Abstract
BACKGROUND
A common single nucleotide polymorphism (C385A) in the human fatty acid amide hydrolase (FAAH) gene has been associated with decreased distress responses in healthy volunteers, but its role in psychiatric disorders remains unknown. Here, we obtained genotypes and carried out a secondary analysis of subjects from a trial of comorbid posttraumatic stress disorder (PTSD) and alcohol dependence (AD). We evaluated the effects of C385A variation on behavioral and biochemical biomarkers of distress responses.
METHODS
Forty-nine patients with PTSD and AD were admitted for 4 weeks to an experimental medicine unit at the NIH Clinical Center. Following detoxification, stress reactivity and peripheral endocannabinoid levels were assessed in response to a challenge session using personalized auditory guided imagery. Over the course of the study, subjects were also evaluated for changes in PTSD symptom severity.
RESULTS
FAAH 385A allele carriers showed a marked increase in serum anandamide levels throughout the stress challenge procedure compared with C allele homozygotes, while levels of endocannabinoids primarily metabolized through other enzymatic activity, such as 2-AG, did not differ between genotype groups. FAAH 385A carriers also had decreased subjective anxiety responses to the stress challenge. Similar effects of FAAH C385A genotype were found at the level of clinical PTSD symptom severity, in particular in the arousal domain.
CONCLUSIONS
This is to our knowledge the first study showing that FAAH 385A variation modulates stress responses in subjects with disorders characterized by increased stress reactivity. These findings point to the endocannabinoid pathway as a promising target for future anti-stress therapeutics.
Keywords: stress, FAAH, endocannabinoids, alcohol use disorders, post-traumatic stress disorder
Introduction
Posttraumatic stress disorder (PTSD) is commonly comorbid with alcohol dependence (AD) (Kessler et al., 1995). Both conditions are associated with high stress reactivity and impaired fear extinction, suggesting potentially overlapping neural substrates. Converging evidence from animal and human studies suggests that brain endocannabinoids (eCB) play an important role in regulating stress and emotional processing, as well as extinction of conditioned fear to aversive stimuli. The eCB system is implicated in several psychiatric disorders marked by emotional–motivational dysfunctions, such as anxiety, addiction, eating disorders, and depression (Monteleone et al., 2009, Parolaro et al., 2010, Sipe et al., 2002).
The neuromodulatory eCB system is primarily comprised of two arachidonic acid derived ligands, anandamide (AEA) and 2-arachidonoylglycerol (2-AG) [reviewed in (Moreira and Wotjak, 2010)], as well as the cannabinoid receptor type 1 and type 2 receptors (CB1R and CB2R, respectively) (Howlett et al., 1990, Matsuda et al., 1990). CB1R is present at high levels in corticolimbic regions mediating anxiety, including the medial prefrontal cortex (mPFC) and hippocampus, as well as the basolateral amygdala (BLA) (Di Marzo V, 2012), whereas CB2R is mainly found in the periphery but also in microglia and select neuronal populations in the central nervous system (CNS) (Xi et al., 2011). Unlike most neurotransmitters, AEA and 2-AG are not stored in releasable pools; instead, they are rapidly synthesized “on-demand” and subserve retrograde regulation of synaptic activity through engaging presynaptic CB1R (Katona et al., 2001, Gulyas et al., 2004), and inhibiting neurotransmitter release (Bisogno et al., 2005, Wilson and Nicoll, 2002). The signaling half-life of eCB molecules is determined by two distinct hydrolytic enzymes, fatty-acid amide hydrolase (FAAH), the primary enzyme capable of AEA hydrolysis, and monoacylglycerol lipase (MAGL), which is the primary, but not exclusive, catabolic enzyme for 2-AG (Deutsch et al., 2002, Ueda, 2002).
eCB signaling, and particularly AEA and the activity of its metabolic enzyme FAAH, is modulated by stress (Patel et al., 2005, Patel et al., 2009, Hill et al., 2009, Hill et al., 2010). Preclinical studies in rats and mice have shown that exposure to several types of acute stress causes a rapid decrease in AEA levels in the amygdala-hippocampal-cortico-striatal circuit (Hill and Gorzalka, 2005). This reduction is mediated by an increase in FAAH activity in response to acute stress, especially within the amygdala (Gray et al., 2015, Hill et al., 2009). Gene deletion or pharmacological inhibition of FAAH prevents stress-induced reductions in AEA and associated anxiety-like behavior (Hill et al., 2013b). Inhibition of FAAH also facilitates fear extinction and rescues deficient fear extinction in mice (Gunduz-Cinar et al., 2013a). Conversely, repeated exposure to the same stressor (chronic homotypic stress), such as restraint or social defeat increases FAAH activity and reduces AEA concentrations. In contrast, the effects of repeated exposure to unpredictable stress on AEA content are less consistent across studies [for a review, see (Morena et al., 2015)].
Human studies show that circulating levels of eCBs are similarly responsive to stress. Two studies have reported that basal levels of AEA in the circulation negatively correlate with anxiety scores on clinical scales, both in a healthy population (Dlugos et al., 2012) and in one composed of individuals with major depression (Hill et al., 2008). More interestingly, a recent report found that circulating levels of AEA are significantly reduced in individuals with PTSD compared to both healthy controls and those exposed to trauma that did not develop PTSD (Hill et al., 2013a).
