Abstract
As a result of our efforts to identify bioactive agents from marine algae, we have isolated and identified one new halogenated monoterpene 1 [(−)-(5E,7Z)-3,4,8-trichloro-7-dichloromethyl-3-methyl-1,5,7-octatriene] in addition to three known compounds (2, 3 and 4) from the red alga Plocamium cartilagineum collected by hand from the eastern coast of South Africa. Compound 1 was found to be active as a cytotoxic agent in human lung cancer (NCI-H460) and mouse neuro-2a cell lines (IC50 4 μg/ml). Two of these compounds (3 and 4) were found to have cytotoxic activity in other cell line assays, especially against human leukemia and human colon cancers (IC50 1.3 μg/ml). None of these metabolites were active as sodium channel blockers or activators. All structures were determined by spectroscopic methods (UV, IR, LRMS, HRMS, 1D NMR and 2D NMR). 1D and 2D NOE experiments were carried out on these compounds to confirm the geometry of the double bonds.
Keywords: Halogenated monoterpenes, human lung cancers, human colon cancers, human leukemia, Plocamium Cartilagineum
1. Introduction
As a result of their predominant growth in temperate and tropical locations, red algae are among the most frequently investigated sources for marine natural products. They include more than 4000 species distributed in different localities around the world (Gerwick et al., 2001; Rovirosa et al., 2013). Plocamium cartilagineum is a species of red algae (family Plocamiaceae, order Gigartinales). This species is characterized by its interesting secondary metabolites, being a rich source of diverse polyhalogenated monoterpenes, with a surprising degree of halogen incorporation (Young et al., 2013; Inés et al., 2004, Palma et al., 2004, Gao et al., 2001, Norton et al., 1977; Capon et al., 1984; Wright et al., 1990; Mynderse and Faulkner, 1975). Polyhalogenated monoterpenes vary for the given species depending on collection, location and season (Fuller et al., 1992). Research on this genus has yielded a number of halogenated metabolites that display considerable biological activities such as cytotoxic activity (Naylor et al., 1983; Ortega et al., 1997; Wessels et al., 2000; Vogel et al., 2014), anti-feedant activity (Argandona et al., 2002), anti-fungal activity, molluscicidal activity and insecticidal activity (Watanabe et al., 1989). These secondary metabolites can be categorized into two predominant skeletal types, the 2,6-dimethyloctanes and cyclohexanes (Mynderse and Faulkner, 1975; Crews 1977).
2. Results and discussion
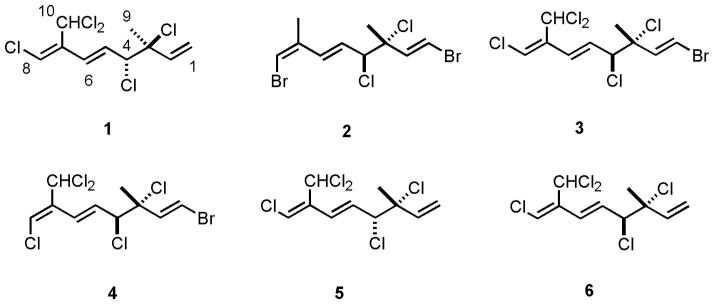
In our continuing efforts using bioassay guided fractionation to characterize the prolific natural products from marine algae, a detailed examination of the crude organic extract of Plocamium cartilagineum from a South African collection was carried out. After extraction of the alcohol-preserved tissue with CH2Cl2/MeOH (2:1), initial fractionation was accomplished by VLC (EtOAc/hexanes gradient) over silica gel. Successive normal phase HPLC fractionations and purifications resulted in the isolation of one new compound (1), in addition to three previously known compounds 2–4 (Mynderse and Faulkner 1975; Crews 1977). (Figure 1)
Figure 1.
Halogenated monoterpenes from P. cartilagineum.
Analysis of different spectroscopic data e.g. UV, IR, LRMS, HRMS, 1D NMR and 2D NMR of the isolated compounds allowed construction of the in planar structures. HMBC and MS fragmentation were used to confirm these statements. 1H-1H coupling constants, 13C NMR, 1D and 2D NOE were used to confirm the double bond geometry.
