Significance
How autoreactive CD4+ T cells recognize their target antigen and induce sustained inflammation in organ-specific autoimmune diseases is incompletely understood. In an experimental model of multiple sclerosis, we show that accumulation of myelin-specific CD4+ T cells within the CNS and subsequent clinical disease development requires autophagy protein (ATG)-dependent phagocytosis in dendritic cells (DCs). Absence of ATG-dependent phagocytosis in DCs abrogates myelin presentation to CD4+ T cells following phagocytosis of oligodendroglial cells, and its pharmacological inhibition delays the onset and reduces the clinical severity of experimental autoimmune encephalomyelitis. Thus, DCs use ATG-dependent phagocytosis for enhanced presentation of myelin antigen during autoimmune CNS inflammation, thereby linking oligodendrocyte injury with antigen processing and autoimmune T cell pathogenicity.
Keywords: autophagy, neuroinflammation, EAE, multiple sclerosis
Abstract
Although reactivation and accumulation of autoreactive CD4+ T cells within the CNS are considered to play a key role in the pathogenesis of multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE), the mechanisms of how these cells recognize their target organ and induce sustained inflammation are incompletely understood. Here, we report that mice with conditional deletion of the essential autophagy protein ATG5 in classical dendritic cells (DCs), which are present at low frequencies in the nondiseased CNS, are completely resistant to EAE development following adoptive transfer of myelin-specific T cells and show substantially reduced in situ CD4+ T cell accumulation during the effector phase of the disease. Endogenous myelin peptide presentation to CD4+ T cells following phagocytosis of injured, phosphatidylserine-exposing oligodendroglial cells is abrogated in the absence of ATG5. Pharmacological inhibition of ATG-dependent phagocytosis by the cardiac glycoside neriifolin, an inhibitor of the Na+, K+-ATPase, delays the onset and reduces the clinical severity of EAE induced by myelin-specific CD4+ T cells. These findings link phagocytosis of injured oligodendrocytes, a pathological hallmark of MS lesions and during EAE, with myelin antigen processing and T cell pathogenicity, and identify ATG-dependent phagocytosis in DCs as a key regulator in driving autoimmune CD4+ T cell-mediated CNS damage.
Multiple sclerosis (MS) is considered to be an antigen-driven, predominantly T cell-mediated autoimmune disease of the CNS. Genome-wide association studies confirmed that HLA class II haplotypes, in particular HLA-DRB1*1501/HLA-DRB5*0101, are the strongest genetic risk factors for MS development (1, 2), a substantial fraction of T cells isolated from CNS lesional tissue and the cerebrospinal fluid from MS patients are derived from clonal expansion (3–5), and inflammatory demyelination in experimental autoimmune encephalomyelitis (EAE), an animal model for MS, is dependent on CD4+ T cells that react to myelin antigen (6, 7).
Before infiltrating the CNS, autoreactive CD4+ T cells are primed in the peripheral immune system. Priming can be targeted to skin draining lymph nodes after s.c. immunization with myelin antigen and complete Freund’s adjuvant (CFA) during active EAE induction. In MS, the site where autoreactive T cells are primed is not known, but the human disease is usually not elicited by vaccinations and persists in the absence of any systemic inflammatory challenges. Local reactivation and sustained accumulation of autoreactive T cells within the CNS are, therefore, considered instrumental in both MS and EAE. This effector phase can be modeled by adoptive transfer of primed myelin-specific CD4+ T cells into naïve mice (adoptive transfer EAE; AT-EAE) and depends on the presence of CD11c+ antigen-presenting cells (APCs) (8, 9). How myelin-reactive CD4+ T cells recognize their target organ and become reactivated to induce sustained CNS tissue damage are, however, incompletely understood.
The autophagic machinery delivers cytoplasmic cargo and substrates for MHC class II presentation to late endosomes and lysosomes (10, 11). In addition, autophagy proteins regulate degradation of extracellular material via the noncanonical use of autophagy-related proteins (ATGs) during ATG8/microtubule-associated protein 1A/1B light chain 3 (LC3)-associated phagocytosis (LAP) (12–18). Here, we report that DCs use ATG-dependent phagocytosis for enhanced presentation of myelin antigen during autoimmune CNS inflammation, thereby linking oligodendrocyte injury with antigen processing and autoimmune T cell pathogenicity.
Results
Primed, Encephalitogenic CD4+ T Cells Require ATG5 Expression by DCs to Induce EAE.
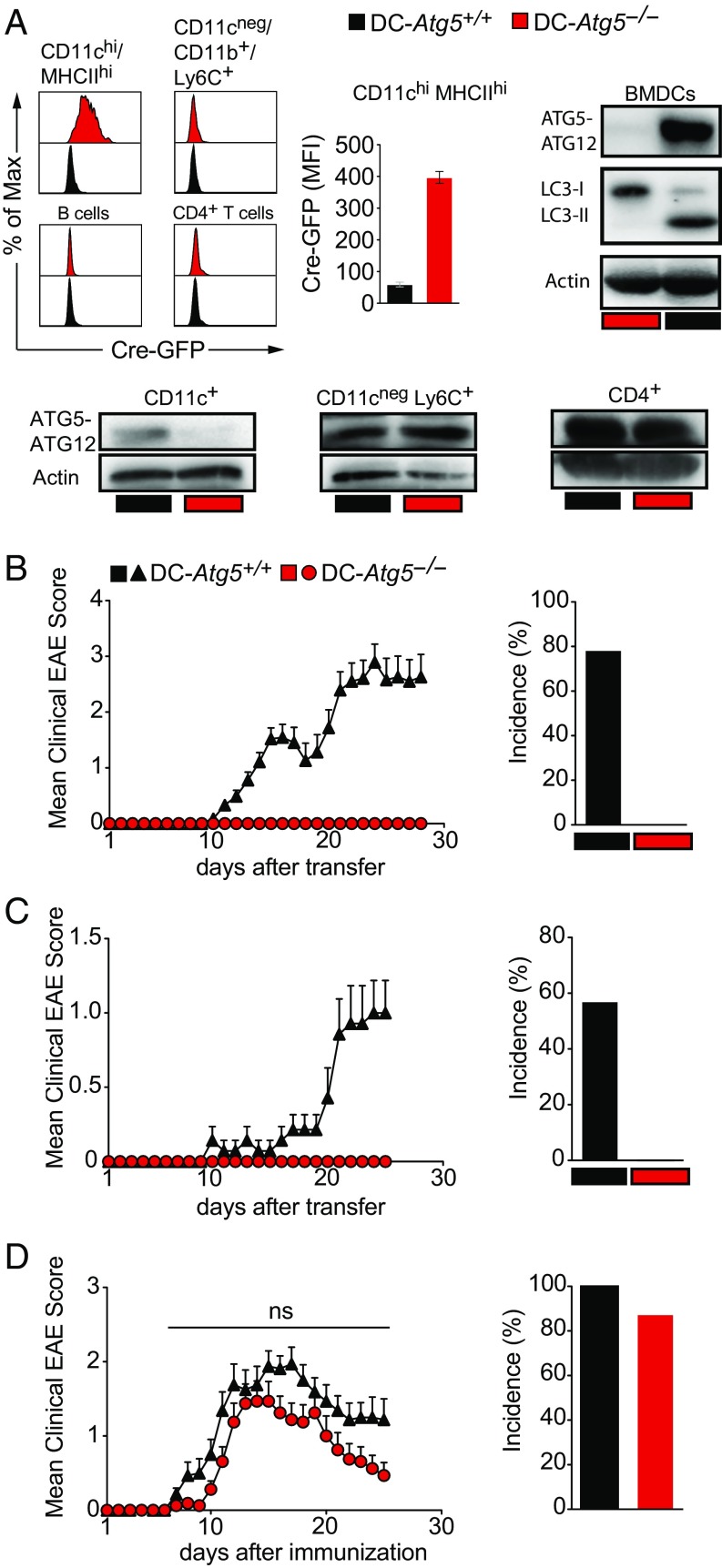
To address whether the autophagy machinery modulates EAE development, we generated conditional knockout mice (C57BL/6) for disruption of Atg5 in CD11c+ APCs (Atg5flox/flox × CD11c-Cre-GFP, designated DC-Atg5−/−) (Fig. 1A). Absence of ATG5 in CD11c+ cells led to complete protection from EAE development upon adoptive transfer of T cell receptor-transgenic (TCR-tg) MOG35–55–specific CD4+ T cells derived from 2D2/TCRMOG animals (Fig. 1B). Protection was also observed after adoptively transferring non–TCR-tg, polyclonal encephalitogenic CD4+ T cells obtained from MOG35–55–immunized C57BL/6 mice (Fig. 1C). In contrast, absence of ATG5 in CD11c+ cells resulted in only minor and not statistically significant differences in incidence rates and clinical severity grades in DC-Atg5−/− mice upon active immunization (Fig. 1D). CD11c-Cre-GFP mice were fully susceptible to EAE development (Fig. S1A).
Fig. 1.
