Abstract
The physiological function of the epidermal growth factor receptor (EGFR) is to regulate epithelial tissue development and homeostasis. In pathological settings, mostly in lung and breast cancer and in glioblastoma, the EGFR is a driver of tumorigenesis. Inappropriate activation of the EGFR in cancer mainly results from amplification and point mutations at the genomic locus, but transcriptional upregulation or ligand overproduction due to autocrine/paracrine mechanisms has also been described. Moreover, the EGFR is increasingly recognized as a biomarker of resistance in tumors, as its amplification or secondary mutations have been found to arise under drug pressure. This evidence, in addition to the prominent function that this receptor plays in normal epithelia, has prompted intense investigations into the role of the EGFR both at physiological and at pathological level. Despite the large body of knowledge obtained over the last two decades, previously unrecognized (herein defined as ‘noncanonical’) functions of the EGFR are currently emerging. Here, we will initially review the canonical ligand‐induced EGFR signaling pathway, with particular emphasis to its regulation by endocytosis and subversion in human tumors. We will then focus on the most recent advances in uncovering noncanonical EGFR functions in stress‐induced trafficking, autophagy, and energy metabolism, with a perspective on future therapeutic applications.
Keywords: cancer, EGFR, membrane trafficking, signal transduction
Abbreviations
- AGO2
Argonaute 2
- AKT
AKT8 virus oncogene cellular homolog
- AP2
adaptor protein 2
- ATG14
autophagy‐related gene 14
- Bcl‐2
B‐cell lymphoma gene 2
- BRAF
v‐Raf murine sarcoma viral oncogene homolog B
- Ca2+
calcium ion
- Cbl
cellular homologue of Cas NS‐1 oncogene
- CCP
clathrin‐coated pit
- CLCb
clathrin light chain b
- CME
clathrin‐mediated endocytosis
- c‐MET
MET proto‐oncogene receptor tyrosine kinase
- CRC
colorectal cancer
- Dyn1
dynamin 1
- EE
early endosome
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ErbB
erythroblastosis oncogene B
- ER
endoplasmic reticulum
- ESCRT
endosomal sorting complex required for transport
- FEME
endophilin‐mediated endocytosis
- FGFR2
fibroblast growth factor receptor 2
- GLUT1
Glucose Transporter Type 1
- GLUT3
Glucose Transporter Type 3
- GPCR
G‐protein‐coupled receptor
- Grb2
growth factor receptor‐bound protein 2
- HK1
hexokinase 1
- ILV
intraluminal vesicle
- KI
knock‐in
- KO
knockout
- LAPTM4B
lysosomal‐associated protein transmembrane 4 beta
- LC3
microtubule‐associated proteins 1A/1B light chain 3B
- LDLR
low‐density lipoprotein receptor
- LIR
LC3‐interacting region
- mAb
monoclonal antibody
- MAPK
mitogen‐activated protein kinase
- miRNA
microribonucleic acid
- mTORC1
mechanistic target of rapamycin complex 1
- mTORC2
mechanistic target of rapamycin complex 2
- MVB
multivesicular body
- MYC
myelocytomatosis oncogene cellular homolog
- NCE
nonclathrin endocytosis
- NSCLC
non‐small‐cell lung cancer
- PDGFR
platelet‐derived growth factor receptor
- PDK1
phosphoinositide‐dependent kinase‐1
- PI3K
phosphoinositide 3 kinase
- PIP2
phosphatidylinositol 4,5‐bisphosphate
- PIPKIγi5
phosphatidylinositol‐4‐Phosphate 5‐kinase type Iγ
- PKC
protein kinase C
- PKCε
protein kinase C ε
- PKM2
pyruvate kinase M2
- PLC
phospholipase C
- PM
plasma membrane
- PTP1B
protein phosphotyrosyl phosphatase 1B
- PUMA
p53‐upregulated modulator of apoptosis
- Rab
Ras analog in the brain
- Ras
retrovirus‐associated DNA sequence
- RNF11
Ring Finger protein 11
- RTK
receptor tyrosine kinase
- RTN3
reticulon 3
- SCD1
stearoyl‐CoA desaturase‐1
- SGLT1
sodium‐glucose cotransporter 1
- Src
Rous sarcoma oncogene cellular homolog
- SREBP‐1
sterol regulatory element‐binding protein 1
- SYNJ2
5′‐inositol lipid phosphatase synaptojanin 2
- TfR
transferrin receptor
- TGF
transforming growth factor α
- TNF‐α
tumor necrosis factor α
- TPC
two‐pore channel
- TXNIP
thioredoxin‐interacting protein
- UV
ultraviolet
- VPS34
vacuolar protein sorting 34
1. Introduction
The epidermal growth factor receptor (EGFR) belongs to the ErbB family of receptor tyrosine kinases (RTKs) and exerts critical functions in epithelial cell physiology (Schlessinger, 2014). It is frequently mutated and/or overexpressed in different types of human cancers and is the target of multiple cancer therapies currently adopted in the clinical practice (Yarden and Pines, 2012).
Early studies of the EGFR pathway started with the discovery of EGF in 1963 by Stanley Cohen and, later in the 1980s, of the EGFR gene. Since then, biochemical, structural, and genetic studies have depicted the molecular mechanisms underlying receptor transphosphorylation, which usually occurs in response to ligand stimulation, and the consequent activation of the intracellular signaling cascade. This cascade consists in the activation of multiple pathways that deliver the information from the cell surface, and the intracellular vesicular compartments, to the nucleus leading to the activation of genes responsible for cell proliferation, survival, and differentiation (Lemmon and Schlessinger, 2010; Schlessinger, 2014).
The best characterized functions of the EGFR are in the context of ligand‐ and kinase‐dependent activation, that is, the ‘canonical’ EGFR signaling pathway (Lemmon and Schlessinger, 2010). However, novel functions, both kinase dependent and independent, have been recently identified. They reveal unexpected roles of the EGFR, such as in the regulation of autophagy and metabolism (Tan et al., 2016a). These noncanonical functions are generally induced by cellular and environmental stresses. Several of these ‘stress pathways’ are activated in cancer cells to provide them with a survival advantage and resistance to therapy (Jutten et al., 2013; Tan et al., 2016a). This has led to an emerging concept that concomitant targeting of EGFR and stress pathways might offer a window of opportunity in cancer treatment.
2. Canonical ligand‐dependent EGFR signaling pathway
Under unstimulated conditions, the EGFR is mainly found in an auto‐inhibited, dimerization‐incompetent, state at the plasma membrane (PM). Ligand binding promotes receptor dimerization, which determines a series of structural rearrangements that are conveyed to the cytoplasmic domain allowing the formation of asymmetric dimers between the two juxtaposed catalytic domains (Zhang et al., 2006; Fig. 1A). These events lead to the allosteric activation of the EGFR kinase and to the trans‐autophosphorylation of critical tyrosine residues in the cytoplasmic receptor tail, thereby triggering the signaling cascade (Lemmon et al., 2014). For in‐depth molecular details of EGFR activation, we refer the reader to recent reviews (Kovacs et al., 2015; Lemmon et al., 2014).
Figure 1.
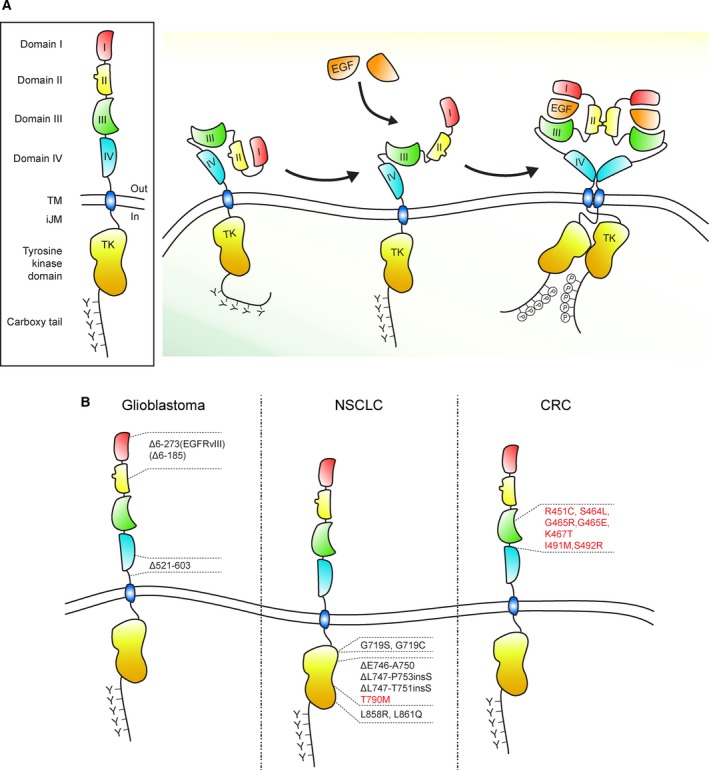
Scheme of EGFR and its mutations in glioblastoma and in lung and colorectal cancer. (A) Schematic representation of the EGFR and EGF‐induced receptor activation. The EGFR extracellular region encompasses domains I, II, III, and IV; following are the transmembrane region (TM), the intracellular juxtamembrane domain (iJM), the tyrosine kinase domain (TK), and the carboxyl‐terminal tail (carboxy tail). EGF binding to the receptor unmasks a dimerization motif and determines structural rearrangements that are conveyed to the cytoplasmic domain allowing the formation of asymmetric dimers between the two juxtaposed catalytic domains. (B) Most frequent EGFR mutations in glioblastoma, in NSCLC (non‐small‐cell lung cancer), and in CRC (colorectal cancer). Mutations found in tumors resistant to EGFR blockade are shown in red. In CRC, the indicated EGFR mutations have been identified in patients that progressed upon cetuximab treatment (Arena et al., 2015, 2016; Montagut et al., 2012; Van Emburgh et al., 2016).
Ligand‐induced EGFR phosphorylation and conformational changes occurring in the intracellular tail lead also to the recruitment of the endocytic machinery that mediates receptor endocytosis, with internalization rates that are ~ 10‐fold higher for ligand‐bound than for unliganded EGFR (Conte and Sigismund, 2016).
The EGFR can heterodimerize with other ErbB family members, ErbB2, ErbB3, and ErbB4 (Lemmon et al., 2014), with critical effects on receptor trafficking and signaling (Lenferink et al., 1998). Indeed, heterodimers have a reduced ligand‐binding strength, leading to ligand dissociation in endosomes, and they are unable to recruit Cbl and the endocytic machinery as efficiently as EGFR homodimers (Baulida et al., 1996; Lenferink et al., 1998; Levkowitz et al., 1998; Waterman et al., 1999). Signaling from heterodimers is therefore enhanced and predicted to be more oncogenic than signaling from homodimers.
