Abstract
Objective
To assess the association of proxies of behavioral adherence to the TODAY lifestyle program with changes in glycemic control and obesity in a multi-ethnic sample of youth with type 2 diabetes.
Methods
The TODAY clinical trial included an intensive lifestyle intervention to promote weight reduction. Adherence was assessed with measures of attendance at intervention sessions and rates of self-monitoring of diet and physical activity by participants and their caregivers. The relation between participant characteristics and consistency of proxies of adherence were examined across three phases of intervention.
Results
234 TODAY youth were randomized to the lifestyle program. Overall rate of session attendance was approximately 60% of planned sessions. Participants with an adequate dose of session attendance (≥75% attended) did not differ from those who attended <75% of sessions in glycemic control, but did have significantly greater reductions in percent overweight compared with those who attended fewer than 75% of sessions. Rates of self-monitoring were low and additional analysis was not possible.
Conclusions
Rates of session attendance were moderate in a lifestyle program for youth with type 2 diabetes, but levels of self-monitoring, considered a key lifestyle change behavior, were low. Glycemic control was not significantly associated with session attendance but reductions in percent overweight were. Given the salience of program attendance and self-monitoring to lifestyle weight management established in other populations, future research is needed to understand, develop, and promote strategies and interventions targeting weight loss to achieve improved glycemic control in youth diagnosed with type 2 diabetes.
Keywords: pediatric obesity, type 2 diabetes, lifestyle intervention, adherence
INTRODUCTION
In response to increases in the prevalence of type 2 diabetes in children and adolescents, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) funded the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) randomized clinical trial, which compared the efficacy of three treatment arms: metformin monotherapy, metformin plus rosiglitazone, and metformin plus an intensive lifestyle intervention. After following the cohort for an average of 3.86 years, the trial found that the addition of rosiglitazone but not lifestyle intervention was associated with durable glycemic control (1). The metformin plus intensive lifestyle intervention was associated with superior short-term weight loss when compared to metformin monotherapy or metformin plus rosiglitazone, but contrary to expectation, this favorable change was not associated with durable glycemic control. However, a secondary analysis of data from the TODAY cohort (irrespective of randomized treatment) confirmed a relation between changes in weight and cardiovascular risk factors over time, suggesting the importance of weight management in youth with type 2 diabetes (2).
Findings in the pediatric obesity literature have documented a relationship between proxies for lifestyle program adherence, such as session attendance and compliance with self-monitoring of food intake and physical activity, and program outcomes. Higher levels of session attendance have been shown to be related to greater decreases in child percent overweight (3–5). Self-monitoring also has been shown to be associated with successful weight management in adolescents with obesity (6) and minority youth with severe obesity (7). Further, there is some evidence that parent self-monitoring is related to short-term weight losses among adolescents (8).
In TODAY, attendance at a pre-planned target of at least 75% of lifestyle program sessions during the first 24 months of the program was not associated with longer-term weight loss or maintenance of glycemic control (1). Given the imperative to enhance the effectiveness of treatments for pediatric type 2 diabetes (9) and initial findings documenting an association between weight changes and risk factor status (2), there is a strong rationale for examining more closely the role of adherence in the intensive lifestyle intervention in TODAY.
In the current analysis we report on two proxies for adherence to the TODAY lifestyle program (TLP), session attendance and self-monitoring of food intake and physical activity, and their association with changes in measures of obesity and glycemic control in a multi-ethnic sample of youth. We hypothesized that attendance at lifestyle intervention sessions and higher frequency of self-monitoring in youth and family participants would be positively associated with treatment outcome. We also examined whether baseline characteristics were related to or predictive of program adherence.
RESEARCH DESIGN AND METHODS
TODAY study design
Design of the TODAY clinical trial has been reported (10). Briefly, the collaborative study group included 15 clinical centers, a data coordinating center, the NIDDK project office, and central laboratories and reading centers (see on-line appendix). Participants (n=699) were enrolled between July 2004 and February 2009, aged 10–17 years with type 2 diabetes duration of less than two years at randomization and a body mass index (BMI) ≥85th percentile. To be eligible, TODAY participants had to identify an adult caregiver who agreed to support the youth in the study and accompany them to all sessions; this individual was typically a family member. Eligible subjects also had to complete successfully a 2–6 month run-in period that included attaining two successive months of glycemic control (HbA1c <8% [<64 mmol/mol]) on1,000 to 2,000 mg of metformin monotherapy, mastering standard diabetes education, demonstrating ≥80% adherence to study medication for at least eight of 12 consecutive weeks, and attending scheduled sessions. Eligible participants were randomized to one of three treatment arms: (1) metformin alone (M), (2) metformin plus rosiglitazone (M+R), and (3) metformin plus an intensive lifestyle program (M+L). The primary objective was to compare the three treatment conditions on time to treatment failure, i.e., loss of glycemic control defined as either HbA1c ≥8% (≥64 mmol/mol) for a six month period or inability to wean from temporary insulin therapy within three months following acute metabolic decompensation.
