Abstract
There is a growing body of literature indicating that lexical retrieval training can result in improved naming ability in individuals with neurodegenerative disease. Traditionally, treatment is administered by a speech-language pathologist, with little involvement of caregivers or carry-over of practice into the home. The current study examined the effects of a lexical retrieval training program that was implemented first by a clinician and, subsequently, by a trained caregiver. Two dyads, each consisting of one individual with anomia caused by neurodegenerative disease (one with mild cognitive impairment and one with logopenic primary progressive aphasia) and their caregiver, participated in the study. Results indicated medium and large effect sizes for both clinician-and caregiver-trained items, with generalization to untrained stimuli. Participants reported improved confidence during communication as well as increased use of trained communication strategies after treatment. This study is the first to document that caregiver-administered speech and language intervention can have positive outcomes when paired with training by a clinician. Caregiver-administered treatment may be a viable means of increasing treatment dosage in the current climate of restricted reimbursement, particularly for patients with progressive conditions.
Keywords: Primary progressive aphasia, Treatment, Lexical retrieval, Anomia, Caregiver
Background
Anomia, or word retrieval difficulty, is the most pervasive feature of stroke-induced aphasia (Goodglass & Wingfield, 1997; Raymer, 2005; Wilshire & Coslett, 2000) and the most prevalent symptom in primary progressive aphasia (PPA; Mesulam, 1982; Westbury & Bub, 1997; Mesulam, 2001). Deficits in retrieving the names of common items and/or proper names result from a breakdown in a complex process that includes the retrieval of a word’s semantic, phonological, and motor components (Maher & Raymer, 2004). Because anomia is a pervasive feature across aphasia subtypes, a great deal of research has addressed the utility of speech-language interventions intended to rehabilitate word retrieval in aphasia caused by stroke (for a review, see Wisenburn & Mahoney, 2009). However, there is far less research examining such treatments for individuals with anomia in the context of PPA (for reviews, see Croot, Nickels, Laurence, & Manning, 2009; Jokel, Graham, Rochon, & Leonard, 2014; Tippett, Hillis, & Tsapkini, 2015) and even fewer studies have addressed naming treatment in other neurodegenerative disorders (for a review, see Creighton, van der Ploeg, & O’Connor, 2013).
In addition to the scant evidence base supporting the efficacy of treatment in individuals with aphasia caused by neurodegenerative disease, there are no guidelines regarding the optimal frequency and duration of treatment. Investigations of treatment dosage in aphasia caused by stroke suggest that increased intensity and frequency of speech and language intervention may lead to enhanced treatment outcomes (Bhogal, Teasell, Foley, & Speechley, 2003; Hinckley & Craig, 1998; Lee, Kaye, & Cherney, 2009; Robey, 1998). However, restrictions on reimbursement for care impose limitations on the intensity and frequency of treatment provided by speech-language pathologists (SLPs), creating a substantial barrier to delivery of the most potent treatment dosage. For this reason, it is incumbent on clinicians to explore methods to extend the training regimen beyond the typical scope of inpatient and outpatient rehabilitation settings. One such avenue may involve training patients’ primary communication partners or caregivers to be partners in treatment administration.
In this study, we investigated the effects of lexical retrieval treatment (Beeson et al., 2011; Henry et al., 2013) administered by both a speech-language pathology graduate student clinician and a caregiver, to two participants with anomia caused by neurodegenerative disease. The goal of the study was to discern whether treatment initiated by a speech-language pathologist could be extended into the home setting by a trained caregiver and whether treatment outcomes from clinician and caregiver-administered training would be comparable. Participant dyads included one individual with mild cognitive impairment (MCI) and his caregiver, as well as one participant with a diagnosis of PPA and her caregiver. In the following sections, we discuss the general behavioural presentation associated with these diagnoses and review interventions for anomia in MCI and PPA.
Mild cognitive impairment
Mild cognitive impairment (MCI), now also referred to as minor neurocognitive disorder, is a condition in which cognitive decline is greater than expected for an individual’s age, but does not interfere significantly with activities of daily living (American Psychiatric Association, 2013; Gauthier et al., 2006; Winblad et al., 2004). Consensus criteria indicate that, for a diagnosis of MCI to be made, an individual must: be neither neurotypical nor demented, show cognitive deterioration (measured objectively or via report), and show intact or minimally altered performance on activities of daily living (Winblad et al., 2004). Cognitive impairment must be evident in one or more domains, including, memory, executive function, attention, visuospatial skills, or language, and MCI is often considered a prodromal stage of frank dementias such as Alzheimer’s dementia (AD; Bäckman, Jones, Berger, Laukka, & Small, 2004).
Studies have documented that individuals with MCI show deficits on word retrieval tasks. For example, individuals with MCI may be distinguished from neurotypical controls on the basis of verbal fluency tasks (Bennett et al., 2002; Bschor, Kuhl, & Reischies, 2001; Dwolatzky et al., 2003; Geslani, Tierney, Herrmann, & Szalai, 2005; Grundman et al., 2004; Petersen et al., 1999; Ribeiro, de Mendonça, & Guerreiro, 2006; Tabert et al., 2006), or on tests of confrontation naming (for a review see Taler & Phillips, 2008). These studies documented impaired naming in individuals with MCI relative to controls (e.g., Dwolatzky et al., 2003; Grundman et al., 2004; Petersen et al., 1999), but less impaired naming relative to individuals with mild AD (e.g., Adlam, Bozeat, Arnold, Watson, & Hodges, 2006; Bschor, Kuhl, & Reischies, 2001). However, this finding is not undisputed, as some studies did not observe differences between the aforementioned groups (i.e., normal elderly versus individuals with MCI; individuals with MCI versus AD; see Taler & Phillips, 2008 for a review).
Given that MCI can present with deficits in one or more cognitive domains (memory, executive function, attention, visuospatial skills, or language), there is heterogeneity in its presentation, which may account for the discrepancy in findings on confrontation naming tests (between individuals with MCI and neurotypical adults or those with mild AD). Moreover, it is likely that only a subset of individuals with MCI will demonstrate deficits in confrontation naming. For example, in a six-year longitudinal study examining cognitive and linguistic skills in 10 MCI patients, Hodges, Erzinçlioglu, and Patterson (2006) found that onset and degree of impairment on confrontation naming measures was more variable than performance on memory or category fluency tasks. Other variables that might contribute to the variability in findings include the use of shortened test versions to assess naming, and the small sample sizes used in these studies.
Whereas studies have yet to examine the effects of lexical retrieval treatment in individuals with MCI, treatment for anomia has proven effective in individuals with AD (Bier et al., 2008; Clare, Wilson, Carter, & Hodges, 2003; Clare, Wilson, Carter, Hodges, & Adams, 2001; Clare, Wilson, Carter, Roth, & Hodges, 2002; Haslam, Moss, & Hodder, 2010; Metzler-Baddeley & Snowden, 2005; Noonan, Pryer, Jones, Burns, & Ralph, 2012; Ousset et al., 2002). Although the majority of naming treatment studies in AD have focused on errorless learning approaches to relearn names (Clare et al., 2001; Clare et al., 2002; Clare et al., 2003), most studies that have compared errorful and errorless approaches for relearning face-name (Bier et al., 2008; Dunn & Clare, 2007) and object-name associations (Noonan et al., 2012) have not found significant differences in the outcomes of these approaches. Although there are no studies to-date which address the utility of lexical retrieval therapy in individuals with MCI specifically, this is a population that may show great benefit from treatment, as cognitive-linguistic and functional abilities are relatively preserved when compared to those with a diagnosis of frank dementia.
Primary progressive aphasia
By definition, individuals with a diagnosis of MCI have relative sparing of activities of daily living and may show impairment of non-linguistic cognitive domains. In contrast, patients with primary progressive aphasia (PPA) suffer a gradual but debilitating deterioration of speech and language abilities with initial preservation of other aspects of cognition (Gorno-Tempini et al., 2011; Mesulam, 1982, 2001). There are three clinical variants of PPA, each of which presents with a unique speech and language profile linked to a specific pattern of neurodegeneration and likely pathological profile. The semantic variant of PPA (svPPA) presents with a loss of conceptual knowledge that manifests as naming and single-word comprehension deficits (Gorno-Tempini et al., 2011; Grossman & Ash, 2004; Hodges & Patterson, 2007; Hodges, Graham, & Patterson, 1995; Kertesz et al., 2010). In this variant, atrophy is prominent in the anterior temporal lobes (typically left greater than right), which are thought to support semantic processing (Hodges & Patterson, 2007; Lambon Ralph, Sage, Jones, & Mayberry, 2010). The logopenic variant of PPA (lvPPA) presents with phonological processing deficits affecting naming and repetition. In lvPPA, atrophy is prominent in the left posterior perisylvian region, which is involved in phonological aspects of language production and comprehension (Gorno-Tempini et al., 2008; Henry & Gorno-Tempini, 2010). Last, the nonfluent/agrammatic variant (nfvPPA) presents with motor speech and/or syntactic impairments (Ash et al., 2010; Gorno-Tempini et al., 2011; Ogar, Dronkers, Brambati, Miller, & Gorno-Tempini, 2007; Rohrer, Rossor, & Warren, 2010; Santos-Santos et al., 2016; Wilson et al., 2010). This variant is associated with atrophy in the left inferior frontal region, which supports grammar and speech production (Wilson et al., 2010).
Established clinical criteria identify anomia as an early and prominent feature in svPPA and lvPPA (Gorno-Tempini et al., 2011). In svPPA, lexical retrieval deficits are thought to result from weakened activation of phonological representations by degraded semantic input (Lambon Ralph, McClelland, Patterson, Galton, & Hodges, 2001). By contrast, in lvPPA, lexical retrieval deficits likely result from phonological storage and assembly deficits (Gorno-Tempini et al., 2008; Wilson et al., 2010).
Unsurprisingly, given the prominence of anomia as a clinical feature in PPA, the bulk of behavioural treatment research in this population has addressed remediation of lexical retrieval deficits. The majority of this work has been conducted in individuals with svPPA (Beales, Cartwright, Whitworth, & Panegyres, 2016; Graham, Patterson, Pratt, & Hodges, 1999; Henry et al., 2013; Heredia et al., 2009; Jokel et al., 2014; Jokel, Rochon, & Anderson, 2010; Jokel, Rochon, & Leonard, 2006; Mayberry, Sage, Ehsan, & Lambon Ralph, 2011; Meyer, Getz, Brennan, Hu, & Friedman, 2015; Savage, Ballard, Piguet, & Hodges, 2013; Savage, Piguet, & Hodges, 2015; Snowden & Neary, 2002). Research examining interventions for naming in svPPA demonstrates the effectiveness of a variety of approaches. Some studies have utilised simple rehearsal of word forms in conjunction with pictured targets, whereas others have used more elaborated training hierarchies that provide semantic, phonological or orthographic cues. Studies that used word-picture rehearsal resulted in improvement for naming of trained items only (Heredia et al., 2009; Jokel et al., 2006; Savage et al., 2015), whereas studies that used cueing hierarchies documented generalisation to items that were not trained in therapy (Henry et al., 2013; Jokel & Anderson, 2012; Jokel et al., 2010). Maintenance of gains was documented with each of these types of approaches, although follow-up schedules varied and none extended beyond six months post-treatment (Dressel et al., 2010; Henry et al., 2013; Jokel & Anderson, 2012; Jokel et al., 2010).