Compound 1 showed in the LRCIMS the presence of fragment ion clusters [M-Cl]+ (m/z 271, 273, 275, 277), [M-Cl-HCl]+ (m/z 235, 237, 239, 241) and [M-Cl-2HCl]+ (m/z 199, 201, 203). HRCIMS showed an ion at m/z 305.93079 [M]+ for a molecular formula of C10H11Cl5, and therefore possessed three degrees of unsaturation. The IR spectrum of 1 showed absorption bands at 2923 cm−1, indicating the presence of an olefinic group functionality. The 1H NMR spectrum in CDCl3 of 1 (Table S1) showed signals corresponding to one dihalomethylene group at δH 6.96 (1H, s) and six olefinic protons at δH 6.3 (1H, s), 6.35 (1H, dd, J = 17, 7 Hz), 6.34 (1H, dd, J = 17, 2 Hz), 5.41 (1H, d, J = 17 Hz), 5.29 (1H, d, J = 11 Hz) and 6.07 (1H, dd, J = 17, 11 Hz). Additionally there was a doublet of doublet at δH 4.55 (1H, dd, J = 7, 2 Hz), attributed to a mono halomethylene group proton and one methyl group geminal to a halogen atom (δH 1.77, 3H, s). The 13C NMR spectrum of 1 in CDCl3 (Table S1) showed signals for 10 carbons. The numbers of attached hydrogen atoms were determined from the HSQC and HMBC spectra: one methyl at δ 25.1, one methylene, at δ 116.0, six methines (four olefinic at δ140.0, δ 131.0, δ 127.0, δ 119.7 two bearing halogen at δ 69.2 and 66.0), and two nonprotonated carbons at δ138.1 and 72.0 were observed. This was in keeping with the three degrees of unsaturation required by the molecular formula.
Chemical shift arguments and 1H-1H COSY correlations supported by MS data and HMBC allowed the assignment of fragments “a” and “b” (Figure 2). From the 1H-1H COSY NMR spectrum of 1, it was possible to differentiate two discrete spin systems. The coupling between signals corresponding to the olefinic protons at δ 5.41, 5.29 and 6.07 established the connectivity of the H-1-H-2 fragment in a. The coupling between one of the protons bearing halogen (δ 4.55) and the methine protons at δ 6.35 and 6.34 established the connectivity of the H-4 to H6 in fragment b. HMBC data were used to confirm the remainder of fragment b from the correlations between H-4 and C-2, C-3, C-5, C-6. The C-5/C-6/C-7 constellation was determined by the correlation between H-6 and C-5, C-7, and also by the correlations of H-8 with C-10, C-7, and C-10. The C-8/C-7/C-10 linkage was confirmed by the correlation of H-8 with C-7 and C-10. The linkage C-2/C-3 was secured by the correlations between H-1 and C-2, C-3 and suggested the overall planar structure 1.
Figure 2.
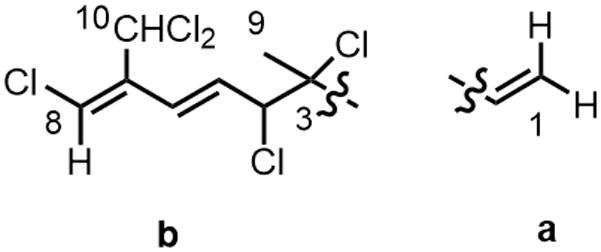
Partial structures of compound 1.
The 5E,7Z stereochemistry of the double bonds were deduced from the 13C NMR chemical shift, 1H-1H coupling constants, 1D NOE and 2D NOE (Table S1). Many trials were carried out to crystallize compound 1 from different solvents at low temperature, but all failed. In order to investigate the stereochemistry of compound 1, we applied the empirical rules of (Mynderse and Faulkner 1975; Crews et al., 1974).
We noticed that the C-3 and C-4 chiral centers of compound 1 must be assigned (3R*, 4S*) according to the previous rules, as C-3 was found to possess δC = 25.1 and δH = 1.77 ppm and this fits the chemical shift range reported in the previous rules for the (3R*, 4S*) stereochemistry of these compounds. The suggested structure of compound 1 was found be not similar to compound 5 (3R*, 4R*) (Table S2). However, the difference in 1H chemical shift and the strong negative sign of the optical rotation of compound 1 ([α]25D = −92.0°) and the positive sign of compound 6 ([α]25D = + 5.1°), strongly supported the suggestion that compound 1 is a diastereoismer to that of the previously reported compound 6 at C-3 and C-4. The 3S*, 4R* relationship at C-3 and C-4 was reinforced by 1D and 2D NOE experiments. Weak NOE effects between H-4 and H3-9 were observed, confirming a relative (R*)-configuration for the C-4 chiral center. Compound 1, which had a negative optical rotation, [α]25D – 92.0 (c 0.07, CHCl3), was thus shown to be a 3,4-erythro compound. However, we can conclude that 1H shifts are not very discriminating as shown by comparing the data of compounds 5, 6 and 1. Hence, the 13C NMR values are more useful for establishing the threo (28 ppm) versus erythro (25 ppm) relationship of substituents at C-3 and C-4 (Crews 1977).
