Significance
The concept that ovarian cycles in female mammals are regulated by gonadal steroids released from the ovary is well-established dogma. The findings of this study, however, showing that the removal of neuroestradiol synthesis in the median eminence of the hypothalamus by the aromatase inhibitor, letrozole, greatly attenuates estrogen-induced gonadotropin releasing hormone and luteinizing hormone surges in ovariectomized female monkeys, indicate that neuroestradiol locally synthesized and released in the hypothalamus is an integral part of the ovary-initiated positive feedback mechanism of estradiol.
Keywords: neuroestradiol, GnRH, kisspeptin, preovulatory surge, aromatase
Abstract
Negative and positive feedback effects of ovarian 17β-estradiol (E2) regulating release of gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) are pivotal events in female reproductive function. While ovarian feedback on hypothalamo–pituitary function is a well-established concept, the present study shows that neuroestradiol, locally synthesized in the hypothalamus, is a part of estrogen’s positive feedback loop. In experiment 1, E2 benzoate-induced LH surges in ovariectomized female monkeys were severely attenuated by systemic administration of the aromatase inhibitor, letrozole. Aromatase is the enzyme responsible for synthesis of E2 from androgens. In experiment 2, using microdialysis, GnRH and kisspeptin surges induced by E2 benzoate were similarly attenuated by infusion of letrozole into the median eminence of the hypothalamus. Therefore, neuroestradiol is an integral part of the hypothalamic engagement in response to elevated circulating E2. Collectively, we will need to modify the concept of estrogen’s positive feedback mechanism.
Estradiol (E2), predominantly synthesized by the ovaries, feeds back to the hypothalamus and anterior pituitary to regulate release of gonadotropin releasing hormone (GnRH) and gonadotropins, respectively. In addition to the ovary, the brain is also a source of E2 synthesis and release. This “neuroestradiol” modulates various neuronal functions (1). Previously, we have shown that neuroestradiol, synthesized and released in the median eminence of the hypothalamus in ovariectomized (OVX) female rhesus monkeys, contributes to control pulsatile GnRH release (2). Neuroestradiol action differs from classical ovarian E2 negative feedback action. Prolonged infusion of E2 benzoate (EB) into the median eminence, mimicking neuroestradiol release, continuously stimulates the release of GnRH and kisspeptin in the median eminence and increases circulating luteinizing hormone (LH) (3), unlike the suppressive effects seen when EB is injected systemically (4). Because the sustained elevation of GnRH and LH release induced by prolonged EB infusion into the median eminence is reminiscent of the preovulatory surge (3), we hypothesized that release of neuroestradiol in the median eminence is an integral part of preovulatory GnRH and LH surges.
During the late follicular phase, the preovulatory gonadotropin surge is initiated by increased levels of circulating E2 and progesterone from the preovulatory follicle. Experimentally, two different E2 replacement strategies are used for inducing GnRH/LH surges in OVX nonhuman primates. An E2 capsule is implanted into OVX females to mimic LH levels during the follicular phase and 2–3 wk later an additional dose of EB is injected systemically (5, 6), or a single large dose of EB alone is injected systemically in OVX females (7, 8). Both approaches are sufficient to induce an LH surge, resembling the preovulatory LH surge (7). GnRH and LH surges induced by systemic administration of EB in primates have a latency of ∼24 h and last 36–60 h (9–11). Using these two GnRH/LH surge models, in the present study we investigated the role of neuroestradiol in estrogen’s positive feedback effects on the release of GnRH, kisspeptin, and LH in OVX female rhesus monkeys.
