Abstract
Melanoma represents a significant clinical problem affecting a large segment of the population with a relatively high incidence and mortality rate. Ultraviolet radiation (UVR) is an important etiological factor in malignant transformation of melanocytes and melanoma development. UVB, while being a full carcinogen in melanomagenesis, is also necessary for the cutaneous production of vitamin D3 (D3). Calcitriol (1,25(OH)2D3) and novel CYP11A1-derived hydroxyderivatives of D3 show anti-melanoma activities and protective properties against damage induced by UVB. The former activities include inhibitory effects on proliferation, plating efficiency and anchorage-independent growth of cultured human and rodent melanomas in vitro, as well as the in vivo inhibition of tumor growth by 20(OH)D3 after injection of human melanoma cells into immunodeficient mice. The literature indicates that low levels of 25(OH)D3 are associated with more advanced melanomas and reduced patient survivals, while single nucleotide polymorphisms of the vitamin D receptor or the D3 binding protein gene affect development or progression of melanoma, or disease outcome. An inverse correlation of VDR and CYP27B1 expression with melanoma progression has been found, with low or undetectable levels of these proteins being associated with poor disease outcomes. Unexpectedly, increased expression of CYP24A1 was associated with better melanoma prognosis. In addition, decreased expression of retinoic acid orphan receptors α and γ, which can also bind vitamin D3 hydroxyderivatives, showed positive association with melanoma progression and shorter disease-free and overall survival. Thus, inadequate levels of biologically active forms of D3 and disturbances in expression of the target receptors or D3 activating or inactivating enzymes, can affect melanomagenesis and disease progression. We therefore propose that inclusion of vitamin D into melanoma management should be beneficial for patients, at least as an adjuvant approach. The presence of multiple hydroxyderivatives of D3 in skin that show anti-melanoma activity in experimental models and which may act on alternative receptors, will be a future consideration when planning which forms of vitamin D to use for melanoma therapy.
Keywords: Melanoma, therapy, vitamin D, vitamin D receptor, retinoic acid orphan receptors
1. Introduction
Exposure to the highly energetic spectrum of ultraviolet radiation (UVB; λ=290–320 nm; 5% of total UVR reaching Earth surface), while representing a major risk factor for basal cell and squamous cell carcinomas (BCC and SCC) and melanomas, is also necessary for cutaneous production of vitamin D3 (D3) (Fig. 1)[1–5]. The transformation of 7-dehydrocholesterol (7DHC) to D3 after absorption of UVB energy by the unsaturated B ring represents the most fundamental reaction in photobiology [6–8], since the biologically active form of D3, 1,25(OH)2D3, not only regulates body calcium homeostasis, but also displays a variety of pleiotropic effects [7, 9, 10]. Importantly, these include radioprotective and anticarcinogenic activities [3, 5, 9, 11–14]. Moreover, skin supplies >90% of the body’s requirement for this prohormone.
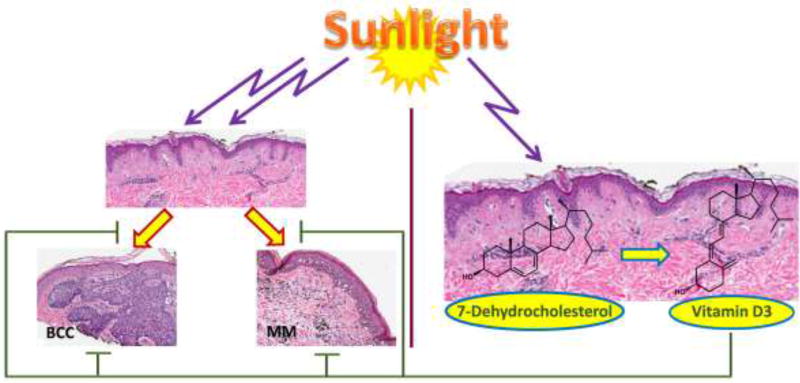
Figure 1.
Ultraviolet B acts as a double edge sword inducing skin cancer and producing vitamin D3 of which bioactive hydroxy-derivatives have photoprotective and anti-cancerogenic effects. BCC: basal cell carcinoma; MM: malignant melanoma.
Over 80% of non-melanoma skin cancers (NMSC) occur in sun-exposed sites, head, neck and the back of hands, attesting to a role for UVR in carcinogenesis. UVR is also considered as a full carcinogen for melanoma (Fig. 1) [4, 15–17]. UVR, depending on its wavelength, penetrates different layers of the skin affecting DNA integrity, cell and tissue homeostasis, inducing mutations, and modulating the expression of a plethora of genes including oncogenes and tumor suppressor genes [18–21]. It can also modify the expression and activity of growth factors/cytokines and their receptors, and has local and systemic immunosuppressive effects [22–27]. UVB absorbed by DNA can induce covalent bond formation between adjacent pyrimidines which leads to the production of mutagenic photoproducts such as cyclobutane pyrimidine dimers (CPD) and pyrimidine-pyrimidine adducts ((6–4)PPs)[19–21]. UVR also augments the production of reactive oxygen species (ROS) with deleterious effects on skin, and induces tumor suppressor factor p53 as a part of the response to DNA damage [28–30]. The net effects are skin aging, solar elastosis, precancerous states such as solar keratosis, and finally skin cancers such as SCC, BCC and melanoma. Also, excessive sun damage can induce a field of cancerization, which may complicate the traditional therapy of skin cancer [31–33].
In this review, we summarize experimental and clinical data from our laboratories and the literature demonstrating that active forms of vitamin D3 show potent anti-melanoma activity in experimental models, and that defects in vitamin D signaling can contribute to melanomagenesis and tumor progression, with implications in melanoma therapy and pathological risk assessment of the disease outcome. We also discuss a hypothesis that endogenously produced active forms of D3 can attenuate or reverse UVB induced damages with net antioxidative, antimutagenic, antigenotoxic and anti-proliferative effects.
2. Activation of vitamin D
2.1 Established (canonical) pathway of vitamin D activation
Vitamin D3 is produced in the skin by the photochemical opening of the B ring of 7-dehydrocholesterol by UVR. The initial product, previtamin D3, undergoes a slow thermal isomerization to vitamin D3 at skin temperature [34]. Vitamin D3 is a prohormone and is activated by two hydroxylations, the first at C25 by CYP2R1 or CYP27A1 in the liver to produce 25(OH)D3, and the second at C1α by CYP27B1 in the kidney to produce the active hormone, 1,25(OH)2D3 [34, 35]. 1,25(OH)2D3 can also be produced locally in the skin with CYP2R1, CYP27A1 and CYP27B1 being expressed in skin cells [35–38]. 1,25(OH)2D3 is inactivated by the action of CYP24A1, primarily in the kidney, which hydroxylates it at C24 then further oxidizes the side chain to the excretory product, calcitroic acid [39–42]. 25(OH)D3 can be metabolized by the same oxidation pathway. CYP24A1 is also expressed in the skin [35, 43].
2.2 CYP11A1-dependent (non-canonical) pathway of vitamin D activation
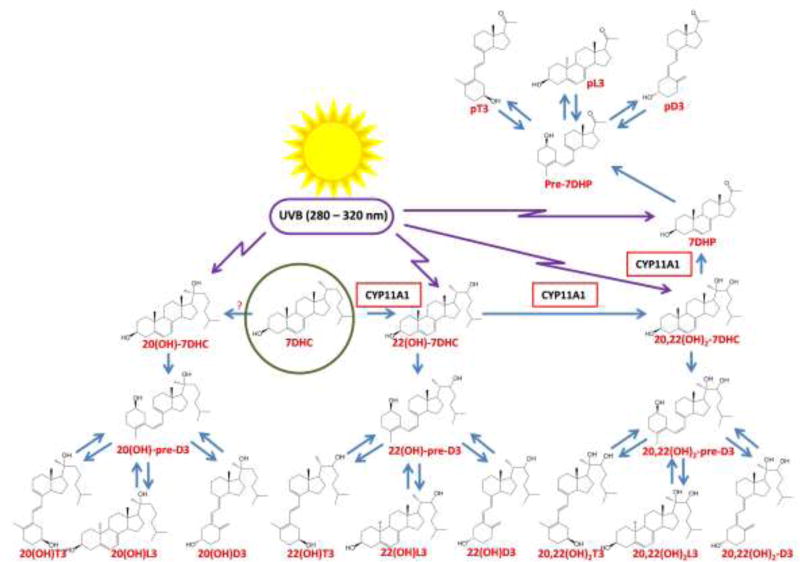
Over 15 years of experimental work has revealed an alternative pathway of vitamin D3 activation by CYP11A1 [44]. CYP11A1 is best known for initiating steroidogenesis where the side chain of cholesterol is hydroxylated and cleaved to produce pregnenolone (reviewed in [45, 46]. CYP11A1 can hydroxylate the side chain of vitamin D3 at C17, C20, C22 and/or C23, but no cleavage occurs. Hydroxylation at C20 is favored with 20S-hydroxyvitamin D3 [20(OH)D3] being the principal product. Other major products are 20,23(OH)2D3, 17,20(OH)2D3, 20,22(OH)2D3, 17,20,23(OH)3D3 and 22(OH)D3 [38, 47–51]. These secosteroids were originally identified as products of the enzyme in vitro. Subsequent studies where skin cells, or fragments of adrenal glands or placentae were incubated with D3 confirmed their production under ex vivo conditions [38]. Importantly, 22(OH)D3 and 20(OH)D3 are present in human serum in the nM range [52]. Many of the CYP11A1-derived hydroxy-secosteroids, including 20(OH)D3 and 20,23(OH)D3, are converted to their 1α-hydroxy derivatives by CYP27B1in vitro [53] and during ex vivo incubations [38] with 1,20,23(OH)3D3 and 1,20(OH)2D3 being present in human serum and/or the epidermis [52]. CYP27A1, CYP24A1 and CYP3A4 can act on 20(OH)D3 producing 20,24(OH)2D3, 20,25(OH)2D3 and/or 20,26(OH)2D3 [54–56] which have also been identified in serum [52]. These dihydroxy-metabolites are good substrates for CYP27B1 which hydroxylates them at C1α [53]. CYP24A1 can also act on 20,23(OH)2D3 producing 20,23,24(OH)3D3 and 20,23,25(OH)3D3 [55]. Thus, CYP11A1 initiates new pathways of vitamin D3 metabolism with initial products being acted on by the well characterized P450s of the canonical pathway, with 16-different hydroxyvitamin D3 metabolites being identified by NMR to date. These pathways can be regarded as activating vitamin D3 since both the primary CYP11A1-derived products and secondary products, including those produced by CYP24A1, display biological activity, as described later. Similarly, CYP11A1 can activate vitamin D2 producing 20(OH)D2, 17,20(OH)2D3 and 17,20,24(OH)3D3, with 20(OH)D2 being converted to 1,20(OH)2D3 by CYP27B1 [57–60].
