Abstract
A number of parental experiences, even when occurring prior to conception, have been shown to induce transgenerational effects beyond the first generation. In the case of exposure to drugs of abuse, studies in rodents suggest that offspring demonstrate significant differences in how they respond to the drug to which their parent was exposed. We have previously observed significant alterations in morphine analgesia, conditioned place preference and self-administration in the offspring of females exposed to morphine during adolescent development. In addition to effects on pain perception and reward, morphine also modulates the hypothalamic pituitary adrenal (HPA) axis. The purpose of the current study was to determine whether female adolescent morphine exposure results in transgenerational effects on regulation of the HPA axis by morphine in future generations. Adolescent morphine was administered to female Sprague Dawley rats using a 10 day, escalating dose regimen of morphine (5–25 mg/kg; from 30–39 days of age). Control animals received saline. Both saline and morphine exposed females (SAL-F0 and MOR-F0, respectively) were mated with drug naïve males beginning at least 3 weeks after the final injection. Plasma corticosterone levels were measured in male and female offspring (F1) during adulthood following 0, 0.1, or 10 mg/kg morphine. In addition, expression of corticotropin releasing hormone (Crh) and mu opioid receptor (Oprm1) in the paraventricular nucleus (PVN) were measured using quantitative PCR. MOR-F1 males, but not females, had blunted morphine-induced corticosterone secretion. This effect was specific to offspring from females exposed to morphine during adolescence as those exposed during adulthood produced offspring in which the effect was absent. In addition, MOR-F1 males had significantly lower levels of PVN Crh following saline. These effects were not driven by PVN oprm1 in the F1 males as there were no differences based on maternal adolescent exposure. To determine the persistence of the blunted morphine-induced corticosterone effect, SAL-F2 and MOR-F2 males were examined. Blunted morphine-induced corticosterone secretion extended into the MOR-F2 generation, as well as effects on Crh. In addition, there was additional dysregulation ofOprm1 expression in the PVN in MOR-F2 compared with SAL-F2 males. These findings suggest that sex-specific alterations in opioid-mediated regulation of the HPA axis are transgenerationally transmitted for at least two generations following female adolescent morphine exposure. These effects may play a role in the previously observed changes in morphine analgesia and reward-related behaviors observed in this phenotype. In addition, alterations in HPA functioning such as these may play a broad role in transgenerational epigenetic transmission.
Keywords: Transgenerational, opioids, hypothalamic pituitary adrenal axis, corticotropin releasing hormone, mu opioid receptor
Introduction
A growing body of evidence suggests that parental experiences, including those that occur prior to conception, can influence the neurodevelopment and subsequent phenotype of future offspring [1–4]. Some of the initial studies in this area focused on maternal effects, and in particular on behavioral differences in rodent maternal care resulting in epigenetic modifications that alter the regulation of the stress axis [5–8]. More recently, findings in both males and females, have implicated stress dysregulation as a potential trigger for phenotypic variations in future generations [9, 10]. Similar to the effects of stress, preconception exposure to drugs of abuse have also been shown to affect future generations [11]. Once again, these effects are reported following exposure in both males and females and in response to a number of different substances including alcohol, cocaine, cannabinoids, and opiates [12]. The nature of these next generation effects suggests modifications in systems that influence reward-related behaviors, with differences in drug-induced locomotor sensitization [13], conditioned place preference [14, 15], and drug self-administration reported in adult offspring [16–18]. Of note, all of these reward-related behaviors can be regulated by stress hormones and stress-related neuropeptides [19–21]. Moreover, both the hypothalamic-pituitary-adrenal (HPA) axis and central corticotropin releasing hormone (CRH) system have been repeatedly implicated in the development and expression of substance abuse, suggesting significant interplay between drugs of abuse and stress physiology [22].
Using a model of female adolescent morphine exposure in rats (F0 generation), we have previously reported significant next generation effects in both male and female offspring [13, 14, 18, 23–25]. In all of our studies females were drug free for several weeks prior to mating. A number of the effects observed using this model suggest a dysregulation of the HPA axis. For example, we observed significantly augmented corticosterone secretion following repeated exposure to the D2/D3 agonist quinpirole in the male offspring of adolescent morphine exposed females (MOR-F1) as compared to male offspring of adolescent saline exposed control females (SAL-F1). Moreover, similar effects were observed in the F2 generation (derived from F1 females) [13]. More recently, we reported decreased corticosterone secretion in response to morphine in prepubertal MOR-F1 males [25]. Of note, no differences in corticosterone were observed in F1 females. In addition, no differences in response to a saline injection were observed in either study [13, 25]. Thus, these findings suggest that one transgenerational consequence of female adolescent morphine exposure may be a shift in drug-mediated effects on the HPA axis.
