-HT neurons in the DR of transgenic DBA/1 mice without photostimulation (n = 36 sections from 3 mice) (Figure 6A), photostimulation (20 ms pulse duration, 20 Hz) at 9 mW for 15 min mainly increased the expression of c-fos in 5-HT neurons that also expressed GFP in the DR of transgenic DBA/1 mice (n = 36 sections from 3 mice) (Figure 6B). c-fos(+)/GFP(+) cells were counted from 4 randomly selected sections in each of 3 brain slices that were sectioned from each mice in two treatment groups of transgenic DBA/1 mice (with or without photostimulation). The average number of c-fos(+)/GFP(+) cells per section in the DR of transgenic DBA/1 mice with photostimulation (35.3 ± 0.4/section) was significantly greater than that in the transgenic DBA/1 mice without photostimulation (11.0 ± 0.1/section) (p < 0.001) (Figure 6C).
Discussion
Accumulating data from animal models relevant to SUDEP show that 5-HT neurotransmission plays an important role in the pathogenesis of S-IRA (Brennan et al., 1997; Buchanan et al., 2014; Feng and Faingold, 2017). Thus, the incidence of S-IRA is selectively reduced by SSRIs, chemicals that enhance synaptic 5-HT levels (Buchanan et al., 2014; Faingold et al., 2011; Tupal and Faingold, 2006; Zeng et al., 2015) and by 5-HTP, a chemical precursor for 5-HT synthesis (Zhang et al., 2016) in several mouse models that are relevant to SUDEP. Consistent with these animal studies, emerging evidence suggests that deficits in 5-HT transmission are potentially involved in seizure-induced respiratory dysfunction in patients with partial seizures (Bateman et al., 2010). In the current study, we observed that selective enhancement of 5-HT transmission via photostimulation of 5-HT neurons in the DR reduces S-IRA in transgenic DBA/1 mice. This observation is independent of seizure models, as the anatomical substrate, seizure initiation and pattern of AGSz evoked by acoustic stimulation is quite different from those of seizures evoked by PTZ. These findings indicate that a deficiency in 5-HT neurotransmission in the DR may be importantly involved in the pathophysiology of S-IRA.
Our data demonstrate at both cellular and behavioral levels that photostimulation-evoked reduction of S-IRA can be attributed to selective activation of 5-HT neurons in the DR. First, our immunohistochemistry study showed that ChR2 is specifically expressed on the surface of 5-HT neurons in transgenic DBA/1 mice, consistent with previous study in C57BL/6J TPH2-ChR2 mice, in which the functionality of surface ChR2 was confirmed by electrophysiology (Tang and Trussell, 2015; Zhao et al., 2011). Second, the expression of c-fos, a widely used marker for neuronal activation in optogenetics studies (Cai et al., 2014; Do-Monte et al., 2015; Ramirez et al., 2015), is significantly elevated by photostimulation in the neurons that express ChR2. An increase in intracellular Na+ triggers the expression of c-fos in 5-HT neurons (Miller and Loewy, 2014), consistent with photostimulation activation of ChR2, a cation channel that is permeable to Na+ (Zhang et al., 2010). Depolarization of 5-HT neurons by ChR2 activation results in 5-HT release in the brain (Miyazaki et al., 2014; Ohmura et al., 2014). Third, photostimulation only suppresses S-IRA in DBA/1 mice that express ChR2 in 5-HT neurons but exerts no effect on S-IRA in those DBA/1 mice that do not express ChR2 in 5-HT neurons. Fourth, enhancing 5-HT synthesis using 5-HTP at a dose that alone produces no effect on S-IRA in DBA/1 mice (Zhang et al., 2016) significantly facilitates the suppressant effect of photostimulation on S-IRA, and administration of a 5-HT3 antagonist, which is known to reverse the effect of the SSRI, fluoxetine, on S-IRA in DBA/1 mice (Faingold et al., 2016b), significantly inhibits the suppressant effect of photostimulation on S-IRA in transgenic DBA/1 mice. Finally, S-IRA evoked by acoustic stimulation in DBA/1 mice is reversibly suppressed by photostimulation of 5-HT neurons in the DR. These data further support the idea that a deficiency of 5-HT transmission in the CNS is involved in the pathogenesis of S-IRA. Clearly, more studies are needed to confirm our findings in other animal models, especially those with spontaneous seizures, as well as in humans.
