Abstract
Atypical and anaplastic meningiomas (AAM) represent 20% of all meningiomas. They are associated with poor outcomes due to their tendency to recur. While surgery and radiation (RT) are first line therapy, no effective systemic medical treatment has been identified. Protein phosphatase 2A (PP2A) is a ubiquitously expressed serine/threonine phosphatase involved in cell cycle regulation and DNA repair. Here, we examined radiosensitizing effects of LB-100, a novel inhibitor of PP2A against AAM as a novel treatment strategy. Three human-derived immortalized meningioma cell lines, IOMM-LEE, GAR, and CH-157, were used to investigate the radio-sensitizing potential of LB-100 in AAM. Survival fraction by clonogenic assay, immunofluorescence, cell cycle analysis and protein expression were evaluated in vitro. The antitumor effects of combining LB-100 with RT were verified in vivo by using intracranial orthotopic xenograft mouse model. Pharmacologic PP2A inhibition with LB-100 prior to RT enhanced the radiosensitivity of meningioma cells and reduced survival fraction in clonogenic assays. LB-100 increased DNA double-strand breakage (measured by γ-H2AX), mitotic catastrophe cell death, and G2/M cell cycle arrest in irradiated meningioma cells. Also, LB-100 decreased activation of STAT3 and expression of its downstream proteins. In vivo, LB-100 and RT combined treatment prolonged the survival of mice with xenografts compared to RT alone. Taken together, these results provide convincing preclinical data to support the use of LB-100 as a radiosensitizing agent for treatment of malignant meningioma. Its potential for clinical application deserves further investigation.
Keywords: LB-100, PP2A, meningioma, radiation, STAT3
1. Introduction
Meningioma is the most common primary central nervous system (CNS) tumor[1]. Although most are benign lesions, 20% are classified as World Health Organization (WHO) atypical grade II or anaplastic grade III[2-4]. Standard of care involves maximal surgical resection followed by radiation for grade III and sub-totally resected grade II tumors[5, 6]. Unfortunately, atypical and anaplastic meningiomas (AAM) tend to recur despite aggressive treatment[7, 8]. For WHO grade II tumors, the 5-year progression free survival (PFS) is 70% after gross total resection and 60% after subtotal resection[9, 10]. For WHO grade III tumors, 5-year PFS survival ranged from 12% to 57%, even after resection and radiotherapy[6, 11]. Progressive tumors are often refractory to further treatment and produce neurologic sequela by local invasion or, in the cases of grade III tumors, metastasis to bone or lung.
Medical therapy thus far has had limited success in the treatment of meningioma While a wide variety of antineoplastic agents have been investigated, limited results were only seen with anti-angiogenic agents, such as bevacizumab, in retrospective studies, [12-15]. However, confirmation of their clinical effectiveness in prospective controlled trials is needed before these agents could be recommended as standard of care. Given the lack of effective treatment for patients without further surgical or radiotherapeutic options, there is an urgent clinical need to develop novel therapies to expand the armamentarium against these hard to treat tumors. Since radiotherapy is the mainstay adjuvant treatment after surgical resection, the addition of a radio-sensitizing agent potentiating the therapeutic efficiency of standard radiotherapy is an attractive strategy.
Protein phosphatase 2A (PP2A) is an ubiquitous serine/threonine phosphatase that is involved in a broad array of regulatory cellular functions including cell survival, apoptosis, mitosis and DNA-damage response[16]. In particular, PP2A has been shown to play a critical role in repairing chemotherapy or radiation induced DNA damage[17, 18]. Therefore, inhibiting PP2A may further worsen DNA damage in cancer cells and accelerate inappropriate entry into S and M phase. In the setting of ionizing radiation this would result in increased disordered replication and cell death[19]. However, while PP2A is an attractive target to sensitize tumor cells to the effect of radiation, no inhibitor of PP2A is FDA-approved. Established chemical inhibitors, such as okadaic acid and cantharidin, are toxic and have limited clinical utility[20]. LB-100 is a novel small molecule inhibitor of PP2A. In a completed Phase 1 study, LB-100 was well tolerated in adult patients with progressive solid tumors[21]. In a number of preclinical studies[22], LB-100 was an effective sensitizing agent of radiation, including pancreatic cancer[18], nasopharyngioma[23] and glioblastoma[24]. However, the effect of PP2A inhibition on meningioma was not explored and none of the studies examined the radio-sensitizing effect of LB-100 in intracranial tumor models. We therefore chose to investigate the radio-sensitizing potential of LB100 in AAM in a pre-clinical setting. Three immortalized meningioma cell lines, IOMM-Lee, GAR, and CH-157 were used for in vitro studies, and IOMM-LEE cells were used for an intracranial skull base xenograft model. In addition to preventing DNA repair, PP2A inhibition also reduces activation of Signal Transducer and Activator of Transcription 3 (STAT3) [25, 26]. The role of STAT3 in tumorigenesis has been extensively studied in many different types of cancer[27]. In meningioma, constitutive activation of STAT3 was greater in tumor compared to normal dura[28] and its expression correlated with tumor grade and VEGF expression, suggesting that it plays a critical role in meningioma pathogenesis[29]. We, therefore, hypothesize that LB-100 could also deactivate STAT3 and enhance radiation induced cell death.
2. Materials and Methods
Reagents and Antibodies
LB-100 was provided by Lixte Biotechnology Holdings, Inc. and was dissolved in PBS at a concentration of 10mmol/L stock solution. Aliquots were prepared and stored at −20°C. Solutions for in vitro treatment and in vivo injections were diluted from stock solution immediately before administration.
Meningioma Cell Cultures
The human immortal meningioma cell lines IOMM-Lee, GAR, and CH-157 were given by Dr. Randy Jensen (University of Utah). All three cell lines were maintained in complete medium, specifically Dulbecco's Modified Eagle Medium (DMEM, PAA) with 10% fetal bovine serum (FBS, Invitrogen) and supplemented with L-glutamine, 1mM sodium pyruvate (PAA), and 1% penicillin/streptomycin (Invitrogen) at 37°C and 5%CO2.
Protein Extraction and Immunoblotting Analysis
Whole-cell pellets were extracted for protein in RIPA lysis buffer (Thermo Fischer Scientific Inc.) enhanced with Complete Protease Inhibitor and Phosphatase Inhibitor Cocktail Tablets (Roche), and sonicated and purified through centrifugation. The Bio-Rad Protein Assay kit (Bio-Rad) was used to quantify protein in the supernatant. Equal amounts of protein were denatured at 85°C for 5 minutes in protein loading buffer prior to being loaded on a NuPAGE 4% to 12% Bis–Tris gel (Invitrogen Life Technologies). Electronic transfer to nitrocellulose membranes (Invitrogen Life Technologies) was performed using iBlot2 dry blotting system (Invitrogen Life Technologies). Membranes were blocked in 5% dried skim milk in PBST and probed with primary antibody overnight. Primary antibodies were as follows: cyclin D1, Mcl-1, c-myc and hsp90 (Cell Signaling). Horseradish peroxidase-conjugated secondary antibodies (species-specific) were visualized by enhanced chemiluminescence substrate (SuperSignal; Pierce).
