Abstract
Chagas disease is the highest impact parasitic disease in the Americas, yet remains virtually unknown and untreated, despite the fact that the infection is curable and the global problem of Chagas disease is manageable. The causes of this situation and how it can be changed are the focus of this communication.
Keywords: Chagas disease, drug discovery, neglected diseases, Trypanosoma cruzi
With the exception of the relative handful of investigators who study Trypanosoma cruzi and the even fewer physicians who might recognize the disease it causes, Chagas disease is unfamiliar or misunderstood. Although infection is infrequently acutely fatal, it is also rarely completely resolved (Boxes 1 and 2). As a result, those with T. cruzi infection are seldom identified before they have life-altering and life-threatening clinical disease and are almost never treated. It is the decades-long persistence of the infection that drives the development of cardiac and other pathologies in many patients, accompanied by disability and premature death. T. cruzi infection and Chagas disease are plagued by poor diagnostics, inadequate treatment options, and the absence of vaccines [1]. In essence, essentially nothing is being done to identify and treat the 20 million or more who are likely to be currently infected, or to prevent new infections throughout South, Central, and North America where the infection is endemic, or in the rest of the world where it is carried via human migration.
Box 1. Trypanosoma cruzi Infection.
T. cruzi is vectored by blood-feeding insects that normally inhabit nests and burrows of various animals but which will also infest houses that offer concealed spaces (thatch, cracks in adobe walls). A bite by these nocturnally feeding insects does not result in infection since the infective stages of T. cruzi develop in the insect gut and are excreted in the feces, often during the course of blood engorgement. Those excreted parasites then have to find entry into the vertebrate host – through a break in the skin or across mucous membranes near the eyes or mouth. Ingestion of infected insects or feces is a very likely route of infection in many cases; humans can also be infected congenitally or by blood transfusion/tissue transplantation. In mammals, T. cruzi continuously cycles between intracellular replicating amastigotes (Figure I; 1 infecting parasite producing approximately 500 progeny in 4–5 days) and extracellular trypomastigotes (Figure II, Box 2) that circulate in the blood to spread to other tissue sites, or are ingested by blood-feeding insects. T. cruzi infects most mammals and invades many different cells types. However, the disease is often most evident in muscle tissue, particularly the heart, associated with parasite persistence in these tissues.
Figure I. Trypanosoma cruzi.
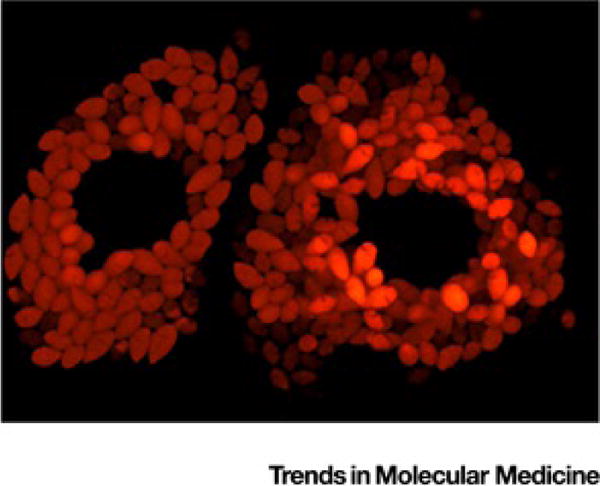
Amastigotes. Live cell imaging of intracellular T. cruzi amastigotes expressing the tandem tomato (tdTomato) fluorescent protein, within two adjacent Vero host cells. Central dark areas are occupied by host cell nuclei.
Box 2. Control of Trypanosoma cruzi Infection.
In immunocompetent hosts, T. cruzi infection is routinely controlled and parasite numbers are reduced to very low levels. This is one of the factors that makes diagnosis of the infection such a challenge; direct detection of parasites after the initial few months of the infection is difficult. This low parasite burden is the result of a highly effective immune response that targets both the infected host cells (Figure I, Box 1) and the extracellular trypomastigotes (Figure II). Although T. cruzi persists for the life of the host in most cases, the infection is spontaneously cured in some instances, suggesting that these immune responses can be curative. Is not known why this is not so in more cases, or how one might induce curative (not just controlling) levels of immune responses. This lack of knowledge, as well as an incomplete understanding of how T. cruzi persists and evades the otherwise effective immune response makes vaccine development a hope, but not a certainty. The drugs benznidazole and nifurtimox have been in use for decades and can also provide parasitological cure. However, the uncertainty over efficacy and the inability to accurately determine if treatment actually cured the infection, coupled with a substantial risk of side effects, have limited the wider use of these compounds. New, less toxic and more consistently effective treatments are needed.
