Abstract
Antimony is the first line drug for treating American tegumentary leishmaniasis (ATL) in Brazil. In this country, Leishmania braziliensis causes at least three distinct forms of disease: localized cutaneous (CL), mucosal (ML) and disseminated leishmaniasis (DL). All forms can be found in Corte de Pedra, Northeast Brazil. ML and DL respond poorly to antimony, in contrast to CL. The L. braziliensis population causing ATL in Corte de Pedra is genetically very diverse, with strains of the parasite associating with the clinical form of leishmaniasis. We tested the hypotheses that antimony refractoriness is associated with L. braziliensis genotypes, and that parasites from ML and DL present greater in vitro resistance to antimony than L. braziliensis from CL. Comparison of geographic coordinates of living sites between antimony responders and non-responders by Cusick and Edward’s test showed that refractoriness and responsiveness to the drug were similarly wide spread in the region (p>0.05). Parasites were then genotyped by sequencing a locus starting at position 425,451 on chromosome 28, which is polymorphic among L. braziliensis of Corte de Pedra. Haplotype CC- in CHR28/425451 was associated with risk of treatment failure among CL patients (Fisher’s exact test, p=0.03, odds ratio=4.65). This haplotype could not be found among parasites from ML or DL. Finally, sensitivity to antimony was evaluated exposing L. braziliensis promastigotes to increasing concentrations of meglumine antimoniate in vitro. Parasites from ML and DL were more resistant to antimony at doses of 2 mg/100 μL and beyond than those isolated from CL (Fisher’s exact test, p=0.02 and p=0.004, respectively). The intrinsically lower susceptibility of L. brazliensis from ML and DL to antimony parallels what is observed for patients’ responsiveness in the field. This finding reinforces that ML and DL patients would benefit from initiating treatment with drugs currently considered as second line, like amphotericin B.
Keywords: Leishmania braziliensis, sensitivity, refractoriness, antimony, mucosal, disseminated, cutaneous, American tegumentary leishmaniasis
Graphical Abstract
1. Introduction
Tegumentary leishmaniasis is caused by protozoa of the genus Leishmania, presenting a worldwide incidence of up to 1.2 million cases per year. Brazil is one of ten countries that concentrate 75% of the global incidence of the disease (Alvar et al., 2012). Leishmania (Viannia) braziliensis is the predominant species causing leishmaniasis in the Americas (Azulay and Azulay Junior, 1995).
Antimony is still the standard first line treatment for American tegumentary leishmaniasis (ATL) in Brazil (Saúde., 2010). However, its efficacy varies depending on the infecting parasite species and even within the same species in different geographic regions (Arevalo et al., 2007; Azeredo-Coutinho et al., 2007; Llanos-Cuentas et al., 2008; Romero et al., 2001). Furthermore, previous studies show that success of antimony therapy in patients infected with L. braziliensis also varies according to form of leishmaniasis (Guimaraes et al., 2009; Machado et al., 2011; Marsden, 1994; Murray et al., 2005).
Localized cutaneous (CL), and the more severe mucosal (ML) and disseminated leishmaniases (DL) are three of the major ATL forms caused by L. braziliensis (Carvalho et al., 1994; Jones et al., 1987; Marsden, 1985; Turetz et al., 2002). It has been reported that a large proportion of ML and DL cases respond poorly to antimony (Machado et al., 2007; Machado et al., 2011; Machado et al., 2015). This, in conjunction to the reports of regional differences in the efficacy of these drugs to treat patients infected with L. braziliensis, is suggestive that parasite strain may be one important determinant for such variability in responses.
To address this hypothesis, we evaluated the in vitro susceptibility to antimony among L. braziliensis isolated from patients with either CL, ML or DL. These parasites derived from human infections acquired in a region of high endemicity for ATL in Northeast Brazil, named Corte de Pedra (Schriefer et al., 2009). The population structure of L. braziliensis that infects the human beings in this region is complex, with a high genetic diversity, presenting several subpopulations of strains (Schriefer et al., 2004) that have been implicated with form of ATL in previous studies (Guimaraes et al., 2016; Queiroz et al., 2012; Schriefer et al., 2004).
2. Materials and Methods
2.1 Study area
The Corte de Pedra region is located in the Northeast of Brazil within the geographic coordinates (latitude, longitude) 14°/39°, 13°/39°, 14°/40°, 13°/40°. Corte de Pedra is composed of 20 municipalities in a rural area previously dominated by an Atlantic rain forest in the southeast part of the state of Bahia. Local residents work mostly in agriculture, often carried out in proximity to primary or secondary forests. Lutzomyia (Nyssomyia) whitmany and Lu. (N.) intermedia sandflies transmit L. braziliensis in the region (Miranda et al., 2002).
