Abstract
Stroke represents the first cause of adult acquired disability. Spontaneous recovery, dependent on endogenous neurogenesis, allows for limited recovery in 50% of patients who remain functionally dependent despite physiotherapy. Here, we propose a review of novel drug therapies with strong potential in the clinic. We will also discuss new avenues of stem cell therapy in patients with a cerebral lesion. A promising future for the development of efficient drugs to enhance functional recovery after stroke seems evident. These drugs will have to prove their efficacy also in severely affected patients. The efficacy of stem cell engraftment has been demonstrated but will have to prove its potential in restoring tissue function for the massive brain lesions that are most debilitating. New answers may lay in biomaterials, a steadily growing field. Biomaterials should ideally resemble lesioned brain structures in architecture and must be proven to increase functional reconnections within host tissue before clinical testing.
1. Introduction
Pathologies such as stroke remain chronically debilitating despite scientific advances in the vast field of CNS injury. Following the acute phase, there are no effective treatments available to patients besides physiotherapy.
It is now well known that various mechanisms of brain plasticity occur after stroke onset, both in the acute phase and beyond [1–6]. They may partially account for the spontaneous recovery of motor function [7]. Therefore, drug treatments have increasingly aimed to enhance these processes in order to improve functional recovery [8].
As for tissue repair of the lesioned area, endogenous neurogenesis does not however produce mature neuronal and glial cells in a sufficient number to completely regenerate lesioned CNS tissue [9]. Over the last decades, this observation has led to intense focus on stem cell therapy for the treatment of acute and focal CNS damage produced by pathologies such as stroke, traumatic brain injury, and spinal cord injury (SCI). Transplanted stem cells are expected to (i) exert trophic effects on host tissue by secretion of beneficial factors and/or (ii) actually replace lost tissue and establish functional short- or long-distance connections with host cells. Numerous neural and nonneural stem cell types have shown promise in experimental rodent models of stroke [10, 11] and nonhuman primate (NHP) models of SCI [12]. This preclinical evidence has allowed stem cell delivery to be clinically tested for safety and efficacy in the treatment of stroke [13, 14], TBI [15, 16], and SCI [17]. However, stem cell trials for brain repair have yet to show consistent results respective to efficacy and functional improvement in man [18].
Indeed, when considering stem cell graft within the lesion site, it is important to stress the inhospitable nature of the tissue. Excitotoxicity, inflammatory processes, glial scar formation, growth-inhibiting factors, abnormal tissue structure, and loss of extracellular matrix components render the lesion site unfavorable to neuroblast survival and differentiation [19, 20]. Stem cells grafted close to the brain lesion may die despite immunosuppressant therapy [21].
A promising way to provide endogenous neuroblasts and grafted cells with a suitable microenvironment may consist in the development of biomaterial ECM replacements and “scaffolds” [22]. Biomaterials aiming to mimic the ECM have enhanced tissue reconstruction in models of stroke [23]. They may also be engineered to deliver trophic factors [24] or to guide axonal growth [25]. Implantation of biomaterial has just reached first-in-man clinical testing in the injured spinal cord [26].
Cotransplantation of biomaterial and stem cells has been successfully tested in preclinical studies for the treatment of stroke in the chronic phase in rodents [27, 28]. Although the translation of such therapies to the clinic presents technical challenges, we believe this technology opens up exciting avenues of treatment for focal chronic brain injury.
Here, we propose to review the most recent innovative drug-, stem cell-, and biomaterial-based therapies for the treatment of CNS injuries such as those caused by stroke and SCI.
1.1. Drugs
1.1.1. Drugs for Axon Repair
Central nervous system axons, unlike those in the peripheral nervous system, were long thought to have lost their capacity for regeneration after being sectioned. This concept now seems outdated. Many recent studies have revealed the existence of proteins, such as NOGO, within the myelin sheath that are capable of inhibiting axonal growth and preventing axonal regeneration after a lesion. Drugs targeting these inhibitory proteins, such as anti-NOGOs, have been successfully tested in rodents and primates. Cramer et al. conducted a double-blinded placebo-controlled pilot study of GSK249320, a monoclonal anti-MAG (myelin-associated glycoprotein) antibody, in patients presenting a moderate walking disability after stroke (0.5 m/sec on average 5 days after stroke). The drug was administered 24 h and 9 days after the stroke onset and was well tolerated at the three doses tested (1, 5, or 15 mg/kg, i.v.). Only the 5 mg/kg (n = 9) dose significantly improved walking speed against placebo (n = 17) in a 112-day period, and recovery was particularly marked in the first 60 days [29]. This result suggests that dose and duration of treatment may be further optimized. Experimental testing in animals also showed that early administration within the first week may be more efficient [30]. Unfortunately, a recent large trial on 134 patients was interrupted for lack of efficacy despite the safety of the humanized monoclonal antibody [31]. However, anti-NOGO or other molecules may prove the efficacy of this strategy in the future.
1.1.2. Growth Factors
Growth factors such as G-CSF (granulocyte colony-stimulating factor), known to recruit hematopoietic stem cells, have been considered for use in stroke therapy based on the rationale that they possess such beneficial properties in the acute phase of stroke such as the inhibition of glutamate secretion, reduction of inflammation, and antiapoptotic and antiedema effects, as well as proangiogenesis and neurogenesis properties in the chronic phase [32]. However, no functional improvement was evidenced in a cohort of 548 patients [33]. Similar results were found for other growth factors, such as bFGF (basic fibroblast growth factor or trafermin), known to increase neurite growth. When administered in the acute phase, bFGF caused systemic adverse effects and mortality. The phase II/III trial was interrupted at 286 patients [34]. Another neurotrophic factor, brain-derived neurotrophic factor, was shown to be toxic. Thus, it is not currently feasible to consider the use of such growth factors for therapy after ischemic stroke.
