Abstract
A significant shortcoming in olefin metathesis, a chemical process that is central to research in several branches of chemistry, is the lack of efficient methods that kinetically favor E-isomers in the product distribution. Here, we show that kinetically E-selective cross-metathesis reactions may be designed to generate thermodynamically disfavored alkenyl chlorides and fluorides in high yield and with exceptional stereoselectivity. With 1.0–5.0 mol % of a molybdenum-based catalyst, which may be delivered in the form of air- and moisture-stable paraffin pills, reactions typically proceed to completion within four hours at ambient temperature. Many isomerically pure E-alkenyl chlorides, applicable to catalytic cross-coupling transformations and found in biologically active entities, thus become easily and directly accessible. Similarly, E-alkenyl fluorides can be synthesized from simpler compounds or more complex molecules.
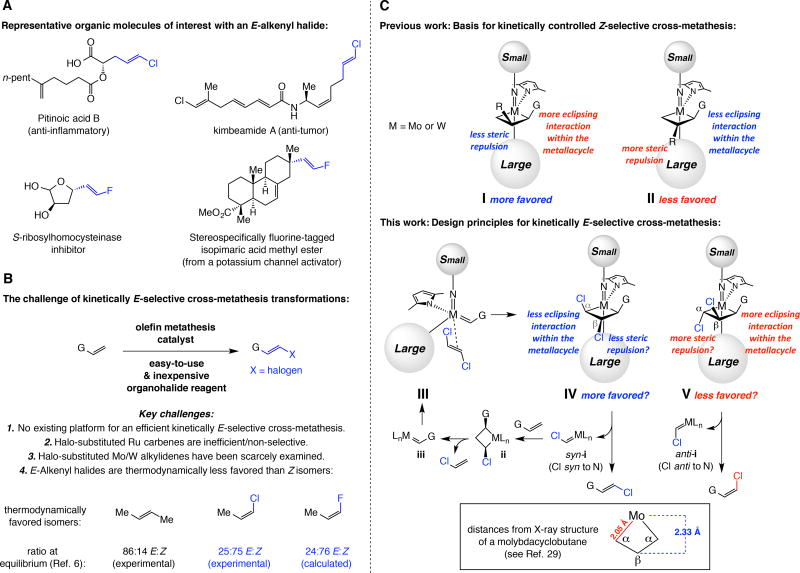
Olefin metathesis is an enormously enabling chemical process for which well-defined catalysts were discovered nearly three decades ago (1,2). Although kinetically controlled Z-selective reactions were introduced in 2009 (3), there are no corresponding transformations that are broadly applicable and through which E isomers can be synthesized in high yield. Just recently were E-selective cross-metathesis (CM) reactions involving Ru catechothiolate complexes (4) reported; however, only the thermodynamically preferred E isomers of simple (unfunctionalized) 1,2-disubstituted aliphatic alkenes could be obtained in 3–31% yield (5). E alkenes are often lower in energy and thus generated preferentially; nonetheless, olefin metathesis strategies that furnish them are needed for several reasons. The energy gap between the geometric forms is often too small to ensure high selectivity, E olefin isomers are not always thermodynamically preferred, and in many cases E and Z alkene isomers cannot be easily separated. Perhaps the most notable instance relates to acyclic alkenyl halides where hyperconjugative donation of electron density from a filled σC–H into a low-lying vacant σ*C–halogen orbital causes stabilization of the Z isomers (6). Herein, we disclose the design of efficient and kinetically E-selective CM reactions through which E-alkenyl chlorides and fluorides can be obtained in good yield and with exceptional stereoselectivity.
E-1,2-Disubstituted chloro-alkenes can be used in catalytic cross-coupling, one of the most influential transformations in modern chemistry (7). They are found in biologically active molecules as well (8); two examples are pitinoic acid B, a potent anti-inflammatory agent (9), and kimbeamide A, a natural product with significant anti-tumor activity (10) (Fig. 1A). E-Alkenyl fluorides are of interest because of the substantial value of organofluorines in medicine (11) and agrochemicals (12). A biologically active fluoro-alkenyl entity that inhibits Bacillus subtilis S-ribosyl-homocysteinase (LuxS) (13) is presented in Fig. 1A; only an E/Z mixture of this compound has been evaluated due to the difficulties associated with accessing a stereoisomerically pure alkenyl fluoride. Functionalization of a stereochemically defined fluoro-substituted olefin would facilitate preparation of other desirable F-containing molecules (14).
Fig. 1. Kinetically E-selective cross-metathesis (CM): potential impact and challenges.
(A) Examples of biologically active organic molecules that contain an E alkenyl chloride or fluoride. (B) Challenges in designing a kinetically E-selective CM to prepare alkenyl halides. (C) Analysis of stereochemical models that suggest a strategy for the design of kinetically E-selective olefin metathesis processes. G, R = functional groups.
