Abstract
Intracellular organelles, including secretory vesicles, emerged when eukaryotic cells evolved some 3 billion years ago. The primordial organelles that evolved in Archaea were similar to endolysosomes, which developed, arguably, for specific metabolic tasks, including uptake, metabolic processing, storage and disposal of molecules. In comparison to prokaryotes, cell volume of eukaryotes increased by several orders of magnitude and vesicle traffic emerged to allow for communication between distant intracellular locations. Lysosomes, first described in 1955, a prominent intermediate of endo- and exocytotic pathways, operate virtually in all eukaryotic cells including astroglia, the most heterogeneous type of homeostatic glia in the central nervous system. Astrocytes support neuronal network activity in particular through elaborated secretion, based on a complex intracellular vesicle network dynamics. Deranged homeostasis underlies disease and astroglial vesicle traffic contributes to the pathophysiology of neurodegenerative (Alzheimer’s disease, Huntington’s disease), neurodevelopmental diseases (intellectual deficiency, Rett’s disease) and neuroinfectious (Zika virus) disorders. This review addresses astroglial cell-autonomous vesicular traffic network, classified into primary and secondary vesicular network defects in diseases, targets for developing new therapies for neurologic conditions.
Keywords: Vesicle network, Endocytosis, Exocytosis, Lysosome, Astrocyte, Secretory vesicle, Traffic, Rett’s disease, Intellectual deficiency, Neurodegeneration
The gliocrine system: astrocytes as secretory elements of the CNS
Astroglia are the class of neural cells, heterogeneous in form and function, primarily responsible for the homeostasis of the central nervous system (CNS). Astroglial functions also include control of synaptogenesis and regulation of synaptic connectivity, integration and synchronization of neuronal networks and the maintenance of blood-brain barrier (BBB) integrity 1–18. In addition, astrocytes represent the fundamental element of defensive system of nervous tissue; insults to the brain or spinal cord trigger reactive astrogliosis, which limits the damage and facilitates post-lesion regeneration of neural networks 3, 11, 13 although it may be also neurotoxic in some circumstances 19. Moreover, astrocytes are part of the glymphatic system, regulating the convective removal of waste accumulated in the extracellular space 20.
Signalling between astrocytes and other cellular elements of the CNS is mainly mediated through the release of chemical messengers. Astroglial cells secrete surprisingly large number of molecules, which include neurotransmitters and their precursors, neurohormones, trophic, plastic and growth factors, scavengers of reactive oxygen species, immunoactive molecules and pro-inflammatory factors and many more 16, 21, 22. Mechanisms of astroglial secretion are complex and include vesicular exocytosis, diffusion through plasmalemmal channels and plasmalemmal transporters 12, 16. Thus astrocytes represent a gliocrine system 22, responsible for regulation of the CNS microenvironment through secretion of neuroactive compounds. The role for secretory organelles is, however, substantially wider than simple export of various cargos; different types of cytosolic vesicles contribute to endocytosis and delivery of proteins to plasmalemma, whereas impairment of vesicular traffic results in multiple neuropathologies.
Evolution of secretory organelles
Life forms belong to the Domains (also known as Superkingdoms) of Bacteria, Archea and Eukarya 23, 24. It is almost universally accepted that secretory organelles exist solely in eukaryotic cells, which notion, however, requires critical assessment. The origin and appearance of eukaryotes remains debatable 25, 26 and their immediate predecessors (for example, archaebacteria or eubacteria 27) are not yet unequivocally identified. Similarly disputed is the geological age of eukaryote emergence; eukaryotic signatures have been recovered in fossils of 3 to 3.5 billion years of age 28, this being substantially older than generally accepted time of eukaryote emergence ~ 2 billion years ago. This may indicate a very early divergence of the Domains of Life. Incidentally, the intermediate form lying in between Archea and Eukarya, the “Chronocyte”, has also been suggested 29.
Cytological structure of Bacteria and Archaea is relatively uncomplicated. How Eukarya, characterized by a significant degree of cellular structural complexity developed from Archaea 30, is unclear, although most hypotheses assume a prokaryote-to-eukaryote transition or else an integration of an archaebacterium into a eubacterium. Conceptually, the eukaryote evolution is linked to the presence of mitochondrion-like structures and/or mitochondrial proteins. It seems, however, possible that aerobic and anaerobic eukaryotes, harbouring mitochondrial homologues of various sorts, have co-existed throughout eukaryote history 31.
The hypothesis that Eukarya emerged from an ancestral Archea has recently gained interest, in particular after subcellular organelles other than mitochondria were recognised in the early life forms. Indeed, the discovery of ‘Lokiarchaeota’, a novel phylum, with genes encoding an array of eukaryotic signature proteins that are required for membrane plasticity supports this view 30. Specifically, this ancestral Archaea expressed genes encoding proteins, which in Eukarya contribute to remodelling of cell shape and membrane deformation including phagocytosis, a form of endocytosis, where molecules or particles are transported into the cell involving vesicles pinching off the plasma membrane 32. This process requires cytoskeletal elements such as actin; the Lokiarchaeum genome encodes five actin homologues with high similarity to eukaryotic actin and actin-related proteins 30. Key regulators of actin cytoskeleton dynamics are small GTPases, which are essential for vesicle traffic 33. A substantial presence of Ras-superfamily GTPases, which account for nearly 2% of the proteome, have been identified in Lokiarchaeota 30. This Archaean phylum also contains the genes for endosomal sorting complexes required for transport (ESCRT) proteins, components of the eukaryotic multivesicular body (MVB) belonging to the endosome pathway required for the biogenesis of lysosomes, directing plasma membrane proteins for lysosomal degradation, acting in viral budding, in cytokinesis, autophagy, and other pathways 34. Identification of archaeal genes involved in membrane remodelling and vesicular trafficking indicates that the emergence of subcellular organelles, resembling endolysosomal structures, was already under way before the acquisition of the mitochondrial endosymbiont 30.
The lysosomal network
Lysosomes are dedicated organelles of endo- and exocytotic pathways, which operate in nearly all animal and plant cells. The term lysosome was introduced in 1955 35 from Ancient Greek “λύσις” – “to loosen” and “soma” - body i.e. “lytic or digestive body” since several degrading enzymes have been found in this structure 36, 37. To visualize this new organelle a staining method for acid phosphatase was used, and the location of these enzymes in the lysosome was confirmed by using electron microscopy 38. An important insight about the degrading function of lysosomes, which contains many hydrolytic enzymes, came from the discoveries that molecules present in the extracellular space can enter the cell via endocytosis. Fragments of the labelled extracellular proteins were found localised in lysosomes 39.
Until recently, it was thought that lysosomes are merely responsible for catabolism, since they contain hydrolytic enzymes. Besides degradation of biomolecules, however, the lysosome is involved in energy metabolism, in secretion, in delivery of membrane receptors to the plasma membrane, in plasma membrane repair and in cell signalling. All this is possible as lysosomes actively fuse (see Figure 1) with other cellular structures including the plasmalemma, late endocytotic vesicles, autophagosomes that haul intracellular material for degradation; in addition membrane transporters translocate metabolites, ions and soluble substrates into and out of lysosomes 37. The central position of lysosome is not only linked to endocytotic entry of molecules into the cell but also to exocytotic exit of molecules (Fig. 1). Incidentally, the terms autophagy (for lysosomal degradation of material of the intracellular origin), endocytosis (for lysosomal digestion of material of the extracellular origin) and exocytosis were all introduced at a meeting in London in 1963 36, which followed lysosomes discovery. The complex lysosomal vesicular network is also present in astrocytes.
Figure 1.
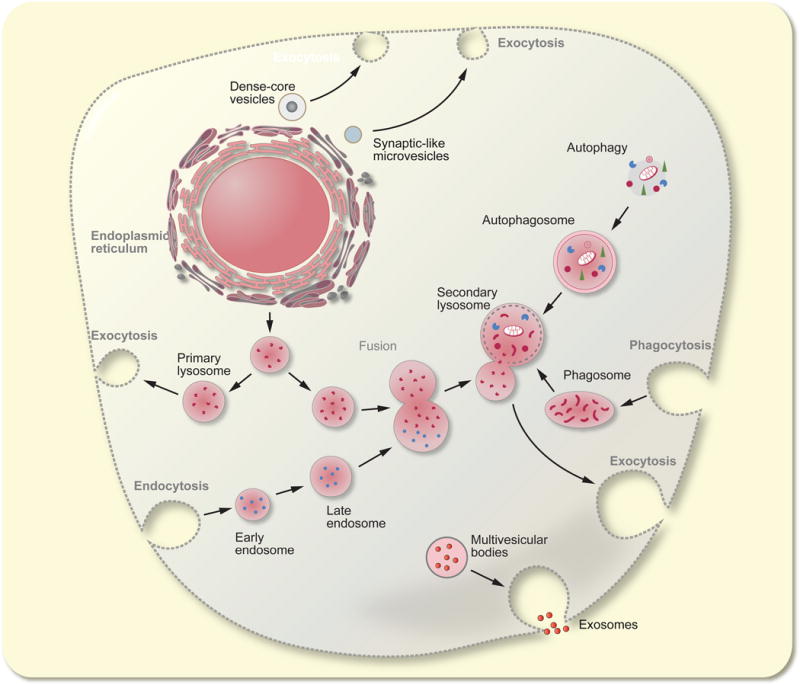
The vesicular-lysosome network.
Intracellular secretory organelles (synaptic-like vesicles, dense-core vesicles and primary lysosomes) originate from the endoplamic reticulum and Golgi complex. Primary lysosomes fuse with endosomes, phagosomes and autopahgosomes and convert to secondary lysosomes which undergo exocytosis thus expelling products of degradation. The multivesicular bosies contain exosomes that may carry various signalling factors.
Astrocytes and vesicle traffic
Astrocytes actively participate in neurochemical signalling in the CNS, a property considered no longer exclusive to neurones. Astroglia express virtually all types of receptors 40, 41 to neurotransmitters and neuromodulators linked to the second messenger excitability involving cytosolic ionic signals 42–45 and cyclic adenosine monophosphate (cAMP) pathways 22, 46. Astrocytes are also capable of secreting numerous signalling molecules.
Neurochemical signalling involves vesicle-related processes and the first observation of these functional subcellular structures in astrocytes was made over 100 years ago by microscopic studies conducted by Hans Held 47 and Jean Nageotte 48, who both proposed that glial cells act as secretory entities in the CNS. Existence of secretory organelles in astroglia has been confirmed by advanced biophysical, electrophysiological and quantitative high-resolution optical microscopy studies 16, 49, 50. In contrast to vesicle-based fast neuronal signalling, vesicular regulated exocytosis in astrocytes occurs in a much slower time-domain, with temporal characteristics closely related to the slow CNS processes including neurodevelopmental plasticity, memory formation and homeostatic metabolic support; all these being strongly influenced by the relatively slow delivery of metabolic precursors for morphological and functional CNS plasticity 51, 52.
