Significance
Invertebrates are productive models for understanding basic molecular principles that link cytokine-mediated inflammatory pathways to aging in humans. Here, we use a high-throughput dsRNA screen to determine that the multifunctional Drosophila cytokine, GBP, is a ligand for the hitherto orphan GPCR, Mthl10. Through genetic manipulation of the GBP/Mthl10 axis in larvae and adult flies, we are able to demonstrate how organismal longevity is interconnected to immunological, metabolic, and stress-protective responses. Our results provide a molecular basis for hypothesizing that a successful defense against environmental insults—be they pathogenic inflammation or noninfective challenges—will ultimately reduce lifespan.
Keywords: stress, longevity, receptor
Abstract
A systems-level understanding of cytokine-mediated, intertissue signaling is one of the keys to developing fundamental insight into the links between aging and inflammation. Here, we employed Drosophila, a routine model for analysis of cytokine signaling pathways in higher animals, to identify a receptor for the growth-blocking peptide (GBP) cytokine. Having previously established that the phospholipase C/Ca2+ signaling pathway mediates innate immune responses to GBP, we conducted a dsRNA library screen for genes that modulate Ca2+ mobilization in Drosophila S3 cells. A hitherto orphan G protein coupled receptor, Methuselah-like receptor-10 (Mthl10), was a significant hit. Secondary screening confirmed specific binding of fluorophore-tagged GBP to both S3 cells and recombinant Mthl10-ectodomain. We discovered that the metabolic, immunological, and stress-protecting roles of GBP all interconnect through Mthl10. This we established by Mthl10 knockdown in three fly model systems: in hemocyte-like Drosophila S2 cells, Mthl10 knockdown decreases GBP-mediated innate immune responses; in larvae, Mthl10 knockdown decreases expression of antimicrobial peptides in response to low temperature; in adult flies, Mthl10 knockdown increases mortality rate following infection with Micrococcus luteus and reduces GBP-mediated secretion of insulin-like peptides. We further report that organismal fitness pays a price for the utilization of Mthl10 to integrate all of these various homeostatic attributes of GBP: We found that elevated GBP expression reduces lifespan. Conversely, Mthl10 knockdown extended lifespan. We describe how our data offer opportunities for further molecular interrogation of yin and yang between homeostasis and longevity.
The field of geroscience takes an interdisciplinary, systems-level approach to the study of aging, by integrating the diverse cellular and organismal stress–response pathways that are activated by intrinsic and extrinsic challenges (1). Pivotal to this approach is the characterization of intertissue signaling cascades that induce adaptive and chronic inflammation (1). Such pathways tend to be highly conserved, and so invertebrates have proved to be productive models for pursuing a molecular understanding of links between inflammation and aging in humans (1, 2). Of special interest to the current study is one particular family of cytokines that are distributed through several insect orders (3, 4); while these peptides are multifunctional, the family is commonly designated by the activity of the founding member: growth-blocking peptide (GBP) (5). This eponymous cytokine was identified from its growth-inhibiting effects in the larval stage of the armyworm, Pseudaletia separata, upon parasitization by the wasp Cotesia kariyai (5). In Drosophila there is a 24-residue, biologically active GBP cytokine that is produced by serine protease cleavage of the C terminus of a larger, precursor protein (3) (Fig. S1). Interestingly, Drosophila GBP shares some sequence similarity with human BD2 (Fig. S1), a member of the immunomodulatory β-defensin family (6).
Biological functions of GBP in Drosophila include protection against certain environmental stresses (3), regulation of humoral and cellular innate immune responses (7), and release of insulin-like peptides (ILPs) from the brain in response to nutrient intake (8). However, there has not previously been a molecular rationalization of this cytokine’s multiple homeostatic properties. We hypothesized that identification of a GBP cell-surface receptor could provide a molecular basis for understanding the molecular pathways that GBP regulates, and explain the nature by which the various biological activities of this cytokine might be interrelated. We further posited that genetic manipulation of a GBP receptor might provide a basis for systems-level insight into general relationships between inflammation and aging.
Results and Discussion
Identification of a GBP Receptor by High-Throughput Screening of Ca2+ Mobilization.
