Abstract
Glaucoma is one of the leading cause of blindness globally, and is characterized by loss of retinal ganglion cells (RGCs). Because vision loss in glaucoma is not reversible, therapeutic interventions early in disease are highly desirable. However, due to the current limitations in evaluating glaucomatous neurodegeneration, it is challenging to monitor the disease severity and progression objectively, and to design rational therapeutic strategies accordingly. Therefore, there is a clear need to identify quantifiable molecular biomarkers of glaucomatous neurodegeneration. As such, in our opinion, molecular biomarker(s) that specifically reflect stress or death of RGCs, and which correlate with disease severity, progression and response to therapy, are highly desirable.
Keywords: Glaucoma, Neurodegeneration, Biomarker, Retinal ganglion cell, Aqueous humor, Growth differentiation factor 15 (GDF15)
Challenges of glaucoma treatment and diagnosis/prognosis
Glaucoma, a group of irreversible, progressive optic neuropathies, is the second leading cause of blindness globally [1, 2]. This neurodegenerative condition results in loss of retinal ganglion cells (RGCs) (see Glossary) leading to blindness. Molecular mechanisms of RGC death followed by axonal degeneration in glaucoma are not fully understood, but a monkey model of intraocular pressure (IOP) elevation showed accumulation of radioactive label at the lamina cribrosa, indicating that blockade of both anterograde and retrograde axonal transport could lead to deprivation of neurotrophic signals [3]. In addition, in vivo imaging of mitochondria has recently shown decreased number of transported mitochondria before RGC death in a mouse model (laser-induced ocular hypertension) of glaucoma [4], indicating that axonal transport of mitochondria may play a key role in glaucoma pathogenesis.
As IOP is the most important and modifiable risk factor for the development of glaucoma [5–8], all currently available medical, laser, and surgical therapies for glaucoma are focused on lowering IOP as a strategy to protect RGCs from cell death [9]. Thus, neuroprotection for glaucoma would be highly desirable, but strategies aimed at protecting RGCs have thus far failed to demonstrate efficacy in clinical trials, with no agents currently approved by regulatory authorities [10]. Recent and extensive experimental approaches aim to regenerate damaged RGC axons, or potentially, replace dead RGCs by transplantation [11]; however, it will take considerable time to be able to apply these concepts to the clinical setting.
Other (non-modifiable) risk factors of primary open angle glaucoma (POAG) include older age [12–14], African descent [15], thinner central corneal thickness [16, 17], and a family history of glaucoma [18, 19]. Genome wide association studies (GWAS) [20] have identified several genomic variants associated with glaucoma [21–24], but this does not currently represent a clinically-relevant assessment for patients [25].
Because vision loss in glaucoma is not reversible, therapeutic interventions that might prevent RGC loss early in the disease process, should be sought after. However, there is a lack of available and reliable methods for screening high-risk populations, established parameters of early diagnosis, as well as the prompt detection of disease progression and stability. This poses significant challenges for the clinician managing patients with glaucoma. Therefore, we posit that there is a clear need to identify specific molecular markers that quantify glaucomatous neurodegeneration by accurately and objectively measuring RGC-specific cell death.
Current methods of monitoring glaucomatous neurodegeneration
Accurate monitoring for evidence of disease stability/progression is vital to preserve visual function of glaucoma patients. Ultimately, the desired goal of any glaucoma therapeutic intervention is neuroprotection, leading to survival of RGCs. Physicians currently have only surrogate measures of glaucomatous neurodegeneration, as described below (Figure 1). Although these surrogate measures often synergize to assist the treating physician in making an informed decision, no single examination or diagnostic test is able to accurately predict disease progression, due to inherent subjectivity, unreliability, and limitations of normative databases [26].
Figure 1.
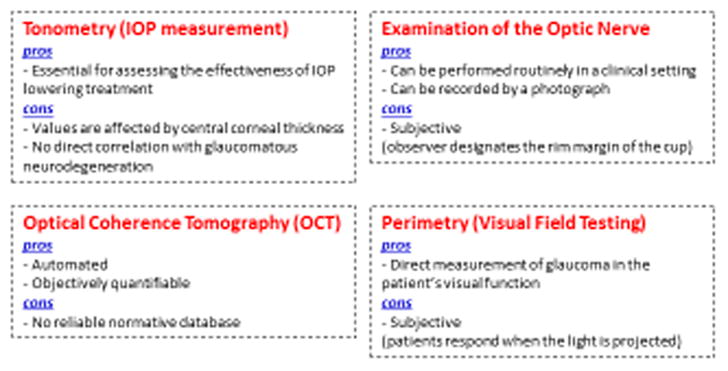
Current methods of detecting/monitoring glaucomatous neurodegeneration: pros and cons.
Tonometry (IOP measurement)
Various instruments are utilized to measure IOP in the clinical setting. The current gold standard instrument is the Goldmann applanation tonometer, that measures IOP by providing force that indents a constant area of the cornea. Notably, variations in the central corneal thickness -- another independent risk factor for glaucoma -- have been shown to affect the accuracy of this tonometer. Indeed, this instrument overestimates IOP with thicker central corneal thickness, and underestimates IOP with thinner central corneal thickness [16, 27]. In addition, there is no proven range of “normal” IOP for a given individual or population. Although IOP is considered “elevated” when greater than 21 mmHg, the distribution is non-Gaussian, and does not correlate with glaucoma pathology [28]. Elevated IOP (ocular hypertension) is a risk factor for glaucoma, but not a definitive characteristic. In fact, some individuals have low tension or normal tension glaucoma with no evidence of IOP elevation [29–31]. Therefore, although IOP is the most significant risk factor for the development of glaucoma and the surrogate endpoint used for assessment and regulatory approval of novel therapies, IOP measurement alone cannot provide precise information of glaucomatous neurodegeneration.
