Abstract
The lung undergoes a striking repair process in response to severe injuries, such as influenza infection. A study now demonstrates that associated stem/progenitor cells are heterogeneous in nature and comprise subpopulations dominated by hypoxia/Notch or Wnt signaling. Modulation of this heterogeneity in favor of functional repair may have therapeutic value.
As a vital organ that is in constant risk of injury from exposure to airborne agents, the lung has evolved multifaceted repair tools using regional stem/progenitor cells 1. One striking example is the formation of large clusters of keratin 5 (KRT5) cells that were originally discovered in a mouse model of severe influenza infection and also observed in fibrotic human lungs 2, 3. Xi et al. now considerably advance our understanding of the cellular origin and molecular regulation of these KRT5 cells 4.
An ideal injury response in the lung should satisfy the short-term need of re-establishing the epithelial barrier to prevent tissue fluid loss and further infection, as well as the long-term goal of restoring the pre-injury cellular structure and function. In animal models of limited injury, the lung mobilizes several cellular sources including basal cells, club cells, and alveolar type 2 (AT2 or AEC2) cells to repair the local damage 1. However, when faced with extensive injury caused by sublethal influenza infection and, to a lesser extent, bleomycin exposure, the lung resorts to a unique distal stem/progenitor cell population that expands rapidly to form KRT5 cell clusters in the damaged alveolar region 2, 3, 5–7. This injury response is remarkable and has generated substantial interest for several reasons. First, these KRT5 cells are believed to arise from immature stem/progenitor cells that are named distal airway stem cells (DASCs) or lineage-negative epithelial progenitors (LNEPs) by different groups 2, 3. Second, although quiescent and rare in a healthy lung, these stem/progenitor cells proliferate dramatically to form clusters of hundreds, if not thousands, of cells in the alveolar region within days after injury. Third, unlike conventional epithelial cells, such activated cells are highly mobile. Finally, although capable of differentiation into functional alveolar cells, the KRT5 cells remain mostly as an undifferentiated dysplastic epithelium in vivo 7. For these reasons, origin, activation, expansion, and differentiation of this stem/progenitor cell population have remained unclear and are now tackled in this study.
Previous lineage tracing experiments demonstrated that the majority of KRT5 cell clusters do not descend from known mature lung epithelial cells, although they can be lineage traced using Sox2CreER, which labels all airway cells 3, 6. The original study by Kumar et al. identified rare transformation related protein 63 (TRP63 or p63) cells in the distal airways that can be cloned as stem cells in culture 2. Hence, the authors suggested that the KRT5 cell clusters, which also express TRP63, arise from these rare P63 cells 2. This possibility is now demonstrated convincingly in the current study using p63CreER, which labels rare distal airway cells in a healthy lung and nearly all KRT5 cells upon influenza infection. By contrast, Krt5CreER lineage tracing labels only a small fraction of the KRT5 cell clusters 3, suggesting that the p63 cells in the distal airways do not consistently express KRT5 and thus are not bona fide basal cells.
Having identified the origin of the KRT5 cell clusters, the authors searched for the signals that lead to their expansion in the alveolar region and observed a remarkable correlation between these clusters and hypoxia. Strikingly, genetic deletion of hypoxia inducible factor 1, alpha subunit (Hif1a), a key mediator of the hypoxia response, largely blocked the formation of the KRT5 cell clusters. Perhaps surprisingly, without these KRT5 cell clusters, which have been considered essential for lung repair after influenza infection 5, the Hif1a knockout mice recovered better, as judged by improvement in body weight, arterial oxygen saturation, and lung barrier function. The authors proposed that although the KRT5 cell clusters quickly resealed the damaged epithelium, they remained dysplastic with limited gas exchange capacity so that blocking their formation by Hif1a deletion activated alternative repair pathways that were better at restoring a functional alveolar epithelium.
Such alternative repair pathways are supported by several lines of evidence in the current and previous studies 3, 4. First, transplantation of LNEPs into injured lungs leads to formation of either KRT5 cell clusters or AT2 cells, the latter of which are considered more important in gas exchange 3. Second, in this study, airway-specific deletion of Hif1a in conjunction with Sox2CreER lineage tracing showed that instead of forming KRT5 cell clusters, lineage traced Hif1a knockout airway cells formed AT2 cells in large numbers upon influenza infection. Third, expression of stabilized beta-catenin (CTNNB1) in airway cells surprisingly activated an AT2 marker, surfactant associated protein C (SFTPC or SPC), in some airway cells even without infection. These airway cells with stabilized CTNNB1 expression also expanded into the alveolar region as AT2 cells instead of KRT5 cells. However, it is unclear from these studies whether such alternative AT2-favoring repair pathways are alternative fates of the same/interconvertible stem/progenitor cells and/or intrinsically distinct populations of stem/progenitor cells. The reason for this is that the described methods to sort and manipulate LNEPs are expected to target a mixed cell population. Future experiments using the newly characterized p63CreER allele should provide further insights. In addition, the frequency of potentially AT2-favoring stem/progenitor cells seemed to be markedly higher than the number of quiescent small airway p63 cells, as suggested by the stabilized CTNNB1 experiments. Further in-depth single cell sequencing of airway cells after exclusion of known lineages might bring more clarity in terms of the proposed stem/progenitor cell heterogeneity.
