Abstract
Treatment with radioactive iodine (RAI) for differentiated thyroid cancer has been associated with alterations in gonadal function in women, including changes in menstrual function and an earlier age at menopause. Our objective was to evaluate associations between RAI and post-diagnosis live birth rates among thyroid cancer survivors diagnosed at ages 15–39 years. We identified women diagnosed with differentiated thyroid cancer between January 2000 and December 2013 in the North Carolina Central Cancer Registry (CCR). CCR records were linked to state birth certificate files to identify livebirths to thyroid cancer survivors through December 2014. Person-years of follow-up were accrued from 6 months after diagnosis to first birth, 46th birthday, death, or December 31, 2014, whichever came first. Cox proportional hazards regression was used to estimate hazards ratios (HR) and 95% confidence intervals (CI) for first livebirth. Among 2,360 women with a differentiated thyroid cancer diagnosis, 53% received RAI. The cumulative incidence of birth at the end of follow-up (maximum 14.5 years) was 30.0% and 29.3% among those who were and were not treated with RAI, respectively. Overall, first birth rates did not significantly differ between groups (HR=1.00; 95% CI: 0.82, 1.23). In our observational cohort, treatment with RAI was not associated with a reduced birth rate. Our findings add to the evidence available for counseling thyroid cancer patients with concerns about future fertility.
Keywords: thyroid cancer, radioactive iodine, birth rates
Introduction
In the United States, thyroid cancer is the fifth most common cancer diagnosed among women of all ages and the most common cancer among women aged 20 to 34 years.1 An estimated 42,470 new cases of thyroid cancer will be diagnosed among women in 2017,1 with more than one-third of these occurring in women younger than 45 years of age.2 Most new diagnoses are differentiated thyroid cancers (papillary or follicular thyroid cancers)3 and are highly curable, with a 5-year relative survival of 98% for all stages combined.1 The standard treatment for differentiated thyroid cancers is thyroidectomy, which is often followed by radioactive iodine (RAI) treatment for delivery of adjuvant therapy or ablation of any postoperative remnant thyroid tissue.4 However, for low-risk patients, who comprise the majority of younger patients with differentiated thyroid cancer,5, 6 RAI may be of little or no benefit for recurrence or survival,4, 5, 7 while increasing risk of second primary malignancies and other adverse health effects.8–11
For younger women with a thyroid cancer diagnosis, an important consideration surrounding treatment with RAI therapy is potential effects on future reproductive function. RAI may affect gonadal tissue and has been associated with transient elevations in serum gonadotrophins and temporary oligomenorrhea or amenorrhea.12, 13 Women treated with RAI may also have an earlier average age at menopause than those not treated with RAI.13 Though prior studies are conflicting,14, 15 an increase in miscarriage rates in the first year following RAI has been reported,15 and clinical guidelines generally recommend that women delay conception for at least 6–12 months after treatment.13, 16, 17 A prior systematic review did not find an increased long-term risk of infertility with RAI treatment.13 However, few large population-based studies have been conducted in contemporary cohorts,18 and the impact of RAI on birth rates among thyroid cancer survivors remains unclear.
In the current study, we used data from the North Carolina Central Cancer Registry and state birth certificate file to evaluate associations between RAI therapy and post-diagnosis childbirth among women diagnosed with thyroid cancer during adolescence and young adulthood.
Materials and methods
We identified all women diagnosed with thyroid cancer at ages 15–39 from January 1, 2000 through December 31, 2013 in the North Carolina Central Cancer Registry (CCR) using ICD-O-3 code C739 (n=2,684). Information recorded in the CCR includes primary site and histology codes, date of diagnosis, stage, tumor size, marital status, and primary treatments (surgery, radiation, and chemotherapy). Dates are also recorded for initiation of primary treatments. We defined differentiated thyroid cancers using the following histology codes: 8050, 8260, 8330, 8331, 8332, 8335, 8340, 8342, 8343, and 8344. All other histology codes, including those for anaplastic or medullary thyroid cancers, were excluded (n=209). We also excluded all women treated with chemotherapy (N=7) or external beam radiation (N=5), and those with missing radiation information (n=24). We classified women as having received RAI if their dominant radiation modality was listed as ‘radio-isotopes, NOS,’ the modality which includes treatment with iodine-131. Those treated with RAI more than six months after diagnosis (the 95th percentile for dates of initiating RAI) were excluded (N=72), as were those who died within six months of diagnosis (n=7). Thus final analyses included 2,360 women with a thyroid cancer diagnosis.
