Abstract
Obstructive sleep apnea (OSA), the most severe form of sleep disordered breathing, is characterized by intermittent hypoxia during sleep (IH), sleep fragmentation, and episodic hypercapnia. OSA is associated with increased risk for morbidity and mortality affecting cardiovascular, metabolic, and neurocognitive systems, and more recently with non-alcoholic fatty liver disease (NAFLD) and cancer-related deaths. Substantial variability in OSA outcomes suggests that genetically-determined and environmental and lifestyle factors affect the phenotypic susceptibility to OSA. Furthermore, OSA and obesity often co-exist and manifest activation of shared molecular end-organ injury mechanisms that if properly identified may represent potential therapeutic targets. A challenge in the development of non-invasive diagnostic assays in body fluids is the ability to identify clinically relevant biomarkers. Circulating extracellular vesicles (EVs) include a heterogeneous population of vesicular structures including exosomes, prostasomes, microvesicles (MVs), ectosomes and oncosomes, and are classified based on their size, shape and membrane surface composition. Of these, exosomes (30–100 nm) are very small membrane vesicles derived from multi-vesicular bodies or from the plasma membrane and play important roles in mediating cell-cell communication via cargo that includes lipids, proteins, mRNAs, miRNAs and DNA. We have recently identified a unique cluster of exosomal miRNAs in both humans and rodents exposed to intermittent hypoxia as well as in patients with OSA with divergent morbid phenotypes. Here we summarize such recent findings, and will focus on exosomal miRNAs in both adult and children which mediate intercellular communication relevant to OSA and endothelial dysfunction, and their potential value as diagnostic and prognostic biomarkers.
Sleep Disordered Breathing
Sleep-disordered breathing (SDB) is a general term referring to the presence of a spectrum of conditions in which partial or complete cessation of airflow along with central or obstructed breathing occurs many times throughout the night. In this setting, obstructive sleep apnea (OSA) is caused by the increased upper airway collapsibility along with insufficiency or loss of upper airway dilating muscle capacity promoting repeated pharyngeal narrowing (hypopnea) or closure (apnea), thereby leading to declines in oxyhemoglobin saturation and increases in the partial pressure of carbon dioxide in arterial blood (Jordan et al., 2014). To restore pharyngeal patency, patients have recurrent arousals from sleep, resulting in sleep fragmentation which in turn leads to daytime sleepiness and reduced quality of life. Moreover, the chronic cycles of hypoxia-re-oxygenation, i.e., intermittent hypoxia (IH), induce oxidative stress (ROS) and promote activation of inflammatory pathways (Lavie, 2003). In addition to fluctuations in oxygenation (IH), sleep fragmentation (SF), and episodic oscillations in carbon dioxide tension, the intermittent changes in upper airway resistance during sleep in OSA patients foster other physiological disturbances including increased intrathoracic pressure swings, altered acid-base status, and disruptions in autonomic function and behavioral state (Dempsey et al., 2010).
Obstructive sleep apnea (OSA), is considered by far the most common form of sleep-disordered breathing, is highly prevalent across the lifespan, and is associated with a myriad of adverse health consequences independent of other traditional cardio-metabolic risk factors and obesity, including an increased risk of death (Al Lawati et al., 2009; Caples et al., 2007; Tahrani et al., 2013; Young et al., 2008). SDB and more specifically OSA have been associated with a large spectrum of neurocognitive, behavioral, cardiovascular, and metabolic adverse consequences, which appear to be particularly prominent in obese patients (Amaddeo et al., 2017; Floras, 2014; Luz Alonso-Alvarez et al., 2011; Marcus et al., 2012; Sforza and Roche, 2016), while also differentially affecting both genders and varying in severity according to chronological age (Mokhlesi et al., 2016).
A large body of evidence has clearly demonstrated that chronic IH is critically involved in the short-term and long-term cardiovascular consequences of OSA, including systemic hypertension, left ventricular hypertrophy, and endothelial dysfunction (Alchanatis et al., 2002; Ameli et al., 2007; Amin et al., 2002; Brooks et al., 1997; Lesske et al., 1997; Varadharaj et al., 2015) (Marin et al., 2005; Mason et al., 2012). Indeed, recent studies have shown clear and robust associations of OSA with the development of hypertension (Nieto et al., 2000; Peppard et al., 2000; Phillips and O’Driscoll, 2013; Ren et al., 2016), type II diabetes (Lai et al., 2016; Plihalova et al., 2016; Reichmuth et al., 2005), stroke (Arzt et al., 2005; Campos-Rodriguez et al., 2014; Ifergane et al., 2016), congestive heart failure (Cowie, 2016; Pearse and Cowie, 2016; Shahar et al., 2001), coronary artery disease (Loo et al., 2014; Marin et al., 2012; Selim et al., 2010), cardiac arrhythmias (Vizzardi et al., 2014), and cancer (Campos-Rodriguez et al., 2013).
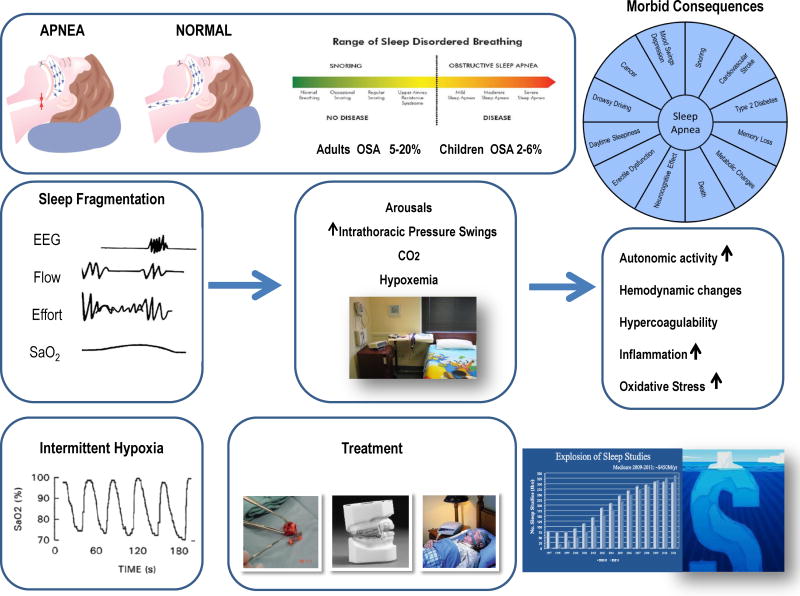
Despite the high prevalence and important morbidity load (Figure 1), OSA remains significantly under recognized, and it is estimated that only 40% of those with OSA have been diagnosed (Banno et al., 2009), with the medical costs of untreated OSA in the United States being estimated at US$ 4 billion/year (Kapur et al., 1999; Young et al., 1997). The total economic impact of OSA is even greater than direct medical costs, in light of the sleepiness-associated work and motor vehicle accident risks, increased job productivity losses, and social disability needs (Hillman et al., 2006).
Figure 1.
Schematic diagram illustrating the clinical spectrum of sleep disordered breathing, the major physiologic alterations in OSA, namely sleep fragmentation and intermittent hypoxia, leading to a variety of downstream pathophysiological pathways ultimately resulting in a large repertoire of morbid phenotypes. The major therapeutic options, namely CPAP, intra-oral appliances or adenotonsillectomy (in children) are shown, and the hidden economic costs of OSA along with escalating healthcare costs involved in the diagnosis and treatment of OSA are displayed.
OSA in children is also a highly prevalent disorder that can result in significant morbidity, and is primarily associated with adenotonsillar hypertrophy (Capdevila et al., 2008; Lumeng and Chervin, 2008). The prominent increases in childhood overweight and obesity rates in the globe even among youngest of children have further translated into parallel increases in the prevalence of OSA, and such trends are undoubtedly associated with deleterious global health outcomes and life expectancy (Bixler et al., 2009; Kheirandish-Gozal and Gozal, 2012; Li et al., 2010; Marcus et al., 2012).
As mentioned above, OSA in both adults and children is not only a prevalent condition but one that is clearly associated with multisystem involvement. However, evidence of morbidity is not a universal finding in patients with OSA. It also remains unclear whether those patients with evidence of one end-organ morbidity are at increased risk for developing another (Mokhlesi and Gozal, 2010). Chronic IH can lead to increased pro-inflammatory cytokine production, endothelial dysfunction, oxidative stress, metabolic dysregulation and insulin resistance (Christou et al., 2003; Ciftci et al., 2004). Several oxidative stress markers are increased in OSAS, and could amplify inflammatory cascades, induce endothelial dysfunction and development of atherosclerosis and promote central nervous system dysfunction (Lavie, 2003; Lavie, 2015; Wang et al., 2010; Zhang et al., 2012; Zhou et al., 2016). However the determinants of injury in any given patient with OSA are not well delineated, and in addition to disease severity and concurrent presence of other underlying conditions such as obesity, they are likely to involve both genetic and environmental contributions (Bhattacharjee et al., 2011; Kheirandish-Gozal and Gozal, 2013; Tan et al., 2014).
Obesity
Obesity has rapidly evolved into a global pandemic, and poses a significant healthcare and socioeconomic burden in both developed and developing countries. Obesity is a multifactorial disease that is often accompanied by other health problems, such as hypertension, dyslipidemia, fatty liver disease, diabetes, polycystic ovary syndrome, sleep-disordered breathing (SDB), psychosocial problems and other associated issues (Estrada et al., 2014; Haslam and James, 2005; James et al., 2004). Obesity affects one of every three individuals in the US with the incremental cost of healthcare related to obesity alone being expected to increase by over US $500 billion by the year 2030 if the current trends are sustained (Finkelstein et al., 2012). According to the National Institutes of Diabetes and Digestive and Kidney Diseases, approximately two-thirds of American adults are either overweight or obese. Obesity in children is a primary risk factor for high blood pressure (BP) and hypertension, and these conditions associate in 13% of cases; moreover, children with hypertension have an increased risk of becoming hypertensive adults (Freedman et al., 2001). Furthermore, when comparing obese with non-obese children who were followed up for a 22-year period, the presence of obesity alone independently predicted the long-term risks of diabetes or adulthood obesity (Liang et al., 2015).
It is well established that obesity plays an important role in the development of OSA in both adults and children (Crummy et al., 2008; Dayyat et al., 2009; Romero-Corral et al., 2010), such that interactions between these 2 conditions facilitate and amplify the risk for end-organ morbidities associated with OSA (Bahrami et al., 2008; Bhattacharjee et al., 2012; Bhattacharjee et al., 2011; Spruyt and Gozal, 2012), with multiple pathways being potentially involved.
Experimental Human Intermittent hypoxia
To investigate the separate contributions of IH and SF to end-organ dysfunction, several experimental IH protocols have been developed (Brugniaux et al., 2011; Champod et al., 2013; Gilmartin et al., 2010; Mateika et al., 2015; Navarrete-Opazo and Mitchell, 2014; Querido et al., 2012; Tamisier et al., 2011; Taylor et al., 2014; Tremblay et al., 2016; Weiss et al., 2015; Xing et al., 2014; Zhang et al., 2014). Studies in healthy human subjects have shown that sleep disruption and intermittent hypoxia can each decrease insulin sensitivity and worsen glucose tolerance. For example, healthy adult human subjects exposed to IH for relatively short durations manifest increase in their circulating plasma glucose levels (Louis and Punjabi, 2009; Newhouse et al., 2017). Similarly, Beaudin et al. (2014) exposed young healthy adults to IH for 4 days, and showed the presence of IH-induced perturbations in endothelial function that are reversible upon termination of the IH exposures (Beaudin et al., 2014; Champod et al., 2013; Foster et al., 2009; Gajos-Michniewicz et al., 2014). Longer exposures up to 4 weeks have resulted in the emergence of systemic elevations in blood pressure (Gilmartin et al., 2010; Tamisier et al., 2011).
