Abstract
Provider interactions other than explicit care coordination, which is challenging to measure, may influence practice and outcomes. We performed a network analysis using claims data from a commercial payor. Networks were identified based on provider pairs billing outpatient care for the same patient. We compared network variables among patients who had and did not have a 30-day readmission after hospitalization for heart failure. After adjusting for comorbidities, high median provider connectedness—normalized degree, which for each provider is the number of connections to other providers normalized to the number of providers in the region—was the network variable associated with reduced odds of readmission after heart failure hospitalization (OR: 0.55; 95% CI: 0.35 – 0.86). We conclude that heart failure patients with high provider connectedness are less likely to require readmission. The structure and importance of provider relationships using claims data merits further study.
Keywords: patient care constellation, patient readmission, practice patterns, physicians’, heart failure
INTRODUCTION
Unplanned readmissions after hospital discharge are commonly treated as indicators of possible lapses in care delivery quality and failure to adhere to best practices (Holt et al., 2015; Psotka & Teerlink, 2013). Though hospital readmission is often used as a quality measure for hospitals (Krumholz et al., 2011; Krumholz et al., 2013; Krumholz et al., 2009), the quality of outpatient care also influences risk of readmission (Martineau, Frenette, Blais, & Sauve, 2004; Maru et al., 2015; O'Connell, Crawford, & Abrams, 2001). While demographic, clinical, and sociological variables have been used to model risk factors for re-hospitalization (Barnett, Hsu, & McWilliams, 2015; Cleland et al., 2014), few studies consider provider characteristics (Betihavas, Newton, Frost, MacDonald, & Davidson, 2013). Specifically, the care provided by any one particular provider tends to be influenced by the practice of other providers caring for their shared patients (Barnett et al., 2012; Landon et al., 2012; Pollack, Weissman, Lemke, Hussey, & Weiner, 2013). Various mechanisms may account for such influence, including the impact of the care other physicians provide, coordination of care, or dissemination of knowledge and practice norms through the physician and shared patient network. Of note, these effects on outcome may occur regardless of whether providers recognize relationships among themselves through their shared patients.
Emerging methods define a role for network analysis in characterizing the influence of provider constellations on care processes and outcomes (Barnett et al., 2012; Effken, Gephart, Brewer, & Carley, 2013; Geva et al., 2011; Gray et al., 2010; Ong et al., 2015; Pollack et al., 2013; Weenink, van Lieshout, Jung, & Wensing, 2011). “Constellation” refers to the subset of a regional network consisting of providers who submitted claims for an individual patient (Mandl, Olson, Mines, Liu, & Tian, 2014). Recreating the complete network of a patient’s outpatient providers is challenging. Payor claims data, which yield a view of a patient’s interactions with providers across care settings, can be used to characterize networks of outpatient providers and, specifically, the constellation of outpatient providers caring for each patient (Barnett, Landon, O'Malley, Keating, & Christakis, 2011; Landon et al., 2013; Mandl et al., 2014).
New Contributions
In contrast to many studies of physician networks based on Medicare data (Barnett et al., 2012; Casalino et al., 2015; Pollack, Weissman, Bekelman, Liao, & Armstrong, 2012), we used claims from a commercial health plan in order to capture the physician networks of younger patients. Although heart failure is typically a disease of older patients, prior studies of Medicare patients fail to account for provider connections through non-Medicare patients. Additionally, we characterized provider constellations through shared patients without regard to recognized patient-provider relationships, as we hypothesized that provider interactions inferred from large data networks would have a measurable impact on patient outcomes even without explicit measurement of care coordination per se. As a model of this effect, we explored how the connectedness of providers affects the risk of 30-day readmission for patients discharged after admission for heart failure.
METHODS
Data
This was a retrospective study of payor claims in the HealthCore Integrated Research Database (HIRD™) (HealthCore, 2015), which contains longitudinal data from 14 states. Claims from four regions in the United States with high plan penetrance were used in the analysis. Further details of the region and network characteristics have been published previously (Mandl et al., 2014). Briefly, there were a total of 10,325 providers with at least 50 patients in the four regions. In all regions, more than 99% of a provider’s patients were within that provider’s primary region, and provider pairs shared on average 2 patients. HIRD plans had high plan penetrance in each study region, representing at least 30% of the local population. An independent institutional review board used by HealthCore approved the study, and the study was designated as exempt by the Boston Children’s Hospital institutional review board.