However, an important question remains to be answered: which brain structures contribute to S-IRA? While 5-HT neurons in the brainstem DR mediate brain functions such as arousal response to hypercapnia via ascending projections to a variety of forebrain structures (Ursin, 2002; Vertes, 1991), those in the medullary raphe nuclei regulate brain functions like respiration via projections to other brainstem regions (Brust et al., 2014; Depuy et al., 2011; Kinney et al., 2009). Our data demonstrate that photostimulation of 5-HT neurons in the DR suppresses S-IRA evoked by either acoustic stimulation or by PTZ, suggesting that deficits in ascending 5-HT circuitry contribute to S-IRA in DBA/1 mice. Interestingly, 5-HT3 receptors are highly expressed in the forebrain structures (Morales and Bloom, 1997). A 5-HT3 receptor antagonist inhibits the suppressant effect of photostimulation of the DR (this study) and the SSRI, fluoxetine (Faingold et al., 2016b), on S-IRA, also supporting the idea that a deficiency in the projection from the DR to the forebrain is involved in the pathophysiology for S-IRA in DBA/1 mice. Although the exact neural circuit mechanisms underlying S-IRA are unknown, functional deficits within the neuronal pathways involved in 5-HT-mediated arousal are good candidates for mediation of S-IRA. 5-HT neurons in the DR have been shown to mediate arousal response to hypercapnia (Buchanan and Richerson, 2010; Sowers et al., 2013). Hypercapnia is commonly observed during seizures (Bateman et al., 2008; Moseley et al., 2010), probably due to seizure-induced hypoventilation (Zhan et al., 2016). Elevation of CO2 can activate 5-HT neurons in the DR to enhance the arousal response (Buchanan and Richerson, 2010), allowing the patient to increase the respiratory function and/or change body position to compensate for the peri-ictal respiratory depression. If the arousal response is defective, the hypoventilation may not be corrected or may even be worsened due to airway occlusion, finally leading to the death of the patient. Consistent with this idea, a meta-analysis found that most SUDEP patients died in the prone position (Liebenthal et al., 2015), in which their airways may be obstructed by bedding. Our previous studies demonstrated that fluoxetine, at a dose that reduces the incidence of S-IRA, does not enhance basal ventilation in the absence of seizures, and respiratory stimulants exert no effect on S-IRA evoked by acoustic stimulation in DBA/1 mice (Zeng et al., 2015), suggesting that fluoxetine does not reduce S-IRA by directly enhancing ventilation in the absence of seizures. The present findings support a potentially important role of the DR in averting S-IRA. These data suggest that the ability of fluoxetine and several other SSRIs to prevent S-IRA in DBA/1 mice (Feng and Faingold, 2017) may be due, at least in part, to increasing the effect of 5-HT released by DR 5-HT neurons. Further studies are needed to test the hypothesis that 5-HT-mediated arousal circuitry is involved in S-IRA.
In line with previous studies (Buchanan et al., 2014; Yan et al., 1995), activation of 5-HT neurons in the DR produces some anticonvulsant effect in DBA/1 mice. Photostimulation alone blocked tonic AGSz without altering wild running and clonic seizures in most transgenic DBA/1 mice. The exact mechanism for selective blockade of tonic AGSz by photostimulation is unknown. Studies indicate that periaqueductal gray is involved in the tonic AGSz (Faingold, 2012). DR is adjacent to periaqueductal gray and sends projections to it (Li et al., 2001). Additionally, periaqueductal gray contains a subset of 5-HT neurons (Johnson et al., 2004). It is possible that photostimulation of DR could have activated the 5-HT pathway from DR to periaqueductal gray and/or directly stimulated 5-HT neurons in the periaqueductal gray, producing a greater activation of periaqueductal gray than other brain structures that are involved in wild running or clonic AGSz. This could lead to more specific blockade of tonic AGSz. However, we do not think that blockade of tonic AGSz is one of the key reasons for the reduction of S-IRA, as systemic administration of 5-HT enhancing agents at moderate dosages specifically suppresses S-IRA without affecting AGSz and at high dosages also blocks tonic AGSz in some DBA/1 mice (Feng and Faingold, 2017; Zhang et al., 2016). Our current study indicates that blockade of tonic AGSz is dependent on the photostimulation parameters, suggesting that S-IRA can be specifically suppressed with “optimal” photostimulation parameters. These data may also indicate that the specific blockade of S-IRA by systemic administration of 5-HT enhancing agents is dependent on other sources of 5-HT as well.
