Abstract
Development of catalyst-controlled stereoselective olefin metathesis processes1 has been a pivotal recent advance in chemistry. Incorporation of appropriate ligands within molybdenum-2, tungsten3 and ruthenium-based complexes4 has made reactivity and selectivity levels that were formerly inaccessible feasible. Here, we show that molybdenum monoaryloxide chloride (MAC) complexes furnish higher energy (Z) isomers of trifluoromethyl-substituted alkenes through cross-metathesis (CM) reactions with commercially available, inexpensive and typically inert Z-1,1,1,4,4,4-hexafluoro-2-butene. Furthermore, otherwise inefficient and non-stereoselective transformations with Z-1,2-dichloro- and 1,2- dibromoethene can be effected with substantially improved efficiency and Z selectivity. Synthesis of representative biologically active molecules and trifluoromethyl analogues of medicinally relevant compounds underscore the importance of the advance. The origins of activity and selectivity levels, which contradict the previously proposed principles5, are elucidated with the aid of DFT calculations.
Substitution of an oxygen-based ligand with a pyrrolide moiety converts a Mo or W alkylidene (e.g., Mo-1a, Fig. 1a) to a uniquely efficient and stereoselective1 olefin metathesis catalyst. In Z-selective processes, an alkene binds trans to the pyrrolide5, generating a metallacyclobutane with sterically differentiated imido (smaller) and aryloxide (larger) ligands6. Kinetically E-selective CM reactions were recently introduced as well7. Nevertheless, critical shortcomings persist. For instance, with Mo monoaryloxide pyrrolide (MAP) catalysts CM of Z-1,2-dihaloalkenes with aryl olefins or 1,3-dienes is often inefficient and non-stereoselective8. In addition, CM reactions generating Z-alkenes that carry a trifluoromethyl group are unknown; these moieties can impart increased bioavailability, metabolic stability, lipophilicity or binding selectivity9,10 to biologically active molecules11 and are needed for future advances in agrochemicals12 and materials research13. Yet, the state-of-the-art for synthesis of trifluoromethyl-substituted olefins is at a primitive stage. The available protocols are either minimally stereoselective14,15 or afford E isomers predominantly16 (e.g., CM with gaseous 3,3,3-trifluoropropene17), and the small number of methods for preparing Z-trifluoromethyl olefins are expensive and/or impractical18,19. Partial hydrogenation of alkynyl substrates is possible but over-reduction can be an issue20.
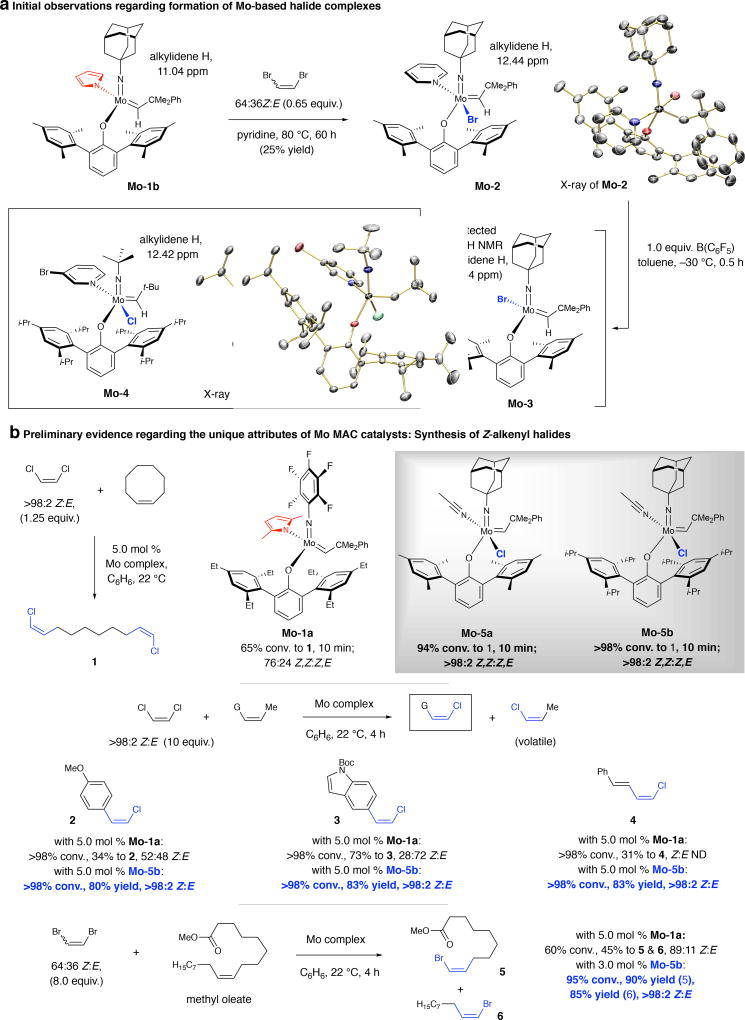
Fig. 1. Initial findings and synthesis of Z-alkenyl halides.