Subjects
Subjects were 381,758 patients enrolled for any length of time between April 1, 2008 and September 31, 2011. Patients were excluded if they did not have an encounter with at least one outpatient provider during the study period. We also excluded patients who were at least 65 years old at time of entry to the HIRD since these patients were likely to have had additional claims billed to Medicare and thus provider associations not captured by the database. From the remaining 297,019 patients, we identified patients with an admission for heart failure when one of the following International Classification of Diseases, Ninth Revision (ICD-9) codes was billed as the primary diagnosis for an inpatient admission: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 415.0, 425.4, 425.5, 425.7, 428.x, or 674.5x. Patients were excluded from analysis if follow-up was not available in the claims dataset for at least 30 days after discharge (based on time from discharge until enrollment end dates recorded in HIRD) and there was no claims evidence of readmission within that period. Claims for these patients still contributed to the provider network described below.
Network Construction
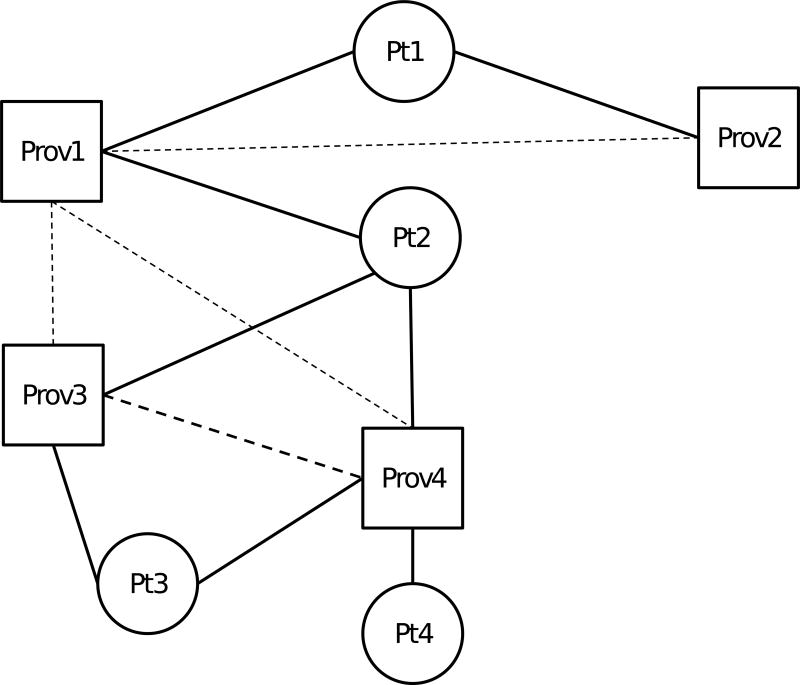
Networks linking patients to providers were created based on outpatient encounters billed by a provider for a given patient. A link between a patient and provider was inferred when a patient had at least one outpatient visit with a provider. Links between patients and providers were analyzed as either present or absent, regardless of the number of claims billed by a provider for a patient. A patient’s care constellation consisted of all providers who billed for face-to-face encounters with a patient during the study period. A provider-provider relationship was inferred if a pair of providers billed outpatient care for the same patient (Figure 1); this represents a one-mode projection of a bipartite network of patients and providers (Borgatti & Everett, 1997). We expressly included all patients’ providers rather than focusing on a select provider type, as heart failure affects a patient’s general health and may influence and be influenced by a broad range of healthcare services. In addition, consistent with the Agency for Healthcare Research and Quality measure, our outcome of interest was all-cause readmission rather than heart failure-specific readmission (National Quality Measures Clearinghouse, 2015), increasing the relevance of a broad array of providers.
Figure 1. Schematic of network construction.
Squares represent providers (Prov1 – Prov4) and circles represent patients (Pt1 – Pt4). Solid lines represent a patient-provider relationship based on billing for an outpatient visit. Dashed lines represent provider-provider relationships inferred by both providers having billed outpatient care for a pair of patients, with heavier lines (between Prov3 and Prov4) representing more such shared patients. Note, as an example, that for Prov1, provider connectedness is 100%, as s/he is connected to all other providers in the diagram, whereas for Prov4, provider connectedness is 66.67%, as s/he is connected to 2 of 3 other providers. The median provider connectedness for Pt2 is then 66.67%, the median of the provider connectedness for Prov1, Prov3, and Prov4, the providers in Pt2’s constellation.
Network measures were constructed for both patient and provider perspectives (Box 1). We examined a variety of network constructs to explore the strength and breadth of provider-provider and patient-provider relationships. In particular, we examined a network metric, normalized degree, with a novel application to healthcare, that we refer to as provider connectedness. In a general network, degree is the number of connections from a given entity to others in the network. For a given provider, provider connectedness is the number of connections from the provider to other providers in the region, divided by the total number of other providers (excluding the index provider) in that region, and expressed as a percent. Normalizing degree to the number of other providers in the region thus accounts for the baseline likelihood of more connections in regions with more providers, in which more potential connections are possible. We used summary measures across each care constellation to analyze how provider network measures affect individual patient outcomes. For example, median provider connectedness is the median provider connectedness among all the providers in a patient’s constellation (Figure 1).
