Abstract
The shape and position of mitochondria are intimately connected to both mitochondrial and cellular function. Mitochondrial anchors play a central role in mitochondrial positioning by exerting spatial, temporal, and contextual control over the cellular position of the organelle. Investigations into the molecular mechanisms of mitochondrial anchoring are still in the early stages, and we are beginning to appreciate the number and variety of anchors that exist. From the insight gained thus far, it is clear that mitochondrial anchoring has functional and physiological consequences that extend beyond mitochondrial positioning to other critical cellular processes.
Introduction
While it is well appreciated that mitochondrial division, fusion, and motility all contribute to the overall distribution of mitochondria within a cell, the critical contributions of actively anchoring the organelle to specific cellular sites and structures are becoming increasingly evident. Mitochondria make many contacts within the cell. These contacts, which are mediated by tether proteins, can be very dynamic and transient or stable and maintained over long periods of time. Here we will focus on our current understanding of the molecular bases, mechanisms, and physiological functions of tethers that function to stably anchor and position mitochondria.
The molecular mechanisms of mitochondrial anchoring
General anchoring mechanisms
While there is evidence of mitochondrial anchoring in various cell types, the molecular basis of this activity is poorly understood. From studies in which the identity of the anchoring protein(s) has been elucidated, it is clear that there is not one specific mechanism used by cells to anchor mitochondria. Tethering proteins may function to anchor mitochondria to other cellular membranes, such as the endoplasmic reticulum and plasma membrane, to cytoskeletal structures, such as microtubules and the actin network, or to motor proteins (Fig. 1) The molecular basis and mechanism for tether protein-mitochondria interactions also differ. Some tethers make direct contact with the mitochondrial membrane, while others interact with proteins anchored in the mitochondrial membrane or themselves are membrane anchored. While the list of anchoring proteins continues to grow and their mechanisms of anchoring are elucidated, the challenge will be to understand how the tethering activity of these proteins is regulated to position mitochondria in the right place at the right time.
Figure 1. General mechanisms for stable anchoring of mitochondria.
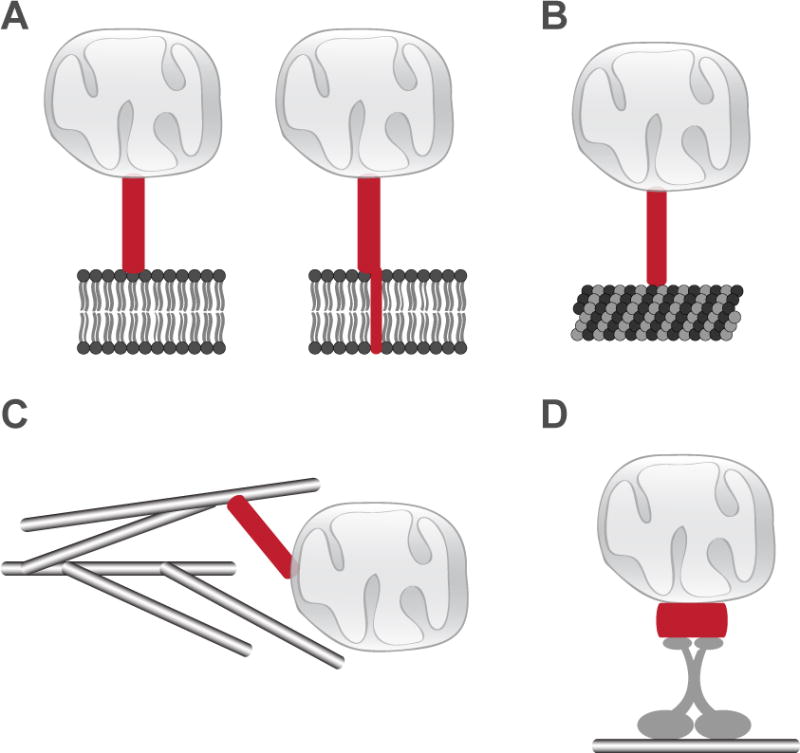
Tethering proteins function to anchor mitochondria to a variety of cellular components using a variety of mechanisms. Mitochondria can be anchored to other cellular membranes via proteins with integral or peripheral membrane binding domains (A). Tethering proteins can also stably anchor mitochondria to cytoskeletal structures, such as microtubules (B) or actin (C), or to motor proteins (D), which drive organelle trafficking.
Insight into the molecular mechanism of mitochondrial anchoring from studies in yeast
Similar to the impact yeast has had on the mitochondrial dynamics field, studies in yeast have and will continue to provide valuable insight into fundamental mechanisms used by cells to construct and regulate anchoring complexes that position mitochondria. Three tethers involved in mitochondrial positioning have been identified in yeast: the mitochondria-ER-cortex anchor (MECA), Mmr1, and Mfb1.
MECA is an extended multi-subunit structure composed of at least two proteins, Num1 and Mdm36, and three organelles, mitochondria, the endoplasmic reticulum (ER), and the plasma membrane [1–3]. Num1, the core protein component of MECA, assembles into clusters of limited mobility and dynamics at the cell cortex in mother cells and large buds and functions to stably tether mitochondria to the plasma membrane at these sites [3–7]. MECA interacts directly with the mitochondrial and plasma membranes via two distinct lipid binding domains within Num1 [8]. An unpredicted membrane binding region within the N-terminal coiled-coil region of Num1 interacts directly with the mitochondrial outer membrane exhibiting a preference for the mitochondrial specific lipid cardiolipin [3,6,8], and a C-terminal PH domain interacts with PI4,5P2 in the plasma membrane [9,10]. The preferential binding of Num1 to cardiolipin and PI4,5P2, organelle-specific lipids that play key structural, regulatory, and signaling roles [11,12], may provide a mechanism to integrate the tethering capacity of MECA with mitochondrial and cellular functions. In contrast to integral membrane domains, the peripheral membrane interactions mediated by Num1’s lipid binding domains allow for rapid membrane association and dissociation. In addition, as Num1 assembly likely enhances interactions with its membrane binding partners and, consequently, the ability of Num1 to robustly tether mitochondria, assembly may be exploited as a mechanism to regulate tether function.