1.1.3. Selective Serotonin Reuptake Inhibitors (SSRIs)
Our team in Toulouse has focused on neuroimaging as a means to develop and adapt biomarker-based therapeutic strategies. We propose candidate biomarkers (1) for the use in motor outcome prediction [35–37] and (2) as therapeutic agents with proven efficacy as evaluated by fMRI [38–44]. Our work, which was confirmed by other teams, has demonstrated that the ipsilesional motor cortex M1 is a key structure of motor recovery and is thus a suitable target for drug-, stem cell-, and noninvasive brain stimulation-based therapies. Functional activations in the primary sensorimotor cortex may be enhanced by the administration of monoaminergic drugs. Drug-induced hyperactivations have been positively correlated with motor improvement, even in unique doses of treatment. However, this result was elicited in small groups of moderately disabled stroke patients, and work must be extended to more severely affected patients, who respond modestly to interventions. Our group demonstrated, in a double-blind placebo-controlled multicentric clinical trial of 118 patients, including heavily affected stroke patients, that fluoxetine (Prozac) treatment significantly improves motor recovery (Fugl-Meyer scale and motor NIHSS) when compared to placebo. Functional improvement was observed, and a higher number of patients regained independence in the treatment group (modified Rankin Score (mRS)) [45]. In a recent study with another SSRI, a similar result was found along with a 50% reduction in the 3-month National Institutes of Health Stroke Scale compared with the baseline scores. This was achieved in 57 patients on the citalopram and 39 patients on the placebo group [46]. Recommendations for the design of clinical drug studies in stroke have been produced [47]. The Cochrane review reported that while SSRIs may improve patient independence, deficit, and neurological status, as well as lessening anxiety and depression, intertrial heterogeneity limits the drawing of meaningful conclusions. Larger clinical trials are needed to validate fluoxetine as stroke treatment before it can be prescribed routinely in the clinic [48] and must confirm the treatment efficacy and determine the optimal dose and length of treatment. To this end, phase III trials have been launched in Australia (http://affinitytrial.org), Sweden (http://www.effects.se), and the United Kingdom (http://focustrial.org.uk) [49] and aim to include 6000 patients, 4530 of which have already been enrolled (FOCUS 3127 (closed), AFFINITY 522, and EFFECT 881). SSRIs induce only minor and well-known adverse effects and are well tolerated in stroke patients. Although clinical evidence of efficacy is pending, the benefit-to-risk ratio seems for now in favor of SSRIs' prescription after ischemic stroke.
When considering the mechanism of action of this antidepressant, it is useful to evoke the historic experiments that first evidenced the concomitant firing of neurons in the raphe nucleus during movement and increased synaptic strength of the sensorimotor synapse and short-term and long-term facilitation, leading Jacobs and Fornal to propose motor facilitation as a primary function of the serotoninergic system [50]. It follows that the benefit of SSRI treatment may be further enhanced by physiotherapy. Furthermore, recent studies have described other biological effects of SSRI drugs such as anti-inflammatory properties through microglial repression and reduction of neutrophil infiltration [51, 52], an increase in BDNF secretion [53], and enhancement of neurogenesis (see the next section) and neural stem cell survival and differentiation [54, 55], even in aged-brain lesioned rats [56]. In line with the neurogenic effect of SSRI, studies have shown that fluoxetine improves declarative memory and increases hippocampal volume in patients suffering from a posttraumatic stress disorder [57, 58].
1.2. Stem Cell Engraftment
Neurogenesis, defined as the capacity of the brain to produce new neurons, has been evidenced in man [59] in neurogenic brain regions, namely, the dentate gyrus of the hippocampus and in the subventricular zone of the cortex. These niches produce stem cells and progenitor cells that are capable of migrating to damaged cortical and/or subcortical brain areas and replacing lost neurons in patients after stroke [1, 9, 60, 61]. However, few neuroblasts survive to reach full neuronal differentiation. Those that do often remain confined to the lesion border and are thus incapable of replacing extensive losses of neuronal tissue. Recent work has shown that as few as 0.2% of lost neurons are replaced [9].
Stem cell-based therapeutic strategies aim to support and/or stimulate endogenous neurogenesis by engraftment of stem cells, most often through intravenous or intracerebral delivery. One benefit of stem cell therapy may be the release of neuroprotective, trophic, or immunomodulatory factors by grafted cells. These so-called trophic effects occur rapidly after engraftment and may stimulate endogenous neurogenesis, angiogenesis, and neovascularization, as well as reducing apoptosis and inflammation [62]. However, for massive brain injury and severely affected patients, trophic effects will unlikely allow sufficient tissue regeneration. In these cases particularly, engraftment of stem cells with a view to not only provide trophic support but also replace damaged neurons and brain tissue could be considered.
The least invasive method of stem cell delivery remains the intravenous method. This procedure is carried out for the delivery of hematopoietic or mesenchymal stem cells. Clinical trials must meet stringent GMP (good manufacturing practice) norms that regulate the quality and safety of cells for engraftment. These regulations dictate all aspects of cell origin, from the composition of cell culture mediums (which should avoid reliance on products of animal origin) to the cell banks from which the cells are selected, which must be genetically stable and homogenous and regularly tested for identity, viability, and sterility.
