Abstract
Long-term treatment of glaucoma, a major leading cause of blindness, is challenging due to poor patient compliance. Therefore, a drug delivery device that can achieve drug release over several months can be highly beneficial for glaucoma management. Here, we evaluate the long-term pharmacokinetics and therapeutic efficacy of polycaprolactone intracameral drug delivery devices in rabbit eyes. Our study showed that a single drug delivery device loaded with a proprietary hypotensive agent, DE-117, reduced intraocular pressure in normotensive rabbits significantly for 23 weeks. In addition, we demonstrated that concentration of DE-117 and its hydrolyzed active form (hDE-117) was maintained in the aqueous humor and the target tissue (iris-ciliary body) up to 24 weeks. Our proof-of-concept glaucoma implant shows potential as a long-term treatment that circumvents patient compliance barriers compared to current treatment via eye drops.
Keywords: ocular drug delivery, glaucoma, implant
Graphical Abstract
1. Introduction
Glaucoma, a group of ocular disorders characterized by progressive optic neuropathy [1], is a major leading cause of irreversible blindness worldwide [2]. A global projection estimated that the number of patients with glaucoma may reach 111.8 million in 2040 [2]. Previous studies have shown that intraocular pressure (IOP) is a major risk factor in glaucoma progression [3] and control of IOP is important in slowing progression of visual field defects [4]. Therefore, glaucoma is often treated with one or more hypotensive drugs, each applied by patients as eye drops up to 3 times daily. However, patient compliance has been a continuing challenge in efficacious glaucoma therapy [5]. For example, a study that assessed patient compliance of a topical once-daily treatment (Travatan, Alcon, Fort Worth, TX, USA) found that the mean adherence rate was 0.71, even though the patients were aware that they were being monitored electronically [6]. Reasons for non-compliance included forgetfulness and difficulty using eye drops [7]. Not surprisingly, poor patient compliance has been linked with worsened visual field defect severity [8].
Due to issues with patient compliance, several drug delivery approaches have been explored to provide a long-term therapy solution. For example, microparticles with a poly(D, L-lactic-co-glycolic acid) (PLGA) core and a poly(lactic acid) (PLA) shell were explored for long-term subconjunctival delivery of brimonidine tartrate, which is typically administered topically three times daily [9]. Alternatively, nanostructured PLGA microparticles with a mucoadhesive polymer additive (polyethylene glycol (PEG)) were used to increase preocular particle retention of drug delivery particles [10]. These brimonidine-loaded particles were able to prolong the activity period of brimonidine more than two-fold when compared to topical eye drops (Alphagan P, Allergan, Irvine, CA, USA) [10]. Furthermore, supraciliary delivery of brimonidine- loaded PLA particles via a hollow microneedle has been shown to reduce IOP in vivo for more than a month [11].
We previously reported in vitro and short-term in vivo evaluation of an intracameral polycaprolactone (PCL) implant for glaucoma therapy that releases a proprietary hypotensive agent (DE-117) with zero-order release kinetics for up to 6 months in vitro [12]. DE-117 is a selective EP2 agonist when converted to its active form (hDE-117) by hydrolysis. Previous studies showed the hypotensive efficacy of topical DE-117 in animal models [13] and in a clinical trial [14]. DE-117 was chosen based on its high potency (0.002%) [14] compared to other glaucoma medications, which reduces the amount of payload necessary for a long-term (6-month) implant [12]. We chose the intracameral space for our implant because the aqueous humor, which fills the intracameral space and serves as the drug elution medium for the implant, is in direct contact with the target tissue in the anterior segment of the eye. PCL, a biodegradable polyester that has been used as a diffusion- limiting barrier in our drug delivery device [12], has been shown to have long-term biocompatibility in the eye [15].
While ocular implants require more invasive insertion procedure compared to eye drops, a study in Singapore found that 62.8% of glaucoma patients were willing to accept a subconjunctival implant instead of eye drops [16]. In addition, a more recent study found that more than half of interviewed glaucoma patients were willing to accept intracameral drug administration for sustained drug delivery systems [17]. Implants may be able to provide better controlled release of drug over an extended period of time [12, 18] due to increased total amount of drug payload and easier control over the diffusive polymer barrier compared to drug delivery via particles. Supporting the potential of implants for glaucoma therapy, there are clinical trials evaluating drug delivery implants that aim to provide long-term treatments for glaucoma including bimatoprost sustained-release (NCT02250651) [19] and ENV515 travoprost extended release (XR) (NCT02371746) [20].
In this study, the IOP reducing effects and pharmacokinetics of DE-117-loaded devices were investigated for 23 and 24 weeks respectively in vivo. We show long-term reduction of IOP in normotensive rabbits upon implantation of a DE-117 loaded device compared to an empty device implantation or no treatment. We also demonstrate ocular tissue distribution of DE-117 and hDE-117 after 5, 12, and 24 weeks of implantation as well as histological analysis of eyes with 24 weeks of device exposure.
2. Material and methods
2.1 Materials
Chemicals were obtained from Sigma-Aldrich Corporation (St. Louis, MO, USA) unless noted otherwise. PCL with Mn = 80 kDa (Sigma-Aldrich Corporation) was used for device fabrication throughout the study. DE-117, its active form (hDE-117), and deuterium- labeled hDE-117 was prepared by Ube Industries, Ltd. (Ube, Japan).
