Abstract
Introduction
Adipose tissues secrete adipokines, peptides with potent effects modulating fibrosis, inflammation, and vascular homeostasis. Dysregulated adipose tissue biology and adipokine balance have been recently implicated in systemic sclerosis (SSc). We sought to determine if altered circulating adipokine levels correlate with SSc disease subsets or clinical manifestations.
Materials and methods
Multiplex assays were used to measure circulating adipokine levels in 198 patients with SSc and 33 healthy controls. Serum adipokine levels were correlated with demographics and clinical features including pulmonary arterial hypertension (PAH). To assess the relevance of adipsin, an adipokine involved in complement pathway activation, in SSc, we analyzed publically available genetic and transcriptomic data.
Results
Levels of adiponectin and adipsin demonstrated significant differences between controls and patients. Adipsin was significantly elevated in patients with limited cutaneous SSc (OR 28.3, 95% C.I. 7.0-113.8, p<0.0001), and its levels were associated with serum autoantibody status, pulmonary function and cardiovascular parameters, and PAH (OR 3.3 95% C.I. 1.3-8.7, p=0.02). Elevated adipsin was more strongly associated with PAH than B-type natriuretic peptide (BNP). Moreover, in SSc patients, adipsin gene single nucleotide polymorphisms were associated with PAH. Transcriptome dataset analysis demonstrated elevated adipsin expression in patients with SSc-PAH.
Conclusions
We identify adipsin as a novel adipose tissue-derived marker of PAH in SSc. Circulating adipsin levels might serve as predictive biomarkers in SSc. Mechanistically, adipsin might represent a pathogenic link between adipocyte dysfunction and complement pathway activation, and play an important role in the pathogenesis of SSc-PAH.
Introduction
Systemic sclerosis (SSc) is a devastating multisystem disorder that causes significant organ dysfunction, has no approved therapy, and has the highest mortality of any connective tissue disease. A hallmark of SSc is its marked heterogeneity, with substantial patient-to-patient variations in clinical manifestations, autoantibody patterns, and disease outcomes. The pathogenesis of SSc is driven by autoimmunity, vascular damage, and tissue fibrosis. Skin fibrosis and Raynaud phenomenon are the most common disease manifestations, while interstitial lung disease (ILD) and pulmonary artery hypertension (PAH) are leading causes of death (1). The prognosis of SSc-associated PAH is worse than idiopathic and other forms of PAH (2).
Recent studies highlight an emerging role for adipose tissue and fat cells (adipocytes) in modulating fibrosis (3). Patients with SSc have markedly attenuated intradermal white adipose tissue, and attrition of this adipose depot correlates inversely with increased skin fibrosis (4). Adipokines, including leptin, adiponectin, resistin, visfatin, and adipsin are peptides secreted from adipose tissue and have systemic paracrine, and autocrine effects, and play key roles in health and disease (5). These adipokines modulate immune cell activation, vascular function and fibrogenesis, processes that are central to SSc pathogenesis (6).
Previous studies have revealed alterations in circulating adipokine levels in SSc patients (7). However, to date only leptin and adiponectin have been thoroughly investigated, and the results have been variable. Moreover, the association of altered adipokines with SSc organ involvement has not been comprehensively assessed. In these studies, we tested the hypothesis that adipokines are dysregulated in SSc, are associated with clinical features, and their levels may have prognostic value as biomarkers.
Our initial studies led us to focus on adipsin (also known as complement Factor D), which was elevated in a subset of patients with limited cutaneous SSc (lcSSc), especially those with prevalent PAH. To assess a potential pathogenic role of adipsin in SSc, we queried adipsin genetic and gene expression data among SSc patients with and without PAH. Our results identify adipsin as a potential biomarker for SSc-PAH, and provide evidence suggesting that by linking adipose tissue dysfunction and complement pathway activation, it may play a role in SSc and SSc-PAH pathogenesis.