a, Formation of a monoaryloxide bromide complex (Mo-2). Lewis acid treatment afforded the four-coordinate species Mo-3. b, Monoaryloxide chloride (MAC) complexes are most effective in promoting Z-selective ROCM (vs. the corresponding pyrrolide or MAP systems). CM of Z-1,2-dichloroethene and various types of olefins are exceptionally efficient and stereoselective with MAC complexes, which can also promote Z-selective CM with a 64:36 Z:E mixture of 1,2-dibromoethene. 1H NMR spectra were recorded in C6D6; stereoselectivities measured by 1H NMR analysis (±2%); yields are for isolated/purified products (±5%). See the Supplementary Information for details. Boc, tert-butoxycarbonyl; G, functional groups; ND, not determined.
As part of an initiative to synthesize halo-substituted Mo alkylidenes, intermediates in stereoselective CM reactions that afford alkenyl halides7,8, we discovered that treatment of Mo-1b with 1,2-dibromoethene and pyridine gives monoaryloxide bromide complex Mo-2 (Fig. 1a). Subjection of Mo-2 to tris(pentafluorophenyl)borane afforded four-coordinate species Mo-3, which is not sufficiently stable to be isolated. Procedures for preparation of multi-gram quantities of monoaryloxide chloride (MAC) derivatives (e.g., Mo-4) from readily accessible and inexpensive materials were subsequently developed (details in the Supplementary Information). Coordination of pyridine trans to chloride in Mo-4 suggests that an alkene substrate would likely bind similarly, reminiscent of the formerly examined MAP systems (olefin trans to pyrrolide)21.
To evaluate the chemistry of MAC complexes, we first examined their effectiveness in promoting the ring-opening/cross-metathesis (ROCM) between 1,2-dichloroethene and cyclooctene (Fig. 1b). Whereas after 10 minutes there was 65% conversion to 1 with Mo-1a (>98% conv. after 1 h), with Mo-5a reaction proceeded to 94% conversion and stereocontrol was considerably higher (>98:2 vs. 76:24 Z,Z:Z,E); ROCM with the bulkier Mo-5b was similarly efficient and stereoselective. There was less than 5% conversion to 1 after one hour with the pyridine-bound Mo-2, implying that the derived four coordinate entity is catalytically active.
CM of terminal alkenes with MAC complexes was inefficient (<10% conv.), the reasons for which remain to be determined. We therefore turned to evaluating CM with (Z)-1,2-disubstituted alkenes, which can be purchased or accessed in one step through catalytic cross-coupling22 between commercially available Z-1-bromo-1-propene and an aryl- or alkenylboronic acid or pinacol ester (see the Supplementary Information for details). In the event, whereas with Mo-1a there was 34% and 73% conversion to 2 and 3, respectively (Fig. 1b), with Mo-5b these compounds was isolated in ≥80% yield. Moreover, although CM with Mo-1a was either non-selective (2, 52:48 Z:E) or E-selective (3, 28:72 Z:E, probably due to post-metathesis isomerization), with Mo-5b only the Z product was detected. The same applies to Z,E-diene 4, where there was 53% conversion to β-chlorostyrene with Mo-1a (from CM with the styrenyl olefin) and stereoselectivity was not determined because of a complicated product mixture. Similarly, CM of 1,2-dibromoethene with methyl oleate was more efficient and Z-selective with Mo-5b (Fig. 1b); use of Mo-5a, a less hindered complex that may generate shorter living alkylidenes, led to diminished efficiency (32% conv. to 5 and 6). The higher Z selectivities in MAC-catalyzed reactions were surprising since Z-to-E isomerization is often an issue with the more active complexes. Consistent with the commonly used stereochemical model1,5,Error! Bookmark not defined., we expected the size difference between the imido and aryloxide moieties to determine stereoselectivity, not the identity of the anionic ligand trans to the metallacyclobutane.