We also isolated from this algal species the previously reported metabolites 2, 3 and 4 (Crews 1977). We carried out a full investigation of the spectroscopic data of these compounds in the course of this work. Our isolation of compounds “2”, “3” and “4” were found to possess the same physical and spectroscopic data as reported in the literature (Mynderse and Faulkner 1975). 1D and 2D NOE experiments on compounds 2, 3 and 4 involving the protons of the methyl group (H-9) at 1.75, 1.77 and 1.78 gave weak enhancements or even no enhancements of the protons at δ 4.59 (H-4), δ 4.53 (H-4) and δ 4.58 (H-4), respectively. This suggested that this methyl group and the proton attached to the chloromethine group at C-4 are directed to the opposite face of the molecule. Our data were found to obey the empirical rules (Crews 1977), but to some extent contradicts (Mynderse and Faulkner 1975) as they mainly depended only on the 1H NMR chemical shifts of H3 -9 beside the sign of the optical rotation to determine the stereochemistry of the chiral centers at C-3 and C-4 of the halogenated monoterpene compounds. For example, of two compounds with H3-9 in the same chemical shift range and with the same sign of optical rotation, one was assigned 3R*, 4S* and the other assigned 3R*, 4R* by (Mynderse and Faulkner). The 13C NMR of compound 2 was at δ 25.6, consistant with an erythro relationship of substituents at C-3 and C-4, and consistant with previous isolates of the compound (Mynderse and Faulkner 1975). Because there were no 13C NMR data published in the previous literature (Mynderse and Faulkner 1975) for compound 3, we recorded and assigned those data here as part of this study (Table S6).
The isolated compounds were evaluated for their biological activity in several systems. Compound 1 was found to have cytotoxic activity (IC50 = 4 μg/ml) to a human lung cancer cell line (NCI-H460) and the mouse neuro-2a neuroblastoma cell line. Upon testing these compounds in a sodium channel modulation assay, none of the compounds were found to have blocking or activating activity (data not shown). Compounds 3 and 4 were found to have cytotoxic activity in human colon cancer (CFU) cell lines (IC50 1.3 μg/ml). None of these metabolites were active as sodium channel blockers or activators (Halogenated monoterpenes have not previously been evaluated for this bioactivity).
3. Experimental
3.1 General Experimental Procedures
Optical rotations were measured on a Perkin-Elmer 141 polarimeter. IR and UV spectra were recorded on Nicolet 510 and Beckman DU640B spectrophotometers, respectively. NMR spectra were recorded on a Bruker DPX400 spectrometer, with the solvent (CDCl3 at δC 77.2, δH 7.26) used as an internal standard. Mass spectra were recorded on a Kratos MS50TC mass spectrometer, and HPLC isolations were performed using Waters Millipore model 515 pumps and a Waters 969 diode array detector.
3.2 Algal Collection
The marine brown alga Plocamium cartilagineum (voucher specimen available from WHG as collection number ZAT-26 -93) was collected intertidally by hand from South Africa eastern coast. The material was stored in 2-propanol at −20 °C until extraction.
3.3 Extraction and Isolation
Approximately 47 g (dry wt.) of the Plocamium cartilagineum was extracted repeatedly with 2:1 CH2Cl2/MeOH to produce 2.8 g of crude organic extract. The crude extract (1.5) g was subjected to Si vacuum liquid chromatography (VLC, hexanes/EtOAc/MeOH) to produce 9 chemically distinct fractions. The fraction eluting with 100% hexanes (1.073 gm) was subjected to normal phase HPLC (0–30% ethyl acetate/hexanes) dual silica, Phenomenex Luna 10u Silica 250×4.6mm to yield 540 mg of compound 3 and 60 mg of compound 4, 2 mg of compound 2 and 4 mg of compound 1.