Endogenous opioids are critical regulators of the HPA axis, although the nature of these effects can be both region and state dependent [26]. In rats, acute morphine administration stimulates corticosterone secretion [27, 28] and these effects are mediated in part via the activation of mu opioid receptors within the paraventricular nucleus (PVN) [29]. Moreover, these effects result in increased levels of both corticotropin releasing hormone (CRH) and adrenocorticotropin hormone (ACTH) in a manner that is both time and dose dependent [30]. Thus, differences in the stimulatory effect of acute morphine on corticosterone secretion may implicate a shift in the mu opioid receptor activity within the PVN and a shift in opioidergic regulation of the HPA axis.
The purpose of the current study was to examine the transgenerational effect of female adolescent morphine exposure on acute morphine-stimulated corticosterone secretion in adult offspring. Initial experiments examined the sex specificity of these effects while subsequent experiments examined the persistence of the effect in the F2 generation. Additionally, quantitative PCR was used to examine potential changes in both Crh and mu-opioid receptor (Oprm1) gene expression in the PVN to begin to identify potential neural mechanisms underlying these effects.
Results
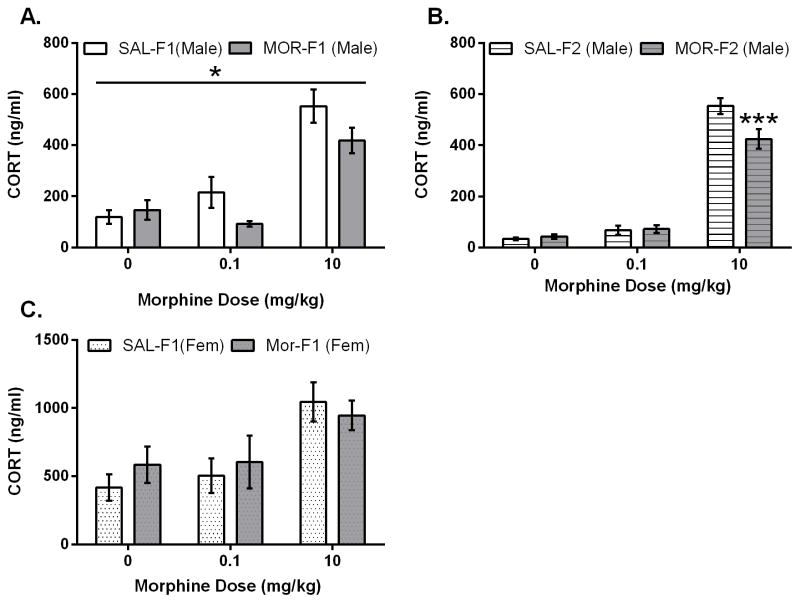
As has been previously shown, an acute morphine injection increases secretion of corticosterone in rats. Shown in Figure 1A, F1 males displayed increased corticosterone levels 30 minutes after an acute injection (10mg/kg) of morphine (F1 animals: main effect dose F[2,66] = 37.3; p<0.0001). However, a significant blunting of morphine-induced corticosterone secretion was observed in the MOR-F1 males. Thus, there was a significant main effect of maternal adolescent exposure (F[1, 66] = 4.1; p=0.05) with MOR-F1 males demonstrating lower corticosterone secretion in response to morphine compared with SAL-F1 males. To determine whether these effects persist in a transgenerational manner, we next examined the F2 generation. As shown in Figure 1B, the expected increased corticosterone secretion 30 min after acute morphine administration was observed in all F2 males (main effect dose; F[2,57]=247.4; p<0.0001) following the 10 mg/kg dose (all p’s < 0.001). There was also a significant main effect of adolescent exposure (F[1,57]=4.4, p<0.05) and a significant interaction (F[2,57]=6.0, p < 0.01). Post hoc analyses indicate that these effects were due to significantly blunted corticosterone secretion observed in MOR-F2 males following the 10 mg/kg injection (p < 0.001). Thus, blunted corticosterone secretion was observed in both the MOR-F1 and MOR-F2 male generations.
Figure 1.
Mean (±SEM) plasma corticosterone levels (ng/ml) as measure 30 minutes after acute (0, 0.1, 10 mg/kg) morphine administration in adult F1 males (panel A), adult F2 males (panel B), and adult F1 females (panel C). F1 males: Main effect of dose (F[2,66]=37.2; p<0.0001). Main effect of maternal adolescent exposure (F[1,66)=4.081; p<0.05). F2 males: main effect of dose (F[2,57]=247.4; p<0.0001); main effect of grandmaternal adolescent exposure (F[1,57]=4.4; p<0.05); significant interaction (F[2,57]=6.0; p<0.01). *p<0.05 as compared SAL-F1; ***p<0.0001 compared to Sal-F1. F1 females: main effect of dose (F[2,54]=8.1; p<0.001). Sample sizes as follows: Male SAL-F1/MOR-F1; 0 mg/kg N=12/11; 0.1 mg/kg N=12/12; 10 mg/kg N=12/13. Male SAL-F1/MOR-F1; 0 mg/kg N=11/10; 0.1 mg/kg N=10/11; 10 mg/kg N=11/10 Female SAL-F1/MOR-F1; 0 m/kg N=10/10; 0.1 mg/kg N=7/10; 10 mg/kg N=12/11.