While MECA functions to tether mitochondria to the plasma membrane at sites relatively evenly distributed along the cell cortex of mothers and large buds, recent work identified the protein Mfb1 in functioning to tether mitochondria to the distal end of the mother cell, also referred to as the mother tip [13]. Unlike MECA-mediated tethering, which occurs in proximity to cortical ER [3], the ER does not appear to be required for Mfb1-mediated anchoring. The mechanism by which Mfb1, a non-canonical F-box protein, tethers mitochondria is unknown, but the localization of the protein to mitochondria at the mother cell tip suggests a direct role in anchoring is possible [13–15].
In contrast to MECA and Mfb1, which function primarily in the mother-specific retention of mitochondria [1,3,7,13], the function of Mmr1 in mitochondrial positioning is daughter specific. Mmr1 serves to tether mitochondria to Myo2 for myosin-driven actin-based transport of mitochondria into the bud [16–18]. Mmr1 is also proposed to function in the retention of mitochondria in buds by tethering mitochondria to cortical ER sheets at the bud tip [19]. If and how Mmr1 switches between its roles in mitochondrial transport and anchoring is unclear. Mmr1 is a soluble protein that interacts with the mitochondrial outer membrane in a peripheral manner [16]. While both the transport and tethering roles of Mmr1 require an interaction with mitochondria, the molecular basis and mechanism of the interaction are unknown.
MECA, Mfb1, and Mmr1 work at spatially distinct locations at specific times to ensure the proper distribution of mitochondria between the mother and daughter over the course of the yeast cell cycle. Mmr1 functions early in the cell cycle to transport mitochondria to and tether mitochondria in buds prior to MECA assembly in and Mfb1 localization to buds late in the cell cycle. In mothers, MECA and Mfb1 function to retain mitochondria throughout the cell cycle. How the localization and activity of these tethers are regulated in space and time to coordinately govern the distribution and inheritance of the essential mitochondrial network is an outstanding question.
Mitochondrial anchoring in neurons
In neurons, roughly one-third of mitochondria are mobile while the remaining two-thirds remain stationary [20]. This distribution is not fixed; the mobility state of one mitochondrion can be rapidly switched, and neuronal activity can alter the fraction of stationary versus mobile mitochondria in specific regions of the cell, such as dendrites, nodes of Ranvier and synaptic boutons. The coordinate regulation of transport and anchoring mechanisms is critical to achieve activity-dependent changes in mitochondrial distribution. In neurons, the kinesin KIF5 is the main motor driving anterograde mitochondrial transport, while the retrograde transport of mitochondria is driven by dynein. Interestingly, both kinesin and dynein are localized to mitochondria regardless of the mobility state of the organelle [21,22]. Thus, it is not simply the absence of one or both of the motor proteins that determines the mobility state of mitochondria. In axons, the activity-dependent immobilization of mitochondria requires the mitochondrial outer membrane protein syntaphilin [23]. In response to neuronal activity, syntaphilin is recruited to axonal mitochondria where it is proposed to function as an activity-regulated brake by stably anchoring mitochondria to microtubules as well as inhibiting KIF5 [23,24]. The interaction between syntaphilin and mitochondria is mediated by two transmembrane domains in the C-terminal tail of the protein, and syntaphilin interacts directly with microtubules via a microtubule binding domain [23]. How syntaphilin targeting to axonal mitochondria and its interaction with microtubules are regulated in an activity-dependent manner is unclear. In addition, the mechanism by which the syntaphilin-mediated brake is released has yet to be determined, although post-translational modifications have been suggested to play a role.
The activity-dependent immobilization of mitochondria in dendrites does not require syntaphilin, indicating that a dendrite-specific tethering mechanism exists [24]. In addition, the actin cytoskeleton may play a role in anchoring mitochondria in neurons. Following treatment of sensory neurons with nerve growth factor at specific sites along an axon, mitochondria accumulate and are retained at the site of treatment. Treatment of the cells with latrunculin B disrupts the site-specific accumulation of mitochondria following nerve growth factor stimulation [25]. Thus, interactions with the actin cytoskeleton are required, but the molecular basis is unknown.
Mitochondrial anchoring in the Drosophila germline
A recent study identified Long Oskar, previously known to help anchor germ plasm to the posterior cortex [26,27], to be required for posterior mitochondrial accumulation in Drosophila melanogaster embryos. During oogenesis, mitochondria become enriched at the oocyte posterior during a period of cytoplasmic streaming. Cytoplasmic streaming is necessary for the efficient posterior accumulation of mitochondria, serving to passively transport the organelle, which then gets trapped at the posterior through a Long Oskar- and actin-dependent mechanism [28]. Interestingly, Long Oskar is one of two isoforms and contains an N-terminal domain that Short Oskar lacks. This N-terminal domain, which mediates an interaction with the actin cytoskeleton, is necessary and sufficient to trap mitochondria. The molecular basis of the Long Oskar-mitochondria association has yet to be determined.