Examination of the Optic Nerve
Biomicroscopic examination in the clinical setting of the optic nerve as it exits the retina provides morphologic cues regarding evidence of optic neuropathy, a requisite for the diagnosis and progression of glaucoma. In humans, the optic nerve head en face is called the disc. There is a recessed area in the central region of the nerve head, the cup, around which the RGC axons are arranged. Thus, an increased “cup-to-disc” ratio represents loss of RGCs, a phenotype that is quantifiable (Figure 2 A, B) [32]. However, there is wide variation of the normal ratio, given that evaluation of the optic nerve is subjective and dependent on precisely where the observer designates the rim margin of the cup [33, 34]. In addition, there are differences in size of the optic disc itself, with larger cup-to-disc ratios identified in eyes without glaucomatous damage [35]. Therefore, examination of the optic nerve is quantifiable but subjective, and thus considered a supplemental method to monitor glaucomatous neurodegeneration.
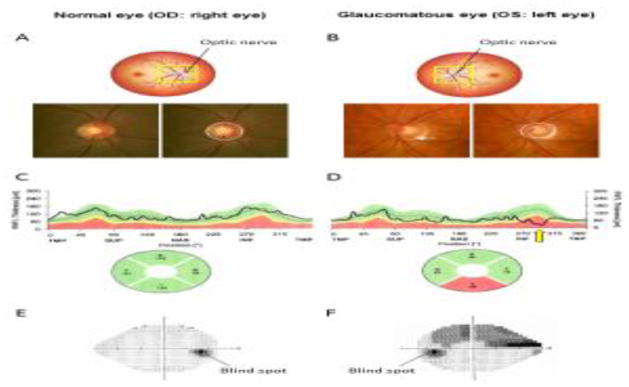
Figure 2. Biometric tools to assess the presence and severity of glaucoma in humans.
(A–B) Cupping (excavation) of the optic nerve head (ONH) of the (A) normal and (B) glaucomatous eye. Loss of retinal ganglion cells is associated with enlargement of the optic cup. Solid circle represents the outline of the ONH, and dashed circle represents the contour of the cup within the ONH. Note that the glaucomatous eye has a larger cup than the normal eye. There is also a hemorrhage at the ONH rim (white arrow), which is another characteristic of glaucoma. Average diameter of ONH is approximately 1.5 mm. (C–D) Representative graphs of retinal nerve fiber layer (RNFL) thickness of the (C) normal and (D) glaucomatous eye measured by optical coherence tomography (OCT) are shown. Note that the inferior RNFL of the glaucomatous eye is thinner (yellow arrow) than normative database. S or SUP: superior. T or TMP: temporal. I or INF: inferior. N or NAS: nasal refer to the thickness of the RNFL in selected quadrants. (E–F) Perimetry of the (E) normal and (F) glaucomatous eye. Dark region in the normal visual field of the right eye indicates “physiological” blind spot corresponding to the reflection of the optic nerve in the temporal visual field. In contrast, perimetry of the glaucomatous eye demonstrates a superior arcuate visual field defect corresponding to loss of RNFL in the inferior quadrant.
Optical Coherence Tomography (OCT)
Substantial technological advances in optic nerve imaging have occurred over the past few decades and have become widely accepted in glaucoma management. Optical Coherence Tomography (OCT) is an extension of a technique known as low-coherence interferometry, a light-based imaging procedure used to non-invasively visualize the anterior and posterior segments of the eye at high resolution [36]. A significant advantage of this technique is that it objectively allows for acquisition of quantifiable data both cross-sectionally and longitudinally, with comparison to a normative database of age-matched control subjects [36]. Rapid acquisition of data scanning around the optic nerve measuring retinal nerve fiber layer (RNFL) thickness provides information of parameters that discriminate glaucomatous eyes from healthy ones by detecting thinning of RNFL in the affected area (Figure 1C, D). However, there are challenges to comparing assessment with the normative database in terms of racial differences, anatomical variants with unreliable measurements (e.g. high myopia), as well as other yet unknown variables [37]. Therefore, although OCT can give us objective and quantifiable data of glaucomatous neurodegeneration, refined and reliable normative databases are required before OCT can become an independent method to monitor glaucomatous neurodegeneration.
Perimetry (Visual Field Testing)
Perimetry, or visual field testing, is a subjective but quantifiable test to directly measure the patient’s visual field (Figure 2E, F) [38]. A light spot is repeatedly presented in different areas of the visual field and the patient responds when the light is perceived. The patient must maintain fixation on a central target and only respond when the light is projected and perceived. Central visual acuity is usually preserved until very advanced stages of glaucoma, making this assessment critical to monitoring progression in early, moderate and severe stages. As a functional measure of RGC loss, therapeutic interventions are aimed at preventing initiation or progression of visual field defects. However, because of inherent subjectivity, interpretations of visual field testing results can be challenging and often require a period of time to reliably detect progression with this modality, thus frequently occurring after documentation of RGC loss has taken place [39]. Therefore, visual field testing alone is not considered a reliable method to monitor glaucomatous neurodegeneration independently.
Molecular biomarkers to monitor neurodegeneration in glaucoma
In light of the dynamic range of acceptable IOP levels for a given patient, the imperfect and developing normative database for OCT interpretation, and the inherent subjectivity of visual field testing, there is a heightened need for molecular biomarkers; especially ones that can predict glaucomatous neurodegeneration prior to its occurrence.
