Abstract
Cancer patients frequently suffer from gastrointestinal complications. In this manuscript, we update our 2013 guideline on the diagnosis and management of gastrointestinal complications in adult cancer patients by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). An expert group was put together by the AGIHO to update the existing guideline. For each sub-topic, a literature search was performed in PubMed, Medline, and Cochrane databases, and strengths of recommendation and the quality of the published evidence for major therapeutic strategies were categorized using the 2015 European Society for Clinical Microbiology and Infectious Diseases (ESCMID) criteria. Final recommendations were approved by the AGIHO plenary conference. Recommendations were made with respect to non-infectious and infectious gastrointestinal complications. Strengths of recommendation and levels of evidence are presented. A multidisciplinary approach to the diagnosis and management of gastrointestinal complications in cancer patients is mandatory. Evidence-based recommendations are provided in this updated guideline.
Keywords: Abdominal complications, Colitis, Diarrhea, Chemotherapy, Cancer, Infection
Introduction
Abdominal complications are a frequent problem in patients with hematological malignancies or solid tumors. In 2013, we published the first version of “Diagnosis and management of gastrointestinal complications in adult cancer patients: evidence-based guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO)”, one of the first comprehensive, practical, and evidence-based guidelines covering the epidemiology, pathophysiology, diagnosis and treatment of most non-infectious and infectious complications as well as the corresponding hygiene measures [1]. The present update takes the evolving basis of evidence into consideration. In addition, the section on infectious complications caused by parasites has been considerably expanded in response to the increasing mobility of our patients. Whenever possible, pre-existent recommendations from other guideline panels were incorporated into this overview.
Methods
Sub-topics of this guideline were assigned to members of the AGIHO and a literature search was performed in PubMed, Medline, and Cochrane databases. The strength of recommendation and the quality of evidence for major therapeutic strategies were categorized using the current criteria of the European Society for Clinical Microbiology and Infectious Diseases (ESCMID, Table 1) which include an index to the level II recommendations, where appropriate [2].
Table 1.
Categories of evidence—ESCMID criteria [2]
| Category, Grade | Definition |
|---|---|
| Strength of recommendation | |
| A | AGIHO strongly supports a recommendation for use |
| B | AGIHO moderately supports a recommendation for use |
| C | AGIHO marginally supports a recommendation for use |
| D | AGIHO supports a recommendation against use |
| Quality of evidence | |
| I | Evidence from at least one properly designed randomized, controlled trial |
| II | Evidence from at least one well-designed clinical trial, without randomization; from cohort or case-control analytic studies (preferably from more than one center); from multiple time series; or from dramatic results of uncontrolled experiments |
| III | Evidence from opinions of respected authorities, based on clinical experience, descriptive case studies, or reports of expert committees |
| Index (for level II quality of evidence, only) | |
| r | Meta-analysis or systematic review of randomized controlled trials |
| t | Transferred evidence, i.e., results from different patient cohorts, or similar immune-status situation |
| h | Comparator group is a historical control |
| u | Uncontrolled trial |
| a | Abstract published at an international meeting |
Consensus discussions were held on each of the topics. After ratification of all topics by this expert group, recommendations were discussed and ratified by the AGIHO plenary.
Treatment-associated anorexia, nausea, and emesis are not included, as this would exceed the scope of this guideline and has already been addressed elsewhere [3].
Guideline
Diarrhea and colitis
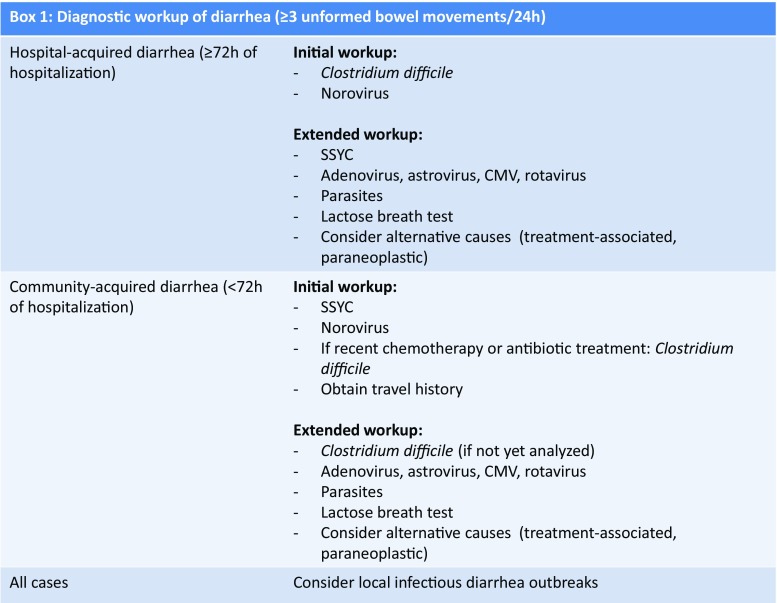
Independent of its cause, diarrhea should always be treated with adequate oral or intravenous fluid and electrolyte replacement (AIII). Patients should be observed for signs of malnutrition and/or catabolic state. If indicated, enteral or parenteral electrolytes, carbohydrates, lipids, amino acids, protein, and vitamins should be supplemented (AIII). Figure 1 provides important facts on the diagnostic work-up of diarrhea in cancer patients. Commonly, repeat testing for the same pathogen should not be performed to avoid false positive results [4, 5].
Fig. 1.
Diagnostic work-up of diarrhea (≥ 3 unformed bowel movements/24 h)
Non-infection-related diarrhea and colitis
Paraneoplastic diarrhea
Paraneoplastic diarrhea is a rare phenomenon which may be triggered by a variety of pathophysiological mechanisms. Secretion of vasoactive intestinal polypeptide (VIP), as typically observed in patients with non-β islet cell tumors of the pancreas, may cause watery diarrhea, hypokalemia, and hypochlorhydria [6]. Flush and diarrhea are the typical symptoms of serotonin-producing carcinoid tumors [7]. Other hormones that may cause paraneoplastic diarrhea include glucagon (glucagonoma), gastrin (gastrinoma or hepatocellular carcinoma), somatostatin (somatostatinoma or pheochromocytoma), and the prostaglandins (hepatocellular carcinoma) [8–12]. In association with small cell lung carcinoma, antibodies directed against neuronal proteins may cause autonomic neuropathy and diarrhea [13]. In patients with thymoma, diarrhea as part of a graft-versus-host-disease-like reaction has been described [14, 15].
In most cases of paraneoplastic diarrhea, diagnosis and treatment of the underlying disease is considered the only effective measure to reduce diarrhea. If a well-differentiated neuroendocrine neoplasia (NEN) has been diagnosed and patients present with typical symptoms of a functional NEN such as diarrhea and flush, treatment with somatostatin analogs is indicated as discussed elsewhere [16].
Therapy-associated diarrhea
In cancer patients, factors related to toxic effects of chemotherapy are the most common cause of abdominal complications. 5-fluorouracil, irinotecan, capecitabine, anthracyclines, and a number of small molecules and monoclonal antibodies have been associated with therapy-associated diarrhea [17–25]. Previous studies have reported incidence rates of diarrhea in 27–76% of neutropenic patients. In only 5–17% of these cases, an infectious agent was identified as the cause of diarrhea, suggesting primarily toxicity-related symptoms [17, 26–28]. Disruption of the gastrointestinal microflora after administration of antibiotics may result in osmotic diarrhea due to alterations in carbohydrate metabolism and impaired absorption of short-chain fatty acids in 5–62% of patients [29–31]. In 7–50% of these cases, overgrowth with Clostridium difficile may ensue, leading to C. difficile-associated diarrhea [32, 33].
Chemotherapy-associated lactose intolerance presenting as diarrhea, bloating, and malabsorption has also been discussed as a cause of non-infectious diarrhea in cancer patients. While up to 35% of patients present with an abnormal lactose breath hydrogen test during chemotherapy, only up to 11% became symptomatic. Generally, test results returned to normal after completion of chemotherapy [34, 35].
Radiotherapy involving the gastrointestinal tract may cause severe mucosal bowel damage resulting in acute or chronic diarrhea. Symptoms usually peak about 7–14 days after initiation of irradiation and may be intensified by combination treatment with chemotherapy. In some patients, surgical resection may result in impairment of physiological gastrointestinal function with diarrhea developing because of accelerated gastric and intestinal transit times, bacterial overgrowth, and altered secretion and absorption of bile acids.
After exclusion of an infectious cause of diarrhea (see Fig. 1), loperamide is recommended for first-line treatment of non-infectious diarrhea (initial dose 4 mg, followed by 2 mg po after each unformed bowel movement, maximum daily dose is 16 mg) [36–39] (AII u). Patients with severe diarrhea persisting for more than 48 h despite administration of antimotility agents should be hospitalized [36] (AIII). Of note, in long-term neutropenic patients, overdosage of antimotility agents may lead to iatrogenic ileus with an increased risk of bacteremia [27].
In patients failing to respond to loperamide, octreotide may be considered with a starting dose of 100 μg tid sc [40–42] (BII u). In patients not responding to the initial dosage, dose increases until symptom control are recommended [40–44] (AIII). An alternative might be the administration of psyllium seeds, although this approach has not been evaluated in patients with chemotherapy-associated diarrhea [45, 46] (BII t). Further options include diphenoxylate plus atropine and opiates such as paregoric tincture of opium, codeine, and morphine [36] (BIII). Primary prevention using octreotide long acting release (LAR) has been evaluated in two randomized controlled trials, but did not prove efficacious [47, 48] (DI). The efficacy of glutamine in preventing treatment-associated diarrhea was assessed in a meta-analysis comprising eight heterogeneous small to medium-sized randomized controlled trials. According to this analysis, glutamine significantly reduced duration of diarrhea, but not severity [49]. Another randomized controlled trial showing no advantage for glutamine was not included into this meta-analysis [50]. Overall, the available evidence does not support the use of glutamine in this indication (DI). Similarly, ReCharge ice cream based on iron-saturated lactoferrin and anhydrous milk failed to control treatment-associated diarrhea in a randomized controlled trial [51] (DI).
Concerning diarrhea associated with specific substances, budesonide or neomycin prophylaxis for late-onset diarrhea after treatment with irinotecan showed no significant advantage [52, 53] (DI). However, addition of budesonide [24] (BII u) or acetorphan [25] (BII u) to loperamide in the treatment of manifest irinotecan-associated diarrhea was effective in two small clinical trials. In contrast, preventive calcium aluminosilicate clay did not reduce incidence and severity of diarrhea [54] (DI).
5-FU-associated diarrhea was targeted by a small randomized pilot study in which lafutidine, a second generation histamine H2 receptor antagonist, or placebo was administered to ten patients with gastric adenocarcinoma. While the results were promising with respect to reduction of diarrhea and nausea, the limited sample size does not allow for generalization [55] (CI). Due to the lack of evaluable data, an expert panel discussed and published treatments for idelalisib-associated diarrhea and recommended budesonide or other oral or intravenous steroids [56] (BIII).
Concerning chemotherapy-associated lactose intolerance, we do not recommend dietary restriction of milk products, unless clinical symptoms of lactose intolerance are observed after ingestion of milk products [34, 35] (BII u).
A large number of trials assessing the protective effect of prophylactic probiotic treatment to avoid antibiotic-associated diarrhea have been conducted. Studies in immunocompetent patients suggest a protective effect for Saccharomyces boulardii, Lactobacillus rhamnosus, and a combination of L. casei, L. bulgaricus, and S. thermophilus [32, 57, 58]. A recent review identified 11 studies in patients with cancer and concluded that the severity and frequency of diarrhea may be reduced by use of probiotics. However, it was also underlined that more studies were needed to assess effect size and safety aspects, as there were five cases of probiotic-related bacteremia or fungemia [59] (CII t,r). Recommendations on therapy-associated diarrhea are summarized in Table 2.
Table 2.
Treatment-related diarrhea
| Clinical situation | Intention | Intervention | SoR | QoE | Reference | Comments |
|---|---|---|---|---|---|---|
| Treatment-associated diarrhea | Primary prevention | Octeotride LAR | D | I | [47, 48] | No sufficient evidence to recommend the use of octreotide LAR for secondary prevention |
| Glutamine | D | I | [49, 50] | |||
| Late-onset diarrhea after irinotecan therapy | Cure | Treatment with loperamide po plus | Stop treatment, if no response after 72 h | |||
| Budesonide 3 mg tid po until resolution of symptoms or | B | IIu | [24] | |||
| Acetorphan 100 mg tid po for 48 h | B | IIu | [25] | |||
| Late-onset diarrhea after irinotecan therapy | Primary prevention | Prophylaxis with budesonide 3 mg tid po or | D | I | [52] | |
| Neomycin 500 mg bid po | D | I | [53] | |||
| Treatment-associated diarrhea, 1st line | Cure | Loperamide, initial dose 4 mg, followed by 2 mg po after each unformed bowel movement | A | IIu | [36–39] | -Only in persisting and severe cases of diarrhea and after exclusion of infectious diarrhea -Careful risk-benefit assessment in neutropenic patients -No further benefit after 16 mg qd -Administer 30 min. before eating for maximum efficacy |
| Treatment-associated diarrhea, 2nd line | Cure | Octreotide 100 μg tid sc; increase to 500 μg qd if no improvement after 24 h | B | IIu | [40–44] | -Only in cases of persisting and severe diarrhea and after exclusion of infectious diarrhea -Titration to higher dosages may be considered if no response to 500 μg qd sc -Careful risk-benefit assessment in neutropenic patients -iv administration at 25–50 μg/h possible |
| Cure | Psyllium seeds | B | IIt | [45, 46] | -Only in persisting and severe cases of diarrhea and after exclusion of infectious diarrhea | |
| Alternatives: Diphenoxylate plus atropine, paregoric tincture of opium, codeine or morphine | B | III | -Only in cases of persisting and severe diarrhea and after exclusion of infectious diarrhea -Careful risk-benefit assessment in neutropenic patients |
|||
| Chemotherapy-associated lactose intolerance | Prevention | Dietary restriction of milk products | B | IIu | [34, 35] | Only if clinical signs and symptoms are present |
| Antibiotic-associated diarrhea | Prevention | Probiotics | C | IIt,r | [59] | No sufficient safety data in immunocompromised patients available |
SoR strength of recommendation, QoE quality of evidence
Infection-related diarrhea and colitis
The diagnosis of infection-related diarrhea should trigger adequate hygiene measures [60] (AII). The regular practice of appropriate hand hygiene is considered a cornerstone in the prevention of hospital-acquired infections [61, 62] and has been discussed in detail elsewhere [63]. Table 3 shows recommended hygiene procedures for most common infectious causes of gastroenteritis. Of note, hygiene measures can be subject to local or national legislation which may differ from these recommendations.
