Abstract
Objective
Current rehabilitation to improve gait symmetry following stroke is based on one of two competing motor learning strategies: minimizing or augmenting symmetry errors. We sought to determine which of those motor learning strategies best improves overground spatiotemporal gait symmetry.
Design
randomized controlled trial
Setting
Rehabilitation research lab
Subjects
47 participants (59±12 years old) with chronic hemiparesis post-stroke and spatiotemporal gait asymmetry were randomized to error augmentation, error minimization, or conventional treadmill training (control) groups.
Interventions
To augment or minimize asymmetry on a step-by-step basis, we developed a responsive, ‘closed loop’ control system, using a split-belt instrumented treadmill that continuously adjusted the difference in belt speeds to be proportional to the patient's current asymmetry.
Main Measures
Overground spatiotemporal asymmetries and gait speeds were collected prior to and following 18 training sessions.
Results
Step length asymmetry reduced after training, but stance time did not. There was no group × time interaction. Gait speed improved after training, but was not affected by type of asymmetry, or group. Of those who trained to modify step length asymmetry, there was a moderately strong linear relationship between the change in step length asymmetry and the change in gait speed.
Conclusions
Augmenting errors was not superior to minimizing errors or providing only verbal feedback during conventional treadmill walking. Therefore, the use of verbal feedback to target spatiotemporal asymmetry, which was common to all participants, appears to be sufficient to reduce step length asymmetry. Alterations in stance time asymmetry were not elicited in any group.
Keywords: gait, stroke, asymmetry, adaptation, learning
Introduction
The slow walking speeds commonly observed following stroke are associated with marked temporal and spatial interlimb asymmetries in 48% and 44% of subjects, respectively.1 Asymmetric gait has been associated with gait energetics,2 gait speed,1 and balance control;3 and may lead to an increased risk of falls, lower extremity musculoskeletal injury,4 and loss of bone mineral density in the paretic limb.5 Because asymmetries are considered movement errors, therapists often use tactile, visual, or verbal cues to minimize asymmetries during gait training (error minimization strategy).6 Indeed, conventional locomotor training on a treadmill has demonstrated improved gait speed,7-9 but no effect on interlimb symmetry.7, 10, 11
In contrast, the approach of augmenting asymmetry (error augmentation strategy) to modify gait 12-14 is based on established motor learning principles, including error-based learning 15 and variability of practice.16 Such an error augmentation strategy may be beneficial for individuals post-stroke due to their deficits in recognizing spatiotemporal asymmetries as movement errors.17 The improved detection of augmented errors by muscle and joint proprioceptors may be the driving signal for adaptation and learning.15 Walking on a split-belt treadmill with belts operating at different fixed speeds (e.g., 2:1 ratio with short step length limb on fast belt) is a prime example of augmenting error, which will initially exaggerate step length asymmetry.18 The adaptation over time and resulting aftereffect suggest that the neuromuscular system retains the ability to produce nearly symmetric step lengths following stroke.18 Long term training (e.g., 4 weeks) with this locomotor adaptation approach elicited significant improvement in step length symmetry in more than half of the trained individuals.19
Rather than using a fixed perturbation, motor learning may be accelerated by transient amplification of movement errors.20, 21 To test this hypothesis, we designed a closed-loop feedback system coupled with an instrumented split-belt treadmill, in which step-by-step performance of spatiotemporal asymmetry determines the ratio of the belt speeds (i.e., error magnitude).22 The system can either augment or minimize error on a step by step basis, by continuously adjusting the difference in belt speed to be proportional to the patient's current asymmetry. For example, if stance time is shorter on the paretic limb, the velocity of the paretic treadmill belt can either be increased (to augment asymmetry) or decreased (to minimize asymmetry) relative to the non-paretic treadmill belt. With our unique system, we can determine which motor learning strategy (error minimization or augmentation) is best for improving overground spatial and temporal symmetry post-stroke.
Given that rehabilitation to improve gait symmetry may be based on one of these two competing motor learning strategies (i.e., minimizing or augmenting symmetry errors), the purpose of this randomized controlled trial was to determine which of those motor learning strategies best improves overground spatial and temporal gait symmetry in individuals with chronic hemiparesis post-stroke. Based on data from split belt adaptation work,18 we hypothesized that training with error augmentation would elicit the largest change in overground gait symmetry, because this mode would enhance awareness of movement errors.
Methods
Setting and Participants
The study was a randomized controlled trial with three parallel groups. The trial was registered with clinicaltrials.gov (NCT01598675) and all subjects received medical clearance from a physician to participate in training and signed an informed consent form approved by the UNC-Chapel Hill IRB (study #11-1240). We recruited potential subjects with hemiparesis of >6 months duration after unilateral, supratentorial, ischemic or hemorrhagic stroke. Participants were recruited from local stroke support groups, email listservs, and referred by local physicians and physical therapists.
Potential subjects were included if they were able to walk >10 m overground without physical assistance, had an overground comfortable gait speed < 1.0 m/s (using assistive devices and bracing below the knee, as needed), were able to walk on a treadmill at >80% of comfortable gait speed using upper extremity support if needed, and exhibited stance time asymmetry and/or step length asymmetry. Asymmetry was defined by a Symmetry Ratio 2 (max[paretic, non-paretic]/(paretic+non-paretic)) ≥ 0.524 for stance time asymmetry and ≥ 0.537 for step length asymmetry. These values represent a doubling of the upper 95% confidence limit of unimpaired individuals.1 Potential subjects were excluded if they had uncontrolled cardiorespiratory/metabolic disease, other neurologic disorders or orthopedic injury that may affect gait, botulinum toxin to the lower limb in the past 6 months, or concurrent physical therapy.