Methods
Patients
The study sample consisted of 198 patients with SSc evaluated at a single center. Patients were sub-classified as limited cutaneous/lcSSc (n=116) and diffuse cutaneous/dcSSc (n=82) based on criteria proposed by Leroy et al. All patients fulfilled the 2013 ACR/EULAR classification criteria. Thirty three healthy control individuals were also included. Patients underwent baseline clinical evaluation, pulmonary function testing, and echocardiograms which were completed within twelve months of serum collection. Pulmonary hypertension was assessed both by echocardiography (echo defined PH was defined as PASP >35 mmHg) and using hemodynamic measures (PAH defined as mean pulmonary artery pressure ≥ 25 mmHg, pulmonary capillary wedge pressure ≤ 15 mmHg, and pulmonary vascular resistance > 3 Wood units). Demographics and clinical characteristics of patients are shown in Table 1. Further description of the patients and assessments are presented under Supplemental Methods.
Table 1. Clinical characteristics of SSc patients included in the study.
| SSc (n=198) Mean ± SD | dcSSc (n=82) Mean ± SD | lcSSc (n=116) Mean ± SD | p-value | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 52.6 ± 11.4 | 50.1 ± 11.2 | 54.3 ± 11.3 | 0.66 |
| BMI (kg/m2) | 26.2 ± 5.5 | 26.0 ± 5.1 | 26.4 ± 5.8 | 0.20 |
| Sex (% female) | 83.3 | 77.3 | 87.9 | 0.05 |
| ANA (% positive) | 95.8 | 97.6 | 94.4 | 0.03 |
| ACA (% positive) | 20.0 | 5.0 | 30.9 | <0.0001 |
| ATA (% positive) | 27.5 | 29.3 | 26.0 | 0.63 |
| RNAP3 (% positive) | 27.4 | 43.4 | 11.3 | <0.0001 |
| Disease Duration (years) | 9.2 ± 8.1 | 3.6 ± 4.2 | 13.2 ± 7.0 | <0.0001 |
| Immunomodulatory treatment (%) | 32.9 | 59.8 | 21.4 | <0.0001 |
| MRSS | 10.4 ± 9.9 | 16.4 ± 12.1 | 4.7± 3.8 | <0.0001 |
| FVC (% predicted) | 79.9 ±19.3 | 75.1 ± 18.7 | 83.4 ± 19.3 | 0.003 |
| DLCO (% predicted) | 60.2 ± 20.0 | 60.2 ± 20.6 | 60.2±19.9 | 0.99 |
| PASP (mmHg) | 33.4 ± 10.7 | 33.2 ± 13.3 | 32.0±7.5 | 0.85 |
| TAPSE (cm) | 2.1 ± 0.5 | 2.2 ± 0.5 | 2.1±0.5 | 0.80 |
| LV Mass (g) | 77.7 ± 20.2 | 80.7 ± 24.0 | 76.0 ± 17.6 | 0.18 |
| Lateral E' Velocity (cm/s) | 12.0 ± 3.7 | 12.2 ± 3.4 | 12.0 ± 3.3 | 0.62 |
| LV Ejection Fraction (%) | 62.0 ± 5.4 | 60.5 ± 4.0 | 62.2 ± 5.0 | 0.12 |
| Pre-capillary PAH (%) | 13.2 | 3.7 | 19.0 | 0.001 |
| Adiponectin (μg/mL) | 7.16 ± 7.32 | 3.41 ± 8.08 | 9.16 ± 3.21 | <0.0001 |
| Adipsin (μg/mL) | 1.18 ± 1.04 | 0.71 ± 0.22 | 1.51 ± 1.25 | <0.0001 |
| Leptin (ng/mL) | 15.38 ± 26.83 | 8.35 ± 10.09 | 20.38 ± 33.19 | 0.002 |
| Resistin (ng/mL) | 18.39 ± 5.76 | 16.97 ± 4.52 | 19.60 ± 6.40 | 0.002 |
| Visfatin (ng/mL) | 6.99 ± 10.75 | 7.15 ± 13.49 | 6.89 ± 8.34 | 0.32 |
Abbreviations: BMI = body mass index. ANA = antinuclear antibodies. ACA = anticentromere antibodies. ATA = anti-topoisomerase antibodies. RNAP3 = RNA polymerase III antibodies. MRSS = modified Rodnan skin score. FVC = forced vital capacity. DLCO = diffusion of lung carbon monoxide. PASP = pulmonary artery systolic pressure. TAPSE = tricuspid annular plane systolic excursion. LV = left ventricle. EF = ejection fraction. PAH = pulmonary arterial hypertension. SSc = Systemic Sclerosis, lcSSc = Limited Cutaneous SSc, dcSSc = Diffuse Cutaneous SSc, SD = Standard Deviation.