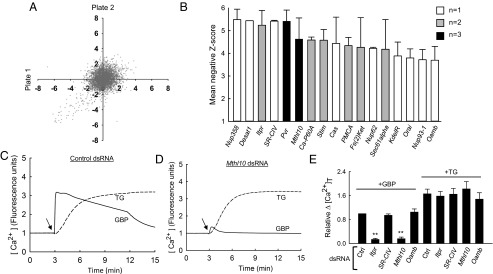
We have previously shown that Drosophila GBP recruits the PLC/Ca2+ signaling pathway to mediate innate immune responses (7, 9). Thus, we conducted a dsRNA library screen for a GBP receptor, using Ca2+ mobilization in Drosophila S3 cells as a biological readout. This screening was facilitated by our creating a Drosophila S3 cell line that hosts a genetically encoded Ca2+ sensor, GCaMP3. These cells (S3GCaMP3) were used to record GBP-stimulated Ca2+ mobilization (7) during screening of a dsRNA library that targets 1,729 genes encoding transmembrane proteins (Fig. 1 A and B and Figs. S2 and S3 and Dataset S1). Each individual dsRNA was screened in replicate plates, generally with good reproducibility (Fig. 1A). We adopted a lenient disambiguation approach: A mean Z score of <−1.5 for any dsRNA pair was considered a hit (Dataset S1). This minimized false negatives, albeit by generating some false positives (see below). The top 17 hits included several genes that encode known Ca2+-signaling proteins (Itpr, Ca-P60A, PMCA, Stim, Orai; Fig. 1B and Fig. S2B) (10, 11), plus four genes encoding cell-surface proteins: SR-CIV (scavenger receptor protein); Pvr (PDGF/VEGF receptor tyrosine kinase); Oamb (octopamine Ca2+ signaling/cAMP receptor), and Mthl10, an orphan GPCR (12). The Mthl10 hit was particularly striking: A high Z score mean of −4.6 was obtained from three separate dsRNA pairs (Fig. 1B and Dataset S1).
Fig. 1.
Application of a dsRNA library to determine that Mthl10 mediates GBP-dependent Ca2+ mobilization in Drosophila S3 cells. (A) Correlation of Z scores from all technical replicates that describe GBP-mediated Ca2+ signaling in S3GCaMP3 cells in the dsRNA library screen. (B) Bar graph depicting the 17 highest Z scores for the indicated genes; the data (mean ± SD) were obtained from the numbers of dsRNA pairs indicated by the key: white, 1; gray, 2; black, 3. (C and D) Secondary screening of Ca2+-signaling dynamics in S3GCaMP3 cells pretreated with control- or Mthl10-dsRNA; arrows show time of addition of either 50 nM GBP or 2 μM TG. (E) Secondary screening of Ca2+ responses to GBP or TG in S3GCaMP3 cells pretreated with either control dsRNA or dsRNA against the indicated genes. All dsRNAs were >80% effective except that for Oamb (60%; qRT-PCR). Bar graphs show total Ca2+ release (i.e., [Ca2+]T) relative to controls (set to unity), calculated by integrating the areas under the Ca2+-mobilization curves (means ± SEM; n = 3–4). **P < 0.01.
We performed secondary screening using independent dsRNAs. The treatment of S3GCaMP3 cells with Mthl10 dsRNA diminished GBP-mediated Ca2+ mobilization by 85% (Fig. 1 C–E). We conducted further experiments with thapsigargin (TG), which, by inhibition of Ca-P60A, exposes Ca2+ leak from the endoplasmic reticulum, thereby promoting Mthl10-independent, Orai-mediated Ca2+ entry into the cell (ref. 10 and Fig. S2B). The results that we obtained (Fig. 1 C–E and Fig. S4) show that Mthl10 dsRNA does not indirectly perturb any aspect of Ca2+ signaling that bypasses the cell-surface GBP receptor.
Mthl10 is one of 12 members of the Drosophila “Mth superclade,” which includes Methuselah (Mth) itself, and 11 Mth-like (Mthl) paralogs, indicative of the possibility of functional redundancy (12, 13). Within this family, ligand-specific functional significance has previously only been ascribed to Mth, which regulates secretion of ILPs in response to the ligand Stunted (14). Nevertheless, in our primary screen, Mthl10 was the only hit from within the Mth superclade (Dataset S1). We also performed follow-up experiments, in which independent dsRNA constructs were used to knock down each member of the Mth superclade in S3GCaMP3 cells (Fig. S5). Only Mthl10 knockdown attenuated GBP-mediated PLC/Ca2+ signaling (Fig. S5). Outside of the Mth superclade, there are four more distantly related paralogs, Mthl1, 5, 14, and 15, which are not viable GBP receptors as they appear not to encode a ligand-binding ectodomain (12). One of these, Mthl5, has been reported to contribute to the development of Drosophila heart tube morphology (15). Nevertheless, knockdown of each of these four paralogs did not inhibit GBP-mediated PLC/Ca2+ signaling (Fig. S5).
In additional secondary screening, knockdown of either SR-CIV or Oamb did not inhibit Ca2+ mobilization (Fig. 1E and Fig. S6 A–C), indicating these genes are false positives in the primary screen (obtained from only one dsRNA pair; Fig. 1B); as stated above, our methodology was expected to yield some false positives. As for loss of Ca2+ signaling following Pvr knockdown in the primary screen (Fig. 1B), that was partly attributed to an “off-target” reduction in cell proliferation (Fig. S5B; see also ref. 16). Additionally, loss of GCaMP3 expression following Pvr knockdown ablated GBP-independent fluorescence responses (Fig. S6D). Secondary screening using Fluo-4 fluorescence (9) showed that equivalent numbers of control and Pvr dsRNA-targeted cells yielded similar GBP-mediated Ca2+ responses (Fig. S6 E and F).