Intermittent Hypoxia Exposures in Animals
Experimental IH patterns utilized in most of the animal-based studies have aimed to replicate OSA cyclical changes in oxygenation. Nevertheless, each laboratory has adapted a slightly different IH protocol such that cycle frequency (from seconds to few minutes), overall duration, FIO2 kinetics and magnitude, and restriction of IH to either the sleep or rest periods have led to huge variability and precluded direct comparisons across studies (Almendros et al., 2014b; Quintero et al., 2013). Notwithstanding the wide repertoire of IH-exposure paradigms, the overall findings have recapitulated the majority of the previously uncovered associations between OSA and end-organ morbidities. Indeed, animal experiments support for example the notion that IH exposures entail reductions in systemic and adipose tissue insulin sensitivity (Carreras et al., 2015; Gozal et al., 2017; Iiyori et al., 2007), facilitate emergence of dyslipidemia with elevations in serum total cholesterol and triglyceride levels that correlates with the degree of hypoxemia (Li et al., 2007), and promote the development and progression of atherosclerosis via multiple mechanistic pathways (Arnaud et al., 2011; Castro-Grattoni et al., 2016; Drager et al., 2012; Drager et al., 2013; Fang et al., 2012; Gautier-Veyret et al., 2013; Gemel et al., 2017; Gileles-Hillel et al., 2014; Jun et al., 2010; Li et al., 2011; Poulain et al., 2014; Tuleta et al., 2014; Wei et al., 2016). Furthermore, longer exposures to IH which reflect the marked delays that normally occur in patients with OSA between onset of their disease and diagnosis and treatment may result in partially irreversible morbidities (Cortese et al., 2017; Gileles-Hillel et al., 2016). The impact of age at which IH exposures occur has also been preliminarily explored. For example, neonatal mice exposed to IH for 4 weeks had increased risk for cardiovascular disease during early adulthood, manifesting as systolic hypertension, altered baroreflex responses, and reduced heart rate variability indicative of altered balance in sympathetic-parasympathetic autonomic nervous system control, implicating epigenetic re-programming (Chu et al., 2015). Similarly, early life exposures to IH result in persistent cognitive deficits and behavioral perturbations (Cai et al., 2012; Kheirandish et al., 2005).
Gestational Intermittent Hypoxia
The Developmental Origins of Health and Disease theory suggests that there are critical periods during perinatal life in which maternal nutrition and other environmental factors can program changes that will affect offspring, and influence its susceptibility to metabolic disease during adulthood (Blackmore and Ozanne, 2013). However, the mechanisms by which early environmental insults can impose long-term effects on offspring through epigenetic modifications remain poorly defined (Reynolds et al., 2015). Although several studies have evaluated the association between OSA symptoms in pregnancy and adverse fetal outcomes, all of these studies were specifically circumscribed to the immediate postnatal period, and did not explore long-term consequences of OSA during gestation on the offspring (Bourjeily et al., 2013; Xu et al., 2014). Pregnancy has been associated with several alterations in sleep patterns and a high incidence of sleep disturbances (Facco et al., 2012; Pien and Schwab, 2004).
IH during late gestation appears to adversely impact the exposed offspring and manifest as metabolic syndrome during adulthood (Khalyfa et al., 2017). Indeed, we found that adult male, but not female offspring of pregnant mice that were exposed to late gestational IH developed body weight and food consumption, along with reduced daily energy expenditure, increased adiposity index, increased insulin resistance and systemic elevations of lipid levels, as well as altered macrophage populations and changes in DNA methylation patterns in visceral adipose tissues. Accordingly, gestational IH leads to a comprehensive array of adverse epigenetic and metabolic consequences associated with OSA during pregnancy in the offspring, and further provide a strong argument to identify the disease during pregnancy and also detect infants at risk for future morbidities.
Intercellular Interactions in Intermittent Hypoxia
As discussed above, there is consistent evidence from animal models and clinical studies suggesting that OSA enhances oxidative and inflammatory processes and leads to CNS, metabolic, autonomic, vascular, and cardiac dysfunction. The existence of a complex network of well-organized interactions between different cell populations that play a pivotal roles in several pathophysiological processes prompts the formulation of a conceptual framework whereby, similar to other disease states, direct cell-to-cell contact, cell-matrix interactions, and extracellular electric, chemical, or biological signals should promote the amplification and expansion of pathological consequences of IH (Sheikh et al., 2009; Tirziu et al., 2010). In this context, discovery of integrative signature responses facilitating the detection of populations at risk of morbidities associated with OSA or identifying new therapeutic targets would be of great interest, particularly considering the wide heterogeneity in the expression of morbid phenotypes as induced by IH. Thus, the study of novel circulating markers as potential candidates would emerge as a promising venue, and among those one of the widely and actively explored include extracellular vesicles (EVs).
Extracellular Vesicle and Intercellular Communications
Cells are known to secrete a large variety of vesicles into the extracellular space. Cell-to-cell communication is necessary for proper coordination during development and among different cell types within adult tissues to allow for seamless and integrated coordination of cellular functions, a critical feature in the development and environmental adaptation of multicellular organisms. On the other hand, dysregulated pathways of communication appear to drive oncogenesis and cancer progression. Cell communication often involves soluble factors, such as cytokines, chemokines, growth factors and neurotransmitters, and their specific recognition by cell-surface receptors. Although there are different ways how intercellular communications can occur, direct cell-to-cell contact including the adhesion junction, release of soluble signaling molecules or exchange of cellular fragments such as EVs are the dominant forms of intercellular interactions (Ahmed and Xiang, 2011; Ogorevc et al., 2013).
Human body fluids contain a multitude of cell-derived vesicles, which are secreted by most cell types, and are commonly referred to as EVs, which can then travel long distances until they are up taken by receptor target cells (Lee et al., 2011). EVs have emerged in recent years as potent vehicles for cell-to-cell communication, particularly since the discovery that they contain functional mRNA, miRNA and DNA molecules that can be taken up by target (acceptor) cells (Baj-Krzyworzeka et al., 2006; Mittelbrunn and Sanchez-Madrid, 2012; Montecalvo et al., 2012; Valadi et al., 2007). A number of different EVs subpopulations have been described, and their classification is usually dependent upon their size and specific biogenesis (Thery et al., 2009). EVs are classified into 3 main classes: (a) microvesicles/microparticles/ectosomes with an approximate size of 100 –1000 nm; (b) exosomes that are formed within the endosomal network and released upon fusion of multi-vesicular bodies with the plasma membrane and size ranging from 30–100 nm; and (c) apoptotic bodies with sizes between 100–5000 nm (Thery et al., 2009; Zhang and Grizzle, 2014). This latter subpopulation of EVs, which arises from cells undergoing apoptosis, is rapidly engulfed by phagocytic cells. EVs in general and especially exosomes have generated substantial excitement in view of their potential as disease biomarkers or as precision carriers for drug delivery.
Exosomes
Exosomes were first discovered in the mid-1980s by Johnstone and colleagues, who found that the transferrin receptor and some other membrane associated elements are selectively released in multi-vesicular body (MVB) derived circulating vesicles (Johnstone et al., 1987; Pan et al., 1985; Trams et al., 1981). There are two steps for exosome biogenesis: (a) the inward budding of membranous vesicles of endosomes, (b) their release into a structure known as a multi-vesicular body (MVB). The formation of MVBs occurs during the maturation of early endosomes into late endosomes with the accumulation of intraluminal vesicles (Hanson and Cashikar, 2012). After maturation, MVBs are directed for fusion with either the lysosome, where their cargo will undergo lysosomal degradation, or to the plasma membrane, where their contents will be released into the extracellular space. When MVBs undergo this process, transmembrane proteins are incorporated into the membrane, maintaining a topological orientation similar to that of the plasma membrane (Escrevente et al., 2011).
Exosomes have been found in essentially all body fluids under both healthy and morbid conditions (Gallo et al., 2012; Jenjaroenpun et al., 2013; Khalyfa A et al., 2016; Simpson et al., 2009). Exosomes have been successfully isolated from cell culture conditioned medium (Balaj et al., 2011) and different body fluids including plasma (Ashcroft et al., 2012), serum (Dalton, 1975), saliva (Keller et al., 2011), amniotic fluid (Keller et al., 2011), breast milk (Hata et al., 2010), and urine (Wiggins et al., 1987). The gold standard and most commonly used protocol for exosome isolation/purification is differential centrifugation, which involves several centrifugation and ultracentrifugation steps. However, such protocols vary across users, leading to inconsistencies in the recovery of exosomes, mainly because of different biofluid viscosity properties (Momen-Heravi et al., 2012; Yuana et al., 2011). Recently, several alternative methods were introduced and successful implemented for isolation and purification of exosomes, including antibody-coated magnetic beads, microfluidic devices, precipitation technologies (ExoQuick™), and filtration technologies. A consensus for characterization of exosomes has been recently adopted and should be therefore followed to enable comparative studies across different laboratories (Lotvall et al., 2014). The composition of exosomes obviously differs from cell type to cell type. According to the most recent version of the exosome content database, exosomes from various organisms and various cell types in the current version of ExoCarta show 41,860 proteins, >7,540 RNA and 1,116 lipid molecules (Keerthikumar et al., 2016; Mathivanan et al., 2012).
Exosomes were initially thought to serve simply as “garbage bags” for cells to get rid of unwanted constituents. However, an increasing body of evidence has demonstrated that exosomes play an important role in cell-to-cell communication and influence both physiological and pathological processes. For example, exosomes were recognized as being closely involved in the function of the immune system. In addition, exosomes were found to function as “communication shuttles” between cells and transduce signals in recent years, and that such interactions could re-encode genes of target cells and play a part in the development, invasion, metastasis and drug resistance of cancer (Azmi et al., 2013).
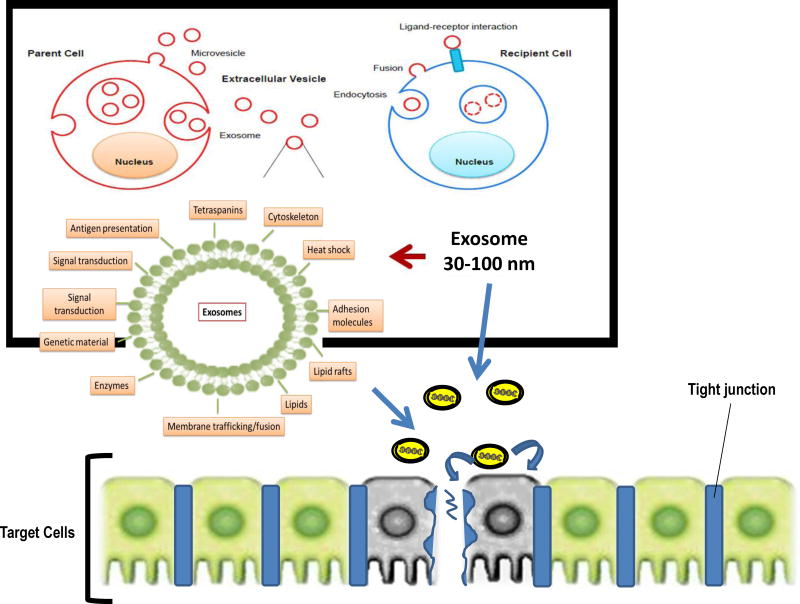
The general composition of exosomes is illustrated in Figure 2. For example, exosomes are enriched in particular cellular proteins, including the tetraspanins CD63, CD9, and CD81, endosomal sorting complexes required for transport (ESCRT)-related proteins Alix and Tsg101, MHCI, and heat shock proteins (Vlassov et al., 2012). Exosomes derived from immune cells are also enriched in MHCII and co-stimulatory molecules (Thery et al., 2009). Exosomes also package cellular RNAs and protect them from degradation (Valadi et al., 2007), such that exosomes isolated from serum contain RNA that represents a subset of the RNA present in the exosome-producing cells (Skog et al., 2008). Thus, exosomes and their contents are potentially promising as biomarkers, particularly for diseases wherein the diseased cells produce many exosomes. For these reasons, diagnostic analysis of exosomal proteins and RNA has received much attention and even been commercialized (Vlassov et al., 2012). In general, exosomes naturally deliver mRNA, miRNA, various noncoding RNA, mitochondrial DNA, genomic DNA, and proteins (Guescini et al., 2010; Valadi et al., 2007; Waldenstrom et al., 2012). Exosome-transferred miRNAs were also suggested to be functional in target cells, as miRNAs from T-cell exosomes caused inhibition of target genes in dendritic cells (Mittelbrunn et al., 2011), and miRNAs contained in exosomes from Epstein-Barr virus infected B-cells affected the expression of target genes in monocytes (Pegtel et al., 2010). Moreover, it remains not clear whether a set of mRNAs is targeted to all exosomes despite their cell of origin, in addition to cell-type-specific mRNAs. On the miRNAs side, it is still unclear whether specific miRNA sequences, rather than the whole set of intracellular miRNAs, are targeted to exosomes.
Figure 2.
Diagram schematically illustrating some of the processes involved in generation of exosomes, their major content and delivery to target cells.