Table 3.
Isolation procedures for the most common causes of infectious diarrhea
| Pathogen | SR | GG | M | Infectious material | Stop | SoR | QoE | Comment |
|---|---|---|---|---|---|---|---|---|
| Clostridium difficile | ● | ○ | Feces | Normalization of clinical symptoms (diarrhea or colitis) | B | III | -Use warm water and plain soap for hand hygiene after patient contact -No precautions for asymptomatically colonized patients -Do not re-test for C. difficile toxin to evaluate further necessity of isolation -Gloves and gown only if contact with infectious material or contaminated surfaces |
|
| Salmonella, Shigella, Yersinia, Campylobacter spp. | ● | ○ | Feces, vomitus, possibly urine | Three negative stool samples | B | III | Gloves and gown only if contact with infectious material or contaminated surfaces | |
| Norovirus | ● | ● | ● | Feces, vomitus | Three negative stool samples | B | III |
● always required, ○ only required under certain circumstances specified in the comment box, SR single room, GG gloves and gown, M mask, SoR strength of recommendation, QoE quality of evidence
Neutropenic enterocolitis
Neutropenic enterocolitis (NEC) is a common chemotherapy-associated complication, particularly in patients with acute leukemia [17, 64–67]. A pooled incidence rate of 5.3% was calculated for hospitalized patients with hematological malignancies, aplastic anemia, or those receiving high-dose chemotherapy for solid tumors [67]. NEC has been associated with mortality rates between < 20 and 82% [65, 68–70]. Administration of high-dose cytarabine plus anthracyclines has been identified as major risk factor. However, many other cytostatic agents and radiotherapy have been identified as triggers of NEC [28, 71–80]. Mucosal barrier damage facilitates infiltration and penetration of the bowel wall by bacteria, viruses, and fungi. From blood cultures drawn during episodes of NEC, Gram-negative Enterobacteriaceae were the most frequently documented organisms [17, 27, 66]. A systematic review on fungal infections related to NEC found a pooled frequency of 6.2% [68].
Clinical signs and symptoms include abdominal pain, diarrhea, nausea, and vomiting. In more severe cases rebound tenderness, decreased bowel sounds or muscular guarding may develop. Proposed diagnostic criteria according to Gorschlüter et al. are the presence of fever, abdominal pain, and a bowel wall thickening of more than 4 mm (transversal scan) over more than 30 mm (longitudinal scan) in any segment by ultrasonography or computed tomography (CT) [67].
Since this definition of NEC describes patients at a late pathophysiological stage of intestinal impairment, a clinical definition identifying neutropenic patients at risk of further clinical deterioration due to abdominal complications was recently developed. Neutropenic patients with chemotherapy-associated bowel syndrome (T ≥ 37.8 °C and abdominal pain and/or lack of bowel movement for ≥ 72 h) were more likely to suffer complications and death [27].
Non-invasive imaging is generally recommended to confirm the diagnosis of NEC and to exclude bowel wall perforation. Blood cultures, stool cultures, and a C. difficile toxin test for exclusion of NEC-associated bacteremia and colitis due to C. difficile, respectively, are recommended. Endoscopy to obtain biopsies is discouraged, due to the increased risk of bowel wall perforation.
Conservative therapy is preferred in most cases, consisting of a bland diet, hydration, and an effective pain treatment (BIII). In accordance with IDSA guidelines for patients with complicated abdominal infections in non-neutropenic patients [81] and the guideline for antimicrobial therapy of unexplained fever in neutropenic patients of the AGIHO [82], we recommend administration of piperacillin/tazobactam or imipenem/cilastatin or meropenem (BIII). There are no studies assessing the effect of additional metronidazole or vancomycin on patient outcome (CIII). Empirical antifungal therapy may be discussed if it has not yet been administered for the indication of persistent febrile neutropenia [68, 83, 84] (BIII). The use of hematopoietic growth factors might be considered, even though corresponding evidence is not available (BIII). Antimicrobial therapy should be administered until resolution of clinical signs and neutropenia. While a surgical consultation should be obtained at an early stage of disease evolution, surgical interventions in the neutropenic and/or thrombocytopenic patient are reserved to severe cases, e.g., patients with bowel wall perforation (BIII).
Clostridium difficile infection
C. difficile is the most common cause of healthcare-associated infectious diarrhea and colitis, in both Europe and the USA [85, 86]. In adult patients with cancer, infections due to C. difficile (CDI) occur in 5–9% of chemotherapy courses and 5–20% of patients, respectively [27, 28, 66, 87–91].
Binding of C. difficile toxins A and B to epithelial cells and subsequent internalization leads to diarrhea by induction of apoptosis [92]. An increase in the frequency of CDI has been reported and attributed to the emergence of a new and hypervirulent strain of C. difficile, named NAP1 (synonymous terms are BI, ribotype 027, and toxinotype III) [93–95]. In NAP1 strains, single-base deletion mutations at position 117 of the tcdC gene, a downregulator of toxin transcription, lead to disinhibition of toxin A and B production, thus contributing to increased intracolonic toxin levels [96]. The most important risk factors for CDI are antibiotic exposure, advanced age, immunosuppression, and chronic kidney disease. However, other factors such as prolonged length of hospital stay, previous CDI, and use of proton pump inhibitors have also been discussed [97–102].
The most recent ESCMID update on treatment guidance differentiates between non-severe and severe disease, whereas severe disease is indicated by signs of colitis identified by clinical examination or imaging. Alternatively, laboratory markers, i.e., marked leucocytosis (leucocyte count > 15 × 109/L) and/or marked left shift (band neutrophils > 20% of leucocytes), rise in serum creatinine (> 50% above the baseline), elevated serum lactate (≥ 5 mM), or markedly reduced serum albumin (< 30 g/L), may also indicate severe disease. In addition, the guideline suggests classification of patients at an increased risk of developing severe CDI into this same category. Relevant risk factors in this context are age ≥ 65 years, serious comorbidities, intensive care unit admission, and immunodeficiency. Based on the recommendations, cancer patients are unlikely to be classified as non-severe cases, unless they are currently not considered immunocompromised [103].
Clinical signs and symptoms of CDI are diarrhea, fever, abdominal pain, and distension. Presentation ranges from mild diarrhea to fulminant pseudomembranous colitis with paralytic ileus, toxic megacolon, or perforation [88, 90, 104]. Onset of diarrhea may occur at any time during and up to 2 weeks after the end of antibiotic treatment [104].
In accordance with ESCMID guidelines, CDI is defined as (i) > 3 unformed stools within 24 h or (ii) ileus or toxic megacolon in combination with evidence of toxin-producing C. difficile in stools and absence of another cause of symptoms, or (iii) pseudomembranous colitis diagnosed by endoscopy, colectomy, or histopathological examination [103]. In neutropenic patients, as well as in patients with severe colitis, diagnostic endoscopy is contraindicated because of the risk of colon perforation or hemorrhage [95].
The proper laboratory specimen is an unformed stool promptly submitted to the laboratory [5]. Processing a single specimen from a patient at onset of a symptomatic episode is sufficient and should not be repeated to avoid false positive results through multiple testing [5]. No single commercial test, but a two-step algorithm should be used to diagnose CDI from fecal samples. Possible combinations include a glutamate dehydrogenase (GDH) enzyme immunoassay (EIA) or a nucleic acid amplification test (NAAT) followed by a toxin A and B EIA [5].
To minimize the risk of developing CDI, antibiotics should cover a spectrum no broader than necessary and should be adapted with respect to results of cultures and/or susceptibility (BIII). If possible, antibiotics not intended for treatment of CDI should be discontinued after diagnosis of CDI [105–107] (AII); however, in febrile neutropenia, this may not always be possible.
There is insufficient evidence to recommend administration of prophylactic antibiotics or probiotics in cancer patients at risk for CDI [59, 94, 108] (CIII, CII t, r ). With the registration of bezlotoxumab, an antibody against C. difficile toxin B which is added to standard treatment and significantly decreases the likelihood of CDI recurrence, a new option for the secondary prevention of CDI in high-risk patients after an initial episode or first recurrence of CDI (BII t) or multiple recurrences (AII t ) has become available [109]. In patients still suffering from recurrent CDI after treatment with vancomycin and fidaxomicin, fecal microbiota transfer (FMT) as secondary prophylaxis may be discussed as an intervention of last resort [110–112] (AII t ). While the current basis of evidence does not suggest safety issues specific to cancer patients [111], administration during neutropenia should be avoided, whenever possible (Table 4).
Table 4.
Prevention and treatment of Clostridium difficile infection
| Clinical situation | Intention | Intervention | SoR | QoE | Reference | Comments |
|---|---|---|---|---|---|---|
| Increased risk of CDI during antimicrobial treatment | Primary prevention | Antimicrobial prophylaxis | C | III | [94, 108] | |
| Increased risk of CDI during antimicrobial treatment | Primary prevention | Probiotic prophylaxis | C | IIr,t | [59] | Insufficient data in immunocompromised patients |
| CDI—first episode or first recurrence | Secondary prevention | Bezlotoxumab 10 mg/kg qd iv | B | IIt | [109] | |
| CDI—multiple recurrences | Secondary prevention | Bezlotoxumab 10 mg/kg qd iv | A | IIt | [109] | |
| Fecal microbiota transfer | A | IIt | [110–112] | Only in case of recurrence after treatment with vancomycin and fidaxomicin | ||
| Diarrhea with CDI suspected—non-severe disease | Cure | Empirical therapy | C | IIu | [113] | |
| Diarrhea with CDI suspected -Severe or complicated clinical disease |
Cure | Empirical therapy | B | III | Only if patient instable and high suspicion of CDI | |
| CDI—non-severe | Cure | Vancomycin 125 mg qid po for 10 days or | A | I | [114, 115] | |
| Fidaxomicin 200 mg bid po for 10 days or | ||||||
| Metronidazole 400 mg tid po for 10 days | B | IIt | [116–118] | If metronidazole 400 mg tablets not available, use 375 mg qid po | ||
| CDI—non-severe, oral administration not possible | Cure | Metronidazole 500 mg tid iv for 10 days | A | IIu | [119, 120] | |
| CDI—severe | Cure | Vancomycin 125 mg qid po for 10 days or | A | IIt | [114, 115, 121] | |
| Fidaxomicin 200 mg bid po for 10 days | ||||||
| Metronidazole | D | I | [122] | |||
| CDI—sever and oral administration not possible | Cure | Metronidazole 500 mg tid iv for 10 days | A | IIu | [119, 120] | |
|
plus vancomycin 500 mg intracolonic every 4–12 h and/or vancomycin 500 mg qid by nasogastric tube |
C | III | [123] | |||
| CDI—refractory | Cure | Combination treatment with vancomycin po plus metronidazole any route or | C | IIh | [124, 125] | |
| Teicoplanin 100 mg bid po or | IIu | [126] | ||||
| Tigecyclin 100 mg loading, followed by 50 mg bid for 3-21d or | IIh | [127–129] | ||||
| Fecal microbiota transfer | IIu | [110, 111] | ||||
| CDI—1st recurrence | Cure | Repeat strategy from 1st episode | C | III | [130, 131] | |
| Vancomycin 125 mg qid po for 10 days or | A | IIt | [114, 115] | |||
| Fidaxomicin 200 mg bid po for 10 days or | ||||||
| Vancomycin pulsed/taper strategya | [132–134] | |||||
| CDI—multiple recurrences | Cure | Fidaxomicin 200 mg bid po for 10 days or | A | IIt | [114, 115] | |
| Vancomycin pulsed/taper strategya | [132–134] |
SoR strength of recommendation, QoE quality of evidence; ae.g., vancomycin 125 mg qid po for 7 to 14 days, 125 mg bid po for 7 days, 125 mg qd po for 7 days, 125 mg qd po every other day, 125 mg qd po every 3 days for 14 days
Results from one small, monocentric observational study in a mixed patient population do not suffice to generally recommend empirical therapy in patients with diarrhea and at risk for CDI [113] (CII u). However, in patients with symptoms compatible with CDI and severe or complicated disease, empirical treatment may be considered (BIII). Antiperistaltic agents, including opiates, are discouraged [135] (DII u).
For non-severe CDI, pooled data of all published randomized controlled trials suggests only a trend towards superior cure rates for oral vancomycin as opposed to metronidazole. However, recent findings indicate an increase in isolates resistant to metronidazole as opposed to vancomycin [116–118] (BII t). In two large randomized controlled trials, fidaxomicin met non-inferiority criteria when compared to vancomycin for treatment of non-severe and severe CDI [114, 115] (AI). Metronidazole should not be used in severe cases [122] (DI).