Interestingly, a single unit recording study showed that seizures in a different epilepsy model markedly suppress the firing of medullary 5-HT neurons (Zhan et al., 2016), suggesting that dysfunction of medullary raphe nuclei may also contribute to S-IRA. Consistent with this, a recent imaging study using manganese-enhanced MRI indicates that several medullary raphe nuclei, such as raphe pallidus and raphe magnus, are involved in S-IRA in DBA/1 mice (Kommajosyula et al., 2017). Further studies that dissect the medullary 5-HT circuitry in S-IRA may shed light on this issue. In addition to 5-HT neurotransmission, it was reported that noradrenergic (Zhang et al., 2017) and adenosinergic (Faingold et al., 2016a; Shen et al., 2010) neurotransmission are involved in the pathogenesis of S-IRA and/or seizure-induced sudden death. It is interesting to determine the relative contribution of these neurotransmission systems to S-IRA in future studies.
Our observation that S-IRA is suppressed by optogenetic stimulation of DR may have significant translational potential to prevent SUDEP. Deep brain stimulation (DBS) has been used to control seizures and drive arousal function (Gooneratne et al., 2016; Kundishora et al., 2017). Stimulation of DR using a DBS device may protect epileptic patients from SUDEP. As DR is involved in sleep-wake regulation, stimulation in the daytime may be more beneficial to minimize the interruption of the physiological sleep-wake rhythms. Application of DBS in the daytime may be adequate to control SUDEP, as our data show that the effect of DR photostimulation is long lasting.
Conclusions
Taken together, our data demonstrate that optogenetic enhancement of 5-HT neurotransmission in the DR of transgenic DBA/1 mice suppresses S-IRA evoked by either acoustic stimulation or a chemoconvulsant, indicating that a deficiency in 5-HT neurotransmission in the DR contributes importantly to S-IRA. Our findings suggest that neurostimulatory activation of 5-HT neurons in the DR is a potential strategy to prevent SUDEP.
Table 1.
Summary of experimental groups of DBA/1 mice
| Experiments | Experimental groups | Number of mice |
|---|---|---|
| Photostimulation (9 mW, ChR2+) | 1-min PS | 8* |
| 5-min PS | 6* | |
| 10-min PS | 6* | |
| 15-min PS | 15* | |
| Photostimulation (15 mW, ChR2+) | 3-min PS | 4* |
| 5-min PS | 6* | |
| Photostimulation (9 or 15 mW, ChR2−) | 15- or 5-min PS | 6 |
| Photostimulation (9 mW, 5-HTP) | 10-min PS | 6* |
| vehicle or 5-HTP | ||
| Photostimulation (9 mW, OND) | 15-min PS | 6* |
| vehicle or OND | ||
| Photostimulation (9 mW, PTZ) | PTZ only | 6 |
| PTZ with 15-min PS | 6 | |
| Injection (i.p.) | Vehicle or 5-HTP | 8* |
| Injection (i.p.) | Vehicle or OND | 6* |
| Immunohistochemistry | ChR2-TPH2 | 3 |
| Immunohistochemistry | ChR2-c-fos with PS | 3 |
| ChR2-c-fos without PS | 3 |
PS, photostimulation; 5-HTP, 5-hydroxytryptophan; OND, ondansetron; PTZ, pentylenetetrazole.
indicates that pre-treatment control or vehicle was performed in the same group of mice as treatment.