Randomization and Intervention
Following confirmation of eligibility and a double baseline (28 ± 16 days between baseline sessions), participants were randomized into one of three groups (Augmentation, Minimization, Control) by drawing a card from one of four envelopes that were stratified based on initial overground gait speed (≤0.5 m/s or >0.5 m/s, to help ensure similar gait speeds amongst groups) 23 and the type of asymmetry (step length or stance time). To avoid excluding subjects who had both stance time asymmetry and step length asymmetry, subjects were assigned, and trained accordingly, based on the larger of these two Symmetry Ratios. Assessors assigned to perform all outcome measures were initially blinded to group allocation, however, due to staffing constraints the final four subjects were assessed by an unblinded assessor. All participants refrained from attending their usual physical therapy, if applicable, during the course of the study.
Subjects completed 18 sessions of walking for up to 20 minutes on a split-belt, instrumented treadmill (Bertec Corp, Worthington, OH) wearing a safety harness that did not restrict lower extremity movements. Subjects were permitted to hold onto the handrail, but were discouraged from doing so, if possible. Therapists did not facilitate limb movements. Verbal instructions, using external focus of attention to cue for increased symmetry (e.g., “ride the belt a little longer on your weak side”, “reach for a spot near the front of the treadmill”) were used for all groups, as necessary.6 Heart rate and oxygen saturation were monitored throughout training and blood pressure was measured prior to, at mid-training (∼10 minutes) and following each training session to ensure that blood pressure remained below 180/110 mm Hg.24 Participants took rest breaks, as needed.
All three groups began each training session with two minutes of Control walking on the treadmill with both belts moving at the same speed, as a warm up. Then, without stopping the treadmill, subjects transitioned to the training phase (Asymmetry Augmentation, Minimization, or Control) for up to 18 minutes of additional walking. Each training session on the treadmill was followed by 10-15 minutes of overground gait training to encourage carryover of training to overground surfaces.6 During overground training, subjects were permitted to use an assistive device, but typically practiced without, when possible. Similar verbal cues were used during overground and treadmill training.
Treadmill Control
The treadmill was controlled by a novel computer-driven control algorithm. Treadmill control began by the therapist setting a base walking speed, vbase, which is the average speed of the two treadmill belts. During the first session, participants began walking at vbase = 80% of comfortable gait speed for overground gait. The difference in speeds between the two belts, vdiff, was determined by the degree of the patient's asymmetry on a step-by-step basis:
where k is a scale factor (default = 3), which can be increased to progress training so that a small amount of asymmetry will yield a larger difference in belt speed. Stance time or step length was calculated on a step-by-step basis from ground reaction force data (treadmill force plates) at 1080 Hz with a Vicon MX40+ system (Vicon/Peak, Los Angeles, CA). Stance time was the time (msec) that the vertical ground reaction force exceeded 10N, and step length was determined from center of pressure data as the anterior/ posterior distance between the feet at successive heel strikes 25, 26. These data were sent to the application computer using VRPN.27 The belt speed difference, vdiff, was applied to the treadmill by speeding up one belt and slowing the other:
Augmenting asymmetry was achieved with a positive “k” value, whereas a negative “k” value was used to minimize asymmetry. Each belt had high and low speed limits applied for safety, such that the belt speeds would not exceed a 2:1 ratio.18 Training step length or stance time was progressed similarly within and between sessions by altering treadmill speed and the “k” value. The “k” value was adjusted independent of treadmill speed (i.e., vbase). Treadmill speed was increased if heart rate remained below 70% of age-predicted maximum 28 or the perceived exertion was less than 14 on the Borg scale (for participants receiving medications that blunt heart rate response).24
Outcomes and Follow-up
We collected overground gait characteristics from both baseline visits. Because of the tendency for gait speed to increase with repeated testing,29 we used the overground data from the second baseline visit, which occurred one week prior to (Pre) training, and the repeated assessments at one week following the final training session (Post), and at one month after training (Follow-up). At each assessment visit, subjects were instructed to “walk at their normal, comfortable walking speed” as they performed 3 passes across a 14 foot GAITRite mat (CIR Systems, Franklin, NJ) with a 4 foot acceleration and deceleration zone at either end of the mat. GAITRite software was used to determine comfortable gait speed and gait asymmetry, as described above, for both step length and stance time variables.29 Given the automated process of calculating outcome measures, we believe that the lack of blinded assessment for the final four subjects was inconsequential.
Statistical Analysis
All data were exported into electronic spreadsheets (Excel 2016, Microsoft Corp) and data analysis was subsequently performed using SPSS (ver 23, IBM). Data residuals were checked for assumptions of normality using the Shapiro-Wilk test, and Mauchly's W was used to test for sphericity with the Huynh-Feldt adjustment, as needed. Primary (gait symmetry ratio) and secondary (comfortable gait speed) variables were analyzed using a two-way, repeated measures ANOVA (repeated for time) to assess the interaction effect (i.e., effect of time on the three groups: Augmentation, Minimization, Control). Tukey (between subjects) and Bonferroni corrected paired-samples t-tests (within subjects) were used for post-hoc tests. For those with step length asymmetry, the shorter step length can occur on either the paretic or non-paretic limb.18, 29 We therefore sorted limbs based on which step was longer and shorter for separate comparison across time using a repeated-measures ANOVA. The relationship between changes in spatiotemporal symmetry and change in gait speed from Pre to Post training was assessed using Pearson correlations. Prior to enrollment, we performed a power analysis based on a non-central F-distribution to satisfy concerns about a potentially asymmetric distribution of our primary outcome measure (spatiotemporal asymmetry). Power was calculated using assumptions based on our review of reported changes in spatiotemporal asymmetry with locomotor training.10, 13, 30 Estimating a standard deviation of 0.019 and a difference in the change in asymmetry of 0.035 from pre-training to post-training, we expected to have greater than 80% power with sample size of 45.
