Significance
Excitation–contraction coupling (ECC) in vertebrate skeletal muscle depends upon specialized junctions at which CaV1.1, a voltage-gated channel in the plasma membrane, interacts with RyR1, a calcium release channel in the sarcoplasmic reticulum. Because calcium flux via CaV1.1 is unnecessary for ECC, CaV1.1 is thought to “conformationally couple” to RyR1. Studies of muscle cells with gene knockouts have shown that additional, junctional proteins are also necessary, but are unable to reveal whether or not these complete the set of required proteins. Here, we show that conformational coupling can be conferred upon tsA201 cells by expressing five junctional proteins (CaV1.1, RyR1, β1a, Stac3, and junctophilin2), thus establishing a minimum set of proteins supporting CaV1.1–RyR1 conformational coupling in skeletal muscle.
Keywords: calcium signaling, excitation–contraction coupling, CaV1.1, RyR1, junctophilin
Abstract
Skeletal muscle contraction is triggered by Ca2+ release from the sarcoplasmic reticulum (SR) in response to plasma membrane (PM) excitation. In vertebrates, this depends on activation of the RyR1 Ca2+ pore in the SR, under control of conformational changes of CaV1.1, located ∼12 nm away in the PM. Over the last ∼30 y, gene knockouts have revealed that CaV1.1/RyR1 coupling requires additional proteins, but leave open the possibility that currently untested proteins are also necessary. Here, we demonstrate the reconstitution of conformational coupling in tsA201 cells by expression of CaV1.1, β1a, Stac3, RyR1, and junctophilin2. As in muscle, depolarization evokes Ca2+ transients independent of external Ca2+ entry and having amplitude with a saturating dependence on voltage. Moreover, freeze-fracture electron microscopy indicates that the five identified proteins are sufficient to establish physical links between CaV1.1 and RyR1. Thus, these proteins constitute the key elements essential for excitation–contraction coupling in skeletal muscle.
In vertebrate skeletal muscle, the coupling of excitation to contraction (EC coupling) functions under demanding conditions, including high firing rates, hypoxia, and metabolic acidosis. A key step in EC coupling is the transduction of electrical excitation into intracellular release of calcium, which in turn triggers muscle contraction. This transduction occurs at triad junctions in which surface membrane invaginations, the transverse tubules, are flanked on two sides by the sarcoplasmic reticulum (SR): Depolarization of the transverse tubules elicits Ca2+ release from the SR. Experiments on muscle cells, which are genetically null for specific triadic proteins (1, 2), have revealed that CaV1.1 is the protein which responds to transverse tubular voltage changes and that RyR1 is the channel that gates SR Ca2+ release, a process that can occur in skeletal muscle without the entry of extracellular calcium (3). More recent work with gene-deletion models showed that functional and structural interactions between CaV1.1 and RyR1 in muscle depend upon the simultaneous presence of at least two additional proteins, the β1a auxiliary subunit (4, 5) of CaV1.1 and the Stac3 adaptor protein (6–9). However, these experiments have not been able to establish the mechanism of the CaV1.1–RyR1 interaction or whether currently unidentified proteins are also essential. This latter possibility is given added emphasis by the fact that, until recently, Stac3 was not known to be a triadic protein (6, 8).
Because they must operate under stringent conditions, triad junctions contain a large complex of proteins, only some of which are directly essential for the transduction process. This complexity, together with the fact that SR Ca2+ release depends on the interaction between two polarized membrane systems, has greatly hindered the use of reductionist approaches for defining the molecular mechanism of EC coupling Ca2+ release. These difficulties are illustrated by a prior attempt to recapitulate such Ca2+ release by means of heterologous expression, which has been a primary method for identifying the sets of proteins required for the function of specific ion channels. In that earlier work, an attempt was made to reconstitute EC coupling Ca2+ release in CHO cells by coexpressing a CaV1 construct, auxiliary CaV subunits, and RyR1 (10). This approach encountered two major problems. The first was that high-level expression of CaV1.1 was unobtainable, forcing the authors to use a chimeric construct containing >90% CaV1.2 sequence. The second problem was that endoplasmic reticulum (ER)–plasma membrane (PM) junctions did not form in the transfected CHO cells. As a result, these cells produced only very slow cytoplasmic Ca2+ increases dependent upon extracellular Ca2+ entry.
Here, we have overcome these problems by (i) obtaining robust CaV1.1 expression in tsA201 cells by coexpression of Stac3, and (ii) demonstrating that, in these cells, junctophilin2 (JP2) promotes ER–PM junctions which contain RyR1 and are morphologically similar to SR–PM junctions in muscle cells. These have allowed us to demonstrate that CaV1.1, β1a, Stac3, RyR1, and JP2 are sufficient to induce ER–PM junctions in which CaV1.1 and RyR1 interact with one another both functionally, producing intracellular Ca2+ release resembling that in skeletal muscle, and structurally, causing CaV1.1 to be arranged into tetrads indicative of physical links to RyR1.
Results
JP2 Promotes ER–PM Junctions.