Equally important, by conversion of an alkene to an E-alkenyl fluoride through catalytic and stereoselective CM a hydrogen may be substituted with a fluorine atom within a complex molecule, such as the methyl ester of potassium channel activator isopimaric acid in Fig. 1A (15); such modification can result in the alteration and/or enhancement of its biological activity (16, 17).
E-1,2-Disubstituted alkenyl chlorides can be prepared in a number of ways (see the Supplementary Material for further bibliography). Terminal alkynes may be transformed to nucleophilic E-alkenylmetal intermediates that are then treated with an electrophilic halogen source (18, 19, 20). E-Alkenyl boronic acids, prepared from a terminal alkyne by a two step procedure (hydroboration, hydrolysis), can be converted by a third step to the corresponding chlorides (21), which may alternatively be synthesized by treatment of an aldehyde with excess chromium chloride and chloroform (22). A small number of procedures furnish E-alkenyl fluorides from aldehydes (23) or alkynes (24,25), but none are catalytic and several require stoichiometric amounts of strongly basic (e.g., t-BuLi) and/or toxic reagents (e.g., hexamethylphosphoramide (HMPA)). In another case, a stereochemically defined fluorinesubstituted alkenyl tosylate must first be secured by the use of n-BuLi and LiAlH4 before it is subjected to a cross-coupling reaction (26). Efficient and E-selective catalytic CM strategies that deliver alkenyl chlorides and fluorides (Fig. 1B), involving robust, abundant and less costly alkenes (vs. aldehydes or alkynes) would complement the aforementioned methods, offering a more direct and economical approach on most occasions.
We recently demonstrated that molybdenum-based monoaryloxide pyrrolide (MAP) complexes possess the unique ability to promote efficient Z-selective CM reactions that afford alkenyl halides (27); transformations probably proceed via olefin-derived alkylidenes that then react with a halo-olefin reagent. Reactions with widely used complexes (e.g., dichloro-Ru carbenes) are either inefficient and/or non-stereoselective (27). Designing a kinetically E-selective variant, however, poses several distinct challenges. Not only was there no blueprint for a high yielding and kinetically controlled E-selective olefin metathesis when these studies were initiated, the desired products would be thermodynamically less favored: The Z-halo-alkenes are the more preferred isomers, as indicated by the relative energies shown in Fig. 1B (6).
In contemplating a strategy for achieving kinetic E selectivity, we evaluated the stereochemical model proposed for the corresponding Z-selective CM reactions (28), realizing an implicit principle. Reaction via metallacyclobutane I is preferred versus II (Fig. 1C) because the eclipsing interaction between the C–R and C–G bonds in I is less destabilizing than the steric repulsion between the C–R unit and the larger catalyst ligand. The question then became: If an alkylidene complex were to react with E-1,2-dichloroethene via III, would metallacycle IV or its corresponding diastereomer V be favored? In IV, the eclipsing interactions between Cα–G or Cα–Cl and Cβ–H bonds in a structurally flat (29) molybdacyclobutane (non-puckered due to the longer C–Mo bonds) would be less severe compared to those involving Cα–G and Cβ–Cl bonds of V (Cα–Cα distance is ~2.75 Å). Whereas in IV, which would release an E-alkene, Cβ–Cl bond is oriented towards the more sizeable ligand, in V it would be a Cα–Cl unit that is so disposed. Moreover, collapse of IV would yield a presumably lower energy syn-alkylidene (Cl pointing towards the imido ligand) (30). The resulting highly active (27) chloro-substituted syn-alkylidene (syn-i) would then react with the olefin substrate via the lower energy α,α’-disubstituted metallacyclobutane ii (minimal eclipsing interaction/steric repulsion with aryloxide ligand with G and Cl oriented towards the smaller imido unit) to generate complex iii, which could be converted to III. It was unclear which of the latter pathways would be dominant and how high the stereoselectivity would be.
In search of more clues, we examined the crystal structure of an unsubstituted molybdacyclobutane derived from a monoaryloxide pyrrolide (MAP) complex (29), noting that the molybdenum–Cβ distance is longer than the metal–Cα bond length (2.33 vs. 2.05 Å; Fig. 1C). This suggested that there might be less steric repulsion between the halogen and the larger aryloxide ligand in IV (vs. V), which could translate to an E-selective process. However, the above analysis pointed to another possible complication: the vinyl chloride byproduct might cause a diminution in selectivity by reaction via metallacyclobutane I, affording the thermodynamically favored Z-alkenyl halide. Use of vacuum (28) would not be an option; although the volatility of E-1,2-dichloroethene allows it to be easily handled and readily removed (boiling point, 48 °C), a substantial amount (if not all) would be lost even under mildly reduced pressure.