Relatively fewer studies have implemented treatments for naming in individuals with lvPPA, with encouraging findings overall (Beales et al., 2016; Beeson et al., 2011; Croot et al., 2015; Henry et al., 2013; Meyer, Snider, Eckmann, & Friedman, 2015 Meyer, Tippett, & Friedman, 2016; Newhart et al., 2009). Outcomes from these studies indicate improvement on trained items, with one study that used a computer-based treatment (Meyer et al., 2016), and other studies that have utilised a self-cueing approach yielding generalisation to untrained items (Beales et al., 2016; Beeson et al., 2011; Henry et al., 2013; Newhart et al., 2009), with maintenance documented up to six-months post-treatment (Beeson et al., 2011; Henry et al., 2013). Particularly promising outcomes were observed with a treatment hierarchy designed to facilitate self-cueing via strategic retrieval of residual semantic, phonological, and orthographic knowledge (Henry et al., 2013). In fact, this treatment proved beneficial not only for the participant with lvPPA, but also for a participant with svPPA. Both individuals demonstrated improvement on trained and untrained items, with maintenance of gains up to six months post-treatment, and patient-reported improvements in word retrieval and increased confidence during communication attempts.
Taken together, these results indicate that lexical retrieval training in PPA may result in significant and lasting gains. Generalisation to untrained items is observed in some but not all studies (Beeson et al., 2011; Henry et al., 2013; Jokel & Anderson, 2012; Jokel et al., 2010; Newhart et al., 2009), and is likely facilitated by self-cueing strategies (Beeson et al., 2011; Henry et al., 2013; Newhart et al., 2009), where patients are not only trained on a specific set of items but are guided to use strategies for retrieving words (e.g., semantic circumlocution or retrieval of partial orthographic/phonological information) beyond the training set. Because the purpose of the present study was to examine the utility of caregiver-administered treatment for individuals with aphasia caused by neurodegenerative disease, we now turn to the literature addressing communication training for caregivers of individuals with aphasia and dementia.
Communication training for caregivers of individuals with aphasia
To-date, studies involving caregivers of individuals with aphasia or dementia have been restricted to caregiver education and conversation partner training programmes (e.g., Dietz & Fergadiotis, 2013; Purdy & Hindenlang, 2005; Ripich, Ziol, Fritsch, & Durand, 2000; Small, Gutman, Makela, & Hillhouse, 2003; for reviews, see Egan, Bérubé, Racine, Leonard, & Rochon, 2010; Cherney, Simmons-Mackie, Raymer, Armstrong, & Holland, 2013; Simmons-Mackie, Raymer, Armstrong, Holland, & Cherney, 2010; Simmons-Mackie, Raymer, & Cherney, 2016; Zientz et al., 2007).
Studies that have focused on training caregivers of individuals with stroke-induced aphasia typically involve training groups of caregivers, dyads consisting of caregivers and individuals with aphasia, or individual caregivers. Some of these studies report training programmes for caregivers using general strategies for facilitating successful communication with individuals with aphasia, whereas others focus on strategies tailored for a given dyad (for reviews, see Cherney et al., 2013; Simmons-Mackie et al., 2010; Simmons-Mackie et al., 2016). The current evidence base suggests that communication partner training is appropriate for caregivers of individuals with chronic aphasia and often results in an increase in appropriate strategy usage (Simmons-Mackie et al., 2016).
In addition to stroke-induced aphasia, researchers have examined the effectiveness of communication strategy training for people with Alzheimer’s dementia (AD) and their caregivers (Ripich et al., 2000; Small et al., 2003; for reviews, see Egan et al., 2010; Zientz et al., 2007). These studies include descriptive research identifying effective communication strategies for caregivers of individuals with AD (Small et al., 2003), as well as studies examining outcomes following caregiver communication training programmes (see Egan et al., 2010). Results suggest that training reduces the frequency of ineffective communication strategies, while increasing the use of effective strategies learned through training.
Overall, the literature indicates that caregivers can be trained to shape communication exchanges in order to maximise inter-dyad communicative success. Although studies have addressed the communication partner’s role in conversational exchanges, they have yet to investigate direct administration of speech or language interventions by the caregiver. Moreover, the majority of these studies have focused on participants with anomia subsequent to stroke, with fewer studies investigating caregiver-focused interventions in progressive language disorders.
This study
In light of research demonstrating that increased frequency and duration of treatment can lead to greater outcomes (Lee et al., 2009; Robey, 1998), and given the fact that reimbursement for care is often limited, methods to extend treatment beyond the traditional clinical setting warrant exploration. In this study, we aimed to determine whether caregivers of persons with word-finding deficits due to neurological disorders could, with training, carry over the work of the clinician into the home. To do so, we examined the effects of lexical retrieval treatment administered in an initial phase by a clinician relative to a subsequent phase of caregiver-administered treatment using a single-subject multiple baseline crossover design. Unlike previous caregiver training studies in aphasia and dementia, we focused on training caregivers to implement word-retrieval treatment themselves, rather than on training strategies to promote successful communication exchanges. The treatment approach administered in both phases involved a treatment hierarchy that emphasises strategic retrieval of residual phonological, semantic, and orthographic information (based on Henry et al., 2013). The approach trains individuals to use self-cueing strategies (semantic and orthographic/phonemic self-cueing) to improve word-retrieval for a functional set of words, with the intent that these strategies will generalise to untrained targets. In this study, we describe implementation and treatment outcomes for two individuals, one with MCI and the other with the logopenic variant of PPA. We predicted that both participants would benefit from treatment in each phase of training, with comparable benefits subsequent to clinician-administered and caregiver-administered treatment. A finding of comparable outcomes following each phase of treatment would provide evidence supporting caregiver-implemented treatment as a means for augmenting and extending therapy.
Method
Participants
Two monolingual English-speaking patient-caregiver dyads participated in this study. Each dyad comprised one participant with progressive anomia and their caregiver/ primary communication partner. Participants were administered an extensive speech-language and cognitive assessment battery. In addition, each individual’s MRI scan (either obtained for the purposes of this study or a previous clinical scan), was examined to determine patterns of underlying brain atrophy. The Institutional Review Board at the University of Texas at Austin approved all procedures in this study.
Dyad 1
Participant with mild cognitive impairment (CT1)
CT1 was a 79-year-old right-handed man (see Table 1 for patient demographics) who first reported memory and language difficulties seven years prior to this study. CT1’s memory and language difficulties gradually worsened, and a formal diagnosis of mild cognitive impairment (MCI) was made four years prior to his participation in this study. His clinical MRI scan collected at the time of MCI diagnosis revealed bilateral hippocampal and temporal lobe volume loss, dilated temporal horns and enlarged ventricles and subarachnoid space (Figure 1). A research scan was not collected for the purpose of this study due to a contraindication for MRI scanning (i.e., pacemaker).
Table 1.
Dyad Demographics
| Participant | CT1 | CT2 |
|---|---|---|
| Age | 79 | 66 |
| Education (years) | 18 | 14 |
| Gender | Male | Female |
| Caregiver’s age | 72 | 61 |
| Caregiver’s education (years) | 14 | 14 |
| Caregiver’s gender | Female | Male |
Figure 1.
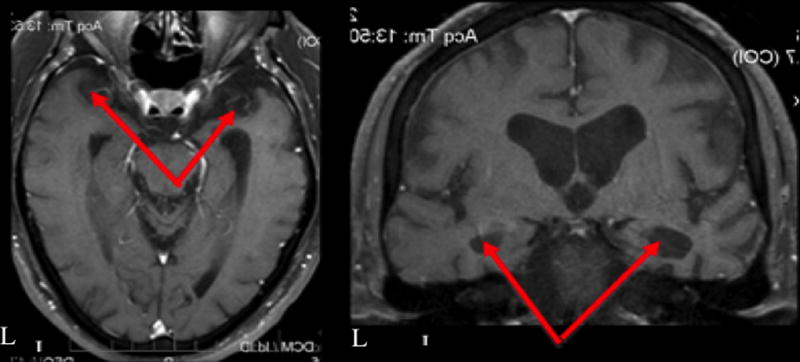
Clinical magnetic resonance images of CT1 acquired three years prior to his participation in this study. Red arrows indicate bilateral hippocampal and temporal lobe atrophy.
At the time of his initial evaluation at the University of Texas at Austin (UT Austin), CT1’s primary complaint was impaired word retrieval. He and his wife reported occasional spontaneous use of self-cueing strategies, including finger spelling, during word retrieval attempts. Despite complaints of word-finding difficulty, his performance on activities of daily living was reported to be minimally altered. CT1 and his caregiver reported that he no longer drove at the time of this study, largely as a precautionary measure at the suggestion of his neurologist.
Notably, CT1 received a diagnosis of bipolar disorder in his forties, for which he took several medications. One medication (Lamotrigine) that he began to take approximately five years prior to this study has a potential side effect of impaired word retrieval. However, lexical retrieval deficits began at least two years prior to the start of this medication and the participant reported no difference in word finding ability with initiation of the medication or with subsequent changes in dosage. During his participation in this study there were no alterations in the dosage of this medication.
CT1 reported that he used his left hand when first learning to write, but was instructed to use his right hand by elementary school teachers. He also reported a history of difficulty with reading and spelling. CT1 received an undergraduate and Master’s degree in Business Administration. Subsequently, he worked as a teacher, a real estate agent, and a pharmaceutical salesman. He had been retired for approximately 13 years at the time of his initial evaluation at UT Austin. CT1 presented with high-frequency hearing loss; thus, a personal sound amplification device was provided throughout assessment and treatment.
CT1’s caregiver
CT1’s caregiver and communication partner was his 72-year-old wife of 27 years. She attended college for approximately two years, where she majored in Elementary Education with a minor in Speech-Language Pathology. Her knowledge of the field of speech-language pathology was limited to a few courses and a few hours of observation. This coursework and observation occurred more than 30 years prior to her participation in this study. After college, she held administrative positions in the fields of library science and education.
Cognitive-linguistic evaluation results for CT1
At the time of his initial evaluation at the University of Texas at Austin, CT1 presented with a mild degree of overall cognitive impairment, as measured by the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975) and Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog; Rosen, Mohs, & Davis, 1984; see Table 2 for results). Impairment was evident on items that tested executive function, memory and language. His language performance on the Western Aphasia Battery-Revised (WAB-R; Kertesz, 2006) was consistent with anomic aphasia (Aphasia Quotient 82.6). Although fluent, there were frequent pauses due to impaired lexical retrieval. Naming impairment was also noted on the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 2001; 29/60). For items that were not named spontaneously, phonemic cues facilitated CT1’s retrieval of nine additional items. Throughout confrontation naming testing, the participant often attempted circumlocution (i.e., “a pin that you throw” for dart) to describe items that he was unable to name. Although most information pertained to the target, CT1 often included extraneous and vague information when describing the object (i.e., “these are the things that make these things tighter or looser” for harp).
Table 2.
Performance on Speech, Language, and Cognitive Measures for Participant CT1
| Assessment | Pre-Tx | Post-Phase 1 Tx |
Post-Tx | 3 Mo. | 6 Mo. | Healthy control mean score (SD) |
|---|---|---|---|---|---|---|
| Cognitive Assessments | ||||||
| MMSE (30) | 25 | 26 | 22 | 23 | 18 | 29 (1.6)c |
| ADAS-Cogj (70)* | 29 | -- | 31 | -- | -- | 6.9 (2.7)f |
| Benson Figure drawingd | ||||||
| Figure Copy (17): | 15 | 16 | 16 | 16 | 13 | 15.6 (1.0)d |
| Figure Recall (17): | 5 | 4 | 2 | 2 | 0 | 10.0 (3.6)d |
| GDSg | 4 | -- | 4 | -- | 5 | |
| Digit Spand Forward | 3 | -- | 3 | -- | 3 | 6.7 (1.0)d |
| Digit Span Backward | 2 | -- | 3 | -- | 3 | 5.4 (1.1)d |
| Speech-Language Assessments | ||||||
| Western Aphasia Battery aphasia quotient (100) | 82.6 | -- | 84.1 | 84.4 | 82.8 | ≥93.8 |
| Information content (10) | 7 | 8 | 8 | 7 | ||
| Fluency (10) | 8 | 8 | 8 | 8 | ||
| Comprehension (10) | 9.6 | 9.95 | 9.3 | 10 | ||
| Repetition (10) | 8.9 | 8.6 | 9.1 | 8.8 | ||
| Naming (10) | 7.8 | 7.5 | 7.8 | 7.6 | ||
| Boston Naming Test | 29 (/60) | 7 (/15) | 29 (/60) | 14 (/30) | 8 (/30) | 54.9 (4.3)a |
| Pyramids and Palm Trees (14) | 14 | -- | 14 | 14 | 14 | 13.9 (.24)b |
| ABRSh reading: words (36) | 34 | -- | 34 | 33 | 33 | 98.8% (3.6)e |
| ABRS reading: nonwords (18) | 8 | -- | 4 | 3 | 4 | 95.6% (7.3)e |
| ABRS spelling: words (20) | 9 | -- | 9 | 8 | 10 | 95.5% (10.4)e |
| ABRS spelling: nonwords (10) | 4 | -- | 4 | 3 | 1 | 91.1% (15.9)e |
| APBi- shortenedi (36) | 8 | -- | 10 | 9 | 8 | |
| MSEk apraxia of speech rating (0–7) | 0 | -- | -- | -- | -- | |
| MSE dysarthria rating (0–7) | 0 | -- | -- | -- | -- |
Note. A larger score represents a greater level of impairment on the ADAS-Cog.
