Significance
Prostate cancer (PC) is the most common cancer affecting males. To date, there is no effective treatment for metastatic PC. Prostate tumors invariably overexpress prostate surface membrane antigen (PSMA). We present a potent therapy, PEI-PEG-DUPA/polyinosinic/polycytosinic acid (PPD/polyIC), which is targeted against PSMA. This treatment leads to tumor cell death and to the recruitment of immune cells, which attack neighboring tumor cells, even those that do not overexpress PSMA. In a mouse model, PPD/polyIC led to shrinkage of all PSMA-overexpressing tumors and to complete regression in half of the animals. The multipronged approach invokes antitumor immunity, and reduces the probability of acquired resistance and disease recurrence. PPD/polyIC is a promising, affordable candidate for clinical development.
Keywords: prostate cancer, DUPA, polyIC, PSMA, PEI-PEG
Abstract
There is an urgent need for an effective treatment for metastatic prostate cancer (PC). Prostate tumors invariably overexpress prostate surface membrane antigen (PSMA). We designed a nonviral vector, PEI-PEG-DUPA (PPD), comprising polyethylenimine–polyethyleneglycol (PEI–PEG) tethered to the PSMA ligand, 2-[3-(1, 3-dicarboxy propyl)ureido] pentanedioic acid (DUPA), to treat PC. The purpose of PEI is to bind polyinosinic/polycytosinic acid (polyIC) and allow endosomal release, while DUPA targets PC cells. PolyIC activates multiple pathways that lead to tumor cell death and to the activation of bystander effects that harness the immune system against the tumor, attacking nontargeted neighboring tumor cells and reducing the probability of acquired resistance and disease recurrence. Targeting polyIC directly to tumor cells avoids the toxicity associated with systemic delivery. PPD selectively delivered polyIC into PSMA-overexpressing PC cells, inducing apoptosis, cytokine secretion, and the recruitment of human peripheral blood mononuclear cells (PBMCs). PSMA-overexpressing tumors in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice with partially reconstituted immune systems were significantly shrunken following PPD/polyIC treatment, in all cases. Half of the tumors showed complete regression. PPD/polyIC invokes antitumor immunity, but unlike many immunotherapies does not need to be personalized for each patient. The potent antitumor effects of PPD/polyIC should spur its development for clinical use.
Prostate cancer (PC) is the most common malignancy among males. PC typically presents prostate-specific membrane antigen (PSMA) on the cell surface. PSMA is a multifunctional transmembrane protein that functions as a glutamate carboxypeptidase and also demonstrates rapid, ligand-induced internalization and recycling (1, 2). Although its exact role in PC is unclear, PSMA expression is 1,000-fold higher in prostate tumors than in noncancerous tissues, and its overexpression increases with progression of the cancer (3, 4). Although a given tumor may be heterogeneous for PSMA expression, completely PSMA-negative primary or metastatic tumors are rare (5). Thus, PSMA is an ideal marker for targeting PC, and there has been much interest in the development of PSMA ligands for diagnostic and therapeutic purposes.
A number of PSMA-targeted therapies have been devised for the treatment of PC, but none has entered the clinic to date (6–10). The standard of care for metastatic PC patients is androgen deprivation therapy (ADT). Although ADT is highly effective at achieving short-term remission, many patients gradually establish resistance to the therapy and proceed to develop castration-resistant PC. Such patients have a median survival of 3 y (11–13).
Cancers are characterized by genomic instability, heterogeneity, and rapid tumor evolution. Targeting a single subpopulation in a heterogeneous tumor is ineffective, because the nontargeted cells can survive and proliferate. To overcome the mutability of cancer, treatments must act before the cancer cells can adapt and acquire resistance. To effectively combat cancer, therefore, we need treatments that can achieve full tumor eradication within a short time frame.
We have developed a promising strategy to meet this requirement. Our strategy is to activate multiple death pathways, leading to the rapid killing of the targeted cells and, simultaneously, to the triggering of “bystander effects,” which lead to the killing of neighboring, untargeted tumor cells. The combined effects create a potent treatment that leads to the eradication of heterogeneous tumors and prevents the development of drug resistance (14). In nature, viral dsRNA triggers multiple antiviral defense mechanisms, leading to the apoptosis of the infected cells and to the activation of an immune response that leads to the killing of neighboring cells, all in an attempt to mitigate the infection (15). We use a synthetic analog of dsRNA, polyinosinic/polycytosinic acid (polyIC), to achieve a similar effect. Following internalization into cancer cells, polyIC induces apoptosis and activates bystander effects, leading to tumor eradication. These bystander effects are both direct and immune mediated. The direct effects are caused by toxic cytokines that are secreted from the targeted cancer cells; these cytokines cause neighboring, untargeted cancer cells to apoptose. The immune-mediated effects are caused by the secretion of cytokines that recruit and activate immune cells, which in turn kill any remaining cancer cells.
PolyIC is used in the clinic as an immune adjuvant. However, polyIC is highly toxic and therefore is confined to a narrow therapeutic window. By targeting polyIC to PC cells, we can take advantage of its potency while using very low doses, avoiding toxicity. We designed a vector consisting of polyethyleneimine–polyethyleneglycol (PP), which binds polyIC and assists in its endosomal release, conjugated to a ligand that homes to PSMA, to deliver the polyIC directly to PSMA-overexpressing cancer cells. We have previously demonstrated the strength of this strategy using PP conjugated to targeting ligands for epidermal growth factor receptor (EGFR) (14, 16, 17) and for Her2 (18).
Our PSMA-targeting vector utilizes the urea-based ligand of PSMA, DUPA (2-[3-(1, 3-dicarboxy propyl)ureido] pentanedioic acid) as its targeting moiety. DUPA is a highly selective ligand that was shown to steer siRNA (19) and chemotherapeutic agents into PC cells (20) and to facilitate the imaging of PC metastases (21, 22). Here, we show that PP-DUPA (PPD) bound to polyIC (PPD/polyIC) exhibits powerful killing of PSMA-overexpressing cancer cells and effectively stimulates both direct and immune-mediated bystander effects. Finally, we show that PPD–polyIC has remarkable efficacy against prostate tumors in NOD/SCID mice that have been reconstituted with a human immune system [peripheral blood mononuclear cells (PBMCs)].