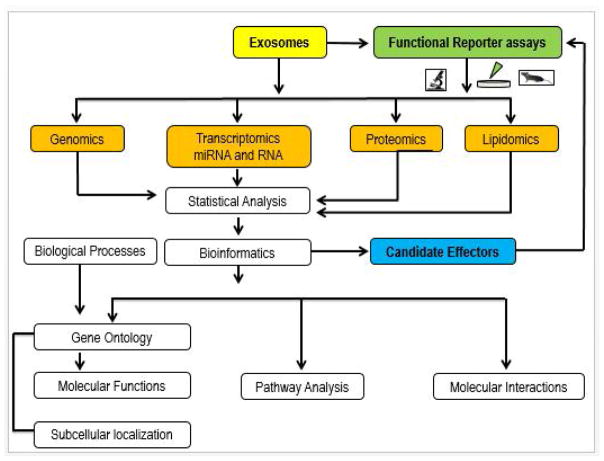
Exosomal RNAs are not only stable and accessible biomarkers, but can also be functionally transferred from parent cells to recipient cells (Valadi et al., 2007). For instance, mRNAs released in exosomes by glioblastoma cells are translated by recipient brain microvascular endothelial cells (Skog et al., 2008). Exosomes secreted by macrophages can deliver miRNAs into breast cancer cells to promote invasion (Yang et al., 2011). Exosomes secreted by human colorectal cancer cells contain mRNAs, miRNAs and natural antisense RNAs that can be transferred into the human hepatoma and lung cancer cell lines (Chiba et al., 2012). Interestingly, breast cancer cells secrete exosomes with specific capacity for cell-independent miRNA biogenesis, while normal cell-derived exosomes lack this ability; these cancer exosomes stimulate non-tumorigenic epithelial cells to form tumors by altering their transcriptome (Melo et al., 2014). Besides immunity and cancer, exosomes have been shown to play important roles in cardiovascular, metabolic and neurological disease, (Danielson and Das, 2014; Khalyfa and Gozal, 2014; Khalyfa et al., 2016b; Yelamanchili et al., 2015). Due to the restricted available evidence on exosome biology and IH, we will focus only the rest of this paper on miRNAs and mRNA. As shown in Figure 3, there are several tools to evaluate exosomes.
Figure 3.
Diagram depicting an integrated bioinformatics analysis of exosomes contents and functional reporter assays. Isolated exosomes can be analyzed to profile the genomic, transcriptomic, proteomic, and lipidomic contents. With the generated datasets, multiple integrated analyses can be performed including Gene Ontology (biological process and molecular function) enrichment, protein interaction network, pathway and domain enrichment, and subcellular localization analysis. Administration of biological fluids-derived exosomes or specific cell source-derived exosomes to target cells or animals enables functional characterization. Selective modification of exosome cargo that is predicated upon bioinformatic predictions of effector genes/molecules and delivery to targeted functional reporter assays enables confirmation of mechanistic assumptions.
Exosomal miRNAs in OSA and Intermittent Hypoxia
Circulating miRNAs have been proposed as attractive candidates as both diagnostic and prognostic biomarkers in various diseases, including a spectrum of cardiovascular conditions, whereby dysregulation of specific miRNAs in response to genetic or environmental factors can contribute to aberrant gene expression patterns underlying vascular or metabolic dysfunction (Hamilton et al., 2013). miRNAs are small noncoding RNAs (approximately 22 nucleotides) that post-transcriptionally regulate gene expression by blocking translation or inducing degradation of the targeted mRNA (Bartel, 2009), and currently more than 2,000 human miRNAs are registered (miRBase Release). There are an estimated ~ 45,000 miRNA-targeting sites in the human genome, affecting the expression of ~ 60% of genes (Friedman et al., 2009). miRNA genes can be coded by the intronic regions of protein-coding genes (intronic miRNAs) or by sequences outside protein-coding genes (intergenic miRNAs) (Inui et al., 2010). Furthermore, miRNAs have been implicated in almost every cardiovascular disorder in which they have been examined, including heart failure, cardiac hypertrophy, remodeling after myocardial infarction, arrhythmias, atherosclerosis, atrial fibrillation, and peripheral artery disease (Adachi et al., 2010; Bonauer et al., 2010; Kawashima and Shioi, 2011; Thai et al., 2010). Since miRNAs can be readily detected in various body fluids, they have been advanced as useful biomarkers for the diagnosis and characterization of systemic diseases including type 2 diabetes mellitus, hypertension, obesity, and cardiovascular disease (D’Alessandra et al., 2010; Ortega et al., 2013; Zampetaki et al., 2010; Zampetaki et al., 2012).
The biological processes of miRNA-regulated gene expression are various including cell proliferation, cell differentiation, apoptosis, signal transduction, immune response, stress resistance, fat metabolism, insulin secretion and hematopoiesis (Ambros, 2003; Bartel, 2004; Wang and Sen, 2011). In addition, miRNAs are involved in a number of metabolic processes, such as maintenance of cellular glucose, cholesterol, triglyceride, and fatty acid metabolism (Rottiers and Naar, 2012).
In the context of OSA, two studies focusing on plasma miRNAs have been recently published from our laboratory. One of such studies explored the potential use of a miRNA panel in patients with resistant hypertension and OSA to predict whether a favorable hypotensive response to CPAP would occur (Sanchez-de-la-Torre et al., 2015). In a subsequent study, we examined children with obesity who were also matched for demographic and anthropometric characteristics but differed in their endothelial functional phenotype. In these children, we identified a subset of miRNAs in their plasma that readily segregated those children with endothelial dysfunction (ED) from those with normal endothelial function (NEF) (Khalyfa et al., 2016a). In a subsequent study we expanded such initial findings to children with OSA or obesity, and examined exosomal miRNA cargo rather than plasma miRNAs (Khalyfa et al., 2016a). In this setting, divergent endothelial phenotypes led to identification of 5 differentially expressed exosomal miRNAs. Among them, miRNA-630 expression in exosomes was significantly reduced in children with ED, regardless of whether they had OSA, obesity, or both. We had transfected a specific mimic of miRNA-630 to exosomes from ED children, and also transfected a miRNA-630 inhibitor to exosomes from NEF children, and treated endothelial cells with these transfected exosomes. As controls, exosomes transfected with scrambled sequences of either the mimic or the inhibitor were used. These experiments showed that increases or decreases in miRNA 630 recapitulated the previously assessed endothelial functional phenotype in the children. Furthermore, we could inject exosomes from ED or NEF children and reproduce the endothelial functional phenotype in naïve healthy mice. Finally, we also performed mRNA transcriptomic analyses of the treated endothelial cells with exosomes treated with either mimic, inhibitor or scrambled controls to determine the gene targets of miRNA-630 in endothelial cells. These experiments revealed a total of 416 miRNA-630 gene targets in endothelial cells, corresponding to 10 major functional pathways many of which have been previously identified as playing important roles in endothelial function and homeostasis (Khalyfa et al., 2016a).
In parallel, we have conducted independent assessments of plasma-derived exosomes in healthy adults exposed to experimental IH for 4 days followed by a normoxic recovery period of similar duration. Exosomes after IH exposures showed not only the ability to disrupt endothelial function in naïve endothelial cells in vitro and recruit the expression of adhesion molecules that promote monocyte adherence, but the presence of several differentially expressed miRNAs in their cargo and the widespread transcriptomic effects of such exosomes in endothelial cells. Moreover, after normoxic recovery, such cargo content and functional properties reverted to baseline (Khalyfa et al., 2016c).
Using an experimental model of IH in mice, we performed similar experiments with the intent to detect exosomal miRNAs that would promote changes in malignant properties of cancer cells (Almendros et al., 2016). Such experiments were a logical sequel to initial observations that IH mimicking OSA can induce changes in the biological characteristics of solid tumors in vivo (Almendros et al., 2014a). We identified 11 distinct miRNAs in IH-exposed mice, and further uncovered their gene targets in TC1 tumor cells (Almendros et al., 2016). These preliminary findings on exosomes in OSA or in human or murine models of OSA consisting of IH provide compelling justification to expand efforts to characterize how IH alters exosome generation and release as well as exosomal cargo and function.
MiRNAs actively participate in the modulation of important cell physiological processes and are involved in the pathogenesis of lung diseases such as lung cancer, pulmonary fibrosis, asthma and chronic obstructive pulmonary disease. Several studies have been indicated that there is association between miRNAs and other form of hypoxia (e.g. acute lung injury or pulmonary hypertension). For example, miR-29-3p was explored to play a role in the activation of pulmonary adventitial fibroblasts suggesting that miR-29a-3p regulates the activation and phenotype of pulmonary adventitial fibroblasts in hypoxia and has preventative and therapeutic potential in hypoxic pulmonary hypertension (Luo et al., 2015).
In this context, several questions have been raised regarding circulating miRNAs and exosomes: First, why are circulating miRNAs stable? In fact, the blood contains high levels of RNase activity that degrades exogenously added mRNA within seconds, indicating that miRNAs are unlikely to exist in a unprotected state (Tsui et al., 2002). Several studies indicated that serum or plasma miRNA levels do not appear to be affected by incubation of the serum or plasma samples at 4°C or room temperature, multiple freeze–thaw cycles, long-term storage or even boiling or treatment with acid or base (Chen et al., 2008; Gilad et al., 2008; Ho et al., 2010; Mitchell et al., 2008). Second, what are the functions of circulating RNAs? A number of studies have demonstrated the presence of mRNA in various microvesicles (Ratajczak et al., 2006; Skog et al., 2008) and when exosomal mRNAs were analyzed bioinformatically, their key functions included cellular development, protein synthesis and RNA post-transcriptional modifications. Third, which cells are targeted by circulating miRNAs? While many of these suggested roles for circulating miRNAs are plausible, elucidation of the functional role requires demonstration of a gene regulatory effect of miRNAs after being transferred to recipient target cells. As exosomes bear the same surface proteins and ligands as their parent cells, receptor mediated interactions could potentially occur with specific recipient cells. Valadi et al. showed that mast cell-derived exosomes transferred labeled RNA to other mast cells, and the mRNA component was translated. As this RNA transfer was to mast cells only, and did not occur to CD4+ cells, this study provides evidence that the exosome exchange represents a controlled and highly specific communication pathway (Valadi et al., 2007). Several studies have since addressed this issue, showing effects of horizontally transferred miRNAs on recipient cell gene expression (Kosaka et al., 2010; Pegtel et al., 2010). Increasing evidences have shown that exosome composition contains both a common and a specific set of RNA, proteins and lipids (Kim et al., 2013; Mathivanan and Simpson, 2009). In addition to exosomes mRNAs and miRNAs cargos, there are other cargoes which including enzymatic cargo (Lozito and Tuan, 2012; Nojima et al., 2016), protein cargo (Rezeli et al., 2016; Simpson et al., 2008; Sinha et al., 2017), and bioactive lipids content (Choi et al., 2013; Frey and Gaipl, 2011) which may play an important function of EVs/exosomes.
Exosome Release
The release of exosomes is a specifically regulated cellular process and cells can release exosomes in response to certain physical, chemical and biological stimuli including low pH, thermos-and-oxidative stress and hypoxia (although the effects of IH have yet to be examined). Also, exosome release is sensitive to the changes in intracellular calcium in some cell types, and depolarization induced by K+ appears to increase the secretion of neuronal exosomes (Eldh et al., 2010; Hedlund et al., 2011; Parolini et al., 2009). Numerous steps could affect the intracellular decision to degrade or release exosomes, including intracellular transport of multivesicular bodies (MVBs) along microtubules to the plasma membrane, creation of docking sites at the plasma membrane, or attachment protein receptor (SNARE) proteins that mediate fusion with either lysosomes or the plasma membrane. Molecular regulators implicated in exosome release include multiple molecules implicated in MVB docking, including the GTPases and Rabs family (Stenmark, 2009). Different Rab proteins play in regulating exosome release are cell-specific, depending on the differential expression and function of particular effectors. In fact, Rab4, Rab5, Rab11, Rab35, Rab27a and Rab27b have been implicated in different steps of exosome release in different cell types (Hsu et al., 2010; Koles et al., 2012; Savina et al., 2002). Other studies have described different mechanisms of exosome secretion that involve the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein YKT6 (Gross et al., 2012). Briefly, the cytoskeleton and the contractile machinery of the cell move, attracting the opposing membranes with the assistance of the SNARE complex before pinching off the membrane connection and releasing the vesicle into the extracellular space (Cocucci et al., 2007; Sudhof and Rothman, 2009). Interestingly, silencing Rab27a by RNA interference disrupted exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can also reduce tumor growth and metastasis (Bobrie et al., 2012). Other Rab proteins such as Rab11 and Rab35 might serve as alternative targets for impairing the release of exosomes by inhibiting the docking of MVBs with the plasma membrane (Hsu et al., 2010; Savina et al., 2005). Furthermore, other processes such as acid sphingomyelinase activation in glial cells (Bianco et al., 2009) or endothelial cells (Serban et al., 2016); neutrophil sphingomyelinase-2 signaling (Kosaka et al., 2010); and higher order oligomerization (Buschow et al., 2009; Muntasell et al., 2007) have been shown to play an important role in EVs release under various hemostatic or stress conditions.
Exosome Uptake
Exosomes have emerged as one of the main players in intercellular communication, and exosomes can carry various cargos such as proteins, mRNAs and miRNAs to recipient cells. Uptake of exosomes and their cargo can induce and/or inhibit different cellular and molecular pathways that lead to the alteration of cell behavior. The most established mechanism by which exosomes can modulate the recipient cells is by direct interaction. There are multiple ways for uptake of exosomal cargo by recipient cells including: phagocytosis, clathrin- or caveolin-mediated endocytosis, lipid raft mediated endocytosis, in which caveolin or clathrin is also involved (Mulcahy et al., 2014). Other mechanisms exosomal uptake include micropinocytosis, where exosomes are enclosed in the so called macropinosomes or exosomes can be trapped in membrane raffles (Nakase et al., 2015).