To determine the role of the eCB system and to examine the effects of elevated AEA on stress responses, several studies have focused on a functional single-nucleotide polymorphism (SNP) in the FAAH gene that results in elevated levels of AEA (Sipe et al., 2010). This polymorphism (C385A; rs324420) leads to a proline > threonine substitution, and has an allele frequency of ~25% in populations of Caucasian ancestry. The 385A allele does not alter catalytic properties of the enzyme, but results in reduced levels of FAAH protein possibly through enhanced sensitivity to proteolytic degradation (Sipe et al., 2002). This variant is thought to enhance AEA signaling via decreased enzymatic degradation, a notion supported by the recent development of a humanized mouse model. FAAH 385A knock-in mice show the predicted reduction in FAAH levels and function, accompanied by increased AEA levels. These biochemical traits are accompanied by decreased anxiety-like behavior, enhanced fear extinction learning, and increased fronto-amygdala connectivity (Dincheva et al., 2015).
In agreement with the observations in humanized mice, human FAAH 385A carriers exhibit reduced activation and accelerated habituation of the amygdala response to threat cues, enhanced extinction of fear memories, and increased prefrontal–amygdala resting-state connectivity (Dincheva et al., 2015, Gunduz-Cinar et al., 2013a, Hariri et al., 2009). A recent PET study reported that FAAH 385A carriers have lower in vivo central binding of the FAAH labeling radioligand [11C]CURB (Boileau et al., 2015), confirming the effect of genetic variation at this locus on central FAAH levels. Taken together, these studies support the role of FAAH activity and AEA levels in buffering stress responses. Moreover, they suggest that the FAAH C385A polymorphism moderates these effects.
Here, we therefore evaluated the effects of FAAH C385A variation in a sample of individuals with comorbid PTSD and AD (alcoholism). We investigated whether the FAAH 385A variation affected circulating AEA concentrations, PTSD symptoms, and subjective anxiety ratings in response to a challenge session using personalized auditory guided imagery scripts.
Materials and methods
Subjects
All subjects were treatment-seeking alcoholics enrolled in a 4-week inpatient experimental medicine study for treating co-morbid AD and PTSD at the National Institutes of Health (NIH) Clinical Center in Bethesda, MD. The parent study was a double-blind placebo controlled study evaluating the efficacy of a neurokinin 1 (NK1) antagonist to reduce craving for alcohol (NCT00896038).The results found no drug effect on craving (Kwako et al., 2015a); thus, we collapsed the groups and included the full sample in our analyses, while controlling for group allocation. For a detailed description of the study, see (Kwako et al., 2015a). A flowchart of study procedures for days 1−19 of the study appears in Table 1.
Table 1.
Timeline of study procedures
| Week | |
|---|---|
| 1 | Placebo lead-in |
| Script development sessions (days 4 and 5) | |
| Rating scales (PSSI) | |
| 2 | Active treatment or placebo |
| Rating scales (ADS, ASI, CTQ, NEO, PSSI) | |
| 3 | Active treatment or placebo continues |
| Rating scales (PSSI) | |
| Trier Social Stress Test/Cue reactivity session | |
| 4 | Rating scales (PSSI) |
| Script presentation (3 consecutive days) |
ADS = alcohol dependence scale; ASI = addiction severity index; CR = cue reactivity; CTQ = childhood trauma questionnaire; NEO = NEO personality inventory-revised; PSSI = posttraumatic stress disorder symptom severity index.
In brief, subjects were recruited through advertisements in local media. Following telephone screening, they were admitted to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) clinical inpatient unit. Upon admission, subjects underwent medically managed detoxification if necessary, after which they were screened for inclusion into the study. Subjects were 21 – 50 years old, diagnosed with AD and PTSD according to the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID) (First et al., 1995) and in good physical health. Individuals were excluded if they presented with complicated medical problems, such as advanced liver disease, were unable to participate in all study procedures or provide informed consent.
Assessments
Following detoxification, subjects were assessed with the Alcohol dependence scale (ADS) (Skinner and Horn, 1984) to assess AD severity, the Time-Line Follow-Back (TLFB) (Sobell and Sobell, 1992), which assesses alcohol consumption over a given time, and the Spielberger State Trait Anxiety Inventory (trait version, STAI-T) (Spielberger et al., 1970). Over the course of the study, participants also completed a semi-structured interview measure of PTSD symptom severity, the PTSD Symptom severity index (PSSI) (Foa et al., 1993). Additional scales administered included the Addiction Severity Index (Skinner and Horn, 1984), the NEO Personality Inventory-revised (Costa and McRae, 2002), which assesses five domains of personality (neuroticism, openness, conscientiousness, agreeableness and extraversion), and the childhood trauma questionnaire (CTQ) (Bernstein et al., 1994) which measures exposure to abusive and neglectful experiences prior to age 18 baseline.