Metronidazole 500 mg tid iv for 10 days is likely to result in effective concentrations in feces and colon and may be an option if oral antimicrobials cannot be administered [119, 120] (AII u).
In severe cases of CDI, additional administration of vancomycin (e.g., 500 mg) by nasogastric tube and/or by rectal catheter may be discussed [123] (CIII). Refractory CDI is defined as lack of clinical response to standard CDI treatment, i.e., vancomycin and fidaxomicin, and should be distinguished from recurrent CDI. For this scenario, no data from randomized controlled trials is available. Therefore, alternatives including combination treatment [124, 125], treatment with teicoplanin [126], tigecycline [127–129], or an FMT [110, 111] cannot be recommended without reservations, due to lack of data (CII). In case of complicated CDI, a surgical evaluation should be obtained at an early stage of disease. However, surgical intervention in the neutropenic and/or thrombocytopenic patient should be reserved to selected complicated cases (BIII). In patients with a first recurrence, previous guidelines have recommended repetition of the initial strategy as the treatment of choice [103]. However, recent findings suggest a key role of the fecal microbiota in the pathophysiology of recurrent CDI [136]. Multiple treatments with standard vancomycin regimens seem to decrease the likelihood of long-term stabilization of these cases, as the diversity of the fecal microbiota is further compromised [130, 137]. In this setting, repetition of the initial strategy does no longer seem warranted (CIII). Alternatively, vancomycin 125 mg qid po for 10 days [114, 115] seems still warranted, if metronidazole was used during the initial episode. Fidaxomicin 200 mg bid po [114, 115] or a vancomycin pulsed/taper [132–134] strategy should be used, if standard vancomycin was used during the initial episode (AII t ).
Other bacterial infections causing diarrhea (non-typhoidal Salmonella, Shigella, Yersinia, and Campylobacter spp.)
In cancer patients, infection-related diarrhea due to non-typhoidal Salmonella, Shigella, Yersinia, or Campylobacter spp. (SSYC) is a rare event (0–2.8%) [4, 138–140]. Clinical signs and symptoms include watery, mucoid, or bloody diarrhea; abdominal tenderness; fever; and nausea. Abdominal pain tends to be particularly severe in Campylobacter enteritis and may mimic appendicitis in Yersinia spp. and Campylobacter spp. infection. Since SSYC are typically community-acquired, testing for these pathogens should be restricted to fecal samples taken within 72 h of hospital admission from symptomatic patients. In case of clinical deterioration, an abdominal ultrasound or x-ray may be performed to detect an ileus or toxic megacolon. A thickened bowel wall may be detected by abdominal ultrasound or CT scan. In this case, the differential diagnosis of NEC should be considered. Perforation rarely occurs in this setting and may be identified by plain abdominal x-ray or abdominal CT scan.
Based on the low incidence of these infectious agents and the possibility of induction of resistance, prophylactic treatment is not recommended [4, 138–141] (DII t,u). While non-severe cases of diarrhea caused by bacteria other than C. difficile may not always require antibiotic treatment, severely ill and/or immunocompromised individuals should receive systemic treatment (BIII). Given the limited data in these populations, treatment recommendations for cancer patients were derived from studies performed in immunocompetent individuals. Immunocompromised patients suffering from non-typhoidal salmonellosis may benefit from therapy with ciprofloxacin. Alternatively, ceftriaxone iv may be administered depending on in vitro susceptibility test results [142] (BIII). In patients with Salmonella spp. bacteremia, treatment with a combination of ceftriaxone plus ciprofloxacin is recommended to avoid initial treatment failure before resistance test results are available and allow de-escalation to a monotherapy [142, 143] (BIII). Two randomized controlled trials on the treatment of shigellosis established ciprofloxacin or another fluoroquinolone as the treatment of choice with azithromycin being an effective alternative [144, 145] (BII t).
For infections with Campylobacter spp., azithromycin has become the drug of choice due to an increase in fluoroquinolone resistance [146] (BII t). Treatment with erythromycin is not considered standard of care, due to its unfavorable toxicity profile. Furthermore, a study performed in a pediatric population showed inferiority of erythromycin to azithromycin [147].
For infections caused by Yersinia spp., treatment with a fluoroquinolone or trimethoprim-sulfamethoxazole is suggested (BIII). For patients with severe disease, the preferred regimen is a third-generation cephalosporin combined with gentamicin [148] (BIII). If feasible, antibiotic treatment in patients with Shigatoxin-producing Escherichia coli should be avoided. However, if an accompanying infection requires treatment, a carbapenem or azithromycin should be preferred, if suitable [149, 150] (CIII). Table 5 summarizes these recommendations.
Table 5.
Treatment of non-typhoidal Salmonella, Shigella, Yersinia, and Campylobacter spp. (SSYC)
| Clinical situation | Intention | Intervention | SoR | QoE | Reference | Comments |
|---|---|---|---|---|---|---|
| Neutropenia or immunosuppression | Prevention | Antimicrobial prophylaxis against Salmonella, Shigella, Yersinia or Campylobacter spp. | D | IIt,u | [4, 138, 140] | |
| Diarrhea caused by non-typhoidal Salmonella spp. | Cure | Ciprofloxacin 500 mg bid po or 400 mg bid iv or | B | III | -Treat only if patient currently immunocompromised or severely ill -Consider local resistance patterns -Treatment duration recommended for immunocompetent patients is 5–7 days and should be extended to 14 days in immunocompromised individuals |
|
| Ceftriaxone 2 g qd iv | ||||||
| Bacteremia caused by non-typhoidal Salmonella spp. | Cure | Ceftriaxone 2 g qd iv plus ciprofloxacin 400 mg bid iv | B | III | Start with combination therapy and de-escalate once resistance data becomes available | |
| Diarrhea caused by Shigella spp. | Cure | Fluoroquinolone, e.g., ciprofloxacin 400 mg bid iv or 500 mg bid po or | B | IIt | [144, 145] | Treatment duration recommended for immunocompetent patients is 3–5 days and may be extended to 5–7 days in immunocompromised individuals |
| Azithromycin 500 mg qd iv/po | ||||||
| Diarrhea caused by Campylobacter spp. | Cure | Azithromycin 500 mg qd iv/po or | A B |
IIt | [145] | -Treat only if patient currently immunocompromised or severely ill -Treatment duration recommended for immunocompetent patients is 3 days and may be extended in immunocompromised individuals -High fluoroquinolone resistance |
| Ciprofloxacin 400 mg bid iv or 500 mg bid po | ||||||
| Diarrhea caused by Yersinia spp. | Cure | Ciprofloxacin 400 mg bid iv or 500 mg bid po or
Trimethoprim–sulfamethoxazole 960/180 mg qd po/iv |
B | III | -Treat only if patient currently immunocompromised or severely ill -Treatment duration recommended for immunocompetent patients is 5–7 days and may be extended in immunocompromised individuals |
|
| Bacteremia caused by Yersinia spp. | Cure | Ceftriaxone 2 g qd iv plus gentamicin 5 mg/kg qd iv | B | III | [148] | -Treat only if patient currently immunocompromised or severely ill -Treatment duration recommended for immunocompetent patients is 7–14 days and may be extended in immunocompromised individuals |
| Diarrhea caused by Shigatoxin producing Escherichia coli | Cure | Carbapenem iv or | C | III | [149, 150] | -Limited data in immunocompromised patients -If possible, restrict to supportive treatment, as antibiotics may be deleterious |
| Azithromycin po |
SoR strength of recommendation, QoE quality of evidence
Viral infections
The most common viral causes of gastroenteritis in cancer patients include norovirus (earlier known as Norwalk-like virus), rotavirus, adenovirus, and cytomegalovirus (CMV). Self-limiting infections with norovirus and rotavirus may affect cancer patients of all risk groups. On the other hand, patients with impaired cellular immunity, e.g., due to a chronic lymphatic malignancy, immunosuppression after allogeneic stem cell transplantation (allo-SCT), treatment with alemtuzumab or other substances compromising T cell function, are at increased risk of developing clinically significant courses of viral gastroenteritis due to CMV or adenovirus, warranting treatment. These infections are unlikely to occur in patients undergoing conventional chemotherapy and those suffering from solid tumors [151–154].
Norovirus is a frequent cause of acute gastroenteritis during the cold season. Transmission occurs by contact with excretions, even in the form of aerosols, and requires only 10–100 viral particles. The incubation period of 12–48 h is typically followed by vomiting, diarrhea, abdominal pain, myalgia, and low fever. Incidence rates of 2.5 and 1.3%, respectively, have been reported from cohorts of neutropenic high-risk and allo-SCT patients presenting with diarrhea, respectively [4, 27, 155]. In the immunocompetent host, the course is self-limiting with symptoms commonly lasting 12–72 h and a mean duration of viral shedding of 4 weeks after onset of illness. In the immunocompromised patient, duration and intensity of clinical signs and symptoms and of viral shedding may be considerably prolonged [156, 157].
Real-time PCR (sensitivity 94%, specificity 92%) is currently considered the gold standard for the detection and typing of norovirus; numerous conventional and real-time norovirus RT-PCR assays have been developed [158, 159]. A number of EIAs are commercially available for the detection of norovirus antigens in stool specimens. EIA is a method for outbreak investigations, particularly in laboratories that lack molecular diagnostic capabilities [152].
A considerably high mortality rate of up to 25% has been attributed to norovirus gastroenteritis in allo-SCT patients [160]. No specific treatment options are currently available; therapeutic management is supportive.
Rotavirus infections are a far less common cause of gastroenteritis in the adult immunocompromised patient. A 3-day course of nitazoxanide significantly reduces the duration of rotavirus disease in immunocompetent pediatric patients [161, 162]. As this therapy has not been assessed in immunocompromised patients and only in very few adult patients [163], further studies are required before a recommendation can be made (CII t). In two patients, oral immunoglobulin has been successfully used to treat rotavirus gastroenteritis [164] (CIII).
Adenovirus is typically associated with gastroenteritis in newborns and children as well as with keratoconjunctivitis epidemica and acute respiratory distress syndrome. In patients with impaired cellular immunity, life-threatening courses of adenovirus disease have been reported [165, 166].
Cidofovir is approved for severe adenovirus infections, even though only limited data from case reports and small series is available. Low-dose cidofovir (1 mg/kg thrice a week) was effective in one adult patient [165], and in a report from a pediatric hematology unit with an adenovirus outbreak, seven patients were successfully treated with cidofovir 5 mg/kg iv once weekly for 2 weeks, then once every other week [167].
Treatment with cidofovir may therefore be discussed in severely ill patients with adenovirus-associated diarrhea (BII u); however, its considerable nephrotoxicity should be taken into account.
CMV is found in blood and excretions of individuals with profound and long-lasting cellular immunosuppression and is a rare cause of gastrointestinal infections in other patient groups [168–171].
Patients may present with nausea, vomiting, bloody or non-bloody diarrhea, fever, abdominal pain, and prolonged anorexia [172]. CMV infection (viremia) is diagnosed by detection of antigen (pp65; antigenemia assay), DNA, or mRNA. Quantification of viral load by PCR is also widely available [173]. However, for diagnosis of CMV disease with organ involvement, such as enteritis, detection of CMV in peripheral blood is not appropriate and may be negative. Similarly, CMV detection in fecal samples does not suffice to establish a diagnosis [174]. In addition, CMV detection in an endoscopically obtained biopsy specimen from suspicious areas in the esophagus, stomach, small bowel, or large intestine is needed [175, 176]. The diagnosis is made by the association of CMV disease with specific mucosa pathology and appropriate symptoms [177].
Recommendations on CMV prophylaxis and pre-emptive treatment are given in the updated European Conference on Infections in Leukemia (ECIL) recommendations on the management of CMV, HHV-6, HHV-7, and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies or those after SCT [178, 179].
We recommend treating gastrointestinal CMV disease with ganciclovir for 2–3 weeks with induction dosing of 5 mg/kg bid iv, followed by several weeks of maintenance therapy at a dose of 5 mg/kg qd iv on 5–6 days per week. The prolonged treatment interval is intended to cover the period of mucosal re-epithelialization [172] (AI). The addition of intravenous immunoglobulins to antiviral therapy may be considered [180–182] (CII u). Concerning antiviral treatment alternatives, the administration of foscarnet [183] (BII t), cidofovir [184–186] (BII u), or the combination of foscarnet and ganciclovir may be considered [187–189] (BII t). Both substances, foscarnet and cidofovir, are associated with significant renal toxicity. Recommendations on the treatment of viral gastroenteritis are summarized in Table 6.
Table 6.