Biomarkers are defined as measurements that can indicate a biological process; these include physiologic measurements, blood testing information, metabolic or genetic data, and image quantification [40]. Biomarkers are further differentiated into biomarkers of exposure, utilized to predict susceptibility to disease [40, 41], and biomarkers of disease, including diagnosis and monitoring of disease progression [40]. To monitor glaucomatous neurodegeneration, we need biomarkers of disease. In addition, prediction of a clinically relevant outcome and the effect of therapies on the outcome determines whether a biomarker can be characterized as a surrogate endpoint [42].
Potential molecular biomarkers for glaucoma, including both biomarkers of exposure and biomarkers of disease, have previously focused on key pathways of disease development [43]. For this, the following fluids have been studied as a potential source of glaucoma biomarkers because the sampling is non-invasive, or minimally invasive.
Tears
Collecting tears for analysis is performed by using a strip of test paper or a microcapillary glass tube, and these collection methods are simple and essentially non-invasive. Brain-derived neurotrophic factor (BDNF) in tears has been reported to be significantly decreased in patients with primary open angle glaucoma compared to control cataract patients without glaucoma [44]. However, there are potential biases caused by topical medications (e.g. eye drops) because topical antiglaucoma medications induce ocular surface inflammation and affect protein profiles in tears [45]. One study investigated only newly diagnosed primary open angle glaucoma patients who did not use any eye-drops, and showed that interleukin IL-12 was significantly lower in primary open angle glaucoma patients compared to patients without glaucoma [46]. Because tears are secreted by the lacrimal gland and coat the ocular surface, but are not in direct contact with the tissues inside of the eye, they may not be the appropriate fluid to detect molecular biomarkers to monitor glaucomatous neurodegeneration.
Urine
Although collecting urine is also essentially non-invasive, few studies of glaucoma patients’ urine biomarkers have been performed. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG), a marker of oxidative DNA damage, has been reported to be significantly increased in patients with progressive normal tension glaucoma, compared to patients with non-progressive normal tension glaucoma [47]. Another study showed elevated urine formaldehyde in elderly patients with primary open angle glaucoma, compared to healthy controls [48]. However, in addition to a lack of contact with the eye, measurement of target molecules in urine can be confounded by the effects of filtration and reabsorption in the kidney. For instance, plasma citrate concentrations have been reported to being significantly lower in glaucoma patients compared to healthy controls, while no significant difference in citrate concentrations between glaucoma patients and healthy controls were found [49]. Therefore, the possibility of robustly and accurately detecting molecular biomarkers of glaucoma in urine seems to be unlikely.
Blood (Serum/Plasma)
The majority of previous glaucoma biomarker studies have used blood (serum/plasma) samples as the source of glaucoma biomarkers, although blood does not inherently contact the eye. The procedure to obtain blood samples is minimally invasive, but in addition, samples are relatively easy to obtain because blood tests are routinely performed at various medical settings. Circulating biomarkers of oxidative stress such as superoxide dismutase 1 (sod1) gene expression in blood have been reported to be significantly decreased in primary open angle glaucoma patients compared to healthy controls [50]. Neuroprotective factors such as brain-derived neurotrophic factor (BDNF) in serum have been found to be decreased in patients with primary open angle glaucoma compared to control cataract patients without glaucoma [44]. In addition, neurotoxic factors such as plasma homocysteine have been reported to be increased in patients with primary open angle glaucoma compared to control cataract patients without glaucoma [51, 52]. However, in the pathogenesis of glaucoma, these markers can be considered as biomarkers of exposure, but not biomarkers of disease. Challenges of sampling blood in order to detect molecular biomarkers to monitor glaucomatous neurodegeneration include the confounding effect of dilution of eye-derived factors and the relative abundance of proteins such as albumin that could mask low-abundant eye-derived proteins. Therefore, blood samples may not be appropriate for detecting molecular biomarkers to monitor glaucomatous neurodegeneration, although they may be useful as biomarkers of exposure, when searching for systemic factors that may lead glaucoma.
Requiring invasive techniques for sampling, other fluids have also been studied as a potential source of glaucoma biomarkers. Although the ease of accessibility of tear, urine and blood samples may be attractive for biomarker screening, analysis of fluids that have contact with the tissues of interest (i.e. RGC layer) may provide more specific information about the disease. Thus, studies of aqueous humor and/or vitreous may be more advantageous in identifying candidates for molecular biomarkers to monitor glaucomatous neurodegeneration (Figure 3).
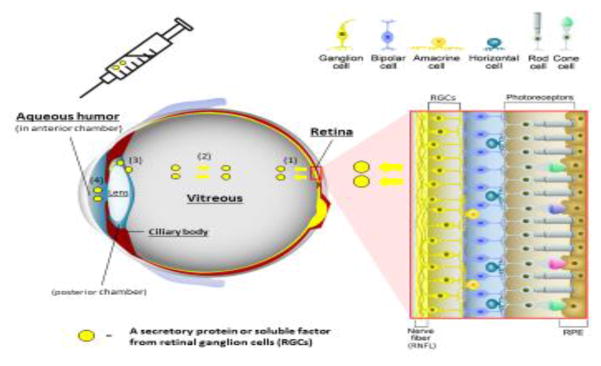
Figure 3 (Key figure). Structure of the neurosensory retina and access to molecular biomarkers of retinal ganglion cell health.
The retina is composed of various types of neurons (red box inset); photoreceptors (rods and cones) in the outer retina, intermediate neurons (bipolar, amacrine, and horizontal cells), and ganglion cells in the inner retina. Axons of retinal ganglion cell (RGC) come together to form the optic nerve that extends processes that synapse in the lateral geniculate nucleus, ultimately sending axons to the occipital (visual) cortex of the brain. Retinal pigmented epithelium (RPE), which is located external to the neurosensory retina, performs essential specialized functions such as phagocytosis of photoreceptor outer segments and recycling photopigments in the visual cycle.