Exosomes can be internalized into recipient cells via a number of potential mechanisms: (i) by endocytosed or internalized into an endocytic compartment or MVB, from which they may undergo back-fusion, by fusing with the limiting membrane and releasing their cargo into the cytoplasm of the recipient cell (Parolini et al., 2009); (ii) by fusing with the plasma membrane, releasing the cargo directly into the cytoplasm of the recipient cell; and (iii) receptor ligand mediated interactions could result in either signal transduction or exosomal internalization (Fitzner et al., 2011; OrLoughlin et al., 2012). Mechanisms for interaction and uptake of exosomes to target cells appear to involve clathrin-dependent and independent pathways that are most likely specific to a given cellular source of exosomes and a given recipient cell type (Colombo et al., 2014). Interactions between exosomes and recipient/target cells can also be mediated through direct signaling interactions via surface-expressed molecules including integrins (Fedele et al., 2015; Hoshino et al., 2015). Several mechanisms of exosomes internalization are reported (Xu et al., 2016) including receptor-mediated endocytosis (LFA1, TIM1 and TIM4), phagocytosis, and direct plasma membrane fusion (Thery et al., 2009). Understanding exosome internalization and transfer of their cargo to target cells will be critically important for IH-related biological processes.
The most common methods for detecting exosomes uptake are the use of fluorescent lipid membrane dyes to stain exosomes membranes. Examples of such dyes include PKH67, PKH26, rhodamine B (also known as R18), DiI and DiD which are lipophilic dyes (Atay et al., 2011; Fitzner et al., 2011; Franzen et al., 2014; Morelli et al., 2004; Tian et al., 2010). Subsequent entry of EVs into recipient cells can be measured using methods such as flow cytometry and confocal microscopy. Instead of labelling whole exosomes, we have shown more recently that exosomal RNA can be labeled by assessing RNA uptake in cell culture (Khalyfa A et al., 2016). Furthermore, exosome uptake into recipient cells appears to be phagocytic processes dependent on dynamin2 and PI3K (phosphatidyl inositol-3-kinase). The uptake process relies on a variety of specific cell surface molecules on the receptor cell (T-cell membrane protein 4 Tim4, but not Tim1, has been shown to be involved in exosomes uptake) as well as proteins like integrins, annexins, galectin, and ICAM1 (inter-cellular adhesion molecule 1) on the exosomal surface. Uptake of exosomes is possibly a non-random event and recent papers suggest a mechanistic basis to target cell selection by invoking tetraspanins as entry selection markers (Rana and Zoller, 2011). This is supported by a defined set of exosome associated tetraspanins that appear to direct targeting to endothelial cells to promote angiogenesis and vasculogenesis. As mentioned above, how IH affects such processes remains virtually unexplored.
Exosomes and Cardiovascular and Metabolic Diseases
Under pathological conditions such as IH, exosomes could contribute to the establishment of a pro-inflammatory phenotype that leads to endothelial dysfunction (Agouni et al., 2008), and our aforementioned work seems to support this concept. In addition, two recent studies have reported the presence of a correlation between exosomes and metabolic dysfunction, and such work could inferentially be adapted to study the impact of OSA on metabolic function. One such study showed that ob/ob mice display elevated numbers of exosomes compared to wild-type mice (Phoonsawat et al., 2014). The second study demonstrated that exosome levels bearing cystatin C were positively related to metabolic complications of obesity in patients with clinically vascular diseases (Kranendonk et al., 2014). These findings uncover a potential role of exosomes in the pathogenesis of metabolic diseases. Several other studies have identified exosomes in the culture supernatants of mouse adipose tissues (Deng et al., 2009), rat primary adipocytes (Muller et al., 2009), and mouse adipocyte cell line 3T3-L1 (Sano et al., 2014) that exhibit biological activity. For example, exosomes isolated from the culture supernatant of visceral adipose tissue excised from mice showed that injection of the exosomes derived from diet-induced or genetically (leptin-deficient ob/ob) obese mice into wild-type lean mice results in macrophage activation and insulin resistance (Deng et al., 2009). Also, isolated exosomes from the supernatants of differentiated 3T3-L1 cells under hypoxic conditions are enriched in enzymes related to lipogenesis and promote lipid accumulation in recipient 3T3-L1 adipocytes (Sano et al., 2014).
Exosomes have pro- as well as anti-angiogenic properties (Yang et al., 2008). Endothelial cell (ECs) cultures release MVs containing metalloproteinase proteins MMP-2 and MMP-9 (Taraboletti et al., 2002). These endothelial-MVs (EMVs) promote matrix degradation and formation of new blood vessels. Also, MVs from platelets (PMVs) promote proliferation, survival, migration, and formation of capillary-like structures of ECs in vitro (Kim et al., 2004). In addition to pro-angiogenic features, EMVs also inhibit angiogenesis as they can stimulate the production of endothelial reactive oxygen species (ROS) (Burger and Kipps, 2006). Lymphocyte-derived MVs generated after actinomycin D treatment in vitro decrease nitric oxide (NO) and increase ROS production by stimulating phosphatidylinositol 3-kinase, xanthine oxidase and nicotinamide adenine dinucleotide phosphate oxidase pathways (Yang et al., 2008). Thus, reduced NO and increased ROS production mediated via exosome activity inhibits angiogenesis.
Exosomes and Neurodegenerative Diseases
The function of human nervous system is critically dependent on proper interneuronal communication. Many cell types including neurons, astrocytes, and glia, have the capacity to release a variety of membranous vesicles into the extracellular space. In the CNS, exosomes have been implicated in neuronal development and several pathologies (Schneider and Simons, 2013; Vella et al., 2008). Secretion of exosomes from neurons and astrocytes was first demonstrated in cultures of primary cortical neurons prepared from rat embryos (Faure et al., 2006). Exosomes from multipotent MSCs mediate the miR-133b transfer to astrocytes and neurons, which regulate gene expression responsible for neurite remodeling and functional recovery after stroke (Xin et al., 2013). Exosomes were also found to be released from cultured cortical cells and hippocampal neurons, which contain receptor molecules that support glutamatergic synaptic activity (Lachenal et al., 2011). When activated, microglia serve as antigen presenting cells by secreting exosomes under pathological conditions (Kettenmann et al., 2011). The main physiological roles of exosomes include eliminating cellular waste, regulating immune response and communicating between neural cells (Gupta and Pulliam, 2014; Sharples et al., 2008). A number of proteins involved in diseases have been identified in their normal and pathogenic states to be associated with exosomes, and include PrP, tau, AB, α-synuclein and SOD1 (Quek et al., 2017). This suggests that exosomes may be involved in the spreading of these misfolded proteins in the brain. We are unaware of any published work on the impact of IH on exosome release and their biological properties in the CNS. Considering the extensive evidence indicating substantial structural and functional changes induced by chronic IH in brain regions cognitive, behavioral, mood, and autonomic functions (Burckhardt et al., 2008; Capone et al., 2012; Cheng et al., 2011; Darnall et al., 2017; Dayyat et al., 2012; Douglas et al., 2010; Goldbart et al., 2003; Gozal et al., 2001; Kanaan et al., 2006; Kim et al., 2015; Knight et al., 2011; Li et al., 2004; Lim et al., 2016; Nair et al., 2011; Row et al., 2007; Sapin et al., 2015; Shan et al., 2007; Xu et al., 2015; Xu et al., 2004; Yagishita et al., 2017; Zhan et al., 2005), it would be important to explore the potential contributions of exosomes in these contextual settings both as biomarkers for CNS morbidity as well as novel therapeutic targets. In addition, circulating EVs/exosomes have been associated with various diseases severity. For examples, these vesicles have been suggested for clinical biomarkers for OSA (Ayers et al., 2009; Kim et al., 2011; Yun et al., 2010), for pulmonary hypertension (Amabile et al., 2008; Bakouboula et al., 2008), for sepsis (Mostefai et al., 2008), and for COPD (Takahashi et al., 2012; Thomashow et al., 2013).
Exosomes in Clinical Medicine
A potential challenge in the development of non-invasive diagnostic assays based on exosome analysis in body fluids is the ability to identify the clinically relevant cluster of exosomes from all the other numerous exosomes. Such approaches are slowly developing and should provide opportunities to explore novel mechanisms of disease pathogenesis that can also be harnessed as clinical tools. Besides the obvious questions of disease and tissue specificity, current techniques used in the isolation and characterization of exosomes remain laborious, and suffer from a lack of standardization, as well as from high variability, and such considerations will have to be resolved before the processing of exosomes from blood and other bodily fluids as “liquid biopsies” becomes economically viable, reproducible and validated. Advances in microfluidics and optical sensing methods should enable greater robustness and selectivity in exosome characterization.
Additionally, exosomes could be harnessed as delivery vehicles since they possess an intrinsic homing ability relative to other synthetic particles, thus avoiding their unwanted accumulation in organs other than the target tissues. They demonstrate no inherent toxicity, are not associated with any long-term differentiation issues among engrafted cells or tumor generation (Thirabanjasak et al., 2010), and carry no apparent risk of aneuploidy (Buyanovskaya et al., 2009) or immune rejection following in vivo allogenic administration (Bruno et al., 2009). The pro-generative effects mediated by exosomes depend on their enrichment in bioactive lipids, the delivery of proteins that improve cell function, and the presence of both the desired restricted sets of mRNAs and miRNAs. In addition, it is possible to introduce drugs into exosomes and convert them to promising candidate drug-delivery vehicles with the ability to carry both hydrophobic and hydrophilic drugs which enable crossing of biological barriers such as the blood brain barrier (Alvarez-Erviti et al., 2011; Batrakova and Kim, 2015).
It has been indicated that biomarkers including DNA, RNA, proteins and metabolites that can reflect an individual’s state of health or disease (Magni et al., 2010). The National Institutes of Health Biomarkers in 1998 defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention (Strimbu and Tavel, 2010). Based on their utility in practice, biomarkers can provide insights on diagnosis, prognosis, regression or response to treatment of a disease (Perera and Weinstein, 2000). Currently, invasive and non-invasive procedures are often employed to identify biomarkers and the sleep field in general, and that of OSA in particular is especially concerned about development and validation of such biomarkers (Mullington et al., 2016). Considering that virtually all bodily fluids contain exosomes, this creates unparalleled opportunities to exploit these vesicles as reservoirs of disease biomarkers in the context of precision medicine as it related to SDB and associated morbidities (Kalra et al., 2013; Pack, 2016; Properzi et al., 2013).
Conclusions
OSA and one of its major pathogenetic components, i.e., IH, have emerged as a most prevalent chronic disorder worldwide, and have been associated with increased risk for multiple organ system morbidities, albeit coupled with an expansive heterogeneity in the phenotypic expression of such adverse consequences. Exosomes contain a rich cargo of proteins, RNA, lipids and metabolites which are specifically sorted and often reflect the biological state of the cell type of origin. Thus, exosomes in body fluids provide a rich source of potential biomarkers. The capacity of exosomes to convey a cargo to distant recipient cells has led the scientific community to consider exosomes as important mechanistic determinants as well as therapeutic vehicles. There is little doubt that future studies focused on exosome biology in the context of IH and OSA should enable important advances in our understanding of the disease and its consequences while opening the door for biomarker discovery and therapeutics.
Highlights.
Obstructive sleep apnea (OSA) is a prevalent disease across the lifespan.
OSA is characterized by intermittent hypoxia during sleep (IH), sleep fragmentation (SF), and episodic hypercapnia.
OSA is associated with increased risk for morbidity and mortality primarily affecting cardiovascular, metabolic, and neurocognitive systems.
Exosomes are very small membrane vesicles derived from nearly every cell in the body.
Exosomes contain a rich cargo of highly regulated proteins, RNA, DNA, lipids and metabolites.
Exosomes play important roles in mediating cell-cell communication via selective cargo transfer to target cells.
Unique subsets of exosomal miRNAs in both adult and children mediate intercellular communication relevant to OSA and endothelial dysfunction.
Exosomes can be used as potential diagnostic and prognostic biomarkers.
Acknowledgments
Funding: Authors are supported by National Institutes of Health grant HL130984 (to LKG).