Normative Data for males ages 25–88 years (Tombaugh & Hubley, 1997)
Normative Data for ages 46–80 (Breining et al., 2015)
Normative Data for ages 75–79 (Crum, Anthony, Bassett, & Folstein, 1993)
Normative Data from UCSF Memory and Aging Center Bedside Neuropsychological Screen (Kramer, et al., 2003)
Normative Data from University of Arizona Aphasia Research Project; 34 healthy adults, mean age= 62.9 (11.4), range 34–85; mean education = 15.8 years (2.8), range 12–22 years. Male: Female 13:21
Normative Data for ages 70–79 (Zec et al., 1992)
Global Deterioration Scale (GDS; Reisberg, Ferris, de Leon & Crook, 1983)
Arizona Battery for Reading and Spelling (ABRS; Beeson, Rising, Him & Rapcsak, 2010)
Arizona Phonological Battery (APB; Beeson et al., 2010)
Alzheimer’s Disease Assessment Scale-Cognitive Scale (ADAS-Cog; Rosen et al., 1984)
Motor Speech Evaluation (MSE; Wertz et al., 1984)
There was no evidence of motor speech impairment, as measured by the Motor Speech Evaluation (MSE; Wertz, LaPointe, & Rosenbek, 1984). He also presented with normal semantic processing, as demonstrated by his performance on the Pyramids and Palm Trees Test (PPT; Howard & Patterson, 1992). On the Arizona Battery for Reading and Spelling (ABRS; Beeson, Rising, Kim, & Rapcsak, 2010), CT1 read regular words accurately (100%) but demonstrated some difficulty reading irregular words (88.89% accuracy) and pseudowords (44.44% accuracy). CT1 also displayed prominent spelling difficulty, with greater impairment on irregular words (30% accuracy) and pseudowords (40% accuracy) relative to regular words (60% accuracy). Reading and spelling errors on pseudowords were often lexicalizations; for example, he read “guest” for the target grest, and spelled “neat” for the target nace.
A shortened version of the Arizona Phonological Battery (APB; Beeson, Rising, Kim, & Rapcsak, 2010) confirmed prominent difficulty with phonological processing tasks (phoneme deletion in real words: 5/6 and pseudowords: 3/6; sound blending in real words: 0/6 and pseudowords: 0/6; phoneme replacement in real words: 0/6 and pseudo-words: 0/6).
In summary, CT1 presented with a mild degree of cognitive impairment without frank dementia. Impaired domains included memory, executive functioning, and language. With regard to language, he presented with relatively spared motor speech and semantic processing skills, whereas impairment was observed on confrontation naming and phonological processing measures.
Dyad 2
Participant with logopenic variant primary progressive aphasia (CT2)
CT2 was a 66-year-old right-handed woman who reported a three-year history of memory and expressive language difficulties (see Table 1 for patient demographics). She received a diagnosis of mixed (logopenic and semantic variants) PPA one year prior to her participation in this study subsequent to an evaluation at an academic medical centre. Her initial diagnostic report highlighted deficits in phonology and repetition as well as semantically based deficits such as surface dyslexia and difficulty recognising famous faces. An MRI scan (Figure 2), collected prior to the start of treatment for this study, revealed left greater than right anterior temporal and inferior parietal lobe atrophy.
Figure 2.
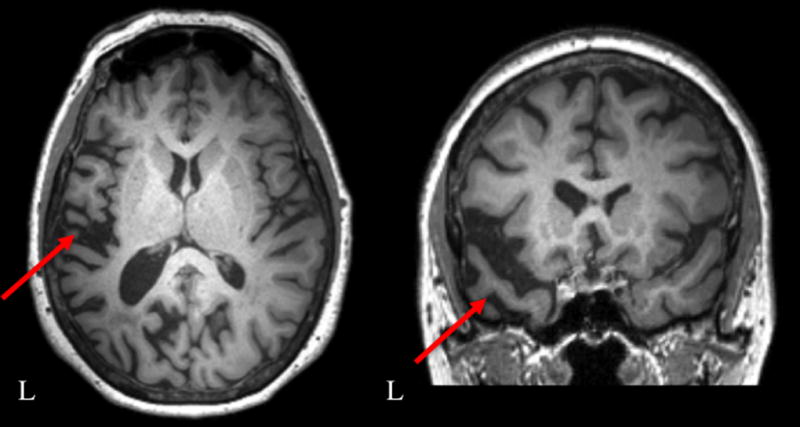
Pre-treatment structural magnetic resonance scan for CT2. Red arrows highlight left greater than right anterior temporal and inferior parietal lobe volume loss.
Impaired word-finding was CT2’s primary complaint at the time of her initial evaluation at the University of Texas at Austin. She also endorsed difficulty with short-term memory, simple calculations, reading, and spelling. She reported feeling socially with-drawn and having decreased interest in hobbies due to communication deficits over the prior two years. She and her caregiver reported that she no longer handled financial matters due to difficulty performing calculations. Further, they had decided to limit her driving as a precautionary measure following a recent relocation to an unfamiliar community. Other activities of daily living such as cooking and self-care were not impacted at the time of this study.
CT2 reported that she did well in school, and that reading and writing were enjoyable prior to the onset of her language difficulties. She graduated from high school and subsequently completed some college. CT2 worked for many years as an office manager for a factory, and retired a few months prior to enrolling in this study. She wore glasses for reading, and presented with high-frequency hearing loss. Therefore, she wore a personal amplification device during all assessment sessions.
CT2’s caregiver
CT2’s primary communication partner and caregiver was her partner of 14 years. He graduated from high school and attended some college. He worked as a police officer for 31 years, and also held jobs as a consultant and paramedic, retiring in 2004.
Cognitive-linguistic evaluation results for CT2
At the time of this study, CT2 presented with minimal overall cognitive impairment, as measured by the ADAS-Cog (see Table 3; note that the initial MMSE score is likely not representative of this participant’s abilities, as she demonstrated a high level of anxiety during this assessment). The ADAS-Cog revealed greatest difficulty on items requiring word retrieval. She received a WAB-R Aphasia Quotient of 80.7, with a fluent but anomic profile. Evidence of word-finding difficulty included frequent pauses in connected speech and impaired performance on the BNT (26/60). The participant correctly named 11 additional items when given a phonemic cue. Semantic naming errors (i.e., “bat” for racket) and phonological paraphasias (i.e., “stefa … stafascope” for stethoscope) were observed on confrontation naming. CT2 spontaneously engaged in semantic circumlocution on the BNT; however, semantic information was often poorly organised or inefficiently conveyed (i.e., “You put it in vegetables” for the target word trellis.)
Table 3.
Performance on Speech, Language, and Cognitive Measures for Participant CT2
| Assessment | Pre-Tx | Post-Phase 1 Tx |
Post-Tx | 3 Mo. | 6 Mo. | 12 Mo. | Healthy control mean score (SD) |
|---|---|---|---|---|---|---|---|
| Cognitive Assessments | |||||||
| MMSE (30) | 18 | 25 | 27 | 22 | 22 | 20 | 29 (1.0)c |
| ADAS-Cogj (70)* | 14 | -- | 15.67 | -- | -- | -- | 4.9 (2.5)f |
| Benson Figure drawingd | |||||||
| Figure Copy (17): | 14 | 16 | 14 | 12 | 13 | 12 | 15.5 (1.1)d |
| Figure Recall (17): | 7 | 7 | 5 | 9 | 9 | 10 | 11.6 (3.0)d |
| GDSg | 4 | -- | 4 | -- | -- | -- | |
| Digit Spand Forward | 4 | -- | 4 | 3 | 3 | 3 | 7.3 (1.1)d |
| Digit Span Backward | 3 | -- | 3 | 2 | 2 | 3 | 5.5 (1.1)d |
| Speech-Language Assessments | |||||||
| Western Aphasia Battery aphasia quotient (100) | 80.7 | -- | 81.4 | 83.3 | 79.4 | 78.3 | ≥93.8 |
| Information content (10) | 9 | 9 | 9 | 9 | 9 | ||
| Fluency (10) | 8 | 8 | 8 | 8 | 8 | ||
| Comprehension (10) | 8.05 | 8.5 | 8.65 | 7.5 | 8.3 | ||
| Repetition (10) | 8.2 | 7.3 | 8.2 | 7.6 | 6.2 | ||
| Naming (10) | 7.1 | 7.9 | 7.8 | 7.6 | 7.7 | ||
| Boston Naming Test | 26 (/60) | 6 (/15) | 23 (/60) | 10 (/30) | 11 (/30) | 16 (/60) | 58.0 (2.0)a |
| Pyramids and Palm Trees (short – 14 pictures; long – 52 pictures) | 49 (52) | -- | 50 (52) | 14 (14) | 14 (14) | 14 (14) | 49.4 (1.46)b |
| ABRSh reading: words (36) | 26 | -- | 28 | 28 | 25 | 25 | 98.8% (3.6)e |
| ABRS reading: nonwords (18) | 11 | -- | 11 | 15 | 11 | 10 | 95.6% (7.3)e |
| ABRS spelling: words (20) | 8 | -- | 9 | 7 | 7 | 6 | 95.5% (10.4)e |
| ABRS spelling: nonwords (10) | 6 | -- | 4 | 5 | 4 | 4 | 91.1% (15.9)e |
| APBi- shortenedi (36) | 20 | -- | 18 | 13 | 14 | 8 | |
| MSEk apraxia of speech rating (0–7) | 0 | -- | -- | -- | -- | -- | |
| MSE dysarthria rating (0–7) | 0 | -- | -- | -- | -- | -- | |
| Famous Faces (20) | 1 | -- | -- | -- | -- | -- |
Note.
Normative Data for males ages 25–88 years (Tombaugh & Hubley, 1997)
Normative Data for ages 46–80 (Breining et al., 2015)
Normative Data for ages 75–79 (Crum et al., 1993)
Normative Data from UCSF Memory and Aging Center Bedside Neuropsychological Screen (Kramer, et al., 2003)
Normative Data from University of Arizona Aphasia Research Project; 34 healthy adults, mean age= 62.9 (11.4), range 34–85; mean education = 15.8 years (2.8), range 12–22 years. Male: Female 13:21
Normative Data for ages 70–79 (Zec et al., 1992)
Global Deterioration Scale (GDS; Reisberg et al., 1983)
Arizona Battery for Reading and Spelling (ABRS; Beeson et al., 2010)
Arizona Phonological Battery (APB; Beeson et al., 2010)
Alzheimer’s Disease Assessment Scale-Cognitive Scale (ADAS-Cog; Rosen et al., 1984)
Motor Speech Evaluation (MSE; Wertz et al., 1984)
There was no evidence of apraxia of speech or dysarthria on the MSE (Wertz et al., 1984), although phonological paraphasias were observed throughout this measure, especially on repetition tasks that were phonologically demanding (e.g., “Arthur was an oozy, oily sneak”). Her performance on the PPT (Howard & Patterson, 1992) (49/52), was slightly below the normal mean score (50.9/52).