2.2 Device fabrication and characterization
Devices were fabricated and evaluated as previously described [12] with minor modifications. To briefly describe, DE-117 powder was encapsulated between stacked PCL thin films, which were made by spin-casting. The edges of the devices were sealed by placing the device on a nichrome wire embedded in PDMS and resistively heating the wire. Devices were slightly miniaturized in both dimensions (approximately 2.5 to 3 mm in width and length) and film thickness (45 μm films stacked 4 times, resulting in 180 μm in thickness) compared to previous devices. Devices underwent in vitro release studies in 1 mL of phosphate-buffered saline (PBS: EMD Millipore, Billerica, MA, USA) with 0.1% Tween 80 (Spectrum Chemical, New Brunswick, NJ, USA) for 10 days before implantation. Release buffer was replaced with fresh buffer every two days. Concentration of DE-117 was measured using high performance liquid chromatography (HPLC) (1260 Infinity Quaternary LC System, Agilent Technologies, Santa Clara, CA, USA) with a gradient of mobile phase A:B = 90:10 to 20:80 in 40 minutes (A = deionized water with 0.03% trifluoroacetic acid and B = HPLC grade acetonitrile (Thermo Fisher Scientific, Waltham, MA, USA)).
2.3 In vivo studies with loaded and empty device implantation
Implantation of PCL devices in the rabbit eye was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research (1995). PCL devices were implanted in New Zealand white rabbits as previously described [12] with minor adjustments. A 3.0 mm slit knife (Alcon Laboratories, Ft. Worth, TX, USA) was used to construct a clear corneal incision to insert the device in the anterior chamber. The incision was closed with 9–0 nylon suture (Alcon Laboratories, Ft. Worth, TX, USA). Surgical procedures were performed under sterile conditions on one eye of each animal and the other eye was kept untreated. Twelve rabbits were implanted with DE-117 loaded devices (euthanized after 5, 12, and 24 weeks, n=4 each) and 4 rabbits were implanted with empty devices as controls (euthanized after 24 weeks).
Clinical ophthalmologic exams of unanesthetized animals were performed after the surgery along with IOP measurements. Exams utilizing the operating microscope were performed immediately after implantation as well as prior to euthanasia and eye photos were taken with a Canon EOS Rebel T4i DSLR (Canon U.S.A, San Jose, CA, USA) camera body and a Zeiss SLR camera-microscope adapter (Carl Zeiss Meditec Inc., Dublin, CA, USA).
Rabbits were anesthetized and euthanized at the above time points post-implantation by intravenous injection of 2 mmol/kg potassium chloride into the marginal ear vein. Aqueous humor was withdrawn prior to euthanasia by limbal paracentesis using a 30-gauge needle on a 1 mL syringe. Blood samples were collected prior to euthanasia in tubes containing EDTA (BD Vacutainer®, BD, Franklin Lakes, NJ, USA) and kept on ice until centrifugation. Whole blood was centrifuged at 1300 × g for 15 minutes in a refrigerated centrifuge to separate cells from the sample. Eyes implanted with drug-loaded devices were enucleated, frozen in dry ice, and stored in −80°C until dissection and analysis.
2.4 IOP measurements
IOP measurements were taken with a handheld rebound tonometer (TonoVet®, Icare, Vantaa, Finland) with three technical replicate measurements per eye between 11am and 5pm with the exception of one time point (measured once per eye due to logistical difficulties). Baseline IOP of rabbits dedicated to the 24-week time point were measured one day before the implantation procedure between 12 to 2 pm. Area under the curve (AUC) of baseline subtracted IOP values was calculated using the trapezoidal rule.
2.5 Histological analysis
Eyes of rabbits with empty device implantation were enucleated immediately after euthanasia and submerged in 60 mL of Hartman’s Fixative. After one day, globes were transferred to PBS (UCSF Cell Culture Facility, San Francisco, CA, USA) for two days and to 70% ethanol until histological analysis. Histological preparation was performed by the Gladstone Histology and Light Microscopy Core (San Francisco, CA, USA). Eyes were cut longitudinally along a plane passing through the center of the cornea and the optic nerve and each half globe was processed, embedded, sectioned, and stained with hematoxylin and eosin (H&E). Images of the sectioned samples were taken with a brightfield microscope equipped with a Nikon DS-Ri2 camera and a Plan Apo 20x/0.75 objective at the Nikon Imaging Center (UCSF, San Francisco, CA, USA).
2.6 Quantification of DE-117 and hDE-117 in rabbit ocular tissues
Concentration of DE-117 and hDE-117 in dissected ocular tissues was measured by liquid chromatography coupled with a tandem mass spectrometry (LC/MS/MS) as previously described [12]. One vitreous sample (5-week time point) was experimentally lost during sample preparation. After tissue dissection, drug delivery devices were retrieved and their residual drug (DE-117) payload was analyzed via ultra performance liquid chromatography (UPLC) (ACQUITY UPLC, Waters, Milford, MA, USA) with a gradient of mobile phase A:B = 100:0 to 10:90 in 4 minutes (A = deionized water with 0.03% trifluoroacetic acid/acetonitrile (9:1) and B = deionized water with 0.03% trifluoroacetic acid/acetonitrile (1:4)).