1.2.1. Mesenchymal Stem Cells
(1) Intravenous Delivery. Mesenchymal stem cells have the advantage of being relatively easy to isolate and amplify from readily accessible tissue samples. In particular, they may be extracted more easily from fat tissue than from bone marrow. Allogenic stem cell transplantation is rendered possible by the fact that these cells do not express the major histocompatibility complex (MHC) antigen. Mesenchymal stem cells can be differentiated into many cell types (chondrocytes, osteoblasts, osteocytes, adipocytes, myocytes, and tendinocytes) and possess capacity for migration toward damaged tissue in the brain [63]. Intravenous administration of adult mesenchymal stem cells has been proven safe thus far [64–66] and potentially efficient. A recent study found that intravenous delivery of multipotent progenitor cells, although well tolerated, did not produce significant improvement [67]. However, the number of patients included (n = 126, intent-to-treat population) may not have provided sufficient statistical power to show modest effects. Clinical trials to evaluate the efficacy of the approach are ongoing. It is likely that any beneficial properties will result from trophic effects, which may reduce neuroinflammation in the acute phase and support the neovascularization within the damaged parenchyma.
(2) Intracerebral Delivery. A recent phase I/2a American trial has demonstrated the safety of an intracerebral graft of mesenchymal stem cells, genetically engineered to transiently express notch-1, a factor known to drive neuronal differentiation [13]. 18 patients with ischemic brain damage (11 of whom were women), with an average age of 61 years, and presenting a stable and chronic motor deficit received the graft between 6 and 20 months after injury and were followed for a year (n = 16). 2.5, 5, or 10 million SB263 cells produced by SanBio were injected into the peri-infarct. Proof-of-concept research showed cell survival 1 month after transplantation in cerebrolesioned animals [13]. One serious adverse event was declared (asymptomatic subdural hematoma). NIHSS neurological scale, European stroke scale, and Fugl-Meyer scale results evidenced significant improvement of recovery in graft recipients. However, for ethical reasons, this study was not controlled by a group of patients receiving a control surgical procedure.
1.2.2. Intraspinal Graft of Olfactory Ensheathing Stem Cells
Autologous engraftment of olfactory ensheathing cells, harvested from the olfactory mucosa of 3 chronic medullar injury patients, produced a quite spectacular improvement in American Spinal Injury Association class (A to B or C) scores in two patients and more local enhancement of motricity and sensitivity in the third patient [17]. Though the mechanisms of action of these cells are far from elucidated, it has been suggested that these “support cells” may reduce glial scar formation, rendering the lesion site more permissive to axonal regeneration.
1.2.3. Intracerebral Graft of Neural Stem Cells
The main challenge in tissue regeneration therapies is not only the replacement of lost neurons but also the establishment of functional reconnections. In this view, selecting a cell source is difficult.
In the first phase 2 randomized clinical trial led by Kondziolka et al., the feasibility of intracerebral stem cell engraftment in 14 stable stroke patients was demonstrated [68, 69]. Although the hNT2 (LBS-Neurons, Layton Bioscience) stem cell line was successfully differentiated into neurons, this line originates from a teratocarcinoma which is no longer authorized for trial in man due to its extremely abnormal karyotype. The study included a small (n = 4) group of control patients, paired for physiotherapy. Six out of eleven PET scans evidenced an improvement of glucose intake at the implantation site (3 injections were performed: above, within, and below the lesion site). Improvement of functional recovery was not significant in the treated group compared to controls. Four treated patients, who presented lesions in the nondominant hemisphere, showed enhanced performance in the figure of Rey test. This suggests improved visuospatial skills and nonverbal memory [70].
A recent phase 1 first-in-man study used the CTX0E03 or ReN001 cell line (ReNeuron) derived from genetically modified embryonic stem cells originating from the human fetal neuroepithelium [14]. In order to control the amplification of cells, they used c-mycERT AM technology to drive expression of an oestradiol receptor under tamoxifen (4-OHT) induction (added to culture medium). Cell division is arrested, and differentiation into neuronal and glial lineages was induced by removal of tamoxifen and growth factors from the medium. It is important to note that the use of tamoxifen for the treatment of breast cancer in women could restart division of the transplanted cells. For this reason, women were excluded from the protocol. Eleven men presenting a moderate-to-severe disability were enrolled for perilesional grafting of 2, 5, 10, or 20 million cells 6 to 60 months after the stroke onset. Patients did not receive any immunosuppressive therapy. Patients were followed for 2 years as part of this noncontrolled trial. No immunological or adverse effects were attributed to the grafted cells. Modest improvements of different motor scales were observed (NIHSS, Barthel index, Ashworth Spasticity Scale for the arm and leg, and a quality of life and health status EuroQoL Five Dimensions questionnaire EQ-5D).
Although the setup of methodologies to control trials with groups of operated-upon but nongrafted patients poses for now unsurmountable technical and ethical difficulties, the true efficacy of stem cell-based interventions cannot be fully validated without this condition and larger patient cohorts. Perilesional injection of cells into healthy tissue is often performed in order to optimize stem cell survival. The rapidly occurring trophic effects of this approach are now well established; however, true functional replacement of lost cells remains to be solidly demonstrated although difficult to test in humans.
While regenerative medicine strategies aim to replace the lesioned neural tissue by intracerebral engraftment, the lesion site microenvironment is unconducive to progenitor survival and differentiation due to the destruction of extracellular matrix (ECM) components which is replaced or isolated by scar tissue [19, 71]. Effectiveness of therapy is limited as only 5% of grafted cells survive. An exciting solution to this problem may be produced by nanotechnology scaffolds.
1.3. Neuro-Implants
Biomaterials may provide a suitable support for cells, replacing the lost extracellular matrix. They may promote cell survival and differentiation, revascularisation, and recolonisation of the lesioned tissue by glial and endothelium cells from the host. More complex biomimetic materials may also guide axonal growth towards their biological targets, restoring effective and even long-distance connections between damaged and healthy tissues. Where stroke is concerned, research in this innovative field remains currently preclinical.