DC-Atg5−/− mice are resistant to EAE induced by primed, myelin-specific T cells. (A) Representative histograms depicting Cre-GFP expression of either CD11c+MHCII+ splenic DCs (cDCs), CD11cnegCD11b+Ly6C+ monocytes, B cells, or CD4+ T cells in DC-Atg5+/+ (black) or DC-Atg5−/− (red) mice in steady state (Upper Left). Quantification of Cre-GFP median fluorescence intensity (MFI; DC-Atg5+/+, n = 7; DC-Atg5−/−, n = 9) (Upper Middle). Western blot analysis for protein expression of the ATG5–ATG12 complex, LC3-I, and LC3-II in CD11c+ BMDCs (Upper Right) and protein expression of the ATG5–ATG12 complex in primary splenic cell populations CD11c+, CD11cnegLy6C+, and CD4+ (Lower) derived from DC-Atg5+/+ or DC-Atg5−/− mice is depicted. Actin served as a loading control. One representative of two experiments is shown. (B) EAE was induced via adoptively transferring 2D2/TCRMOG-derived encephalitogenic CD4+ T cells into DC-Atg5+/+ (black triangles) or DC-Atg5−/− mice (red circles). Each data point represents the mean of 44 or more animals. Pooled data of eight independent experiments are shown (Left). Quantification of disease incidence is shown (Right). (C) EAE was induced via adoptively transferring C57BL/6 wild type-derived encephalitogenic CD4+ T cells into DC-Atg5+/+ or DC-Atg5−/− mice. Each data point represents the mean of seven animals (Left). One representative of two independent experiments is shown. Quantification of disease incidence is shown (Right). (D) EAE was induced via active immunization with MOG35–55 peptide in DC-Atg5+/+ or DC-Atg5−/− mice. Each data point represents the mean of 15 or more animals. Pooled data of three independent experiments are shown (Left). Quantification of disease incidence is shown (Right). Statistical analysis: Mean ± SEM is depicted. Two-way ANOVA (D, Left) was applied. ns, not significant: P > 0.05.
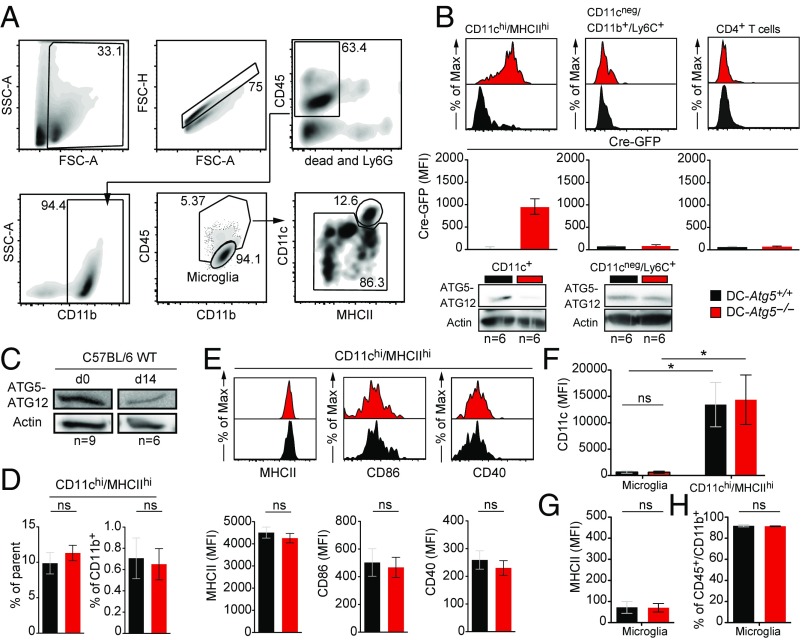
Since loss of ATG5 in CD11c+ cells completely prevented the development of AT-EAE, we next profiled CD11c promoter-driven Cre-GFP expression in MHC class II+ CNS-resident APCs before EAE induction in naïve mice. A minor population of CNS CD11b+ myeloid cells coexpressed high levels of CD11c and MHC class II, indicative of classical DCs (cDCs), and was efficiently targeted by Cre-mediated recombination (Fig. 2 A and B). By comparison, Cre-GFP expression in CD11cintMHC class IIhi cells derived from DC-Atg5−/− mice was minor and not statistically different from DC-Atg5+/+ mice (Fig. S1B). CD11cneg myeloid populations such as CD11cnegLy6Chi monocytes, precursors of CNS-invading monocyte-derived DCs and required for the initiation of tissue inflammation in actively induced EAE (19–23), were not targeted by Cre-mediated recombination (Fig. 2B), indicating that site-specific deletion of Atg5 was confined to CD11c+MHC class II+ DCs. However, we observed that up-regulation of MHC class II in DC-Atg5−/− bone marrow-derived myeloid cells upon GM-CSF incubation precedes Cre-mediated recombination and loss of ATG5 (Fig. S1C), suggesting a delayed targeting of ATG5 in monocyte-derived DCs in DC-Atg5−/− mice.
Fig. 2.
CD11c+ Cre-GFP–expressing DCs in the CNS before EAE induction. (A) Gating strategy for flow cytometry analysis in the CNS of naïve DC-Atg5+/+ and DC-Atg5−/− mice. First, single leukocytes were defined by applying the respective gates (leukocytes: SSC-A vs. FSC-A; single cells: FSC-H vs. FSC-A). Next, CD45+ cells were gated on, while excluding Ly6G+ neutrophils and dead cells. After gating on CD11b+ myeloid cells, microglia were defined as CD45loCD11b+. Inside the CD45hiCD11b+ cell gate, it was further gated on CD11chiMHCIIhi cells. Within the remainder of the cells it was subgated on CD11cnegCD11b+Ly6C+ monocytes (not shown). (B) Representative histograms (Upper) depicting Cre-GFP expression of either CNS-resident CD11chiMHCIIhi cells (Upper Left) or CNS-derived CD11cnegCD11b+Ly6C+ monocytes (Upper Middle) in naïve mice. Representative histogram depicting Cre-GFP expression in CNS-infiltrating CD4+ T cells at the peak of disease (day 21 ± 1) (Upper Right). Quantification of Cre-GFP MFI is depicted (Middle). Western blot analysis for protein expression of the ATG5–ATG12 complex in CNS-derived CD11c+ cells (Lower Left) and CD11cnegLy6C+ cells (Lower Right) (DC-Atg5+/+, n = 6; DC-Atg5−/−, n = 6). Actin served as a loading control. (C) Western blot analysis for protein expression of the ATG5–ATG12 complex in CNS-derived CD11c+ cells of C57BL/6 wild-type (WT) mice before induction of adoptive transfer EAE (n = 9) or on day 14 after induction of adoptive transfer EAE (n = 6). Actin served as a loading control. (D) Quantified frequencies (percentage of parent, Left; percentage of CD11b+ cells, Right) of CNS-resident CD11chiMHCIIhi cells in naïve DC-Atg5+/+ and DC-Atg5−/− mice. (E) Representative histograms (Upper) depicting either MHCII (Left), CD86 (Middle), or CD40 (Right) MFI of CNS-resident CD11chiMHCIIhi cells. Quantification of MFI values is shown (Lower). (F) Microglia versus CD11chiMHCIIhi cell phenotypes in DC-Atg5−/− mice before EAE induction. CD45lo/intCD11b+ microglial cells do not express CD11c to a substantial level at steady state compared with CD11chiMHCIIhi CNS cells. (G) Surface expression levels of MHCII are similar on microglia when comparing DC-Atg5+/+ and DC-Atg5−/− mice. (H) Frequencies of CD45lo/intCD11b+ microglial cells are unchanged in DC-Atg5−/− mice. Pooled data of two independent experiments are shown (DC-Atg5+/+, n = 5 and DC-Atg5−/−, n = 5 for naïve myeloid compartments; DC-Atg5+/+, n = 10 and DC-Atg5−/−, n = 12 for peak of disease CD4+ T cell analysis). Statistical analysis: Mean ± SEM is depicted. Unpaired two-tailed Student t test was applied. ns, not significant: P > 0.05; *P < 0.05.
In C57BL/6 wild-type mice, ATG5 protein expression was detectable in CNS-derived CD11c+ cells in naïve mice as well as after induction of AT-EAE (Fig. 2C). In DC-Atg5−/− mice, frequencies of CD11chiMHC class IIhi CNS DCs were similar compared with DC-Atg5+/+ littermates (Fig. 2D). Moreover, ATG5-deficient CNS-derived CD11chiMHC class IIhi DCs did not differ from their ATG5-competent counterparts in expression levels of MHC class II, CD40 and CD86 (Fig. 2E). Microglial cells, in which expression of CD11c and MHC class II can be induced upon activation, did not express CD11c, exhibited no detectable levels of MHC class II molecules (Fig. 2 F and G), and were observed at similar frequencies in naïve mice when comparing DC-Atg5−/− with DC-Atg5+/+ (Fig. 2H). Furthermore, frequencies of splenic DC and monocyte subsets, CD4+ T cells, CD8+ T cells, and CD4+ Foxp3+ regulatory T cells and B cells (Fig. S2A), MHC class II and costimulatory molecule expression on splenic cDCs (Fig. S2B), and CD4+ T cell inflammatory cytokine expression (IFNγ, IL-17, and GM-CSF) upon activation were unchanged in DC-Atg5−/− mice compared with their DC-Atg5+/+ control littermates (Fig. S2C). Using the identical CD11c-Cre and Atg5flox/flox strains for DC-specific deletion of Atg5, Lee et al. (11) previously demonstrated that Atg5-deficient DCs show intact migratory capacities, similar expression levels of MHC class II, CD40, and CD86 in the steady state, and upon immune activation along with similar secretion levels of IL-12p40, IL-6, and TNF-α.