2.7 Statistical analysis
Statistical analysis was performed using Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA). Repeated-measures analysis of variance (ANOVA) was used to determine statistical significance of baseline-subtracted IOP measurements between treated eyes and contralateral untreated eyes over time. Two-tailed student’s t-test with Bonferroni correction (number of comparisons = 3) and one-way ANOVA were used to evaluate statistically significant differences among AUC. Data is presented as mean ± standard deviation.
3. Results
3.1 Device characterization before implantation and after euthanasia
In vitro release studies of devices were performed before implantation to confirm their release rates. The analysis showed that the implanted devices released DE-117 at a rate of 0.49 ± 0.11 μg/day (n=12, linear regression, R2 ranging from 0.97 to 1.00 for each device), which was consistent with our previous study [12]. Remaining drug payload in the retrieved devices after euthanasia was also analyzed. While DE-117 was detected in the remaining drug payload (146 ± 79 μg per device), hDE-117 was not detected by HPLC. This showed that DE-117 was protected from hydrolysis inside the device.
3.2 Clinical evaluation of implanted PCL devices over 24 weeks
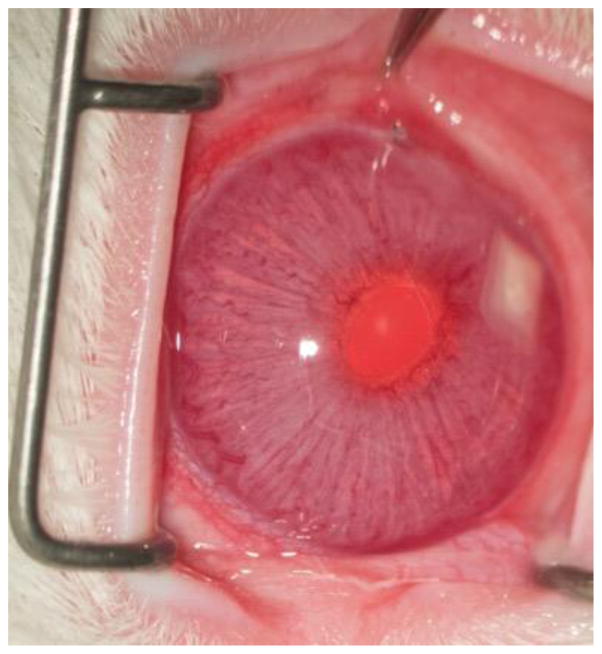
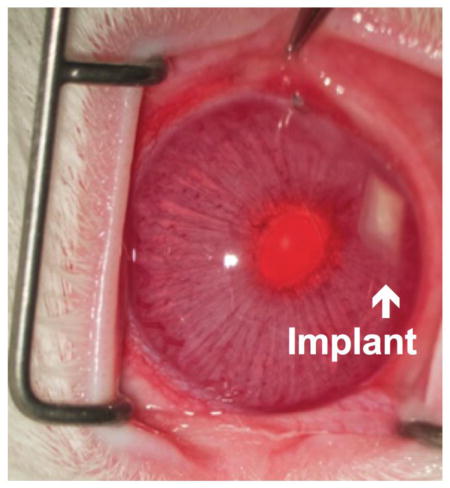
The devices were generally tolerated in the intracameral space with the exception of complications arising from the surgery itself (Fig. 1). There was no cataract formation or obvious signs of ocular inflammation. We have previously reported a relatively high rate (4 out of 9) of iatrogenic iris trauma and/or hyphema during implantation procedures [12]. In this study, the rate of iris trauma and/or hyphema was reduced to 3 out of 16. In the previous work, we also noted the possibility of the device being stuck in the anterior chamber angle (between the root of the iris and the peripheral cornea [21]) [12]. Since the trabecular meshwork located in the angle is the site of aqueous humor outflow [21], device migration to this location is generally not preferred. In this study, one device (drug loaded device implantation, euthanized after 12 weeks) migrated to the angle of the eye. However, device migration did not have an observable effect on device biocompatibility in the intracameral space. Of the 16 rabbits, one rabbit (empty device implantation, euthanized after 24 weeks) developed partial corneal opacification and neovascularization in the treated eye. This eye underwent histological analysis to assess potential corneal damage. Also, one case of transient subconjunctival hemorrhage was observed in a device implanted eye.
Fig 1.