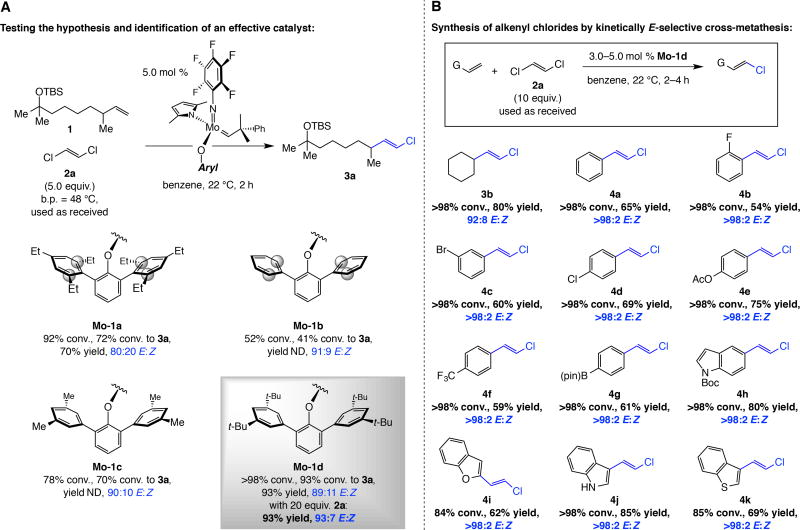
The above reasoning implied that an entirely distinct catalyst construct would not be needed for achieving high kinetic E selectivity and could be validated experimentally: CM of alkene 1 and E-1,2-dichloroethene 2a with 5.0 mol % Mo-1a (Fig. 2A), affords E-alkenyl chloride 3a with 80:20 E:Z ratio. Equally encouraging was that the products were obtained in 70% yield after purification without resorting to long reaction times or elevated temperatures (2 h, 22 °C).
Fig. 2. Kinetically controlled E-selective cross-metathesis reactions that generate alkenyl chlorides.
(A) Model studies involving commercially available and easy-to-handle E-dichloroethene (2a) indicated that structural adjustment of the catalyst’s aryloxide ligand is needed for maximal efficiency and stereoselectivity. (B) A range of alkyl-, aryl, as well as heteroaryl-substituted E-alkenyl chlorides can be obtained in up to >98:2 E:Z selectivity. Yields are for purified products. TBS = tert-butyldimethylsilyl. G = functional group. Ac = acetyl. pin = pinacolato. Boc = tert-butoxycarbonyl. See the Supplementary Materials for details.
We envisioned that removing the ortho aryl substituents within the aryloxide ligand (highlighted in Mo-1a,b, Fig. 2A) might alleviate steric repulsion with the Cβ chlorine atom, favoring reaction via IV. We therefore prepared and examined the selectivity of the CM reaction with Mo-1b, which led to significantly improved stereoselectivity (91:9 E:Z) but lower efficiency (41% vs. 72% conv. with Mo-1a) probably due to competitive bimolecular alkylidene decomposition (30). To retain high selectivity without efficiency loss, we evaluated the performance of Mo-1c and Mo-1d and established that either complex can produce 3a with appreciable efficiency in ~90% E selectivity (Fig. 2A). With Mo-1d and 20 equivalents of 2a selectivity improved to 93% E likely due to diminished competitiveness of non-selective CM involving the vinyl chloride byproduct (via I, Fig. 1C). Excess 2a was easily removed in vacuo.
An array of E-alkenyl chlorides was accessed by reaction between a terminal olefin and commercially available 2a, which was used as received (no purification). Transformations may be performed with sterically congested alkenes: cyclohexyl-substituted 3b was obtained in 80% yield and 92:8 E:Z selectivity. CM with various aryl (4a–g) as well as heteroaryl α-olefins (4h–k) gave the products in 54–85% yield after four hours at room temperature; the difference between the conversion and yield is largely due to the formation of homocoupling products. In none of the cases did we detect the Z isomer (by analysis of 400 MHz 1H NMR spectra of the unpurified product mixtures). Product 4a was obtained from the reaction with E-β-methyl styrene. Aryl halides 4c and 4d and arylboronate 4g are noteworthy because their synthesis through cross-coupling could pose chemoselectivity issues (31,32).
