Abstract
N-cadherin (Ncad) mediates cell-cell interactions, regulates β-catenin (βcat) signaling, and promotes the chondrogenic differentiation of mesenchymal lineage cells. Here, we utilized confocal imaging to investigate the influence of Ncad interactions on single mesenchymal stem cell (MSC) behavior within 3-dimensional hydrogel environments under conditions that promote chondrogenic differentiation. Human MSCs were photoencapsulated in hyaluronic acid (HA) hydrogels functionalized with Ncad mimetic peptides and compared to cells in environments with control non-active peptides (Ctrl). Using single-cell imaging, we observed a significant increase in membrane βcat, nuclear βcat, and cell roundness after 3 days in Ncad hydrogels compared to Ctrl hydrogels. The extent of membrane and nuclear βcat localization and MSC roundness decreased to Ctrl hydrogel levels via pre-treatment with Ncad-specific antibodies prior to encapsulation in the Ncad hydrogels, confirming the activity of the peptide. Interestingly, there was a pronounced (>80% increase) in βcat nuclear localization in two-cell clusters within the Ctrl hydrogels, which was much greater than the increase (~30%) in βcat nuclear localization in two-cell clusters within the Ncad hydrogels. In summary, we utilized fluorescent imaging to demonstrate Ncad-mediated single cell responses to developmental cues within hydrogels towards chondrogenesis.
Keywords: Hyaluronic acid, N-cadherin, Biomaterials, Tissue Engineering
Introduction
Due to limitations in the natural healing capacity of cartilage and given the increasing incidence of osteoarthritis, there exists a growing demand for cell-based therapies for cartilage repair. Tissue engineering, and particularly approaches based on autologous mesenchymal stem cells (MSCs), has evolved to yield clinically relevant techniques that are translating into patient therapies.19 To increase the clinical efficacy of these approaches, it is important to understand the interface between MSCs and their microenvironment, particularly as it is becoming clear that biochemical and biophysical signals within stem cell environments can play an important role in controlling differentiation.10 For example, the extent of chondrogenesis within hydrogels can be regulated by signals that mimic components of native cartilage, either during development or within adult tissues.7,16
Towards stem-cell based cartilage tissue engineering, we have used hydrogels formed from hyaluronic acid (HA) precursors, due to the role that HA has on cell behavior via receptor interactions (i.e., CD44, CD168),4,6,17 including during development.15 Beyond interactions with HA, other features of the microenvironment are important, including cell-cell interactions. N-cadherin (Ncad), a transmembrane protein that mediates cell-cell adhesion, is widely considered to be a key factor in directing cell-cell interactions during mesenchymal condensation.1,22,29 Indeed, overexpression of Ncad in the developing limb bud promotes early chondrogenic differentiation of the mesenchyme, while expression of Ncad dominant negative isoforms delays or prevents this differentiation lineage. Despite the importance of such interactions, most hydrogel-based approaches encapsulate single MSCs that are separated from one another by the biomaterial and inherently limit cell-cell contact. The His-Ala-Val (HAV) motif in the first extracellular domain of Ncad was previously identified as a critical regulator of cell-cell interactions,5 and we have shown that the incorporation of an HAV motif into HA hydrogels results in both an upregulation of chondrogenic genes within the first 3 days of culture and enhanced matrix production by photoencapsulated MSCs.4
Importantly, this study identified Ncad peptides as a potentially important target for the engineering of hydrogels for cartilage repair; however, additional information is needed regarding these interactions between Ncad peptide mimics presented in the hydrogel and encapsulated MSCs. For example, it is well known that β-catenin (βcat) plays a central role in both cell-cell junctions and Wnt signaling.14 Upon engagement of the extracellular domain of Ncad, the cytoplasmic domain of Ncad forms complexes with βcat, recruiting βcat to the membrane and reducing βcat phosphorylation, which leads to ubiquitin-dependent degradation.25 Membrane βcat recruitment also results in an increase of available βcat in the cell, which permits βcat localization to the nucleus and the activation of numerous pathways. Notably, several studies have reported that cells in densely populated environments that recapitulate mesenchymal condensation exhibit an increase in nuclear βcat. For example, Tuli et al. showed that pellet cultures in culture media supplemented with TGF-β exhibited an increase in nuclear βcat.30 Additionally, Modarresi et al. reported that overexpression of Ncad in micromass cultures resulted in an increase of nuclear βcat localization.21 Taken together, the presence of βcat in both the membrane and in the nucleus indicates cellular Ncad engagement with the Ncad peptides presented in the hydrogels.
To observe such interactions, techniques must be used that probe outcomes in single cells, rather than total population analyses. Additionally, it is well understood that MSC cultures are innately heterogeneous and variant cell behavior and differentiation potential can be observed across cells within the same cultures.3,24 For instance, after 7 days in culture, bovine MSCs photoencapsulated in hydrogels supplemented with chondrogenic induction media displayed variable proteoglycan production, indicating that some cells are more efficient at secreting matrix than others under identical microenvironments.8,12 Again, imaging techniques are needed to understand cell signaling in the context of MSC heterogeneity within hydrogels.
