Abstract
Background: There are variable recommendations regarding initiating monotherapy or dual therapy in patients with newly diagnosed type 2 diabetes (T2D). Clear initial strategies are of particular importance in underserved settings where access to care and financial burdens are significant barriers. Objectives: To provide descriptive data of metabolic outcomes to therapy regimens for low-income individuals with newly diagnosed T2D placed on oral hypoglycemic agents (OAs). Methods: We conducted a retrospective chart review of low-income individuals with newly diagnosed T2D initiated on OAs. We provided descriptive data and then evaluated the effects of OA regimens (ie, mono-, dual-, transition [from mono to dual or vice versa] therapy) on hemoglobin A1c (A1c) (baseline to 12 months). Results: A total of 309 patients were included in the study. At 12 months, the mean decrease in A1c for the entire sample was −2.36% (9.37% to 7.01%). Patients prescribed dual therapy had a greater change of A1c compared to those taking monotherapy with metformin (−1.11%, P < .01). Patients who transitioned therapies did not differ in change of A1c compared to monotherapy. Conclusion: Initiation of dual therapy was superior to metformin monotherapy or transitioning therapies and may be preferred for low-income individuals with newly diagnosed T2D.
Keywords: diabetes, low-income settings, underserved, chronic disease, oral hypoglycemic agent, community health centers, medications, newly diagnosed
There is ongoing debate regarding the strategies for oral hypoglycemic agents (OAs) in the treatment of type 2 diabetes (T2D). Hemoglobin A1c (A1c) levels often dictates these strategies. For example, the American College of Endocrinology recommends dual or 2-drug therapy for A1c levels ≥7.5%, while the American Diabetes Association suggests dual therapy for levels ≥9%.1,2 A meta-analysis of 35 randomized control trials revealed that metformin monotherapy or 1-drug therapy decreased A1c by 1.12%, whereas dual therapy with metformin was not as effective in lowering A1c levels (0.95% reduction).3 Another meta-analysis of 15 randomized controlled trials found metformin monotherapy inferior to combination therapy and less attainment of American Diabetes Association target A1c levels (ie, <7%)1 for a wide range of baseline A1c levels (7.2%-9.9%).4 However, studies often evaluate individuals with a long history of diabetes—a group with vast variations in pancreatic β-cell function than those newly diagnosed.3-5
There are advantages to monotherapy including simplicity of dosing and, thereby, improved adherence, lower treatment costs, and less potential for adverse reactions.6 However, published literature has suggested that there is a glucose-lowering synergistic effect related to dual hypoglycemic agent therapy.7 This has contributed to the ongoing evaluation of the efficacy of monotherapy and dual therapy. Though more streamlined medication protocols would benefit all with newly diagnosed with T2D, they would be particularly advantageous in low-income individuals. Low socioeconomic status has been associated with a higher prevalence of diabetes and its complications.8 Poor health literacy, communication barriers, and limited access to care are disproportionate in low-income areas and have a direct effect on patient outcomes.9,10 Initiating precise therapy on diagnosis could decrease the risk of adverse drug events associated with switching medication regimens.11
Previous prospective studies have demonstrated multiple variations of OA treatment recommendations,1-4 but there is limited retrospective data, including information on the medications classes, available from low-income settings. This results in a narrow view of patient populations, making it difficult to view the efficacy of these agents in clinical practice for low-income patients.12 One study revealed that the addition of metformin to insulin or a sulfonylurea as combination therapy significantly improved A1c levels and promoted weight loss.13 Another study showed that therapy regimens were not predictive of A1c change (P = .905).14 Though these studies were both retrospective, they did not provide information regarding exclusive OA use, medications available to low-income individuals, or newly diagnosed T2D.13,14
There are 11 classes of OAs. Because of their cost-effectiveness, OAs in the biguanide (ie, metformin) and sulfonylurea classes are the most commonly used dual therapies in low-income settings.15 Some investigators have encouraged avoiding sulfonylureas because of their risks of hypoglycemia and weight gain.16 However, studies have shown that severe hypoglycemia (glucose <50 mg/dL) is rare with sulfonylureas and weight gain is less than that of insulin.16,17
To our knowledge, there are no reported studies evaluating real-life data of therapy regimens for low-income individuals with newly diagnosed T2D. In order to improve the understanding in this area, we conducted a retrospective chart review of low-income adults with newly diagnosed T2D. Specifically, the objectives of the study were to provide descriptive data of individuals placed on OAs (ie, prescription practices) and analyze which therapy regimen (ie, monotherapy, dual therapy, transition therapy [from monotherapy to dual therapy or vice versa]) results in the greatest change of A1c from baseline to 12 months. We hypothesized that one of these 3 regimens would be superior for glycemic control.