2.2 Patients; definitions of disease type and of treatment response
Enrollment was carried out from August 2008 to July 2011. CL was defined as a disease with <10 ulcerative skin lesions without evidence of mucosal involvement. DL was defined as a disease with >10 acneiform, papular, or ulcerative lesions spread over the skin of >2 body areas. ML was defined as a disease with metastatic mucosal lesions affecting the nose, palate, pharynx or larynx, not contiguous with primary cutaneous lesions. Some DL patients also presented lesions in the nasal mucosa. All patients had their diagnosis initially determined by at least two of the following criteria: (1) positive culture of parasites from lesion aspirates; (2) identification of amastigotes in lesion histopathology; and (3) positive skin test with L. braziliensis antigen (Montenegro test). All subjects had their diagnosis subsequently confirmed by parasite DNA detection in lesion biopsy specimens by PCR (Weirather et al., 2011).
All patients were initially treated with intravenous pentavalent antimony (Glucantime ®) at a dose of 20mg/kg/day for 20 days for individuals with CL, and 30 days for those with ML or DL. Patients presenting complete healing of tegumentary lesions after one course with antimony were classified as responsive to treatment. Patients that failed antimony therapy were classified as refractory and underwent rescue treatment with amphotericin B. Complete healing was defined as re-epitelization of cutaneous and / or mucosal lesions accompanied by improvement of local inflammatory aspect, without recurrence of disease up to day 180 of monthly follow-up.
2.3 Parasite culturing, DNA extraction, and species confirmation
All clinical specimens used for parasite cultivation and isolation were collected at diagnosis, before patients’ treatments started. Leishmania isolation was performed by culturing the material aspirated from the borders of skin or mucosal lesions. Aspirate material was immediately suspended in biphasic liver infusion tryptose/Novy, McNeal, Nicolle (LIT/NNN) medium and incubated at 26ºC for one to three weeks. At weekly analyses, turbid cultures presenting observable promastigotes were considered positive.
Positive cultures had the species of Leishmania confirmed by PCR as follows. Suspensions were transferred to Schneider’s medium complemented with 10% heat inactivated fetal calf serum and 2 mM L-glutamine, and incubated at 26ºC until they reached a density of 107 parasite cells / mL. Then 1.7 mL aliquots of the suspensions were centrifuged at 2,000 rpm, the supernatants discarded and the pellets re-suspended in 150 μL of TELT (50mM Tris-HCl pH 8.0, 62.5mM EDTA pH 9.0, 2.5M LiCl, 4% v/v Triton x 100) buffer. After 5 min incubation at room temperature, 150 μL of phenol-chloroform was added to each suspension, followed by brief vortexing and centrifugation at 13,000 rpm for 5 min. The supernatants were transferred to new tubes, 300 μL of ethanol were added to each tube and the entire contents centrifuged 10 min at 13,000 rpm. The supernatants were discarded, 1mL of ethanol was added and the suspensions incubated 5 min at room temperature. After a final 5 min centrifugation at 13,000 rpm, the supernatants were discarded, and then the pellets were air dried and re-suspended in 100 μL of TE buffer. Long-term storage DNA aliquots were kept at −70°C, while test samples were maintained at −20°C. Detection and confirmation of Leishmania species in test DNA samples was performed by a serial real-time qPCR assay previously reported (Weirather et al., 2011).
For detecting parasite DNA in patients’ skin and nasal mucosa for diagnostic purposes, the specimens obtained by biopsy of the lesions borders during initial consultations were stored in RNA later solution (Ambion, Life Technologies, Thermo Fisher Scientific, USA) immediately after the procedure was carried out in the field. The fragment thus conserved was kept at room temperature for approximately six hours during the travel back from field area, and then stored at 4ºC in the laboratory. Upon two to three days of specimens’ arrival from the ATL endemic region, the nucleic acids were extracted from the skin and mucosal fragments using the DNA Purification kit (Promega Co., USA), according the manufacturer recomendations.