Results
Letrozole Attenuates the Estrogen-Induced LH Surge (Experiment 1).
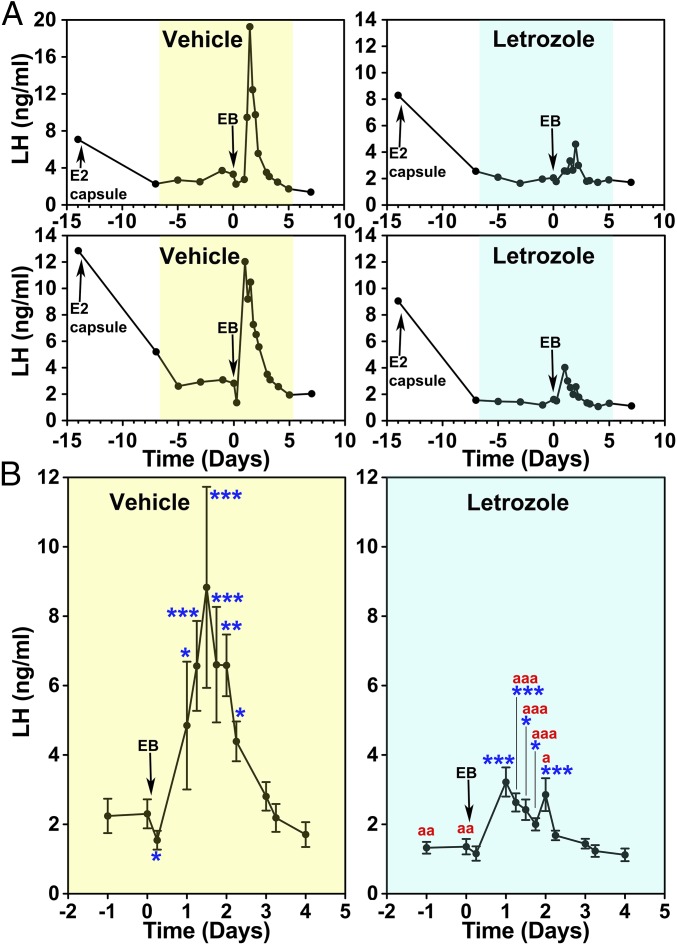
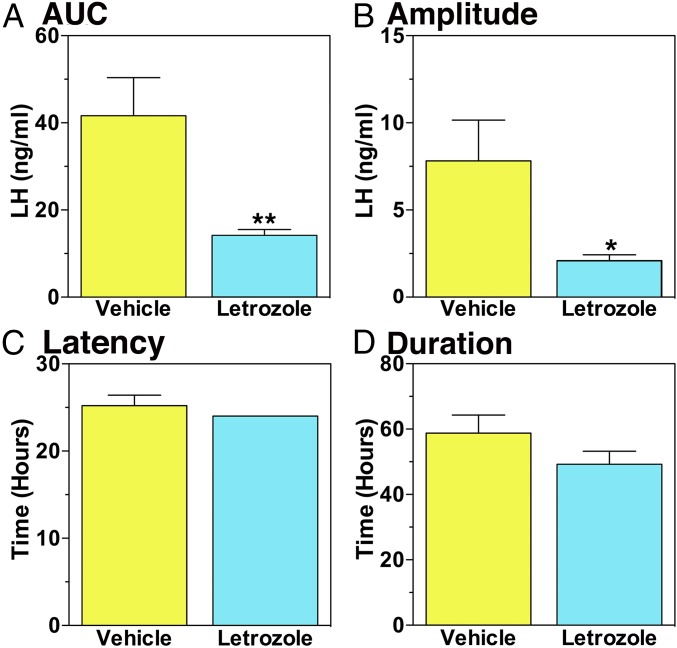
The effects of letrozole or vehicle on the E2-induced LH surge were examined with a protocol schematically shown in Fig. S1A. All animals received s.c. implantation of an E2 capsule 14 d before systemic EB (day 0, the day of EB injection), and the E2 capsule remained throughout the entire experiment. As shown previously (6), E2 capsule implantation in OVX female monkeys suppresses LH levels within a week (compare day −14 to day −7, Fig. 1A). Starting at day −7, they were injected daily with letrozole or vehicle through day 5. Before EB injection (days −5, −3, −1, and 0) letrozole, not vehicle, treatment caused a slight, but significant, suppression of baseline LH levels (Fig. 1B). In the vehicle group, EB injection on day 0 suppressed LH at 6 h (negative feedback phase), followed by stimulation of LH surge starting at 24 h and peaking at 30–48 h after EB injection (positive feedback phase, Fig. 1). The elevated LH release continued up to 54 h after EB. In contrast, letrozole treatment significantly attenuated the LH surge (Fig. 1). Analysis indicated that both area under the curve (AUC) and LH surge amplitude in the letrozole group had 67% and 73% reductions compared with the vehicle group, respectively (Fig. 2 A and B). Letrozole treatment did not alter the latency to or duration of the LH surge (Fig. 2 C and D). Letrozole treatment did not change elevated circulating E2 levels resulting from estrogen capsule implantation or EB injection (Fig. S2).
Fig. 1.
Letrozole attenuated the EB-induced LH surge in OVX female monkeys. (A) Changes in LH release from two examples in each (vehicle- and letrozole-treated groups shown in yellow and blue, respectively) during the entire course of the experiment and (B) group data (mean ± SEM) highlighting the EB-induced LH surge are shown. All animals were implanted with capsules containing estradiol (E2) on day −14 and received EB injection on day 0. In both groups, E2 capsule lowered LH levels, similar to those during the early follicular phase. Analysis indicated that EB induced significant LH elevations over baseline LH levels before EB injection in both vehicle (n = 5)- and letrozole (n = 5)-treated animals (for both P < 0.001) and that the EB-induced LH surge with letrozole was significantly (P < 0.001) smaller than that with vehicle. Bonferroni post hoc analysis indicated that the LH levels in the vehicle group measured at day 0.25 were significantly (*P < 0.05) lower than before EB and LH levels at days 1, 1.25, 1.5, 1.75, 2, and 2.25 were (*P < 0.05, **P < 0.01, and ***P < 0.001) significantly higher than before EB treatment. Similarly, LH levels in the letrozole-treated group at days 1, 1.25, 1.5, 1.75, and 2 were (*P < 0.05 and ***P < 0.001) higher than before treatment. Importantly, LH levels on days −3, −1, 0, 1.25, 1.5, 1.75, and 2 in the letrozole treatment were significantly (aP < 0.5, aaP < 0.01, and aaaP < 0.001) lower than those in the vehicle group.
Fig. 2.
Letrozole attenuated the area under the curve (AUC, A, **P = 0.01) and amplitude (B, *P = 0.04) of the EB-induced LH surge, but not the latency (C) and duration (D) of the EB-induced LH surge.
Letrozole Infusion into the Median Eminence Attenuates the EB-Induced GnRH and Kisspeptin Surge (Experiment 2).