2.3. Phototransformation of Δ7- hydroxysterols into hydroxy-secosteroids
Previous studies have demonstrated that CYP11A1 can convert 7DHC to 22(OH)-7DHC and 20,22(OH)2-7DHC with subsequent cleavage of the side chain producing 7-dehydropregnenolone (7DHP), in vitro [47, 61] and ex-vivo [62, 63], with some of the products detectable in the human epidermis and serum [52]. 7DHP can also be hydroxylated to Δ7-steroids by steroidogenic enzymes or have the remaining 2C side chain cleaved producing Δ7-androsta-steroids [62, 63]. In the skin, the breakage of the unsaturated B ring of these compounds by the absorption of UVB energy [6] causes their transformation into the corresponding secosteroids, with or without a full-length side chain, vitamin D-like and tachysterol-like, as well as lumisterol-like formed when the broken Bring reseals in a different stereochemical configuration to 7DHC (Fig. 2) [61, 64–69].
Figure 2.
UVB-induced transformation of 7DHC, its hydroxy-derivatives and 7DHP to their vitamin D-like and tachysterol-like secosteroids, as well as lumisterol-like configurations. Although the enzyme transforming 7DHC to 20(OH)7DHC must be identified there is a high probability that it is the same enzyme which transforms cholesterol to 20-hydroxycholesterol (CYP11A1 has very low activity for this). Not shown are the Δ7-products of 7DHP metabolism by steroidogenic enzymes, which are detectable in the steroidogenic tissues or serum under normal and pathological conditions [52, 62, 63, 164–166]. These Δ7-steroids can be transformed to their corresponding secosteroids after exposure to UVB [64–67, 69, 125].
3. Malignant melanoma
3.1. Introduction
Cutaneous melanomas represent a challenge for patients, clinicians and researchers because of the relatively high incidence and high mortality rates, as well as the relative resistance of metastatic disease to the therapy [70–74]. For example, for 2016 in the USA 7,630 new cases of melanoma were reported for this year with an estimated 10,130 death associated with this disease [70, 71]. Therefore, this disease deserves serious attention including its prevention, early and precise diagnosis, and adequate surgical removal of the tumor which may be curable for melanoma in situ or the invasive tumor at the radial growth phase (RGP) [73, 75, 76]. The pathologist plays a crucial role in this process by providing adequate diagnosis supported by synoptic reporting of the necessary prognostic factors as well as by performing ancillary studies relating to the therapy or for predicting prognosis.
Over the last decade, there has been a significant advancement in our understanding of the immune and molecular principles regulating melanoma behavior [74, 77–81]. This led to development of new therapeutic approaches including targeted therapy using BRAF and MEK inhibitors or immunotherapy also encompassing checkpoint inhibitor antibodies against CTLA-4, PD1 and PDL-1. Unfortunately, targeted therapy retains efficacy only for a short period and the disease relapses leading to the death of patients. In addition, checkpoint immunotherapy is effective in only a certain subset of patients, has toxic side effects and is very expensive. Thus, a new creative approach is required such as the use of natural products like vitamin D, in combination with the above strategies, to control tumor growth and to obtain a positive disease outcome [82, 83].
Such an approach appears to be supported by reports in the literature of an inverse relationship between 25(OH)D serum levels, melanoma thickness and patient survival, and polymorphisms in the genes encoding the vitamin D receptor (VDR) and the vitamin D binding protein [83–87]. This is consistent with the documented anti-cancer activity of vitamin D [9, 13, 88]. Furthermore, experiments on VDR or RXR knock-out mice have suggested a role for these receptors in the development of melanocytic tumors [89–91]. Below we summarize clinic-pathological correlations between the expression of VDR, CYP27B1, CYP24A1 and retinoic acid orphan receptors (RORs) with the progression of malignant melanoma.
3.2. Defects in vitamin signaling during melanomagenesis and melanoma progression
Clinical-based studies on melanoma patients have revealed that vitamin D plays an important role in the attenuation of melanoma development and tumor progression. Several studies show that the serum level of 25(OH)D3 is deficient in melanoma patients, and that there is a negative correlation between serum 25(OH)D concentrations and prognostic markers of melanoma such as tumor thickness [85, 92, 93], ulceration [84, 94], mitotic rate and histological type [94]. Consequently, lower 25(OH)D3 is accompanied by poorer melanoma prognosis [85, 87, 92, 93]. Recently published data also reveal that patients with melanomas formed on sun-exposed sites have higher levels of 25(OH)D than patients with melanomas on shielded-sites [95]. Some of these studies show that the 25(OH)D3/D2 concentration at the time of diagnosis is important for the prognosis of melanoma patients [84, 92, 94]. Others suggested that changes in the 25(OH)D3 concentration after diagnosis during follow-up are more important and could be a prognostic marker [96], or that both the initial 25(OH)D3 concentration and its changes during follow-up are predictors of melanoma patient prognosis [87]. Serum 25(OH)D levels can also influence the relationship between VDR polymorphisms and melanoma patient outcome [92]. This study also partly explained some inconsistencies in reports related to VDR polymorphisms and melanoma risk and outcome [83, 86, 93, 97–102].
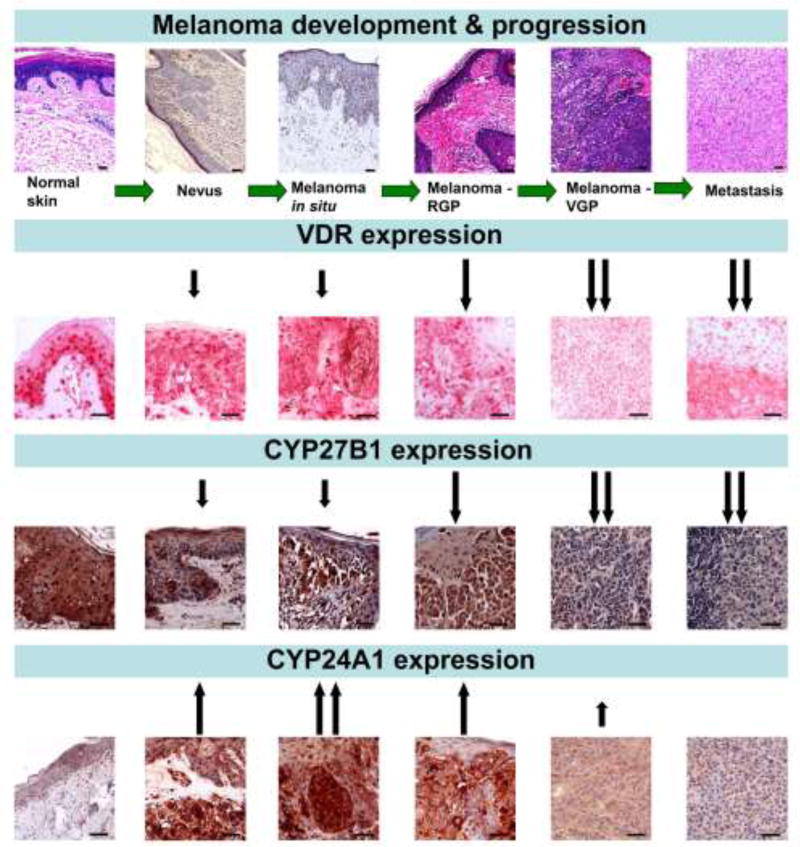
In our studies on clinical melanocytic tumor samples we have shown that changes of VDR expression correlate with clinico-pathomorphological features. Both cytoplasmic and nuclear VDR immunostaining decreased with the development de novo, or progression of tumors from nevi to primary melanomas and to metastatic disease (Fig. 3). Similarly, in primary melanomas increasing tumor advancement (thickness, depth, pT stage) correlates with decreasing VDR expression [103, 104]. Moreover, the presence of other poor prognostic markers such as ulceration, higher mitotic rate, absence of tumor-infiltrating lymphocytes, nodular histological type, vertical growth phase and the presence of local and distant metastases are also accompanied by decreased VDR expression [103, 104]. Consequently, patients with low or undetectable VDR expression in melanoma cells had a higher overall stage of the disease and showed shorter overall survival (Table 1). The statistically significant relationship between overall survival and VDR was seen for nuclear VDR but not cytoplasmic immunostaining (Table 1) [103].
Figure 3.
Changes in VDR, CYP27B1 and CYP24A1 expression during the progression of melanocytic tumors. Upper panel presents the routine H&E sections of normal skin, junctional melanocytic nevus and melanomas at different stages of progression. VDR expression was detected using rat antibody (clone 9A7; Abcam, Cambridge, MA, USA; a dilution 1:75) and visualized with Red AP Substrate (Vector Laboratories, Burlingame, CA, USA) [104]. CYP27B1 expression was immunostained using rabbit antibody (clone H-90, Santa Cruz Biotechnology, Santa Cruz, CA, USA, a dilution of 1:75) and visualized with ImmPACT NovaRED substrate (Vector Laboratories, Burlingame, CA, USA) [111]. CYP24A1 expression was detected with mouse antibody (Abcam, Cambridge, UK, dilution 1:40) and visualized with ImmPACT NovaRED substrates (Vector Laboratories, Burlingame, CA, USA) [112]. Arrows indicate the changes of the expression in relation to normal skin, scale bars - 50 µm.
Table 1.
Overall survival (OS) and disease-fee survival (DFS) of melanoma patients in relation to VDR, CYP27B1 and CYP24A1 expression in primary melanomas.
| Antigen | DFS time (median) [days] | OS time (median) [days] |
|---|---|---|
|
| ||
| Nuclear VDR | P>0.05 | P<0.05 |
| Absent | 539 | 810 |
| Present | 1607 | 2297 |
|
| ||
| Cytoplasmic VDR | P>0.05 | P>0.05 |
| Absent | 539 | 912 |
| Present | 1607 | 1018 |
|
| ||
| CYP27B1 | P<0.001 | P<0.01 |
| Absent | 471 | 748 |
| Present | 2520 | 1449 |
|
| ||
| CYP24A1 | P>0.05 | P<0.05 |
| Absent | 459 | 581 |
| Present | 1607 | 1807 |
Since epidemiological studies showed that melanoma patients had serum concentrations of 25(OH)D3 in the insufficient range at the time of diagnosis and/or during follow-up, we analyzed the expression of CYP27B1 in clinical samples of melanomas. Aside from kidney, CYP27B1 is widely expressed in peripheral tissues including skin [105–110]. In our study, similarly to VDR, CYP27B1 expression was higher in nevi, decreased in primary melanomas and was the lowest in metastatic lesions (Fig. 3). Also, the advancement of primary lesions (Breslow thickness, Clark’s level and pT stage) correlated with decreasing CYP27B1 expression. Lack of or low CYP27B1 expression was associated with the presence of markers of poor prognosis. Melanoma at the vertical growth phase and metastasizing melanomas had lower CYP27B1 expression than in the radial growing phase and non-metastasizing tumors, respectively. In melanomas with low CYP27B1 expression the proliferative activity was also elevated. Finally, better disease-free and overall survival was seen in patients with higher tumor CYP27B1 expression (Table 1) [111].