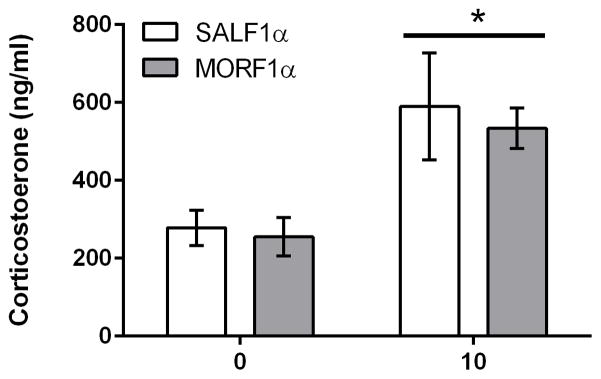
In F1 females, morphine also induced a significant increase in corticosterone (Figure 1C; main effect dose: F[2, 54] = 8.1; p<0.001), with higher levels of corticosterone observed following the 10 mg/kg dose (p’s < 0.001 when compared to all other doses). However, there was no main effect of maternal adolescent exposure (F[1,54] = 0.2; p=.6) and no interaction (F2,54 ] = 0.53; p=.58), indicating that the effect of adolescent morphine exposure on morphine-induced corticosterone secretion in future offspring is sex-specific, with significant differences only observed in male offspring. In addition, male offspring from adult exposed females were also examined for their corticosterone secretion following 0 or 10 mg/kg morphine. There were no differences in morphine-induced plasma corticosterone indicating that the effect is specific to adolescent exposure (Figure 2).
Figure 2.
Mean (±SEM) plasma corticosterone levels (ng/ml) as measure 30 minutes after acute (0, 10 mg/kg) morphine administration in adult F1 males derived from adult-exposed F0 females. Main effect of dose (F[1,23]=8.8; p<0.01) N=6/group.
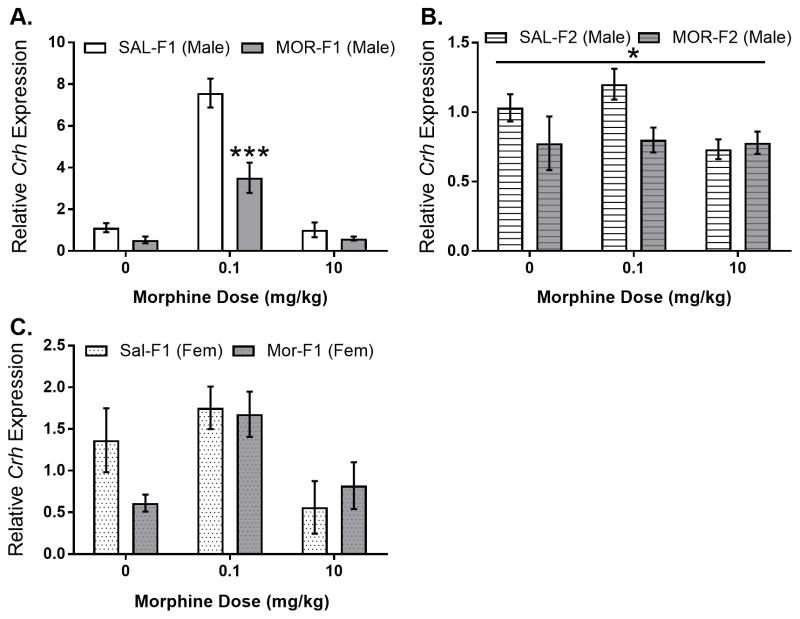
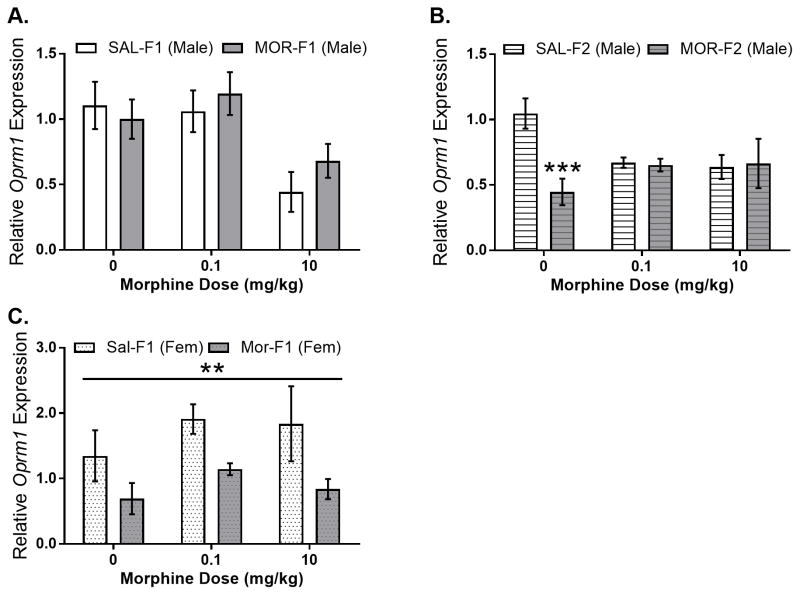
To determine whether changes in neural systems that regulate morphine-stimulated corticosterone secretion are modified by maternal adolescent opioid exposure, expression of Crh in the PVN was examined. As shown in Figure 3A, Crh gene expression in males was regulated by dose (main effect dose; F[2,36]=96.28, p<0.0001) and maternal adolescent exposure (main effect maternal adolescent exposure; F[1,36]=32.43, p<0.0001). In addition, there was also a significant dose by maternal adolescent exposure interaction (F[2,36]=13.68; p<0.0001). Sidak’s multiple comparisons test revealed a significant decrease in Crh gene expression in MOR-F1 males at the 0.1 mg/kg dose (p<0.0001). In both SAL-F1 and MOR-F1 males, the 0.1 mg/kg morphine dose significantly increased Crh gene expression when compared to either acute saline or 10 mg/kg morphine (all p’s<0.01). Again, this effect extended into the subsequent generation with MOR-F2 males demonstrating significantly blunted Crh expression levels in the MOR-F2 males compared with SAL-F2 males (main effect of maternal adolescent exposure F[1,40]=4.41; p<0.05), shown in Figure 3B. In female F1 animals, there was a significant effect of morphine dose on Crh gene expression in the PVN (main effect dose; F[2,31]=6.47, p<0.01), but no effect of maternal adolescent exposure nor an interaction (Figure 3C). In addition to examining PVN Crh, relative expression of Oprm1 in the PVN was also determined. In male F1 animals, there was no effect of maternal adolescent exposure (F[1,35]=0.45, p=0.51) and no F0 condition by dose interaction (F[2,35]=.69, p=0.51). There was, however, a main effect of morphine dose (F[2,35]=7.5, p<0.01) which was due to significantly lower Oprm1 gene expression levels following administration of 10 mg/kg morphine (Figure 4A). Differences in Oprm1 expression were also observed in F2 males with a main effect of grandmaternal adolescent exposure (F[1, 40]=4.31, p<0.05) as well as a significant interaction (F[2,40]=6.12; p<0.01). Post hoc analysis revealed that there was significantly lower Oprm1 mRNA expression in saline injected MOR-F2 males compared with SAL-F2 males (t40=4.1; p<0.0001), Figure 4B. Data from female F1 animals revealed a significant main effect of maternal adolescent treatment (F[1,31]=9.07; p<0.01), see Figure 4C.