Thus, a minor population of CD11chiMHC class IIhi DCs within the nondiseased CNS is targeted by Cre-mediated Atg5 deletion in DC-Atg5−/− mice. ATG5 deficiency does not affect their frequency or expression levels of MHC class II and costimulatory molecules, but prevents EAE development induced by primed, myelin-specific CD4+ T cells.
ATG5 in DCs Does Not Impair Priming of Myelin-Specific CD4+ T Cells but Regulates Their Accumulation Within the CNS.
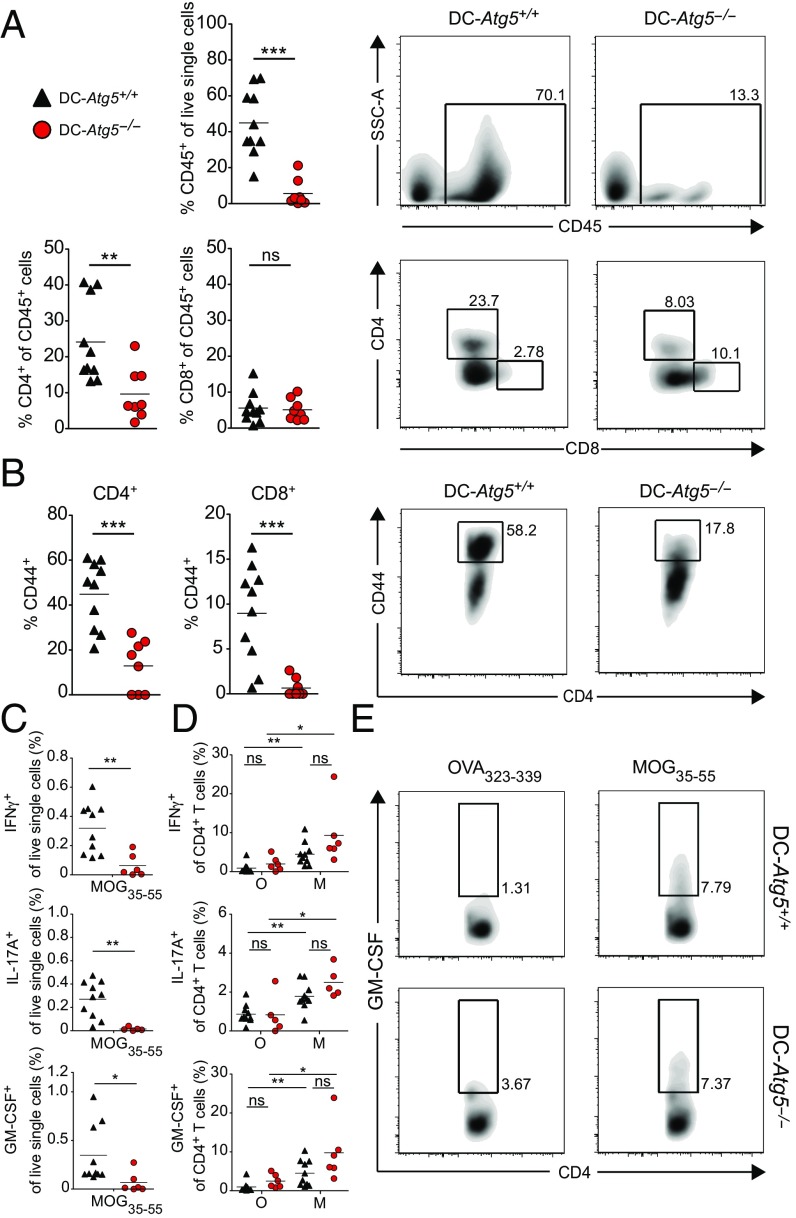
We next determined frequencies and effector functions of CNS-infiltrating T cells in DC-Atg5−/− compared with DC-Atg5+/+ littermates. To this end, mice were killed on day 21 (±1) upon adoptive transfer of MOG35–55–specific CD4+ T cells, at the peak of disease in DC-Atg5+/+ animals. DC-Atg5−/− mice showed substantially lower proportions of CNS-infiltrating CD4+ but not CD8+ T cells (Fig. 3A). In addition, frequencies of activated CNS CD44hi T cells were markedly reduced in DC-Atg5−/− mice (Fig. 3B). Reduced accumulation of MOG-specific CD4+ T cells was also observed at an earlier time point (day 12 post transfer) after AT-EAE induction (Fig. S3). In line with the pivotal role of CD4+ T cells in EAE (24, 25), the proportion of CD4+ but not CD8+ T cells positively correlated with the severity of EAE in DC-Atg5+/+ animals (Fig. S4). These data indicate that protection from EAE development in DC-Atg5−/− mice is associated with reduced activation and accumulation of CD4+ T cells within the CNS.
Fig. 3.
Lack of ATG5 in CD11c+ DCs limits accumulation of encephalitogenic CD4+ T cells within the CNS. (A) Twenty-one days (±1) after adoptive transfer EAE induction, DC-Atg5−/− mice exhibit significantly fewer CD45+ infiltrates in the CNS. Furthermore, quantification of immune cell subsets shows significantly lower proportions of CD4+ T cells in DC-Atg5−/− compared with DC-Atg5+/+ mice at the peak of disease in the CNS, whereas no difference is observed in the CD8+ T cell compartment. (B) At the peak of disease, DC-Atg5−/− mice exhibit significantly lower frequencies of activated CD44+/CD4+ and CD8+ T cells in the CNS. (C) The ability of CNS-invading CD4+ T cells to produce proinflammatory cytokines was determined. Leukocytes were isolated and purified from the CNS at the peak of disease (day 21 ± 1). For each animal the CNS-derived cell suspension was divided into two groups, and cells were restimulated for 4 h with either MOG35–55 peptide (M) or OVA323–339 peptide (O). CNS-infiltrating CD4+ T cells in DC-Atg5−/− mice maintain their capacity to produce effector cytokines. DC-Atg5+/+ mice contain significantly more (percentage of live single cells) cytokine-producing MOG35–55–specific CD4+ T cells in the CNS than DC-Atg−/− mice. (D) Both DC-Atg5−/−– and DC-Atg5+/+–derived CD4+ T cells are capable of secreting cytokines (IFNγ, IL-17A, and GM-CSF) upon restimulation with MOG35–55 (but not with OVA323–339) to similar degrees. (E) Representative density plot for GM-CSF+ CD4+ T cells in the CNS of DC-Atg5−/− or DC-Atg5+/+ mice at the peak of disease. Pooled data of two independent experiments are shown. Statistical analysis: Mean is depicted. Unpaired two-tailed Student t test was applied. ns, not significant: P > 0.05; *P < 0.05, **P < 0.01, ***P < 0.001.
Since the overall frequencies of CNS-infiltrating leukocytes producing proinflammatory cytokines upon ex vivo restimulation with MOG35–55 were significantly reduced in DC-Atg5−/− compared with DC-Atg5+/+ mice at the peak of disease (Fig. 3C), we next determined the ability of CNS-invading CD4+ T cells to produce proinflammatory cytokines. To this end, leukocytes were isolated and purified from the CNS at the peak of disease (day 21 ± 1). For each animal the CNS-derived cell suspension was divided into two groups, and cells were restimulated for 4 h either with MOG35–55 peptide or OVA323–339 peptide. Production of IFNγ+, IL-17+, and GM-CSF+ by CNS CD4+ T cells upon rechallenge with MOG35–55 was preserved in DC-Atg5−/− mice (Fig. 3 D and E).