2.4 In vitro antimony (Glucantime®) sensitivity assay
N-methyl glucamine antimoniate (meglumine antimoniate) provided by the Ministry of Health was used to test susceptibility of promastigotes in vitro, with dose range and procedures based on previous reports (Azeredo-Coutinho et al., 2007; Zauli-Nascimento et al., 2010). One hundred microliter aliquotes of logarithmic phase suspensions with 1×107 promastigotes/mL, containing L. braziliensis obtained from patients responsive or refractory to antimony, were exposed to increasing amounts of meglumine antimoniate: 0.0, 0.25, 0.5, 1, 2, 4, 8 and 16 mg per well. These suspensions were kept at 25°C for 72 hours. After incubation, 20μL of 5mg/mL MTT were added to each well and the suspensions were further incubated for 3 hours. The MTT assay is based on the cleavage of the tetrazolium salt MTT [3- (4, 5- dimethylthiazol-2-yl)- 2,5 diphenyl tetrazolium bromide; Sigma-Aldrich] by the mitochondrial dehydrogenase of viable cells. The reaction was stopped adding 40 μL of 20% sodium dodecyl sulfate to each well. Reading was performed in a multi-well scanning spectrophotometer at the wavelength of 540nm. All experiments were performed in triplicate.
2.5 Parasite genotyping
L. braziliensis isolated from the ATL patients were genotyped according to the nucleic acid sequence of a segment with approximately 400 base-pairs, starting at position 425,451 on parasite’s chromosome 28 (i.e. locus CHR28/425451), as previously described(Queiroz et al., 2012). Briefly, the locus CHR28/425451 was amplified from genomic DNA of L. braziliensis cultured from each ATL subject, using primers 5':TAAGGTGAACAAGAAGAATC and 5':CTGCTCGCTTGCTTTC. Amplicons were electrophoresed, and their bands extracted from agarose gels then cloned into pCR 2.1-TOPO vectors (Invitrogen Inc.), according to manufacturer’s instructions. Plasmid mini-preps were generated from four representative bacterial clones, for each L. braziliensis isolate (Sambrook et al., 1989). Plasmid inserts were sequenced with primers complementary to bacteriophage M13 sequences flanking the vector’s cloning site. Sequencing employed Sanger’s method at Macrogen Inc. (Seoul, S. Korea). The consensus sequence and haplotypes of SNP and indel polymorphisms (i.e. alleles) at the CHR28/425451 locus were determined across the different L. braziliensis in the sample with the Mega 4.0 software package (Tamura et al., 2007).
2.6 Mapping ATL patients in the study area
High-resolution distribution of ATL cases was determined by acquisition of geographic coordinates of likely places of disease transmission by global positioning system. Geographic coordinates were obtained using a Brunton Multi-Navigator GPS apparatus (Brunton Company, Riverton, WY, USA), which has a precision range of 15 m. Because leishmaniasis is believed to be transmitted mostly within plantations, where residents of the region live and work, patients’ residences were used as reference points for standardization purposes. Collected data were statistically evaluated as described below, and plotted for visual inspection onto a high-definition satellite photograph of Corte de Pedra region (ENGESAT, Curitiba, Brazil), using ArcGis version 10 software (Environmental Systems Research Institute Inc., Redlands, CA, USA).
2.7 Statistical analyses
Non-linear correlations between MTT assay absorbance values, reflecting parasite survival rates, and meglumine antimoniate concentrations in vitro were performed using Graphpad Prism version 5 (Graphpad Software Inc. La Jolla, CA, USA). In vitro sensitivity to antimony among CL, ML and DL derived parsites, as well as differences in the distribution frequencies of SNPs / indels haplotypes in the L. braziliensis locus CHR28/425451 between antimony responsive and refractory ATL cases were compared by Fisher’s exact test, with Graphpad Prism. Odds ratios and p values were determined at confidence levels of 95%. Comparison of geographic distributions between antimony responsive and refractory ATL patients over the affected area employed the Cuzick and Edward's test, using Clusterseer version 2.3 software (Terraseer Inc., Ann Arbor, MI, USA). Values of p<0.05 were considered significant.
2.8 Human subjects' approvals
The procedures used in this study were approved by the Ethics Committee of the University Hospital at Universidade Federal da Bahia (document of approval: CAAE – 3041.0.000.054.07) and by the National Council on Ethics in Research (document of approval: CONEP-128/2008, 17/03/2008).
3. Results
3.1 Antimony responsive and refractory ATL patients distribute similarly over the affected region
A total of 278 ATL patients were included in the study. One-hundred eighty-nine (68%) subjects responded (i.e. presented complete healing of lesions) and eighty-nine (32%) were refractory to antimony treatment. To understand whether antimony refractoriness tends to cluster in the study region, the distribution of ATL patients responsive to Glucantime® was compared to that of non-responders. Figure one shows that both groups spread broadly over the area, with a larger frequency of cases occurring in the west sectors of the region. Furthermore, there was no global difference between distributions of responders and non-responders (Cusick and Edward’s p>0.05), indicating that they were similarly exposed to eco-environmental risk factors influencing ATL outcomes in Corte de Pedra.
Figure 1.