Figure 3.
Mean (±SEM) relative expression of Crh mRNA in the PVN of adult F1 males (panel A), adult F2 males (panel B), and adult F1 females (panel C) as measured 30 minutes after morphine administration (0, 0.1, 10 mg/kg). F1 males: main effect of dose (F[2,36]=96.28; p<0.0001); main effect of maternal adolescent exposure (F[1,36)=32.43; p<0.0001); interaction (F[2,36]=13.68; p<0.0001). Sidak’s post hoc analysis (p<0.0001 at 0.1 mg/kg). F2 males: maternal adolescent exposure (F[1,40]=4.413; p<0.05). F1 females: Main effect of dose (F[2,31)=6.47; p<0.001). All N’s = 6–8 per group.
Figure 4.
Mean (±SEM) relative expression of Oprm1 mRNA in the PVN of adult F1 males (panel A), adult F2 males (panel B), and adult F1 females (panel C) as measure 30 minutes after morphine administration. F1 males: main effect of dose (F[2,35]=7.5; p<0.01). F2 males: main effect of grandmaternal adolescent exposure (F[1,40]=5.71; p<0.05), significant interaction (F[2,40]=6.12; p<0.01). *** p<0.0001 as compared to SAL-F2 males at same dose. F1 females: Main effect of maternal adolescent exposure (F[1,31]=9.07; p<0.01) All N’s = 6–8 per group.
Discussion
The current findings indicate that female exposure to morphine during adolescent development, even in the absence of continued use during pregnancy, can affect the physiological response of their future offspring to morphine in a sex-specific manner. Moreover, this effect extends into the grandoffspring (F2) generation indicating a transgenerational phenotype. Thus, MOR-F1 and MOR-F2 males demonstrated blunted corticosterone secretion in response to morphine. No significant effects on corticosterone secretion were observed following a saline injection in either MOR-F1 or MOR-F2 males, suggesting that these effects are not due to a general reduction in HPA activity. Indeed, previous findings using this same adolescent morphine paradigm reported no significant effects on corticosterone levels between SAL-F1/F2 and MOR-F1/F2 males following either an acute injection of saline or the D2 agonist quinpirole [13]. In addition, the effect is specific to the female exposure during the adolescent window of development as exposure during adulthood did not produce the same blunted corticosterone response in F1 offspring. Together, these findings suggest that adolescent morphine exposure in females induces transgenerational effects on the regulation of the HPA axis which may result in different outcomes depending upon the nature of the stimulus. Indeed, previous work has shown a similar blunted cort response following acute morphine injections in juvenile F1 animals [25] as well as alterations in anxiety-like behavior measured using open field as well as elevated plus maze [23, 24]. Moreover, we also showed alterations to the rewarding and motivational properties of morphine across multiple generations [14, 31]. Interestingly, male animals responded differently for lower doses, and not the 10mg/kg dose, when tested in conditioned place preference [14]. This is likely due to divergent effects of morphine on HPA activation and reward. Unlike the effects observed in males, no differences between SAL-F1 and MOR-F1 females with corticosterone secretion were observed. Thus, maternal adolescent exposure modified the effects of morphine on the regulation of corticosterone secretion in a sex-specific manner.