The integrating role of glial communication in the CNS resembles, to some extent, the function of the endocrine system, a master regulator of bodily functions, which operates substantially slower than rapid, often sub-millisecond, synaptic neuronal signalling. Therefore, astroglia were proposed to constitute the gliocrine system within the CNS 16, 22. Another similarity between the endocrine and the gliocrine systems lies in the manner of signalling molecules delivery to the appropriate targets. Hormones are transported by blood circulation, while gliosignalling molecules, secreted by astroglia into the interstitium, can be convectively transferred by the recently described glymphatic apparatus 20. Thus, astroglial vesicular networks contribute to the tissue-wide array of processes, all fundamental to normal and pathological CNS conditions.
Astroglial endocytosis
Endocytosis consists of many processes, and contributes to a multitude of cellular functions including uptake of extracellular nutrients, regulation of cell-surface receptor presence, which shapes the plasmalemmal signalling landscape, and antigen presentation 32. Astrocytes are facultative antigen presenting cells 53, especially during pathological conditions, when they become reactive. Astrogliosis, an active defensive response of astrocytes is associated with various diseases/disorders; astrogliotic remodelling facilitates resolution of the pathology, although under some circumstances it may be maladaptive and deleterious 13, 19. Discrimination of stages of astrogliosis is difficult, since there is a gradient of changes in structure, expression of genes and function. However, exposure of astrocytes to interferon-γ (IFN-γ) induces the expression of major histocompatibility complex II (MHC II) molecules. These molecules traffic within astrocytes by endolysosomal vesicles along the cytoskeletal intermediate filaments 53 and reach the plasmalemmal surface following the fusion of these organelles with the plasma membrane. Lysosomal destination for fusion with the plasma membrane is to some degree determined by their previous interaction with autophagosomes 37 and is regulated by β-adrenergic receptor signalling, which inhibits the expression of MHC II molecules in astrocytes 54, 55. Whether this process provides toxic or beneficial effects in the pathological process in the damaged tissue remains to be determined.
Extracellular debris removal by astroglial endocytosis
The removal of waste materials and debris from the extracellular space is in part the function of endocytosis coupled with the convective flow of the glymphatic system, an important clearance mechanism, depending on aquaporin 4 (AQP4) water channel 56–58. Although the position of AQP4 in the brain appears to be important in determining water transport in the brain 59, it is still unclear how AQP4 participates in the convective flow of extracellular solution through the brain and spinal cord parenchyma, which appears key in ensuring CNS homeostasis and contributes to pathologic conditions as occurring in cerebral ischemia, neurotrauma, cytotoxic oedema, epilepsy, Parkinson’s and Alzheimer’s diseases, in which the BBB is compromised 60–62. Debris that accumulates in the CNS due to transfer from the systemic circulation through the BBB or due to pathological events within the CNS itself, coupled to diffusion, fluxes along the relatively long tortuous extracellular space of the brain interstitium and may be taken into astroglial vesicle network by endocytosis.
Astroglial endocytic and lysosomal network can be fundamental for the removal of cellular detritus and toxic macromolecules both in physiological and pathological context. In a multitude of diseases/conditions associated with myelin destruction, which include multiple sclerosis, progressive multifocal leukoencephalopathy, metachromatic leukodystrophy and subacute infarct, astrocytes accumulate myelin debris by means of cell surface scavenger receptor low density lipoprotein receptor-related protein 1 (LRP1) receptor-mediated endocyosis 63. Myelin fragments were rapidly (within 120 min) translocated from endosomes to lysosomes, where they underwent degradation. Astrocytes can remove myelin in physiological context, for example during developmentally regulated shortening of optic nerve in Xenopus laevis 64. In Drosophila larvae astroglial cells act as primary phagocytotic elements critical for the removal of neurites during neuropil development 65 as well as pruning of axons in developing, mushroom bodies 66, with the removed material entering edno-lysosomal degrading pathway. Astrocytes also employ endocytosis for the accumulation of signalling molecules, for example neurokinin B 67 or pro-brain derived neurotrophic factor (BDNF) 68.
In neurotrauma, for example, cytoplasmic proteins exit into the extracellular space. This may result in additional toxicity, with these proteins exerting further damaging effects on neighbouring cells. The capacity of internalization of S100B, a protein secreted by astrocytes 69, was studied recently 70. Fluorescently labelled S100B (S100B-Alexa Fluor®488) was added to the cultured astrocytes and cells were monitored by time-lapse confocal microscopy. The S100B was found to enter cells, with subsequent trafficking with endocytotic vesicles. The entry of S100B-Alexa488 involved, at least in part, the “scavenger receptor” RAGE, since pre-treatment with anti-RAGE antibody partially prevented the uptake of S100B-Alexa Fluor®488. Endocytotic uptake of S100B-Alexa Fluor®488 was also suppressed by a dynamin inhibitor Dynole 34-2. As described previously 71, endolysosomal traffic exhibits directional and non-directional vesicle mobility, and this was also observed for endocytotic vesicles taking up S100B-Alexa Fluor®488 70. Directional mobility of S100B-Alexa Fluor®488-positive vesicles increased over time as did the co-localization of this protein with a lysosomal marker, indicating the transport of the internalized protein into this compartment 70.
Exocytotic vesicles in astroglia
Although astroglial vesicle-mediated release of chemical messengers is well documented, this mechanism, however, is distinct from that operational in neurones, especially when the kinetics is considered 16, 49, 72, 73. Following the pioneering studies which revealed astroglial release of glutamate in a Ca2+-dependent manner 74, the role of vesicular secretion of gliosignalling molecules was analysed in vesicles containing fluorescently labelled atrial natriuretic peptide (ANP) 50 and by monitoring membrane capacitance, a parameter linearly related to membrane area 75 and sufficiently sensitive to detect unitary vesicle fusion/fission events in real-time in isolated 76, 77 and also in astrocytes in tissue slices 78. While there has been comprehensive coverage on the size and nature of vesicles in astrocytes elsewhere 10, 16, 18, using electron microscopy and fluorescence markers 68, 79–82. Here, we focus on a recent study, where the super-resolution fluorescence microscopy was combined with the high-resolution membrane capacitance monitoring 83, compellingly demonstrated the existence of two populations of secretory vesicles (distinct in their size) in cultured and freshly isolated rat astrocytes. Small vesicles (~ 70 nm in diameter) mainly contain amino acids glutamate (based on labelling of the vesicular glutamate transporter 1; V-GLUT1) and D-serineand/or peptides (brain derived neurotrophic factor - BDNF and ANP), whereas ATP and some peptides are present mainly in larger (~ 200 nm in diameter) lysosome-associated compartments (marked by antibody against the lysosomal associated membrane protein LAMP-1 and by quinacrine (which binds to adenine nucleotides), as was already reported previously 84. This separation of vesicular populations was not dependent on animal age, since similar results were obtained in astrocytes deriving from post-natal as well as from adult animals. The larger vesicles that contain peptides and ATP may well be generated by the fusion of peptidergic vesicles that arise from the Golgi cisternae with some endosomes and/or lysosomes. Vesicles of all sizes in astrocytes interact with the plasma membrane, as was monitored by discrete increases in membrane capacitance. As was already noted for endocrine cells 85, transient exocytotic fusions were more abundant than the full-fusion exocytotic events, especially after stimulation of cells, which facilitates conversion of transient fusion events to full-fusion predominantly in larger vesicles 83. This vesicle size-dependent mechanism of unitary exocytotic events is not due to a different density of SNARE molecules in larger and smaller vesicles 86, but it more likely reflects vesicle size-dependent stability of fusion pores, which appear more stable in smaller vesicles 87.
Regulation of vesicle network dynamics in astrocytes
First measurements of single vesicle peptidergic release from astrocytes 50, found that individual vesicles exhibit high mobility with elongated directional and contorted non-directional tracks. This was further corroborated by quantification of vesicle paths 88. Similar dual-modal vesicle mobility has been observed in other cell types, such as chromaffin and neuroendocrine cells 88–91. In contrast to other cell types, however, vesicle traffic in astrocytes exhibited a remarkable regulation by changes in [Ca2+]i 92, 93. Increase in [Ca2+]i reduced mobility of peptides- and ATP-containing vesicles 71, while increasing the mobility of vesicles carrying amino acids and labelled by VGLUT1 94. Recycling vesicles, which take-up extracellular markers (such as antibodies that bind to vesicle cargo or vesicle luminal markers) and subsequently release them by exocytosis, also demonstrate similar vesicle dynamics, both in cultured astrocytes 95 and in the brain slices 96. Increase in astroglial [Ca2+]i, arrests movements of recycling vesicles 95. These vesicle type dependent, regulatory mechanisms may well change in pathologic conditions 2, 92.
Astroglia as a central element of neuropathology
Astroglia contribution to neuropathology is multifaceted and most likely disease-specific. The very nature of astrocytes as homeostatic cells makes them central elements in neural tissue response to various insults. In the context of neurological diseases astroglia may undergo pathological remodelling (for example, in Alexander disease - AxD), degeneration with loss of function (in psychiatric diseases or at the early stages of neurodegenerative diseases such as amyotrophic lateral sclerosis - ALS, or Alzheimer’s disease - AD) or reactive astrogliotic remodelling instigated by neurotrauma or accumulation of extracellular pathological protein deposits such as Levy bodies of senile plaques 11, 13, 19, 21, 40, 97–102. These principal pathological metamorphoses can develop separately or in combination, and moreover distinct astrogliopathological changes may emerge at different stages of neurological disorders. Degeneration and atrophy of astrocytes, which is manifested by a decrease in the number of cells and dwindling of their morphological profiles is often associated with compromised glutamate clearance that seems to be a converging point in many diseases associated with glutamate neurotoxicity. The impaired astroglial glutamate uptake plays a critical role in neurological disorders with broad clinical manifestations, which include ALS, Wernicke’s encephalopathy, Huntington’s disease (HD) and some psychiatric disorders 3, 103. Astrogliotic response is a similarly widespread feature of neuropathology 98. Astrogliosis represents a conserved defensive reprogramming of astroglial cells, which involves complex biochemical and functional remodelling and produces multiple reactive cellular phenotypes. Reactive astrocytes boost neuroprotection and are critical for post-lesion regeneration of the nervous tissue; inhibition of astroglial reactivity usually exacerbates neuropathology. The common denominator of astrogliopathological changes are associated with morphological remodelling aimed towards atrophy or hypertrophy 104, 105. These relatively rapid morphological changes may involve modified vesicular trafficking 46, 92; similarly, both astrodegeneration and astroglial reactivity result in changes in astroglial vesicular intracellular networks.