Compound 1
Colorless oil; [α]25D – 92.0 (c 0.07, CHCl3); UV (CHCl3) max 242 (ε 3623) nm; IR (neat) 2923, 2853, 1459, 1375, 1215, 961, 932, 819, 749, 720 cm−1; 1H NMR and 13C NMR (400 MHz, CDCl3) see Tables S1, S2 and S3; HRCIMS showed an ion at m1z 305.93079 [M]+ for a molecular formula of C10H11Cl5, and therefore possessed three degrees of unsaturation. (Calculated mass is 306 for C10H11Cl5).
Compound 2
Colorless oil; [α]25D – 11.0 (c 0.1, CHCl3) (literature - 4.4 Mynderse and Faulkner 1975). 1H and 13C NMR data see Table S4 and Table S5; with remaining physical and spectroscopic properties identical to those previously reported (Mynderse and Faulkner 1975).
Compound 3
Colorless oil; [α]25D – 26.0 (c 0.1, CHCl3) literature – 22.9 (Mynderse and Faulkner 1975) UV (CHCl3)max 248 (ε 1973) nm; IR (neat) 3086, 2990, 2931, 1617, 1577, 1448, 1379, 1207, 1052, 964, 936, 854, 721cm−1; 1H NMR and 13C NMR data see Table S6 and Table S7; HRCIMS m/z [M]+ 383.8398 (Calculated mass is 384 for for C10H11BrCl5).
Compound 4
Colorless oil; [α]25D – 37.7 (c 0.07, CHCl3) literature – 46.0 (Mynderse and Faulkner 1975) 1H and 13C NMR data see Table S8; with remaining physical and spectroscopic properties identical to those previously reported (Mynderse and Faulkner 1975).
3.4 Cytotoxicity against NCI-H460 human lung cancer and neuro-2a neuroblastoma cell line (Alley et al., 1988)
Cytotoxicity was measured to NCI-H460 human lung tumor cells and mouse neuro-2a blastoma cells using the method of the method of Alley et al. (1988) with cell viability being determined by MTT reduction. Cells were seeded in 96-well plates at 5000 and 8000 cells/well in 180 μl for H460 and neuro-2a cells, respectively. Twenty-four hours later, the test chemical dissolved in DMSO and diluted into medium without fetal bovine serum was added at 20 μg/well. Final DMSO concentration was less than 1% (v/v). After 48 hours, the medium was removed and cell viability determined.
3.5 Sodium channel modulation (Manger and Leja 1995)
Isolated compounds were evaluated for their capacity to either activate or block sodium channels using the following modifications to the cell-based bioassay of Manger and Leja (1995). Twenty-four hours prior to chemical testing, mouse neuro-2a blastoma cells were seeded in 96-well plates at 8 X 104 cells/well in a volume of 200 μl. Test chemicals dissolved in DMSO were serially diluted in medium without fetal bovine serum and added at 10 μl/well. Final DMSO concentration was less than 1% (v/v). Plates to evaluate sodium channel activating activity received 20 μl/well of either a mixture of 3 mM ouabain and 0.3 mM veratridine (Sigma Chemical Co.) in 5 mM HCl in addition to the test chemical. Plates were incubated for 18 hr and results compared to similarly treated solvent controls with 10 μl medium added in lieu of the test chemical., The sodium channel activator brevetoxin PbTx-1 (Calbiochem) was used as the positive control and added at 10 ng/well in 10 μl medium. Sodium channel blocking activity was assessed in a similar manner except that ouabain and veratridine were 5.0 and 0.5 mM, respectively, and the sodium channel blocker saxitoxin (Calbiochem) was used as the positive control. Plates were incubated for approximately 22 hour.
4. Conclusions
One new halogenated monoterpene [(−)-(5E,7Z)-3,4,8-trichloro-7-dichloromethyl-3-methyl-1,5,7-octatriene] in addition to three known compounds were isolated from the red alga Plocamium cartilagineum collected by hand from the eastern coast of South Africa. Structures of these compounds were determined by spectroscopic methods (UV, IR, LRMS, HRMS, 1D NMR and 2D NMR). Compound 1 has cytotoxic activity against human lung cancer (NCI-H460) and mouse neuro-2a cell lines (IC50 4 μg/ml). Compound 3 and compound 4 were found to have cytotoxic activity against human leukemia and human colon cancers (IC50 1.3 μg/ml). None of these metabolites were active as sodium channel blockers or activators (Halogenated monoterpenes have not previously been evaluated for this bioactivity).