Livebirths to thyroid cancer survivors were identified through a linkage between CCR data and North Carolina statewide vital records from January 1, 2000 through December 31, 2014. Birth certificate files were linked to CCR records using a probabilistic linkage strategy in Link Plus.19 Variables used in the linkage included maternal name, date of birth, and social security number. We included the first, post-diagnosis livebirth to each woman, except those for which we assumed that the mother’s thyroid cancer diagnosis occurred during pregnancy, defined as an infant’s gestational age longer than the interval between the mother’s diagnosis date and the infant’s date of birth.
Statistical analysis
Person-years at risk of giving birth were accrued from 6 months after diagnosis until first livebirth, death, 46th birthday, or December 31, 2014, whichever came first. Cox proportional hazards regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for first birth, comparing women who received RAI to those who did not. Models were adjusted for age at diagnosis (15–24, 25–29, 30–34, 35–39) and disease stage (localized, regional/distant). In stratified analyses, we estimated rate ratios according to age at diagnosis and disease stage. The proportional hazards assumption was checked by visual inspection of log-log plots. Assessment of log-log plots suggested evidence of non-proportional hazards for the association between RAI and time to first birth, both overall and in stratified analyses. Thus all hazards ratios presented should be interpreted as time-averaged summary measures. In sensitivity analyses, we estimated HRs separately for <6 years and ≥6 years of follow-up, the point at which hazards for the RAI and non-RAI groups appeared to cross. We also performed sensitivity analyses varying the start of follow-up from 6 to 12 months. Due to the high proportion of women with missing information for tumor size (22%), we conducted further sensitivity analyses in which we imputed tumor size using multiple imputation. Because estimates remained similar when the imputed data were used, we present results without adjustment for tumor size. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Of the 2,360 identified women with a diagnosis of differentiated thyroid cancer between ages 15 and 39 years, 53% received RAI. The median follow-up was 4.9 years (IQR: 2.5, 7.5) from 6 months after diagnosis. The distribution of age at diagnosis was similar between those who did and did not receive RAI; in each group the median age was 32 years (IQR: 27, 36) (Table 1). Those treated with RAI were more likely to have been diagnosed with regional or distant stage disease (p<0.001). The median tumor size was larger among those who received RAI (20 mm; IQR: 12, 30) than among those who did not (11 mm; IQR: 5, 22; p<0.001).
Table 1.
Patient characteristics according to receipt of radioactive iodine (RAI)
| No RAI | RAI | ||||
|---|---|---|---|---|---|
|
| |||||
| N | % | N | % | p-valuea | |
|
|
|||||
| Total | 1108 | 1252 | |||
| Age at diagnosis | 0.563 | ||||
| 15–19 | 51 | 5% | 64 | 5% | |
| 20–24 | 122 | 11% | 158 | 13% | |
| 25–29 | 230 | 21% | 270 | 22% | |
| 30–34 | 305 | 28% | 341 | 27% | |
| 35–39 | 400 | 36% | 419 | 33% | |
| Median (IQR) | 32 | (27, 36) | 32 | (27, 36) | 0.101 |
| Race | 0.070 | ||||
| White | 877 | 80% | 1000 | 81% | |
| Black | 168 | 15% | 157 | 13% | |
| Other | 58 | 5% | 85 | 7% | |
| Missing/unknown | 5 | 10 | |||
| Marital status at diagnosis | 0.878 | ||||
| Single (never married) | 299 | 32% | 362 | 33% | |
| Marriedb | 566 | 61% | 657 | 60% | |
| Separated/Widowed/Divorced | 64 | 7% | 79 | 7% | |