Seven EGFR ligands have been described to date, which have been shown to induce specific cellular responses and intracellular trafficking events both in vitro and in vivo (Roepstorff et al., 2009; Wilson et al., 2012; Yang et al., 2017). In some cases, these differences are determined by the different strength of the ligand–receptor interaction, which dictates whether the ligand dissociates (as in the case of TGFα transforming growth factor α) or not (as in the case of EGF) from the receptor in the mild acidic pH of the endosomes, favoring EGFR recycling or degradation, respectively. In other instances, the different signaling properties of the various ligands have been attributed to their ability to differentially stabilize the EGFR dimers, therefore determining specific signaling outputs (Freed et al., 2017).
Once activated at the PM, the EGFR also undergoes ubiquitination by the E3 ligase Cbl in complex with the adaptor molecule Grb2 (Levkowitz et al., 1998; Sigismund et al., 2013; Waterman et al., 2002). EGFR ubiquitination is threshold controlled by EGF concentration (Sigismund et al., 2013) and occurs on several lysine residues within the kinase domain (Huang et al., 2006). In specific cell contexts, EGFR ubiquitination works as a signal for receptor internalization into the nonclathrin endocytic (NCE) pathway. At later stages of trafficking, ubiquitination becomes a common requirement to target receptors to lysosomal degradation (see Section 2.1).
Ligand‐dependent EGFR activation transduces multiple signaling pathways, including the Ras/MAPK pathway, the PI3K/AKT pathway, and the phospholipase C (PLC)/protein kinase C (PKC) signaling cascade (Lemmon and Schlessinger, 2010). Canonical EGFR signaling is critical for several cellular functions including survival, proliferation, differentiation, and motility.
The quality, the amplitude, and the duration of these signaling events are tightly regulated by compartmentalization and trafficking of the EGFR along the endocytic pathway, as discussed in the following paragraphs.
2.1. Temporal regulation of EGFR signaling by endocytosis
The first step in the regulation of EGFR signaling takes place at the PM, where the EGFR is internalized through multiple endocytic pathways with different morphological, molecular, and kinetic features that influence receptor activity and fate (Barbieri et al., 2016; Bergeron et al., 2016). Both clathrin‐mediated endocytosis (CME) and several NCE pathways are involved in EGFR internalization (Barbieri et al., 2016). EGFR‐CME is active at all ligand concentrations in all type of cells (Carpentier et al., 1982; Goh et al., 2010; Hanover et al., 1984; Jiang et al., 2003; Sigismund et al., 2008; Sorkin and Carpenter, 1993). Conversely, the EGFR‐NCE pathways – despite their molecular and morphological differences – are generally activated at higher, but still physiologically relevant, EGF doses (≥ 10 ng·mL−1) and their significance is cell context dependent (Boucrot et al., 2015; Caldieri et al., 2017; Orth et al., 2006; also reviewed in Barbieri et al., 2016).
The molecular mechanisms underlying CME are well defined, with clathrin, adaptor protein 2 (AP2) and the large GTPase dynamin being the major players (see Kirchhausen et al., 2014; McMahon and Boucrot, 2011 for recent reviews). CME controls EGFR signaling through various mechanisms. At the PM, clustering of EGFR in clathrin‐coated pits (CCPs) is required to optimize receptor phosphorylation, and to amplify and spatially constrain EGFR signaling (Garay et al., 2015; Ibach et al., 2015). AP2 exerts a critical function during the assembly of CCPs and it is essential to maintain the right vesicle size, with predictable consequences for receptor clustering and signaling (Aguet et al., 2013; Kadlecova et al., 2017; Miller et al., 2015). In addition to AP2, dynamin and the cargo itself tightly regulate the timing of CCP assembly allowing for receptor clustering and productive signaling (Loerke et al., 2009). Some non‐small‐cell lung cancer (NSCLC) cells show an aberrantly accelerated CME, with deregulated CCP initiation and maturation (Chen et al., 2017). This phenotype has been linked to the activation of the neuronal dynamin isoform, dynamin1 (dyn1), in non‐neuronal cells, and/or to the overexpression of the clathrin light chain b (CLCb; Chen et al., 2017). The increased uncontrolled CME rate causes increased EGFR recycling and signaling through AKT, promoting cancer cell survival (Chen et al., 2017). Interestingly, both dyn1 and CLCb are upregulated in NSCLC and breast cancer (reviewed in Schmid, 2017).
In instances in which NCE is activated in parallel to CME, the integration of the two pathways is critical in determining the final signaling response. For instance, in HeLa and in other epithelial cells, CME and NCE determine opposing receptor fates (Sigismund et al., 2008): CME mainly induces receptor recycling (with limited EGFR degradation), while NCE – which requires EGFR ubiquitination as an internalization signal (Sigismund et al., 2005, 2013) – targets the majority of internalized EGFRs to degradation in the lysosome. In this way, CME, which is active at low EGF concentrations, directs the EGFR/EGF complex away from degradation and toward recycling to maintain signaling when ligand is limited. In addition, through recycling, CME also serves to prolong EGFR signaling, a requirement critical to achieve a productive proliferative response, and to polarize EGFR signaling to specific regions of the PM (Bisi et al., 2013; Sigismund et al., 2012). Polarized trafficking of cargo proteins to regions of the PM represents one of the most frequently altered functions of endo/exocytosis in cancer as it is primarily involved in migration and invasion of metastatic cells and in maintenance of epithelial cell polarity (reviewed in Lanzetti and Di Fiore, 2017).
EGFR‐NCE is activated only at high EGF concentrations and is critical for long‐term attenuation of EGFR signaling by directing EGFRs to lysosomal degradation. Recently, the mechanism governing EGFR‐NCE has been elucidated. This endocytic route depends on the function of an endoplasmic reticulum (ER)‐resident protein, reticulon 3 (RTN3), which is involved in the formation of contact sites between the ER and regions of the PM where EGFR‐NCE occurs (Caldieri et al., 2017, also discussed in Section 3.1). This modality of EGFR‐NCE appears to act as a safeguard against excessive EGFR signaling, and might represent a mechanism for modulating EGFR signaling at specific PM regions where polarized functions take place, an issue that deserves further investigation.
Other types of EGFR‐NCE occur at specific PM locations and are connected with cell migration. They include (a) the macropinocytic‐like pathway that originates, in mouse and human fibroblasts, from actin‐based membrane ruffles, defined as circular or dorsal ruffles (Orth et al., 2006), implicated in three‐dimensional cell motility and extracellular matrix degradation (Suetsugu et al., 2003), and (b) the fast endophilin‐mediated endocytosis (FEME). This latter pathway is involved in the internalization of several G‐protein‐coupled receptors and RTKs, including the EGFR, and is active at the leading edge of migrating cells, suggesting its involvement in polarized signaling during cell migration (Boucrot et al., 2015).
Once internalized, EGFRs reach the early endosomes (EEs), a further ‘level’ in the regulation of EGFR signaling. At this station, EGFRs are sorted toward different fates, recycling or degradation (reviewed in Wandinger‐Ness and Zerial, 2014). Receptor recycling is usually the default pathway. Escape from recycling is determined by EGFR ubiquitination, which is an active signal recognized by the ESCRT (endosomal sorting complexes required for transport) complexes that, through a stepwise process, sort receptors into multivesicular bodies (MVBs) and into lysosomes for degradation (reviewed in Raiborg and Stenmark, 2009; Wollert et al., 2009).
Besides sorting, endosomes work as platforms for EGFR signaling. Here, signals originating at the PM can be prolonged – in order to achieve a productive signaling response – and/or diversified – by assembling specific signaling complexes (reviewed in Villasenor et al., 2016). Furthermore, the endosome fusion and fission machinery tightly controls EGFR signaling by keeping the number of EGFR clusters per endosome constant over a wide range of EGF concentrations (Villasenor et al., 2015), thus conferring robustness to the system. Varying the number of EGFR clusters per endosome through alteration of the endosome fission/fusion rate critically impacts the EGFR signaling output, for example, proliferation vs. differentiation (Villasenor et al., 2015).
A novel regulatory mechanism occurring at the EEs has been recently described, which is able to sense the amount of EGFRs trafficking toward the endosomes and to induce de novo receptor biosynthesis and exocytosis, in order to preserve EGFR levels at the PM (Scharaw et al., 2016). When cells are continuously stimulated with high EGF doses, the transcription factor RNF11 translocates from the EEs to the nucleus where it induces transcription of genes required for EGFR transport to the PM (Scharaw et al., 2016). How RNF11 senses the amount of internalized EGFR at the EEs remains an open question.
2.2. EGFR cancer mutants divert from the normal trafficking itinerary
EGFR signaling is frequently altered in several human cancers due to EGFR gene amplification and/or protein overexpression, mutations or in‐frame deletions (Roskoski, 2014). The most frequent mutations in glioblastoma and lung cancer are illustrated in Fig. 1B; this figure also includes mutations found in colorectal cancers that are resistant to antibody‐mediated EGFR blockade]. These genetic lesions often occur concomitantly with increased EGFR ligand production due to autocrine or paracrine loops (Wilson et al., 2009, 2012). In many cases, EGFR genetic alterations determine abnormal EGFR trafficking, which contributes to increased signaling and tumor development. For instance, the increase in EGFR density at the PM due to EGFR amplification/overexpression was shown to stimulate receptor homo‐ and heterodimerization leading to kinase activation (Chung et al., 2010; Sawano et al., 2002; Wiley, 1988; Wilson et al., 2009). In particular, heterodimers with the ligand‐orphan receptor ErbB2 are constitutively active, evade receptor ubiquitination and degradation, and are mostly recycled back to the PM, thereby producing sustained signaling and cell proliferation (Mellman and Yarden, 2013; Schneider and Yarden, 2016). In agreement, saturation of the endocytic and/or the ubiquitination machinery has been proposed as a mechanism underlying sustained signaling in EGFR‐overexpressing cancer cells (Capuani et al., 2015; French et al., 1994; Wiley, 1988).