Determination of serum adipokine levels
Serum was collected during a standard of care blood draw. Levels of leptin, resistin, visfatin, adipsin and adiponectin were determined using multiplex assay kits (Bio-Rad, Hercules, CA) according to the manufacturer's protocol, samples were run in duplicate and the coefficient of variation (Cv) for adipsin was 6.7%. Measurement was performed on a Luminex 100 platform (Luminex Corporation, Austin, TX).
Correlation of serum adipokine levels with clinical parameters
We first compared clinical characteristics and levels of each adipokine between lcSSc and dcSSc subroups using χ2 and Fisher exact tests (for categorical variables) and the Mann-Whitney U test (for continuous variables). In addition, we further subdivided lcSSc into high and low adipsin levels, with the high adipsin cut-off value defined as 2 SD above the mean in the control group. Next, we performed a multivariable logistic regression analysis to determine the independent association between adipsin and PAH. For additional description of statistical methods, see Supplemental Methods.
Assessment of adipsin genetic polymorphisms and gene expression
Adipsin gene single nucleotide polymorphisms (SNPs) were assessed using publicly available data from a GWAS that included SSc patients with and without echocardiographically-defined pulmonary hypertension (pulmonary artery systolic pressure (PASP >40 mmHg) (clinical data obtained with authorized access from dbGAP, accession phs000357.v1.p1). SNPs associated with SSc-PAH were then assessed for presence of eQTLs using expression data from the GTeX portal (www.gtexportal.org). Additionally, adipsin expression was assessed using publicly available data from both peripheral blood mononuclear cells (PBMCs) and lung tissue from SSc patients with and without PAH (GEO accessions GSE19617and GSE22356). Further description of the datasets used and methods of analysis is presented in the Supplemental Methods.
Results
Adipokine levels in SSc
We found significantly higher levels of adiponectin (9.16 ± 8.09 μg/mL vs 3.41 ± 3.21 μg/mL, p < 0.0001), adipsin (1.51 ± 1.25 μg/mL vs 0.71 ± 0.22 μg/mL, p<0.0001), leptin (20.38 ± 33.19 ng/mL vs 8.35 ± 10.09 ng/mL, p=0.002), and resistin (19.60 ± 6.40 ng/mLvs 16.97 ± 4.52 ng/mL, p = 0.002) in patients with lcSSc compared to patients with dcSSc (Table 1). Similar differences in adipokine levels were noted between lcSSc patients and controls. After correction for multiple hypothesis testing and adjustment for age, sex, race, body mass index (BMI), and disease duration (defined as interval between first non-Raynaud SSc symptom and serum collection), only levels of adiponectin (p=0.003) and adipsin (p=0.001) remained significantly different between the lcSSc and dcSSc subsets (Supplemental Table 1). Further analysis focused on adipsin also known as complement factor D, which has been implicated in the alternative complement pathway, but has not previously been linked to SSc.
Stratification of adipsin levels
Adipsin was substantially elevated in a subset of SSc patients, almost exclusively those with lcSSc (Fig. 1A). Using the cutoff of 1.49 μg/mL (Fig 1B, blue dashed line), 25.8% (30/116) of patients with lcSSc but only 1.2% (1/82) of patients with dcSSc (OR 28.3, 95% C.I. 7.0-113.8, p<0.0001, OR 38.2, 95% C.I. 4.1-333.3, p=0.001 after adjustment for clinical covariates), and 6.1% (2 out of 33) of controls had elevated adipsin levels. Moreover, elevated adipsin was associated with a greater frequency of anti-centromere (OR 2.85, 95% C.I. 1.25-6.51, p=0.01), and lower frequency of anti-Scl70 (OR 0.09, 95% C.I. 0.01-0.69, p=0.004) antibodies (Figure 1C-D). The overall comparison of adipsin in SSc vs controls was not significant (p = 0.66, supplemental table 1), largely reflecting the bimodal distribution in which adipsin was increased in lcSSc patients and decreased in dcSSc patients compared to controls.