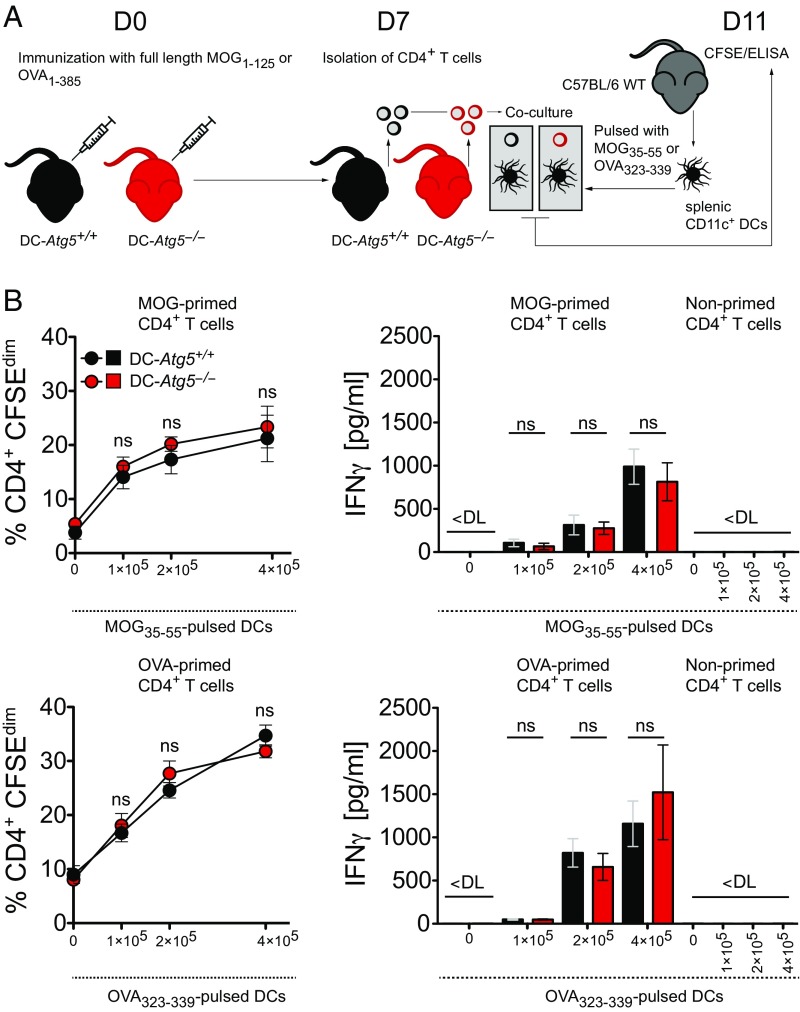
To additionally determine whether ATG5 deficiency in DCs impairs CD4+ T cell priming, we immunized DC-Atg5−/− mice and DC-Atg5+/+ littermates with full-length MOG protein/CFA (or full-length OVA protein/CFA) and restimulated splenic CD4+ T cells with titrated numbers of C57BL/6 wild type-derived peptide-pulsed DCs 7 d after immunization (Fig. 4A). Antigen-specific proliferation and IFNγ cytokine production of CD4+ T cells primed in DC-Atg5−/− mice were similar to those primed in DC-Atg5+/+ littermates (Fig. 4B).
Fig. 4.
Loss of ATG5 in DCs does not impair priming of antigen-specific CD4+ T cells. (A) Experimental setup. (B) Quantification of DC-Atg5−/−– and DC-Atg5+/+–derived CD4+ T cell proliferation via carboxyfluorescein succinimidyl ester (CFSE) dilution in the presence of increasing amounts of wild type-derived peptide-pulsed DCs (Upper and Lower Left). CD4+ T cell response (IFNγ secretion) is unchanged upon coculture with peptide-pulsed DCs (Upper and Lower Right). Pooled data of two independent experiments are shown. Statistical analysis: Mean ± SEM is depicted. Unpaired two-tailed Student t test was applied. ns, not significant: P > 0.05. DL, detection limit.
Thus, ATG5 in DCs is not required for priming myelin-specific CD4+ T cells upon active immunization. Lack of ATG5 in DCs does not affect the encephalitogenic capacity of primed, CNS-infiltrating CD4+ T cells, but restrains their in situ reactivation and accumulation.
Absence of ATG5 in DCs Abrogates Endogenous Myelin Peptide Presentation Following Phagocytosis of Injured Oligodendroglial Cells.
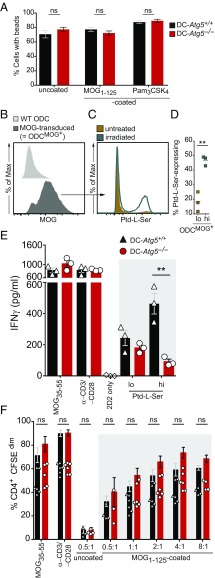
Canonical autophagy delivers intracellular antigens for MHC class II presentation, while EAE development is driven by an antigen not intrinsically expressed by professional APCs and therefore requires endocytosis, followed by myelin antigen processing and presentation. ATGs may contribute to extracellular antigen processing through phagosome maturation, regulated through cytosolic attachment of ATG8/LC3 in a process called ATG-dependent or LC3-associated phagocytosis. We therefore determined whether Atg5−/− DCs are impaired in phagocytosis and/or in their capability to present myelin antigen for CD4+ T cell activation. First, we assessed general phagocytosis by loading CD11c+ bone marrow-derived DCs (BMDCs) with polystyrene beads decorated with and without MOG1–125 full-length protein and beads decorated with the LAP-triggering Toll-like receptor (TLR)2 agonist Pam3CSK4 (26). Ingested beads were quantified by laser scanning confocal microscopy. Both DC-Atg5−/−– and DC-Atg5+/+–derived BMDCs phagocytosed similar numbers of either naked polystyrene beads, beads that had been decorated with MOG protein, and beads decorated with Pam3CSK4 (Fig. 5A), indicating that the general capacity of ATG5-deficient DCs to phagocytose extracellular material is not compromised.
Fig. 5.
Endogenous myelin presentation by CD11c+ DCs is abrogated in the absence of ATG5. (A) DC-Atg5−/−– and DC-Atg5+/+–derived BMDCs were incubated for 4 h with either uncoated, MOG1–125–coated, or Pam3CSK4–coated polystyrene beads, and the percentage of bead-containing cells was quantified via confocal microscopy. (B) Representative histogram comparing MOG-transduced oligodendroglial cell line MO3.13 (ODCMOG+) with wild-type MO3.13 cells (WT ODC) for surface MOG expression. (C) ODCMOG+ were either UVB–irradiated (870 mJ/cm2) or left untreated, which resulted in Ptd-l-Serhi– and Ptd-l-Serlo–expressing ODCMOG+. (D) Quantification via annexin V staining. Pooled data of three independent experiments are shown. (E) Coculture of MOG-specific 2D2/TCRMOG-derived CD4+ T cells with splenic CD11c+ DCs that had previously been pulsed with either Ptd-l-Serhi– or Ptd-l-Serlo–expressing ODCMOG+. CD4+ T cell response (IFNγ secretion) is augmented upon coculture with ODCMOG+-pulsed Ptd-l-Serhi DCs. Absence of ATG5 in DCs abrogates CD4+ T cell response upon coculture with ODCMOG+-pulsed Ptd-l-Serhi DCs. One representative of >3 independent experiments is shown. (F) Coculture of 2D2/TCRMOG-derived CD4+ with DC-Atg5−/−– and DC-Atg5+/+–derived FAC-sorted splenic DCs in the presence of MOG1–125–coated beads. Different bead:DC ratios are depicted. CD4+ T cell proliferation was quantified via CFSE dilution. Pooled data of three independent experiments are shown. Statistical analysis: Mean ± SEM is depicted. Unpaired two-tailed Student t test was applied. ns, not significant: P > 0.05; **P < 0.01.
ATG-dependent phagocytosis of extracellular material requires triggering through receptor-mediated antigen uptake such as phosphatidylserine (Ptd-l-Ser)-recognizing receptors, danger-associated molecular pattern (DAMP) receptors, TLR1/2, TLR2/6, TLR4, TLR9, and Dectin-1, or Fc receptors recognizing DNA immune complexes (12, 14, 17, 18, 27). Ptd-l-Ser can be exposed on membrane debris derived from damaged cells or specifically flipped to the outer cell-membrane leaflet during apoptosis (28). Oligodendrocyte injury and concomitant focal demyelination constitute unique pathological hallmarks of MS lesions and during EAE development (29, 30), and can even precede the formation of inflammatory infiltrates (31–33). We therefore hypothesized that uptake of damaged Ptd-l-Ser–exposing oligodendroglial cells by CD11c+ DCs triggers myelin-specific T cell activation in an ATG5-dependent manner.
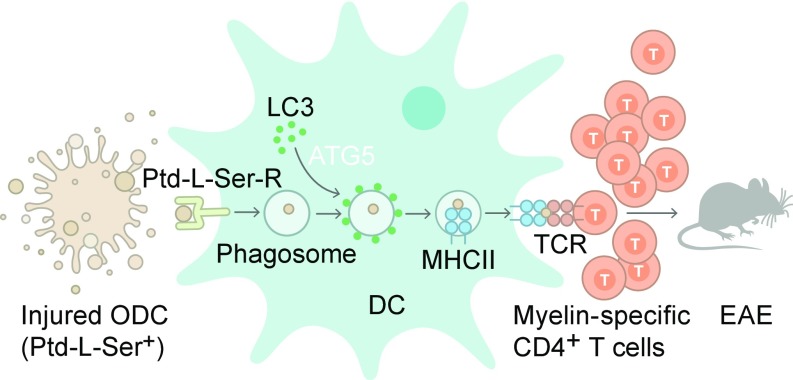
To test our hypothesis, irradiated Ptd-l-Serhi compared with nonirradiated Ptd-l-Serlo MOG-expressing oligodendroglial cells (Fig. 5 B–D) were loaded onto DC-Atg5−/−– or DC-Atg5+/+–derived primary splenic DCs. Endogenous myelin-derived peptide presentation was assessed by IFNγ production of cocultured MOG35–55–specific CD4+ T cells. T cell IFNγ production upon presentation of endogenous myelin antigen derived from Ptd-l-Serhi oligodendroglial cells was abrogated in ATG5-deficient DCs (Fig. 5E). ATG5-dependent activation of myelin-specific T cells required processing of oligodendroglial cell components exposing Ptd-l-Ser, since loading of DC-Atg5−/−– or DC-Atg5+/+–derived DCs with MOG protein-decorated polystyrene beads resulted in similar T cell proliferation upon coculture with myelin-specific CD4+ T cells (Fig. 5F). These data indicate that recognition and engulfment of injured oligodendroglial cells by DCs induce myelin peptide presentation on MHC class II molecules and subsequent activation of primed myelin-specific CD4+ T cells through ATG5-regulated phagocytosis (Fig. 6).