Other examples of mitochondrial anchoring
We are still in the early stages of our investigations into mitochondrial anchors and are just beginning to appreciate the number and variety of anchors that exist. Electron microscopy (EM) analyses of neurons and myocytes have revealed physical structures that link mitochondria to the plasma membrane and/or endoplasmic/sarcoplasmic reticulum [29–32]. In pancreatic acinar cells, a population of mitochondria is stably maintained in close apposition to the plasma membrane [33,34], and in T-cells, interactions between mitochondria and a rotating microtubule network are thought to help position mitochondria at the immune synapse [35,36]. Mitochondrial anchoring at specific regions in these polarized cells likely serves to meet the energy demands and calcium buffering or signaling needs of these active cellular locations. In Plasmodium berghei, an obligate parasite that causes malaria, EM tomography reveals a long tethering molecule connecting the inner membrane complex (IMC) to mitochondria, microtubules, and other organelles with a fixed periodicity along the periphery of the cell. [37]. In Toxoplasma gondii, a related pathogenic eukaryote that causes toxoplasmosis, a single lasso-shaped mitochondrion is stably anchored to the cell periphery, specifically the IMC, when the organism is replicating in a host cell [38–40]. A uniform distance between the organellar membranes is maintained at sites of close juxtaposition between mitochondria and the IMC, and the sites are stable over time, both of which are consistent with protein-mediated tethering between the two organelles [40].
In these many examples of mitochondrial anchoring, we still await the identification of the proteins involved. The greater accessibility of cutting-edge live cell imaging and EM techniques paired with advances in genetic screening and proteomics approaches will greatly facilitate the identification and characterization of novel mitochondrial anchors in a variety of cell types and organisms. Once identified, structure-function studies along with biochemical reconstitution can be used to dissect the mechanism of anchoring.
Functional and physiological roles of mitochondrial anchoring
Anchors position mitochondria
An obvious function of a mitochondrial anchor is to keep the organelle at a specific place for a specific length of time. Anchors can function to keep mitochondria at regions where there is a high demand for mitochondrial activity. In addition, anchors can influence the overall structure and cellular distribution of the mitochondrial network. However, not all anchors may function to stably position mitochondria within the cell. Anchors may also function to stably attach mitochondria to cellular structures that themselves are trafficked resulting in a hitchhiking scenario that functions to relocate mitochondria. While positioning mitochondria within a cell is a critical function of anchors, mitochondrial anchoring has functional and physiological consequences that extend beyond mitochondrial positioning to other critical cellular processes.
Anchors in organelle crosstalk
By stably anchoring mitochondria to other membrane compartments, anchors may facilitate inter-organellar crosstalk. Indeed, tether protein-mediated contact between organelles has been proposed to facilitate the exchange of lipid, calcium, and other small molecules [41,42]. While some anchoring proteins solely serve to physically bridge organelles, others actively participate in inter-organelle communication and function. As the role of tether proteins in the formation and function of membrane contact sites has been recently and thoroughly reviewed, we will refrain from going into detail here (for detailed reviews please [43,44]).
Anchors may influence the activity of other cellular machines
Num1, the core protein component of MECA, has been implicated in mitochondrial division, which requires the function of the dynamin related protein Dnm1 [1,2,45]. Mitochondrial division rates are reduced in cells that lack Num1 [1–3]. Decreased division rates in the absence of MECA-mediated tethering may be a consequence of disrupting the tension along a mitochondrial tubule that is required for dynamin related protein-mediated membrane scission [46]. Interestingly, Dnm1 co-localizes with MECA, and the population of Dnm1 associated with MECA is distinct from the population that participates in mitochondrial division [1–3]. Consistently, Dnm1’s association with MECA requires the Dnm1 adaptor Caf4 [3]. While structurally similar to a Dnm1 adaptor required for Dnm1-driven membrane fission, Caf4 promotes the assembly of Dnm1, but not its membrane fission activity [47]. Thus, MECA may harnesses the membrane remodeling activity of Dnm1 in a manner distinct from its role in membrane fission, the functional significance of which is at this point unclear. Num1 also serves as a cortical anchor for dynein, and, as such, functions to harness dynein-mediated microtubule sliding along the cell cortex for nuclear migration into buds [5,48]. While dynein is not required for Num1-dependent mitochondrial anchoring [3,6], the requirement of mitochondria for Num1-dependent dynein anchoring is currently being investigated.
Anchors in mitochondrial quality control and cellular aging
Studies in yeast highlight the importance of mitochondrial positioning not only for the inheritance of the organelle, but, more specifically, the asymmetric inheritance of higher functioning mitochondria by daughter cells. Yeast cells exhibit a mother-daughter age asymmetry in which daughter cells are born young despite the age of the mother [49]. Functional asymmetry of the mitochondrial compartment between the mother and daughter has been proposed to be a contributing factor to mother-daughter age asymmetry [50,51]. In yeast, mitochondria retained in the mother cell have overall lower membrane and redox potential and higher superoxide levels than those inherited by the daughter [51]. Interestingly, however, mother cell mitochondria located at the distal end of the mother cell, which colocalize with Mfb1, are more reduced and higher functioning than the rest of the mother cell mitochondrial network [13]. How these asymmetries in mitochondrial function between the mother and bud and within the mother are achieved is not clear; however, retention mechanisms likely play a role (Fig. 2A). Indeed, in cells lacking Mmr1, mother-daughter age asymmetry is disrupted [51], and disruption of the Mfb1-dependent enrichment of higher functioning mitochondria at the distal end of the mother cell correlates with an overall reduction in mitochondrial fitness and reductions in cellular healthspan and replicative life span [13]. The defects in mitochondrial function and replicative life span observed in cells lacking Mfb1 can be rescued by the deletion of Mmr1, and the premature aging observed in cells lacking Mmr1 can be prevented by deleting Mfb1 [13]. Therefore, Mmr1 and Mfb1 work antagonistically at spatially distinct locations to ensure daughters receive high functioning mitochondria while not fully sacrificing the function of mitochondria in mothers.