Vesicular dysfunction in neurologic diseases
Vesicle traffic associates with numerous functions of astrocytes including regulation of the plasma membrane density of receptors, pumps, transporters, is involved in changes in morphological plasticity and in cell degradation pathways. Impairment of the vesicle network dynamics hence may contribute to astrogliopathies 92.
Primary and secondary vesicle network defects
In relation to vesicle network disturbances, in principle, there are two main groups of disorders, depending on the defect within the astroglial vesicular network. First, vesicle traffic may be primarily affected due to altered regulation of vesicle mobility including altered association of vesicles with the cytoskeletal elements, mutated cytoskeletal elements, and/or disturbances in the vesicle membrane merger. These alterations are becoming uncovered only recently when experimental approaches enabling direct monitoring of vesicle traffic at single vesicle level in real-time in astrocytes have been introduced 76, 88, 106 and due to advanced genetic and posttranslational tubulin studies 107. Examples of neurological diseases with primary vesicle network defects are listed in Table 1. Impaired vesicle mobility may result from alterations in cytoskeleton fabrics. Such alterations accompany reactive gliosis with overexpressed intermediate filaments 108. Similarly, cytoskeleton is impaired in AxD, associated with sporadic mutations of glial fibrillary acidic protein 109. Unstable microtubules contribute to the Rett’s disease 110. General defects in vesicle traffic possibly are observed in neurodegeneration including AD 111, HD 112, and in ALS 113, 114. Mutated regulatory proteins affect vesicle dynamics 115 in intellectual deficiency (ID) 116. Vesicle dynamics may be also altered by virus infection such as the Tick borne encephalitis virus (TBEV) infection 117 or autoimmune Devic’s disease (neuromyelitis optica) 118, 119 where vesicle dynamics of AQP4 water channel is affected 120, 121. Alterations in vesicle dynamics may develop as side effects of medication, for example, in multiple sclerosis treated with FTY 720 122 or when using ketamine, an anaesthetic and antidepressant 106, which also affects vesicle merger with the plasma membrane 123.
Table 1.
Neurological diseases associated with primary and secondary vesicle network defects
| Astroglial Vesicle Network Defect | ||||
|---|---|---|---|---|
| Diseases and neuropathological states | Primary | Secondary | Mechanism/Comment | Reference |
| Tay Sachs disease, a member of the lysosomal storage diseases | X | Hexosaminidase A deficiency | 129 | |
| Sandhoff disease, a member of the lysosomal storage diseases | X | Hexosaminidase A and B deficiency | 127 | |
| Nieman-Pick’s Type A disease, a lipid storage disorder | X | Sphyngomyelinase deficiency | 131 | |
| Alexander’s disease | X | Cytoskeletal defect | 109 | |
| Devic’s disease (Neuromyelitis optica) | X? | Autoantibodies against AQP4, affecting AQP4 vesicle dynamics | 118–120 | |
| Alzheimer’s Disease | X | General vesicle traffic defect | 111 | |
| Intellectual Deficiency | X | General vesicle traffic defect | 116 | |
| Rett’s disease | X | General vesicle traffic defect/cytoskeletal defect | 110 | |
| Glycogen Storage Disease | X | Glucose-6- phosphatase deficiency | 177. | |
| Neuroinfection by viruses (Tick-borne encephalitis virus - TBEV) | X? | Affecting endocytosis and endolysosomal traffic dynamics | 117 | |
| Amyotrophic lateral sclerosis (ALS) | X? | Signalling and vesicle traffic defect | 113, 114 | |
| Huntington’s Disease | X | Mutated huntingtin affects vesicle traffic | 112 | |
| Ketamine treated psychiatric disease | X | Affecting vesicle merger with the plasma membrane/signalling | 106, 123 | |
| Fingolimod (FTY 720) treated multiple sclerosis | X | Affecting vesicle dynamics | 122 | |
| Reactive gliosis | X | Overexpression of intermediate filaments affects vesicle dynamics | 108 | |
| Microcephaly, lissencephay | X | Tubulin cytoskeleton alterations in radial glia leading to abnormal cell division and migration | 107 | |
| Gaucher disease | X | deficient acid-beta-glucosidase, leading to accumulation of glucosylceramide | 132 | |
| Krabbe disease, a form of leukodystrophy | X | deficiency of β-galactocerebrosidase leads to build-up of unmetabolized lipids affecting the growth of the myelin sheath | 127 | |
| Infantile neuronal ceroid lipofuscinosis (INCL), neurodegenerative disease | X | Deficiency of palmitoyl protein thioesterase 1, a lysosomal enzyme | 124 | |
| Batten disease, neuronal ceroid lipofuscinoses, a form of lysosomal storage diseases | X | Lipofuscin materials build up in neuronal cells and many organs | 133 | |
The other vesicle network defects, termed secondary, are associated with abnormalities of carried cargo. Enzymatic deficits result in impaired processing of metabolic intermediates, which are transported in vesicles leading to accumulation of the non-degraded cargo in the vesicular compartment that may disrupt (jam) the vesicle network as a whole (Figure 1). In support that the vesicle network defects are of two kinds, primary and secondary, are experiments, where a combination of cytoskeleton alteration and an enzymatic deficit was introduced to study infantile neuronal ceroid lipofuscinosis (INCL), an inherited neurodegenerative disorder, caused by mutations in the lysosomal hydrolase, palmitoyl protein thioesterase 1 (PPT1), revealed that if both the intermediate filament cytoskeleton (deleting GFAP and vimentin) and the the PPT1 were impaired, the INCL progressed more rapidly 124.
Malfunction of hydrolitic enzymes, such as PPT1, impairs lysosomal degrading pathway; this impairment lies at the core of extended (more than 60 members) family of lysosomal storage diseases 125, 126. Roughly two thirds to three quarters of lysosomal storage diseases are neuropathic 127. Neurological disorders resulting from lysosomal pathology include neuronal ceroid lipofuscinoses (or Batten disease), Krabbe disease, Gaucher disease, Sandhoff disease, Niemann-Pick (NP) type C, Tay-Sachs disease, etc. 128. In Tay-Sachs disease there is a deficiency of hexosaminidase A, a vital lysosomal hydrolytic enzyme, degrading glycolipids 129. As a result, these lipids accumulate, and this intracellular accumulation affects normal cell function and interferes with normal tissue processes, eventually leading to premature cell death. Sandhoff disease is characterized by deficiency of hexosaminidases A and B, resulting in excessive lysosomal accumulation of GM2 gangliosides and oligosaccharides containing glucosamine residues 130. The Niemann–Pick (NP) diseases are a subfamily of lipid storage disorders in which harmful quantities of lipids or fatty acids accumulate in the liver, bone marrow, lungs, spleen, and CNS. In the type A of NP diseases there is a deficiency of sphingomyelinase 131, hence sphingomyelin accumulates in lysosomes, leading to the enlargement of cells. The Gaucher disease reflects upon deficiency of acid-beta-glucosidase, leading to accumulation of glucosylceramide 132, whereas Krabbe disease is associated with the deficiency of β-galactocerebrosidase 127. In the context of lysosomal storage diseases, astrocytes demonstrate either reactive phenotypes or apoptotic death, both of which possibly associate with abnormalities of the lysosomal network 127. Astroglial reactive remodelling sometimes occurs at the early pathological stages preceding (and possibly precipitating) neuronal death; such an early activation is observed, for example, in juvenile Batten disease 133. Considerable lysosomal pathology was observed in astrocytes in the context of multiple sulfatase deficiency that cause severe neurodegeneration. Deficient astroglial lysosomal network affects autophagy and is linked to decreased neuronal survival 134.
In contrast to secondary vesicle network impairments, vesicle dynamics in primary network defects, may be directly affected by changes in cytoskeleton along which vesicles are transported. As mentioned previously, vesicles exhibit directional and non-directional mobility. The former depends on intact cytoskeleton 108 and impairments in the linking of vesicles to cytoskeleton as well as changes in cytoskeleton may lead to pathology. This means that vesicle network dynamics may be a target for treatments.
Neurotropic viruses use endocytosis to enter astrocytes
The rise of Zika virus (ZIKV) epidemic in the Central and South Americas enticed refreshed interest into how the virus, linked with microcephaly (severe under-development of the cerebral cortex in newborn babies from infected mothers 135), instigates this neurodevelopmental disorder. As shown previously the tick-borne encephalitis virus (TBEV), a human pathogen that causes neuroinfections in Europe and Asia 136–138, infects astroglia, after entering astrocytes by endocytosis 117. The TBEV, as well as ZIKV are members of the Flaviviridae family 135, 139. A similar mechanism of entry into astrocytes, (although it is yet to be fully characterised) underlies ZIKV neurotropism. Again, similar mechanism underlies astroglial infection with human immunodeficiency virus 1 (HIV-1), which enters astroglia by endocytosis, however the HIV-1 virus seems to be effectively destroyed in the astroglial lysosomal pathway 140.
Mechanisms of TBEV entry into rodent astrocytes are complex 117. First, to invade the CNS, a neurotropic virus must negotiate the BBB, which protects the CNS tissue from the direct ingress of viruses circulating in the blood 138, 141. Astrocytes are a key component of the BBB, with astroglial endfeet providing almost complete coverage of the intracranial vasculature. Astrocytes are positioned between synapses and blood vessels, contributing to neurovascular coupling 142, 143. Infection with TBEV does not affect astroglial viability; to the contrary, astrocytes likely serve as a reservoir for TBEV supporting re-infection 117. Second, preferential viral infection of astroglia likely reflects the fact that astrocytes provide a favourable environment for virus replication, possibly because since they are the site with a special adaptation of glycolysis. Aerobic glycolysis, characteristic for astroglia, also exists in rapidly dividing cells and in cells undergoing plastic morphological changes, despite the presence of adequate levels of oxygen, a phenomenon known as “the Warburg effect” 144. This form of metabolism is far from being efficient for ATP production; however, it provides biosynthetic intermediates thus being advantageous for developing and growing tissues 145. Aerobic glycolysis, primarily taking place in astrocytes in the CNS, is operative during neurodevelopment and even in adulthood in some areas of the CNS 146. Therefore, this property of astrocytes may be associated with the preferential infection of astrocytes by neurotropic viruses, such as ZIKV.