Figure 1. Association of serum adipsin levels with lcSSc and pulmonary arterial hypertension.
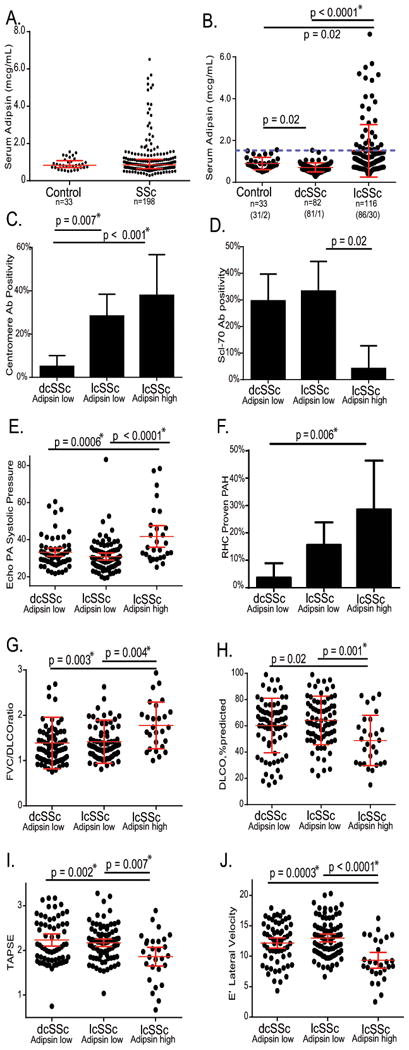
A. Serum levels of adipsin are not significantly different in patients than controls. B. Limited (lcSSc) patients have higher adipsin levels than controls (p=0.02) or diffuse (dcSSc) patients (p<0.0001) The blue dotted line represents the upper cutoff value for adipsin. Association of SSc groups stratified by limited/diffuse status and adipsin level with C. centromere, D. Scl70 antibodies, E. Pulmonary artery systolic pressure measured by echocardiography, F. Right heart catheterization-proven PAH. G. FVC/DLCO ratio H. DLCO I. TAPSE J. E' Lateral velocity. In panels C-H “dcSSc” group represents 82 patients with 81/82 having low adipsin, “lcSSc adipsin low” comprises 86 patients and “lcSSc adipsin high” represents 30 patients. Red bars indicate the mean ± 95% C.I. for each group. Mann-Whitney p-values are listed for each comparison. *p<0.01
Abbreviations. SSc = systemic sclerosis, lcSSc = limited cutaneous systemic sclerosis, dcSSc = diffuse cutaneous systemic sclerosis. PA = pulmonary artery. RHC = right heart catheterization. PAH = pulmonary arterial hypertension. FVC = forced vital capacity. DLCO = diffusion of lung carbon monoxide. TAPSE = tricuspid annular plane systolic excursion.
Adipsin levels are not associated with markers of fibrosis in SSc
Adipsin was assessed for association with severity of SSc-associated skin and lung disease. These analyses showed no association between levels of adipsin (or any other adipokines) and the modified Rodnan skin score (MRSS), radiographically defined ILD, or pulmonary function parameters (FVC, FEV1, or TLC) (Supplementary Figure 1).