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institute of Health (GM 63554 and CA52955)
We gratefully acknowledge Jeff. Moore (Chemistry Department, OSU) for mass spectral data, the Government of South Africa for permission to make these collections. Financial support for this work came from the National Institute of Health (GM 63554 and CA52955).
Footnotes
Supplementary materials relating to this paper are available online.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abreu PM, Galindro JM. A new polyhalogenated epoxymonoterpene from Plocamium cartilagineum. Ind J Chem. 1998;37B:610–611. [Google Scholar]
- Abreu Pedro M, Galindro Jose M. Polyhalogenated Monoterpenes from Plocamium cartilagineum from the Portuguese Coast. J Nat Prod. 1996;59:1159–1162. [Google Scholar]
- Alley MC, Scudiero DA. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Research. 1988;48:589–601. [PubMed] [Google Scholar]
- Argandona VH, Rovirosa J, San-Martin A, Riquelme A, Diaz-Marrero AR, Cueto M, Darias J, Santana O, Guadano A, Gonzalez-Coloma A. Antifeedant Effects of Marine Halogenated Monoterpenes. J Agr Food Chem. 2002;50:7029–7033. doi: 10.1021/jf025857p. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Bowman NJ, Munro MHG, Parsons MJ, Wright GJ, Kon YK. Polyhalogenated Monoterpenes of the New Zealand Marine Red Alga Plocamium cartilagineum. Aust J Chem. 1985;38:519–25. [Google Scholar]
- Burreson BJ, Woolard FX, Moore RE. Evidence for the biogenesis of halogenated myrcenes from the red alga Chondrococcus hornemanni. Chemistry Letters. 1975;11:1111–14. [Google Scholar]
- Capon RJ, Engelhardt LM, Ghisalberti EL, Jefferies PR, Patrick VA, White AH. Structural studies of polyhalogenated monoterpenes from Plocamium species. Aust J Chem. 1984;37:537–544. [Google Scholar]
- Vogel CV, Pietraszkiewicz H, Sabry OM, Gerwick WH, Valeriote FA, Vanderwal CD. Enantioselective Divergent Syntheses of Several Polyhalogenated Plocamium Monoterpenes and Evaluation of Their Selectivity for Solid Tumors. Angewandte Chemie. 2014;126(45):12401–12405. doi: 10.1002/anie.201407726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews P, Kho-Wiseman E, Cartilagineal An Unusual Monoterpene Aldehyde from Marine Alga. J Org Chem. 1974;39:3303–3304. [Google Scholar]
- Crews P, Naylor S, Hanke FJ, Hogue ER, Kho E, Braslau R. Halogen Regiochemistry and Substituent Stereochemistry Determination in Marine Monoterpenes by 13C NMR. J Org Chem. 1984;49:1371–1377. [Google Scholar]
- Crews P, Ourers BL, Naylor S, Clason EL, Jacobs RS, Staal GB. Bio-active monoterpenes from red seaweeds. Phytochem. 1984;23:1449–51. [Google Scholar]
- Crews Phillip. Monoterpene Halogenation by the Red Alga Plocamium oregonum. J Org Chem. 1977;42:2634–6. doi: 10.1021/jo00435a024. [DOI] [PubMed] [Google Scholar]
- Inés CD, Argandoña VH, Rovirosa J, San-Martín A, Díaz-Marrero AR, Cueto M, González-Coloma A. Cytotoxic activity of halogenated monoterpenes from Plocamium cartilagineum. Zeitschrift für Naturforschung C. 2004;59:339–344. doi: 10.1515/znc-2004-5-609. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Cardellina JH, II, Kato Y, Brinen LS, Clardy J, Snader KM, Boyd MR. A Pentahalogenated Monoterpene from the Red Alga Portieria hornemannii Produces a Novel Cytotoxicity Profile against a Diverse Panel of Human Tumor Cell Lines. J Med Chem. 1992;35:3007–3011. doi: 10.1021/jm00094a012. [DOI] [PubMed] [Google Scholar]
- Gao D, Okuda R, Lopez-Avila V. Supercritical fluid extraction of halogenated monoterpenes from the red alga Plocamium cartilagineum. Journal of AOAC International. 2001;84:1313–133. [PubMed] [Google Scholar]