Methods
This was a retrospective chart review of low-income individuals initiated on OAs who received their healthcare through the Harris Health System. Harris Health is a single system with multiple community clinics sites for low-income individuals in Harris County. Its county seat is in Houston, Texas. Individuals living at or below 150% of the federal poverty line and who are a Harris County resident, quality for Harris Health services. The majority of patients in Harris Health are uninsured (60.1%) while others have Medicaid or Children’s Health Insurance Program (CHIP) (20.6%), Medicare (9.7%), or other funding/commercial insurance (9.5%).
Inclusion criteria were (1) adults (≥18 years old) in the Harris Health system and (2) a documented diagnosis of newly diagnosed T2D with a confirmatory provider note. Exclusion criteria were (1) A1c at goal without medications (ie, A1c <7%), (2) exclusionary disease or condition (ie, gestational diabetes, chronic steroid therapy), (3) exclusionary medication-related condition (ie, not on OAs, on insulin, or medication nonadherence), and (4) incomplete electronic medical record (EMR) (ie, missing A1c levels). The study design was evaluated and approved by the institutional review board at Baylor College of Medicine and the Harris Health system.
An electronic search of all the medical records of individuals in the Harris Health/Baylor College of Medicine electronic medical database (n = 6 clinics) identified patients who received a new diagnosis code of diabetes between the years of 2010 and 2015 using International Classification of Diseases (ICD) codes 250.00 and 250.02. This search resulted in identification of 25 763 individuals diagnosed with diabetes. To avoid coding errors (ie, a new diagnosis in the Harris Health/Baylor College of Medicine system but diagnosed previously at an outside clinic) and to assess medication adherence, a physician investigator performed a secondary EMR review that included reading provider notes and medication records (ie, prescriptions prior to 2010).
To appropriately power the study, we determined that we would need 300 patients. An initial feasibility study of 100 patients revealed that 13.97% met inclusion criteria, resulting in 2147 charts that needed to be reviewed to achieve a N = 300. A pseudo-automatic random number generator developed a list of random numbers and randomly selected 2147 EMRs for chart review. Clinical information (ie, A1c, height, weight), ethnicity, medications, and past medical history were extracted from each patient’s EMR. A1c was collected at baseline (date of OA initiation), 3, 6, 9, and 12 months. Figure 1 describes the inclusion/exclusion of the study population.
Figure 1.