The protocol was approved by an External Evaluation Committee convened by the NIDDK and by the Institutional Review Boards of each participating institution. All participants provided written informed consent and children confirmed assent according to local guidelines.
Lifestyle program elements
Details of the TLP have been described (11). The program was designed to incorporate evidence-supported components used in numerous studies of family-based pediatric obesity treatment (12–13) and was conducted separately from the medical management provided to all TODAY participants. The program was administered in three consecutive phases. In phase 1, lifestyle change (LC) was delivered over the first 6–8 months in weekly sessions focused on achieving weight loss, physical activity, and behavior change goals. Lessons followed a set curriculum to ensure that a standard intervention was delivered. Weekly in-person sessions were held at the clinic, home, community location, or via telemedicine. A weight loss goal of one pound per week was encouraged and incentives were provided based on the attainment of goals. In phase 2, lifestyle maintenance (LM) was delivered during in-person sessions scheduled every two weeks for 6–8 months and phone contact with the participant in the intervening week; the purpose was to continue to reinforce and review the concepts introduced in LC. In the continuing contact (CC) phase 3, the youth selected topics covered during monthly in-person sessions over one year with phone contact between sessions.
The lifestyle program was administered to individual participants by a trained Personal Activity-nutrition Leader (PAL) who was supervised by a doctoral level psychologist. Lifestyle program sessions lasted approximately one hour and included: measuring weight; reviewing participant self-monitoring logs of activity and dietary intake; reviewing the status of participant goals; presenting program materials; and meeting with the youth alone, the adult support person alone, and both together.
The lifestyle program was designed to be administered in two years, the minimum duration of follow-up in TODAY. Participants with follow-up greater than two years were considered to be in ‘extended’ CC and continued to meet quarterly with their PAL until the end of the study. The current paper reports on activities during the first two years only.
Proxies for program adherence
Adherence to the lifestyle program was defined by session attendance and self-monitoring of diet and physical activity (keeping a recorded log) by the youth participant and family member.
Attendance
Per protocol, a session included face-to-face contact at the clinic, a community location, the home, or via telemedicine. The LC phase was designed to be delivered in 24 weekly sessions; participants were given up to eight months to complete the phase to accommodate missed sessions, so actual number of weeks in LC could exceed 24. The LM phase included one session biweekly for six months, for a total of 12 in-person sessions; participants were given up to eight months to complete the phase. The CC phase was one session per month for 12 months, for a total of 12 in-person sessions.
Self-monitoring of diet and physical activity
Participants were instructed to self-monitor by recording targeted diet modification and physical activity behaviors in lifestyle logs between sessions and to bring the logs to each session. Diet modification involved self-monitoring ‘red’ (or ‘stop and think’) foods containing five or more grams of fat, sugary cereals, energy-dense, non-nutritious snacks, and soft drinks. Participants were considered adherent to self-monitoring of diet if they had recorded red foods on at least one day during the period since the last session. Physical activity targets of minutes per week of moderate-to-vigorous intensity activity were set for participants and minutes per day were entered into the log; adherence to self-monitoring was defined as at least one day of activity minutes recorded in the log. Adherence to self-monitoring of red foods and activity were calculated as number of times the participant met adherence criteria across sessions for each program phase.
Family member self-monitoring
The family member supporting the participant was also directed to self-monitor using a lifestyle log; adherence was defined as having made at least one entry for one of the target behaviors in the log between sessions.
Measurement of adherence
Adherence to lifestyle treatment was expressed by phase as the percent of the session target goal accomplished for each of the four measures (participant session attendance, participant self-monitoring logs of red foods and physical activity, and self-monitoring by a family member). Only sessions that occurred before study endpoint (occurrence of treatment failure or end of protocol participation) were included. Session attendance was dichotomized using a cut-off of 75% (<75% versus ≥75%), the same criterion used to monitor adequacy of attendance during the trial. For intent-to-treat purposes, a participant was assigned counts of zero for measures of attendance in a phase if no sessions were documented, but date of study endpoint was after the anticipated time period for the phase; for example, a participant could be a ‘no-show’ for LM but return months later to be placed in CC without experiencing study endpoint, and counts of zero were given for adherence measures during LM.
Baseline characteristics
Race-ethnicity was determined by self-report on two separate items; categories that were too small for separate analysis were combined into ‘other’. Tanner stage was evaluated by physical examination of breasts and pubic hair for girls and genitalia and pubic hair for boys. Household education was the highest education level attained by parent/guardian or current spouse/partner; 15 categories were collapsed into four for purposes of analysis. Presence of depressive symptoms was based on cut-offs applied to scores calculated from the Children’s Depression Inventory (14) for individuals ≤15 years (cut-off ≥13) or the Beck Depression Inventory (15) for individuals ≥16 years (cut-off ≥14). Impaired quality of life was based on the Pediatric Quality of Life Inventory (cut-off ≥71.19) (16). Percent overweight was calculated as BMI minus BMI at 50th percentile for age and sex divided by 50th percentile; percent overweight is the preferred metric for growing children (17,18).