Oncogenic EGFR mutations and large genetic rearrangements (as observed in glioblastoma, brain, lung, breast, and ovarian cancers) often cause altered receptor endocytosis, which contributes to increased signaling properties (Yarden and Pines, 2012). In some cases, mutations directly disrupt the recruitment site of the E3 ligase, Cbl, in the intracellular domain of the receptor (i.e., EGFRvIV and EGFRvV mutants), thereby affecting receptor ubiquitination and lysosomal degradation (Roskoski, 2014). In other instances, mutations are located in the extracellular domain (i.e., EGFRvIII), leading to ligand‐independent receptor activation (Grandal et al., 2007; Han et al., 2006; Schmidt et al., 2003). Unexpectedly, these mutations also caused hypophosphorylation of the intracellular tyrosine residue 1045, the direct Cbl‐binding site, via an unknown mechanism. In this way, receptor ubiquitination and turnover are affected, resulting in sustained signaling (Grandal et al., 2007; Han et al., 2006; Schmidt et al., 2003). Somatic EGFR activating mutations have been detected in ~ 15–20% of NSCLC patients (Yun et al., 2007). One of the most frequent mutations, L858R, despite having a more highly phosphorylated Cbl‐binding site than the wild‐type receptor, is impaired in Cbl recruitment and receptor ubiquitination, again affecting trafficking toward the lysosome and receptor degradation, with consequent signal upregulation (Kon et al., 2014; Shtiegman et al., 2007). Increased heterodimerization of this mutant with ErbB2 has been proposed to cause this behavior (Kon et al., 2014).
Finally, it is important to stress that besides oncogenic alterations, inappropriate activation of the EGFR in cancer can originate from derailed receptor endocytosis and trafficking (Mellman and Yarden, 2013). This is achieved by two mechanisms: either mutated RTKs hijack the endocytic apparatus, which, in turn, fosters their signaling properties, or altered endocytic/trafficking genes potentiate the duration and the amplitude of the signal (Sigismund et al., 2012). Indeed, alterations in the balance between receptor recycling and degradation have been found in several aggressive cancers (Belle et al., 2015; Boulay et al., 2016). This latter mechanism largely relies on the overexpression and amplification of genes that are involved in RTKs endocytosis and recycling, including several GTPases belonging to the Rab family which control vesicular trafficking (Caswell et al., 2007; Cheng et al., 2004; Frittoli et al., 2014; Kajiho et al., 2016; Wheeler et al., 2015). Increased expression of endocytic/recycling molecules prolongs propagation of the signal and/or re‐locates RTKs and adhesive receptors at specific membrane sites, mainly involved in cancer cell invasion (Caswell et al., 2008; Eppinga et al., 2012; also reviewed in Lanzetti and Di Fiore, 2017; Mellman and Yarden, 2013; Mills et al., 2009; Mosesson et al., 2008; Sigismund et al., 2012). Among these molecules, copy number gain and overexpression of the 5′‐inositol lipid phosphatase synaptojanin 2 (SYNJ2) in breast cancer provides a paradigmatic example of sustained EGFR activation by altered trafficking pathways. Elevation of SYNJ2 promotes EGFR recycling at lamellipodia, stimulating cell motility and the formation of invadopodia (Ben‐Chetrit et al., 2015).
3. Noncanonical kinase‐dependent and kinase‐independent EGFR functions
In this section, we will discuss both kinase‐dependent and kinase‐independent functions of the EGFR that have recently emerged and that diverge from the canonical EGFR signaling pathway. For what concerns kinase‐independent roles, their existence has been known for many years. Indeed, while EGFR‐knockout mice are mid‐gestation or perinatal lethal (depending on the genetic background), due to gross developmental defects (Miettinen et al., 1995; Sibilia and Wagner, 1995; Threadgill et al., 1995), kinase‐dead EGFR‐knock‐in mice are viable, displaying only mild defects in the eye and skin (Luetteke et al., 1994). In addition, the EGFR is able to promote cell survival pathways through both kinase‐dependent and kinase‐independent mechanisms (Ewald et al., 2003; Tan et al., 2016a). These EGFR kinase‐independent functions could result from the heterodimerization of the EGFR with other ErbB family members or could be mediated by kinases that crosstalk with the EGFR pathway (e.g., Src or p38‐MAPK, see Section 3.2). Moreover, inactivation of phosphatases (e.g., PTP1B, see Sections 3.1 and 3.2) might contribute to activation of EGFR signaling. More work is needed to address whether these mechanisms are at play in living cells and whether they are mutually exclusive or coexisting in the regulation of EGFR function.
3.1. ER contact sites regulate EGFR signaling at different steps of the endocytic pathway
Communication between organelles is critical for several fundamental cellular processes, including organelle positioning and function, organelle fission, lipid transport, and Ca2+ signaling (van Bergeijk et al., 2016; Phillips and Voeltz, 2016; Saheki and De Camilli, 2017). Communication occurs through so‐called contact sites: regions of juxtaposition (≤ 20 nm) between two heterologous membranes, tethered by in trans protein–protein interactions (Eisenberg‐Bord et al., 2016; Phillips and Voeltz, 2016). In particular, the ER, due to its tubular organization that extends all over the cell, has been shown to make contact and to exchange materials with all of the other cellular organelles (Phillips and Voeltz, 2016).
ER contact sites have a critical role in controlling EGFR signaling and trafficking at multiple steps. During the initial phase of endocytosis, high doses of EGF are able to induce tubulation of cortical ER and the formation of ER contact sites with the PM, at regions where the EGFR is internalizing via NCE (Caldieri et al., 2017; Fig. 2). The formation of these contact sites is critical to induce local Ca2+ signaling at ER–PM interface, which is in turn required for the fission of NCE tubular intermediates and, thus, for completion of the internalization process (Caldieri et al., 2017). This mechanism ultimately leads to EGFR endocytosis via NCE, receptor degradation, and signal termination (Caldieri et al., 2017; Sigismund et al., 2008). Polarized Ca2+ waves might also be critical in specifying the final EGFR‐NCE signaling output, given the role of Ca2+ in growth factor‐induced cell migration (Tsai et al., 2014), an issue that requires further investigation.
Figure 2.
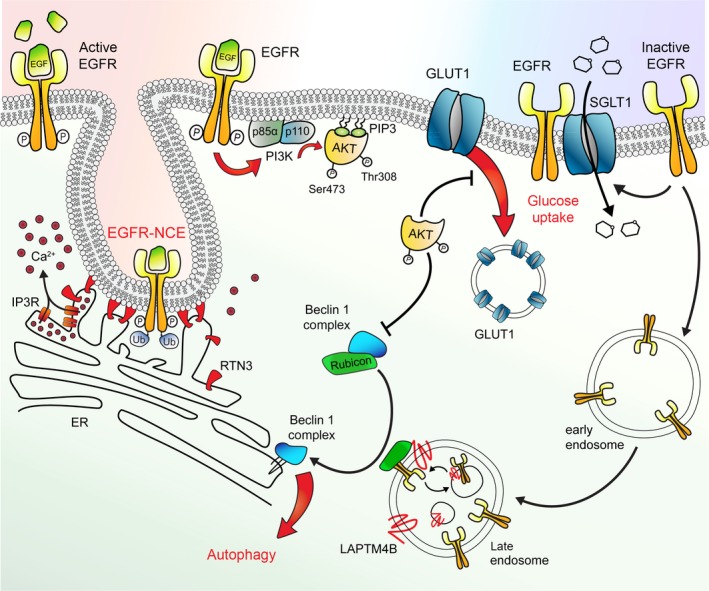
Active and inactive EGFR‐related functions. This picture schematizes some noncanonical EGFR functions. From left to right: EGFR stimulated with high EGF doses (active EGFR) is phosphorylated (P) and ubiquitinated (Ub) and undergoes both clathrin‐mediated endocytosis (not depicted) and nonclathrin‐dependent endocytosis (NCE), the latter dependent on the formation of RTN3‐mediated ER–PM contact sites. This is accompanied by calcium release in the proximity of contact sites, which likely controls fission of the tubular invagination. It is still unclear whether RTN3 is the tethering factor between the ER and the PM (as depicted), or it is just involved the tubulation of cortical ER, but not directly engaged at contact sites. EGFR ligand stimulation elicits the classical signaling cascade based on the recruitment of PI3K (made of its p85 regulatory subunit and p110 catalytic subunit) that catalyzes the formation of PIP3s. PIP3s bind to the PH domain of AKT and of phosphoinositide‐dependent kinase‐1, PDK1. PDK1 phosphorylates AKT on Thr308, while mammalian target of rapamycin complex 2, mTORC2 (not depicted here), is responsible for phosphorylation on Ser 473, leading to full AKT activation. Active AKT inhibits autophagy and blocks GLUT1 endocytosis. This latter function leads to higher levels of GLUT1 at the plasma membrane, increasing the uptake of glucose. In addition, ligand‐independent direct interaction of EGFR (inactive EGFR) and SGLT1 stabilizes the glucose transporter at the cell surface promoting high glucose uptake. Ligand‐unbound EGFR constitutively internalizes into early and late endosomes where it is sequestered by LAPTM4B. Here, the inactive EGFR interacts with Rubicon causing its dissociation from Beclin‐1. Beclin‐1 complex can now initiates autophagy on the ER membrane.
All along the endocytic route, the ER makes contact with the endosomes and these contact sites increase as endosomes traffic and mature (Friedman et al., 2013). ER–endosomal contact sites are critical in defining the timing and position of endosome fission during cargo sorting (Rowland et al., 2014), but they also have a direct role in the regulation of EGFR signaling. Indeed, a major RTK phosphatase, PTP1B, localizes to the cytosolic face of the ER and dephosphorylates the EGFR in trans during its trafficking to the endosomes/MVBs (Eden et al., 2010; Haj et al., 2002). Of note, PTP1B regulates constitutively internalized EGFR, thereby restricting spurious EGFR kinase activation, as well as ligand‐activated receptor that is dephosphorylated by PTP1B on the way to the lysosome (Baumdick et al., 2015). The formation of ER–MVB contact sites is mediated by annexin‐1 and is Ca2+ dependent (Eden et al., 2016; Kilpatrick et al., 2017). The release of Ca2+ occurs through the two‐pore channel that is localized on the endolysosomal membrane at ER contact sites (Kilpatrick et al., 2017). Disrupting these contact sites has been shown to delay PTP1B‐mediated EGFR dephosphorylation, causing delayed receptor degradation and enhanced signaling (Eden et al., 2016; Kilpatrick et al., 2017).
3.2. Stress‐induced EGFR trafficking pathways
Different stresses applied to cells have been shown to stimulate EGFR endocytosis and trafficking in a ligand‐independent fashion. For instance, UV radiation, cisplatin, inflammatory cytokines (tumor necrosis factor α), and the antibiotic anisomycin all trigger p38‐MAPK activation, required for ligand‐independent EGFR internalization (reviewed in Tan et al., 2016a; Tomas et al., 2014).