CYP24A1 expression changed during the progression of melanocytic tumors, however, the expression pattern was different from that of VDR and CYP27B1. In nevi and primary melanomas CYP24A1 expression was elevated when compared to normal skin, with the highest expression found in nevi and early stage melanomas (Breslow thickness ≤2mm, Clark level ≤ III, pT ≤ 2) [112]. Advanced melanomas and melanoma metastases showed decreased CYP24A1 expression (Fig. 3). CYP24A1 expression decreased in melanomas developing local and distant metastases (pN1–3, pM1) and melanomas at an advanced overall stage (stage 2–3). Furthermore, CYP24A1 expression was lower in melanomas showing a more aggressive phenotype such as nodular histological type, melanomas with higher proliferative index and with ulceration. Similarly to VDR and CYP27B1, lack of or decreased CYP24A1 expression was accompanied by shorter overall and disease-free survival (Table 1) [112]
Summarizing these findings, the disruption of the expression of VDR and of the vitamin D activating enzyme, CYP27B1, is associated with melanoma development and progression. The surprising positive correlation between the 1,25(OH)2D3 inactivating enzyme, CYP24A1, and disease outcome could either be a reflection of activation of the VDR that would stimulate CYP24A1 expression, as expected, or it may be secondary to the recently described additional function for CYP24A1 as an activator of 20(OH)D3 [40, 54].
3.3. Retinoic orphan acid receptors (RORs) and melanoma
Vitamin D metabolites can also function as inverse agonists for the retinoic acid-related orphan receptors (RORs), RORα and RORγ, and therefore provide an alternative mechanism by which vitamin D metabolites affect melanoma development [113, 114]. Both RORα and RORγ were found to be expressed in human melanocytic tumors samples, but their expression varied and was dependent on the clinico-pathomorphological features of the tumors [113, 115]. Both RORα and RORγ expression decreased with the progression of the tumors from nevi to primary melanomas to metastases. In addition, increasing advancement of primary melanomas, as assessed by Clark’s level, Breslow thickness and pT stage, was accompanied by decreasing levels of RORα and RORγ expression. Similarly, melanomas developing metastases were also characterized by the reduced expression of RORα and RORγ [115]. The lack of or decreased expression of these receptors was also related to the presence of negative prognostic melanoma markers such as nodular histological type of melanoma, the lack of tumor-infiltrating lymphocytes, the presence of ulceration, and a high proliferation index. Immunohistochemistry showed that cytoplasmic and nuclear RORα and RORγ levels were dependent on the clinico-pathomorphological features of the melanomas. Correspondingly, expression of RORα and RORγ correlated with less advanced overall stage, better prognosis and longer disease-free and overall survival (Table 2). The relationship with overall survival (OS) and disease-free survival (DFS) was observed for both cytoplasmic and nuclear RORγ and for nuclear RORα (only for OS). This trend was also observed after adjustment for Breslow thickness (OS and DFS) and overall stage (DFS) in Cox proportional-hazards regression analysis [115].
Table 2.
Expression of RORα and RORγ in primary melanomas can serve as a prognostic factor for the disease outcome.
| Antigen | DFS time (median) [days] | OS time (median) [days] |
|---|---|---|
|
| ||
| Nuclear RORα | P>0.05 | P<0.01 |
| Absent | 780 | 530 |
| Present | 1607 | 1748 |
|
| ||
| Cytoplasmic RORα | P>0.05 | P>0.05 |
| Absent | 1076 | 972 |
| Present | 1607 | 1499 |
|
| ||
| Nuclear RORγ | P<0.05 | P<0.05 |
| Absent | 539 | 912 |
| Present | 1607 | 2297 |
|
| ||
| Cytoplasmic RORγ | P<0.01 | P<0.01 |
| Absent | 471 | 912 |
| Present | 1607 | 2297 |
The data were extracted from [115]. P value - Log-rank (Mantel-Cox) Test
OS: overall survival, DFS: disease fee survival
3. Testing the anti-melanoma activity of active forms of vitamin D
3.1. Overview on canonical active forms of vitamin D
The work of Colston and colleagues originally demonstrated the anti-melanoma activity of 1,25(OH)2D3 and the presence of VDR receptors in melanoma cells [116]. Shortly thereafter, the inhibitory effects of 1,24,25(OH)3D3 and 1,25,26(OH)3D3 on melanoma cells were also demonstrated [117] and production of 1,25(OH)2D3 and of 24,25(OH)2D3 by melanoma cells was shown [118]. Since then several papers have demonstrated the anti-melanoma activity of 1,25(OH)2D3 and its analogs in cell culture, and the expression of VDR and vitamin D activating- and inactivating enzymes in different melanomas (for most recent reviews see [2, 83, 97, 119]). Most frequently used in these studies were human melanoma lines and rodent melanomas including murine and hamster lines. The responsiveness of melanoma cells to active forms of vitamin D was cell-type specific and dependent on the culture conditions. Interestingly, some human melanomas were not responsive to vitamin D analogs and there were conflicting results on vitamin D effects in B16 and Cloudman murine melanomas (reviewed in [83]). Furthermore, active forms of vitamin D have been shown to display protection against UVR-induced damage, including in melanocytes and against photocarcinogenesis [3, 11, 120–122]. CYP11A1-derived 20(OH)D3 also shows photoprotective properties in model systems of both cultured human skin cells (at 10−7 M [12] and 10−7 M [123]) and murine skin in vivo (23 or 46 pmol/cm2), comparable to equivalent concentrations 1,25(OH)2D3 [12, 124]. Finally, results from Dr Indra’s laboratory clearly demonstrate the protective role of VDR against UVR-induced damage in murine melanocytes in vivo [91].
3.2. Antimelanoma activity of CYP11A1-derived secosteroids
Novel vitamin D derivatives with a full length or short side chain, as listed in Table 3, have been tested for their in vitro anti-melanoma activity using established lines of human (SKMEL-188, WM35, WM1341, WM164, WM98D, SBCE2, YUROB, YUKSI and YULAC and hamster (Bomirski AbC1 and Ab) melanomas [53, 54, 59, 62, 65–67, 125–131]. Among the compounds with a complete side chain the most extensively studied are 20(OH)D2, 20(OH)D3, 20,23(OH)2D3 and 1,20(OH)2D3. They show inhibition of cell proliferation in monolayer, inhibition of plating efficiency (colony formation in monolayer) and inhibition of anchorage-independent cell growth (ability to grow in soft agar) with potencies similar to that of 1,25(OH)2D3 (Table 3). Inhibition of cell proliferation by 20(OH)D was related to arrest in the G1/G0 phase of the cell cycle and a decrease in S and G2/M phases, with no effect on subG1 (an indicator of apoptosis) [59]. Daily intraperitoneal injections of 20(OH)D3, 20,23(OH)2D3 and 20(OH)D2 into rodents have shown that these secosteroids are non-calcemic at the highest doses tested, 60, 3 and 4 µg/kg, respectively [59, 132–134]. The CYP27B1-catalysed hydroxylation product, 1,20(OH)2D3, also shows reduced calcemic activity in comparison to 1,25(OH)2D3 [134].
Table 3.
Anti-melanoma activities of novel secosteroids
| Compound | in vitro inhibitory effects | in vivo inhibitory effects |
|---|---|---|
| 20(OH)D2 | Colony formation in monolayer [59] | ND |
| Proliferation [59, 129] | ||
| 20(OH)D3 | Anchorage-independent growth [53, 54, 128, 130] | Tumor volume and geometric mean of tumor dimensions (NSG mice on a vitamin D-deficient diet) [128] |
| Chemotactic capacity [128] | ||
| Colony formation in monolayer [128, 130] | ||
| Migratory capability, cell-ECM interactions and cell-cell interactions [128] | ||
| Proliferation [129, 130, 139, 167] | ||
| 1,20(OH)2D3 | Colony formation in monolayer [125, 130] | ND |
| Proliferation [125, 130] | ||
| 20,23(OH)2D3 | Anchorage-independent growth [130] | ND |
| Colony formation in monolayer [130] | ||
| Proliferation [125, 127, 130] | ||
| 20,24(OH)2D3 | Anchorage-independent growth [54] | ND |
| Proliferation [126] | ||
| 20,25(OH)2D3 | Anchorage-independent growth [53, 54] | ND |
| 20,26(OH)2D3 | Anchorage-independent growth [53, 54] | ND |
| 1,20,23(OH)3D3 | Proliferation [127] | ND |
| 1,20,24(OH)3D3 | Proliferation [126] | ND |
| 1,20,25(OH)3D3 | Anchorage-independent growth [53] | ND |
| 1,20,26(OH)3D3 | Anchorage-independent growth [53] | ND |
| 17,20,23(OH)3D3 | Proliferation [125] | ND |
| pD3 | Anchorage-independent growth [62, 67, 129] | ND |
| Proliferation [125, 129] | ||
| 21(OH)pD | Anchorage-independent growth [66] | ND |
| Proliferation [66, 129] | ||
| 20(OH)pD | Anchorage-independent growth [65, 129] | ND |
| Proliferation [65, 67, 125] |
The in vitro effects of the compounds tested were measured in comparison to 1,25(OH)2D3. The in vivo effect of 1,25(OH)2D3 in the experimental model described in [128] was not tested due to its toxicity at the testing dose.
Abbreviations: NSG: NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice; ND: not determined
It is important here to discuss the dietary supply of vitamin D to laboratory animals through commercially available chow. Vitamin D3 from the diet is easily converted to the active forms in the liver, kidney and other tissues, including malignant ones, equipped with enzymatic machinery [135]. Although the affinity of 25(OH)D3 for the VDR is approximately 500-fold less than that of 1,25(OH)2D3, its presence at high concentrations may affect the outcome of in vivo experiments, either directly or by increased conversion to1,25(OH)2D3 [136]. As shown in a preclinical study, a 5-fold rise in vitamin D3 levels in the mouse chow led to an increase of 50% and 145% in serum 25(OH)D serum levels and resulted in significant (50–60%) shrinkage of xenograft breast and prostate tumors [136]. Therefore, in order to properly establish the role of vitamin D in melanoma progression it is crucial to eliminate any external sources of vitamin D (diet and UVB exposure) to avoid compromising the interpretation of results. This should allow for better assessment of the true extent of tumor inhibition due to treatment with the secosteroids under study. According to the American Institute of Nutrition guidelines, the optimal content of vitamin D in rodent chow is 0.025 mg/kg (0.65 µmol or 1.0 IU/g) [137]. Nowadays the regular rodent chows contain 2.39 – 4.6 IU/g of vitamin D3, with the average daily chow intake of 5 g/mouse [138] (LabDiet, St. Louis, MO; Harlan Lab., Madison, WI). In mice, hypercalcemia, manifested as calcium depositions in vital organs, has been observed at a dose as low as 5.0 mg/kg for vitamin D3 and 25(OH)D3, and 0.1 µg/kg for 1,25(OH)2D3 [135]. In our experiments employing 20(OH)D3 we used 30 µg/kg/day [128] (Table 3), with 60 µg/kg still being non-calcemic [133].