Secreted proteins or soluble factors are released from RGCs into vitreous (1) and can diffuse throughout the vitreous body (2). In addition, they can be detected in the aqueous humor (AH) in the posterior chamber (3), and in the anterior chamber (4) of the eye. AH in anterior chamber is collected by a micro-invasive procedure called paracentesis through the peripheral cornea.
Cerebrospinal fluid (CSF)
CSF is an ultra-filtrate of blood in direct contact with the optic nerve, which is collected by lumbar puncture. Although interest in CSF biomechanics has increased with findings that low CSF pressure is associated with glaucomatous optic neuropathy in normal tension glaucoma [53], no studies of glaucoma biomarkers in CSF have been performed to date. Therefore, CSF is not likely an appropriate target fluid for the detection of putative molecular biomarkers to monitor glaucomatous neurodegeneration.
Vitreous
Vitreous is a transparent gelatinous substance, which fills the posterior space of the ocular globe, between the lens and the retina, and in direct contact with RGCs (Figure 3). In a mouse glaucoma model (IOP elevation model induced by episcleral vein injection of hypertonic saline), dying or damaged RGCs have been found to release key molecules such as catalase, which are significantly increased in both the retina and vitreous [54]; thus, it is possible that such molecules may be exploited as biomarkers of glaucomatous neurodegeneration. However, vitreous collection, often performed as a surgery called vitrectomy, is an invasive procedure with the inherent possibility of complications [55], and only performed in an operating room setting. Although there is a report of office-based vitreous sampling [56], the safety of this method for routine diagnostic testing has not yet been established. Therefore, although vitreous may offer the possibility of detecting biomarkers to monitor glaucomatous neurodegeneration, it is relatively invasive for routine sampling, unless a new minimally-invasive collection method is brought forth.
Aqueous humor (AH)
Aqueous humor (AH), secreted by the ciliary body, is a transparent fluid that fills the anterior segment of the eye, supplying nutrients and removing waste from surrounding avascular tissues (Figure 3). Analysis of human vitreous and aqueous humor of the same eyes with ischemic retinal diseases such as diabetic retinopathy, has indicated that a vitreous-to-aqueous gradient promotes the anterior diffusion of vascular endothelial growth factor (VEGF) [57]. This finding potentially accounts for the occurrence of anterior segment neovascularization in association with retinal ischemia [57]. Similar results have been shown for IL-6 levels in vitreous and aqueous humor sampled from a same eye of patients with diabetic retinopathy [58, 59]. Based on these findings, soluble factors such as cytokines are now considered to be able to diffuse from vitreous into AH. Thus, AH might be considered to be indirectly in contact with RGCs (Figure 3).
AH is harvested by a procedure called paracentesis through the peripheral cornea, a micro-invasive procedure that may be performed in an outpatient clinic setting. Because of the putative contacts with fluid bathing RGCs, and relative ease of access to obtain the sample, we believe AH currently represents the best target for measuring candidate molecular biomarkers to monitor glaucomatous neurodegeneration. Numerous glaucoma biomarker candidates have been identified in AH of patients with primary open angle glaucoma, including inflammatory proteins such as IL-8 [60–62] and TNF-alpha [63, 64], hypoxia-related proteins such as erythropoietin [65–67], and other proteins, including endothelin-1 [68, 69].
In addition, previous studies have focused on the role of transforming growth factor beta 2 (TGF-B2) in the pathogenesis of AH of patients with primary open angle glaucoma and in animal models of glaucoma. Specifically, in these studies, TGF-B2 concentrations in AH were found to be significantly elevated in primary open angle glaucoma patients compared to control cataract patients without glaucoma [70–76]. However, one limitation of using AH as a target fluid to detect candidate molecular biomarkers to monitor glaucomatous neurodegeneration has been that AH is in contact with ocular tissues such as the lens and ciliary body (Figure 3), and these tissues can secrete or modify target molecules [77, 78]. In fact, primary cultures of human, rat, and bovine ciliary epithelial cells [79], and primary cultures of rat lens epithelial cells have been demonstrated to be capable of producing TGF-B2 [80]. In addition, following RGC death induced by optic nerve crush in mice and rats, tgfb2 gene expression was not significantly increased in the eye, when compared to sham-operated eyes [81]. Furthermore, TGF-B2 concentrations in AH did not correlate with the severity of glaucomatous neurodegeneration in a cross-sectional human study that compared various stages of disease severity in primary open angle glaucoma patients [81]. Moreover, studies have shown that TGF-B2 concentrations are linked to the decreased ability of the trabecular meshwork to effectively drain AH and maintain IOP at a normal range, leading to elevation of IOP [82]. These findings indicate that TGF-B2 in AH might be used as a biomarker of exposure, but not as a biomarker of disease, and the latter would be preferable for monitoring glaucomatous neurodegeneration..
We recently reported that growth differentiation factor 15 (GDF15) in AH is elevated in both mouse and rat following RGC death induced by optic nerve crush, and in patients with primary open angle glaucoma compared to control cataract patients with glaucoma [81]. Unlike TGF-B2, which appears to a biomarker of exposure, we hypothesize that an ideal molecular biomarker to monitor glaucomatous neurodegeneration would be one that reflects damage to RGCs (cell stress and/or cell death) so that we may infer RGC death by measuring levels of these molecules. In order to identify such retinal secretory proteins specific to RGC death, we carefully selected GDF15, choosing a factor that was upregulated in the retina following RGC death induced by optic nerve crush, but which was not upregulated in a mouse inflammation model (endotoxin-induced uveitis) or in a mouse photoreceptor-specific cell death model (light-induced retinal degeneration) [81]. Furthermore, these findings were validated retrospectively in a small cohort of patients with various stages of severity of primary open angle glaucoma [81]; GDF15 concentrations were significantly increased in the AH of patients with primary open angle glaucoma compared to control cataract patients without glaucoma; furthermore, GDF15 concentrations were significantly associated with increasing severity of disease, as measured by visual field testing [81]. Although prospective studies in larger patient populations will be evidently needed to validate the utility of GDF15 in clinical practice, and to determine whether it can robustly predict disease progression or response to treatment, we believe that GDF15 may hold significant promise as a potential molecular marker of glaucomatous neurodegeneration.