While the mechanism is similar for all these treatments, it has been most extensively characterized in the case of UV treatment. UV‐stimulated EGFR endocytosis occurs via CME and depends on the phosphorylation of serine/threonine residues in the C‐terminal receptor tail mediated by p38‐MAPK activity (Oksvold et al., 2004; Tomas et al., 2017; Tong et al., 2014; Vergarajauregui et al., 2006; Zwang and Yarden, 2006). Interestingly, other receptors, such as the insulin receptor, c‐MET, and the transferrin receptor, are not internalized upon UV treatment, suggesting the existence of some level of specificity (Zwang and Yarden, 2006). Once internalized, EGFRs accumulate in a subpopulation of MVBs, distinct from the EGF‐induced MVB pool, where they are entrapped into intraluminal vesicles (ILVs) without being degraded (Oksvold et al., 2002; Tomas et al., 2015). The process is reversible as, upon p38‐MAPK inhibition, ILV‐localized EGFRs can be recovered to the limiting MVB membrane from which they are recycled back to the PM (Tomas et al., 2015).
EGFR also responds to hypoxia, which, on the one hand, upregulates the transcription of the EGFR gene, providing a mechanism for EGFR overexpression in the absence of genetic alterations (Franovic et al., 2007); on the other hand, it triggers EGFR Src‐dependent, caveolae‐dependent endocytosis (Shen et al., 2013). At endosomes, EGFRs bind and phosphorylate the endosomal membrane‐associated protein, argonaute 2, a molecule involved in micro‐RNA (miRNA) maturation, causing inhibition of the maturation of tumor suppressor miRNAs, thus promoting cancer cell survival (Shen et al., 2013). A similar mechanism of EGFR internalization and endosomal accumulation appears to be at work also in the case of oxidative stress induced by H2O2 (Filosto et al., 2011; Khan et al., 2006; Ravid et al., 2002). In this case, the generation of reactive oxygen species inactivates redox‐sensitive, cysteine‐based, tyrosine phosphatases, including PTP1B, causing the activation of Src and, possibly, of the EGFR itself (Denu and Tanner, 1998; Lee et al., 1998). Src‐dependent caveolae‐mediated EGFR endocytosis is also activated by ionizing radiation. Importantly, mechanisms of resistance to ionizing radiation depend on the EGFR (Dittmann et al., 2008). Indeed, this treatment increases EGFR expression, induces Src activation and caveolae‐mediated EGFR endocytosis. Phosphorylation of threonine 654 in the EGFR juxtamembrane region by PKCε negatively regulates Cbl‐dependent ubiquitination and promotes EGFR nuclear translocation, leading to enhanced DNA repair and cell survival (Dittmann et al., 2008; Wanner et al., 2008). In agreement, EGFR nuclear localization has been associated with radiation resistance and poor clinical outcome (Tan et al., 2016a; Tomas et al., 2014).
In conclusion, it is emerging that multiple mechanisms of ligand‐independent trafficking are activated under stress conditions and that these mechanisms can promote cancer cell survival. However, more work is needed to molecularly dissect these pathways, in order to clarify how they are regulated, how they interplay with the canonical EGFR pathway, and whether they can be hijacked to prevent resistance to anti‐EGFR therapies.
3.3. Role of EGFR in autophagy
Autophagy is critical in maintaining cellular homeostasis and is finely regulated under physiological conditions to allow cells to rapidly respond to environmental changes. It is deregulated in different pathologies, including neurodegenerative diseases, aging, and cancer, and is one of the major mechanisms promoting resistance to cancer therapies (for recent reviews see, for instance Galluzzi et al., 2015, 2017; Goldsmith et al., 2014; Menzies et al., 2015; Rubinsztein et al., 2012).
The EGFR is a crucial regulator of autophagy. In nutrient‐rich growth conditions, ligand‐activated EGFR has a dual activity: on the one hand, it stimulates cell proliferation; on the other, it inhibits autophagy. Inhibition of autophagy is achieved: (a) directly, through the phosphorylation and consequent inhibition of Beclin‐1, a core subunit of the VPS34/autophagy initiation complex (Wei et al., 2013), and (b) indirectly, through the activation of AKT. In turn, AKT activates the mechanistic target of the rapamycin complex 1 (mTORC1) pathway, which ultimately inhibits autophagy (Tan et al., 2016a).
In contrast, under serum‐starved conditions, inactive EGFR is emerging as a promoter of autophagy. In this case, ligand‐unbound receptors, which constitutively traffic toward the endosomes, are sequestered by the lysosomal‐associated protein transmembrane 4 beta (LAPTM4B), localized in a subpopulation of early and late endosomes. The increased EGFR endosomal pool interacts with the autophagy inhibitor, Rubicon, causing its dissociation from Beclin‐1, leading to Beclin‐1 activation and autophagy initiation (Tan et al., 2015a,2015b; Fig. 2). This function is maintained by the kinase‐dead EGFR mutant, confirming that it is indeed independent of kinase activation (Tan et al., 2015b). The loss of EGFR generates cells defective in autophagy initiation, at variance with the loss of other RTKs, including c‐MET, PDGFR, and FGFR2 (Tan et al., 2015b), suggesting that this is an EGFR‐specific function.
Autophagy initiation seems to occur at ER–endosome contact sites. In particular, to initiate autophagy, autophagy‐related gene 14 on the ER surface has to interact with PIPKIγi5 kinase (PIPKIγi5K), an enzyme localized on endosomal membranes in complex with inactive EGFR and LAPTM4B. This binding stimulates phosphatidylinositol 4,5‐bisphosphate (PIP2) production by PIPKIγi5 and autophagy (Tan et al., 2016b). Thus, ER contact sites seem to provide a platform for autophagic complex assembly.
Interestingly, the ER‐resident protein RTN3, which is required for the establishment of ER–PM contact sites needed for EGFR endocytosis via NCE (Caldieri et al., 2017), has also been implicated in ER turnover by selective autophagy (Grumati et al., 2017). A specific RTN3 isoform, which possesses multiple LC3‐interacting regions, has been found to exert this function (Grumati et al., 2017). Whether these two functions of RTN3 are related, and how they are integrated within the cell, is not yet known; however, they might unveil connections between ligand‐dependent and ligand‐independent EGFR trafficking pathways.
Activation of autophagy has been found to promote resistance and survival of cancer cells treated with EGFR kinase inhibitors (Tan et al., 2016b). The mechanism seems to resemble the one induced by LAPTM4B in the physiological context. Indeed, these compounds promote endosomal accumulation of the EGFR, enhancing its association with Rubicon and favoring the dissociation of Rubicon/Beclin‐1 complex, thereby initiating the autophagic flux (Tan et al., 2015a). It is possible that other stresses causing EGFR endosomal accumulation (discussed in Section 3.2) might also activate autophagy as a part of their survival response, a scenario that deserves further investigation.
3.4. Mitochondrial functions of EGFR
The EGFR is usually considered to act at the PM and on vesicles mainly belonging to the endosomal compartment. However, it also localizes to the nucleus and mitochondria. Translocation of full‐length EGFR into the nucleus has long been documented and the functions it has at this location have been extensively investigated; we therefore refer the readers to detailed reviews (Brand et al., 2011; Han and Lo, 2012). Differently, the role of EGFR in mitochondria is more elusive and has been connected with antiapoptotic and metabolic functions.
In NSCLC cells, high levels of EGFR expression have been detected in the mitochondria (Che et al., 2015). In these cells, artificially mitochondria‐targeted EGFR redistributes these organelles to lamellipodia, increasing cell motility, possibly through the localized increase in energy (Che et al., 2015). In addition, translocation of wild‐type EGFR and of the EGFRvIII mutant into mitochondria has also been observed in cells treated with kinase inhibitors, or following proapoptotic stimuli (Cao et al., 2011). This translocation correlates with resistance to apoptosis and decreased sensitivity to EGFR inhibition (Cao et al., 2011). The latter function might be related to the ability of both wild‐type EGFR and EGFRvIII to constitutively bind to p53‐upregulated modulator of apoptosis (PUMA), a proapoptotic member of the Bcl‐2 family of proteins primarily located in the mitochondria (Zhu et al., 2010).
In breast cancer cells, translocation of EGFR to mitochondria has been shown to occur upon EGF stimulation resulting in phosphorylation of the cytochrome c oxidase subunit II (Boerner et al., 2004; Demory et al., 2009). The biological outcome of this modification is not clear. However, this event requires phosphorylation of the EGFR on tyrosine 845 by Src, which also undergoes mitochondrial translocation with similar kinetics to that of the EGFR (Demory et al., 2009). Of note, EGF stimulation also induces palmitoylation of mitochondrial EGFR, which, in turn, favors fusion of mitochondria (Bollu et al., 2014). EGFR, independently of its kinase activity, interacts with the fatty acid synthase, stimulating de novo synthesis of palmitate (Bollu et al., 2014). This finding points to the involvement of the EGFR in the regulation of cell metabolism and supports the existence of a signaling‐metabolic wiring that plays a critical role in cancer.
3.5. Role of EGFR in cancer cell metabolism
Oncogenic signaling pathways induce metabolic reprogramming in cancer cells supporting tumor growth (Cairns et al., 2011). In this context, EGFR signaling has been involved in the regulation of several metabolic processes that are critical for cancer cell proliferation: from the biosynthesis of fatty acids and pyrimidines, to glucose catabolism (Guo et al., 2009; Makinoshima et al., 2014). The EGFR promotes these metabolic pathways both directly by phosphorylating rate‐limiting enzymes (Lim et al., 2016; Zhang et al., 2017), or indirectly through activation of the MYC transcription factor and of the AKT signaling cascade (Babic et al., 2013; Guo et al., 2009; Makinoshima et al., 2014, 2015, and reviewed in DeBerardinis and Chandel, 2016; Masui et al., 2014).
In glioblastoma multiforme, oncogenic EGFR signaling by EGFRvIII stimulates the PI3K/AKT‐dependent nuclear translocation of sterol regulatory element‐binding protein 1 (SREBP‐1) and the expression of the low‐density lipoprotein receptor (LDLR). Increased LDLR, in turn, allows for the uptake of cholesterol bypassing negative feedback regulation (Guo et al., 2009). This represents a point of metabolic vulnerability as these cells depend on cholesterol uptake and are highly sensitive to inhibitors of fatty acid and cholesterol biosynthesis (Guo et al., 2011).
Furthermore, the EGFR has been recently found to directly phosphorylate and, thereby, stabilize stearoyl‐CoA desaturase‐1 (SCD1), resulting in the upregulation of monounsaturated fatty acid production (Zhang et al., 2017). Notably, phosphorylation of SDC1 correlates with poor prognosis of glioblastoma multiforme (Zhang et al., 2017), suggesting that it might have a causative role in these tumors.