Antimony refractory and antimony responsive American tegumentary leishmaniasis patients distribute similarly in Corte de Pedra, Northeast Brazil. Antimony responsive (N= 189) and non-responsive (N= 89) patients diagnosed between 2008 and 2011 in Corte de Pedra were mapped, and the resulting sets of geographic events were statistically compared. White and Red dots correspond to responders and non-responders, respectively. Total number of dots plotted is smaller than the actual number of corresponding cases due to overlap of some patients' geographic coordinates. The two groups of patients presented similar distributions in the region, with Cuzick and Edward’s comparison yielding non-significant (p>0.05). For details refer to Methods section.
3.2 Antimony refractory CL patients tend to be infected with particular L. braziliensis strains
L. braziliensis could be isolated from 92 subjects. All ninety-two isolates were employed in the L. braziliensis genotype versus antimony response phenotype association analyses. Sixty-six isolates were drawn from responders and 26 from patients that were refractory to one course of antimony treatment. According to disease form this sample was comprised of parasites drawn from 68 CL, 16 DL and 8 ML patients. Alignment of CHR28/425451 among parasite isolates displayed four different haplotypes of nucleotides in polymorphic positions 425480, 425736, 425995 of chromosome 28: CC-, CCT, TT- and TTT. Haplotype counts in the sample consisted of 10 CC-, 78 CCT, 53 TT- and 11 TTT. The number of haplotypes is greater than that of L. braziliensis isolates tested because of heterozygozity in CHR28/425451, previously reported (Queiroz et al., 2012). Parasites bearing CC- haplotype were significantly associated with host antimony refractoriness (p=0.027), causing an odds ratio of 4.65 (95% CI: 1.19 to 18.16) to treatment failure. Besides, parasites bearing CC-/CCT genotypes just missed association (p=0.052), causing an odds ratio of 3.84 (95% CI: 0.98 to 15.81) to antimony refractoriness by the host. Noteworthy, the presence of CC- in locus CHR28/425451 was associated with a higher risk of treatment failure specifically among CL patients, since this genotype could not be found in the parasites isolated from ML or DL cases in this sample. These observations suggest that, in CL, part of non-responders to antimony treatment may be infected with particular strains of L. braziliensis.
3.3 L. braziliensis isolated from more severe ATL cases are more resistant to antimony in vitro than those drawn from CL patients
Figure two shows the dose-response viability curve derived from average values for seven randomly chosen L. braziliensis isolates of Corte de Pedra patients, exposed in vitro to incremental concentrations of antimony. Parasite survival progressively decreased upon exposure from 0.0 to 2.0mg of Glucantime® per 100 μL of medium, leveling thereafter, except for a consistent spike affecting most parasite isolates at 8.0mg/100 μL that subsides back to the original trend at the 16 mg/100 μL data point.
Figure 2.
Sensitivity of L. braziliensis promastigotes to increasing amounts of antimony (Glucantime®) in vitro. One hundred microliter aliquotes of early logarithmic phase suspensions with 1×107 promastigotes/mL, containing L. braziliensis obtained from seven ATL patients of Corte de Pedra were exposed to increasing amounts of meglumine antimoniate: 0.0, 0.25, 0.5, 1, 2, 4, 8, 16 mg per well. After 72 hours incubation, 5mg/ml MTT was added to each well then suspensions were incubated for another 3 hours. Reactions were stopped with sodium dodecyl sulfate, and cell viability evaluated by spectrophotometry at 540nm wavelength. Curve consists in non-linear correlation between absorbance values reflecting parasite survival rates and meglumine antimoniate concentrations in vitro. Data points consist of mean optical density (O.D.540 nm) values. Bars consist of standard error of means (SEM).
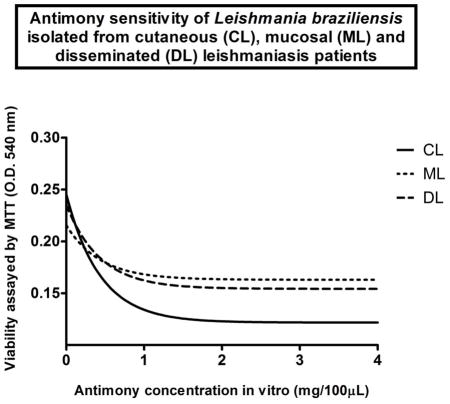
The in vitro dose-response curves of sixty-five study isolates were then determined within the dose range of 0 to 4 mg/100 μL of Glucantime®. Results were similar for parasites obtained from antimony refractory and antimony responsive patients (Student’s t test p>0.05 for antimony responsive versus refractory comparisons at all data points tested; i.e. 0, 0.25, 0.5, 1, 2, 4 mg/100 μL). Stratification by disease form revealed that parasites isolated from DL and ML displayed equivalent responses, and an overall greater resistance to antimony in vitro than those from CL patients (Figure 3).