XTT Cell Viability Assay
Cell viability was assessed with XTT Assay (ATCC), which contains 4 tetrazolium salt. 96-well plates were seeded with ∼ 1 × 104 IOMM-LEE, GAR and CH-157. After overnight culture in complete medium, cells were treated with various concentrations of LB-100. The XTT assays were carried out according to the manufacturer's instructions after 48 hours of treatment. Absorbance values were determined at 490 and 650 nanometers on an ELx800 spectrophotometer (BioTek). All the XTT assays were performed in triplicate.
Clonogenic Assay and Sensitizer Enhancement Ratio Analysis
Cellular suspensions were seeded into 6-well tissue culture plates. The cells were given 6 hours to attach prior to initiation of treatment regimen. Pretreatment with LB100 was conducted (2.5 mmol/L LB100) and after 4 hours of incubation, the cells were irradiated (5 Gy). Ten days after seeding, the colonies were stained with 0.1% crystal violet solution. Colonies with over 50 cells were counted. The cell survival curves were obtained by fitting 3 surviving fractions into the linear-quadratic model using CS-Cal clonogenic survival calculation software (http://angiogenesis.dkfz.de/oncoexpress/software/cs-cal/index.htm). The sensitizer enhancement ratio (SER) was calculated as the ratio of the radiation dose required to achieve surviving fraction (SF) values of 0.5 in the absence of LB-100 to that in the presence of LB-100.
PP2A phosphatase activity assay
Cells were grown to 80% confluence in 100-mm dishes and treated as indicated. LB-100 was given three hours prior to RT. 3 hours after RT, cells were washed twice with cold TBS (pH 7.4) and lysed in RIPA lysis buffer (Thermo Scientific) supplemented with protease inhibitors (Roche) for 30 minutes on ice. Cell lysates were sonicated for 10 seconds then centrifuged at maximum speed for 15 minutes. Supernatants containing 50 ug of total cellular protein were assayed with the PP2A Phosphatase Assay Kit (Millipore) according to the manufacturer's instructions. Experiments were performed in triplicate, and the data are presented as a percentage mean of relative PP2A activity compared with control ± SEM.
Phospho-STAT3 (Y705) sandwich enzyme-linked immunosorbent assay
Cells were grown to 80% confluence in 100-mm dishes and treated as indicated. LB-100 was given three hours prior to RT. 24 hours after RT, cells were harvested for protein extraction using RIPA lysis buffer (Thermo Fischer Scientific Inc.) with Complete Protease Inhibitor and Phosphatase Inhibitor Cocktail Tablets (Roche). Lysates was quantified with Bio-Rad Protein Assay kit (Bio-Rad) for equal protein amounts were used for the assay. Levels of phosphorylated STAT3 (Y705) in organ lysate was measuring using a PathScan Phospho-STAT3 (Y705) Sandwich ELISA kit (Cell Signaling, 7149C, 7149) as per the manufacturer's instructions. The data are represented in relative chemiluminescence light units.
γ-H2AX Assay
Immunofluorescent cytochemical staining for g-H2AX foci was performed. Cells were grown in chamber slides and exposed LB100 (2.5 mmol/L) for 4 hours prior to administration of 5Gy or sham radiation. Cells were fixed with 2% paraformaldehyde, washed with PBS, permeabilized with 1% Triton X-100, washed again with PBS, and blocked with 1% BSA. Mouse anti-g-H2AX antibody (Millipore) was added at 1:500 and incubated overnight at 4C. Cells were washed with 1% BSA and goat anti-mouse-FITC antibody (Jackson ImmunoResearch) was added at 1:100 and incubated 1 hour at room temperature. Nuclei were counterstained with DAPI (Sigma). Coverslips were mounted with VectaShield anti-fade solution (Vector Labs) and cells were examined on a Leica DMRXA fluorescent microscope (Leica Microsystems). γ-H2AX foci were quantitated in 50 cells per condition.
Mitotic Catastrophe
The presence of fragmented nuclei was used to delineate cells undergoing mitotic catastrophe. Cells were grown on chamber slides under identical treatment conditions as above, with LB-100 treated groups given 3 hours prior to radiation treatment. At 48 hours after radiation, cells were fixed with methanol, blocked with 1% BSA, and stained overnight at 4C with mouse anti-a-tubulin antibody (Sigma) followed by goat anti-mouse-Texas Red antibody (Jackson ImmunoResearch) for 2 hours at room temperature. The nuclei were then counterstained with DAPI (Sigma). Coverslips were mounted with VectaShield antifade solution (Vector Labs) and the cells were visualized on a Leica DMRXA fluorescent microscope (Leica Microsystems). Cells were manually counted and criteria for defining cells undergoing mitotic catastrophe were the presence of 2 or more lobes of fragmented nuclei. For each condition, at least 100 cells were scored.
Apoptosis Assay and Cell Cycle Analysis
About 1 × 106 cells were plated 6-well plates. After 48-hour of treatment, cells were then fixed and permeabilized using the Click-iT EdU Plus flow cytometry assay kit (Thermo Scientific) according to the manufacturer's instructions. Briefly, cells were pulse labeled with 10 μM EdU for 1.5 hours prior to performing he Click-iT reaction. The cells were then fixed and permeabilized, before stained with cleaved Caspase-3 (cC3) (Cell Signaling) and cleaved PARP (cPARP) (Cell Signaling) antibodies for one hour then stained with appropriate secondary antibody for 30 minutes. Finally, cells were incubated in DAPI (1 μg/ml). Cells were then analyzed by flow cytometry (MoFlo Astrios cell sorter with Summit acquisition software, Beckman Coulter). Data analysis was completed with Kaluza software (Beckman Coulter). All data are presented as a percentage mean ± SEM.
Luciferase-expressing IOMM-LEE Cell Line
The luciferase-expressing IOMM-LEE cell line (IOMM-Luc) was produced by transduction with LV-CMV-FLuc-IRES-GFP lentivirus (MOI 10) to label IOMM-LEE cells with Firefly-Luciferase and GFP (Capital Bioscience). Transduced cells were maintained in puromycin selection. Prior to implantation, FACS was performed to ensure at least > 80% GFP-positive cells.
Intracranial Orthotopic Skull Base Meningioma Mouse Model
All animal studies were conducted in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Animals and approved by the Animal Care and Use Committee of the National Institute of Health. The skull base meningioma model was described previously[30]. To implant the tumors, 8-week old female BALB/c nude mice (Charles River) were used. The mouse head was fixated in a stereotactic apparatus. The skin over the skull was cleaned with antimicrobial solution. A longitudinal incision was made between the occiput and forehead. A high-speed drill was used to cannulate a bur hole in the frontal bone 3 mm anterior and 2 mm lateral to the bregma. The 1 × 105 cells were resuspended in 3 μl of media and drawn into a 10-μl Hamilton syringe (Hamilton), which was mounted onto the stereotactic mouse frame. Stereotactically, the needle was passed through the bur hole and slowly dropped to the floor of the middle fossa. The cell suspension was injected over 45 seconds and left in place for 30 seconds, after which the needle was withdrawn over 30 seconds. This process aids in preventing leakage of cells along the needle track. After implantation, the animals were independently monitored daily by the animal facility staff. The animals were euthanized when they displayed neurologic symptoms or lost > 20% of peak body weight.