Concluding remarks
There is a clear need for molecular biomarkers of glaucomatous neurodegeneration, potentially providing clinicians objective and predictive information about the stability and severity of a patient’s disease. Currently, the AH may represent a superior avenue to detect such biomarkers, although there is a clear limitation in that AH is in touch with ocular tissues such as lens and ciliary body, which can secrete or modify target molecules. With the recent development of comprehensive multiplex protein arrays or mass spectrometry, it is now possible to analyze various targets in the AH of glaucoma patients. However, non-targeted exhaustive analyses tend to be associative, with low specificity and sensitivity. Targeted analysis using animal models that can lead to the identification of novel candidate molecular biomarkers with a clear causal relationship to disease, as well prospective human studies will be needed to identify sensitive and specific biomarkers of disease severity, progression and response to therapy (See box 1 and Outstanding Questions). Once this is achieved, technologies may be harnessed to facilitate the development of a test that can be routinely used to assess disease status in glaucoma patients.
Box 1. Clinician’s corner.
Glaucoma is characterized by loss of retinal ganglion cells (RGCs), visual field loss, and, if untreated, may result in blindness. Because vision loss in glaucoma is not reversible, therapeutic interventions early in the disease are highly desirable.
Among the verified risk factors of glaucoma, only high intraocular pressure (IOP) is modifiable. All currently available therapies for glaucoma are focused on lowering IOP as a strategy to protect RGCs from cell death.
Due to the current limitations in evaluating glaucomatous neurodegeneration, it is challenging to monitor glaucoma severity and progression objectively and design rational therapeutic strategies accordingly.
Physicians could treat glaucoma patients more appropriately if we had sensitive and specific molecular biomarker(s) of glaucoma severity, progression and response to therapy.
Outstanding Questions.
What is the underlying molecular mechanism of glaucomatous retinal ganglion cell (RGC) loss? Can we identify novel pathways --either dependent or independent-- of intraocular pressure (IOP) elevation?
Although IOP lowering is the only treatment option for glaucoma, can neuroprotective or regenerative therapies be on the horizon?
Can reliable normative databases be developed to accurately enable the latest imaging techniques (e.g. optical coherence tomography (OCT)),so as to objectively evaluate glaucomatous neurodegeneration?
Can growth differentiation factor (GDF15) in aqueous humor (AH) predict the development and progression of glaucoma in prospective, longitudinal studies?
Can we reveal any correlation between elevation of GDF15 and the quantitative retinal nerve fiber layer (RNFL) alterations seen by imaging using OCT in glaucoma?
What is the molecular function of GDF15, especially in RGCs?
Trends Box.
There is a clear need to identify objective molecular biomarker(s) to monitor glaucomatous neurodegeneration due to the subjectivity of clinical biomicroscopy, visual field testing, and current limitations of normative databases of other imaging techniques such as optical coherence tomography (OCT).
Although it is now possible to analyze various targets in aqueous humor (AH) from glaucoma patients with comprehensive multiplex protein arrays and mass spectrometry, non-targeted exhaustive analyses tend to be associative, with low specificity and sensitivity.
Growth differentiation factor 15 (GDF-15) in AH, which is elevated in mice and rats following retinal ganglion cell injury and in primary open angle glaucoma patients, might have the potential of being exploited as a molecular marker to assess the severity of glaucomatous neurodegeneration.
Acknowledgments
Washington University in St. Louis School of Medicine has filed intellectual property based on these findings, with R.S. Apte as inventor. This work was supported by NIH grants R01 EY019287 (RA), R01 EY021515 (CS), and P30EY02687 (Vision Core Grant); the Starr Foundation; the Kuzma Family, Research to Prevent Blindness (RPB) Physician Scientist Award (RSA) RPB Nelson trust Award (RSA), and an unrestricted grant from RPB to the Department of Ophthalmology, Washington University in St. Louis School of Medicine. We thank Tae Jun Lee (Washington University in St. Louis) for graphic support.
Glossary
- Aqueous humor
Transparent fluid secreted by the ciliary epithelium, filling the anterior segment of the eye
- Axonal degeneration
Degeneration or loss of axons after damage to cell bodies and/or axons of the neuron; one of the characteristic events of neurodegenerative disease.
- Biomarkers of disease
Measurable characteristics objectively measured and evaluated as indicators of pathogenic processes that can be used in screening, diagnosis, and monitoring of disease.
- Biomarkers of exposure
Measurable characteristics used in risk prediction and/or susceptibility to disease.
- Ciliary body
Composed of the ciliary muscle that controls accommodation of the lens, and the ciliary epithelium that secretes AH into the posterior chamber
- Cup
The size of the recessed area in the central region of the nerve head when viewed from the front of the eye
- Disc
The optic nerve head en face where the RGC axons converge to exit the eye to form the optic nerve.
- Cup-to-disc ratio
Ratio of recessed area in the central region of the nerve head (cup) to the optic nerve head (disc). Increased cup-to disc ratio generally reflects loss of RGC axons.