We hypothesize that the introduction of Ncad mimetic peptides (i.e., HAV) into hydrogels will increase both membrane and nuclear βcat. To assess Ncad-mediated signaling in the context of membrane and nuclear βcat localization, MSCs were photoencapsulated in HA hydrogels from methacrylated HA (MeHA) macromers functionalized with either Ncad mimetic (Ncad) or non-active control peptides (Ctrl) (Fig. 1). Confocal imaging was then utilized to assess membrane βcat, cell morphology, and nuclear βcat (defined as the volume ratio of βcat in the nucleus to that of the cytosol). Furthermore, this approach allowed us to compare single cells versus two-cell clusters within hydrogels, which would not be possible with population-based measurement techniques.
Figure 1.
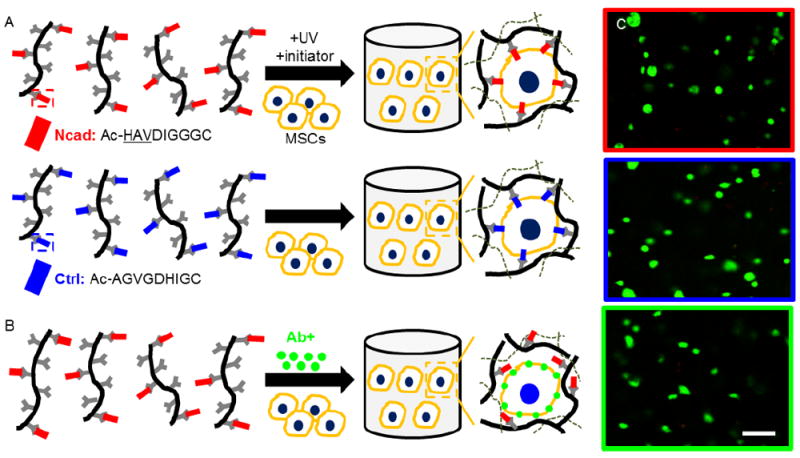
Experimental design and viability. (A) MeHA macromers were functionalized with either Ncad mimetic peptides (Ncad, red) or non-active scrambled control peptides (Ctrl, blue) and used to encapsulate MSCs in the presence of initiator and light. (B) Schematic of blocking Ncad transmembrane proteins prior to the encapsulation of MSCs within Ncad modified hydrogels by exposure of MSCs to Ncad antibodies (Ab+, green). (C) Live/dead imaging (live cells green, dead cells red) of MSC-laden hydrogels at 3 days. MSC viability was high in all groups (>95%). (Scale bar, C = 200 μm).
Materials and Methods
MeHA synthesis and peptide coupling
MeHA was synthesized as previously described.28 Briefly, methacrylic anhydride was reacted with 1% (wt/v) sodium hyaluronate powder (molecular weight ~75 kDa, Lifecore) in deionized water at a pH of 8. Next, MeHA was purified via dialysis (SpectraPor, 6-8 kDa molecular weight cutoff) for 4 days and lyophilized for at least 3 days. The extent of MeHA methacrylation was confirmed by 1H NMR to be ~29%. Ncad (Ac-HAVDIGGGC) and Ctrl (Ac-AGVGDHIGC) peptides (827.9 Da) with a cysteine residue at the C-terminal end were obtained from GenScript. For Michael-type addition peptide coupling, 9.28 mg of peptide and 100 mg MeHA were dissolved in triethanolamine-buffered saline (TEOA buffer, pH 8) and reacted at 37 °C overnight, followed by dialysis (4 days) and lyophilization (3 days). This coupling ratio was used to obtain a final concentration of peptide of 2 mM in a 3 wt% hydrogel.
Cell culture and hydrogel encapsulation
Human MSCs (Lonza) expanded to passage 3 in growth media (α-MEM supplemented with 16.7% (v/v) FBS (Gibco), 1% (v/v) L-glutamine (Invitrogen), 1% (v/v) penicillin-streptomycin (Invitrogen)) were used in all experiments. For Ncad blocking studies, 2.5 million MSCs were suspended in 2 mL buffer (2% (v/v) FBS in PBS) or 2 mL anti-Ncad antibody in buffer (50 μg/mL, clone GC-4, Sigma) for 45 min on ice, followed by two centrifugation washes (500 RCF) in buffer and resuspension in PBS prior to photoencapsulation.
HA hydrogels containing MSCs (5 million cells/mL) were formed by exposing MeHA macromer solutions (3 wt%) to light (365 nm, 2 mW/cm2, 10 min) in the presence of 0.05% (w/v) photoinitiator (I2959 Irgacure, Ciba). Hydrogel disks (5 mm diameter, 2.6 mm thickness) contained either the Ncad mimetic peptide (Ncad) or the scrambled non-active control peptide (Ctrl). MSC-laden hydrogels were cultured in chondrogenic media (α-MEM, 1% (v/v) ITS+Premix, 50 μg/ml L-proline, 0.1 μM dexamethasone, 0.9 mM sodium pyruvate, 50 μg/ml ascorbate, 1% (v/v) penicillin-streptomycin) supplemented with 10 ng/ml TGF-β3 for 3 days.