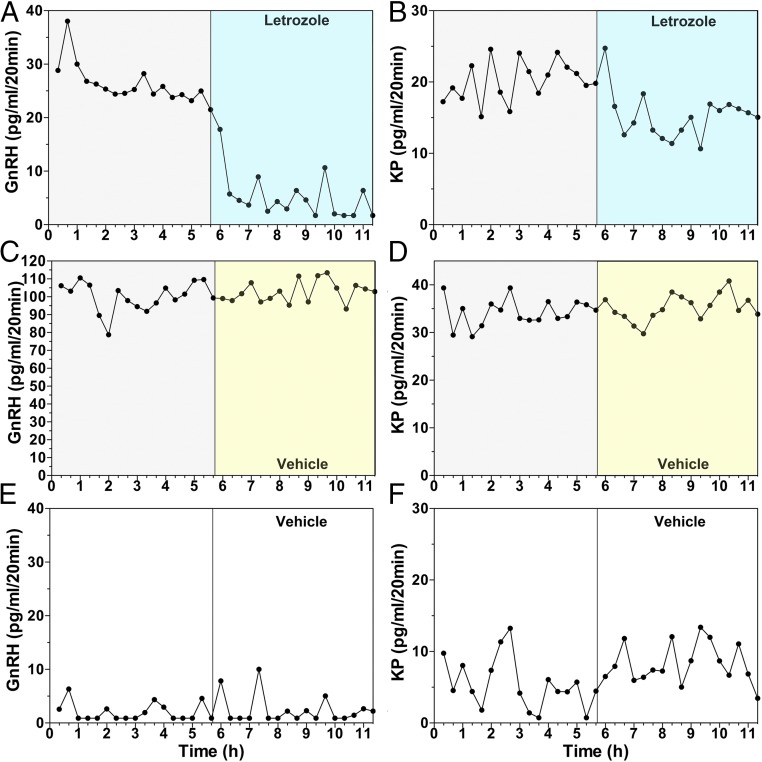
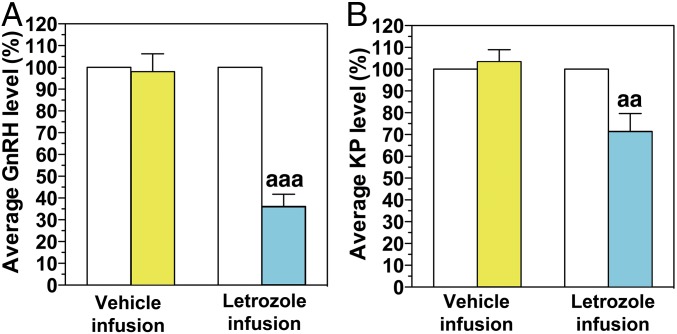
The attenuation of the AUC and amplitude of the LH surge by letrozole in experiment 1 suggested that neuroestradiol locally synthesized in the hypothalamus may play a role in the EB-induced GnRH surge. To determine this possibility, we examined the effects of letrozole infusion into the median eminence on the EB-induced surge of GnRH and kisspeptin using a microdialysis method. The rationale for kisspeptin measurement is based on its role in the E2 positive feedback effects (12). Because the microdialysis experiments in conscious monkeys were limited to <12 h, we collected dialysates between 36 and 48 h after EB or oil injection, during which vehicle was infused for the first 5.7 h followed by a 5.7-h letrozole infusion into the median eminence (Fig. S1B). As seen in Fig. 3 and Table S1, EB injection induced GnRH surges: GnRH levels were higher over oil control during the corresponding period (compare the first 5.7 h in Fig. 3 A and C vs. Fig. 3E and Table S1). Similarly, EB induced kisspeptin surges. Kisspeptin levels during the first 5.7 h in the EB-treated group were significantly higher than the oil control group during the corresponding period (Fig. 3 B and D vs. Fig. 3F and Table S1). Letrozole infusion into the median eminence suppressed GnRH (Figs. 3A and 4A and Table S1) and kisspeptin release (Figs. 3B and 4B and Table S1), whereas vehicle infusion had no effect on GnRH (Figs. 3C and 4A and Table S1) or kisspeptin release (Figs. 3D and 4B and Table S1).
Fig. 3.
Examples showing the effects of letrozole (A and B) or vehicle (C and D) infusion into the median eminence on EB-induced GnRH (Left column) and kisspeptin (Right column) surges, examined using microdialysis in OVX female monkeys. While in A–D, EB was injected 36 h before sample collection, in E and F, only oil (vehicle for EB) was injected 36 h before the start of dialysate collection. Both GnRH and kisspeptin levels during the first 5.7 h in EB-injected animals (A–D) were higher than those in oil controls (E and F, see also Table S1), indicating EB induced GnRH and kisspeptin surges. In EB-treated animals letrozole infusion (A and B) suppressed both GnRH and kisspeptin release within 20 min, whereas vehicle infusion (C and D) did not change release of GnRH and kisspeptin. Note that there was considerable individual variation in elevated GnRH and kisspeptin levels after EB injection.
Fig. 4.
Letrozole infusion into the median eminence suppressed EB-induced GnRH (A) and kisspeptin (KP, B) surges. The data are shown as the % change relative to the peptide level before letrozole or vehicle infusion. Letrozole infusion significantly suppressed GnRH release (aaaP < 0.001; n = 7, A) and kisspeptin release (aaP < 0.01; n = 7, B), whereas vehicle infusion did not induce any effects.