Results
We recruited 48 subjects to participate (26 in the step length symmetry training and 22 in the stance time symmetry training). As seen in the CONSORT diagram (figure 1), 37 subjects completed gait training (over a mean (SD) of 48 (10) days) and testing. The 3 groups did not differ on key baseline characteristics (table 1). The 11 subjects who dropped out were split between groups (Augmentation: N=4; Minimization: N=4; Control: N=3). Reasons for drop-out varied (transportation issues = 6; exercise intolerance = 2; blood pressure/cardiovascular issues = 2; none given = 1).
Figure 1.
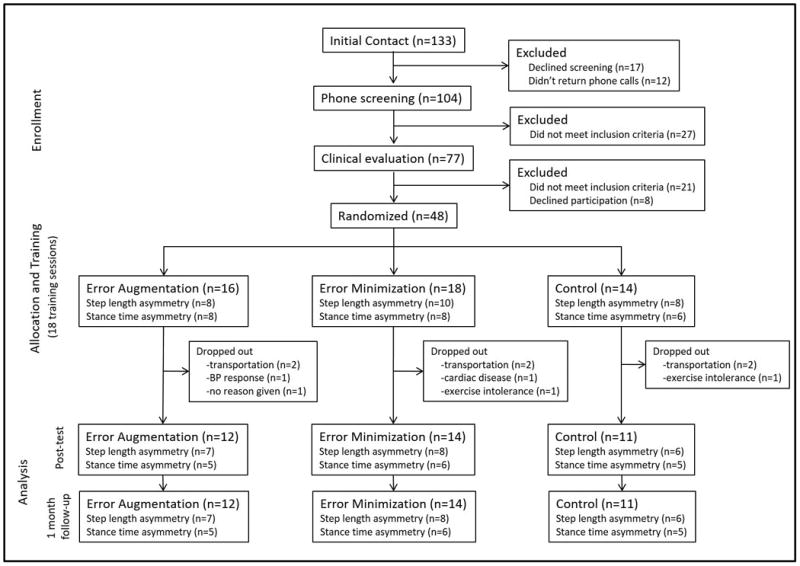
CONSORT diagram depicting subject allocation through study flow.
Table 1. Subject Demographics.
| Error Augmentation (n = 12) | Error Minimization (n=14) | Control (n=11) | ||
|---|---|---|---|---|
| Age | 57.5 (10.5) years | 60.7 (13.0) years | 57.6 (13.2) years | p = 0.764 |
| Sex | 5 F / 7 M | 5F / 9 M | 5 F / 6 M | p = 0.916 |
| Paretic Side | 4 L / 8 R | 9 L / 5 R | 8 L / 3 R | p = 0.181 |
| Time Post-Stroke | 36.3 (21.7) months | 54.4 (55.8) months | 28.1 (27.9) months | p = 0.228 |
| Overground gait speed (Pre) | 0.42 (0.21) m/s | 0.44 (0.28) m/s | 0.36 (0.22) m/s | p = 0.710 |
| Assistive Device at pre-test | 6 canes, 6 none | 10 canes, 4 none | 9 canes, 2 none | p = 0.275 |
| Ankle bracing at pre-test | 3 yes, 9 no | 7 yes, 7 no | 9 yes, 2 no | p = 0.026 |
Values represent mean (SD).
Overall, we observed a significant interaction (p = 0.026; η2p = 0.140) between time and the selected asymmetry parameter (i.e., step length or stance time asymmetry) indicating that those who were trained to alter step length vs stance time asymmetries responded differently over time. For this reason, all further analyses were separated by the participant's training parameter. No other interaction effects were significant (all p>0.453).
Step Length Asymmetry
Twenty six subjects were assigned to perform training to improve step length asymmetry. Of those subjects, 21 (81%) completed testing and training (N=7: Augmentation; N=8: Minimization; N=6: Control). We observed no significant interaction effect (group × time: p = 0.472; η2p = 0.086; figure 2) among the subjects who trained to improve step length asymmetry. Likewise, we observed no significant effect for group (p = 0.183; η2p = 0.172). We did, however note a significant effect of time on step length asymmetry (p = 0.005; η2p = 0.311). Across all groups, step length asymmetry was significantly higher (i.e., worse) at pre-test compared to post-test (p=0.014) and at follow-up (p=0.045). No difference was observed between post-test and follow-up (p=1.000).
Figure 2.