Stress procedure
During the fourth and final week of the study, participants underwent auditory guided imagery script challenge sessions, using personalized stress-, alcohol-associated or neutral stimuli as described (Sinha et al., 2011a). Scripts, which lasted approximately 5 minutes, were presented in randomized, counterbalanced order on three consecutive days in the final week of the study. No alcohol content was permitted in the stress and neutral scripts, and the cue and neutral scripts were free of stressful content. Many subjects had experienced multiple traumatic events, therefore they were asked to describe the most traumatic event they could recall in detail.
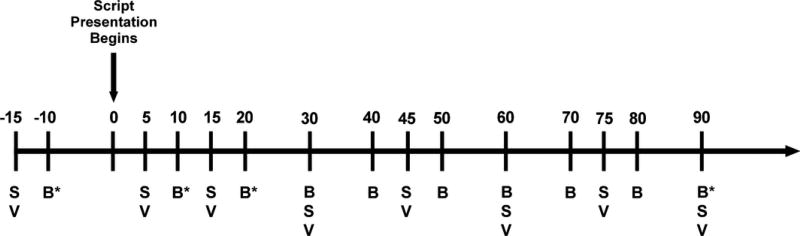
During the challenge session, the Subjective Units of Distress Scale (SUDS), a visual analog scale (1–100), and the Spielberger State Trait Anxiety Inventory (state version, STAI-S) were used to assess self-reported distress and anxiety, respectively. Behavioral measures were collected at eight timepoints (−15, +5, +15, +30, +45, +60, +75, +90 minutes), while blood samples for endocrine measures were collected at 10 timepoints (−10, +10, +20, +30, +40, +50, +60, +70, +89, +90 minutes). Peripheral endocannabinoid signaling markers were assessed at four timepoints (−10, +10, +20, +90 minutes). The 0 timepoint represents the initiation of the procedure (see Fig. 1 for a graphical representation of these timepoints and their relationship to study procedures.)
Figure 1.
Timeline of stress script procedures. B = blood samples, * = sample assayed for peripheral endocannabinoids; S = assessment scales; V = vital signs
Genotyping
Genomic DNA was extracted from whole blood using standard protocols. DNA samples were genotyped using the Illumina OmniExpress BeadChip array (Illumina, San Diego, CA, USA) including more than 700, 000 SNPs. Assessment of population stratification was performed using ancestry informative markers (AIMs). Ancestry informative markers (n = 2500) were extracted from the Illumina array to calculate ancestral proportions for all study participants. Using methods described previously for an AIMs panel including 186 markers (Hodgkinson et al., 2008), which were not available for the current data set, the ancestry assessment identified six ethnic factors (Africa, Europe, Asia, Far East Asia, Oceania, and Americas).
Measurement of serum endocannabinoid contents
Serum (0.4 ml) was thawed and placed into an Eppendorf tube with 0.6 ml saline, 0.176 ml 100% ethyl alcohol and deuterated standards (8.1 nmol [2H8]2-AG and 675 pmol [2H8]AEA (Cayman Chemical Company)). After vortexing and a brief centrifugation, the supernatants were added to 1 ml C18 solid phase extraction columns (Bond Elute) that had previously been washed with 1 ml 100% ethyl alcohol and 3 × 1 ml double distilled H2O (ddH2O). The sample-loaded column was washed with 5 ml ddH2O, with 1 ml ethyl acetate. After drying under a stream of N2, the lipid extract was taken up into mobile phase B (100% methanol with 5 µl acetic acid and 0.0075 g ammonium acetate per 100 ml) and placed into vials for liquid chromatography: mass spectrometry (LC/MS/MS) using an Agilent Technologies 6460 Triple Quad LC/MS with a 1290 Infinity liquid chromatography unit, and were analyzed using Agilent MassHunter Qualitative Analysis B.04.00 software. A Chromasil, 5 µ C18 column with dimensions of 250 × 2.00 mm was used. The elution ramped from 15% mobile phase A (100% ddH2O with 5 µl acetic acid and 0.0075 g ammonium acetate per 100 ml) and 85% mobile phase B to 100% B over 8 min; remained at 100% B until 23 min then returned to 85% mobile phase B.
The concentrations of AEA, 2-AG and two additional lipid analogs that are also substrates of FAAH (palmitoylethanolamide - PEA, N-oleoylethanolamine - OEA) were determined using isotope dilution. Standard curves re constructed from ten concentrations of each analyte and [2H8]AEA and [2H8]2-AG. Peaks corresponding to the product (daughter ions) were measured; the molecular weights for these ions were [2H8]2-AG-293.1; 2-AG-287.1; 2-OG-265.1; [2H8]AEA, AEA, OEA and PEA-62. The ratio of the peak areas of the analyte to [2H8] standard was calculated and the concentration of analyte was determined from the standard curve.