In the current set of findings, we observed significant effects on both Crh and Oprm1 expression within the PVN as a function of F0 condition, with some of these effects being dose-dependent. When compared to SALF1 males, MORF1 males displayed decreased expression of CRH in the PVN following a saline injection, with a similar trend towards reduced expression following a subthreshold dose of morphine (0.1 mg/kg). These differences were not discerned following the higher dose of morphine (10 mg/kg). It is unclear whether the down-regulation of CRH mRNA observed in the absence of morphine (i.e. following saline) plays a role in the decreased morphine-stimulated corticosterone secretion observed in MORF1 males. Unexpectedly, the 0.1 mg/kg dose of morphine significantly increased CRH expression in both SALF1 and MORF1 males while having no statistically significant effect on corticosterone secretion. The 0.1 mg/kg dose was not anticipated to have any discernable effects on any measures and the large effect it had on CRH expression was quite surprising. In addition, the effect on crh in F1 animals in response to this subthreshold dose was much larger than that of F2 animals. This may indicate an effect due to direct exposure of the oocytes to either an injection stress or morphine. A more complete time course evaluation would be needed to determine whether increased corticosterone secretion would be observed at later time points following this low dose. The mechanism underlying the stimulatory effect of this dose on Crh transcription is unknown. Indeed, we could not find another article that attempted to utilize such a small dose. This may represent a more physiological level agonism of the mu opioid receptor that may be important for neuronal-endocrine effects. Further studies are necessary to follow up on this finding. We also examined the effects of morphine administration on Oprm1 in the PVN. In males, morphine down regulated Oprm1 expression regardless of maternal adolescent exposure. In contrast, MOR-F2 males accumulated additional dysregulations showing significantly lower levels of the mu opiate receptor mRNA in the PVN following saline. The observation of accumulated dysregulations is not uncommon in the transgenerational field[31–33]. Indeed, we previously showed that gene expression changes within the nucleus accumbens accumulate in F2 males [31]. This phenomenon represents a spiraling of effects that may become enhanced across generations as dysregulations in one generation cause more irregularities in the next.
While female morphine exposure occurred several weeks prior to conception, direct effects of the drug on germline cells could underlie the observed reduction in morphine-induced corticosterone secretion in F1 males. The presence of dysregulated HPA-related genes in the F2 males indicates a transgenerational transmission of this physiological phenotype, which is particularly striking when it is considered that F2 males were generated along the maternal line, given that F1 females do not demonstrate the effect. Moreover, these results might indicate divergent mechanisms for the observed blunting of corticosterone secretion following morphine between F1 and F2 generations with crh expression in the PVN playing a role in the F1 males and both Crh and Oprm1 acting in the F2 males.
In rats, morphine-stimulated corticosterone secretion is centrally mediated [34] with mu-opioid receptor activation increasing CRH content, as well as stimulating ACTH and corticosterone release both in vivo and in vitro [35, 36]. Acute morphine administration increases the immediate early gene Fos in the PVN [37]; however, there is some suggestion that these effects may not be mediated solely by effects on CRH but may involve additional central regulators of ACTH [38], including vasopressin, which has been shown to be modified by mu opioid receptor activation at the level of the PVN [39].
These findings support the hypothesis that adolescent morphine exposure prior to pregnancy results in transgenerational effects on the regulation of the HPA axis. They do not, however, provide a clear mechanism of action. Direct injections of morphine into the PVN would be needed to confirm whether this region plays a critical role regarding blunted morphine-induced corticosterone. A number of other brain regions may be involved in modulating this effect, such as the nucleus of the solitary tract, central amygdala or bed nucleus of the stria terminalis, all of which express mu opioid receptors and are regulators of the HPA axis [22]. We have previously reported significant differences in MOR-F1 males and females on a number of parameters following repeated morphine exposure; these include differences the development of analgesic tolerance [24], conditioned place preference [14], and self-administration [31]. These effects are often sex-specific and some have been documented in the F2 generation as well. As all of these parameters can be influenced by the HPA axis, further investigations into the potential role played by both glucocorticoids and CRH in mediating the observed transgenerational effects of adolescent morphine exposure are warranted.