Adipsin levels are associated with PAH in SSc
High adipsin levels were significantly associated with (1) pulmonary hypertension (PH), determined by echocardiography (OR 4.6, 95% C.I. 1.8-11.3, p=0.001); and (2) PAH, defined by invasive hemodynamic testing (OR 3.3 95% C.I. 1.3-8.7, p=0.02) (Figure 1 E and F). After adjustment for age, sex, BMI, disease duration and disease subtype, the PH association by echocardiography remained significant (OR 7.7, 95% C.I. 2.1-21.0, p<0.001), but the association with PAH did not (OR 2.7, (95% C.I. 0.8-8.9, p=0.10). Elevated adipsin was also associated with reduction in DLCO, increased ratio of FVC/DLCO, and lower values of tricuspid annular plane systolic excursion (TAPSE) and Doppler E' lateral velocity, which are indicative of right ventricular dysfunction and diastolic dysfunction, respectively (Figure 1 G-J). Patients with elevated adipsin levels had increased frequency of both RV dysfunction (TAPSE<1.5 cm) (OR 19.9, 95% C.I. 3.8-104.5, p<0.001), and LV diastolic dysfunction (OR 5.1, 95%, C.I. 2.1-12.4) (Figure 1 I-J).
Elevated adipsin levels are more strongly associated with SSc-PAH than BNP levels
B-type natriuretic peptide (BNP) is widely used as a biomarker for SSc-PAH(8). Serum levels of BNP were elevated (>100 pg/mL) in 21% of SSc patients, while levels of adipsin were elevated in 16%. Elevated BNP was not significantly associated with PH defined by echocardiogram (p = 0.91) or PAH defined invasively (p = 0.23); in contrast to elevated adipsin levels which were significantly associated with both echocardiographic PH (p < 0.0001) and invasively defined PAH (p = 0.001). High adipsin levels were more specific for PAH than BNP levels (specificity of 85% vs 57% for invasively-proven PAH) and receiver operator curve (ROC) analysis demonstrated that adipsin had greater area under the curve (AUC) for both echo-PH and invasively proven PAH relative to BNP (AUC of 0.73 vs 0.51 for echo PASP>35 and 0.65 vs 0.62 for RHC-proven PAH, Supplementary Figure 2).
Adipsin gene variants are associated with SSc-PAH and modulate gene expression
To determine if elevated adipsin levels may be genetically determined in SSc-PAH, we assessed association of polymorphisms in the CFD (adipsin) gene with SSc-PAH using GWAS data (dbGAP phs000357.v1.p1). We analyzed data from 7 SNPs in the CFD region and assessed SNP allele frequencies from patients stratified either as “normal” (PASP<40, n=167) or as elevated (PASP > 40, n=91) PA systolic pressure by echocardiography. This strategy revealed that 4 of 7 CFD region SNPs (rs1683591, rs2365702, rs106044, and rs2965292) were associated with elevated PA pressure (Figure 2A). To determine the potential functional consequences of the CFD-PAH genetic association, we queried the GTeX portal. This analysis showed a significant expression quantitative trait locus (eQTL) effect in whole blood with the SSc-PAH associated CFD alleles with increased adipsin expression in individuals carrying the minor allele for the PAH-associated SNPs (Figure 2B).
Figure 2. Genetic and expression studies demonstrate dysregulated adipsin in SSc-PAH.
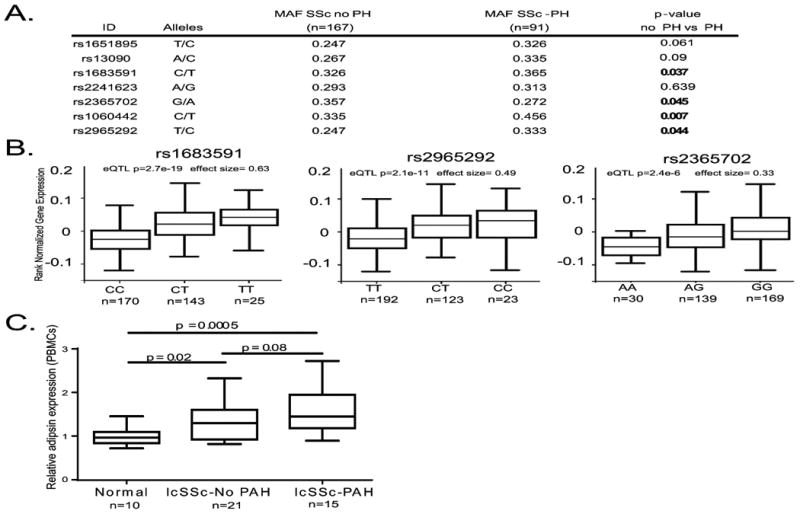
A. List of 7 SNPs in the adipsin (CFD) region on chromosome 19 genotyped in a SSc GWA study (dbGAP accession phs000357.v1.p1). Minor allele frequencies for patients who underwent echocardiography are grouped by presence of echo PASP >40 or not. Note that 4/7 SNPs in the region are associated with SSc-PAH. Data derived from dbGAP accession phs000357.v1.p1 B. 3 of 4 associated SNPs are whole blood eQTLs. C. Expression of adipsin is elevated in lcSSc patients and more so in lcSSc patients with PAH (data derived from GEO GSE19617)
Abbreviations. SNP = single nucleotide polymorphism. CFD = complement factor D. PASP = pulmonary artery systolic pressure. eQTL = expression quantitative trait loci SSc = systemic sclerosis, lcSSc = limited cutaneous systemic sclerosis. PAH = pulmonary arterial hypertension. PBMCs = peripheral blood mononuclear cells. FVC = forced vital capacity. DLCO = diffusion of lung carbon monoxide.