The lack of hypercalcemia at very high doses of 20(OH)D3 led us to test this compound more extensively. Specifically, 20(OH)D3 inhibited nuclear factor-kappa B (NF-κB) activity in human SKMEl-188 melanoma cells with concomitant reduction of melanoma proliferation [139]. It should be noted that in human melanomas, NF-κB is upregulated leading to a poor disease outcome [140, 141]. In addition, 20(OH)D3 caused inhibition of the migratory capabilities of the cells and cell-cell and cell-extracellular matrix interactions using transwell cell migration and spheroid toxicity assays [128]. Importantly, 20(OH)D3 at intraperitoneal daily doses of 30 µg/kg inhibited melanoma tumor growth in immunocompromised old female NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice kept on a vitamin D-deficient diet (TD.89123, Harlan Laboratories, Madison, WI) [128], without visible signs of toxicity. Interestingly, the total body score was higher in the 20(OH)D3 treatment group as compared to the control group (2.8 vs. 2.55) [128]. The body score (BS) scale is a 5-point practical, rapid, noninvasive methods to assess the overall health condition of an animal [142]. A score of 3 indicates the optimal health state and any deviation from this value (above or below) indicates worsening of body condition. Precursor vitamin D3 at the same dose had no effect on tumor growth (Skobowiat et al., unpublished) indicating the requirement for vitamin D activation in order for it to exert anti-melanoma activity.
20(OH)D3 is not only further hydroxylated by CYP11A1 producing other bioactive secosteroids [49, 143] but is also hydroxylated by CYP27A1, CYP24A1 and CYP3A4 to produce 20,24(OH)2D3, 20,25(OH)2D3 and 20,26(OH)2D3, as described above [53, 54, 56]. These are more potent and efficacious than their precursor 20(OH)D3 at inhibiting melanoma growth in soft agar [53, 54]. These secosteroids are excellent substrates for CYP27B1 [53, 126]. However, they and their 1α-hydroxyderivatives show similar antiproliferative potencies against keratinocytes [114]. Also, hydroxylation at C1α had rather minimal effect on their anti-melanoma activity [53, 54].
With respect to the mechanism of action of CYP11A1-derived vitamin D compounds with a full-length side chain, they inhibit melanoma growth in a similar manner independent of the presence or absence of hydroxyl at C1α. The involvement of VDR in this process is indicated by amplification of growth inhibition by 20(OH)D2 in melanoma cells overexpressing VDR [59]. 20(OH)D3 and its hydroxyderivatives, with or without C1α(OH), cause translocation of the VDR from the cytoplasm to the nucleus in melanoma and other skin cells with high potency, comparable to that of 1,25(OH)2D3. This is consistent with their high docking scores predicted by molecular modeling, indicating high affinity for binding to the genomic site of the VDR, as reported previously [114, 143, 144]. However, their interaction with VDR-ligand binding domain using the LanthaScreen TR-FRET Vitamin D receptor Coactivator kit required the presence of the C1α(OH). Thus, for the synthetic VDRE used in this coactivator binding assay, the absence of the C1α(OH) presumably prevents the full conformational change necessary to promote binding of the coactivator. This indicates that the appropriate VDRE and complete receptor complex in the cellular environment are necessary for proper interaction of the VDR with ligands having hydroxyl groups solely on the side chain and not at C1α [114]. Therefore, we suggest that CYP11A1-derived D3 hydroxyderivatives with hydroxyl groups placed only on the side chain can act as biased agonists (the term “biased” was defined by Kenakin [145, 146] for selective activity on specific receptors), or as partial agonists (as we proposed previously [143]) on VDR in melanoma cells, selectively causing some but not all of the phenotypic effects of 1,25(OH)2D3 [114, 143]. Examples of the latter are the relatively poor activation of CYP24A1 expression and the lack of calcemia by 20(OH)D3. An example of the former (similar potency and efficacy to 1,25(OH)2D3) is the inhibition of melanoma cell colony formation by 20(OH)D3 [54, 128, 130]. We are in the process of further addressing these exciting observations through microarray and genomic technology, as well as crystallographic studies aimed at obtaining the structure of 20(OH)D3 bound to the VDR ligand binding domain (see also section 3.3).
As described earlier, there are alternative nuclear receptors for CYP11A1-derived secosteroids, the RORs [113, 114]. In the above context, the use of melanoma cell lines with silenced or overexpressed VDR or RORs should provide an indication of which particular receptor is involved in the regulation of selective phenotypic traits of melanoma by the CYP11A1-derived secosteroids.
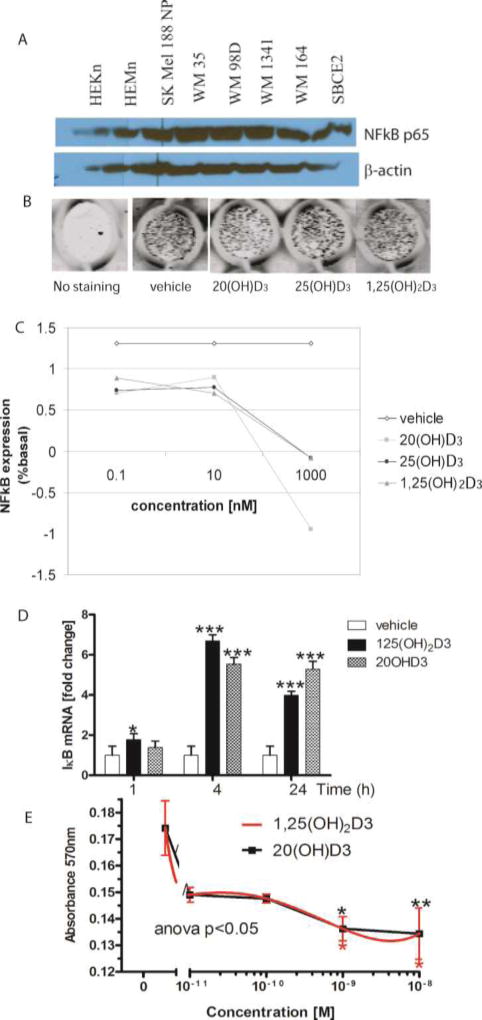
Another downstream mechanism of inhibition of skin cell proliferation, including melanoma, by 20(OH)D3 and 20,23(OH)2D3 is down-regulation of NF-κB activity [139, 147, 148] (Fig. 4). The mechanism of action of these secosteroids on NF-κB is very similar to that mediated by 1,25(OH)2D3, i.e., stimulation of the expression of IkappaBalpha (IκBα) with subsequent sequestration of NF-κB in the cytoplasm and consequent attenuation of transcriptional and phenotypic activities [139, 147, 148] (Fig. 4). Although recent data suggest that the VDR is involved in the upstream regulation of NF-κB signaling [149, 150], the involvement of RORs cannot be entirely ruled out, since 1,25(OH)2D3 may also act as an inverse agonist on RORα and RORγ [114], with the latter being placed upstream of pro-inflammatory responses [151, 152]. Of note, it has been proposed that upregulation of NF-κB can be secondary to a deregulation in upstream pathways including Ras/Raf, PI3K/Akt, and NIK that play a role in melanoma genesis [140, 141]. NF-κB, being involved in the regulation of apoptosis, angiogenesis, tumor cell invasion and tumor progression, is a druggable target for melanoma therapy [139–141, 153, 154].
Figure 4. NF-kB as a target for bioregulation by hydroxyvitamin D3 in melanoma.
A. Basal expression of NF-kB in human melanoma lines and normal epidermal melanocytes and keratinocytes. Whole cell extracts were prepared from human melanoma lines or skin cells and were subjected to Western blotting as described previously [139, 148]. B-actin served as an internal control for equal amounts of proteins loaded onto the gel.
B, C. 20(OH)D3 decreases protein expression of NF-κB in keratinocytes in a dose-dependent manner. HaCaT keratinocytes were plated in a 96-well plate and incubated for 24 h with 1,25(OH)2D3, 20(OH)D3 or 25(OH)D3. Cells were fixed and stained with NF-κB antibody [148], followed by incubation with secondary IR antibody. The plate was scanned using an Odyssey infrared scanner (B) and data were analyzed using software for in-cell western (LI COR) (C).
D. Expression of the gene for IκBα (NF-kB inhibitor) is increased in human melanoma upon treatment with 1,25(OH)2D3 or 20(OH)D3. SKMel-188 cells were treated with 1,25(OH)2D3, 20(OH)D3 or ethanol (vehicle) for 1, 4 or 24 h. Cells were harvested for RNA isolation. cDNA was used for RT-PCR to test the expression of IκBα as described previously [139, 147, 148]. Reactions were performed using TaqMan. Cyclophillin B was used as internal control. Data were analyzed using the delta-delta CT method and presented as a fold change. Statistical analysis was performed using the t-test (*p<0.05; ***p<0.001) in comparison to vehicle.
E. 20(OH)D3 and 1,25(OH)2D3 inhibit growth of human melanoma cells. SKMel-188 cells were treated with 1,25(OH)2D3 or 20(OH)D3 at different concentrations (10−8–10−11 M) for 48 h. After incubation, the MTT assay was performed and absorbance was recorded at 570 nm. P<0.05*, p<0.01**, p<0.001***.
Analogs of pregna- or androsta-calciferols (secosteroidal or lumisterol compounds with a short or no side chain) also show therapeutically desirable anti-melanoma activities comparable to 1,25(OH)2D3 [62, 65–67, 125, 129] (Table 3). Interestingly, their antiproliferative activities, at least under certain experimental conditions (in vitro melanoma models) did not require translocation of VDR to the nucleus [129], consistent with previous molecular modeling predicting weak binding [144]. In addition, the pregnacalciferol compounds lack calcemic effects [155]. Thus, they may be considered as good candidates for treatment of VDR-negative melanomas, however the details of their mechanism of action require further investigation.
3. 3. Towards pre-clinical and clinical testing of novel forms of vitamin D3
Patient-derived orthotopic xenografts (PDOX) models of melanoma have been used to test their sensitivity to molecularly-targeted drugs, standard chemotherapeutics, as well as live therapeutics such as tumor-targeting bacteria [156–160]. For example, a BRAF-V600E-mutant melanoma obtained from the right chest wall of a patient was grown orthotopically in the right chest wall of nude mice to establish a PDOX model. Trametinib (TRA), a MEK inhibitor, was the only agent of the 4 tested that caused tumor regression. In contrast, another MEK inhibitor, cobimetinib (COB), could slow but not arrest growth or cause regression of the melanoma. First-line therapy with temozolomide could slow, but not arrest tumor growth or cause regression. Since the patient had a BRAF-V600E-mutant melanoma, the patient would be considered as a strong candidate for vemurafenib (VEM) as first-line therapy, since VEM targets this mutation. However, VEM was not effective in the PDOX. The PDOX model thus helped identify the very-high efficacy of TRA against the melanoma PDOX and is a promising drug for this patient. These results demonstrate the powerful precision of the PDOX model for cancer therapy, not achievable by genomic analysis alone [156–160]. Therefore, these models are the most suitable for preclinical testing the efficacy of novel non-or low-calcemic vitamin D analogs discussed above in melanoma therapy and whether high doses of vitamin D, applied either orally or parenterally, can slow tumor progression or improve efficacy of the BRAF or MEK inhibitors.