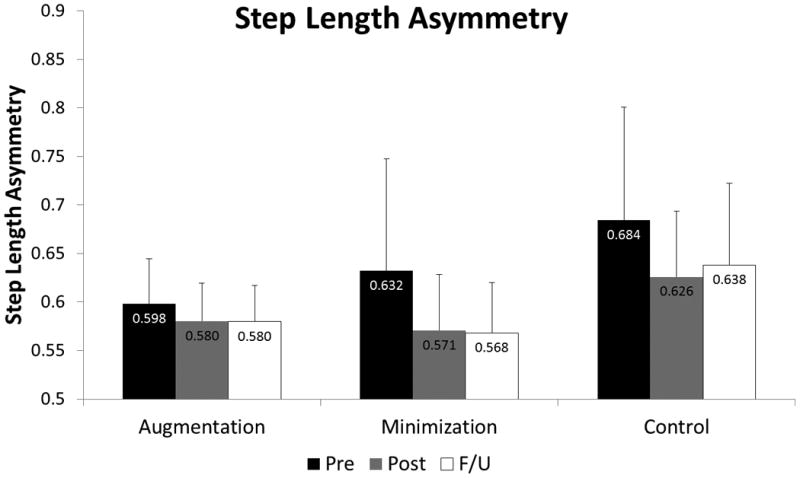
Step length asymmetry ratio (N=21) at the Pre-test (black), Post-test (gray), and Follow-up (white) time points for the Augmentation, Minimization, and Control groups. A significant effect of time was observed with step length asymmetry reducing from pre-test to post-test and persisting at follow-up.
To confirm that subjects did not simply improve their symmetry ratio by reducing the longer step length, we compared the step lengths of both limbs (table 3). Importantly, we observed that subjects significantly increased both their shorter (p < 0.001; η2p = 0.621) and longer (p<0.001; η2p = 0.701) step lengths as a result of training, and maintained those improvements at follow-up (figure 3). Nine subjects (of 21) exhibited changes in step length asymmetry from pre- to post-training that exceeded the minimal detectable change (MDC) of 0.035.29 Of those, one trained with error augmentation, five were in the error minimization group, and three were in the control group (p=0.185). Within this group that trained to improve step length asymmetry, we observed no change in stance time asymmetry (p=0.774; η2p = 0.012, table 2).
Table 3. Step Lengths (cm) Stratified by Shorter and Longer Steps.
| Pre-training | Post-training | Follow-up | ||
|---|---|---|---|---|
| Shorter Steps | Augmentation | 26.8 (11.2) | 33.5 (13.7) | 33.3 (13.6) |
| Minimization | 26.7 (17.3) | 38.8 (16.0) | 39.5 (15.1) | |
| Control | 20.2 (13.3) | 30.5 (13.1) | 28.6 (13.1) | |
| Longer Steps | Augmentation | 39.1 (12.4) | 45.2 (14.6) | 45.2 (15.3) |
| Minimization | 41.1 (13.9) | 49.9 (15.3) | 50.4 (14.1) | |
| Control | 38.9 (7.9) | 48.3 (8.5) | 47.1 (7.4) |
Values represent mean (SD)
Figure 3.
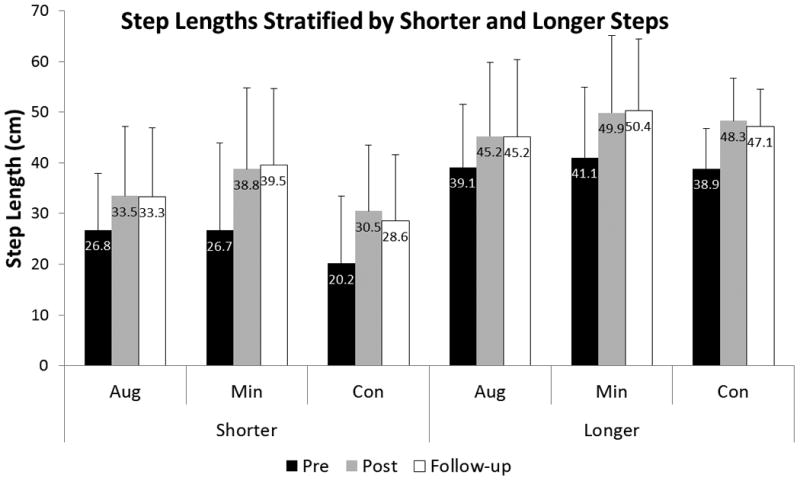
Step lengths significantly increased from pre-test to post-test on both the limb taking the shorter step as well as the limb taking the longer step. These improvements persisted at follow-up on both legs.
Table 2. Spatiotemporal Gait Asymmetry.
| Pre-training | Post-training | Follow-up | |||
|---|---|---|---|---|---|
| Step Length Asymmetry Trained (N=21) | Step Length Asymmetry | Augmentation | 0.598 (0.046) | 0.580 (0.039) | 0.580 (0.037) |
| Minimization | 0.632 (0.115) | 0.571 (0.058) | 0.568 (0.052) | ||
| Control | 0.684 (0.116) | 0.626 (0.068) | 0.638 (0.084) | ||
| Stance Time Asymmetry | Augmentation | 0.528 (0.013) | 0.526 (0.013) | 0.525 (0.016) | |
| Minimization | 0.536 (0.022) | 0.542 (0.028) | 0.541 (0.028) | ||
| Control | 0.539 (0.020) | 0.539 (0.017) | 0.536 (0.019) | ||
| Stance Time Asymmetry Trained (N=16) | Stance Time Asymmetry | Augmentation | 0.544 (0.011) | 0.545 (0.008) | 0.547 (0.010) |
| Minimization | 0.542 (0.016) | 0.535 (0.018) | 0.536 (0.016) | ||
| Control | 0.569 (0.014) | 0.565 (0.018) | 0.561 (0.014) | ||
| Step Length Asymmetry | Augmentation | 0.527 (0.022) | 0.531 (0.015) | 0.517 (0.017) | |
| Minimization | 0.521 (0.009) | 0.517 (0.013) | 0.520 (0.014) | ||
| Control | 0.533 (0.027) | 0.539 (0.024) | 0.533 (0.029) |
Values represent mean (SD)
Stance Time Asymmetry
We assigned 22 subjects to train to improve stance time symmetry and 16 (73%) completed training (N=5: Augmentation; N=6: Minimization; N=5: Control). Of those who trained to improve stance time asymmetry, there was no significant effect of time (p = 0.302; η2p = 0.088) on stance time asymmetry. Likewise, we observed no significant interaction effect (group × time: (p = 0.374; η2p = 0.146; figure 4). These subjects were trained to improve stance time asymmetry, and thus we observed no influence of training on step length asymmetry (p=0.134; η2p = 0.143).