Figure 3.
Promastigotes from localized cutaneous leishmaniasis patients are more sensitive to antimony in vitro than promastigotes from mucosal and disseminated leishmaniasis cases. Parasite samples were grouped according to form of ATL they derived, and then treated in vitro in parallel, as in figure two. Concentrations of meglumine antimoniate tested: 0, 0.25, 0.5, 1, 2, 4 mg per 100 uL of parasite suspension. Circles consist of localized cutaneous leishmaniasis patients (CL); squares, mucosal leishmaniasis patients (ML); triangles, disseminated leishmaniasis patients (DL). Data points consist of mean optical density (O.D.540 nm) values, reflecting parasite survival rates upon exposure to meglumine antimoniate increasing concentrations in vitro, as detected by MTT assay (for details, please refer to the methods section). Bars consist of standard error of means (SEM). Student’s t test p values for CL versus ML comparisons of L. braziliensis promastigotes viability upon exposure to 0, 0.25, 0.5, 1, 2 and 4 mg of antimony per 100 uL parasite suspensjion: 0.2; 0.4; 0.3; 0.2; 0.05; 0.02; respectively. Student’s t test p values for CL versus DL comparisons of L. braziliensis promastigotes viability upon exposure to 0, 0.25, 0.5, 1, 2 and 4 mg of antimony per 100 uL parasite suspensjion: 0.2; 0.3; 0.3; 0.2; 0.08; 0.02; respectively. There was no statistical difference when ML derived parasites were compared to those from DL at any condition tested.
In order to reinforce the above impression, we compared the frequencies of L. braziliensis isolates sensitive to 2 mg Glucantime® between responders and non-responders to antimony treatment, and among the different forms of ATL. We defined as sensitive those isolates that would present a drop in viability below 50% from base-line observed among unexposed parasites cultured in parallel.
Sixteen (41%) of the 39 L. braziliensis isolated from antimony responsive ATL patients, and fifteen (57.7%) of the 26 isolates from antimony refractory patients were sensitive to 2mg of Glucantime® in vitro (Fisher’s exact test, p=0,21), reflecting the similar dose-response curves observed in figure 3. When L. braziliensis were stratified by form of ATL, sixty-six percent of CL isolates (25/38) were sensitive to 2mg antimony in vitro. In contrast, only 22% of DL (4/18) and of ML (2/9) derived parasites were sensitive to 2mg of the drug (Fisher’s exact test; CL x DL, p=0.004; CL x ML, p=0.02).
4. Discussion
In the present study, we compared the susceptibility profiles of L. braziliensis promastigotes to antimony in vitro. We found that parasites from both antimony responders and non-responders presented similar susceptibility patterns. However, stratification of the analysis per form of ATL revealed that parasites from more severe forms of leishmaniasis were significantly more resistant to antimony than parasites from localized cutaneous leishmaniasis patients.
The reasons for antimony response or failure are probably many. For example, patient’s age, disease duration and number or location of skin lesions have all been shown as a risk factors involved in treatment outcome (Llanos-Cuentas et al., 2008; Unger et al., 2009). Clinical form of ATL has also been extensively associated with therapeutic success. ML and DL patients tend to fail antimony therapy much more frequently than CL patients (Machado et al., 2007; Machado et al., 2011; Machado et al., 2015). This epidemiologic observation parallels what we found in vitro, with parasites from patients with metastatic disease being significantly more resistant to Glucantime® than those from localized disease.
The fact that we used cultured promastigotes in our evaluation is one limitation of this study. Even though it is not clear whether promastigotes participate in the continuous infection of new phagocytes at later times after the initial inoculation of the parasite in the host by sand flies, Leishmania infection in vertebrates is maintained primarily by the multiplication of intracellular amastigotes (Sacks and Sher, 2002). So, this must be the form of the parasite most affected by the drugs used to treat the patients. This certainly renders our approach somewhat artificial.
Three options to overcome this limitation would be to use axenic amastigote cultures (Bates, 1993; Callahan et al., 1997), amastigote infected macrophage cell lines (de Souza et al., 2016) or human volunteer monocyte derived macrophages infected with L. braziliensis (Carneiro et al., 2016). Axenic amastigotes and infected cell lines would be approaches probably as artificial as the use of cultured promastigotes. On the other hand, human monocyte derived macrophages infected with parasites in vitro might mimic well what would happen during treatment in natural infections. However, it would be difficult to evaluate a large sample of L. braziliensis isolates tested at different concentrations of antimony, in triplicate experiments employing this approach. Besides, more than one donor of macrophages would be necessary per experiment, adding variables difficult to control, like consistency of experiment-to-experiment and intra-experiment rates of phagocyte infection by promastigotes of different strains, and host-to-host genetic background variability.