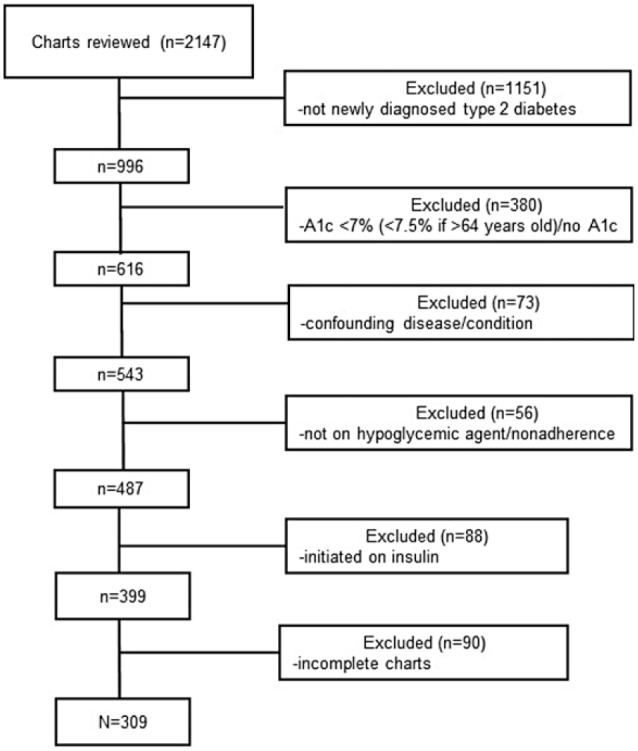
Flowchart of patients who met inclusion/exclusion criteria for the study population.
Subpopulation
To evaluate the effects of OA regimens on A1c, we analyzed a subpopulation that excluded individuals without complete 12-month OA data (ie, transitioned to insulin, treated with diet therapy, missing 12-month medication record). These individuals were placed into 3 subgroups according to their baseline OA regimen: (1) metformin monotherapy, (2) dual therapy (metformin with a sulfonylurea), or (3) transition therapy (monotherapy to dual therapy or vice versa before month 12). The 3 categories of OAs were investigated with metformin monotherapy being the reference category. Clinical and demographic characteristics were included as covariates in all models to control for factors that may be related to being prescribed OAs and A1c levels. Characteristics included: OA regimen, age at diagnosis, gender, race/ethnicity (Hispanic, non-Hispanic black, Asian, white), and body mass index (BMI).18
Data Analysis
Using STATA SE 13 statistical software (StataCorp LP), descriptive analyses and ordinary least squares (OLS) regression models were performed. Descriptive analyses were first conducted on the full analytic sample. Then a subpopulation was grouped according to the three OA categories. Bivariate associations between the OAs and the various A1c measures and each control variable were investigated using either analysis of variance for continuous variables or chi-square tests for dichotomous variables.
OLS regression models were conducted to assess which OA predicted the greatest change in A1c over 12 months. Specifically, change in A1c was regressed on the OA and on the control variables. A change score accounted for different baseline A1c levels. Regression analyses were conducted on a sample of 193 cases that had full data on the independent and control variables. If a subject did not have 12-month data (n = 34), the 9-month A1c value was imputed. Results did not differ between the non-imputed and imputed models. The models with the imputed outcome were presented. In these models, metformin monotherapy was the reference category as it is the recommended first-line agent in T2D.1
Results
Descriptive Statistics
Table 1 displays the descriptive statistics for the full sample (N = 309) and subpopulation (n = 193). In the full sample, patients had a baseline A1c of 9.37% and averaged a −2.36% change at 12 months. In the full sample, the majority (n = 159, 51%) of patients were placed on metformin monotherapy, 24% (n = 74) were prescribed dual therapy, 18% (n = 55) transitioned therapies, and 7% (n = 21) discontinued OAs (ie, started insulin, changed to lifestyle modifications). The patient average age was 49.1 years, and there were 42% male, a majority Hispanic population (73%), and most were overweight (27%) or obese (63%). Patients typically gained (44%) or lost (51%) weight. Comparing the full sample to the total subpopulation, the percentage of individuals placed on metformin, dual, and transition therapy were similar and had minimal overall outcome variations.
Table 1.