On the ABRS, CT2 read regular words with 88.89% accuracy, irregular words with 55.59% accuracy and pseudowords with 61.11% accuracy. On spelling tasks of the ABRS, CT2 spelled regular words with 70% accuracy, irregular words with 10% accuracy and pseudowords with 60% accuracy. Reading and spelling errors on irregular words were often regularisation errors (i.e., she read “borrow” for the target borough, and spelled “quire” for the target choir).
On the APB, CT2 showed greater impairment for tasks involving pseudowords (phoneme deletion in real words: 6/6 and pseudowords: 3/6; sound blending in real words: 4/6 and pseudowords: 2/6; phoneme replacement in real words: 3/6 and pseudo-words: 2/6).
Overall, CT2 presented with relatively spared cognitive function and a fluent aphasia with pronounced anomia and phonological processing deficits. Despite her earlier diagnosis of mixed sv and lvPPA, evaluation results from this study confirmed a profile consistent with lvPPA, with less prominent impairment of semantic knowledge (Gorno-Tempini et al., 2011).
Treatment approach
A Lexical Retrieval Cascade Treatment was implemented with both participants. This treatment, developed at the University of Arizona (Henry et al., 2013), utilises a hierarchy of tasks that are designed to capitalise on strategic recruitment of spared semantic, orthographic, and phonological knowledge in order to facilitate word retrieval via self-cueing. Table 4 summarises the general approach used with each participant.
Table 4.
Lexical Retrieval Hierarchy Used in Both Treatment Conditions (Henry et al., 2013)
| 1. (Picture is presented) Semantic self-cue | Clinician prompts semantic description with, “Tell me about it.” Additional prompting follows as needed, involving “Where would you find this? What is it used for? Do you have any memories about this?” (If the item is named in this step, the clinician proceeds to step 5.) |
| 2. Orthographic self-cue | Clinician requests written form of the word with, “Can you write the word?” If unable to, the participant is encouraged to think of the first letter and/or sound of the word and any other characteristics about the word (i.e., “Is it a long or a short word?”). If the participant cannot come up with the first letter, the clinician writes the first grapheme. |
| 3. Phonemic self-cue | Clinician asks the participant to make the sound that the letter makes, in hopes that this helps the participant name the word. (If the item is named in this step, the clinician proceeds to step 5.) |
| 4. Oral reading | If the item is not yet named, the clinician writes out the remainder of the word and the participant reads it out loud. |
| 5. Written and Spoken Repetition | The participant writes and says the word three times. |
| 6. Semantic Plausibility Judgments | Clinician asks three yes/no questions regarding semantic features of the item (e.g., “would you find this in a toolbox?”) |
| 7. Recall | Clinician asks the participant to provide the most important semantic features and write and say the word one time. |
Modified Copy and Recall Treatment (CART; Beeson & Egnor, 2006) was assigned as daily homework. CART requires participants to view a picture of the target, copy its orthographic form and verbally produce the word 10 times. Subsequently, participants recall the spoken and written word from memory. Each participant was provided with a homework log to record completion of CART homework. The graduate clinician reviewed completed homework with the participant at the start of each treatment session. Participants were also provided flashcards, which contained an image of the object on one side, and the orthographic form of the target on the opposite side. Flashcards were intended to provide additional practice, and their use during homework was encouraged but optional.
Stimulus selection
Thirty-six imageable nouns were targeted for training and twelve items were selected to remain untrained. Items were eligible for inclusion in the treatment or control sets if the participant was unable to name the item correctly on at least two of three pre-treatment probing opportunities. Items were divided into six trained and two untrained sets, which were matched for relevant linguistic characteristics, including familiarity, imageability, frequency, number of letters, and number of syllables. Stimulus characteristics were obtained from the Corpus of Contemporary American English (Davies, 2009) and the MRC Psycholinguistic Database (Coltheart, 1981). Sets were compared using two-tailed t-tests, which revealed no significant differences between sets (all p-values > 0.05).
Training criteria and data collection
A multiple baseline crossover design was used for each participant. The thirty-six items that were selected for treatment were trained in six sets of six items; the clinician trained four sets during phase 1 of treatment, and the caregiver trained the remaining two sets during phase 2. Performance on the items in the set currently receiving training was probed at the beginning of each treatment session. Criterion for mastery of each set was 83.33% (5/6 items) on probes collected across two consecutive sessions, with a maximum of three sessions on a given set. All remaining items were probed once per week: half of the items were probed at the start of the first session for a given week, and the remaining half at the start of the second session. Items that the participant named either spontaneously or with participant-initiated self-cues were scored as correct. The clinician did not provide cues or feedback to the participant during probing. The short form of the BNT was administered at the end of phase 1 (clinician-administered treatment) and the full BNT was administered at the end of phase 2 (caregiver-administered treatment) to assess stability in general naming ability across phases, as well as potential generalisation of strategy use. Alternate 30-item versions of the BNT were used at follow-up visits. A common set of items from across BNT administrations was used for all reported statistical analyses. All probes and assessments were administered by the graduate student clinician, regardless of the treatment phase. Maintenance of gains and generalisation were assessed at three and six months post-treatment for CT1 (CT1 passed away after his 6-month follow up), whereas follow-up data were collected at three, six and 12 months post-treatment for CT2. It is important to note that, following treatment, participants were allowed to keep their homework materials and could therefore practice between post-treatment and follow-up visits. By allowing for practice to take place after treatment, we hoped to approximate the maintenance to be expected following standard clinical care with a speech-language pathologist (where patients are allowed to keep their training materials and are typically encouraged to continue with practice).
Caregiver training and administration of treatment
Each caregiver observed the last two clinician-administered treatment sets (sets 3–4). During these observations, the caregiver read along with a printed copy of the steps of the cueing hierarchy. At the end of these sessions, the clinician answered any questions posed by the caregiver regarding treatment administration. Upon completion of set four, the caregiver-administered phase of therapy (phase 2), began. The clinician attended the first caregiver-administered session with each participant. This allowed the caregiver to ask questions, as needed, and to receive online feedback. In subsequent sessions, the clinician observed via a video system or in an observation corridor, which allowed for feedback to be provided as needed or at the end of the session. Throughout the remainder of phase 2, clinician support was gradually decreased and therapy moved toward a naturalistic setting. At the end of phase 2, treatment was administered at home with no clinician present. Equivalent therapy materials (only modified for targets in a given set) were used in both phases of treatment.
Treatment fidelity
Two trained undergraduate raters independently reviewed videos of 25% of clinician-and caregiver-administered sessions to assess treatment fidelity. The raters were instructed to determine whether the clinician administered each step in the treatment hierarchy correctly. If the total fidelity rating for a treatment session was not identical across raters, the two raters watched the session together to resolve any discrepancies. Fidelity scores revealed that during clinician-administered sessions, therapy steps were correctly administered 100% of the time for CT1, and 99.54% of the time for CT2. For caregiver-administered sessions that took place with the clinician observing remotely, treatment steps were correctly administered 81.54% of the time for CT1 and 84.62% of the time for CT2.
Calculation of effect sizes
Effect sizes (d-statistics) were calculated for clinician-trained sets (sets one through four), caregiver-trained sets (five through six), all trained sets (one through six), and untrained sets (seven through eight) for each participant (Beeson & Robey, 2006). To estimate the treatment effect size, the first three probes were compared to the maintenance phase for each set. Only the first three probes were calculated in this measure of effect size to minimise any effect of baseline drift caused by generalisation of treatment strategies. Because caregiver-administered treatment occurred after clinician-administered treatment, we collected two and four week post-treatment probes following caregiver-administered treatment. These data points were included in the calculation of the d-statistic for caregiver-trained sets. This allowed for the inclusion of equivalent post-treatment data points from the end of each treatment phase. Z-tests were conducted to compare the effects of clinician versus caregiver trained sets and trained versus untrained sets. The z-test was calculated by taking the difference between weighted effect sizes from each condition (d-statistics) and dividing this difference by the square root of their combined variance. We hypothesised that, following treatment, participants’ performance on trained sets would be significantly better than their performance on untrained sets. Therefore, we evaluated results from these z-tests using one-tailed tests. On the other hand, we did not have a priori hypotheses regarding performance on clinician versus caregiver-administered sets; therefore, we evaluated our results for this comparison using a two-tailed test. Changes in participant scores on the BNT were analyzed using McNemar tests.
Self-and caregiver-assessment of change
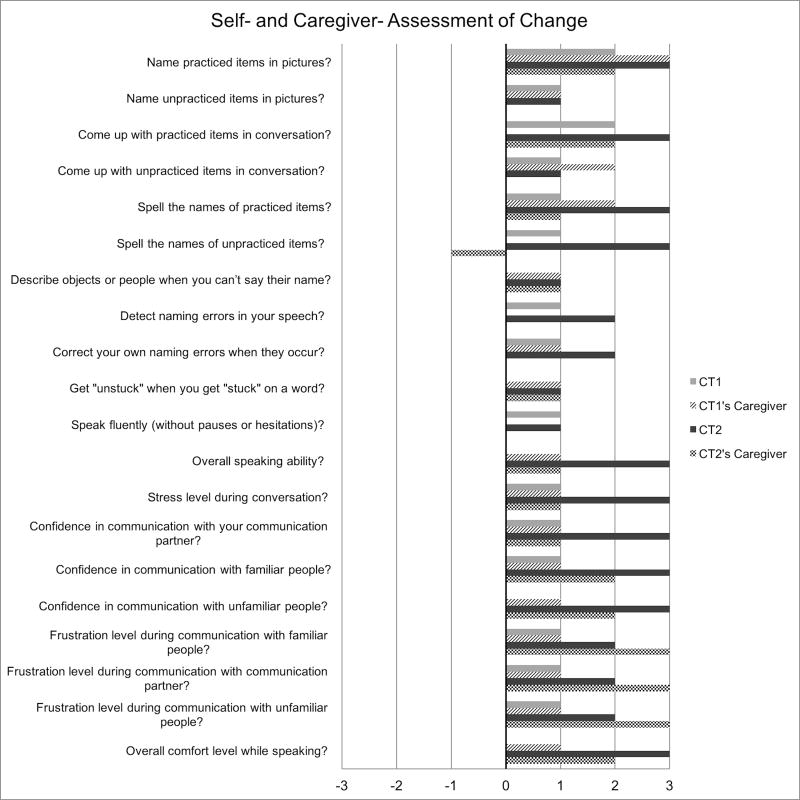
A post-treatment assessment survey (modified from Henry et al., 2013) was administered to both dyads to assess participant and caregiver perceptions regarding the effectiveness of treatment. This survey was administered at the start of the initial post-treatment session, and involved a qualitative rating scale. Each participant and caregiver was asked to rate items concerning the participant’s communication post-treatment relative to pre-treatment. These surveys were completed independently, unless the caregiver or participant required clarification. The 7-point qualitative rating scale was as follows: 3 = “A lot better,” 2 = “Better,” 1 = “Somewhat better,” 0 = “Unchanged,” −1 = “Somewhat worse,” −2 = “Worse,” and −3 = “A lot worse.” Twenty questions were answered using this scale. The questions, along with each dyad’s responses, are provided in Figure 3.
Figure 3.
Post-treatment survey data for CT1, CT2 and their caregivers. The x-axis displays ratings for each item on the survey, on the following scale: 3 = “A lot better,” 2 = “Better,” 1 = “Somewhat better,” 0 = “Unchanged,” −1 = “Somewhat worse,” −2 = “Worse,” and −3 = “A lot worse.”