Results
Specific Binding and Internalization of DUPA–Linker–DyLight 680 to PSMA-Overexpressing Cells.
We began our investigation by confirming that DUPA can selectively home to PSMA-overexpressing cancer cells, and deliver cargo into the cells. To this end, we synthesized DUPA according to Kularatne et al. (23). To avoid steric hindrance, the DUPA was conjugated via a linker consisting of a hydrocarbon chain of 8-aminooctanoic acid and a short peptide to the fluorescent dye DyLight 680 (Thermo Scientific). We first constructed DUPA–linkerA as described (23) conjugated to DyLight 680 (SI Materials and Methods) and confirmed that this selectively bound PSMA overexpressing cells (Fig. S1). We then modified the linker of Kularatne et al. (23) to incorporate the peptide Cys-Gly-Trp-Trp-Gly-Phe (Fig. 1A). The Cys residue would later allow us to conjugate the DUPA–linker to polyethyleneimine–polyethyleneglycol (PEI–PEG, or PP), and the Trp residues would allow us to quantify PPD by UV spectra.
Fig. 1.
Internalization of DUPA ligand into PSMA-overexpressing cells is selective. (A) Molecular structure of DUPA–linker. (B) Selective internalization of DUPA–linker–DyLight 680 into PSMA-overexpressing cells. PC3-PSMA, LNCaP, or MCF7 cells were treated with 70 nM DUPA–linker–DyLight 680 for 5 h and visualized in a laser scanning confocal fluorescence microscope.
We confirmed the structure of the modified DUPA–linker–DyLight 680 conjugate (Fig. S2) and analyzed its binding to cancer cells using confocal fluorescence microscopy (Fig. 1B). After 5-h incubation, DUPA–linker–DyLight 680 successfully bound and internalized into LNCaP and PC3–PSMA cells (which overexpress PSMA), but did not enter MCF7 cells (which do not), indicating that DUPA–linker–DyLight 680 is indeed selective for PSMA.
PPD/polyIC Selectively Kills PSMA-Overexpressing PC Cells.
Next, we prepared the chemical vector, PPD (Fig. S3), designed to selectively kill PSMA-overexpressing PC cells. To generate PPD, we conjugated the DUPA–linker to PP, which binds polyIC, as described previously (24).
We bound polyIC to PPD (PPD/polyIC) and measured the complex by dynamic light scattering as described in Joubran et al. (24). The complex was found to be 105 ± 16.7 nm, a size that is well within the 200-nm limit for endosomal entry (25).
We next tested the complex for potency and selectivity. In a 3-d treatment, PPD–polyIC efficiently killed 80–95% of LNCaP, VCaP, and PC3-PSMA cells, all of which overexpress PSMA, and left MCF7 and PC3 cells, which do not express PSMA, unharmed (Fig. 2A). Addition of the PSMA inhibitor 2-(phosphonomethyl)pentanedioic acid (PMPA) (26) protected the cells from PPD/polyIC-induced killing (Fig. S4), implying that PPD/polyIC is endocytosed into the cells in a PSMA-dependent fashion (1, 27).
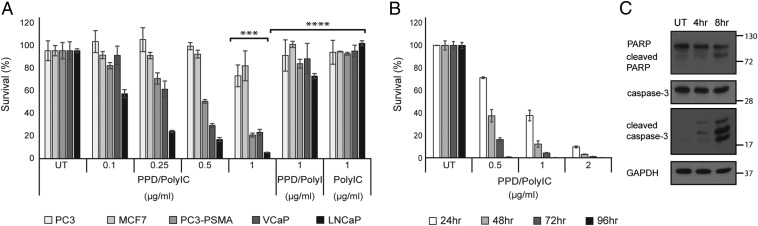
Fig. 2.
PPD/polyIC induces selective killing of PSMA-overexpressing cells by activating apoptosis pathways. (A) PPD/polyIC complexes selectively kill PSMA-overexpressing cells. LNCaP, VCaP, PC3-PSMA, PC3, and MCF7 cells were seeded in triplicate onto 96-well plates, grown overnight, and treated with PPD/polyIC at the indicated concentrations of polyIC, or with PPD/polyI or polyIC alone as controls. Viability was measured by the CellTiter-Glo Luminescent Cell Viability Assay (Promega) 72 h after the treatment was initiated (***P ≤ 0.001 LNCaP or VCaP or PC3-PSMA vs. MCF7 or PC3, ****P ≤ 0.001 1 µg/mL PPD/polyIC vs. 1 µg/mL PPD/polyI or 1 µg/mL polyIC alone). (B) PPD/polyIC complexes rapidly kill LNCaP Cells. LNCaP cells were seeded in triplicate onto 96-well plates, grown overnight, and treated with PPD/polyIC at the indicated concentrations of polyIC. Viability was measured as above at the indicated times after initiation of treatment. The graphs in A and B show means plus SDs. (C) PPD/polyIC triggers apoptotic signaling pathways. Whole-cell lysates were prepared from LNCaP cells treated with PPD/polyIC (2 µg/mL) at the indicated times and analyzed by Western blot, using antibodies against full-length caspase 3, cleaved caspase 3, and PARP.
We incubated LNCaP cells with PPD/polyIC–rhodamine and used time-lapse confocal microscopy to follow the polyIC–rhodamine. As shown in Fig. S5A, polyIC–rhodamine spread throughout the cytoplasm within 2 min of entry into the cells. Little if any polyIC entered the nucleus. Although it is likely that PPD/polyIC enters by endocytosis, the process was so rapid that it was not possible to identify endosomes. PolyIC–rhodamine that was not complexed with PPD barely entered the cells (Fig. S5B), confirming the role of PPD in targeting the cells.