Descriptive Statistics for the Full Analytic Sample and Subpopulation by Oral Hypoglycemic Agent (SD or %).
| Full Sample (N = 300) | Subpopulation (n = 193) | ||||
|---|---|---|---|---|---|
| Total | Metformin Monotherapy (n = 102) | Dual Therapy (n = 55) | Transitiona (n = 36) | ||
| A1c (%) | |||||
| Baseline | 9.37 (2.09) | 9.40 (2.09) | 8.48 (1.65) | 10.67 (2.15)b | 10.09 (1.84)b |
| 12 months | 7.01 (1.31) | 6.96 (1.27) | 6.74 (0.90) | 7.22 (1.27)b | 7.21 (1.93) |
| Change in A1c (over 12 months) | −2.36 (2.31) | −2.44 (2.17) | −1.74 (1.75) | −3.45 (2.31)b | −2.88 (3.67)b |
| Oral hypoglycemic agent, % | |||||
| Metformin monotherapy | 51 | 53 | 100 | 0 | 0 |
| Dual therapy | 24 | 29 | 0 | 100 | 0 |
| Transition between mono/dual | 18 | 18 | 0 | 0 | 100 |
| Discontinued OHA (ie, due to insulin initiation) | 7 | n/a | 0 | 0 | 0 |
| Control variables | |||||
| Age at diagnosis (years) | 49.1 (10.83) | 49.9 (10.49) | 50.4 (11.26) | 50.2 (10.02) | 47.8 (8.79) |
| Gender (male), % | 42 | 40 | 44 | 35 | 39 |
| Race/ethnicity, % | |||||
| Hispanic | 73 | 73 | 68 | 76 | 81 |
| Non-Hispanic black | 16 | 15 | 20 | 11 | 6b |
| Asian | 7 | 7 | 7 | 9 | 3 |
| White | 4 | 5 | 6 | 4 | 11 |
| Body mass index, % | |||||
| Normal (18.5-24.9 kg/m2) | 10 | 9 | 5 | 16b | 8 |
| Overweight (25-29.9 kg/m2) | 27 | 29 | 24 | 25 | 47%b,c |
| Obese (>30 kg/m2) | 63 | 63 | 72 | 58 | 44b |
| Weight change, % | |||||
| Weight loss | 51 | 50 | 65 | 22b | 50c |
| Weight maintenance | 4 | 5 | 4 | 9 | 0 |
| Weight gain | 44 | 46 | 31 | 69b | 50 |
Abbreviation: n/a, not applicable.
Transition—from mono/dual therapy.
Significantly different from metformin monotherapy, P < .05.
Significantly different from dual therapy, P < .05.
Subpopulation
There were a number of significant findings as described below. Patients who were prescribed dual therapy and those that transitioned therapies had a greater change in A1c during the 12 months compared with patients who were prescribed metformin monotherapy (−3.45%, −2.88%, −1.74%, respectively; P < .05). Fewer non-Hispanic blacks were placed on transition therapy (6%) compared with monotherapy (20%) (P < .05). Additionally, more individuals prescribed dual therapy (16%) were normal weight and fewer patients that transitioned therapies were obese (44%) compared with those prescribed monotherapy (normal, 5%; obese, 72%; P < .05). Also, a greater percentage of individuals who transitioned therapies (47%) were overweight compared with both monotherapy (24%) and dual therapy (25%) (P < .05). Furthermore, fewer dual therapy patients lost weight (22%) and more (69%) gained weight compared with those on monotherapy (loss, 65%; gain, 31%; P < .05), and a greater percentage of transition therapy patients lost weight (50%) than individuals taking dual therapy (22%) (P < .05). There were more patients that transitioned from monotherapy to dual therapy (n = 20) than from dual therapy to monotherapy (n = 15) or from monotherapy to dual therapy back to monotherapy (n = 1).
Using multivariate regression models, we investigated how OAs, specifically dual therapy and transitioning between dual and metformin therapy, compared with metformin monotherapy contributed to changes in A1c from baseline to 12 months (Table 2). Patients prescribed dual therapy resulted in a significantly greater change in A1c at 12 months compared with patients prescribed metformin monotherapy (−1.11%, P < .01). Patients who transitioned between dual and metformin therapy compared with patients prescribed a metformin monotherapy did not significantly differ in A1c levels during the 12-month period (−0.45%, P > .05). Furthermore, patients who gained weight significantly increased A1c levels (1.17%, P < .001). To account for different baseline A1c levels between groups, we calculated a change score as the dependent variable.