Statistical analysis
Descriptive statistics are presented as means, standard deviations, and distributional percentiles. Comparisons between those with <75% adherence versus ≥75% session attendance were performed using chi-square or t-test. Analysis of session attendance examined whether characteristics differed with consistency of adherence across the three lifestyle phases. The analysis was performed in the 234 who started the LC phase comparing those with ≥75% in LC phase (n=105) versus <75% (n=129), in the 187 who started LM phase comparing those with ≥75% in both LC and LM phase (n=76) versus <75% (n=111), and in the 155 who started CC phase comparing those with ≥75% in all three phases LC, LM, and CC (n=43) versus <75% (n=112). Adequate adherence to study medication (dose taken ≥80% by pill count) was tested as a covariate, but was not significantly related to program adherence (p=0.4374 in LC phase) and was dropped. Survival analysis tested for differences in time to primary outcome in those who started LM phase, comparing those who had <75% LC phase session adherence to those who had ≥75%. Longitudinal analysis used general linear mixed models to account for the repeated measures. All results are considered exploratory and p<0.05 is considered statistically significant without adjustment for multiple comparisons; the study was powered for the primary outcome only.
RESULTS
Of 699 eligible participants in the TODAY cohort, 234 were randomized to M+L. Table 1 gives baseline characteristics. Of the 234 who started LC phase, 47 either reached the primary endpoint (treatment failure) (40) or dropped out (7) while in LC. Of the 187 who started LM phase, 32 either failed (25) or dropped out (7) while in LM, leaving 155 who started CC phase.
Table 1.
Baseline Characteristics in N=234 Randomized to the TODAY Combination Metformin + Lifestyle Program Treatment Group
| Baseline Characteristics and Measurements statistics are mean (SD) or percent | Overall (n=234) | Participant Session Attendance in LC Phase | ||
|---|---|---|---|---|
| < 75% (n=129) | ≥ 75% (n=105) | p-value | ||
| Age (years) | 13.8 (2.0) | 13.9 (1.9) | 13.7 (2.1) | 0.3967 |
|
| ||||
| Sex female | 65.8% | 72.1% | 58.1% | 0.0248 |
|
| ||||
| Race-ethnicity | 0.1826 | |||
| Non-Hispanic Black | 36.8% | 40.3% | 32.4% | |
| Hispanic | 36.8% | 37.2% | 36.2% | |
| Non-Hispanic White | 19.6% | 18.6% | 20.9% | |
| Other | 6.8% | 3.9% | 10.5% | |
|
| ||||
| Tanner stage 4–5 | 87.2% | 89.9% | 83.8% | 0.1642 |
|
| ||||
| Household annual income (self-report) | 0.1721 | |||
| < $25,000 | 43.6% | 44.0% | 43.2% | |
| $25,000–49,999 | 31.3% | 26.7% | 36.8% | |
| ≥ $50,000 | 25.1% | 29.3% | 20.0% | |
|
| ||||
| Household highest level of education | 0.8951 | |||
| 12th grade or less | 27.1% | 26.4% | 27.9% | |
| High school grad, GED, business, technical | 28.8% | 28.0% | 29.8% | |
| Some college or associates degree | 27.5% | 29.6% | 25.0% | |
| Bachelor’s degree or higher | 16.6% | 16.0% | 17.3% | |
|
| ||||
| Lives with | 0.0032 | |||
| Both biological parents | 41.0% | 31.0% | 53.4% | |
| Biological mother only | 44.4% | 51.9% | 35.0% | |
| Biological father only | 4.7% | 7.0% | 1.9% | |
| Neither biological parent | 9.9% | 10.1% | 9.7% | |
|
| ||||
| Depressive symptoms | 17.5% | 21.6% | 12.5% | 0.0709 |
|
| ||||
| Depressive symptoms in adult caregiver | 19.6% | 14.5% | 25.7% | 0.0347 |
|
| ||||
| Impaired quality of life | 23.6% | 21.4% | 26.2% | 0.3961 |
|
| ||||
| Months since diagnosis | 7.7 (5.8) | 8.0 (5.8) | 7.2 (5.8) | 0.2709 |
|
| ||||
| HbA1c (%) | 6.00 (0.81) | 6.09 (0.78) | 5.89 (0.85) | 0.0609 |
|
| ||||
| BMI (kg/m2) | 34.1 (7.1) | 34.2 (6.5) | 33.9 (7.8) | 0.7036 |
|
| ||||
| Percent overweight (%) | 75.6 (35.3) | 75.9 (31.9) | 75.4 (39.2) | 0.9148 |
|
| ||||
| Change in % overweight during run-in | −2.8 (6.8) | −2.7 (7.1) | −3.0 (6.4) | 0.7593 |
|
| ||||
| Length of run-in (days) | 80.8 (31.2) | 87.0 (35.4) | 73.1 (23.0) | 0.0007 |
Measures of adherence proxies by program phase