Box 1. Definitions of network variables.
Provider measures
Maximum number of patients shared by provider pairs
Size of providers’ patient panels; a patient panel is the set of patients for whom a provider billed outpatient care.
Percent of patients in provider pairs’ combined panels that is shared
Provider degree = number of connections from each provider to other providers in region
Provider connectedness = normalized degree = the number of connections from a provider to other providers in the region divided by the number of other providers (excluding the index provider) in the region, expressed as a percent
Patient measures
Constellation = subset of a regional network consisting of providers who submitted claims for an individual patient
Care density = average number of shared patients by the provider pairs in a patient’s constellation
Presence of a primary care provider or cardiologist in patient’s constellation
Number of providers in patient’s constellation
From a patient perspective, variables describing the care constellation included the total number of providers in the constellation and care density, defined as the average number of patients shared by the provider pairs in each patient’s constellation (Pollack et al., 2013). Care density is a measure of the strength of network ties among a patient’s providers, since care density will increase when those providers have more shared patients. We also examined whether a primary care provider (defined as providers in family practice, general practice, internal medicine, or pediatric medicine) or cardiologist was part of the patient’s care constellation.
Statistical Analysis
The outcome of interest was readmission for any diagnosis up to 30 days after hospital discharge among patients hospitalized for heart failure. Only the first readmission was analyzed for each patient. Readmission was identified based on a claim for an inpatient hospitalization in the HIRD, regardless of whether the patient was admitted from the emergency department, outpatient clinic, or other site, if the date of admission for an inpatient claim was no more than 30 days after the date of discharge associated with an inpatient claim for heart failure, as defined based on the ICD-9 codes above. Preliminary analyses used t-tests for continuous variables and chi-square tests for categorical variables to examine relations between individual variables and the dichotomous readmission outcome variable. Specific comorbidities known to increase the risk of readmission for heart failure—chronic obstructive pulmonary disease (COPD), chronic kidney disease, diabetes, and hypertension—were identified based on HIRD data and treated as binomial variables (Cleland et al., 2014). Charlson comorbidity index (CCI) (Charlson, Pompei, Ales, & MacKenzie, 1987) was treated as a categorical variable. Median household income in the patient’s ZIP code was used as a proxy for socioeconomic status. Because the distributions of most continuous covariates were highly non-normal, these variables were dichotomized by choosing optimal cut points that maximized areas under the receiver operating characteristic (ROC) curves of separate univariate logistic regression models fitted with each variable. Variables with p-values less than 0.2 in univariate analysis were considered in the multivariate model. For the final multivariate analysis, forward stepwise logistic regression was used to identify independent predictors of 30-day readmission. A p-value < 0.05 was considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
To assess the robustness of the primary model, we used propensity score matching to balance covariates that may confound the relationship between high median provider connectedness and 30-day readmission. A logistic regression model was created to estimate the propensity (probability) of a subject having a high median provider connectedness (greater than 27.5 based on the ROC analysis) conditional on the following covariates: comorbid disorders (COPD, chronic kidney disease, diabetes, and hypertension), age, gender, CCI, median household income, and having a cardiologist, primary care provider, or rehabilitation specialist in the subject’s care constellation. Subjects with a high median provider connectedness were then matched 1:1 to subjects with a low median provider connectedness having the closest propensity score. Forward stepwise logistic regression was then repeated as above using the matched subset of patients (N = 582). This analysis was performed in R version 3.3.2 using the MatchIt package (Ho, Imai, King, & Stuart).
RESULTS
We identified 1,429 patients meeting inclusion criteria who had at least one admission for heart failure, of whom 82 (5.7%) were excluded for having less than 30 days of follow-up in the claims dataset (Figure 2). Of the remaining 1,347 patients, 333 (25%) were readmitted at least once within 30 days of discharge. As shown in Table 1, patients readmitted after discharge for heart failure were more likely to be older (54.9 y ± 9.6 y v. 53.1 y ± 10.3 y, p = 0.01) and to live in a ZIP code with lower median household income ($54,600 ± $19,800 v. $58,200 ± $58,200, p = 0.01). They also had more comorbidities, with a higher CCI (median 6 (interquartile range (IQR) 4 – 8) v. 3 (IQR 2 – 6), p < 0.001) and more distinct ICD-9 codes (110 ± 56 v. 67 ± 41), p < 0.001). Care constellations of readmitted patients included more provider ties (36 ± 50 v. 28 ± 41, p = 0.01), but having a PCP (91% v. 92% of patients, p = 0.68) or cardiologist (74% v. 74%, p > 0.99) in the constellation was not associated with risk of readmission.