Fig. 6.
Provision of injured oligodendrocyte-derived antigenic material via ATG5-dependent phagocytosis. Schematic depiction summarizing how ATG5-dependent phagocytosis contributes to the provision of injured oligodendrocyte (ODC)-derived antigenic material to the MHC class II antigen presentation pathway in CNS DCs. Parts of compromised, Ptd-l-Ser+ ODCs are phagocytosed upon ligation of Ptd-l-Ser receptors (Ptd-l-Ser-Rs) triggering ATG5-dependent phagocytosis. LC3-I is converted into LC3-II in an ATG5-dependent manner and recruited to the single-membrane phagosome, which fuses with MHC class II-containing compartments. Myelin-derived antigenic material will be subsequently presented to encephalitogenic T cells, facilitating the development and maintenance of neuroinflammation.
The Cardiac Glycoside Neriifolin Inhibits ATG-Dependent Phagocytosis, Delays Onset, and Reduces Clinical Severity of EAE.
Although several interventions are available to inhibit autophagy at the nucleation, elongation, fusion, or degradation phase (34), pharmacological inhibitors of ATG-dependent phagocytosis have not been identified so far. The autophagy protein beclin 1/ATG6 initiates autophagosome formation via interaction with the class III type phosphoinositide 3-kinase (PI3K3)/Vps34. Vps34, along with its regulatory protein kinase Vps15, is a critical regulator of endocytic sorting in yeast and mammalian cells (35), and both kinases form a heterotrimeric complex along with beclin 1, referred to as the “beclin 1–Vps34 complex” (36). This complex, in addition to its role in initiating autophagosome formation, was shown to be recruited to phagosomes, responsible for phagosomal maturation (37), and suggested to mediate LC3 lipidation of phagosomal membranes during LAP (16).
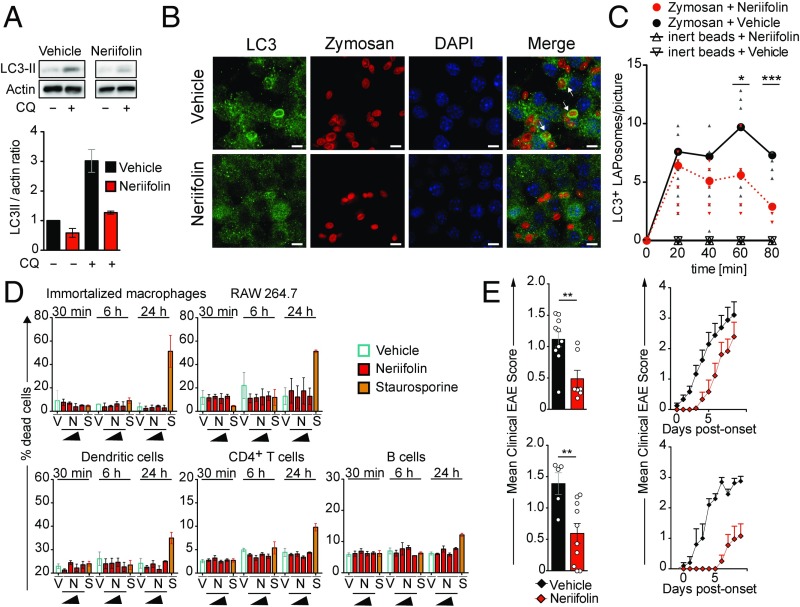
The CNS-penetrating cardiac glycoside neriifolin, an inhibitor of the Na+, K+-ATPase, a plasma membrane pump that generates Na+ and K+ gradients across the membrane, has recently been identified to inhibit ATG-dependent autophagy processes through interaction with beclin 1 (38). In rats, treatment with neriifolin prevented the increase in autophagy after hypoxia–ischemia injury as measured by decreased levels of LC3 lipidation (38). We first tested if exposure to neriifolin regulates autophagic activity by virtue of LC3 lipidation in RAW 264.7 cells, which are known to carry out high levels of ATG-dependent phagocytosis (12, 16). In line with the aforementioned data (38), LC3-II levels were decreased upon 24 h of neriifolin treatment compared with the vehicle control (Fig. 7A). Next, we investigated whether neriifolin inhibits ATG-dependent phagocytosis. Recruitment of LC3 to zymosan-containing phagosomes, a hallmark of ATG-dependent phagocytosis (16), was significantly reduced by exposure of RAW 264.7 cells to neriifolin (Fig. 7 B and C). The compound did not exhibit any cytotoxicity at the range of concentration tested (0.1 to 1,000 nM) (Fig. 7D). In vivo, neriifolin treatment significantly delayed the onset and reduced disease severity of EAE upon active immunization and following transfer of myelin-specific CD4+ T cells (Fig. 7E).
Fig. 7.
Cardiac glycoside neriifolin inhibits ATG-dependent phagocytosis and delays onset of EAE. (A) Western blot analysis for protein expression of LC3-II in RAW 264.7 cells in the absence (vehicle, 0.5% ethanol in PBS) or presence of neriifolin (1 µM). Actin served as a loading control. One representative of eight independent experiments (Upper) and quantification of Western blot analyses (Lower) are shown. (B) Immunofluorescence confocal laser scanning microscopy of RAW 264.7 cells (neriifolin, 1 µM; vehicle, 0.5% ethanol in PBS) visualizing LC3 (green), zymosan (red), and DAPI (blue). Representative pictures from the 80-min time point are depicted. Original magnification with 63×, 1.4 N.A. oil immersion lens. White arrows indicate LC3-decorated, zymosan-containing LAPosomes. (Scale bars, 5 µm.) (C) Quantification and kinetics of zymosan-triggered LAPosome formation in the absence (vehicle, 0.5% ethanol in PBS) or presence of neriifolin (1 µM). Non–LAP-triggering inert polystyrene beads were used as a control. (D) FACS analysis of neriifolin cytotoxicity using myeloid cell lines and primary (splenic) CD11chiMHCIIhi dendritic cells, CD3+CD4+ T cells, and CD19+MHCII+ B cells. Cells were exposed to increasing amounts of neriifolin (N; 0.1, 1, 10, and 1,000 nM) for either 30 min, 6 h, or 24 h. The protein kinase inhibitor staurosporine (S; 1 µM) was used as a positive and vehicle (V; 0.5% ethanol in PBS) as a negative control. (E) Neriifolin treatment (0.25 mg/kg; vehicle, 0.5% ethanol in PBS) delays onset and ameliorates disease severity of adoptive transfer (Upper) and active (Lower) EAE. Pooled data of two independent experiments are depicted. Each dot represents one individual animal. Statistical analysis: Mean ± SEM is depicted. Unpaired two-tailed Student t test was applied. *P < 0.05, **P < 0.01, ***P < 0.001. CQ, chloroquine.