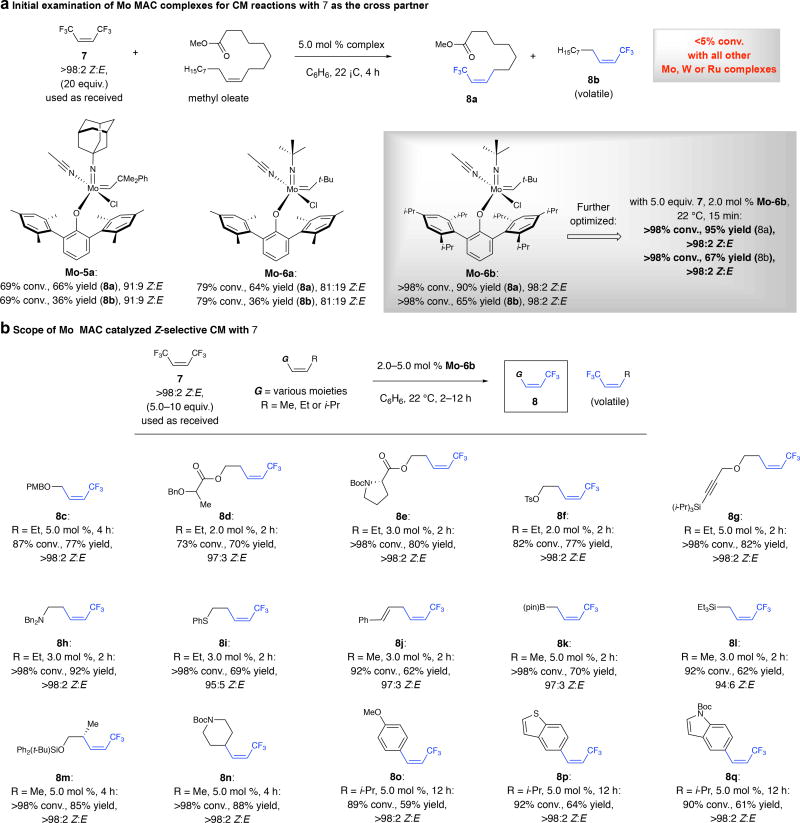
The main question then was whether a MAC complex can catalyse CM reactions with Z-1,1,1,4,4,4-hexafluoro-2-butene (7; >98% Z), a hydrofluoroolefin that can be bought in small amounts (USD 295/5 g from Synquest) or bulk quantities as a foam-blowing agent and that has ozone depleting potential (ODP) and global warming potential (GWP) values of zero and low, respectively23. Furthermore, 7 is a non-flammable liquid at ambient temperature and convenient to use (boiling point, +33 °C vs. –22 °C for 3,3,3-trifluoropropene). However, compound 7, which is utilized in industrial applications is generally inert probably because of its hindered and severely electron deficient alkene. To the best of our knowledge, this organofluoride has not been used in organic chemistry; our efforts to access Z-trifluoromethyl-substituted alkenes (e.g., CM of methyl oleate with 7) with known Mo complexes or Ru carbenes were completely unsuccessful (no desired products detected).
In sharp contrast and remarkably, with Mo-5a and Mo-6a CM of methyl oleate and 7 afforded appreciable amounts of 8a and 8b (Fig. 2a). In considering ways that Z selectivity might be improved, we reasoned that, other than post-metathesis isomerization, formation of the undesired E isomer might originate from initial isomerization of the olefin substrate7. Accordingly, with Mo-6b, a more congested and longer living MAC complex, CM was complete in four hours, furnishing 8a and 8b in 98:2 Z:E selectivity and 90% and 65% yield, respectively. Further study indicated that with 2.0 mol % Mo-6b and five equivalents of 7 the transformation was complete in only 15 minutes with nearly the same yields and Z selectivities (slightly lower yields with Mo-5b).
Fig. 2. Mo MAC complexes engage a typically inert trifluoromethyl-substituted alkene.
a, Several Mo MAC complexes can catalyze CM of Z-1,2-disubstituted alkenes and reagent 7 with exceptional Z selectivity (Mo-6b). b, Various alkyl and aryl olefins, including those containing Lewis basic esters, carbamates and amines or α-branched moieties, may be used in efficient and exceptionally Z-selective CM reactions. The requisite Z-1,2-disubstituted alkene starting materials may either be purchased or prepared easily in one step from commercially available compounds. PMB, para-methoxybenzyl; Bn, benzyl; Boc, tert-butoxycarbonyl; pin, pinacolato; Ts, tosyl group. Stereoselectivities measured by 1H NMR analysis (±2%); yields are for isolated and purified products (±5%). See the Supplementary Information for details.
Many (Z)-1,2-disubstituted alkenes, commercially available or accessible in one step from naturally occurring Z-olefins (e.g., Z-3-hexen-1-ol) or through cross-coupling, can be used (Fig. 2b). Products containing an ether (8c), an α-alkoxy ester capable of chelating to the Mo center (8d), or a carbamate (8e) were easily accessed. CM with alkenes containing a tosylate (8f), an alkyne (8g), a tertiary amine (8h), or a sulfide (8i) was efficient and Z-selective. A 1,4-diene (8j), a crotyl–B(pin) (8k) or a crotylsilane (8l) were suitable substrates. Transformations with hindered α-branched 1,2-disubstituted alkenes (8m,n) and β-substituted styrenes (8o-q) proceeded smoothly. CM with aryl olefins needed (Z)-β-isopropylstyrenyl substrates so that homocoupling would be less competitive. Paraffin tablets containing a MAC species7 may be used (no glove box); for instance, with a pellet containing Mo-6b (~3.0 mol %; toluene, 35 °C, 2 h) 8e was obtained in 74% yield and >98:2 Z:E ratio.