Immunostaining and imaging
After 3 days in culture, MSC-laden hydrogels were fixed in 10% formalin overnight at 4 °C. Samples were then permeabilized with 0.1% Triton X-100 (30 min) and blocked with 3% normal goat serum (1 hour) in PBS. Primary βcat antibody (Abcam, 1:100) was added overnight at 4 °C, followed by Alexa Fluor 488 secondary antibody (Life Technologies, 1:200) for 2 hours at room temperature. To visualize individual cells and nuclei, samples were stained for actin (rhodamine phalloidin, 1 hour, 1:100) and double-stranded DNA (DAPI, 30 min, 1:500), respectively. After staining, samples were washed and imaged with a 20X objective using a Leica SP8 inverted confocal microscope. Using this setup, 3-dimensional image stacks of xy-plane cross-sections were acquired (thickness ~150 μm, step-size 0.69 μm). To confirm MSC viability under these conditions, a live/dead assay was used per the manufacturer’s instructions (Life Technologies). Briefly, this assay simultaneously stains cells with green-fluorescent calcein-AM in viable cells and red-fluorescent ethidium homodimer in compromised cells to indicate intracellular esterase activity and loss of plasma membrane integrity, respectively.
Image Analysis
All image analysis was performed using ImageJ NIH image processing software (Bethesda, MD). To quantify membrane βcat, cross-sectional images at the centroid from 3-dimensional βcat channel image stacks were used to calculate horizontal average pixel intensity profiles of two-pixel thick rectangles spanning the length of the cell (Fig. 2A). To account for differences in cell size, horizontal distances were normalized so they range from 0 to 1, and pixel intensity values were subtracted and divided by the average pixel intensity of the rectangles to account for cell-to-cell variability in total pixel intensity.
Figure 2.
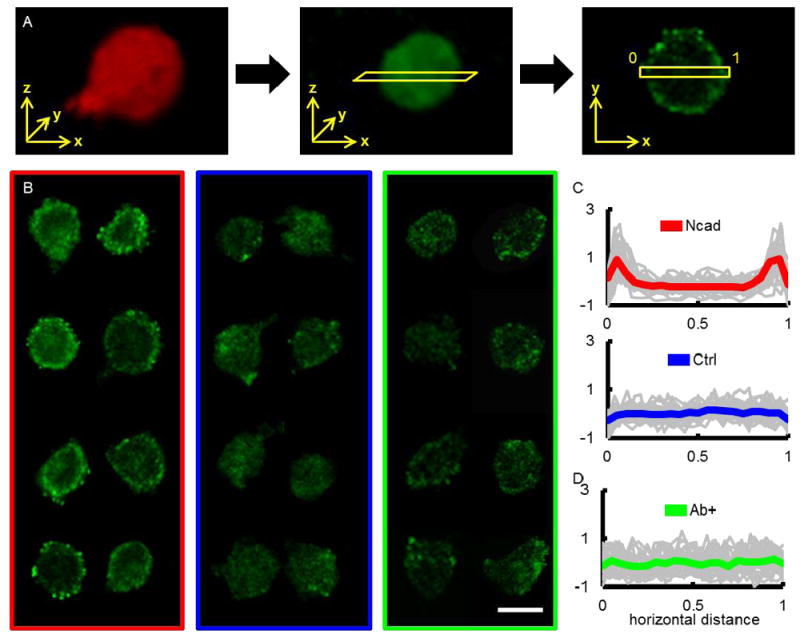
Membrane βcat determination and staining of single MSCs in Ncad and Ctrl hydrogels. (A) Overview of semi-quantitative assessment of membrane βcat. 3-dimensional reconstruction of single MSCs stained for actin (red) and βcat (green) were used to determine the centroid of the cell. A two-pixel thick rectangle was then used to calculate a horizontal pixel intensity profile that spanned the width of the cell. (B) Representative cross-section images of single MSCs in Ncad (red rectangle, left), Ctrl (blue rectangle, middle), and Ab+ MSCs in Ncad (green rectangle, right) hydrogels stained for βcat at day 3. Normalized βcat intensity profiles (grey lines for individual cells, thick colored line for average) for MSCs in (C) Ncad (top, red) and Ctrl (bottom, blue) hydrogels, as well as (D) when antibodies were used to block Ncad interactions prior to MSC encapsulation (Ab+, green). (Scale bar, B = 200 μm).
For each cell, the nuclear and cytosolic domains were determined by generating binary masks of 3-dimensional image stacks of double-stranded DNA and actin using Otsu’s intensity-based thresholding method (Fig. 3A).23 Actin staining was only used to assess cellular morphology and not actin distribution throughout the cells. Image J’s 3D Objects Counter function was then used to calculate volume and surface area for each domain. These values were used to define cellular roundness by determining the ratio between the cell’s surface area divided by the surface area of a sphere with the cell’s volume, resulting in a value ranging from 0 to 1, where 0 is a straight line and 1 is a perfect sphere.
Figure 3.