Validation of GBP-Binding to Mthl10.
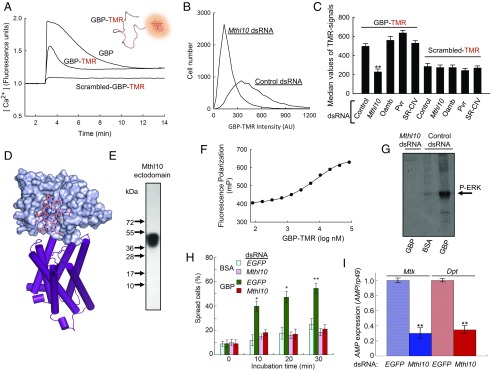
We used orthologous methodology to interrogate the validity of Mthl10 as a hit from our primary screen. S3GCaMP3 cells were incubated with GBP that was C-terminally tagged with tetramethylrhodamine (TMR; Fig. 2A and Fig. S1). GBP-TMR retained the ability to mobilize Ca2+, albeit with reduced potency (Fig. 2A). Analysis by flow cytometry showed that dsRNA-mediated Mthl10 knockdown reduced the intensity of the cell-associated fluorophore signal to the level at which a scrambled-GBP-TMR construct (Fig. S1) nonspecifically bound to S3 cells (Fig. 2 B and C). These data are consistent with Mthl10 being a GBP receptor. Significantly, knockdown of either Pvr, SR-CIV, or Oamb did not affect specific binding of GBP-TMR to S3 cells, consistent with our conclusion that these genes are false positives in the primary screen (Fig. 2C).
Fig. 2.
Mthl10 is a cell-surface receptor for GBP. (A) Representative Ca2+-signaling dynamics in S3GCaMP3 cells upon addition of 200 nM of either GBP, GBP-TMR, or “scrambled-GBP”-TMR. (B) Representative analysis by flow cytometry of GBP-TMR association with S3 cells pretreated with either control- or Mthl10-dsRNA. (C) Analysis of the association of either GBP-TMR or scrambled-GBP-TMR with S3 cells pretreated with the indicated dsRNA (data are means ± SEM; n = 3). (D) Homology model of the extracellular domain of Mthl10 based on Mth (PDB ID code 1FJR), into which is docked GBP (which is also presented in ribbon format in the A Inset). The ribbon structure for the transmembrane domain comes from homology modeling of the β-2 adrenergic receptor (PDB ID code 3SN6). (E) SDS/PAGE of purified recombinant Mthl10 ectodomain. (F) Representative analysis by fluorescence polarization of GBP-TMR binding to the recombinant extracellular domain of Mthl10. (G) Representative Western assay of ERK phosphorylation (“P-ERK”), detected using anti-phospho-ERK1/2 antibody, following treatment of Drosophila S2 cells with either 50 nM GBP or BSA (control) for 3 min. (H) Drosophila S2 cells were pretreated with either Mthl10 dsRNA or control (EGFP) dsRNA, then 50 nM GBP or BSA control was added for the indicated times, and cell spreading was assayed (data are means ± SEM; n = 8). (I) Control- and Mthl10-dsRNA treated S2 cells were incubated with 50 nM GBP for 60 min and AMP expression was determined (data are means ± SEM, n = 7). As indicated, *P < 0.05, **P < 0.01, versus corresponding control.
Since Drosophila Mthl10 and Mth are paralogs (12, 13), we used the crystal structure of Mth (17) as a template to model the Mthl10 ectodomain; the Mthl10 surface is predicted to have a shallow groove, into which we docked GBP (Fig. 2D). This model aided our design of a gene construct for expression of recombinant, epitope-tagged Mthl10 ectodomain, which we purified to apparent homogeneity; the single smeared band around 45 kDa is indicative of glycosylation (Fig. 2E). Specific binding of GBP-TMR to Mthl10 was confirmed by fluorescence polarization (Fig. 2F). The affinity of binding was estimated to be 6 ± 0.07 μM (n = 3; Fig. 2F), the value likely reflecting a reduction in true GBP affinity due to the addition of the TMR tag (Fig. 2A).
Mthl10 Integrates Immunological and Metabolic Functions of GBP.