Data analysis
Because there were only three subjects homozygous for the 385A allele, analyses were conducted using two genotype groups: 385A carriers (AX) and C385 homozygotes (CC). FAAH genotype effect on baseline measures was assessed using one-way ANOVA with sex as a covariate. Genotype effects on behavioral and peripheral endocannabinoid response to the stress script were analyzed using PROC MIXED for mixed-effect modeling in SAS version 9.3 (SAS Institute, Cary, NC), with genotype (CC/AX) as the between-subjects factor and time point as the within-subjects factor. PTSD symptom severity measures were similarly analyzed using PROC MIXED, with genotype (CC/AX) as the between-subjects factor and day as the within-subjects factor. The Kenward-Roger correction (Kenward and Roger, 1997) was used in all models, as the use of this correction is highly recommended in repeated measures models with more complex covariance structures, especially when there is an unbalanced design (Littell, 2006). We note that this correction may result in atypical denominator degrees of freedom compared to traditional repeated measures models (e.g., denominator degrees of freedom may actually be higher than the number of subjects).The level of statistical significance was set at p < .05 for all tests, and all post hoc comparisons were conducted using Tukey’s Honestly Significant Difference (HSD) test. All models included treatment assignment as a covariate (although no treatment effects were observed in the original study), as well as the ancestry scores for African and European descent determined from ancestry informative markers; additional covariates were evaluated for inclusion on a model-by-model basis such that covariates that significantly predicted the outcome measure were retained in the model. Covariates that were evaluated included sex, race, age, years of education, ADS score, STAI-T score, average drinks per day and the number of heavy drinking days from the TLFB, total score from the CTQ, neuroticism score from the NEO, and the total score from the ASI. Model-specific covariates are noted in the relevant figure legends.
Results
Subjects characteristics
Demographics, recent drinking histories, and psychological characteristics are shown in Table 2. There were no significant differences between genotype groups in demographics or baseline characteristics. The mean age of study subjects was 40 years [standard deviation = 7.9, range 21–51], and 43% were Caucasian. Subjects drank an average of 15 drinks per day in the 90 days preceding study enrollment; AD severity (as measured by the ADS) was in the severe range (M = 21.8, SD = 7.8). Women reported greater frequency and severity of childhood trauma than did men on the CTQ, t(46) = 2.06, p=0.04; otherwise, there were no significant gender differences in demographic, alcohol-related or psychosocial characteristics.
Table 2.
Participant demographics, drinking histories, and psychological characteristics
| Full Sample (n = 49)1 |
Genotype CC (n = 24) |
Genotype AX2 (n = 25) |
|
|---|---|---|---|
| Demographics | |||
|
| |||
| Age | 40.8 (8.1) | 39.5 (7.9) | 42.2 (8.2) |
| Female | 24 (49.0%) | 11 (45.8%) | 13 (52.0%) |
| Caucasian3 | 22 (44.9%) | 12 (50.0%) | 10 (40.0%) |
| Education (years) | 13.1 (2.7) | 13.3 (3.0) | 13.0 (2.4) |
| Smoker | 40 (81.6%) | 19 (79.2%) | 21 (84%) |
|
| |||
| Alcohol Use (past 90 days) | |||
|
| |||
| Average Drinks/Day | 15.0 (8.1) | 14.5 (7.2) | 15.3 (9.0) |
| Heavy Drinking Days | 67.2 (23.2) | 63.0 (23.2) | 71.3 (22.9) |
| ADS Score | 22.1 (7.5) | 21.5 (7.7) | 22.7 (7.4) |
|
| |||
| Psychological Characteristics | |||
|
| |||
| STAI-T trait score | 48.5 (9.8) | 49.3 (10.5) | 47.8 (9.3) |
| CTQ total score | 54.0 (19.2) | 55.9 (18.8) | 52.3 (19.9) |
| Neuroticism score | 62.1 (10.7) | 62.8 (11.0) | 61.3 (10.4) |
| PSSI total severity | 35.5 (7.6) | 36.1 (6.7) | 35.0 (8.5) |
| ASI total score | 1.9 (0.7) | 2.0 (0.8) | 1.9 (0.7) |
Of the total subject sample, 47 subjects completed the stress script challenge, 44 subjects (including the 2 subjects who did not complete the script challenge) completed all weekly PSSI assessments, and 43 subjects hds plasma samples assayed for peripheral endocannabinoid levels; thus the sample size varies for each analysis.
AC and AA genotypes combined into a single group
The majority of the remaining subjects were Black/African American
FAAH 385A allele frequencies in our sample were higher compared to prior reports in healthy volunteers (C= 0.71; A= 0.29). The genotype distribution (C/C=24; C/A=22; A/A=3) deviated significantly from Hardy Weinberg Equilibrium (χ2=16.4, p=0.0003).
Endocannabinoid levels
We first examined whether FAAH C385A variation would be reflected in peripheral endocannabinoid levels during the stress script procedure, providing an objective, biochemical demonstration that the SNP is functional in our study participants. We measured levels of AEA, which is primarily degraded by FAAH, and those of 2AG, an eCB primarily metabolized through a distinct pathway not involving FAAH. In addition, we measured two cannabinoid receptor-inactive, biosynthetically related congeners of AEA, OEA and PEA.