Treatment of viral gastroenteritis
| Clinical situation | Intention | Intervention | SoR | QoE | Reference | Comments |
|---|---|---|---|---|---|---|
| Rotavirus enteritis | Cure | Nitazoxanide 7.5 mg/kg bid po | C | IIt | [161, 162] | Mainly assessed in immunocompetent pediatric patients |
| Oral immunoglobulin | C | III | [164] | No sufficient evidence to recommend dosage | ||
| Adenovirus enteritis | Cure | Cidofovir 5 mg/kg iv once weekly for 2 weeks, then once every other week | B | IIu | [165, 167] | To reduce cidofovir toxicity, add at least 2 L of iv prehydration and probenecid 2 g po 3 h prior and 1 g 2 and 8 h following cidofovir |
| CMV enteritis | Cure | Ganciclovir 5 mg/kg bid iv for 2–3 weeks followed by several weeks of 5 mg/kg qd iv on 5 days per week | A | I | [172] | |
| Foscarnet 90 mg/kg bid iv over 2 h or 60 mg/kg tid iv over 1 h or | B | IIt | [183] | Used in a pre-emptive setting | ||
| Cidofovir 5 mg/kg iv once weekly for 2 weeks, then once every other week or | B | IIu | [184–186] | To reduce cidofovir toxicity, add at least 2 L of iv prehydration and probenecid 2 g po 3 h prior and 1 g 2 and 8 h following cidofovir | ||
| Foscarnet 90 mg/kg bid iv over 2 h or 60 mg/kg tid iv over 1 h plus ganciclovir 5 mg/kg bid iv for 2–3 weeks followed by several weeks of 5 mg/kg qd iv on 5 days per week | B | IIt | [187–189] | Alternatively, the dosage of both combination partners may be reduced by 50% | ||
| Addition of iv immunoglobulin | C | IIu | [180–182] | No sufficient evidence to recommend dosage |
SoR strength of recommendation, QoE quality of evidence
Parasitic infections
Given extensive travels, and growing populations of migrants, rising incidence rates of gastrointestinal infections with parasites are to be expected. In cancer patients with persistent diarrhea in spite of a complete work-up for bacterial and viral pathogens, examination of stools for parasites may therefore be warranted. Potentially causative pathogens include Blastocystis spp., Cryptosporidium, Cyclospora cayetanensis, Entamoeba histolytica, Giardia lamblia, Isospora belli, Sarcocystis hominis, S. suihominis, and Strongyloides stercoralis. For a diagnostic work-up, three fresh stool samples should be analyzed at a sufficiently qualified laboratory. Treatment options have not been specifically assessed in immunocompromised cancer patients and were extrapolated from studies in other patient populations. While many randomized controlled trials were performed in this area of research, most of them are of limited size or quality.
Based on results from a randomized controlled trial, metronidazole has been established as the treatment of choice for Blastocystis spp. infection [190] (AII t). Alternatives, the majority of them supported by only limited clinical evidence, include nitazoxanide, trimethoprim–sulfamethoxazole, tinidazole, and paromomycin [191–193] (BII t).
Nitazoxanide is the only FDA-registered drug for the treatment of Cryptosporidium spp. infections. Studies leading to registration were performed mainly in HIV-infected patients and children [194–197]. A meta-analysis performed for immunocompromised HIV and non-HIV patients confirmed improved oocyte clearance, but did not confirm any impact of nitazoxanide treatment on resolution of diarrhea. However, the subpopulation of HIV seronegative patients experienced a significant clinical benefit [198]. Similarly, data from a compassionate use program in which nitazoxanide was administered over variable periods of time to patients with HIV-related cryptosporidiosis does suggest a considerable clinical benefit [199] (BII r,t). As a non-registered alternative, paromomycin was assessed in the same meta-analysis, but no advantages over placebo treatment could be confirmed. However, all patients included into the analysis were suffering from advanced HIV, such that the efficacy in patients immunocompromised for other reasons is not evaluable [198]. In immunocompetent individuals, however, improvement in 60–70% of patients has been documented [197, 200] (CII r,t).
Intestinal E. histolytica amebiasis without dysentery (blood, mucus in stools) may be treated with paromomycin, an intraluminal agent, alone [201] (BII t). Patients with E. histolytica colitis should be treated with tinidazole (AII r,t) or metronidazole (BII r,t), followed by an intraluminal agent, e.g., paromomycin, diiodohydroxyquin, or diloxanide furoate (BIII), intended to eliminate intraluminal cysts [202].
Several randomized controlled trials support the use of 5-nitroimidazoles (metronidazole or tinidazole) [203, 204] or benzimidazoles (albendazole or mebendazole) [204] for the treatment of Giardia spp. infections (AII r,t). In case of resistance, nitazoxanide may be a suitable alternative [205] (BII t).
A small randomized trial in HIV-infected patients showed efficient treatment of Isospora belli or Cyclospora spp. infections by use of trimethoprim-sufamethoxazole (AII t) and alternatively oral ciprofloxacin [206, 207] (BII t).
A small number of patients with Isospora belli infection have also been treated successfully with nitazoxanide, but there is not sufficient evidence to recommend use [208] (CIII).
According to a recent Cochrane analysis, ivermectin (AII r,t) is superior to albendazole (BII r,t) in the treatment of infection with Strongyloides stercoralis [209].
Gastrointestinal disease with Sarcocystis hominis and S. suihominis is usually self-limiting and does therefore not require any treatment apart from supportive care. Table 7 summarizes all recommendations on the treatment of parasitic infections.
Table 7.
Treatment of parasitic diarrhea/colitis
| Clinical situation | Intention | Intervention | SoR | QoE | References | Comment |
|---|---|---|---|---|---|---|
| Blastocystis spp. Infection | Cure | Metronidazole 500 mg tid po for 7 days | A | IIt | [190] | Treatment of colonization without compatible signs and symptoms not recommended |
| Alternatives: nitazoxanide, trimethoprim–sulfamethoxazole, tinidazole, paromomycin | B | IIt | [191–193] | |||
| Cryptosporidium spp. Infection | Cure | Nitazoxanide 500 mg bid po for 3 days or | B | IIr,t | [194–199] | Higher dosages (e.g., 1000 mg bid for up to 14 days) might be required in severely immunocompromised patients |
| Paromomycin 25 to 35 mg/kg/day po in 2 to 4 divided doses for 10 to 14 days | C | IIr,t | [197, 198, 200] | |||
| Cyclospora spp. Infection | Cure | Trimethoprim–sulfamethoxazole 160/800 mg bid po for 7 days or | A | IIt | [206, 210, 211] | |
| Ciprofloxacin 500 mg bid po for 7 days | B | IIt | ||||
| Entamoeba histolytica infection, non-invasive | Cure | Paromomycin 30 mg/kg qd po in three divided doses for 7 days | B | IIt | [201] | |
| Entamoeba histolytica, invasive colitis | Cure | Tinidazole 2 g qd po for 3 days or | A | IIr,t | [202] | |
| Metronidazole 500 mg tid po for 10 days followed by: | B | IIr,t | [202] | |||
| Paromomycin 25–30 mg/kg qd po in three divided doses for 7 days or | B | III | [202] | |||
| Diiodohydroxyquin 650 mg tid po for 20 days or | ||||||
| Diloxanide furoate 500 mg tid po for 10 days | ||||||
| Giardia spp. Infection | Cure | Metronidazole 250 mg tid po for 5–10 days or | A | IIr,t | [203, 204] | |
| Tinidazole 2 g po as a single dose | ||||||
| Albendazole 400 mg qd po for 5–10 days or | A | IIr,t | [204] | |||
| Mebendazole 200 mg tid po for 3–7 days | ||||||
| Nitazoxanide 500 mg bid for 3 days | B | IIt | [205] | Higher dosages (e.g., 1000 mg bid for up to 14 days) might be required in severely immunocompromised patients | ||
| Isospora belli infection | Cure | Trimethoprim–sulfamethoxazole 160/800 mg bid po for 7 days or | A | IIt | [206, 207] | |
| Ciprofloxacin 500 mg bid po for 7 days or | B | IIt | [206] | |||
| Nitazoxanide 500 mg bid po for 7 days | C | III | [208] | |||
| Strongyloides stercoralis infection | Cure | Ivermectin 200 μg/kg qd po for 2 days | A | IIr,t | [209] | |
| Albendazole 400 mg bid po for 3–10 days | B | IIr,t |
Acknowledgments
We would like to thank Angelina Lagodny, the team assistant at the Clinical Trials Unit Infectious Diseases II at the University Hospital Cologne, for her reliable and timely assistance during the literature search.
Funding
Consensus meetings held in the context of this guideline were funded by the AGIHO.
Compliance with ethical standards
No patient data was collected or animal experiments conducted to complete this work.
Conflict of interest
DB is a consultant to Gilead Sciences and Merck Sharp & Dohme/Merck, received research grants from Gilead Sciences and Pfizer, honoraria for lectures from Astellas, Gilead Sciences, Merck Sharp & Dohme/Merck, Pfizer, and TEVA and travel grants from Astellas, Merck Sharp & Dohme/Merck, and Pfizer.
OAC is supported by the German Federal Ministry of Research and Education (BMBF grant 01KN0706), has received research grants from Astellas Pharma, Bayer, Basilea, Genzyme, Gilead Sciences, Pfizer, Merck, Optimer, Schering-Plough, Vicuron, has worked as a consultant for Astellas Pharma, Basilea, F2G, Gilead Sciences, Pfizer, Merck, Mölnlycke Health Care, Nektar Schering-Plough, Zeneus, and served at the speakers’ bureau of Schering-Plough, Astellas Pharma, Gilead Sciences, Merck, Pfizer, SpePharm and United Medical.
MH has served at the speakers` bureau of MSD, Roche, Novartis, Gilead Sciences, Boehringer Ingelheim, has received travels grants from MSD, Roche, Novartis, Gilead Sciences, Pfizer, Janssen-Cilag, and has served as a consultant for Takeda Pharma.
GM has served as a consultant for Gilead Sciences, MSD, Pfizer, Essex (Schering-Plough), Novartis and Sanofi-Aventis and has been on the Speakers’ Bureau for Gilead Sciences, MSD, Pfizer and Cephalon.
ES has received travel grants from Essex/Schering-Plough, Gilead, Janssen-Cilag, Merck/MSD, Novartis, Pfizer, Roche and Shire.
JV has received grants from Schering-Plough and Astellas Pharma, worked as a consultant for Pfizer and Schering-Plough, and served at the speakers’ bureau of Astellas Pharma, Gilead Sciences, Schering-Plough, Merck and Pfizer.
MJGTV is a consultant to: Berlin Chemie, MSD/Merck and Astellas Pharma; has served at the speakers’ bureau of: Astellas Pharma, Basilea, Gilead Sciences, Merck/MSD, Organobalance and Pfizer; received research funding from: 3M, Astellas Pharma, DaVolterra, Gilead Sciences, Merck/MSD, Organobalance, and Seres Therapeutics.
All remaining authors have declared no conflicts of interest.