| Missing/unknown | 179 | 154 | |||
| Summary stage | <0.001 | ||||
| Localized | 908 | 82% | 762 | 61% | |
| Regional | 171 | 15% | 457 | 37% | |
| Distant | 7 | 1% | 21 | 2% | |
| Unstaged | 22 | 2% | 11 | 1% | |
| Missing/unknown | 0 | 1 | |||
| Tumor size, mm | <0.001 | ||||
| 0–9 | 344 | 42% | 134 | 13% | |
| 10–19 | 225 | 27% | 358 | 35% | |
| 20–39 | 189 | 23% | 381 | 38% | |
| 40+ | 64 | 8% | 143 | 14% | |
| Missing/unknown | 286 | 236 | |||
| Median (IQR) | 11 | (5, 22) | 20 | (12, 30) | <0.001 |
p-value from Wilcoxon or chi-squared test
Includes 'married (including common law)' and 'unmarried or domestic partner (same sex or opposite sex, registered or unregistered, other than common law marriage)'
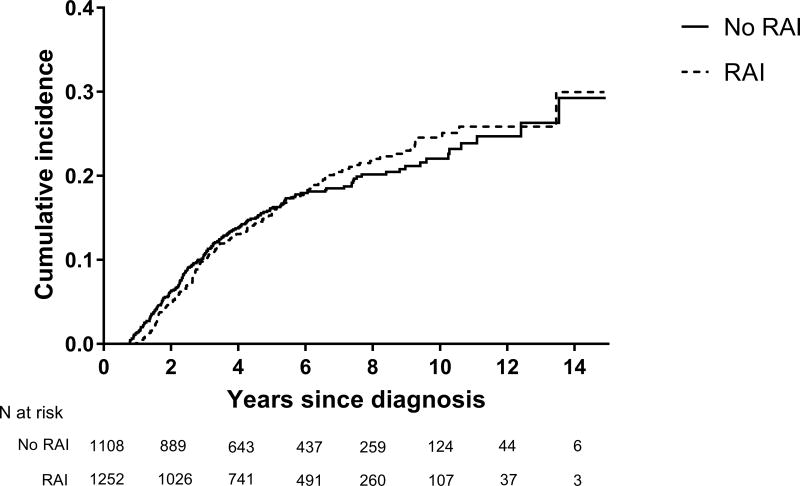
Overall, birth rates were similar between those who did and did not receive RAI (HR=1.00; 95% CI: 0.82, 1.23) (Table 2). The cumulative incidence of birth over a maximum of 14.5 years of follow-up was 30.0% and 29.3% among those who were and were not treated with RAI, respectively (Figure 1). Among those treated with RAI who subsequently had at least one livebirth, the median time from the date of starting RAI to the date of first livebirth was 2.6 years (IQR: 1.67, 4.62). Overall, 43% of the included post-diagnosis births were to women who were nulliparous at treatment. The estimated HR for livebirth for the period ≥ 6 years from start of follow-up (HR=1.31; 95% CI: 0.71, 2.42) was higher than that estimated for <6 years (HR=0.97; 95% CI: 0.78–1.20), although the difference was not statistically significant (p=0.551). Patterns were not sensitive to varying the start of follow-up from 6 to 12 months after diagnosis (data not shown).
Table 2.
Hazard ratios for first birth according to receipt of radioactive iodine treatment (RAI)
| N women | N births | Unadjusted HR | Adjusted HRa | |
|---|---|---|---|---|
| Overall | ||||
|
| ||||
| No RAI | 1108 | 188 | 1 | 1 |
| RAI | 1252 | 218 | 1.02 (0.84, 1.24) | 1.00 (0.82, 1.23) |
|
| ||||
| Age at diagnosis | ||||
|
| ||||
| 15–24 | ||||
| No RAI | 173 | 60 | 1 | 1 |
| RAI | 222 | 51 | 0.62 (0.43, 0.91) | 0.71 (0.48, 1.05) |
| 25–29 | ||||
| No RAI | 230 | 68 | 1 | 1 |
| RAI | 270 | 81 | 1.08 (0.78, 1.49) | 1.06 (0.76, 1.50) |
| 30–34 | ||||
| No RAI | 305 | 40 | 1 | 1 |
| RAI | 341 | 68 | 1.47 (0.99, 2.17) | 1.45 (0.97, 2.15) |
| 35–39 | ||||
| No RAI | 400 | 20 | 1 | 1 |
| RAI | 419 | 18 | 0.85 (0.45, 1.60) | 0.76 (0.39, 1.48) |
|
| ||||
| Stage | ||||
|
| ||||
| Localized | ||||
| No RAI | 908 | 149 | 1 | 1 |
| RAI | 762 | 131 | 1.01 (0.80, 1.28) | 1.01 (0.80, 1.28) |
| Regional/distant | ||||
| No RAI | 178 | 33 | 1 | 1 |
| RAI | 478 | 85 | 1.01 (0.68, 1.52) | 1.00 (0.67, 1.49) |
Adjusted for age at diagnosis and stage
Figure 1.
Cumulative incidence of post-diagnosis birth according to receipt of radioactive iodine treatment (RAI)
Associations between RAI and birth rates appeared to differ by age at thyroid cancer diagnosis (Table 2). Among those ages 15–24 years, the birth rate was non-significantly lower among those who received RAI (HR=0.71; 95% CI: 0.48, 1.05). Birth rates did not differ significantly according to RAI in the 25–29 year age group (HR=1.06; 95% CI: 0.76, 1.50). For those 30–34 years at diagnosis, the birth rate appeared to be higher among those who received RAI than among those who did not (HR=1.45; 95% CI: 0.97, 2.15). Among those ages 35–39, the birth rate was non-significantly lower among those treated with RAI (HR=0.76; 95% CI: 0.39, 1.48), though few births occurred to women in this age group (no RAI: n=20 births; RAI: n=18 births). Associations between RAI and birth rates did not differ according to disease stage.