- Gerwick WH, Tan LT, Sitachitta N. Nitrogen-containing metabolites from marine cyanobacteria. Alkaloids. 2001;57:75–184. doi: 10.1016/s0099-9598(01)57003-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez AG, Arteaga JM, Martin JD, Rodriguez ML, Fayos J, Martinez-Ripolls M. Two new polyhalogenated monoterpenes from the red alga Plocamium cartilagineum. Phytochemistrry. 1978;17:947–8. [Google Scholar]
- Higgs MD, Vanderah DJ, Faulkner DJ. Polyhalocenated monoterpenes from Plocamium cartilagineum from the British coast. Tetrahedron. 1977;33:2775–80. [Google Scholar]
- Manger RL, Leja LS. Detection of sodium channel toxins: directed cytotoxicity assays of purified ciguatoxins, brevetoxins, saxitoxins, and seafood extracts. Journal of AOAC International. 1995;78:521–527. [PubMed] [Google Scholar]
- Mynderse JS, Faulkner DJ. Violacene a Polyhalogenated Monocyclic Monoterpene from the Red Alga Plocamium uioluceum. JACS. 1974;96:6771–6772. [Google Scholar]
- Mynderse JS, Faulkner DJ. Polyhalogenated monoterpenes from the red alga Plocamium cartilagineum. Tetrahedron. 1975;31:1963–1967. [Google Scholar]
- Naylor S, Hanke FJ, Manes LV, Crews P. Chemical and biological aspects of marine monoterpenes. Prog Chem Org Nat Prod. 1983;44:189–241. [Google Scholar]
- Norton RS, Warren RG, Wells RJ. Three new polyhalogenated monoterpenes from Plocamium species. Tetrahedron Lett. 1977;18:3905–3908. [Google Scholar]
- Ortega MJ, Zubia E, Salva J. A New Cladiellane Diterpenoid from Eunicella labiata. J Nat Prod. 1997;60:482–484. [Google Scholar]
- Palma R, Edding M, Rovirosa J, San-Martín A, Argandoña VH. Effect of photon flux density and temperature on the production of halogenated monoterpenes by Plocamium cartilagineum (Plocamiaceae, Rhodophyta) Zeitschrift für Naturforschung C. 2004;59:679–683. doi: 10.1515/znc-2004-9-1012. [DOI] [PubMed] [Google Scholar]
- Rovirosa J, Soler A, Blanc V, Leon R, San-Martin A. Bioactive monoterpenes from antarctic Plocamium cartilagineum. Journal of the Chilean Chemical Society. 2013;58:2025–2026. [Google Scholar]
- San-Martin A, Rovirosa J. Variations in the Halogenated Monoterpene Metabolites of Plocamium cartiagineum of the Chilean Coast. Biochem System Ecol. 1986;14:459–61. [Google Scholar]
- Thomas SG, Beveridge AA. The novel marine natural product plocamadiene a causes histamine release from mast cells of the guinea-pig and rat in vitro. Clin Exp Pharm Phys. 1993;20:223–9. doi: 10.1111/j.1440-1681.1993.tb01674.x. [DOI] [PubMed] [Google Scholar]
- Young RM, Von Salm JL, Amsler MO, Lopez-Bautista J, Amsler CD, McClintock JB, Baker BJ. Site-specific variability in the chemical diversity of the Antarctic red alga Plocamium cartilagineum. Marine drugs. 2013;11:2126–2139. doi: 10.3390/md11062126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Miyakado M, Ohno N, Okada A, Yanagi K, Moriguchi K. A polyhalogenated insecticidal monoterpene from the red alga, Plocamium telfairiae. Phytochemisty. 1989;28:77–78. [Google Scholar]
- Wessels Matthias, Koenig Gabriele M, Wright Anthony D. New Natural Product Isolation and Comparison of the Secondary Metabolite Content of Three Distinct Samples of the Sea Hare Aplysia dactylomela from Tenerife. J Nat Prod. 2000;63:920–928. doi: 10.1021/np9905721. [DOI] [PubMed] [Google Scholar]
- Wright AD, Coll JC, Price IR. Tropical marine algae, vii. The chemical composition of marine algae from north queensland waters. J Nat Prod. 1990;53:845–861. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.