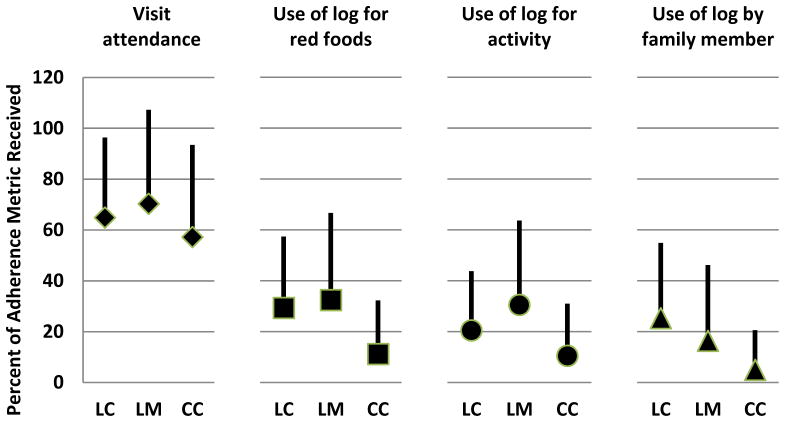
Figure 1 shows mean percent for each of the four adherence metrics. Average session attendance in LC was 65%, LM 70%, and CC 57%. Within each phase, session attendance rates were much higher than the other metrics of adherence; self-monitoring by either the participant or family member occurred in less than half the sessions attended. In LM and CC phases, participant self-monitoring of red foods and physical activity were at similar levels, but the frequency of family member self-monitoring was about half that of youth participants. Comparing rates of adherence across phases, session attendance stayed at approximately 60%, but family member self-monitoring steadily declined from LC to CC, and participant self-monitoring declined from LM to CC. By CC phase, a little over 60% of participants and 86% of family members had no self-monitoring entries between sessions. Session attendance was significantly correlated (p<.0001) with the other three measures (r=0.73 with self-monitoring red food, r=0.69 with self-monitoring physical activity, r=0.56 with self-monitoring by family member). The lack of distribution in the self-monitoring of food intake and physical activity data rendered these measures insufficient for productive analysis. Therefore, session attendance alone was used to analyze program adherence further and the analysis focused on the effects of session attendance in the intensive LC phase when the program was implemented in weekly sessions.
Figure 1.
Percent Adherence for 4 Measures Across Program Phase
Percent adherence (mean and 1 SD) to 4 measures (participant session attendance, participant self-monitoring logs of red foods and physical activity, and self-monitoring by a family member) are shown across the 3 consecutive program phases: Lifestyle Change (LC), Lifestyle Maintenance (LM), and Continuing Contact (CC). All data prior to occurrence of treatment failure (primary outcome) while participants were being administered the randomized study treatment.
Session attendance and treatment failure
To test for an effect of session attendance on subsequent treatment failure (loss of glycemic control), the analysis was performed on the 187 participants who started the LM phase; those who did not start LM either failed or dropped out prior to having the opportunity to achieve ≥75% attendance. Of the 98 with ≥75% session attendance in the LC phase, 39.8% failed to maintain glycemic control versus 33.7% of the 89 with <75% (p=0.3889). Survival analysis showed no difference in time to treatment failure in those who did versus those who did not achieve adequate session adherence in the LC phase (p=0.4263).
The analysis was repeated for the 155 participants who started the CC program phase, i.e., had complete session adherence data for both LC and LM phases. Of the 73 who achieved ≥75% session attendance in both LC and LM phases, 26.0% failed to maintain glycemic control versus 30.5% of the 82 who had <75% in one or both phases (p=0.5387).
Session attendance and weight change
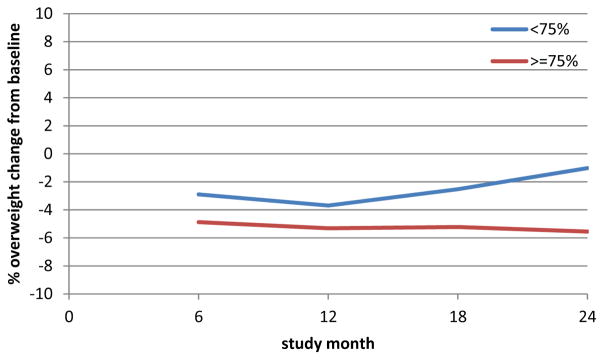
Percent overweight was used to test for an effect of session attendance on change in weight. Figure 2 shows mean change from baseline in percent overweight for participants who had <75% LC phase session attendance versus ≥75%. There was a significant difference between the groups (p=0.0026) that occurred by month six and persisted without a trend over time (i.e., the lines were flat and parallel).
Figure 2.
Change in % Overweight from Baseline to Follow-up Study Month by LC Phase Session Adherence
Trend in change in % overweight from baseline is shown. Change is calculated as the difference between follow-up minus baseline (negative indicates a decrease from baseline). Means are plotted across study month (session) by LC phase session attendance < or ≥ 75%. The two lines are significantly different (p=0.0056) and parallel (interaction with study month not significant), i.e., difference at 6 months was sustained.