Finally, the mechanism of transmission underlying these effects remains unknown. As all animals were reared by their biological mothers, a shift in maternal behavior cannot be ruled out as a factor in transmission. We have previously conducted a detailed examination of maternal responding in these animals and observed few effects [40]. Indeed, the only significant difference between SAL-F0 and MOR-F0 dams was a decreased frequency of nursing in the dark period on postnatal day 4 and yet no difference in time spent nursing was observed when using continuous video recording during the dark period on postnatal day 5 [40]. Moreover, at the time of weaning on postnatal day 21, MOR-F1 animals had gained more weight than their SAL-F1 counterparts [40]. Thus, the changes in maternal behavior are quite subtle. Nonetheless, other alterations in the postnatal environment could contribute to the observed transgenerational effects, including differences in milk components. Interestingly, a number of the bioactive peptides present in milk, which includes opioid peptides, could influence neurodevelopment [41]. Future studies utilizing untreated donor mothers will be needed to begin to parse pre- versus postnatal effects. Moreover, direct epigenetic modifications on the germline that are maintained across generations cannot be ruled out as a contributing factor in the observed effects.
In summary, the current findings support the premise that adolescent morphine exposure alters the physiological response of future offspring and grandoffspring to morphine, and that these effects may be mediated by alterations in opioidergic regulation of the HPA axis.
Material and Methods
Animals
Female Sprague-Dawley rats (23 days of age for adolescent exposure and 200–225g for adult exposure) were purchased from Charles River Breeding Laboratories [Crl:CD(SD)BR; Kingston, NY, USA]. Upon arrival animals were group-housed in light-(on 0700–1900 hours) and temperature- (21–24°C) controlled rooms and provided with food and water ad libitum. All animals were maintained in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals and all procedures were approved by the Institutional Animals Care and Use Committee of Tufts University.
Adolescent Morphine Exposure
Beginning at 30 days of age (or 200–225 grams for adult exposure) females received daily subcutaneous (s.c.) injections of saline or morphine (morphine sulfate; Butler-Schein, Dublin, OH, USA) for a total of 10 days using an increasing dose regimen. The doses used in this paradigm are based on allometric scaling to approximate human use [42] and the increasing dose paradigm is used to model human use patterns, which typically include rising and falling levels of opiates. On day 1, animals received 5 mg/kg morphine sulfate (s.c.) between 0900 and 1100 h. Injections continued daily with the dose of morphine increased by 5 mg/kg every other day, such that by the final day of treatment subjects received 25 mg/kg. Age-matched control animals received saline vehicle (0.9% NaCl, s.c.). Bodyweights were recorded daily throughout the treatment and during drug withdrawal. Adolescent morphine exposed females are referred to as MOR-F0 while saline-treated controls are referred to as SAL-F0. Adult morphine and saline exposed females are referred to as MOR-F0α and SAL-F0α, respectively.
Generation of F1 and F2 Animals
Beginning three weeks after morphine exposure, females were placed in breeding cages with drug naïve colony males at a ratio of 2 females to 1 male. Females were single housed when they were visibly pregnant. To generate F2 males, drug-naïve F1 females were mated with colony males. Litters were culled to 10 pups (5 male and 5 female) on post-natal day 1 (PND1, parturition = PND0). On PND21 animals were weened and housed with same sex littermates until adulthood (>PND60). In order to avoid potential litter effects, only one pup of each sex per litter was used in any experiment. Previous studies using this morphine exposure paradigm have not observed any effects on fertility or fecundity. Additionally, no differences in litter birth weights, litter size, or gender distribution have been observed [13, 24, 40]; however, for completeness all of these measures were recorded in the current set of studies with no significant effects observed (data not shown).
Experimental Design
To measure corticosterone secretion in response to acute morphine administration adult F1 and F2 animals (60–90 days of age) were injected with morphine (0, 0.1, or 10 mg/kg; s.c.) in their home cage and sacrificed 30 min later. All injections were made between 0900–1000 h to avoid fluctuations in diurnal corticosterone secretion. Additional F1 and F2 animals generated from adolescent exposed females were examined for potential alterations in gene expression. In these studies, subjects were injected with morphine (0, 0.1, 10 mg/kg) and sacrificed 30 min later. Expression of Crh and Oprm1 mRNA in the PVN were examined using qPCR. Only one subject per litter per dose was used to minimize potential litter effects.
Collection of trunk blood and brains
All animals were sacrificed by rapid decapitation following brief (60 sec) exposure to CO2 as required by current IACUC protocols. Trunk blood was collected into heparinized tubes and immediately stored on ice. Brains were removed and rapidly frozen in −20°C 2-methylbutane. Brains were stored at −80°C until further processing. Blood was centrifuged at 2500 × g for 20 minutes at 4°C after which plasma was frozen at −20°C prior to assay.
Corticosterone Radioimmunoassay
Plasma corticosterone was determined using a standard radioimmunoassay according to manufacturer’s directions (Corticosterone Coat-a-Count, Seimens, CA). All samples were run in duplicate.