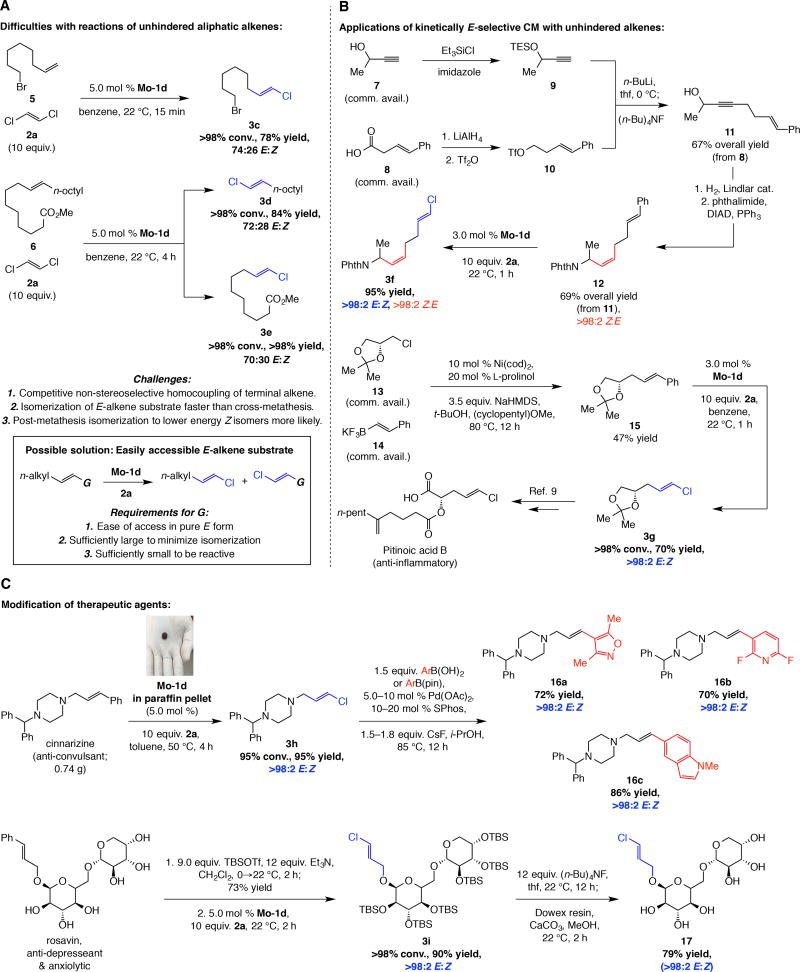
Preparation of E-1,2-disubstituted alkenyl chlorides with unhindered aliphatic alkenes (vs. those with an allylic substituent as in 3a,b) presented another complication (Fig. 3A). CM was efficient with olefin 5, affording chloroalkene 3c in 78% yield, but selectivity was moderate (74% E). Other than the possibility of a more facile post-metathesis isomerization of the relatively uncongested olefin, this discrepancy might originate from a more competitive and moderately selective generation of homocoupling products, which then can react with 2a to afford isomeric mixtures. Further, the finding that CM with E-1,2-disubstituted olefin 6 gave 3d and 3e with similar selectivity (~70:30 E:Z) implies that 1,2-dialkyl olefins might undergo rapid non-stereoselective isomerization by self-metathesis prior to reaction with 2a.
Fig. 3. Synthesis of unhindered E-alkenyl chlorides by catalytic and stereoselective substituent swapping.
(A) With unhindered aliphatic α-olefins catalytic CM with 2a is efficient but moderately E-selective. This may be attributed to rapid formation of E/Z mixture of 1,2-disubstituted alkenes from homocoupling that react with 2a. Thus, readily available E-1,2-disubstituted alkenes may be used instead (G = Ph). (B) Through the use of easily accessible β-alkyl styrenes, a number of biologically active compounds can be readily prepared. (C) The catalytic method in combination with catalytic cross-coupling may be used to access a variety of potentially valuable derivatives readily. An air- and moisture-resistant paraffin tablet containing a Mo MAP complex can be easily used. Yields are for purified products. TBS = tert-butyldimethylsilyl. TES = triethylsilyl. Tf = trifluoromethanesulfonyl. DIAD = di(iso-propyl)azodicarboxylate. Phth = phthalyl. cod = cyclooctadiene. HMDS = hexamethyldisilazide. SPhos = 2-dicyclohexylphosphino-2’,6’-dimethoxybiphenyl. See the Supplementary Materials for details.
The above data show that alkylidenes derived from Mo-1d can catalyze CM between 1,2-disubstituted alkenes with E-dichloroethene 2a: within four hours at room temperature 3d was obtained in 84% yield despite its volatility and 3e was isolated in >98% yield. Such high activity recommended a strategy for accessing alkenyl chlorides with better stereocontrol. We surmised that by using an appropriate E-1,2-disubstituted olefin, chloro-olefins might become accessible with kinetic E selectivity by what amounts to an efficient catalytic and stereospecific group exchange. The ideal substituent would possess the following attributes: i) Easily and efficiently accessible. ii) Sufficiently large to discourage self-metathesis and adventitious E-to-Z isomerization, but not too large to inhibit CM with 2a. We selected E-β-substituted styrenes because these comparatively robust compounds can be prepared by different methods, as showcased by the findings in Fig. 3B–C.
The first example (Fig. 3B) is a concise synthesis of the amine segment of kimbeamide A (cf. Fig. 1A). The substrate (12) was synthesized from commercially available (racemic or as either enantiomeric form) propargyl alcohol 7 and carboxylic acid 8. Partial hydrogenation of enyne 11 followed by a Mitsunobu reaction afforded diene 12 (>98% E-β-alkylstyrene, >98% Z allylic amine; 69% overall yield). Subsequent treatment with 3.0 mol % Mo-1d and 2a generated 3f in 95% yield and >98:2 E:Z selectivity after one hour at room temperature. The Z-alkene was left untouched in the course of CM (>98% Z at the allylic amine site), highlighting exceptional chemoselectivity. Another case corresponds to pitinoic acid B (Fig. 3B); here, catalytic cross-coupling of commercially available and enantiomerically pure alkyl chloride 13 and trifluoroborate 14 afforded 15 (33) directly in 47% yield. E-β-Substituted styrene 15 was then converted to E-alkenyl chloride 3g, which has been formerly transformed to the anti-inflammatory agent (9).