In vivo Bioluminescent Imaging and Survival Analysis
Mice were anesthetized in a chamber with an oxygen-enhanced air gas mixture containing 3–5% isoflurane. Animals were then given an ip injection with 150 mg/kg of D-luciferin. After 15 minutes, the animals were imaged using a IVIS 200 imaging station (Caliper Life Sciences). Regions of interest were defined using living image software, and the total photons/s/sr/cm2 (photons per second per steradian per square cm) were recorded to monitor tumor growth and therapy response. The tumor growth was measured and the tumor burden was approximated using the total bioluminescence. Five days after injection of IOMM-LEE-Luc cells, the mice with established tumor with BLI signal greater than 5 × 104 photons/s/sr/cm2 were randomized into 4 groups (n = 10 for each group): saline (control), LB-100-treated group, RT-treated group, and combination (LB-100 + RT). Treatment was given on day 5, 7, 9 and 12 after tumor implantation. Mice in the LB-100 treated group were given i.p. injection of 1.5 mg/kg LB-100. Four treatments were given. For RT treatment, 2Gy of cranial radiation was given for each session for a total radiation dose of 8Gy. In the combination treatment group, LB-100 was given about 3 hours prior to radiation of tumors. Animals were restrained in lead jigs custom made by the Radiation Biology Branch of the National Cancer Institute. The mice were sacrificed for histological analysis at the survival end point.
Histological characterization
At the endpoint of the survival study, animals were euthanized using CO inhalation. The cerebrum was excised from the cranium and then 2 formalin- red and sectioned (10 μm). Hematoxylin and eosin (H & E) staining was performed.
Statistical Analysis
Statistics were performed on results from at least two independent replicates. The two-sided Student's t-test was applied to determine statistical significance between two groups. Ordinary one-way ANOVA test was used for comparison between more than two groups. A P value of ≤ 0.05 (*), was considered statistically significant. Data are presented as mean ±SEM.
3. Results
LB-100 has direct cytotoxic effect on meningioma cells at high concentration
To investigate the utility of LB-100, a small molecule inhibitor of PP2A, in the treatment of AAM, we first tested the effect of LB-100 on the cell viability of three different meningioma cell lines in vitro: IOMM-LEE, GAR and CH-157. XTT assays were performed after 48 hours of LB-100 treatment (Fig. 1A). While there was a dose dependent inhibition of cell growth with increasing LB-100 concentration, the cytotoxic effect is only observed in high doses (IC50>10 uM) of LB-100. Based on the pharmacokinetics study performed in rats (unpublished, Lixte Biotechnology Investigator Brochure), the maximum achievable plasma concentration of LB-100 is likely <5 uM when given the tolerated dose of 1.5 mg/kg used in previous pre-clinical studies. Therefore, LB-100 as a mono-therapy is unlikely to be effective in treatment of meningioma. We thereby chose the dose of 2.5 uM in subsequent in vitro studies to examine the effect of LB-100 in sensitizing cells to the effect of radiation.
Figure 1.
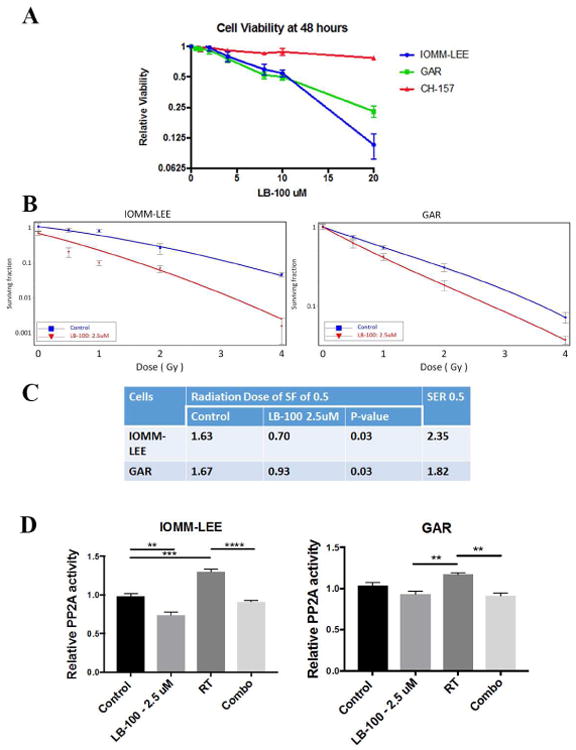
LB-100 sensitized effects of radiation in vitro. (A) Three meningioma cell lines IOMM-LEE, GAR and CH-157 were used in vitro. XTT assays were performed after 48 hours of treatment. Increasing concentrations of LB-100 reduced cell viability, but cytotoxic effect was seen only at high drug concentration with IC50>10uM. (B) The effects of LB-100 on radiosensitivity of IOMM-LEE and GAR cells were demonstrated by clonogenic assay. Cells were seeded as a single-cell suspension with a specified number of cells. After allowing cells time to attach (6 hours), 2.5 uM of LB-100 was added and the plates were irradiated 3 hours later. Ten days after seeding, survival curves were generated by counting colonies with >50 cells. The surviving fractions of meningioma cells at various doses of irradiation ± 2.5 uM of LB-100 were compared. Data are representative of 2 independent experiments. (C) SER was calculated as the radiation dose needed for radiation only divided by the dose needed for 2.5 uM of LB-10 with radiation at surviving fraction of 0.5 (SF0.5). Data are the mean ± SEM from two independent experiments. P values were determined by student t-test. (D) In vitro PP2A activity of IOMM-LEE and GAR cells are shown. LB-100 (2.5 uM) was given 3 hours prior to RT (5Gy). PP2A activity was then assessed 3 hours after RT. PP2A activity was measured based on phosphatase assay and expressed as ratio relative to control. LB-100 inhibited RT induced increase in PP2A activity. Data are representative of two independent experiments.
LB-100 sensitized meningioma cells to effect of radiation in vitro
We performed clonogenic assays using only IOMM and GAR cells. CH-157 cells were not used since they do not form colonies. To determine whether LB-100 enhances the sensitivity of the cells to RT, clonogenic assay was performed (Fig. 1B). The SER at SF of 0.5 (SER0.5) was 2.35 in IOMM-LEE and 1.82 in GAR (Fig. 1C). The clonogenic assay showed that the LB-100+RT combination treatment significantly reduced colonies compared with single-treated groups. These results convincingly demonstrate that LB-100 acts as a radiosensitizer against meningioma cells in-vitro.
LB-100 inhibited radiation induced increase of PP2A activity
PP2A has been shown to be involved in ATM/ATR-mediated activation of the G2–M cell-cycle checkpoint following radiation induced DNA damage[31]. Consistent with earlier reports in other cell types, radiation induced an increase in PP2A activity[23, 24], (130% and 117% compared to control in IOMM and GAR cells respectively). Pre-treatment with 2.5 uM LB-100 3 hours prior to radiation abrogated the increase in PP2A activity (90% and 91% compared to control in IOMM and GAR cells respectively) (Fig. 1C).