1.3.1. Injectable Nanometric Biomaterials
(1) Nanofibers. Fibrous biomaterials of nanometric dimension were injected in scar tissue in a rat model of medullar lesion. They were composed of peptides that autoassemble to form fibers and contain epitopes of laminin, an ECM component involved in processes such as cell adhesion. Axons of the descending corticospinal tract and those of the ascending sensory neurons that could not previously cross the fibrous glial scar were able to penetrate the biomaterial and cross the lesion. Importantly, motor recovery was significantly enhanced in treated animals [72]. A biodegradable and biocompatible block copolymer of poly-lactic-co-glycolic acid and poly-L-lysine improves functional recovery of rats and nonhuman primates after a partial and complete lateral hemisection of the thoracic spinal cord [73]. INSPIRE, a clinical trial, is ongoing, and the safety of this approach in man has been published in one case [26].
(2) Hydrogels. Polymer hydrogels are another candidate biomaterial for the support of grafted cells. For instance, polyglycolic acid (PGA) is often used as it is porous, biodegradable, and entirely synthetic, meaning its exact composition can be easily controlled. Park et al. included neural stem cells in a soluble hydrogel which then polymerizes within the lesion site [74]. They demonstrated convincing tissue reconstruction in a rodent model of ischemic stroke (middle cerebral artery occlusion (MCAo)) which produces massive lesions. The biomaterial is conducive to neurite growth, and connections were evidenced between the host and grafted cells. Vascularisation and reduction of the glial scar and of monocyte infiltration were also found. This type of approach has shown promising results for sensorimotor and cognitive recovery [75].
1.3.2. Micrometric Injectable Biomaterials
(1) Microbeads. Easily injectable micrometric biomaterial beads have also been developed. When injected in a rat model of Parkinson disease, they improved motricity, decreased striatal lesion volume, and reduced substantia nigra degeneration [76].
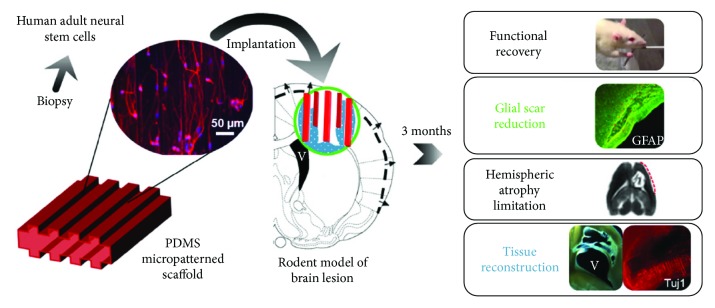
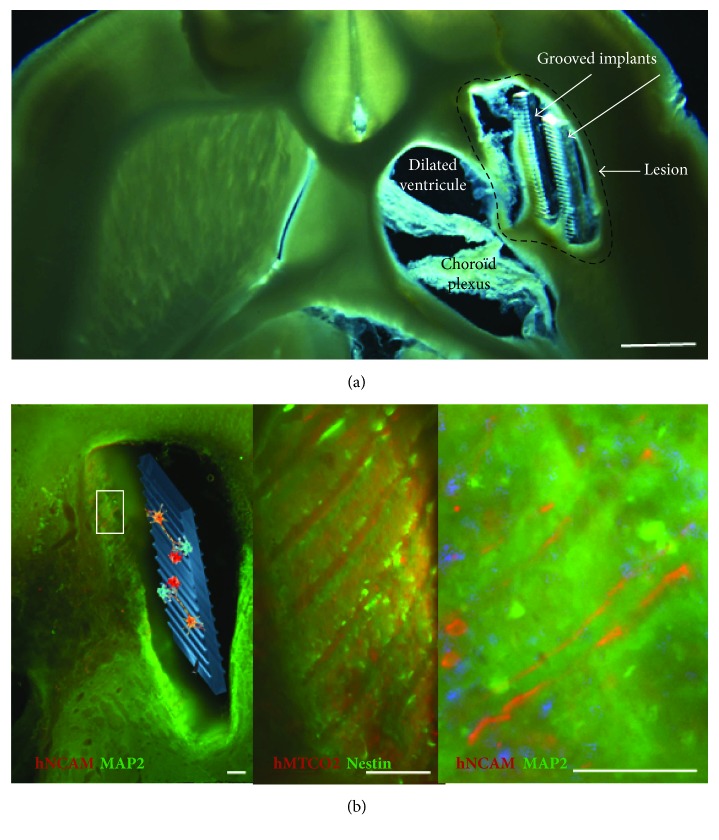
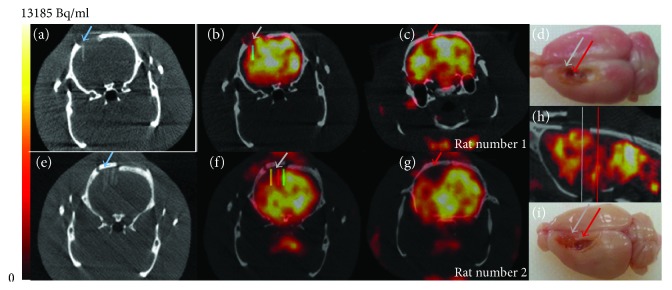
(2) Structured and Guiding Biomaterial Implants. Our team has proposed a strategy for the long-distance bridging of brain regions using biomaterials seeded with neural stem cells, called neuro-implants, in collaboration with LAAS-CNRS (Figure 1). They are made with PDMS (polydimethylsiloxane) and microstructured to guide axonal growth in predefined directions (Figure 2). We have conducted a proof-of-concept study of the efficacy of neuro-implants compared to implants alone in a rat model of corticostriatal lesion impacting the corticospinal tract, which produces loss of forelimb strength and dexterity [77]. The implants did not increase reactive astrogliosis, scarring, or inflammatory responses. They improved the survival of grafted cells, their maturation, and partial tissue reconstruction within the lesion site around the implants. Reconstructed tissue around the neuro-implants was vascularized as assessed by the HMPAO radiotracer perfusion with SPECT imaging (Figure 3). In contrast, lesioned tissue without implants evolved in a cystic cavity (Figure 3, red arrows). The increase in number of surviving grafted cells may also have trophic effects on cerebral plasticity, such as growth factor and anti-inflammatory factor secretion [78].