Adipsin expression is increased in lcSSc patients with SSc-PAH
Next, to determine if the genetic association of CFD SNPs with SSc-PAH leads to dysregulated adipsin expression in tissue, we analyzed data from publicly available PBMC and lung tissue microarrays. Data from two independent SSc cohorts (GEO accessions GSE19617 and GSE22356) demonstrated that circulating PBMCs from SSc patients with PH had higher adipsin expression compared to patients without PH (Figure 2C, Supplemental Figure 3). In lung tissue obtained at the time of lung transplant, patients transplanted for SSc-PAH also demonstrated elevated adipsin expression when compared to those transplanted for SSc-ILD (GSE48149, data not shown).
Discussion
We found that levels of multiple adipokines were significantly dysregulated in SSc. Our detailed analysis focused on adipsin for a number of reasons. First, adipsin levels demonstrated the largest difference between lcSSc and dcSSc patients. Second, adipsin, also known as Factor D, has not previously been associated with SSc or vascular disease. Most intriguing, adipisin has a unique role in linking adipose tissue function and complement pathway activation. In this analysis, we uncovered a robust association between elevated circulating level of adipsin and SSc-PAH.
Patients with elevated adipsin levels were more likely to have the limited cutaneous form of SSc and were at significantly increased risk for PAH and related cardiac dysfunction. The association with PAH was confirmed by both echo and hemodynamic data, and further bolstered by associations with DLCO, FVC/DLCO ratio, and measures of right ventricular function. While levels of leptin, resistin, and adiponectin were also elevated in lcSSc patients, they were not significantly associated with clinical markers of fibrosis or vascular disease, suggesting that while multiple adipokines are dysregulated in SSc, adipsin may be unique in its relationship with vascular outcomes such as PAH.
To begin to explore a potential involvement of adipsin in SSc pathogenesis, we queried publicly available datasets. The results indicate that adipsin gene variants are associated with increased PAH susceptibility. Moreover, SSc-PAH-associated SNPs were associated with increased adipsin expression, SSc patients (particularly lcSSc) had elevated adipsin expression compared to controls, and SSc-PAH patients showed increased adipsin expression compared to SSc patients without PAH. Taken together, these findings suggest that elevated adipsin production may play a pathogenic role in SSc-PAH. While we are unaware of studies to evaluate adipsin in PAH, an intriguing study showed that a protease implicated in the pathogenesis of monocrotaline induced PAH in rats is related to adipsin(9).
Our analyses have certain limitations. First, in view of its cross-sectional design, the study is limited in its ability to assess adipsin's prognostic significance. Future studies are planned to examine the prognostic value of baseline adipsin levels for predicting future outcomes such as clinical worsening, development of PAH, and the implications of changes in adipsin over time. Moreover, because the results of this study are luminex-based, future studies might utilize confirmatory ELISA or other sensitive assays. Additionally, while we studied a relatively large and well characterized cohort of SSc patients, the association of serum adipsin with SSc-PAH will need to be validated in independent SSc cohorts. Moreover, the relatively small number of control individuals (n=33) limits power to detect adipokine differences between SSc and normal. Additionally although genetic and gene expression data support a possible mechanistic relationship between adipsin, SSc, and PAH, functional studies will be required to demonstrate the role of adipsin in disease pathogenesis.