Figure II.
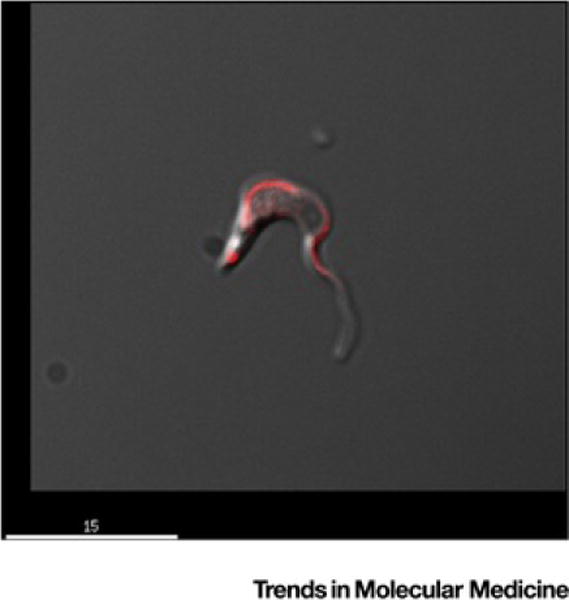
Trypomastigote of Trypanosoma cruzi. Merged differential interference contrast and fluorescent image of a trypomastigote of T. cruzi stained with anti-Par4 antibody that highlights the flagellum [16] (scale bar = 15 μm).
One reason for this unacceptable state of affairs is that Chagas disease is critically understudied. There are perhaps approximately 15 laboratories in Europe and the United States that have undertaken Chagas as a primary emphasis of their research. Chagas disease is a more common research focus of academic and public health facilities in Central and South America where it has its greatest impact on human health, but even there, the studies are limited in scope, and decreasing in numbers. Moreover, they have always been woefully underfunded. As a result, studies often focus on what is ‘doable’ with limited resources and under less-than-optimal circumstances, rather than on what ‘should be done’ in order to reduce the impact of the disease. The resulting literature generated is often conflicting and not readily translational. For example, determining how to predict or moderate disease development in already infected individuals often takes precedent over studying better ways to prevent or eliminate the actual cause of disease – the parasitic infection itself. Chagas disease must be among the few – if not the only –infectious disease for which several largely effective and non-life-threatening treatments exist, but are rarely used. This is not to say that treatment options are perfect – they are far from it. The course of treatment is long – 30–60 days – has side effects, and too often does not cure the infection. Chagas disease is also unique in that it requires multiple, similar serodiagnostic tests in order to arrive at a ‘conclusive’ diagnosis. In addition, a lack of concurrence on these tests leads to a high degree of uncertainty with respect to infection status, which is a consequence of poor diagnostics that mostly depend on archaic technology [2].
Chagas is ignored because it can be. Those infected tend to be in the lowest socioeconomic class in their respective countries, including those living with Chagas disease in the United States and in Europe. Death from Chagas disease is also not typically dramatic; it rarely occurs in the very young and only in exceptional cases, in large outbreaks. T. cruzi is not rapidly spreading into new populations or locales. Rather, it is slow and insidious, both in populations and within the individual. It is not AIDS [3] or tuberculosis or malaria; it does not even exist in Africa, the hotbed of the ‘big three’ and other neglected diseases. Chagas disease does not make the headlines, except occasionally, in the form of hyperbolic jabber.
Although the World Health Organization (WHO) and its affiliates distribute information on the prevalence of T. cruzi infection and Chagas disease, these data are poorly documented and thus largely undependable, making estimates of the true impact impossible (e.g., see [4]). Likewise, data on access to treatment, the cure rate when treatments are used, and the ultimate impact of treated or untreated infection are either unattainable or nonverifiable.
Chagas Disease Is Ignored In Part Because It Is Advertised As Being Intractable
This simply is not true. Yes, T. cruzi will never be eradicated because it cannot be eliminated from the many mammalian species that it naturally infects. However, some of these naturally infected hosts, including mice, dogs, and various non-human primates make outstanding models for assessing the activity of vaccines and drugs. Very few infectious diseases have this wealth of excellent animal hosts – certainly this has been a major impediment for the study of the big three, all of which lack high-quality animal models. Additionally, transmission of T. cruzi is impressively inefficient, because it is released from its insect vectors in their feces rather than being injected during the taking of a blood meal, the case for many insect-borne pathogens. Thus, the opportunities to disrupt transmission are numerous – they are just not being widely utilized [5].