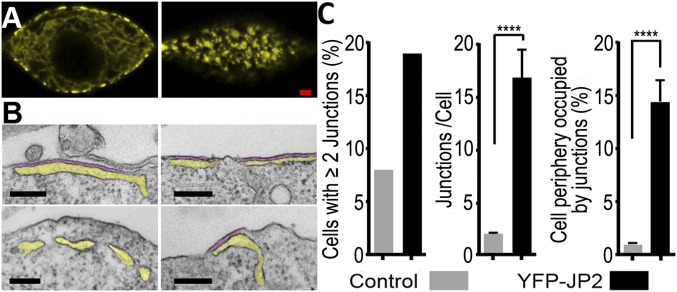
As a first step toward identifying proteins sufficient to enable functional and structural interactions between CaV1.1 and RyR1, it was important (i) to be able to identify the minority of cells transiently expressing multiple cDNA constructs, and (ii) to establish the conditions that would cause the formation of ER–PM junctions. Taking both into account, we determined whether JP2 tagged on its amino terminus with YFP (YFP–JP2) would produce ER–PM junctions identifiable by yellow fluorescence. Previous work had shown that the junctophilin family of proteins has a short, C-terminal segment anchored in the ER and repeated “MORN” motifs which are nearer to the N terminus and associate with the PM, thereby causing the junctional association of these two membrane systems (11). Of the two junctophilin isoforms expressed in skeletal muscle, we selected JP2 because it is expressed earlier during development than JP1 and is able to support the EC coupling of limb muscle in the absence of JP1 (12). As shown in Fig. 1A, tsA201 cells expressing YFP–JP2 displayed prominent, ∼0.5- to 2.0-µm yellow patches of fluorescence associated with the surface. Representative thin-section electron micrographs (Fig. 1B) provided strong evidence that these patches correspond to ER–PM junctions, which are numerous in cells expressing YFP–JP2 and relatively rare in nontransfected cells (Fig. 1C and Table S1). Thus, the fluorescent patches at the periphery of cells transfected with tagged JP2 appear to provide a useful indicator for likely sites of ER–PM junctions.
Fig. 1.
Expression of YFP–JP2 induces the formation of extensive ER–PM junctions in tsA201 cells. (A) Midlevel (Left) and surface (Right) confocal sections of a transiently transfected tsA201 cell reveal clustering of YFP–JP2 at the cell periphery. (Scale bar, 2 µm.) (B) Thin-section electron micrographs reveal that the peripherally clustered YFP–JP2 likely corresponds to ER–PM junctions. Representative images are shown of cells transfected with YFP–JP2 (Upper) or nontransfected cells (Lower) with yellow and purple shading to indicate ER lumen and junctional gap, respectively. (Scale bars, 200 nm.) (C) Quantitative morphometry reveals that transfection increased the fraction of cells displaying ER–PM junctions, the average length of the junctions, and the percentage of the cellular periphery occupied by those junctions. Transfection efficiency was ∼20%, and the analysis of junctions per cell and percentage of cellular periphery occupied were carried out for cells with two or more junctions (Table S1). Based on Welch’s adjusted t test, the bracketed values were significantly different. ****P < 0.0001.
CaV1.1 Traffics to JP2-Induced Junctions.
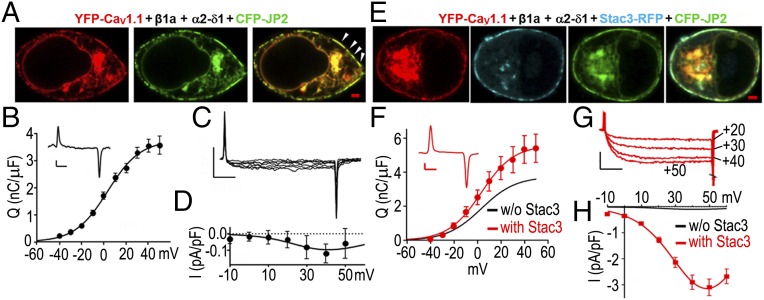
Having found that transfection with JP2 effectively induces the formation of ER–PM junctions, we next tested whether these junctions shared properties with the SR–PM junctions found in muscle cells. One of these properties is that CaV1.1 can traffic to SR–PM junctions in the absence of RyR1, since junctions containing CaV1.1 are present in “dyspedic” muscle cells genetically null for RyR1 (13). Thus, we determined whether CaV1.1 targeted to junctions in tsA201 cells which lack RyR1. Previously, it was shown that in tsA201 cells transfected with YFP–CaV1.1, β1a, and α2–δ1 only, CaV1.1 fails to traffic to the surface, as indicated both by the intracellular retention of yellow fluorescence and the absence of gating charge movements that result when CaV1.1 is inserted into the PM (14). By contrast, when CFP–JP2 was also present, there were numerous colocalized fluorescent patches of CaV1.1 and JP2 at the periphery (Fig. 2A). Moreover, gating charge movements in such cells (Fig. 2B) demonstrated that CaV1.1 had actually been inserted into the PM. Thus, in tsA201 cells expressing JP2, as in muscle, the presence of RyR1 did not seem to be required for CaV1.1 to traffic efficiently to the PM–ER junctions. Despite this, the calcium currents were of very small size in these cells (Fig. 2 C and D). Thus, we tested the effects of the additional presence of Stac3 because it had been shown to affect CaV1.1 expression and function in both muscle and tsA201 cells (7, 9, 14). The additional presence of Stac3 did not alter the coclustering of CaV1.1 and JP2 at the cell surface (Fig. 2E) and only slightly increased the magnitude of gating charge movement (Fig. 2F). However, Stac3 caused a very large (∼30-fold) increase in the amplitude of the L-type Ca2+ current (Fig. 2G), which could not be attributed to the ∼1.5-fold increase in the magnitude of charge movement (Table S2). Because Stac3 is of evident importance for the function of CaV1.1 in muscle (7, 9), it was one of the constructs used in all of the experiments described below. However, to limit the total number of transfected cDNAs, α2–δ1 was omitted because its knockdown by siRNA has minor effects on Ca2+ current and EC coupling in skeletal myotubes (15, 16). Similarly, its omission had little effect on Ca2+ currents in tsA201 cells (Fig. S1).
Fig. 2.