Figure 2. Flow diagram of patients included and excluded from the study.
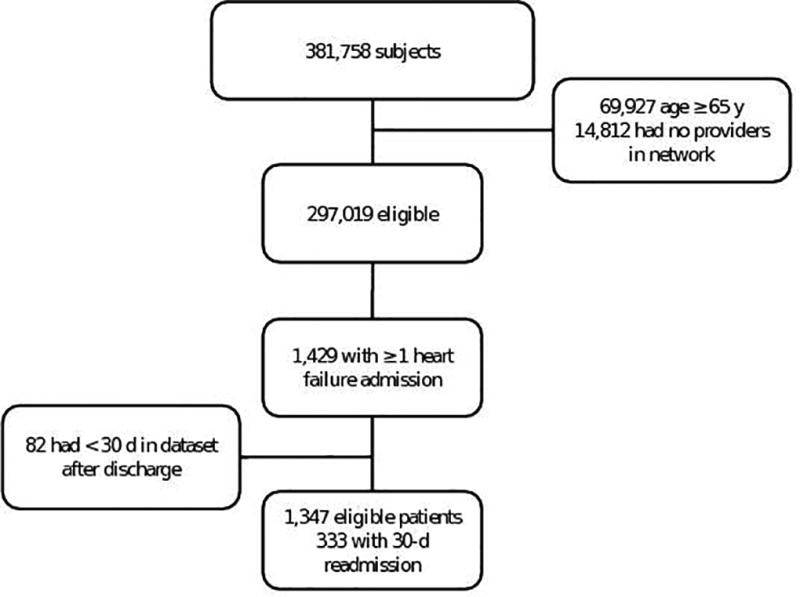
Table 1.
Cohort characteristics and univariate analysis.
| Variable | 30-d readmission (N = 333) |
No 30-d readmission (N = 1,014) |
p-value | |
|---|---|---|---|---|
| Age in years, mean (sd) | 54.9 (9.6) | 53.1 (10.3) | 0.01 | |
|
| ||||
| Female gender, n (%) | 135 (41) | 401 (40) | 0.75 | |
|
| ||||
| Median household income in $1000, mean (sd) | 54.6 (19.8) | 58.2 (21.8) | 0.01 | |
|
| ||||
| Comorbidity index, n (%) | <0.001 | |||
| 0 | 0 (0) | 7 (0.69) | ||
| 1 | 13 (3.9) | 154 (15) | ||
| 2 | 35 (11) | 171 (17) | ||
| 3 | 30 (9.0) | 187 (18) | ||
| 4 | 34 (10) | 142 (14) | ||
| >4 | 221 (66) | 353 (35) | ||
|
| ||||
| Number of distinct diagnostic codes, mean (sd) | 110 (56.3) | 66.8 (40.6) | <0.001 | |
|
| ||||
| PCP in care constellation, n (%) | 303 (91) | 927 (92) | 0.68 | |
|
| ||||
| Cardiologist in care constellation, n (%) | 245 (74) | 746 (74) | >0.99 | |
|
| ||||
| Chronic kidney disease, n (%) | 167 (50) | 270 (27) | <0.001 | |
|
| ||||
| Diabetes, n (%) | 189 (57) | 410 (40) | <0.001 | |
|
| ||||
| Hypertension, n (%) | 303 (91) | 866 (85) | 0.01 | |
|
| ||||
| COPD, n (%) | 105 (32) | 159 (16) | <0.001 | |
|
| ||||
| Number of ties in provider constellation, mean (sd) | 35.9 (50.2) | 27.9 (40.7) | 0.01 | |
|
| ||||
| Care density, mean (sd) | 11.5 (27.4) | 13.1 (24.1) | 0.37 | |
|
| ||||
| Maximum number of shared patients, mean (sd)* | 75.4 (109) | 78.0 (123) | 0.72 | |
|
| ||||
| Mean percent of providers’ patients that are shared, mean (sd)* | 2.3 (3.0) | 2.6 (3.9) | 0.18 | |
|
| ||||
| Mean number of patients in providers’ panels, mean (sd)* | 294 (147) | 298 (153) | 0.71 | |
|
| ||||
| Median provider degree, mean (sd)* | 558 (202) | 548 (201) | 0.49 | |
|
| ||||
| Median provider connectedness, mean (sd)* | 18.3 (9.7) | 19.2 (10.9) | 0.18 | |
Summary measures among providers within a care constellation, reported as mean (sd) among patients in each group.