One of the best‐studied metabolic drifts in cancer cells is the elevation of glycolysis in the presence of oxygen: the Warburg effect. Cancer cells are generally characterized by the avid uptake of glucose, which occurs through increased expression and membrane localization of glucose transporters, mainly GLUT1 and GLUT3 (Barron et al., 2016). Intracellular glucose is metabolized to pyruvate that, in cancer cells, is preferentially converted into lactate (Cairns et al., 2011).
The EGFR has been shown to foster aerobic glycolysis through several, both kinase‐dependent and kinase‐independent, mechanisms (Fig. 2). Physical association of EGFR with SGLT1 stabilizes the sodium‐glucose cotransporter at the cell surface increasing the glucose influx (Weihua et al., 2008). This kinase‐independent function provides survival advantages to cells, helping them escape autophagic cell death when grown in the presence of low glucose concentrations (Weihua et al., 2008).
In response to EGF stimulation, the EGFR controls expression of hexokinase (HK1) and phosphorylation of the pyruvate kinase M2 (PKM2), two glycolytic enzymes that catalyze key steps in the pathway, thus increasing aerobic glycolysis of breast cancer cells (Lim et al., 2016). One relevant ‘side effect’ of increased aerobic glycolysis is the production of high levels of lactate that, in these tumors, inhibits the cytotoxic activity of T cells, supporting their immune escape (Lim et al., 2016).
In lung adenocarcinoma cells bearing oncogenic EGFR mutations, deregulated signaling has been shown to stabilize GLUT1 at the cell surface through the activation of the PI3K/AKT/mTOR pathway (Makinoshima et al., 2015). Indeed, activation of AKT in response to cytokine stimulation has long been known to inhibit endocytosis of GLUT1 in lymphoid cells (Wieman et al., 2007; Wofford et al., 2008). Recent findings showing that AKT phosphorylates and inhibits thioredoxin‐interacting protein (TXNIP), the endocytic adaptor responsible for CME of GLUT1 (Hong et al., 2016; Waldhart et al., 2017), suggest that this might be the mechanism at work.
Of note, inhibition of the PI3K/AKT/mTOR pathway in lung cancer cells harboring EGFR mutations affects the glycolytic flux impairing their viability (Makinoshima et al., 2015). In line with these findings, combined inhibition of EGFR and glycolysis has been shown to synergistically suppress proliferation of triple‐negative breast cancer cells (Lim et al., 2016), further supporting the relevance of EGFR signaling in cancer cell metabolism.
3.6. Membrane trafficking influences the efficacy of EGFR‐targeted therapies
Given its critical role in cancer, several EGFR‐targeted therapies have been developed, including monoclonal humanized antibodies (mAbs) directed against the receptor extracellular domain, as well selective small‐molecule inhibitors targeting the tyrosine kinase domain. Small‐molecule EGFR inhibitors (e.g., gefitinib, erlotinib, and afatinib) have been approved for lung cancer treatment as a first‐line therapy in those cases where EGFR mutations have been confirmed (Cohen et al., 2005; Hirsch et al., 2013; Thatcher et al., 2005). Interestingly, in addition to kinase inhibition, gefitinib was shown to increase the formation of inactive EGFR dimers through some form of communication between the kinase domain and the extracellular dimerization domain, suggesting the possibility that gefitinib‐induced dimers could be more rapidly endocytosed and degraded (Arteaga et al., 1997; Gan et al., 2007), an issue that warrants further studies.
Cetuximab and panitumumab are the most widely employed EGFR‐neutralizing monoclonal antibodies, used for the treatment of head and neck cancer and metastatic colon cancer (Licitra et al., 2013; Peeters et al., 2015; Pierotti et al., 2010). Mechanistically, these compounds act by preventing ligand binding, thereby inhibiting receptor activation and downstream signaling (Bou‐Assaly and Mukherji, 2010; Dubois and Cohen, 2009; Vincenzi et al., 2008). They also favor EGFR dimerization, which, in turn, causes internalization of antibody‐bound dimers. These complexes are internalized at a lower rate and are more efficiently recycled to the PM compared with EGF‐bound dimers (Jaramillo et al., 2006). The combined use of anti‐EGFR antibodies directed against nonoverlapping antigens appears to be a more efficient strategy than the use of single antibodies, as it increases EGFR endocytosis and degradation (Ferraro et al., 2013; Friedman et al., 2005; Pedersen et al., 2010), raising the possibility of improving antitumor efficacy through the regulation of EGFR trafficking.
Currently, however, EGFR antibody‐based therapies, as well as small‐molecule inhibitors, have been shown to exert a limited response and to frequently evoke resistance in patients due to (a) secondary mutations within the EGFR itself (e.g., T790M in NSCLC, and mutations found in the extracellular domain of cetuximab‐resistant colorectal cancers, Fig. 1B), (b) alterations in other kinases (e.g., c‐MET, PIK3CA, BRAF, MAPK1), or (c) the emergence of feedback regulatory loops and mechanisms that overcome EGFR kinase inhibition (reviewed in Mancini and Yarden, 2016). In the latter case, the effect of therapies might be dampened by the activation of ligand‐independent EGFR trafficking pathways and functions, such as increased autophagy and elevated aerobic glycolysis (discussed in Sections 3.3 and 3.5). In addition, mechanisms that likely contribute to the emergence of drug resistance include also (a) relocalization of the EGFR to the nucleus following ionizing irradiation to promote DNA repair (Liccardi et al., 2011; Szumiel, 2006) and (b) translocation to mitochondria upon kinase inhibitor treatment to exert antiapoptotic effects (Cao et al., 2011; detailed in Section 3.4).
Concluding remarks
The EGFR has long been considered the prototype of all RTKs. Indeed, most of the knowledge accumulated on signal transduction cascades in general and on the mechanisms underlying receptor endocytosis, recycling, and degradation has derived from studies focused on the EGFR. Nevertheless, novel unexpected functions of this receptor continue to emerge, some of which are linked to previously unrecognized subcellular localizations. Thus, despite the large body of knowledge already accumulated, this receptor still holds a number of surprises.
An emerging aspect that could be exploited for cancer treatment is the study of how membrane trafficking can influence the outcome of EGFR‐targeted therapies. Findings in this area could increase efficacy and overcome or delay the occurrence of resistance to treatments, an adverse event that invariably occurs in the patient population. Recently, in an attempt to overcome tumor resistance, simultaneous targeting of driver mutations and basic cellular processes has been proposed as a promising therapeutic perspective (Nagel et al., 2016). In this framework, endocytosis/recycling, autophagy, and metabolism might represent targets for the development of inhibitory tools to be tested in combination with EGFR inhibitors (Mellman and Yarden, 2013). A similar approach is currently being undertaken in tumors where the oncogenic EGFR signaling promotes metabolic reprogramming with promising results.
Acknowledgements
We thank Rosalind Gunby for critically reading the manuscript. This work was supported by grants from WWCR (Worldwide Cancer Research) to SS (16‐1245), the Associazione Italiana per la Ricerca sul Cancro (AIRC) Investigator Grant, Project 15180 to LL, and Fondo Ricerca Locale 2017 (University of Turin) to LL.
Contributor Information
Sara Sigismund, Email: sara.sigismund@ifom.eu.
Letizia Lanzetti, Email: letizia.lanzetti@ircc.it.
References
- Aguet F, Antonescu CN, Mettlen M, Schmid SL and Danuser G (2013) Advances in analysis of low signal‐to‐noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev Cell 26, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena S, Bellosillo B, Siravegna G, Martínez A, Cañadas I, Lazzari L, Ferruz N, Russo M, Misale S, González I et al (2015) Emergence of multiple EGFR extracellular mutations during cetuximab treatment in colorectal cancer. Clin Cancer Res 21, 2157–2166. [DOI] [PubMed] [Google Scholar]
- Arena S, Siravegna G, Mussolin B, Kearns JD, Wolf BB, Misale S, Lazzari L, Bertotti A, Trusolino L, Adjei AA et al (2016) MM‐151 overcomes acquired resistance to cetuximab and panitumumab in colorectal cancers harboring EGFR extracellular domain mutations. Sci Transl Med 8, 324ra314. [DOI] [PubMed] [Google Scholar]
- Arteaga CL, Ramsey TT, Shawver LK and Guyer CA (1997) Unliganded epidermal growth factor receptor dimerization induced by direct interaction of quinazolines with the ATP binding site. J Biol Chem 272, 23247–23254. [DOI] [PubMed] [Google Scholar]
- Babic I, Anderson ES, Tanaka K, Guo D, Masui K, Li B, Zhu S, Gu Y, Villa GR, Akhavan D et al (2013) EGFR mutation‐induced alternative splicing of Max contributes to growth of glycolytic tumors in brain cancer. Cell Metab 17, 1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri E, Di Fiore PP and Sigismund S (2016) Endocytic control of signaling at the plasma membrane. Curr Opin Cell Biol 39, 21–27. [DOI] [PubMed] [Google Scholar]
- Barron CC, Bilan PJ, Tsakiridis T and Tsiani E (2016) Facilitative glucose transporters: implications for cancer detection, prognosis and treatment. Metabolism 65, 124–139. [DOI] [PubMed] [Google Scholar]
- Baulida J, Kraus MH, Alimandi M, Di Fiore PP and Carpenter G (1996) All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem 271, 5251–5257. [DOI] [PubMed] [Google Scholar]
- Baumdick M, Bruggemann Y, Schmick M, Xouri G, Sabet O, Davis L, Chin JW, Bastiaens PI (2015) EGF‐dependent re‐routing of vesicular recycling switches spontaneous phosphorylation suppression to EGFR signaling. Elife 4, e12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle L, Ali N, Lonic A, Li X, Paltridge JL, Roslan S, Herrmann D, Conway JR, Gehling FK, Bert AG et al (2015) The tyrosine phosphatase PTPN14 (Pez) inhibits metastasis by altering protein trafficking. Sci Signal 8, ra18. [DOI] [PubMed] [Google Scholar]
- Ben‐Chetrit N, Chetrit D, Russell R, Körner C, Mancini M, Abdul‐Hai A, Itkin T, Carvalho S, Cohen‐Dvashi H, Koestler WJ et al (2015) Synaptojanin 2 is a druggable mediator of metastasis and the gene is overexpressed and amplified in breast cancer. Sci Signal 8, ra7. [DOI] [PubMed] [Google Scholar]
- van Bergeijk P, Hoogenraad CC and Kapitein LC (2016) Right time, right place: probing the functions of organelle positioning. Trends Cell Biol 26, 121–134. [DOI] [PubMed] [Google Scholar]
- Bergeron JJ, Di Guglielmo GM, Dahan S, Dominguez M and Posner BI (2016) Spatial and temporal regulation of receptor tyrosine kinase activation and intracellular signal transduction. Annu Rev Biochem 85, 573–597. [DOI] [PubMed] [Google Scholar]
- Bisi S, Disanza A, Malinverno C, Frittoli E, Palamidessi A and Scita G (2013) Membrane and actin dynamics interplay at lamellipodia leading edge. Curr Opin Cell Biol 25, 565–573. [DOI] [PubMed] [Google Scholar]