Results
Participant 1: CT1
CT1 participated in nine weeks of treatment, with sessions occurring twice weekly for approximately one hour each. There was a total of 12 hours of direct contact with the clinician in phase 1 (a total of four sets trained, and 11 treatment sessions), and a total of approximately six hours of training with the caregiver in phase 2 (a total of two sets trained, and a total of 5 treatment sessions). Homework was completed on all days that therapy did not occur, for a total of five days per week. Homework took approximately 30 minutes per day; totalling approximately 15 hours in phase 1 and 7.5 hours in phase 2. Two optional homework packets were given to the participant each week for the days on which therapy took place. However, optional homework packets were rarely completed, turned in to the clinician, or recorded on the homework log.
Treatment effects in CT1
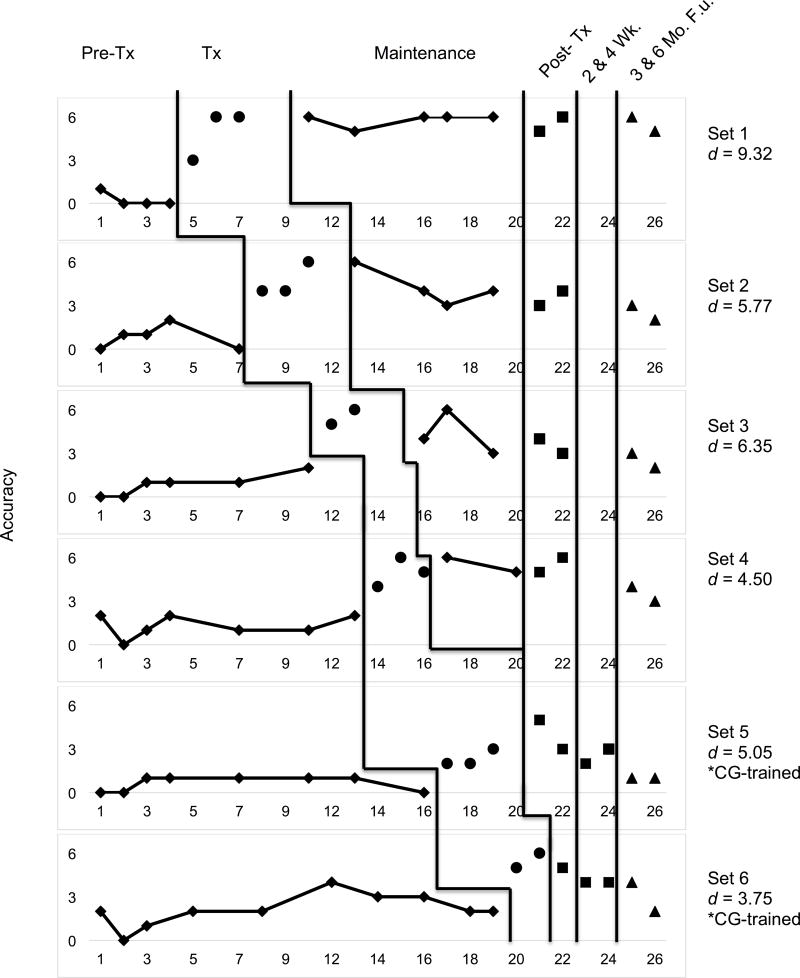
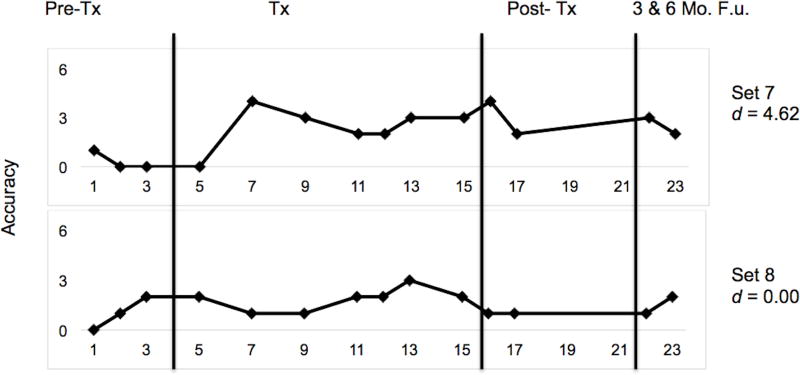
Multiple baseline data for CT1 are presented in Figure 4. CT1 met criterion (83.33%; 5/6 items) in three of four clinician-trained sets, and in one out of two caregiver-trained sets. Criterion was met after two treatment sessions for one clinician and one caregiver-trained set. For the remaining two clinician-administered sets, criterion was met after three treatment sessions. His performance was relatively stable across the three pre-treatment observations. As treatment began, however, minimal upward drift was observed for untrained, and to-be-trained items, suggesting possible generalisation of lexical retrieval strategies. Performance on maintenance, post-treatment, and follow-up probes was consistently higher than baseline performance.
Figure 4.
Multiple-baseline data for CT1’s performance during and after lexical retrieval training for clinician and caregiver-trained sets. Additional post-treatment probes were collected at 2 and 4 weeks posttreatment for caregiver-trained sets. Phases of treatment are indicated by vertical lines, including baseline, treatment, maintenance, post-treatment, and follow-up visits. Tx = Treatment, Wk = Week, F.u. = Follow-up, and CG = Caregiver.
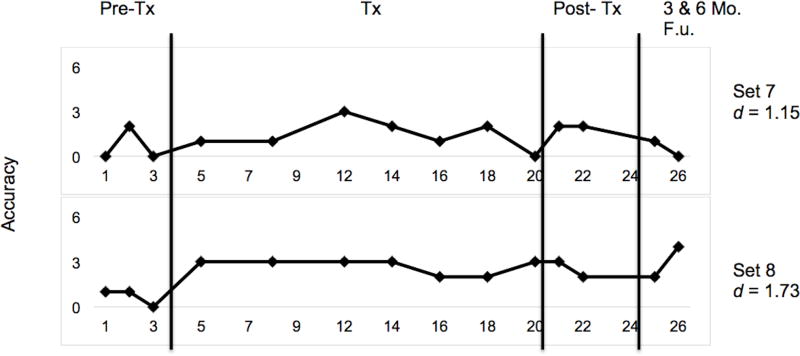
Effect sizes in the form of d-statistics were calculated for each set and are displayed in Figure 4. The weighted effect size for all trained sets was 6.02 (Beeson & Robey, 2006). The effect size for clinician-trained sets was 6.69, and was 4.40 for caregiver-trained sets. Benchmarks for evaluating the magnitude of effect sizes put forth by Robey, Schultz, Crawford, and Sinner (1999) were used to interpret our d-statistics. The overall effect size (d = 6.02) and the effect size for clinician-trained sets (d = 6.69) were large, and in caregiver-trained sets (d = 4.40), a medium effect size was observed. An effect size of 1.44 was observed for the two untrained sets, indicating that generalisation to untrained items was minimal.
A z-test was conducted to compare the effect sizes from each treatment condition from pre-to post-treatment. This test revealed no significant difference between clinician-trained and caregiver-trained sets (z = 1.49, p = 0.12, two-tailed). However, a significant difference was observed for performance on all trained relative to untrained sets (z = 4.17, p < 0.0001, one-tailed).
Performance at three and six months post-treatment indicated a gradual decline in accuracy for trained and untrained sets (Figures 4 and 5). For CT1, caregiver-administered sets showed a slightly greater decline relative to clinician-administered sets. However, performance for clinician and caregiver-trained sets at both follow-up visits was above baseline performance.
Figure 5.
Multiple-baseline data for CT1’s performance during and after lexical retrieval training for untrained sets. Phases of treatment are indicated by vertical lines, including baseline, treatment, post-treatment, and follow-up time points. Tx = Treatment and F.u. = Follow-up.
Post-treatment and follow-up assessments for CT1
Results of CT1’s performance on post-treatment and follow-up assessment measures are shown in Table 2. His performance on cognitive measures remained stable through-out the course of treatment and at three months post-treatment. However, at six months post-treatment CT1 demonstrated a decline in overall cognitive function (as evidenced by a lower MMSE score and higher rating on the GDS), consistent with his diagnosis of MCI.
CT1’s performance on the WAB remained stable throughout the course of treatment and at follow-up visits. His performance on the BNT also remained stable throughout treatment (score of 29/60 at pre-and post-treatment). The short version of the BNT was administered at the end of phase 1, prior to the start of the caregiver-trained phase. CT1 received a score of 7/15 between phases, indicating stability of performance relative to his pre-treatment performance on this subset of items (7/15).
Despite CT1’s identical score on the BNT at pre-and post-phase 2, functional changes were observed. For unnamed items, CT1’s semantic descriptions focused on more relevant information at the post-treatment administration. For example, his description of the item “scroll” changed from “two objects that are wrapped up that will allow a person to increase the size” at pre-treatment, to “two pieces that hold a bunch of paper together and you can pull one end down and write a note.”
For follow-up visits at three and six months, we alternated administration of equivalent 30-item short forms of the BNT. Thus, separate statistical analyses were conducted to account for the different item sets that were administered. Changes on the BNT between post-, and three months post-treatment were not significant as measured by a McNemar test (p = .100). However, decline in performance between post-treatment and six months post-treatment was significant (p = 0.008). Nonverbal semantic knowledge, as measured by PPT score, did not change across time points (14/14 at all observations).
The reading and spelling of both words and pseudowords remained a challenge for CT1 throughout the course of treatment. Although he showed a slight decrease in each of these categories at his three-month follow-up, his scores did not decrease between three and six months post-treatment. Performance on the phonological battery (APB) also remained relatively stable over time.
Self- and caregiver-assessment of change in CT1
The post-treatment qualitative rating scale, along with CT1 and his caregiver’s responses, are depicted in Figure 3. CT1 rated two items as “better” (e.g., ability to name practiced items, ability to come up with the names of practiced items in conversation), thirteen items as “somewhat better” (e.g., ability to name unpracticed items in pictures, ability to spell the name of unpracticed items, stress level during conversation), and six items as “unchanged” (e.g., overall speaking ability, confidence in communication with unfamiliar people, overall number of hesitations or pauses while speaking). Additionally, he stated that his approach to communication was different post-treatment in that he was more likely to describe an object or to use the first sound or letter of a word during instances of word retrieval difficulty. He also reported that he was less likely to give up if he couldn’t think of a word.
A survey was also administered to CT1’s caregiver to explore any perceived changes in the participant’s communication. She rated one item as “a lot better” (ability to name practiced items in pictures), two items as “better” (e.g., ability to name unpracticed items in conversation, ability to spell the names of practiced items), fourteen as “somewhat better” (e.g., ability to describe objects or people when they can’t say their name, confidence when communicating with their primary communication partner, and four items as “unchanged” (e.g., ability to detect naming errors in their speech, ability to speak in complete sentences). Additionally, she stated that CT1 was more likely to talk around words that he couldn’t retrieve in conversation as a result of treatment, and that his descriptions were more precise. Her report also indicated that he was less likely to give up if he couldn’t think of a word. Importantly, after playing the role of clinician, the caregiver reported that she was better able to guide CT1 in his descriptions of objects during naming difficulty.
Participant 2: CT2
CT2 participated in six weeks of treatment, with sessions occurring twice weekly for an average of one hour. There was a total of approximately eight hours of direct contact with the clinician throughout phase 1 (for a total of four sets), and about four hours of treatment with the caregiver in phase 2 (for a total of two sets). Homework was completed on all days that therapy did not occur, for a total of five days each week. Optional homework packets were given for the two days a week on which therapy did occur, and CT2 reported completing these optional packets. Homework took approximately 30 minutes each day, totalling approximately 14 hours throughout phase 1 and seven hours throughout phase 2.