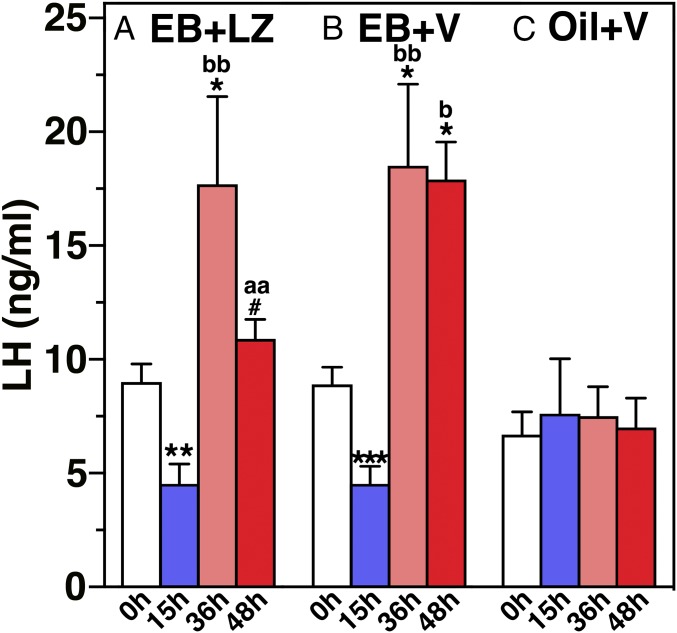
Changes in LH levels in blood samples obtained during the course of experiment 2 (Fig. S1B) suggested that EB suppressed LH levels at 15 h (negative feedback phase, Fig. 5), whereas it stimulated LH release at 36 h EB over baseline LH levels (positive feedback phase, Fig. 5) in both the EB-treated vehicle and letrozole infusion groups. Importantly, LH levels after letrozole infusion in the EB-treated group at 48 h were significantly lower than those in the EB-treated vehicle infusion group at the corresponding time (Fig. 5). In contrast, the oil-injected group had no changes (Fig. 5).
Fig. 5.
Letrozole infusion into the median eminence suppressed LH release. Blood samples were obtained at 0 h (before), 15, 36, and 48 h after s.c. EB (A and B) or oil (C) injection. In A, letrozole (LZ) was infused 42–48 h after EB, whereas in B, vehicle (V) was similarly infused 42–48 h after EB. Analysis indicated that EB injection induced biphasic changes in LH release (negative and positive feedback effects) (P < 0.0001 for both A and B). LH levels in the EB-injected and letrozole-infused group were also statistically different from EB-injected vehicle infusion (P = 0.01). Oil injection did not induce any LH change. Post hoc analysis indicated that LH levels at 15 h after EB were significantly lower (**P < 0.01 for A, ***P < 0.001 for B) than before EB (0 h). LH levels at 36 h after EB in both EB-treated groups and 48 h in the EB + V group were significantly higher (*P < 0.05 for all) than before EB in respective EB-treated group. Similarly, LH levels at 36 h after EB in EB + LZ and EB + V groups and 48 h after EB + V group were higher than corresponding time in the oil + V group (bP < 0.05; bbP < 0.01). LH levels in the EB-injected letrozole-infused group at 48 h were lower than values at 36 h within the group (#P < 0.05) and at the corresponding time in the EB-injected vehicle-infused group (aaP < 0.01), but values were no longer different from before EB.
Discussion
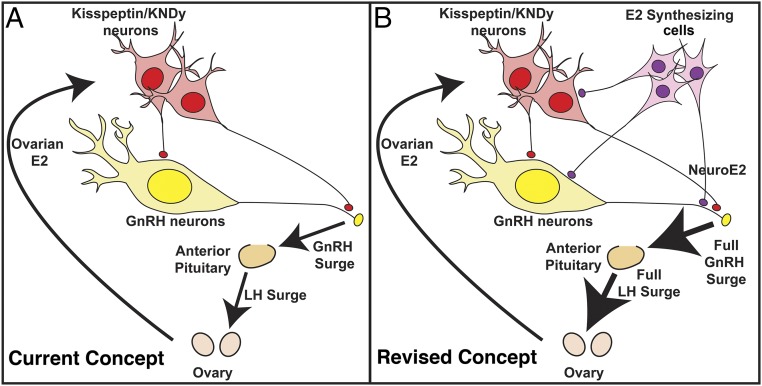
In the present study, we found that letrozole attenuated the EB-induced LH and GnRH surge by approximately two-thirds in OVX female monkeys. In experiment 1, the latency and duration of the LH surges were not affected by letrozole treatment. This indicates that an elevation of circulating E2, presumably from a preovulatory follicle in the ovary, is necessary to trigger GnRH and LH surges. Once the surge starts, it lasts over 24 h. The results further indicate that a sustained increase in E2 in the hypothalamus is required for full GnRH/LH surges: While elevated E2 in general circulation lasts only ∼30 h (4, 6), the amplitude and the area under the curve (total release) of the LH surge were severely attenuated in the presence of letrozole. Despite the removal of the ovarian source of E2, cells in the hypothalamus and anterior pituitary synthesize E2 (13–15). As such, we speculated that suppression of neuroestradiol synthesis in the median eminence with letrozole during the EB-induced GnRH/LH surge would modify GnRH levels. Indeed, in experiment 2, letrozole infusion into the median eminence reduced release of GnRH and kisspeptin. Collectively, the results of this study suggest that, while a rise in circulating E2 stimulates interneurons, such as kisspeptin neurons, resulting in GnRH neuronal activation inducing the GnRH surge (12), circulating E2 also stimulates E2 synthesizing cells in the hypothalamus. This locally synthesized E2 (i.e., neuroestradiol) further augments the GnRH surge directly or indirectly through interneurons (Fig. 6B). This view is significantly different from the traditional concept of the ovarian centric positive feedback loop (Fig. 6A).