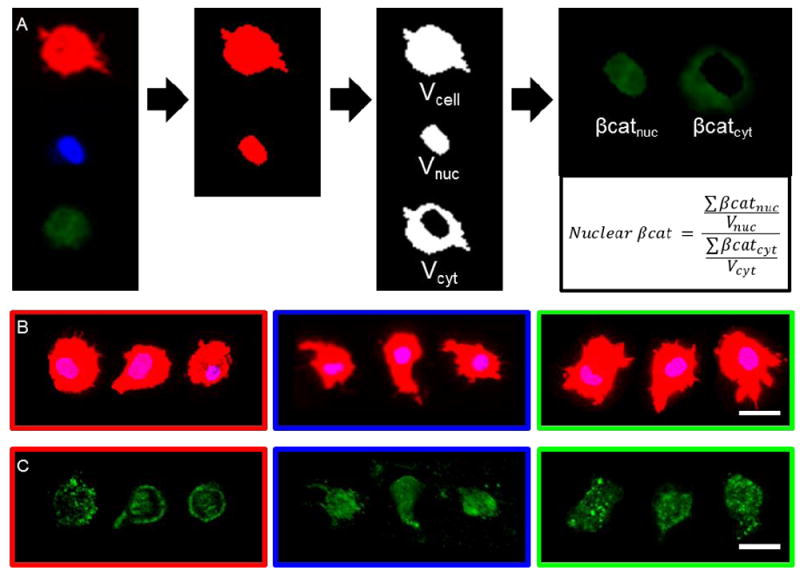
Nuclear βcat quantification workflow and images of single MSCs in Ncad and Ctrl hydrogels. (A) To assess how single MSCs interact with Ncad mimetic peptides, actin (red), nucleus (blue), and βcat (green) channels were separated, and Otsu’s intensity thresholding method was used to generate 3-dimensional masks (Vcell, Vnuc, Vcyt) for each channel. Next, βcat image stacks were superimposed with Vnuc and Vcyt to obtain βcat intensity in the cytosolic (βcatcyt) and nuclear (βcatnuc) domains. Vcell was used to determine cell roundness (1 = perfect sphere) and nuclear βcat was calculated as the ratio (nuclear βcat) between βcatnuc and βcatcyt normalized to Vnuc and Vcyt, respectively. Representative (B) maximum and (C) average projections of single MSCs photoencapsulated in MeHA hydrogels (Ncad: red, left; Ctrl: blue, middle; Ab+: green, right) after 3 days and stained for (B) actin (red), nucleus (blue), and (C) βcat (green). (Scale bars, B,C = 25 μm).
To quantify nuclear βcat, nuclei masks were inverted and superimposed with corresponding actin masks to generate masks that encompass the cytosol yet exclude the nucleus. 3-dimensional βcat image stacks of the same cells were then superimposed with these masks, resulting in new image stacks containing cytosolic βcat. Similar methods were employed using nuclei masks to obtain nuclear βcat image stacks. These image stacks were then subject to Image J’s Plot Z-axis Profile function to calculate total pixel intensity for each cross-section in the 3-dimensional image stacks. Using this data, the ratio between nuclear and cytosolic βcat was calculated and normalized to the volumes of the nuclear and cytosolic domains within the cell (Fig. 3A). For multiple cells (two-cell clusters), the same approach for determining nuclear βcat was utilized with multiple nuclei.
Statistical analysis
Statistical analyses were performed with one-way ANOVA with Tukey’s post hoc test using Excel software with Daniel’s XL Toolbox add-in. Differences between culture conditions were considered significant for p-values less than 0.01, and error bars indicate standard mean error around the mean. At least 30 cells were analyzed per group and condition.
Results
Ncad mimetic peptides recruit βcat to the cell membrane
HA hydrogels were formulated from MeHA macromers that contained covalently bound peptide - either HAV, which was identified from the extracellular domain of Ncad (Ncad), or the same sequence scrambled into a non-active form (Ctrl). To confirm cell viability when MSCs were photoencapsulated in 3 wt% hydrogels containing either peptide, a live/dead assay was performed after 3 days. High viability (>95%) was observed across all hydrogel formulations with no noticeable differences between groups (Fig. 1C). To assess membrane βcat differences of MSCs in Ncad and Ctrl hydrogels, βcat cross-sections were first taken at the centroid of single MSCs (Fig. 2A). Next, horizontal average intensity profiles were obtained from these cross-sections, in a two-pixel thick rectangle that crossed the centroid and spanned the length of the cell. Prior to plotting, distance and pixel intensity were normalized, such that distance ranged from 0 (left side of rectangle) to 1 (opposite end), and pixel values were subtracted and divided by the average pixel intensity of the rectangle to account for cell-to-cell variability in pixel intensity.
Representative βcat images of single MSCs in Ncad hydrogels (Fig. 2B, left collage) featured more membrane βcat than those in the Ctrl hydrogels (Fig. 2B, center collage). A normalized average pixel intensity versus horizontal distance plot of single MSCs in Ncad hydrogels (gray lines denote single cell profiles and thick red line denotes population average) features intensity peaks near the ends of the graph, which correspond to the cell periphery (Fig. 2C, top plot). In contrast, the images and plot of MSCs in the Ctrl group (thick blue line denotes population average) do not display regions of high intensity and βcat was fairly uniform throughout the span of the cell (Fig. 2C, bottom plot).
Ncad mimetic peptides enhance MSC roundness and nuclear βcat localization
To assess the influence of Ncad peptides on cellular roundness and nuclear βcat, single MSCs were stained for βcat, actin, and double-stranded DNA, and Otsu’s intensity-based thresholding method was used to generate 3-dimensional masks of the cell (Vcell) and nucleus (Vnuc) (Fig. 3A). Cell volume and surface area were calculated using cell masks, and these values were used to calculate cell roundness, which is the surface area ratio between the cell and a sphere with the same volume as the cell. To create cytosol masks that include the cell body and exclude the nuclear domain (Vcyt), nuclei masks were inverted and superimposed with corresponding cell masks. 3-dimensional βcat image stacks were then superimposed with Vnuc and Vcyt to obtain image stacks of βcat pixel intensity in the nuclear (βcatnuc) and cytosolic (βcatcyt) space. Nuclear localization of βcat (nuclear βcat) was determined by taking the ratio between total βcatnuc and βcatcyt pixel intensities normalized to Vnuc and Vcyt volumes, respectively.