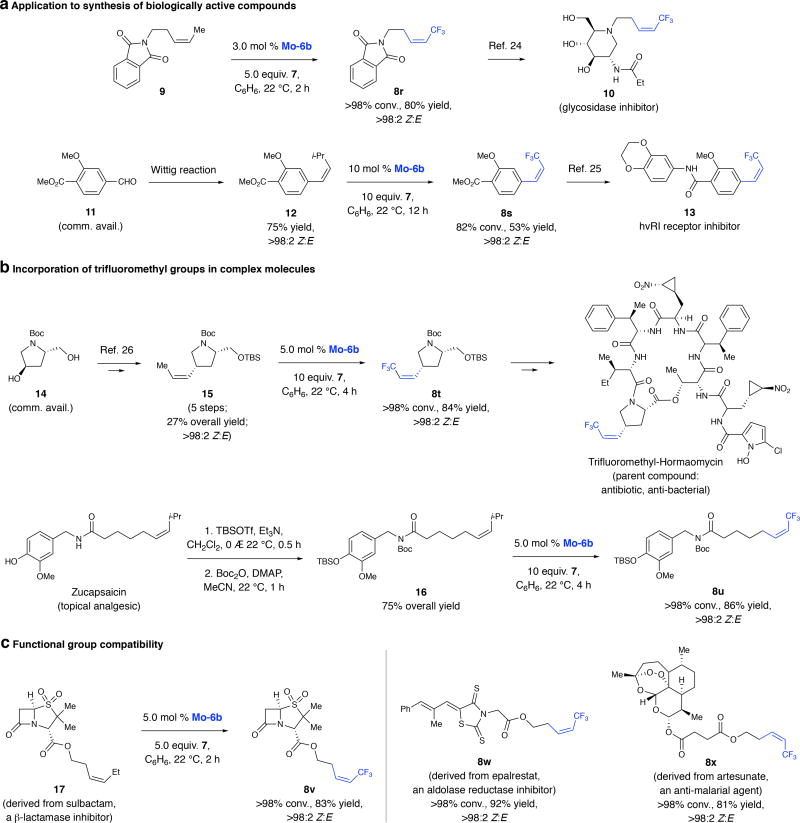
Product 8r has been transformed to glycosidase inhibitor 1024 (Fig. 3a). Conversion of the commercially available aldehyde 11 to Z-alkene 12 followed by CM with 7 afforded 8s, an intermediate en route to hvRI receptor inhibitor 1325. Previously, 8s was prepared by Wittig reaction with aldehyde 11 and 2,2,2-trifluoroethyl diphenylphosphine oxide (not commercially available), affording a mixture of E/Z isomers (exact ratio and yield not reported25). Severl examples show that synthesis of trifluoromethyl analogues of medicinally relevant agents can be facilitated (Fig. 3b). Z-Alkene 15, formerly accessed in five steps and 27% overall yield from commercially available enantiomerically pure 14, was transformed to 8t in 84% yield and >98% Z selectivity, allowing for synthesis of a trifluoromethyl analogue of hormaomycin26. CM of 16, derived from analgesic zucapsaicin27, delivered 8u (86% yield, >98% Z). Transformation of 17, obtained from sulbactam28 (β-lactamase inhibitor), to 8v and syntheses of 8w (from epalrestat29, aldolase reductase inhibitor) and 8x (from artesunate30, anti-malarial agent), underscore the compatibility of Mo MAC complexes with polar functional groups.
Fig. 3. Utility and functional group compatibility.
a, Mo MAC-catalyzed CM provides direct access to biologically active molecules. Synthesis of 13 is notable as it involves reaction between severely hindered alkenes. b, MAC complexes can be used to prepare and probe the activity of Z-trifluoromethyl derivatives of new drug candidates, benefitting from advantages of a trifluoromethyl unit. c, Despite their high Lewis acidity, Mo MAC complex tolerate Lewis basic functional groups that regularly appear in therapeutic agents (e.g., 8v–8x). TBS, tert-butyldimethylsilyl; Boc, tert-butoxycarbonyl; Tf, trifluoromethylsulfonyl; DMAP, 4-dimethylaminopyridine. Stereoselectivities measured by 1H NMR analysis (±2%); yields are for isolated/purified products (±5%). See the Supplementary Information for details.
Two central points merit further brief discussion: 1) CM reactions with terminal alkenes would be more desirable but, as mentioned earlier, the (Z)-1,2-disubstituted alkenes utilized here are readily accessed. Considering the high value of the Z-trifluoromethyl-substituted alkenes, ease of their preparation together with the paucity of alternative methods, the present approach offers a compelling solution to a longstanding problem. For instance, the Z-allyl–B(pin) 8k (see Fig. 2b), a product that may be used to access an assortment of desirable trifluoromethyl-containing products through future developments in diastereo- and/or enantioselective additions to electrophiles, was obtained by reaction of commercially available Z-crotyl–B(pin). 2) Development of compounds that contain a Z-trifluoromethyl-substituted olefin and/or a related derivative with desirable biological activity has probably been hampered because of the absence of direct and practical methods to obtain such species. Still, as indicated by the examples mentioned above, the considerable potential of such entities is well-appreciated9,10.