Highlights.
Photostimulation of dorsal raphe reduces seizure-induced respiratory arrest (S-IRA)
Photostimulation of dorsal raphe produces anticonvulsant effect
Suppression of S-IRA by optogenetics is promoted by 5-hydroxytryptophan
Suppression of S-IRA by optogenetics is inhibited by a 5-HT3 receptor antagonist
Optogenetic suppression effect on S-IRA is independent of seizure models
Acknowledgments
We thank Kunpeng Liu for the help with genotyping.
Funding
This work was supported by R03NS078591, R21NS101311 and CURE (Citizens United for Research in Epilepsy) foundation to HJF. We thank the support from the Massachusetts General Hospital Department of Anesthesia, Critical Care and Pain Medicine. Honghai Zhang is a recipient of fund from the Zhejiang Provincial Branch of Natural Science Foundation of China (LY14H090005) and from National Natural Science Foundation of China (NSFC 81771403), Chang Zeng is a recipient of Young Scientists Fund from National Natural Science Foundation of China (NSFC 81501130), and Haiting Zhao is a recipient of fellowship from China Scholarship Council (CSC 201506370075). The authors declare no competing financial interests.
Abbreviations
- AGSz
Audiogenic seizures
- ChR2
Channelrhodopsin-2
- DBS
Deep brain stimulation
- DR
Dorsal raphe
- 5-HT
Serotonin
- 5-HTP
5-Hydroxytryptophan
- OND
Ondansetron
- PTZ
Pentylenetetrazole
- S-IRA
Seizure-induced respiratory arrest
- SSRI(s)
Selective serotonin reuptake inhibitor(s)
- SUDEP
Sudden unexpected death in epilepsy
- TPH2
Tryptophan hydroxylase-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bateman LM, et al. Serotonin reuptake inhibitors are associated with reduced severity of ictal hypoxemia in medically refractory partial epilepsy. Epilepsia. 2010;51:2211–4. doi: 10.1111/j.1528-1167.2010.02594.x. [DOI] [PubMed] [Google Scholar]
- Bateman LM, et al. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131:3239–45. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum AS. Respiratory physiology of seizures. J Clin Neurophysiol. 2009;26:309–15. doi: 10.1097/WNP.0b013e3181b7f14d. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, et al. Sound-induced seizures in serotonin 5-HT2c receptor mutant mice. Nat Genet. 1997;16:387–90. doi: 10.1038/ng0897-387. [DOI] [PubMed] [Google Scholar]
- Brust RD, et al. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Rep. 2014;9:2152–65. doi: 10.1016/j.celrep.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, et al. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol. 2014;592:4395–410. doi: 10.1113/jphysiol.2014.277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–9. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YQ, et al. Optogenetic activation of brainstem serotonergic neurons induces persistent pain sensitization. Mol Pain. 2014;10:70. doi: 10.1186/1744-8069-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525–30. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuy SD, et al. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981–90. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, et al. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15:1075–88. doi: 10.1016/S1474-4422(16)30158-2. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, et al. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci. 2015;35:3607–15. doi: 10.1523/JNEUROSCI.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL. Brainstem Networks: Reticulo-Cortical Synchronization in Generalized Convulsive Seizures. In: Noebels JL, et al., editors. Jasper’s Basic Mechanisms of the Epilepsies. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- Faingold CL, Randall M. Effects of age, sex, and sertraline administration on seizure-induced respiratory arrest in the DBA/1 mouse model of sudden unexpected death in epilepsy (SUDEP) Epilepsy Behav. 2013;28:78–82. doi: 10.1016/j.yebeh.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Faingold CL, et al. Susceptibility to seizure-induced sudden death in DBA/2 mice is altered by adenosine. Epilepsy Res. 2016a;124:49–54. doi: 10.1016/j.eplepsyres.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Faingold CL, et al. DBA/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy Behav. 2010;17:436–40. doi: 10.1016/j.yebeh.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Faingold CL, et al. Serotonergic agents act on 5-HT3 receptors in the brain to block seizure-induced respiratory arrest in the DBA/1 mouse model of SUDEP. Epilepsy Behav. 2016b;64:166–170. doi: 10.1016/j.yebeh.