JP2 causes CaV1.1 to insert into discrete domains of the PM, but channel function is minimal without Stac3. (A) Midlevel confocal section of a tsA201 cell transiently transfected with YFP–CaV1.1 (red), β1a, α2–δ1, and CFP–JP2 (green), but not Stac3. A tight colocalization between CaV1.1 and JP2 is visible both at the internal (ER) level and at the periphery of the cell where the two proteins are concentrated in discrete foci (some indicated by arrowheads) likely representing ER–PM junctions. (Scale bar, 2 µm.) (B and C) Evidence that the peripherally localized CaV1.1, visible in cells like those in A, was inserted into the PM is provided by the presence of gating charge movements (B; representative trace at +40 mV and average ON charge, n = 11, as a function of test potential from a holding potential of −80 mV) and small, but detectable, Ca2+ currents (C; representative currents for test potentials of −10 to +50 mV in 10-mV steps). (D) Average peak currents, n = 7, as a function of test potential. (E) Midlevel confocal section of a cell transfected with YFP–CaV1.1 (red), β1a, α2–δ1, Stac3–RFP (cyan), and CFP–JP2 (green): All three tagged proteins colocalize at the cell periphery. (Scale bar, 2 µm.) (F) The additional presence of Stac3 caused only a small increase in the magnitude of gating charge movement (representative trace at +40 mV and average ON charge, n = 14, as a function of test potential). (G and H) However, the additional presence of Stac3 caused a very large increase in the amplitude of the Ca2+ current: representative currents at the indicated test potentials (G) and average peak current vs. voltage (H) (n = 22). The smooth black curves in F and H are replotted from B and D, respectively. Vertical calibration: 1 pA/pF (B, C, and F) or 2 pA/pF (G). Horizontal: 5 ms (B and F) or 50 ms (C and G).
RyR1 Extends from ER to PM.
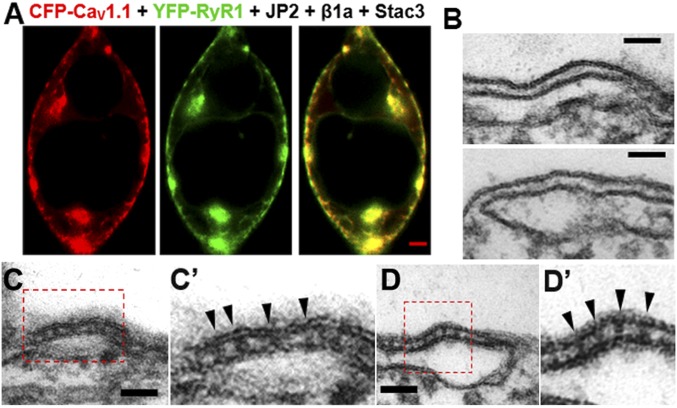
After the demonstration that JP2 caused CaV1.1 to traffic to PM junctions with the ER in tsA201 cells, it was next necessary to determine whether RyR1 would also traffic to these junctions. In myotubes, junctional targeting of RyR1 occurs in the absence of CaV1.1 (17). In a somewhat similar manner, some RyR1-containing ER was localized near the surface of cells cotransfected with JP2 and not CaV1.1, although a large fraction of RyR1 in such cells remained in the cell interior (Fig. S2). By contrast, in cells transfected not only with RyR1 and JP2, but also with CaV1.1, β1a, and Stac3, the majority of RyR1 was localized near the surface, where it colocalized with CaV1.1 (Fig. 3A), raising the possibility that these two key proteins could functionally and physically interact with one another. Given the resolution limits of fluorescence microscopy, thin-section electron microscopy was used to determine whether electron-dense “feet,” which correspond to the cytoplasmic domain of RyR1 (18), could be observed spanning between the ER and PM. Before this, we created a cell line (“RyR1-stable cells”), in which expression of RyR1 was nearly 100% (Fig. S3) and which thus meant that the number of transiently transfected cDNAs could be reduced by one. These RyR1-stable cells and naïve tsA201 cells were then compared after transient transfection of both cell types with YFP–CaV1.1, β1a, Stac3, and JP2. Both the naïve and RyR1-stable cells displayed frequent, extended junctions between the ER and PM (Fig. 3 B–D). In the naïve cells, the junctional gaps were relatively uniform and electron lucent (Fig. 3B), consistent with the hypothesis that only JP2, which is a small protein, filled those gaps. On the other hand, electron-dense particles with a semiregular spacing were clearly visible in some of the junctions in the RyR1-stable cells (Fig. 3 C and D). These particles closely resembled the foot structures described in skeletal muscle triads (19) and peripheral dyads in myotubes (20).
Fig. 3.
RyR1 traffics to ER–PM junctions induced by JP2. (A) In cells transiently transfected with CFP–CaV1.1 (red), β1a, Stac3, YFP–RyR1 (green), and JP2, the RyR1 fluorescence was colocalized with that of CaV1.1, partly in the ER, but mostly at the cell periphery. (Scale bar, 2 µm.) (B–D) Thin-section electron micrographs are shown for naïve tsA201 cells (B) and tsA201 cells stably expressing RyR1 (C and D) obtained after both types of cells were transiently transfected with YFP-CaV1.1, β1a, Stac3, and JP2. C′ and D′ present magnified (2×) views of the indicated areas in C and D. The ER–PM gaps of the junctions in the naïve cells were essentially free of electron-dense material, whereas those in the RyR1-stable cells displayed periodic, electron-dense structures spanning the gap between the ER and PM (arrowheads). These densities resemble in shape and size the feet structures which are observed in triads of skeletal muscle (and dyads of developing skeletal muscle) and which have been attributed to the cytoplasmic domain of RyR1. (Scale bars, 50 nm.)
EC Coupling Ca2+ Release Recapitulated.
For the measurement of Ca2+ transients, we switched from conventional whole-cell clamping to the perforated patch technique (21), which was applied to cells that had been loaded with Fluo-3 AM. The perforated patch had the disadvantage of increased access resistance, which compromised measurement of membrane currents. However, it had the advantage of minimizing perturbations of intracellular calcium handling, which seemed important given that tsA201 cells lack the adaptations which equip skeletal muscle to efficiently move Ca2+ into the ER/SR and to store it there. Even with perforated patch, we found that Ca2+ transients were small and that restoration of the ER store was so slow that the second of two responses to identical stimuli was smaller than the first. Thus, it was necessary to compare populations of cells which raised a second issue: A significant fraction of cells not producing a detectable response might simply not have been expressing the entire set of transfected constructs. Because it was not possible to determine whether or not an individual cell was expressing all of the transfected constructs, we calculated average Ca2+ transients only from cells in which the responses exceeded a minimum threshold unlikely to have been exceeded in nontransfected cells (ΔF ≥ 1.5 in the 200-ms interval after onset of the test pulse).