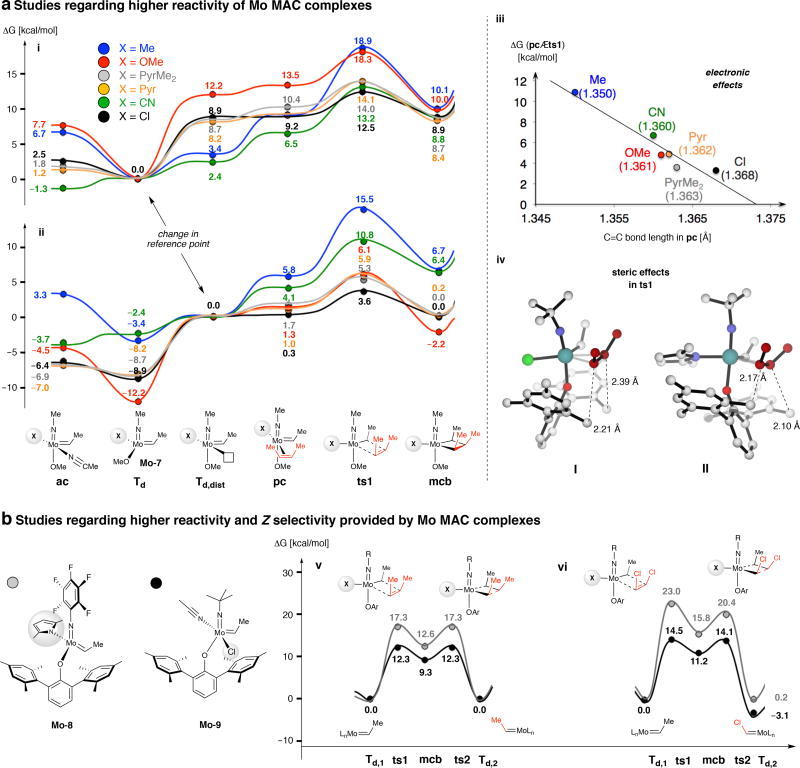
DFT calculations shed light on why MAC complexes are singularly effective. We first probed the influence of several anionic ligands on the reaction of Z-2-butene with Mo-7 (Fig. 4a, i). While the energy for distortion of the chloro complex is relatively high (8.9 kcal/mol), the ensuing metallacyclobutane (mcb) formation (Td,dist/pc → ts1) is the most facile. There is strong correlation between the barrier to ts1 and the extent of C–C double bond activation in the Mo π-complex (pc), a characteristic more evident in Fig. 4a, diagram ii where Td,dist is the reference point. Whereas methyl–Mo complex emerges as the least activated (C=C, 1.350 Å) the more Lewis acidic chloro species has the longest (most tightly) chelated alkene (C=C, 1.368 Å), a trend consistent with the lowest unoccupied molecular orbital (LUMO) energies for the distorted ground state complexes (Td,dist). The overall energy requirement appears to be derived from a combination of the cost of structural distortion (Td → Td,dist) and mcb formation (Td,dist/pc → ts1); the model MAC system has the smallest barrier (12.5 kcal/mol) and the largest is for the methyl and methoxy derivatives (Fig. 4a, i). These principles are distinct from those of a previous study, which involved less substituted mcb intermediates, where methyl-substituted complexes were assigned higher reactivity (vs. methoxy) based on the principle that a stronger σ-donating ligand helps make available a trans ligation site5. The present work shows that neither a methoxy- nor a methyl-substituted species can deliver the activity level of a Mo chloride species.
Fig. 4. Computational/mechanistic studies.
a, Electronic effect of the anionic ligand on degenerate olefin metathesis of Z-2-butene with model complexes Mo-7 (i–iii) and the influence of steric factors with larger aryloxide moieties (iv). b, Comparison of the reactivity and selectivity of Mo-8,9 with Z-2-butene (v) and Z-1,2-dichloroethene (vi). Energy values correspond to the free energy (ΔG in kcal/mol) determined at the MN12SX/Def2TZVPP//ω−B97XD/Def2SVP level in benzene as solvent (SMD solvation model). Abbreviations: PyrMe2 = 2,5-dimethylpyrrolide; Pyr = pyrrolide; ac = acetonitrile complex; Td, tetrahedral complex; Td,dist, distorted tetrahedral complex; pc, π-complex; ts1, transition state for metallacylobutane formation; mcb, metallacyclobutane; ts2; transition state for metallacyclobutane cleavage; R, aryl or alkyl group; Ar, aryl group. See the Supplementary Information for details.