In Drosophila embryos, larvae, and adults, Mthl10 is expressed in a variety of tissues, including the CNS and the fat body (ref. 13 and Fig. S7A). As a consequence, multiple biological activities of the Mthl10/GBP axis can be anticipated. To assign a specific physiological function for Mthl10 within a single cell type, we knocked down the expression of this receptor in the S2 cell line, a hemocyte-like model (18). In this cell type, GBP regulates both cellular and humoral innate immune activities (7). A key cellular immune response in S2 cells, ERK-dependent cell spreading, was severely attenuated by Mthl10 knockdown (Fig. 2 G and H). Additionally, the GBP-mediated humoral response, i.e., the expression of genes that encode antimicrobial proteins (AMP) such as Metchnikowin (Mtk) and Diptericin (Dpt), was also strongly blocked by Mthl10 knockdown (Fig. 2I).
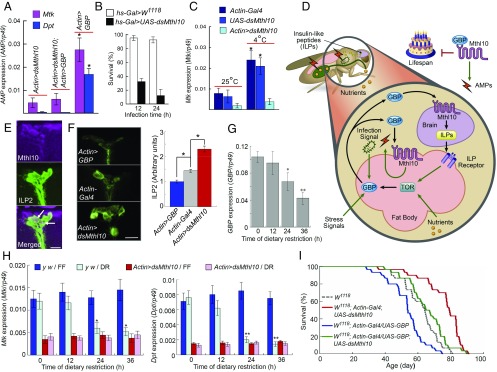
Adult flies exhibit increased expression of Mtk and Dpt in response to GBP overexpression (4). We found that this immunological response is ablated by knockdown of Mthl10 in Drosophila adults (Fig. 3A). These observations suggested to us that loss of Mthl10 might increase susceptibility to infection. Indeed, Mthl10 knockdown dramatically increases mortality rates following infection with the pathogenic bacterium, Micrococcus luteus (Fig. 3B).
Fig. 3.
GBP signaling through Mthl10 ties lifespan to environmental stress. (A) AMP expression (either Mtk or Dpt, as indicated) in female Drosophila adults (data are means ± SEM; n = 8). (B) Fly survival after bacterial infection; in each experiment, 20 male adults were stabbed with a thin tungsten needle previously dipped into a concentrated culture of M. luteus. Values shown are mean ± SEM (n = 13). (C) Mtk expression in Drosophila larvae maintained at either 25 °C, or 4 °C for 16 h (data are means ± SEM; n = 8). *P < 0.05 vs. Actin > dsMthl10. (D) Graphic depicting the participation of the GBP/Mthl10 axis in sensing environmental stress, and the impact upon lifespan. (E) Representative images of Mthl10 (Top) and ILP2 (Middle) in ILP-producing cells of adult female Drosophila. The arrows in the merged image (Bottom) show the overlap of both signals. (Scale bar: 15 μm.) (F) Reporter assay for ILP2 secretion (8). (Left) Representative images of mean ILP2 immunofluorescence in the ILP-producing cells of Drosophila female adult brains. (Scale bar: 15 μm.) (Right) Relative, mean fluorescence intensities quantified by ImageJ (means ± SEM; n = 5; *P < 0.05 vs. Actin-Gal4). (G) GBP expression levels in dietary restricted female Drosophila adult brains (data are means ± SEM; n = 6). *P < 0.05, **P < 0.01, vs. zero time. (H) Expression of Mtk (Left) and Dpt (Right) in dietary restricted (DR) and fully fed (FF) female y w and Mthl10 RNAi strains of Drosophila (data are means ± SEM; n = 8). *P < 0.05, **P < 0.01, vs. zero time. (I) Effect of GBP overexpression and Mthl10 knockdown on lifespans of Drosophila female flies. Lifespan curves of W1118; Actin-Gal4;UAS-dsMthl10 (P = 1.93 × 10−8) and W1118;Actin-Gal4/UAS-GBP (P = 3.43 × 10−4) were significantly different from control (W1118) (log rank test).
We found that many other members of the Mth superclade are expressed in the brain and eviscerated abdomen of Drosophila adults (Fig. S7B). However, none of these paralogs showed off-target changes in their degree of expression following Mthl10 knockdown (Fig. S7B).
GBP has also been shown to promote AMP production in response to noninfectious stress such as low temperature (4). We exposed Drosophila larvae to 4 °C for 16 h, which normally elevates Mtk expression; this adaptive response was substantially attenuated by Mthl10 knockdown (Fig. 3C).
In addition to immunological and stress–protection responses, recent work (8) has described a vital metabolic role for GBP in Drosophila larvae. For example, nutrient status sensing by Drosophila target of rapamycin (TOR) (19) stimulates the fat body to secrete GBP, which releases insulin-like peptides (ILP) from the brain (ref. 8 and Fig. 3D). It is therefore significant that we identified ILP-producing cells within the brain in which both ILP2 and Mthl10 are coexpressed (Fig. 3E). Moreover, Mthl10 knockdown decreased ILP2 secretion, as evidenced (14) by the resulting increase in its cellular levels (Fig. 3F). We further discovered that GBP overexpression promotes ILP2 secretion (Fig. 3F). In control experiments, we found ILP2 expression in the brain was unaffected by either GBP overexpression or by Mthl10 knockdown (Fig. S8).