A major metabolic modification to the D3 structure in the noncanonical pathway is the hydroxylation at the C20 position to stereo-specifically form 20S(OH)D3. Both 20S(OH)D3 and its non-natural stereo-isomer 20R(OH)D3 can be chemically synthesized, and they show comparable potency with 1,25(OH)2D3 in stimulating VDR gene expression [68, 131, 161]. Further hydroxylations to the 20(OH)D3 scaffold, performed chemically or enzymatically, produced a number of di-hydroxylated analogs such as 20S,23S(OH)2D3, 20S,23R(OH)2D3, 20S,24S(OH)2D3, 20S,24R(OH)2D3, and their 1α(OH)-tri-hydroxylated derivatives [126, 127]. While all these derivatives demonstrate similar anti-melanoma and anti-inflammatory activities, the addition of the 1α(OH) was found to significantly enhance downstream VDR transcriptional activity of CYP24A1 and TRPV6, and VDR translocation to the nucleus, presumably because of the increased binding affinity/altered conformational change contributed by the 1α(OH) (discussed in section 3.2) [114, 126, 127]. Clarification of their interactions with the VDR will require obtaining crystal structures of ligand bound to the VDR-LBD, which for 20S(OH)D3 and 1,20S(OH)2D3 are being generated in our laboratories (Li et al., in preparation). In addition to hydroxylation to the parental 20(OH)D3 scaffold, chemically synthesizing Gemini 20(OH)D3 analogs by adding a second identical side chain, or changing the composition of the side chain has also been explored [133, 162]. Interestingly, there were no clear correlations between the Gemini chain length or side chain composition and their VDR activation or anti-melanoma activities, suggesting high flexibility in the ligand-binding pocket of the VDR [133, 162]. The above compounds will be tested for their anti-melanoma activity in the PDOX models described above.
Most recently a pilot placebo-controlled randomized phase II trial has been started by the Australia and New Zealand Melanoma Trials Group (ANZMTG 02.09) to assess the feasibility, safety and toxicity of an oral loading dose of 500,000 IU of vitamin D, followed by an oral dose of 50,000 IU of vitamin D monthly for 2 years in patients who have been treated for cutaneous melanoma by wide excision of the primary tumor [163]. Patients with stage IIb, IIc, IIIa (N1a, N2a) or IIIb (N1a, N2a) disease were included for randomization 2:1 to vitamin D treatment or placebo. The study will determine whether vitamin D is both safe and tolerable under these conditions and whether it will prolong time to recurrence within 5 years, or improve overall survival at 5 years [163]. Although limited in patient numbers (75), this is an important step in testing vitamin D as an adjuvant agent in melanoma therapy. Results of safety will be presented at World Melanoma Congress in Brisbane Australia, October 2017.
4. Perspective and conclusion
Based on the above, it is clear that vitamin D deficiency, defects in vitamin D activation, transport, activation of corresponding receptors and downstream signaling play a role in melanoma development and progression. Aside from avoidance of solar radiation, the preventive measures could include use of high doses of precursor vitamin D or its active forms that would fulfill the definition of endogenous or natural products. In addition to the oral route of delivery of vitamin D, the active forms could be applied topically to increase their efficacy in radioprotection and/or anti-cancerogenic effects. It must be noted that the vast majority of orally delivered vitamin D will be hydroxylated in position 25 in the liver making it unavailable for biotransformation by CYP11A1, because 25(OH)D is not acted on by this enzyme [44, 48]. Therefore, in order for vitamin D to be metabolized by the non-canonical pathway it would have to be of skin origin, or applied topically or parenterally to be metabolized in the skin or in steroidogenic tissues including adrenals, for systemic use [38, 44].
With respect to melanoma therapy or prevention of metastatic disease after removal of the primary tumor, high doses of vitamin D could be applied either orally or parenterally. Definitively, vitamin D could be included into any therapeutic regime as the adjuvant, since melanoma patients are under strict physician supervision that would identify any early signs of toxicity. This could be an economical measure to possibly amplify the therapeutic efficacy of the first line drugs [82]. As optimal melanoma therapy represents a challenge for the patients [73], testing of new naturally derived active forms of vitamin D, alone, or in combination using appropriate preclinical models may represent a dawn of a new inexpensive and less toxic therapy. Thus, while UVB is a full melanoma carcinogen, products of its activity could be used in prevention or treatment of this devastating disease.
Highlights.
-
-
Active forms of vitamin D inhibit growth of melanomas in vitro and in vivo
-
-
Defects in vitamin D receptors and activating enzymes affect melanomagenesis and melanoma progression
-
-
Active forms of vitamin D warrant pre- and clinical testing against melanoma
-
-
Vitamin D can be used as an adjuvant factor in melanoma therapy
Acknowledgments
Writing of this mini-review and cited studies were supported by grants from NIH (R21AR066505, 1R01AR056666, 2R01AR052190 and 1 R01AR071189 to AS) and (1R21AR063242, 1S10OD010678, 1S10RR026377 to WL), 1I01BX003395-01 from VA to CE, the University of Western Australia to RCT; and the Intramural Research Program of the NIEHS, NIH (Z01-ES-101586 to AMJ), 2014/15/B/ NZ4/00751 from National Science Centre, Poland and CO/ZPNiP/1/2013 from Oncology Centre-Prof. Franciszek Łukaszczyk Memorial Hospital to AAB, N402 662840 from the Polish Ministry of Science and Higher Education to MAZ, APP1070688 NHMRC, Australia to RSM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest
References
- 1.Holick MF. Biological Effects of Sunlight, Ultraviolet Radiation, Visible Light, Infrared Radiation and Vitamin D for Health. Anticancer Res. 2016;36:1345–1356. [PubMed] [Google Scholar]
- 2.Reichrath J, Rass K. Ultraviolet damage, DNA repair and vitamin D in nonmelanoma skin cancer and in malignant melanoma: an update. Adv Exp Med Biol. 2014;810:208–233. doi: 10.1007/978-1-4939-0437-2_12. [DOI] [PubMed] [Google Scholar]
- 3.Dixon KM, Tongkao-On W, Sequeira VB, Carter SE, Song EJ, Rybchyn MS, Gordon-Thomson C, Mason RS. Vitamin D and death by sunshine. Int J Mol Sci. 2013;14:1964–1977. doi: 10.3390/ijms14011964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikle DD. Vitamin D receptor, UVR, and skin cancer: a potential protective mechanism. J Invest Dermatol. 2008;128:2357–2361. doi: 10.1038/jid.2008.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Bikle DD. Vitamin D: an ancient hormone. Exp Dermatol. 2011;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 9.Bikle DD, Oda Y, Tu CL, Jiang Y. Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J Steroid Biochem Mol Biol. 2015;148:47–51. doi: 10.1016/j.jsbmb.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song EJ, Gordon-Thomson C, Cole L, Stern H, Halliday GM, Damian DL, Reeve VE, Mason RS. 1alpha,25-Dihydroxyvitamin D3 reduces several types of UV-induced DNA damage and contributes to photoprotection. J Steroid Biochem Mol Biol. 2013;136:131–138. doi: 10.1016/j.jsbmb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Slominski AT, Janjetovic Z, Kim TK, Wasilewski P, Rosas S, Hanna S, Sayre RM, Dowdy JC, Li W, Tuckey RC. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J Steroid Biochem Mol Biol. 2015;148:52–63. doi: 10.1016/j.jsbmb.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant WB. Roles of Solar UVB and Vitamin D in Reducing Cancer Risk and Increasing Survival. Anticancer Res. 2016;36:1357–1370. [PubMed] [Google Scholar]
- 14.Reichrath J, Reichrath S, Heyne K, Vogt T, Roemer K. Tumor suppression in skin and other tissues via cross-talk between vitamin D- and p53-signaling. Front Physiol. 2014;5:166. doi: 10.3389/fphys.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 16.Kauvar ANB, Cronin TJ, Roenigk R, Hruza G, Bennett R. Consensus for Nonmelanoma Skin Cancer Treatment: Basal Cell Carcinoma, Including a Cost Analysis of Treatment Methods. Dermatologic Surgery. 2015;41:550–571. doi: 10.1097/DSS.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 17.Slominski AT, Zmijewski MA, Semak I, Zbytek B, Pisarchik A, Li W, Zjawiony J, Tuckey RC. Cytochromes p450 and skin cancer: role of local endocrine pathways. Anticancer Agents Med Chem. 2014;14:77–96. doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Runger TM. How different wavelengths of the ultraviolet spectrum contribute to skin carcinogenesis: the role of cellular damage responses. J Invest Dermatol. 2007;127:2103–2105. doi: 10.1038/sj.jid.5700988. [DOI] [PubMed] [Google Scholar]
- 19.Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci U S A. 2006;103:13765–13770. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeifer GP, Besaratinia A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci. 2012;11:90–97. doi: 10.1039/c1pp05144j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wondrak GT, Roberts MJ, Cervantes-Laurean D, Jacobson MK, Jacobson EL. Proteins of the extracellular matrix are sensitizers of photo-oxidative stress in human skin cells. J Invest Dermatol. 2003;121:578–586. doi: 10.1046/j.1523-1747.2003.12414.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 23.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34:827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kripke ML. Ultraviolet radiation and immunology: something new under the sun--presidential address. Cancer Res. 1994;54:6102–6105. [PubMed] [Google Scholar]
- 25.Skobowiat C, Postlethwaite AE, Slominski AT. Skin Exposure to Ultraviolet B Rapidly Activates Systemic Neuroendocrine and Immunosuppressive Responses. Photochem Photobiol. 2016 doi: 10.1111/php.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norval M, Halliday GM. The consequences of UV-induced immunosuppression for human health. Photochem Photobiol. 2011;87:965–977. doi: 10.1111/j.1751-1097.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- 27.Slominski AT. Ultraviolet radiation (UVR) activates central neuro-endocrine-immune system. Photodermatol Photoimmunol Photomed. 2015;31:121–123. doi: 10.1111/phpp.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heck DE, Vetrano AM, Mariano TM, Laskin JD. UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J Biol Chem. 2003;278:22432–22436. doi: 10.1074/jbc.C300048200. [DOI] [PubMed] [Google Scholar]