We then investigated the transformation between Z-2-butene with Mo-8 (see Fig. 4b) with the methoxy ligand replaced by a much larger 2,6-dimesityl-phenoxy moiety. We find that in transition state I (Fig. 4a, iv), the aryloxide ligand tilts toward the Cl ligand with longer C–H⋯·C–H distances (2.21 and 2.39 Å). In the MAP complex II the aryloxy group and the reacting alkene are forced into closer contact (2.10 and 2.17 Å). The increased steric pressure has stronger impact on the activation barriers (ts1 = 12.1 and 20.4 kcal/mol for the chloro and dimethylpyrrolide complexes, respectively) compared to the more diminutive methoxy complexes (ts1 = 12.5 and 14.0 kcal/mol for the chloro and dimethylpyrrolide systems, respectively; Fig. 4a, ii).
The improved efficiency and Z selectivity in generating alkenyl halides with MAC complexes arise from differences in chemoselectivity. This is indicated by a larger gap in the energy required for ts1 in reactions of Mo-8 (MAP) with Z-2-butene (17.3 kcal/mole; Fig. 4b, v) and Z-1,2-dichloroethene (23.0 kcal/mol; Fig. 4b, vi) compared to those for the transformation with Mo-9 (MAC; 12.3 and 14.5 kcal/mole, respectively). Alkyl-substituted MAP alkylidenes are more prone to react with an aliphatic alkene (vs. less Lewis basic 1,2-dichloroalkene) to afford homocoupling products and thus Z-to-E isomerization/CM becomes an issue. With excess dihaloalkene CM becomes more favourable and homocoupling is less competitive. With a MAC species, capable of reacting with either alkene at comparable rates, adventitious alkene homocoupling and E isomer generation is minimal, especially with excess dihaloalkene; control experiments indicate that Z-to-E interconversion of these reagents is slow.
Similar arguments may be extended to reactions that deliver Z-alkenyl bromides (Fig. 1b). Despite a more active MAC complex, capable of causing post-metathesis isomerization, and the presence of 36% E-1,2-dibromoethene, CM is exceptionally Z-selective. This may be attributed to lower reactivity of the E isomer, which is supported by the diminished Z:E ratio (41:59) of recovered reagent after CM of methyl oleate with 2.3 equivalents of 1,2-dibromoethene (~1.5 equiv. Z isomer) with 5.0 mol % of Mo-5b (4 h). Mo MAC complexes do not promote efficient CM reactions with E-1,2-dichloroethene or E-1,1,1,4,4,4-hexafluoro-2-butene (<10% conv.); we attribute this to rapid decomposition of the derived metallacyclobutanes. Subjection of Z-methyl oleate to a 3:2 mixture of Z- and E-1,2-dichloroethene and 3.0 mol % Mo-6a led only to 20% conversion to the CM products (C6H6, 22 °C, 4 h vs. >98% conv. and 97% yield with the pure Z isomer). This is unlike the case with the bulkier 1,2-dibromoethene, where the E isomer reacts at a sufficiently slower rate so that CM can proceed to completion.
The importance of Mo MAC complexes is evidenced by their ability to catalyse – with unprecedented efficiency and selectivity – the formation of three types of products that are of considerable importance in the preparation and identification of potential medicines and functional small molecules. The ability to promote transformations with Z-1,1,1,4,4,4-hexafluoro-2-butene (7), a compound not previously utilized in a chemical transformation, is particularly noteworthy. Computational studies teach us a key lesson as well: contrary to expectations based on former studies5, the chloride complexes exhibit higher activity compared to MAP species due to enhanced Lewis acidity and diminution in steric repulsion within a trigonal bipyramidal intermediate.
Methods
General Procedure for CM with a MAC complex
In a N2-filled glove box, an oven-dried 8 mL vial equipped with a magnetic stir bar was charged with alkene substrate and the corresponding organohalogen reagent (Z-1,1,1,4,4,4-hexafluoro-2-butene, Z-1,2-dichloroethene or 1,2-dibromoethene). A solution of an appropriate MAC complex in benzene was then added. The resulting mixture was allowed to stir for 15 min-12 h at 22 °C, after which the reaction was quenched by the addition of wet (undistilled) CDCl3 (percent conversion was determined by 1H NMR analysis of the unpurified mixture). Purification was performed through silica gel chromatography, preparative thin layer chromatography and/or Kugelrohr distillation.
General Procedure for CM with a paraffin tablet containing a MAC complex
An oven-dried 8 mL vial equipped with a magnetic stir bar was charged with a paraffin tablet (9 wt% in Mo-6b, 20.0 mg, 2.2 µmol) and (S,Z)-1-t-butyl 2-hex-3-enyl pyrrolidine-1,2-dicarboxylate (22.0 mg, 0.0740 mmol). The vial was sealed with a septum, then evacuated and back-filled with N2 three times to remove oxygen. Z-1,1,1,4,4,4-Hexafluoro-2-butene (7; 43 µL, 0.370 mmol) and toluene (74 µL) were added by syringe and the resulting mixture was allowed to stir at 35 °C for 2 hours under N2 atmosphere. The reaction was quenched by addition of MeCN (1.5 mL) and the mixture was allowed to stir at 22 °C for 10 minutes. The slurry was filtered through a short plug of silica gel and eluted with MeCN (2 mL). The filtrate was concentrated and analysis of the unpurified mixture revealed 98% consumption of (S,Z)-1-tert-butyl 2-hex-3-enyl pyrrolidine-1,2-dicarboxylate. The resulting green oil was purified by silica gel chromatography (4% Et2O/pentane to 20% Et2O/pentane) to afford 8e (18.5 mg, 0.0548 mmol, 74% yield) in >98:2 Z:E ratio as colourless oil.