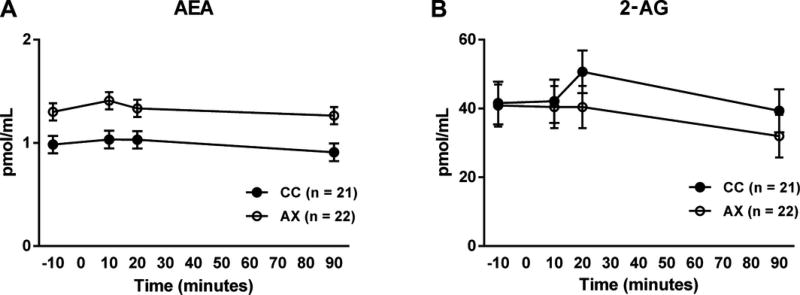
There was a robust main effect of genotype on AEA levels, with 385A carriers showing increased serum AEA levels throughout the course of the procedure [F(1,42) = 10.54, p = 0.002] (Fig. 2a). Similar results were found for two other FAAH metabolized molecular species, OEA [F(1,59) = 34.93, p < 0.0001] and PEA [F(1,56) = 11.45, p =0.001] (data not shown). In contrast, there was no main effect of C385A genotype on serum 2-AG levels [F(1,43) = 0.43, p = 0.51] (Fig. 2b). This demonstrates the biochemical specificity of the genotype effect, as 2-AG is mainly metabolized by the catabolic enzyme MAGL.
Figure 2.
Effects of FAAH C385A variation on peripheral endocannabinoid levels during the stress script procedure. (a) FAAH 385A SNP effects on anandamide (AEA) levels. 385A carriers showing increased serum AEA levels throughout the course of the procedure [F(1,43) = 12.16, p = 0.001]. Ancestry scores and treatment (not significant) were the only covariates in the model. (b) Effects of FAAH C385A on serum 2-AG levels. Ancestry scores and treatment (not significant) were the only covariates in the model.
Stress and anxiety
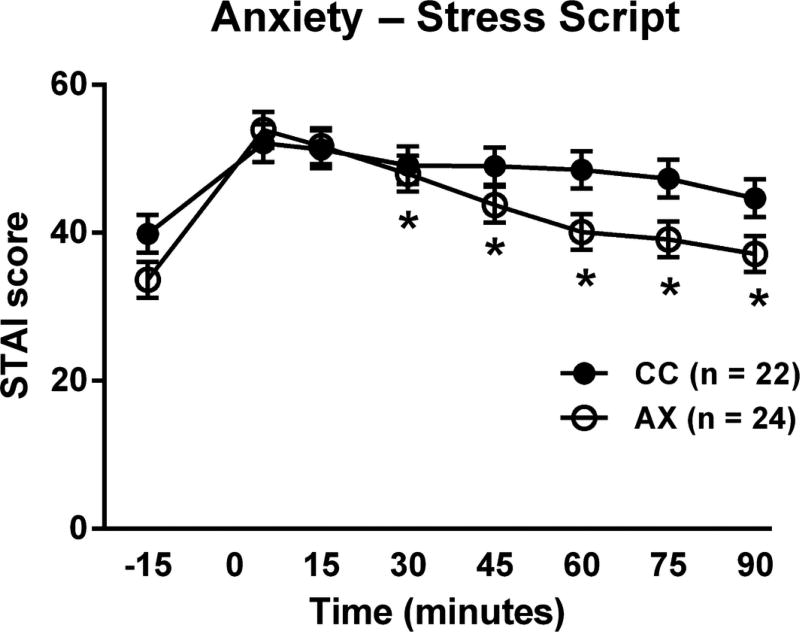
We first examined the effects of FAAH 385A carrier status on several baseline measures of stress and anxiety. As stated above, there were no differences between genotype groups on baseline measures of trait anxiety, personality traits or early life adversity, as previously reported (Hariri et al., 2009, Conzelmann et al., 2012). We then investigated the influence of FAAH C385A variation on subjective anxiety ratings in response to the script challenge procedures. On the basis of our previous findings (Kwako et al., 2015b), we focused the analysis on the stress script, which induces significant distress responses. We found a significant genotype × time interaction on subjective anxiety responses after the stress scripts [F(7,304) = 2.00, p = 0.05], such that the initial anxiety response was virtually identical between the groups, but declined more rapidly in 385A carriers [Fig. 3]. There was no effect of genotype on subjective stress response measured by the SUDS.
Figure 3.
Effects of FAAH C385A on subjective anxiety ratings in response to the stress script. Anxiety response in 385A carriers declined more rapidly than in 385C carriers over the course of the procedure [F(7,303) = 2.01, p < 0.05; * = significantly different from the 5 minute time point in the AX group only]. Model covariates included gender, treatment (not significant), ancestry scores, and neuroticism.