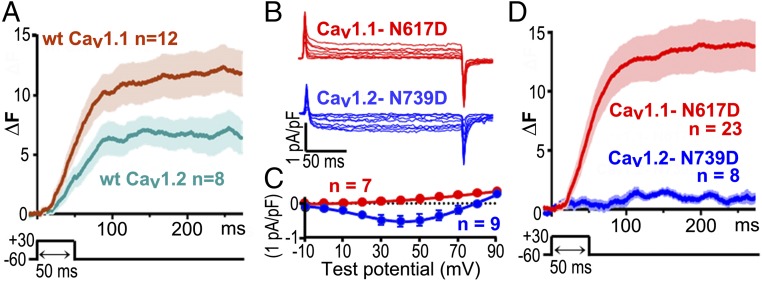
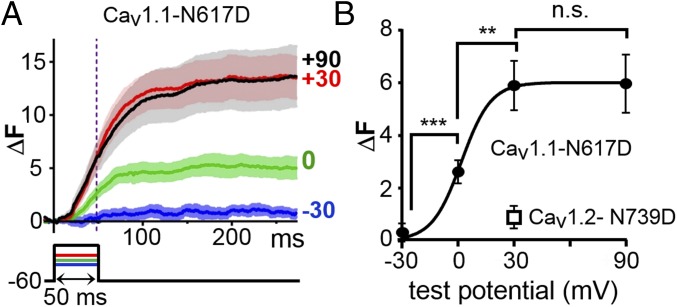
Fig. 4A compares the average Ca2+ transients elicited by a 50-ms depolarization to +30 mV which was applied to RyR1-stable cells transfected with β1a, Stac3–RFP, JP2, and either YFP–CaV1.1 (brown trace) or YFP–CaV1.2 (teal trace). The transients were quite similar to one another. Furthermore, both CaV1.1 and CaV1.2 colocalized with RyR1 (Fig. 3A and Fig. S4A, respectively), raising the question of whether these transients resulted from the same underlying mechanism. Previous work had shown that transients triggered by CaV1.2, both in native tissue (22) and after expression in dysgenic myotubes (23), depend on entry of external Ca2+, and this also appeared to be the case for RyR1-stable cells transfected with YFP–CaV1.2, β1a, Stac3–RFP, and JP2. In such cells, cytoplasmic Ca2+ remained unchanged during a depolarization to +90 mV, a potential at which Ca2+ entry did not occur, and only increased upon subsequent repolarization to −60 mV, which generated an inward tail current (Fig. S4B, which also illustrates the threshold used to include/exclude data for subsequent analysis). To determine whether Ca2+ entry was also required for the transients in cells expressing CaV1.1, we used two approaches. The first was to measure Ca2+ transients in single cells before and after the addition of Cd2+ and La3+ to the bathing solution. After this addition, inward Ca2+ current was effectively eliminated, but Ca2+ transients were still present (Fig. S5). As a second approach, we introduced a point mutation into the pore of CaV1.1 (N617D) and at the homologous position of CaV1.2 (N739D). The N617D mutation eliminates Ca2+ permeability of CaV1.1 (Fig. 4 B and C) without affecting its function in EC coupling (24), and the N739D mutation caused a large reduction of inward Ca2+ current via CaV1.2 (Fig. 4 B and C). Ca2+ transients were greatly reduced for CaV1.2–N739D (Fig. 4D) compared with WT CaV1.2 (Fig. 4A), as expected if the transients for CaV1.2 depended on entry of extracellular Ca2+. However, the transients were essentially identical for CaV1.1–N617D (Fig. 4D) and WT CaV1.1 (Fig. 4A), despite the complete loss of extracellular Ca2+ entry. Thus, it seems likely that these transients resulted, as in skeletal muscle, from the conformational activation of RyR1 by CaV1.1.
Fig. 4.
Five triad-junction proteins are sufficient to produce voltage-gated Ca2+ release that does not require entry of extracellular Ca2+. (A) Average cytoplasmic Ca2+ transients elicited by a 50-ms depolarization to +30 mV applied, via the perforated patch technique, to RyR-stable cells transiently transfected with β1a, Stac3–RFP, and JP2 together with either YFP–CaV1.1 (brown line) or YFP–CaV1.2 (teal line). Data are shown as Fluo-3 fluorescence increase (ΔF) ± SEM. (B and C) Representative currents and average peak I–V relationships recorded from naïve tsA201 cells transfected with YFP–CaV1.1–N617D (red) or CaV1.2–N739D, together with β1a, Stac3–RFP, and JP2. Mutation of the conserved IIS6 asparagine to aspartate completely eliminated inward Ca2+ current via CaV1.1 and left only a small inward Ca2+ current via CaV1.2. (D) The average Ca2+ transient for CaV1.2–N739D (blue) was much smaller than that for WT CaV1.2, as expected if it depended on the entry of extracellular Ca2+. By contrast, the amplitude of the average transient for YFP–CaV1.1–N617D (red) was comparable to that of WT CaV1.1, consistent with the hypothesis that, as in skeletal muscle, CaV1.1 activates intracellular Ca2+ release without requiring extracellular Ca2+ entry. Except for the CaV constructs used, conditions were identical for the data shown in A and D.