Fig. 6.
Schematic illustration of the positive feedback effects of E2. The current concept shown in A is that an increase in E2 from ovarian follicles during the late follicular phase gives a signal to interneurons expressing estrogen receptor (ER)α, such as kisspeptin and/or KNDy (kisspeptin, neurokinin B, and dynorphin) neurons, in the hypothalamus, which stimulates perikarya and neuroterminals of GnRH neurons resulting in GnRH surge and subsequent LH surge from the pituitary. However, the results of this study indicate that the current concept is insufficient, as the removal of locally synthesized E2 (neuroestradiol) in the median eminence by the aromatase inhibitor, letrozole, reduces the amount of GnRH and LH by over 65%. Therefore, we propose to revise the concept (B). That is, an increase in E2 from the ovary stimulates not only the interneurons such as kisspeptin neurons, but also cells that locally synthesize E2 (neuroestradiol), which stimulates GnRH neurons directly or indirectly resulting in a full GnRH and LH surge (B). The characteristics of E2 synthesizing cells are yet to be determined.
In humans, clinical treatment with letrozole increases LH levels (16, 17), because suppression of E2 synthesis in gonads results in a reduction in negative feedback. However, we observed that letrozole treatment in OVX animals suppressed baseline LH levels beyond the negative feedback action induced by E2 capsules (4, 6), i.e., unlike in control animals, EB injection did not suppress the LH level at 6 h in letrozole-treated animals. The baseline LH suppression with letrozole treatment before EB injection indicates that nonovarian E2 stimulates the GnRH neurosecretory system and/or the pituitary gonadotropes, as animals were OVX. This view is consistent with our previous findings that neuroestradiol in the hypothalamus stimulates GnRH release in OVX monkeys (2). The question of whether low baseline LH/GnRH levels influence the magnitude of the LH/GnRH surge remains to be addressed in the future. The dynamics between neuroestradiol synthesis and circulating E2, including those during ovulatory cycles in intact females, are also unknown.
The role of neuroestradiol in the preovulatory GnRH surge might be specific to primates or perhaps species with a luteal phase. This speculation is based on two distinct differences in primates/ruminants and rodents. First, preovulatory GnRH surges continue well after the end of LH surges in sheep and monkeys (9, 10, 18), both known to have a luteal phase. In contrast, in rats, which lack a true luteal phase, GnRH surges complete at the same time as LH surges, despite secondary GnRH increases several hours after the first surge (19). Second, aromatase-deficient humans and mice are infertile (20), but ovariectomized aromatase knockout mice respond to E2 challenge with full LH surges (21), unlike the monkeys treated with letrozole in this study. There is no study in aromatase-deficient humans in this context. Although the importance of pulsatile GnRH in maintenance of luteal function has been demonstrated (22), deprivation of elevated GnRH during the prolonged phase of GnRH surge has not been examined. Together, the prolonged GnRH surge stimulated by local neuroestradiol seems to be critical for establishment of the corpus luteum.
The finding that neuroestradiol release is required for full GnRH surges is quite exciting, as suppression of neuroestradiol synthesis would result in a failure of ovulation. Among clinical reports using aromatase inhibitors for estrogen receptor (ER)-sensitive breast cancer in postmenopausal women (23), reproductive assistance for induction of ovulation in anovulatory patients with polycystic ovarian syndrome (24–26), or treatment in patients with endometriosis (27), two papers reported that letrozole-treated patients for induction of ovulation had lower LH surge levels (28) and a lower pregnancy rate (29). While those reports are consistent with our finding, more systematic studies in humans are necessary to confirm the observation. Nonetheless, theoretically, the findings from this study raise the possibility that a new drug for contraception may be developed, because the human aromatase gene has multiple tissue-specific promoters/exons, including exon 1, which is brain specific (30).
The positive feedback effect of E2 on GnRH release has been extensively investigated in rodents. Because GnRH neurons express ERβ, not ERα (31, 32), whereas kisspeptin neurons, which directly innervate GnRH neurons (33), express ERα (34), and selective deletion of ERα specific to kisspeptin neurons results in a persistent estrous, anovulatory state (35), kisspeptin neurons in the anteroventral periventricular (AVPV) region have been regarded as a mediator of estrogen action (12). Recent studies also indicate that activation of kisspeptin neurons in the AVPV region occurs at the time of the E2-induced LH surge in nonhuman primates (36, 37). Unlike in rodents, however, participation of AVPV kisspeptin neurons may be redundant in primates, and kisspeptin neurons in the arcuate nucleus may be sufficient for estrogen’s positive feedback effects, as surgical isolation of the medial basal hypothalamus from the rest of the brain does not interfere with cyclic ovulations in monkeys (38). Our findings demonstrating that during the EB-induced GnRH surge, kisspeptin release was not only elevated but also attenuated by letrozole, suggest that kisspeptin neurons, especially their neuroterminal fibers, are a part of estrogen’s positive feedback loop. Perhaps, kisspeptin neurons are a mediator of neuroestradiol.