The PPD/polyIC-induced killing of LNCaP cells was rapid. Cell death was evident as soon as 24 h following treatment initiation (Fig. 2B). With prolonged treatment, survival decreased further. Treating LNCaP cells with 0.5 µg/mL polyIC bound to PPD for 96 h sufficed to kill nearly 100% of the cells (Fig. 2B). Cleavage of caspase 3 was evident within 4 h and cleavage of PARP [Poly (ADP-ribose) polymerase] within 8 h of exposure to PPD/polyIC, indicating that cell death occurred by apoptosis (Fig. 2C).
PPD/polyIC Treatment Leads to Massive Tumor Cell Death by Inducing Direct and Indirect Bystander Effects.
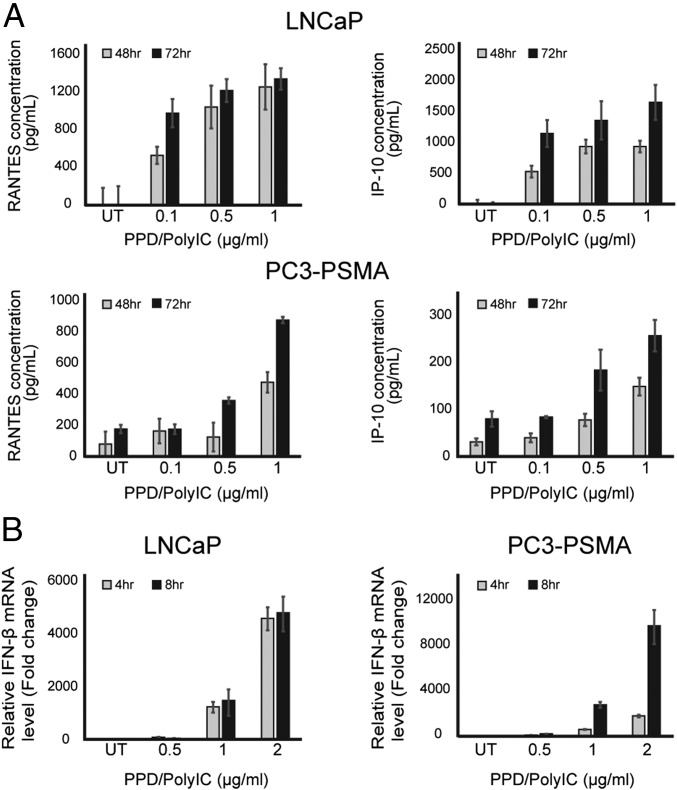
The presence of dsRNA in cells activates pathways leading to the secretion of chemotactic cytokines, which activate immune cells and recruit them to the infected area. We explored the effect of PPD/polyIC treatment on cytokine production in LNCaP and PC3-PSMA cells. PPD/polyIC treatment led to time-dependent and dose-dependent secretion of RANTES/CCL5 (regulated on activation, normal T cell expressed and secreted) and IP-10/CXCL10 (interferon gamma-induced protein 10) (Fig. 3A), as detected by ELISA. Using qRT-PCR, we detected induction of the cytotoxic cytokine IFN-β as early as 4 h after treatment (Fig. 3B).
Fig. 3.
PPD/polyIC induces expression of proinflammatory and cytotoxic cytokines in LNCaP and PC3-PSMA cells. (A) PPD/polyIC leads to secretion of chemotactic cytokines. LNCaP and PC3-PSMA cells were treated as indicated for 48 or 72 h, after which the medium was collected and the levels of RANTES and IP-10 were measured using ELISAs. (B) PPD/polyIC leads to the production of IFN-β. LNCaP and PC3-PSMA cells were treated as indicated for 4 or 8 h, after which IFN-β mRNA was measured by qRT-PCR. The graphs show means plus SDs.
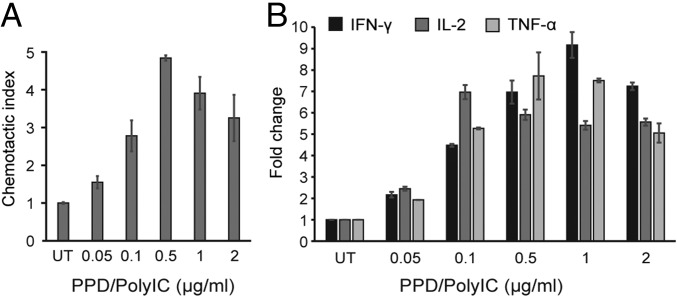
We next evaluated whether PPD/polyIC could lead to the recruitment of immune cells. Conditioned medium from LNCaP cells that had been treated with PPD/polyIC for 48 h led to a fivefold induction of chemotaxis of PBMCs relative to medium obtained from untreated cells (Fig. 4A). PBMCs that were exposed to conditioned medium from treated cells showed increased IL-2 expression, indicating that these PBMCs were activated (28) (Fig. 4B). The conditioned medium from treated cells also induced the PBMCs to express the toxic, proinflammatory cytokines IFN-γ and TNFα (Fig. 4B). Notably, expression of IL-2, IFN-γ, and TNF-α was elevated following treatment with doses as low as 0.05 µg/mL polyIC (Fig. 4B).
Fig. 4.