A characteristic feature of EC coupling in skeletal muscle is that Ca2+ release increases in magnitude as a sigmoidal function of test potential and saturates for strong depolarizations. Thus, we characterized the voltage dependence of Ca2+ release in RyR1-stable cells transfected with YFP–CaV1.1–N617D (to prevent possible contributions of Ca2+ entry), β1a, Stac3–RFP, and JP2. Due to the weak Ca2+ reuptake ability of tsA201 cells, it was not possible to reliably test multiple depolarizations in single cells. Therefore, we measured transients in individual cells in response to a single potential of −30, 0, +30, or +90 mV and included that response as part of the average for that potential if it met the criterion described earlier (ΔF ≥ 1.5 within 200 ms of the onset of depolarization). Transients at −30 mV which did not meet this criterion (four cells) were also included in the average if the transient for a subsequent, stronger depolarization did meet it. Average transients obtained in this fashion are illustrated in Fig. 5A, and their magnitude measured just before repolarization is plotted as a function of test potential in Fig. 5B. Although the number of test potentials was limited, the voltage dependence appeared to be similar to that in skeletal myotubes (25), in regard to both midpoint and slope factor (Table S2).
Fig. 5.
The voltage dependence of Ca2+ release in cells expressing five triad-junction proteins is similar to that of Ca2+ release in skeletal muscle. (A) RyR1-stable cells were transiently transfected with YFP–CaV1.1–N617D, β1a, Stac3–RFP, and JP2, loaded with Fluo-3 AM, and depolarized to the indicated potentials with the perforated patch technique. Data are presented as mean (solid line) ± SEM. Number of cells averaged was 10, 13, 24, and 22 for −30, 0, +30, and +90 mV, respectively, is shown. (B) Average ΔF, measured at 48 ms after the onset of depolarization (vertical dotted line in A), as a function of test potential. Based on Welch’s adjusted t test, the bracketed values were statistically different: ***P = 0.0006; **P = 0.002; n.s., not significantly different (P = 0.949). The data point for CaV1.2–N739D was obtained from the eight cells used to generate the average transient in Fig. 4D.
Interestingly, the fluorescence signals illustrated in Figs. 4 and 5 continued to increase for tens of milliseconds after repolarization to the holding potential. The interpretation of these signals depends upon knowing the flux rates and subcellular localization of the Ca2+ release sites, the transport rates and subcellular localization of the Ca2+ removal sites, and the concentration and affinities of cellular Ca2+ buffers. Although detailed information was lacking, it can be said that the overall rate of removal of Ca2+ from the cytoplasm was extremely slow (Fig. S6A). Additionally, the majority of Ca2+ release would be expected to occur at the periphery where most of RyR1 is located (e.g., Fig. 3A). Consequently, the rate of fluorescence change at the periphery should be approximately related to the rate of Ca2+ release there. On the other hand, the fluorescence change in the cellular interior, where the bulk of the Ca2+ indicator is located, would lag because it depends on the diffusion of Ca2+ from the release sites, and this diffusion would be slowed by the cellular Ca2+ buffers. Evidence that these ideas may be correct was provided by the cell illustrated in Fig. S6B. The fluorescence within the interior continued to increase for ∼75 ms after repolarization, whereas the fluorescence at the edge of the cell ceased to increase shortly after repolarization. Thus, it appears that repolarization may cause a rapid termination of release at the ER–PM junctions containing CaV1.1 and RyR1.
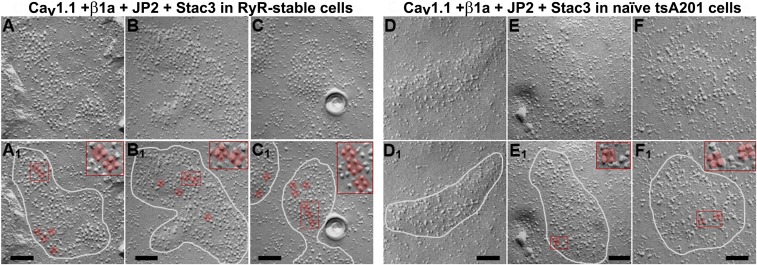
CaV1.1 Physically Links to RyR1.
In freeze-fracture replicas of skeletal muscle cells, CaV1.1 appears as large particles arranged in groups of four (“tetrads”), with each particle aligned with one of the four monomers comprising the RyR1 homotetramer (26). Moreover, the tetradic arrangement depends on the presence of RyR1 (27), thus providing evidence that CaV1.1 and RyR1 are physically linked to one another in skeletal muscle, either directly or via intervening proteins. To determine whether RyR1 caused CaV1.1 to be arranged as tetrads in tsA201 cells, we analyzed freeze fractures of RyR1-stable and naïve cells that had been transfected with YFP–CaV1.1, β1a, Stac3, and CFP–JP2. In both groups of cells, PM domains at sites of junction with the ER (circled in Fig. 6 A1–F1) could be recognized by a slightly raised appearance and the presence of large particles, having the expected size for CaV1.1 (15, 28). In the RyR1-stable cells, the large particles were spaced fairly evenly and sometimes arrayed as tetrad-like groups containing three or four particles (Fig. 6 A–C). Some examples of likely tetrads in the transfected RyR1-stable cells have been overlaid with a ∼30- × 30-nm square (Fig. 6 A1–C1), which corresponds to the outer dimensions of a single tetrad in muscle cells (29, 30). In addition to size, other features resembling those of tetrads in muscle included the clear separation of the individual particles within each apparent tetrad and the generally similar orientation of the apparent tetrads throughout each junction. By contrast, the large particles in the naïve cells were interspersed with smaller ones, and the large particles appeared to be randomly distributed with occasional clumps (Fig. 6 D–F). Of course, randomly distributed particles in the naïve cells would be expected occasionally to be arranged in groups of four, some examples of which are indicated in Fig. 6 E1 and F1. However, these differ from the apparent tetrads in the RyR1-stable cells in that they did not have a clear separation between all four particles. Nonetheless, as an independent method for determining whether there was a difference in the prevalence of tetrad-like structures in the naïve and RyR1-stable cells, we asked three investigators to determine the number of tetrads present in unidentified micrographs (Fig. S7). They were 79%, 95%, and 96% accurate in identifying tetrads as preferentially present in the RyR1-stable cells.
Fig. 6.