sd = standard deviation
COPD = chronic obstructive pulmonary disease
PCP = primary care provider
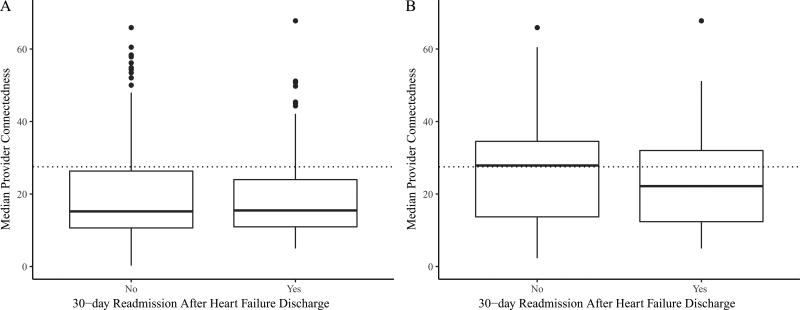
In multivariate logistic regression analysis, a higher median provider connectedness was independently associated with reduced 30-day readmission after hospitalization for heart failure (OR = 0.69, 95% CI 0.49 – 0.98, p = 0.04). However, as shown in Figure 3A, readmitted and not readmitted patients differed only in the high tail of median provider connectedness. In the propensity-matched model, readmitted patients had a lower median provider connectedness (23 ± 12 v. 26 ± 12, p = 0.02) than patients who were not readmitted within 30 days (Figure 3B). The multivariatee model identified similar associated variables as the model based on the full data (Table 2), and the point estimate for median provider connectedness was also similar (OR = 0.55, 95% CI 0.35 – 0.86, p = 0.008). Other network variables, including care density, were not associated with 30-day readmission in this cohort. Median household income in the beneficiary’s ZIP code was not associated with readmission when other covariates were introduced, and thus not included in the final model.
Figure 3. Distribution of median provider connectedness.
Propensity score matching adjusts for variables that may explain patients’ tendency to have a high median provider connectedness. These box plots demonstrate that only the tails of the distributions in the unmatched data (A) fall above the median provider connectedness cutoff of 27.5 (dotted line) used to dichotomize the predictor. After matching using propensity scores, patients who were readmitted had a lower median provider connectedness than those who were not (B).
Table 2.
Multivariate analysis.
| Unmatched Data | Propensity-Matched Data | |||
|---|---|---|---|---|
| Variable | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value |
| Charlson comorbidity index > 4 | 1.59 (1.16 – 2.18) | 0.004 | -- | -- |
|
| ||||
| Age > 51 y | -- | -- | 1.68 (0.97 – 3.03) | 0.07 |
|
| ||||
| Number of distinct diagnostic codes | 1.01 (1.01 – 1.02) | <0.001 | 1.02 (1.01 – 1.02) | <0.001 |
|
| ||||
| COPD | 1.49 (1.08 – 2.05) | 0.02 | 1.95 (1.12 – 3.36) | 0.02 |
|
| ||||
| Median provider connectedness > 27.5 | 0.69 (0.49 – 0.98) | 0.04 | 0.55 (0.35 – 0.86) | 0.008 |
DISCUSSION
This study adds to a growing body of literature demonstrating the impact of provider constellations on patient outcomes (Effken et al., 2013; Geva et al., 2011; Gray et al., 2010; Ong et al., 2015; Weenink et al., 2011). Measuring care coordination at the patient or the population level remains challenging, and the relationship between outpatient provider networks and quality of inpatient care is complex (Bynum & Ross, 2013). Our results highlight the influence of provider constellations on 30-day readmission for patients with heart failure. In particular, our results suggest that the constellations of providers involved in patients’ care may affect outcomes regardless of whether providers in the constellations recognize relationships among themselves. We found that higher care density, which has been suggested to reflect provider relationships and has previously although not consistently been associated with improved outcomes (Pollack, Lemke, Roberts, & Weiner, 2015; Pollack et al., 2012; Pollack et al., 2013), was not associated with 30-day readmission for heart failure. Instead, a higher provider connectedness, which reflects the normalized number of regional providers with whom a provider shares at least one patient, was associated with lower odds of 30-day readmission.