Overall, our data indicate that a close relationship exists between the immunological and metabolic effects of the GBP/Mthl10 axis (Fig. 3D). This is significant because the mounting of an immune response is bioenergetically expensive (20); it takes a considerable energy investment by innate immune cells to synthesize and secrete a battery of cytokines. Coordinating release of ILP2 during stress can mobilize nutrients to satisfy the energetic demands of increased AMP production.
The GBP/Mthl10 Axis Influences Lifespan.
An intrinsic property of AMP production is its age-dependent up-regulation (21). It is therefore notable that dietary restriction, which is known to extend lifespan in animals (22–24), is associated with reduced GBP expression in Drosophila (ref. 8 and Fig. 3G), which is an antiinflammatory adaptation. Furthermore, GBP-mediated AMP expression is reduced in dietary-restricted, adult flies (Fig. 3H). It is intriguing that we found this response to dietary restriction to be phenocopied by Mthl10 knockdown (Fig. 3H). Moreover, the levels of Mtk and Dpt expression in Mthl10 knockdown flies are equivalent to those observed after 24 h of dietary restriction (Fig. 3H). Conversely, our model (Fig. 3D) predicts that TOR hyperactivation by excess nutrient intake (19) could recruit GBP/Mthl10 to exacerbate metabolic inflammation; this may be one of the reasons that nutrient excess in Drosophila can model human metabolic syndrome (19).
These considerations of some of the potential, negative impacts of GBP/Mthl10 signaling led us to study the lifespan of Mthl10 knockdown flies (Fig. 3I). We found that these flies lived significantly longer than did control W1118 lines; in females, Mthl10 knockdown was associated with a 25% increase in half-survival time compared with control flies. This phenomenon exhibited some sexual dimorphism; the benefit in longevity was less in males (12%; Fig. S9). Interestingly, the well-known impact of dietary restriction upon lifespan is also greatest in female flies (25). Additionally, we found that GBP overexpression significantly shortened lifespan compared with the control lines (16% in females; 10% in males; Fig. 3I and Fig. S9). The GBP-mediated, shorter-lived phenotype was not observed in a strain with simultaneous knockdown of Mthl10 (Fig. 3I). Thus, we conclude that Drosophila lifespan can be shortened by stress-activated, GBP-Mthl10 signaling pathways (Fig. 3D).
Concluding Comments.
The most important development to emerge from this study is the deorphanization of Mthl10, through the placement of this GPCR at the epicenter of a molecular pathway that pits stress responses against lifespan. We show how various immunological and metabolic properties of a single cytokine, GBP, are integrated through its interactions with Mthl10. In particular, we show how the operation of the GBP/Mthl10 axis (Fig. 3D) usefully matches nutrient supply to the degree of a metabolically expensive inflammatory response; this is an important topic in immunology. Our model for GBP/Mthl10 functionality (Fig. 3D) also shows how it has the potential to exacerbate metabolic inflammation; this may be one of the reasons that nutrient excess in Drosophila can model human metabolic syndrome (19). Furthermore, we link these homeostatic functions for Mthl10 to its strong influence upon longevity. This provides a molecular foundation for a theory of aging, namely, that a shortened lifespan can be the ultimate price that a young organism pays to successfully combat short-term environmental stresses (26).
We have also considered our findings in relation to previous work (13) that provides a detailed analysis of the expression pattern of Mthl10 in Drosophila embryos and larvae. For example, due to extensive expression of Mthl10 in imaginal discs, it has been proposed this gene may influence organogenesis (13). It is therefore relevant that cytokines—including the Mthl10 ligand, GBP—are well-known to regulate tissue remodeling and development (27). Additionally, our determination that Mthl10 regulates GBP-mediated innate immune responses (Fig. 2 G and H) seems pertinent to earlier observations (13) that Mthl10 is expressed in hematopoietic tissue (which has immunological functions) and also crystal cells, which encapsulate foreign material. Nevertheless, we cannot exclude the possibility that other ligands for Mthl10 remain to be identified, perhaps as a consequence of the expression of alternate Mthl10 transcripts (13).
The significance of Mthl10 to longevity and metabolism (Fig. 3 D, H, and I and Figs. S8 and S9) is shared by Mth (28, 29). In fact, it was the first gene duplication within the Mth superclade that is believed to have given rise to Mthl10, which did not then undergo any further expansion in Drosophila (12, 13). In contrast, five further rounds of gene duplication apparently occurred before Mth emerged (12, 13). Thus, we conclude that the connection between lifespan and metabolic homeostasis that we observed for Mthl10 is an ancestral trait rather than adaptive specifically to Mth.