LB-100 enhanced radiation induced DNA damage in meningioma cells
To assess the effects of PP2A inhibition on DNA damage and repair, γ-H2AX expression was assessed by immunofluorescence in IOMM-LEE, GAR and CH157 cells as a measure of DNA double-strand breaks (Fig. 2A). Cells were pre-treated with LB-100 for three hours before receiving 5Gy of radiation. γ-H2AX was stained 24 hours after treatment. The average number of γ-H2AX foci per nucleus was quantified. LB-100 alone caused no significant change in γ-H2AX levels; Radiation as expected increased γ-H2AX levels after 24 hours. However, combination treatment significantly enhanced the amount γ-H2AX expression in all 3 cell lines (Fig. 2B), suggesting that PP2A inhibition impaired the cellular repair of radiation induced DNA double-strand breaks.
Figure 2.
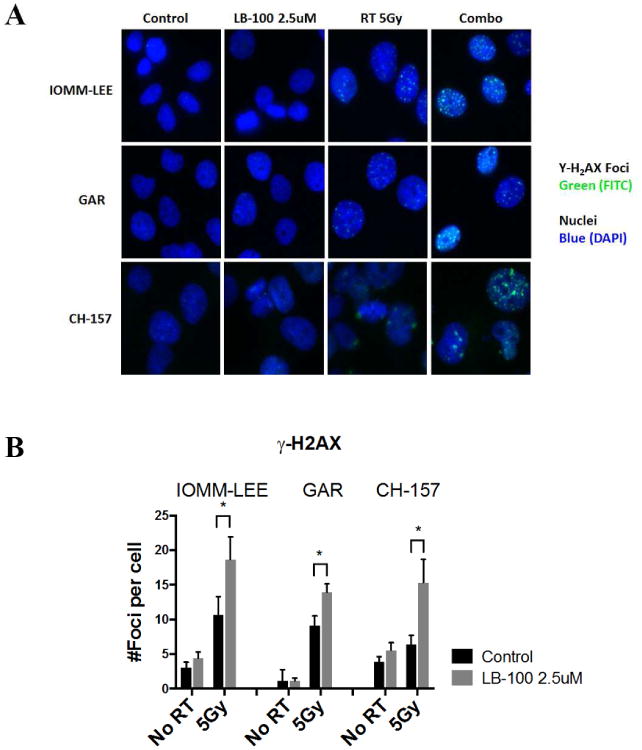
LB-100 enhanced radiation induced DNA damage. (A) Immunofluorescence staining to detect γ-H2AX foci was performed in IOMM-LEE, GAR and CH-157 cells 48 hours after treatment. Cells were plated in 4-well chamber slides and allowed to attach (6-12 hours), and then treated with LB-100 (2.5 uM) for 3 hours prior to radiation (5 Gy). Foci were counted in at least 50 cells per experiment. Representative images for each cell line in each treatment group are shown. (B) Quantitative assessment of γ-H2AX foci per cell at 48 hours after radiation is shown. Data are the mean ± SEM from three independent experiments. *, P < 0.05 (student t-test comparing with or without LB-100 in the RT -treated groups.
LB-100 augmented mitotic catastrophe cell death
To assess whether the radiation sensitization effect of LB-100 is mediated through increased apoptosis, we measured expression of cleaved C3 and cleaved PARP by flow cytometry 48 hours after treatment (Fig. 3A). There was no significant increase in apoptotic cells in combination treatment compared to RT alone in all three cell lines (Fig. 3B). Previous work has suggested that LB-100 treatment may promote mitotic catastrophe (MC), a specific form of cell death distinct from apoptosis, characterized by appearance of abnormal mitotic figures. To assess the degree of MC in LB-100 and/or radiation, the relative number of cells in MC was quantified by immunofluorescence after 48 hours of treatment. The percentages of MC cells were significantly greater in combination compared to RT treatment alone in all three cell lines. We therefore conclude that the predominate form of cell death promoted by LB-100 and radiation combination was through MC and not apoptosis.
Figure 3.
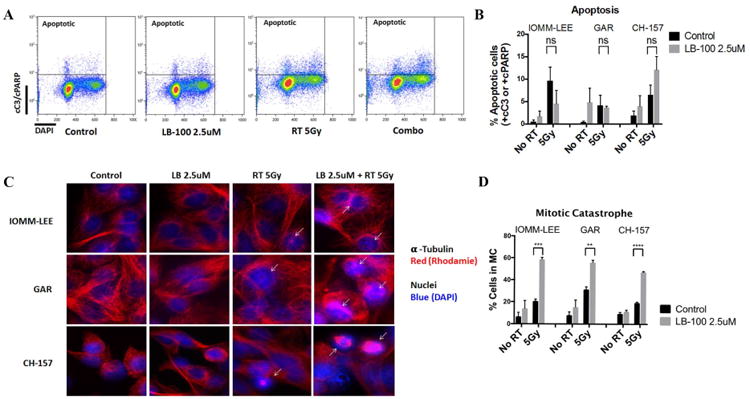
LB-100 enhanced radiation induced mitotic catastrophe but not apoptosis. (A) Flow cytometry analysis was performed to determine rate of apoptosis in IOMM-LEE, GAR and CH-157 cells 48 hours after treatment. LB-100 was given 3 hours prior to RT. Double staining with cC3/cPARP-DAPI was used to label apopototic cells. Representative FACS plots were shown. (B). Quantification of the data showed no enhancement of apoptosis with LB-100 in RT treated groups (student t-test) (C) IOMM-LEE, GAR and CH-157 cells growing in chamber slides were exposed to LB 100 (2.5 umol/L) for 3 hours, irradiated (5 Gy), and 48 hours after radiation immunofluorescence staining was performed to assess the level of mitotic catastrophe. Nuclear fragmentation (defined as the presence of two or more distinct lobes within a single cell) was evaluated in at least 100 cells per treatment per experiment. Representative images for each cell line in each treatment group are shown. D, Quantitative assessment of percentage of cells in MC at 48 hours after radiation is shown. Data represents the mean ± SEM from three independent experiments. *, P < 0.05 (student t-test comparing with or without LB-100 in the RT -treated groups).
LB-100 and radiation combination induced accumulation of G/2-M phase of cell cycle
To further investigate the underlying mechanism of LB100-mediated radio-sensitization, cell cycle analysis was performed to demonstrate the impact of PP2A inhibition on cell cycle control. PP2A has been shown to be necessary for G2–M arrest in some cancer cell lines, and that inhibition of PP2A in the setting of DNA damage would induce accumulation of cells in G2/M phase[23]. Flow cytometry was performed 48 hours after treatment to assess the cell cycle distribution of meningioma cells (Fig. 4A-B). LB-100 treatment alone showed no significant difference in the distribution of cells in G0/G1 and S phases. However, when treated with LB-100 and RT combination, there were a significantly higher proportion of cells in G2/M phase compared to single treatment and control cells (Fig. 4C). This result is consistent with previous findings in other tumor types in which PP2A inhibition led to an increase in cells in G2/M as a result of persistent unrepaired DNA damage.
Figure 4.