Figure 2. Asymmetric inheritance of mitochondria.
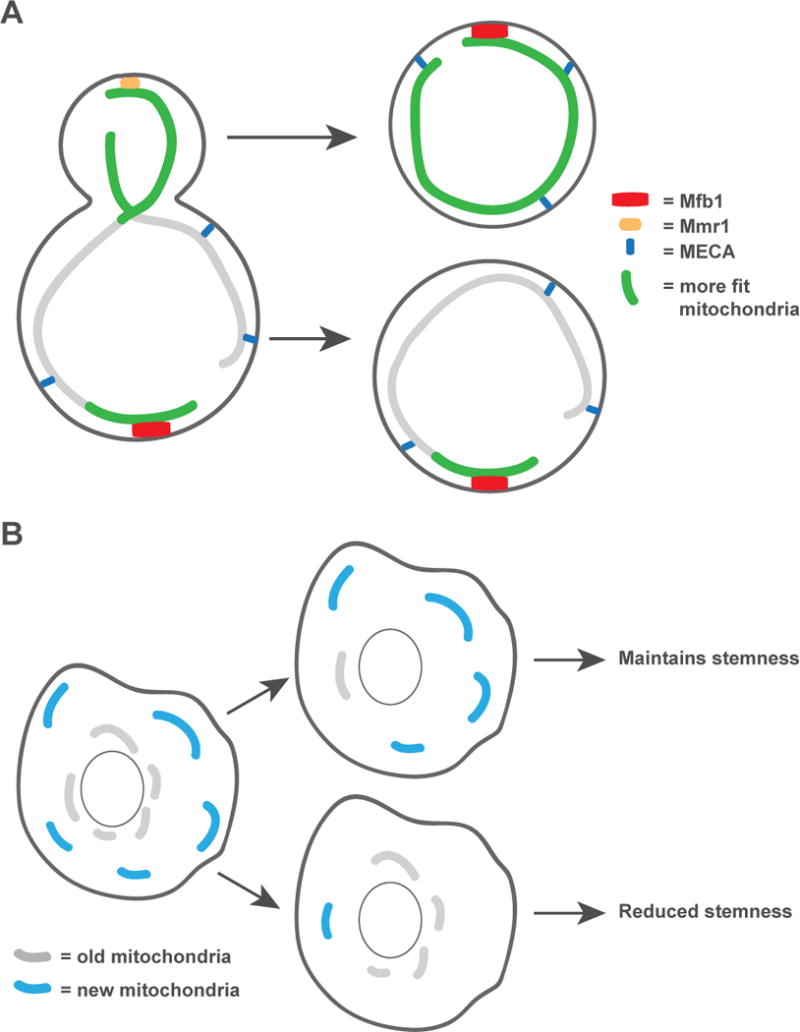
A) During yeast cell division, daughter cells inherit fitter mitochondria, which are more energetic and less oxidized. More fit portions of the mitochondrial network are also retained in the distal end of the mother. In cells lacking tether proteins, the asymmetric inheritance of fit mitochondria is disrupted and defects in the healthspan and replicative lifespan of cells are observed. B) Dividing mammalian stem-like cells asymmetrically inherit old and new mitochondria. Cells that inherit more new mitochondria maintain their stemness, while cells that inherit more old mitochondria lose stem-like properties.
Gametogenesis, also called sporulation, has also been shown to reset the aging clock in yeast [52]. During gametogenesis dramatic restructuring and repositioning of the mitochondrial network is observed with the end result of each newly formed gamete inheriting part of the mitochondrial compartment [53–55]. In the earlier stages of sporulation, mitochondria move away from the cell cortex and associate with the ER-enveloped nuclear membrane. In later stages of sporulation, the tight association of mitochondria with the nuclear membrane is no longer observed, and eventually mitochondria re-associate with the cell cortex. Interestingly, these stages of mitochondrial repositioning temporally correlate with the depletion and reestablishment of cortical ER [54], suggesting a role for ER tethering in the repositioning of mitochondria. The repositioning of the mitochondrial network observed during sporulation suggests that the function of tethers such as MECA must be regulated to release mitochondria from and re-anchor mitochondria to the cell cortex. Interestingly, only ~40–50% of the mitochondrial compartment is inherited by gametes with the remainder being left behind in the mother cell ascus [54]. It is not known if there is an asymmetry between the functionality of mitochondria inherited in gametes versus those left behind in the mother cell and, if an asymmetry exists, what role mitochondrial anchoring may play in this functional segregation.
Mitochondria also contribute to the asymmetric inheritance of both age- and stress-induced protein aggregates, which influences the healthspan and replicative life span of cells [56]. In yeast, protein aggregates are asymmetrically retained in mother cells [57–59], and recent work demonstrates that tethering of aggregates to the ER and mitochondria, often at contact sites between the two organelles, contributes to the asymmetric retention of the aggregates [60]. As MECA and Mfb1 both function primarily in mother cell mitochondrial retention, it will be interesting to determine what role, if any, these mitochondrial anchors play in the asymmetric retention of protein aggregates.