- Genome wide association studies (GWAS)
unbiased genome search method to detect small variations in genes occurring more frequently in individuals with a specific disease.
- Goldmann applanation tonometer
Instrument used to measure intraocular pressure (IOP) by providing force that indents a constant area of the cornea. This device is the most widely accepted method of IOP measurement.
- Growth differentiation factor 15 (GDF15)
Secreted protein (cytokine) belonging to the TGF-B superfamily. GDF15 has a role in regulating cell/tissue stress and apoptotic pathways.
- Intraocular pressure (IOP)
Fluid pressure inside the eye, usually measured by a tonometer.
- Lamina cribrosa
Mesh-like structure that allows nerve fibers of the optic nerve to pass through the sclera.
- Normal tension glaucoma
Type of glaucoma in which glaucomatous neurodegeneration occurs without eye pressure exceeding the normal range (not greater than 21 mmHg).
- Normative databases
A benchmark against which a given subject can be compared in order to determine its relative standing relative to a group of known healthy subjects.
- Primary open angle glaucoma (POAG)
Type of glaucoma defined by an open and normal appearing anterior chamber angle with no other underlying disease. POAG is the most common type of glaucoma.
- Retinal nerve fiber layer (RNFL) thickness
Thickness of the retinal nerve fiber layer, reflecting the number of RGC axons, and is measured in the region around the optic nerve (peripapillary) to assess glaucomatous damage.
- Retinal ganglion cells (RGCs)
Type of neuron located near the inner layer of the retina RGCs receive visual information from photoreceptor cells via bipolar cells and amacrine cells.
- Paracentesis
Procedure to sample body fluid. In ophthalmology, it refers to the procedure by which peripheral cornea are punctured by a needle to sample AH.
- Trabecular meshwork
Tissue located in the anterior chamber angle around the base of the cornea; responsible for draining AH from the eye.
- Transforming growth factor beta 2 (TGF-B2)
Secreted cytokine belonging to the TGF-B superfamily.
- Visual field testing
or perimetry; directly measures the patient’s central and peripheral vision. A light spot is repeatedly presented in different areas of the visual field and then the patient responds when the light is perceived.
- Vitreous
Transparent gelatinous substance filling the posterior space of the globe between the lens and the retina.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Resnikoff S, et al. Global data on visual impairment in the year 2002. Bulletin of the World Health Organization. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Kingman S. Glaucoma is second leading cause of blindness globally. Bulletin of the World Health Organization. 2004;82:887–888. [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, et al. Blockade of rapid axonal transport. Effect of intraocular pressure elevation in primate optic nerve. Archives of ophthalmology. 1979;97:525–531. doi: 10.1001/archopht.1979.01020010269018. [DOI] [PubMed] [Google Scholar]
- 4.Takihara Y, et al. In vivo imaging of axonal transport of mitochondria in the diseased and aged mammalian CNS. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:10515–10520. doi: 10.1073/pnas.1509879112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. American journal of ophthalmology. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 6.Musch DC, et al. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology. 2009;116:200–207. doi: 10.1016/j.ophtha.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kass MA, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Archives of ophthalmology. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- 8.Leske MC, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Garway-Heath DF, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–1304. doi: 10.1016/S0140-6736(14)62111-5. [DOI] [PubMed] [Google Scholar]
- 10.Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119:979–986. doi: 10.1016/j.ophtha.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laha B, et al. Regenerating optic pathways from the eye to the brain. Science. 2017;356:1031–1034. doi: 10.1126/science.aal5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudnicka AR, et al. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Investigative ophthalmology & visual science. 2006;47:4254–4261. doi: 10.1167/iovs.06-0299. [DOI] [PubMed] [Google Scholar]
- 13.Heijl A, et al. Natural History of Open-Angle Glaucoma. Ophthalmology. 2009;116:2271–2276. doi: 10.1016/j.ophtha.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 14.Kim KE, et al. Prevalence, Awareness, and Risk Factors of Primary Open-Angle Glaucoma: Korea National Health and Nutrition Examination Survey 2008–2011. Ophthalmology. 2016;123:532–541. doi: 10.1016/j.ophtha.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Kosoko-Lasaki O, et al. Race, ethnicity and prevalence of primary open-angle glaucoma. J Natl Med Assoc. 2006;98:1626–1629. [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon MO, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Archives of ophthalmology. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- 17.Brandt JD. Central corneal thickness, tonometry, and glaucoma risk--a guide for the perplexed. Can J Ophthalmol. 2007;42:562–566. [PubMed] [Google Scholar]
- 18.Sack J, et al. The problem of overlapping glaucoma families in the Glaucoma Inheritance Study in Tasmania (GIST) Ophthalmic genetics. 1996;17:209–214. doi: 10.3109/13816819609057895. [DOI] [PubMed] [Google Scholar]
- 19.McNaught AI, et al. Accuracy and implications of a reported family history of glaucoma: experience from the Glaucoma Inheritance Study in Tasmania. Archives of ophthalmology. 2000;118:900–904. [PubMed] [Google Scholar]
- 20.Visscher PM, et al. Five years of GWAS discovery. American journal of human genetics. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burdon KP, et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet. 2011;43:574–578. doi: 10.1038/ng.824. [DOI] [PubMed] [Google Scholar]