Intra-astrocytic traffic of TBEV within the endosomal system was monitored in real-time 117. As depicted on Figure 2, TBEV particles associate with early endosomes and later, during the post-infection time, also with lysosomes as revealed by co-localization of TBEV particles with endosomal (early endosome antigen 1, EEA1) and lysosomal LAMP1 markers. Similarly to secretory vesicles 88, endocytosed TBEV-containing vesicles exhibit two forms of mobility in living astrocytes, directional and non-directional. The former likely reflects vesicular transport with protein motors along the cytoskeletal elements, such as microtubules, actin and intermediate filaments, including astroglia-specific glial acidic fibrillary protein (GFAP) 108. In contrast, the mobility of non-directional vesicles is determined by free diffusion 88. Prolongation of the post-infection time not only increased the number of TBEV particles within an astrocyte, but it also resulted in increased TBEV particle mobility 117.
Figure 2.

Time-dependence of the number of endosomes and lysosomes associated with DiD-TBEV particles in astrocytes.
(Ai,ii). An astrocyte with DiD-labelled TBEV vesicles (TBEV) incubated at 37 °C for 4 h and 18 h and with labelled early endosomes (EEA1) Overlays represents overlapped TBEV and EEA1 fluorescent signals, indicating the association between TBEV and endosomes. Bars: 5 μm. (Aiii) Prolonged incubation increased the average number of TBEV labelled vesicles per cell and also the average number of vesicles co-labelled with TBEV and EEA1. Black bars - TBEV labelled vesicles, white bars - TBEV and EEA1 co-labelled vesicles, *P<0.05. (Bi,ii) An astrocyte with DiD-labelled TBEV vesicles (TBEV) incubated at 37 °C for 4 h and 18 h and with LAMP1-labelled lysosomes (LAMP1-lysosomal associated membrane protein 1). Overlays represent overlapped TBEV and LAMP1 fluorescent signals, indicating the association between TBEV and lysosomes. Bars: 5 μm. (Biii) Prolonged incubation increased the average number of TBEV labelled vesicles per cell and the average number of vesicles co-labelled with TBEV and LAMP1. Black bars - TBEV labelled vesicles, white bars - TBEV and LAMP1 co-labelled vesicles, ***P<0.001. (C) Astrocyte co-labelled with anti-LAMP1 and anti-EEA1. In a single, 1 μm thick optical slice, lysosomes and early endosomes appear to be largely different in size due to different position in z-axis and variable antibody attachments. Arrowheads point to large late lysosomes (LAMP1) and early endosomes (EEA1). Bar (bar inset): 5 μm (2.5 μm). n (n) = number of cells (number of vesicles). Reprinted with permission 117.
Endocytosis was recently confirmed to be the mechanism of ZIKV infection of astrocytes and microglia 147. The membrane protein Axl, a member of the Tyro3 Axl Mer (TAM) family of proteins, exhibiting tyrosine kinase receptor activity, regulating innate immunity and the removal of apoptotic cells 148, plays a role in targeting glia 147. Since Axl is abundantly expressed in glial but not in neural progenitor cells, this indicates a preferential cellular tropism for ZIKV infection 147. Future experiments should aim to confirm that neurovirulence of ZIKV and other flaviviruses is associated mainly with astroglia, including radial glia, which are fundamental for the development of the human neocortex, which involves the activity of locus coeruleus (LC)149. The release of noradrenaline form LC neurones activates aerobic glycolysis in astrocytes, monitored as an increase in cytosolic glucose concentration 150, leading to the production of L-lactate 151. The increased levels of L-lactate feed back to the LC neurons 152, an interesting glio-neuronal communication where L-lactate serves as a signal.
Neurodegeneration and vesicle traffic deficits
Neurodegeneration starts decades before clinical presentation, and may involve defective vesicle traffic. The initial changes might be small, but over a long time-period, significant transformations of cells and, subsequently, of tissue function develop. Small initial changes have been detected in astrocytes from 3xTg-AD mice, an animal model for AD, expressing three mutant genes for amyloid precursor protein (APPSwe), presenilin 1 (PS1M146V) and microtubule-associated protein Tau (TauP301L, 153. These astrocytes, which (according to the disease model genetic design) express only the PS1M146V mutation, exhibit impaired vesicle traffic 111. Mobility of peptidergic and endolysosomal vesicles in astrocytes, isolated during early pre-symptomatic state, revealed that spontaneous as well as stimulated, Ca2+- dependent, vesicle mobility was diminished. The impaired vesicle traffic was observed also in wild type rat astrocytes transfected with familial AD-associated mutated PS1M146V gene. Eventually, altered properties of vesicle mobility may result in reduced peptide secretion, contributing to the development of neurodegeneration 111. Moreover, a recent study where mutated huntingtin caused impaired peptidergic secretion in HD 112, is supporting the view that vesicle traffic defects may lead to neurodegeneration 92.
In addition to a reduced capacity of vesicular release of peptidergic and various chemical messengers from astrocytes, other vesicle-based mechanisms might contribute to the evolution of neurodegeneration. A prominent histopathological sign of AD is the extracellular accumulation of β-amyloid, which may be associated with a reduced capacity for removal or degrading mechanisms 154. An important factor that may decrease β-amyloid deposits is the release of proteolytic enzymes from exocytotic lysosomes, which degrade the β-amyloid peptide in the extracellular space. Insulin degrading enzyme (IDE) is such an enzyme, secreted from neurones in the healthy brain 155. In astrocytes the secretion of IDE can be increased by an autophagy-dependent way by statins, a process likely leading to statin-induced degradation of extracellular β-amyloid peptide Aβ40, which depends on IDE secretion from primary astrocytes 156. Therefore, it appears, that in AD the function of IDE secretion by astrocytes is reduced in comparison to astrocytes in healthy subjects, consequently leading to increased deposits of β-amyloid. The mechanism of reduced secretion of IDE in AD is possibly mediated by an impaired interaction between autophagosomes and lysosomes 154, an interaction needed to facilitate lysosomes to become competent for exocytosis 37. The reduced capacity for release of IDE by astrocytes may mirror a general vesicle traffic defect associated with a lysosomal involvement in injury repair in astrocytes 157.
In the vicinity of plaques and β-amyloid deposits astrocytes attain a reactive phenotype with over-expressed intermediate filaments 158. Astrocytes exposed to IFN-γ, a pro-inflammatory cytokine, start to expresses in their surface membrane MHC-II molecules, turning astrocytes into antigen-presenting cells. These molecules are delivered to the plasma membrane by endolysosomes 53, 159. Vesicle delivery of MHC-II molecules to the plasma membrane involves a cytoskeletal network that exhibits upregulated intermediate filaments, a characteristic of astrogliosis 13. However, microtubules 160, 161, actin 162 and their motor proteins 160, 163 have all been reported to play a role in MHC-II-vesicle traffic. Up-regulation of cytoskeletal structures, in particular intermediate filaments, speeds up vesicle mobility and makes antigen presentation more efficient 53. Noradrenaline, released from LC neurones is an endogenous suppressor of antigen presentation by astrocytes 54; whether this inhibition involves reduced vesicle traffic, remains to be studied.
Neurodevelopment-related diseases and vesicle traffic
Vesicle traffic is strongly involved in neurodevelopment 164, for example, by regulating cell migration during the neocortex formation 165. Thus, when a neurotoxic attack or a viral infection impairs vesicle dynamics, neurodevelopmental defects (such as the microcephaly associated with ZIKV infection) may follow 135.
In neurodevelopmental diseases, such as in intellectual deficiency (ID), a form of mental retardation characterised with low (< 85) IQ, vesicle traffic disturbances may be the key cellular defect. The only manifest sign in nonspecific ID is a reduced cognitive potential, which when presented together with other clinical signs and symptoms may compose a syndrome (e.g., Down syndrome), or may be associated with mitochondrial, developmental or metabolic deficits 166. Symptoms of X-linked forms of ID (XLID), which appear early in life, are affecting a fair number of subjects; XLID is present in around 2% of the population 167. One of the first mutated genes discovered in patients with XLID is GDI1 168, which encodes the αGDI, a regulatory protein of monomeric GTP-ases, such as the family of Rab proteins. The αGDI protein retrieves the inactive, GDP-bound form of Rab proteins, from the membrane. Rab GTPases are important vesicle traffic regulators 169. Therefore, when the causal role for GDI1 mutated gene in ID was identified, this suggested that vesicular traffic in neuronal synapses is affected 170, 171. Astrocytes express αGDI, and vesicle traffic in astrocytes carrying a mutation in GDI revealed an impaired vesicle phenotype; vesicle directionality index was reduced, revealing a less efficient coupling of vesicles to the cytoskeleton 116. These results are consistent with previously published data, where the role of mutated Rab 4 and Rab 5 monomeric GTP-ases, both regulated by αGDI protein, were studied (Potokar et al., 2012). How the impaired endolysosomal traffic affects the function of astrocytes in ID remains to be clarified.
Abnormal astrocytic vesicle traffic underlies another autistic spectrum disorder, the Rett’s syndrome, a rare X-linked neurodevelopmental disease 110. Mutations in the methyl-CpG-binding protein 2 (Mecp2), encoding a multifunctional protein that binds to methylated DNA, acting as a transcriptional regulator are believed to be responsible. When Mecp2 is re-expressed specifically in astrocytes in the Mecp2-null mice, locomotion and anxiety levels were significantly improved, respiratory abnormalities were restored to a normal pattern and lifespan was greatly prolonged compared with knock-out mice 172. Microtubule-dependent vesicle transport is altered in Mecp2-deficient astrocytes isolated from newborn Mecp2-deficient mice and from human MECP2 p.Arg294* iPSC-derived astrocytes 110. Administration of microtubule-stabilizing drug (Epothilone D), that passes BBB restored microtubule dynamics in Mecp2-deficient astrocytes and in human MECP2 p.Arg294* iPSC-derived astrocytes in vitro. Therefore, drugs that target not only the fusion/fission of vesicles with the plasma membrane as does ketamine, but also molecules that target the integrity of the cytoskeleton are valid targets for new innovative treatments of impaired vesicle networks to be used in future therapies.
Vesicle network dynamics, a target of drugs for treating neurological disturbances
Several drugs, currently used to treat neurological disease, have an effect on astrocytic vesicle dynamics. Vesicle mobility in astrocytes is decreased by fingolimod or FTY720 122. This drug (Gilenya, Novartis), which is hydrophobic in nature, accumulates in the white matter in the CNS and is used for the treatment of multiple sclerosis 173. The marketing authorization holder claims that the mechanism of action of this drug is an inhibition of the egress of lymphocytes into the CNS in its phosphorylated form, acting via the sphingosin 1 receptor (SP1R) 174. However, this molecule also acts in the unphosphorylated form directly on astrocytes where it affects astrocytic vesicle dynamics 122 and likely also antigen presentation by astrocytes, that associates with neuroinflammation 53. FTY 720 treatment revealed that traffic of all vesicle types studied were affected 122, hence this drug may modulate the release of other proinflammatory factors from astrocytes, including eicosanoids, which are released in the CNS by an ATP-dependent mechanism 175. Regulated release of ATP from astrocytes 84 participates in the neuroinflammatory CNS states and alterations of traffic of ATP carrying organelles may additionally affect neuroinflammatory response in diseased conditions.