- Boerner JL, Demory ML, Silva C and Parsons SJ (2004) Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol Cell Biol 24, 7059–7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollu LR, Ren J, Blessing AM, Katreddy RR, Gao G, Xu L, Wang J, Su F and Weihua Z (2014) Involvement of de novo synthesized palmitate and mitochondrial EGFR in EGF induced mitochondrial fusion of cancer cells. Cell Cycle 13, 2415–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou‐Assaly W and Mukherji S (2010) Cetuximab (Erbitux). AJNR Am J Neuroradiol 31, 626–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucrot E, Ferreira AP, Almeida‐Souza L, Debard S, Vallis Y, Howard G, Bertot L, Sauvonnet N and McMahon HT (2015) Endophilin marks and controls a clathrin‐independent endocytic pathway. Nature 517, 460–465. [DOI] [PubMed] [Google Scholar]
- Boulay PL, Mitchell L, Turpin J, Huot‐Marchand J, Lavoie C, Sanguin‐Gendreau V, Jones L, Mitra S, Livingstone JM, Campbell S et al (2016) Rab11‐FIP1C Is a critical negative regulator in ErbB2‐mediated mammary tumor progression. Cancer Res 76, 2662–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand TM, Iida M, Li C and Wheeler DL (2011) The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov Med 12, 419–432. [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Harris IS and Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11, 85–95. [DOI] [PubMed] [Google Scholar]
- Caldieri G, Barbieri E, Nappo G, Raimondi A, Bonora M, Conte A, Verhoef L, Confalonieri S, Malabarba MG, Bianchi F et al (2017) Reticulon 3‐dependent ER‐PM contact sites control EGFR nonclathrin endocytosis. Science 356, 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Zhu H, Ali‐Osman F and Lo HW (2011) EGFR and EGFRvIII undergo stress‐ and EGFR kinase inhibitor‐induced mitochondrial translocalization: a potential mechanism of EGFR‐driven antagonism of apoptosis. Mol Cancer 10, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuani F, Conte A, Argenzio E, Marchetti L, Priami C, Polo S, Di Fiore PP, Sigismund S and Ciliberto A (2015) Quantitative analysis reveals how EGFR activation and downregulation are coupled in normal but not in cancer cells. Nat Commun 6, 7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier JL, Gorden P, Anderson RG, Goldstein JL, Brown MS, Cohen S and Orci L (1982) Co‐localization of 125I‐epidermal growth factor and ferritin‐low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol 95, 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D and Norman JC (2008) Rab‐coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol 183, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI et al (2007) Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell 13, 496–510. [DOI] [PubMed] [Google Scholar]
- Che TF, Lin CW, Wu YY, Chen YJ, Han CL, Chang YL, Wu CT, Hsiao TH, Hong TM and Yang PC (2015) Mitochondrial translocation of EGFR regulates mitochondria dynamics and promotes metastasis in NSCLC. Oncotarget 6, 37349–37366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PH, Bendris N, Hsiao YJ, Reis CR, Mettlen M, Chen HY, Yu SL and Schmid SL (2017) Crosstalk between CLCb/Dyn1‐mediated adaptive clathrin‐mediated endocytosis and epidermal growth factor receptor signaling increases metastasis. Dev Cell 40(278–288), e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, Liu J, Smith‐McCune K, Lu KH, Fishman D et al (2004) The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med 10, 1251–1256. [DOI] [PubMed] [Google Scholar]
- Chung I, Akita R, Vandlen R, Toomre D, Schlessinger J and Mellman I (2010) Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature 464, 783–787. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Johnson JR, Chen YF, Sridhara R and Pazdur R (2005) FDA drug approval summary: erlotinib (Tarceva) tablets. Oncologist 10, 461–466. [DOI] [PubMed] [Google Scholar]
- Conte A and Sigismund S (2016) Chapter Six – The ubiquitin network in the control of EGFR endocytosis and signaling. Prog Mol Biol Transl Sci 141, 225–276. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ and Chandel NS (2016) Fundamentals of cancer metabolism. Sci Adv 2, e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demory ML, Boerner JL, Davidson R, Faust W, Miyake T, Lee I, Hüttemann M, Douglas R, Haddad G and Parsons SJ (2009) Epidermal growth factor receptor translocation to the mitochondria: regulation and effect. J Biol Chem 284, 36592–36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu JM and Tanner KG (1998) Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry 37, 5633–5642. [DOI] [PubMed] [Google Scholar]
- Dittmann K, Mayer C, Kehlbach R and Rodemann HP (2008) Radiation‐induced caveolin‐1 associated EGFR internalization is linked with nuclear EGFR transport and activation of DNA‐PK. Mol Cancer 7, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois EA and Cohen AF (2009) Panitumumab. Br J Clin Pharmacol 68, 482–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden ER, Sanchez‐Heras E, Tsapara A, Sobota A, Levine TP and Futter CE (2016) Annexin A1 tethers membrane contact sites that mediate ER to endosome cholesterol transport. Dev Cell 37, 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden ER, White IJ, Tsapara A and Futter CE (2010) Membrane contacts between endosomes and ER provide sites for PTP1B‐epidermal growth factor receptor interaction. Nat Cell Biol 12, 267–272. [DOI] [PubMed] [Google Scholar]
- Eisenberg‐Bord M, Shai N, Schuldiner M and Bohnert M (2016) A tether is a tether is a tether: tethering at membrane contact sites. Dev Cell 39, 395–409. [DOI] [PubMed] [Google Scholar]
- Eppinga RD, Krueger EW, Weller SG, Zhang L, Cao H and McNiven MA (2012) Increased expression of the large GTPase dynamin 2 potentiates metastatic migration and invasion of pancreatic ductal carcinoma. Oncogene 31, 1228–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald JA, Wilkinson JC, Guyer CA and Staros JV (2003) Ligand‐ and kinase activity‐independent cell survival mediated by the epidermal growth factor receptor expressed in 32D cells. Exp Cell Res 282, 121–131. [DOI] [PubMed] [Google Scholar]
- Ferraro DA, Gaborit N, Maron R, Cohen‐Dvashi H, Porat Z, Pareja F, Lavi S, Lindzen M, Ben‐Chetrit N, Sela M et al (2013) Inhibition of triple‐negative breast cancer models by combinations of antibodies to EGFR. Proc Natl Acad Sci USA 110, 1815–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosto S, Khan EM, Tognon E, Becker C, Ashfaq M, Ravid T and Goldkorn T (2011) EGF receptor exposed to oxidative stress acquires abnormal phosphorylation and aberrant activated conformation that impairs canonical dimerization. PLoS One 6, e23240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franovic A, Gunaratnam L, Smith K, Robert I, Patten D and Lee S (2007) Translational up‐regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci USA 104, 13092–13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed DM, Bessman NJ, Kiyatkin A, Salazar‐Cavazos E, Byrne PO, Moore JO, Valley CC, Ferguson KM, Leahy DJ, Lidke DS et al (2017) EGFR ligands differentially stabilize receptor dimers to specify signaling kinetics. Cell 171, 683–695.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AR, Sudlow GP, Wiley HS and Lauffenburger DA (1994) Postendocytic trafficking of epidermal growth factor‐receptor complexes is mediated through saturable and specific endosomal interactions. J Biol Chem 269, 15749–15755. [PubMed] [Google Scholar]
- Friedman JR, Dibenedetto JR, West M, Rowland AA and Voeltz GK (2013) Endoplasmic reticulum‐endosome contact increases as endosomes traffic and mature. Mol Biol Cell 24, 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LM, Rinon A, Schechter B, Lyass L, Lavi S, Bacus SS, Sela M and Yarden Y (2005) Synergistic down‐regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy. Proc Natl Acad Sci USA 102, 1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frittoli E, Palamidessi A, Marighetti P, Confalonieri S, Bianchi F, Malinverno C, Mazzarol G, Viale G, Martin‐Padura I, Garré M et al (2014) A RAB5/RAB4 recycling circuitry induces a proteolytic invasive program and promotes tumor dissemination. J Cell Biol 206, 307–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo‐San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI et al (2017) Molecular definitions of autophagy and related processes. EMBO J 36, 1811–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Pietrocola F, Bravo‐San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V et al (2015) Autophagy in malignant transformation and cancer progression. EMBO J 34, 856–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan HK, Walker F, Burgess AW, Rigopoulos A, Scott AM and Johns TG (2007) The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor AG1478 increases the formation of inactive untethered EGFR dimers. Implications for combination therapy with monoclonal antibody 806. J Biol Chem 282, 2840–2850. [DOI] [PubMed] [Google Scholar]
- Garay C, Judge G, Lucarelli S, Bautista S, Pandey R, Singh T and Antonescu CN (2015) Epidermal growth factor‐stimulated Akt phosphorylation requires clathrin or ErbB2 but not receptor endocytosis. Mol Biol Cell 26, 3504–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh LK, Huang F, Kim W, Gygi S and Sorkin A (2010) Multiple mechanisms collectively regulate clathrin‐mediated endocytosis of the epidermal growth factor receptor. J Cell Biol 189, 871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith J, Levine B and Debnath J (2014) Autophagy and cancer metabolism. Methods Enzymol 542, 25–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandal MV, Zandi R, Pedersen MW, Willumsen BM, van Deurs B and Poulsen HS (2007) EGFRvIII escapes down‐regulation due to impaired internalization and sorting to lysosomes. Carcinogenesis 28, 1408–1417. [DOI] [PubMed] [Google Scholar]
- Grumati P, Morozzi G, Holper S, Mari M, Harwardt MI, Yan R, Muller S, Reggiori F, Heilemann M, Dikic I (2017) Full length RTN3 regulates turnover of tubular endoplasmic reticulum via selective autophagy. Elife 6, e25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Prins RM, Dang J, Kuga D, Iwanami A, Soto H, Lin KY, Huang TT, Akhavan D, Hock MB et al (2009) EGFR signaling through an Akt‐SREBP‐1‐dependent, rapamycin‐resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal 2, ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, Kuga D, Amzajerdi AN, Soto H, Zhu S et al (2011) An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP‐1/LDLR‐dependent pathway. Cancer Discov 1, 442–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj FG, Verveer PJ, Squire A, Neel BG and Bastiaens PI (2002) Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science 295, 1708–1711. [DOI] [PubMed] [Google Scholar]
- Han W and Lo HW (2012) Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett 318, 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Zhang T, Yu H, Foulke JG and Tang CK (2006) Hypophosphorylation of residue Y1045 leads to defective downregulation of EGFRvIII. Cancer Biol Ther 5, 1361–1368. [DOI] [PubMed] [Google Scholar]