Infusion of EB directly into the median eminence stimulates GnRH release within minutes (2, 3), whereas systemic injection of EB results in the negative feedback effects on LH and GnRH release for the first 2–18 h (4), followed by the positive feedback effects for the subsequent 24–60 h (5, 7–11). The difference in the two modes of EB administration is likely attributable to the difference in neurocircuitries involved in estrogen action. While direct EB infusion is limited to GnRH and kisspeptin neuroterminals and perhaps a small number of their cell bodies (2, 3), EB injected systemically diffuses widely into the preoptic area and basal hypothalamus, where neurons expressing ERα, including kisspeptin neurons, are distributed. Activation of AVPV kisspeptin neurons by E2 resulting in the GnRH surge requires DNA transcription (12). In contrast, a series of our in vitro studies suggests that direct, rapid action of E2 on GnRH neurons mediated by a non-ERα mechanism occurs at the membrane level (39). Collectively, we hypothesize that the two modes of estrogen action are sequentially involved in the positive feedback effect of E2, hence the preovulatory GnRH surge. First, increased E2 from the ovaries stimulates ERα in AVPV kisspeptin neurons as well as neuroestradiol synthesizing cells in the hypothalamus. Second, neuroestradiol released in the median eminence further augments the GnRH surge through rapid membrane action at GnRH neuroterminals.
Previously, we found that letrozole infusion into the median eminence quickly and completely suppressed neuroestradiol release (2). Nevertheless, one might raise concern as to the specificity of the aromatase inhibitor letrozole, as letrozole may interfere with other P450 enzymes, such as CYP2A6, which would reduce letrozole clearance (40). Alternatively, others might raise concern as to whether the change induced by letrozole is the consequence of reduction in estrogens or elevation of androgens. Direct measurement of estradiol along with other steroids in the hypothalamus during the surge with liquid chromatography-mass spectrometry will provide a clear picture.
In conclusion, the results from this study indicate that a nonovarian source of E2, namely neuroestradiol, stimulates GnRH release. Moreover, the augmentation of GnRH release by neuroestradiol, locally synthesized in the median eminence, is an integral part of the positive feedback effects of E2. Nonetheless, the current finding may well lead to therapeutic approaches for treatments of anovulation and tools for contraception in women.
Materials and Methods
Animals and Experimental Design.
A total of 14 OVX female Rhesus macaques (Macaca mulatta) were used in this study. All animals were OVX at least 1 mo before experimentation, and pair-housed under standard housing conditions in the Wisconsin National Primate Research Center. Experiments were approved by the Animal Care and Use Committee at the University of Wisconsin-Madison and conducted under the guidelines of the NIH and US Department of Agriculture.
In experiment 1, we tested the hypothesis that a nonovarian source of E2 plays a role in positive feedback action of E2 on LH surges using the aromatase inhibitor, letrozole, in OVX monkeys. A total of seven animals (age: 11.0 ± 1.7 y) were implanted with estrogen capsules for 2 wk, mimicking the hormonal conditions seen during the midfollicular phase, and subsequently 30 µg EB in sesame oil was s.c. injected (designated day 0) to induce LH surges (Fig. S1A). Beginning on day −7 and continuing through day 5, animals received a daily s.c. injection of either a 1 mg/kg dose of letrozole in 2.5% carboxy methylcellulose (CMC) solution or 2.5% CMC solution alone (vehicle control). Letrozole in CMC solution was previously described (41). The protocol for the letrozole treatment was based on human studies showing that a similar treatment was sufficient to suppress aromatase activity (40). To assess changes in LH and E2 levels, serum samples were periodically obtained (Fig. S1A). First, a set of three animals was examined with a criss-cross design by reversing the treatment with letrozole and vehicle. Second, four animals were examined by treatment with either letrozole or vehicle. In experiment 2, we tested the hypothesis that neuroestradiol released in the median eminence plays a role in estrogen’s positive feedback effects on release of GnRH and kisspeptin using the microdialysis method. Because animals are allowed to stay in a chair up to 12 h, we were not able to conduct microdialysate collection experiments for the entire GnRH surge period, which lasts over 48 h. Similarly, due to the nature of microdialysis experiments, we chose a high (50 µg/kg) single EB injection protocol for GnRH surge induction. A new set of seven OVX female monkeys (age: 7.5 ± 0.8 y) implanted with cranial pedestals was used in this experiment. To examine the effects of letrozole in the EB-induced GnRH and kisspeptin release, three different treatments were applied to the animals in random order (Fig. S1B). In treatment 1, EB was injected at time 0 and microdialysates were collected at 36–48 h after EB, during which vehicle was infused for the first 5.7 h, followed by 5.7 h of 100 nM letrozole infusion through the microdialysis probe. The dose of letrozole was based on our previous study (2). Of note, because of the permeability of the microdialysis membrane, the median eminence was exposed to letrozole concentrations at approximately one-tenth of the infused dose (42). In treatment 2, EB was injected at time 0 and microdialysates were continuously collected for 11.4 h at 36–48 h after EB. In treatment 3, sesame oil (vehicle for EB) alone was injected at time 0 and microdialysates were continuously collected for 11.4 h at 36–48 h after oil injection. To determine the changes in LH levels in experiment 2, serum samples were also obtained as shown in Fig. S1B.
Cranial Pedestal Implantation, Guide Cannula Insertion, and Microdialysis Procedure.
Animals were well adapted to primate chairs, experimental conditions, and researchers before experimentation. Before microdialysis experiments, animals were implanted with cranial pedestals under anesthesia as previously described (2, 42). On the day of the experiment, a microdrive unit was secured to the cranial pedestal and a guide cannula with inner stylet was inserted into the brain above the median eminence (2, 42). X-ray ventriculography confirmed the site of sample collection within the median eminence. Microdialysis experiments were conducted as previously described (2, 42). Dialysates were collected in 20-min fractions, and the sample was split into two 20-µL aliquots for GnRH and kisspeptin RIA and stored at −80 °C.