Drug Discovery Is Too Difficult
Nonsense! Yes, we have endured several failed clinical trials of prospective treatments (ClinicalTrials.goviii identifiers NCT01489228, NCT01377480, and NCT01162967), but these failures are the result of rushing to human trials with poorly vetted compounds [6–8]. For the most part, drug discovery efforts have been half - hearted and ill-directed. We have two drugs that were discovered approximately 50 years ago and that are still effective: benznidazole and nifurtimox. There is no evidence of induced drug resistance (although natural resistance to these particular drugs exists), and the ecology of the infection makes the likelihood of drug resistance to be seemingly low (humans with chronic infections – who would be the primary target of drug treatment – are mostly poor transmitters to insect vectors). Consequently, a single, highly effective drug is likely to last a long time – if not forever. Additionally, current drugs may be able to be more effectively delivered (e.g., with a reduced dosing schedule), thus minimizing adverse events [9].
A rare bright spot in Chagas disease research has been the interest taken by both small and large drug companies in the discovery of new drugs [10,11]. Despite the good intentions of some companies and especially their willingness to share compound libraries, the lack of long-term industry commitment, the convoluted and often secretive nature of industry collaborations, and the absence of substantial funding to the right partners often hinder and ultimately cripple these efforts before they have had a real chance to succeed. As already noted, we are blessed with outstanding models for screening and testing drugs to ensure they do not fail in human trials. Indeed, the recent addition of Chagas disease to the Food and Drug Administration Priority Review Voucher program should provide additional fuel for the drug discovery fire. Another fortunate positive point for Chagas disease has been the recent interest taken by the Wellcome Trust, particularly with respect to drug discovery and the tools required for the validation of new drugs. We have to do a better job of capitalizing on these resources, recognizing the opportunities and expanding the pool of involved entities.
It Is Too Difficult And Too Dangerous to Work with T. cruzi
I do not think so. We have multiple sequenced genomes with excellent omics data and resources. All four life cycle stages can be readily cultured in large numbers. Mice are a natural host for T. cruzi and unmatched as a model for dissecting host–pathogen interactions. With the development of the CRISPR/Cas9-based gene editing tool in T. cruzi, genetic manipulations that used to take months can now be done in days [12]. A few hurdles obviously remain, but all are surmountable. Unlike its related protozoan pathogens, the African trypanosomes, Leishmania, Plasmodium, etc., T. cruzi is classified as a biohazard level III agent in Europe, thus making it disproportionately more expensive and cumbersome to work with than these equally dangerous relatives. Otherwise, the major limitation to more rapid progress is good ideas and the resources to evaluate those ideas.
Another positive sign in the Chagas landscape is its inclusion in the London Declaration on Neglected Tropical Diseases (http://www.unitingtocombatntds.org/ii), a proposed blueprint for the control or elimination of ten neglected diseases. Unfortunately, the goals established for Chagas disease in the original Declaration were extremely poorly conceived and the yearly scorecards show lackluster progress. The Chagas community has rallied to address the shortcomings of the London Declaration, as well as the lack of leadership on the part of WHO and Pan American Health Organization on Chagas disease, and the failure of health ministers in the Americas to make Chagas disease a priority issue [13]. The suggested approach is relatively straightforward and begins very inexpensively, with the collection of data on the size of the problem and a cataloging of the efforts to address the problem. Revealing the actual scope of the problem will incentivize public health officials and international agencies to acknowledge and address the problem rather than sweeping it under the rug. Unfortunately, no funder has answered the call to support this modest data-collection step. That is remarkable.
Chagas Disease Is a Solvable Problem
Many of those with the capacity to contribute to a solution are not stepping up to do so, because they have failed to understand the problem and to realize the opportunities. Two of the countries in which Chagas disease is a major public health problem, Brazil and Mexico, last year had the 8th and 13th largest economies in the world, respectively. An additional four endemic countries are among the top 50 economies. These are in addition to the United States, where T. cruzi has circulated for centuries. All these countries have substantial, well-trained, and dedicated scientific communities and prosperous citizens and philanthropies. The Carlos Slim Foundation, endowed by the richest man in the world, is headquartered in Mexico but has done little to address the Chagas disease problem there or elsewhere in Latin America. The Bill and Melinda Gates Foundation has generously attacked neglected diseases around the world but has largely sat on the sidelines with respect to Chagas disease.