Another established drug, ketamine, an anaesthetic and an antidepressant, affects the vesicle traffic and BDNF release from astroglia 106. High-resolution membrane capacitance measurements revealed that in the presence of clinically relatively low concentrations, ketamine induces fusion pores to enter into a stable, narrow flickering state 123. In the presence of ketamine, the fusion pore attains a diameter that is too narrow to permit the discharge of the peptides contained in the vesicle lumen 106, 176. These results indicate that the fusion pore, an intermediate stage of the secretory vesicle traffic, is a target for therapy.
Conclusions
Vesicular network consists of many pathways, where endocytotic and exocytotic vesicles merge and give rise to new vesicles, essentially intersecting with the lysosome, present in nearly all eukaryotic cells including astrocytes. Thus lysosomes, being at the crossroad of many vesicle-based processes, not only contribute to their classically asigned function – degradation of molecules - but instead, control the whole vesicle network, contributing to important subcellular physiological events, including the modulation of the metabolic and signalling capacity of astrocytes, as well as determining the morphological plasticity and adaptability to pathologic changes. By understanding more fully the mechanisms of vesicle network traffic and the interaction of vesicles with the cytoskeleton, at various stages, from how endocytotic mechanisms prime some lysosomes to becoming exocytotic vesicles, to how vesicles containing peptidergic cargo, emerging from Golgi, interact with antigen presentation systems and phagocytosis. Clearly, as addressed in this review, all these processes may well form the basis for neurological dysfunction and represent targets for development of new therapies.
Figure 3.

Secretory vesicles studied by STED and SIM microscopies in acutely isolated rat astrocytes
(A) Confocal and STED microscopy images of immunostained D-serine-, V-GLUT1-, ANP- and BDNF-positive vesicles in acutely isolated astrocytes. Histograms display STED-acquired vesicle diameter distributions for 1788 (D-serine), 6787 (V-GLUT1), 1747 (ANP) and 798 (BDNF) vesicles (2 cells per staining). The black curves show Gaussian fits of the diameter distributions; the numbers next to the distribution peaks indicate the average vesicle diameter (expectation value ± SEM). Recalculated values taking into account the microscope’s optical resolution (45 nm) are 80.8 nm for D-serine, 88.4 nm for V-GLUT1, 85.9 nm for ANP and 86.8 nm for BDNF. Scale bar, 500 nm.
(B) Wide-field microscopy and SIM were used to determine the vesicle diameter of immunostained LAMP1 endolysosomes and ATP-loaded vesicles (quinacrine dihydrochloride). Histograms show SIM-acquired vesicle diameter distributions for 557 (LAMP1, 2 cells) and 445 (quinacrine, 2 cells) vesicles in acutely isolated astrocytes (upper two panels) and 338 (LAMP1, 3 cells) and 333 (quinacrine, 6 cells) vesicles in astrocytes isolated from 7- to 8-week-old rats (lower two panels). The black curves show Gaussian fits of the diameter distributions; the average vesicle diameter (expectation value ± SEM) is labelled next to the distribution peaks. Scale bar, 500 nm. Modified with permission 83.
Acknowledgments
This work was supported by grants P3 310, J3 6790, J3 6789 and J3 7605 from the Slovenian Research Agency (ARRS), CipKeBip, COST Action BM1002, EU COST Action CM1207 - GLISTEN (to RZ). VP’s work is supported by the National Institutes of Health (The Eunice Kennedy Shriver National Institute of Child Health and Human Development award HD078678 to VP).
Abbreviations
- AD
Alzheimer’s disease
- AQP4
aquaporin 4 ALS, amyotrophic lateral sclerosis
- ANP
atrial natriuretic peptide
- AxD
Alexander’s disease
- BDNF
brain derived neurotrophic factor
- BBB
blood-brain barrier
- CNS
central nervous system
- cAMP
cyclic adenosine monophosphate
- EEA1
early endosome antigen 1
- ESCRT
endosomal sorting complexes required for transport
- FTY 720
fingolimod
- HIV-1
human immunodeficiency virus 1
- HD
Huntington’s disease
- ID
intellectual deficiency
- IDE
Insulin degrading enzyme
- IFN-γ
interferon-γ
- INCL
infantile neuronal ceroid lipofuscinosis
- iPSC
inducible pluripotent stem cells
- LAMP-1
lysosome associated membrane protein 1
- LC
locus coeruleus
- LRP1
low density lipoprotein receptor-related protein 1
- Mecp2
methyl-CpG-binding protein 2
- MHC II
major histocompatibility complex II
- MVB
multivesicular body
- NP
Niemann–Pick disease
- PPT1
palmitoyl protein thioesterase 1
- SNARE
soluble NSF attachment protein receptor
- TBEV
Tick borne encephalitis virus
- V-GLUT1
vesicular glutamate transporter
- ZIKV
Zika virus
- XLID
X-linked forms of ID
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Verkhratsky A, Nedergaard M. The homeostatic astroglia emerges from evolutionary specialization of neural cells. Philos Trans R Soc Lond B Biol Sci. 2016;371 doi: 10.1098/rstb.2015.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potokar M, Stenovec M, Kreft M, Gabrijel M, Zorec R. Physiopathologic dynamics of vesicle traffic in astrocytes. Histol Histopathol. 2011;26:277–284. doi: 10.14670/HH-26.277. [DOI] [PubMed] [Google Scholar]
- 3.Verkhratsky A, Zorec R, Rodriguez JJ, Parpura V. Astroglia dynamics in ageing and Alzheimer’s disease. Curr Opin Pharmacol. 2016;26:74–79. doi: 10.1016/j.coph.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Abbott N, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 5.Christopherson K, Ullian E, Stokes C, Mullowney C, Hell J, Agah A, Lawler J, Mosher D, Bornstein P, Barres B. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haydon P. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 8.Martin D. Synthesis and release of neuroactive substances by glial cells. Glia. 1992;5:81–94. doi: 10.1002/glia.440050202. [DOI] [PubMed] [Google Scholar]
- 9.Nedergaard M, Ransom B, Goldman S. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Parpura V, Baker B, Jeras M, Zorec R. Regulated exocytosis in astrocytic signal integration. Neurochem Int. 2010 doi: 10.1016/j.neuint.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF, Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. J Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Rev. 2010;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131:323–345. doi: 10.1007/s00401-015-1513-1. [DOI] [PubMed] [Google Scholar]
- 14.Sultan S, Li L, Moss J, Petrelli F, Casse F, Gebara E, Lopatar J, Pfrieger FW, Bezzi P, Bischofberger J, Toni N. Synaptic Integration of Adult-Born Hippocampal Neurons Is Locally Controlled by Astrocytes. Neuron. 2015;88:957–972. doi: 10.1016/j.neuron.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Tao-Cheng J, Nagy Z, Brightman M. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci. 1987;7:3293–3299. doi: 10.1523/JNEUROSCI.07-10-03293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R. Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J. 2016 doi: 10.15252/embj.201592705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolburg H, Neuhaus J, Kniesel U, Krauss B, Schmid E, Ocalan M, Farrell C, Risau W. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci. 1994;107(Pt 5):1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- 18.Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro. 2012;4 doi: 10.1042/AN20110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thrane AS, Rangroo Thrane V, Nedergaard M. Drowning stars: reassessing the role of astrocytes in brain edema. Trends Neurosci. 2014;37:620–628. doi: 10.1016/j.tins.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zorec R, Verkhratsky A, Rodriguez JJ, Parpura V. Astrocytic vesicles and gliotransmitters: Slowness of vesicular release and synaptobrevin2-laden vesicle nanoarchitecture. Neuroscience. 2016;323:67–75. doi: 10.1016/j.neuroscience.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Vardjan N, Zorec R. Excitable Astrocytes: Ca(2+)- and cAMP-Regulated Exocytosis. Neurochem Res. 2015;40:2414–2414. doi: 10.1007/s11064-015-1545-x. [DOI] [PubMed] [Google Scholar]
- 23.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding F, O’Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium. 2013;54:387–394. doi: 10.1016/j.ceca.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gribaldo S, Brochier-Armanet C. The origin and evolution of Archaea: a state of the art. Philos Trans R Soc Lond B Biol Sci. 2006;361:1007–1022. doi: 10.1098/rstb.2006.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spang A, Martijn J, Saw JH, Lind AE, Guy L, Ettema TJ. Close encounters of the third domain: the emerging genomic view of archaeal diversity and evolution. Archaea. 2013;2013:202358. doi: 10.1155/2013/202358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalier-Smith T. The neomuran origin of archaebacteria, the negibacterial root of the universal tree and bacterial megaclassification. Int J Syst Evol Microbiol. 2002;52:7–76. doi: 10.1099/00207713-52-1-7. [DOI] [PubMed] [Google Scholar]
- 28.Brocks JJ, Logan GA, Buick R, Summons RE. Archean molecular fossils and the early rise of eukaryotes. Science. 1999;285:1033–1036. doi: 10.1126/science.285.5430.1033. [DOI] [PubMed] [Google Scholar]
- 29.Hartman H, Fedorov A. The origin of the eukaryotic cell: a genomic investigation. Proc Natl Acad Sci U S A. 2002;99:1420–1425. doi: 10.1073/pnas.032658599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spang A, Saw JH, Jorgensen SL, Zaremba-Niedzwiedzka K, Martijn J, Lind AE, van Eijk R, Schleper C, Guy L, Ettema TJ. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature. 2015;521:173–179. doi: 10.1038/nature14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Embley TM, Martin W. Eukaryotic evolution, changes and challenges. Nature. 2006;440:623–630. doi: 10.1038/nature04546. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 33.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 34.Hurley JH. The ESCRT complexes. Crit Rev Biochem Mol Biol. 2010;45:463–487. doi: 10.3109/10409238.2010.502516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Duve C, Pressman BC, Gianetto R, Wattiaux R, Appelmans F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955;60:604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Duve C. The lysosome turns fifty. Nat Cell Biol. 2005;7:847–849. doi: 10.1038/ncb0905-847. [DOI] [PubMed] [Google Scholar]