References
- 1.Vehreschild MJ, Vehreschild JJ, Hubel K, Hentrich M, Schmidt-Hieber M, Christopeit M, et al. Diagnosis and management of gastrointestinal complications in adult cancer patients: evidence-based guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Oncol. 2013;24:1189–1202. doi: 10.1093/annonc/mdt001. [DOI] [PubMed] [Google Scholar]
- 2.Cornely OA, Cuenca-Estrella M, Meis JF, Ullmann AJ. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study Group (EFISG) and European Confederation of Medical Mycology (ECMM) 2013 joint guidelines on diagnosis and management of rare and emerging fungal diseases. Clin Microbiol Infect. 2014;20(Suppl 3):1–4. doi: 10.1111/1469-0691.12569. [DOI] [PubMed] [Google Scholar]
- 3.Einhorn LH, Rapoport B, Navari RM, Herrstedt J, Brames MJ. 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following multiple-day chemotherapy, high-dose chemotherapy, and breakthrough nausea and vomiting. Support Care Cancer. 2017;25:303–308. doi: 10.1007/s00520-016-3449-y. [DOI] [PubMed] [Google Scholar]
- 4.Kamboj M, Mihu CN, Sepkowitz K, Kernan NA, Papanicolaou GA. Work-up for infectious diarrhea after allogeneic hematopoietic stem cell transplantation: single specimen testing results in cost savings without compromising diagnostic yield. Transpl Infect Dis. 2007;9:265–269. doi: 10.1111/j.1399-3062.2007.00230.x. [DOI] [PubMed] [Google Scholar]
- 5.Crobach MJ, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2016;22(Suppl 4):S63–S81. doi: 10.1016/j.cmi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Nasir A, Gardner NM, Strosberg J, Ahmad N, Choi J, Malafa MP, et al. Multimodality management of a polyfunctional pancreatic endocrine carcinoma with markedly elevated serum vasoactive intestinal polypeptide and calcitonin levels. Pancreas. 2008;36:309–313. doi: 10.1097/MPA.0b013e31815b321c. [DOI] [PubMed] [Google Scholar]
- 7.Udenfriend S, Weissbach H, Sjoerdsma A. Studies on tryptophan and serotonin in patients with malignant carcinoid. Science. 1956;123:669. doi: 10.1126/science.123.3199.669. [DOI] [PubMed] [Google Scholar]
- 8.Strohm WD. Paraneoplastic spastic tetraparesis in glucagonoma syndrome. Successful therapy with octreotide, dacarbazine and interferon-alpha. Z Gastroenterol. 1996;34:438–445. [PubMed] [Google Scholar]
- 9.Saban J, Boixeda D, Moreno A, Barcena R, Serrano-Rios M. Long survival of diarrhea-associated hepatocarcinoma treated with Adriamycin and indomethacin. A case report. Am J Clin Pathol. 1986;86:241–247. doi: 10.1093/ajcp/86.2.241. [DOI] [PubMed] [Google Scholar]
- 10.Domen RE, Shaffer MB, Jr, Finke J, Sterin WK, Hurst CB. The glucagonoma syndrome. Report of a case. Arch Intern Med. 1980;140:262–263. doi: 10.1001/archinte.1980.00330140120030. [DOI] [PubMed] [Google Scholar]
- 11.Interlandi JW, Hundley RF, Kasselberg AG, Orth DN, Salmon WD, Jr, Sullivan JN. Hypercortisolism, diarrhea with steatorrhea, and massive proteinuria due to pheochromocytoma. South Med J. 1985;78:879–883. doi: 10.1097/00007611-198507000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Steiner E, Velt P, Gutierrez O, Schwartz S, Chey W. Hepatocellular carcinoma presenting with intractable diarrhea. A radiologic-pathologic correlation. Arch Surg. 1986;121:849–851. doi: 10.1001/archsurg.1986.01400070119025. [DOI] [PubMed] [Google Scholar]
- 13.Winkler AS, Dean A, Hu M, Gregson N, Chaudhuri KR. Phenotypic and neuropathologic heterogeneity of anti-Hu antibody-related paraneoplastic syndrome presenting with progressive dysautonomia: report of two cases. Clin Auton Res. 2001;11:115–118. doi: 10.1007/BF02322055. [DOI] [PubMed] [Google Scholar]
- 14.Ge F, Li ZJ, Cao ZL. Thymoma associated with severe diarrhoea and anaemia. Chin Med J (Engl) 2006;119:526–528. [PubMed] [Google Scholar]
- 15.Sleijfer S, Kaptein A, Versteegh MI, Hegt VN, Snels DG, van Tilburg AJ. Full-blown graft-versus-host disease presenting with skin manifestations, jaundice and diarrhoea: an unusual paraneoplastic phenomenon of a thymoma. Eur J Gastroenterol Hepatol. 2003;15:565–569. doi: 10.1097/01.meg.0000059131.68845.65. [DOI] [PubMed] [Google Scholar]
- 16.O'Toole D, Kianmanesh R, Caplin M. ENETS 2016 consensus guidelines for the management of patients with digestive neuroendocrine tumors: an update. Neuroendocrinology. 2016;103:117–118. doi: 10.1159/000443169. [DOI] [PubMed] [Google Scholar]
- 17.Aksoy DY, Tanriover MD, Uzun O, Zarakolu P, Ercis S, Erguven S, et al. Diarrhea in neutropenic patients: a prospective cohort study with emphasis on neutropenic enterocolitis. Ann Oncol. 2007;18:183–189. doi: 10.1093/annonc/mdl337. [DOI] [PubMed] [Google Scholar]
- 18.Delaunoit T, Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Findlay BP, et al. Mortality associated with daily bolus 5-fluorouracil/leucovorin administered in combination with either irinotecan or oxaliplatin: results from Intergroup Trial N9741. Cancer. 2004;101:2170–2176. doi: 10.1002/cncr.20594. [DOI] [PubMed] [Google Scholar]
- 19.Kuebler JP, Colangelo L, O'Connell MJ, Smith RE, Yothers G, Begovic M, et al. Severe enteropathy among patients with stage II/III colon cancer treated on a randomized trial of bolus 5-fluorouracil/leucovorin plus or minus oxaliplatin: a prospective analysis. Cancer. 2007;110:1945–1950. doi: 10.1002/cncr.23013. [DOI] [PubMed] [Google Scholar]
- 20.Sloan JA, Goldberg RM, Sargent DJ, Vargas-Chanes D, Nair S, Cha SS, et al. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol. 2002;20:1491–1498. doi: 10.1200/JCO.2002.20.6.1491. [DOI] [PubMed] [Google Scholar]
- 21.Abigerges D, Chabot GG, Armand JP, Herait P, Gouyette A, Gandia D. Phase I and pharmacologic studies of the camptothecin analog irinotecan administered every 3 weeks in cancer patients. J Clin Oncol. 1995;13:210–221. doi: 10.1200/JCO.1995.13.1.210. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22:3238–3247. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 23.Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 24.Lenfers BH, Loeffler TM, Droege CM, Hausamen TU. Substantial activity of budesonide in patients with irinotecan (CPT-11) and 5-fluorouracil induced diarrhea and failure of loperamide treatment. Ann Oncol. 1999;10:1251–1253. doi: 10.1023/A:1008390308416. [DOI] [PubMed] [Google Scholar]
- 25.Saliba F, Hagipantelli R, Misset JL, Bastian G, Vassal G, Bonnay M, et al. Pathophysiology and therapy of irinotecan-induced delayed-onset diarrhea in patients with advanced colorectal cancer: a prospective assessment. J Clin Oncol. 1998;16:2745–2751. doi: 10.1200/JCO.1998.16.8.2745. [DOI] [PubMed] [Google Scholar]
- 26.Avery R, Pohlman B, Adal K, Bolwell B, Goldman M, Kalaycio M, et al. High prevalence of diarrhea but infrequency of documented Clostridium difficile in autologous peripheral blood progenitor cell transplant recipients. Bone Marrow Transplant. 2000;25:67–69. doi: 10.1038/sj.bmt.1702086. [DOI] [PubMed] [Google Scholar]
- 27.Vehreschild MJ, Meissner AM, Cornely OA, Maschmeyer G, Neumann S, von Lilienfeld-Toal M, et al. Clinically defined chemotherapy-associated bowel syndrome predicts severe complications and death in cancer patients. Haematologica. 2011;96:1855–1860. doi: 10.3324/haematol.2011.049627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorschluter M, Glasmacher A, Hahn C, Schakowski F, Ziske C, Molitor E, et al. Clostridium difficile infection in patients with neutropenia. Clin Infect Dis. 2001;33:786–791. doi: 10.1086/322616. [DOI] [PubMed] [Google Scholar]
- 29.Wistrom J, Norrby SR, Myhre EB, Eriksson S, Granstrom G, Lagergren L, et al. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. J Antimicrob Chemother. 2001;47:43–50. doi: 10.1093/jac/47.1.43. [DOI] [PubMed] [Google Scholar]
- 30.Owens RC, Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S19–S31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 31.McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3:563–578. doi: 10.2217/17460913.3.5.563. [DOI] [PubMed] [Google Scholar]
- 32.Hickson M, D'Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plummer S, Weaver MA, Harris JC, Dee P, Hunter J. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol. 2004;7:59–62. [PubMed] [Google Scholar]
- 34.Parnes HL, Fung E, Schiffer CA. Chemotherapy-induced lactose intolerance in adults. Cancer. 1994;74:1629–1633. doi: 10.1002/1097-0142(19940901)74:5<1629::AID-CNCR2820740523>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Osterlund P, Ruotsalainen T, Peuhkuri K, Korpela R, Ollus A, Ikonen M, et al. Lactose intolerance associated with adjuvant 5-fluorouracil-based chemotherapy for colorectal cancer. Clin Gastroenterol Hepatol. 2004;2:696–703. doi: 10.1016/S1542-3565(04)00293-9. [DOI] [PubMed] [Google Scholar]
- 36.Benson AB, 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA, Jr, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004;22:2918–2926. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 37.Rothenberg ML, Eckardt JR, Kuhn JG, Burris HA, 3rd, Nelson J, Hilsenbeck SG, et al. Phase II trial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer. J Clin Oncol. 1996;14:1128–1135. doi: 10.1200/JCO.1996.14.4.1128. [DOI] [PubMed] [Google Scholar]
- 38.Abigerges D, Armand JP, Chabot GG, Da Costa L, Fadel E, Cote C, et al. Irinotecan (CPT-11) high-dose escalation using intensive high-dose loperamide to control diarrhea. J Natl Cancer Inst. 1994;86:446–449. doi: 10.1093/jnci/86.6.446. [DOI] [PubMed] [Google Scholar]
- 39.Rougier P, Bugat R, Douillard JY, Culine S, Suc E, Brunet P, et al. Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol. 1997;15:251–260. doi: 10.1200/JCO.1997.15.1.251. [DOI] [PubMed] [Google Scholar]
- 40.Wadler S, Haynes H, Wiernik PH. Phase I trial of the somatostatin analog octreotide acetate in the treatment of fluoropyrimidine-induced diarrhea. J Clin Oncol. 1995;13:222–226. doi: 10.1200/JCO.1995.13.1.222. [DOI] [PubMed] [Google Scholar]
- 41.Barbounis V, Koumakis G, Vassilomanolakis M, Demiri M, Efremidis AP. Control of irinotecan-induced diarrhea by octreotide after loperamide failure. Support Care Cancer. 2001;9:258–260. doi: 10.1007/s005200000220. [DOI] [PubMed] [Google Scholar]
- 42.Gebbia V, Carreca I, Testa A, Valenza R, Curto G, Cannata G, et al. Subcutaneous octreotide versus oral loperamide in the treatment of diarrhea following chemotherapy. Anti-Cancer Drugs. 1993;4:443–445. doi: 10.1097/00001813-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Kornblau S, Benson AB, Catalano R, Champlin RE, Engelking C, Field M, et al. Management of cancer treatment-related diarrhea. Issues and therapeutic strategies. J Pain Symptom Manag. 2000;19:118–129. doi: 10.1016/S0885-3924(99)00149-9. [DOI] [PubMed] [Google Scholar]
- 44.Goumas P, Naxakis S, Christopoulou A, Chrysanthopoulos C, Nikolopoulou VV, Kalofonos HP. Octreotide acetate in the treatment of fluorouracil-induced diarrhea. Oncologist. 1998;3:50–53. [PubMed] [Google Scholar]
- 45.Murphy J, Stacey D, Crook J, Thompson B, Panetta D. Testing control of radiation-induced diarrhea with a psyllium bulking agent: a pilot study. Can Oncol Nurs J. 2000;10:96–100. doi: 10.5737/1181912x10396100. [DOI] [PubMed] [Google Scholar]
- 46.Qvitzau S, Matzen P, Madsen P. Treatment of chronic diarrhoea: loperamide versus ispaghula husk and calcium. Scand J Gastroenterol. 1988;23:1237–1240. doi: 10.3109/00365528809090197. [DOI] [PubMed] [Google Scholar]
- 47.Hoff PM, Saragiotto DF, Barrios CH, del Giglio A, Coutinho AK, Andrade AC, et al. Randomized phase III trial exploring the use of long-acting release octreotide in the prevention of chemotherapy-induced diarrhea in patients with colorectal cancer: the LARCID trial. J Clin Oncol. 2014;32:1006–1011. doi: 10.1200/JCO.2013.50.8077. [DOI] [PubMed] [Google Scholar]
- 48.Zachariah B, Gwede CK, James J, Ajani J, Chin LJ, Donath D, et al. Octreotide acetate in prevention of chemoradiation-induced diarrhea in anorectal cancer: randomized RTOG trial 0315. J Natl Cancer Inst. 2010;102:547–556. doi: 10.1093/jnci/djq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun J, Wang H, Hu H. Glutamine for chemotherapy induced diarrhea: a meta-analysis. Asia Pac J Clin Nutr. 2012;21:380–385. [PubMed] [Google Scholar]
- 50.Rotovnik Kozjek N, Kompan L, Soeters P, Oblak I, Mlakar Mastnak D, Mozina B, et al. Oral glutamine supplementation during preoperative radiochemotherapy in patients with rectal cancer: a randomised double blinded, placebo controlled pilot study. Clin Nutr. 2011;30:567–570. doi: 10.1016/j.clnu.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Perez D, Sharples KJ, Broom R, Jeffery M, Proctor J, Hinder V, et al. A randomised phase IIb trial to assess the efficacy of ReCharge ice cream in preventing chemotherapy-induced diarrhoea. Support Care Cancer. 2015;23:3307–3315. doi: 10.1007/s00520-015-2755-0. [DOI] [PubMed] [Google Scholar]
- 52.Karthaus M, Ballo H, Abenhardt W, Steinmetz T, Geer T, Schimke J, et al. Prospective, double-blind, placebo-controlled, multicenter, randomized phase III study with orally administered budesonide for prevention of irinotecan (CPT-11)-induced diarrhea in patients with advanced colorectal cancer. Oncology. 2005;68:326–332. doi: 10.1159/000086971. [DOI] [PubMed] [Google Scholar]