Other than facilitating synthesis of biologically active compounds, the approach offers a convenient route for their modification; two cases are shown in Fig. 3C. We were able to convert 0.74 gram of cinnarizine, a potent anti-convulsant agent, to E-alkenyl chloride 3h in 94% yield and with >98% E selectivity (5.0 mol % Mo-1d, 10 equiv. 2a, toluene, 22 °C, 4 h). Importantly, the Mo MAP complexes can be delivered in the form of air- and moisture-resistant paraffin tablets. For example, with a pellet containing Mo-1d (see Fig. 3C; ~5 mg in ~95 mg paraffin wax; 5.0 mol % loading), reaction of cinnarizine with 2a cleanly afforded 3h in 95% yield (>98% E). To ensure complete release of the MAP species, the transformation was performed at 50 °C in toluene under nitrogen atmosphere (Fig. 5B). After four hours, the resulting mixture was purified by routine silica gel chromatography (see the Supplementary Material for further details). The entire procedure was carried out in a fume hood.
Compounds such as 3h are a convenient entry to analogues that cannot be accessed efficiently or with high E selectivity by alternative methods, including direct CM; the three examples shown in Fig. 3C (16a–c), obtained by catalytic cross-coupling with commercially available boronic acids or pinacol esters (34), are illustrative. In another case, a persilylated derivative of anti-depressant rosavin was converted to E-alkenyl chloride 3i in 90% yield and >98% E selectivity. As with cinnarizine-derived 3h, analogues may be easily synthesized via 3i or its deprotected form 17. The applications to pitinoic acid B, cinnarizine and rosavin underscore the advantages of the CM approach to synthesis of E alkenyl chlorides versus the existing methods involving terminal alkynes or aldehydes (see above). Reactions with 1,2-dibromoethene, which can only be purchased as an isomeric mixture (64:36 Z:E), were inefficient and non-selective; for example, CM of alkene 1 and 1,2-dibromoethene (8.0 equiv.) with 5.0 mol % Mo-1d proceeded to 16% conversion after four hours with 61:39 E:Z selectivity.
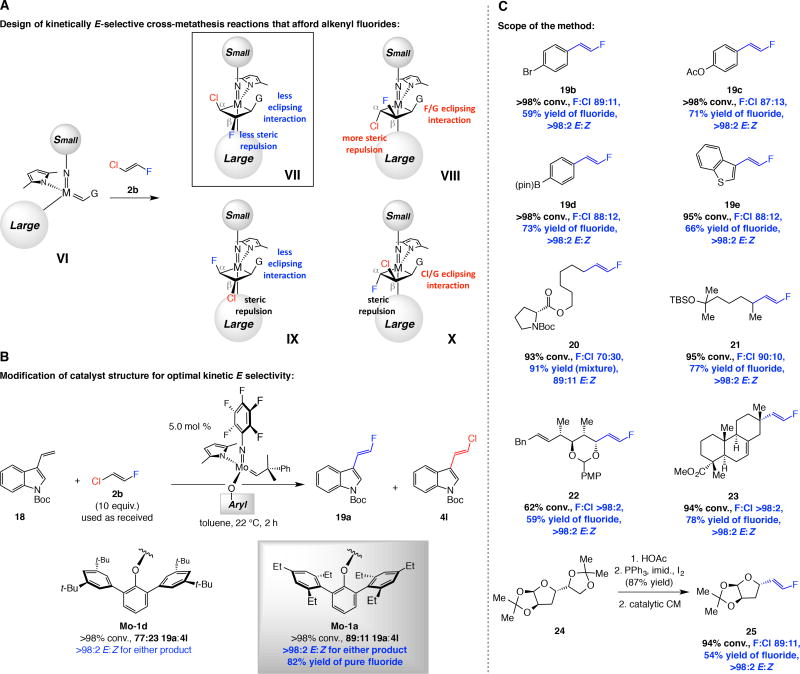
Kinetically E-selective reactions that might furnish fluoro-substituted olefins present the additional issue of cost and practicality: E-1,2-difluoroethene is too expensive and volatile (boiling point, −42 °C). One option would be to use E-1-chloro-2-fluoroethene (2b), which is commercially available, economically far more viable and easier to use (boiling point, −4 °C). The issue was whether with 2b the transformations would be appreciably product-selective (alkenyl fluoride vs. chloride) as CM can proceed via four distinct metallacyclobutane isomers (VII–X, Fig. 4A), only one of which would produce the desired E-fluoro-alkene (VII). We posited that CM might be preferentially channeled via VII for several reasons: i) Overall, there should be less steric strain in VII and IX (vs. VIII and X, respectively). There are no severe eclipsing interactions in VII and IX, and steric repulsion between a halogen atom and the larger aryloxide ligand would be more costly at the Cα position (cf. VIII and X). ii) Matching polarity of the Mo=C and C=C bonds of the dihaloethene, as indicated by the distinct chemical shifts in the 1H NMR spectrum of the latter [δ 6.90 and δ 6.15 ppm for CH(F) and CH(Cl), respectively, in CDCl3], as well as the smaller size of a fluorine atom (0.42 Å atomic radius vs. 0.79 Å for Cl) should favor VII over IX. In the event (Fig. 4B), CM between aryl alkene 18 and 2b (solution in toluene) with 5.0 mol % Mo-1d favored the formation of alkenyl fluoride 19a over the chloro-substituted alkene 4l (77:23); both products were generated with >98% E selectivity.