In contrast to adipokines such as leptin and adiponectin, adipsin has not been well studied, and its role in autoimmune/inflammatory/fibrotic disease has not been evaluated. Adipsin is one of the major adipocyte products and was the first adipokine described(10). Subsequently, it was recognized to function as the serine protease complement factor D which catalyzes the rate-limiting step of the alternative complement pathway, promotes the formation of the membrane attack complex, and generates complement signaling molecules including the anaphylatoxins C3a and C5a(11). Serum levels of adipsin decline in obesity, pregnancy, and in type II diabetes, while elevated levels are seen in pre-eclampsia, but have not previously been assessed in patients with SSc or other rheumatic disease(10).
The pathogenic role of the complement cascade remains incompletely characterized in SSc. However, previous studies have described dysregulated complement activity in a subset of patients, particularly those with vascular disease. SSc patients have increased complement activation(12), and activated complement complex C5b-9 and CD46 are present on arterioles in SSc skin biopsies (13). Moreover, SSc patients with vascular damage have elevated circulating levels of complement C3f-des-arginine (DRC3f) which enhances endothelial cell proliferation (14).
Importantly, adipsin and complement cascade activation represent potential drug targets. In particular, lampalizumab, a pharmacologic inhibitor of adipsin, is currently in phase III clinical trials for macular degeneration, while eculizumab, a terminal complement inhibitor, is approved for the treatment of paroxysmal nocturnal hemoglobinuia (PNH) and atypical hemolytic uremic syndrome (aHUS). Remarkably, eculizumab has been reported to induce remission in scleroderma renal crisis (a thrombotic microangiopathy that resembles aHUS), and to ameliorate pulmonary hypertension in patients with PNH(15). If adipsin-mediated activation of the alternative complement pathway is shown to be pathogenic in SSc, in addition to marking a set of patients with severe vascular manifestations, this work may also identify a novel personalized and much needed treatment approach for this set of patients.
Conclusions
We demonstrate that circulating adipsin levels are elevated in patients with lcSSc, and identify a significant association between elevated adipsin levels and PAH in this population. Genetic variants of adipsin are associated with SSc-PAH, and adipsin gene expression was elevated in patients with SSc-PAH. Taken together, these findings suggest a previously unrecognized pathogenic role for adipsin in SSc and SSc-PAH, and highlight adipsin as a novel marker of vascular manifestations with important prognostic and potentially therapeutic value. Moreover, in light of the essential role of adipsin in modulating complement pathway activity, our results implicate the complement system in SSc-associated PAH.
Supplementary Material
Acknowledgments
We graciously thank members of the Scleroderma Program including Kathleen Aren, Esperanza Arroyo, and Lauren Beussink-Nelson for their help with clinical, echocardiographic, and serum data collection. We thank the Northwestern Metabolic Hormone Core for performing Luminex assays.