Representative actin and nuclei images of single MSCs in the Ncad (Fig. 3B left) and Ctrl (Fig. 3B center) conditions showed that cells were similar in size, but exhibited differences in cellular morphologies between groups. In the Ncad condition there was generally more membrane and nuclear βcat compared to the Ctrl hydrogels, which did not contain the active Ncad mimetic peptides (Fig. 3C left and center).
Roundness was significantly lower for MSCs in the Ctrl condition, suggesting some degree of localized cell spreading into the hydrogel (Fig. 4A). Likewise, nuclear βcat was significantly lower in the Ctrl condition (Fig. 4B). To assess nuclear βcat population heterogeneity of MSCs in Ncad hydrogels, a nuclear βcat histogram was plotted (Fig. 4C). Correlations between cell morphology and nuclear βcat localization were also determined by plotting nuclear βcat versus roundness (Fig. 4D). In both cases, these plots showed that single cells in the Ctrl condition formed a cluster that was distinguishable from the cells in the Ncad hydrogels, and that both groups exhibited heterogeneity.
Figure 4.
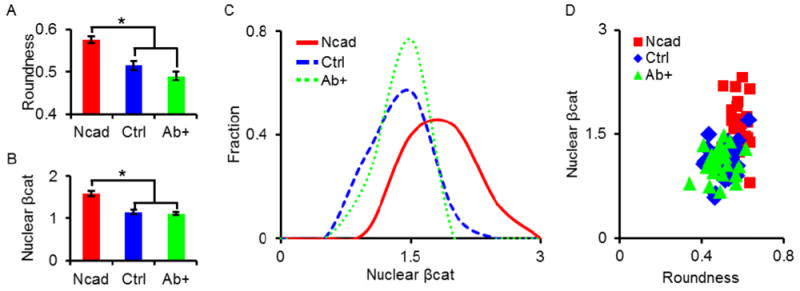
Roundness and βcat signaling of single MSCs in Ncad and Ctrl hydrogels after 3 days of culture. Outcomes of average (A) cell roundness and (B) βcat Ratio between groups (Ncad: red; Ctrl: blue; Ab+: green). The groups containing the Ncad peptide had statistically greater cell roundness and nuclear βcat compared to Ctrl hydrogels and Ab+ MSCs in Ncad hydrogels; no statistical differences were observed between the Ctrl and Ab+ groups. To better illustrate heterogeneity in groups, nuclear βcat is also plotted as (C) histograms and (D) scatter plots in correlation with cell roundness. * indicates statistical significance (p < 0.01) versus the Ncad group.
Blocking Ncad transmembrane proteins mitigates effects of Ncad mimetic peptides
To ascertain whether cell-biomaterial interactions between Ncad transmembrane proteins on cells and Ncad peptides in hydrogels led to changes in βcat membrane/nuclear translocation and roundness, MSCs were treated with a monoclonal Ncad antibody (Ab+) prior to photoencapsulation in Ncad hydrogels (Fig. 1B) and remained highly viable for the duration of the study (Fig. 1C). By doing so, a comparison could be made between untreated MSCs in Ncad hydrogels which were able to interact with Ncad peptides in the hydrogel and MSCs in the Ab+ group that were unable to interact with the Ncad peptides due to Ncad blocking.
Representative βcat images of MSCs in the Ab+ group (Fig. 2B, right collage) feature significantly less membrane βcat than untreated MSCs in the same Ncad formulation (Fig. 2B, left collage). A normalized pixel intensity versus horizontal distance plot of the Ab+ group (gray lines denote single cell profiles and thick green line denotes population average) shows a uniform pixel intensity distribution throughout the cells (Fig. 2D), which contrasts observations seen in untreated MSCs in Ncad hydrogels (Fig. 2C, top), yet is similar to MSCs in the Ctrl hydrogels (Fig. 2C, bottom).
MSCs in the Ab+ group exhibited a larger cell size (11,000 ± 600 μm3) when compared to untreated MSCs in Ncad (7,600 ± 500 μm3) and Ctrl (8,000 ± 300 μm3) hydrogels (Fig. 3B). Interestingly, similar to the membrane βcat findings, Ab+ MSCs adopted βcat distributions similar to untreated MSCs in Ctrl hydrogels (Fig. 3C). Roundness values between Ab+ and Ctrl groups were not significantly different and MSCs were significantly less round than untreated MSCs in Ncad hydrogels (Fig. 4A). Nuclear βcat of Ab+ MSCs was also significantly lower than MSCs in the Ncad group, and were indistinguishable from MSCs in the Ctrl group (Fig. 4B). Additionally, the nuclear βcat histogram curve of MSCs in the Ab+ group followed a similar trend to the curve from the Ctrl group (Fig. 4C), and the nuclear βcat versus cell roundness scatter plot showed Ab+ MSCs clustering in a similar manner to MSCs in the Ctrl hydrogels (Fig. 4D).