It is not unusual for gene regulatory networks to be widely conserved, even when certain components might undergo evolutionary turnover (12). Indeed, recent work (30) has shown that although selection pressure has caused GPCR ectodomains and their ligands to codiversify (e.g., Fig. S1), there has nevertheless been considerable conservation of the receptor’s intracellular interactions with G proteins; as a result, flies and mammals share many of the same downstream signaling cascades (30). Indeed, GBP exhibits some sequence similarity with the human defensin BD2 (Fig. S1); both are small, cationic cytokines produced by protease action upon larger, precursor proteins (6). Furthermore, human BD2 acts through an uncharacterized GPCR to stimulate PLC/Ca2+ signaling to initiate inflammatory responses (31); the current study demonstrates that GBP is also a GPCR ligand that initiates PLC/Ca2+ signaling (7, 9). Thus, we propose that there is general applicability to the concepts that emerge from our integration of immunological, metabolic, and lifespan functions for the GBP/Mthl10 axis.
Materials and Methods
Animals and Cells.
Drosophila melanogaster were normally reared at 25 ± 1 °C on artificial food containing 8.7% (wt/wt) cornmeal, 5.2% (wt/wt) glucose, 3.5% (wt/wt) dried yeast, 0.3% ethyl p-hydroxybenzoate, and 1.0% (wt/wt) agar. Under restricted diet condition, flies were reared on special food containing 5.0% (wt/wt) cornmeal, 5.0% (wt/wt) glucose, 1.0% (wt/wt) dried yeast, 0.3% ethyl p-hydroxybenzoate, and 1.0% (wt/wt) agar (22). The UAS-GBP strain was generated and as described previously (4). The UAS-dsMthl10 strain was supplied by NIG-FLY (National Institute of Genetics). Actin-gal4 and hs-Gal4 strains were derived as described elsewhere (32).
Drosophila S2 cells were supplied by Riken BRC and maintained as previously described (7). Drosophila S3 cells were obtained from Karen Adelman, National Institute of Environmental Health Sciences (NIEHS), Research Triangle Park, NC. To prepare S3GCaMP3 cells, we subcloned a GCaMP3 transcript (obtained from Karen Adelman) into pAc5.1/V5-His A vectors (Invitrogen) using the EcoRI and XbaI restriction sites (for primers, see Dataset S2); the vector was transfected into S3 cells along with the pCoBlast vector, using a Drosophila Expression System (Invitrogen). Both S3 and S3GCaMP3 cells were maintained and utilized at 25 °C in Schneider’s medium (Gibco) supplemented with 10% heat-inactivated FBS (Invitrogen), 100 units/mL penicillin, and 100 μg/mL streptomycin (Gibco); 25 μg/mL blasticidin (Invitrogen) was added to the culture medium for S3GCaMP3 cells.
Primary dsRNA Screen.
The dsRNA library was purchased from Harvard/Howard Hughes Medical Institute Drosophila RNAi Screening Center (https://fgr.hms.harvard.edu/); 0.25 μg/well of dsRNA was used to target each of 1,729 genes that are annotated or computationally predicted to encode transmembrane proteins that it is targeted. The library is provided in duplicate (34 × 384-well plates). The average number of unique dsRNA pairs per gene is two. Each plate is setup with “spare” wells (no added dsRNA), which we utilized for the following controls (Fig. S2): (i) gain of function, using dsRNA against either Tsr or Atx2; (ii) loss of function controls, using dsRNA against Itpr; (iii) bland controls, using dsRNA against an irrelevant gene, LacZ.
Approximately 104 cells were plated in each well of a dsRNA library plate and incubated in 40 μL of culture medium (see above). After 5 d, GBP-induced fluorescence changes were recorded using a FLIPRTETRA (Molecular Devices) at 25 °C. The excitation wavelength was 488 nm. Fluorescence emission was selected with a 510- to 575-nm bandpass filter and monitored simultaneously in all wells of a single plate with a cooled charge-coupled device camera.
Total Ca2+ released ([Ca2+]T) was quantified by integrating the area under each “Ca2+ trace.” Z scores were calculated as the mean value of every unique dsRNA pair. A hit was defined by a Z score of less than −1.5 (the mean value for a single dsRNA, assayed in duplicate); Z score = ([[Ca2+]T of each dsRNA] – [average [Ca2+]T of plate]) / [SD of plate [Ca2+]T]). The list of Z scores for every gene is available at https://fgr.hms.harvard.edu/.
Quantitative Real-Time PCR Analysis.