Figure 1.
Neuro-implant concept. Guiding scaffolds located in the lesion of the corticospinal tract may improve tissue reconstruction and appropriate direction of regenerated tracts.
Figure 2.
Representative horizontal brain section of the lesioned area of rats with implants alone (a) (scale bar: 1 mm) and neuro-implants (b) under brightfield illumination. The newly generated tissue was mostly located around the PDMS implants. (b) Human neural stem cells were identified by a specific human marker hNCAM or hMTCO2, in combination with a marker (in green) of immature (nestin) and mature (MAP2) neurons. Low magnification is provided on the left and higher magnifications on the right (scale bars: 100 μm). Grafted cell neurites were aligned along the grooves of the implant.
Figure 3.
Measurement of cerebral blood flow by nanoSPECT Plus-CT Bioscan with [99mTc]-HMPAO. Fifteen minutes after intravenous injection of 50 MBq of [99mTc]-HMPAO in the tail vein of Sprague-Dawley anesthetized rats, data were acquired during 7 min for SPECT (48 sec and 100000 cps per projection, image size 276 × 276 × 164, 0.1 mm) and 1 min for CT (55 kVp, 500 msec, pitch 0.5, binning 1 : 4). Following the reconstruction, the CT images were spatially aligned to match the SPECT images. Processing of reconstructed images was performed with the in-house Sysiphe software [79]. Brain implants were identified on CT (blue arrows), and 3D volumes of interest (VOIs) were drawn on either side of the implants (colored rectangles) and symmetric ROIs were drawn on the contralateral side as a control (not shown). Images of two rats 20 days after a corticostriatal lesion and 7 days after implantation of neuro-implants. (a, e) CT scan of the brain implants (blue arrows). One implant was inserted in rat number 1 brain and 5 implants in rat number 2 brain. (b, f) SPECT-CT with HMPAO radiotracer on the area of the brain implant. (c, g) SPECT-CT with HMPAO radiotracer on the area of brain damage (located behind the implantation zone). We observed major hypoperfusion (red arrow). The presence of implants limited the hypoperfusion: for rat number 1, −13% in (b) compared to −25% in (c) (ROI volume was 0.4 mm3) and for rat number 2, −18% in (f) compared to −57% in (g) (ROI volume was 1.5 mm3). (h) Sagittal view of rat number 1. Coronal views (b, c) are located with grey and red lines. (d, i) Rat brain perfused and extracted 3 months after the lesion showing the lesion area where neuro-implants were inserted (grey arrows) or not (red arrows).
2. Conclusion
In summary, effective drug therapies are gradually becoming available to improve functional recovery after stroke. However, these will unlikely allow spectacular gains in patients with severe brain damage. Many research teams currently strive to demonstrate the efficacy of stem cell transplantation, which has shown promise in many preclinical models of brain injury. Nonetheless, stem cells alone may not repair the most extensive and debilitating lesions. Much hope has arisen from the development of biomaterial scaffolds, a rapidly growing field of research. These would ideally resemble the architecture of the brain in structure [80] and be proven to allow adequate reconnections with host tissue if possible. If not, given the complexity of this approach, they must at least provide a very high benefit before they can be considered in a clinical setting.
Acknowledgments
The authors thank Carine Pestourie who carried out the rat SPECT/CT experiments (Noninvasive Exploration Service, US006/CREFRE Inserm/UPS/ENVT, Toulouse, France). They also thank Laurence Vaysse who managed the cell culture and immunohistology. This work has been in part supported by a grant from the French National Agency for Research (“Investissements d'Avenir” no. ANR-11-LABEX-0018-01).