Figure 4.
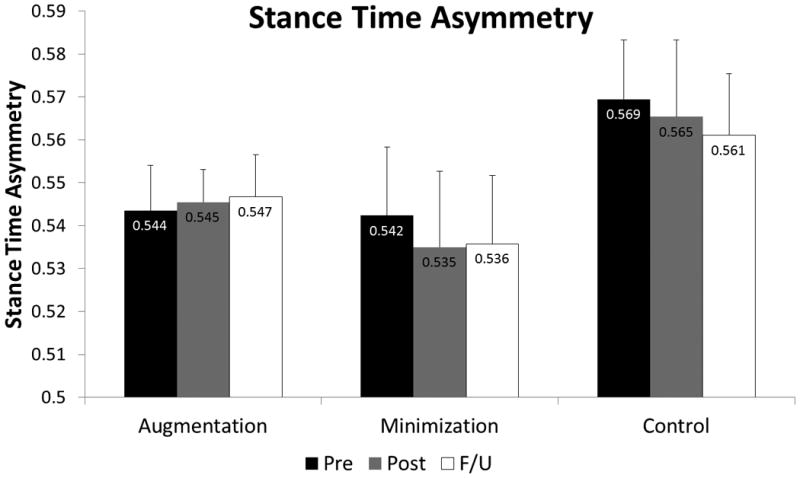
Stance time asymmetry ratio (N=16) at the Pre-test (black), Post-test (gray), and Follow-up (white) time points for the Augmentation, Minimization, and Control groups. There was no effect of time, and no interaction effect to indicate that one group changed more than another.
Gait Speed
Because there were no significant interaction effects (all p>0.342), the post-hoc analysis for gait speed was collapsed across all subjects. Gait speed significantly increased over time (p<0.001; η2p = 0.617). Specifically, we observed that subjects walked significantly faster after training (mean (SD) gait speed was 0.53 (0.28) m/s) compared to pre-training speeds (0.41 (0.24) m/s; p<0.001; d = 0.52). Gait speed continued to improve from the post-test to the follow-up session (0.56 (0.29) m/s; p = 0.018; d = 0.08).
Of those who trained to improve their step length asymmetry, we observed a moderate relationship between the change in step length asymmetry from pre- to post-training and the change in gait speed from pre- to post-training (r = -0.45; p = 0.040; figure 5). There was no such relationship between the change in stance time asymmetry and gait speed for those who trained to improve stance time asymmetry (r = -0.153; p = 0.571).
Figure 5.
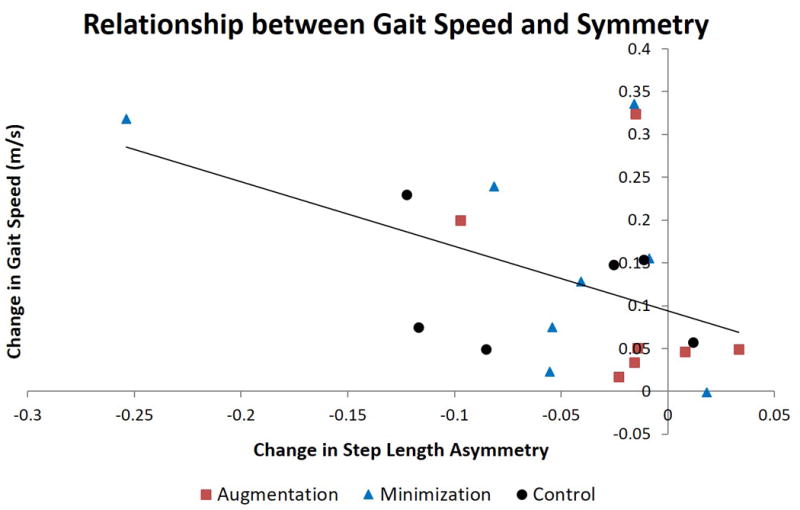
We observed the presence of a moderate relationship between the change in step length asymmetry and the change in gait speed from pre-training to post-training. Group allocation of individual subjects is shown.
We performed one additional comparison because we were particularly concerned about the potential for training with error minimization to increase (i.e., make worse) subject's asymmetry.18 Indeed, during training, our paradigm has the potential to augment step length symmetry while minimizing stance time symmetry, or vice versa. For example, a participant assigned to augment step length asymmetry during training may also have concomitantly minimized stance time asymmetry during training. For that reason, subjects were recoded as to whether step length asymmetry or stance time asymmetry was minimized or augmented (regardless of group assignment). In fact, we observed that the subjects (N=13) who had their step length asymmetry errors minimized (either intentionally or not) showed a tendency to reduce their step length asymmetry from 0.592 (0.103) at pre-test to 0.556 (0.049) at post-test (p=0.097; d = 0.48). Those who had their stance time asymmetry errors minimized (N=13, regardless of intentional or unintentional) did not alter stance time asymmetry from pre-test (0.537 (0.014)) to post-test (0.539 (0.029); p = 0.682; d = 0.12). These data suggest that adaptive minimization of movement errors did not lead to an increase (worsening) in gait asymmetry following training.