Intramembranous particles in groups of four (tetrads) indicate that CaV1.1 is physically linked to RyR1 in tsA201 cells expressing five triad-junction proteins. Freeze-fracture electron micrographs are shown of RyR1-stable (A–C) and naïve (D–F) tsA201 cells transiently transfected with YFP–CaV1.1, β1a, Stac3, and CFP–JP2. ER–PM junctions in both cell types appear as slightly “domed” structures (encircled by white lines in A1–F1), containing clusters of large particles. Some of the large particles in A1–C1, E1, and F1 have been overlaid with a ∼30 × 30-nm red square, and subregions containing some of these squares are magnified 2× in Insets. A 30 × 30-nm square was chosen because tetrads in native muscle cells fit well inside, with one particle at each corner. Such well-fitting groups of particles were evident in junctions of the transfected RyR1-stable cells (A1–C1). In some instances, only three particles were present (e.g., A1, Inset), as also occurs in native skeletal muscle, and in other instances the particles appeared to have been deformed during the fracture process. Particles that would fit within a 30 × 30-nm square were occasionally found in the transfected, naïve tsA201 (E1 and F1), but the fit was generally poorer, and there was a lack of clear separation between individual particles as occurs in skeletal muscle and as was evident for many of the presumptive tetrads in the transfected RyR1-stable cells. (Scale bars, 100 nm.)
Discussion
We have shown here that five proteins, CaV1.1, β1a, Stac3, JP2, and RyR1, when expressed in tsA201 cells, are sufficient to support Ca2+ release which resembles that in skeletal muscle, in that it does not require the entry of external Ca2+ and has a saturating dependence on voltage. Moreover, in cells expressing these five proteins, it appears likely that CaV1.1 and RyR1 are physically linked to one another. Of course, triad junctions in muscle contain many more proteins, some of which interact with one or more of the proteins analyzed in our work. Nonetheless, our results indicate that CaV1.1, β1a, Stac3, JP2, and RyR1 represent fundamental components of the Ca2+ release mechanism and are the locus of protein–protein interactions sufficient for functional and structural coupling between CaV1.1 and RyR1.
As touched on in the introduction, experiments on myotubes that are null for endogenous CaV1.1 (1), RyR1 (2), β1a (4, 5), or Stac3 (7, 9) have shown that EC coupling Ca2+ release requires the simultaneous presence of all four proteins. Because JP2 would have been present in these null myotubes, these experiments were not informative about whether JP2 is also directly involved in linking CaV1.1 to RyR1 or functions only to induce the formation of junctions. In this regard, it would be of interest to determine the extent to which JP1 or the neuronal junctophilins (JP3 and JP4) can substitute for JP2 in producing the CaV1.1–RyR1 coupling in the tsA201 cell system.
Clearly, elucidating the molecular mechanism of EC coupling Ca2+ release will require high-resolution structure. In this regard, the structures of the isolated components are likely to be of limited value because previous work has indicated that the absence of RyR1 alters the structures of CaV1.1 and β1a, and that the absence of CaV1.1 alters the structure of RyR1. In particular, the absence of RyR1 in muscle cells results in altered gating of CaV1.1 (31) and, based on FRET measurements, causes rearrangement of the CaV1.1 cytoplasmic domains (32) and likely the N terminus of β1a (33). Conversely, the absence of CaV1.1 appears to alter RyR1 structure in that resting Ca2+ leak via RyR1 is increased in muscle cells lacking CaV1.1 (34). Furthermore, the absence of Stac3 significantly affects the function of CaV1.1, including its ability to activate RyR1 (7, 9). Thus, it would seem necessary to obtain the structure of CaV1.1, β1a, Stac3, and RyR1 arranged as a working complex. Toward this end, the transfected tsA201 cells provide a system of potentially great value. For this approach to succeed, it will likely be necessary to develop clonal cell lines expressing all five proteins and perhaps also to use cell-sorting techniques. However, these difficulties may be compensated by the greatly simplified protein content of the transfected tsA201 cells and the ability to dock structures of the assembled complex with those already determined for the large portions of CaV1.1, β1a, and RyR1.
Materials and Methods
Cell Culture and Cell Lines.
tsA201 cells were cultured in high-glucose DMEM (Mediatech Inc.) supplemented with 10% (vol/vol) FBS (Gemini Bio Products), 2 mM glutamine (Mediatech Inc.), 100 U/mL penicillin (Gemini Bio Products), and 100 μg/mL streptomycin (Gemini Bio Products) in a humidified incubator with 5% (vol/vol) CO2. Cells at ∼70% confluence were transfected according to manufacturer’s instructions for 3.5 h by using the jetPRIME reagent (Polyplus-transfection Inc.) containing either 1 µg/µL cDNA (RyR and CaV constructs) or 0.5 µg/µL cDNA (all other constructs). The cells were then detached from the dish with 0.05% trypsin EDTA diluted from a 0.25% stock (Gibco) and replated at ∼1.5 × 104 cells per dish in 35-mm culture dishes for electrophysiology or at ∼2.5 × 104 per cm2 in ECL (Upstate Biotechnology)-coated, glass-bottomed dishes (14-mm microwell diameter; MatTek) for imaging or freeze-fracture. To generate tsA201 cells stably expressing RyR1, the cells were transfected as described with RyR1–pCEP4 and propagated in medium (described above) supplemented with 300 µg/mL hygromycin B (Invitrogen) for selection. After establishment of a polyclonal culture, the cells were replated at low density (5,000 cells per 35-mm dish) and maintained for 2–3 d until the isolated single cells had expanded into monoclonal colonies of at least 15–20 cells. The cells were then loaded with Fluo-3 AM (Molecular Probes) (see below), and the monoclonal colonies were tested for their response to localized application of 0.5 mM RyR1 agonist 4-chloro-m-cresol (Pfaltz & Bauer, Inc.). Three colonies showing high, uniform response were isolated, subcultured, and expanded before freezing. Clone 1 was used for all of the experiments described here (denoted RyR1-stable cells).
Molecular Biology.