Cell-cell interactions enhance nuclear βcat localization
Since Ncad interactions result in the formation of adherens junctions between two cells and initiate downstream signaling,27 we next sought to determine whether cell-cell interactions also enhance nuclear βcat localization. Although the majority of cells photoencapsulated within hydrogels were distributed as single cells, a small percentage of cells were also encapsulated as two-cell clusters (Fig. 5A). As such, the same culture conditions were used to investigate the role of cell-cell interactions on nuclear βcat localization in two-cell clusters of MSCs photoencapsulated in either Ncad or Ctrl hydrogels in comparison to single cell outcomes (Fig. 5B). Representative actin and nuclei images of two-cell clusters in the Ncad and Ctrl conditions featured similar cellular and nuclear morphologies and were otherwise indistinguishable from one another (Fig. 5C), following the same trend observed for single cells in those same groups (Fig. 3B). However, unlike single cells in Ncad and Ctrl groups, where a large decrease in nuclear βcat was observed in Ctrl hydrogels (Fig. 3C), no differences in βcat signal for the same two-cell clusters were observed, regardless of peptide condition (Fig. 5D).
Figure 5.
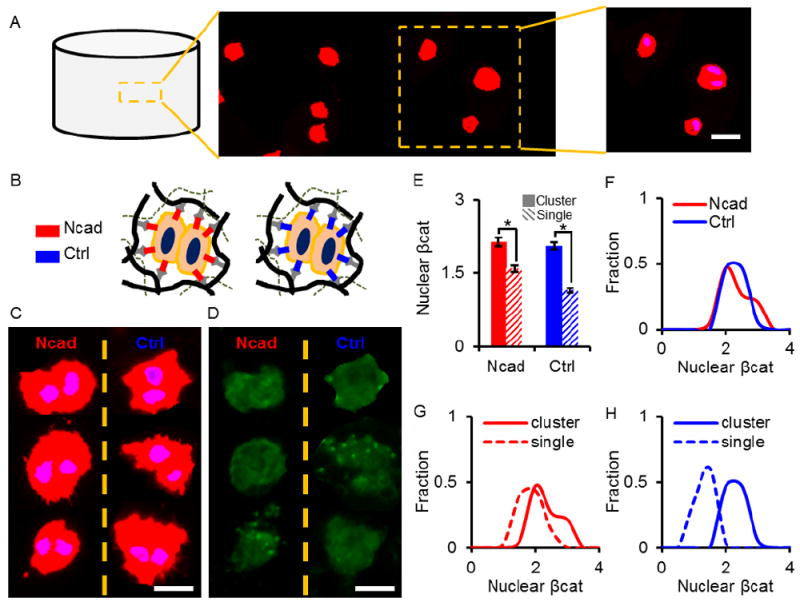
Comparison of single- and two-cell clusters. (A) Representative maximum projection (actin: red, nuclei: blue) of single- and two-cell clusters within a hydrogel. (B) Schematic of multiple cells interacting with each other as well as with hydrogels containing either Ncad or Ctrl peptides. (C) Maximum projections derived from 3-dimensional image stacks of two-cell clusters in Ncad (left) and Ctrl (right) hydrogels stained for actin (red) and nuclei (blue). (D) Average projections of the same clusters immunostained for βcat (green). (E) Nuclear βcat of single cells and two-cell clusters in the Ncad and Ctrl hydrogels. There is no significant difference in nuclear βcat between two-cell clusters in the Ncad and Ctrl hydrogels; however, there is a slight increase in nuclear βcat between single and two-cell clusters in the Ncad hydrogels and an almost two-fold increase in nuclear βcat in the clusters when compared to single cells in the Ctrl hydrogels. Histograms of (F) two-cell clusters in Ncad (red) and Ctrl (blue) hydrogels and two-cell clusters (solid lines) versus single MSCs (dashed lines) in (G) Ncad hydrogels and in (H) Ctrl hydrogels. (Scale bars, A,C,D = 25 μm). * indicates statistical significance (p < 0.01) versus single cells group.
Nuclear βcat was determined for single and two-cell clusters in Ncad and Ctrl hydrogels (Fig. 5E). There was a 20% increase in nuclear βcat between single cells and two-cell clusters in the Ncad group. Similarly, there was an almost a two-fold increase in nuclear βcat in two-cell clusters in Ctrl hydrogels, relative to single cells in this gel. Additionally, no statistical differences were observed between two-cell clusters cultured in Ncad and Ctrl hydrogels. Nuclear βcat histograms of two-cell clusters in the Ncad and Ctrl groups show similar nuclear βcat distributions (Fig. 5F). Histograms of single versus two-cell clusters show similar trends for the Ncad hydrogels (Fig. 5G); however, more pronounced differences in nuclear βcat distributions between single cells and two-cell clusters were observed in Ctrl hydrogels (Fig. 5H).
Discussion
Hydrogels engineered to contain cues that mimic developmental and native microenvironments can significantly enhance chondrogenesis.4 However, the molecular mechanisms conducive to chondrogenesis within hydrogels remain largely unexplored, beyond population analyses of gene expression and resulting matrix formation. This is particularly important as MSC heterogeneity has been implicated in such systems and a better understanding of this phenomenon may lead to further improvements in MSC-based approaches for cartilage repair. To address these limitations, we utilized confocal imaging to investigate single-cell behavior within hydrogels.