Discussion
Our hypothesis that gait training with error augmentation would improve spatiotemporal gait asymmetry more than minimizing errors was not supported by these data. Subjects were able to improve step length (spatial) asymmetry, but did not show an appreciable change in stance time (temporal) asymmetry following training. Despite this overall improvement in step length asymmetry after training, we did not observe one method of training to be superior to another. The common training component across all groups was the deliberate focus on spatiotemporal asymmetry and the verbal feedback from therapists during gait training. This attention to specific training variables through verbal feedback during functional practice may, therefore, have been the active ingredient that promoted improvements in step length asymmetry.
We are excited at the prospect that step length asymmetry was significantly improved, and that the improvement was retained for at least a month. Although the training was performed on a treadmill, all testing was performed overground, emphasizing the context-independent nature of the improvements. Although we were initially surprised that the error augmentation algorithm did not produce greater gains, from a clinical perspective this represents a more promising finding. Rather than requiring a split-belt instrumented treadmill with computer controlled algorithms, it appears possible to elicit substantial changes to step length symmetry in individuals with chronic stroke during “conventional” gait training; provided that appropriate focus, attention,31 and variability in practice are provided.
Based on previous literature,19 we were surprised that the error augmentation group did not outperform the other two groups. Although some have noted that error augmentation is more beneficial than error minimization during a single session,32 others have reported that error augmentation did not improve the immediate retention of a locomotor balance task.33 In our cohort, the type of error manipulation during training (either minimization or augmentation) did not appear to differentially influence spatial asymmetry changes; comparable changes occurred in all three groups. Importantly, however, we observed that step length symmetry was only improved in the subjects who trained to improve step length symmetry. We can think of two possible reasons for this. First, we couldn't train subjects to improve stance time asymmetry and expect to see an improvement in step length asymmetry. Despite the known relationship between spatial and temporal asymmetry,1 the specific attention to step length asymmetry during training appeared important for eliciting change. Second, by the nature of the inclusion criteria, the subjects who trained to improve step length asymmetry exhibited greater step length asymmetry than those who trained to improve stance time asymmetry. Likewise, Reisman and colleagues demonstrated that responders appeared to have a larger baseline asymmetry than the non-responders.19 Nevertheless, there were many individuals in our stance time asymmetry trained group (n= 9 of 16 who completed training) who exhibited substantial step length asymmetry, but happened to have stance time asymmetry that was larger in magnitude than their step length asymmetry. Therefore, although the difference in baseline step length asymmetry may have contributed to this finding, we think it is more likely that the change in step length asymmetry after training was due to the attention focused on that particular parameter.
We observed, similar to others,7, 10, 11, 13 that stance time asymmetry is particularly resistant to change. As a rapidly adapting parameter,26 stance time asymmetry is highly responsive to the speed of the treadmill belts, and thus did not change in response to training. Stance time is a reactive parameter, which relies heavily on peripheral feedback from hip flexor afferents, limb load receptors, and cutaneous feedback. The fact that after-effects are not observed after manipulation of stance time asymmetry suggests that this is a parameter that may not be modified using error manipulation.34 Interestingly, even though stance time asymmetry and step length asymmetry are related to each other,1 there was no concomitant change in stance time asymmetry in our group who trained to improve step length asymmetry. Thus, changing one parameter does not necessitate a change in another.35
Consistent with current neurorehabilitation strategies, which encourage spatiotemporal symmetry during training,6 our “closed loop”, error minimization system was intended to produce nearly symmetric gait. There has been concern that such training may exacerbate gait asymmetry after training.18 Instead, we observed that those who had their asymmetry errors minimized tended to reduce their asymmetry after 18 training sessions. Although this observation would appear to conflict with previous literature,18 it is important to note the inherent difference in control schemes between our paradigm and that of others. Previous work has used open-loop systems, which are unresponsive to the step-by-step changes in movement that occur during training.18 Instead, our “closed-loop” system puts the subject in the loop to ensure that the manipulation of errors is responsive to the subject's stepping. Our subjects in the error minimization and error augmentation groups, therefore, received real-time, proprioceptive feedback of gait asymmetry. Despite the likely presence of deficits in spatiotemporal asymmetry perception,17 our subjects following stroke did not appear to need the altered proprioceptive feedback from the changing treadmill belt speeds, as subjects in all three groups were able to improve step length asymmetry.
The change in gait speed observed following training was comparable to previously published work.7, 36 Although gait speed was increased within and across sessions, we focused our subject's attention on spatiotemporal gait symmetry rather than gait speed. Additionally, the gait speed change of our subjects was likely an underestimate compared to previous work. Because gait speed is known to increase substantially without the presence of an intervention,29, 37 we performed a double baseline and used the second (i.e., faster) session's speed as the Pre-training speed. Notably, the change in step length asymmetry was related to the change in comfortable gait speed. Although this finding would seem to suggest that step length asymmetry is a therapeutic target for improving gait speed following stroke, there is evidence to the contrary. In short, we observed that several subjects experienced sizable increases in gait speed, with small changes in step length symmetry. Likewise, others have noted significant improvements in step length symmetry, without a concomitant change in gait speed.19 Thus, we must question whether changing step length asymmetry is really necessary to elicit improvements in gait speed.