PPD/poly-IC attracts and activates PBMCs. (A) PPD/polyIC induces chemotactic migration of human PBMCs. LNCaP cells were grown in 24-well plates in duplicates and treated as indicated. Forty-eight hours after treatment, cell medium was transferred to the lower chamber of a Transwell chemotaxis plate with 0.5-μm pores. The 106 PBMCs in 100 µL medium were added to the upper chamber, and plates were incubated for 3.5 h at 37 °C. Migrated cells were collected from the lower chamber and quantified by FACS, scatter gating on lymphocytes. The chemotactic index is the ratio of the number of lymphocytes that migrated in the presence of conditioned medium from treated cells to the number that migrated in the presence of conditioned medium from untreated cells. (B) Quantitative analysis of IL-2, TNF-α, and INF-γ mRNA expression in PBMCs after incubation with conditioned medium from treated LNCaP cells. Total cellular RNA was isolated from PBMCs after 24-h incubation with conditioned medium derived from LNCaP cells that had been treated with PPD/polyIC for 48 h. Aliquots of RNA (1 μg) were subjected to qRT-PCR. All results were normalized to the mRNA levels of the housekeeping gene HuPO. The graphs show means plus SDs.
To study the bystander effects induced by PPD/polyIC treatment, we utilized coculture systems. These systems utilized LNCaP-Luc, PC3-Luc, and MCF7-Luc cells, which stably express luciferase (Luc). Having determined that Luc activity was proportional to the number of Luc-expressing cells, Luc activity was used as a proxy for the number of Luc-expressing cells.
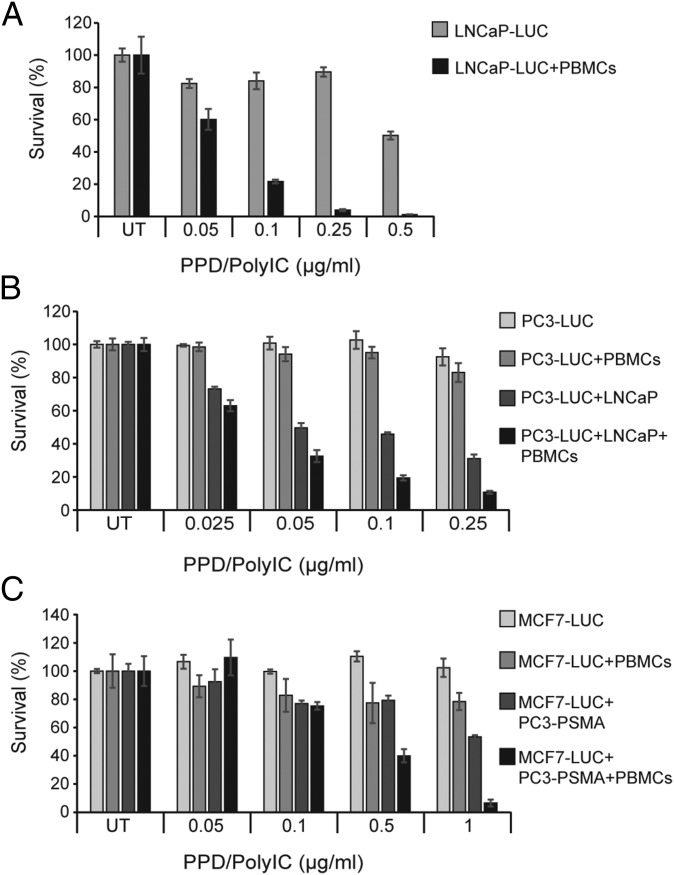
The PBMC-mediated bystander effect was demonstrated by coculturing PBMCs with LNCaP-Luc cells that had been pretreated with PPD/polyIC. We first treated LNCaP-Luc cells with low doses of PPD/polyIC (up to 0.5 µg/mL polyIC). These low doses led to a modest effect, with up to 50% cell death following 72 h of treatment (Fig. 5A). Remarkably, when PBMCs were added to the pretreated LNCaP-Luc cells for 48 h, the cancer cells were completely eradicated (Fig. 5A). PBMCs had no effect on untreated LNCaP-Luc cells.
Fig. 5.
PPD/polyIC induces bystander effects. (A) Low doses of PPD/polyIC eradicated LNCaP cells by the PBMC-mediated bystander effect. LNCaP-Luc cells were treated with PPD/polyIC at the indicated concentrations of polyIC. After 24 h, PBMCs (or medium) were added to the culture for 48 additional hours. The survival of LNCaP-Luc cells was measured by luciferase assay (Promega). (B and C) PPD/polyIC led to death of the untreated cells by combined direct and PBMC-mediated bystander effects. (B) LNCaP cells were treated with PPD/polyIC at the indicated concentrations of polyIC. After 16 h, PC3-Luc cells were added to the culture. PBMCs (or medium) were added 6 h later. The coculture was incubated for 72 additional hours. The survival of PC3-Luc was measured as above. (C) PC3-PSMA cells were treated with PPD/polyIC at the indicated concentrations of polyIC. After 16 h, MCF7-Luc cells were added to the culture. PBMCs (or medium) were added 6 h later. The coculture was incubated for 72 h longer and MCF7-Luc survival measured as above. The graphs show means plus SDs.
The direct bystander effect was demonstrated by coculturing PC3-Luc or MCF7-Luc cells (which do not overexpress PSMA) with PPD/polyIC-treated LNCaP or PC3-PSMA cells (which overexpress PSMA). Neither PC3-Luc (Fig. 5B) nor MCF7-Luc cells (Fig. 5C) were affected by PPD/polyIC alone. Coculturing PC3-Luc with PPD/polyIC-treated LNCaP cells resulted in the death of up to 70% of the PC3-Luc cells (Fig. 5B), and coculturing MCF7-Luc with PPD/polyIC-treated PC3-PSMA cells resulted in the death of up to 40% of the MCF7-Luc cells (Fig. 5C). We infer that the proapoptotic cytokines from the PPD/polyIC-treated PSMA-overexpressing cells triggered the death of the cocultured PPD/polyIC-insensitive Luc-expressing cells.