For expression plasmids, see SI Materials and Methods.
Imaging Analysis.
Cells were bathed in physiological saline (in mM: 146 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, and 10 Hepes, pH 7.4, with NaOH) and optical slices were obtained by using a Zeiss 710 confocal microscope with a 40× (1.3 NA) or 63× (1.4 NA) objective. Fluorescence excitation (Ex) and emission (Em) (nanometers) were DAPI (Ex, 405; Em, 410−585), GFP (Ex, 488; Em, 493−586), CFP (Ex, 440; Em, 454−503 ± 5), YFP (Ex, 514; Em, 520 ± 5−596 ± 23), mCherry (Ex, 543; Em, 578−696), RFP (Ex, 543; Em, 597−745), and Alexa Fluor 568 (Ex, 543; Em, 568−712).
Measurement of Currents and Cytoplasmic Ca2+ Transients.
All experiments were performed at room temperature (∼25 °C). Cells were voltage-clamped with either the whole-cell or perforated patch techniques. For whole-cell recording of ionic currents or charge movements, the pipettes had a resistance of ∼3 MΩ when filled with internal solution of (in mM) 140 Cs–aspartate, 10 Cs–EGTA, 5 MgCl2, and 10 Hepes (pH 7.4), with CsOH. After entry into whole-cell mode, electronic compensation was used to reduce the effective series resistance to <8 MΩ (time constant < 500 μs). The bath solution contained (in mM) 145 tetraethylammonium (TEA)–Cl, 10 CaCl2, and 10 Hepes (pH 7.4, with TEA-OH). For measurement of charge movements, the bath additionally contained 0.1 mM LaCl3 and 0.5 mM CdCl2.
For perforated patch recording (21) of ionic currents or Ca2+ transients, the pipette tip was front-filled by immersing it for ∼5 s in internal solution consisting of (in mM): 70 Cs–Aspartate, 10 NaCl, 1 CaCl2, 1 MgCl2, and 10 Hepes (pH 7.2), with CsOH. The pipette was then back-filled with a 100-fold dilution (in the same solution) of amphotericin stock solution: 20 mg/mL amphotericin (APExBIO Technology LLC) and 0.5% (wt/vol) pluronic in DMSO. After giga-seal formation (≥2 GΩ) with a cell, negative pressure on the pipette was released, and cell recording began after access resistance had fallen to ≤20 MΩ. The bath contained (in mM): 137 NaCl, 5.6 KCl, 2.6 CaCl2, 1.2 MgCl2, 10 glucose, and 10 Hepes (pH 7.4), with NaOH.
Depolarizing test steps were applied from a holding potential of −60 mV, unless otherwise noted. Linear leak and capacity currents were corrected by –P/4 (whole-cell) or –P/3 (perforated patch) subtraction. Signals were analog filtered 1–2 or 5 kHz for ionic currents and charge movements, respectively, and sampled at 20 kHz.
For measurement of cytoplasmic Ca2+ transients, cells were loaded for 20 min at 37 °C with Fluo-3 AM added to the medium (3.5 µM Fluo-3 AM, 0.035% pluronic, and 0.35% DMSO). The cells were then washed and clamped with the perforated patch technique (see above), with candidate cells selected on the basis of red fluorescence arising from Stac3-tagRFP. A fluorometer apparatus (Biomedical Instrumentation Group, University of Pennsylvania) equipped with fluorescein optics was used to measure changes in Fluo-3 fluorescence (ΔF) from baseline, in response to 50-ms depolarizations. The baseline was taken as the average fluorescence in the 2- to 4-ms interval immediately preceding the test depolarization.
Electron Microscopy.
See SI Materials and Methods for sample preparation. To quantify ER–PM junctions, all cells displaying two or more junctions (“positive cells”) were identified in random areas of microscope grids. The fraction of all cells that were positive was recorded, and each positive cell was subsequently analyzed with ImageJ (National Institutes of Health) to determine the lengths of all of the junctions within the cell and the length of the cell perimeter. The percentage of cell perimeter occupied by junctions was calculated by dividing the total junction length by the perimeter for each positive cell, while average junctional length, maximum length, and minimum length were determined from all junctions imaged in the positive cells.
Quantification and Statistical Analysis.
Statistical parameters including the exact value of n, dispersion and precision measures (mean ± SEM), and statistical significance are reported in the figures and figure legends. Data were judged to be statistically significant when P < 0.05 by Welch’s adjusted unpaired t test. In figures, asterisks denote statistical significance as calculated by Student’s t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). GraphPad Prism 6 software was used for constructing data plots, curve fitting, and statistical analysis.
Supplementary Material
Acknowledgments
We thank Drs. Alex Polster, Symeon Papadopoulos, and Eric Olson for providing cDNA constructs; Rock Levinson, William Sather, and John Bankston for counting tetrads; Catherine Proenza for commenting on the manuscript; and Clara Franzini-Armstrong for providing use of the freeze-fracture machine. This work was supported by NIH Grants AR070298 and AR052354; and Muscular Dystrophy Association Grant 277475 (to K.G.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716461115/-/DCSupplemental.