A limitation of this work is the large number of drop-outs. Getting participants to the lab for 18 training sessions proved more difficult than we anticipated given our budget limitations. Ultimately we had 77% (37 of 48) of our participants complete training, which was consistent with some recently reported gait training trials 7, but a smaller percentage than others.38 Despite the somewhat high drop-out rate, there were similar numbers of participants who did not complete training in each group. Additionally, the small sample size produced small cell counts within each stratification, which limited our ability to examine potential differences between individuals with a slow vs a fast walking speed. An additional limitation of this work is that the final four participants were not measured by a blinded assessor. Finally, additional information about specific lesion location might have helped with the interpretation of our data.
The differential response to training suggests that better selection criteria are needed to identify responders to treatment. We observed that nine of the 21 subjects trained to improve step length asymmetry exceeded the minimal detectable change.29 Likewise, Reisman and colleagues 19 demonstrated improvement in 7 of 13 subjects using a more liberal definition of “responder”. Although some individuals clearly had the capacity to improve their step length asymmetry, there are also some individuals who did not improve as a result of training. For those individuals who did demonstrate improvement in step length symmetry, only one reached the threshold for having symmetric gait. Presently, thresholds of minimally important clinical changes remain unknown for step length asymmetry. If further work to restore symmetric gait following stroke is to continue, it is important to determine the characteristics that makes someone successful at altering their step length asymmetry. Because the locomotor adaptation literature suggests that many individuals have the neuromotor capacity to produce improved symmetry, 18 perhaps verifying this capacity would improve subject selection for future work.
Clinical Message.
In patients more than six months after stroke and able to walk, persistent step length asymmetry was improved following training, whereas stance time asymmetry was not.
A specific emphasis on either minimizing or augmenting asymmetry during treadmill training did not appear to influence these results.
Verbal instruction and feedback were common across all groups and may have contributed to the noted improvement in step length asymmetry.
Acknowledgments
The authors acknowledge Jeff Feasel, MS for the treadmill control algorithms; Samantha Hansen, Mary O'Brien, Donna Dean, and Robert Sykes III for assistance with scheduling, recruiting, training, and data management; Prue Plummer for helpful insight including review of the manuscript prior to submission.
Competing Interests: This work was supported by the National Institutes of Health (R21 – HD068805 to Lewek).
Contributor Information
Michael D Lewek, Division of Physical Therapy, Department of Allied Health Sciences, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; Phone: 919-966-9732.
Carty H Braun, UNC Health Care System, Chapel Hill, NC 27599.
Clint Wutzke, Human Movement Science Program, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599.
Carol Giuliani, Division of Physical Therapy, Department of Allied Health Sciences, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599.
Literature Cited
- 1.Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait & posture. 2010;31:241–6. doi: 10.1016/j.gaitpost.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabilitation and neural repair. 2015;29:416–23. doi: 10.1177/1545968314552528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewek MD, Bradley CE, Wutzke CJ, Zinder SM. The relationship between spatiotemporal gait asymmetry and balance in individuals with chronic stroke. J Appl Biomech. 2014;30:31–6. doi: 10.1123/jab.2012-0208. [DOI] [PubMed] [Google Scholar]
- 4.Norvell DC, Czerniecki JM, Reiber GE, Maynard C, Pecoraro JA, Weiss NS. The prevalence of knee pain and symptomatic knee osteoarthritis among veteran traumatic amputees and nonamputees. Archives of physical medicine and rehabilitation. 2005;86:487–93. doi: 10.1016/j.apmr.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 5.Jorgensen L, Crabtree NJ, Reeve J, Jacobsen BK. Ambulatory level and asymmetrical weight bearing after stroke affects bone loss in the upper and lower part of the femoral neck differently: bone adaptation after decreased mechanical loading. Bone. 2000;27:701–7. doi: 10.1016/s8756-3282(00)00374-4. [DOI] [PubMed] [Google Scholar]
- 6.Duncan PW, Sullivan KJ, Behrman AL, et al. Protocol for the Locomotor Experience Applied Post-stroke (LEAPS) trial: a randomized controlled trial. BMC neurology. 2007;7:39. doi: 10.1186/1471-2377-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced Gait-Related Improvements After Therapist- Versus Robotic-Assisted Locomotor Training in Subjects With Chronic Stroke. A Randomized Controlled Study. Stroke; a journal of cerebral circulation. 2008;39:1786–92. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan KJ, Brown DA, Klassen T, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Physical therapy. 2007;87:1580–602. doi: 10.2522/ptj.20060310. discussion 603-7. [DOI] [PubMed] [Google Scholar]
- 9.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. The New England journal of medicine. 2011;364:2026–36. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patterson SL, Rodgers MM, Macko RF, Forrester LW. Effect of treadmill exercise training on spatial and temporal gait parameters in subjects with chronic stroke: a preliminary report. J Rehabil Res Dev. 2008;45:221–8. doi: 10.1682/jrrd.2007.02.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silver KH, Macko RF, Forrester LW, Goldberg AP, Smith GV. Effects of aerobic treadmill training on gait velocity, cadence, and gait symmetry in chronic hemiparetic stroke: a preliminary report. Neurorehabilitation and neural repair. 2000;14:65–71. doi: 10.1177/154596830001400108. [DOI] [PubMed] [Google Scholar]