LH, GnRH, Kisspeptin, and E2 RIAs.
GnRH and kisspeptin in dialysates and LH and E2 in serum samples were measured by RIA as previously described (3, 19, 41). Assay sensitivity and intra- and interassay coefficients of variation for those assays were not significantly different from those previously reported (2–4, 42).
Statistical Analysis.
In experiment 1, the results from the criss-cross and non–criss-cross designs were combined. As such, the number of animals was five in each letrozole- and vehicle-treated group. For experiment 1, mean LH levels were calculated for each time point. To determine statistical differences based on time and treatment, two-way ANOVA with repeated measures followed by Bonferroni’s post hoc analysis was used. The surge was defined as continuous LH elevation greater than the average baseline (days −3, −1, and 0) after EB injection. Mean AUC (the addition of LH values during the surge), amplitude (the maximum peak LH value from the average baseline level), duration (the total time of the LH surge), and latency to the LH surge (the period between EB injection and the time at which the first significant LH elevation above the baseline occurred) were calculated. Student’s t test was applied for comparison. In experiment 2, both GnRH and kisspeptin levels during the surge were highly variable among individual cases, as previously reported in monkeys and sheep (9–11, 18). Thus, to determine whether letrozole modifies GnRH and kisspeptin surges, (i) the average of the 5.7-h periods preceding and following initiation of letrozole or vehicle treatment was calculated, (ii) the value before the treatment was set to 100%, (iii) the percent change after treatment was calculated in each case, and (iv) the means from each treatment group were compared using one-way ANOVA with repeated measures followed by Bonferroni’s post hoc analysis. The effects of EB on mean GnRH and kisspeptin surges were also tested by Kruskal–Wallis nonparametric test using raw values. Changes in LH by EB and letrozole were also compared using two-way ANOVA followed by Bonferroni’s post hoc analysis. For all statistical analyses, differences between treatments or groups were considered significant at P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank Dr. David Abbott for his critical comments on this manuscript. This work is supported by R01HD015433 and R21HD07747 from the Eunice K. Shriver National Institute of Child Health and Human Development (to E.T.). The work was made possible by support from the NIH Office of the Director for the Wisconsin National Primate Research Center (OD011106).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716097115/-/DCSupplemental.
References
- 1.Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: Steroid synthesis and action at the synapse. Endocr Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenealy BP, et al. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33:19051–19059. doi: 10.1523/JNEUROSCI.3878-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenealy BP, Keen KL, Garcia JP, Richter DJ, Terasawa E. Prolonged infusion of estradiol benzoate into the stalk median eminence stimulates release of GnRH and kisspeptin in ovariectomized female rhesus macaques. Endocrinology. 2015;156:1804–1814. doi: 10.1210/en.2014-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizuno M, Terasawa E. Search for neural substrates mediating inhibitory effects of oestrogen on pulsatile luteinising hormone-releasing hormone release in vivo in ovariectomized female rhesus monkeys (Macaca mulatta) J Neuroendocrinol. 2005;17:238–245. doi: 10.1111/j.1365-2826.2005.01295.x. [DOI] [PubMed] [Google Scholar]
- 5.Karsch FJ, et al. Positive and negative feedback control by estrogen of luteinizing hormone secretion in the rhesus monkey. Endocrinology. 1973;92:799–804. doi: 10.1210/endo-92-3-799. [DOI] [PubMed] [Google Scholar]
- 6.Terasawa E, Noonan J, Bridson WE. Anaesthesia with pentobarbitone blocks the progesterone-induced luteinizing hormone surge in the ovariectomized rhesus monkey. J Endocrinol. 1982;92:327–339. doi: 10.1677/joe.0.0920327. [DOI] [PubMed] [Google Scholar]
- 7.Yamaji T, et al. Estrogen induction of LH release in the rhesus monkey. Endocrinology. 1971;89:1034–1041. doi: 10.1210/endo-89-4-1034. [DOI] [PubMed] [Google Scholar]
- 8.Schultz NJ, Terasawa E. Posterior hypothalamic lesions advance the time of the pubertal changes in luteinizing hormone release in ovariectomized female rhesus monkeys. Endocrinology. 1988;123:445–455. doi: 10.1210/endo-123-1-445. [DOI] [PubMed] [Google Scholar]
- 9.Levine JE, et al. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology. 1985;117:711–721. doi: 10.1210/endo-117-2-711. [DOI] [PubMed] [Google Scholar]
- 10.Xia L, Van Vugt D, Alston EJ, Luckhaus J, Ferin M. A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology. 1992;131:2812–2820. doi: 10.1210/endo.131.6.1446619. [DOI] [PubMed] [Google Scholar]
- 11.Pau KY, Berria M, Hess DL, Spies HG. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology. 1993;133:1650–1656. doi: 10.1210/endo.133.4.8404606. [DOI] [PubMed] [Google Scholar]
- 12.Herbison AE. Physiology of the adult gonadotropin-releaseing hormone neuronal network. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction. Academic; San Diego: 2015. pp. 399–467. [Google Scholar]