Supplementary Material
Acknowledgments
This research was supported by the United States National Institutes of Health, Institute of General Medical Sciences (GM-59426). M. J. K. acknowledges support through LaMattina and BristolMyers-Squibb Graduate Fellowships. We thank Dr. Peter Muller for helping obtain various X-ray structures and Dr. Xiao Shen for valuable advice and experimental assistance. We are grateful to XiMo, AG for gifts of paraffin tablets.
Footnotes
The authors declare no competing financial interests.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions M. J. K. and T. T. N. were involved in the discovery, design and development of the new Z-selective cross-metathesis strategies and their applications. J. K. L., J. H. and R. R. S. were involved in the synthesis and characterization of Mo MAC complexes. S. T. designed and performed the computational investigations, developed the models for the observed levels and patterns in reactivity and stereoselectivity. A.H.H. and R. R. S. designed and directed the investigation. A. H. H. wrote the manuscript with revisions provided by M. J. K., T. T. N., J. K. L., S. T. and R. R. S.
References
- 1.Hoveyda AH, Khan RKM, Torker S, Malcolmson SJ. Catalyst-controlled stereoselective olefin metathesis. In: Grubbs RH, Wenzel AG, O’Leary DJ, Khosravi E, editors. Handbook of Metathesis. Wiley–VCH; Weinheim: 2014. pp. 503–562. [Google Scholar]
- 2.Malcolmson SJ, Meek SJ, Sattely ES, Schrock RR, Hoveyda AH. A new class of chiral catalysts for enantioselective alkene metathesis. Nature. 2008;456:933–937. doi: 10.1038/nature07594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu M, Wang C, Kyle AF, Jakubec P, Dixon DJ, Schrock RR, Hoveyda AH. Synthesis of macrocyclic natural products by catalyst-controlled stereoselective ring-closing metathesis. Nature. 2011;479:88–93. doi: 10.1038/nature10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myles MB, Grubbs RH. Z-Selective cross metathesis with ruthenium catalysts: Synthetic applications and mechanistic implications. Angew. Chem. Int. Ed. 2015;54:5018–5024. doi: 10.1002/anie.201411588. [DOI] [PubMed] [Google Scholar]
- 5.Poater A, Solans-Monfort X, Copéret C, Eisenstein O. Understanding d0-olefin metathesis catalysts: Which metal, which ligands? J. Am. Chem. Soc. 2007;129:8207–8216. doi: 10.1021/ja070625y. [DOI] [PubMed] [Google Scholar]
- 6.Meek SJ, O’Brien RV, Llaveria J, Schrock RR, Hoveyda AH. Catalytic Z-selective olefin cross-metathesis for natural product synthesis. Nature. 2011;471:461–466. doi: 10.1038/nature09957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen TT, Koh MJ, Shen X, Romiti F, Schrock RR, Hoveyda AH. Kinetically E-selective olefin metathesis reactions. Science. 2016;552:569–575. doi: 10.1126/science.aaf4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh MJ, Nguyen TT, Zhang H, Schrock RR, Hoveyda AH. Direct synthesis of Z-alkenyl halides through catalytic cross-methathesis. Nature. 2016;531:459–465. doi: 10.1038/nature17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015;58:8315–8359. doi: 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- 10.Innocenti P, et al. Design of potent and selective hybrid inhibitors of the mitotic kinase Nek2: Structure–activity relationship, structural biology, and cellular activity. J. Med. Chem. 2012;55:3228–3241. doi: 10.1021/jm201683b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Shimizu M, Hiyama T. A facile stereocontrolled approach to CF3-substituted triarylethenes: Synthesis of panomifene. Angew. Chem. Int. Ed. 2004;43:879–882. doi: 10.1002/anie.200353032. [DOI] [PubMed] [Google Scholar]
- 12.Fujita M, Hiyama T, Kondo K. Practical and stereocontrolled synthesis of both (1R*,3S*)- and (1R*,3R*)-3-(2-chloro-3,3,3,-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylates. Tetrahedron Lett. 1986;27:2139–2142. [Google Scholar]
- 13.Shimizu M, Takeda Y, Higashi M, Hiyama T. Synthesis and photophysical properties of dimethoxybis(3,3,3-trifluoropropen-1-yl)benzenes: Compact chromophores exhibiting violet fluorescence in the solid state. Chem. Asian J. 2011;6:2536–2544. doi: 10.1002/asia.201100176. [DOI] [PubMed] [Google Scholar]