Representative ocular photo after device implantation in the anterior chamber taken before euthanasia
3.3 Drug distribution in ocular tissues
Concentration of DE-117 and hDE-117 was measured in the aqueous humor, vitreous, and iris-ciliary body of device implanted eyes (Fig. 2). Concentration of DE-117 in the aqueous humor (Fig. 2A) indicated sustained release of DE-117 in the anterior chamber through 24 weeks. In addition, a previous study on in vitro hydrolysis of latanoprost (a prostaglandin analog prodrug used for glaucoma treatment) reported that conversion of latanoprost to its active form may be limited in the aqueous humor compared to conversion in other ocular tissues, such as the cornea and the ciliary body [22]. Because conversion of DE-117 to hDE-117 is important for its therapeutic effect, we wanted to confirm that DE-117 is sufficiently hydrolyzed to hDE-117 in the aqueous humor. Our analysis showed a concentration of hDE-117 in the aqueous humor (Fig. 2B) similar to that of DE-117 (Fig. 2A), indicating conversion of released DE-117 to hDE-117 in the aqueous humor up to 24 weeks after implantation. Both DE-117 and hDE-117 were detected at a similar concentration in the aqueous humor and the iris-ciliary body likely due to the relatively slow nature of prodrug hydrolysis in the anterior chamber compared to the rate of drug release from the device. Concentration of hDE-117 in the vitreous humor was noticeably lower than that in the aqueous humor (Fig. 2B), which is expected based on the location of device placement. DE-117 in the vitreous humor was below the limit of quantification. Both DE-117 and hDE-117 concentration in the target tissue (iris-ciliary body) was maintained at a relatively steady level at all time points (Fig. 2C, D). Considering the relatively small device variation characterized by pre-implantation in vitro release studies, variations in tissue drug concentrations are expected to be due to difference among each animal.
Fig 2.
Concentration of DE-117 and hDE-117 in the aqueous humor and vitreous (A, B) and iris-ciliary body (C, D) 5, 12, and 24 weeks after DE-117-loaded device implantation. Concentration of DE-117 in the vitreous was below the limit of quantification.
In addition, concentration of DE-117 and hDE-117 in the aqueous humor of untreated eyes was measured to check if the released drug can cross over to the contralateral eye. Analysis indicated that DE-117 and hDE-117 concentration in the aqueous humor of untreated eyes was below the limit of quantification (0.100 ng/mL). DE-117 and hDE-117 were also below the limit of quantification in the blood samples.
3.4 IOP following loaded or empty device implantation
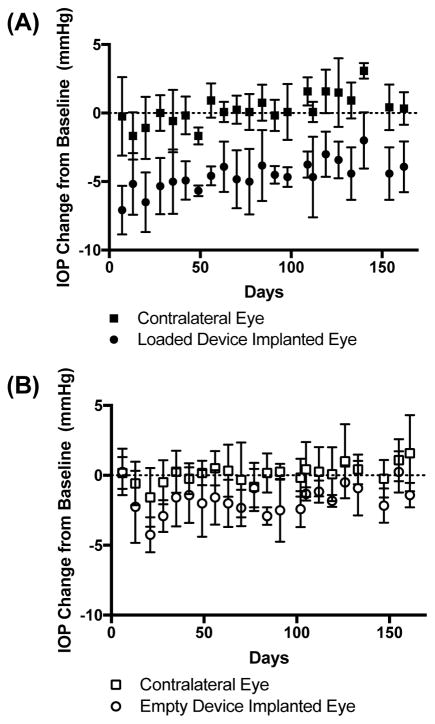
IOP measurements of the device implanted eyes were compared to those of untreated, contralateral eyes for approximately 23 weeks after treatment (Fig. 3). One week after implantations, IOP was reduced 7.1 ± 1.8 mmHg from baseline with DE-117-loaded devices while the contralateral eyes experienced an IOP reduction of 0.3 ± 2.9 mmHg. When averaging over all IOP measurements after implantation, loaded device implanted eyes experienced a −4.6 mmHg change in IOP while the contralateral eyes experienced a 0.3 mmHg change (repeated-measures ANOVA, p < 0.005). To evaluate the effect of device implantation independent of the effects of released drug, IOP was evaluated with empty device implantation (Fig. 3B). Empty device implanted eyes did not experience measurable IOP reduction 6 days after the implantation procedure (change of 0.3 ± 1.7 mmHg) compared to contralateral eyes (0.2 ± 1.1 mmHg). However, when considering an average over all IOP measurements, empty device implantation did slightly lower IOP over time (−1.7 mmHg) compared to untreated eyes (0.1 mmHg) (repeated-measures ANOVA, p < 0.05), although to a lesser degree than eyes with drug-loaded devices.
Fig 3.
IOP change from baseline for eyes with (A) DE-117 loaded or (B) empty device implantation compared to their contralateral (untreated) eyes. Data is shown as mean ± standard deviation.
In addition, a previous study evaluated the relationship between IOP fluctuation to visual field progression and concluded that long-term IOP fluctuation is linked with visual field progression in glaucoma patients with low mean IOP [23]. In this study, long-term IOP fluctuation was defined as the standard deviation of IOP in mmHg at all time points [23]. Following this observation, we evaluated the long-term IOP fluctuation in our study using the standard deviation of all IOP measurements after the implantation procedure. Lack of noticeable difference in long-term IOP variation was observed across all experimental groups (Supplementary Fig. 1).