- 13.MacLusky NJ, Naftolin F, Goldman-Rakic PS. Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proc Natl Acad Sci USA. 1986;83:513–516. doi: 10.1073/pnas.83.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roselli CE, Klosterman S, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J Comp Neurol. 2001;439:208–223. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- 15.Galmiche G, Richard N, Corvaisier S, Kottler ML. The expression of aromatase in gonadotropes is regulated by estradiol and gonadotropin-releasing hormone in a manner that differs from the regulation of luteinizing hormone. Endocrinology. 2006;147:4234–4244. doi: 10.1210/en.2005-1650. [DOI] [PubMed] [Google Scholar]
- 16.Hayes FJ, Seminara SB, Decruz S, Boepple PA, Crowley WF., Jr Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. J Clin Endocrinol Metab. 2000;85:3027–3035. doi: 10.1210/jcem.85.9.6795. [DOI] [PubMed] [Google Scholar]
- 17.Kucherov A, et al. Aromatase inhibition causes increased amplitude, but not frequency, of hypothalamic-pituitary output in normal women. Fertil Steril. 2011;95:2063–2066. doi: 10.1016/j.fertnstert.2011.01.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology. 1990;127:1375–1384. doi: 10.1210/endo-127-3-1375. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar DK, Chiappa SA, Fink G, Sherwood NM. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature. 1976;264:461–463. doi: 10.1038/264461a0. [DOI] [PubMed] [Google Scholar]
- 20.Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Prog Brain Res. 2010;181:209–232. doi: 10.1016/S0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- 21.Szymanski L, Bakker J. Aromatase knockout mice show normal steroid-induced activation of gonadotrophin-releasing hormone neurones and luteinising hormone surges with a reduced population of kisspeptin neurones in the rostral hypothalamus. J Neuroendocrinol. 2012;24:1222–1233. doi: 10.1111/j.1365-2826.2012.02334.x. [DOI] [PubMed] [Google Scholar]
- 22.Hutchison JS, Zeleznik AJ. The corpus luteum of the primate menstrual cycle is capable of recovering from a transient withdrawal of pituitary gonadotropin support. Endocrinology. 1985;117:1043–1049. doi: 10.1210/endo-117-3-1043. [DOI] [PubMed] [Google Scholar]
- 23.Iveson TJ, et al. Phase I study of the oral nonsteroidal aromatase inhibitor CGS 20267 in healthy postmenopausal women. J Clin Endocrinol Metab. 1993;77:324–331. doi: 10.1210/jcem.77.2.8345035. [DOI] [PubMed] [Google Scholar]
- 24.Klein KO, et al. Use of ultrasensitive recombinant cell bioassay to measure estrogen levels in women with breast cancer receiving the aromatase inhibitor, letrozole. J Clin Endocrinol Metab. 1995;80:2658–2660. doi: 10.1210/jcem.80.9.7673408. [DOI] [PubMed] [Google Scholar]
- 25.Fisher SA, Reid RL, Van Vugt DA, Casper RF. A randomized double-blind comparison of the effects of clomiphene citrate and the aromatase inhibitor letrozole on ovulatory function in normal women. Fertil Steril. 2002;78:280–285. doi: 10.1016/s0015-0282(02)03241-7. [DOI] [PubMed] [Google Scholar]
- 26.Legro RS, et al. NICHD Reproductive Medicine Network Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119–129. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duhan N, Madaan S, Sen J. Role of the aromatase inhibitor letrozole in the management of uterine leiomyomas in premenopausal women. Eur J Obstet Gynecol Reprod Biol. 2013;171:329–332. doi: 10.1016/j.ejogrb.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Jirge PR, Patil RS. Comparison of endocrine and ultrasound profiles during ovulation induction with clomiphene citrate and letrozole in ovulatory volunteer women. Fertil Steril. 2010;93:174–183. doi: 10.1016/j.fertnstert.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Diamond MP, et al. NICHD Reproductive Medicine Network Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373:1230–1240. doi: 10.1056/NEJMoa1414827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda S, Harada N, Takagi Y. Novel exon 1 of the aromatase gene specific for aromatase transcripts in human brain. Biochem Biophys Res Commun. 1994;198:1153–1160. doi: 10.1006/bbrc.1994.1163. [DOI] [PubMed] [Google Scholar]
- 31.Abrahám IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci. 2004;24:6326–6333. doi: 10.1523/JNEUROSCI.1006-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franceschini I, et al. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Mayer C, et al. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107:22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe Y, et al. Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J Neuroendocrinol. 2014;26:909–917. doi: 10.1111/jne.12227. [DOI] [PubMed] [Google Scholar]
- 37.Vargas Trujillo M, Kalil B, Ramaswamy S, Plant TM. Estradiol upregulates kisspeptin expression in the preoptic area of both the male and female rhesus monkey (Macaca mulatta): Implications for the hypothalamic control of ovulation in highly evolved primates. Neuroendocrinology. 2017;105:77–89. doi: 10.1159/000448520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology. 1975;96:1073–1087. doi: 10.1210/endo-96-5-1073. [DOI] [PubMed] [Google Scholar]
- 39.Terasawa E, Kenealy BP. Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front Neuroendocrinol. 2012;33:364–375. doi: 10.1016/j.yfrne.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haynes BP, Dowsett M, Miller WR, Dixon JM, Bhatnagar AS. The pharmacology of letrozole. J Steroid Biochem Mol Biol. 2003;87:35–45. doi: 10.1016/s0960-0760(03)00384-4. [DOI] [PubMed] [Google Scholar]
- 41.Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frost SI, Keen KL, Levine JE, Terasawa E. Microdialysis methods for in vivo neuropeptide measurement in the stalk-median eminence in the Rhesus monkey. J Neurosci Methods. 2008;168:26–34. doi: 10.1016/j.jneumeth.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.