Table 2.
Ordinary Least Squares Regression Predicting the Association Between Oral Hypoglycemic Agent and Change in A1c During 12 Months (n = 193).
| B | Standard Error | |
|---|---|---|
| Oral hypoglycemic agents | ||
| Metformin monotherapy (reference) | — | — |
| Dual therapy | −1.11* | 0.38 |
| Transition between metformin mono and dual therapy | −0.45 | 0.42 |
| Control variables | ||
| Oral hypoglycemic agent regimen | −0.62 | 0.42 |
| Age at diagnosis (years) | −0.004 | 0.01 |
| Gender (male) | 0.48 | 0.31 |
| Race/ethnicity | ||
| Hispanic (reference) | — | — |
| Non-Hispanic black | −0.58 | 0.42 |
| Asian | −0.42 | 0.60 |
| White | 0.13 | 0.60 |
| Weight change | ||
| Weight loss | — | — |
| Weight maintenance | -0.49 | 0.69 |
| Weight gain | 1.17** | 0.31 |
| Constant | 1.34 | 0.77 |
P < .01; **P < .001.
Discussion
Though clinical trials are a vital part of the scientific process, they lack the ability to establish continuous relationships and to assess complex interactions within a study arm.12 This may lead to an emphasis of the efficacy for simple therapies and internal validity rather than delivery of care practices and external validity.12 This is why retrospective analyses that portray real world data are critical. This retrospective review provided clarity for the optimal treatment regimens upon diagnosis of diabetes. Specifically, we found that dual therapy significantly reduced A1c levels from baseline to 12 months when compared with monotherapy or transitional therapy. The primary and most critical outcome in diabetes is glycemic control, and it is vital that health care providers choose regimens that most effectively and efficiently achieve this.
Patient adherence plays an important part in diabetes outcomes. This is not to be misunderstood with compliance, which infers patient cooperation. For example, approximately 50% of individuals have poor medication adherence that relate to the patient (eg, disease understanding), the physician (eg, understanding of patient financial burden), or the health care system (eg, fragmentation).10 Clear initial OA regimens have the potential to markedly improve these issues. For instance, provider-patient encounters could focus on diabetes education and prevention (eg, obesity) rather than a new pharmacotherapy.
Another key factor relating to patient adherence is BMI. In the current study, individuals who gained weight resulted in a significant increase in A1c. Similar to medications, this does not always depend on patient will. Achieving an optimal weight is extremely challenging, requiring shifting paradigms around the causes.19 Individuals living in poverty face additional challenges including limited food availability and financial constraints to purchase healthy food.20 Emerging evidence suggests that diabetes therapy may need to incorporate BMI levels.2 However, further research is needed for clear standard of care practices related to BMI and to determine if weight-based dosing, such as in antibiotics, would be appropriate.