We also looked at the combined direct and indirect bystander effects on cells that do not overexpress PSMA. As in the direct bystander samples, we cocultured PC3-Luc cells with PPD/polyIC-treated LNCaP, and we cocultured MCF7-Luc cells with PPD/polyIC-treated PC3-PSMA cells. However, we now added PBMCs to these samples. The addition of PBMCs as well as PPD/polyIC-treated PSMA-overexpressing cells led to the massive killing of cells that do not overexpress PSMA (Fig. 5 B and C). We noted a slight activation of PBMCs that led to killing of up to 20% of the Luc-expressing cells, when we cocultured the PBMCs with PPD/polyIC-treated PC3-Luc or MCF7-Luc cells, in the absence of PSMA-overexpressing cells (Fig. 5 B and C). We believe that PPD/polyIC accumulates in the medium of PC3-Luc or MCF7-Luc cells, because the vector cannot internalize into these cells. The polyIC that remains in the culture medium leads to the slight activation of the PBMCs, resulting in the killing of 20% of the Luc-expressing cells.
These experiments show that PPD/polyIC leads to strong direct and indirect bystander effects—killing cocultured cancer cells that are not themselves targeted by PPD/polyIC.
Systemic Application of PPD/polyIC Combined with PBMCs Induces Regression of Prostate Tumor Xenografts.
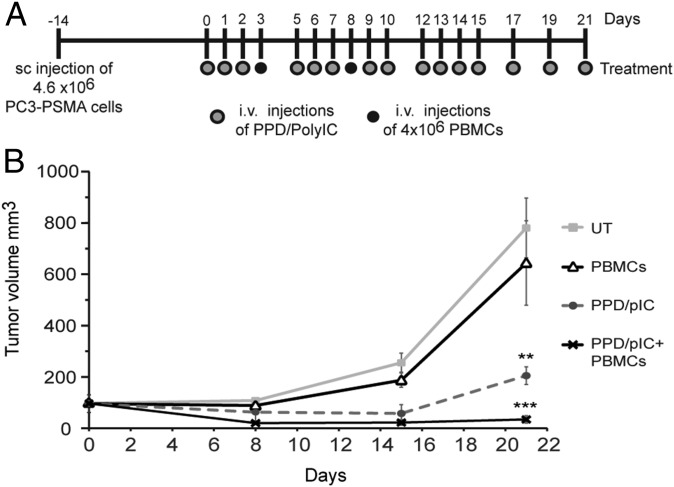
Finally, we investigated the effect of PPD/polyIC on prostate tumors in mice. We used a PC model, injecting NOD/SCID male mice s.c. with PC3-PSMA cells, which overexpress human PSMA. Treatment began when the tumors reached ∼100 mm3. To test the effect of the immune system on PPD/polyIC treatment, we partially reconstituted the animals’ immune systems using human PBMCs. The mice were treated with PPD/polyIC repeatedly over the course of 3 wk, and injected with PBMCs twice during the treatment (Fig. 6A). Although untreated mice and mice that were treated with PBMCs alone developed large tumors and were killed 3 wk after the initiation of treatment, the mice that were treated with PPD/polyIC showed a strong delay in tumor growth. Remarkably, the combination of PPD/polyIC treatment and PBMCs led to a reduction in tumor size. Even more impressively, in four of seven mice that received the combination treatment, the tumors were no longer detectable. The effect of the combination treatment was significantly better than the effect of PPD/polyIC administered alone, indicating that the combination of the direct tumor-killing effect of PPD/polyIC and the bystander effect elicited in the presence of immune cells leads to marked tumor regression (Fig. 6B).
Fig. 6.
PPD/polyIC led to the regression of PSMA-overexpressing tumors grown in NOD/SCID mice that were engrafted with human PBMCs. (A) Schematic representation of the treatment schedule. Gray dots indicate PPD/polyIC injections; black dots indicate PBMC injections. (B) Effect of PPD/polyIC on PC3-PSMA tumors. PC3-PSMA cells (4.6 × 106) were inoculated s.c. into male NOD/SCID mice. On day 0, mice bearing tumors of ∼100 mm3 in size were randomized and divided into four groups (seven mice per group). Mice were injected with PPD/polyIC alone, PBMCs alone or PPD/polyIC and PBMCs as shown in A. The graph shows means plus SEs (***P ≤ 0.001, PPD/polyIC plus PBMC treatment vs. untreated mice; **P ≤ 0.01, PPD/polyIC plus PBMC treatment vs. PPD/polyIC alone).
Discussion
PSMA is an ideal target antigen for the prognosis and treatment of advanced PC. It is present in nearly all prostate carcinomas at all stages of malignancy, and is elevated in late-stage hormone refractory tumors following androgen deprivation therapy (5, 29, 30). Despite the emergence of highly effective PSMA-targeted PET tracers for the detection of metastatic tumors (31), no PSMA-targeted therapy has entered the clinic to date.
Although PSMA is evident in virtually every prostate tumor, it is not actually overexpressed by all tumor cell subpopulations. An effective therapy should attack all tumor subpopulations—those that overexpress PSMA and those that do not. We present a PSMA-targeted therapy that delivers a viral dsRNA analog—namely, polyIC—to prostate tumors. Just as viral dsRNA activates an antiviral immune response to eradicate all infected cells in the area, PSMA-targeted polyIC directly attacks the PSMA-overexpressing cells and triggers an immune response that kills neighboring, untargeted cancer cells. Targeted polyIC is effective at low concentrations, and should avoid the toxic effects and strong systemic immune reactions caused by systemic polyIC application (32).
As a PSMA targeting moiety we chose the PSMA ligand DUPA. An analog of DUPA showed excellent targeting ability in the PET tracer 68Ga-PSMA HBED-CC, which accurately detected early lymph node, bone, and liver metastases that could not be detected by other methods (21, 22, 33). Access of DUPA to its PSMA binding site is through a deep, gradually narrowing tunnel with two hydrophobic pockets (23). To meet this structural requirement, when conjugating DUPA to the polyIC-carrying moiety, we had to expand the space between them using a linker. We therefore conjugated DUPA to the polyIC-binding moiety PP with the linker Cys-Gly-Trp-Trp-Gly-Phe (Fig. 1A), which provides optimal fit to the length and chemistry of the entry tunnel. The resulting compound, which we dubbed PPD (Fig. S3), successfully guided polyIC selectively to PSMA-overexpressing cells (Fig. 2A). The PEI moiety of PPD acts as a proton sponge, leading to endosome rupture (34–36). This process was extremely rapid (Fig. S5A). PolyIC is apparently released from the PPD complex owing to competition from RNAs in the cytoplasm (37), freeing the polyIC to activate dsRNA-dependent signaling proteins and downstream apoptotic pathways within 8 h and leading to complete cell eradication after 96 h (Fig. 2 B and C). The combination of high selectivity and rapid killing is expected to eradicate tumor cells before they are able to develop resistance, while minimizing toxic side effects.