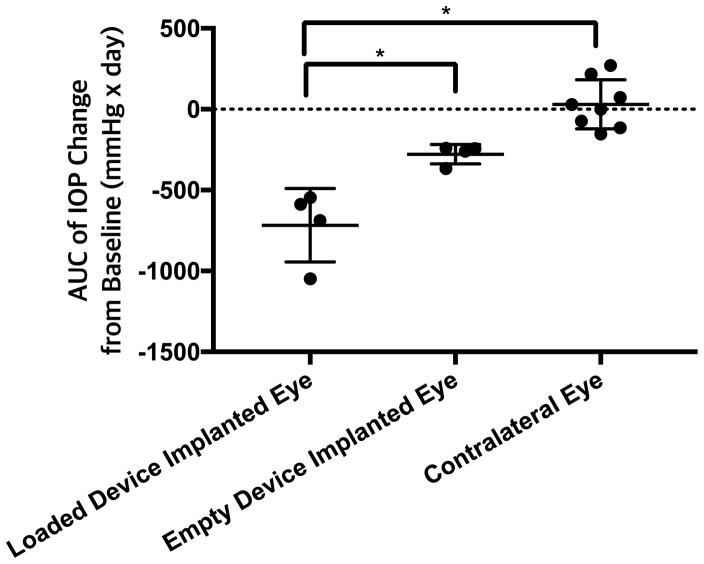
3.5 Cumulative IOP reduction evaluated by AUC
Cumulative IOP reduction upon device implantation was evaluated by the AUC of IOP measurements from baseline. There was one-day difference in final IOP measurements between experimental groups (162 days for drug-loaded devices versus 161 days for empty devices), which will affect the precise AUC value. However, when normalized to the overall duration, the magnitude of the trend is largely unaffected, so the AUC values were compared without accounting for this effect. Furthermore, while most time points were measured weekly on the same day of the week, some time points were adjusted to accommodate unavailability throughout the long-term evaluation; this sampling variability was accounted for during integration. Specific time points of IOP measurements are as shown in Fig. 3. With these caveats in mind, there was a statistically significant difference for IOP AUC among loaded device implanted, empty device implanted, and untreated eyes (ANOVA, p < 0.001) (Fig. 4). AUC upon drug-loaded device implantation was significantly different from that with empty device implantation (two-tailed t-test with Bonferroni correction, p < 0.05) (Fig. 4). Also, AUC of treated eyes was significantly different compared to that of untreated contralateral eyes for drug-loaded implantation (p < 0.05) but not with empty device implantation (p > 0.05) (Fig. 4).
Fig 4.
Cumulative IOP reduction represented by the AUC of IOP change from baseline. Reduction in IOP from baseline was calculated as negative area. Data is shown as mean ± standard deviation.
3.6 Histological analysis after 24 weeks of device implantation
Device-implanted eyes (without drug) and their contralateral (control, unimplanted) eyes (total 8 eyes) underwent histological analysis after 24 weeks of implantation. As corneal opacification and neovascularization were observed clinically in one of the implanted eyes, histological evidence of corneal damage was assessed. The eye with clinical corneal opacification and neovascularization exhibited a relatively thick layer of proteinaceous exudate, which appeared as an eosinophilic deposit, on the posterior corneal surface (black arrow in Fig. 5A). Furthermore, this eye showed anterior synechia, adhesions between the anterior iris surface and the posterior cornea (Fig. 5B, white asterisk). The cause of anterior synechia is postulated to be of mechanical nature, as the implanted device pushed the iris against the cornea. In regions of anterior synechia, localized disruption of the corneal endothelium and presence of inflammatory cells in the corneal stroma were noted (white arrows in Fig. 5B). These histological findings correlate with the clinically apparent partial corneal opacification and neovascularization (white arrowhead in Fig. 5B). Accumulation of exudate and inflammatory cells was either noticeably less or not present in other device-implanted eyes (Fig. 5C) and untreated eyes (Fig. 5D) and the corneal endothelium of those eyes generally appeared to be healthy (dotted black arrow in Fig. 5C, D). One device-implanted eye showed evidence of corneal endothelial metaplasia (dotted white arrow in Fig. 5E), which refers to the change of corneal endothelial cells to a fibrocyte phenotype. Corneal endothelial metaplasia is likely a long-term consequence of exudate accumulation on the posterior cornea.
Fig 5.
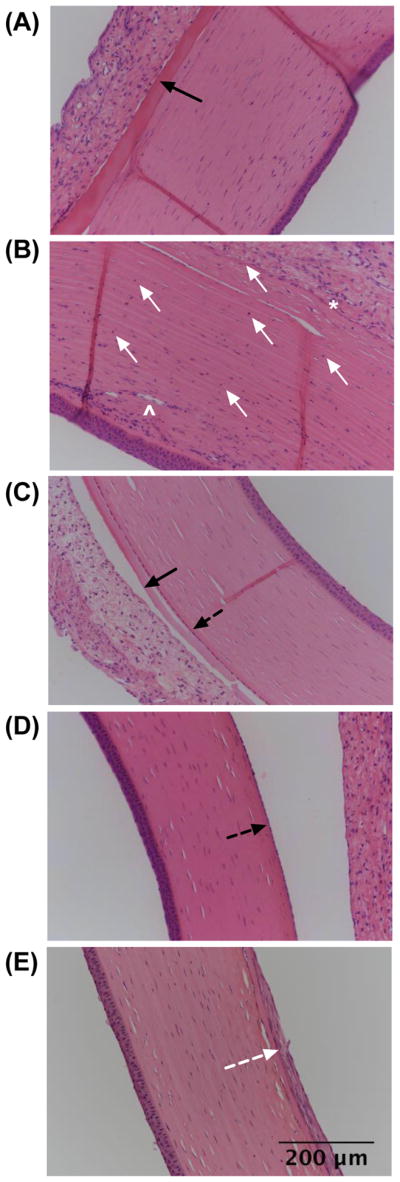
Histological images of the rabbit cornea (A, B, C, E) after 24 weeks of device (without drug) implantation and (D) without implantation (contralateral eyes). Eye shown in (A, B) (n=1) exhibited partial corneal opacification and neovascularization, which was not observed in other eyes (n=7) (C, D, E). Black arrow = accumulation of eosinophilic exudate, white arrow = inflammatory cells and corneal endothelial attenuation, black dotted arrow = corneal endothelium, white asterick = anterior synechiae, white arrowhead = stromal neovascularization, and white dotted arrow = corneal endothelial metaplasia.