The study findings are of particular importance for low-income individuals where issues including limited access to care, low-literacy rates, and financial constraints are magnified.21 Limited access to care hinders low-income patients from follow-up visits, such as for medication adjustments.2,22 This is concerning given an analysis revealing that 58% of sulfonylurea and 65% of metformin patients had no titration of their initial regimen.23 In addition, the high prevalence of poor literacy levels in many low-income populations increases the risk of provider-patient miscommunication when drug regimens are changed.24,25 To fully understand most health information, patients must read at a level greater than 10th grade.24 However, 30% to 50% of individuals have not achieved this level.24 Furthermore, frequent titration rates lead to greater out-of-pocket costs for patients.26
Though the benefits of precise OA protocols are clear, the physiology of dual therapy is not. Some investigators suggest a synergistic relationship between the metformin and sulfonylureas, resulting in additive glucose-lowering effects.7 This effect may be due to the differing mechanisms of actions in each drug. Metformin’s antihyperglycemic effect is attributed to mitochondrial inhibition leading to hepatic gluconeogenesis suppression.27 On the other hand, sulfonylureas stimulate pancreatic β-cells for insulin release and reduce hepatic clearance of insulin.16 Further investigations are needed to determine whether a small dose of a sulfonylurea is needed for these additive effects. If so, sulfonylurea doses may be lowered in dual therapy in conjunction with higher metformin doses to avoid β-cell exhaustion from long-term overstimulation with sulfonylureas.28 Additionally, other considerations including adherence, inability to tolerate a medication, and adverse reactions are important variables when comparing therapy efficacy.
As with all retrospective chart reviews, there are limitations to the study. Unaccounted confounding variables may be present. For instance, some participants may have started an exercise program while others did not. Also, some clinics may have offered a nutrition education or weight loss classes, whereas others did not. Furthermore, we could not systematically gather information including as exercise or diet behaviors as in a prospective trial since we were dependent on what healthcare providers recorded in their notes. Prospective, randomized controlled trials are needed to further evaluate and compare OA therapy strategies.
Summary and Conclusions
Diabetes control in low-income settings is a difficult task and is often overwhelming.6,29,30 Because of significant barriers to care, choosing optimal initial therapy has critical implications in these settings. Our findings suggest that patients initiated on dual therapy consisting of metformin and a sulfonylurea may have better A1c outcomes. Future studies are warranted to establish OA dosing protocols to clarify additive effects between sulfonylureas and metformin.
Author Biographies
Elizabeth M. Vaughan is an Assistant Professor of Medicine (Division of General Internal Medicine) at Baylor College of Medicine. Her research involves increasing access to care for low-income populations, specifically in diabetes.
Craig A. Johnston is an Associate Professor in the Department of Health and Human Performance at the University of Houston. He has extensively researched the treatment of obesity and its comorbidities in both adults and children. He has participated in multiple grants that focus on both the treatment and prevention of diabetes.
David J. Hyman is Professor of Medicine (Division of General Internal Medicine) and Family & Community Medicine and the Chief of General Internal Medicine at Baylor College of Medicine. He has done extensive research of patient and physician factors involved in blood pressure control.
Daphne C. Hernandez is an Assistant Professor of Nutrition, Obesity Studies, and Health Disparities at the University of Houston. She has extensive experience studying gender and race/ethnic health disparities resulting from poverty-related issues including food insecurity and obesity across the life span.
Vagish Hemmige is an Assistant Professor of Medicine (Division of Infectious Diseases) at Baylor College of Medicine. His clinical interest is in infectious diseases in transplant patients and in HIV. In addition, he has a research interest in the use of statistical methods to analyze clinical data.
John P. Foreyt is Professor, Department of Medicine and Department of Psychiatry and Behavioral Sciences at Baylor College of Medicine. He also is the Director of the Behavioral Medicine Research Center at Baylor.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institutes of Health, National Institute of Diabetes, Digestive, and Kidney Disorders, Federal Award Identification Number (FAIN) K23DK110341 (Elizabeth Vaughan, principal investigator).