Previous studies indicate that promastigote susceptibility pattern is a good proxy of amastigote resistance to antimony in vitro (Azeredo-Coutinho et al., 2007). The paired comparison of cultured promastigotes and axenic amastigotes showed that the former are intrinsically less susceptible to the drug (Ephros et al., 1999). However, exposure to increasing doses of antimony showed that promastigote survival accompanies survival rates of amastigotes of the same strain (Azeredo-Coutinho et al., 2007).
Global distributions of Glucantime® responsive and non-responsive patients in the Corte de Pedra region indicate they faced similar exposure to environmental variables. Such variables include, for example, species of sand fly vectors transmitting L. braziliensis and strains of the parasite, which might potentially present focal aggregation and spread heterogeneously in the region. In respect of parasite strains, we have previously shown that certain L. braziliensis genotypes defined by allele contents in locus CHR 28/425451 were significantly associated with disseminated (Queiroz et al., 2012) and atypical cutaneous leishmaniasis (Guimaraes et al., 2016). A large proportion of these patients do not respond to antimony and require subsequent treatment with second line drugs like amphotericin B (Guimaraes et al., 2009; Guimaraes et al., 2016; Machado et al., 2007).
Association between genotype of L. braziliensis and response to treatment in the current study was significant, but merits further assessment. Alleles of CHR 28/425451 involved in this observation presented low frequency in the sample with only ten subjects being infected with parasites presenting the implicated allele or combination of alleles (i.e. CC- or CC-/TTT, respectively). Nevertheless, this finding helps reinforce the overall conclusion stemming from in vitro evaluations that different strains of the parasite are intrinsically more susceptible or resistant to antimony.
The standard first line treatment of American tegumentary leishmaniasis (ATL) in Brazil consists of intravenous pentavalent antimony at a dose of 20mg/kg/day for 20 days for individuals with CL, and 30 days for those with ML, DL or ACL (Saúde., 2010). Our findings, showing an intrinsically lower susceptibility of L. brazliensis from ML and DL to Glucantime®, indicate that such patients would benefit from immediate treatment with second line drugs like amphotericin B. As a matter of fact, ML, DL and ACL cases often need rescue treatment with amphotericin B after one or two failed antimony courses in the study region. In future research, we will try to address the sensitivity of intra-cellular amastigotes of L. braziliensis from Corte de Pedra to antimony, and to two other drugs we conducted clinical trials with opposing results in that region: Fluconazole and Miltefosine, which showed lower and higher efficacy than Glucantime®, respectively.
Table 1.
Frequency distribution of heterozygozity in the locus CHR28/425451 of Leishmania braziliensis isolated from American tegumentary leishmaniasis subjects of Corte de Pedra-Brazil, stratified according to disease form: localized cutaneous (CL), mucosal (ML) or disseminated leishmaniasis (DL). Counts of haplotypes of polymorphic nucleotides (i.e. alleles) found in positions 425480, 425736, 425995 of chromosome 28: 10 CC-, 78 CCT, 53 TT- and 11 TTT.
| CL | ML | DL | Total | |
|---|---|---|---|---|
| Heterozygous isolates counts | 40 | 6 | 6 | 52 |
| Homozygous isolates counts | 28 | 2 | 10 | 40 |
| Total | 68 | 8 | 16 | 92 |
Highlights.
Comparison of geographic coordinates between antimony responders and non-responders showed that refractoriness and responsiveness to antimony were similarly wide spread in the Corte de Pedra region, Northeast Brazil.
Parasites genotyping at the locus starting in position 425,451 on L. braziliensis chromosome 28 showed that haplotype CC- was associated with higher risk of antimony treatment failure among cutaneous leishmaniasis patients.
Parasites from mucosal and disseminated leishmaniases were more resistant to antimony in vitro than those isolated from cutaneous leishmaniasis patients.
Acknowledgments
We deeply thank all personnel of the Jackson Costa Health Post in Corte de Pedra, Bahia, Brazil, for their careful help with patient management; and Angela Giudice for laboratory support with parasite isolation and management. This work was supported in part by the National Institutes of Health (NIH), USA, through grant NIH P50-AI30639. SS, VM, JS, LSM, AQS and LHSG were recipients of CAPES MS and PhD scholarships.