Footnotes
Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of interest: The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, Nonogi H, Iwai N. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem. 2010;56:1183–1185. doi: 10.1373/clinchem.2010.144121. [DOI] [PubMed] [Google Scholar]
- Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, Heymes C, Martinez MC, Andriantsitohaina R. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. The American journal of pathology. 2008;173:1210–1219. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed KA, Xiang J. Mechanisms of cellular communication through intercellular protein transfer. Journal of cellular and molecular medicine. 2011;15:1458–1473. doi: 10.1111/j.1582-4934.2010.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Progress in cardiovascular diseases. 2009;51:285–293. doi: 10.1016/j.pcad.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Alchanatis M, Tourkohoriti G, Kosmas EN, Panoutsopoulos G, Kakouros S, Papadima K, Gaga M, Jordanoglou JB. Evidence for left ventricular dysfunction in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2002;20:1239–1245. doi: 10.1183/09031936.02.00278002. [DOI] [PubMed] [Google Scholar]
- Almendros I, Khalyfa A, Trzepizur W, Gileles-Hillel A, Huang L, Akbarpour M, Andrade J, Farre R, Gozal D. Tumor Cell Malignant Properties Are Enhanced by Circulating Exosomes in Sleep Apnea. Chest. 2016;150:1030–1041. doi: 10.1016/j.chest.2016.08.1438. [DOI] [PubMed] [Google Scholar]
- Almendros I, Wang Y, Becker L, Lennon FE, Zheng J, Coats BR, Schoenfelt KS, Carreras A, Hakim F, Zhang SX, Farre R, Gozal D. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2014a;189:593–601. doi: 10.1164/rccm.201310-1830OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol. 2014b;307:L129–140. doi: 10.1152/ajplung.00089.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Amabile N, Heiss C, Real WM, Minasi P, McGlothlin D, Rame EJ, Grossman W, De Marco T, Yeghiazarians Y. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1268–1275. doi: 10.1164/rccm.200710-1458OC. [DOI] [PubMed] [Google Scholar]
- Amaddeo A, de Sanctis L, Olmo Arroyo J, Giordanella JP, Monteyrol PJ, Fauroux B. Obesity and obstructive sleep apnea in children. Arch Pediatr. 2017;24(Suppl 1):S34–S38. doi: 10.1016/j.arcped.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Ameli F, Brocchetti F, Semino L, Fibbi A. Adenotonsillectomy in obstructive sleep apnea syndrome. Proposal of a surgical decision-taking algorithm. Int J Pediatr Otorhinolaryngol. 2007;71:729–734. doi: 10.1016/j.ijporl.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Amin RS, Kimball TR, Bean JA, Jeffries JL, Willging JP, Cotton RT, Witt SA, Glascock BJ, Daniels SR. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1395–1399. doi: 10.1164/rccm.2105118. [DOI] [PubMed] [Google Scholar]
- Arnaud C, Beguin PC, Lantuejoul S, Pepin JL, Guillermet C, Pelli G, Burger F, Buatois V, Ribuot C, Baguet JP, Mach F, Levy P, Dematteis M. The inflammatory preatherosclerotic remodeling induced by intermittent hypoxia is attenuated by RANTES/CCL5 inhibition. Am J Respir Crit Care Med. 2011;184:724–731. doi: 10.1164/rccm.201012-2033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atay S, Gercel-Taylor C, Taylor DD. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory IL-1beta production by macrophages. American journal of reproductive immunology. 2011;66:259–269. doi: 10.1111/j.1600-0897.2011.00995.x. [DOI] [PubMed] [Google Scholar]
- Ayers L, Ferry B, Craig S, Nicoll D, Stradling JR, Kohler M. Circulating cell-derived microparticles in patients with minimally symptomatic obstructive sleep apnoea. Eur Respir J. 2009;33:574–580. doi: 10.1183/09031936.00107408. [DOI] [PubMed] [Google Scholar]
- Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer metastasis reviews. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. Journal of the American College of Cardiology. 2008;51:1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Branski P, Ratajczak MZ, Zembala M. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer immunology, immunotherapy: CII. 2006;55:808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakouboula B, Morel O, Faure A, Zobairi F, Jesel L, Trinh A, Zupan M, Canuet M, Grunebaum L, Brunette A, Desprez D, Chabot F, Weitzenblum E, Freyssinet JM, Chaouat A, Toti F. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:536–543. doi: 10.1164/rccm.200706-840OC. [DOI] [PubMed] [Google Scholar]
- Banno K, Ramsey C, Walld R, Kryger MH. Expenditure on health care in obese women with and without sleep apnea. Sleep. 2009;32:247–252. doi: 10.1093/sleep/32.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. Journal of controlled release: official journal of the Controlled Release Society. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin AE, Pun M, Yang C, Nicholl DD, Steinback CD, Slater DM, Wynne-Edwards KE, Hanly PJ, Ahmed SB, Poulin MJ. Cyclooxygenases 1 and 2 differentially regulate blood pressure and cerebrovascular responses to acute and chronic intermittent hypoxia: implications for sleep apnea. Journal of the American Heart Association. 2014;3:e000875. doi: 10.1161/JAHA.114.000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee R, Kim J, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141:682–691. doi: 10.1378/chest.11-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee R, Kim J, Kheirandish-Gozal L, Gozal D. Obesity and obstructive sleep apnea syndrome in children: a tale of inflammatory cascades. Pediatr Pulmonol. 2011;46:313–323. doi: 10.1002/ppul.21370. [DOI] [PubMed] [Google Scholar]
- Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28:1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A, Fedok F, Vlasic V, Graff G. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32:731–736. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore HL, Ozanne SE. Maternal diet-induced obesity and offspring cardiovascular health. Journal of developmental origins of health and disease. 2013;4:338–347. doi: 10.1017/S2040174412000761. [DOI] [PubMed] [Google Scholar]
- Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Thery C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer research. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- Bonauer A, Boon RA, Dimmeler S. Vascular microRNAs. Current drug targets. 2010;11:943–949. doi: 10.2174/138945010791591313. [DOI] [PubMed] [Google Scholar]
- Bourjeily G, El Sabbagh R, Sawan P, Raker C, Wang C, Hott B, Louis M. Epworth sleepiness scale scores and adverse pregnancy outcomes. Sleep Breath. 2013;17:1179–1186. doi: 10.1007/s11325-013-0820-9. [DOI] [PubMed] [Google Scholar]
- Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106–109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugniaux JV, Pialoux V, Foster GE, Duggan CT, Eliasziw M, Hanly PJ, Poulin MJ. Effects of intermittent hypoxia on erythropoietin, soluble erythropoietin receptor and ventilation in humans. Eur Respir J. 2011;37:880–887. doi: 10.1183/09031936.00156009. [DOI] [PubMed] [Google Scholar]
- Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Journal of the American Society of Nephrology: JASN. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt IC, Gozal D, Dayyat E, Cheng Y, Li RC, Goldbart AD, Row BW. Green tea catechin polyphenols attenuate behavioral and oxidative responses to intermittent hypoxia. Am J Respir Crit Care Med. 2008;177:1135–1141. doi: 10.1164/rccm.200701-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- Buschow SI, Nolte-‘t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, Ossendorp F, Melief CJ, Raposo G, Wubbolts R, Wauben MH, Stoorvogel W. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10:1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- Buyanovskaya OA, Kuleshov NP, Nikitina VA, Voronina ES, Katosova LD, Bochkov NP. Spontaneous aneuploidy and clone formation in adipose tissue stem cells during different periods of culturing. Bulletin of experimental biology and medicine. 2009;148:109–112. doi: 10.1007/s10517-009-0647-3. [DOI] [PubMed] [Google Scholar]
- Cai J, Tuong CM, Zhang Y, Shields CB, Guo G, Fu H, Gozal D. Mouse intermittent hypoxia mimicking apnoea of prematurity: effects on myelinogenesis and axonal maturation. The Journal of pathology. 2012;226:495–508. doi: 10.1002/path.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, Duran-Cantolla J, de Pena ML, Masdeu MJ, Gonzalez M, Campo F, Gallego I, Marin JM, Barbe F, Montserrat JM, Farre R, Spanish Sleep N. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187:99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nunez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189:1544–1550. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric Obstructive Sleep Apnea: Complications, Management, and Long-term Outcomes. Proc Am Thorac Soc. 2008;5:274–282. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- Capone C, Faraco G, Coleman C, Young CN, Pickel VM, Anrather J, Davisson RL, Iadecola C. Endothelin 1-dependent neurovascular dysfunction in chronic intermittent hypoxia. Hypertension. 2012;60:106–113. doi: 10.1161/HYPERTENSIONAHA.112.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras A, Zhang SX, Almendros I, Wang Y, Peris E, Qiao Z, Gozal D. Resveratrol attenuates intermittent hypoxia-induced macrophage migration to visceral white adipose tissue and insulin resistance in male mice. Endocrinology. 2015;156:437–443. doi: 10.1210/en.2014-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Grattoni AL, Alvarez-Buve R, Torres M, Farre R, Montserrat JM, Dalmases M, Almendros I, Barbe F, Sanchez-de-la-Torre M. Intermittent Hypoxia-Induced Cardiovascular Remodeling Is Reversed by Normoxia in a Mouse Model of Sleep Apnea. Chest. 2016;149:1400–1408. doi: 10.1016/j.chest.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Champod AS, Eskes GA, Foster GE, Hanly PJ, Pialoux V, Beaudin AE, Poulin MJ. Effects of acute intermittent hypoxia on working memory in young healthy adults. Am J Respir Crit Care Med. 2013;187:1148–1150. doi: 10.1164/rccm.201209-1742LE. [DOI] [PubMed] [Google Scholar]
- Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- Cheng F, Xie S, Guo M, Fang H, Li X, Yin J, Lu G, Li Y, Ji X, Yu S. Altered glucose metabolism and preserved energy charge and neuronal structures in the brain of mouse intermittently exposed to hypoxia. J Chem Neuroanat. 2011;42:65–71. doi: 10.1016/j.jchemneu.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Chiba M, Kimura M, Asari S. Exosomes secreted from human colorectal cancer cell lines contain mRNAs, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncology reports. 2012;28:1551–1558. doi: 10.3892/or.2012.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–1571. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- Christou K, Markoulis N, Moulas AN, Pastaka C, Gourgoulianis KI. Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath. 2003;7:105–110. doi: 10.1007/s11325-003-0105-9. [DOI] [PubMed] [Google Scholar]
- Chu A, Gozal D, Cortese R, Wang Y. Cardiovascular dysfunction in adult mice following postnatal intermittent hypoxia. Pediatr Res. 2015;77:425–433. doi: 10.1038/pr.2014.197. [DOI] [PubMed] [Google Scholar]
- Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G, Podini P, Meldolesi J. Enlargeosome traffic: exocytosis triggered by various signals is followed by endocytosis, membrane shedding or both. Traffic. 2007;8:742–757. doi: 10.1111/j.1600-0854.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual review of cell and developmental biology. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Cortese R, Gileles-Hillel A, Khalyfa A, Almendros I, Akbarpour M, Khalyfa AA, Qiao Z, Garcia T, Andrade J, Gozal D. Aorta macrophage inflammatory and epigenetic changes in a murine model of obstructive sleep apnea: Potential role of CD36. Scientific reports. 2017;7:43648. doi: 10.1038/srep43648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie MR. Sleep-Disordered Breathing-Do We Have to Change Gears in Heart Failure? Current heart failure reports. 2016;13:255–265. doi: 10.1007/s11897-016-0304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crummy F, Piper AJ, Naughton MT. Obesity and the lung: 2. Obesity and sleep-disordered breathing. Thorax. 2008;63:738–746. doi: 10.1136/thx.2007.086843. [DOI] [PubMed] [Google Scholar]