- 29.Oda K, Arakawa H, Tanaka T, Matsuda K, Tanikawa C, Mori T, Nishimori H, Tamai K, Tokino T, Nakamura Y, Taya Y. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell. 2000;102:849–862. doi: 10.1016/s0092-8674(00)00073-8. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Chen J, Gan S, Guan H, Zhou Y, Ouyang Q, Shi J. DNA damage strength modulates a bimodal switch of p53 dynamics for cell-fate control. BMC Biol. 2013;11:73. doi: 10.1186/1741-7007-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philipp-Dormston WG. Field cancerization: from molecular basis to selective field-directed management of actinic keratosis. Curr Probl Dermatol. 2015;46:115–121. doi: 10.1159/000366547. [DOI] [PubMed] [Google Scholar]
- 32.Dika E, Fanti PA, Misciali C, Vaccari S, Crisman G, Barisani A, Baraldi C, Ribero S, Patrizi A. Risk of skin cancer development in 672 patients affected by actinic keratosis. G Ital Dermatol Venereol. 2016;151:628–633. [PubMed] [Google Scholar]
- 33.Stockfleth E. The importance of treating the field in actinic keratosis. J Eur Acad Dermatol Venereol. 2017;31(Suppl 2):8–11. doi: 10.1111/jdv.14092. [DOI] [PubMed] [Google Scholar]
- 34.Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol. 2013;5:51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skrede S, Bjorkhem I, Kvittingen EA, Buchmann MS, Lie SO, East C, Grundy S. Demonstration of 26-hydroxylation of C27-steroids in human skin fibroblasts, and a deficiency of this activity in cerebrotendinous xanthomatosis. J Clin Invest. 1986;78:729–735. doi: 10.1172/JCI112633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vantieghem K, Overbergh L, Carmeliet G, De Haes P, Bouillon R, Segaert S. UVB-induced 1,25(OH)2D3 production and vitamin D activity in intestinal CaCo-2 cells and in THP-1 macrophages pretreated with a sterol Delta7-reductase inhibitor. J Cell Biochem. 2006;99:229–240. doi: 10.1002/jcb.20910. [DOI] [PubMed] [Google Scholar]
- 38.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, Tuckey RC. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–3915. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Tieu EW, Tang EK, Tuckey RC. Kinetic analysis of human CYP24A1 metabolism of vitamin D via the C24-oxidation pathway. FEBS J. 2014;281:3280–3296. doi: 10.1111/febs.12862. [DOI] [PubMed] [Google Scholar]
- 41.Makin G, Lohnes D, Byford V, Ray R, Jones G. Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem J. 1989;262:173–180. doi: 10.1042/bj2620173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy GS, Tserng KY. Calcitroic acid, end product of renal metabolism of 1,25-dihydroxyvitamin D3 through C-24 oxidation pathway. Biochemistry. 1989;28:1763–1769. doi: 10.1021/bi00430a051. [DOI] [PubMed] [Google Scholar]
- 43.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39:365–379. doi: 10.1016/j.ecl.2010.02.010. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2015;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26:273–281. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine Reviews. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci U S A. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, Miller D, Slominski A. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275:2585–2596. doi: 10.1111/j.1742-4658.2008.06406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuckey RC, Nguyen MN, Slominski A. Kinetics of vitamin D3 metabolism by cytochrome P450scc (CYP11A1) in phospholipid vesicles and cyclodextrin. Int J Biochem Cell Biol. 2008;40:2619–2626. doi: 10.1016/j.biocel.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, Chen J, Howell DE, Benson HA, Sweatman T, Baldisseri DM, Slominski A. Production of 22-hydroxy-metabolites of vitamin D3 by cytochrome P450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos. 2011 doi: 10.1124/dmd.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EK, Tuckey RC. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep. 2015;5:14875. doi: 10.1038/srep14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang EK, Chen J, Janjetovic Z, Tieu EW, Slominski AT, Li W, Tuckey RC. Hydroxylation of CYP11A1-derived products of vitamin D3 metabolism by human and mouse CYP27B1. Drug Metab Dispos. 2013;41:1112–1124. doi: 10.1124/dmd.113.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tieu EW, Tang EK, Chen J, Li W, Nguyen MN, Janjetovic Z, Slominski A, Tuckey RC. Rat CYP24A1 acts on 20-hydroxyvitamin D(3) producing hydroxylated products with increased biological activity. Biochem Pharmacol. 2012;84:1696–1704. doi: 10.1016/j.bcp.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tieu EW, Li W, Chen J, Kim TK, Ma D, Slominski AT, Tuckey RC. Metabolism of 20-hydroxyvitamin D3 and 20,23-dihydroxyvitamin D3 by rat and human CYP24A1. J Steroid Biochem Mol Biol. 2015;149:153–165. doi: 10.1016/j.jsbmb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng CY, Slominski AT, Tuckey RC. Hydroxylation of 20-hydroxyvitamin D3 by human CYP3A4. J Steroid Biochem Mol Biol. 2016;159:131–141. doi: 10.1016/j.jsbmb.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, Tuckey RC. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–2901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen MN, Slominski A, Li W, Ng YR, Tuckey RC. Metabolism of vitamin D2 to 17,20,24-trihydroxyvitamin D2 by cytochrome p450scc (CYP11A1) Drug Metab Dispos. 2009;37:761–767. doi: 10.1124/dmd.108.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, Li W, Chen J, Nguyen MN, Tang EK, Miller D, Chen TC, Holick M. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300:C526–541. doi: 10.1152/ajpcell.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slominski AT, Kim TK, Shehabi HZ, Tang EK, Benson HA, Semak I, Lin Z, Yates CR, Wang J, Li W, Tuckey RC. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol. 2014;383:181–192. doi: 10.1016/j.mce.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, R CT. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, Zjawiony JK, Tuckey RC. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One. 2009;4:e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, Sweatman T, Janjetovic Z, Tuckey RC. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol. 2012;44:2003–2018. doi: 10.1016/j.biocel.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives. Photochem Photobiol Sci. 2008;7:1570–1576. doi: 10.1039/b809005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74:218–228. doi: 10.1016/j.steroids.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, Miller DD, Slominski AT. Synthesis and photochemical transformation of 3beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76:193–203. doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slominski A, Kim TK, Zmijewski MA, Janjetovic Z, Li W, Chen J, Kusniatsova EI, Semak I, Postlethwaite A, Miller DD, Zjawiony JK, Tuckey RC. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinol. 2013;5:7–19. doi: 10.4161/derm.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, Slominski A. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids. 2010;75:926–935. doi: 10.1016/j.steroids.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J, Slominski AT, Miller DD, Li W. Effects of sidechain length and composition on the kinetic conversion and product distribution of vitamin D analogs determined by real-time NMR. Dermatoendocrinol. 2013;5:142–149. doi: 10.4161/derm.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 71.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 72.Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol. 2014;170:11–19. doi: 10.1111/bjd.12492. [DOI] [PubMed] [Google Scholar]
- 73.Slominski AT, Carlson JA. Melanoma resistance: a bright future for academicians and a challenge for patient advocates. Mayo Clin Proc. 2014;89:429–433. doi: 10.1016/j.mayocp.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rajkumar S, Watson IR. Molecular characterisation of cutaneous melanoma: creating a framework for targeted and immune therapies. Br J Cancer. 2016;115:145–155. doi: 10.1038/bjc.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 76.Carlson JA, Ross JS, Slominski A, Linette G, Mysliborski J, Hill J, Mihm M., Jr Molecular diagnostics in melanoma. J Am Acad Dermatol. 2005;52:743–775. doi: 10.1016/j.jaad.2004.08.034. quiz 775–748. [DOI] [PubMed] [Google Scholar]
- 77.Schadendorf D, Fisher DE, Garbe C, Gershenwald JE, Grob J-J, Halpern A, Herlyn M, Marchetti MA, McArthur G, Ribas A, Roesch A, Hauschild A. Melanoma. Nature Reviews Disease Primers. 2015 doi: 10.1038/nrdp.2015.3. [DOI] [PubMed] [Google Scholar]
- 78.Lo JA, Fisher DE. The melanoma revolution: from UV carcinogenesis to a new era in therapeutics. Science. 2014;346:945–949. doi: 10.1126/science.1253735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwong LN, Davies MA. Targeted therapy for melanoma: rational combinatorial approaches. Oncogene. 2014;33:1–9. doi: 10.1038/onc.2013.34. [DOI] [PubMed] [Google Scholar]
- 80.Su MY, Fisher DE. Immunotherapy in the Precision Medicine Era: Melanoma and Beyond. PLoS Med. 2016;13:e1002196. doi: 10.1371/journal.pmed.1002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levesque MP, Cheng PF, Raaijmakers MI, Saltari A, Dummer R. Metastatic melanoma moves on: translational science in the era of personalized medicine. Cancer Metastasis Rev. 2017;36:7–21. doi: 10.1007/s10555-017-9658-0. [DOI] [PubMed] [Google Scholar]