Discussion
Our study shows that myelin-specific CD4+ T cells require ATG-dependent phagocytosis in DCs to induce sustained inflammation and EAE development. CD11c+ cells within the CNS alone, that is, in the absence of secondary lymphoid tissues, are sufficient to present antigen in vivo to primed myelin-reactive T cells to mediate CNS inflammation (8, 9, 39). In the steady state, CD11c+MHC class II+ DCs within the CNS are enriched in the choroid plexus (40, 41) which, along with the meningeal vasculature, is an active site for immune trafficking into and out of the CNS (42–44) and a first port of entry for pathogenic T cells during EAE (45). Choroid plexus DCs resemble splenic cDCs in morphology, gene expression profile, antigen-presenting function, and their shared intrinsic requirement for Fms-related tyrosine kinase (Flt)3 ligand (46). We identified a small population of CD11chiMHC class IIhi DCs that are specifically targeted by Cre-mediated recombination within the nondiseased CNS. Targeted deletion of ATG5 in these cells abrogated CD4+ T cell accumulation and completely prevented clinical disease development following adoptive transfer of primed, encephalitogenic T cells, which reflects the effector phase of EAE. Following active EAE induction with s.c. immunization with antigen/CFA, differences in incidence rates and clinical severity grades in DC-Atg5−/− compared with control mice were minor and not statistically significant. These data indicate distinct functions of ATG5 in cDCs in active vs. AT-EAE. While reactivation of encephalitogenic T cells within the CNS is required in both induction protocols, they differ in their requirement for cDCs for disease initiation. CD11cnegLy6ChiCCR2+ monocytes, precursors of CNS-invading monocyte-derived DCs, but not cDCs, are required for the initiation of tissue inflammation in actively induced EAE (19–23). Studies over the past years that employed inducible ablation of cDCs through CD11c promoter-driven human diphtheria toxin (DT) receptor (DTR) expression additionally investigated whether CD11c+ cells are required for T cell priming during EAE development following active immunization. Isaksson et al. (22) reported that DT-treated mice still develop EAE after active immunization, indicating that a population of APCs other than cDCs executed initial priming of encephalitogenic T cells. However, DT treatment did not ablate CD11c+ APCs in the CNS during the peak of disease, and CD11c+ APCs present in the CNS still could present myelin to invading encephalitogenic T cells (22). Yogev et al. (23) also used CD11c-DTR mice to deplete cDCs including dermal DCs, and found that mice lacking these DC populations are even hypersusceptible to EAE induction, supporting the concept that DCs maintain peripheral tolerance. However, they demonstrated that after T cell priming and during T cell invasion into the CNS (effector phase), DC-less mice showed reduced clinical scores compared with control mice. Along these lines, adoptive transfer of primed, encephalitogenic CD4+ T cells into CD11c+-depleted recipients leads to dramatically reduced disease incidence and scores (9). Thus, while the aforementioned studies reflect the complexity of APCs during T cell priming and indicate that, after active EAE induction, priming synapses can be formed between non–cDC-APC populations such as inflammatory CD11cnegLy6ChiCCR2+ monocytes (19, 20), they do not contradict but support the concept that CD11c+ cDCs are the most efficient APCs in driving the reactivation of primed myelin-specific CD4+ T cells during the effector phase of EAE (47).
Lack of Cre-mediated recombination in CD11cnegLy6C+ monocytes and the delayed targeting of ATG5 in CNS-invading monocyte-derived DCs might have contributed to the nonsignificant and minor differences in incidence rates and clinical severity grades in DC-Atg5−/− mice upon active immunization. Bhattacharya et al. (48) previously reported that CD11c-Cre–driven deletion of ATG7 ameliorates actively induced EAE. Different efficacies in targeting ATG7 in inflammatory monocyte and monocyte-derived DC subsets, which were not analyzed in the aforementioned study, might have contributed to the more distinctive phenotype during actively induced EAE compared with our study. Treatment with neriifolin, which inhibits ATG-dependent phagocytosis and does not specifically target either inflammatory monocytes and their progeny or cDCs, ameliorated both active and adoptive transfer EAE, indicating that ATG-dependent phagocytosis contributes to both disease models.
In addition to cDCs, myeloid cells within the CNS include parenchymal microglia and nonparenchymal meningeal, perivascular, and choroid-plexus macrophages (49). Assessing the precise immunological and antigen-presenting functions of the aforementioned subsets located at CNS–periphery interfaces in vivo is still hampered by their scarcity and the dearth of specific methods of isolation and targeting (49). While we found Cre-mediated site-specific deletion of Atg5 to be confined to CD11c+MHC class II+ DCs, leptomeningeal, perivascular-space, and choroid-plexus myeloid cells might have potential antigen presentation capacity during the course of EAE. Whether ATG-dependent phagocytosis in these subsets additionally contributes to myelin-specific CD4+ T cell reactivation and EAE development remains to be clarified.
Both ATG5 and ATG7 are part of the ubiquitin-like protein conjugation system that mediates LC3 lipidation, required for the formation of double-membrane autophagosomes and the decoration of single-membrane phagosomes following extracellular antigen uptake during ATG-dependent phagocytosis (12–14, 16). It has previously been suggested that loss of ATG7 in DCs reduces in vivo priming of myelin-specific CD4+ T cells following MOG/CFA immunization (48). The conclusion made in the aforementioned study is based on the finding that splenocytes derived from MOG-immunized DC-Atg7−/− mice produce less IL-2 5 to 6 d following restimulation with MOG peptide compared with ATG7-proficient mice (48). While these data indicate a reduced recall response in DC-Atg7−/− mice upon T cell reactivation, they do not specifically address the capability of ATG7-deficient DCs to prime CD4+ T cells. We demonstrate that absence of ATG5 in DCs does not impair their ability to prime myelin-specific CD4+ T cells in vivo. Instead, absence of ATG5 in DCs abrogates endogenous myelin peptide presentation to already-primed myelin-specific CD4+ T cells following phagocytosis of injured Ptd-l-Ser+ oligodendroglial cells, indicating that ATG-dependent phagocytosis of extracellular antigen contributes to MHC class II presentation but requires receptor-mediated antigen uptake (12, 14, 17, 26, 27).
CNS-infiltrating, myelin-specific CD4+ T cells require de novo phagocytosis and processing from intact myelin to induce sustained demyelination (8, 39, 50, 51). Similar to its function during canonical autophagy, ATG5 forms a heterodimer with ATG12 that is bound to ATG16L1 to produce the ATG5–ATG12–ATG16L1 complex (52). The E3 ligase activity of this complex finally mediates LC3 lipidation at the phagosomal membrane of late phagosomes (12, 14, 18, 53). Thus, closure of the plasma membrane around extracellular cargo and early phagosome formation are upstream of LC3 lipidation and independent of ATG5 (Fig. 6). In line with these data, we found that the general capacity of ATG5-deficient DCs to phagocytose extracellular material is not compromised. In contrast, their capacity to stimulate myelin-specific CD4+ T cells following processing of damaged Ptd-l-Ser–exposing oligodendroglial cells was greatly diminished. Although exposure of Ptd-l-Ser is primarily associated with nonimmunogenic clearance of apoptotic cells, it also occurs during nonapoptotic cell death, where externalized Ptd-l-Ser can be attributed to rupture of the plasma membrane rather than being an active exposure process, and DAMPs released from damaged cells can additionally activate inflammatory programs (54). DAMP receptors additionally identified to trigger ATG-dependent phagocytosis include TLRs such as TLR2 and TLR9, whose genetic deletion ameliorates EAE development induced by myelin-specific T cells, that is, in the absence of any apparent microbial involvement (55, 56). While the receptors and signaling pathways involved remain to be identified, our data indicate that ATG-dependent phagocytosis of injured oligodendrocytes critically augments CD4+ T cell pathogenicity during autoimmune CNS demyelination.
Autophagosome formation, maturation, and lysosomal degradation can be targeted by pharmacological compounds whose therapeutic efficacies could be demonstrated in various autophagy-associated disease conditions (57). We found that the Na+, K+-ATPase inhibitor neriifolin, reported to target beclin 1 (38), inhibits the formation of LC3-associated phagosomes and ameliorates EAE development. In addition to its previously reported inhibitory effect on hypoxia-induced LC3 lipidation in vivo (38), neriifolin might exert autophagy-independent, including neuroprotective, functions (58), as multiple chemical agents that are currently available to activate or inhibit autophagy have limited specificity for the autophagic process. Consistent with our findings, treatment with chloroquine, which inhibits degradation of autolysosomes, before EAE onset was reported to delay disease progression and attenuate its severity (48). While the aforementioned effects might not be exclusively mediated through ATG-dependent phagocytosis and antigen presentation, they support the concept that pharmacological inhibition of the autophagy machinery in DCs should be further explored for its potential therapeutic merit to limit autoimmune CD4+ T cell-mediated CNS inflammation.
Absence of ATG-dependent phagocytosis in myeloid cells by lysozyme M-Cre–mediated gene deletion, which targets macrophages, monocytes, some neutrophils, and cDCs, was recently shown to lead to development of a systemic autoinflammatory syndrome in mice with increased expression of IFN signature genes, occurrence of anti–double-stranded DNA and nuclear antibodies, and signs of kidney damage, commonly associated with systemic lupus erythematosus (18). The aforementioned study showed that macrophages deficient in ATG7 or Rubicon, both required for ATG-dependent phagocytosis, did engulf, but not effectively clear, dying cells and produced proinflammatory cytokines, including IL-1β and IL-6, upon challenge with apoptotic cell material (18). An increased propensity of ATG7−/− macrophages to produce proinflammatory cytokines after engulfment of apoptotic cells has also been described in vitro (14), indicating that defective ATG-dependent phagocytosis in macrophages results in a failure to digest engulfed dying cells, leading to elevated inflammatory cytokine production and the development of a lupus-like syndrome. In our study, DC-Atg5−/− mice were protected from the development of a CD4+ T cell-mediated autoimmune disease, and ATG5 was required for DCs to efficiently present antigen derived from injured oligodendroglial cells to myelin-specific CD4+ T cells. We find our data in line with in vitro studies that identified ATG-dependent phagocytosis in professional APCs supporting downstream CD4+ T cell responses by promoting sustained MHC class II antigen presentation (26, 27). ATG-dependent phagocytosis/LAP was shown to be required for retinoid recycling by phagocytic retinal pigment epithelial cells, which contributes to maintaining vision in mice (13), and to occur on macropinosomes (15) and at the ruffled border in osteoclasts (59), suggesting that this pathway contributes significantly to physiology in multiple contexts (60). During CD4+ T cell-driven CNS inflammation, DCs use ATG5 for enhanced presentation of endocytosed cargo derived from damaged oligodendrocytes on MHC class II molecules, thus linking oligodendrocyte injury with myelin antigen processing and T cell pathogenicity.