Total RNA was prepared from either S2 cells, adult Drosophila, or adult Drosophila tissue, as described previously (33). First-strand cDNA was synthesized with oligo(dT)12–18 primer using ReverTra Ace RT-PCR kit (Toyobo), according to the manufacturer’s protocol. Real-time quantitative PCR analysis was carried out by using the Light-Cycler 1.3 instrument and software (Roche Applied Science). PCR specificity was confirmed by sequencing of the PCR products and melting curve analysis at each data point.
For assay of gene expression in S3GCaMP3 cells, total RNA was isolated from either control- or Mthl10-dsRNA treated cells using RNeasy Mini Kit (Qiagen). cDNA was synthesized using SuperScript III First-Strand (Invitrogen) and analyzed by IQ SYBR Green Supermix and IQ5 RT-PCR Detection System (Bio-Rad).
All samples were analyzed in duplicate or triplicate, and assay variation was typically within 10%. Data were normalized according to the expression level of, as indicated, either αTub84D (Tubulin) or rp49, determined in duplicate by reference to a serial dilution calibration curve. All primers are listed in Dataset S2.
Statistics.
For comparison of tested activities of culture cells and gene expression levels of flies or larvae, Tukey’s honest significant difference tests were carried out. The Shapiro–Wilk test, showed that data sets do not deviate from the normality. These statistical analyses were performed using JMP 9.0.2 (SAS Institute). Survival ratio comparison was made with the log rank test, using R version 3.2.2 (34). Statistical analysis of Ca2+-signaling dynamics and GBP-TMR binding was performed with a paired t test. Where representative figures are provided, these are one of at least three biological replicates.
Secondary Screening and Orthologous Methodologies.
All secondary screening procedures are described in SI Materials and Methods.
Mthl10 Ectodomain Expression, Purification, and Analysis of Ligand Binding Using Fluorescence Polarization.
These procedures are described in SI Materials and Methods.
ERK Phosphorylation, Confocal Immunofluorescence, and Cell-Spreading Assays.
These procedures are described in SI Materials and Methods.
Lifespan Determination.
This analysis was performed as described in SI Materials and Methods.
Structural Modeling.
These procedures are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the following for their advice and generosity with their resources: Drs. J. Z. Sexton (from North Carolina Central University), and K. Kim, D. E. Malarkey, G. Travlos, C. D. Bortner, and K. Jeon (from NIEHS). This research was supported by the Intramural Research Program of the NIH, NIEHS, and by Grant-in-Aid for Scientific Research (A) 16H0259 from Japan Society for the Promotion of Science (to Y.H.). The Drosophila RNAi Screening Center at Harvard University has received support from NIH Grant NIGMS R01 GM067761.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712453115/-/DCSupplemental.
References
- 1.Kennedy BK, et al. Geroscience: Linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irazoqui JE, Urbach JM, Ausubel FM. Evolution of host innate defence: Insights from Caenorhabditis elegans and primitive invertebrates. Nat Rev Immunol. 2010;10:47–58. doi: 10.1038/nri2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumoto H, Tsuzuki S, Date-Ito A, Ohnishi A, Hayakawa Y. Characteristics common to a cytokine family spanning five orders of insects. Insect Biochem Mol Biol. 2012;42:446–454. doi: 10.1016/j.ibmb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Tsuzuki S, et al. Drosophila growth-blocking peptide-like factor mediates acute immune reactions during infectious and non-infectious stress. Sci Rep. 2012;2:210. doi: 10.1038/srep00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayakawa Y. Juvenile hormone esterase activity repressive factor in the plasma of parasitized insect larvae. J Biol Chem. 1990;265:10813–10816. [PubMed] [Google Scholar]
- 6.Shafee TM, Lay FT, Phan TK, Anderson MA, Hulett MD. Convergent evolution of defensin sequence, structure and function. Cell Mol Life Sci. 2017;74:663–682. doi: 10.1007/s00018-016-2344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuzuki S, et al. Immunophysiological polarization: Switching between humoral and cellular innate immune responses is guided by Drosophila cytokine dGBP. Nat Commun. 2014 doi: 10.1038/ncomms5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koyama T, Mirth CK. Growth-blocking peptides as nutrition-sensitive signals for insulin secretion and body size regulation. PLoS Biol. 2016;14:e1002392. doi: 10.1371/journal.pbio.1002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, et al. Activation of PLC by an endogenous cytokine (GBP) in Drosophila S3 cells and its application as a model for studying inositol phosphate signalling through ITPK1. Biochem J. 2012;448:273–283. doi: 10.1042/BJ20120730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trebak M, Putney JW., Jr ORAI calcium channels. Physiology (Bethesda) 2017;32:332–342. doi: 10.1152/physiol.00011.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brini M, Carafoli E, Calì T. The plasma membrane calcium pumps: Focus on the role in (neuro)pathology. Biochem Biophys Res Commun. 2017;483:1116–1124. doi: 10.1016/j.bbrc.2016.07.117. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich M, Jones JW. Gene ages, nomenclatures, and functional diversification of the Methuselah/Methuselah-like GPCR family in Drosophila and Tribolium. J Exp Zool B Mol Dev Evol. 2017;326:453–463. doi: 10.1002/jez.b.22721. [DOI] [PubMed] [Google Scholar]