Baseline characteristics associated with session attendance
Table 1 compares baseline demographics and characteristics in participants who had <75% LC phase session attendance versus ≥75%. Characteristics that were not significant in LC phase were not significant in either of the other analyses that looked at consistently high adherence in LC+LM and in LC+LM+CC. Of the four characteristics that were significantly different in LC phase – sex (p=0.0248), living with biological parents (0.0032), presence of depressive symptoms in adult caregiver (p=0.0347), and length of pre-randomization run-in period (p=0.0007) – only sex and living with biological parents maintained statistical significance across all three comparisons. Males were more likely to achieve higher levels of session adherence; 37.9% of males consistently attended ≥75% of sessions across LC, LM, and CC phases compared to 21.6% of females (p=0.0285). Participants with ≥75% session attendance were more likely to be living with both biological parents and participants with <75% session attendance were more likely to be living with the biological mother but not father.
CONCLUSIONS
In the TODAY randomized clinical trial, the intensive family-based lifestyle program added to metformin was associated with greater short-term decreases in percent overweight but not the maintenance of glycemic control in youth with T2D compared with youth treated with metformin monotherapy (1). The present analysis examined two proxies of adherence to the lifestyle program - attendance at intervention contacts and self-monitoring - in order to understand more fully the outcomes associated with the lifestyle program. Findings confirmed that session attendance was moderately high as further described below and was related to weight loss, but rates of self-monitoring were poor and insufficient for additional analysis.
Session attendance has been used in several pediatric obesity trials as a proxy for program adherence (3–5). The current analysis indicates that attendance rates in each of the three phases of the TODAY intervention averaged about 60% of the planned sessions; further, more than half of the sample attended 75% or more of the intervention sessions. These rates of attendance are similar to those obtained in other pediatric obesity clinical trials that have focused on youth without diabetes (3–5). For example, Kalarchian et al. (3) observed an attendance rate of 63% at sessions over a six month period, with ≥75% attendance by 50% of the youth, and attendance was significantly associated with weight loss. In another trial, Berkowitz et al. (5) found that the median attendance rate at intervention sessions was 75% and youth with higher attendance had significantly greater reductions in BMI at one year compared with those who attended ≤75% of the meetings.
Also consistent with findings from the pediatric obesity trials noted above, program attendance was associated with successful weight loss in all phases of the TODAY lifestyle intervention. TODAY participants with ≥75% session attendance had significantly greater reductions in percent overweight compared to those with session attendance <75%. Indeed, TODAY youth with adequate session attendance in the intensive LC phase averaged a 5.5% decrease from baseline in percent overweight at the end of two years, a decrease that approaches the pre-planned threshold of 7% for clinically meaningful decreases in percent overweight. In contrast, youth with session attendance of <75% of sessions had only a 1.02% decrease in percent overweight. Session attendance was not associated with maintenance of adequate glycemic control; rates of loss of glycemic control over time did not differ between those with and without adequate attendance. Nevertheless, another TODAY report found that decreases in percent overweight ≥7% in participants across all TODAY intervention arms were associated with small but sustained improvements in HbA1c, HDL, and C-peptide oral disposition index (a measure of β-cell function) over two years of the trial (2). Differences in the relation between weight change and diabetes status in the two reports based on the TODAY study probably reflect differences in the research questions and samples studied. Clarification of the relationship between weight loss and cardiometabolic outcomes in youth with type 2 diabetes requires additional study.
There were few significant baseline predictors of session attendance in the current study. The length of time spent in run-in and self-reported parent symptoms of depression were associated with session attendance during the period of intensive intervention requiring weekly sessions and may reflect barriers to attending the scheduled sessions. The only baseline factors associated with sustained attendance over time were non-modifiable, i.e., male sex and living with biological parent. TODAY did not collect data that would help understand the reasons for lower rates of session attendance by girls and this finding requires replication. Youth living with both biological parents may face fewer logistical barriers to attendance. TODAY incorporated numerous recommended strategies to facilitate participation including home sessions and the use of telemedicine. Nevertheless, future work to reduce barriers is warranted.
The rates of self-monitoring in the TODAY lifestyle intervention were disappointing. Youth participants were considered adherent to monitoring of red foods and physical activity if there was one day or more of recording in the log between sessions. Even given this lenient threshold for adherence, average rates of self-monitoring during the first six months of treatment were approximately 30% for red foods and lower yet for activity. Rates of self-monitoring for family members were only about 20% during the first six months. Moreover, rates of self-monitoring by youth and participating family member decreased across phases of the lifestyle intervention; by the end of the two year intervention rates averaged about 10% for youth and fell nearly to 0% among the adults. In a previous study of child and parent self-monitoring in the management of low-income minority youth with severe obesity (7), 80.3% of the youth participants self-monitored for more than one day per week and 43.9% for at least 3.5 days during the first three months of treatment. Although rates of self-monitoring decreased over time, youth who averaged more than 3.5 days of self-monitoring over six months of treatment (representing approximately 25% of the remaining sample) lost significantly more weight than those who did not. However, similar to the current findings, parent monitoring in the study by Germann et al. (7) were low; only 2% self-monitored for 3.5 days per week and the investigators were not able to analyze the relation between parent self-monitoring and treatment outcome.