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL, et al. Prevention of seizure-induced sudden death in a chronic SUDEP model by semichronic administration of a selective serotonin reuptake inhibitor. Epilepsy Behav. 2011;22:186–90. doi: 10.1016/j.yebeh.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Feldman JL, et al. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–66. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HJ. Partners against mortality in epilepsy conference summary. Epilepsy Curr. 2014;14:17. doi: 10.5698/1535-7597-14.s6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HJ, Faingold CL. Synaptic plasticity in the pathway from the medial geniculate body to the lateral amygdala is induced by seizure repetition. Brain Res. 2002;946:198–205. doi: 10.1016/s0006-8993(02)02884-6. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Faingold CL. Abnormalities of serotonergic neurotransmission in animal models of SUDEP. Epilepsy Behav. 2017;71:174–180. doi: 10.1016/j.yebeh.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AM, et al. Sudden unexpected death in epilepsy genetics: Molecular diagnostics and prevention. Epilepsia. 2016;57(Suppl 1):17–25. doi: 10.1111/epi.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooneratne IK, et al. Comparing neurostimulation technologies in refractory focal-onset epilepsy. J Neurol Neurosurg Psychiatry. 2016;87:1174–1182. doi: 10.1136/jnnp-2016-313297. [DOI] [PubMed] [Google Scholar]
- Hirsch LJ. Is sudden unexpected death in epilepsy due to postictal brain shutdown? Ann Neurol. 2010;68:773–5. doi: 10.1002/ana.22242. [DOI] [PubMed] [Google Scholar]
- Hughes JR. A review of sudden unexpected death in epilepsy: prediction of patients at risk. Epilepsy Behav. 2009;14:280–7. doi: 10.1016/j.yebeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Johnson PL, et al. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann N Y Acad Sci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- Kinney HC, et al. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–50. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen TL, et al. High-resolution molecular genomic autopsy reveals complex sudden unexpected death in epilepsy risk profile. Epilepsia. 2014;55:e6–12. doi: 10.1111/epi.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommajosyula SP, et al. Specific subcortical structures are activated during seizure-induced death in a model of sudden unexpected death in epilepsy (SUDEP): A manganese-enhanced magnetic resonance imaging study. Epilepsy Res. 2017;135:87–94. doi: 10.1016/j.eplepsyres.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Kundishora AJ, et al. Restoring Conscious Arousal During Focal Limbic Seizures with Deep Brain Stimulation. Cereb Cortex. 2017;27:1964–1975. doi: 10.1093/cercor/bhw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan Y, et al. Sudden unexpected death in epilepsy: a series of witnessed deaths. J Neurol Neurosurg Psychiatry. 2000;68:211–3. doi: 10.1136/jnnp.68.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhatoo S, et al. Sudden unexpected death in epilepsy: Identifying risk and preventing mortality. Epilepsia. 2015;56:1700–6. doi: 10.1111/epi.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, et al. Morphological features and electrophysiological properties of serotonergic and non-serotonergic projection neurons in the dorsal raphe nucleus. An intracellular recording and labeling study in rat brain slices. Brain Res. 2001;900:110–8. doi: 10.1016/s0006-8993(01)02272-7. [DOI] [PubMed] [Google Scholar]
- Liebenthal JA, et al. Association of prone position with sudden unexpected death in epilepsy. Neurology. 2015;84:703–9. doi: 10.1212/WNL.0000000000001260. [DOI] [PubMed] [Google Scholar]
- Liu X, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–5. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Loewy AD. 5-HT neurons of the area postrema become c-Fos-activated after increases in plasma sodium levels and transmit interoceptive information to the nucleus accumbens. Am J Physiol Regul Integr Comp Physiol. 2014;306:R663–73. doi: 10.1152/ajpregu.00563.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, et al. Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Curr Biol. 2014;24:2033–40. doi: 10.1016/j.cub.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–67. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley BD, et al. How common is ictal hypoxemia and bradycardia in children with partial complex and generalized convulsive seizures? Epilepsia. 2010;51:1219–24. doi: 10.1111/j.1528-1167.2009.02490.x. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, et al. Optogenetic activation of serotonergic neurons enhances anxiety-like behaviour in mice. Int J Neuropsychopharmacol. 2014;17:1777–83. doi: 10.1017/S1461145714000637. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 2013. [Google Scholar]