Treatment effects in CT2
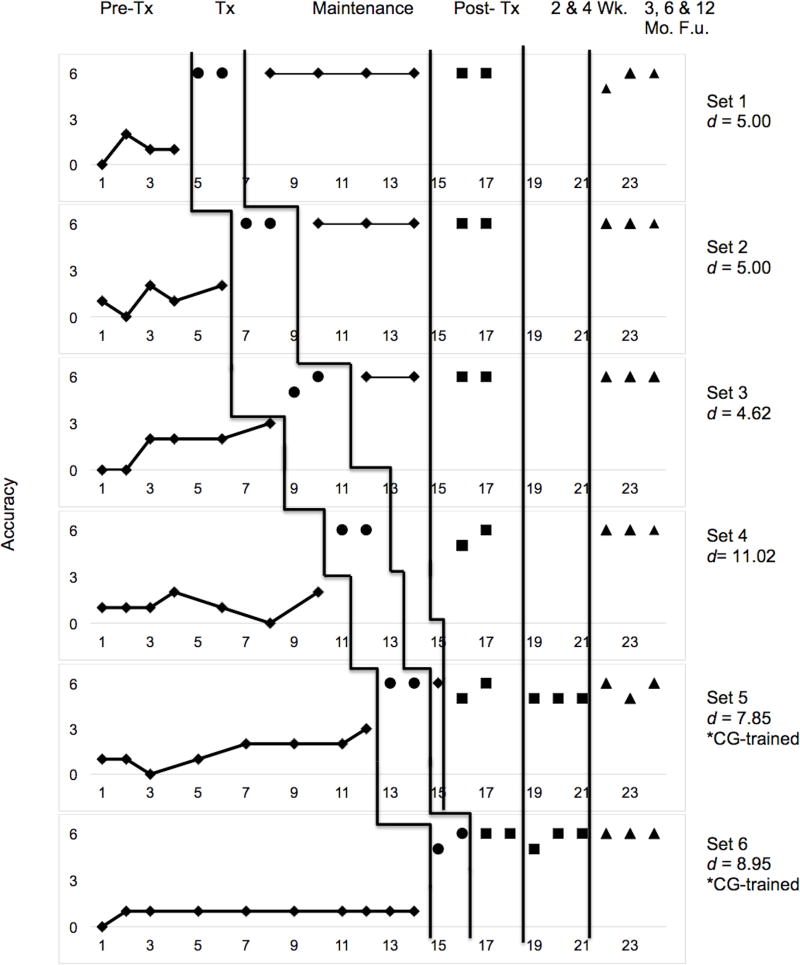
Multiple baseline data for CT2 are presented in Figure 6. Her naming performance was relatively stable across the first three pre-treatment probes, with a slight upward drift observed in several sets after treatment began, suggesting generalisation of lexical retrieval strategies. The participant consistently met criterion in the minimum number of two sessions. This occurred in both the clinician-and caregiver-trained sets. CT2 demonstrated maintenance of gains for the duration of treatment, post-treatment, and follow-up phases.
Figure 6.
Multiple-baseline data for CT2’s performance during and after lexical retrieval training for clinician and caregiver-trained sets. Additional post-treatment probes were collected at 2 and 4 weeks posttreatment for caregiver-trained sets. Phases of treatment are indicated by vertical lines, including baseline, treatment, maintenance, post-treatment, and follow-up visits. Tx = Treatment, Wk = Week, F.u. = Follow-up and CG = Caregiver.
Effect sizes (d-statistics) were calculated for each set and are displayed in Figures 6 and 7 (Beeson & Robey, 2006). The weighted effect size for CT2 across all trained sets was 6.87 (a large treatment effect; Robey et al., 1999). Clinician-trained sets had an effect size of 5.95 and caregiver-trained sets had an effect size of 8.43, each corresponding to large effect sizes. Untrained sets yielded an effect size of 2.89, which is considered to be a small effect. A z-test was conducted to compare the effect sizes from each treatment condition. This test revealed no significant difference between clinician-trained and caregiver-trained sets (z = −1.19, p = 0.233, two-tailed). Conversely, a significant difference was observed for performance on trained versus untrained sets (z = 2.75, p = 0.002, one-tailed).
Figure 7.
Multiple-baseline data for CT2’s performance during and after lexical retrieval training for untrained sets. Phases of treatment are indicated by vertical lines, including baseline, treatment, post-treatment, and follow-up time points. Tx = Treatment and F.u. = Follow-up.
Performance at three, six, and twelve months post-treatment indicated maintenance of treatment gains for trained sets as well as stability of performance for untrained sets (Figures 6 and 7). Caregiver-and clinician-treated sets showed equivalent degrees of maintenance, and performance at all follow-up visits remained above baseline.
Post- treatment and follow-up assessments for CT2
CT2’s post-treatment and follow-up assessment scores are outlined in Table 3. Her performance on the MMSE increased substantially from pre-treatment to post-treatment (from 18 to 27); this change was likely due to the participant’s reported and observed test anxiety throughout the first testing session (she would not answer a question if she was not confident in her response). However, by the post-treatment testing sessions, she was more willing to attempt challenging items and to guess when she was not completely confident in her answers. At follow-up time points CT2 exhibited stability in performance on cognitive assessments.
CT2’s performance on the WAB remained relatively stable throughout treatment and at follow-up visits. BNT score was stable from pre-to post phase 2 treatment (26/60 to 23/60; p = .549), as indicated by a McNemar’s test. Performance on the short version of the BNT at the end of phase 1 (7/15 pre-treatment, 6/15 post phase 1) documented stability in performance. Following our protocol with CT1, we also alternated administration of equivalent 30-item short forms of the BNT at follow-up visits with CT2. Pairwise McNemar tests revealed no significant difference between post-treatment and any follow-up time point (three-months, six months, and one-year; all p-values > .710). Nevertheless, moderate changes in functional performance were observed. For example, CT2 was more likely to engage in repeated phonemic approximations post-treatment, and to provide more efficient and informative semantic information during circumlocutions (i.e., pre-treatment she responded, “I have one” for the target wreath; post-treatment she responded “I always have them at Christmas.”) Performance on the PPT was stable across all time points, indicating minimal change in nonverbal semantic processing.
At post relative to pre-treatment, the reading and spelling of both words and pseudowords remained difficult for CT2. In terms of follow-up visits, her ability to read and spell both real words and pseudowords showed a small decline at one-year post-treatment. A gradual decline was also noted in her ability to manipulate phonological information, as measured by performance on the APB at post-treatment and subsequent follow-ups.
Self- and caregiver-assessment of change in CT2
CT2 completed a post-treatment self-assessment questionnaire to evaluate her perceptions of change in her lexical retrieval ability (Figure 3). CT2 rated five items as “somewhat better” (e.g., ability to name unpracticed items in pictures, ability to come up with the name of unpracticed items in conversation, ability to describe objects or people when you can’t say their name), five items as “better” (e.g., ability to detect naming errors, ability to correct naming errors when they occur, frustration level during communication exchanges), and eleven items as “a lot better” (e.g., ability to come up with the names of practiced items in conversation, ability to spell the names of practiced items, overall comfort level when speaking). She stated that, after the completion of treatment, she was more likely to talk around words that she couldn’t retrieve in conversation, and that she used the first sound or letter of words to help her think of words in conversation. She also stated that she communicated more with her friends and family than she did prior to the onset of treatment.
CT2’s caregiver also completed a survey at the completion of treatment to evaluate how treatment impacted her communicative abilities. He rated six items as “unchanged” (e.g., ability to name unpracticed items, ability to detect naming errors, ability to speak in complete sentences), nine items as “somewhat better” (e.g., ability to spell the names of practiced items, ability to describe objects when they can’t be named, frustration level when communicating), and five items as “better” (e.g., ability to come with names of practiced items in conversation, confidence in communication with familiar and unfamiliar people, overall comfort level while speaking). CT2’s caregiver also reported that she was more likely to speak to others after participating in treatment. Additionally, he stated that, after playing the role of the clinician, he had insight regarding how to better help CT2 during instances of word-finding difficulty. Lastly, he responded that CT2 was less frustrated when communicating with him when he employed strategies learned in their treatment sessions.
Discussion
In this study, we sought to build upon the literature base documenting positive treatment outcomes for individuals with language impairment in the context of neurodegenerative disease. Secondly, we aimed to discern whether caregiver-administered treatment for anomia is both feasible and effective. To our knowledge, this is the first study to train caregivers of individuals with aphasia to directly administer behavioural speech and language treatment. We have presented outcomes for lexical retrieval training for two individuals: one with a diagnosis of MCI and the other with logopenic variant PPA. With each participant, we observed non-significant differences in the magnitude of treatment effects for clinician-and caregiver-administered treatment phases. There was, however, a significant difference between trained and untrained sets for both participants, indicating that treatment gains were greatest for directly trained items.
The treatment approach included a hierarchy of tasks that involved guided retrieval and rehearsal of residual semantic, orthographic, and phonological information (Henry et al., 2013). The treatment is intended to be strategic in nature, with the goal of training functional approaches to word-retrieval such as semantic and orthographic/phonemic self-cueing, which may promote generalised improvement in naming. Importantly, this treatment hierarchy allows for on-line modification to fit the needs of individual participants. For example, with CT1, the semantic self-cueing step focused on describing relevant features of each item, as his semantic descriptions tended to include largely irrelevant information. With CT2, this step focused on organisation and prioritisation of semantic information for each item, as the information was often relevant but disorganised, limiting its utility to aid in listener comprehension.
Subsequent to the initial phase of clinician-administered treatment, each participant’s caregiver was trained to administer the treatment hierarchy. With this second phase of treatment, our goal was to explore the possibility of increasing the dosage of traditional language therapy through caregiver involvement. Treatment outcomes revealed that both participants improved their ability to consistently name targeted nouns as a result of training with the clinician, and additional treatment targets were mastered in the subsequent phase of caregiver-administered treatment. Following the intervention, each participant reported functional benefit from treatment, including increased frequency of self-cueing via circumlocution and retrieval of the first letter or sound of words during naming difficulty. Additionally, both participants reported feeling more confident when communicating with others. Each caregiver noted functional changes and qualitative differences in their own approach to communication with their partner. Both caregivers also reported an improved ability to guide their communication partners during instances of anomia.
Despite the progressive nature of their language difficulties, both participants demonstrated maintenance following completion of structured intervention (documented at CT1’s final follow-up (six months post) and up to twelve months post-treatment in CT2) and generalisation of gains, although to differing degrees. In order to approximate current clinical practice, participants were allowed to keep their homework materials and reported their weekly practice at each follow up visit. CT1 did not practice at all between his post-treatment and three-month follow-up; however, he practiced a handful of times (5) between his three-month and six-month follow-up. CT2, on the other hand, consistently practiced three times per week between her post-treatment and follow-up visits. In the following sections, we will discuss differential patterns of treatment response and maintenance in the context of each individual’s cognitive-linguistic profile.
The participant diagnosed with MCI, CT1, was generally able to relearn the names of targeted items and to recall item names in subsequent probes. However, this was not always the case, as there were two sets of words in which he did not meet criterion. The semantic and phonologic steps in the treatment hierarchy appeared to be most helpful to CT1, as these steps often led to correct naming of the lexical item. However, he struggled to recall the lexical retrieval strategies and exhibited difficulty in comprehending that a goal of treatment was to apply word-retrieval strategies beyond the training set in a generalised manner. Consistent with his diagnosis of MCI, CT1 demonstrated a greater degree of non-linguistic cognitive impairment (as evidenced by a higher ADAS score), which likely resulted in difficulty with retaining and generalising strategic aspects of the training. In addition, this participant presented with a lack of motivation to complete tasks outside of treatment sessions. He was pleasant and compliant in therapy, but was reported to need convincing to complete homework activities.
Additional caregiver and patient factors should be considered in the interpretation of our results. With regard to caregiver-administered sets, CT1’s caregiver quickly mastered the treatment hierarchy and required relatively little constructive feedback as she began leading treatment sessions. The relative ease with which she undertook this role may relate to her brief background in elementary education and prior observation of speech therapy sessions as a student. It is also important to note that CT1 displayed a less robust response to the first caregiver-administered set (set 5). This attenuation in performance between treatment phases may have been due to a fall that occurred at the start of the phase 2 (caregiver-administered treatment). The fall resulted in a knee injury; however, there was no loss of consciousness or injury to the head. Nonetheless, he reported that he felt fatigued after his fall and needed time to rest before returning to his daily activities.