PTSD symptoms
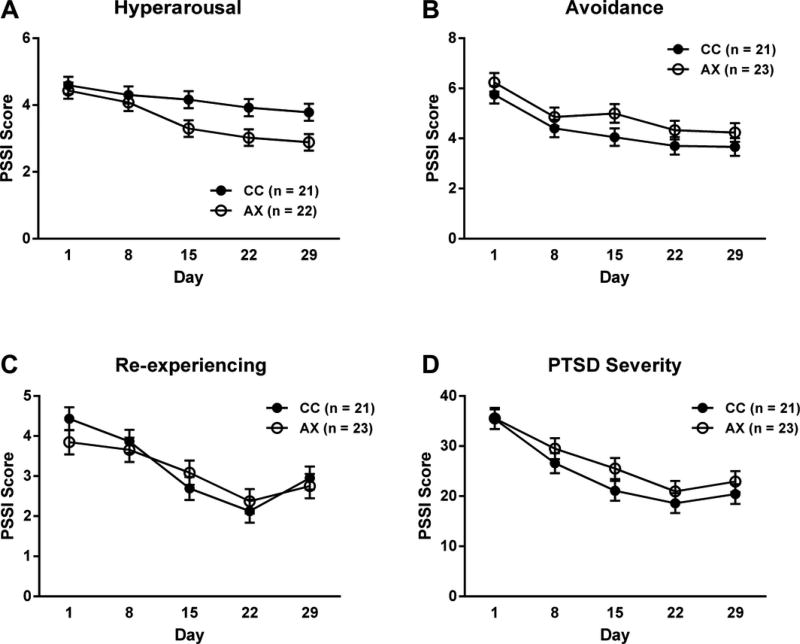
PTSD total symptom severity was assessed weekly over the course of the study using the PSSI (see Fig. 4). There was a main effect of time, such that total PSSI scores declined over the course of the inpatient stay [F(4,162)=22.41, p<0.0001], but no main effect of genotype [F(1,42)=1.18, p=0.28] or time×genotype interaction [F(4,162)=0.62, p=0.65]. The PSSI consists of three subscales that assess different PTSD symptom domains: Hyperarousal, Avoidance and Re-experiencing Symptoms. Because our findings on anxiety levels suggested that possible genotype effects might primarily be on symptoms of hyperarousal, we analyzed each sub-scale separately. There was a main effect of genotype on hyperarousal, such that subjects carrying the low-expressing 385A variant exhibited decreased arousal compared to C homozygotes [F(1,47)=6.04, p=0.02] (Figure 4). There were no significant genotype effects on Avoidance and Re-experiencing Symptoms.
Figure 4.
FAAH 385A SNP effects on hyperarousal. Subjects carrying the lesser-expressing 385A variant exhibit lower arousal scores over time [F(1,51)=5.72, p=0.02]. Model covariates included gender, treatment (not significant), ancestry scores, and neuroticism.
Discussion
This study is to our knowledge the first showing the impact of FAAH C385A variation on biochemistry, behavior and clinical symptoms in subjects with disorders characterized by increased stress reactivity. Specifically, we show that in subjects with comorbid PTSD and alcoholism, FAAH 385A carrier status was associated with marked increase in serum anandamide plasma levels and a concomitant decrease in anxiety level throughout a stress challenge session using personalized auditory guided imagery. Moreover, carriers of the FAAH 385A allele reported decreased PTSD-related hyperarousal over the course of the study in comparison to C homozygotes.
These findings parallel studies showing that anxiety inversely correlates with peripheral AEA content in human subjects (Dlugos et al., 2012, Hill et al., 2008), and that among individuals with PTSD, those with lower peripheral AEA content exhibit higher symptom severity (Hill et al., 2013a). Our data are also consistent with preclinical research demonstrating that genetic deletion or pharmacological inhibition of FAAH during stress exposure is able to prevent some of the adverse physiological and behavioral effects of stress (Hill et al., 2013b, Gunduz-Cinar et al., 2013a).
The putative role of AEA in regulating anxiety was initially demonstrated by the Kathuria and colleagues, who showed that inhibition of AEA-hydrolysis by FAAH resulted in a reduction of anxiety (Kathuria et al., 2003). This ability of AEA signaling to reduce anxiety was then determined to be highly specific to the stressful nature of the environmental context, such that inhibition of FAAH through either pharmacological or genetic means is more effective at reducing anxiety-related behaviors under challenging environmental conditions or after overt stressor exposure (Bluett et al., 2014, Dincheva et al., 2015, Hill et al., 2013b). A potential interpretation of this phenomenon is that stress or aversive experiences produce anxiety in part through a rapid reduction in AEA signaling within anxiety-regulating brain circuits, involving nodes such as the amygdala and hippocampus.
It has been suggested that stress-induced depletion in AEA content is in part a consequence of increased CRH signaling at the CRHR1 within the amgydala (Gray et al., 2015, Hill et al., 2009, Patel et al., 2005). The stress-induced release of CRH rapidly triggers FAAH activity in the BLA to reduce AEA signaling, which in turn promotes the generation of anxiety (Gray et al., 2015). Further studies have shown that central AEA levels are negatively correlated with anxiety-like behaviors (Bluett et al., 2014), and elevating AEA signaling can effectively curb anxiety induced by both acute and chronic stress (Bluett et al., 2014, Hill et al., 2013b, Rossi et al., 2010). Together with the effects on AEA signaling, exposure to stress also results in a transient decline in CB1 receptor signaling that facilitates the emergence of an anxiety state, and a delayed elevation in 2-AG signaling, that instead appears to contribute to termination of the stress response (Morena et al., 2015).
Taken together, these findings demonstrate that eCB signaling is an important modulator of stress, and also suggest the utility of AEA augmentation as a therapeutic approach for stress-related affective and anxiety disorders, lending support for a model in which increased FAAH works to mitigate the effects of stress.