- 22.Gharahkhani P, et al. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nat Genet. 2014;46:1120–1125. doi: 10.1038/ng.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, et al. A common variant near TGFBR3 is associated with primary open angle glaucoma. Hum Mol Genet. 2015;24:3880–3892. doi: 10.1093/hmg/ddv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiggs JL, et al. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet. 2012;8:e1002654. doi: 10.1371/journal.pgen.1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiggs JL. Genotypes need phenotypes. Arch Ophthalmol. 2010;128:934–935. doi: 10.1001/archophthalmol.2010.108. [DOI] [PubMed] [Google Scholar]
- 26.Realini T, et al. Normative Databases for Imaging Instrumentation. Journal of glaucoma. 2015;24:480–483. doi: 10.1097/IJG.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandt JD, et al. Adjusting intraocular pressure for central corneal thickness does not improve prediction models for primary open-angle glaucoma. Ophthalmology. 2012;119:437–442. doi: 10.1016/j.ophtha.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davanger M, et al. Frequency distribution of IOP. Analysis of a material using the gamma distribution. Acta Ophthalmol (Copenh) 1991;69:561–564. doi: 10.1111/j.1755-3768.1991.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 29.Shields MB. Normal-tension glaucoma: is it different from primary open-angle glaucoma? Current opinion in ophthalmology. 2008;19:85–88. doi: 10.1097/ICU.0b013e3282f3919b. [DOI] [PubMed] [Google Scholar]
- 30.The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 31.Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. American journal of ophthalmology. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 32.Quigley HA, et al. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992;99:19–28. doi: 10.1016/s0161-6420(92)32018-4. [DOI] [PubMed] [Google Scholar]
- 33.Bowd C, et al. Evaluating the optic disc and retinal nerve fiber layer in glaucoma. I: Clinical examination and photographic methods. Seminars in ophthalmology. 2000;15:194–205. doi: 10.3109/08820530009037871. [DOI] [PubMed] [Google Scholar]
- 34.Lichter PR. Variability of expert observers in evaluating the optic disc. Transactions of the American Ophthalmological Society. 1976;74:532–572. [PMC free article] [PubMed] [Google Scholar]
- 35.Greenfield DS. Glaucomatous versus nonglaucomatous optic disc cupping: clinical differentiation. Seminars in ophthalmology. 1999;14:95–108. doi: 10.3109/08820539909056069. [DOI] [PubMed] [Google Scholar]
- 36.Gabriele ML, et al. Optical coherence tomography: history, current status, and laboratory work. Investigative ophthalmology & visual science. 2011;52:2425–2436. doi: 10.1167/iovs.10-6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatham AJ, et al. Strategies for improving early detection of glaucoma: the combined structure-function index. Clin Ophthalmol. 2014;8:611–621. doi: 10.2147/OPTH.S44586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Moraes CG, et al. Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Progress in retinal and eye research. 2017;56:107–147. doi: 10.1016/j.preteyeres.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerrigan-Baumrind LA, et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investigative ophthalmology & visual science. 2000;41:741–748. [PubMed] [Google Scholar]
- 40.Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical pharmacology and therapeutics. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 41.Galasko D. Biological markers and the treatment of Alzheimer’s disease. J Mol Neurosci. 2001;17:119–125. doi: 10.1385/JMN:17:2:119. [DOI] [PubMed] [Google Scholar]
- 42.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 43.Golubnitschaja O, Flammer J. What are the biomarkers for glaucoma? Surv Ophthalmol. 2007;52(Suppl 2):S155–161. doi: 10.1016/j.survophthal.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Shpak AA, et al. Brain-Derived Neurotrophic Factor in Patients with Primary Open-Angle Glaucoma and Age-related Cataract. Current eye research. 2017:1–8. doi: 10.1080/02713683.2017.1396617. [DOI] [PubMed] [Google Scholar]
- 45.Wong TT, et al. Proteomic profiling of inflammatory signaling molecules in the tears of patients on chronic glaucoma medication. Investigative ophthalmology & visual science. 2011;52:7385–7391. doi: 10.1167/iovs.10-6532. [DOI] [PubMed] [Google Scholar]
- 46.Gupta D, et al. Cytokine biomarkers in tear film for primary open-angle glaucoma. Clinical ophthalmology. 2017;11:411–416. doi: 10.2147/OPTH.S125364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuki K, Tsubota K. Increased urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG)/creatinine level is associated with the progression of normal-tension glaucoma. Current eye research. 2013;38:983–988. doi: 10.3109/02713683.2013.800889. [DOI] [PubMed] [Google Scholar]
- 48.Cui Y, et al. Elevated urine formaldehyde in elderly patients with primary open angle glaucoma. International journal of ophthalmology. 2016;9:411–416. doi: 10.18240/ijo.2016.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fraenkl SA, et al. Plasma citrate levels as a potential biomarker for glaucoma. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2011;27:577–580. doi: 10.1089/jop.2011.0062. [DOI] [PubMed] [Google Scholar]
- 50.Canizales L, et al. Low-level expression of SOD1 in peripheral blood samples of patients diagnosed with primary open-angle glaucoma. Biomarkers in medicine. 2016;10:1218–1223. doi: 10.2217/bmm-2016-0167. [DOI] [PubMed] [Google Scholar]
- 51.Roedl JB, et al. Homocysteine levels in aqueous humor and plasma of patients with primary open-angle glaucoma. Journal of neural transmission. 2007;114:445–450. doi: 10.1007/s00702-006-0556-9. [DOI] [PubMed] [Google Scholar]
- 52.Ghanem AA, et al. Homocysteine and hydroxyproline levels in patients with primary open-angle glaucoma. Current eye research. 2012;37:712–718. doi: 10.3109/02713683.2012.669512. [DOI] [PubMed] [Google Scholar]