- 13.Patel MV, et al. Dramatic expansion and developmental expression diversification of the methuselah gene family during recent Drosophila evolution. J Exp Zoolog B Mol Dev Evol. 2012;318:368–387. doi: 10.1002/jez.b.22453. [DOI] [PubMed] [Google Scholar]
- 14.Géminard C, Rulifson EJ, Léopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Patel MV, et al. Gia/Mthl5 is an aorta specific GPCR required for Drosophila heart tube morphology and normal pericardial cell positioning. Dev Biol. 2016;414:100–107. doi: 10.1016/j.ydbio.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sims D, Duchek P, Baum B. PDGF/VEGF signaling controls cell size in Drosophila. Genome Biol. 2009;10:R20. doi: 10.1186/gb-2009-10-2-r20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West AP, Jr, Llamas LL, Snow PM, Benzer S, Bjorkman PJ. Crystal structure of the ectodomain of Methuselah, a Drosophila G protein-coupled receptor associated with extended lifespan. Proc Natl Acad Sci USA. 2001;98:3744–3749. doi: 10.1073/pnas.051625298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koppen T, et al. Proteomics analyses of microvesicles released by Drosophila Kc167 and S2 cells. Proteomics. 2011;11:4397–4410. doi: 10.1002/pmic.201000774. [DOI] [PubMed] [Google Scholar]
- 19.Owusu-Ansah E, Perrimon N. Modeling metabolic homeostasis and nutrient sensing in Drosophila: Implications for aging and metabolic diseases. Dis Model Mech. 2014;7:343–350. doi: 10.1242/dmm.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazzaro BP. Adenosine signaling and the energetic costs of induced immunity. PLoS Biol. 2015;13:e1002136. doi: 10.1371/journal.pbio.1002136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kounatidis I, et al. NF-κB immunity in the brain determines fly lifespan in healthy aging and age-related neurodegeneration. Cell Rep. 2017;19:836–848. doi: 10.1016/j.celrep.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bass TM, et al. Optimization of dietary restriction protocols in Drosophila. J Gerontol A Biol Sci Med Sci. 2007;62:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatar M, Post S, Yu K. Nutrient control of Drosophila longevity. Trends Endocrinol Metab. 2014;25:509–517. doi: 10.1016/j.tem.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magwere T, Chapman T, Partridge L. Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2004;59:3–9. doi: 10.1093/gerona/59.1.b3. [DOI] [PubMed] [Google Scholar]
- 26.Gladyshev VN. Aging: Progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell. 2016;15:594–602. doi: 10.1111/acel.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayakawa Y. Insect cytokine growth-blocking peptide (GBP) regulates insect development. Appl Entomol Zool (Jpn) 2006;41:545–554. [Google Scholar]
- 28.Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 29.Delanoue R, et al. Drosophila insulin release is triggered by adipose Stunted ligand to brain Methuselah receptor. Science. 2016;353:1553–1556. doi: 10.1126/science.aaf8430. [DOI] [PubMed] [Google Scholar]
- 30.Flock T, et al. Selectivity determinants of GPCR-G-protein binding. Nature. 2017;545:317–322. doi: 10.1038/nature22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niyonsaba F, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 32.Takehana A, et al. Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J. 2004;23:4690–4700. doi: 10.1038/sj.emboj.7600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ninomiya Y, Kurakake M, Oda Y, Tsuzuki S, Hayakawa Y. Insect cytokine growth-blocking peptide signaling cascades regulate two separate groups of target genes. FEBS J. 2008;275:894–902. doi: 10.1111/j.1742-4658.2008.06252.x. [DOI] [PubMed] [Google Scholar]
- 34.Linford NJ, Ro J, Chung BY, Pletcher SD. Gustatory and metabolic perception of nutrient stress in Drosophila. Proc Natl Acad Sci USA. 2015;112:2587–2592. doi: 10.1073/pnas.1401501112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Y, Jasper H. Studying aging in Drosophila. Methods. 2014;68:129–133. doi: 10.1016/j.ymeth.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yi NY, et al. Development of a cell-based fluorescence polarization biosensor using preproinsulin to identify compounds that alter insulin granule dynamics. Assay Drug Dev Technol. 2015;13:558–569. doi: 10.1089/adt.2015.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto Y, Oda Y, Uryu M, Hayakawa Y. Insect cytokine growth-blocking peptide triggers a termination system of cellular immunity by inducing its binding protein. J Biol Chem. 2003;278:38579–38585. doi: 10.1074/jbc.M305986200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.