Discussion
The use of RAI after surgery for differentiated thyroid cancers is controversial, particularly for patients with low-risk, localized disease. While younger women may have concerns about the impact of RAI on future fertility, little evidence exists to counsel patients about reproductive outcomes following therapy. In this population-based study of adolescent and young adult women diagnosed with differentiated thyroid cancer, overall birth rates were similar between women who did and did not receive RAI, suggesting little effect of RAI on subsequent fertility.
Treatment with RAI has been associated with alterations in gonadal function in women, as reflected by transient changes in serum gonadotrophins and menstrual function. In a systematic review of 16 studies of clinical cohorts, Sawka et al. reported that approximately 12–31% of women experience changes in menstrual timing or flow after RAI, with associated elevations in serum FSH and LH.13 Transient amenorrhea occurred among 8–27% of RAI-treated women across included studies. These changes were reportedly more common among women treated in their mid-thirties or older compared to women treated at younger ages. The review authors noted that in general, alterations in menstrual function were expected to resolve within one year of receiving RAI. However, a slightly earlier onset of menopause was also reported for women who were treated with RAI compared to those who were not. Though studies included in this review did not indicate an association between RAI and long-term infertility, the paucity of large scale studies in unselected cohorts leaves uncertainty surrounding the impact of RAI on rates of livebirths among thyroid cancer survivors.
Our overall results suggested that birth rates did not differ appreciably between women treated and not treated with RAI. These findings are largely similar to those in a recent report using data from the California Cancer Registry linked to birth records. For female patients diagnosed with differentiated thyroid cancer between 1999 and 2008, their overall results suggested little difference in birth rates according to receipt of RAI. However, in subgroup analyses, RAI was associated with a significant 29% reduction in birth rate among women ages 35–39 (11.5 vs 16.3 livebirths/1000 person-years, p<0.01).18
Our subgroup analyses suggested a potential reduction in birth rates associated with RAI among those in the youngest (15–24 years) and oldest (35–39 years) age groups at diagnosis, though estimates were relatively imprecise, particularly in the latter group. Conversely, those treated with RAI in the 30–34 year age group had a higher incidence of livebirths than those not treated with RAI. Taken together, our findings and those of the California study do not suggest an adverse effect of RAI on fertility, but rather may reflect characteristics associated with reproductive choice that vary with age and likelihood of receiving RAI, but are not fully captured in administrative data sources.
Strengths of the current study include the population-based cohort with up to 15 years of follow-up for livebirths after a thyroid cancer diagnosis. Some limitations should also be considered. Adjuvant therapies such as radiotherapy may be underreported in cancer registries,20 leading to some misclassification of exposure in our analyses. However, any underreporting would have occurred prior to childbirth; therefore we do not expect radiotherapy misclassification to be differentially related to the primary study outcome. Personal characteristics associated with reproductive choice and childbearing potential were not available in our administrative data sources. Thus we were unable to directly address potential confounding by these factors. Reliable dosing information was also not available for RAI, so we could not evaluate whether birth rates differed according to RAI dosage. However, our stratified analyses did not suggest a lower birth rate associated with RAI among women with regional or distant stage disease, who may be more likely to receive a higher dose. Additionally, we had small numbers of births within strata of age at thyroid cancer diagnosis, leading to imprecise estimates of birth rates in our subgroup analyses.
For younger women with a differentiated thyroid cancer diagnosis, concerns over future reproductive health may influence treatment decisions surrounding RAI. In our analysis of women diagnosed with differentiated thyroid cancer at ages 15–39 years, the proportion of women who had a child after diagnosis did not significantly differ between those treated and not treated with RAI, suggesting little impact of RAI on future reproductive potential.
Novelty and impact.
In young women with differentiated thyroid cancer, treatment with radioactive iodine therapy (RAI) has been associated with alterations in gonadal function. However, few population-based studies have evaluated the impact of RAI on rates of post-diagnosis childbirth among thyroid cancer survivors. In this observational study of 2,360 women with a thyroid cancer diagnosis, childbirth rates did not significantly differ according to receipt of RAI, suggesting little effect of RAI on future reproductive potential.