References
- 1.Tanabe T, Beam KG, Powell JA, Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336:134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- 2.Takeshima H, et al. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994;369:556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N′-tetracetic acid. Biochim Biophys Acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- 4.Beurg M, et al. Involvement of the carboxy-terminus region of the dihydropyridine receptor beta1a subunit in excitation-contraction coupling of skeletal muscle. Biophys J. 1999;77:2953–2967. doi: 10.1016/S0006-3495(99)77128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schredelseker J, Dayal A, Schwerte T, Franzini-Armstrong C, Grabner M. Proper restoration of excitation-contraction coupling in the dihydropyridine receptor beta1-null zebrafish relaxed is an exclusive function of the beta1a subunit. J Biol Chem. 2009;284:1242–1251. doi: 10.1074/jbc.M807767200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horstick EJ, et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat Commun. 2013;4:1952. doi: 10.1038/ncomms2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linsley JW, et al. Congenital myopathy results from misregulation of a muscle Ca2+ channel by mutant Stac3. Proc Natl Acad Sci USA. 2017;114:E228–E236. doi: 10.1073/pnas.1619238114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson BR, et al. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc Natl Acad Sci USA. 2013;110:11881–11886. doi: 10.1073/pnas.1310571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polster A, Nelson BR, Olson EN, Beam KG. Stac3 has a direct role in skeletal muscle-type excitation-contraction coupling that is disrupted by a myopathy-causing mutation. Proc Natl Acad Sci USA. 2016;113:10986–10991. doi: 10.1073/pnas.1612441113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suda N, et al. Ca2+-induced Ca2+ release in Chinese hamster ovary (CHO) cells co-expressing dihydropyridine and ryanodine receptors. J Gen Physiol. 1997;109:619–631. doi: 10.1085/jgp.109.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: A novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, et al. Deficiency of triad junction and contraction in mutant skeletal muscle lacking junctophilin type 1. J Cell Biol. 2001;154:1059–1067. doi: 10.1083/jcb.200105040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takekura H, Franzini-Armstrong C. Correct targeting of dihydropyridine receptors and triadin in dyspedic mouse skeletal muscle in vivo. Dev Dyn. 1999;214:372–380. doi: 10.1002/(SICI)1097-0177(199904)214:4<372::AID-AJA9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 14.Polster A, Perni S, Bichraoui H, Beam KG. Stac adaptor proteins regulate trafficking and function of muscle and neuronal L-type Ca2+ channels. Proc Natl Acad Sci USA. 2015;112:602–606. doi: 10.1073/pnas.1423113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gach MP, et al. Alpha2delta1 dihydropyridine receptor subunit is a critical element for excitation-coupled calcium entry but not for formation of tetrads in skeletal myotubes. Biophys J. 2008;94:3023–3034. doi: 10.1529/biophysj.107.118893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obermair GJ, et al. The Ca2+ channel alpha2delta-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of alpha1S or excitation-contraction coupling. J Biol Chem. 2005;280:2229–2237. doi: 10.1074/jbc.M411501200. [DOI] [PubMed] [Google Scholar]
- 17.Franzini-Armstrong C, Pincon-Raymond M, Rieger F. Muscle fibers from dysgenic mouse in vivo lack a surface component of peripheral couplings. Dev Biol. 1991;146:364–376. doi: 10.1016/0012-1606(91)90238-x. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto RM, Brunschwig JP, Kim KC, Caswell AH. Isolation, characterization, and localization of the spanning protein from skeletal muscle triads. J Cell Biol. 1986;103:1405–1414. doi: 10.1083/jcb.103.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzini-Armstrong C. STUDIES OF THE TRIAD: I. Structure of the junction in frog twitch fibers. J Cell Biol. 1970;47:488–499. doi: 10.1083/jcb.47.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Protasi F, et al. RYR1 and RYR3 have different roles in the assembly of calcium release units of skeletal muscle. Biophys J. 2000;79:2494–2508. doi: 10.1016/S0006-3495(00)76491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Näbauer M, Callewaert G, Cleemann L, Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989;244:800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- 23.Tanabe T, Mikami A, Numa S, Beam KG. Cardiac-type excitation-contraction coupling in dysgenic skeletal muscle injected with cardiac dihydropyridine receptor cDNA. Nature. 1990;344:451–453. doi: 10.1038/344451a0. [DOI] [PubMed] [Google Scholar]
- 24.Dayal A, et al. The Ca(2+) influx through the mammalian skeletal muscle dihydropyridine receptor is irrelevant for muscle performance. Nat Commun. 2017;8:475. doi: 10.1038/s41467-017-00629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García J, Beam KG. Measurement of calcium transients and slow calcium current in myotubes. J Gen Physiol. 1994;103:107–123. doi: 10.1085/jgp.103.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988;107:2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Protasi F, Franzini-Armstrong C, Allen PD. Role of ryanodine receptors in the assembly of calcium release units in skeletal muscle. J Cell Biol. 1998;140:831–842. doi: 10.1083/jcb.140.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takekura H, et al. Differential contribution of skeletal and cardiac II-III loop sequences to the assembly of dihydropyridine-receptor arrays in skeletal muscle. Mol Biol Cell. 2004;15:5408–5419. doi: 10.1091/mbc.E04-05-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franzini-Armstrong C, Kish JW. Alternate disposition of tetrads in peripheral couplings of skeletal muscle. J Muscle Res Cell Motil. 1995;16:319–324. doi: 10.1007/BF00121140. [DOI] [PubMed] [Google Scholar]
- 30.Paolini C, Protasi F, Franzini-Armstrong C. The relative position of RyR feet and DHPR tetrads in skeletal muscle. J Mol Biol. 2004;342:145–153. doi: 10.1016/j.jmb.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 31.Nakai J, et al. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- 32.Polster A, Ohrtman JD, Beam KG, Papadopoulos S. Fluorescence resonance energy transfer (FRET) indicates that association with the type I ryanodine receptor (RyR1) causes reorientation of multiple cytoplasmic domains of the dihydropyridine receptor (DHPR) α(1S) subunit. J Biol Chem. 2012;287:41560–41568. doi: 10.1074/jbc.M112.404194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papadopoulos S, Leuranguer V, Bannister RA, Beam KG. Mapping sites of potential proximity between the dihydropyridine receptor and RyR1 in muscle using a cyan fluorescent protein-yellow fluorescent protein tandem as a fluorescence resonance energy transfer probe. J Biol Chem. 2004;279:44046–44056. doi: 10.1074/jbc.M405317200. [DOI] [PubMed] [Google Scholar]
- 34.Eltit JM, et al. Orthograde dihydropyridine receptor signal regulates ryanodine receptor passive leak. Proc Natl Acad Sci USA. 2011;108:7046–7051. doi: 10.1073/pnas.1018380108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.