- 53.de Jong FA, Kehrer DF, Mathijssen RH, Creemers GJ, de Bruijn P, van Schaik RH, et al. Prophylaxis of irinotecan-induced diarrhea with neomycin and potential role for UGT1A1*28 genotype screening: a double-blind, randomized, placebo-controlled study. Oncologist. 2006;11:944–954. doi: 10.1634/theoncologist.11-8-944. [DOI] [PubMed] [Google Scholar]
- 54.Kee BK, Morris JS, Slack RS, Crocenzi T, Wong L, Esparaz B, et al. A phase II, randomized, double blind trial of calcium aluminosilicate clay versus placebo for the prevention of diarrhea in patients with metastatic colorectal cancer treated with irinotecan. Support Care Cancer. 2015;23:661–670. doi: 10.1007/s00520-014-2402-1. [DOI] [PubMed] [Google Scholar]
- 55.Namikawa T, Munekage E, Maeda H, Kitagawa H, Kobayashi M, Hanazaki K. Feasibility study of supportive care using lafutidine, a histamine H2 receptor antagonist, to prevent gastrointestinal toxicity during chemotherapy for gastric cancer. Anticancer Res. 2014;34:7297–7301. [PubMed] [Google Scholar]
- 56.Coutre SE, Barrientos JC, Brown JR, de Vos S, Furman RR, Keating MJ, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56:2779–2786. doi: 10.3109/10428194.2015.1022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Surawicz CM. Role of probiotics in antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, and recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2008;42(Suppl 2):S64–S70. doi: 10.1097/MCG.0b013e3181646d09. [DOI] [PubMed] [Google Scholar]
- 58.Doron SI, Hibberd PL, Gorbach SL. Probiotics for prevention of antibiotic-associated diarrhea. J Clin Gastroenterol. 2008;42(Suppl 2):S58–S63. doi: 10.1097/MCG.0b013e3181618ab7. [DOI] [PubMed] [Google Scholar]
- 59.Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919–1929. doi: 10.1093/annonc/mdu106. [DOI] [PubMed] [Google Scholar]
- 60.Ejemot RI, Ehiri JE, Meremikwu MM, Critchley JA (2008) Hand washing for preventing diarrhoea. Cochrane Database Syst Rev. CD004265 [DOI] [PMC free article] [PubMed]
- 61.Mortimer EA, Jr, Lipsitz PJ, Wolinsky E, Gonzaga AJ, Rammelkamp CH., Jr Transmission of staphylococci between newborns. Importance of the hands to personnel. Am J Dis Child. 1962;104:289–295. doi: 10.1001/archpedi.1962.02080030291012. [DOI] [PubMed] [Google Scholar]
- 62.Semmelweis IP. Die Aetiologie, der Begriff und die Prophylaxis des Kindbettfiebers. CA Hartleben’s Verlags-Expedition: Pest, Wien und Leipzig; 1861. [Google Scholar]
- 63.Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23:S3–40. doi: 10.1086/503164. [DOI] [PubMed] [Google Scholar]
- 64.Picardi M, Selleri C, Camera A, Catalano L, Rotoli B. Early detection by ultrasound scan of severe post-chemotherapy gut complications in patients with acute leukemia. Haematologica. 1999;84:222–225. [PubMed] [Google Scholar]
- 65.Cartoni C, Dragoni F, Micozzi A, Pescarmona E, Mecarocci S, Chirletti P, et al. Neutropenic enterocolitis in patients with acute leukemia: prognostic significance of bowel wall thickening detected by ultrasonography. J Clin Oncol. 2001;19:756–761. doi: 10.1200/JCO.2001.19.3.756. [DOI] [PubMed] [Google Scholar]
- 66.Gorschluter M, Marklein G, Hofling K, Clarenbach R, Baumgartner S, Hahn C, et al. Abdominal infections in patients with acute leukaemia: a prospective study applying ultrasonography and microbiology. Br J Haematol. 2002;117:351–358. doi: 10.1046/j.1365-2141.2002.03434.x. [DOI] [PubMed] [Google Scholar]
- 67.Gorschluter M, Mey U, Strehl J, Ziske C, Schepke M, Schmidt-Wolf IG, et al. Neutropenic enterocolitis in adults: systematic analysis of evidence quality. Eur J Haematol. 2005;75:1–13. doi: 10.1111/j.1600-0609.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 68.Gorschluter M, Mey U, Strehl J, Schmitz V, Rabe C, Pauls K, et al. Invasive fungal infections in neutropenic enterocolitis: a systematic analysis of pathogens, incidence, treatment and mortality in adult patients. BMC Infect Dis. 2006;6:35. doi: 10.1186/1471-2334-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sachak T, Arnold MA, Naini BV, Graham RP, Shah SS, Cruise M, et al. Neutropenic enterocolitis: new insights into a deadly entity. Am J Surg Pathol. 2015;39:1635–1642. doi: 10.1097/PAS.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 70.Gomez L, Martino R, Rolston KV. Neutropenic enterocolitis: spectrum of the disease and comparison of definite and possible cases. Clin Infect Dis. 1998;27:695–699. doi: 10.1086/514946. [DOI] [PubMed] [Google Scholar]
- 71.Vlasveld LT, Zwaan FE, Fibbe WE, Tjon RT, Tham TA, Kluin PM, et al. Neutropenic enterocolitis following treatment with cytosine arabinoside-containing regimens for hematological malignancies: a potentiating role for amsacrine. Ann Hematol. 1991;62:129–134. doi: 10.1007/BF01702926. [DOI] [PubMed] [Google Scholar]
- 72.Cunningham SC, Fakhry K, Bass BL, Napolitano LM. Neutropenic enterocolitis in adults: case series and review of the literature. Dig Dis Sci. 2005;50:215–220. doi: 10.1007/s10620-005-1585-1. [DOI] [PubMed] [Google Scholar]
- 73.Oehadian A, Fadjari TH. Neutropenic enterocolitis in breast cancer patient after taxane-containing chemotherapy. Acta Med Indones. 2008;40:29–33. [PubMed] [Google Scholar]
- 74.Kouroussis C, Samonis G, Androulakis N, Souglakos J, Voloudaki A, Dimopoulos MA, et al. Successful conservative treatment of neutropenic enterocolitis complicating taxane-based chemotherapy: a report of five cases. Am J Clin Oncol. 2000;23:309–313. doi: 10.1097/00000421-200006000-00021. [DOI] [PubMed] [Google Scholar]
- 75.Geisler JP, Schraith DF, Manahan KJ, Sorosky JI. Gemcitabine associated vasculitis leading to necrotizing enterocolitis and death in women undergoing primary treatment for epithelial ovarian/peritoneal cancer. Gynecol Oncol. 2004;92:705–707. doi: 10.1016/j.ygyno.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 76.Gadducci A, Gargini A, Palla E, Fanucchi A, Genazzani AR. Neutropenic enterocolitis in an advanced epithelial ovarian cancer patient treated with paclitaxel/platinum-based chemotherapy: a case report and review of the literature. Anticancer Res. 2005;25:2509–2513. [PubMed] [Google Scholar]
- 77.Ferrazzi E, Toso S, Zanotti M, Giuliano G. Typhlitis (neutropenic enterocolitis) after a single dose of vinorelbine. Cancer Chemother Pharmacol. 2001;47:277–279. doi: 10.1007/s002800000218. [DOI] [PubMed] [Google Scholar]
- 78.Kronawitter U, Kemeny NE, Blumgart L. Neutropenic enterocolitis in a patient with colorectal carcinoma: unusual course after treatment with 5-fluorouracil and leucovorin—a case report. Cancer. 1997;80:656–660. doi: 10.1002/(SICI)1097-0142(19970815)80:4<656::AID-CNCR2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 79.Hayes D, Jr, Leonardo JM. Neutropenic enterocolitis in a woman treated with 5-fluorouracil and leucovorin for colon carcinoma. N C Med J. 2002;63:132–134. [PubMed] [Google Scholar]
- 80.Blijlevens NM, Donnelly JP, De Pauw BE. Mucosal barrier injury: biology, pathology, clinical counterparts and consequences of intensive treatment for haematological malignancy: an overview. Bone Marrow Transplant. 2000;25:1269–1278. doi: 10.1038/sj.bmt.1702447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solomkin JS, Mazuski JE, Baron EJ, Sawyer RG, Nathens AB, DiPiro JT, et al. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin Infect Dis. 2003;37:997–1005. doi: 10.1086/378702. [DOI] [PubMed] [Google Scholar]
- 82.Link H, Bohme A, Cornely OA, Hoffken K, Kellner O, Kern WV, et al. Antimicrobial therapy of unexplained fever in neutropenic patients—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO), Study Group Interventional Therapy of Unexplained Fever, Arbeitsgemeinschaft Supportivmassnahmen in der Onkologie (ASO) of the Deutsche Krebsgesellschaft (DKG-German Cancer Society) Ann Hematol. 2003;82(Suppl 2):S105–S117. doi: 10.1007/s00277-003-0764-4. [DOI] [PubMed] [Google Scholar]
- 83.Micozzi A, Cartoni C, Monaco M, Martino P, Zittoun R, Mandelli F. High incidence of infectious gastrointestinal complications observed in patients with acute myeloid leukemia receiving intensive chemotherapy for first induction of remission. Support Care Cancer. 1996;4:294–297. doi: 10.1007/BF01358883. [DOI] [PubMed] [Google Scholar]
- 84.Wade JC, Rubenstein EB. NCCN: fever and neutropenia. Cancer Control. 2001;8:16–21. [PubMed] [Google Scholar]
- 85.Control ECfDPa . Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals. Stockholm: ECDC; 2013. [Google Scholar]
- 86.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Altclas J, Requejo A, Jaimovich G, Milovic V, Feldman L. Clostridium difficile infection in patients with neutropenia. Clin Infect Dis. 2002;34:723. doi: 10.1086/338721. [DOI] [PubMed] [Google Scholar]
- 88.Bilgrami S, Feingold JM, Dorsky D, Edwards RL, Bona RD, Khan AM, et al. Incidence and outcome of Clostridium difficile infection following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1999;23:1039–1042. doi: 10.1038/sj.bmt.1701773. [DOI] [PubMed] [Google Scholar]
- 89.Dettenkofer M, Ebner W, Bertz H, Babikir R, Finke J, Frank U, et al. Surveillance of nosocomial infections in adult recipients of allogeneic and autologous bone marrow and peripheral blood stem-cell transplantation. Bone Marrow Transplant. 2003;31:795–801. doi: 10.1038/sj.bmt.1703920. [DOI] [PubMed] [Google Scholar]
- 90.Panichi G, Pantosti A, Gentile G, Testore GP, Venditti M, Martino P, et al. Clostridium difficile colitis in leukemia patients. Eur J Cancer Clin Oncol. 1985;21:1159–1163. doi: 10.1016/0277-5379(85)90008-2. [DOI] [PubMed] [Google Scholar]
- 91.Schalk E, Bohr UR, Konig B, Scheinpflug K, Mohren M. Clostridium difficile-associated diarrhoea, a frequent complication in patients with acute myeloid leukaemia. Ann Hematol. 2009;89:9–14. doi: 10.1007/s00277-009-0772-0. [DOI] [PubMed] [Google Scholar]
- 92.Reineke J, Tenzer S, Rupnik M, Koschinski A, Hasselmayer O, Schrattenholz A, et al. Autocatalytic cleavage of Clostridium difficile toxin B. Nature. 2007;446:415–419. doi: 10.1038/nature05622. [DOI] [PubMed] [Google Scholar]
- 93.Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 94.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 95.Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol. 2009;15:1554–1580. doi: 10.3748/wjg.15.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 97.Kim SC, Seo MY, Lee JY, Kim KT, Cho E, Kim MG, et al. Advanced chronic kidney disease: a strong risk factor for Clostridium difficile infection. Korean J Intern Med. 2016;31:125–133. doi: 10.3904/kjim.2016.31.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Keddis MT, Khanna S, Noheria A, Baddour LM, Pardi DS, Qian Q. Clostridium difficile infection in patients with chronic kidney disease. Mayo Clin Proc. 2012;87:1046–1053. doi: 10.1016/j.mayocp.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rogala BG, Malat GE, Lee DH, Harhay MN, Doyle AM, Bias TE. Identification of risk factors associated with Clostridium difficile infection in liver transplantation recipients: a single-center analysis. Transplant Proc. 2016;48:2763–2768. doi: 10.1016/j.transproceed.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 100.Ramos A, Ortiz J, Asensio A, Martinez-Ruiz R, Munez E, Cantero M, et al. Risk factors for Clostridium difficile diarrhea in patients with solid organ transplantation. Prog Transplant. 2016;26:231–237. doi: 10.1177/1526924816655073. [DOI] [PubMed] [Google Scholar]
- 101.Vehreschild MJ, Weitershagen D, Biehl LM, Tacke D, Waldschmidt D, Tox U, et al. Clostridium difficile infection in patients with acute myelogenous leukemia and in patients undergoing allogeneic stem cell transplantation: epidemiology and risk factor analysis. Biol Blood Marrow Transplant. 2014;20:823–828. doi: 10.1016/j.bbmt.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 102.Abramowitz J, Thakkar P, Isa A, Truong A, Park C, Rosenfeld RM. Adverse event reporting for proton pump inhibitor therapy: an overview of systematic reviews. Otolaryngol Head Neck Surg. 2016;155:547–554. doi: 10.1177/0194599816648298. [DOI] [PubMed] [Google Scholar]
- 103.Debast SB, Bauer MP, Kuijper EJ, European Society of Clinical M, Infectious D European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 104.Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S12–S18. doi: 10.1086/521863. [DOI] [PubMed] [Google Scholar]
- 105.Pear SM, Williamson TH, Bettin KM, Gerding DN, Galgiani JN. Decrease in nosocomial Clostridium difficile-associated diarrhea by restricting clindamycin use. Ann Intern Med. 1994;120:272–277. doi: 10.7326/0003-4819-120-4-199402150-00003. [DOI] [PubMed] [Google Scholar]
- 106.Climo MW, Israel DS, Wong ES, Williams D, Coudron P, Markowitz SM. Hospital-wide restriction of clindamycin: effect on the incidence of Clostridium difficile-associated diarrhea and cost. Ann Intern Med. 1998;128:989–995. doi: 10.7326/0003-4819-128-12_Part_1-199806150-00005. [DOI] [PubMed] [Google Scholar]