- 37.Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14:283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Essner E, Novikoff AB. Localization of acid phosphatase activity in hepatic lysosomes by means of electron microscopy. J Biophys Biochem Cytol. 1961;9:773–784. doi: 10.1083/jcb.9.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straus W. Cytochemical Observations on the Relationship between Lysosomes and Phagosomes in Kidney and Liver by Combined Staining for Acid Phosphatase and Intravenously Injected Horseradish Peroxidase. J Cell Biol. 1964;20:497–507. doi: 10.1083/jcb.20.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verkhratsky A. Physiology of neuronal-glial networking. Neurochem Int. 2010;57:332–343. doi: 10.1016/j.neuint.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Mol Neurobiol. 2009;39:190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- 42.Rusakov DA, Zheng K, Henneberger C. Astrocytes as regulators of synaptic function: a quest for the Ca2+ master key. Neuroscientist. 2011;17:513–523. doi: 10.1177/1073858410387304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- 44.Rose CR, Verkhratsky A. Principles of sodium homeostasis and sodium signalling in astroglia. Glia. 2016;64:1611–1627. doi: 10.1002/glia.22964. [DOI] [PubMed] [Google Scholar]
- 45.Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 2012;35:497–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Vardjan N, Kreft M, Zorec R. Dynamics of β-adrenergic/cAMP signaling and morphological changes in cultured astrocytes. Glia. 2014;62:566–579. doi: 10.1002/glia.22626. [DOI] [PubMed] [Google Scholar]
- 47.Held H. Über die Neuroglia marginalis der menschlichen Grosshirnrinde. Monatschr f Psychol u Neurol. 1909;26:360–416. Rdg.-Heft. [Google Scholar]
- 48.Nageotte J. Phenomenes de secretion dans le protoplasma des cellules nevrogliques de la substance grise. Paris: 1910. [Google Scholar]
- 49.Kreft M, Stenovec M, Rupnik M, Grilc S, Krzan M, Potokar M, Pangrsic T, Haydon PG, Zorec R. Properties of Ca(2+)-dependent exocytosis in cultured astrocytes. Glia. 2004;46:437–445. doi: 10.1002/glia.20018. [DOI] [PubMed] [Google Scholar]
- 50.Krzan M, Stenovec M, Kreft M, Pangrsic T, Grilc S, Haydon PG, Zorec R. Calcium-dependent exocytosis of atrial natriuretic peptide from astrocytes. J Neurosci. 2003;23:1580–1583. doi: 10.1523/JNEUROSCI.23-05-01580.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vardjan N, Kreft M, Zorec R. Regulated Exocytosis in Astrocytes is as Slow as the Metabolic Availability of Gliotransmitters: Focus on Glutamate and ATP. Adv Neurobiol. 2014;11:81–101. doi: 10.1007/978-3-319-08894-5_5. [DOI] [PubMed] [Google Scholar]
- 52.Zorec R, Horvat A, Vardjan N, Verkhratsky A. Memory Formation Shaped by Astroglia. Front Integr Neurosci. 2015;9:56. doi: 10.3389/fnint.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vardjan N, Gabrijel M, Potokar M, Svajger U, Kreft M, Jeras M, de Pablo Y, Faiz M, Pekny M, Zorec R. IFN-γ-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments. J Neuroinflammation. 2012;9:144. doi: 10.1186/1742-2094-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frohman EM, Vayuvegula B, Gupta S, van den Noort S. Norepinephrine inhibits gamma-interferon-induced major histocompatibility class II (Ia) antigen expression on cultured astrocytes via beta-2-adrenergic signal transduction mechanisms. Proc Natl Acad Sci U S A. 1988;85:1292–1296. doi: 10.1073/pnas.85.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laureys G, Clinckers R, Gerlo S, Spooren A, Wilczak N, Kooijman R, Smolders I, Michotte Y, De Keyser J. Astrocytic beta(2)-adrenergic receptors: from physiology to pathology. Prog Neurobiol. 2010;91:189–199. doi: 10.1016/j.pneurobio.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35:518–526. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei F, Zhang C, Xue R, Shan L, Gong S, Wang G, Tao J, Xu G, Zhang G, Wang L. The pathway of subarachnoid CSF moving into the spinal parenchyma and the role of astrocytic aquaporin-4 in this process. Life Sci. 2017 doi: 10.1016/j.lfs.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 59.Vindedal GF, Thoren AE, Jensen V, Klungland A, Zhang Y, Holtzman MJ, Ottersen OP, Nagelhus EA. Removal of aquaporin-4 from glial and ependymal membranes causes brain water accumulation. Mol Cell Neurosci. 2016;77:47–52. doi: 10.1016/j.mcn.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia. 2013;61:1939–1958. doi: 10.1002/glia.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pangrsic T, Potokar M, Haydon PG, Zorec R, Kreft M. Astrocyte swelling leads to membrane unfolding, not membrane insertion. J Neurochem. 2006;99:514–523. doi: 10.1111/j.1471-4159.2006.04042.x. [DOI] [PubMed] [Google Scholar]
- 62.Vardjan N, Horvat A, Anderson JE, Yu D, Croom D, Zeng X, Luznik Z, Kreft M, Teng YD, Kirov SA, Zorec R. Adrenergic activation attenuates astrocyte swelling induced by hypotonicity and neurotrauma. Glia. 2016;64:1034–1049. doi: 10.1002/glia.22981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponath G, Ramanan S, Mubarak M, Housley W, Lee S, Sahinkaya FR, Vortmeyer A, Raine CS, Pitt D. Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain. 2017;140:399–413. doi: 10.1093/brain/aww298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mills EA, Davis CH, Bushong EA, Boassa D, Kim KY, Ellisman MH, Marsh-Armstrong N. Astrocytes phagocytose focal dystrophies from shortening myelin segments in the optic nerve of Xenopus laevis at metamorphosis. Proc Natl Acad Sci U S A. 2015;112:10509–10514. doi: 10.1073/pnas.1506486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tasdemir-Yilmaz OE, Freeman MR. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 2014;28:20–33. doi: 10.1101/gad.229518.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hakim Y, Yaniv SP, Schuldiner O. Astrocytes play a key role in Drosophila mushroom body axon pruning. PLoS One. 2014;9:e86178. doi: 10.1371/journal.pone.0086178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shahzad R, Jones MR, Viles JH, Jones CE. Endocytosis of the tachykinin neuropeptide, neurokinin B, in astrocytes and its role in cellular copper uptake. J Inorg Biochem. 2016;162:319–325. doi: 10.1016/j.jinorgbio.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 68.Bergami M, Santi S, Formaggio E, Cagnoli C, Verderio C, Blum R, Berninger B, Matteoli M, Canossa M. Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J Cell Biol. 2008;183:213–221. doi: 10.1083/jcb.200806137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I. S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta. 2009;1793:1008–1022. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Lasic E, Galland F, Vardjan N, Sribar J, Krizaj I, Leite MC, Zorec R, Stenovec M. Time-dependent uptake and trafficking of vesicles capturing extracellular S100B in cultured rat astrocytes. J Neurochem. 2016;139:309–323. doi: 10.1111/jnc.13754. [DOI] [PubMed] [Google Scholar]
- 71.Potokar M, Stenovec M, Gabrijel M, Li L, Kreft M, Grilc S, Pekny M, Zorec R. Intermediate filaments attenuate stimulation-dependent mobility of endosomes/lysosomes in astrocytes. Glia. 2010;58:1208–1219. doi: 10.1002/glia.21000. [DOI] [PubMed] [Google Scholar]
- 72.Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Rev. 2010;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kreft M, Krizaj D, Grilc S, Zorec R. Properties of exocytotic response in vertebrate photoreceptors. J Neurophysiol. 2003;90:218–225. doi: 10.1152/jn.01025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 75.Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kreft M, Zorec R. Cell-attached measurements of attofarad capacitance steps in rat melanotrophs. Pflugers Arch. 1997;434:212–214. doi: 10.1007/s004240050387. [DOI] [PubMed] [Google Scholar]
- 77.Rituper B, Gucek A, Jorgacevski J, Flasker A, Kreft M, Zorec R. High-resolution membrane capacitance measurements for the study of exocytosis and endocytosis. Nat Protoc. 2013;8:1169–1183. doi: 10.1038/nprot.2013.069. [DOI] [PubMed] [Google Scholar]
- 78.Jorgacevski J, Potokar M, Kreft M, Gucek A, Mothet JP, Zorec R. Astrocytic Vesicle-based Exocytosis in Cultures and Acutely Isolated Hippocampal Rodent Slices. J Neurosci Res. 2017 doi: 10.1002/jnr.24051. [DOI] [PubMed] [Google Scholar]
- 79.Bergersen LH, Morland C, Ormel L, Rinholm JE, Larsson M, Wold JF, Roe AT, Stranna A, Santello M, Bouvier D, Ottersen OP, Volterra A, Gundersen V. Immunogold detection of L-glutamate and D-serine in small synaptic-like microvesicles in adult hippocampal astrocytes. Cereb Cortex. 2012;22:1690–1697. doi: 10.1093/cercor/bhr254. [DOI] [PubMed] [Google Scholar]
- 80.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 81.Bowser DN, Khakh BS. Two forms of single-vesicle astrocyte exocytosis imaged with total internal reflection fluorescence microscopy. Proc Natl Acad Sci U S A. 2007;104:4212–4217. doi: 10.1073/pnas.0607625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malarkey EB, Parpura V. Temporal characteristics of vesicular fusion in astrocytes: examination of synaptobrevin 2-laden vesicles at single vesicle resolution. J Physiol. 2011;589:4271–4300. doi: 10.1113/jphysiol.2011.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gucek A, Jorgacevski J, Singh P, Geisler C, Lisjak M, Vardjan N, Kreft M, Egner A, Zorec R. Dominant negative SNARE peptides stabilize the fusion pore in a narrow, release-unproductive state. Cell Mol Life Sci. 2016;73:3719–3731. doi: 10.1007/s00018-016-2213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pangrsic T. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- 85.Jorgacevski J, Potokar M, Grilc S, Kreft M, Liu W, Barclay JW, Buckers J, Medda R, Hell SW, Parpura V, Burgoyne RD, Zorec R. Munc18-1 tuning of vesicle merger and fusion pore properties. J Neurosci. 2011;31:9055–9066. doi: 10.1523/JNEUROSCI.0185-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flasker A, Jorgacevski J, Calejo AI, Kreft M, Zorec R. Vesicle size determines unitary exocytic properties and their sensitivity to sphingosine. Mol Cell Endocrinol. 2013;376:136–147. doi: 10.1016/j.mce.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 87.Jorgacevski J, Fosnaric M, Vardjan N, Stenovec M, Potokar M, Kreft M, Kralj-Iglic V, Iglic A, Zorec R. Fusion pore stability of peptidergic vesicles. Mol Membr Biol. 2010;27:65–80. doi: 10.3109/09687681003597104. [DOI] [PubMed] [Google Scholar]