Malignant tumors develop mechanisms that inhibit immune surveillance, so they successfully avoid elimination by the immune system (38). Our therapy acts against the tumor by several mechanisms, leading to tumor cell apoptosis and reinstating immune surveillance against the tumor. Although other therapies similarly target PSMA to deliver toxic compounds to overexpressing cells (39–42), our therapy also kills neighboring, untargeted cancer cells through the mediation of powerful bystander effects. PolyIC activates dsRNA-binding proteins, such as Toll-like receptor 3 (TLR3), dsRNA-dependent protein kinase (PKR), retinoic acid-inducible gene I (RIG-1), and melanoma differentiation-associated gene 5 (MDA5) (43). These signaling proteins simultaneously trigger a number of proapoptotic pathways, and also induce the tumor to secrete toxic and immunostimulatory cytokines.
Our results demonstrate that PPD/polyIC leads to the production of IFN-β, IP-10, and RANTES (Fig. 3). Type I family interferons (IFN-I), including IFN-α and IFN-β, are strongly activated by polyIC (44). IFN-I, either through endogenous production or through exogenous administration, is already in use as anticancer therapy (45). In PC, it has been shown that treatment with IFN-α and IFN-β can increase androgen receptor levels and potentially restore androgen sensitivity in androgen-independent tumors (46). IFN-I inhibits the proliferation and induces the apoptosis of cancer cells, and activates immune cells, stimulating an anticancer immune response (47). IP-10 and RANTES are key chemokines responsible for the attraction and extravasation of natural killer (NK) cells and of T lymphocytes (48, 49).
In our experiments, PPD/polyIC led to PBMC activation and chemotaxis (Fig. 4). Activation of the PBMCs was evident from the strong expression of IL-2 (Fig. 4B), which is mainly produced by activated CD4+ T-helper cells (50). The activated PBMCs secreted high levels of TNF-α and IFN-γ (Fig. 4B). TNF-α is cytotoxic toward several PC cell lines (PC-3, DU-145, and LNCaP) (51). IFN-γ, produced predominantly by activated CD4+ lymphocytes and NK cells, can increase the cell surface display of the MHC-1 and tumor-associated antigens, strongly increasing tumor immunogenicity (45). In PC cells, treatment with IFN-γ was shown to increase Fas-mediated apoptosis by up-regulating Fas expression (52).
It is likely that the local secretion of cytokines and chemokines from PPD/polyIC-treated tumors will trigger strong, localized bystander effects against untargeted tumor cells. In vitro, we demonstrated strong direct and immune-mediated effects on cocultured tumor cells. The direct bystander effect resulting from the toxic cytokines secreted by the treated cells killed up to 70% of neighboring untargeted cancer cells. Adding PBMCs, to model the immune cell-mediated bystander effect, led to a much stronger effect (Fig. 5 B and C). Compared with targeted delivery of polyIC alone, addition of PBMCs achieved higher levels of killing with much smaller doses. This suggests that PPD/PolyIC can avoid the toxic effects of systemic polyIC treatment.
In a xenograft model of PC, treatment with PPD/polyIC led to a dramatic reduction in tumor load. The combination of targeted polyIC and PBMCs halted tumor growth and, in more than half of the mice, led to tumor eradication within 2 wk (Fig. 6B). To avoid toxicity, we used only a small number of PBMCs. The powerful effect of such a small number of PBMCs implies that they were recruited directly to the tumor site. The speed and potency of this treatment should forestall the development of resistance. Moreover, the bystander effect should allow the treatment to eradicate heterogeneous tumors, as we have previously shown with EGFR-targeted polyIC (17). In human patients, with an active immune system, we anticipate that PSMA-targeted polyIC may be even more effective. Our treatment does not need to be personalized for each patient and can be prepared for a fraction of the price of autologous antigen presentings cells (APCs) (53, 54).
PC is an excellent candidate for effective targeted therapy because it expresses specific markers. PSMA is a promising antigen for this purpose, but hitherto it has entered the clinic only for imaging. The importance of engaging the immune system against cancer is now recognized. PPD/polyIC was designed as a targeted therapy, which leads both to the direct destruction of the tumor and to the recruitment of the immune system against the tumor. The preclinical data presented here show that this double-edged approach has strong potential to improve the outlook for PC patients.
Materials and Methods
Cancer cell lines and growth conditions are described in SI Materials and Methods. Cell survival was assayed using CellTiter-Glo (Promega) according to the manufacturer’s instructions. For confocal microscopy, cells were grown in µ-Slides (Ibidi) and visualized using a FluoView FV1000 Olympus microscope. Chemotaxis assays used Transwell plates (Corning).
PPD/PolyIC Complex Formation.
Low molecular weight polyIC (InvivoGen) was used for all experiments. PPD vector was complexed with polyIC at a ratio of N/P = 8 [nitrogen (from PPD)/phosphate (from polyIC)] in HBG buffer (20 mM Hepes, pH 7.4, 5% glucose, wt/vol) for in vivo experiments, or in HBS (20 mM Hepes, pH 7.4, 150 mM NaCl) for in vitro experiments. PPD was incubated with polyIC for 45 min at room temperature.
Xenograft PC Model with Reconstituted Immune System.