- Pezzella M, et al. Severe pulmonary congestion in a near miss at the first seizure: further evidence for respiratory dysfunction in sudden unexpected death in epilepsy. Epilepsy Behav. 2009;14:701–2. doi: 10.1016/j.yebeh.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Ramirez S, et al. Activating positive memory engrams suppresses depression-like behaviour. Nature. 2015;522:335–9. doi: 10.1038/nature14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–77. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- Sarkis RA, et al. Autonomic changes following generalized tonic clonic seizures: An analysis of adult and pediatric patients with epilepsy. Epilepsy Res. 2015;115:113–8. doi: 10.1016/j.eplepsyres.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Shen HY, et al. A novel mouse model for sudden unexpected death in epilepsy (SUDEP): role of impaired adenosine clearance. Epilepsia. 2010;51:465–8. doi: 10.1111/j.1528-1167.2009.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So EL, et al. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia. 2000;41:1494–7. doi: 10.1111/j.1528-1157.2000.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Sowers LP, et al. Sudden unexpected death in epilepsy: fatal post-ictal respiratory and arousal mechanisms. Respir Physiol Neurobiol. 2013;189:315–23. doi: 10.1016/j.resp.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ZQ, Trussell LO. Serotonergic regulation of excitability of principal cells of the dorsal cochlear nucleus. J Neurosci. 2015;35:4540–51. doi: 10.1523/JNEUROSCI.4825-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NE, et al. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A. 2016;113:12826–31. doi: 10.1073/pnas.1614340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, et al. Mapping brain metabolic connectivity in awake rats with muPET and optogenetic stimulation. J Neurosci. 2013;33:6343–9. doi: 10.1523/JNEUROSCI.4997-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman DJ, et al. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia. 2014;55:1479–85. doi: 10.1111/epi.12666. [DOI] [PubMed] [Google Scholar]
- Tomson T, et al. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol. 2008;7:1021–31. doi: 10.1016/S1474-4422(08)70202-3. [DOI] [PubMed] [Google Scholar]
- Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia. 2006;47:21–6. doi: 10.1111/j.1528-1167.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- Van Dort CJ, et al. Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc Natl Acad Sci U S A. 2015;112:584–9. doi: 10.1073/pnas.1423136112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–68. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- White HS. Clinical significance of animal seizure models and mechanism of action studies of potential antiepileptic drugs. Epilepsia. 1997;38(Suppl 1):S9–17. doi: 10.1111/j.1528-1157.1997.tb04523.x. [DOI] [PubMed] [Google Scholar]
- Yan QS, et al. Further evidence of anticonvulsant role for 5-hydroxytryptamine in genetically epilepsy-prone rats. Br J Pharmacol. 1995;115:1314–8. doi: 10.1111/j.1476-5381.1995.tb15042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, et al. Fluoxetine prevents respiratory arrest without enhancing ventilation in DBA/1 mice. Epilepsy Behav. 2015;45:1–7. doi: 10.1016/j.yebeh.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Q, et al. Impaired Serotonergic Brainstem Function during and after Seizures. J Neurosci. 2016;36:2711–22. doi: 10.1523/JNEUROSCI.4331-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–56. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. Atomoxetine, a norepinephrine reuptake inhibitor, reduces seizure-induced respiratory arrest. Epilepsy Behav. 2017;73:6–9. doi: 10.1016/j.yebeh.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. 5-Hydroxytryptophan, a precursor for serotonin synthesis, reduces seizure-induced respiratory arrest. Epilepsia. 2016;57:1228–35. doi: 10.1111/epi.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, et al. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods. 2011;8:745–52. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]