Our laboratory recently engineered HA hydrogels that inherently interact with receptors, including CD44 and CD168, through the native biopolymer backbone to stimulate chondrogenesis (e.g., upregulation of chondrogenic genes including type 2 collagen and aggrecan),4,26,31 as well as to mimic cell-cell interactions through inclusion of an Ncad peptide that presents signals found during embryonic limb bud development.15 In addition to promoting cellular condensation,2,11 Ncad recruits βcat to the membrane and helps prevent it from being degraded by the GSK3 complex in the cytosol, which increases available βcat and allows for its translocation to the nucleus.18 Several studies also cite the importance of cell shape during stem cell differentiation.9,13,20 For instance, Chen and coworkers showed that in the presence of TGF-β3, MSCs preferentially differentiate towards a chondrogenic lineage when confined to a round morphology.9
With these features in mind, single cell analyses were performed after 3 days in culture, since we previously showed that Ncad mimetic peptides induced increased expression of chondrogenic genes at this time point.4 Likewise, modifications to hydrogels are likely to influence cells at early times, prior to extensive tissue production that can lead to signaling from produced matrix molecules. The hydrogels were designed so that all features were similar, including hydrogel mechanics and peptide concentration, and the only difference between groups was the peptide sequence included. The non-active peptide was a scrambled sequence of the active domain, so that other features of the peptide such as charge were normalized across groups. With this approach, the groups that contained Ncad peptides led to a significant increase in cell roundness and βcat localization at both the membrane and nucleus. It is important to note that in this study only one concentration of active and control peptide was investigated; however, this concentration led to differences in cell response. Using the imaging technique presented, a more broad range of concentrations could be investigated to determine whether there are peptide concentrations that induce even greater signaling and chondrogenesis.
To assess the extent of heterogeneity in stem cell responses, nuclear βcat histograms were plotted. Although histogram peaks for nuclear βcat values in Ncad hydrogels were higher than in Ctrl hydrogels, there was a high degree of heterogeneity under all conditions. For instance, some cells in the Ctrl condition had nuclear βcat values representative of MSCs in Ncad hydrogels and vice versa. To identify potential relationships between cell morphology and βcat nuclear localization, nuclear βcat versus roundness scatter plots were created, showing a positive correlation between nuclear βcat localization and cell roundness.
To show that Ncad mimetic peptide-induced roundness and βcat localization at the membrane and in the nucleus was due to Ncad peptide interactions with Ncad transmembrane proteins, two experiments were performed. First, Ncad transmembrane proteins were blocked by treating MSCs with an anti-GC-4 Ncad antibody prior to photoencapsulation in Ncad hydrogels. The GC4 clone was chosen due to its selective binding affinity to the N-terminal half of the extracellular domain of human Ncad.4 Thus, the goal of the antibody was to block cellular interactions with the Ncad peptides, resulting in MSC behavior of membrane/nuclear βcat and roundness similar to that of MSCs in Ctrl hydrogels without Ncad peptides. At a concentration that did not compromise cell viability, antibody-treated MSCs exhibited a significant drop in roundness and membrane/nuclear βcat localization, indicating that Ncad peptides interact with Ncad transmembrane proteins. Furthermore, a nuclear βcat versus roundness scatter plot showed a clear separation between the two conditions and a positive correlation between these metrics, analogous to trends observed between untreated MSCs in Ctrl and Ncad hydrogels.
As another indication of the peptide induction of cell-cell interactions, we separated out findings of single cells with two-cell clusters observed within the hydrogels. In the images analyzed at 3 days, about 15% of cells formed clusters (data not shown). This low percentage of clusters is similar to what we observe immediately after encapsulation; thus, we believe this to be due to the encapsulation technique rather than proliferation. Two-cell clusters in both control and Ncad hydrogels reached the same levels of βcat nuclear localization. The nuclear βcat achieved was slightly higher than levels attained by single MSCs in Ncad hydrogels, suggesting that there is room for improvement in the signaling from the peptide modified hydrogels to reach values of cell-cell contact. In contrast, comparisons of single cells and two-cell clusters in the Ctrl hydrogels (without the Ncad peptides) showed a much larger increase in nuclear βcat, indicating that cell-cell contacts alone significantly enhanced βcat nuclear localization when the Ncad signal was not included. Thus, the peptide modification is a significant improvement when single cells are encapsulated and in combination with the benefits of using hydrogels in cartilage repair represents and advance for the field.
To summarize, we utilized confocal imaging to probe single cell responses to Ncad mimetic peptides of photoencapsulated MSCs. We found that the presence of Ncad mimetic peptides increased βcat accumulation at the cell membrane and within nucleus as well as MSC roundness. Using histograms, we were able to identify the extent of heterogeneity in these hydrogels, highlighting the potential use of this method as a screening tool to identify conditions that reduce heterogeneity. We also found that situations of multiple cells (two-cell clusters) rather than single cells resulted in enhanced nuclear βcat in both Ncad and Ctrl hydrogels, with a much more pronounced effect in the Ctrl group. Varying cell cluster size and Ncad peptide concentrations may further enhance nuclear βcat localization and downstream Ncad-mediated cartilage production. In conclusion, the methods presented show promise in identifying candidate biomaterials to further the use of stem cells in cell-based chondrocyte repair strategies.
Acknowledgments
This work was supported by a graduate research fellowship from the National Science Foundation (MK) and grants from the National Institutes of Health (R01 EB008722) and the Department of Veterans Affairs (I01 RX000700).