The 4.6 × 106 PC3-PSMA cells were injected s.c. into NOD/SCID male mice (Harlan Laboratories, Inc.). When the tumors reached ∼100 mm3 in size, the mice were randomized and divided into four groups (seven mice per group). On the days indicated, two groups were treated with i.v. injections of PPD/polyIC (0.25 mg/kg, N/P ratio 8), and two groups were left untreated. In two groups, one treated and one untreated, an immune system was partially reconstituted by i.v. injection of 4 × 106 human PBMCs on days 3 and 8. Tumor volumes were calculated from measurements of tumor length (L) and width (W), using digital calipers, by the formula W2 × L/2. All animal protocols were approved by the Hebrew University of Jerusalem Institutional Animal Care and Use Committee.
The sources of additional reagents and detailed methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Prof. Michel Sadelain for providing PC3-PSMA-expressing cells; Aviva Petcho for assistance with cell culture; Dr. Naomi Book for assistance with confocal imaging; and Dr. Mario Lebendiker for assistance with purification. This study was partially supported by European Research Council Advanced Grant 249898 (to A.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714587115/-/DCSupplemental.
References
- 1.Liu H, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58:4055–4060. [PubMed] [Google Scholar]
- 2.Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol. 2005;288:C975–C981. doi: 10.1152/ajpcell.00506.2004. [DOI] [PubMed] [Google Scholar]
- 3.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 4.Wright GL, Jr, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 5.Mannweiler S, et al. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15:167–172. doi: 10.1007/s12253-008-9104-2. [DOI] [PubMed] [Google Scholar]
- 6.Fu W, Madan E, Yee M, Zhang H. Progress of molecular targeted therapies for prostate cancers. Biochim Biophys Acta. 2012;1825:140–152. doi: 10.1016/j.bbcan.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galsky MD, et al. Phase I trial of the prostate-specific membrane antigen-directed immunoconjugate MLN2704 in patients with progressive metastatic castration-resistant prostate cancer. J Clin Oncol. 2008;26:2147–2154. doi: 10.1200/JCO.2007.15.0532. [DOI] [PubMed] [Google Scholar]
- 8.Kuroda K, et al. Saporin toxin-conjugated monoclonal antibody targeting prostate-specific membrane antigen has potent anticancer activity. Prostate. 2010;70:1286–1294. doi: 10.1002/pros.21164. [DOI] [PubMed] [Google Scholar]
- 9.Ejadi S, et al. Phase 1 study of the PSMA-tubulysin small-molecule drug conjugate EC1169 in pts with metastatic castrate-resistant prostate cancer (mCRPC) J Clin Oncol. 2016 doi: 10.1200/jco.2015.33.15_suppl.e13527. [DOI] [Google Scholar]
- 10.Petrylak DP, et al. A phase 2 study of prostate specific membrane antigen antibody drug conjugate (PSMA ADC) in patients (pts) with progressive metastatic castration-resistant prostate cancer (mCRPC) following abiraterone and/or enzalutamide (abi/enz) J Clin Oncol. 2015 doi: 10.1200/jco.2015.33.7_suppl.144. [DOI] [Google Scholar]
- 11.Luo J, Beer TM, Graff JN. Treatment of nonmetastatic castration-resistant prostate cancer. Oncology (Williston Park) 2016;30:336–344. [PubMed] [Google Scholar]
- 12.Attard G, et al. Improving the outcome of patients with castration-resistant prostate cancer through rational drug development. Br J Cancer. 2006;95:767–774. doi: 10.1038/sj.bjc.6603223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javidan J, Deitch AD, Shi XB, de Vere White RW. The androgen receptor and mechanisms for androgen independence in prostate cancer. Cancer Invest. 2005;23:520–528. doi: 10.1080/07357900500202721. [DOI] [PubMed] [Google Scholar]
- 14.Shir A, Ogris M, Roedl W, Wagner E, Levitzki A. EGFR-homing dsRNA activates cancer-targeted immune response and eliminates disseminated EGFR-overexpressing tumors in mice. Clin Cancer Res. 2011;17:1033–1043. doi: 10.1158/1078-0432.CCR-10-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders LR, Barber GN. The dsRNA binding protein family: Critical roles, diverse cellular functions. FASEB J. 2003;17:961–983. doi: 10.1096/fj.02-0958rev. [DOI] [PubMed] [Google Scholar]
- 16.Schaffert D, et al. Poly(I:C)-mediated tumor growth suppression in EGF-receptor overexpressing tumors using EGF-polyethylene glycol-linear polyethylenimine as carrier. Pharm Res. 2011;28:731–741. doi: 10.1007/s11095-010-0225-4. [DOI] [PubMed] [Google Scholar]
- 17.Shir A, Ogris M, Wagner E, Levitzki A. EGF receptor-targeted synthetic double-stranded RNA eliminates glioblastoma, breast cancer, and adenocarcinoma tumors in mice. PLoS Med. 2006;3:e6. doi: 10.1371/journal.pmed.0030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zigler M, et al. HER2-targeted polyinosine/polycytosine therapy inhibits tumor growth and modulates the tumor immune microenvironment. Cancer Immunol Res. 2016;4:688–697. doi: 10.1158/2326-6066.CIR-15-0203. [DOI] [PubMed] [Google Scholar]
- 19.Thomas M, et al. Ligand-targeted delivery of small interfering RNAs to malignant cells and tissues. Ann N Y Acad Sci. 2009;1175:32–39. doi: 10.1111/j.1749-6632.2009.04977.x. [DOI] [PubMed] [Google Scholar]
- 20.Kularatne SA, et al. Synthesis and biological analysis of prostate-specific membrane antigen-targeted anticancer prodrugs. J Med Chem. 2010;53:7767–7777. doi: 10.1021/jm100729b. [DOI] [PubMed] [Google Scholar]
- 21.Rauscher I, et al. Intrapatient comparison of 111In-PSMA I&T SPECT/CT and hybrid 68Ga-HBED-CC PSMA PET in patients with early recurrent prostate cancer. Clin Nucl Med. 2016;41:e397–e402. doi: 10.1097/RLU.0000000000001273. [DOI] [PubMed] [Google Scholar]
- 22.Rauscher I, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: Comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57:1713–1719. doi: 10.2967/jnumed.116.173492. [DOI] [PubMed] [Google Scholar]