- 82.Slominski AT, Brozyna A, Jozwicki W, Tuckey RC. Vitamin D as an adjuvant in melanoma therapy. Melanoma Manag. 2015;2:1–4. doi: 10.2217/mmt.14.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slominski AT, Brozyna AA, Zmijewski MA, Jozwicki W, Jetten AM, Mason RS, Tuckey RC, Elmets CA. Vitamin D signaling and melanoma: role of vitamin D and its receptors in melanoma progression and management. Lab Invest. 2017 doi: 10.1038/labinvest.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Newton-Bishop JA, Davies JR, Latheef F, Randerson-Moor J, Chan M, Gascoyne J, Waseem S, Haynes S, O’Donovan C, Bishop DT. 25-Hydroxyvitamin D2 /D3 levels and factors associated with systemic inflammation and melanoma survival in the Leeds Melanoma Cohort. Int J Cancer. 2015;136:2890–2899. doi: 10.1002/ijc.29334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wyatt C, Lucas RM, Hurst C, Kimlin MG. Vitamin D deficiency at melanoma diagnosis is associated with higher Breslow thickness. PLoS One. 2015;10:e0126394. doi: 10.1371/journal.pone.0126394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Orlow I, Reiner AS, Thomas NE, Roy P, Kanetsky PA, Luo L, Paine S, Armstrong BK, Kricker A, Marrett LD, Rosso S, Zanetti R, Gruber SB, Anton-Culver H, Gallagher RP, Dwyer T, Busam K, Begg CB, Berwick M G.E.M.S. Group. Vitamin D receptor polymorphisms and survival in patients with cutaneous melanoma: a population-based study. Carcinogenesis. 2016;37:30–38. doi: 10.1093/carcin/bgv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Timerman D, McEnery-Stonelake M, Joyce CJ, Nambudiri VE, Hodi FS, Claus EB, Ibrahim N, Lin JY. Vitamin D deficiency is associated with a worse prognosis in metastatic melanoma. Oncotarget. 2017;8:6873–6882. doi: 10.18632/oncotarget.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 89.Coleman DJ, Garcia G, Hyter S, Jang HS, Chagani S, Liang X, Larue L, Ganguli-Indra G, Indra AK. Retinoid-X-receptors (alpha/beta) in melanocytes modulate innate immune responses and differentially regulate cell survival following UV irradiation. PLoS Genet. 2014;10:e1004321. doi: 10.1371/journal.pgen.1004321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coleman DJ, Chagani S, Hyter S, Sherman AM, Lohr CV, Liang X, Ganguli-Indra G, Indra AK. Loss of keratinocytic RXRalpha combined with activated CDK4 or oncogenic NRAS generates UVB-induced melanomas via loss of p53 and PTEN in the tumor microenvironment. Mol Cancer Res. 2015;13:186–196. doi: 10.1158/1541-7786.MCR-14-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chagani S, Kyryachenko S, Yamamoto Y, Kato S, Ganguli-Indra G, Indra AK. In vivo role of Vitamin D Receptor (VDR) signaling in UVB induced DNA damage and melanocyte homeostasis. J Invest Dermatol. 2016 doi: 10.1016/j.jid.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 92.Newton-Bishop JA, Beswick S, Randerson-Moor J, Chang YM, Affleck P, Elliott F, Chan M, Leake S, Karpavicius B, Haynes S, Kukalizch K, Whitaker L, Jackson S, Gerry E, Nolan C, Bertram C, Marsden J, Elder DE, Barrett JH, Bishop DT. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27:5439–5444. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Randerson-Moor JA, Taylor JC, Elliott F, Chang YM, Beswick S, Kukalizch K, Affleck P, Leake S, Haynes S, Karpavicius B, Marsden J, Gerry E, Bale L, Bertram C, Field H, Barth JH, Silva Idos S, Swerdlow A, Kanetsky PA, Barrett JH, Bishop DT, Bishop JA. Vitamin D receptor gene polymorphisms, serum 25-hydroxyvitamin D levels, and melanoma: UK case-control comparisons and a meta-analysis of published VDR data. Eur J Cancer. 2009;45:3271–3281. doi: 10.1016/j.ejca.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lim A, Shayan R, Varigos G. High serum vitamin D level correlates with better prognostic indicators in primary melanoma: A pilot study. Australas J Dermatol. 2017 doi: 10.1111/ajd.12648. [DOI] [PubMed] [Google Scholar]
- 95.Paolino G, Moliterni E, Didona D, Garelli V, Corsetti P, Lopez T, Richetta AG, Cantisani C, Bottoni U, Calvieri S. Clinicopathological features, vitamin D serological levels and prognosis in cutaneous melanoma of shield-sites: an update. Med Oncol. 2015;32:451. doi: 10.1007/s12032-014-0451-4. [DOI] [PubMed] [Google Scholar]
- 96.Saiag P, Aegerter P, Vitoux D, Lebbe C, Wolkenstein P, Dupin N, Descamps V, Aractingi S, Funck-Brentano E, Autier P, Dragomir M, Boniol M. Prognostic Value of 25-hydroxyvitamin D3 Levels at Diagnosis and During Follow-up in Melanoma Patients. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv264. djv264. [DOI] [PubMed] [Google Scholar]
- 97.Szyszka P, Zmijewski MA, Slominski AT. New vitamin D analogs as potential therapeutics in melanoma. Expert Rev Anticancer Ther. 2012;12:585–599. doi: 10.1586/era.12.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uitterlinden AG, Fang Y, van Meurs JB, van Leeuwen H, Pols HA. Vitamin D receptor gene polymorphisms in relation to Vitamin D related disease states. J Steroid Biochem Mol Biol. 2004;89–90:187–193. doi: 10.1016/j.jsbmb.2004.03.083. [DOI] [PubMed] [Google Scholar]
- 99.Orlow I, Roy P, Reiner AS, Yoo S, Patel H, Paine S, Armstrong BK, Kricker A, Marrett LD, Millikan RC, Thomas NE, Gruber SB, Anton-Culver H, Rosso S, Gallagher RP, Dwyer T, Kanetsky PA, Busam K, From L, Begg CB, Berwick M G.E.M.S. Group. Vitamin D receptor polymorphisms in patients with cutaneous melanoma. Int J Cancer. 2012;130:405–418. doi: 10.1002/ijc.26023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee YH, Gyu Song G. Vitamin D receptor FokI, BsmI, TaqI, ApaI, and EcoRV polymorphisms and susceptibility to melanoma: a meta-analysis. J BUON. 2015;20:235–243. [PubMed] [Google Scholar]
- 101.Li C, Liu Z, Wang LE, Gershenwald JE, Lee JE, Prieto VG, Duvic M, Grimm EA, Wei Q. Haplotype and genotypes of the VDR gene and cutaneous melanoma risk in non-Hispanic whites in Texas: a case-control study. Int J Cancer. 2008;122:2077–2084. doi: 10.1002/ijc.23357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li C, Liu Z, Zhang Z, Strom SS, Gershenwald JE, Prieto VG, Lee JE, Ross MI, Mansfield PF, Cormier JN, Duvic M, Grimm EA, Wei Q. Genetic variants of the vitamin D receptor gene alter risk of cutaneous melanoma. J Invest Dermatol. 2007;127:276–280. doi: 10.1038/sj.jid.5700544. [DOI] [PubMed] [Google Scholar]
- 103.Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum Pathol. 2011;42:618–631. doi: 10.1016/j.humpath.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brozyna AA, Jozwicki W, Slominski AT. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: new data and analyses. Anticancer Res. 2014;34:2735–2743. [PMC free article] [PubMed] [Google Scholar]
- 105.Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347:80–89. doi: 10.1016/j.mce.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–992. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- 107.Diesel B, Radermacher J, Bureik M, Bernhardt R, Seifert M, Reichrath J, Fischer U, Meese E. Vitamin D(3) metabolism in human glioblastoma multiforme: functionality of CYP27B1 splice variants, metabolism of calcidiol, and effect of calcitriol. Clin Cancer Res. 2005;11:5370–5380. doi: 10.1158/1078-0432.CCR-04-1968. [DOI] [PubMed] [Google Scholar]
- 108.Flanagan JN, Whitlatch LW, Chen TC, Zhu XH, Holick MT, Kong XF, Holick MF. Enhancing 1 alpha-hydroxylase activity with the 25-hydroxyvitamin D-1 alpha-hydroxylase gene in cultured human keratinocytes and mouse skin. J Invest Dermatol. 2001;116:910–914. doi: 10.1046/j.1523-1747.2001.01360.x. [DOI] [PubMed] [Google Scholar]
- 109.Radermacher J, Diesel B, Seifert M, Tilgen W, Reichrath J, Fischer U, Meese E. Expression analysis of CYP27B1 in tumor biopsies and cell cultures. Anticancer Res. 2006;26:2683–2686. [PubMed] [Google Scholar]
- 110.Tangpricha V, Flanagan JN, Whitlatch LW, Tseng CC, Chen TC, Holt PR, Lipkin MS, Holick MF. 25-hydroxyvitamin D-1alpha-hydroxylase in normal and malignant colon tissue. Lancet. 2001;357:1673–1674. doi: 10.1016/S0140-6736(00)04831-5. [DOI] [PubMed] [Google Scholar]
- 111.Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of the vitamin D-activating enzyme 1alpha-hydroxylase (CYP27B1) decreases during melanoma progression. Hum Pathol. 2013;44:374–387. doi: 10.1016/j.humpath.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brozyna AA, Jochymski C, Janjetovic Z, Jozwicki W, Tuckey RC, Slominski AT. CYP24A1 expression inversely correlates with melanoma progression: clinic-pathological studies. Int J Mol Sci. 2014;15:19000–19017. doi: 10.3390/ijms151019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey RC, Jetten AM. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–2789. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Slominski AT, Kim TK, Hobrath JV, Oak AS, Tang EK, Tieu EW, Li W, Tuckey RC, Jetten AM. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as "biased" agonists on VDR and inverse agonists on RORalpha and RORgamma. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brozyna AA, Jozwicki W, Skobowiat C, Jetten A, Slominski AT. RORalpha and RORgamma expression inversely correlates with human melanoma progression. Oncotarget. 2016;7:63261–63282. doi: 10.18632/oncotarget.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–1086. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- 117.Frampton RJ, Omond SA, Eisman JA. Inhibition of human cancer cell growth by 1,25-dihydroxyvitamin D3 metabolites. Cancer Res. 1983;43:4443–4447. [PubMed] [Google Scholar]
- 118.Eisman JA, Sher E, Suva LJ, Frampton RJ, McLean FL. 1 alpha, 25-Dihydroxyvitamin D3 specifically induces its own metabolism in a human cancer cell line. Endocrinology. 1984;114:1225–1231. doi: 10.1210/endo-114-4-1225. [DOI] [PubMed] [Google Scholar]
- 119.Reichrath J, Reichrath S. Sunlight, vitamin D and malignant melanoma: an update. Adv Exp Med Biol. 2014;810:390–405. doi: 10.1007/978-1-4939-0437-2_22. [DOI] [PubMed] [Google Scholar]
- 120.Mason RS, Reichrath J. Sunlight vitamin D and skin cancer. Anticancer Agents Med Chem. 2013;13:83–97. [PubMed] [Google Scholar]
- 121.Gordon-Thomson C, Tongkao-on W, Song EJ, Carter SE, Dixon KM, Mason RS. Protection from ultraviolet damage and photocarcinogenesis by vitamin D compounds. Adv Exp Med Biol. 2014;810:303–328. doi: 10.1007/978-1-4939-0437-2_17. [DOI] [PubMed] [Google Scholar]
- 122.Dixon KM, Deo SS, Wong G, Slater M, Norman AW, Bishop JE, Posner GH, Ishizuka S, Halliday GM, Reeve VE, Mason RS. Skin cancer prevention: a possible role of 1,25dihydroxyvitamin D3 and its analogs. J Steroid Biochem Mol Biol. 2005;97:137–143. doi: 10.1016/j.jsbmb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 123.Tonkgao-on A. Role of Vitamin D and Other Compounds in the Protection of Skin Cells from UV. University of Sydney, Sydney., University of Sydney, Sydney; 2015. [Google Scholar]
- 124.Tongkao-On W, Carter S, Reeve VE, Dixon KM, Gordon-Thomson C, Halliday GM, Tuckey RC, Mason RS. CYP11A1 in skin: an alternative route to photoprotection by vitamin D compounds. J Steroid Biochem Mol Biol. 2015;148:72–78. doi: 10.1016/j.jsbmb.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 125.Slominski A, Tuckey RC, Zmijewski MA, Li W, Zjawiony J, Janjetovic Z, Zbytek B, Nguyen MN, Miller D, Chen J. Enzymatic production or chemical synthesis and uses for 5,7-dienes and UVB conversion products thereof. 2009 PCT/US2009/001324. [Google Scholar]