- D’Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, Rubino M, Carena MC, Spazzafumo L, De Simone M, Micheli B, Biglioli P, Achilli F, Martelli F, Maggiolini S, Marenzi G, Pompilio G, Capogrossi MC. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. European heart journal. 2010;31:2765–2773. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson KM, Das S. Extracellular Vesicles in Heart Disease: Excitement for the Future ? Exosomes and microvesicles. 2014:2. doi: 10.5772/58390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall RA, Chen X, Nemani KV, Sirieix CM, Gimi B, Knoblach S, McEntire BL, Hunt CE. Early post-natal exposure to intermittent hypoxia in rodents is pro-inflammatory, impairs white matter integrity and alters brain metabolism. Pediatr Res. 2017 doi: 10.1038/pr.2017.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, Maarafeya MM, Gozal D. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136:137–144. doi: 10.1378/chest.08-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayyat EA, Zhang SX, Wang Y, Cheng ZJ, Gozal D. Exogenous erythropoietin administration attenuates intermittent hypoxia-induced cognitive deficits in a murine model of sleep apnea. BMC Neurosci. 2012;13:77. doi: 10.1186/1471-2202-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiological reviews. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y, Wang J, Xiang X, Zhang S, Zhuang X, Shah SV, Sun D, Michalek S, Grizzle WE, Garvey T, Mobley J, Zhang HG. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498–2505. doi: 10.2337/db09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RM, Ryu J, Kanaan A, Del Carmen Rivero M, Dugan LL, Haddad GG, Ali SS. Neuronal death during combined intermittent hypoxia/hypercapnia is due to mitochondrial dysfunction. Am J Physiol Cell Physiol. 2010;298:C1594–1602. doi: 10.1152/ajpcell.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager LF, Li J, Shin MK, Reinke C, Aggarwal NR, Jun JC, Bevans-Fonti S, Sztalryd C, O’Byrne SM, Kroupa O, Olivecrona G, Blaner WS, Polotsky VY. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. European heart journal. 2012;33:783–790. doi: 10.1093/eurheartj/ehr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans-Fonti S, Gay J, Sussan TE, Jun JC, Myers AC, Olivecrona G, Schwartz AR, Halberg N, Scherer PE, Semenza GL, Powell DR, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med. 2013;188:240–248. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, Lotvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5:e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC cancer. 2011;11:108. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada E, Eneli I, Hampl S, Mietus-Snyder M, Mirza N, Rhodes E, Sweeney B, Tinajero-Deck L, Woolford SJ, Pont SJ Children’s Hospital A. Children’s Hospital Association consensus statements for comorbidities of childhood obesity. Childhood obesity. 2014;10:304–317. doi: 10.1089/chi.2013.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facco FL, Liu CS, Cabello AA, Kick A, Grobman WA, Zee PC. Sleep-Disordered Breathing: A Risk Factor for Adverse Pregnancy Outcomes? Amer J Perinatol. 2012;29:277–282. doi: 10.1055/s-0031-1295658. [DOI] [PubMed] [Google Scholar]
- Fang G, Song D, Ye X, Mao SZ, Liu G, Liu SF. Chronic intermittent hypoxia exposure induces atherosclerosis in ApoE knockout mice: role of NF-kappaB p50. The American journal of pathology. 2012;181:1530–1539. doi: 10.1016/j.ajpath.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R. Exosomes are released by cultured cortical neurones. Molecular and cellular neurosciences. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The alphavbeta6 integrin is transferred intercellularly via exosomes. J Biol Chem. 2015;290:4545–4551. doi: 10.1074/jbc.C114.617662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. American journal of preventive medicine. 2012;42:563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK, Simons M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. Journal of cell science. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- Floras JS. Sleep apnea and cardiovascular risk. Journal of cardiology. 2014;63:3–8. doi: 10.1016/j.jjcc.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Foster GE, Brugniaux JV, Pialoux V, Duggan CT, Hanly PJ, Ahmed SB, Poulin MJ. Cardiovascular and cerebrovascular responses to acute hypoxia following exposure to intermittent hypoxia in healthy humans. The Journal of physiology. 2009;587:3287–3299. doi: 10.1113/jphysiol.2009.171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen CA, Simms PE, Van Huis AF, Foreman KE, Kuo PC, Gupta GN. Characterization of uptake and internalization of exosomes by bladder cancer cells. BioMed research international. 2014;2014:619829. doi: 10.1155/2014/619829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001;108:712–718. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- Frey B, Gaipl US. The immune functions of phosphatidylserine in membranes of dying cells and microvesicles. Seminars in immunopathology. 2011;33:497–516. doi: 10.1007/s00281-010-0228-6. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome research. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajos-Michniewicz A, Duechler M, Czyz M. MiRNA in melanoma-derived exosomes. Cancer letters. 2014;347:29–37. doi: 10.1016/j.canlet.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier-Veyret E, Arnaud C, Back M, Pepin JL, Petri MH, Baguet JP, Tamisier R, Levy P, Stanke-Labesque F. Intermittent hypoxia-activated cyclooxygenase pathway: role in atherosclerosis. Eur Respir J. 2013;42:404–413. doi: 10.1183/09031936.00096512. [DOI] [PubMed] [Google Scholar]
- Gemel J, Su Z, Gileles-Hillel A, Khalyfa A, Gozal D, Beyer EC. Intermittent hypoxia causes NOX2-dependent remodeling of atrial connexins. BMC cell biology. 2017;18:7. doi: 10.1186/s12860-016-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed N, Bentwich Z, Hod M, Goren Y, Chajut A. Serum microRNAs are promising novel biomarkers. PLoS One. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gileles-Hillel A, Almendros I, Khalyfa A, Nigdelioglu R, Qiao Z, Hamanaka RB, Mutlu GM, Akbarpour M, Gozal D. Prolonged Exposures to Intermittent Hypoxia Promote Visceral White Adipose Tissue Inflammation in a Murine Model of Severe Sleep Apnea: Effect of Normoxic Recovery. Sleep. 2016 doi: 10.1093/sleep/zsw074. [DOI] [PubMed] [Google Scholar]
- Gileles-Hillel A, Almendros I, Khalyfa A, Zhang SX, Wang Y, Gozal D. Early intermittent hypoxia induces proatherogenic changes in aortic wall macrophages in a murine model of obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190:958–961. doi: 10.1164/rccm.201406-1149LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin GS, Lynch M, Tamisier R, Weiss JW. Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2010;299:H925–931. doi: 10.1152/ajpheart.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbart A, Row BW, Kheirandish L, Schurr A, Gozal E, Guo SZ, Payne RS, Cheng Z, Brittian KR, Gozal D. Intermittent hypoxic exposure during light phase induces changes in cAMP response element binding protein activity in the rat CA1 hippocampal region: water maze performance correlates. Neuroscience. 2003;122:585–590. doi: 10.1016/j.neuroscience.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Gileles-Hillel A, Cortese R, Li Y, Almendros I, Qiao Z, Khalyfa AA, Andrade J, Khalyfa A. Visceral White Adipose Tissue Following Chronic Intermittent and Sustained Hypoxia in Mice. Am J Respir Cell Mol Biol. 2017 doi: 10.1165/rcmb.2016-0243OC. [DOI] [PubMed] [Google Scholar]
- Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nature cell biology. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. Journal of neural transmission. 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. Journal of neuroinflammation. 2014;11:68. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MP, Rajapakshe K, Hartig SM, Reva B, McLellan MD, Kandoth C, Ding L, Zack TI, Gunaratne PH, Wheeler DA, Coarfa C, McGuire SE. Identification of a pan-cancer oncogenic microRNA superfamily anchored by a central core seed motif. Nature communications. 2013;4:2730. doi: 10.1038/ncomms3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annual review of cell and developmental biology. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva-Nilsson L. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS One. 2011;6:e16899. doi: 10.1371/journal.pone.0016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman DR, Murphy AS, Pezzullo L. The economic cost of sleep disorders. Sleep. 2006;29:299–305. doi: 10.1093/sleep/29.3.299. [DOI] [PubMed] [Google Scholar]
- Ho AS, Huang X, Cao H, Christman-Skieller C, Bennewith K, Le QT, Koong AC. Circulating miR-210 as a Novel Hypoxia Marker in Pancreatic Cancer. Transl Oncol. 2010;3:109–113. doi: 10.1593/tlo.09256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, Barr FA, Simons M. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. The Journal of cell biology. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifergane G, Ovanyan A, Toledano R, Goldbart A, Abu-Salame I, Tal A, Stavsky M, Novack V. Obstructive Sleep Apnea in Acute Stroke: A Role for Systemic Inflammation. Stroke; a journal of cerebral circulation. 2016;47:1207–1212. doi: 10.1161/STROKEAHA.115.011749. [DOI] [PubMed] [Google Scholar]
- Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O’Doherty RM, Polotsky VY, O’Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–857. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nature reviews. Molecular cell biology. 2010;11:252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- James PT, Rigby N, Leach R International Obesity Task F. The obesity epidemic, metabolic syndrome and future prevention strategies. European journal of cardiovascular prevention and rehabilitation: official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2004;11:3–8. doi: 10.1097/01.hjr.0000114707.27531.48. [DOI] [PubMed] [Google Scholar]
- Jenjaroenpun P, Kremenska Y, Nair VM, Kremenskoy M, Joseph B, Kurochkin IV. Characterization of RNA in exosomes secreted by human breast cancer cell lines using next-generation sequencing. PeerJ. 2013;1:e201. doi: 10.7717/peerj.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209:381–386. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, Hulett MD, Mathivanan S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013;13:3354–3364. doi: 10.1002/pmic.201300282. [DOI] [PubMed] [Google Scholar]
- Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1105–1114. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- Kapur V, Blough DK, Sandblom RE, Hert R, de Maine JB, Sullivan SD, Psaty BM. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22:749–755. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Shioi T. MicroRNA, emerging role as a biomarker of heart failure. Circulation journal: official journal of the Japanese Circulation Society. 2011;75:268–269. doi: 10.1253/circj.cj-10-1254. [DOI] [PubMed] [Google Scholar]
- Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. ExoCarta: A Web-Based Compendium of Exosomal Cargo. Journal of molecular biology. 2016;428:688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiological reviews. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Khalyfa A, Kheirandish-Gozal L, Khalyfa AA, Philby MF, Alonso-Alvarez ML, MM, Bhattacharjee R, Teran Santos J, Huang L, Andrade J, GD Circulating plasma extracellular microvesicle miRNA cargo and endothelial dysfunction in OSA children. Am J Resp Crit Care Med. 2016 doi: 10.1164/rccm.201602-0323OC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalyfa A, Cortese R, Qiao Z, Ye H, Bao R, Andrade J, Gozal D. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. The Journal of physiology. 2017 doi: 10.1113/JP273570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalyfa A, Gozal D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. Journal of translational medicine. 2014;12:162. doi: 10.1186/1479-5876-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalyfa A, Kheirandish-Gozal L, Bhattacharjee R, Khalyfa AA, Gozal D. Circulating microRNAs as Potential Biomarkers of Endothelial Dysfunction in Obese Children. Chest. 2016a;149:786–800. doi: 10.1378/chest.15-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalyfa A, Kheirandish-Gozal L, Khalyfa AA, Philby MF, Alonso-Alvarez ML, Mohammadi M, Bhattacharjee R, Teran-Santos J, Huang L, Andrade J, Gozal D. Circulating Plasma Extracellular Microvesicle MicroRNA Cargo and Endothelial Dysfunction in Children with Obstructive Sleep Apnea. American journal of respiratory and critical care medicine. 2016b;194:1116–1126. doi: 10.1164/rccm.201602-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalyfa A, Zhang C, Khalyfa AA, Foster GE, Beaudin AE, Andrade J, Hanly PJ, Poulin MJ, Gozal D. Effect on Intermittent Hypoxia on Plasma Exosomal Micro RNA Signature and Endothelial Function in Healthy Adults. Sleep. 2016c;39:2077–2090. doi: 10.5665/sleep.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirandish-Gozal L, Gozal D. Obesity, asthma, and sleep-disordered breathing. J Pediatr. 2012;160:713–714. doi: 10.1016/j.jpeds.2011.11.036. [DOI] [PubMed] [Google Scholar]
- Kheirandish-Gozal L, Gozal D. Genotype-phenotype interactions in pediatric obstructive sleep apnea. Respir Physiol Neurobiol. 2013;189:338–343. doi: 10.1016/j.resp.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirandish L, Gozal D, Pequignot JM, Pequignot J, Row BW. Intermittent hypoxia during development induces long-term alterations in spatial working memory, monoamines, and dendritic branching in rat frontal cortex. Pediatr Res. 2005;58:594–599. doi: 10.1203/01.pdr.0000176915.19287.e2. [DOI] [PubMed] [Google Scholar]
- Kim DK, Kang B, Kim OY, Choi DS, Lee J, Kim SR, Go G, Yoon YJ, Kim JH, Jang SC, Park KS, Choi EJ, Kim KP, Desiderio DM, Kim YK, Lotvall J, Hwang D, Gho YS. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. Journal of extracellular vesicles. 2013:2. doi: 10.3402/jev.v2i0.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. British journal of haematology. 2004;124:376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Gozal D. Circulating microparticles in children with sleep disordered breathing. Chest. 2011;140:408–417. doi: 10.1378/chest.10-2161. [DOI] [PubMed] [Google Scholar]
- Kim LJ, Martinez D, Fiori CZ, Baronio D, Kretzmann NA, Barros HM. Hypomyelination, memory impairment, and blood-brain barrier permeability in a model of sleep apnea. Brain Res. 2015;1597:28–36. doi: 10.1016/j.brainres.2014.11.052. [DOI] [PubMed] [Google Scholar]
- Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/DeltaFosB in central autonomic regions. Am J Physiol Regul Integr Comp Physiol. 2011;301:R131–139. doi: 10.1152/ajpregu.00830.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, Budnik V. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287:16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranendonk ME, de Kleijn DP, Kalkhoven E, Kanhai DA, Uiterwaal CS, van der Graaf Y, Pasterkamp G, Visseren FL, Group SS. Extracellular vesicle markers in relation to obesity and metabolic complications in patients with manifest cardiovascular disease. Cardiovascular diabetology. 2014;13:37. doi: 10.1186/1475-2840-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Molecular and cellular neurosciences. 2011;46:409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Lai AY, Ip MS, Lam JC, Weaver TE, Fong DY. A pathway underlying the impact of CPAP adherence on intimate relationship with bed partner in men with obstructive sleep apnea. Sleep Breath. 2016;20:543–551. doi: 10.1007/s11325-015-1235-6. [DOI] [PubMed] [Google Scholar]
- Lavie L. Obstructive sleep apnoea syndrome--an oxidative stress disorder. Sleep medicine reviews. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep medicine reviews. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Lee TH, D’Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular ‘debris’. Seminars in immunopathology. 2011;33:455–467. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia--influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593–1603. doi: 10.1097/00004872-199715120-00060. [DOI] [PubMed] [Google Scholar]
- Li AM, So HK, Au CT, Ho C, Lau J, Ng SK, Abdullah VJ, Fok TF, Wing YK. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax. 2010;65:991–997. doi: 10.1136/thx.2010.134858. [DOI] [PubMed] [Google Scholar]
- Li J, Savransky V, Nanayakkara A, Smith PL, O’Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. Journal of applied physiology. 2007;102:557–563. doi: 10.1152/japplphysiol.01081.2006. [DOI] [PubMed] [Google Scholar]
- Li RC, Haribabu B, Mathis SP, Kim J, Gozal D. Leukotriene B4 receptor-1 mediates intermittent hypoxia-induced atherogenesis. Am J Respir Crit Care Med. 2011;184:124–131. doi: 10.1164/rccm.201012-2039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Row BW, Kheirandish L, Brittian KR, Gozal E, Guo SZ, Sachleben LR, Jr, Gozal D. Nitric oxide synthase and intermittent hypoxia-induced spatial learning deficits in the rat. Neurobiol Dis. 2004;17:44–53. doi: 10.1016/j.nbd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Liang Y, Hou D, Zhao X, Wang L, Hu Y, Liu J, Cheng H, Yang P, Shan X, Yan Y, Cruickshank JK, Mi J. Childhood obesity affects adult metabolic syndrome and diabetes. Endocrine. 2015;50:87–92. doi: 10.1007/s12020-015-0560-7. [DOI] [PubMed] [Google Scholar]
- Lim DC, Brady DC, Soans R, Kim EY, Valverde L, Keenan BT, Guo X, Kim WY, Park MJ, Galante R, Shackleford JA, Pack AI. Different cyclical intermittent hypoxia severities have different effects on hippocampal microvasculature. Journal of applied physiology. 2016;121:78–88. doi: 10.1152/japplphysiol.01040.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo G, Tan AY, Koo CY, Tai BC, Richards M, Lee CH. Prognostic implication of obstructive sleep apnea diagnosed by post-discharge sleep study in patients presenting with acute coronary syndrome. Sleep Med. 2014;15:631–636. doi: 10.1016/j.sleep.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. Journal of extracellular vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. Journal of applied physiology. 2009;106:1538–1544. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozito TP, Tuan RS. Endothelial cell microparticles act as centers of matrix metalloproteinsase-2 (MMP-2) activation and vascular matrix remodeling. Journal of cellular physiology. 2012;227:534–549. doi: 10.1002/jcp.22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Dong HY, Zhang B, Feng Z, Liu Y, Gao YQ, Dong MQ, Li ZC. miR-29a-3p attenuates hypoxic pulmonary hypertension by inhibiting pulmonary adventitial fibroblast activation. Hypertension. 2015;65:414–420. doi: 10.1161/HYPERTENSIONAHA.114.04600. [DOI] [PubMed] [Google Scholar]
- Luz Alonso-Alvarez M, Canet T, Cubell-Alarco M, Estivill E, Fernandez-Julian E, Gozal D, Jurado-Luque MJ, Lluch-Rosello MA, Martinez-Perez F, Merino-Andreu M, Pin-Arboledas G, Roure N, Sanmarti FX, Sans-Capdevila O, Segarra-Isern F, Tomas-Vila M, Teran-Santos J Sociedad Espanola de, S, Area de Sueno de la Sociedad Espanola de Neumologia y Cirugia T. Consensus document on sleep apnea-hypopnea syndrome in children (full version). Sociedad Espanola de Sueno. El Area de Sueno de la Sociedad Espanola de Neumologia y Cirugia Toracica(SEPAR) Archivos de bronconeumologia. 2011;47(Suppl 5:0):2–18. doi: 10.1016/S0300-2896(11)70026-6. [DOI] [PubMed] [Google Scholar]
- Magni F, Van Der Burgt YE, Chinello C, Mainini V, Gianazza E, Squeo V, Deelder AM, Kienle MG. Biomarkers discovery by peptide and protein profiling in biological fluids based on functionalized magnetic beads purification and mass spectrometry. Blood transfusion = Trasfusione del sangue. 2010;8(Suppl 3):s92–97. doi: 10.2450/2010.015S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, Schechter MS, Ward SD, Sheldon SH, Shiffman RN, Lehmann C, Spruyt K American Academy of P. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbe F, Vicente E, Wei Y, Nieto FJ, Jelic S. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA: the journal of the American Medical Association. 2012;307:2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- Mason RH, West SD, Kiire CA, Groves DC, Lipinski HJ, Jaycock A, Chong VN, Stradling JR. High prevalence of sleep disordered breathing in patients with diabetic macular edema. Retina. 2012;32:1791–1798. doi: 10.1097/IAE.0b013e318259568b. [DOI] [PubMed] [Google Scholar]
- Mateika JH, El-Chami M, Shaheen D, Ivers B. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. Journal of applied physiology. 2015;118:520–532. doi: 10.1152/japplphysiol.00564.2014. [DOI] [PubMed] [Google Scholar]
- Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature communications. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Sanchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nature reviews. Molecular cell biology. 2012;13:328–335. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhlesi B, Gozal D. Update in sleep medicine 2009. Am J Respir Crit Care Med. 2010;181:545–549. doi: 10.1164/rccm.200912-1948UP. [DOI] [PubMed] [Google Scholar]
- Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J. 2016;47:1162–1169. doi: 10.1183/13993003.01618-2015. [DOI] [PubMed] [Google Scholar]
- Momen-Heravi F, Balaj L, Alian S, Trachtenberg AJ, Hochberg FH, Skog J, Kuo WP. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Frontiers in physiology. 2012;3:162. doi: 10.3389/fphys.2012.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD, Jr, Thomson AW. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- Mostefai HA, Meziani F, Mastronardi ML, Agouni A, Heymes C, Sargentini C, Asfar P, Martinez MC, Andriantsitohaina R. Circulating microparticles from patients with septic shock exert protective role in vascular function. Am J Respir Crit Care Med. 2008;178:1148–1155. doi: 10.1164/rccm.200712-1835OC. [DOI] [PubMed] [Google Scholar]
- Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. Journal of extracellular vesicles. 2014:3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller G, Jung C, Straub J, Wied S, Kramer W. Induced release of membrane vesicles from rat adipocytes containing glycosylphosphatidylinositol-anchored microdomain and lipid droplet signalling proteins. Cellular signalling. 2009;21:324–338. doi: 10.1016/j.cellsig.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Abbott SM, Carroll JE, Davis CJ, Dijk DJ, Dinges DF, Gehrman PR, Ginsburg GS, Gozal D, Haack M, Lim DC, Macrea M, Pack AI, Plante DT, Teske JA, Zee PC. Developing Biomarker Arrays Predicting Sleep and Circadian-Coupled Risks to Health. Sleep. 2016;39:727–736. doi: 10.5665/sleep.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007;26:4263–4272. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One. 2011;6:e19847. doi: 10.1371/journal.pone.0019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase I, Kobayashi NB, Takatani-Nakase T, Yoshida T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Scientific reports. 2015;5:10300. doi: 10.1038/srep10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1181–1197. doi: 10.1152/ajpregu.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse LP, Joyner MJ, Curry TB, Laurenti MC, Man CD, Cobelli C, Vella A, Limberg JK. Three hours of intermittent hypoxia increases circulating glucose levels in healthy adults. Physiological reports. 2017:5. doi: 10.14814/phy2.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA: the journal of the American Medical Association. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, Lentsch AB. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. Journal of hepatology. 2016;64:60–68. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Loughlin AJ, Woffindale CA, Wood MJ. Exosomes and the emerging field of exosome-based gene therapy. Current gene therapy. 2012;12:262–274. doi: 10.2174/156652312802083594. [DOI] [PubMed] [Google Scholar]
- Ogorevc E, Kralj-Iglic V, Veranic P. The role of extracellular vesicles in phenotypic cancer transformation. Radiology and oncology. 2013;47:197–205. doi: 10.2478/raon-2013-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega FJ, Mercader JM, Catalan V, Moreno-Navarrete JM, Pueyo N, Sabater M, Gomez-Ambrosi J, Anglada R, Fernandez-Formoso JA, Ricart W, Fruhbeck G, Fernandez-Real JM. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59:781–792. doi: 10.1373/clinchem.2012.195776. [DOI] [PubMed] [Google Scholar]
- Pack AI. Application of Personalized, Predictive, Preventative, and Participatory (P4) Medicine to Obstructive Sleep Apnea. A Roadmap for Improving Care? Ann Am Thorac Soc. 2016;13:1456–1467. doi: 10.1513/AnnalsATS.201604-235PS. [DOI] [PubMed] [Google Scholar]
- Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. The Journal of cell biology. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse SG, Cowie MR. Sleep-disordered breathing in heart failure. European journal of heart failure. 2016;18:353–361. doi: 10.1002/ejhf.492. [DOI] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. The New England journal of medicine. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Perera FP, Weinstein IB. Molecular epidemiology: recent advances and future directions. Carcinogenesis. 2000;21:517–524. doi: 10.1093/carcin/21.3.517. [DOI] [PubMed] [Google Scholar]
- Phillips CL, O’Driscoll DM. Hypertension and obstructive sleep apnea. Nature and science of sleep. 2013;5:43–52. doi: 10.2147/NSS.S34841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoonsawat W, Aoki-Yoshida A, Tsuruta T, Sonoyama K. Adiponectin is partially associated with exosomes in mouse serum. Biochem Biophys Res Commun. 2014;448:261–266. doi: 10.1016/j.bbrc.2014.04.114. [DOI] [PubMed] [Google Scholar]
- Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27:1405–1417. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- Plihalova A, Westlake K, Polak J. Obstructive sleep apnoea and type 2 diabetes mellitus. Vnitrni lekarstvi. 2016;62:79–84. [PubMed] [Google Scholar]
- Poulain L, Thomas A, Rieusset J, Casteilla L, Levy P, Arnaud C, Dematteis M. Visceral white fat remodelling contributes to intermittent hypoxia-induced atherogenesis. Eur Respir J. 2014;43:513–522. doi: 10.1183/09031936.00019913. [DOI] [PubMed] [Google Scholar]
- Properzi F, Logozzi M, Fais S. Exosomes: the future of biomarkers in medicine. Biomark Med. 2013;7:769–778. doi: 10.2217/bmm.13.63. [DOI] [PubMed] [Google Scholar]
- Quek C, Bellingham SA, Jung CH, Scicluna BJ, Shambrook MC, Sharples RA, Cheng L, Hill AF. Defining the purity of exosomes required for diagnostic profiling of small RNA suitable for biomarker discovery. RNA Biol. 2017;14:245–258. doi: 10.1080/15476286.2016.1270005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querido JS, Sheel AW, Cheema R, Van Eeden S, Mulgrew AT, Ayas NT. Effects of 10 days of modest intermittent hypoxia on circulating measures of inflammation in healthy humans. Sleep Breath. 2012;16:657–662. doi: 10.1007/s11325-011-0555-4. [DOI] [PubMed] [Google Scholar]
- Quintero M, del Gonzalez-Martin MC, Vega-Agapito V, Gonzalez C, Obeso A, Farre R, Agapito T, Yubero S. The effects of intermittent hypoxia on redox status, NF-kappaB activation, and plasma lipid levels are dependent on the lowest oxygen saturation. Free Radic Biol Med. 2013;65:1143–1154. doi: 10.1016/j.freeradbiomed.2013.08.180. [DOI] [PubMed] [Google Scholar]
- Rana S, Zoller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011;39:559–562. doi: 10.1042/BST0390559. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R, Li Y, Zhang J, Zhou J, Sun Y, Tan L, Li T, Wing YK, Tang X. Obstructive Sleep Apnea With Objective Daytime Sleepiness Is Associated With Hypertension. Hypertension. 2016;68:1264–1270. doi: 10.1161/HYPERTENSIONAHA.115.06941. [DOI] [PubMed] [Google Scholar]
- Reynolds CM, Gray C, Li M, Segovia SA, Vickers MH. Early Life Nutrition and Energy Balance Disorders in Offspring in Later Life. Nutrients. 2015;7:8090–8111. doi: 10.3390/nu7095384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezeli M, Gidlof O, Evander M, Bryl-Gorecka P, Sathanoori R, Gilje P, Pawlowski K, Horvatovich P, Erlinge D, Marko-Varga G, Laurell T. Comparative Proteomic Analysis of Extracellular Vesicles Isolated by Acoustic Trapping or Differential Centrifugation. Analytical chemistry. 2016;88:8577–8586. doi: 10.1021/acs.analchem.6b01694. [DOI] [PubMed] [Google Scholar]
- Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711–719. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottiers V, Naar AM. MicroRNAs in metabolism and metabolic disorders. Nature reviews. Molecular cell biology. 2012;13:239–250. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D. Impaired spatial working memory and altered choline acetyltransferase (CHAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep. Behav Brain Res. 2007;177:308–314. doi: 10.1016/j.bbr.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-de-la-Torre M, Khalyfa A, Sanchez-de-la-Torre A, Martinez-Alonso M, Martinez-Garcia MA, Barcelo A, Lloberes P, Campos-Rodriguez F, Capote F, Diaz-de-Atauri MJ, Somoza M, Gonzalez M, Masa JF, Gozal D, Barbe F, Spanish Sleep N. Precision Medicine in Patients With Resistant Hypertension and Obstructive Sleep Apnea: Blood Pressure Response to Continuous Positive Airway Pressure Treatment. Journal of the American College of Cardiology. 2015;66:1023–1032. doi: 10.1016/j.jacc.2015.06.1315. [DOI] [PubMed] [Google Scholar]
- Sano S, Izumi Y, Yamaguchi T, Yamazaki T, Tanaka M, Shiota M, Osada-Oka M, Nakamura Y, Wei M, Wanibuchi H, Iwao H, Yoshiyama M. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun. 2014;445:327–333. doi: 10.1016/j.bbrc.2014.01.183. [DOI] [PubMed] [Google Scholar]
- Sapin E, Peyron C, Roche F, Gay N, Carcenac C, Savasta M, Levy P, Dematteis M. Chronic Intermittent Hypoxia Induces Chronic Low-Grade Neuroinflammation in the Dorsal Hippocampus of Mice. Sleep. 2015;38:1537–1546. doi: 10.5665/sleep.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. Journal of cell science. 2002;115:2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- Schneider A, Simons M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013;352:33–47. doi: 10.1007/s00441-012-1428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim B, Won C, Yaggi HK. Cardiovascular consequences of sleep apnea. Clinics in chest medicine. 2010;31:203–220. doi: 10.1016/j.ccm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Serban KA, Rezania S, Petrusca DN, Poirier C, Cao D, Justice MJ, Patel M, Tsvetkova I, Kamocki K, Mikosz A, Schweitzer KS, Jacobson S, Cardoso A, Carlesso N, Hubbard WC, Kechris K, Dragnea B, Berdyshev EV, McClintock J, Petrache I. Structural and functional characterization of endothelial microparticles released by cigarette smoke. Scientific reports. 2016;6:31596. doi: 10.1038/srep31596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforza E, Roche F. Chronic intermittent hypoxia and obstructive sleep apnea: an experimental and clinical approach. Hypoxia. 2016;4:99–108. doi: 10.2147/HP.S103091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Shan X, Chi L, Ke Y, Luo C, Qian S, Gozal D, Liu R. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol Dis. 2007;28:206–215. doi: 10.1016/j.nbd.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples RA, Vella LJ, Nisbet RM, Naylor R, Perez K, Barnham KJ, Masters CL, Hill AF. Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 2008;22:1469–1478. doi: 10.1096/fj.07-9357com. [DOI] [PubMed] [Google Scholar]
- Sheikh F, Ross RS, Chen J. Cell-cell connection to cardiac disease. Trends in cardiovascular medicine. 2009;19:182–190. doi: 10.1016/j.tcm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert review of proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- Sinha A, Alfaro J, Kislinger T. Characterization of Protein Content Present in Exosomes Isolated from Conditioned Media and Urine. Curr Protoc Protein Sci. 2017;87:24 29 21–24 29 12. doi: 10.1002/cpps.23. [DOI] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature cell biology. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruyt K, Gozal D. A mediation model linking body weight, cognition, and sleep-disordered breathing. Am J Respir Crit Care Med. 2012;185:199–205. doi: 10.1164/rccm.201104-0721OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature reviews. Molecular cell biology. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Strimbu K, Tavel JA. What are biomarkers? Current opinion in HIV and AIDS. 2010;5:463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahrani AA, Ali A, Stevens MJ. Obstructive sleep apnoea and diabetes: an update. Curr Opin Pulm Med. 2013;19:631–638. doi: 10.1097/MCP.0b013e3283659da5. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kobayashi S, Fujino N, Suzuki T, Ota C, He M, Yamada M, Suzuki S, Yanai M, Kurosawa S, Yamaya M, Kubo H. Increased circulating endothelial microparticles in COPD patients: a potential biomarker for COPD exacerbation susceptibility. Thorax. 2012;67:1067–1074. doi: 10.1136/thoraxjnl-2011-201395. [DOI] [PubMed] [Google Scholar]
- Tamisier R, Pepin JL, Remy J, Baguet JP, Taylor JA, Weiss JW, Levy P. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011;37:119–128. doi: 10.1183/09031936.00204209. [DOI] [PubMed] [Google Scholar]
- Tan HL, Kheirandish-Gozal L, Gozal D. The promise of translational and personalised approaches for paediatric obstructive sleep apnoea: an ‘Omics’ perspective. Thorax. 2014;69:474–480. doi: 10.1136/thoraxjnl-2013-204640. [DOI] [PubMed] [Google Scholar]
- Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. The American journal of pathology. 2002;160:673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT, Kent BD, Crinion SJ, McNicholas WT, Ryan S. Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem Biophys Res Commun. 2014;447:660–665. doi: 10.1016/j.bbrc.2014.04.062. [DOI] [PubMed] [Google Scholar]
- Thai TH, Christiansen PA, Tsokos GC. Is there a link between dysregulated miRNA expression and disease? Discovery medicine. 2010;10:184–194. [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews. Immunology. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Thirabanjasak D, Tantiwongse K, Thorner PS. Angiomyeloproliferative lesions following autologous stem cell therapy. Journal of the American Society of Nephrology: JASN. 2010;21:1218–1222. doi: 10.1681/ASN.2009111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MA, Shimbo D, Parikh MA, Hoffman EA, Vogel-Claussen J, Hueper K, Fu J, Liu CY, Bluemke DA, Ventetuolo CE, Doyle MF, Barr RG. Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. The Multi-Ethnic Study of Atherosclerosis Chronic Obstructive Pulmonary Disease study. Am J Respir Crit Care Med. 2013;188:60–68. doi: 10.1164/rccm.201209-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. Journal of cellular biochemistry. 2010;111:488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122:928–937. doi: 10.1161/CIRCULATIONAHA.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams EG, Lauter CJ, Salem N, Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica et biophysica acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- Tremblay JC, Boulet LM, Tymko MM, Foster GE. Intermittent hypoxia and arterial blood pressure control in humans: role of the peripheral vasculature and carotid baroreflex. Am J Physiol Heart Circ Physiol. 2016;311:H699–706. doi: 10.1152/ajpheart.00388.2016. [DOI] [PubMed] [Google Scholar]
- Tsui NB, Ng EK, Lo YM. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin Chem. 2002;48:1647–1653. [PubMed] [Google Scholar]
- Tuleta I, Franca CN, Wenzel D, Fleischmann B, Nickenig G, Werner N, Skowasch D. Hypoxia-induced endothelial dysfunction in apolipoprotein E-deficient mice; effects of infliximab and L-glutathione. Atherosclerosis. 2014;236:400–410. doi: 10.1016/j.atherosclerosis.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Varadharaj S, Porter K, Pleister A, Wannemacher J, Sow A, Jarjoura D, Zweier JL, Khayat RN. Endothelial nitric oxide synthase uncoupling: a novel pathway in OSA induced vascular endothelial dysfunction. Respir Physiol Neurobiol. 2015;207:40–47. doi: 10.1016/j.resp.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella LJ, Sharples RA, Nisbet RM, Cappai R, Hill AF. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. European biophysics journal: EBJ. 2008;37:323–332. doi: 10.1007/s00249-007-0246-z. [DOI] [PubMed] [Google Scholar]
- Vizzardi E, Sciatti E, Bonadei I, D’Aloia A, Curnis A, Metra M. Obstructive sleep apnoea-hypopnoea and arrhythmias: new updates. Journal of cardiovascular medicine. 2014 doi: 10.2459/JCM.0000000000000043. [DOI] [PubMed] [Google Scholar]
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et biophysica acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Waldenstrom A, Genneback N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One. 2012;7:e34653. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sen S. MicroRNA functional network in pancreatic cancer: from biology to biomarkers of disease. Journal of biosciences. 2011;36:481–491. doi: 10.1007/s12038-011-9083-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174:307–316. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Bian Y, Yu F, Zhang Q, Zhang G, Li Y, Song S, Ren X, Tong J. Chronic intermittent hypoxia induces cardiac inflammation and dysfunction in a rat obstructive sleep apnea model. Journal of biomedical research. 2016;30:490–495. doi: 10.7555/JBR.30.20160110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JW, Tamisier R, Liu Y. Sympathoexcitation and arterial hypertension associated with obstructive sleep apnea and cyclic intermittent hypoxia. Journal of applied physiology. 2015;119:1449–1454. doi: 10.1152/japplphysiol.00315.2015. [DOI] [PubMed] [Google Scholar]
- Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Pilowsky PM, Fong AY. Mechanism of sympathetic activation and blood pressure elevation in humans and animals following acute intermittent hypoxia. Prog Brain Res. 2014;209:131–146. doi: 10.1016/B978-0-444-63274-6.00007-2. [DOI] [PubMed] [Google Scholar]
- Xu LH, Xie H, Shi ZH, Du LD, Wing YK, Li AM, Ke Y, Yung WH. Critical Role of Endoplasmic Reticulum Stress in Chronic Intermittent Hypoxia-Induced Deficits in Synaptic Plasticity and Long-Term Memory. Antioxidants & redox signaling. 2015;23:695–710. doi: 10.1089/ars.2014.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Feng Y, Peng H, Guo D, Li T. Obstructive sleep apnea and the risk of perinatal outcomes: a meta-analysis of cohort studies. Scientific reports. 2014;4:6982. doi: 10.1038/srep06982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience. 2004;126:313–323. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- Yagishita S, Suzuki S, Yoshikawa K, Iida K, Hirata A, Suzuki M, Takashima A, Maruyama K, Hirasawa A, Awaji T. Treatment of intermittent hypoxia increases phosphorylated tau in the hippocampus via biological processes common to aging. Mol Brain. 2017;10:2. doi: 10.1186/s13041-016-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Mwaikambo BR, Zhu T, Gagnon C, Lafleur J, Seshadri S, Lachapelle P, Lavoie JC, Chemtob S, Hardy P. Lymphocytic microparticles inhibit angiogenesis by stimulating oxidative stress and negatively regulating VEGF-induced pathways. Am J Physiol Regul Integr Comp Physiol. 2008;294:R467–476. doi: 10.1152/ajpregu.00432.2007. [DOI] [PubMed] [Google Scholar]
- Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang JD, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelamanchili SV, Lamberty BG, Rennard DA, Morsey BM, Hochfelder CG, Meays BM, Levy E, Fox HS. MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease. PLoS pathogens. 2015;11:e1005032. doi: 10.1371/journal.ppat.1005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thrombosis and haemostasis. 2011;105:396–408. doi: 10.1160/TH10-09-0595. [DOI] [PubMed] [Google Scholar]
- Yun CH, Jung KH, Chu K, Kim SH, Ji KH, Park HK, Kim HC, Lee ST, Lee SK, Roh JK. Increased circulating endothelial microparticles and carotid atherosclerosis in obstructive sleep apnea. J Clin Neurol. 2010;6:89–98. doi: 10.3988/jcn.2010.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circulation research. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Willeit P, Drozdov I, Kiechl S, Mayr M. Profiling of circulating microRNAs: from single biomarkers to re-wired networks. Cardiovasc Res. 2012;93:555–562. doi: 10.1093/cvr/cvr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan G, Fenik P, Pratico D, Veasey SC. Inducible nitric oxide synthase in long-term intermittent hypoxia: hypersomnolence and brain injury. Am J Respir Crit Care Med. 2005;171:1414–1420. doi: 10.1164/rccm.200411-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. The American journal of pathology. 2014;184:28–41. doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Downey HF, Chen S, Shi X. Two-week normobaric intermittent hypoxia exposures enhance oxyhemoglobin equilibrium and cardiac responses during hypoxemia. Am J Physiol Regul Integr Comp Physiol. 2014;307:R721–730. doi: 10.1152/ajpregu.00191.2014. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Wang Y, Gozal D. Pathological consequences of intermittent hypoxia in the central nervous system. Compr Physiol. 2012;2:1767–1777. doi: 10.1002/cphy.c100060. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chen P, Peng Y, Ouyang R. Role of Oxidative Stress in the Neurocognitive Dysfunction of Obstructive Sleep Apnea Syndrome. Oxidative medicine and cellular longevity. 2016;2016:9626831. doi: 10.1155/2016/9626831. [DOI] [PMC free article] [PubMed] [Google Scholar]