This analysis does not explain the mechanism by which provider connectedness protects against 30-day readmission, but supports its positive influence on patient outcomes. Possible mechanisms may include greater engagement with or awareness of the practice of other providers, which might improve care quality. Provider connectedness, a normalized measure of regional connections, was more predictive of the outcome than the raw number of connections, perhaps because indexing to the number of possible connections better reflects engagement with the medical community as opposed to patterns of patient care-seeking alone. Other network measures, including the number of patients providers billed for and the number of shared patients between provider pairs, were not associated with readmission. Provider connectedness remained associated with 30-day readmission even in a propensity-matched model that accounted for available clinical and demographic variables that could confound the relationship between provider connectedness and readmission. To our knowledge, this is the first study linking the normalized degree of providers to patient outcomes.
In contrast to our prior study, in which increased care density was associated with lower risk of overlapping benzodiazepine prescriptions (Ong et al., 2015), here, care density was not significantly associated with 30-day readmission. While the mechanism by which coordination of outpatient providers would prevent duplicate benzodiazepine prescriptions is relatively direct and apparent, the mechanism by which a coordinated constellation of outpatient providers prevents short-term hospital readmission is perhaps more multifaceted. Patient-level factors not assessed in this study may also differentially influence the relationship between provider networks and outcomes. Our results are similar to those of Pollack et al. (2015), who found that higher care density was associated with decreased readmission rates for diabetes but not for heart failure or for COPD, perhaps reflecting differences in the influence of outpatient care for different diseases. The impact of care density may also be affected by the structure of the local delivery system, with higher care density potentially reflective of unmeasured confounders independently associated with higher risk of worse outcomes (Bynum & Ross, 2013). We therefore specifically designed this study to explore the impact on patient outcomes of different network constructs, including ones such as provider connectedness that do not presuppose an explicitly recognized relationship between providers based on shared patients.
As a retrospective study of claims data, this study has several limitations. We included all provider relationships across the study period in creating the network because recreating the network and recalculating network statistics at each outcome time point was computationally prohibitive, and because the apparent network at any single point in time is not necessarily representative of the overall sum of provider linkage over time. As such, while some connections may have formed after the occurrence of the outcome, other connections may have pre-dated the study period. We attempted to minimize the impact of this limitation by analyzing a relatively short 4-year period of provider-patient connections, a time over which significant linkage patterns are more likely to be stable. The limited information in claims data meant that we could not control for all clinical variables. In particular, inpatient variables that may be associated with risk of readmission after discharge for heart failure patients could not be assessed. As a proxy for severity of underlying illness and underlying risk of readmission, we included the CCI in our final multivariate model. While other methods to control for comorbidity burden have been used (for example, Elixhauser, Steiner, Harris, & Coffey, 1998), and although CCI was originally designed to predict the risk of mortality in longitudinal studies (Charlson et al., 1987), CCI has been found to be predictive of 30-day hospital readmission in a large cohort of Medicare beneficiaries (Dattalo et al., 2017) and has been used to control for severity of illness in a wide range of studies of other outcomes (Baris, Onyilmaz, Basyigit, Boyaci, & Yildiz, 2017; Bischoff-Ferrari et al., 2017; Hung et al., 2017; Josephson et al., 2017; Melzer & Welch, 2017). Since our data include only claims from a single commercial health plan, we likely have patients shared with other insurers for whom claims were not available. We minimized the risk of incomplete data by limiting our analysis to regions with high market penetration by the study health plan. We note that studies of provider networks based on Medicare claims share this limitation, as provider connections through non-Medicare patients would be missed. Such limitations might be overcome in future analyses of All Payers Claims Databases. Some providers practicing in more than one location may have different provider identification for billing purposes at different sites. We did not have data on clinics with which providers were affiliated to assess the potential confounding of group practices on shared patients and outcomes. Finally, some of the provider relationships identified through billing for shared patients may not indicate meaningful exchange of information between those provider pairs. While others have demonstrated that number of shared patients between providers is associated with increased chance of a recognized relationship between them (Barnett et al., 2011), the optimal threshold for determining such a relationship likely varies by provider and specialty (Landon et al., 2013).
CONCLUSIONS
Patients whose providers have high connectedness are less likely to require readmission after hospitalization for heart failure. Future research should focus on associating network metrics of connectedness with directly measured aspects of team-based care and on relating network metrics to patient outcomes.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health through the National Institute of General Medical Sciences [grant number R21GM107645]; and the Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant number T32HD040128].
Footnotes
DECLARATION OF CONFLICTING INTEREST
The authors declare that there is no conflict of interest.