4. Discussion
Though glaucoma patients can greatly benefit from IOP control using hypotensive eye drops [24], barriers to patient compliance [5, 6] pose a great challenge in efficacious glaucoma treatment. Furthermore, hypotensive eye drops must overcome ocular physiological barriers to reach the target sites. For example, once a drug is topically administered, it is cleared from the ocular surface via the lacrimal fluid flow and systemic absorption [25]. Then, the drug needs to pass through the cornea, which is lined with corneal epithelial cells. Surface corneal epithelial cells, joined by tight junctional complexes, act as a barrier for drug permeation [26]. Also, transporters expressed in the corneal epithelium may further influence drug absorption [26]. For these reasons, anterior tissue concentration of a topically administered drug is typically expected to be orders of magnitude lower than the eye drop concentration [25, 27]. Finally, ocular surface toxicity can arise with long-term use of various topical glaucoma medications [28–30]. A long-term intracameral drug delivery implant can be substantially beneficial for glaucoma treatment because it requires minimal patient intervention after the implant is administered. In addition, when delivered from an intracameral implant, the therapeutic agent bypasses the corneal and lacrimal barriers to reach the target tissues.
We previously presented a PCL reservoir drug delivery device designed for the intracameral space [12]. The devices were able to deliver DE-117, a proprietary hypotensive agent, with controlled release kinetics over 6 months in vitro [12]. In this study, we investigated the long-term in vivo efficacy of the devices for glaucoma treatment. IOP measurements showed that DE-117 loaded devices are able to provide a persistent IOP drop up to approximately 23 weeks. While we observed that empty device implantation also reduced IOP compared to non-implanted contralateral eyes over the studied period, cumulative IOP reduction with drug-loaded devices was significantly greater than that with either an empty device or no treatment. We hypothesized that the surgical procedure, post-operative healing, and/or mechanical effects may have played a role in IOP reduction with empty device implantation.
While this study demonstrates promising IOP reduction with DE-117 releasing implants, there are some limitations that should be noted. First, there is concern in implanting a bulky device in the anterior chamber that may potentially damage the corneal endothelium. For example, we noted one case of partial corneal opacification and neovascularization, which may have resulted due to endothelial damage during device insertion. Since the effect of PCL on corneal endothelial cell viability has been shown to be negligible compared to cells grown on a culture plate [31], we expect that the corneal endothelial damage is due to the surgical procedure or mechanical effects rather than the material (PCL). While the incidence of this complication was low (one of 16 surgeries in this study), the rabbit corneal endothelium is known to have more regenerative potential than that of human [32]. Other animal models that better mimic the human cornea should be explored in the future, including cats [32] and non-human primates [33].
Moreover, we observed one case of device migration to the angle of the eye. Though device migration did not have an obvious effect on device biocompatibility, it resulted in lower drug distribution in ocular tissues than expected. To prevent device migration, device size may be reduced so that the device implantation site can be optimized. Furthermore, reduction in device size can allow device insertion through a smaller corneal incision, which may result in less IOP change upon empty device implantation. A smaller device may also reduce the incidence of anterior synechia since we suspect that the bulky proof-of-concept device can push the iris against the cornea, causing iris adherence to the cornea. In addition, a more thorough evaluation of ocular inflammation using standardized grading scales will be beneficial in future studies to validate the lack of apparent ocular inflammation observed in the clinical assessments of this study.
In summary, long-term biocompatibility and IOP reducing efficacy of the devices in vivo support the potential of our prototype intracameral implants for glaucoma therapy. In addition, analysis of drug distribution in ocular tissues showed that DE-117 and hDE-117 concentration was maintained up to 24 weeks in both the aqueous humor and the iris-ciliary body. With further optimization of device shape and thorough testing in larger animals, the intracameral drug delivery devices may provide a new mode of glaucoma therapy that eliminates the heavy burden of daily patient compliance.
Supplementary Material
Acknowledgments
Funding
This work was partially supported by R01EY021574 from the National Institutes of Health (NIH), a research grant from Santen Pharmaceutical Co., Ltd., and the Mary Anne Koda-Kimble seed award. The laboratory for in vivo studies was supported by an unrestricted grant from Research to Prevent Blindness, a NIH/NEI Core Grant (EY002162), and That Man May See foundation.