AEA signaling and FAAH activity have also been shown to play a critical role in extinction of fear memories. Gunduz-Cinar and colleagues found that intra-BLA infusions of the FAAH inhibitor AM3506 potentiated memory extinction through the activation of CB1 receptors (Gunduz-Cinar et al., 2013a). Similarly, mouse studies of FAAH 385A carriers also support the notion that elevated AEA signaling is associated with accelerated fear extinction (Dincheva et al., 2015). Human carriers of the 385A allele exhibit reduced activation of the amygdala in response to threat cues, accelerated habituation of the amygdala to these cues, reduced trait anxiety, increased extinction of fear memories, and enhanced prefrontal–amygdala resting-state coupling (Dincheva et al., 2015, Gunduz-Cinar et al., 2013b, Hariri et al., 2009). Thus the evidence obtained to date strongly suggests that inhibiting FAAH to selectively boost endogenously recruited anandamide in corticolimbic circuits could not only attenuate acute stress responses, but also facilitate the extinction of aversive emotional memories.
Although our study was not designed to test the effects of FAAH C385A polymorphism on fear extinction, we observed decreased stress-induced anxiety, and also reduced PTSD-related hyperarousal, over the four week course of the study. Together, these data may suggest that increased AEA signaling, as observed in 385A carriers, promotes habituation to repeated threat, an effect likely mediated by dampened activity of the amygdala. More generally, these findings suggest that FAAH inhibition represents a promising pharmacological approach to treat psychopathologies hallmarked by an inability to extinguish maladaptive behaviors, such as PTSD. A question for future studies is whether FAAH inhibitors will be able to normalize impairments in fear extinction produced by environmental insults other than stress, such as chronic alcohol exposure (Izquierdo et al., 2006, Holmes et al., 2012).
It has been suggested that FAAH inhibitors might have utility for treatment of drug and alcohol addiction (Panlilio et al., 2013), conditions in which stress plays a major role in inducing craving and relapse (Sinha et al., 2011b, Mantsch et al., 2015). This proposition is indirectly supported by the high rate of comorbidity between alcohol use disorders and anxiety disorders, and more specifically by the downregulation of central CB1 receptors found in alcoholism (Hirvonen et al., 2013). Results of preclinical studies to date are, however, complex. In genetically selected alcohol-preferring rats, FAAH blockade did not influence ethanol self-administration (Cippitelli et al., 2008), while other studies have reported that genetic or pharmacological FAAH inactivation results in increased alcohol intake, possibly through localized effects in the prefrontal cortex (Blednov et al., 2007). In humans, a post-mortem study found reduced expression and activity of FAAH in the ventral striatum of patients with AD, and these changes in FAAH were correlated with enhanced tissue content of AEA and reduced expression of CB1 receptors (Vinod et al., 2010).
Taken together, these data indicate that the eCB system may be involved in alcohol consumption but the role of eCBs is complicated by their involvement in modulation of alcohol-induced anxiety and stress-induced alcohol craving and relapse, as well as acute positively reinforcing actions of alcohol. We have previously proposed a framework that integrates and reconciles these multiple effects of eCBs in alcoholism (Hirvonen et al., 2013). This framework suggests that increased eCB activity is likely to drive increased alcohol intake in early stages of alcohol use, by potentiating acute alcohol reward; while an allostatic shift occurs over time such that deficient eCB signaling in severe, late stage alcoholism promotes alcohol use through negative reinforcement. This framework generates testable hypotheses about personalized medicine approaches to the use of FAAH inhibitors in alcoholism.
Supplementary Material
Acknowledgments
We thank Monte Phillips, Hui Sun, Christine Diamond, Jacqueline Goodson and NIH Clinical Center nursing staff for technical support.
Role of the Sponsor: This study was supported by the US Government, NIH/NIAAA/DICBR.
Footnotes
Conflict of interest
None of the authors has any potential financial conflict of interest related to this manuscript.