If someone wants to back a successful effort to eliminate a major human health problem in a reasonable timeframe, put your money behind Chagas disease. Yes, there are limitations. Vaccines do not protect from infection [14]. However, that is the case with nearly all parasitic infections. Vaccines may not be the answer to everything and are unnecessary if one can adequately limit transmission, routinely screen to detect new infections, and effectively treat those new infections before they cause damage [15]. Valid markers of cure are needed but there are reasonable candidates for these already. It is time to stop ignoring and start addressing this solvable problem. The infectious disease community could use a good success story.
Acknowledgments
Research in the Tarleton laboratory has been supported primarily by grants from the US National Institutes of Health and the Wellcome Trust. Many thanks to the Tarleton Research Group and its collaborators for this hard work and special thanks to Sam Kurup and Adrianna Canavici for the figures included herein.
Footnotes
References
- 1.Tarleton RL, et al. The challenges of Chagas disease – grim outlook or glimmer of hope. PLoS Med. 2007;4:e332. doi: 10.1371/journal.pmed.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonso AM, et al. A systematic review of high quality diagnostic tests for Chagas disease. PLoS Negl Trop Dis. 2012;6:e1881. doi: 10.1371/journal.pntd.0001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarleton RL, Curran JW. Is Chagas disease really the “new HIV/AIDS of the Americas”? PLoS Negl Trop Dis. 2012;6:e1861. doi: 10.1371/journal.pntd.0001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carabarin-Lima A, et al. Chagas disease (American trypanosomiasis) in Mexico: an update. Acta Trop. 2013;127:126–135. doi: 10.1016/j.actatropica.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Gürtler RE. Sustainability of vector control strategies in the Gran Chaco region: current challenges and possible approaches. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):52–59. doi: 10.1590/s0074-02762009000900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina I, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med. 2014;370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 7.Khare S, et al. Antitrypanosomal treatment with benznidazole is superior to posaconazole regimens in mouse models of Chagas disease. Antimicrob Agents Chemother. 2015;59:6385–6394. doi: 10.1128/AAC.00689-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bustamante JM, Tarleton RL. Potential new clinical therapies for Chagas disease. Expert Rev Clin Pharmacol. 2014;7:317–325. doi: 10.1586/17512433.2014.909282. [DOI] [PubMed] [Google Scholar]
- 9.Bustamante JM, et al. New, combined, and reduced dosing treatment protocols cure Trypanosoma cruzi infection in mice. J Infect Dis. 2014;209:150–162. doi: 10.1093/infdis/jit420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bustamante JM, Tarleton RL. Methodological advances in drug discovery for Chagas disease. Expert Opin Drug Discov. 2011;6:653–661. doi: 10.1517/17460441.2011.573782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bustamante JM, et al. Report of the 2nd Chagas Drug Discovery Consortium meeting, held on 3 November 2010; Atlanta GA, USA. Expert Opin Drug Discov. 2011;6:965–973. doi: 10.1517/17460441.2011.602063. [DOI] [PubMed] [Google Scholar]
- 12.Peng D, et al. CRISPR-Cas9-mediated single-gene and gene family disruption in Trypanosoma cruzi. MBio. 2014;6:e02097–e02114. doi: 10.1128/mBio.02097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarleton RL, et al. Chagas disease and the london declaration on neglected tropical diseases. PLoS Negl Trop Dis. 2014;8:e3219. doi: 10.1371/journal.pntd.0003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bustamante J, Tarleton R. Reaching for the Holy Grail: insights from infection/cure models on the prospects for vaccines for Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz. 2015;110:445–451. doi: 10.1590/0074-02760140440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viotti R, et al. Towards a paradigm shift in the treatment of chronic Chagas disease. Antimicrob Agents Chemother. 2014;58:635–639. doi: 10.1128/AAC.01662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurup SP, Tarleton RL. The Trypanosoma cruzi flagellum is discarded via asymmetric cell division following invasion and provides early targets for protective CD8+ T cells. Cell Host Microbe. 2014;16:439–449. doi: 10.1016/j.chom.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