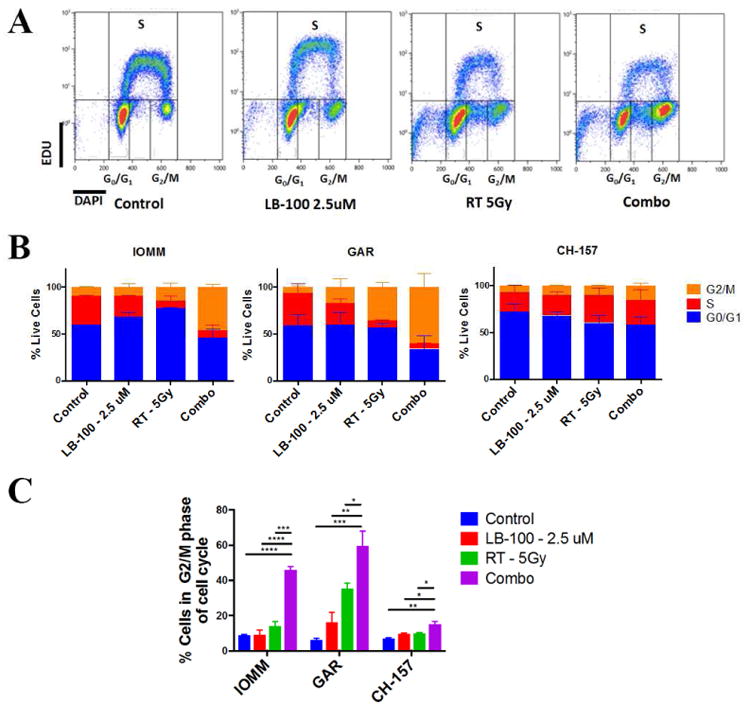
LB-100 and radiation induced accumulation of G2/M phase of cell cycle. (A) Cell cycle analysis using flow cytometry was performed to determine the relative percentages of IOMM-LEE, GAR and CH-157 cells in G0/G1, S and G2/M phased of cell cycle using EDU and DAPI staining 48 hours after treatment. LB-100 was given 3 hours prior to RT. Representative FACS plots were shown. (B) Quantification of data shown in panel A demonstrating the cell cycle distribution of the 3 cell lines in the respective treatment groups (C) The percentages of cells in G2/M phase of cell cycle are shown. LB-100 significantly enhanced the proportion of cells in G2/M. Data represents the mean ± SEM from at least two independent experiments. *P < 0.05, **P < 0.01< ***P<0.001 and ****P < 0.0001. One way ANOVA with Tukey's multiple comparison test)
LB-100 treatment was associated with down-regulated STAT3 phosphorylation at tyrosine 705 (Y705)
Previous work has shown that inhibiting PP2A could lead to inactivation of STAT3 via modulation of serine-727 (S727) phosphorylation[25]. The primary activation phosphorylation site in Y705 had been found to be inversely correlated with S727 phosphorylation [32]. Phosphorylation of STAT3 (Y705) was quantified using Phospho-STAT3 (Y705) sandwich enzyme-linked immunosorbent assay after 24 hours of LB-100 treatment. There was a dose-dependent decrease in STAT3(Y705) phosphorylation with LB-100 treatment (Fig. 5A). At 2.5 uM of LB-100, STAT3 activation was inhibited by about 30% compared to control in all 3 cell lines. To further confirm down-regulation of STAT3 axis, the protein expression of three STAT3 downstream targets, including cyclin D1, c-myc and mcl-1, were examined with immunoblotting in all 3 cell lines (Fig. 5B). All three downstream targets were significantly inhibited in a dose-dependent manner with LB-100 treatment.
Figure 5.
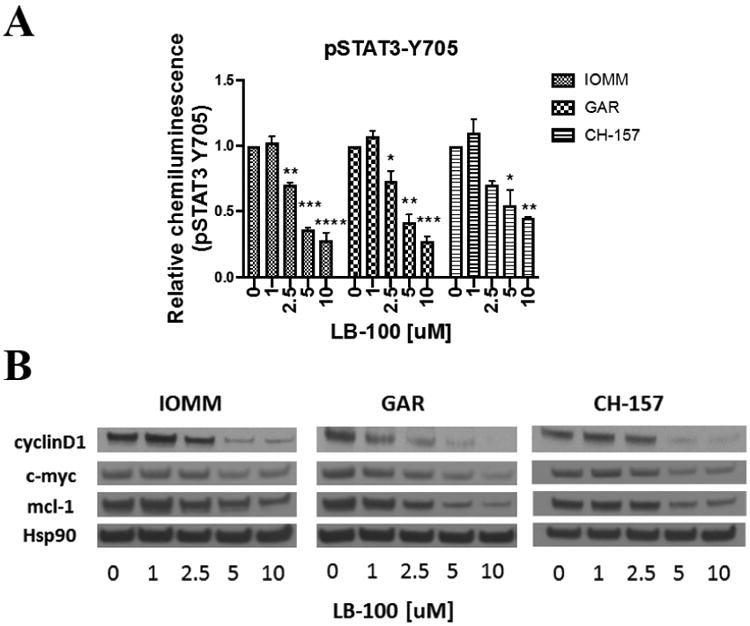
LB-100 inhibits STAT3 activation in vitro. (A) Activation of STAT3 was quantified by phosphorylation level of STAT3(Y705) levels using enzyme-linked immunosorbent assay. IOMM-LEE, GAR and CH-157 cells were treated with LB-100 for 24 hours. Data represents the mean ± SEM from two independent experiments. *P < 0.05, **P < 0.01< ***P<0.001 and ****P < 0.0001. One way ANOVA with Tukey's multiple comparison test. (B) LB-100 down-regulates expression of STAT3 target genes. Western blot analysis of IOMM-LEE, GAR and CH-157 cells treated with increasing LB100 concentration demonstrates dose-dependent decrease in STAT3 target genes, including mcl-1, c-myc, and cyclin D.
LB-100 enhanced therapeutic efficiency of radiation in vivo
To confirm the radiosensitizing effect of LB-100 in vivo, an intracranial orthotopic skull base meningioma mouse model using firefly-luciferase labeled IOMM-LEE (IOMM-Luc) cell line was used as described previously [30]. Five days after tumor implantation, mice with established tumors (BLI signal greater than 5×104 photons/s/sr/cm2) were randomized into four treatment groups. The tumor burden, as measured by BLI at day 5, was similar between all four groups. After randomization, treatment schedule is indicated in (Fig. 6A). Tumor growth was then assessed at day 11 and day 18 after tumor implantation. Representative BLI images were shown in Fig. 6B. At day 18, there was a trend towards decreased tumor growth between RT and combo groups as measured by BLI (p=0.15) (Fig. 6C). Although RT treatment significantly increased median survival compared to control (23 v 27 days, p<0.01), the addition of LB-100 to RT significantly increased median survival compared to RT alone (27 v 31 days, p ≤ 0.05) (Fig. 6D), demonstrating that the addition of LB-100 doubled the survival benefit of RT as measured by median survival. Gross histologic examination of sacrificed mice confirmed skull base location of the implanted tumor (Fig. 6E). H&E staining also demonstrated histologic findings consistent with invasive anaplastic meningioma with high cellularity and brain invasion (Fig. 6F-G).