CT2, the participant with logopenic variant PPA, was highly motivated throughout the course of treatment. She reported rehearsing names of pictured items on her homework flashcards for each set more than was stipulated, and completed the optional homework packets on a weekly basis. CT2 generally provided relevant semantic information about each item during training. In this step of the hierarchy, emphasis was placed on presenting semantic information in an organised and efficient manner in order to best help a listener to determine which item was being described (i.e., saying “it’s a reptile” first for the word lizard, rather than beginning with non-specific descriptive features). CT2 often reported finding the phonemic and orthographic cues most helpful. Her caregiver, who did not have a background that involved education or speech-language pathology, required additional direct feedback compared to CT1’s caregiver. After feedback during initial sessions, he demonstrated mastery of the hierarchy steps and provided positive reinforcement, as appropriate. He reported improved insight regarding strategies to help CT2 in moments of word-finding difficulty as a direct benefit from playing the part of the clinician and from receiving feedback during the caregiver-administered treatment phase.
A strength of this study was its inclusion of participants with clinical diagnoses that are not typically addressed in the aphasia treatment literature. This work highlights the variety of diagnoses that may benefit from this type of treatment, inclusive of diverse participant and caregiver ages, genders, and educational backgrounds. Our observation of comparable outcomes from the clinician and caregiver-administered phases of treatment provides preliminary and encouraging evidence suggesting that caregiver-administered therapy is a viable method for extending the length of treatment in a medical system that often places limits on the duration of restitutive services.
This study did, however, have some limitations. The caregiver-trained portion of the study could have been extended with a greater number of caregiver-trained sets to This study was designed to approximate the implementation of clinician and caregiver-trained phases of treatment as they would likely occur in actual clinical practice. A caregiver-administered phase followed by a clinician-administered phase is simply not practical or feasible, as a key component of training the caregivers in this study was caregiver observation of clinician-administered treatment sessions. A treatment design that reversed the phases of treatment would not be clinically relevant nor would it allow for observation of clinician-administered treatment, a core component of caregiver training. It is also important to note that this approach may not be appropriate for all dyads. To implement this approach, both parties must be comfortable with their respective roles in treatment. If either individual is uncomfortable with the idea of caregiver-administered treatment, this is neither a feasible nor appropriate approach. In addition, the dynamics of each dyad should be evaluated on an individual basis, and should serve as the foundation for tailoring this approach to the specific interaction style of a given dyad.
Directions for future research based on this study include the addition of an educational programme for caregivers regarding the nature of each participant’s cognitive-linguistic impairments. This could potentially maximise treatment gains as a result of a better understanding of the goals and objectives for treatment in participants with aphasia with or without concomitant cognitive deficits. There could also be an added focus on carry-over of strategies outside of the treatment setting. Additionally, a caregiver training component could be extended to other populations and treatment approaches. In doing so, more populations could be reached that typically receive limited speech and language therapy, including those with other types of neurodegenerative disease.
In sum, results from this study add to the growing literature base that documents the positive benefits of language intervention in aphasia caused by neurodegenerative disease (Creighton et al., 2013; Jokel et al., 2014). Additionally, our findings indicate that traditional, clinician-administered treatment for language impairment may be augmented by carefully structured caregiver-administered intervention. Caregiver-administered treatment may help to mitigate the limitations on treatment dosage that often result from a finite reimbursement schedule, with the potential to expand therapy options for individuals with diagnoses that are not traditional treatment candidates.
Acknowledgments
Funding
This work was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award number R03DC01340 and by funding provided to the University of Texas at Austin by the Darrell K Royal Research Fund for Alzheimer’s Disease.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
Contributor Information
Stephanie M. Grasso, Communication Sciences and Disorders, University of Texas at Austin, Austin, United States of America, 2504-A Whitis Ave., A1100, Austin, TX 78741, (512) 471-3420, smgrasso@utexas.edu
Kaleigh M. Shuster, Communication Sciences and Disorders, University of Texas at Austin, Austin, United States of America, kshuster@utexas.edu
Maya L. Henry, Communication Sciences and Disorders, University of Texas at Austin, Austin, United States of America, 2504-A Whitis Ave., A1100, Austin, TX 78741, (512) 471-3420, maya.henry@austin.utexas.edu
References
- Adlam ALR, Bozeat S, Arnold R, Watson P, Hodges JR. Semantic knowledge in mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2006;42:675–684. doi: 10.1016/s0010-9452(08)70404-0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Ash S, McMillan C, Gunawardena D, Avants B, Morgan B, Khan A, Grossman M. Speech errors in progressive non-fluent aphasia. Brain and Language. 2010;113(1):13–20. doi: 10.1016/j.bandl.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer’s disease. Journal of Internal Medicine. 2004;256(3):195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- Beales A, Cartwright J, Whitworth A, Panegyres PK. Exploring generalisation processes following lexical retrieval intervention in primary progressive aphasia. International Journal of Speech-Language Pathology. 2016;18(3):299–314. doi: 10.3109/17549507.2016.1151936. [DOI] [PubMed] [Google Scholar]
- Beeson PM, Egnor H. Combining treatment for written and spoken naming. Journal of the International Neuropsychological Society. 2006;12(06):816–827. doi: 10.1017/S1355617706061005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson PM, King RM, Bonakdarpour B, Henry ML, Cho H, Rapcsak SZ. Positive effects of language treatment for the logopenic variant of primary progressive aphasia. Journal of Molecular Neuroscience. 2011;45:724–736. doi: 10.1007/s12031-011-9579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson PM, Rising K, Kim ES, Rapcsak SZ. A treatment sequence for phonological alexia/agraphia. Journal of Speech Language and Hearing Research. 2010;53(2):450–468. doi: 10.1044/1092-4388(2009/08-0229). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson PM, Robey RR. Evaluating single-subject treatment research: Lessons learned from the aspasia literature. Neuropsychology Review. 2006;16(4):161–169. doi: 10.1007/s11065-006-9013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Bhogal SK, Teasell RW, Foley NC, Speechley MR. Rehabilitation of aphasia: More is better. Topics in Stroke Rehabilitation. 2003;10:66–76. doi: 10.1310/RCM8-5TUL-NC5D-BX58. [DOI] [PubMed] [Google Scholar]
- Bier N, Van der Linden M, Gagnon L, Desrosiers SA, Adam S, Louveaux S, Saint-Mleux J. Face-name association learning in early Alzheimer’s disease: A comparison of learning methods and their underlying mechanisms. Neuropsychological Rehabilitation. 2008;18:343–371. doi: 10.1080/09602010701694723. [DOI] [PubMed] [Google Scholar]
- Breining BL, Lala T, Martínez Cuitiño M, Manes F, Peristeri E, Tsapkini K, Hillis AE. A brief assessment of object antics in primary progressive aphasia. Aphasiology. 2015;29(4):488–505. [Google Scholar]
- Bschor T, Kuhl KP, Reischies FM. Spontaneous speech of patients with dementia of the Alzheimer type and mild cognitive impairment. International Psychogeriatrics. 2001;13:289–298. doi: 10.1017/s1041610201007682. [DOI] [PubMed] [Google Scholar]
- Cherney LR, Simmons-Mackie N, Raymer A, Armstrong E, Holland A. Systematic review of communication partner training in aphasia: Methodological quality. International Journal of Speech-Language Pathology. 2013;15(5):535–545. doi: 10.3109/17549507.2013.763289. [DOI] [PubMed] [Google Scholar]
- Clare L, Wilson BA, Carter G, Hodges JR. Cognitive rehabilitation as a component of early intervention in Alzheimer’s disease: A single case study. Aging & Mental Health. 2003;7:15–21. doi: 10.1080/1360786021000045854. [DOI] [PubMed] [Google Scholar]
- Clare L, Wilson BA, Carter G, Hodges JR, Adams M. Long-term maintenance of treatment gains following a cognitive rehabilitation intervention in early dementia of Alzheimer type: A single case study. Neuropsychological Rehabilitation. 2001;11:477–494. [Google Scholar]
- Clare L, Wilson BA, Carter G, Roth I, Hodges JR. Relearning face-name associations in early Alzheimer’s disease. Neuropsychology. 2002;16:538–547. doi: 10.1037//0894-4105.16.4.538. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. The Quarterly Journal of Experimental Psychology Section A. 1981;33(4):497–505. [Google Scholar]
- Creighton AS, van der Ploeg ES, O’Connor DW. A literature review of spaced-retrieval interventions: A direct memory intervention for people with dementia. International Psychogeriatrics. 2013;25(11):1743–1763. doi: 10.1017/S1041610213001233. [DOI] [PubMed] [Google Scholar]
- Croot K, Nickels L, Laurence F, Manning M. Impairment-and activity/participation-directed interventions in progressive language impairment: Clinical and theoretical issues. Aphasiology. 2009;23(2):125–160. [Google Scholar]
- Croot K, Taylor C, Abel S, Jones K, Krein L, Hameister I, Nickels L. Measuring gains in connected speech following treatment for word retrieval: A study with two participants with primary progressive aphasia. Aphasiology. 2015;29(11):1265–1288. [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level. JAMA: The Journal of the American Medical Association. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- Davies M. The 385+ million word corpus of contemporary American English (1990–2008+): design, architecture, and linguistic insights. International Journal of Corpus Linguistics. 2009;14(2):159–190. [Google Scholar]
- Dietz A, Fergadiotis G. This study into the perceptions of caregivers and people with aphasia following a course on total communication yielded promising results, but there are methodological concerns. Evidence-Based Communication Assessment and Intervention. 2013;7(1):46–51. [Google Scholar]
- Dressel K, Huber W, Frings L, Kummerer D, Saur D, Mader I, Abel S. Model-oriented naming therapy in semantic dementia: A single-case fMRI study. Aphasiology. 2010;24(12):1537–1558. [Google Scholar]
- Dunn J, Clare L. Learning face-name associations in early-stage dementia: Comparing the effects of errorless learning and effortful processing. Neuropsychological Rehabilitation. 2007;17:735–754. doi: 10.1080/09602010701218317. [DOI] [PubMed] [Google Scholar]
- Dwolatzky T, Whitehead V, Doniger GM, Simon ES, Schweiger A, Jaffe D, Chertkow H. Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatrics. 2003;3:1–12. doi: 10.1186/1471-2318-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M, Bérubé D, Racine G, Leonard C, Rochon E. Methods to enhance verbal communication between individuals with Alzheimer’s disease and their formal and informal caregivers: A systematic review. International Journal of Alzheimer’s Disease. 2010;2010:1–12. doi: 10.4061/2010/906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive status of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, Peterson RC, Ritchie K, Broich K, Winblad B. Mild cognitive impairment. The Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Geslani D, Tierney M, Herrmann N, Szalai J. Mild cognitive impairment: An operational definition and its conversion rate to Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2005;19:383–389. doi: 10.1159/000084709. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Wingfield A. Word-finding deficits in aphasia: Brain-behavior relations and clinical symptomology. In: Goodglass H, Wingfield A, editors. Anomia: Neuroanatomical and cognitive correlates. San Diego: Academic Press; 1997. pp. 3–30. [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Miller BL. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71(16):1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz M, Mendez M, Cappa MD, Grossman MD. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Patterson K, Pratt KH, Hodges JR. Relearning and subsequent forgetting of semantic category exemplars in a case of semantic dementia. Neuropsychology. 1999;13(3):359–380. doi: 10.1037//0894-4105.13.3.359. [DOI] [PubMed] [Google Scholar]