Self-monitoring of food and, to a lesser extent physical activity, is a key aspect of behavioral weight management in adults (20), and self-monitoring frequency has been shown to be associated with short- and long-term weight loss in adults (21) and children (6). The dose of self-monitoring required for success, however, is not clear (22). Nevertheless, the dose of self-monitoring by youth in TODAY was lower than that observed in other youth weight management studies and reasons for low rates of self-monitoring adoption are not clear. Low rates might be associated with numerous methodological factors that affect the reliability and validity of behavioral interventions (23). In TODAY, study design, PAL training, lifestyle intervention delivery, and participant characteristics are just a few of the factors that could have affected whether youth understood and were able to perform self-monitoring correctly. We do not have the data to evaluate most aspects of treatment fidelity, but it is possible that despite intensive PAL training and supervision, there was insufficient emphasis on barriers to self-monitoring or the importance of self-monitoring was not stressed sufficiently by the PALs. It also is possible that the family support person did not understand the importance of self-monitoring or have the skills to complete the logs.
Other aspects of TODAY may have affected rates of self-monitoring, and adherence to the lifestyle program in general. In contrast to studies designed to evaluate obesity interventions, TODAY was designed to examine the efficacy of treatment approaches for type 2 diabetes in youth; thus TODAY participants did not volunteer to participate in a weight management intervention. Moreover, TODAY was designed to evaluate the utility of lifestyle intervention as an adjunct to medical care for youth not as an integral part of the medical regiment. Families already were already encumbered by a complex medical regimen for their children, often in the context of challenging life circumstances and, frequently, an inter-generational burden of diabetes. TODAY participants were required to attend clinic sessions, comply with taking medication, self-monitor blood glucose, and complete study assessments. Full participation in the lifestyle intervention, including self-monitoring of food intake and physical activity may have proven too demanding. It also is possible that youth were not experiencing positive results from making the desired behavior changes and rates of self-monitoring suffered as a consequence. Given the positive findings regarding the relation between self-monitoring and weight changes in previous work that focused on youth with severe obesity and demographic characteristics comparable to those of the TODAY cohort (7), future work designed to examine the role self-monitoring of food and physical activity among youth with type 2 diabetes is warranted.
In summary, the present analysis adds to our understanding of the role of behavioral adherence in explaining the performance of the TODAY intensive lifestyle intervention. Findings indicated that the efforts of study personnel to promote lifestyle session attendance were reasonably successful; moreover, adequate attendance was associated with decreases in percent overweight (but not glycemic control) over a period of two years. However, despite the importance of self-monitoring of lifestyle behaviors in behavioral weight management, rates of self-monitoring among TODAY youth were low. Future work is needed to understand how to utilize self-monitoring in the service of long-term weight management in youth with diabetes. Research also is needed to determine whether the integration of lifestyle intervention with overall medical regimen in youth with type 2 diabetes minimizes burden or enhances overall outcome.
Acknowledgments
This work was completed with funding from NIDDK/NIH grant numbers U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254; from the National Center for Research Resources General Clinical Research Centers Program grant numbers M01-RR00036 (Washington University School of Medicine), M01-RR00043-45 (Childrens Hospital Los Angeles), M01-RR00069 (University of Colorado Denver), M01-RR00084 (Children’s Hospital of Pittsburgh), M01-RR01066 (Massachusetts General Hospital), M01-RR00125 (Yale University), and M01-RR14467 (University of Oklahoma Health Sciences Center); and from the NCRR Clinical and Translational Science Awards grant numbers UL1-RR024134 (Children’s Hospital of Philadelphia), UL1-RR024139 (Yale University), UL1-RR024153 (Children’s Hospital of Pittsburgh), UL1-RR024989 (Case Western Reserve University), UL1-RR024992 (Washington University), UL1-RR025758 (Massachusetts General Hospital), and UL1-RR025780 (University of Colorado Denver).
The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; Sanofi-aventis. We also gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service; the opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the respective Tribal and Indian Health Service Institution Review Boards or their members.
