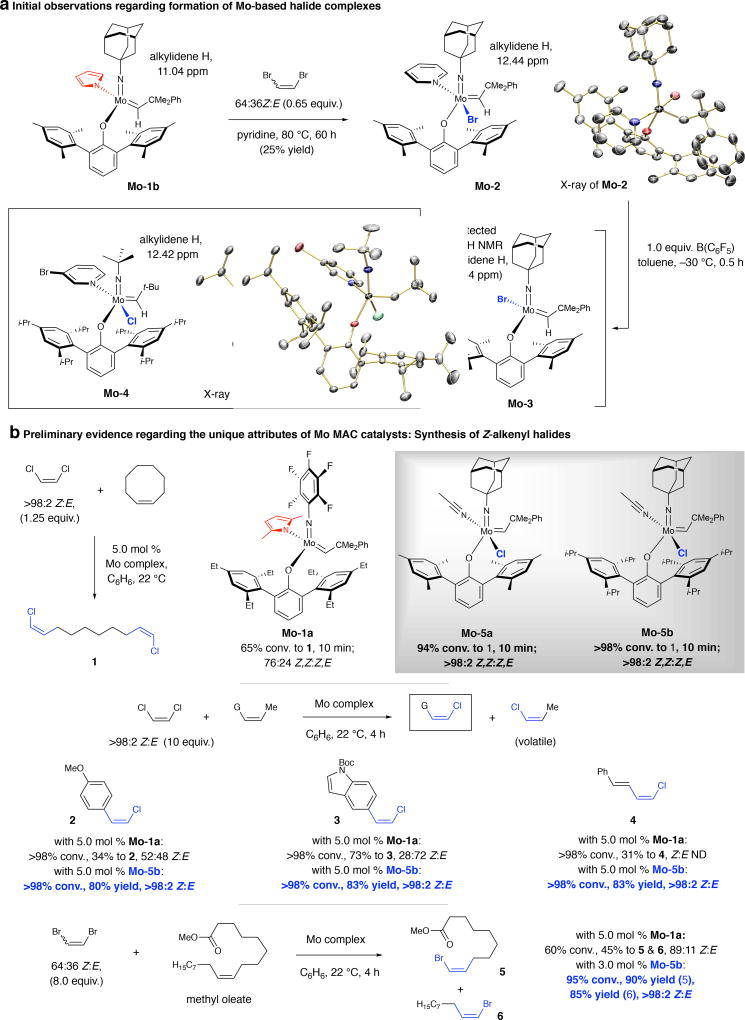
Fig. 1. Initial findings and synthesis of Z-alkenyl halides.
a, Formation of a monoaryloxide bromide complex (Mo-2). Lewis acid treatment afforded the four-coordinate species Mo-3. b, Monoaryloxide chloride (MAC) complexes are most effective in promoting Z-selective ROCM (vs. the corresponding pyrrolide or MAP systems). CM of Z-1,2-dichloroethene and various types of olefins are exceptionally efficient and stereoselective with MAC complexes, which can also promote Z-selective CM with a 64:36 Z:E mixture of 1,2-dibromoethene. 1H NMR spectra were recorded in C6D6; stereoselectivities measured by 1H NMR analysis (±2%); yields are for isolated/purified products (±5%). See the Supplementary Information for details. Boc, tert-butoxycarbonyl; G, functional groups; ND, not determined.