Loss of oligodendrocytes is a distinctive feature in tissue adjacent to rapidly expanding MS lesions (61), and can precede the formation of inflammatory infiltrates (31–33). Moreover, epigenomic changes in genes affecting oligodendrocyte susceptibility to damage and decreased expression of genes regulating oligodendrocyte survival were recently detected in pathology-free areas of MS-affected brains (62), compatible with the concept that oligodendrocyte injury might trigger or augment myelin protein processing if professional APCs are present. Future immunohistochemical studies will determine whether ATG-dependent phagocytosis can be visualized in APCs associated with MS lesions. We conclude from our data that ATG-regulated phagocytosis of injured oligodendrocytes for antigen presentation is required for encephalitogenic T cells to induce sustained CNS inflammation and disease development in mice. ATG-regulated phagocytosis in DCs might also be relevant for the perpetuation of neuroinflammation in patients with MS and, therefore, a potential therapeutic target to limit inflammatory CNS damage.
Materials and Methods
Mice.
Wild-type C57BL/6 mice were purchased from Janvier Labs. Congenic C57BL/6-CD45.1 mice were purchased from Charles River Laboratories. Atg5flox/flox mice were a kind gift of Noboru Mizushima, University of Tokyo, Tokyo, Japan (63). Tg(Itgax-cre,-EGFP)4097Ach mice designated CD11c-Cre were purchased from Jackson Laboratory. MOG-specific TCR transgenic mice C57BL/6-Tg(Tcra2D2,Tcrb2D2)1Kuch/J (designated 2D2/TCRMOG) were a kind gift from Vijay K. Kuchroo, Harvard Institutes of Medicine, Boston, MA. 2D2/TCRMOG mice were backcrossed to CD45.1 congenic C57BL/6 mice in our facility. All animals were bred and housed in the University of Zurich animal facility in individually ventilated cages on a 12-h light/dark cycle with food and water available ad libitum according to institutional guidelines and Swiss animal laws. CD11c-Cre mice were crossed with Atg5flox/flox mice to obtain CD11c-Cre × Atg5flox/flox mice (designated DC-Atg5−/−) on a C57BL/6 background. Atg5flox/flox mice (designated DC-Atg5+/+) were used as littermate controls. All animal protocols were approved by and conducted in accordance with the veterinary office of the canton of Zurich (protocol ZH210/2014).
Genotyping.
CD11c-Cre and Atg5flox/flox mouse genotypes were determined via PCR analysis of DNA from tail or ear biopsies (Table 1).
Table 1.
Primers for genotyping
| Primer | Source | Description | Sequence, 5′-3′ |
| Atg5flox/flox primer 1 | (63) | exon3-1 | GAATATGAAGGCACACCCCTGAAATG |
| Atg5flox/flox primer 2 | (63) | short2 | GTACTGCATAATGGTTTAACTCTTGC |
| Atg5flox/flox primer 3 | (63) | check2 | ACAACGTCGAGCACAGCTGCGCAAGG |
| Atg5flox/flox primer 4 | (63) | 5L2 | CAGGGAATGGTGTCTCCCAC |
| CD11c-Cre primer 1 oIMR1084 | Jackson | Transgene forward | GCGGTCTGGCAGTAAAAACTATC |
| CD11c-Cre primer 2 oIMR1085 | Jackson | Transgene reverse | GTGAAACAGCATTGCTGTCACTT |
| CD11c-Cre primer 3 oIMR7338 | Jackson | Internal positive control forward | CTAGGCCACAGAATTGAAAGATCT |
| CD11c-Cre primer 4 oIMR7339 | Jackson | Internal positive control reverse | GTAGGTGGAAATTCTAGCATCATCC |
2D2/TCRMOG transgenic mice carry the Vα3.2 Jα18 and Vβ11DJβ1.1 regions of the MOG-specific mouse T cell clone 2D2 (64). By virtue of their transgenic MOG-specific TCR expressed within the CD4+ T cell compartment, 2D2/TCRMOG transgenic mice were genotyped via flow cytometry using Vβ11-specific antibodies and peripheral blood obtained from the tail vein. Transgenic animals were identified due to the overrepresentation of this β-chain in their CD4+ T cell repertoire (64). Samples were acquired on a BD FACSCanto II using FACSDiva software v6.1.3 (BD Biosciences) and analyzed with FlowJo software v9.3.1 (Tree Star).
Induction of EAE.
For induction of adoptively transferred EAE, C57BL/6 or 2D2/TCRMOG mice were used as donor mice. On day 0, donor mice were actively induced with EAE via s.c. immunization in the flank region with 200 μg MOG35–55 (MEVGWYRSPFSRVVHLYRNGK; RP10245; GenScript) in CFA (263810; BD Difco). Pertussis toxin (200 ng) from Bordetella pertussis (179B; List Biological Laboratories) in PBS was administered i.p. After induction of EAE, mice were observed daily for weight loss, disability, and availability of food and water. On day 7 after immunization with MOG35–55/CFA, donor mice were euthanized with CO2. Spleen and draining lymph nodes were harvested and leukocytes were purified. Bulk leukocytes were cultured in R10 medium supplemented with recombinant IL-23 (10 ng/mL; 14-8231-63; eBioscience), MOG35–55 (10 µg/mL), and 1% penicillin/streptomycin (P/S) for 48 h at 37 °C and 5% CO2. Bulk 2D2/TCRMOG cells (10 × 106) or wild-type cells (10 to 15 × 106) were injected i.p. into each recipient mouse, which had been sublethally irradiated with 550 rad (RS 2000; Rad Source Technologies) 1 d before cell transfer. Clinical manifestations of EAE and weight loss were monitored and documented daily. Mice were scored as follows: 0, no detectable signs of EAE; 0.5, distal limp tail; 1, complete limp tail; 1.5, limp tail and hindlimb weakness; 2, unilateral partial hindlimb paralysis; 2.5, bilateral partial hindlimb paralysis; 3, complete bilateral hindlimb paralysis; 3.5, complete bilateral hindlimb paralysis and partial forelimb paralysis; 4, moribund (animal unable to move due to paralysis); and 5, animal found dead. In the following instances, animals were immediately euthanized with CO2 upon evaluation: disease score of 3 for more than 7 d, disease score of 3.5 for more than 3 d, and reaching disease score of 4. The last documented score of euthanized or dead animals was carried forward for statistical analysis.
Ptd-l-Ser–Expressing Cell Phagocytosis and Coculture Assay.
ODCMOG+ were either UVB-irradiated (870 mJ/cm2) or left untreated. DC-Atg5−/−– and DC-Atg5+/+–derived splenocytes were isolated, and the frequency of CD11c+MHCII+ DCs was determined via FACS using a small aliquot of the splenocyte suspension. Bulk splenocytes from DC-Atg5−/− and DC-Atg5+/+ mice were cocultured overnight (37 °C, 5% CO2) in cell-culture dishes with either Ptd-l-Serhi or Ptd-l-Serlo ODCMOG+ (10:1 ratio of ODCMOG+ to CD11c+MHCII+ DCs based on their frequencies within the splenocyte suspension determined earlier) in R10 medium supplemented with 1% P/S. The next day, CD11c+MHCII+ DCs were magnetic activated cell sorting (MACS)-purified from all conditions (DC-Atg5−/− + Ptd-l-Serlo ODCMOG+; DC-Atg5−/− + Ptd-l-Serhi ODCMOG+; DC-Atg5+/+ + Ptd-l-Serlo ODCMOG+; DC-Atg5+/+ + Ptd-l-Serhi ODCMOG+). CD11c-enriched fractions were further cocultured at a 1:5 ratio (DC:T cell) in a 96-well U-bottom plate overnight (37 °C, 5% CO2) with 2D2/TCRMOG-derived MACS-purified CD4+ T cells in a total volume of 200 µL R10 supplemented with 1% P/S and IL-2 (10 ng/mL, 402-ML-100; R&D Systems). Wells containing 2D2/TCRMOG CD4+ T cells only, MOG35–55 peptide (20 μg/mL), or anti-CD3 and anti-CD28 antibodies (5 µg/mL each) were also included. All conditions were performed in triplicate. After 24 h of incubation, cell-culture supernatants were collected for analysis of IFNγ concentration by ELISA (88-7314-76; eBioscience).
Statistics.
Statistical tests applied are indicated in the respective figure legends. Unpaired, two-tailed Student t test, two-way ANOVA, and two-tailed Pearson correlation test were performed. A P value <0.05 was considered statistically significant. The asterisks depicted in the figures translate into the following groupings: *P < 0.05, **P < 0.01, ***P < 0.001. All quantitative analyses were performed with Prism v5.0a for Mac OSX (GraphPad Software).