- 88.Potokar M, Kreft M, Pangrsic T, Zorec R. Vesicle mobility studied in cultured astrocytes. Biochem Biophys Res Commun. 2005;329:678–683. doi: 10.1016/j.bbrc.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 89.Burke N, Han W, Li D, Takimoto K, Watkins S, Levitan E. Neuronal peptide release is limited by secretory granule mobility. Neuron. 1997;19:1095–1102. doi: 10.1016/s0896-6273(00)80400-6. [DOI] [PubMed] [Google Scholar]
- 90.Tvaruskó W, Bentele M, Misteli T, Rudolf R, Kaether C, Spector D, Gerdes H, Eils R. Time-resolved analysis and visualization of dynamic processes in living cells. Proc Natl Acad Sci U S A. 1999;96:7950–7955. doi: 10.1073/pnas.96.14.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duncan R, Greaves J, Wiegand U, Matskevich I, Bodammer G, Apps D, Shipston M, Chow R. Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nature. 2003;422:176–180. doi: 10.1038/nature01389. [DOI] [PubMed] [Google Scholar]
- 92.Vardjan N, Verkhratsky A, Zorec R. Pathologic potential of astrocytic vesicle traffic: new targets to treat neurologic diseases? Cell transplantation. 2015;24:599–612. doi: 10.3727/096368915X687750. [DOI] [PubMed] [Google Scholar]
- 93.Potokar M, Vardjan N, Stenovec M, Gabrijel M, Trkov S, Jorgacevski J, Kreft M, Zorec R. Astrocytic vesicle mobility in health and disease. International journal of molecular sciences. 2013;14:11238–11258. doi: 10.3390/ijms140611238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stenovec M, Kreft M, Grilc S, Potokar M, Kreft ME, Pangrsic T, Zorec R. Ca2+-dependent mobility of vesicles capturing anti-VGLUT1 antibodies. Experimental cell research. 2007;313:3809–3818. doi: 10.1016/j.yexcr.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 95.Potokar M, Stenovec M, Kreft M, Kreft ME, Zorec R. Stimulation inhibits the mobility of recycling peptidergic vesicles in astrocytes. Glia. 2008;56:135–144. doi: 10.1002/glia.20597. [DOI] [PubMed] [Google Scholar]
- 96.Potokar M, Kreft M, Lee SY, Takano H, Haydon PG, Zorec R. Trafficking of astrocytic vesicles in hippocampal slices. Biochem Biophys Res Commun. 2009;390:1192–1196. doi: 10.1016/j.bbrc.2009.10.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp Neurol. 2016;275(Pt 3):305–315. doi: 10.1016/j.expneurol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Giaume C, Kirchhoff F, Matute C, Reichenbach A, Verkhratsky A. Glia: the fulcrum of brain diseases. Cell Death Differ. 2007;14:1324–1335. doi: 10.1038/sj.cdd.4402144. [DOI] [PubMed] [Google Scholar]
- 100.Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev. 2014;94:1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 101.Verkhratsky A, Parpura V. Astrogliopathology in neurological, neurodevelopmental and psychiatric disorders. Neurobiol Dis. 2015;85:254–261. doi: 10.1016/j.nbd.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zorec R, Vardjan N, Verkhratsky A. Locus coeruleus noradrenergic neurons and astroglia in health and disease. In: VARDJAN N, ZOREC R, editors. Noradrenergic Signaling and Astroglia. New York: Elsevier; 2017. pp. 1–10. [Google Scholar]
- 103.Pekny M, Pekna M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta. 2016;1862:483–491. doi: 10.1016/j.bbadis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 104.Olabarria M, Noristani HN, Verkhratsky A, Rodríguez JJ. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia. 2010;58:831–838. doi: 10.1002/glia.20967. [DOI] [PubMed] [Google Scholar]
- 105.Jones VC, Atkinson-Dell R, Verkhratsky A, Mohamet L. Aberrant iPSC-derived human astrocytes in Alzheimer’s disease. Cell Death Dis. 2017;8:e2696. doi: 10.1038/cddis.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stenovec M, Lasic E, Bozic M, Bobnar ST, Stout RF, Jr, Grubisic V, Parpura V, Zorec R. Ketamine Inhibits ATP-Evoked Exocytotic Release of Brain-Derived Neurotrophic Factor from Vesicles in Cultured Rat Astrocytes. Mol Neurobiol. 2016;53:6882–6896. doi: 10.1007/s12035-015-9562-y. [DOI] [PubMed] [Google Scholar]
- 107.Chakraborti S, Natarajan K, Curiel J, Janke C, Liu J. The emerging role of the tubulin code: From the tubulin molecule to neuronal function and disease. Cytoskeleton (Hoboken) 2016;73:521–550. doi: 10.1002/cm.21290. [DOI] [PubMed] [Google Scholar]
- 108.Potokar M, Kreft M, Li L, Daniel Andersson J, Pangrsic T, Chowdhury HH, Pekny M, Zorec R. Cytoskeleton and vesicle mobility in astrocytes. Traffic. 2007;8:12–20. doi: 10.1111/j.1600-0854.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 109.Moody LR, Barrett-Wilt GA, Sussman MR, Messing A. Glial Fibrillary Acidic Protein Exhibits Altered Turnover Kinetics in a Mouse Model of Alexander Disease. J Biol Chem. 2017 doi: 10.1074/jbc.M116.772020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Delepine C, Meziane H, Nectoux J, Opitz M, Smith AB, Ballatore C, Saillour Y, Bennaceur-Griscelli A, Chang Q, Williams EC, Dahan M, Duboin A, Billuart P, Herault Y, Bienvenu T. Altered microtubule dynamics and vesicular transport in mouse and human MeCP2-deficient astrocytes. Hum Mol Genet. 2016;25:146–157. doi: 10.1093/hmg/ddv464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stenovec M, Trkov S, Lasič E, Terzieva S, Kreft M, Rodríguez Arellano JJ, Parpura V, Verkhratsky A, Zorec R. Expression of familial Alzheimer disease presenilin 1 gene attenuates vesicle traffic and reduces peptide secretion in cultured astrocytes devoid of pathologic tissue environment. Glia. 2016;64:317–329. doi: 10.1002/glia.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hong Y, Zhao T, Li XJ, Li S. Mutant Huntingtin Impairs BDNF Release from Astrocytes by Disrupting Conversion of Rab3a-GTP into Rab3a-GDP. J Neurosci. 2016;36:8790–8801. doi: 10.1523/JNEUROSCI.0168-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stenovec M, Milosevic M, Petrusic V, Potokar M, Stevic Z, Prebil M, Kreft M, Trkov S, Andjus PR, Zorec R. Amyotrophic lateral sclerosis immunoglobulins G enhance the mobility of Lysotracker-labelled vesicles in cultured rat astrocytes. Acta Physiol (Oxf) 2011;203:457–471. doi: 10.1111/j.1748-1716.2011.02337.x. [DOI] [PubMed] [Google Scholar]
- 114.Milosevic M, Stenovec M, Kreft M, Petrusic V, Stevic Z, Trkov S, Andjus PR, Zorec R. Immunoglobulins G from patients with sporadic amyotrophic lateral sclerosis affects cytosolic Ca2+ homeostasis in cultured rat astrocytes. Cell Calcium. 2013;54:17–25. doi: 10.1016/j.ceca.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 115.Potokar M, Lacovich V, Chowdhury HH, Kreft M, Zorec R. Rab4 and Rab5 GTPase are required for directional mobility of endocytic vesicles in astrocytes. Glia. 2012;60:594–604. doi: 10.1002/glia.22293. [DOI] [PubMed] [Google Scholar]
- 116.Potokar M, Jorgačevski J, Lacovich V, Kreft M, Vardjan N, Bianchi V, D’Adamo P, Zorec R. Impaired αGDI function in the X-linked intellectual disability: the impact on astroglia vesicle dynamics. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9834-1. in press. [DOI] [PubMed] [Google Scholar]
- 117.Potokar M, Korva M, Jorgacevski J, Avsic-Zupanc T, Zorec R. Tick-borne encephalitis virus infects rat astrocytes but does not affect their viability. PLoS One. 2014;9:e86219. doi: 10.1371/journal.pone.0086219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marignier R, De Seze J, Vukusic S, Durand-Dubief F, Zephir H, Vermersch P, Cabre P, Cavillon G, Honnorat J, Confavreux C. NMO-IgG and Devic’s neuromyelitis optica: a French experience. Mult Scler. 2008;14:440–445. doi: 10.1177/1352458507084595. [DOI] [PubMed] [Google Scholar]
- 119.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 120.Potokar M, Stenovec M, Jorgacevski J, Holen T, Kreft M, Ottersen OP, Zorec R. Regulation of AQP4 surface expression via vesicle mobility in astrocytes. Glia. 2013;61:917–928. doi: 10.1002/glia.22485. [DOI] [PubMed] [Google Scholar]
- 121.Potokar M, Jorgacevski J, Zorec R. Astrocyte Aquaporin Dynamics in Health and Disease. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17071121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Trkov S, Stenovec M, Kreft M, Potokar M, Parpura V, Davletov B, Zorec R. Fingolimod–a sphingosine-like molecule inhibits vesicle mobility and secretion in astrocytes. Glia. 2012;60:1406–1416. doi: 10.1002/glia.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lasic E, Rituper B, Jorgacevski J, Kreft M, Stenovec M, Zorec R. Subanesthetic doses of ketamine stabilize the fusion pore in a narrow flickering state in astrocytes. J Neurochem. 2016;138:909–917. doi: 10.1111/jnc.13715. [DOI] [PubMed] [Google Scholar]
- 124.Macauley SL, Pekny M, Sands MS. The role of attenuated astrocyte activation in infantile neuronal ceroid lipofuscinosis. J Neurosci. 2011;31:15575–15585. doi: 10.1523/JNEUROSCI.3579-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Platt FM, Boland B, van der Spoel AC. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J Cell Biol. 2012;199:723–734. doi: 10.1083/jcb.201208152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boustany RM. Lysosomal storage diseases–the horizon expands. Nat Rev Neurol. 2013;9:583–598. doi: 10.1038/nrneurol.2013.163. [DOI] [PubMed] [Google Scholar]
- 127.Rama Rao KV, Kielian T. Astrocytes and lysosomal storage diseases. Neuroscience. 2016;323:195–206. doi: 10.1016/j.neuroscience.2015.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prada CE, Grabowski GA. Neuronopathic lysosomal storage diseases: clinical and pathologic findings. Dev Disabil Res Rev. 2013;17:226–246. doi: 10.1002/ddrr.1116. [DOI] [PubMed] [Google Scholar]