- 23.Kularatne SA, Zhou Z, Yang J, Post CB, Low PS. Design, synthesis, and preclinical evaluation of prostate-specific membrane antigen targeted (99m)Tc-radioimaging agents. Mol Pharm. 2009;6:790–800. doi: 10.1021/mp9000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joubran S, et al. Optimization of liganded polyethylenimine polyethylene glycol vector for nucleic acid delivery. Bioconjug Chem. 2014;25:1644–1654. doi: 10.1021/bc500252a. [DOI] [PubMed] [Google Scholar]
- 25.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Neale JH, Pomper MG, Kozikowski AP. NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov. 2005;4:1015–1026. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]
- 27.Kularatne SA, Wang K, Santhapuram HK, Low PS. Prostate-specific membrane antigen targeted imaging and therapy of prostate cancer using a PSMA inhibitor as a homing ligand. Mol Pharm. 2009;6:780–789. doi: 10.1021/mp900069d. [DOI] [PubMed] [Google Scholar]
- 28.Kruse N, Moriabadi NF, Toyka KV, Rieckmann P. Characterization of early immunological responses in primary cultures of differentially activated human peripheral mononuclear cells. J Immunol Methods. 2001;247:131–139. doi: 10.1016/s0022-1759(00)00316-1. [DOI] [PubMed] [Google Scholar]
- 29.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–640. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 30.Ross JS, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–6362. [PubMed] [Google Scholar]
- 31.Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur J Nucl Med Mol Imaging. 2012;39:1085–1086. doi: 10.1007/s00259-012-2069-0. [DOI] [PubMed] [Google Scholar]
- 32.Krown SE, Kerr D, Stewart WE, 2nd, Field AK, Oettgen HF. Phase I trials of poly(I,C) complexes in advanced cancer. J Biol Response Mod. 1985;4:640–649. [PubMed] [Google Scholar]
- 33.Perera M, et al. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. Eur Urol. 2016;70:926–937. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Boussif O, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 36.Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 37.Bertschinger M, et al. Disassembly of polyethylenimine-DNA particles in vitro: Implications for polyethylenimine-mediated DNA delivery. J Control Release. 2006;116:96–104. doi: 10.1016/j.jconrel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henry MD, et al. A prostate-specific membrane antigen-targeted monoclonal antibody-chemotherapeutic conjugate designed for the treatment of prostate cancer. Cancer Res. 2004;64:7995–8001. doi: 10.1158/0008-5472.CAN-04-1722. [DOI] [PubMed] [Google Scholar]
- 40.Wolf P, Gierschner D, Bühler P, Wetterauer U, Elsässer-Beile U. A recombinant PSMA-specific single-chain immunotoxin has potent and selective toxicity against prostate cancer cells. Cancer Immunol Immunother. 2006;55:1367–1373. doi: 10.1007/s00262-006-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C, Hasegawa K, Russell SJ, Sadelain M, Peng KW. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate. 2009;69:1128–1141. doi: 10.1002/pros.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bander NH, et al. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–4601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 43.Levitzki A. Targeting the immune system to fight cancer using chemical receptor homing vectors carrying polyinosine/cytosine (PolyIC) Front Oncol. 2012;2:4. doi: 10.3389/fonc.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Zhang J, Yu FS. Toll-like receptor 2-mediated expression of beta-defensin-2 in human corneal epithelial cells. Microbes Infect. 2006;8:380–389. doi: 10.1016/j.micinf.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hastie C. Interferon gamma, a possible therapeutic approach for late-stage prostate cancer? Anticancer Res. 2008;28:2843–2849. [PubMed] [Google Scholar]
- 46.Basrawala Z, et al. Androgen receptor levels are increased by interferons in human prostate stromal and epithelial cells. Oncogene. 2006;25:2812–2817. doi: 10.1038/sj.onc.1209304. [DOI] [PubMed] [Google Scholar]
- 47.Parker BS, Rautela J, Hertzog PJ. Antitumour actions of interferons: Implications for cancer therapy. Nat Rev Cancer. 2016;16:131–144. doi: 10.1038/nrc.2016.14. [DOI] [PubMed] [Google Scholar]
- 48.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 49.Huang H, Xiang J. Synergistic effect of lymphotactin and interferon gamma-inducible protein-10 transgene expression in T-cell localization and adoptive T-cell therapy of tumors. Int J Cancer. 2004;109:817–825. doi: 10.1002/ijc.20043. [DOI] [PubMed] [Google Scholar]
- 50.Bachmann MF, Oxenius A. Interleukin 2: From immunostimulation to immunoregulation and back again. EMBO Rep. 2007;8:1142–1148. doi: 10.1038/sj.embor.7401099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherwood ER, Pitt Ford TR, Lee C, Kozlowski JM. Therapeutic efficacy of recombinant tumor necrosis factor alpha in an experimental model of human prostatic carcinoma. J Biol Response Mod. 1990;9:44–52. [PubMed] [Google Scholar]
- 52.Selleck WA, et al. IFN-gamma sensitization of prostate cancer cells to Fas-mediated death: A gene therapy approach. Mol Ther. 2003;7:185–192. doi: 10.1016/s1525-0016(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 53.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 54.Karan D. Prostate immunotherapy: Should all guns be aimed at the prostate-specific antigen? Immunotherapy. 2013;5:907–910. doi: 10.2217/imt.13.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 56.Kelderhouse LE, et al. Development of tumor-targeted near infrared probes for fluorescence guided surgery. Bioconjug Chem. 2013;24:1075–1080. doi: 10.1021/bc400131a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.