We thank Dr. Daniel A. Bernards, who provided valuable feedback and assistance on manuscript preparation, and the Nikon Imaging Center at UCSF for their imaging support.
Footnotes
Author contributions
J.K. and T.A.D. conceptualized device design. J.K., M.K., S.M., H.A., R.B.B., and T.A.D. designed the experiments. J.K. developed and fabricated the devices. M.K. performed IOP measurements and assisted with implantation and euthanasia procedures. N.R.K.S. and R.B.B. performed in vivo implantation surgeries. E.A. performed measurement and analysis of drug distribution in ocular tissues. M.M.B. provided histological analysis. J.K. wrote the manuscript. All authors reviewed and confirmed the manuscript.
Competing interests
H.A. and E.A. are employees of Santen Pharmaceutical Co., Ltd. and S.M. is an employee of Santen, Inc. T.A.D and R.B.B. received a research grant from Santen Pharmaceutical Co., Ltd. and consult for Santen, Inc. T.A.D and R.B.B. are scientific founders of Zordera, a company focused on ocular drug delivery. The University of California, San Francisco (UCSF) has filed a patent application on this technology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allingham RR, Bruce SM. Shields’ textbook of glaucoma. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; c2011. [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Bengtsson B, Leske MC, Hyman L, Heijl A. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:205–209. doi: 10.1016/j.ophtha.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 4.Gaasterland DE, Ederer F, Beck A, Costarides A, Leef D, Closek J, Banks J, Jackson S, Moore K, Vela A, Brown RH, Lynch M, Gunsby J, Lober K, Marsh T, Stepka C, Montgomery R, Clagett D, Ashburn F, Schacht K, Coyle E, Garland MK, Lauber S, Michelitsch K, Plavnieks S, Vayer L, Burt E, Hundley M, Rae A, Allen RC, Miller E, Sporn A, Fendley CK, Hoyle LS, Weber PA, Derick R, McKinney K, Moore D, Lauderbaugh T, Baker ND, Kapetansky F, Lehmann D, Black L, Gloeckner B, Coleman K, Cassady M, Sharf LJ, Romans B, Satterwhite Y, Simmons L, Vela MA, Harbin J, Brannon TSL, Wright J, LaSalle J, Degenhardt G, Bridgman SA, Gunsby J, Ozment RR, Hooper M, Goldstein S, Butler L, Perry M, Eckel A, Martin A, Session C, Nummerdor D, Wille L, Cyrlin MN, Dubay H, Fazio R, Corbin PS, Wilensky JT, Lindenmuth K, Hillman D, Carroll CA, Hatton J, Sonty S, Higginbotham EJ, Scholes G, Uva R, Fiene J, Frohlichstein D, Gates V, Pappas L, Rathbone D, Tadelman M, Hopkins G, Lichter PR, Bergstrom TJ, Moroi SE, Pollack-Rundle CJ, Standardi C, Abt L, Van Heck T, Skuta GL, Schertzer RM, Wicker D, Van Veldhuisen PC The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/S0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53:57–68. doi: 10.1016/j.survophthal.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Okeke CO, Quigley HA, Jampel HD, Ying G, Plyler RJ, Jiang Y, Friedman DS. Adherence with topical glaucoma medication monitored electronically. The travatan dosing aid study. Ophthalmology. 2009;116:191–199. doi: 10.1016/j.ophtha.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Chawla A, Mcgalliard JN, Batterbury M. Use of eyedrops in glaucoma: How can we help to reduce non-compliance? Acta Ophthalmol Scand. 2007;85:464. doi: 10.1111/j.1600-0420.2007.00882.x. [DOI] [PubMed] [Google Scholar]
- 8.Sleath B, Blalock S, Covert D, Stone JL, Skinner AC, Muir K, Robin AL. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118:2398–2402. doi: 10.1016/j.ophtha.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pek YS, Wu H, Mohamed ST, Ying JY. Long-term subconjunctival delivery of brimonidine tartrate for glaucoma treatment using a microspheres/carrier system. Adv Healthc Mater. 2016 doi: 10.1002/adhm.201600780. [DOI] [PubMed] [Google Scholar]
- 10.Park CG, Kim YK, Kim MJ, Park M, Kim MH, Lee SH, Choi SY, Lee WS, Chung YJ, Jung YE, Park KH, Choy YB. Mucoadhesive microparticles with a nanostructured surface for enhanced bioavailability of glaucoma drug. J Control Release. 2015;220:180–188. doi: 10.1016/j.jconrel.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Chiang B, Kim YC, Doty AC, Grossniklaus HE, Schwendeman SP, Prausnitz MR. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J Control Release. 2016;228:48–57. doi: 10.1016/j.jconrel.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Kudisch M, Mudumba S, Asada H, Aya-Shibuya E, Bhisitkul RB, Desai TA. Biocompatibility and pharmacokinetic analysis of an intracameral polycaprolactone drug delivery implant for glaucoma. Investig Opthalmology Vis Sci. 2016;57:4341–4346. doi: 10.1167/iovs.16-19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirihara T, Iwamura R, Yoneda K, Kawabata-Odani N, Shimazaki A, Kawazu K. DE-117, a selective EP2 agonist, lowered intraocular pressure in animal models. Investig Opthalmology Vis Sci (ARVO Annual Meeting Abstract) 2015;56(7):5709. [Google Scholar]