Footnotes
Conflict of interest. The authors of the manuscript do not present any conflict of interests, commercial or otherwise, that might interfere with the study, its results and analyses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verastegui C, Lazo M, Loayza-Muro R, De Doncker S, Maurer A, Chappuis F, Dujardin JC, Llanos-Cuentas A. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis. 2007;195:1846–1851. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- Azeredo-Coutinho RB, Mendonca SC, Callahan H, Portal AC, Max G. Sensitivity of Leishmania braziliensis promastigotes to meglumine antimoniate (glucantime) is higher than that of other Leishmania species and correlates with response to therapy in American tegumentary leishmaniasis. J Parasitol. 2007;93:688–693. doi: 10.1645/GE-1031R.1. [DOI] [PubMed] [Google Scholar]
- Azulay RD, Azulay DR., Junior Immune-clinical-pathologic spectrum of leishmaniasis. Int J Dermatol. 1995;34:303–307. doi: 10.1111/j.1365-4362.1995.tb03608.x. [DOI] [PubMed] [Google Scholar]
- Bates PA. Axenic culture of Leishmania amastigotes. Parasitol Today. 1993;9:143–146. doi: 10.1016/0169-4758(93)90181-e. [DOI] [PubMed] [Google Scholar]
- Callahan HL, Portal AC, Devereaux R, Grogl M. An axenic amastigote system for drug screening. Antimicrob Agents Chemother. 1997;41:818–822. doi: 10.1128/aac.41.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro PP, Conceicao J, Macedo M, Magalhaes V, Carvalho EM, Bacellar O. The Role of Nitric Oxide and Reactive Oxygen Species in the Killing of Leishmania braziliensis by Monocytes from Patients with Cutaneous Leishmaniasis. PLoS One. 2016;11:e0148084. doi: 10.1371/journal.pone.0148084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho EM, Barral A, Costa JM, Bittencourt A, Marsden P. Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop. 1994;56:315–325. doi: 10.1016/0001-706x(94)90103-1. [DOI] [PubMed] [Google Scholar]
- de Souza LD, Vendrame CM, de Jesus AR, Carvalho MD, Magalhaes AS, Schriefer A, Guimaraes LH, Carvalho EM, Goto H. Insulin-like growth factor-I serum levels and their biological effects on Leishmania isolates from different clinical forms of American tegumentary leishmaniasis. Parasites & vectors. 2016;9:335. doi: 10.1186/s13071-016-1619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephros M, Bitnun A, Shaked P, Waldman E, Zilberstein D. Stage-specific activity of pentavalent antimony against Leishmania donovani axenic amastigotes. Antimicrob Agents Chemother. 1999;43:278–282. doi: 10.1128/aac.43.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes LH, Machado PR, Lago EL, Morgan DJ, Schriefer A, Bacellar O, Carvalho EM. Atypical manifestations of tegumentary leishmaniasis in a transmission area of Leishmania braziliensis in the state of Bahia, Brazil. Trans R Soc Trop Med Hyg. 2009;103:712–715. doi: 10.1016/j.trstmh.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes LH, Queiroz A, Silva JA, Silva SC, Magalhaes V, Lago EL, Machado PR, Bacellar O, Wilson ME, Beverley SM, Carvalho EM, Schriefer A. Atypical Manifestations of Cutaneous Leishmaniasis in a Region Endemic for Leishmania braziliensis: Clinical, Immunological and Parasitological Aspects. PLoS Negl Trop Dis. 2016;10:e0005100. doi: 10.1371/journal.pntd.0005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TC, Johnson WD, Jr, Barretto AC, Lago E, Badaro R, Cerf B, Reed SG, Netto EM, Tada MS, Franca TF, et al. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J Infect Dis. 1987;156:73–83. doi: 10.1093/infdis/156.1.73. [DOI] [PubMed] [Google Scholar]
- Llanos-Cuentas A, Tulliano G, Araujo-Castillo R, Miranda-Verastegui C, Santamaria-Castrellon G, Ramirez L, Lazo M, De Doncker S, Boelaert M, Robays J, Dujardin JC, Arevalo J, Chappuis F. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin Infect Dis. 2008;46:223–231. doi: 10.1086/524042. [DOI] [PubMed] [Google Scholar]
- Machado PR, Lessa H, Lessa M, Guimaraes LH, Bang H, Ho JL, Carvalho EM. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clin Infect Dis. 2007;44:788–793. doi: 10.1086/511643. [DOI] [PubMed] [Google Scholar]