Quantitative PCR
Brains were cryostat-mounted and bilateral tissue punches (1 mm3) were taken from the PVN. Total RNA was extracted using RNeasy (Qiagen) followed by cDNA conversion using RETROscript®. Real time PCR was performed on an ABI Prism 7700 (Applied Biosystems, Foster City, CA). Taqman® primers were purchased from Applied Biosystems and were as follows: Crh – Rn01462137; Oprm1 – Rn01430371_m1; GusB – Rn00566655_m1. GusB was used as the housekeeping gene based on preliminary analysis demonstrating similar expression across all treatment groups. Final quantification of mRNA was obtained using the comparative cycle threshold (Ct) method [43] with data relative to the housekeeping gene GusB (ΔCt) and then to the mean of the SA-LF1 or SAL-F2 subjects injected acutely with saline (ΔΔCt). The data is then expressed as 2^ ΔΔCt to obtain relative expression levels.
Statistical analyses
Data were analyzed using two-way analyses of variances (ANOVA) with maternal adolescent exposure and drug dose as factors. Males and females were analyzed separately. Significance was defined as p < 0.05 and post hoc analyses were conducted using Bonferroni’s correction.
Highlights.
Preconception adolescent morphine exposure in females results in attenuated morphine-induced corticosterone secretion in first (F1) and second (F2) generation male offspring.
Preconception adolescent morphine exposure in females results in altered Crh and Oprm1 gene expression within the PVN of both first (F1) and second (F2) generation male offspring.
Observed effects are specific to the female adolescent exposure as offspring from adult exposed females did not show a similar blunted corticosterone response.
Acknowledgments
These studies were supported by National Institutes of Health Grant DA025674.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Skinner MK. Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Res C Embryo Today. 2011;93(1):51–5. doi: 10.1002/bdrc.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rachdaoui N, Sarkar DK. Transgenerational epigenetics and brain disorders. Int Rev Neurobiol. 2014;115:51–73. doi: 10.1016/B978-0-12-801311-3.00002-0. [DOI] [PubMed] [Google Scholar]
- 3.Szyf M. Nongenetic inheritance and transgenerational epigenetics. Trends Mol Med. 2015;21(2):134–44. doi: 10.1016/j.molmed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Desai M, Jellyman JK, Ross MG. Epigenomics, gestational programming and risk of metabolic syndrome. Int J Obes (Lond) 2015;39(4):633–41. doi: 10.1038/ijo.2015.13. [DOI] [PubMed] [Google Scholar]
- 5.Francis D, et al. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 6.Francis DD, et al. The role of corticotropin-releasing factor--norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 1999;46(9):1153–66. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- 7.Francis DD, et al. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- 8.Francis DD, Meaney MJ. Maternal care and the development of stress responses. Curr Opin Neurobiol. 1999;9(1):128–34. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- 9.Blaze J, Roth TL. Evidence from clinical and animal model studies of the long-term and transgenerational impact of stress on DNA methylation. Semin Cell Dev Biol. 2015;43:76–84. doi: 10.1016/j.semcdb.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saavedra-Rodriguez L, Feig LA. Chronic social instability induces anxiety and defective social interactions across generations. Biol Psychiatry. 2013;73(1):44–53. doi: 10.1016/j.biopsych.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vassoler FM, Byrnes EM, Pierce RC. The impact of exposure to addictive drugs on future generations: Physiological and behavioral effects. Neuropharmacology. 2014;76(Pt B):269–75. doi: 10.1016/j.neuropharm.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog Biophys Mol Biol. 2015;118(1–2):21–33. doi: 10.1016/j.pbiomolbio.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrnes JJ, et al. Multigenerational effects of adolescent morphine exposure on dopamine D2 receptor function. Psychopharmacology (Berl) 2013;227(2):263–72. doi: 10.1007/s00213-012-2960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassoler FM, Wright SJ, Byrnes EM. Exposure to opiates in female adolescents alters mu opiate receptor expression and increases the rewarding effects of morphine in future offspring. Neuropharmacology. 2016;103:112–21. doi: 10.1016/j.neuropharm.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrnes JJ, et al. Cannabinoid exposure in adolescent female rats induces transgenerational effects on morphine conditioned place preference in male offspring. J Psychopharmacol. 2012;26(10):1348–54. doi: 10.1177/0269881112443745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szutorisz H, et al. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology. 2014;39(6):1315–23. doi: 10.1038/npp.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vassoler FM, et al. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16(1):42–7. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassoler FM, et al. Transgenerational attenuation of opioid self-administration as a consequence of adolescent morphine exposure. Neuropharmacology. 2016;113(Pt A):271–280. doi: 10.1016/j.neuropharm.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts AJ, Lessov CN, Phillips TJ. Critical role for glucocorticoid receptors in stress- and ethanol-induced locomotor sensitization. J Pharmacol Exp Ther. 1995;275(2):790–7. [PubMed] [Google Scholar]