- 129.Hechtman P, Kaplan F. Tay-Sachs disease screening and diagnosis: evolving technologies. DNA Cell Biol. 1993;12:651–665. doi: 10.1089/dna.1993.12.651. [DOI] [PubMed] [Google Scholar]
- 130.Itoh H, Tanaka J, Morihana Y, Tamaki T. The fine structure of cytoplasmic inclusions in brain and other visceral organs in Sandhoff disease. Brain Dev. 1984;6:467–474. doi: 10.1016/s0387-7604(84)80029-7. [DOI] [PubMed] [Google Scholar]
- 131.Brady RO, Kanfer JN, Mock MB, Fredrickson DS. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann-Pick diseae. Proc Natl Acad Sci U S A. 1966;55:366–369. doi: 10.1073/pnas.55.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jmoudiak M, Futerman AH. Gaucher disease: pathological mechanisms and modern management. Br J Haematol. 2005;129:178–188. doi: 10.1111/j.1365-2141.2004.05351.x. [DOI] [PubMed] [Google Scholar]
- 133.Burkovetskaya M, Karpuk N, Xiong J, Bosch M, Boska MD, Takeuchi H, Suzumura A, Kielian T. Evidence for aberrant astrocyte hemichannel activity in Juvenile Neuronal Ceroid Lipofuscinosis (JNCL) PLoS One. 2014;9:e95023. doi: 10.1371/journal.pone.0095023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Di Malta C, Fryer JD, Settembre C, Ballabio A. Autophagy in astrocytes: a novel culprit in lysosomal storage disorders. Autophagy. 2012;8:1871–1872. doi: 10.4161/auto.22184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 136.Gritsun T, Lashkevich V, Gould E. Tick-borne encephalitis. Antiviral Res. 2003;57:129–146. doi: 10.1016/s0166-3542(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 137.Chu J, Ng M. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J Virol. 2004;78:10543–10555. doi: 10.1128/JVI.78.19.10543-10555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mandl C. Steps of the tick-borne encephalitis virus replication cycle that affect neuropathogenesis. Virus Res. 2005;111:161–174. doi: 10.1016/j.virusres.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 139.Růzek D, Bell-Sakyi L, Kopecký J, Grubhoffer L. Growth of tick-borne encephalitis virus (European subtype) in cell lines from vector and non-vector ticks. Virus Res. 2008;137:142–146. doi: 10.1016/j.virusres.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 140.Chauhan A, Tikoo A, Patel J, Abdullah AM. HIV-1 endocytosis in astrocytes: a kiss of death or survival of the fittest? Neurosci Res. 2014;88:16–22. doi: 10.1016/j.neures.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bernacki J, Dobrowolska A, Nierwińska K, Małecki A. Physiology and pharmacological role of the blood-brain barrier. Pharmacol Rep. 2008;60:600–622. [PubMed] [Google Scholar]
- 142.Carmignoto G, Gómez-Gonzalo M. The contribution of astrocyte signalling to neurovascular coupling. Brain Res Rev. 2010;63:138–148. doi: 10.1016/j.brainresrev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 143.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tech K, Gershon TR. Energy metabolism in neurodevelopment and medulloblastoma. Transl Pediatr. 2015;4:12–19. doi: 10.3978/j.issn.2224-4336.2015.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meertens L, Labeau A, Dejarnac O, Cipriani S, Sinigaglia L, Bonnet-Madin L, Le Charpentier T, Hafirassou ML, Zamborlini A, Cao-Lormeau VM, Coulpier M, Misse D, Jouvenet N, Tabibiazar R, Gressens P, Schwartz O, Amara A. Axl Mediates ZIKA Virus Entry in Human Glial Cells and Modulates Innate Immune Responses. Cell Rep. 2017;18:324–333. doi: 10.1016/j.celrep.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 148.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 150.Prebil M, Vardjan N, Jensen J, Zorec R, Kreft M. Dynamic monitoring of cytosolic glucose in single astrocytes. Glia. 2011;59:903–913. doi: 10.1002/glia.21161. [DOI] [PubMed] [Google Scholar]
- 151.Dienel GA, Cruz NF. Aerobic glycolysis during brain activation: Adrenergic regulation and influence of norepinephrine on astrocytic metabolism. J Neurochem. 2016 doi: 10.1111/jnc.13630. [DOI] [PubMed] [Google Scholar]
- 152.Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S, Teschemacher AG. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Commun. 2014;5:3284. doi: 10.1038/ncomms4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 154.Son SM, Cha MY, Choi H, Kang S, Lee MS, Park SA, Mook-Jung I. Insulin-degrading enzyme secretion from astrocytes is mediated by an autophagy-based unconventional secretory pathway in Alzheimer disease. Autophagy. 2016;12:784–800. doi: 10.1080/15548627.2016.1159375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, Rosner MR, Selkoe DJ. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci. 2000;20:1657–1665. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Son SM, Kang S, Choi H, Mook-Jung I. Statins induce insulin-degrading enzyme secretion from astrocytes via an autophagy-based unconventional secretory pathway. Mol Neurodegener. 2015;10:56. doi: 10.1186/s13024-015-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sreetama SC, Takano T, Nedergaard M, Simon SM, Jaiswal JK. Injured astrocytes are repaired by Synaptotagmin XI-regulated lysosome exocytosis. Cell Death Differ. 2016;23:596–607. doi: 10.1038/cdd.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M. Glia in the pathogenesis of neurodegenerative diseases. Biochem Soc Trans. 2014;42:1291–1301. doi: 10.1042/BST20140107. [DOI] [PubMed] [Google Scholar]
- 159.Soos JM, Morrow J, Ashley TA, Szente BE, Bikoff EK, Zamvil SS. Astrocytes express elements of the class II endocytic pathway and process central nervous system autoantigen for presentation to encephalitogenic T cells. J Immunol. 1998;161:5959–5966. [PubMed] [Google Scholar]
- 160.Wubbolts R, Fernandez-Borja M, Jordens I, Reits E, Dusseljee S, Echeverri C, Vallee RB, Neefjes J. Opposing motor activities of dynein and kinesin determine retention and transport of MHC class II-containing compartments. J Cell Sci. 1999;112(Pt 6):785–795. doi: 10.1242/jcs.112.6.785. [DOI] [PubMed] [Google Scholar]
- 161.Vyas JM, Kim YM, Artavanis-Tsakonas K, Love JC, Van der Veen AG, Ploegh HL. Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells. J Immunol. 2007;178:7199–7210. doi: 10.4049/jimmunol.178.11.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barois N, Forquet F, Davoust J. Actin microfilaments control the MHC class II antigen presentation pathway in B cells. J Cell Sci. 1998;111(Pt 13):1791–1800. doi: 10.1242/jcs.111.13.1791. [DOI] [PubMed] [Google Scholar]
- 163.Vascotto F, Lankar D, Faure-André G, Vargas P, Diaz J, Le Roux D, Yuseff MI, Sibarita JB, Boes M, Raposo G, Mougneau E, Glaichenhaus N, Bonnerot C, Manoury B, Lennon-Duménil AM. The actin-based motor protein myosin II regulates MHC class II trafficking and BCR-driven antigen presentation. J Cell Biol. 2007;176:1007–1019. doi: 10.1083/jcb.200611147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sheen VL, Ganesh VS, Topcu M, Sebire G, Bodell A, Hill RS, Grant PE, Shugart YY, Imitola J, Khoury SJ, Guerrini R, Walsh CA. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat Genet. 2004;36:69–76. doi: 10.1038/ng1276. [DOI] [PubMed] [Google Scholar]
- 165.Barber M, Arai Y, Morishita Y, Vigier L, Causeret F, Borello U, Ledonne F, Coppola E, Contremoulins V, Pfrieger FW, Tissir F, Govindan S, Jabaudon D, Proux-Gillardeaux V, Galli T, Pierani A. Migration Speed of Cajal-Retzius Cells Modulated by Vesicular Trafficking Controls the Size of Higher-Order Cortical Areas. Curr Biol. 2015;25:2466–2478. doi: 10.1016/j.cub.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 166.Luckasson R, Reeve A. Naming, defining, and classifying in mental retardation. Ment Retard. 2001;39:47–52. doi: 10.1352/0047-6765(2001)039<0047:NDACIM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 167.Turner G. Finding genes on the X chromosome by which homo may have become sapiens. Am J Hum Genet. 1996;58:1109–1110. [PMC free article] [PubMed] [Google Scholar]
- 168.D’Adamo P, Menegon A, Lo Nigro C, Grasso M, Gulisano M, Tamanini F, Bienvenu T, Gedeon AK, Oostra B, Wu SK, Tandon A, Valtorta F, Balch WE, Chelly J, Toniolo D. Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat Genet. 1998;19:134–139. doi: 10.1038/487. [DOI] [PubMed] [Google Scholar]
- 169.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 170.D’Adamo P, Welzl H, Papadimitriou S, Raffaele di Barletta M, Tiveron C, Tatangelo L, Pozzi L, Chapman PF, Knevett SG, Ramsay MF, Valtorta F, Leoni C, Menegon A, Wolfer DP, Lipp HP, Toniolo D. Deletion of the mental retardation gene Gdi1 impairs associative memory and alters social behavior in mice. Hum Mol Genet. 2002;11:2567–2580. doi: 10.1093/hmg/11.21.2567. [DOI] [PubMed] [Google Scholar]
- 171.Bianchi V, Farisello P, Baldelli P, Meskenaite V, Milanese M, Vecellio M, Muhlemann S, Lipp HP, Bonanno G, Benfenati F, Toniolo D, D’Adamo P. Cognitive impairment in Gdi1-deficient mice is associated with altered synaptic vesicle pools and short-term synaptic plasticity, and can be corrected by appropriate learning training. Hum Mol Genet. 2009;18:105–117. doi: 10.1093/hmg/ddn321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, Mandel G. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chun J, Brinkmann V. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya) Discov Med. 2011;12:213–228. [PMC free article] [PubMed] [Google Scholar]
- 174.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 175.Murphy S, Pearce B, Jeremy J, Dandona P. Astrocytes as eicosanoid-producing cells. Glia. 1988;1:241–245. doi: 10.1002/glia.440010402. [DOI] [PubMed] [Google Scholar]
- 176.Kreft M, Jorgacevski J, Vardjan N, Zorec R. Unproductive exocytosis. J Neurochem. 2016;137:880–889. doi: 10.1111/jnc.13561. [DOI] [PubMed] [Google Scholar]
- 177.Chou JY, Jun HS, Mansfield BC. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase/glucose-6-phosphate transporter complexes. J Inherit Metab Dis. 2013;38:511–519. doi: 10.1007/s10545-014-9772-x. [DOI] [PubMed] [Google Scholar]