- Machado PR, Rosa ME, Costa D, Mignac M, Silva JS, Schriefer A, Teixeira MM, Bacellar O, Carvalho EM. Reappraisal of the immunopathogenesis of disseminated leishmaniasis: in situ and systemic immune response. Trans R Soc Trop Med Hyg. 2011;105:438–444. doi: 10.1016/j.trstmh.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado PR, Rosa ME, Guimaraes LH, Prates FV, Queiroz A, Schriefer A, Carvalho EM. Treatment of Disseminated Leishmaniasis With Liposomal Amphotericin B. Clin Infect Dis. 2015;61:945–949. doi: 10.1093/cid/civ416. [DOI] [PubMed] [Google Scholar]
- Marsden PD. Clinical presentations of Leishmania braziliensis braziliensis. Parasitol Today. 1985;1:129–133. doi: 10.1016/0169-4758(85)90057-2. [DOI] [PubMed] [Google Scholar]
- Marsden PD. Mucosal leishmaniasis due to Leishmania (Viannia) braziliensis L(V)b in Tres Bracos, Bahia-Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 1994;27:93–101. doi: 10.1590/s0037-86821994000200007. [DOI] [PubMed] [Google Scholar]
- Miranda JC, Reis E, Schriefer A, Goncalves M, Reis MG, Carvalho L, Fernandes O, Barral-Netto M, Barral A. Frequency of infection of Lutzomyia phlebotomines with Leishmania braziliensis in a Brazilian endemic area as assessed by pinpoint capture and polymerase chain reaction. Memorias do Instituto Oswaldo Cruz. 2002;97:185–188. doi: 10.1590/s0074-02762002000200006. [DOI] [PubMed] [Google Scholar]
- Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- Queiroz A, Sousa R, Heine C, Cardoso M, Guimaraes LH, Machado PR, Carvalho EM, Riley LW, Wilson ME, Schriefer A. Association between an emerging disseminated form of leishmaniasis and Leishmania (Viannia) braziliensis strain polymorphisms. J Clin Microbiol. 2012;50:4028–4034. doi: 10.1128/JCM.02064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero GA, Guerra MV, Paes MG, Macedo VO. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. Am J Trop Med Hyg. 2001;65:456–465. doi: 10.4269/ajtmh.2001.65.456. [DOI] [PubMed] [Google Scholar]
- Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat Immunol. 2002;3:1041–1047. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor Laboratory Press; New York, USA: 1989. [Google Scholar]
- Saúde Md. Saúde, S.d.V.d, editor. Manual de Vigilância da Leishmaniose Tegumentar Americana. 2 2010. [Google Scholar]
- Schriefer A, Guimaraes LH, Machado PR, Lessa M, Lessa HA, Lago E, Ritt G, Goes-Neto A, Schriefer AL, Riley LW, Carvalho EM. Geographic clustering of leishmaniasis in northeastern Brazil. Emerg Infect Dis. 2009;15:871–876. doi: 10.3201/eid1506.080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriefer A, Schriefer AL, Goes-Neto A, Guimaraes LH, Carvalho LP, Almeida RP, Machado PR, Lessa HA, de Jesus AR, Riley LW, Carvalho EM. Multiclonal Leishmania braziliensis population structure and its clinical implication in a region of endemicity for American tegumentary leishmaniasis. Infect Immun. 2004;72:508–514. doi: 10.1128/IAI.72.1.508-514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular biology and evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Turetz ML, Machado PR, Ko AI, Alves F, Bittencourt A, Almeida RP, Mobashery N, Johnson WD, Jr, Carvalho EM. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J Infect Dis. 2002;186:1829–1834. doi: 10.1086/345772. [DOI] [PubMed] [Google Scholar]
- Unger A, O'Neal S, Machado PR, Guimaraes LH, Morgan DJ, Schriefer A, Bacellar O, Glesby MJ, Carvalho EM. Association of treatment of American cutaneous leishmaniasis prior to ulcer development with high rate of failure in northeastern Brazil. Am J Trop Med Hyg. 2009;80:574–579. [PMC free article] [PubMed] [Google Scholar]
- Weirather JL, Jeronimo SM, Gautam S, Sundar S, Kang M, Kurtz MA, Haque R, Schriefer A, Talhari S, Carvalho EM, Donelson JE, Wilson ME. Serial quantitative PCR assay for detection, species-discrimination and quantification of Leishmania spp. in human samples. Journal of clinical microbiology. 2011 doi: 10.1128/JCM.r00764-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli-Nascimento RC, Miguel DC, Yokoyama-Yasunaka JK, Pereira LI, Pelli de Oliveira MA, Ribeiro-Dias F, Dorta ML, Uliana SR. In vitro sensitivity of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis Brazilian isolates to meglumine antimoniate and amphotericin B. Trop Med Int Health. 2010;15:68–76. doi: 10.1111/j.1365-3156.2009.02414.x. [DOI] [PubMed] [Google Scholar]