Fig. 4. Preparation of E-alkenyl fluorides by catalytic cross-metathesis.
(A) Mechanistic analysis that serves as the basis for the development of the catalytic processes. (B) Examination of a model process and identification of an effective complex. (C) With Mo-1a and commercially available E-1-chloro-2-fluoroethene 2b an assortment of E-1,2-alkenyl fluorides can be synthesized efficiently and with E:Z ratios that are typically >98:2. Yields are for purified products. Ac = acetyl. pin = pinacolato. Bn = benzyl. PMP = p-methoxyphenyl. Boc = tert-butoxycarbonyl. See the Supplementary Materials for details.
While contemplating ways by which we could improve the fluoro-:chloro-alkene ratio, we noted that because 19a and 4l are formed with >98% E selectivity, it is probably reaction via IX that is the major competing pathway. If so, reverting to the original MAP complex Mo-1a, containing 2,6-disubstituted aryl moieties [vs. 3,5-(t-Bu)2 in Mo-1d], could prove beneficial. Our hope was that this alteration would exacerbate steric repulsion between the substituent at Cβ and the aryloxide more so in IX (Cβ–Cl) than in VII (Cβ–F) because of the smaller size of a fluorine atom and the shorter C–F bond length (1.35 Å vs. 1.80 Å for C–Cl). Indeed, with Mo-1a, under otherwise identical conditions, CM of 18 and 2b proceeded to >98% conversion in two hours at ambient temperature (Fig. 4B), generating 19a with improved selectivity (89% vs. 77% 19a with Mo-1d). Pure E-fluoro-alkene 19a could be isolated in 82% yield (silica gel chromatography). Use of bulkier aryloxides (e.g., i-Pr vs. Et substituents) led to lower conversion.
A variety of E-alkenyl fluorides may be directly accessed (Fig. 4C); 2b was used without purification and reactions generated up to >98:2 fluoro:chloro selectivity. As with 19a, pure alkenyl fluorides could be easily obtained in most cases (54–78% yield). In only one instance (20) did we detect any of the Z-alkene. Different styrenes, including those with versatile functional groups (e.g., 19b and 19d), were effective cross partners. Several examples involving transformations with alkyl-substituted α-olefins are presented in Fig. 4C. Similar to the cases in Fig. 3, reactions with the less congested alkyl-substituted olefins were less E-selective (cf. 20 vs. 21). Unlike when symmetrical 2a was used, CM between E-β-alkyl styrenes and 2b led to substantial amounts of β-fluorostyrene and aliphatic chloro-alkene products. Formation of 22 (>98% fluoro product, 59% yield, >98% E) shows that acid-sensitive moieties such as a para-methoxyphenyl (PMP) acetal are tolerated. The transformation leading to fluorine-tagged isopimaric acid methyl ester (23) involves a particularly congested alkene. A precursor to the aforementioned LuxS inhibitor, alkenyl-fluoride 25, was prepared in three steps from 24 in 51% overall yield with >98% E selectivity; the present approach affords the E-fluoro-alkene in stereoisomerically pure form and is substantially more efficient than the previously reported route (7% overall yield from 24) (13).
We have therefore devised strategies that allow for olefin metathesis processes to proceed with high efficiency and kinetic E selectivity, delivering a valuable set of organic halides where Z isomers are thermodynamically favored. The possibility of utilizing easy-to-handle paraffin tablets, soon to be commercially available, further enhances the potential impact of the approach (35). The strategies delineated above are expected to lead to the development of other efficient, practical and kinetically E-selective olefin metathesis transformations; this is especially relevant to cases where there is minimal energy difference between the two stereoisomeric forms and/or the Z-alkene is preferentially generated with the more commonly used catalysts (e.g., alkenyl sulfides, alkenyl nitriles or enynes) (36).