References
- Baris SA, Onyilmaz T, Basyigit I, Boyaci H, Yildiz F. Frequency of exacerbations and hospitalizations in copd patients who continue to smoke. Acta Medica Okayama. 2017;71(1):11–17. doi: 10.18926/AMO/54820. [DOI] [PubMed] [Google Scholar]
- Barnett ML, Christakis NA, O'Malley J, Onnela JP, Keating NL, Landon BE. Physician patient-sharing networks and the cost and intensity of care in US hospitals. Medical Care. 2012;50(2):152–160. doi: 10.1097/MLR.0b013e31822dcef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803–1812. doi: 10.1001/jamainternmed.2015.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett ML, Landon BE, O'Malley AJ, Keating NL, Christakis NA. Mapping physician networks with self-reported and administrative data. Health Services Research. 2011;46(5):1592–1609. doi: 10.1111/j.1475-6773.2011.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betihavas V, Newton PJ, Frost SA, MacDonald PS, Davidson PM. Patient, provider and system factors influencing rehospitalisation in adults with heart failure. Contemporary Nurse. 2013;43(2):244–256. doi: 10.5172/conu.2013.43.2.244. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Fischer K, Orav EJ, Dawson-Hughes B, Meyer U, Chocano-Bedoya PO, Wilson NM. Statin use and 25-Hydroxyvitamin D blood level response to Vitamin D treatment of older adults. Journal of the American Geriatrics Society. 2017 doi: 10.1111/jgs.14784. [DOI] [PubMed] [Google Scholar]
- Borgatti SP, Everett MG. Network analysis of 2-mode data. Social Networks. 1997;19(3):243–269. doi: 10.1016/S0378-8733(96)00301-2. [DOI] [Google Scholar]
- Bynum JP, Ross JS. A measure of care coordination? Journal of General Internal Medicine. 2013;28(3):336–338. doi: 10.1007/s11606-012-2269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino LP, Pesko MF, Ryan AM, Nyweide DJ, Iwashyna TJ, Sun X, Moody J. Physician networks and ambulatory care-sensitive admissions. Medical Care. 2015;53(6):534–541. doi: 10.1097/MLR.0000000000000365. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Chiswell K, Teerlink JR, Stevens S, Fiuzat M, Givertz MM, O'Connor CM. Predictors of postdischarge outcomes from information acquired shortly after admission for acute heart failure: A report from the placebo-controlled randomized study of the selective a1 adenosine receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function (PROTECT) study. Circulation: Heart Failure. 2014;7(1):76–87. doi: 10.1161/circheartfailure.113.000284. [DOI] [PubMed] [Google Scholar]
- Dattalo M, DuGoff E, Ronk K, Kennelty K, Gilmore-Bykovskyi A, Kind AJ. Apples and oranges: Four definitions of multiple chronic conditions and their relationship to 30-day hospital readmission. Journal of the American Geriatrics Society. 2017 doi: 10.1111/jgs.14539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effken JA, Gephart SM, Brewer BB, Carley KM. Using *ORA, a network analysis tool, to assess the relationship of handoffs to quality and safety outcomes. Computers, Informatics, Nursing. 2013;31(1):36–44. doi: 10.1097/NXN.0b013e3182701082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Geva A, Wright SB, Baldini LM, Smallcomb JA, Safran C, Gray JE. Spread of methicillin-resistant Staphylococcus aureus in a large tertiary nicu: Network analysis. Pediatrics. 2011;128(5):e1173–1180. doi: 10.1542/peds.2010-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JE, Davis DA, Pursley DM, Smallcomb JE, Geva A, Chawla NV. Network analysis of team structure in the neonatal intensive care unit. Pediatrics. 2010;125(6):e1460–1467. doi: 10.1542/peds.2009-2621. [DOI] [PubMed] [Google Scholar]
- HealthCore. Research environment - HealthCore. 2015 Retrieved from http://healthcore.com/research-environment.