- Hanover JA, Willingham MC and Pastan I (1984) Kinetics of transit of transferrin and epidermal growth factor through clathrin‐coated membranes. Cell 39, 283–293. [DOI] [PubMed] [Google Scholar]
- Hirsch FR, Janne PA, Eberhardt WE, Cappuzzo F, Thatcher N, Pirker R, Choy H, Kim ES, Paz‐Ares L, Gandara DR et al (2013) Epidermal growth factor receptor inhibition in lung cancer: status 2012. J Thorac Oncol 8, 373–384. [DOI] [PubMed] [Google Scholar]
- Hong SY, Yu FX, Luo Y and Hagen T (2016) Oncogenic activation of the PI3K/Akt pathway promotes cellular glucose uptake by downregulating the expression of thioredoxin‐interacting protein. Cell Signal 28, 377–383. [DOI] [PubMed] [Google Scholar]
- Huang F, Kirkpatrick D, Jiang X, Gygi S and Sorkin A (2006) Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell 21, 737–748. [DOI] [PubMed] [Google Scholar]
- Ibach J, Radon Y, Gelleri M, Sonntag MH, Brunsveld L, Bastiaens PI and Verveer PJ (2015) Single particle tracking reveals that EGFR signaling activity is amplified in clathrin‐coated pits. PLoS One 10, e0143162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K and Takaoka A (2006) Comparing antibody and small‐molecule therapies for cancer. Nat Rev Cancer 6, 714–727. [DOI] [PubMed] [Google Scholar]
- Jaramillo ML, Leon Z, Grothe S, Paul‐Roc B, Abulrob A and O'Connor McCourt M (2006) Effect of the anti‐receptor ligand‐blocking 225 monoclonal antibody on EGF receptor endocytosis and sorting. Exp Cell Res 312, 2778–2790. [DOI] [PubMed] [Google Scholar]
- Jiang X, Huang F, Marusyk A and Sorkin A (2003) Grb2 regulates internalization of EGF receptors through clathrin‐coated pits. Mol Biol Cell 14, 858–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutten B, Keulers TG, Schaaf MB, Savelkouls K, Theys J, Span PN, Vooijs MA, Bussink J and Rouschop KM (2013) EGFR overexpressing cells and tumors are dependent on autophagy for growth and survival. Radiother Oncol 108, 479–483. [DOI] [PubMed] [Google Scholar]
- Kadlecova Z, Spielman SJ, Loerke D, Mohanakrishnan A, Reed DK and Schmid SL (2017) Regulation of clathrin‐mediated endocytosis by hierarchical allosteric activation of AP2. J Cell Biol 216, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiho H, Kajiho Y, Frittoli E, Confalonieri S, Bertalot G, Viale G, Di Fiore PP, Oldani A, Garre M, Beznoussenko GV et al (2016) RAB2A controls MT1‐MMP endocytic and E‐cadherin polarized Golgi trafficking to promote invasive breast cancer programs. EMBO Rep 17, 1061–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan EM, Heidinger JM, Levy M, Lisanti MP, Ravid T and Goldkorn T (2006) Epidermal growth factor receptor exposed to oxidative stress undergoes Src‐ and caveolin‐1‐dependent perinuclear trafficking. J Biol Chem 281, 14486–14493. [DOI] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Hockey LN, Yates E, Futter CE and Patel S (2017) An endosomal NAADP‐sensitive two‐pore Ca2+ channel regulates ER‐endosome membrane contact sites to control growth factor signaling. Cell Rep 18, 1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T, Owen D and Harrison SC (2014) Molecular structure, function, and dynamics of clathrin‐mediated membrane traffic. Cold Spring Harb Perspect Biol 6, a016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon S, Kobayashi N and Satake M (2014) Altered trafficking of mutated growth factor receptors and their associated molecules: implication for human cancers. Cell Logist 4, e28461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E, Zorn JA, Huang Y, Barros T and Kuriyan J (2015) A structural perspective on the regulation of the epidermal growth factor receptor. Annu Rev Biochem 84, 739–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetti L, Di Fiore PP (2017) Behind the scenes: endo/exocytosis in the acquisition of metastatic traits. Cancer Res 77, 1813–1817. [DOI] [PubMed] [Google Scholar]
- Lee SR, Kwon KS, Kim SR and Rhee SG (1998) Reversible inactivation of protein‐tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem 273, 15366–15372. [DOI] [PubMed] [Google Scholar]
- Lemmon MA and Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J and Ferguson KM (2014) The EGFR family: not so prototypical receptor tyrosine kinases. Cold Spring Harb Perspect Biol 6, a020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenferink AE, Pinkas‐Kramarski R, van de Poll ML, van Vugt MJ, Klapper LN, Tzahar E, Waterman H, Sela M, van Zoelen EJ and Yarden Y (1998) Differential endocytic routing of homo‐ and hetero‐dimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J 17, 3385–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B and Yarden Y (1998) c‐Cbl/Sli‐1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev 12, 3663–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liccardi G, Hartley JA and Hochhauser D (2011) EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res 71, 1103–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licitra L, Storkel S, Kerr KM, Van Cutsem E, Pirker R, Hirsch FR, Vermorken JB, von Heydebreck A, Esser R, Celik I et al (2013) Predictive value of epidermal growth factor receptor expression for first‐line chemotherapy plus cetuximab in patients with head and neck and colorectal cancer: analysis of data from the EXTREME and CRYSTAL studies. Eur J Cancer 49, 1161–1168. [DOI] [PubMed] [Google Scholar]
- Lim SO, Li CW, Xia W, Lee HH, Chang SS, Shen J, Hsu JL, Raftery D, Djukovic D, Gu H et al (2016) EGFR signaling enhances aerobic glycolysis in triple‐negative breast cancer cells to promote tumor growth and immune escape. Cancer Res 76, 1284–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loerke D, Mettlen M, Yarar D, Jaqaman K, Jaqaman H, Danuser G and Schmid SL (2009) Cargo and dynamin regulate clathrin‐coated pit maturation. PLoS Biol 7, e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetteke NC, Phillips HK, Qiu TH, Copeland NG, Earp HS, Jenkins NA and Lee DC (1994) The mouse waved‐2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev 8, 399–413. [DOI] [PubMed] [Google Scholar]
- Makinoshima H, Takita M, Matsumoto S, Yagishita A, Owada S, Esumi H and Tsuchihara K (2014) Epidermal growth factor receptor (EGFR) signaling regulates global metabolic pathways in EGFR‐mutated lung adenocarcinoma. J Biol Chem 289, 20813–20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinoshima H, Takita M, Saruwatari K, Umemura S, Obata Y, Ishii G, Matsumoto S, Sugiyama E, Ochiai A, Abe R et al (2015) Signaling through the phosphatidylinositol 3‐kinase (PI3K)/mammalian target of rapamycin (mTOR) axis is responsible for aerobic glycolysis mediated by glucose transporter in epidermal growth factor receptor (EGFR)‐mutated lung adenocarcinoma. J Biol Chem 290, 17495–17504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M and Yarden Y (2016) Mutational and network level mechanisms underlying resistance to anti‐cancer kinase inhibitors. Semin Cell Dev Biol 50, 164–176. [DOI] [PubMed] [Google Scholar]
- Masui K, Cavenee WK and Mischel PS (2014) mTORC2 in the center of cancer metabolic reprogramming. Trends Endocrinol Metab 25, 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT and Boucrot E (2011) Molecular mechanism and physiological functions of clathrin‐mediated endocytosis. Nat Rev Mol Cell Biol 12, 517–533. [DOI] [PubMed] [Google Scholar]
- Mellman I and Yarden Y (2013) Endocytosis and cancer. Cold Spring Harb Perspect Biol 5, a016949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies FM, Fleming A and Rubinsztein DC (2015) Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 16, 345–357. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z and Derynck R (1995) Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 376, 337–341. [DOI] [PubMed] [Google Scholar]
- Miller SE, Mathiasen S, Bright NA, Pierre F, Kelly BT, Kladt N, Schauss A, Merrifield CJ, Stamou D, Honing S et al (2015) CALM regulates clathrin‐coated vesicle size and maturation by directly sensing and driving membrane curvature. Dev Cell 33, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills GB, Jurisica I, Yarden Y and Norman JC (2009) Genomic amplicons target vesicle recycling in breast cancer. J Clin Invest 119, 2123–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, Salido M, Gallen M, Marsters S, Tsai SP et al (2012) Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med 18, 221–223. [DOI] [PubMed] [Google Scholar]
- Mosesson Y, Mills GB and Yarden Y (2008) Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer 8, 835–850. [DOI] [PubMed] [Google Scholar]
- Nagel R, Semenova EA and Berns A (2016) Drugging the addict: non‐oncogene addiction as a target for cancer therapy. EMBO Rep 17, 1516–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksvold MP, Huitfeldt HS, Ostvold AC and Skarpen E (2002) UV induces tyrosine kinase‐independent internalisation and endosome arrest of the EGF receptor. J Cell Sci 115, 793–803. [DOI] [PubMed] [Google Scholar]
- Oksvold MP, Thien CB, Widerberg J, Chantry A, Huitfeldt HS and Langdon WY (2004) UV‐radiation‐induced internalization of the epidermal growth factor receptor requires distinct serine and tyrosine residues in the cytoplasmic carboxy‐terminal domain. Radiat Res 161, 685–691. [DOI] [PubMed] [Google Scholar]
- Orth JD, Krueger EW, Weller SG and McNiven MA (2006) A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res 66, 3603–3610. [DOI] [PubMed] [Google Scholar]
- Pedersen MW, Jacobsen HJ, Koefoed K, Hey A, Pyke C, Haurum JS and Kragh M (2010) Sym004: a novel synergistic anti‐epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res 70, 588–597. [DOI] [PubMed] [Google Scholar]
- Peeters M, Karthaus M, Rivera F, Terwey JH and Douillard JY (2015) Panitumumab in metastatic colorectal cancer: the importance of tumour RAS status. Drugs 75, 731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MJ and Voeltz GK (2016) Structure and function of ER membrane contact sites with other organelles. Nat Rev Mol Cell Biol 17, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierotti MA, Negri T, Tamborini E, Perrone F, Pricl S and Pilotti S (2010) Targeted therapies: the rare cancer paradigm. Mol Oncol 4, 19–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C and Stenmark H (2009) The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445–452. [DOI] [PubMed] [Google Scholar]
- Ravid T, Sweeney C, Gee P, Carraway KL 3rd and Goldkorn T (2002) Epidermal growth factor receptor activation under oxidative stress fails to promote c‐Cbl mediated down‐regulation. J Biol Chem 277, 31214–31219. [DOI] [PubMed] [Google Scholar]
- Roepstorff K, Grandal MV, Henriksen L, Knudsen SL, Lerdrup M, Grovdal L, Willumsen BM and van Deurs B (2009) Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic 10, 1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R Jr (2014) The ErbB/HER family of protein‐tyrosine kinases and cancer. Pharmacol Res 79, 34–74. [DOI] [PubMed] [Google Scholar]
- Rowland AA, Chitwood PJ, Phillips MJ and Voeltz GK (2014) ER contact sites define the position and timing of endosome fission. Cell 159, 1027–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Codogno P and Levine B (2012) Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11, 709–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki Y and De Camilli P (2017) Endoplasmic reticulum‐plasma membrane contact sites. Annu Rev Biochem 86, 659–684. [DOI] [PubMed] [Google Scholar]