Disclosure
The abstract of this paper had been presented in a SOFAMEA (Société Francophone d'Analyse du Mouvement chez l'Enfant et l'Adulte) meeting.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Lindvall O., Kokaia Z. Neurogenesis following stroke affecting the adult brain. Cold Spring Harbor Perspectives in Biology. 2015;7(11, article a019034) doi: 10.1101/cshperspect.a019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy T. H., Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nature Reviews Neuroscience. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 3.Jin K., Wang X., Xie L., et al. Evidence for stroke-induced neurogenesis in the human brain. Proceedings of the National Academy of Sciences. 2006;103(35):13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermann D. M., Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurology. 2012;11(4):369–380. doi: 10.1016/S1474-4422(12)70039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grefkes C., Fink G. R. Connectivity-based approaches in stroke and recovery of function. Lancet Neurology. 2014;13(2):206–216. doi: 10.1016/S1474-4422(13)70264-3. [DOI] [PubMed] [Google Scholar]
- 6.Liu H., Tian T., Qin W., Li K., Yu C. Contrasting evolutionary patterns of functional connectivity in sensorimotor and cognitive regions after stroke. Frontiers in Behavioral Neuroscience. 2016;10:p. 72. doi: 10.3389/fnbeh.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alia C., Spalletti C., Lai S., Panarese A., Micera S., Caleo M. Reducing GABAA-mediated inhibition improves forelimb motor function after focal cortical stroke in mice. Scientific Reports. 2016;6(1, article 37823) doi: 10.1038/srep37823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chollet F., Tardy J., Albucher J.-F., et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurology. 2011;10(2):123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 9.Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature Medicine. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 10.Muñetón-Gómez V. C., Doncel-Pérez E., Fernandez A. P., et al. Neural differentiation of transplanted neural stem cells in a rat model of striatal lacunar infarction: light and electron microscopic observations. Frontiers in Cellular Neuroscience. 2012;6 doi: 10.3389/fncel.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaysse L., Conchou F., Demain B., et al. Strength and fine dexterity recovery profiles after a primary motor cortex insult and effect of a neuronal cell graft. Behavioral Neuroscience. 2015;129(4):423–434. doi: 10.1037/bne0000067. [DOI] [PubMed] [Google Scholar]
- 12.Yamane J., Nakamura M., Iwanami A., et al. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. Journal of Neuroscience Research. 2010;88(7):1394–1405. doi: 10.1002/jnr.22322. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg G. K., Kondziolka D., Wechsler L. R., et al. Clinical outcomes of transplanted modified bone marrow–derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47(7):1817–1824. doi: 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalladka D., Sinden J., Pollock K., et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. The Lancet. 2016;388(10046):787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- 15.Kota D. J., Prabhakara K. S., van Brummen A. J., et al. Propranolol and mesenchymal stromal cells combine to treat traumatic brain injury. Stem Cells Translational Medicine. 2016;5(1):33–44. doi: 10.5966/sctm.2015-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox C. S., Jr., Hetz R. A., Liao G. P., et al. Treatment of severe adult traumatic brain injury using bone marrow mononuclear cells. Stem Cells. 2017;35(4):1065–1079. doi: 10.1002/stem.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabakow P., Jarmundowicz W., Czapiga B., et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplantation. 2013;22(9):1591–1612. doi: 10.3727/096368912X663532. [DOI] [PubMed] [Google Scholar]
- 18.Boncoraglio G. B., Bersano A., Candelise L., Reynolds B. A., Parati E. A. Stem cell transplantation for ischemic stroke. Cochrane Database of Systematic Reviews. 2010;9, article CD007231 doi: 10.1002/14651858.CD007231.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Burda J. E., Sofroniew M. V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coyne T. M., Marcus A. J., Woodbury D., Black I. B. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24(11):2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- 21.Jablonska A., Janowski M., Lukomska B. Different methods of immunosuppresion do not prolong the survival of human cord blood-derived neural stem cells transplanted into focal brain-injured immunocompetent rats. Acta Neurobiologiae Experimentalis. 2013;73(1):88–101. doi: 10.55782/ane-2013-1924. [DOI] [PubMed] [Google Scholar]
- 22.Boisserand L. S. B., Kodama T., Papassin J., et al. Biomaterial applications in cell-based therapy in experimental stroke. Stem Cells International. 2016;2016:14. doi: 10.1155/2016/6810562.6810562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghuman H., Massensini A. R., Donnelly J., et al. ECM hydrogel for the treatment of stroke: characterization of the host cell infiltrate. Biomaterials. 2016;91:166–181. doi: 10.1016/j.biomaterials.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emerich D. F., Silva E., Ali O., et al. Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplantation. 2010;19(9):1063–1071. doi: 10.3727/096368910X498278. [DOI] [PubMed] [Google Scholar]
- 25.Béduer A., Vieu C., Arnauduc F., Sol J.-C., Loubinoux I., Vaysse L. Engineering of adult human neural stem cells differentiation through surface micropatterning. Biomaterials. 2012;33(2):504–514. doi: 10.1016/j.biomaterials.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 26.Theodore N., Hlubek R., Danielson J., et al. First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: a clinical pilot study for safety and feasibility. Neurosurgery. 2016;79(2):E305–E312. doi: 10.1227/NEU.0000000000001283. [DOI] [PubMed] [Google Scholar]