Anchors in cellular differentiation and development
The asymmetric inheritance of specific mitochondrial populations likely extends beyond yeast to other cell types that undergo asymmetric cell divisions. Indeed, age dependent asymmetric inheritance of mitochondria has been observed in mammalian mammary epithelial stemlike cells in vitro [61]. Following cytokinesis, the two daughter cells produced contain similar amounts of mitochondria, however, the proportion of old versus young mitochondria differs between the two and correlates with differences in cell fate (Fig. 2B). The daughter that inherits more old mitochondria begins to show signs of differentiation, while the daughter that inherits fewer old mitochondria maintains stem cell traits. Interestingly, old mitochondria are restricted to the perinuclear region of the mother cell, indicating an age-dependent positioning mechanism exists. Mitochondrial division and quality control pathways have been implicated in the age-dependent asymmetric inheritance of the organelle, and it remains to be determined if and how mitochondrial anchoring contributes.
The function of mitochondrial anchors in facilitating the inheritance of mitochondria from mothers to daughters also extends beyond yeast. In Drosophila melanogaster, the anchor-mediated trapping of mitochondria at the posterior of the embryo is required for the efficient incorporation of mitochondria into primordial germ cells. Specifically, Long Oskar traps mitochondria at the posterior end of the embryo near the site of primordial germ cell (PGC) formation, and posteriorly-localized mitochondria are taken up by the PGCs [28]. In the absence of Long Oskar, there is a significant reduction in the number of mitochondria anchored at the posterior and a concomitant reduction in the number of mitochondrial genomes in PGCs. Therefore, Long Oskar-mediated anchoring facilitates the incorporation of mitochondrial DNA into PGCs and, consequently, transmission of mitochondrial DNA to the next generation. It remains to be determined if the mitochondria that are anchored to the posterior and/or get inherited by the PGCs are selected on the basis of fitness or some other criteria and if Long Oskar actively influences such a selection.
Anchors in disease
While the functions of mitochondria are required for viability in almost all cell types, certain cells rely more heavily on specific mitochondrial functions for overall cellular function. In neurons, mitochondrial ATP generation and Ca2+ buffering are critical for synapse assembly and action potential generation and transmission. Due to high demands for energy and Ca2+ buffering at specific, yet quite distant cellular locations, neurons possess sophisticated mechanisms to ensure mitochondria are trafficked to and maintained at the cellular locations they are needed [21,22]. As discussed above, syntaphilin is required for the activity-dependent anchoring of mitochondria in axons. In the absence of syntaphilin, activity-mediated anchoring is disrupted and defects in synaptic transmission are observed, indicating anchoring is important for normal neuronal function [23]. Interestingly, disrupting syntaphilin-mediated anchoring has been shown to have beneficial effects in certain circumstances. As neurons mature, axonal mitochondrial transport declines, which limits the regenerative capacity of axons. An increase in syntaphilin-mediated anchoring is responsible for the decline in transport, and in the absence of syntaphilin, mitochondrial transport and axon regrowth are both enhanced [62]. Increasing mitochondrial transport by disrupting syntaphilin anchoring has also been shown to have beneficial effects in a CNS demyelinating mouse that serves as a model for progressive multiple sclerosis [63]. In contrast, syntaphilin-mediated anchoring has been shown to facilitate the survival of axons following acute demyelination [64].
It has long been appreciated that mitochondrial dynamics influence cancer cell metabolism and physiology [65,66], and a recent study implicates syntaphilin-mediated mitochondrial anchoring in cancer metastasis [67]. Syntaphilin, originally described as a neuronal specific mitochondrial anchor, is expressed in a variety of non-neuronal cell types, including tumor cells. When syntaphilin is depleted by siRNA, mitochondria move faster, farther, and with higher processivity to relocate from the perinuclear to cortical cytoskeleton. Increased rates of mitochondrial fusion and fission are also observed. The invasiveness of tumor cells increases when syntaphilin is depleted and decreases when KIF5B or Miro1, the mitochondrial receptor for kinesin, is depleted. These contrasting phenotypes suggest that, similar to neurons, motor-driven transport and syntaphilin-mediated anchoring mechanisms are coordinately regulated to position mitochondria to meet cellular needs, in this case the spatial and temporal energy needs of a tumor cell. Consistently, in clinical sample databases, syntaphilin is downregulated in certain cancers where Miro2 is upregulated, and the downregulation of syntaphilin correlates with a poor prognosis in some cancers [67]. Together these findings suggest that cancer cells exploit mitochondrial transport and anchoring mechanisms. Specifically, by downregulating the expression of a protein that puts a brake on mitochondrial movement, mitochondria are more rapidly trafficked to the cortical cytoskeleton to meet the incredible energy demands of migrating tumor cells.
In Toxoplasma gondii, dramatic changes in mitochondrial morphology are observed when the cell transitions from an intracellular to extracellular environment during infection [40]. As discussed earlier, T. gondii cells have a single mitochondrion that is anchored to the periphery when inside a host cell. During the transition to an extracellular environment, the mitochondrion retracts from the periphery, correlating with fewer areas of contact with the IMC. After invading a new cell, the mitochondrion re-establishes a peripheral localization. Thus, the anchor that tethers mitochondria to the IMC is regulated during the T. gondii infection cycle. This dynamic rearrangement of mitochondria is suggested to be critical for the cells to transition between the extracellular and intracellular stages of their infection cycle. Therefore, a better understanding of the mitochondrial re-positioning mechanism may provide insight into how these mitochondrial changes affect T. gondii physiology and pathogenesis.