Figure 6.
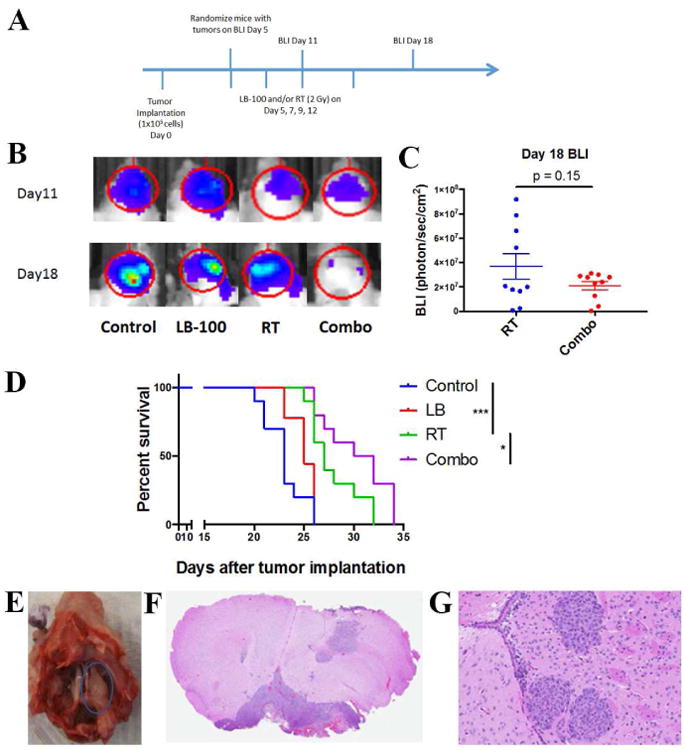
LB-100 enhanced therapeutic efficiency of radiation in vivo. (A) Mice were implanted with 1 × 105 IOMM-LEE cells on the right skull base. Five days after implantation, mice with established tumors on bioluminescence images (BLI) (>5 × 104 photons/s/sr/cm2) were randomized into 4 treatment groups – control, LB-100, RT and combination. Treatments (LB-100 - 1.5 mg/kg and/or RT - 2Gy) were given on day 5, 7, 9 and 12. BLI was obtained on day 11 and 18. (B) Representative BLI images obtained on day 11 and 18 from each treatment group. (C) Quantification of BLI data obtained on day 18 after tumor implantation. There was a trend towards decrease in tumor burden in the combination group compared to RT alone, but it was not statistically significant (p=0.15, student t-test comparing between RT and combination group). (D) Kaplan–Meier survival curves of different treatment groups and compared using the log-rank test. LB-100 + RT combination therapy showed survival benefit compared to RT alone. *P < .05 and ***P < .001. (E) Representative images of gross examination of mice cranium at the survival end point demonstrated expected skull base location of the implanted tumors. (F) Representative images of Hematoxylin and eosin (H&E) coronal section of a mouse skull showing meningioma situated between the brain and skull base. (G) Representative H&E image of implanted tumor showing histologic features consistent with aggressive meningiomas. Tumors are highly cellular with large pleomorphic nuclei, with evidence of brain invasion. Original magnification × 20.
4. Discussion
In this study, we showed the utility of LB-100, a novel small molecule inhibitor of PP2A, in sensitizing the therapeutic effect of radiation in treatment of malignant meningioma, both in vitro and in vivo using an aggressive orthotropic xenograft of skull base meningioma. While PP2A inhibition as a potential strategy for radiosensitization has been suggested in preclinical studies of several tumor types including, nasopharyngeal carcinoma[23], pancreatic adenocarcinoma[18] and glioblastoma[24], to our knowledge, this is the first study demonstrating its radiosensitizing potential in meningioma or any intracranial tumor models.
We have shown that LB-100 alone has limited utility against meningioma growth with IC50 >10 uM in all 3 cell lines, a concentration that is likely unachievable physiologically. However, when combined with RT, 2.5 uM of LB-100 achieved a SER0.5 of 1.8-2.4 in clonogenic assays. We confirmed that LB-100 inhibited the RT-induced increase in PP2A activity, which is critical in mediating homologous recombination repair of radiation-induced DNA damage[33]. In turn, the LB-100 and RT combination significantly enhanced γ-H2AX foci retention, a sign of more unrepaired DNA damage. Consistent with earlier reports, PP2A inhibition in this context resulted in deactivation of the G2/M checkpoint and decreased stabilization of microtubules leading to development of mitotic catastrophe[19, 23, 24], a specific form of cell death. We observed a significant increase in G2/M phases of cell cycle and marked increase in mitotic catastrophe as seen on immunofluorescence staining in the group treated with the combination. LB-100 did not appear to enhance apoptotic cell death, as there was no significant difference in percentage of cC3+ or cPARP+ cells between the RT alone and combination treatment, suggesting the enhanced cytotoxicity of LB-100 and RT combination predominately resulted from increased MC cell death. In our in-vivo skull base meningioma model using IOMM-Luc cells, tumors were aggressive and exhibited histologic characteristics of malignant meningioma by insinuating along arachnoid planes and invading surrounding brain. In the absence of treatment, the median survival was only 23 days. Using BLI measurement as a proxy for tumor size, we have shown that by day 18, there was a trend towards decreased tumor burden in the combination group compared to RT alone. However, the difference was not significant (p=0.15) due to the high variance of growth trajectories, despite a well-randomized baseline tumor burden between groups. On survival analysis, combination treatment did significantly increase median survival by 4 days compared to RT alone (p≤0.05), the same survival benefit conferred by RT compared to control (p<0.01). This is a remarkable result since it suggests LB-100 can double the survival benefit of RT in an aggressive orthotropic tumor model.
In addition to its effect on DNA repair and cell cycle control, PP2A inhibition has also been shown to inhibit activation of STAT3, a critical transcription factor that is constitutively activated in many cancer types[27]. STAT3 activation is also known to play a central role in inducing epithelial–mesenchymal transition (EMT)[34] that enables tumor cells to become metastatic and resistant to chemo- or radiotherapies[35]. STAT3 inhibition has been shown to reverse EMT and radioresistance[36, 37]. In meningiomas, STAT3 is over-activated relative to normal dura[28] and its expression correlates with tumor grade and VGEF expression[29]. We demonstrated that LB-100 could reduce STAT3 activation as reflected by decreased in phosphorylation of the primary activation site at Y705. To confirm decreased signaling of the STAT3 axis, several STAT3 downstream targets were also shown to be down regulated with LB-100, including mcl-2, c-myc and cyclinD1. However the mechanism by which PP2A inhibition decreases STAT3(Y705) is not completely understood. PP2A, being a serine/threonine phosphatase, does not directly modulate STAT3(Y705), but rather is known to de-phosphorylate the secondary phosphorylation site at S727. Inhibition of PP2A induces redistribution of STAT3 to the cytoplasm and functional inhibition of STAT3 [25]. The relative contribution of PP2A-mediated STAT3 inhibition to the radiosensitizing effect in meningioma cells was not assessed in this study.