- 27.Bible E., Dell’Acqua F., Solanky B., et al. Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by 19F- and diffusion-MRI. Biomaterials. 2012;33(10):2858–2871. doi: 10.1016/j.biomaterials.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moshayedi P., Nih L. R., Llorente I. L., et al. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials. 2016;105:145–155. doi: 10.1016/j.biomaterials.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cramer S. C., Abila B., Scott N. E., Simeoni M., Enney L. A., on behalf of the MAG111539 Study Investigators Safety, pharmacokinetics, and pharmacodynamics of escalating repeat doses of GSK249320 in patients with stroke. Stroke. 2013;44(5):1337–1342. doi: 10.1161/STROKEAHA.111.674366. [DOI] [PubMed] [Google Scholar]
- 30.Cash D., Easton A. C., Mesquita M., et al. GSK249320, a monoclonal antibody against the axon outgrowth inhibition molecule myelin-associated glycoprotein, improves outcome of rodents with experimental stroke. Journal of Neurology and Experimental Neuroscience. 2016;2(2):28–33. [PMC free article] [PubMed] [Google Scholar]
- 31.Cramer S. C., Enney L. A., Russell C. K., Simeoni M., Thompson T. R. Proof-of-concept randomized trial of the monoclonal antibody GSK249320 versus placebo in stroke patients. Stroke. 2017;48(3):692–698. doi: 10.1161/STROKEAHA.116.014517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abe K., Yamashita T., Takizawa S., Kuroda S., Kinouchi H., Kawahara N. Stem cell therapy for cerebral ischemia: from basic science to clinical applications. Journal of Cerebral Blood Flow & Metabolism. 2012;32(7):1317–1331. doi: 10.1038/jcbfm.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bath P. M., Sprigg N., England T. Colony stimulating factors (including erythropoietin, granulocyte colony stimulating factor and analogues) for stroke. Cochrane Database of Systematic Reviews. 2013;12, article CD005207 doi: 10.1002/14651858.CD005207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogousslavsky J., Victor S. J., Salinas E. O., et al. Fiblast (trafermin) in acute stroke: results of the European-Australian phase II/III safety and efficacy trial. Cerebrovascular Diseases. 2002;14(3-4):239–251. doi: 10.1159/000065683. [DOI] [PubMed] [Google Scholar]
- 35.Loubinoux I. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. NeuroImage. 2003;20(4):2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Tombari D., Loubinoux I., Pariente J., et al. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. NeuroImage. 2004;23(3):827–839. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 37.Loubinoux I., Dechaumont-Palacin S., Castel-Lacanal E., et al. Prognostic value of fMRI in recovery of hand function in subcortical stroke patients. Cerebral Cortex. 2007;17(12):2980–2987. doi: 10.1093/cercor/bhm023. [DOI] [PubMed] [Google Scholar]
- 38.Pariente J., Loubinoux I., Carel C., et al. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Annals of Neurology. 2001;50(6):718–729. doi: 10.1002/ana.1257. [DOI] [PubMed] [Google Scholar]
- 39.Loubinoux I., Pariente J., Rascol O., Celsis P., Chollet F. Selective serotonin reuptake inhibitor paroxetine modulates motor behavior through practice. A double-blind, placebo-controlled, multi-dose study in healthy subjects. Neuropsychologia. 2002;40(11):1815–1821. doi: 10.1016/S0028-3932(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 40.Loubinoux I., Pariente J., Boulanouar K., et al. A single dose of the serotonin neurotransmission agonist paroxetine enhances motor output: double-blind, placebo-controlled, fMRI study in healthy subjects. NeuroImage. 2002;15(1):26–36. doi: 10.1006/nimg.2001.0957. [DOI] [PubMed] [Google Scholar]
- 41.Loubinoux I., Tombari D., Pariente J., et al. Modulation of behavior and cortical motor activity in healthy subjects by a chronic administration of a serotonin enhancer. NeuroImage. 2005;27(2):299–313. doi: 10.1016/j.neuroimage.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 42.Gerdelat-Mas A., Loubinoux I., Tombari D., Rascol O., Chollet F., Simonetta-Moreau M. Chronic administration of selective serotonin reuptake inhibitor (SSRI) paroxetine modulates human motor cortex excitability in healthy subjects. NeuroImage. 2005;27(2):314–322. doi: 10.1016/j.neuroimage.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Loubinoux I., Chollet F. Brain Repair Stroke. Cambridge: Cambridge University Press; 2010. Neuropharmacology in stroke recovery. [DOI] [Google Scholar]
- 44.Tardy J., Pariente J., Leger A., et al. Methylphenidate modulates cerebral post-stroke reorganization. NeuroImage. 2006;33(3):913–922. doi: 10.1016/j.neuroimage.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Chollet F. Fluoxetine and motor recovery after ischaemic stroke – author’s reply. Lancet Neurology. 2011;10(6):500–501. doi: 10.1016/S1474-4422(11)70113-2. [DOI] [PubMed] [Google Scholar]
- 46.Savadi Oskouie D., Sharifipour E., Sadeghi Bazargani H., et al. Efficacy of citalopram on acute ischemic stroke outcome: a randomized clinical trial. Neurorehabilitation and Neural Repair. 2017;31(7):638–647. doi: 10.1177/1545968317704902. [DOI] [PubMed] [Google Scholar]
- 47.Chollet F., Cramer S. C., Stinear C., et al. Pharmacological therapies in post stroke recovery: recommendations for future clinical trials. Journal of Neurology. 2014;261(8):1461–1468. doi: 10.1007/s00415-013-7172-z. [DOI] [PubMed] [Google Scholar]
- 48.Mead G., Hsieh C., Lee R., et al. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database of Systematic Reviews. 2012;11, article CD009286 doi: 10.1002/14651858.CD009286.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mead G., Hackett M. L., Lundström E., Murray V., Hankey G. J., Dennis M. The FOCUS, AFFINITY and EFFECTS trials studying the effect(s) of fluoxetine in patients with a recent stroke: a study protocol for three multicentre randomised controlled trials. Trials. 2015;16(1):p. 369. doi: 10.1186/s13063-015-0864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobs B. L., Fornal C. A. Serotonin and motor activity. Current Opinion in Neurobiology. 1997;7(6):820–825. doi: 10.1016/S0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- 51.Lim C.-M., Kim S.-W., Park J.-Y., Kim C., Yoon S. H., Lee J.-K. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. Journal of Neuroscience Research. 2009;87(4):1037–1045. doi: 10.1002/jnr.21899. [DOI] [PubMed] [Google Scholar]