Supplementary Material
Acknowledgments
This research was financially supported by the NIH (grant GM-59426). We are grateful to Dr. Sebastian Torker for helpful discussions. We thank Dr. Levente Ondi, Dr. Janos Balazs Czirok and Mr. Gergely Mate Nagy for their support and helpful advice and are grateful to XiMo, AG for gifts of paraffin tablets.
Footnotes
References
- 1.Hoveyda AH, Zhugralin AR. Nature. 2007;450:243–251. doi: 10.1038/nature06351. [DOI] [PubMed] [Google Scholar]
- 2.Grubbs RH, Wenzel AG, O’Leary DJ, Khosravi E, editors. Handbook of Metathesis. Wiley–VCH; Weinheim: 2014. [Google Scholar]
- 3.Ibrahem I, Yu M, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2009;131:3844–3845. doi: 10.1021/ja900097n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh MJ, Khan RKM, Torker S, Yu M, Mikus MS, Hoveyda AH. Nature. 2015;517:181–186. doi: 10.1038/nature14061. [DOI] [PubMed] [Google Scholar]
- 5.Johns AM, Ahmed TS, Jackson BW, Grubbs RH, Pederson RL. Org. Lett. 2016;18:772–775. doi: 10.1021/acs.orglett.6b00031. [DOI] [PubMed] [Google Scholar]
- 6.Wiberg KB, Wang Y, Petersson GA, Bailey WF. J. Chem. Theory Comput. 2009;5:1033–1037. doi: 10.1021/ct900059e. [DOI] [PubMed] [Google Scholar]
- 7.Johansson Seechurn CCC, Kitching MO, Colacot TJ, Sniekus V. Angew. Chem. Int. Ed. 2010;51:5062–5085. doi: 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- 8.Chung W-j, Vanderwal CD. Angew. Chem. Int. Ed. 2016;55:4396–4434. doi: 10.1002/anie.201506388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montaser R, Paul VJ, Luesch H. Org. Lett. 2013;15:4050–4053. doi: 10.1021/ol401396u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunnery JK, et al. J. Org. Chem. 2012;77:4198–4208. doi: 10.1021/jo300160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller K, Faeh C, Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara T, O’Hagan D. J. Fluor. Chem. 2014;167:16–29. [Google Scholar]
- 13.Wnuk SF, et al. Bioorg. Med. Chem. 2008;16:5090–5102. doi: 10.1016/j.bmc.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fustero S, Simón-Fuentes A, Barrio P, Haufe G. Chem. Rev. 2014;115:871–930. doi: 10.1021/cr500182a. [DOI] [PubMed] [Google Scholar]
- 15.Imaizumi Y, et al. Mol. Pharmacol. 2002;62:836–846. doi: 10.1124/mol.62.4.836. [DOI] [PubMed] [Google Scholar]
- 16.Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA. J. Med. Chem. 2015;58:8315–8359. doi: 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- 17.Campbell MG, Ritter T. Org. Proc. Res. Dev. 2014;18:474–480. doi: 10.1021/op400349g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller RB, McGarvey G. J. Org. Chem. 1978;43:4424–4431. [Google Scholar]
- 19.Molander GA, Cavalcarti LN. J. Org. Chem. 2011;76:7195–7203. doi: 10.1021/jo201313a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao F, Hoveyda AH. J. Am. Chem. Soc. 2010;132:10961–10963. doi: 10.1021/ja104896b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petasis NA, Zavialov IA. Tetrahedron Lett. 1996;37:567–570. [Google Scholar]
- 22.Takai K, Nitta K, Utimoto K. J. Am. Chem. Soc. 1986;108:7408–7410. doi: 10.1021/ja00279a068. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L, Ni C, Zhao Y, Hu J. Tetrahedron. 2010;66:5089–5100. [Google Scholar]
- 24.Lee SH, Schwartz J. J. Am. Chem. Soc. 1986;108:2445–2447. doi: 10.1021/ja00269a052. [DOI] [PubMed] [Google Scholar]
- 25.Furuya T, Ritter T. Org. Lett. 2009;11:2860–2863. doi: 10.1021/ol901113t. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Zhou C-B, Chen Q-Y, Xiao J-C, Hong R. Org. Lett. 2010;13:560–563. doi: 10.1021/ol102645g. [DOI] [PubMed] [Google Scholar]
- 27.Koh MJ, Nguyen TT, Zhang H, Schrock RR, Hoveyda AH. Nature. 2016;531:459–465. doi: 10.1038/nature17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meek SJ, O’Brien RV, Llaveria J, Schrock RR, Hoveyda AH. Nature. 2011;471:461–466. doi: 10.1038/nature09957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinescu SC, Schrock RR, Müller P, Hoveyda AH. J. Am. Chem. Soc. 2009;131:10840–10841. doi: 10.1021/ja904786y. [DOI] [PubMed] [Google Scholar]