The current study has also touched upon additional interesting questions. For example, what triggers the activation of p63 cells in the small airways? Although the Hif1a knockout mouse failed to form KRT5 cell clusters in the alveoli, numerous KRT5 cells accumulated within the airways upon influenza infection, suggesting that their initial activation is independent of hypoxia. Systematic profiling and mapping of known signaling pathways in the epithelial cells and surrounding mesenchymal, immune, and endothelial cells might identify molecular candidates driving the activation, migration, and expansion of the p63 cells. In addition, future clonal analyses should reveal the lineage relationship among the airway and alveolar KRT5 cells.
Another focus was on the molecular interactions between the identified hypoxia, Notch, and Wnt signaling pathways. Using primary cell culture, the authors provided evidence for a physical interaction between HIF1A and activated NOTCH; this interaction was required for Notch signaling, which in turn was antagonized by Wnt activation. However, given that signaling pathways likely function in a cell type dependent manner, it is critical to first determine whether the cultured primary cells represent the activated small airway p63 cells or the proposed alternative AT2-favoring p63 negative cells.
Another arising question is how the findings in mice are related to lung injuries in human. The authors found exciting evidence of similar KRT5 cell clusters in multiple types of injured human lungs, including those affected by influenza infection, acute respiratory distress syndrome, scleroderma, dyskeratosis congenital, and Idiopathic pulmonary fibrosis. Different from the mice, these clustered KRT5 cells in human also expressed SPC, suggesting differentiation toward the AT2 lineage or an alternative origin, such as AT2 cells. Another possible and readily testable explanation for such p63/SFTPC hybrid cells would be that repeated injuries in humans lead to repeated activation but alterative differentiation of stem/progenitor cells.
Finally, although it is often emphasized that distinct stem/progenitor cells are activated depending on the type, location, and severity of the injury, the lung is likely to mobilize all possible and appropriate repair mechanisms to combat any given injury. In the case of severe influenza infection, although the KRT5 cell clusters have received the most attention, there is no reason to exclude the contribution of more conventional stem/progenitor cells, including AT2 and club cells, even if, or precisely because, they do not give rise to the dysplastic KRT5 cell clusters. Furthermore, the elephant in the room seem to be the ultrathin alveolar type 1 (AT1) cells, which provide the actual gas exchange surface and are able to adjust their surface area by more than 10-fold without proliferation 8.
In summary, the current study has pinpointed the cellular origin of the KRT5 cell clusters in lungs recovering from severe influenza infection, identified multiple underlying molecular pathways, and implicated the existence of novel alternative stem/progenitor cells.
Figure.
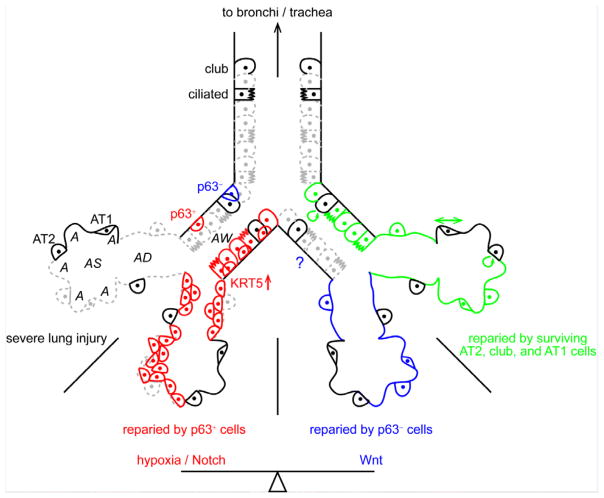
Severe lung injury such as influenza infection causes wide-spread epithelial cell death (grey dashes), which might be repaired by rare small airway p63+ cells (red), p63− cells (blue), or more conventional cells (green). Hypoxia/Notch and Wnt signaling pathways control the balance between p63+ and p63− cell-mediated repair. The p63+ cell-mediated repair results in a more dysplastic epithelium (cell clustering). KRT5 is upregulated when the p63+ cells are activated. The contribution of p63− cells to airway repair is unknown (question mark). AT1 cells might contribute to repairing by stretching their cell surface (double arrow). AW, airway; AD, alveolar duct; AS, alveolar sac; A, alveolus; AT, alveolar type.
References
- 1.Hogan BL, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar PA, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughan AE, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xi Y, et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat Cell Biol. 2017;19:904–914. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuo W, et al. p63Krt5 distal airway stem cells are essential for lung regeneration. Nature. 2014 doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray S, et al. Rare SOX2+ Airway Progenitor Cells Generate KRT5+ Cells that Repopulate Damaged Alveolar Parenchyma following Influenza Virus Infection. Stem cell reports. 2016;7:817–825. doi: 10.1016/j.stemcr.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanegai CM, et al. Persistent Pathology in Influenza-Infected Mouse Lungs. Am J Respir Cell Mol Biol. 2016;55:613–615. doi: 10.1165/rcmb.2015-0387LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, et al. The development and plasticity of alveolar type 1 cells. Development. 2016;143:54–65. doi: 10.1242/dev.130005. [DOI] [PMC free article] [PubMed] [Google Scholar]