References
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signalling system: biochemical aspects. Pharmacol Biochem Behav. 2005;81:224–38. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–82. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Bluett RJ, Gamble-George JC, Hermanson DJ, Hartley ND, Marnett LJ, Patel S. Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl Psychiatry. 2014;4:e408. doi: 10.1038/tp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Tyndale RF, Williams B, Mansouri E, Westwood DJ, Le Foll B, Rusjan PM, Mizrahi R, De Luca V, Zhou Q, Wilson AA, Houle S, Kish SJ, Tong J. The fatty acid amide hydrolase C385A variant affects brain binding of the positron emission tomography tracer [(11)C]CURB. J Cereb Blood Flow Metab. 2015;35:1237–40. doi: 10.1038/jcbfm.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology (Berl) 2008;198:449–60. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Conzelmann A, Reif A, Jacob C, Weyers P, Lesch KP, Lutz B, Pauli P. A polymorphism in the gene of the endocannabinoid-degrading enzyme FAAH (FAAH C385A) is associated with emotional-motivational reactivity. Psychopharmacology (Berl) 2012;224:573–9. doi: 10.1007/s00213-012-2785-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McRae AL. NEO Personality Inventory-Revised (NEO PI-R) Washington DC: APA; 2002. [Google Scholar]
- Deutsch DG, Ueda N, Yamamoto S. The fatty acid amide hydrolase (FAAH) Prostaglandins Leukot Essent Fatty Acids. 2002;66:201–10. doi: 10.1054/plef.2001.0358. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, DP L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2012;367:3216–3228. doi: 10.1098/rstb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, Ra S, Gray JM, Yang R, DeGruccio AM, Huang C, Cravatt BF, Glatt CE, Hill MN, Casey BJ, Lee FS. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos A, Childs E, Stuhr KL, Hillard CJ, de Wit H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology. 2012;37:2416–27. doi: 10.1038/npp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Patient Edition. Biometric Research; 1995. [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6:459–473. [Google Scholar]
- Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, McLaughlin RJ, Hassan KI, Kuhne C, Wotjak CT, Deussing JM, Patel S, Hill MN. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci. 2015;35:3879–92. doi: 10.1523/JNEUROSCI.2737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–58. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci. 2013a;34:637–44. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, Godlewski G, Ramikie TS, Gorka AX, Alapafuja SO, Nikas SP, Makriyannis A, Poulton R, Patel S, Hariri AR, Caspi A, Moffitt TE, Kunos G, Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013b;18:813–23. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, Hillard CJ, Yehuda R. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. 2013a;38:2952–61. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. 2005;15:593–9. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM, Cravatt BF, Hillard CJ, Chattarji S, McEwen BS. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry. 2013b;18:1125–35. doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, Hillard CJ, Gorzalka BB, Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A. 2010;107:9406–11. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34:2733–45. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Zanotti-Fregonara P, Umhau JC, George DT, Rallis-Frutos D, Lyoo CH, Li CT, Hines CS, Sun H, Terry GE, Morse C, Zoghbi SS, Pike VW, Innis RB, Heilig M. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Molecular Psychiatry. 2013;18:916–21. doi: 10.1038/mp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43:505–15. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–61. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Bidaut-Russell M, Devane WA, Melvin LS, Johnson MR, Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13:420–3. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–8. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–18. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–97. [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kwako LE, George DT, Schwandt ML, Spagnolo PA, Momenan R, Hommer DW, Diamond CA, Sinha R, Shaham Y, Heilig M. The neurokinin-1 receptor antagonist aprepitant in co-morbid alcohol dependence and posttraumatic stress disorder: a human experimental study. Psychopharmacology (Berl) 2015a;232:295–304. doi: 10.1007/s00213-014-3665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Schwandt ML, Sells JR, Ramchandani VA, Hommer DW, George DT, Sinha R, Heilig M. Methods for inducing alcohol craving in individuals with co-morbid alcohol dependence and posttraumatic stress disorder: behavioral and physiological outcomes. Addict Biol. 2015b;20:733–46. doi: 10.1111/adb.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC. Encyclopedia of Environmetrics. John Wiley & Sons, Ltd; 2006. SAS. [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Bifulco M, Di Filippo C, Gazzerro P, Canestrelli B, Monteleone F, Proto MC, Di Genio M, Grimaldi C, Maj M. Association of CNR1 and FAAH endocannabinoid gene polymorphisms with anorexia nervosa and bulimia nervosa: evidence for synergistic effects. Genes Brain Behav. 2009;8:728–32. doi: 10.1111/j.1601-183X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Wotjak CT. Cannabinoids and anxiety. Curr Top Behav Neurosci. 2010;2:429–50. doi: 10.1007/7854_2009_16. [DOI] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, Hill MN. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Goldberg SR. Inhibition of FAAH and activation of PPAR: new approaches to the treatment of cognitive dysfunction and drug addiction. Pharmacol Ther. 2013;138:84–102. doi: 10.1016/j.pharmthera.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolaro D, Realini N, Vigano D, Guidali C, Rubino T. The endocannabinoid system and psychiatric disorders. Exp Neurol. 2010;224:3–14. doi: 10.1016/j.expneurol.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–69. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Sacchetti L, Cantarella C, Castelli M, Cavasinni F, Motta C, Studer V, Bernardi G, Cravatt BF, Maccarrone M, Usiello A, Centonze D. Preservation of striatal cannabinoid CB1 receptor function correlates with the antianxiety effects of fatty acid amide hydrolase inhibition. Mol Pharmacol. 2010;78:260–8. doi: 10.1124/mol.110.064196. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011a;68:942–52. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology. 2011b;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A. 2002;99:8394–9. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Scott TM, Murray S, Harismendy O, Simon GM, Cravatt BF, Waalen J. Biomarkers of endocannabinoid system activation in severe obesity. PLoS One. 2010;5:e8792. doi: 10.1371/journal.pone.0008792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H, Horn J. Alcohol Dependence Scale: User's Guide. Toronto: Addiction Research Foundation; 1984. [Google Scholar]
- Sobell LC, Sobell MB. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa: Humana Press; 1992. Timeline follow-back: a technique for assessing self reported ethanol consumption. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1970. [Google Scholar]
- Ueda N. Endocannabinoid hydrolases. Prostaglandins Other Lipid Mediat. 2002;68–69:521–34. doi: 10.1016/s0090-6980(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Kassir SA, Hungund BL, Cooper TB, Mann JJ, Arango V. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J Psychiatr Res. 2010;44:591–7. doi: 10.1016/j.jpsychires.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–82. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB(2) receptors modulate cocaine's actions in mice. Nat Neurosci. 2011;14:1160–6. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.