- 30.Schrock RR, Hoveyda AH. Angew. Chem. Int. Ed. 2003;42:4592–4633. doi: 10.1002/anie.200300576. [DOI] [PubMed] [Google Scholar]
- 31.Billingsley KL, Buchwald SL. J. Org. Chem. 2008;73:5589–5591. doi: 10.1021/jo800727s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barluenga J, Moriel P, Aznar P, Valdés C. Adv. Synth. Catal. 2006;348:347–353. [Google Scholar]
- 33.Molander GA, Argintaru OA. Org. Lett. 2014;16:1904–1907. doi: 10.1021/ol500408a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thakur A, Zhang K, Louie J. Chem. Commun. 2012;48:203–205. doi: 10.1039/c1cc15990a. [DOI] [PubMed] [Google Scholar]
- 35.Sather AC, Lee HG, Colombe JR, Zhang A, Buchwald SL. Nature. 2015;524:208–211. doi: 10.1038/nature14654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torker S, Koh MJ, Khan RKM, Hoveyda AH. Organometallics. 2016;35:543–562. [Google Scholar]
- 37.Kuang C, Senboku H, Tokuda M. Tetrahedron. 2005;61:637–642. [Google Scholar]
- 38.Hayashi S, Nakai T, Ishikawa N, Burton DJ, Naae DG, Kesling HS. Chem. Lett. 1979;8:983–986. [Google Scholar]
- 39.Petasis NA, Yudin AK, Zavialov IA, Prakash GKS, Olah GA. Synlett. 1997;1997:606–608. [Google Scholar]
- 40.Prakash GKS, Shakhmin A, Zibinsky Ml, Ledneczki I, Chacko S, Olah GA. J. Fluor. Chem. 2010;131:1192–1197. [Google Scholar]
- 41.Maity S, Manna S, Rana S, Nveen T, Mallick A, Maiti D. J. Am. Chem. Soc. 2013;135:3355–3358. doi: 10.1021/ja311942e. [DOI] [PubMed] [Google Scholar]
- 42.Falk A, Cavalieri A, Nichol GS, Vogt D, Schmalz H. Adv. Synth. Catal. 2015;357:3317–3320. [Google Scholar]
- 43.Daun J, Field S, Kobayashi S. 20040186127. U.S. Pat. Appl. Publ. 2004 Sep 23;
- 44.Gioia C, Hauville A, Bernardi L, Fini F, Ricci A. Angew. Chem. Int. Ed. 2008;47:9236–9239. doi: 10.1002/anie.200804275. [DOI] [PubMed] [Google Scholar]
- 45.Silva LF, Jr, Craveiro MV, Gambardella MTP. Synthesis. 2007;2007:3851–3857. [Google Scholar]
- 46.Aponick A, Li C-Y, Palmes JA. Org. Lett. 2009;11:121–124. doi: 10.1021/ol802491m. [DOI] [PubMed] [Google Scholar]
- 47.González MA, Zaragozá RJ. J. Nat. Prod. 2014;77:2114–2117. doi: 10.1021/np500569y. [DOI] [PubMed] [Google Scholar]
- 48.Gillingham DG, Kataoka O, Garber SB, Hoveyda AH. J. Am. Chem. Soc. 2004;126:12288–12290. doi: 10.1021/ja0458672. [DOI] [PubMed] [Google Scholar]
- 49.Barder TE, Walker SD, Martinelli JR, Buchwald SL. J. Am. Chem. Soc. 2005;127:4685–4696. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
- 50.Hussein L, Purkait N, Biyikal M, Tausch E, Roesky PW, Blechert S. Chem. Commun. 2014;50:3862–3864. doi: 10.1039/c3cc48874h. [DOI] [PubMed] [Google Scholar]
- 51.Ito H, Nagahara T, Ishihara K, Yamamoto H. Angew. Chem. Int. Ed. 2004;43:994–997. doi: 10.1002/anie.200352809. [DOI] [PubMed] [Google Scholar]
- 52.Yuan J, Schrock RR, Müller P, Axtell JC, Dobereiner GE. Organometallics. 2012;31:4650–4653. [Google Scholar]
- 53.Bull JA, Mousseau JA, Charette AB. Org. Lett. 2008;10:5485–5488. doi: 10.1021/ol802315k. [DOI] [PubMed] [Google Scholar]
- 54.Sodré LA, Esteves PM, de Mattos MCS. J. Braz. Chem. Soc. 2013;24:212–218. [Google Scholar]
- 55.Kaburagi Y, Kishi Y. Org. Lett. 2007;9:723–726. doi: 10.1021/ol063113h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merayala HB, Goud PM, Radikota RR, Reddy KR. J. Carbohydr. Chem. 2000;19:1211–1222. [Google Scholar]
- 57.Ghosh AK, Veitschegger AM, Sheri VR, Effenberger KA, Prichard BE, Jurica MS. Org. Lett. 2014;16:6200–6203. doi: 10.1021/ol503127r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spivey AC, Laraia L, Bayly AR, Rzepa HS, White AJP. Org. Lett. 2010;12:900–903. doi: 10.1021/ol9024259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.