- 53.Morgan WH, et al. The role of cerebrospinal fluid pressure in glaucoma pathophysiology: the dark side of the optic disc. J Glaucoma. 2008;17:408–413. doi: 10.1097/IJG.0b013e31815c5f7c. [DOI] [PubMed] [Google Scholar]
- 54.Walsh MM, et al. Gene and protein expression pilot profiling and biomarkers in an experimental mouse model of hypertensive glaucoma. Experimental biology and medicine. 2009;234:918–930. doi: 10.3181/0811-RM-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maguire JI. Postoperative endophthalmitis: optimal management and the role and timing of vitrectomy surgery. Eye. 2008;22:1290–1300. doi: 10.1038/eye.2008.51. [DOI] [PubMed] [Google Scholar]
- 56.Ghodasra DH, et al. Safety and Feasibility of Quantitative Multiplexed Cytokine Analysis From Office-Based Vitreous Aspiration. Investigative ophthalmology & visual science. 2016;57:3017–3023. doi: 10.1167/iovs.15-18721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aiello LP, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. The New England journal of medicine. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 58.Funatsu H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2005;243:3–8. doi: 10.1007/s00417-004-0950-7. [DOI] [PubMed] [Google Scholar]
- 59.Funatsu H, et al. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. American journal of ophthalmology. 2002;133:70–77. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 60.Takai Y, et al. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Investigative ophthalmology & visual science. 2012;53:241–247. doi: 10.1167/iovs.11-8434. [DOI] [PubMed] [Google Scholar]
- 61.Kokubun T, et al. Characteristic Profiles of Inflammatory Cytokines in the Aqueous Humor of Glaucomatous Eyes. Ocular immunology and inflammation. 2017:1–12. doi: 10.1080/09273948.2017.1327605. [DOI] [PubMed] [Google Scholar]
- 62.Kuchtey J, et al. Multiplex cytokine analysis reveals elevated concentration of interleukin-8 in glaucomatous aqueous humor. Investigative ophthalmology & visual science. 2010;51:6441–6447. doi: 10.1167/iovs.10-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sawada H, et al. Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma. Investigative ophthalmology & visual science. 2010;51:903–906. doi: 10.1167/iovs.09-4247. [DOI] [PubMed] [Google Scholar]
- 64.Balaiya S, et al. Tumor necrosis factor-alpha (TNF-alpha) levels in aqueous humor of primary open angle glaucoma. Clinical ophthalmology. 2011;5:553–556. doi: 10.2147/OPTH.S19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang ZY, et al. Erythropoietin is increased in aqueous humor of glaucomatous eyes. Current eye research. 2010;35:680–684. doi: 10.3109/02713681003778780. [DOI] [PubMed] [Google Scholar]
- 66.Mokbel TH, et al. Erythropoietin and soluble CD44 levels in patients with primary open-angle glaucoma. Clinical & experimental ophthalmology. 2010;38:560–565. doi: 10.1111/j.1442-9071.2010.02318.x. [DOI] [PubMed] [Google Scholar]
- 67.Nassiri N, et al. Erythropoietin levels in aqueous humor of patients with glaucoma. Molecular vision. 2012;18:1991–1995. [PMC free article] [PubMed] [Google Scholar]
- 68.Choritz L, et al. Correlation of endothelin-1 concentration in aqueous humor with intraocular pressure in primary open angle and pseudoexfoliation glaucoma. Investigative ophthalmology & visual science. 2012;53:7336–7342. doi: 10.1167/iovs.12-10216. [DOI] [PubMed] [Google Scholar]
- 69.Iwabe S, et al. Aqueous humor endothelin-1 (Et-1), vascular endothelial growth factor (VEGF) and cyclooxygenase-2 (COX-2) levels in Mexican glaucomatous patients. Current eye research. 2010;35:287–294. doi: 10.3109/02713680903545315. [DOI] [PubMed] [Google Scholar]
- 70.Tripathi RC, et al. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Experimental eye research. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 71.Inatani M, et al. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2001;239:109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- 72.Picht G, et al. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2001;239:199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- 73.Ochiai Y, Ochiai H. Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Japanese journal of ophthalmology. 2002;46:249–253. doi: 10.1016/s0021-5155(01)00523-8. [DOI] [PubMed] [Google Scholar]
- 74.Ozcan AA, et al. The aqueous levels of TGF-beta2 in patients with glaucoma. International ophthalmology. 2004;25:19–22. doi: 10.1023/b:inte.0000018524.48581.79. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto N, et al. Concentration of transforming growth factor beta2 in aqueous humor. Ophthalmic research. 2005;37:29–33. doi: 10.1159/000083019. [DOI] [PubMed] [Google Scholar]
- 76.Min SH, et al. Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean journal of ophthalmology: KJO. 2006;20:162–165. doi: 10.3341/kjo.2006.20.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aketa N, et al. Iris Damage Is Associated With Elevated Cytokine Levels in Aqueous Humor. Investigative ophthalmology & visual science. 2017;58:BIO42–BIO51. doi: 10.1167/iovs.17-21421. [DOI] [PubMed] [Google Scholar]
- 78.Aketa N, et al. Elevated aqueous cytokine levels in eyes with ocular surface diseases. American journal of ophthalmology. 2017 doi: 10.1016/j.ajo.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 79.Helbig H, et al. Mammalian ciliary-body epithelial cells in culture produce transforming growth factor-beta. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1991;229:84–87. doi: 10.1007/BF00172268. [DOI] [PubMed] [Google Scholar]
- 80.Gordon-Thomson C, et al. Differential cataractogenic potency of TGF-beta1, -beta2, and -beta3 and their expression in the postnatal rat eye. Investigative ophthalmology & visual science. 1998;39:1399–1409. [PubMed] [Google Scholar]
- 81.Ban N, et al. GDF15 is elevated in mice following retinal ganglion cell death and in glaucoma patients. JCI insight. 2017:2. doi: 10.1172/jci.insight.91455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fuchshofer R, Tamm ER. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]