The major limitation of this study is the reliance on immortalized cell lines for both in vitro and in vivo experiments. While the three cell lines in this study are widely used in meningioma research and well characterized genetically [38, 39], our experimental conclusions are still prone to the usual pitfalls of using human cancer cell lines[40]. However, this is largely ameliorated by the consistent results across multiple cell lines as well as similar findings in other tumor types. The use of nude mice with xenograft also precludes the study of RT and LB-100 effects in animals with competent immune systems. A recent study found that inhibiting PP2A in immune cells could enhance anti-tumor immune response by activating of CD4 and CD8 T cells[41]. Our study would fail to show the effects of LB-100 treatment on the anti-tumor response mediated by adaptive immunity. However, the lack of readily available transgenic or syngeneic models of meningiomas would make such a study difficult to accomplish.
In conclusion, we have demonstrated convincing pre-clinical evidence that a novel inhibitor of PP2A, LB-100, can effectively sensitize meningioma cells to the therapeutic effects of radiation via multiple mechanisms. These findings and the favorable toxicity profile of LB-100 shown in a recent phase I clinical trial[21], support further clinical investigation of concurrent LB-100 and radiotherapy in patients with AAM.
Pharmacologic inhibition of Protein Phosphatase 2A Inhibitor (PP2A), with a small molecule inhibitor LB-100, enhances the cytotoxic effect of radiation in meningioma cells
PP2A inhibition increases radiation induced DNA double-strand breakage, mitotic catastrophe cell death and G2/M cell cycle arrest
PP2A inhibition also decreases activation of STAT3 and expression of its downstream proteins
Combining PP2A inhibition with radiation significant increased survival of mice with meningioma xenograft
Acknowledgments
We give special thanks to Dr. Randy Jensen (University of Utah) for providing IOMM-LEE, CH-157 and GAR meningioma cell lines.
Funding. All co-authors were supported by the Intramural Research Program at the National Cancer Institute and the National Institute of Neurological Disorders and Stroke at the NIH. Saman Sizdahkhani was additionally supported by the NIH Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, The Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as other private donors. For a complete list, please visit http://fnih.org/work/ education-training-0/medical-research-scholars-program.
Footnotes
Conflict of interest: None
Conflict of interest: The authors report no actual, potential, or potentially perceived conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeMonte F, McDermott MW, Al-Mefty O. Al-Mefty's meningiomas, Thieme. Vol. 1. New York: 2011. online resource (xvi, 432 p.) [Google Scholar]
- 2.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14(5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SJ, Boddu S, Macarthur DC. Atypical meningiomas: WHO moved the goalposts? Br J Neurosurg. 2007;21:588–592. doi: 10.1080/02688690701684246. [DOI] [PubMed] [Google Scholar]
- 4.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99:307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moazzam AA, Wagle N, Zada G. Recent developments in chemotherapy for meningiomas: a review. Neurosurg Focus. 2013;35:E18. doi: 10.3171/2013.10.FOCUS13341. [DOI] [PubMed] [Google Scholar]
- 6.Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, von Deimling A, Stavrinou P, Lefranc F, Lund-Johansen M, Moyal EC, Brandsma D, Henriksson R, Soffietti R, Weller M. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383–391. doi: 10.1016/S1470-2045(16)30321-7. [DOI] [PubMed] [Google Scholar]
- 7.Chan RC, Thompson GB. Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. A retrospective study in 257 cases. J Neurosurg. 1984;60:52–60. doi: 10.3171/jns.1984.60.1.0052. [DOI] [PubMed] [Google Scholar]
- 8.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL, Curry WT, Jr, Barker FG., 2nd Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64:56–60. doi: 10.1227/01.NEU.0000330399.55586.63. discussion 60. [DOI] [PubMed] [Google Scholar]
- 10.Hammouche S, Clark S, Wong AH, Eldridge P, Farah JO. Long-term survival analysis of atypical meningiomas: survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir (Wien) 2014;156:1475–1481. doi: 10.1007/s00701-014-2156-z. [DOI] [PubMed] [Google Scholar]
- 11.Sughrue ME, Sanai N, Shangari G, Parsa AT, Berger MS, McDermott MW. Outcomeand survival following primary and repeat surgery for World Health Organization Grade IIImeningiomas. J Neurosurg. 2010;113:202–209. doi: 10.3171/2010.1.JNS091114. [DOI] [PubMed] [Google Scholar]
- 12.Furtner J, Schopf V, Seystahl K, Le Rhun E, Ruda R, Roelcke U, Koeppen S, Berghoff AS, Marosi C, Clement P, Faedi M, Watts C, Wick W, Soffietti R, Weller M, Preusser M. Kinetics of tumor size and peritumoral brain edema before, during, and after systemic therapy in recurrent WHO grade II or III meningioma. Neuro Oncol. 2016;18:401–407. doi: 10.1093/neuonc/nov183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lou E, Sumrall AL, Turner S, Peters KB, Desjardins A, Vredenburgh JJ, McLendon RE, Herndon JE, 2nd, McSherry F, Norfleet J, Friedman HS, Reardon DA. Bevacizumab therapy for adults with recurrent/progressive meningioma: a retrospective series. J Neurooncol. 2012;109:63–70. doi: 10.1007/s11060-012-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nayak L, Iwamoto FM, Rudnick JD, Norden AD, Lee EQ, Drappatz J, Omuro A, Kaley TJ. Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol. 2012;109:187–193. doi: 10.1007/s11060-012-0886-4. [DOI] [PubMed] [Google Scholar]
- 15.Puchner MJ, Hans VH, Harati A, Lohmann F, Glas M, Herrlinger U. Bevacizumab-induced regression of anaplastic meningioma. Ann Oncol. 2010;21:2445–2446. doi: 10.1093/annonc/mdq634. [DOI] [PubMed] [Google Scholar]