Another eukaryotic pathogen uses mitochondrial anchoring to ensure organelle inheritance. Extensive contact between mitochondria and the apicoplast has been observed in Plasmodium falciparum, a pathogen that causes a majority of malaria cases in humans [68]. The contacts between mitochondria and the apicoplast become more extensive as the cell progresses towards division, and the two organelles are simultaneously inherited by daughter cells [69]. It is thought that tethering the organelles and thereby coupling their trafficking into daughter cells serves as a mechanism to link the inheritance of the two essential organelles. In addition to coordinating inheritance, the physical attachment between mitochondria and apicoplasts has been suggested to be important for the metabolism of the pathogen.
The insight we have gained thus far indicates that positioning mitochondria at specific places and specific times is intimately tied to cellular function and physiology and can be co-opted in disease states. The exact contributions of mitochondrial anchors to the progression and pathogenesis of human diseases remains to be determined.
There is still a lot to learn about mitochondrial anchors
It is clear that mitochondrial anchors play critical roles in mitochondrial positioning and can influence many aspects of cellular function and homeostasis. While great progress in our understanding of a subset of mitochondrial anchors has been recently made, fundamental questions regarding the majority of mitochondrial anchoring events remain unanswered: 1) What are the molecular bases and mechanisms of mitochondrial anchoring? 2) How are mitochondrial anchors regulated to properly position mitochondria in the right place at the right time to meet cellular needs? and 3) How does mitochondrial anchoring both impact and respond to changes in mitochondrial and cellular function? Even when we have insight into the molecular basis and mechanism of anchoring, the mechanisms that regulate the formation and placement and the release or disassembly of the anchor are unknown. The more we learn about the functional and physiological impacts of mitochondrial anchoring, the more evident it becomes that studies on mitochondrial anchors will have significant impact in many areas of biology. In addition, the number of diseases associated with defects in mitochondrial distribution continues to grow [70,71]. Thus, understanding fundamental mechanisms used by cells to position mitochondria will be critical to develop strategies for the treatment of diseases in which the manipulation of mitochondrial position can be beneficial.
Highlights.
Mitochondrial anchors play a central role in mitochondrial positioning
Molecular mechanisms and functions of mitochondrial anchors are discussed
Mitochondrial anchors influence many aspects of cellular function and homeostasis
Acknowledgments
We thank the members of the Lackner Lab for helpful discussions and comments, and we apologize to colleagues whose outstanding work on mitochondrial anchors and positioning could not be including due to space limitations. L.M.K. is supported by the NIH NIGMS Training Grant T32GM008061. L.L.L is supported by the NIH NIGMS grant R01GM120303 and the Robert H. Lurie Comprehensive Cancer Center – The Lefkofsky Family Foundation/Liz and Eric Lefkofsky Innovation Research Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cerveny KL, Studer SL, Jensen RE, et al. Yeast mitochondrial division and distribution require the cortical num1 protein. Dev Cell. 2007;12:363–375. doi: 10.1016/j.devcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Hammermeister M, Schodel K, Westermann B. Mdm36 is a mitochondrial fission-promoting protein in Saccharomyces cerevisiae. Mol Biol Cell. 2010;21:2443–2452. doi: 10.1091/mbc.E10-02-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lackner LL, Ping H, Graef M, et al. Endoplasmic reticulum-associated mitochondria-cortex tether functions in the distribution and inheritance of mitochondria. Proc Natl Acad Sci USA. 2013;110:E458–467. doi: 10.1073/pnas.1215232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farkasovsky M, Kuntzel H. Yeast Num1p associates with the mother cell cortex during S/G2 phase and affects microtubular functions. J Cell Biol. 1995;131:1003–1014. doi: 10.1083/jcb.131.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heil-Chapdelaine RA, Oberle JR, Cooper JA. The cortical protein Num1p is essential for dynein-dependent interactions of microtubules with the cortex. J Cell Biol. 2000;151:1337–1344. doi: 10.1083/jcb.151.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang X, Germain BS, Lee WL. A novel patch assembly domain in Num1 mediates dynein anchoring at the cortex during spindle positioning. J Cell Biol. 2012;196:743–756. doi: 10.1083/jcb.201112017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klecker T, Scholz D, Fortsch J, et al. The yeast cell cortical protein Num1 integrates mitochondrial dynamics into cellular architecture. J Cell Sci. 2013;126:2924–2930. doi: 10.1242/jcs.126045. [DOI] [PubMed] [Google Scholar]
- 8.Ping HA, Kraft LM, Chen W, et al. Num1 anchors mitochondria to the plasma membrane via two domains with different lipid binding specificities. J Cell Biol. 2016;213:513–524. doi: 10.1083/jcb.201511021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang X, Punch JJ, Lee WL. A CAAX motif can compensate for the PH domain of Num1 for cortical dynein attachment. Cell Cycle. 2009;8:3182–3190. doi: 10.4161/cc.8.19.9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu JW, Mendrola JM, Audhya A, et al. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol Cell. 2004;13:677–688. doi: 10.1016/s1097-2765(04)00083-8. [DOI] [PubMed] [Google Scholar]