Grant support: K12 HD055884, 5R03AR066343-02, Dixon Young Investigator Award, Northwestern University Clinical and Translational Sciences Institute
References
- 1.Allanore Y, Simms R, Distler O, Trojanowska M, Pope J, Denton CP, et al. Systemic Sclerosis. Nat Rev Dis Primers. 2015;1:15002. doi: 10.1038/nrdp.2015.2. [DOI] [PubMed] [Google Scholar]
- 2.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, et al. Hemodynamic Predictors of Survival in Scleroderma-Related Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2010;182(2):252–60. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez A, Ezquerro S, Mendez-Gimenez L, Becerril S, Fruhbeck G. Revisiting the Adipocyte: A Model for Integration of Cytokine Signaling in the Regulation of Energy Metabolism. Am J Physiol Endocrinol Metab. 2015 doi: 10.1152/ajpendo.00297.2015. ajpendo 00297 2015. [DOI] [PubMed] [Google Scholar]
- 4.Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, et al. Myofibroblasts in Murine Cutaneous Fibrosis Originate from Adiponectin-Positive Intradermal Progenitors. Arthritis Rheumatol. 2015;67(4):1062–73. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falcao-Pires I, Castro-Chaves P, Miranda-Silva D, Lourenco AP, Leite-Moreira AF. Physiological, Pathological and Potential Therapeutic Roles of Adipokines. Drug Discov Today. 2012;17(15-16):880–9. doi: 10.1016/j.drudis.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Ezure T, Amano S. Negative Regulation of Dermal Fibroblasts by Enlarged Adipocytes through Release of Free Fatty Acids. J Invest Dermatol. 2011;131(10):2004–9. doi: 10.1038/jid.2011.145. [DOI] [PubMed] [Google Scholar]
- 7.Lakota K, Wei J, Carns M, Hinchcliff M, Lee J, Whitfield ML, et al. Levels of Adiponectin, a Marker for Ppar-Gamma Activity, Correlate with Skin Fibrosis in Systemic Sclerosis: Potential Utility as Biomarker? Arthritis Res Ther. 2012;14(3):R102. doi: 10.1186/ar3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathai SC, Bueso M, Hummers LK, Boyce D, Lechtzin N, Le Pavec J, et al. Disproportionate Elevation of N-Terminal Pro-Brain Natriuretic Peptide in Scleroderma-Related Pulmonary Hypertension. Eur Respir J. 2010;35(1):95–104. doi: 10.1183/09031936.00074309. [DOI] [PubMed] [Google Scholar]
- 9.Zhu L, Wigle D, Hinek A, Kobayashi J, Ye C, Zuker M, et al. The Endogenous Vascular Elastase That Governs Development and Progression of Monocrotaline-Induced Pulmonary Hypertension in Rats Is a Novel Enzyme Related to the Serine Proteinase Adipsin. J Clin Invest. 1994;94(3):1163–71. doi: 10.1172/JCI117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR, et al. Adipsin: A Circulating Serine Protease Homolog Secreted by Adipose Tissue and Sciatic Nerve. Science. 1987;237(4813):402–5. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Ma M, Ippolito GC, Schroeder HW, Jr, Carroll MC, Volanakis JE. Complement Activation in Factor D-Deficient Mice. Proc Natl Acad Sci U S A. 2001;98(25):14577–82. doi: 10.1073/pnas.261428398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senaldi G, Lupoli S, Vergani D, Black CM. Activation of the Complement System in Systemic Sclerosis. Relationship to Clinical Severity. Arthritis Rheum. 1989;32(10):1262–7. doi: 10.1002/anr.1780321011. [DOI] [PubMed] [Google Scholar]
- 13.Sprott H, Muller-Ladner U, Distler O, Gay RE, Barnum SR, Landthaler M, et al. Detection of Activated Complement Complex C5b-9 and Complement Receptor C5a in Skin Biopsies of Patients with Systemic Sclerosis (Scleroderma) J Rheumatol. 2000;27(2):402–4. [PubMed] [Google Scholar]
- 14.Xiang Y, Matsui T, Matsuo K, Shimada K, Tohma S, Nakamura H, et al. Comprehensive Investigation of Disease-Specific Short Peptides in Sera from Patients with Systemic Sclerosis: Complement C3f-Des-Arginine, Detected Predominantly in Systemic Sclerosis Sera, Enhances Proliferation of Vascular Endothelial Cells. Arthritis Rheum. 2007;56(6):2018–30. doi: 10.1002/art.22645. [DOI] [PubMed] [Google Scholar]
- 15.Hill A, Rother RP, Wang X, Morris SM, Jr, Quinn-Senger K, Kelly R, et al. Effect of Eculizumab on Haemolysis-Associated Nitric Oxide Depletion, Dyspnoea, and Measures of Pulmonary Hypertension in Patients with Paroxysmal Nocturnal Haemoglobinuria. Br J Haematol. 2010;149(3):414–25. doi: 10.1111/j.1365-2141.2010.08096.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