- 126.Lin Z, Marepally SR, Ma D, Myers LK, Postlethwaite AE, Tuckey RC, Cheng CY, Kim TK, Yue J, Slominski AT, Miller DD, Li W. Chemical Synthesis and Biological Activities of 20S,24S/R-Dihydroxyvitamin D3 Epimers and Their 1alpha-Hydroxyl Derivatives. J Med Chem. 2015;58:7881–7887. doi: 10.1021/acs.jmedchem.5b00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin Z, Marepally SR, Ma D, Kim TK, Oak AS, Myers LK, Tuckey RC, Slominski AT, Miller DD, Li W. Synthesis and Biological Evaluation of Vitamin D3 Metabolite 20S,23S–Dihydroxyvitamin D3 and Its 23R Epimer. J Med Chem. 2016;59:5102–5108. doi: 10.1021/acs.jmedchem.6b00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Skobowiat C, Oak AS, Kim TK, Yang CH, Pfeffer LM, Tuckey RC, Slominski AT. Noncalcemic 20-hydroxyvitamin D3 inhibits human melanoma growth in in vitro and in vivo models. Oncotarget. 2017;8:9823–9834. doi: 10.18632/oncotarget.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wasiewicz T, Szyszka P, Cichorek M, Janjetovic Z, Tuckey RC, Slominski AT, Zmijewski MA. Antitumor effects of vitamin D analogs on hamster and mouse melanoma cell lines in relation to melanin pigmentation. Int J Mol Sci. 2015;16:6645–6667. doi: 10.3390/ijms16046645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slominski AT, Janjetovic Z, Kim TK, Wright AC, Grese LN, Riney SJ, Nguyen MN, Tuckey RC. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–3742. [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Q, Lin Z, Kim TK, Slominski AT, Miller DD, Li W. Total synthesis of biologically active 20S-hydroxyvitamin D3. Steroids. 2015 doi: 10.1016/j.steroids.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, Postlethwaite AE, Miller D, Li W. 20-hydroxyvitamin D(3) inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–746. [PMC free article] [PubMed] [Google Scholar]
- 133.Chen J, Wang J, Kim TK, Tieu EW, Tang EK, Lin Z, Kovacic D, Miller DD, Postlethwaite A, Tuckey RC, Slominski AT, Li W. Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res. 2014;34:2153–2163. [PMC free article] [PubMed] [Google Scholar]
- 134.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, Holick MF. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deluca HF, Prahl JM, Plum LA. 1,25-Dihydroxyvitamin D is not responsible for toxicity caused by vitamin D or 25-hydroxyvitamin D. Arch Biochem Biophys. 2011;505:226–230. doi: 10.1016/j.abb.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 136.Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA, Feldman D. Dietary vitamin D(3) and 1,25-dihydroxyvitamin D(3) (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology. 2012;153:2576–2587. doi: 10.1210/en.2011-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Subcommittee on Laboratory Animal Nutrition, B.o.A. Committee on Animal Nutrition, N.R. Council. Nutrient Requirements of Laboratory Animals: Fourth Revised Edition, 1995. NATIONAL ACADEMY PRESS, 2101 Constitution Avenue; N.W. Washington, D.C: 1995. p. 20418. [Google Scholar]
- 138.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, Pfeffer SR, Pfeffer LM, Slominski AT. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–1884. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ueda Y, Richmond A. NF-kappaB activation in melanoma. Pigment Cell Res. 2006;19:112–124. doi: 10.1111/j.1600-0749.2006.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Amiri KI, Richmond A. Role of nuclear factor-kappa B in melanoma. Cancer Metastasis Rev. 2005;24:301–313. doi: 10.1007/s10555-005-1579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ullman-Cullere MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci. 1999;49:319–323. [PubMed] [Google Scholar]
- 143.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol. 2014;144(Pt A):28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim TK, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, Tang EK, Miller D, Li W, Slominski AT. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol Cell Endocrinol. 2012;361:143–152. doi: 10.1016/j.mce.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kenakin T. What is pharmacological ‘affinity’? Relevance to biased agonism and antagonism. Trends Pharmacol Sci. 2014;35:434–441. doi: 10.1016/j.tips.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 146.Kenakin T. Biased agonism. F1000 Biol Rep. 2009;1:87. doi: 10.3410/B1-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, Slominski AT. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr, Slominski AT. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol. 2010;223:36–48. doi: 10.1002/jcp.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen YH, Yu Z, Fu L, Wang H, Chen X, Zhang C, Lv ZM, Xu DX. Vitamin D3 inhibits lipopolysaccharide-induced placental inflammation through reinforcing interaction between vitamin D receptor and nuclear factor kappa B p65 subunit. Sci Rep. 2015;5:10871. doi: 10.1038/srep10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Martinez-Moreno JM, Herencia C, Montes de Oca A, Munoz-Castaneda JR, Rodriguez-Ortiz ME, Diaz-Tocados JM, Peralbo-Santaella E, Camargo A, Canalejo A, Rodriguez M, Velasco-Gimena F, Almaden Y. Vitamin D modulates tissue factor and protease-activated receptor 2 expression in vascular smooth muscle cells. FASEB J. 2016;30:1367–1376. doi: 10.1096/fj.15-272872. [DOI] [PubMed] [Google Scholar]
- 151.Jetten AM, Kang HS, Takeda Y. Retinoic acid-related orphan receptors alpha and gamma: key regulators of lipid/glucose metabolism, inflammation, and insulin sensitivity. Front Endocrinol (Lausanne) 2013;4:1. doi: 10.3389/fendo.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cook DN, Kang HS, Jetten AM. Retinoic Acid-Related Orphan Receptors (RORs): Regulatory Functions in Immunity, Development, Circadian Rhythm, and Metabolism. Nucl Receptor Res. 2015;2 doi: 10.11131/2015/101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marton A, Kusz E, Kolozsi C, Tubak V, Zagotto G, Buzas K, Quintieri L, Vizler C. Vanillin Analogues o-Vanillin and 2,4,6-Trihydroxybenzaldehyde Inhibit NFkB Activation and Suppress Growth of A375 Human Melanoma. Anticancer Res. 2016;36:5743–5750. doi: 10.21873/anticanres.11157. [DOI] [PubMed] [Google Scholar]
- 154.Takeda T, Tsubaki M, Sakamoto K, Ichimura E, Enomoto A, Suzuki Y, Itoh T, Imano M, Tanabe G, Muraoka O, Matsuda H, Satou T, Nishida S. Mangiferin, a novel nuclear factor kappa B-inducing kinase inhibitor, suppresses metastasis and tumor growth in a mouse metastatic melanoma model. Toxicol Appl Pharmacol. 2016;306:105–112. doi: 10.1016/j.taap.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 155.Holick MF, Garabedian M, Schnoes HK, DeLuca HF. Relationship of 25-hydroxyvitamin D3 side chain structure to biological activity. J Biol Chem. 1975;250:226–230. [PubMed] [Google Scholar]
- 156.Yamamoto M, Zhao M, Hiroshima Y, Zhang Y, Shurell E, Eilber FC, Bouvet M, Noda M, Hoffman RM. Efficacy of Tumor-Targeting Salmonella A1-R on a Melanoma Patient-Derived Orthotopic Xenograft (PDOX) Nude-Mouse Model. PLoS One. 2016;11:e0160882. doi: 10.1371/journal.pone.0160882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kawaguchi K, Murakami T, Chmielowski B, Igarashi K, Kiyuna T, Unno M, Nelson SD, Russell TA, Dry SM, Li Y, Eilber FC, Hoffman RM. Vemurafenib-resistant BRAF-V600E–mutated melanoma is regressed by MEK-targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2016;7:71737–71743. doi: 10.18632/oncotarget.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kawaguchi K, Igarashi K, Murakami T, Chmielowski B, Kiyuna T, Zhao M, Zhang Y, Singh A, Unno M, Nelson SD, Russell TA, Dry SM, Li Y, Eilber FC, Hoffman RM. Tumor-targeting Salmonella typhimurium A1-R combined with temozolomide regresses malignant melanoma with a BRAF-V600E mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget. 2016;7:85929–85936. doi: 10.18632/oncotarget.13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kawaguchi K, Igarashi K, Murakami T, Zhao M, Zhang Y, Chmielowski B, Kiyuna T, Nelson SD, Russell TA, Dry SM, Li Y, Unno M, Eilber FC, Hoffman RM. Tumor-targeting Salmonella typhimurium A1-R Sensitizes Melanoma with a BRAF-V600E Mutation to Vemurafenib in a Patient-derived Orthotopic Xenograft (PDOX) Nude Mouse Model. J Cell Biochem. 2017 doi: 10.1002/jcb.25886. [DOI] [PubMed] [Google Scholar]
- 160.Kawaguchi K, Igarashi K, Chmielowski B, Murakami T, Kiyuna T, Zhao M, Zhang Y, Nelson SD, Russell TA, Dry SM, Li Y, Unno M, Eilber FC, Hoffman RM. Salmonella typhimurium A1-R targeting of a chemotherapy resistant BRAF-V600E melanoma in a patient-derived orthotopic xenograft (PDOX) model is enhanced in combination with either vemurafenib-temozlomide. Cell Cycle. 2017 doi: 10.1080/15384101.2017.1314420. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lu Y, Chen J, Janjetovic Z, Michaels P, Tang EK, Wang J, Tuckey RC, Slominski AT, Li W, Miller DD. Design, Synthesis, and Biological Action of 20R-Hydroxyvitamin D3. J Med Chem. 2012;55:3573–3577. doi: 10.1021/jm201478e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lin Z, Marepally SR, Kim TK, Janjetovic Z, Oak AS, Postlethwaite AE, Myers LK, Tuckey RC, Slominski AT, Miller DD, Li W. Design, Synthesis and Biological Activities of Novel Gemini 20S-Hydroxyvitamin D3 Analogs. Anticancer Res. 2016;36:877–886. [PMC free article] [PubMed] [Google Scholar]
- 163.Saw RP, Armstrong BK, Mason RS, Morton RL, Shannon KF, Spillane AJ, Stretch JR, Thompson JF. Adjuvant therapy with high dose vitamin D following primary treatment of melanoma at high risk of recurrence: a placebo controlled randomised phase II trial (ANZMTG 02.09 Mel-D) BMC Cancer. 2014;14:780. doi: 10.1186/1471-2407-14-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shackleton C, Roitman E, Guo LW, Wilson WK, Porter FD. Identification of 7(8) and 8(9) unsaturated adrenal steroid metabolites produced by patients with 7-dehydrosterol-delta7-reductase deficiency (Smith-Lemli-Opitz syndrome) J Steroid Biochem Mol Biol. 2002;82:225–232. doi: 10.1016/s0960-0760(02)00155-3. [DOI] [PubMed] [Google Scholar]
- 165.Shackleton CH. Role of a disordered steroid metabolome in the elucidation of sterol and steroid biosynthesis. Lipids. 2012;47:1–12. doi: 10.1007/s11745-011-3605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guo LW, Wilson WK, Pang J, Shackleton CH. Chemical synthesis of 7- and 8-dehydro derivatives of pregnane-3,17alpha,20-triols, potential steroid metabolites in Smith-Lemli-Opitz syndrome. Steroids. 2003;68:31–42. doi: 10.1016/s0039-128x(02)00113-7. [DOI] [PubMed] [Google Scholar]
- 167.Wang Q, Lin Z, Kim TK, Slominski AT, Miller DD, Li W. Total synthesis of biologically active 20S-hydroxyvitamin D3. Steroids. 2015;104:153–162. doi: 10.1016/j.steroids.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]