- 12.Savin DN, Tseng SC, Whitall J, Morton SM. Poststroke hemiparesis impairs the rate but not magnitude of adaptation of spatial and temporal locomotor features. Neurorehabilitation and neural repair. 2013;27:24–34. doi: 10.1177/1545968311434552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn JH, Hornby TG. Rapid and long-term adaptations in gait symmetry following unilateral step training in people with hemiparesis. Physical therapy. 2009;89:474–83. doi: 10.2522/ptj.20080237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam T, Luttmann K, Houldin A, Chan C. Treadmill-based locomotor training with leg weights to enhance functional ambulation in people with chronic stroke: a pilot study. J Neurol Phys Ther. 2009;33:129–35. doi: 10.1097/NPT.0b013e3181b57de5. [DOI] [PubMed] [Google Scholar]
- 15.Kawato M. Feedback-error-learning neural network for supervised learning. In: Eckmiller R, editor. Advanced neural computers. North-Holland, Amsterdam: 1990. pp. 365–72. [Google Scholar]
- 16.Schmidt RA. A schema theory of discrete motor skill learning. Psychological Review. 1975;82:225–60. [Google Scholar]
- 17.Wutzke CJ, Faldowski RA, Lewek MD. Individuals Poststroke Do Not Perceive Their Spatiotemporal Gait Asymmetries as Abnormal. Physical therapy. 2015;95:1244–53. doi: 10.2522/ptj.20140482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–72. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabilitation and neural repair. 2013;27:460–8. doi: 10.1177/1545968312474118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emken JL, Reinkensmeyer DJ. Robot-enhanced motor learning: accelerating internal model formation during locomotion by transient dynamic amplification. IEEE Trans Neural Syst Rehabil Eng. 2005;13:33–9. doi: 10.1109/TNSRE.2004.843173. [DOI] [PubMed] [Google Scholar]
- 21.Gordon KE, Ferris DP. Learning to walk with a robotic ankle exoskeleton. J Biomech. 2007;40:2636–44. doi: 10.1016/j.jbiomech.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Feasel J, Whitton MC, Kassler L, Brooks FP, Lewek MD. The integrated virtual environment rehabilitation treadmill system. IEEE Trans Neural Syst Rehabil Eng. 2011;19:290–7. doi: 10.1109/TNSRE.2011.2120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plummer P, Behrman AL, Duncan PW, et al. Effects of stroke severity and training duration on locomotor recovery after stroke: a pilot study. Neurorehabilitation and neural repair. 2007;21:137–51. doi: 10.1177/1545968306295559. [DOI] [PubMed] [Google Scholar]
- 24.ACSM. ACSM's Guidelines for Exercise Testing and Prescription. 7th ed. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 25.Noble JW, Prentice SD. Adaptation to unilateral change in lower limb mechanical properties during human walking. Exp Brain Res. 2006;169:482–95. doi: 10.1007/s00221-005-0162-3. [DOI] [PubMed] [Google Scholar]
- 26.Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94:2403–15. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- 27.Taylor RM, II, Hudson TC, Seeger A, Weber H, Juliano J, Helser AT. Proceedings of the ACM symposium on virtual reality software and technology. Banff Alberta, Canada: ACM; 2001. VRPN: a device-independent, network-transparent VR peripheral system. [Google Scholar]
- 28.Gordon NF, Gulanick M, Costa F, et al. Physical activity and exercise recommendations for stroke survivors: an American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Circulation. 2004;109:2031–41. doi: 10.1161/01.CIR.0000126280.65777.A4. [DOI] [PubMed] [Google Scholar]
- 29.Lewek MD, Randall EP. Reliability of spatiotemporal asymmetry during overground walking for individuals following chronic stroke. J Neurol Phys Ther. 2011;35:116–21. doi: 10.1097/NPT.0b013e318227fe70. [DOI] [PubMed] [Google Scholar]
- 30.Lewek MD, Cruz TH, Moore JL, Roth HR, Dhaher YY, Hornby TG. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Physical therapy. 2009;89:829–39. doi: 10.2522/ptj.20080180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Vliet PM, Wulf G. Extrinsic feedback for motor learning after stroke: what is the evidence? Disabil Rehabil. 2006;28:831–40. doi: 10.1080/09638280500534937. [DOI] [PubMed] [Google Scholar]
- 32.Kao PC, Srivastava S, Agrawal SK, Scholz JP. Effect of robotic performance-based error-augmentation versus error-reduction training on the gait of healthy individuals. Gait & posture. 2013;37:113–20. doi: 10.1016/j.gaitpost.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domingo A, Ferris DP. The effects of error augmentation on learning to walk on a narrow balance beam. Exp Brain Res. 2010;206:359–70. doi: 10.1007/s00221-010-2409-x. [DOI] [PubMed] [Google Scholar]
- 34.Helm EE, Reisman DS. The Split-Belt Walking Paradigm: Exploring Motor Learning and Spatiotemporal Asymmetry Poststroke. Physical medicine and rehabilitation clinics of North America. 2015;26:703–13. doi: 10.1016/j.pmr.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malone LA, Bastian AJ, Torres-Oviedo G. How does the motor system correct for errors in time and space during locomotor adaptation? J Neurophysiol. 2012;108:672–83. doi: 10.1152/jn.00391.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Awad LN, Reisman DS, Kesar TM, Binder-Macleod SA. Targeting paretic propulsion to improve poststroke walking function: a preliminary study. Archives of physical medicine and rehabilitation. 2014;95:840–8. doi: 10.1016/j.apmr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37:75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 38.Awad LN, Reisman DS, Pohlig RT, Binder-Macleod SA. Reducing The Cost of Transport and Increasing Walking Distance After Stroke: A Randomized Controlled Trial on Fast Locomotor Training Combined With Functional Electrical Stimulation. Neurorehabilitation and neural repair. 2016;30:661–70. doi: 10.1177/1545968315619696. [DOI] [PMC free article] [PubMed] [Google Scholar]