- 21.Deroche V, et al. Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15(11):7181–8. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Perez D, et al. Glucocorticoids regulation of FosB/DeltaFosB expression induced by chronic opiate exposure in the brain stress system. PLoS One. 2012;7(11):e50264. doi: 10.1371/journal.pone.0050264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrnes EM. Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 2005;182(4):537–44. doi: 10.1007/s00213-005-0122-4. [DOI] [PubMed] [Google Scholar]
- 24.Byrnes JJ, et al. Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res. 2011;218(1):200–5. doi: 10.1016/j.bbr.2010.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vassoler FM, et al. Next generation effects of female adolescent morphine exposure: sex-specific alterations in response to acute morphine emerge before puberty. Behav Pharmacol. 2014;25(2):173–81. doi: 10.1097/FBP.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodnar RJ. Endogenous opiates and behavior: 2012. Peptides. 2013;50:55–95. doi: 10.1016/j.peptides.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Olszewski PK, et al. Opioids affect acquisition of LiCl-induced conditioned taste aversion: involvement of OT and VP systems. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1504–11. doi: 10.1152/ajpregu.2000.279.4.R1504. [DOI] [PubMed] [Google Scholar]
- 28.Koranyi L, Endroczi E. Piracetam modifies morphine and related drug-induced elevation of serum corticosterone concentration in rats. Acta Physiol Hung. 1983;62(1):75–83. [PubMed] [Google Scholar]
- 29.Kiem DT, Bartha L, Makara GB. Effect of dexamethasone implanted in different brain areas on the morphine-induced PRL, GH and ACTH/corticosterone secretion. Brain Res. 1991;563(1–2):107–13. doi: 10.1016/0006-8993(91)91521-2. [DOI] [PubMed] [Google Scholar]
- 30.el Daly ES. Influence of acute and chronic morphine or stadol on the secretion of adrenocorticotrophin and its hypothalamic releasing hormone in the rat. Life Sci. 1996;59(22):1881–90. doi: 10.1016/s0024-3205(96)00535-8. [DOI] [PubMed] [Google Scholar]
- 31.Vassoler FM, et al. Transgenerational attenuation of opioid self-administration as a consequence of adolescent morphine exposure. Neuropharmacology. 2017;113(Pt A):271–280. doi: 10.1016/j.neuropharm.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarrey JR, et al. Tertiary Epimutations - A Novel Aspect of Epigenetic Transgenerational Inheritance Promoting Genome Instability. PLoS One. 2016;11(12):e0168038. doi: 10.1371/journal.pone.0168038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vassoler FM, Sadri-Vakili G. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience. 2014;264:198–206. doi: 10.1016/j.neuroscience.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pechnick RN. Effects of opioids on the hypothalamo-pituitary-adrenal axis. Annu Rev Pharmacol Toxicol. 1993;33:353–82. doi: 10.1146/annurev.pa.33.040193.002033. [DOI] [PubMed] [Google Scholar]
- 35.Buckingham JC, Cooper TA. Effects of naloxone on hypothalamo-pituitary-adrenocortical activity in the rat. Neuroendocrinology. 1986;42(5):421–6. doi: 10.1159/000124481. [DOI] [PubMed] [Google Scholar]
- 36.Buckingham JC, Cooper TA. Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinology. 1984;38(5):411–7. doi: 10.1159/000123927. [DOI] [PubMed] [Google Scholar]
- 37.Laorden ML, et al. Activation of c-fos expression in hypothalamic nuclei by mu- and kappa-receptor agonists: correlation with catecholaminergic activity in the hypothalamic paraventricular nucleus. Endocrinology. 2000;141(4):1366–76. doi: 10.1210/endo.141.4.7407. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, et al. Hypothalamic-pituitary-adrenal activity and pro-opiomelanocortin mRNA levels in the hypothalamus and pituitary of the rat are differentially modulated by acute intermittent morphine with or without water restriction stress. J Endocrinol. 1999;163(2):261–7. doi: 10.1677/joe.0.1630261. [DOI] [PubMed] [Google Scholar]
- 39.Lessard A, Bachelard H. Tonic inhibitory control exerted by opioid peptides in the paraventricular nuclei of the hypothalamus on regional hemodynamic activity in rats. Br J Pharmacol. 2002;136(5):753–63. doi: 10.1038/sj.bjp.0704780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson NL, et al. Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Front Psychiatry. 2011;2:29. doi: 10.3389/fpsyt.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lonnerdal B. Bioactive proteins in breast milk. J Paediatr Child Health. 2013;49(Suppl 1):1–7. doi: 10.1111/jpc.12104. [DOI] [PubMed] [Google Scholar]
- 42.Chiou WL, et al. Correlation of plasma clearance of 54 extensively metabolized drugs between humans and rats: mean allometric coefficient of 0.66. Pharm Res. 1998;15(9):1474–9. doi: 10.1023/a:1011974226596. [DOI] [PubMed] [Google Scholar]
- 43.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]