- 14.Hafner A, Fischer TS, Bräse S. Synthesis of CF3-substituted olefins by Julia–Kocienski olefination using 2-[(2,2,2-trifluoroethyl)sulfonyl]benzo[d]thiazole as trifluoromethylation agent. Eur. J. Org. Chem. 2013:7996–8003. [Google Scholar]
- 15.Choi S, Kim YJ, Kim SM, Yang JW, Kim SW, Cho EJ. Hydrotrifluoromethylation and iodotrifluoromethylation of alkenes and alkynes using an inorganic electride as a radical generator. Nat. Commun. 2014;5:4881–4887. doi: 10.1038/ncomms5881. [DOI] [PubMed] [Google Scholar]
- 16.Choi WJ, Choi S, Ohkubo K, Fukuzumi S, Cho EJ, You Y. Mechanisms and applications of cyclometalated Pt(II) complexes in photoredox catalytic trifluoromethylation. Chem. Sci. 2015;6:1454–1464. doi: 10.1039/c4sc02537g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imhof S, Randl R, Blechert S. Ruthenium catalysed cross metathesis with fluorinated olefins. Chem. Commun. 2001:1692–1693. doi: 10.1039/b105031c. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran PV, Mitsubishi W. (Z)- or (E)-Selective hydrogenation of potassium (3,3,3-trifluoroprop-1-yn-1-yl)trifluoroborate: Route to either isomer of β-trifluoromethylstyrenes. Org. Lett. 2015;17:1252–1255. doi: 10.1021/acs.orglett.5b00235. [DOI] [PubMed] [Google Scholar]
- 19.Lin Q-Y, Xu X-H, Qing F-L. Chemo-, regio-, and stereoselective trifluoromethylation of styrenes via visible light-driven single-electron transfer (SET) and triplet–triplet energy transfer (TTET) processes. J. Org. Chem. 2014;79:10434–10446. doi: 10.1021/jo502040t. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa T, Kawasaki-Takasuka T, Yamada S, Yamazaki T. Construction of chiral trifluoromethylated materials by combination of stereochemically predictable SN2’ reaction and Ireland-Claisen rearrangement. J. Fluor. Chem. 2013;152:38–45. [Google Scholar]
- 21.Marinescu SC, Schrock RR, Li B, Hoveyda AH. Inversion of configuration at the metal in diastereomeric imido alkylidene monoaryloxide monopyrrolide complexes of molybdenum. J. Am. Chem. Soc. 2009;131:58–59. doi: 10.1021/ja808308e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson Seechurn CCC, Kitching MO, Colacot TJ, Sniekus V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012;51:5062–5085. doi: 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- 23.Baasandorj M, Ravishankara AR, Burkholder JB. Atmospheric chemistry of (Z)-CF3CH═CHCF3: OH Radical reaction rate coefficient and global warming potential. J. Phys. Chem. A. 2011;115:10539–10549. doi: 10.1021/jp206195g. [DOI] [PubMed] [Google Scholar]
- 24.Mceachern EJ, Vocadlo DJ, Zhou Y, Selnick HG. Glycosidase inhibitors and uses thereof. WO2014/032187 A1. Patent. 2014 Mar 6;
- 25.Kelly M, et al. Amide derivatives as ion-channel ligands and pharmaceutical compositions and methods of using the same. WO2006/093832 A2. Patent. 2006 Sep 8;
- 26.Zlatopolskiy BD, Kroll H-P, Melotto E, de Meijere A. Convergent syntheses of N-Boc-protected (2S,4R)-4-(Z)-propenylproline and 5-chloro-1-(methoxymethoxy)pyrrol-2-carboxylic acid – Two essential building blocks for the signal metabolite hormaomycin. Eur. J. Org. Chem. 2004:4492–4502. [Google Scholar]
- 27.Hua X-Y, Chen P, Hwang J, Yaksh TL. Antinociception induced by civamide, an orally active capsaicin analogue. Pain. 1997;71:313–322. doi: 10.1016/s0304-3959(97)00003-1. [DOI] [PubMed] [Google Scholar]
- 28.English AR, Girard D, Jasys VJ, Martingano RJ, Kellogg MS. Orally effective acid prodrugs of the β-lactamase inhibitor sulbactam. J. Med. Chem. 1990;33:344–347. doi: 10.1021/jm00163a055. [DOI] [PubMed] [Google Scholar]
- 29.Ramirez MA, Borja & NL. Epalrestat: An aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy. 2008;28:646–655. doi: 10.1592/phco.28.5.646. [DOI] [PubMed] [Google Scholar]
- 30.Luo X-D, Shen C-C. The chemistry, pharmacology, and clinical applications of qinghaosu (artemisinin) and its derivatives. Med. Res. Rev. 1987;7:29–52. doi: 10.1002/med.2610070103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.