- Ho DE, Imai K, King G, Stuart EA. Matchit : Nonparametric preprocessing for parametric causal inference. Journal of Statistical Software. 2011;42(8):1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- Holt JB, Huston SL, Heidari K, Schwartz R, Gollmar CW, Tran A, Croft JB. Indicators for chronic disease surveillance - United States, 2013. MMWR: Recommendations and Reports. 2015;64(Rr-01):1–246. [PubMed] [Google Scholar]
- Hung C-H, Wang C-J, Tang T-C, Chen L-Y, Peng L-N, Hsiao F-Y, Chen L-K. Recurrent falls and its risk factors among older men living in the veterans retirement communities: A cross-sectional study. Archives of Gerontology and Geriatrics. 2017;70:214–218. doi: 10.1016/j.archger.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Josephson CB, Lowerison M, Vallerand I, Sajobi TT, Patten S, Jette N, Wiebe S. Association of depression and treated depression with epilepsy and seizure outcomes. JAMA Neurology. 2017 doi: 10.1001/jamaneurol.2016.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz HM, Lin Z, Drye EE, Desai MM, Han LF, Rapp MT, Normand SL. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circulation: Cardiovascular Quality and Outcomes. 2011;4(2):243–252. doi: 10.1161/circoutcomes.110.957498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz HM, Lin Z, Keenan PS, Chen J, Ross JS, Drye EE, Normand SL. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593. doi: 10.1001/jama.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Drye EE. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circulation: Cardiovascular Quality and Outcomes. 2009;2(5):407–413. doi: 10.1161/circoutcomes.109.883256. [DOI] [PubMed] [Google Scholar]
- Landon BE, Keating NL, Barnett ML, Onnela JP, Paul S, O'Malley AJ, Christakis NA. Variation in patient-sharing networks of physicians across the United States. JAMA. 2012;308(3):265–273. doi: 10.1001/jama.2012.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon BE, Onnela JP, Keating NL, Barnett ML, Paul S, O'Malley AJ, Christakis NA. Using administrative data to identify naturally occurring networks of physicians. Medical Care. 2013;51(8):715–721. doi: 10.1097/MLR.0b013e3182977991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl KD, Olson KL, Mines D, Liu C, Tian F. Provider collaboration: Cohesion, constellations, and shared patients. Journal of General Internal Medicine. 2014;29(11):1499–1505. doi: 10.1007/s11606-014-2964-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau P, Frenette M, Blais L, Sauve C. Multidisciplinary outpatient congestive heart failure clinic: Impact on hospital admissions and emergency room visits. Canadian Journal of Cardiology. 2004;20(12):1205–1211. [PubMed] [Google Scholar]
- Maru S, Byrnes J, Carrington MJ, Chan YK, Thompson DR, Stewart S, Scuffham PA. Cost-effectiveness of home versus clinic-based management of chronic heart failure: Extended follow-up of a pragmatic, multicentre randomized trial cohort - The WHICH? study (Which Heart Failure Intervention Is Most Cost-Effective & Consumer Friendly in Reducing Hospital Care) International Journal of Cardiology. 2015;201:368–375. doi: 10.1016/j.ijcard.2015.08.066. [DOI] [PubMed] [Google Scholar]
- Melzer M, Welch C. Does the presence of a urinary catheter predict severe sepsis in a bacteraemic cohort? The Journal of Hospital Infection. 2017 doi: 10.1016/j.jhin.2017.01.003. [DOI] [PubMed] [Google Scholar]
- National Quality Measures Clearinghouse. Heart failure (HF): Hospital 30-day, all-cause, unplanned risk-standardized readmission rate (RSRR) following HF hospitalization. 2015 Retrieved from https://www.qualitymeasures.ahrq.gov/summaries/summary/49196.
- O'Connell AM, Crawford MH, Abrams J. Heart failure disease management in an indigent population. American Heart Journal. 2001;141(2):254–258. doi: 10.1067/mhj.2001.111956. [DOI] [PubMed] [Google Scholar]
- Ong MS, Olson KL, Cami A, Liu C, Tian F, Selvam N, Mandl KD. Provider patient-sharing networks and multiple-provider prescribing of benzodiazepines. Journal of General Internal Medicine. 2015;31(2):164–171. doi: 10.1007/s11606-015-3470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack CE, Lemke KW, Roberts E, Weiner JP. Patient sharing and quality of care: Measuring outcomes of care coordination using claims data. Medical Care. 2015;53(4):317–323. doi: 10.1097/MLR.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack CE, Weissman G, Bekelman J, Liao K, Armstrong K. Physician social networks and variation in prostate cancer treatment in three cities. Health Services Research. 2012;47(1 Pt 2):380–403. doi: 10.1111/j.1475-6773.2011.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack CE, Weissman GE, Lemke KW, Hussey PS, Weiner JP. Patient sharing among physicians and costs of care: A network analytic approach to care coordination using claims data. Journal of General Internal Medicine. 2013;28(3):459–465. doi: 10.1007/s11606-012-2104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psotka MA, Teerlink JR. Strategies to prevent postdischarge adverse events among hospitalized patients with heart failure. Heart Failure Clinics. 2013;9(3):303–320. vi. doi: 10.1016/j.hfc.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Weenink JW, van Lieshout J, Jung HP, Wensing M. Patient care teams in treatment of diabetes and chronic heart failure in primary care: An observational networks study. Implementation Science: IS. 2011;6:66. doi: 10.1186/1748-5908-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]