Manuscript Appendix
The following individuals and institutions constitute the TODAY Study Group (* indicates principal investigator or director):
CLINICAL CENTERS Baylor College of Medicine: S. McKay*, B. Anderson, C. Bush, S. Gunn, M. Haymond, H. Holden, K. Hwu, S.M. Jones, S. McGirk, B. Schreiner, S. Thamotharan, M. Zarate Case Western Reserve University: L. Cuttler*, E. Abrams, T. Casey, W. Dahms (deceased), A. Davis, A. Haider, S. Huestis, C. Ievers-Landis, B. Kaminski, M. Koontz, S. MacLeish, P. McGuigan, S. Narasimhan, D. Rogers Childrens Hospital Los Angeles: M. Geffner*, V. Barraza, N. Chang, B. Conrad, D. Dreimane, S. Estrada, L. Fisher, E. Fleury-Milfort, S. Hernandez, B. Hollen, F. Kaufman, E. Law, V. Mansilla, D. Miller, C. Muñoz, R. Ortiz, J. Sanchez, A. Ward, K. Wexler, Y.K. Xu, P. Yasuda Children’s Hospital of Philadelphia: L. Levitt Katz*, R. Berkowitz, K. Gralewski, B. Johnson, J. Kaplan, C. Keating, C. Lassiter, T. Lipman, G. McGinley, H. McKnight, B. Schwartzman, S. Willi Children’s Hospital of Pittsburgh: S. Arslanian*, F. Bacha, S. Foster, B. Galvin, T. Hannon, A. Kriska, I. Libman, M. Marcus, K. Porter, T. Songer, E. Venditti Columbia University Medical Center: R. Goland*, R. Cain, I. Fennoy, D. Gallagher, P. Kringas, N. Leibel, R. Motaghedi, D. Ng, M. Ovalles, M. Pellizzari, R. Rapaport, K. Robbins, D. Seidman, L. Siegel-Czarkowski, P. Speiser Joslin Diabetes Center: L. Laffel*, A. Goebel-Fabbri, M. Hall, L. Higgins, M. Malloy, K. Milaszewski, L. Orkin, A. Rodriguez-Ventura Massachusetts General Hospital: D. Nathan*, L. Bissett, K. Blumenthal, L. Delahanty, V. Goldman, A. Goseco, M. Larkin, L. Levitsky, R. McEachern, K. Milaszewski, D. Norman, B. Nwosu, S. Park-Bennett, D. Richards, N. Sherry, B. Steiner Saint Louis University: S. Tollefsen*, S. Carnes, D. Dempsher, D. Flomo, V. Kociela, T. Whelan, B. Wolff State University of New York Upstate Medical University: R. Weinstock*, D. Bowerman, S. Bristol, J. Bulger, J. Hartsig, R. Izquierdo, J. Kearns, R. Saletsky, P. Trief University of Colorado Denver: P. Zeitler* (Steering Committee Chair), N. Abramson, A. Bradhurst, N. Celona-Jacobs, J. Higgins, A. Hull, M. Kelsey, G. Klingensmith, K. Nadeau, T. Witten University of Oklahoma Health Sciences Center: K. Copeland* (Steering Committee Vice-Chair), E. Boss, R. Brown, J. Chadwick, L. Chalmers, S. Chernausek, C. Macha, R. Newgent, A. Nordyke, D. Olson, T. Poulsen, L. Pratt, J. Preske, J. Schanuel, J. Smith, S. Sternlof, R. Swisher University of Texas Health Science Center at San Antonio: J. Lynch*, N. Amodei, R. Barajas, C. Cody, D. Hale, J. Hernandez, C. Ibarra, E. Morales, S. Rivera, G. Rupert, A. Wauters Washington University School of Medicine: N. White*, A. Arbeláez, J. Jones, T. Jones, M. Sadler, M. Tanner, A. Timpson, R. Welch Yale University: S. Caprio*, M. Grey, C. Guandalini, S. Lavietes, M. Mignosa, P. Rose, A. Syme, W. Tamborlane
COORDINATING CENTER George Washington University Biostatistics Center: K. Hirst*, S. Edelstein, P. Feit, N. Grover, C. Long, L. Pyle
PROJECT OFFICE National Institute of Diabetes and Digestive and Kidney Diseases: B. Linder*
CENTRAL UNITS Central Blood Laboratory (Northwest Lipid Research Laboratories, University of Washington): S. Marcovina*, J. Chmielewski, M. Ramirez, G. Strylewicz DEXA Reading Center (University of California at San Francisco): J. Shepherd*, B. Fan, L. Marquez, M. Sherman, J. Wang Diet Assessment Center (University of South Carolina): M. Nichols*, E. Mayer-Davis, Y. Liu Lifestyle Program Core (Washington University): D. Wilfley*, D. Aldrich-Rasche, K. Franklin, D. Laughlin, G. Leibach, C. Massmann, M. Mills, D. O’Brien, J. Patterson, T. Tibbs, D. Van Buren, A. Vannucci
OTHER Centers for Disease Control: P. Zhang State University of New York at Buffalo: L. Epstein University of Florida: J. Silverstein
Footnotes
ClinicalTrials.gov Identifier: NCT00081328
Disclosures
Robert I Berkowitz is scientific consultant to Eisai and to Takeda. Barbara J Anderson is on the Scientific Advisory Board of Sanofi. Marsha D Marcus is on the Scientific Advisory Board of Weight Watchers International, Inc. Patrice Yasuda was once a paid presenter for Novo Nordisk. None of the other authors have anything to disclose.
Kathryn Hirst had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Robert I Berkowitz, Children’s Hospital of Philadelphia and Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA, 19104.