References
- 1.Ahrens PB, Solursh M, Reiter RS. Stage-related capacity for limb chondrogenesis in cell culture. Developmental biology. 1977;60:69–82. doi: 10.1016/0012-1606(77)90110-5. [DOI] [PubMed] [Google Scholar]
- 2.Balsamo J, Leung T, Ernst H, Zanin MK, Hoffman S, Lilien J. Regulated binding of ptp1b-like phosphatase to n-cadherin: Control of cadherin-mediated adhesion by dephosphorylation of beta-catenin. The Journal of cell biology. 1996;134:801–813. doi: 10.1083/jcb.134.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beane OS, Darling EM. Isolation, characterization, and differentiation of stem cells for cartilage regeneration. Annals of Biomedical Engineering. 2012;40:2079–2097. doi: 10.1007/s10439-012-0639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bian L, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and n-cadherin interactions enhance msc chondrogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10117–10122. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaschuk OW, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Developmental biology. 1990;139:227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- 6.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 7.Connelly JT, Garcia AJ, Levenston ME. Inhibition of in vitro chondrogenesis in rgd-modified three-dimensional alginate gels. Biomaterials. 2007;28:1071–1083. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Cote AJ, Mcleod CM, Farrell MJ, Mcclanahan PD, Dunagin MC, Raj A, Mauck RL. Single cell differences in matrix gene expression do not predict matrix deposition. Nature Communications. 2016 doi: 10.1038/ncomms10865. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao L, Mcbeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through rac1 and n-cadherin. Stem cells. 2010;28:564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guvendiren M, Burdick JA. Engineering synthetic hydrogel microenvironments to instruct stem cells. Current opinion in biotechnology. 2013;24:841–846. doi: 10.1016/j.copbio.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoschuetzky H, Aberle H, Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. The Journal of cell biology. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. Journal of biomechanics. 2010;43:128–136. doi: 10.1016/j.jbiomech.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim W, Kim M, Jho EH. Wnt/beta-catenin signalling: From plasma membrane to nucleus. The Biochemical journal. 2013;450:9–21. doi: 10.1042/BJ20121284. [DOI] [PubMed] [Google Scholar]
- 15.Knudson CB, Toole BP. Hyaluronate-cell interactions during differentiation of chick embryo limb mesoderm. Developmental biology. 1987;124:82–90. doi: 10.1016/0012-1606(87)90462-3. [DOI] [PubMed] [Google Scholar]
- 16.Lee HJ, Yu C, Chansakul T, Hwang NS, Varghese S, Yu SM, Elisseeff JH. Enhanced chondrogenesis of mesenchymal stem cells in collagen mimetic peptide-mediated microenvironment. Tissue engineering Part A. 2008;14:1843–1851. doi: 10.1089/ten.tea.2007.0204. [DOI] [PubMed] [Google Scholar]
- 17.Lisignoli G, Cristino S, Piacentini A, Cavallo C, Caplan AI, Facchini A. Hyaluronan-based polymer scaffold modulates the expression of inflammatory and degradative factors in mesenchymal stem cells: Involvement of cd44 and cd54. Journal of cellular physiology. 2006;207:364–373. doi: 10.1002/jcp.20572. [DOI] [PubMed] [Google Scholar]
- 18.Ma B, Landman EB, Miclea RL, Wit JM, Robanus-Maandag EC, Post JN, Karperien M. Wnt signaling and cartilage: Of mice and men. Calcified Tissue International. 2013;92:399–411. doi: 10.1007/s00223-012-9675-5. [DOI] [PubMed] [Google Scholar]
- 19.Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nature reviews Rheumatology. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mcbeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and rhoa regulate stem cell lineage commitment. Developmental cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 21.Modarresi R, Lafond T, Roman-Blas JA, Danielson KG, Tuan RS, Seghatoleslami MR. N-cadherin mediated distribution of beta-catenin alters map kinase and bmp-2 signaling on chondrogenesis-related gene expression. Journal of cellular biochemistry. 2005;95:53–63. doi: 10.1002/jcb.20396. [DOI] [PubMed] [Google Scholar]
- 22.Oberlender SA, Tuan RS. Spatiotemporal profile of n-cadherin expression in the developing limb mesenchyme. Cell adhesion and communication. 1994;2:521–537. doi: 10.3109/15419069409014216. [DOI] [PubMed] [Google Scholar]
- 23.Otsu N. Threshold selection method from gray-level histograms. Ieee Transactions on Systems Man and Cybernetics. 1979;9:62–66. [Google Scholar]
- 24.Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O’connor KC. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem cells. 2010;28:788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 25.Sadot E, Simcha I, Shtutman M, Ben-Ze’ev A, Geiger B. Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15339–15344. doi: 10.1073/pnas.95.26.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz Z, Griffon DJ, Fredericks LP, Lee HB, Weng HY. Hyaluronic acid and chondrogenesis of murine bone marrow mesenchymal stem cells in chitosan sponges. American journal of veterinary research. 2011;72:42–50. doi: 10.2460/ajvr.72.1.42. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 28.Smeds KA, Grinstaff MW. Photocrosslinkable polysaccharides for in situ hydrogel formation. Journal of Biomedical Materials Research. 2001;54:115–121. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Tavella S, Raffo P, Tacchetti C, Cancedda R, Castagnola P. N-cam and n-cadherin expression during in vitro chondrogenesis. Experimental cell research. 1994;215:354–362. doi: 10.1006/excr.1994.1352. [DOI] [PubMed] [Google Scholar]
- 30.Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves n-cadherin and mitogen-activated protein kinase and wnt signaling cross-talk. The Journal of biological chemistry. 2003;278:41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 31.Wu SC, Chang JK, Wang CK, Wang GJ, Ho ML. Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials. 2010;31:631–640. doi: 10.1016/j.biomaterials.2009.09.089. [DOI] [PubMed] [Google Scholar]