Supplementary Material
Acknowledgments
We thank Dr. Noboru Mizushima (University of Tokyo) for providing the Atg5flox/flox mice, Dr. Fabienne Brilot (University of Sydney) for providing the MOG-overexpressing and wild-type MO3.13 oligodendroglial cell lines, Anne Müller (University of Zurich) for excellent technical assistance, Dr. Melanie Greter (University of Zurich) for valuable discussions, and the flow cytometry facility of the University of Zurich for cell-sorting support. We thank Tara von Grebel of the Scientific Visualization and Visual Communication Department at the University of Zurich for her help with cartoon design. C.W.K. was supported by a scholarship provided by the German Research Foundation (DFG Grant KE 1831/1-1) and a scholarship from the University of Zurich (Forschungskredit FK-14-021). J.D.L. was supported by the Swiss National Foundation (31003A-169664), Novartis Foundation for medical-biological research, Sassella Foundation, Hartmann Müller Foundation, and Swiss Multiple Sclerosis Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713664114/-/DCSupplemental.
References
- 1.Sawcer S, et al. International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2 Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beecham AH, et al. International Multiple Sclerosis Genetics Consortium (IMSGC); Wellcome Trust Case Control Consortium 2 (WTCCC2); International IBD Genetics Consortium (IIBDGC) Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45:1353–1360. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babbe H, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sospedra M, et al. Redundancy in antigen-presenting function of the HLA-DR and -DQ molecules in the multiple sclerosis-associated HLA-DR2 haplotype. J Immunol. 2006;176:1951–1961. doi: 10.4049/jimmunol.176.3.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Planas R, et al. Central role of Th2/Tc2 lymphocytes in pattern II multiple sclerosis lesions. Ann Clin Transl Neurol. 2015;2:875–893. doi: 10.1002/acn3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamvil SS, et al. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Nun A, Wekerle H, Cohen IR. Vaccination against autoimmune encephalomyelitis with T-lymphocyte line cells reactive against myelin basic protein. Nature. 1981;292:60–61. doi: 10.1038/292060a0. [DOI] [PubMed] [Google Scholar]
- 8.Greter M, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 9.Paterka M, et al. Gatekeeper role of brain antigen-presenting CD11c+ cells in neuroinflammation. EMBO J. 2016;35:89–101. doi: 10.15252/embj.201591488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid D, Pypaert M, Münz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HK, et al. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanjuan MA, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 13.Kim J-Y, et al. Noncanonical autophagy promotes the visual cycle. Cell. 2013;154:365–376. doi: 10.1016/j.cell.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez J, et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci USA. 2011;108:17396–17401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol. 2011;13:1335–1343. doi: 10.1038/ncb2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez J, et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol. 2015;17:893–906. doi: 10.1038/ncb3192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Henault J, et al. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012;37:986–997. doi: 10.1016/j.immuni.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez J, et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature. 2016;533:115–119. doi: 10.1038/nature17950. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Croxford AL, et al. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity. 2015;43:502–514. doi: 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Mildner A, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki R, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaksson M, Lundgren BA, Ahlgren KM, Kämpe O, Lobell A. Conditional DC depletion does not affect priming of encephalitogenic Th cells in EAE. Eur J Immunol. 2012;42:2555–2563. doi: 10.1002/eji.201142239. [DOI] [PubMed] [Google Scholar]
- 23.Yogev N, et al. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity. 2012;37:264–275. doi: 10.1016/j.immuni.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Nun A, Wekerle H, Cohen IR. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981;11:195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamoorthy G, Wekerle H. EAE: An immunologist’s magic eye. Eur J Immunol. 2009;39:2031–2035. doi: 10.1002/eji.200939568. [DOI] [PubMed] [Google Scholar]
- 26.Romao S, et al. Autophagy proteins stabilize pathogen-containing phagosomes for prolonged MHC II antigen processing. J Cell Biol. 2013;203:757–766. doi: 10.1083/jcb.201308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J, Becker C, Lowell CA, Underhill DM. Dectin-1-triggered recruitment of light chain 3 protein to phagosomes facilitates major histocompatibility complex class II presentation of fungal-derived antigens. J Biol Chem. 2012;287:34149–34156. doi: 10.1074/jbc.M112.382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata S, Suzuki J, Segawa K, Fujii T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016;23:952–961. doi: 10.1038/cdd.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frohman EM, Racke MK, Raine CS. Multiple sclerosis—The plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 30.Lucchinetti C, et al. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain. 1999;122:2279–2295. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- 31.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 32.Prineas JW, Parratt JDE. Oligodendrocytes and the early multiple sclerosis lesion. Ann Neurol. 2012;72:18–31. doi: 10.1002/ana.23634. [DOI] [PubMed] [Google Scholar]
- 33.Traka M, Podojil JR, McCarthy DP, Miller SD, Popko B. Oligodendrocyte death results in immune-mediated CNS demyelination. Nat Neurosci. 2016;19:65–74. doi: 10.1038/nn.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: Therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinchen JM, et al. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat Cell Biol. 2008;10:556–566. doi: 10.1038/ncb1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juhász G, et al. The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol. 2008;181:655–666. doi: 10.1083/jcb.200712051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger SB, et al. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat Immunol. 2010;11:920–927. doi: 10.1038/ni.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci USA. 2013;110:20364–20371. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kivisäkk P, et al. Localizing central nervous system immune surveillance: Meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prodinger C, et al. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011;121:445–458. doi: 10.1007/s00401-010-0774-y. [DOI] [PubMed] [Google Scholar]
- 41.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- 42.Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 43.Mohammad MG, et al. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clin Invest. 2014;124:1228–1241. doi: 10.1172/JCI71544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schläger C, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530:349–353. doi: 10.1038/nature16939. [DOI] [PubMed] [Google Scholar]
- 45.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 46.Anandasabapathy N, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208:1695–1705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becher B, Greter M. Acquitting an APC: DCs found “not guilty” after trial by ablation. Eur J Immunol. 2012;42:2551–2554. doi: 10.1002/eji.201242928. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharya A, Parillon X, Zeng S, Han S, Eissa NT. Deficiency of autophagy in dendritic cells protects against experimental autoimmune encephalomyelitis. J Biol Chem. 2014;289:26525–26532. doi: 10.1074/jbc.M114.575860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prinz M, Erny D, Hagemeyer N. Ontogeny and homeostasis of CNS myeloid cells. Nat Immunol. 2017;18:385–392. doi: 10.1038/ni.3703. [DOI] [PubMed] [Google Scholar]
- 50.Tompkins SM, et al. De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:4173–4183. doi: 10.4049/jimmunol.168.8.4173. [DOI] [PubMed] [Google Scholar]
- 51.Kawakami N, et al. The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J Exp Med. 2004;199:185–197. doi: 10.1084/jem.20031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizushima N, et al. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci. 2003;116:1679–1688. doi: 10.1242/jcs.00381. [DOI] [PubMed] [Google Scholar]
- 53.Hanada T, et al. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem. 2007;282:37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 54.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: Role of proteases. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 55.Prinz M, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miranda-Hernandez S, et al. Role for MyD88, TLR2 and TLR9 but not TLR1, TLR4 or TLR6 in experimental autoimmune encephalomyelitis. J Immunol. 2011;187:791–804. doi: 10.4049/jimmunol.1001992. [DOI] [PubMed] [Google Scholar]
- 57.Morel E, et al. Autophagy: A druggable process. Annu Rev Pharmacol Toxicol. 2017;57:375–398. doi: 10.1146/annurev-pharmtox-010716-104936. [DOI] [PubMed] [Google Scholar]
- 58.Wang JKT, et al. Cardiac glycosides provide neuroprotection against ischemic stroke: Discovery by a brain slice-based compound screening platform. Proc Natl Acad Sci USA. 2006;103:10461–10466. doi: 10.1073/pnas.0600930103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeSelm CJ, et al. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev Cell. 2011;21:966–974. doi: 10.1016/j.devcel.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bandyopadhyay U, Overholtzer M. LAP: The protector against autoimmunity. Cell Res. 2016;26:865–866. doi: 10.1038/cr.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henderson APD, Barnett MH, Parratt JDE, Prineas JW. Multiple sclerosis: Distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–753. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- 62.Huynh JL, et al. Epigenome-wide differences in pathology-free regions of multiple sclerosis-affected brains. Nat Neurosci. 2014;17:121–130. doi: 10.1038/nn.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 64.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dale RC, et al. Antibodies to MOG have a demyelination phenotype and affect oligodendrocyte cytoskeleton. Neurol Neuroimmunol Neuroinflamm. 2014;1:e12. doi: 10.1212/NXI.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuenzle S, et al. Pathogen specificity and autoimmunity are distinct features of antigen-driven immune responses in neuroborreliosis. Infect Immun. 2007;75:3842–3847. doi: 10.1128/IAI.00260-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.