Acknowledgments
Funding: This research was supported in part by the National Center for Advancing Translational Sciences (KL2-TR001109), and by a Faculty Development Award from the University of North Carolina Office of the Provost
Abbreviations
- RAI
radioactive iodine
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society; 2017. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 3.Patel SS, Goldfarb M. Well-differentiated thyroid carcinoma: the role of post-operative radioactive iodine administration. Journal of surgical oncology. 2013;107:665–72. doi: 10.1002/jso.23295. [DOI] [PubMed] [Google Scholar]
- 4.Nixon IJ, Ganly I, Patel SG, Palmer FL, Di Lorenzo MM, Grewal RK, Larson SM, Tuttle RM, Shaha A, Shah JP. The results of selective use of radioactive iodine on survival and on recurrence in the management of papillary thyroid cancer, based on Memorial Sloan-Kettering Cancer Center risk group stratification. Thyroid : official journal of the American Thyroid Association. 2013;23:683–94. doi: 10.1089/thy.2012.0307. [DOI] [PubMed] [Google Scholar]
- 5.Schvartz C, Bonnetain F, Dabakuyo S, Gauthier M, Cueff A, Fieffe S, Pochart JM, Cochet I, Crevisy E, Dalac A, Papathanassiou D, Toubeau M. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. The Journal of clinical endocrinology and metabolism. 2012;97:1526–35. doi: 10.1210/jc.2011-2512. [DOI] [PubMed] [Google Scholar]
- 6.Goldfarb M, Sener SF. Comparison of radioiodine utilization in adolescent and young adult and older thyroid cancer patients. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2014;20:405–11. doi: 10.4158/EP13343.OR. [DOI] [PubMed] [Google Scholar]
- 7.Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL, Powell CC, van Heerden JA, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World journal of surgery. 2002;26:879–85. doi: 10.1007/s00268-002-6612-1. [DOI] [PubMed] [Google Scholar]
- 8.Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–46. doi: 10.1002/cncr.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawka AM, Thabane L, Parlea L, Ibrahim-Zada I, Tsang RW, Brierley JD, Straus S, Ezzat S, Goldstein DP. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid : official journal of the American Thyroid Association. 2009;19:451–7. doi: 10.1089/thy.2008.0392. [DOI] [PubMed] [Google Scholar]
- 10.Van Nostrand D. The benefits and risks of I-131 therapy in patients with well-differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2009;19:1381–91. doi: 10.1089/thy.2009.1611. [DOI] [PubMed] [Google Scholar]
- 11.Alexander C, Bader JB, Schaefer A, Finke C, Kirsch CM. Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1998;39:1551–4. [PubMed] [Google Scholar]
- 12.Souza Rosario PW, Alvarenga Fagundes T, Villas-Boas Fagundes AS, Barroso AL, Lamego Rezende L, Lanza Padrao E, Guimaraes VC, Horta AC, Franco M, Purisch S. Ovarian function after radioiodine therapy in patients with thyroid cancer. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2005;113:331–3. doi: 10.1055/s-2005-837666. [DOI] [PubMed] [Google Scholar]
- 13.Sawka AM, Lakra DC, Lea J, Alshehri B, Tsang RW, Brierley JD, Straus S, Thabane L, Gafni A, Ezzat S, George SR, Goldstein DP. A systematic review examining the effects of therapeutic radioactive iodine on ovarian function and future pregnancy in female thyroid cancer survivors. Clinical endocrinology. 2008;69:479–90. doi: 10.1111/j.1365-2265.2008.03222.x. [DOI] [PubMed] [Google Scholar]
- 14.Garsi JP, Schlumberger M, Rubino C, Ricard M, Labbe M, Ceccarelli C, Schvartz C, Henri-Amar M, Bardet S, de Vathaire F. Therapeutic administration of 131I for differentiated thyroid cancer: radiation dose to ovaries and outcome of pregnancies. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49:845–52. doi: 10.2967/jnumed.107.046599. [DOI] [PubMed] [Google Scholar]
- 15.Schlumberger M, De Vathaire F, Ceccarelli C, Delisle MJ, Francese C, Couette JE, Pinchera A, Parmentier C. Exposure to radioactive iodine-131 for scintigraphy or therapy does not preclude pregnancy in thyroid cancer patients. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1996;37:606–12. [PubMed] [Google Scholar]
- 16.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 17.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu JX, Young S, Ro K, Li N, Leung AM, Chiu HK, Harari A, Yeh MW. Reproductive outcomes and nononcologic complications after radioactive iodine ablation for well-differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2015;25:133–8. doi: 10.1089/thy.2014.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, National Program of Cancer Registries. Registry Plus Link Plus. Available from: http://www.cdc.gov/cancer/npcr/tools/registryplus/lp.htm.
- 20.He Y, Zaslavsky AM. Combining information from cancer registry and medical records data to improve analyses of adjuvant cancer therapies. Biometrics. 2009;65:946–52. doi: 10.1111/j.1541-0420.2008.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]