- 16.Perrotti D, Neviani P. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol. 2013;14:e229–238. doi: 10.1016/S1470-2045(12)70558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DH, Chowdhury D. What goes on must come off: phosphatases gate-crash the DNA damage response. Trends Biochem Sci. 2011;36:569–577. doi: 10.1016/j.tibs.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei D, Parsels LA, Karnak D, Davis MA, Parsels JD, Marsh AC, Zhao L, Maybaum J, Lawrence TS, Sun Y, Morgan MA. Inhibition of protein phosphatase 2A radiosensitizes pancreatic cancers by modulating CDC25C/CDK1 and homologous recombination repair. Clin Cancer Res. 2013;19:4422–4432. doi: 10.1158/1078-0432.CCR-13-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Kovach JS, Johnson F, Chiang J, Hodes R, Lonser R, Zhuang Z. Inhibition of serine/threonine phosphatase PP2A enhances cancer chemotherapy by blocking DNA damage induced defense mechanisms. Proc Natl Acad Sci U S A. 2009;106:11697–11702. doi: 10.1073/pnas.0905930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssens V, Rebollo A. The role and therapeutic potential of Ser/Thr phosphatase PP2A in apoptotic signalling networks in human cancer cells. Curr Mol Med. 2012;12:268–287. doi: 10.2174/156652412799218930. [DOI] [PubMed] [Google Scholar]
- 21.Chung V, Mansfield AS, Braiteh F, Richards D, Durivage H, Ungerleider RS, Johnson F, Kovach JS. Safety, Tolerability, and Preliminary Activity of LB-100, an Inhibitor of Protein Phosphatase 2A, in Patients with Relapsed Solid Tumors: An Open-Label, Dose Escalation, First-in-Human, Phase I Trial. Clin Cancer Res. 2017;23:3277–3284. doi: 10.1158/1078-0432.CCR-16-2299. [DOI] [PubMed] [Google Scholar]
- 22.Hong CS, Ho W, Zhang C, Yang C, Elder JB, Zhuang Z. LB100, a small molecule inhibitor of PP2A with potent chemo- and radio-sensitizing potential. Cancer Biol Ther. 2015;16:821–833. doi: 10.1080/15384047.2015.1040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv P, Wang Y, Ma J, Wang Z, Li JL, Hong CS, Zhuang Z, Zeng YX. Inhibition of protein phosphatase 2A with a small molecule LB100 radiosensitizes nasopharyngeal carcinoma xenografts by inducing mitotic catastrophe and blocking DNA damage repair. Oncotarget. 2014;5:7512–7524. doi: 10.18632/oncotarget.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon IK, Lu J, Graves CA, Huntoon K, Frerich JM, Hanson RH, Wang X, Hong CS, Ho W, Feldman MJ, Ikejiri B, Bisht K, Chen XS, Tandle A, Yang C, Arscott WT, Ye D, Heiss JD, Lonser RR, Camphausen K, Zhuang Z. Protein Phosphatase 2A Inhibition with LB100 Enhances Radiation-Induced Mitotic Catastrophe and Tumor Growth Delay in Glioblastoma. Mol Cancer Ther. 2015;14:1540–1547. doi: 10.1158/1535-7163.MCT-14-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woetmann A, Nielsen M, Christensen ST, Brockdorff J, Kaltoft K, Engel AM, Skov S, Brender C, Geisler C, Svejgaard A, Rygaard J, Leick V, Odum N. Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc Natl Acad Sci U S A. 1999;96:10620–10625. doi: 10.1073/pnas.96.19.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho WS, Feldman MJ, Maric D, Amable L, Hall MD, Feldman GM, Ray-Chaudhury A, Lizak MJ, Vera JC, Robison RA, Zhuang Z, Heiss JD. PP2A inhibition with LB100 enhances cisplatin cytotoxicity and overcomes cisplatin resistance in medulloblastoma cells. Oncotarget. 2016;7:12447–12463. doi: 10.18632/oncotarget.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 28.Magrassi L, De-Fraja C, Conti L, Butti G, Infuso L, Govoni S, Cattaneo E. Expression of the JAK and STAT superfamilies in human meningiomas. J Neurosurg. 1999;91:440–446. doi: 10.3171/jns.1999.91.3.0440. [DOI] [PubMed] [Google Scholar]
- 29.Zhang MX, Zhao X, Wang ZG, Zhao WM, Wang YS. Constitutive activation of signal transducer and activator of transcription 3 regulates expression of vascular endothelial growth factor in human meningioma differentiation. J Cancer Res Clin Oncol. 2010;136:981–988. doi: 10.1007/s00432-009-0743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragel BT, Elam IL, Gillespie DL, Flynn JR, Kelly DA, Mabey D, Feng H, Couldwell WT, Jensen RL. A novel model of intracranial meningioma in mice using luciferase-expressing meningioma cells. Laboratory investigation. J Neurosurg. 2008;108:304–310. doi: 10.3171/JNS/2008/108/2/0304. [DOI] [PubMed] [Google Scholar]
- 31.Yan Y, Cao PT, Greer PM, Nagengast ES, Kolb RH, Mumby MC, Cowan KH. Protein phosphatase 2A has an essential role in the activation of gamma-irradiation-induced G2/M checkpoint response. Oncogene. 2010;29:4317–4329. doi: 10.1038/onc.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandal T, Bhowmik A, Chatterjee A, Chatterjee U, Chatterjee S, Ghosh MK. Reduced phosphorylation of Stat3 at Ser-727 mediated by casein kinase 2 - protein phosphatase 2A enhances Stat3 Tyr-705 induced tumorigenic potential of glioma cells. Cell Signal. 2014;26:1725–1734. doi: 10.1016/j.cellsig.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Mamouni K, Cristini A, Guirouilh-Barbat J, Monferran S, Lemarie A, Faye JC, Lopez BS, Favre G, Sordet O. RhoB promotes gammaH2AX dephosphorylation and DNA double-strand break repair. Mol Cell Biol. 2014;34:3144–3155. doi: 10.1128/MCB.01525-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendt MK, Balanis N, Carlin CR, Schiemann WP. STAT3 and epithelial-mesenchymal transitions in carcinomas. JAKSTAT. 2014;3:e28975. doi: 10.4161/jkst.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiery JP, Lim CT. Tumor dissemination: an EMT affair. Cancer Cell. 2013;23:272–273. doi: 10.1016/j.ccr.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Zang C, Liu X, Li B, He Y, Jing S, He Y, Wu W, Zhang B, Ma S, Dai W, Li S, Peng Z. IL-6/STAT3/TWIST inhibition reverses ionizing radiation-induced EMT and radioresistance in esophageal squamous carcinoma. Oncotarget. 2017;8:11228–11238. doi: 10.18632/oncotarget.14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han TJ, Cho BJ, Choi EJ, Kim DH, Song SH, Paek SH, Kim IA. Inhibition of STAT3 enhances the radiosensitizing effect of temozolomide in glioblastoma cells in vitro and in vivo. J Neurooncol. 2016;130:89–98. doi: 10.1007/s11060-016-2231-9. [DOI] [PubMed] [Google Scholar]
- 38.Ragel BT, Couldwell WT, Gillespie DL, Wendland MM, Whang K, Jensen RL. A comparison of the cell lines used in meningioma research. Surg Neurol. 2008;70:295–307. doi: 10.1016/j.surneu.2007.06.031. discussion 307. [DOI] [PubMed] [Google Scholar]
- 39.Mei Y, Bi WL, Greenwald NF, Agar NY, Beroukhim R, Dunn GP, Dunn IF. Genomic profile of human meningioma cell lines. PLoS One. 2017;12:e0178322. doi: 10.1371/journal.pone.0178322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masters JR. Human cancer cell lines: fact and fantasy. Nat Rev Mol Cell Biol. 2000;1:233–236. doi: 10.1038/35043102. [DOI] [PubMed] [Google Scholar]
- 41.Zhou P, Shaffer DR, Alvarez Arias DA, Nakazaki Y, Pos W, Torres AJ, Cremasco V, Dougan SK, Cowley GS, Elpek K, Brogdon J, Lamb J, Turley SJ, Ploegh HL, Root DE, Love JC, Dranoff G, Hacohen N, Cantor H, Wucherpfennig KW. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature. 2014;506:52–57. doi: 10.1038/nature12988. [DOI] [PMC free article] [PubMed] [Google Scholar]