- 107.Carling P, Fung T, Killion A, Terrin N, Barza M. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol. 2003;24:699–706. doi: 10.1086/502278. [DOI] [PubMed] [Google Scholar]
- 108.Gerding DN, Muto CA, Owens RC., Jr Measures to control and prevent Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S43–S49. doi: 10.1086/521861. [DOI] [PubMed] [Google Scholar]
- 109.Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376:305–317. doi: 10.1056/NEJMoa1602615. [DOI] [PubMed] [Google Scholar]
- 110.Hagel S, Fischer A, Ehlermann P, Frank T, Tueffers K, Sturm A, et al. Fecal microbiota transplant in patients with recurrent clostridium difficile infection. Dtsch Arztebl Int. 2016;113:583–589. doi: 10.3238/arztebl.2016.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med. 2016;165:609–616. doi: 10.7326/M16-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vasa CV, Glatt AE. Effectiveness and appropriateness of empiric metronidazole for Clostridium difficile-associated diarrhea. Am J Gastroenterol. 2003;98:354–358. doi: 10.1111/j.1572-0241.2003.07227.x. [DOI] [PubMed] [Google Scholar]
- 114.Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K et al (2012) Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis [DOI] [PubMed]
- 115.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 116.Di X, Bai N, Zhang X, Liu B, Ni W, Wang J, et al. A meta-analysis of metronidazole and vancomycin for the treatment of Clostridium difficile infection, stratified by disease severity. Braz J Infect Dis. 2015;19:339–349. doi: 10.1016/j.bjid.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peng Z, Jin D, Kim HB, Stratton CW, Wu B, Tang YW, et al. Update on antimicrobial resistance in Clostridium difficile: resistance mechanisms and antimicrobial susceptibility testing. J Clin Microbiol. 2017;55:1998–2008. doi: 10.1128/JCM.02250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cornely OA, Nathwani D, Ivanescu C, Odufowora-Sita O, Retsa P, Odeyemi IA. Clinical efficacy of fidaxomicin compared with vancomycin and metronidazole in Clostridium difficile infections: a meta-analysis and indirect treatment comparison. J Antimicrob Chemother. 2014;69:2892–2900. doi: 10.1093/jac/dku261. [DOI] [PubMed] [Google Scholar]
- 119.Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut. 1986;27:1169–1172. doi: 10.1136/gut.27.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Friedenberg F, Fernandez A, Kaul V, Niami P, Levine GM. Intravenous metronidazole for the treatment of Clostridium difficile colitis. Dis Colon Rectum. 2001;44:1176–1180. doi: 10.1007/BF02234641. [DOI] [PubMed] [Google Scholar]
- 121.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 122.Johnson S, Louie TJ, Gerding DN, Cornely OA, Chasan-Taber S, Fitts D, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345–354. doi: 10.1093/cid/ciu313. [DOI] [PubMed] [Google Scholar]
- 123.Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clin Infect Dis. 2002;35:690–696. doi: 10.1086/342334. [DOI] [PubMed] [Google Scholar]
- 124.Rokas KE, Johnson JW, Beardsley JR, Ohl CA, Luther VP, Williamson JC. The addition of intravenous metronidazole to oral vancomycin is associated with improved mortality in critically ill patients with Clostridium difficile infection. Clin Infect Dis. 2015;61:934–941. doi: 10.1093/cid/civ409. [DOI] [PubMed] [Google Scholar]
- 125.Li R, Lu L, Lin Y, Wang M, Liu X. Efficacy and safety of metronidazole monotherapy versus vancomycin monotherapy or combination therapy in patients with Clostridium difficile infection: a systematic review and meta-analysis. PLoS One. 2015;10:e0137252. doi: 10.1371/journal.pone.0137252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Popovic N, Korac M, Nesic Z, Milosevic B, Urosevic A, Jevtovic D, et al. Oral teicoplanin for successful treatment of severe refractory Clostridium difficile infection. J Infect Dev Ctries. 2015;9:1062–1067. doi: 10.3855/jidc.6335. [DOI] [PubMed] [Google Scholar]
- 127.Thomas A, Khan F, Uddin N, Wallace MR. Tigecycline for severe Clostridium difficile infection. Int J Infect Dis. 2014;26:171–172. doi: 10.1016/j.ijid.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 128.Britt NS, Steed ME, Potter EM, Clough LA. Tigecycline for the treatment of severe and severe complicated Clostridium difficile infection. Infect Dis Ther. 2014;3:321–331. doi: 10.1007/s40121-014-0050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Metan G, Ture Z, Kaynar L, Berk E, Gursoy S, Alp E, et al. Tigecycline for the treatment of Clostridium difficile infection refractory to metronidazole in haematopoietic stem cell transplant recipients. J Chemother. 2015;27:354–357. doi: 10.1179/1973947814Y.0000000225. [DOI] [PubMed] [Google Scholar]
- 130.Fischer M, Kao D, Mehta SR, Martin T, Dimitry J, Keshteli AH, et al. Predictors of early failure after fecal microbiota transplantation for the therapy of clostridium difficile infection: a multicenter study. Am J Gastroenterol. 2016;111:1024–1031. doi: 10.1038/ajg.2016.180. [DOI] [PubMed] [Google Scholar]
- 131.Waye A, Atkins K, Kao D. Cost averted with timely fecal microbiota transplantation in the management of recurrent Clostridium difficile infection in Alberta, Canada. J Clin Gastroenterol. 2016;50:747–753. doi: 10.1097/MCG.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 132.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 133.Tedesco FJ, Gordon D, Fortson WC. Approach to patients with multiple relapses of antibiotic-associated pseudomembranous colitis. Am J Gastroenterol. 1985;80:867–868. [PubMed] [Google Scholar]
- 134.Hota SS, Sales V, Tomlinson G, Salpeter MJ, McGeer A, Coburn B et al (2016) Oral vancomycin followed by fecal transplantation versus tapering oral vancomycin treatment for recurrent Clostridium difficile infection: an open-label, randomized controlled trial. Clin Infect Dis 64(3):265–271. 10.1093/cid/ciw731. Epub 2016 Nov 9 [DOI] [PubMed]
- 135.Kato H, Iwashima Y, Nakamura M, Nakamura A, Ueda R. Inappropriate use of loperamide worsens Clostridium difficile-associated diarrhoea. J Hosp Infect. 2008;70:194–195. doi: 10.1016/j.jhin.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 136.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lewis BB, Buffie CG, Carter RA, Leiner I, Toussaint NC, Miller LC, et al. Loss of microbiota-mediated colonization resistance to Clostridium difficile infection with oral vancomycin compared with metronidazole. J Infect Dis. 2015;212:1656–1665. doi: 10.1093/infdis/jiv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gorschluter M, Hahn C, Ziske C, Mey U, Schottker B, Molitor E, et al. Low frequency of enteric infections by Salmonella, Shigella, Yersinia and Campylobacter in patients with acute leukemia. Infection. 2002;30:22–25. doi: 10.1007/s15010-002-1178-2. [DOI] [PubMed] [Google Scholar]
- 139.Delaloye J, Merlani G, Petignat C, Wenger A, Zaman K, Monnerat C, et al. Nosocomial nontyphoidal salmonellosis after antineoplastic chemotherapy: reactivation of asymptomatic colonization? Eur J Clin Microbiol Infect Dis. 2004;23:751–758. doi: 10.1007/s10096-004-1206-5. [DOI] [PubMed] [Google Scholar]
- 140.Yuen KY, Woo PC, Liang RH, Chiu EK, Chen FF, Wong SS, et al. Clinical significance of alimentary tract microbes in bone marrow transplant recipients. Diagn Microbiol Infect Dis. 1998;30:75–81. doi: 10.1016/S0732-8893(97)00213-7. [DOI] [PubMed] [Google Scholar]
- 141.Gentile G, Venditti M, Micozzi A, Caprioli A, Donelli G, Tirindelli C, et al. Cryptosporidiosis in patients with hematologic malignancies. Rev Infect Dis. 1991;13:842–846. doi: 10.1093/clinids/13.5.842. [DOI] [PubMed] [Google Scholar]
- 142.Wain J, Hoa NT, Chinh NT, Vinh H, Everett MJ, Diep TS, et al. Quinolone-resistant Salmonella typhi in Viet Nam: molecular basis of resistance and clinical response to treatment. Clin Infect Dis. 1997;25:1404–1410. doi: 10.1086/516128. [DOI] [PubMed] [Google Scholar]
- 143.Wang JH, Liu YC, Yen MY, Chen YS, Wann SR, Cheng DL. Mycotic aneurysm due to non-typhi salmonella: report of 16 cases. Clin Infect Dis. 1996;23:743–747. doi: 10.1093/clinids/23.4.743. [DOI] [PubMed] [Google Scholar]
- 144.Bennish ML, Salam MA, Haider R, Barza M. Therapy for shigellosis. II. Randomized, double-blind comparison of ciprofloxacin and ampicillin. J Infect Dis. 1990;162:711–716. doi: 10.1093/infdis/162.3.711. [DOI] [PubMed] [Google Scholar]
- 145.Khan WA, Seas C, Dhar U, Salam MA, Bennish ML. Treatment of shigellosis: V. Comparison of azithromycin and ciprofloxacin. A double-blind, randomized, controlled trial. Ann Intern Med. 1997;126:697–703. doi: 10.7326/0003-4819-126-9-199705010-00004. [DOI] [PubMed] [Google Scholar]
- 146.Tribble DR, Sanders JW, Pang LW, Mason C, Pitarangsi C, Baqar S, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338–346. doi: 10.1086/510589. [DOI] [PubMed] [Google Scholar]
- 147.Vukelic D, Trkulja V, Salkovic-Petrisic M. Single oral dose of azithromycin versus 5 days of oral erythromycin or no antibiotic in treatment of campylobacter enterocolitis in children: a prospective randomized assessor-blind study. J Pediatr Gastroenterol Nutr. 2010;50:404–410. doi: 10.1097/MPG.0b013e3181a87104. [DOI] [PubMed] [Google Scholar]
- 148.Gayraud M, Scavizzi MR, Mollaret HH, Guillevin L, Hornstein MJ. Antibiotic treatment of Yersinia enterocolitica septicemia: a retrospective review of 43 cases. Clin Infect Dis. 1993;17:405–410. doi: 10.1093/clinids/17.3.405. [DOI] [PubMed] [Google Scholar]
- 149.Menne J, Nitschke M, Stingele R, Abu-Tair M, Beneke J, Bramstedt J, et al. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ. 2012;345:e4565. doi: 10.1136/bmj.e4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nitschke M, Sayk F, Hartel C, Roseland RT, Hauswaldt S, Steinhoff J, et al. Association between azithromycin therapy and duration of bacterial shedding among patients with Shiga toxin-producing enteroaggregative Escherichia coli O104:H4. JAMA. 2012;307:1046–1052. doi: 10.1001/jama.2012.264. [DOI] [PubMed] [Google Scholar]
- 151.Sugata K, Taniguchi K, Yui A, Nakai H, Asano Y, Hashimoto S, et al. Analysis of rotavirus antigenemia in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2012;14:49–56. doi: 10.1111/j.1399-3062.2011.00668.x. [DOI] [PubMed] [Google Scholar]
- 152.Robilotti E, Deresinski S, Pinsky BA. Norovirus. Clin Microbiol Rev. 2015;28:134–164. doi: 10.1128/CMR.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bok K, Prevots DR, Binder AM, Parra GI, Strollo S, Fahle GA, et al. Epidemiology of norovirus infection among immunocompromised patients at a tertiary care research hospital, 2010–2013. Open Forum Infect Dis. 2016;3:ofw169. doi: 10.1093/ofid/ofw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ghosh N, Malik FA, Daver RG, Vanichanan J, Okhuysen PC. Viral associated diarrhea in immunocompromised and cancer patients at a large comprehensive cancer center: a 10-year retrospective study. Infect Dis (Lond) 2017;49:113–119. doi: 10.1080/23744235.2016.1224384. [DOI] [PubMed] [Google Scholar]
- 155.Ueda R, Fuji S, Mori S, Hiramoto N, Hashimoto H, Tanaka T, et al. Characteristics and outcomes of patients diagnosed with norovirus gastroenteritis after allogeneic hematopoietic stem cell transplantation based on immunochromatography. Int J Hematol. 2015;102:121–128. doi: 10.1007/s12185-015-1804-2. [DOI] [PubMed] [Google Scholar]
- 156.van Beek J, van der Eijk AA, Fraaij PL, Caliskan K, Cransberg K, Dalinghaus M, et al. Chronic norovirus infection among solid organ recipients in a tertiary care hospital, the Netherlands, 2006–2014. Clin Microbiol Infect. 2017;23:265 e9–265 e13. doi: 10.1016/j.cmi.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 157.Lemes LG, Correa TS, Fiaccadori FS, Cardoso D, Arantes Ade M, Souza KM, et al. Prospective study on norovirus infection among allogeneic stem cell transplant recipients: prolonged viral excretion and viral RNA in the blood. J Clin Virol. 2014;61:329–333. doi: 10.1016/j.jcv.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 158.Hohne M, Schreier E. Detection and characterization of norovirus outbreaks in Germany: application of a one-tube RT-PCR using a fluorogenic real-time detection system. J Med Virol. 2004;72:312–319. doi: 10.1002/jmv.10573. [DOI] [PubMed] [Google Scholar]
- 159.Rabenau HF, Clarici AM, Muhlbauer G, Berger A, Vince A, Muller S, et al. Rapid detection of enterovirus infection by automated RNA extraction and real-time fluorescence PCR. J Clin Virol. 2002;25:155–164. doi: 10.1016/S1386-6532(01)00257-8. [DOI] [PubMed] [Google Scholar]
- 160.Schwartz S, Vergoulidou M, Schreier E, Loddenkemper C, Reinwald M, Schmidt-Hieber M, et al. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood. 2011;117:5850–5856. doi: 10.1182/blood-2010-12-325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rossignol JF, Abu-Zekry M, Hussein A, Santoro MG. Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial. Lancet. 2006;368:124–129. doi: 10.1016/S0140-6736(06)68852-1. [DOI] [PubMed] [Google Scholar]