Marsha D Marcus, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA, 15261.
Barbara J Anderson, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA, 77030.
Linda Delahanty, Department of Medicine, Massachusetts General Hospital Diabetes Center and Harvard Medical School, Boston, MA, USA, 02114.
Nisha Grover, George Washington University Biostatistics Center, Rockville, MD, USA, 20852.
Andrea Kriska, Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA, USA, 15260.
Lori Laffel, Joslin Diabetes Center, Harvard Medical School, Boston, MA, USA, 02215.
Amy Syme, Pediatric Endocrinology, Yale University, New Haven, CT, USA, 06520.
Elizabeth Venditti, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA, 15261.
Dorothy J. Van Buren, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA, 63110.
Denise E. Wilfley, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA, 63110.
Patrice Yasuda, Department of Pediatrics, Keck School of Medicine, University of Southern California, Los Angeles, CA & Children’s Hospital Los Angeles, Los Angeles, CA, USA, 90033.
Kathryn Hirst, George Washington University Biostatistics Center, Rockville, MD, USA, 20852.
References
- 1.TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. NEJM. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcus MD, Wilfley DE, Elghormli L, Zeitler P, Linder B, Hirst K, Ievers-Landis C, van Buren DJ, Walders-Abramson N, Elghormli L for the TODAY Study Group. Weight change in the management of youth-onset diabetes: TODAY clinical trial experience. Pediatric Obesity. 2016 May 10; doi: 10.1111/ijpo.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalarchian MA, Levine MD, Arslanian SA, Ewing LJ, Houck PR, Cheng Y, Ringham RM, Sheets CA, Marcus MD. Family-based treatment of severe pediatric obesity: randomized, controlled trial. Pediatrics. 2009;124:1060–1068. doi: 10.1542/peds.2008-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theim KR, Sinton MM, Goldschmidt AB, Van Buren DJ, Doyle AC, Saelens BE, Stein RI, Epstein LH, Wilfley DE. Adherence to behavioral targets and treatment attendance during a pediatric weight control trial. Obesity (Silver Spring) 2013;21(2):394–397. doi: 10.1038/oby.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkowitz RI, Rukstalis MR, Bishop-Gilyard CT, Moore RH, Gehrman CA, Xanthopoulos MS, et al. Treatment of adolescent obesity comparing self-guided and group lifestyle modification programs: a potential model for primary care. Journal of Pediatric Psychology. 2013:1–9. doi: 10.1093/jpepsy/jst035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkowitz RI, Wadden TA, Tershakovec AM, Cronquist JL. Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA. 2003;289:1805–1812. doi: 10.1001/jama.289.14.1805. [DOI] [PubMed] [Google Scholar]
- 7.Germann JN, Kirschenbaum DS, Rich BH. Child and parental self-monitoring as determinants of success in the treatment of morbid obesity in low-income minority children. Journal of Pediatric Psychology. 2007;32:111–121. doi: 10.1093/jpepsy/jsl007. [DOI] [PubMed] [Google Scholar]
- 8.Kirschenbaum DS, Germann JN, Rich BH. Treatment of morbid obesity in low-income adolescents: effects of parental self-monitoring. Obesity Research. 2005;13:1527–1529. doi: 10.1038/oby.2005.187. [DOI] [PubMed] [Google Scholar]
- 9.Cefalu WT. TODAY reflects on the changing faces of type 2 diabetes. Diabetes Care. 2013;36(6):1732–1734. doi: 10.2337/dc13-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copeland KC, Janet Silverstein J, Moore KR, Prazar GE, Raymer T, Shiffman RN, Springer SC, Thaker VV, Anderson M, Spann SJ, Flinn SK. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131:364–382. doi: 10.1542/peds.2012-3494. [DOI] [PubMed] [Google Scholar]
- 11.TODAY Study Group. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatric Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.TODAY Study Group. Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. International Journal of Obesity. 2010;34:217–226. doi: 10.1038/ijo.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein L, Paluch R, Roemmich J, Beecher M. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26:381–391. doi: 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilfley D, Stein R, Saelens B, Mockus D, Matt G, Hayden-Wade H, et al. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA. 2007;298:1661–1673. doi: 10.1001/jama.298.14.1661. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs M. Children’s Depression Inventory. New York: Multi-Health Systems; 1992. [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 17.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL™. Health and Quality of Life Outcomes. 2007;5:43. doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score, or BMI centile? Eur J Clin Nutr. 2005;59(3):419–425. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- 19.Paluch RA, Epstein LH, Roemmich JN. Comparison of methods to evaluate changes in relative body mass index in pediatric weight control. AmJ Hum Biol. 2007;19(4):487–494. doi: 10.1002/ajhb.20608. [DOI] [PubMed] [Google Scholar]
- 20.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:226–238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 21.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, Hesson LA, Osei SY, Kaplan R, Stunkard AJ. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 22.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D Czajkowski Susan Treatment Fidelity Workgroup of the NIH Behavior Change Consortium. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology. 2005;23(5):443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]