- 11.Claypool SM, Koehler CM. The complexity of cardiolipin in health and disease. 2012;37:32–41. doi: 10.1016/j.tibs.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strahl T, Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1771:353–404. doi: 10.1016/j.bbalip.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pernice WM, Vevea JD, Pon LA. A role for Mfb1p in region-specific anchorage of high-functioning mitochondria and lifespan in Saccharomyces cerevisiae. Nat Commun. 2016;7:10595. doi: 10.1038/ncomms10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durr M, Escobar-Henriques M, Merz S, et al. Nonredundant roles of mitochondria-associated F-box proteins Mfb1 and Mdm30 in maintenance of mitochondrial morphology in yeast. Mol Biol Cell. 2006;17:3745–3755. doi: 10.1091/mbc.E06-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondo-Okamoto N, Ohkuni K, Kitagawa K, et al. The novel F-box protein Mfb1p regulates mitochondrial connectivity and exhibits asymmetric localization in yeast. Mol Biol Cell. 2006;17:3756–3767. doi: 10.1091/mbc.E06-02-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh T, Toh EA, Matsui Y. Mmr1p is a mitochondrial factor for Myo2p-dependent inheritance of mitochondria in the budding yeast. EMBO J. 2004;23:2520–2530. doi: 10.1038/sj.emboj.7600271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chernyakov I, Santiago-Tirado F, Bretscher A. Active segregation of yeast mitochondria by Myo2 is essential and mediated by Mmr1 and Ypt11. Curr Biol. 2013;23:1818–1824. doi: 10.1016/j.cub.2013.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eves PT, Jin Y, Brunner M, et al. Overlap of cargo binding sites on myosin V coordinates the inheritance of diverse cargoes. J Cell Biol. 2012;198:69–85. doi: 10.1083/jcb.201201024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swayne TC, Zhou C, Boldogh IR, et al. Role for cER and Mmr1p in anchorage of mitochondria at sites of polarized surface growth in budding yeast. Curr Biol. 2011;21:1994–1999. doi: 10.1016/j.cub.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris RL, Hollenbeck PJ. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 1993;104:917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- 21.Lin MY, Sheng ZH. Regulation of mitochondrial transport in neurons. Exp Cell Res. 2015;334:35–44. doi: 10.1016/j.yexcr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz TL. Mitochondrial trafficking in neurons, Cold Spring Harb. Perspect Biol. 2013;5:a011304. doi: 10.1101/cshperspect.a011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang JS, Tian JH, Pan PY, et al. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Sheng ZH. Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport. J Cell Biol. 2013;202:351–364. doi: 10.1083/jcb.201302040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Vanzo N, Oprins A, Xanthakis D, et al. Stimulation of endocytosis and actin dynamics by Oskar polarizes the Drosophila oocyte. Dev Cell. 2007;12:543–555. doi: 10.1016/j.devcel.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Vanzo NF, Ephrussi A. Oskar anchoring restricts pole plasm formation to the posterior of the Drosophila oocyte. Development. 2002;129:3705–3714. doi: 10.1242/dev.129.15.3705. [DOI] [PubMed] [Google Scholar]
- 28.Hurd TR, Herrmann B, Sauerwald J, et al. Long Oskar Controls Mitochondrial Inheritance in Drosophila melanogaster. Dev Cell. 2016;39:560–571. doi: 10.1016/j.devcel.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Csordas G, Renken C, Varnai P, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boncompagni S, Rossi AE, Micaroni M, et al. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol Biol Cell. 2009;20:1058–1067. doi: 10.1091/mbc.E08-07-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkins GA, Sosinsky GE, Ghassemzadeh S, et al. Electron tomographic analysis of cytoskeletal cross-bridges in the paranodal region of the node of Ranvier in peripheral nerves. J Struct Biol. 2008;161:469–480. doi: 10.1016/j.jsb.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins GA, Tjong J, Brown JM, et al. The micro-architecture of mitochondria at active zones: electron tomography reveals novel anchoring scaffolds and cristae structured for high-rate metabolism. J Neurosci. 2010;30:1015–1026. doi: 10.1523/JNEUROSCI.1517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson PR, Dolman NJ, Pope M, et al. Non-uniform distribution of mitochondria in pancreatic acinar cells. Cell Tissue Res. 2003;313:37–45. doi: 10.1007/s00441-003-0741-1. [DOI] [PubMed] [Google Scholar]
- 34.Park MK, Ashby MC, Erdemli G, et al. Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. The EMBO J. 2001;20:1863–1874. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maccari I, Zhao R, Peglow M, et al. Cytoskeleton rotation relocates mitochondria to the immunological synapse and increases calcium signals. Cell Calcium. 2016;60:309–321. doi: 10.1016/j.ceca.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Quintana A, Schwindling C, Wenning AS, et al. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci USA. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudryashev M, Lepper S, Stanway R, et al. Positioning of large organelles by a membrane-associated cytoskeleton in Plasmodium sporozoites. Cell Microbiol. 2010;12:362–371. doi: 10.1111/j.1462-5822.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- 38.Melo EJ, Attias M, De Souza W. The single mitochondrion of tachyzoites of Toxoplasma gondii. J Struct Biol. 2000;130:27–33. doi: 10.1006/jsbi.2000.4228. [DOI] [PubMed] [Google Scholar]
- 39.Seeber F, Ferguson DJ, Gross U. Toxoplasma gondii: a paraformaldehyde-insensitive diaphorase activity acts as a specific histochemical marker for the single mitochondrion. Exp Parasitol. 1998;89:137–139. doi: 10.1006/expr.1998.4266. [DOI] [PubMed] [Google Scholar]