ORCID iD: Daphne C. Hernandez  https://orcid.org/0000-0002-5232-749X
https://orcid.org/0000-0002-5232-749X
References
- 1. American Diabetes Association. Approach to glycemic treatment. Diabetes Care. 2016;39(suppl.1):S52-S59. [DOI] [PubMed] [Google Scholar]
- 2. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology: clinical practice guidelines for developing a diabetes mellitus comprehensive care plan-2015. Endocr Pract. 2015;21(suppl. 1):1S-87S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirst JA, Farmer AJ, Ali R, Roberts NW, Stevens RJ. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care. 2012;35:446-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2013;16:410-417. [DOI] [PubMed] [Google Scholar]
- 5. DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36(suppl 2):S127-S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perucca E. Pharmacologic advantages of antiepileptic drug monotherapy Epilepsia. 1997;38(5 suppl):S6-S8. [Google Scholar]
- 7. Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Am Intern Med. 2011;154:602-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rabi DM, Edwards AL, Southern DA, et al. Association of socio-economic status with diabetes prevalence and utilization of diabetes care services. BMC Health Serv Res. 2006;6:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loignon C, Hudon C, Goulet E, et al. Perceived barriers to healthcare for persons living in poverty in Quebec, Canada: the EQUIhealThY project. Int J Equity Health. 2015;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown MR, Bussell JK. Medication adherence: who cares? Mayo Clin Proc. 2011;86:304-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boockvar K, Fishman E, Kyriacou CK, Monias A, Gavi S, Cortes T. Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long-term care facilities. Arch Intern Med. 2004;164:545-550. [DOI] [PubMed] [Google Scholar]
- 12. Mahajan R. Real world data: additional source for making clinical decisions. Int J Appl Basic Med Res. 2015;5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson M KA, Carson P, McDade AM, Laraway K. A retrospective chart review of uncontrolled use of metformin as an add-on therapy in type 2 diabetes. Clin Ther. 1998;20:691-698. [DOI] [PubMed] [Google Scholar]
- 14. Meyer SL, Hoffman RP. Retrospective chart review of children with type 2 diabetes mellitus evaluating the efficacy of metformin vs. insulin vs. combination insulin/metformin. South Med J. 2011;104:684-688. [DOI] [PubMed] [Google Scholar]
- 15. Aguayo-Royas LB, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sola D, Rossi L, Carnevale-Schianca GP, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11:840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shorr R, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157:1681-1686. [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. About adult BMI. 2017. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html. Accessed September 14, 2017.
- 19. Frood S, Johnston LM, Matteson CL, Finegood DT. Obesity, complexity, and the role of the health system. Curr Obes Rep. 2013;2:320-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaskin DJ, Thorpe RJ, Jr, McGinty EE, et al. Disparities in diabetes: the nexus of race, poverty, and place. Am J Public Health. 2014;104:2147-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kripalani S, Henderson LE, Jacobson TA, Vaccarino V. Medication use among inner-city patients after hospital discharge: patient-reported barriers and solutions. Mayo Clin Proc. 2008;83:529-535. [DOI] [PubMed] [Google Scholar]
- 22. Ogedegbe G, Plange-Rhule J, Gyamfi J, et al. A cluster-randomized trial of task shifting and blood pressure control in Ghana: study protocol. Implement Sci. 2014;9:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maclean JR, Chapman RH, Ferrufino CP, Krishnarajah G. Drug titration patterns and HbA1c levels in type 2 diabetes. Int Clin Pract. 2009;63:1008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plimpton S, Root J. Materials and strategies that work in low literacy health communication. Public Health Rep. 1994;109:86-92. [PMC free article] [PubMed] [Google Scholar]
- 25. Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The prevalence of limited health literacy. J Gen Intern Med. 2005;20:175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nrishnarajah G, Bhosle M, Chapman R. Health care costs associated with treatment modification in type 2 diabetes mellitus patients taking oral anti-diabetic drugs. Manag Care. 2011;20:42-48. [PubMed] [Google Scholar]
- 27. Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56:1898-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rustenbeck I. Desensitization of insulin secretion. Biochem Pharmacol. 2002;63:1921-1935. [DOI] [PubMed] [Google Scholar]
- 29. Bohlen K, Scoville E, Shippee ND, May CR, Montori VM. Overwhelmed patients. Diabetes Care. 2012;35:47-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. García-Pérez LE, Álvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4:175-194. [DOI] [PMC free article] [PubMed] [Google Scholar]