- Sawano A, Takayama S, Matsuda M and Miyawaki A (2002) Lateral propagation of EGF signaling after local stimulation is dependent on receptor density. Dev Cell 3, 245–257. [DOI] [PubMed] [Google Scholar]
- Scharaw S, Iskar M, Ori A, Boncompain G, Laketa V, Poser I, Lundberg E, Perez F, Beck M, Bork P et al (2016) The endosomal transcriptional regulator RNF11 integrates degradation and transport of EGFR. J Cell Biol 215, 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J (2014) Receptor tyrosine kinases: legacy of the first two decades. Cold Spring Harb perspect Biol 6, a008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL (2017) Reciprocal regulation of signaling and endocytosis: implications for the evolving cancer cell. J Cell Biol 216, 2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MH, Furnari FB, Cavenee WK and Bogler O (2003) Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization. Proc Natl Acad Sci USA 100, 6505–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR and Yarden Y (2016) The EGFR‐HER2 module: a stem cell approach to understanding a prime target and driver of solid tumors. Oncogene 35, 2949–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, Lim SO, Du Y, Wang Y, Chang WC, Chen CH et al (2013) EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature 497, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtiegman K, Kochupurakkal BS, Zwang Y, Pines G, Starr A, Vexler A, Citri A, Katz M, Lavi S, Ben‐Basat Y et al (2007) Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene 26, 6968–6978. [DOI] [PubMed] [Google Scholar]
- Sibilia M and Wagner EF (1995) Strain‐dependent epithelial defects in mice lacking the EGF receptor. Science 269, 234–238. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Algisi V, Nappo G, Conte A, Pascolutti R, Cuomo A, Bonaldi T, Argenzio E, Verhoef LG, Maspero E et al (2013) Threshold‐controlled ubiquitination of the EGFR directs receptor fate. EMBO J 32, 2140–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S and Di Fiore PP (2008) Clathrin‐mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell 15, 209–219. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Confalonieri S, Ciliberto A, Polo S, Scita G and Di Fiore PP (2012) Endocytosis and signaling: cell logistics shape the eukaryotic cell plan. Physiol Rev 92, 273–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP and Polo S (2005) Clathrin‐independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA 102, 2760–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A and Carpenter G (1993) Interaction of activated EGF receptors with coated pit adaptins. Science 261, 612–615. [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Yamazaki D, Kurisu S and Takenawa T (2003) Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev Cell 5, 595–609. [DOI] [PubMed] [Google Scholar]
- Szumiel I (2006) Epidermal growth factor receptor and DNA double strand break repair: the cell's self‐defence. Cell Signal 18, 1537–1548. [DOI] [PubMed] [Google Scholar]
- Tan X, Lambert PF, Rapraeger AC and Anderson RA (2016a) Stress‐induced EGFR trafficking: mechanisms, functions, and therapeutic implications. Trends Cell Biol 26, 352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Sun Y, Thapa N, Liao Y, Hedman AC and Anderson RA (2015a) LAPTM4B is a PtdIns(4,5)P2 effector that regulates EGFR signaling, lysosomal sorting, and degradation. EMBO J 34, 475–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Thapa N, Liao Y, Choi S and Anderson RA (2016b) PtdIns(4,5)P2 signaling regulates ATG14 and autophagy. Proc Natl Acad Sci USA 113, 10896–10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Thapa N, Sun Y and Anderson RA (2015b) A kinase‐independent role for EGF receptor in autophagy initiation. Cell 160, 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V et al (2005) Gefitinib plus best supportive care in previously treated patients with refractory advanced non‐small‐cell lung cancer: results from a randomised, placebo‐controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366, 1527–1537. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC et al (1995) Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269, 230–234. [DOI] [PubMed] [Google Scholar]
- Tomas A, Futter CE and Eden ER (2014) EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol 24, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas A, Jones S, Vaughan SO, Hochhauser D, Futter CE (2017) Stress‐specific p38 MAP kinase activation is sufficient to drive EGF receptor endocytosis but not nuclear translocation. J Cell Sci 130, 2481–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas A, Vaughan SO, Burgoyne T, Sorkin A, Hartley JA, Hochhauser D and Futter CE (2015) WASH and Tsg101/ALIX‐dependent diversion of stress‐internalized EGFR from the canonical endocytic pathway. Nat Commun 6, 7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Taylor P and Moran MF (2014) Proteomic analysis of the epidermal growth factor receptor (EGFR) interactome and post‐translational modifications associated with receptor endocytosis in response to EGF and stress. Mol Cell Proteomics 13, 1644–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai FC, Seki A, Yang HW, Hayer A, Carrasco S, Malmersjo S and Meyer T (2014) A polarized Ca2+, diacylglycerol and STIM1 signalling system regulates directed cell migration. Nat Cell Biol 16, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Emburgh BO, Arena S, Siravegna G, Lazzari L, Crisafulli G, Corti G, Mussolin B, Baldi F, Buscarino M, Bartolini A et al (2016) Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat Commun 7, 13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergarajauregui S, San Miguel A and Puertollano R (2006) Activation of p38 mitogen‐activated protein kinase promotes epidermal growth factor receptor internalization. Traffic 7, 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasenor R, Kalaidzidis Y and Zerial M (2016) Signal processing by the endosomal system. Curr Opin Cell Biol 39, 53–60. [DOI] [PubMed] [Google Scholar]
- Villasenor R, Nonaka H, Del Conte‐Zerial P, Kalaidzidis Y, Zerial M (2015) Regulation of EGFR signal transduction by analogue‐to‐digital conversion in endosomes. Elife 4, e06156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenzi B, Schiavon G, Silletta M, Santini D and Tonini G (2008) The biological properties of cetuximab. Crit Rev Oncol Hematol 68, 93–106. [DOI] [PubMed] [Google Scholar]
- Waldhart AN, Dykstra H, Peck AS, Boguslawski EA, Madaj ZB, Wen J, Veldkamp K, Hollowell M, Zheng B, Cantley LC et al (2017) Phosphorylation of TXNIP by AKT mediates acute influx of glucose in response to insulin. Cell Rep 19, 2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger‐Ness A and Zerial M (2014) Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 6, a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner G, Mayer C, Kehlbach R, Rodemann HP and Dittmann K (2008) Activation of protein kinase Cepsilon stimulates DNA‐repair via epidermal growth factor receptor nuclear accumulation. Radiother Oncol 86, 383–390. [DOI] [PubMed] [Google Scholar]
- Waterman H, Alroy I, Strano S, Seger R and Yarden Y (1999) The C‐terminus of the kinase‐defective neuregulin receptor ErbB‐3 confers mitogenic superiority and dictates endocytic routing. EMBO J 18, 3348–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman H, Katz M, Rubin C, Shtiegman K, Lavi S, Elson A, Jovin T and Yarden Y (2002) A mutant EGF‐receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J 21, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Zou Z, Becker N, Anderson M, Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW et al (2013) EGFR‐mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell 154, 1269–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ and Hung MC (2008) Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 13, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DB, Zoncu R, Root DE, Sabatini DM and Sawyers CL (2015) Identification of an oncogenic RAB protein. Science 350, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieman HL, Wofford JA and Rathmell JC (2007) Cytokine stimulation promotes glucose uptake via phosphatidylinositol‐3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell 18, 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley HS (1988) Anomalous binding of epidermal growth factor to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J Cell Biol 107, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KJ, Gilmore JL, Foley J, Lemmon MA and Riese DJ 2nd (2009) Functional selectivity of EGF family peptide growth factors: implications for cancer. Pharmacol Ther 122, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KJ, Mill C, Lambert S, Buchman J, Wilson TR, Hernandez‐Gordillo V, Gallo RM, Ades LM, Settleman J and Riese DJ 2nd (2012) EGFR ligands exhibit functional differences in models of paracrine and autocrine signaling. Growth Factors 30, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford JA, Wieman HL, Jacobs SR, Zhao Y and Rathmell JC (2008) IL‐7 promotes Glut1 trafficking and glucose uptake via STAT5‐mediated activation of Akt to support T‐cell survival. Blood 111, 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert T, Yang D, Ren X, Lee HH, Im YJ and Hurley JH (2009) The ESCRT machinery at a glance. J Cell Sci 122, 2163–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YP, Ma H, Starchenko A, Huh WJ, Li W, Hickman FE, Zhang Q, Franklin JL, Mortlock DP, Fuhrmann S et al (2017) A Chimeric Egfr protein reporter mouse reveals Egfr localization and trafficking in vivo. Cell Rep 19, 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y and Pines G (2012) The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer 12, 553–563. [DOI] [PubMed] [Google Scholar]
- Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M and Eck MJ (2007) Structures of lung cancer‐derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 11, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gureasko J, Shen K, Cole PA and Kuriyan J (2006) An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 125, 1137–1149. [DOI] [PubMed] [Google Scholar]
- Zhang J, Song F, Zhao X, Jiang H, Wu X, Wang B, Zhou M, Tian M, Shi B, Wang H et al (2017) EGFR modulates monounsaturated fatty acid synthesis through phosphorylation of SCD1 in lung cancer. Mol Cancer 16, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Cao X, Ali‐Osman F, Keir S and Lo HW (2010) EGFR and EGFRvIII interact with PUMA to inhibit mitochondrial translocalization of PUMA and PUMA‐mediated apoptosis independent of EGFR kinase activity. Cancer Lett 294, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwang Y and Yarden Y (2006) p38 MAP kinase mediates stress‐induced internalization of EGFR: implications for cancer chemotherapy. EMBO J 25, 4195–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]