- 40.Ovciarikova J, Lemgruber L, Stilger KL, et al. Mitochondrial behaviour throughout the lytic cycle of Toxoplasma gondii. Sci Rep. 2017;7:42746. doi: 10.1038/srep42746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helle SC, Kanfer G, Kolar K, et al. Organization and function of membrane contact sites. Biochim Biophys Acta. 2013;1833:2526–2541. doi: 10.1016/j.bbamcr.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 42.Toulmay A, Prinz WA. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr Opin Cell Biol. 2011;23:458–463. doi: 10.1016/j.ceb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisenberg-Bord M, Shai N, Schuldiner M, et al. A Tether Is a Tether Is a Tether: Tethering at Membrane Contact Sites. Dev Cell. 2016;39:395–409. doi: 10.1016/j.devcel.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Gatta AT, Levine TP. Piecing Together the Patchwork of Contact Sites. Trends Cell Biol. 2016:214–229. doi: 10.1016/j.tcb.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Bleazard W, McCaffery JM, King EJ, et al. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roux A, Uyhazi K, Frost A, et al. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- 47.Griffin EE, Graumann J, Chan DC. The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria. J Cell Biol. 2005;170:237–248. doi: 10.1083/jcb.200503148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farkasovsky M, Kuntzel H. Cortical Num1p interacts with the dynein intermediate chain Pac11p and cytoplasmic microtubules in budding yeast. J Cell Biol. 2001;152:251–262. doi: 10.1083/jcb.152.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longo VD, Shadel GS, Kaeberlein M, et al. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012;16:18–31. doi: 10.1016/j.cmet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higuchi-Sanabria R, Charalel JK, Viana MP, et al. Mitochondrial anchorage and fusion contribute to mitochondrial inheritance and quality control in the budding yeast Saccharomyces cerevisiae. Mol Biol Cell. 2016;27:776–787. doi: 10.1091/mbc.E15-07-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McFaline-Figueroa JR, Vevea J, Swayne TC, et al. Mitochondrial quality control during inheritance is associated with lifespan and mother-daughter age asymmetry in budding yeast. Aging Cell. 2011;10:885–895. doi: 10.1111/j.1474-9726.2011.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Unal E, Kinde B, Amon A. Gametogenesis eliminates age-induced cellular damage and resets life span in yeast. Science. 2011;332:1554–1557. doi: 10.1126/science.1204349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyakawa I, Aoi H, Sando N, et al. Fluorescence microscopic studies of mitochondrial nucleoids during meiosis and sporulation in the yeast, Saccharomyces cerevisiae. J Cell Sci. 1984;66:21–38. doi: 10.1242/jcs.66.1.21. [DOI] [PubMed] [Google Scholar]
- 54.Suda Y, Nakanishi H, Mathieson EM, et al. Alternative modes of organellar segregation during sporulation in Saccharomyces cerevisiae. Eukaryot Cell. 2007;6:2009–2017. doi: 10.1128/EC.00238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorsich SW, Shaw JM. Importance of mitochondrial dynamics during meiosis and sporulation. Mol Biol Cell. 2004;15:4369–4381. doi: 10.1091/mbc.E03-12-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinkraus KA, Kaeberlein M, Kennedy BK. Replicative aging in yeast: the means to the end. Annu Rev Cell Dev Biol. 2008;24:29–54. doi: 10.1146/annurev.cellbio.23.090506.123509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aguilaniu H, Gustafsson L, Rigoulet M, et al. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 58.Liu B, Larsson L, Caballero A, et al. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 59.Zhou C, Slaughter BD, Unruh JR, et al. Motility and segregation of Hsp104-associated protein aggregates in budding yeast. Cell. 2011;147:1186–1196. doi: 10.1016/j.cell.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou C, Slaughter BD, Unruh JR, et al. Organelle-based aggregation and retention of damaged proteins in asymmetrically dividing cells. Cell. 2014;159:530–542. doi: 10.1016/j.cell.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katajisto P, Dohla J, Chaffer CL, et al. Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science. 2015;348:340–343. doi: 10.1126/science.1260384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou B, Yu P, Lin MY, et al. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J Cell Biol. 2016;214:103–119. doi: 10.1083/jcb.201605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joshi DC, Zhang CL, Lin TM, et al. Deletion of mitochondrial anchoring protects dysmyelinating shiverer: implications for progressive MS. J Neurosci. 2015;35:5293–5306. doi: 10.1523/JNEUROSCI.3859-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohno N, Chiang H, Mahad DJ, et al. Mitochondrial immobilization mediated by syntaphilin facilitates survival of demyelinated axons. Proc Natl Acad Sci USA. 2014;111:9953–9958. doi: 10.1073/pnas.1401155111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Senft D, Ronai ZA. Regulators of mitochondrial dynamics in cancer. Curr Opin Cell Biol. 2016;39:43–52. doi: 10.1016/j.ceb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vyas S, Zaganjor E, Haigis MC. Mitochondria and Cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caino MC, Seo JH, Aguinaldo A, et al. A neuronal network of mitochondrial dynamics regulates metastasis. Nat Commun. 2016;7:13730. doi: 10.1038/ncomms13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hopkins J, Fowler R, Krishna S, et al. The plastid in Plasmodium falciparum asexual blood stages: a three-dimensional ultrastructural analysis. Protist. 1999;150:283–295. doi: 10.1016/S1434-4610(99)70030-1. [DOI] [PubMed] [Google Scholar]
- 69.van Dooren GG, Marti M, Tonkin CJ, et al. Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle of Plasmodium falciparum. Mol Microbiol. 2005;57:405–419. doi: 10.1111/j.1365-2958.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- 70.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Labbe K, Murley A, Nunnari J. Determinants and functions of mitochondrial behavior. Annu Rev Cell Dev Biol. 2014;30:357–391. doi: 10.1146/annurev-cellbio-101011-155756. [DOI] [PubMed] [Google Scholar]