- 52.Lee J. Y., Lee H. E., Kang S. R., Choi H. Y., Ryu J. H., Yune T. Y. Fluoxetine inhibits transient global ischemia-induced hippocampal neuronal death and memory impairment by preventing blood–brain barrier disruption. Neuropharmacology. 2014;79:161–171. doi: 10.1016/j.neuropharm.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Lee C. H., Park J. H., Yoo K.-Y., et al. Pre- and post-treatments with escitalopram protect against experimental ischemic neuronal damage via regulation of BDNF expression and oxidative stress. Experimental Neurology. 2011;229(2):450–459. doi: 10.1016/j.expneurol.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 54.Li W.-L., Cai H.-H., Wang B., et al. Chronic fluoxetine treatment improves ischemia-induced spatial cognitive deficits through increasing hippocampal neurogenesis after stroke. Journal of Neuroscience Research. 2009;87(1):112–122. doi: 10.1002/jnr.21829. [DOI] [PubMed] [Google Scholar]
- 55.Taguchi N., Nakayama S., Tanaka M. Fluoxetine has neuroprotective effects after cardiac arrest and cardiopulmonary resuscitation in mouse. Resuscitation. 2012;83(5):652–656. doi: 10.1016/j.resuscitation.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Buga A.-M., Ciobanu O., Bădescu G. M., et al. Up-regulation of serotonin receptor 2B mRNA and protein in the peri-infarcted area of aged rats and stroke patients. Oncotarget. 2016;7(14):17415–17430. doi: 10.18632/oncotarget.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santarelli L., Saxe M., Gross C., et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 58.Vermetten E., Vythilingam M., Southwick S. M., Charney D. S., Bremner J. D. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biological Psychiatry. 2003;54(7):693–702. doi: 10.1016/S0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eriksson P. S., Perfilieva E., Björk-Eriksson T., et al. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 60.Chen J., Magavi S. S. P., Macklis J. D. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(46):16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magavi S. S., Leavitt B. R., Macklis J. D. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405(6789):951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 62.Dihne M., Hartung H.-P., Seitz R. J. Restoring neuronal function after stroke by cell replacement: anatomic and functional considerations. Stroke. 2011;42(8):2342–2350. doi: 10.1161/STROKEAHA.111.613422. [DOI] [PubMed] [Google Scholar]
- 63.Li Y., McIntosh K., Chen J., et al. Allogeneic bone marrow stromal cells promote glial–axonal remodeling without immunologic sensitization after stroke in rats. Experimental Neurology. 2006;198(2):313–325. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 64.Lee J. S., Hong J. M., Moon G. J., Lee P. H., Ahn Y. H., Bang O. Y. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 65.Chen L., Zhang G., Khan A. A., Guo X., Gu Y. Clinical efficacy and meta-analysis of stem cell therapies for patients with brain ischemia. Stem Cells International. 2016;2016:8. doi: 10.1155/2016/6129579.6129579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prasad K., Sharma A., Garg A., et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke. Stroke. 2014;45(12):3618–3624. doi: 10.1161/STROKEAHA.114.007028. [DOI] [PubMed] [Google Scholar]
- 67.Hess D. C., Wechsler L. R., Clark W. M., et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurology. 2017;16(5):360–368. doi: 10.1016/S1474-4422(17)30046-7. [DOI] [PubMed] [Google Scholar]
- 68.Kondziolka D., Steinberg G. K., Wechsler L., et al. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. Journal of Neurosurgery. 2005;103(1):38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 69.Kondziolka D., Wechsler L., Goldstein S., et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55(4):565–569. doi: 10.1212/WNL.55.4.565. [DOI] [PubMed] [Google Scholar]
- 70.Stilley C. S., Ryan C. M., Kondziolka D., Bender A., DeCesare S., Wechsler L. Changes in cognitive function after neuronal cell transplantation for basal ganglia stroke. Neurology. 2004;63(7):1320–1322. doi: 10.1212/01.WNL.0000140700.44904.53. [DOI] [PubMed] [Google Scholar]
- 71.Silver J., Miller J. H. Regeneration beyond the glial scar. Nature Reviews Neuroscience. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 72.Tysseling-Mattiace V. M., Sahni V., Niece K. L., et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. The Journal of Neuroscience. 2008;28(14):3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slotkin J. R., Pritchard C. D., Luque B., et al. Biodegradable scaffolds promote tissue remodeling and functional improvement in non-human primates with acute spinal cord injury. Biomaterials. 2017;123:63–76. doi: 10.1016/j.biomaterials.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 74.Park K. I., Teng Y. D., Snyder E. Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nature Biotechnology. 2002;20(11):1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 75.Jin K., Mao X., Xie L., et al. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. Journal of Cerebral Blood Flow & Metabolism. 2010;30(3):534–544. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Delcroix G. J.-R., Garbayo E., Sindji L., et al. The therapeutic potential of human multipotent mesenchymal stromal cells combined with pharmacologically active microcarriers transplanted in hemi-parkinsonian rats. Biomaterials. 2011;32(6):1560–1573. doi: 10.1016/j.biomaterials.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 77.Vaysse L., Beduer A., Sol J. C., Vieu C., Loubinoux I. Micropatterned bioimplant with guided neuronal cells to promote tissue reconstruction and improve functional recovery after primary motor cortex insult. Biomaterials. 2015;58:46–53. doi: 10.1016/j.biomaterials.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 78.Vaysse L., Labie C., Canolle B., et al. Adult human progenitor cells from the temporal lobe: another source of neuronal cells. Brain Injury. 2012;26(13-14):1636–1645. doi: 10.3109/02699052.2012.700084. [DOI] [PubMed] [Google Scholar]
- 79.Tensaouti F., Lotterie J. A. European Society for Magnetic Resonance Medicine and Biology 2008 Congress. Berlin: Springer; 2008. Sysiphe-Neuroimaging software toolbox; pp. 2–4. [Google Scholar]
- 80.Álvarez Z., Castaño O., Castells A. A., et al. Neurogenesis and vascularization of the damaged brain using a lactate-releasing biomimetic scaffold. Biomaterials. 2014;35(17):4769–4781. doi: 10.1016/j.biomaterials.2014.02.051. [DOI] [PubMed] [Google Scholar]