- 162.Mahapatro S, Mahilary N, Satapathy AK, Das RR. Nitazoxanide in acute rotavirus diarrhea: a randomized control trial from a developing country. J Trop Med. 2017;2017:7942515. doi: 10.1155/2017/7942515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rossignol JF, El-Gohary YM. Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial. Aliment Pharmacol Ther. 2006;24:1423–1430. doi: 10.1111/j.1365-2036.2006.03128.x. [DOI] [PubMed] [Google Scholar]
- 164.Kanfer EJ, Abrahamson G, Taylor J, Coleman JC, Samson DM. Severe rotavirus-associated diarrhoea following bone marrow transplantation: treatment with oral immunoglobulin. Bone Marrow Transplant. 1994;14:651–652. [PubMed] [Google Scholar]
- 165.Nicolasora NP, Reddy P, Kaul DR. Biopsy-proven adenoviral diarrhea responding to low-dose cidofovir. Transpl Infect Dis. 2008;10:346–350. doi: 10.1111/j.1399-3062.2008.00303.x. [DOI] [PubMed] [Google Scholar]
- 166.Schofield KP, Morris DJ, Bailey AS, de Jong JC, Corbitt G. Gastroenteritis due to adenovirus type 41 in an adult with chronic lymphocytic leukemia. Clin Infect Dis. 1994;19:311–312. doi: 10.1093/clinids/19.2.311. [DOI] [PubMed] [Google Scholar]
- 167.Leruez-Ville M, Chardin-Ouachee M, Neven B, Picard C, Le Guinche I, Fischer A, et al. Description of an adenovirus A31 outbreak in a paediatric haematology unit. Bone Marrow Transplant. 2006;38:23–28. doi: 10.1038/sj.bmt.1705389. [DOI] [PubMed] [Google Scholar]
- 168.Ng AP, Worth L, Chen L, Seymour JF, Prince HM, Slavin M, et al. Cytomegalovirus DNAemia and disease: incidence, natural history and management in settings other than allogeneic stem cell transplantation. Haematologica. 2005;90:1672–1679. [PubMed] [Google Scholar]
- 169.Lee MY, Chiou TJ, Hsiao LT, Yang MH, Lin PC, Poh SB, et al. Rituximab therapy increased post-transplant cytomegalovirus complications in non-Hodgkin’s lymphoma patients receiving autologous hematopoietic stem cell transplantation. Ann Hematol. 2008;87:285–289. doi: 10.1007/s00277-007-0397-0. [DOI] [PubMed] [Google Scholar]
- 170.Cheung WW, Tse E, Leung AY, Yuen KY, Kwong YL. Regular virologic surveillance showed very frequent cytomegalovirus reactivation in patients treated with alemtuzumab. Am J Hematol. 2007;82:108–111. doi: 10.1002/ajh.20780. [DOI] [PubMed] [Google Scholar]
- 171.Holmberg LA, Boeckh M, Hooper H, Leisenring W, Rowley S, Heimfeld S, et al. Increased incidence of cytomegalovirus disease after autologous CD34-selected peripheral blood stem cell transplantation. Blood. 1999;94:4029–4035. [PubMed] [Google Scholar]
- 172.Reed EC, Wolford JL, Kopecky KJ, Lilleby KE, Dandliker PS, Todaro JL, et al. Ganciclovir for the treatment of cytomegalovirus gastroenteritis in bone marrow transplant patients. A randomized, placebo-controlled trial. Ann Intern Med. 1990;112:505–510. doi: 10.7326/0003-4819-112-7-505. [DOI] [PubMed] [Google Scholar]
- 173.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113:5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun YQ, Xu LP, Han TT, Zhang XH, Wang Y, Han W, et al. Detection of human cytomegalovirus (CMV) DNA in feces has limited value in predicting CMV enteritis in patients with intestinal graft-versus-host disease after allogeneic stem cell transplantation. Transpl Infect Dis. 2015;17:655–661. doi: 10.1111/tid.12420. [DOI] [PubMed] [Google Scholar]
- 175.Page MJ, Dreese JC, Poritz LS, Koltun WA. Cytomegalovirus enteritis: a highly lethal condition requiring early detection and intervention. Dis Colon Rectum. 1998;41:619–623. doi: 10.1007/BF02235271. [DOI] [PubMed] [Google Scholar]
- 176.Hackman RC, Wolford JL, Gleaves CA, Myerson D, Beauchamp MD, Meyers JD, et al. Recognition and rapid diagnosis of upper gastrointestinal cytomegalovirus infection in marrow transplant recipients. A comparison of seven virologic methods. Transplantation. 1994;57:231–237. doi: 10.1097/00007890-199401001-00014. [DOI] [PubMed] [Google Scholar]
- 177.Kakugawa Y, Kami M, Matsuda T, Saito Y, Kim SW, Fukuda T, et al. Endoscopic diagnosis of cytomegalovirus gastritis after allogeneic hematopoietic stem cell transplantation. World J Gastroenterol. 2010;16:2907–2912. doi: 10.3748/wjg.v16.i23.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ljungman P, de la Camara R, Cordonnier C, Einsele H, Engelhard D, Reusser P, et al. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 2008;42:227–240. doi: 10.1038/bmt.2008.162. [DOI] [PubMed] [Google Scholar]
- 179.Ljungman P, de la Camara R, Cordonnier C, Einsele H, Engelhard D, Reusser P et al (2008) Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 6(4):227–240. 10.1038/bmt.2008.162 [DOI] [PubMed]
- 180.Ljungman P, Cordonnier C, Einsele H, Bender-Gotze C, Bosi A, Dekker A, et al. Use of intravenous immune globulin in addition to antiviral therapy in the treatment of CMV gastrointestinal disease in allogeneic bone marrow transplant patients: a report from the European Group for Blood and Marrow Transplantation (EBMT). Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 1998;21:473–476. doi: 10.1038/sj.bmt.1701113. [DOI] [PubMed] [Google Scholar]
- 181.Machado CM, Dulley FL, Boas LS, Castelli JB, Macedo MC, Silva RL, et al. CMV pneumonia in allogeneic BMT recipients undergoing early treatment of pre-emptive ganciclovir therapy. Bone Marrow Transplant. 2000;26:413–417. doi: 10.1038/sj.bmt.1702526. [DOI] [PubMed] [Google Scholar]
- 182.Crippa F, Corey L, Chuang EL, Sale G, Boeckh M. Virological, clinical, and ophthalmologic features of cytomegalovirus retinitis after hematopoietic stem cell transplantation. Clin Infect Dis. 2001;32:214–219. doi: 10.1086/318447. [DOI] [PubMed] [Google Scholar]
- 183.Reusser P, Einsele H, Lee J, Volin L, Rovira M, Engelhard D, et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood. 2002;99:1159–1164. doi: 10.1182/blood.V99.4.1159. [DOI] [PubMed] [Google Scholar]
- 184.Ljungman P, Deliliers GL, Platzbecker U, Matthes-Martin S, Bacigalupo A, Einsele H, et al. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. The Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2001;97:388–392. doi: 10.1182/blood.V97.2.388. [DOI] [PubMed] [Google Scholar]
- 185.Platzbecker U, Bandt D, Thiede C, Helwig A, Freiberg-Richter J, Schuler U, et al. Successful preemptive cidofovir treatment for CMV antigenemia after dose-reduced conditioning and allogeneic blood stem cell transplantation. Transplantation. 2001;71:880–885. doi: 10.1097/00007890-200104150-00010. [DOI] [PubMed] [Google Scholar]
- 186.Cesaro S, Zhou X, Manzardo C, Buonfrate D, Cusinato R, Tridello G, et al. Cidofovir for cytomegalovirus reactivation in pediatric patients after hematopoietic stem cell transplantation. J Clin Virol. 2005;34:129–132. doi: 10.1016/j.jcv.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 187.Bacigalupo A, Bregante S, Tedone E, Isaza A, Van Lint MT, Moro F, et al. Combined foscarnet-ganciclovir treatment for cytomegalovirus infections after allogeneic hemopoietic stem cell transplantation (Hsct) Bone Marrow Transplant. 1996;18(Suppl 2):110–114. [PubMed] [Google Scholar]
- 188.Mattes FM, Hainsworth EG, Geretti AM, Nebbia G, Prentice G, Potter M, et al. A randomized, controlled trial comparing ganciclovir to ganciclovir plus foscarnet (each at half dose) for preemptive therapy of cytomegalovirus infection in transplant recipients. J Infect Dis. 2004;189:1355–1361. doi: 10.1086/383040. [DOI] [PubMed] [Google Scholar]
- 189.Salzberger B, Stoehr A, Heise W, Fatkenheuer G, Schwenk A, Franzen C, et al. Foscarnet and ganciclovir combination therapy for CMV disease in HIV-infected patients. Infection. 1994;22:197–200. doi: 10.1007/BF01716702. [DOI] [PubMed] [Google Scholar]
- 190.Nigro L, Larocca L, Massarelli L, Patamia I, Minniti S, Palermo F, et al. A placebo-controlled treatment trial of Blastocystis hominis infection with metronidazole. J Travel Med. 2003;10:128–130. doi: 10.2310/7060.2003.31714. [DOI] [PubMed] [Google Scholar]
- 191.Armentia A, Mendez J, Gomez A, Sanchis E, Fernandez A, de la Fuente R, et al. Urticaria by Blastocystis hominis. Successful treatment with paromomycin. Allergol Immunopathol (Madr) 1993;21:149–151. [PubMed] [Google Scholar]
- 192.Ok UZ, Girginkardesler N, Balcioglu C, Ertan P, Pirildar T, Kilimcioglu AA. Effect of trimethoprim-sulfamethaxazole in Blastocystis hominis infection. Am J Gastroenterol. 1999;94:3245–3247. doi: 10.1111/j.1572-0241.1999.01529.x. [DOI] [PubMed] [Google Scholar]
- 193.Rossignol JF, Kabil SM, Said M, Samir H, Younis AM. Effect of nitazoxanide in persistent diarrhea and enteritis associated with Blastocystis hominis. Clin Gastroenterol Hepatol. 2005;3:987–991. doi: 10.1016/S1542-3565(05)00427-1. [DOI] [PubMed] [Google Scholar]
- 194.Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of Nitazoxanide. J Infect Dis. 2001;184:103–106. doi: 10.1086/321008. [DOI] [PubMed] [Google Scholar]
- 195.Rossignol JF, Hidalgo H, Feregrino M, Higuera F, Gomez WH, Romero JL, et al. A double-‘blind’ placebo-controlled study of nitazoxanide in the treatment of cryptosporidial diarrhoea in AIDS patients in Mexico. Trans R Soc Trop Med Hyg. 1998;92:663–666. doi: 10.1016/S0035-9203(98)90804-5. [DOI] [PubMed] [Google Scholar]
- 196.Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360:1375–1380. doi: 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- 197.Hussien SM, Abdella OH, Abu-Hashim AH, Aboshiesha GA, Taha MA, El-Shemy AS, et al. Comparative study between the effect of nitazoxanide and paromomycine in treatment of cryptosporidiosis in hospitalized children. J Egypt Soc Parasitol. 2013;43:463–470. doi: 10.12816/0006403. [DOI] [PubMed] [Google Scholar]
- 198.Abubakar I, Aliyu SH, Arumugam C, Usman NK, Hunter PR. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br J Clin Pharmacol. 2007;63:387–393. doi: 10.1111/j.1365-2125.2007.02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rossignol JF. Nitazoxanide in the treatment of acquired immune deficiency syndrome-related cryptosporidiosis: results of the United States compassionate use program in 365 patients. Aliment Pharmacol Ther. 2006;24:887–894. doi: 10.1111/j.1365-2036.2006.03033.x. [DOI] [PubMed] [Google Scholar]
- 200.Vandenberg O, Robberecht F, Dauby N, Moens C, Talabani H, Dupont E, et al. Management of a Cryptosporidium hominis outbreak in a day-care center. Pediatr Infect Dis J. 2012;31:10–15. doi: 10.1097/INF.0b013e318235ab64. [DOI] [PubMed] [Google Scholar]
- 201.Kikuchi T, Koga M, Shimizu S, Miura T, Maruyama H, Kimura M. Efficacy and safety of paromomycin for treating amebiasis in Japan. Parasitol Int. 2013;62:497–501. doi: 10.1016/j.parint.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 202.Gonzales ML, Dans LF, Martinez EG (2009) Antiamoebic drugs for treating amoebic colitis. Cochrane Database Syst Rev. CD006085 [DOI] [PMC free article] [PubMed]
- 203.Canete R, Escobedo AA, Gonzalez ME, Almirall P, Cantelar N. A randomized, controlled, open-label trial of a single day of mebendazole versus a single dose of tinidazole in the treatment of giardiasis in children. Curr Med Res Opin. 2006;22:2131–2136. doi: 10.1185/030079906X132497. [DOI] [PubMed] [Google Scholar]
- 204.Granados CE, Reveiz L, Uribe LG, Criollo CP. Drugs for treating giardiasis. Cochrane Database Syst Rev. 2012;12:CD007787. doi: 10.1002/14651858.CD007787.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Escobedo AA, Alvarez G, Gonzalez ME, Almirall P, Canete R, Cimerman S, et al. The treatment of giardiasis in children: single-dose tinidazole compared with 3 days of nitazoxanide. Ann Trop Med Parasitol. 2008;102:199–207. doi: 10.1179/136485908X267894. [DOI] [PubMed] [Google Scholar]
- 206.Verdier RI, Fitzgerald DW, Johnson WD, Jr, Pape JW. Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients. A randomized, controlled trial. Ann Intern Med. 2000;132:885–888. doi: 10.7326/0003-4819-132-11-200006060-00006. [DOI] [PubMed] [Google Scholar]
- 207.Pape JW, Verdier RI, Johnson WD., Jr Treatment and prophylaxis of Isospora belli infection in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1989;320:1044–1047. doi: 10.1056/NEJM198904203201604. [DOI] [PubMed] [Google Scholar]
- 208.Doumbo O, Rossignol JF, Pichard E, Traore HA, Dembele TM, Diakite M, et al. Nitazoxanide in the treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa. Am J Trop Med Hyg. 1997;56:637–639. doi: 10.4269/ajtmh.1997.56.637. [DOI] [PubMed] [Google Scholar]
- 209.Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC Jr, Terashima A, Samalvides F et al (2016) Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. CD007745 [DOI] [PMC free article] [PubMed]
- 210.Hoge CW, Shlim DR, Ghimire M, Rabold JG, Pandey P, Walch A, et al. Placebo-controlled trial of co-trimoxazole for Cyclospora infections among travellers and foreign residents in Nepal. Lancet. 1995;345:691–693. doi: 10.1016/S0140-6736(95)90868-4. [DOI] [PubMed] [Google Scholar]
- 211.Madico G, McDonald J, Gilman RH, Cabrera L, Sterling CR. Epidemiology and treatment of Cyclospora cayetanensis infection in Peruvian children. Clin Infect Dis. 1997;24:977–981. doi: 10.1093/clinids/24.5.977. [DOI] [PubMed] [Google Scholar]