- 14.Ihekoromadu N, Lu F, Iwamura R, Yoneda K, Kawabata-Odani N, Shams NK. Safety and efficacy of DE-117, a selective EP2 agonist in a phase 2a study. Investig Opthalmology Vis Sci (ARVO Annual Meeting Abstract) 2015;56(7):5708. [Google Scholar]
- 15.Bernards DA, Bhisitkul RB, Wynn P, Steedman MR, Lee OT, Wong F, Thoongsuwan S, Desai TA. Ocular biocompatibility and structural integrity of micro- and nanostructured poly(caprolactone) films. J Ocul Pharmacol Ther. 2013;29:249–257. doi: 10.1089/jop.2012.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foo RCM, Lamoureux EL, Wong RCK, Ho SW, Chiang PPC, Rees G, Aung T, Wong TT. Acceptance, attitudes, and beliefs of singaporean Chinese toward an ocular implant for glaucoma drug delivery. Investig Ophthalmol Vis Sci. 2012;53:8240–8245. doi: 10.1167/iovs.12-10393. [DOI] [PubMed] [Google Scholar]
- 17.Chan HH, Wong TT, Lamoureux E, Perera S. A survey on the preference of sustained glaucoma drug delivery systems by singaporean Chinese patients: A comparison between subconjunctival, intracameral, and punctal plug routes. J Glaucoma. 2015;24:485–492. doi: 10.1097/IJG.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 18.Lance KD, Good SD, Mendes TS, Ishikiriyama M, Chew P, Estes LS, Yamada K, Mudumba S, Bhisitkul RB, Desai TA. In vitro and in vivo sustained zero-order delivery of rapamycin (sirolimus) from a biodegradable intraocular device. Investig Opthalmology Vis Sci. 2015;56:7331–7337. doi: 10.1167/iovs.15-17757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allergan Safety and efficacy of bimatoprost sustained-release (SR) in patients with open-angle glaucoma or ocular hypertension. ClinicalTrials.gov. < https://www.clinicaltrials.gov/ct2/show/NCT02250651?term=NCT02250651&rank=1>.
- 20.Envisia Therapeutics. Safety and efficacy of ENV515 travoprost extended release (XR) in patients with bilateral ocular hypertension or primary open angle glaucoma. ClinicalTrials.gov. < https://www.clinicaltrials.gov/ct2/show/NCT02371746?term=NCT02371746&rank=1>.
- 21.Rumelt S. Glaucoma: Basic and clinical concepts. Rijeka, Croatia: InTech; 2011. [DOI] [Google Scholar]
- 22.Guerra FL, Rager J, Bhoopathy S, Hidalgo I, Castermans K, Defert O, Boland S, Defert O. In vitro hydrolysis of latanoprost by human ocular tissues. Investig Opthalmology Vis Sci (ARVO Annual Meeting Abstract) 2012:5319. [Google Scholar]
- 23.Caprioli J, Coleman AL. Intraocular pressure fluctuation. A risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008;115:1123–1129. doi: 10.1016/j.ophtha.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 24.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, II, Wilson MR, Gordon MO. The ocular hypertension treatment study. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 25.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood-retina barrier: Emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 2006;58:1136–1163. doi: 10.1016/j.addr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Urtti A, Pipkin JD, Rork G, Sendo T, Finne U, Repta AJ. Controlled drug delivery devices for experimental ocular studies with timolol 2. Ocular and systemic absorption in rabbits. Int J Pharm. 1990;61:241–249. doi: 10.1016/0378-5173(90)90215-P. [DOI] [Google Scholar]
- 28.Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23:490–496. doi: 10.1097/01.ico.0000116526.57227.82. [DOI] [PubMed] [Google Scholar]
- 29.Servat JJ, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface eyelids and periorbital tissue. Drugs and Aging. 2011;28:267–282. doi: 10.2165/11588830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Baudouin C. Side effects of antiglaucomatous drugs on the ocular surface. Curr Opin Ophthalmol. 1996;7:80–86. doi: 10.1097/00055735-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Natu MV, Gaspar MN, Ribeiro CAF, Correia IJ, Silva D, de Sousa HC, Gil MH. A poly(ε-caprolactone) device for sustained release of an anti-glaucoma drug. Biomed Mater. 2011;6:25003. doi: 10.1088/1748-6041/6/2/025003. [DOI] [PubMed] [Google Scholar]
- 32.Van Horn DL, Sendele DD, Seideman S, Buco PJ. Regenerative capacity of the corneal endothelium in rabbit and cat. Investig Opthalmology Vis Sci. 1977;16:597–613. [PubMed] [Google Scholar]
- 33.Van Horn DL, Hyndiuk RA. Endothelial wound repair in primate cornea. Exp Eye Res. 1975;21:113–124. doi: 10.1016/0014-4835(75)90076-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.