- Grossman M, Ash S. Primary progressive aphasia: A review. Neurocase. 2004;10:3–18. doi: 10.1080/13554790490960440. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Archives of Neurology. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Haslam C, Moss Z, Hodder K. Are two methods better than one? Evaluating the effectiveness of combining errorless learning with vanishing cues. Journal of Clinical and Experimental Neuropsychology. 2010;32:973–985. doi: 10.1080/13803391003662686. [DOI] [PubMed] [Google Scholar]
- Henry ML, Gorno-Tempini ML. The logopenic variant of primary progressive aphasia. Current Opinion in Neurology. 2010;23(6):633–637. doi: 10.1097/WCO.0b013e32833fb93e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Rising K, DeMarco AT, Miller BL, Gorno-Tempini ML, Beeson PM. Examining the value of lexical retrieval treatment in primary progressive aphasia: Two positive cases. Brain and Language. 2013;127:145–156. doi: 10.1016/j.bandl.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia CG, Sage K, Ralph MAL, Green C, Sage K, Ralph MAL, Berthier ML. Relearning and retention of verbal labels in a case of semantic dementia. Aphasiology. 2009;23:192–209. [Google Scholar]
- Hinckley JJ, Craig HK. Influence of rate of treatment on the naming abilities of adults with chronic aphasia. Aphasiology. 1998;12(11):989–1006. [Google Scholar]
- Hodges JR, Erzinçlioglu S, Patterson K. Evolution of cognitive deficits and conversion to dementia in patients with mild cognitive impairment: A very long-term follow-up study. Dementia and Geriatric Cognitive Disorders. 2006;21:380–391. doi: 10.1159/000092534. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Semantic dementia: A unique clinicopathological syndrome. The Lancet Neurology. 2007;6(11):1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham N, Patterson K. Charting the progression in semantic dementia: Implications for the organisation of semantic memory. Memory. 1995;3:463–495. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and palm trees: A test of semantic access from pictures and words. Bury St. Edmunds: Thames Valley Test Company; 1992. [Google Scholar]
- Jokel R, Anderson ND. Quest for the best: Effects of errorless and active encoding on word re-learning in semantic dementia. Neuropsychological Rehabilitation. 2012;22(2):187–214. doi: 10.1080/09602011.2011.639626. [DOI] [PubMed] [Google Scholar]
- Jokel R, Graham NL, Rochon E, Leonard C. Word retrieval therapies in primary progressive aphasia. Aphasiology. 2014;28:1038–1068. [Google Scholar]
- Jokel R, Rochon E, Anderson ND. Errorless learning of computer-generated words in a patient with semantic dementia. Neuropsychological Rehabilitation. 2010;20(1):16–41. doi: 10.1080/09602010902879859. [DOI] [PubMed] [Google Scholar]
- Jokel R, Rochon E, Leonard C. Treating anomia in semantic dementia: Improvement, maintenance, or both? Neuropsychological Rehabilitation. 2006;16(3):241–256. doi: 10.1080/09602010500176757. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston naming test. Philadelphia: Lippincott, Williams and Wilkins; 2001. [Google Scholar]
- Kertesz A. Western aphasia battery-revised. Austin, TX: Pro-Ed; 2006. [Google Scholar]
- Kertesz A, Jesso S, Harciarek M, et al. What is semantic dementia? A cohort study of diagnostic features and clinical boundaries. Neurology. 2010;67(4):483–489. doi: 10.1001/archneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland J, Patterson K, Galton C, Hodges J. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13(3):341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. Coherent concepts are computer in the anterior temporal lobes. Proceedings of the National Academy of Sciences. 2010;107(6):2717–2722. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JB, Kaye RC, Cherney LR. Conversational script performance in adults with non-fluent aphasia: Treatment intensity and aphasia severity. Aphasiology. 2009;23(7):885–897. [Google Scholar]
- Maher LM, Raymer AM. Management of anomia. Topics in Stroke Rehabilitation. 2004;11:10–21. doi: 10.1310/318R-RMD5-055J-PQ40. [DOI] [PubMed] [Google Scholar]
- Mayberry EJ, Sage K, Ehsan S, Lambon Ralph MA. Relearning in semantic dementia reflects contributions from both medial temporal lobe episodic and degraded neocortical semantic systems: Evidence in support of the complementary learning systems theory. Neuropsychologia. 2011;49(13):3591–3598. doi: 10.1016/j.neuropsychologia.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Annals of Neurology. 1982;11(6):592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Annals of Neurology. 2001;49:425–432. [PubMed] [Google Scholar]
- Metzler-Baddeley C, Snowden J. Brief report: Errorless versus errorful learning as a memory rehabilitation approach in Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology. 2005;27:1070–1079. doi: 10.1080/13803390490919164. [DOI] [PubMed] [Google Scholar]
- Meyer AM, Getz HR, Brennan DM, Hu TM, Friedman RB. Telerehabilitation of anomia in primary progressive aphasia. Aphasiology. 2015;30(4):1–25. doi: 10.1080/02687038.2015.1081142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Snider SF, Eckmann CB, Friedman RB. Prophylactic treatments for anomia in the logopenic variant of primary progressive aphasia: Cross-language transfer. Aphasiology. 2015;29(9):1062–1081. doi: 10.1080/02687038.2015.1028327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Tippett DC, Friedman RB. Prophylaxis and remediation of anomia in the semantic and logopenic variants of primary progressive aphasia. Neuropsychological Rehabilitation. 2016;7:1–17. doi: 10.1080/09602011.2016.1148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhart M, Davis C, Kannan V, Heidler-Gary J, Cloutman L, Hillis A. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7–8):823–834. [Google Scholar]
- Noonan KA, Pryer LR, Jones RW, Burns AS, Ralph ML. A direct comparison of errorless and errorful therapy for object name relearning in Alzheimer’s disease. Neuropsychological Rehabilitation. 2012;22(2):215–234. doi: 10.1080/09602011.2012.655002. [DOI] [PubMed] [Google Scholar]
- Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive non-fluent aphasia and its characteristic motor speech deficits. Alzheimer Disease and Associated Disorders. 2007;21(4):S23–S30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- Ousset PJ, Viallard G, Puel M, Celsis P, Demonet JF, Cardebat D. Lexical therapy and episodic word learning in dementia of the Alzheimer type. Brain and Language. 2002;80(1):14–20. doi: 10.1006/brln.2001.2496. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Purdy M, Hindenlang J. Educating and training caregivers of persons with aphasia. Aphasiology. 2005;19(3/4/5):377–388. [Google Scholar]
- Raymer AM. Naming and word retrieval problems. In: LaPointe LL, editor. Aphasia and related neurogenic language disorders. 3. New York: Thieme; 2005. pp. 72–86. [Google Scholar]
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. American Journal of Psychiatry. 1982;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- Ribeiro F, de Mendonça A, Guerreiro M. Mild cognitive impairment: Deficits in cognitive domains other than memory. Dementia and Geriatric Cognitive Disorders. 2006;21:284–290. doi: 10.1159/000091435. [DOI] [PubMed] [Google Scholar]
- Ripich DN, Ziol E, Fritsch T, Durand EJ. Training Alzheimer’s disease caregivers for successful communication. Clinical Gerontologist. 2000;21(1):37–56. [Google Scholar]
- Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. Journal of Speech Language and Hearing Research. 1998;41:172–187. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- Robey RR, Schultz MC, Crawford AB, Sinner CA. Single-subject clinical-outcome research: Designs, data, effect sizes, and analyses. Aphasiology. 1999;13:445–473. [Google Scholar]
- Rohrer JD, Rossor MN, Warren JD. Syndromes of nonfluent primary progressive aphasia: A clinical and neurolinguistic analysis. Neurology. 2010;75:603–610. doi: 10.1212/WNL.0b013e3181ed9c6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. American Journal of Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Santos-Santos MA, Mandelli ML, Binney RJ, Ogar J, Wilson SM, Henry ML, Gorno-Tempini ML. Features of patients with nonfluent/agrammatic primary progressive aphasia With underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurology. 2016;73(6):733–742. doi: 10.1001/jamaneurol.2016.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Ballard KJ, Piguet O, Hodges JR. Bringing words back to mind-improving word production in semantic dementia. Cortex. 2013;49(7):1823–1832. doi: 10.1016/j.cortex.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Savage SA, Piguet O, Hodges JR. Cognitive intervention in semantic dementia: Maintaining words over time. Alzheimer Disease and Associated Disorders. 2015;29(1):55–62. doi: 10.1097/WAD.0000000000000053. [DOI] [PubMed] [Google Scholar]
- Simmons-Mackie N, Raymer A, Armstrong E, Holland A, Cherney LR. Communication partner training in aphasia: A systematic review. Archives of Physical Medicine and Rehabilitation. 2010;91(12):1814–1837. doi: 10.1016/j.apmr.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Simmons-Mackie N, Raymer A, Cherney LR. Communication partner training in aphasia: An updated systematic review. Archives of Physical Medicine and Rehabilitation. 2016;97(12):2202–2221.e8. doi: 10.1016/j.apmr.2016.03.023. [DOI] [PubMed] [Google Scholar]
- Small J, Gutman G, Makela S, Hillhouse B. Effectiveness of communication strategies used by caregivers of persons with Alzheimer’s disease during activities of daily living. Journal of Speech Language and Hearing Research. 2003;46:353–367. doi: 10.1044/1092-4388(2003/028). [DOI] [PubMed] [Google Scholar]
- Snowden JS, Neary D. Relearning of verbal labels in semantic dementia. Neuropsychologia. 2002;40(10):1715–1728. doi: 10.1016/s0028-3932(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, Devanand DP. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Archives of General Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- Taler V, Phillips NA. Language performance in Alzheimer’s disease and mild cognitive impairment: A comparative review. Journal of Clinical and Experimental Neuropsychology. 2008;30(5):501–556. doi: 10.1080/13803390701550128. [DOI] [PubMed] [Google Scholar]
- Tippett D, Hillis AE, Tsapkini K. Treatment of primary progressive aphasia. Current Treatment Options in Neurology. 2015;17:1–11. doi: 10.1007/s11940-015-0362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Hubley aM. The 60-item Boston naming test: Norms for cognitively intact adults aged 25 to 88 years. Journal of Clinical and Experimental Neuropsychology. 1997;19(6):922–932. doi: 10.1080/01688639708403773. [DOI] [PubMed] [Google Scholar]
- Wertz L, LaPointe L, Rosenbek JC. Apraxia of speech in adults: The disorder and its management. New York: Grune and Stratton; 1984. [Google Scholar]
- Westbury C, Bub D. Primary progressive aphasia: A review of 112 cases. Brain and Language. 1997;60:381–406. doi: 10.1006/brln.1997.1840. [DOI] [PubMed] [Google Scholar]
- Wilshire CE, Coslett HB. Disorders of word retrieval in aphasia: Theories and potential applications. In: Gonzalez-Rothi L, Crosson B, Nadeau S, editors. Aphasia and language: Theory to practice. New York: Guildford Press; 2000. pp. 82–107. [Google Scholar]
- Wilson SM, Henry ML, Bebris M, Ogar JM, Dronkers NF, Jarrold W, Gorno-Tempini ML. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133(7):2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Petersen RC. Mild cognitive impairment—beyond controversies, towards a consensus: Report of the international working group on mild cognitive impairment. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wisenburn B, Mahoney K. A meta-analysis of word-finding treatments for aphasia. Aphasiology. 2009;23:1338–1352. [Google Scholar]
- Zec RF, Landreth ES, Vicari SK, Feldman E, Belman J, Andrise A, Becker R. Alzheimer disease assessment scale: Useful for both early detection and staging of dementia of the Alzheimer type. Alzheimer’s Disease and Associated Disorders. 1992;6:89–102. [PubMed] [Google Scholar]
- Zientz J, Rackley A, Chapman SB, Hopper T, Mahendra N, Kim ES, Cleary S. Evidence-based practice recommendations for dementia: Educating caregivers on Alzheimer’s disease and training communication strategies. Journal of Medical Speech-Language Pathology. 2007;15(1):iii–lxiv. [Google Scholar]