Abstract
The functional heterogeneity of HDL is attributed to its diverse bioactive components. We evaluated whether the vasodilatory effects of HDL differed across HDL subpopulations, reflecting their distinct molecular composition. The capacity of five major HDL subfractions to counteract the inhibitory effects of oxidized LDL on acetylcholine-induced vasodilation was tested in a rabbit aortic rings model. NO production, an essential pathway in endothelium-dependent vasorelaxation, was studied in simian vacuolating virus 40-transformed murine endothelial cells (SVECs). Small dense HDL3 subfractions displayed potent vasorelaxing activity (up to +31% vs. baseline, P < 0.05); in contrast, large light HDL2 did not induce aortic-ring relaxation when compared on a total protein basis. HDL3 particles were enriched with sphingosine-1-phosphate (S1P) (up to 3-fold vs. HDL2), with the highest content in HDL3b and -3c that concomitantly revealed the strongest vasorelaxing properties. NO generation was enhanced by HDL3c in SVECs (1.5-fold, P < 0.01), a phenomenon that was blocked by the S1P receptor antagonist, VPC 23019. S1P-enriched reconstituted HDL (rHDL) was a 1.8-fold (P < 0.01) more potent vasorelaxant than control rHDL in aortic rings. Small dense HDL3 particles displayed potent protective effects against oxidative stress-associated endothelium dysfunction, potentially reflecting their elevated content of S1P that might facilitate interaction with S1P receptors and ensuing NO generation.
Keywords: atherosclerosis, vasodilation, oxidized low density lipoprotein, endothelium, nitric oxide, high density lipoprotein
Strategically located between the blood and the vessel wall, endothelial cells possess several vasoprotective functions, including induction of vasodilation (1, 2). Such endothelium-dependent vasoprotective mechanisms are mainly mediated by NO production (3). Oxidative stress induced by local inflammatory processes can reduce endothelial NO production through modification of LDL to oxidized LDL (oxLDL), a potent inhibitor of endothelium-dependent vasorelaxation (4).
In contrast to oxLDL, HDL displays vasoprotective effects. Indeed, HDL may directly improve endothelial function (5) or indirectly reverse the inhibitory effects of oxLDL on vasorelaxation (6). However, HDLs obtained from type 2 diabetic patients (7) or abdominally obese subjects (8) are incapable of counteracting the inhibitory effect of oxLDLs on vasorelaxation. Interestingly, such HDL dysfunctionality was independent of HDL-cholesterol levels, as raising of HDL-cholesterol by glitazone did not restore the vasodilatory activity of HDL (9). Such loss-of-function can be attributed to compositional remodeling of HDL, as evidenced by increased content of apoC-III (10) and/or reduced content of apoJ (10, 11) and sphingosine-1-phosphate (S1P) in HDL isolated from coronary artery disease patients in whom the lipoprotein is functionally deficient (12–16). Furthermore, alterations in the conformation and/or the content of apoA-I, which is the major protein constituent of HDL, can be associated with deficient HDL functionality (17–22).
Several studies aiming to elucidate the molecular determinants of HDL-mediated vasodilation reveal that S1P plays a pivotal role in endothelial cell integrity via activating NO production (23–27). Although HDL carries the majority of S1P in plasma (28), there is a considerable variation in S1P content among different HDL subclasses, which is elevated in small dense protein-rich HDL3 relative to large light lipid-rich HDL2 (29–31). These data suggest that HDL particles may differ in their capacity to induce vasorelaxation, with small dense HDL3 displaying potent vasorelaxing properties as a result of elevated S1P content, enhanced activation of S1P receptors, and increased NO production.
In order to evaluate this hypothesis, we examined five HDL subpopulations isolated from healthy normolipidemic subjects for their ability to counteract the inhibitory effects of oxLDL on acetylcholine-induced vasodilation in isolated rabbit aortic rings. In a candidate-based approach to define specific constituents associated with the vasodilatory response, we assessed the impact of reconstituted HDLs (rHDLs) containing apoA-I and phosphatidylcholine (PC) in the absence or presence of other biologically active components known to be enriched or depleted in HDL3 (32, 33). Our data reveal that small dense HDL3 particles displayed potent protective effects against oxidative stress-induced endothelium dysfunction, whereas large light HDL2 particles did not induce aortic-ring relaxation. Consistent with these data, HDL-associated S1P was a potent vasorelaxant in aortic rings. These findings indicate that elevated content of S1P may account for the potent vasodilatory activity of HDL3.
METHODS
Blood samples
Blood samples were obtained from four healthy blood donors. EDTA plasma was prepared by 15 min low-speed centrifugation at 4°C. Sucrose, at a final concentration of 0.6%, was added to the plasma as a cryoprotectant for lipoproteins; samples were aliquoted and frozen at −80°C under nitrogen; each aliquot was thawed only once directly before use.
HDL subfractionation and characterization
First, total HDL was isolated from plasma by sequential flotation ultracentrifugation, as described previously (34). Subsequently, plasma HDL subfractions, including HDL2b, -2a, -3a, -3b, and -3c, were isolated by a single-step isopycnic density gradient ultracentrifugation of the total HDL fraction at 15°C at 274,000 g for 44 h, as described previously (35). All HDL subfractions were extensively dialyzed against PBS treated with Chelex 100 ion exchange resin (pH 7.4) at 4°C in the dark, stored at 4°C, and used within 10 days (35).
All HDL subfractions were analyzed for the content of major HDL lipid components, including total cholesterol (TC), free cholesterol (FC), phospholipid, and triglyceride (TG), using commercially available enzymatic colorimetric kits (CHOP-PAP; Biomerieux, France). Cholesterol ester content was calculated by multiplying the difference between TC and FC by 1.67 (35). Total protein was measured in HDL using the BCA assay. apoA-I was assessed by immunoturbidimetry using an automated Konelab 60i analyzer (Thermo Fisher Scientific, Villebon-sur-Yvette, France). Paraoxonase-1 (PON1) activity was determined photometrically in the presence of CaCl2 using phenylacetate as a substrate (36). Albumin and apoM content were evaluated in HDL subpopulations by LC/MS/MS, as described previously (32).
S1P quantification
An HPLC-based method was used to determine the S1P content of HDL, as previously described (30). Briefly, 100 μl of isolated HDL subfractions containing 0.5 to 2.0 mg of total HDL mass per milliliter of buffer were added to 1 ml of acidified methanol and supplemented with 15 pmol of an internal standard [D-erythro-S1P (C17 base); Avanti, InstruChemie]. The S1P-containing phase was isolated with 1 ml chloroform, 200 μl NaCl (4 M), and 100 μl NaOH (3 M) in a two-step procedure. Subsequently, the organic phase was evaporated and dissolved in 50 μl of methanol and 0.07 M K2HPO4 (9:1 v/v). Five microliters of the derivatization mixture containing 10 mg o-phthaldialdehyde, 200 μl ethanol, 10 μl 2-mercaptoethanol, and 10 ml boric acid (3% v/w) were added to the lipid followed by a 15 min incubation at room temperature. One microliter of the derivatized sample was analyzed with a Hewlett Packard HPLC system using an RP 18 Kromasil column (2.1 mm inner diameter × 150 mm) and an isocratic eluent containing methanol:K2HPO4 (0.07 M) (78:22 v/v) at a flow rate of 0.25 ml/min maintained at 45°C. Derivatives were detected using a Hewlett Packard spectrofluorometer at 340 nm and 456 nm as excitation and emission wavelengths, respectively. S1P quantification was done by comparison of its fluorescent signal with that of the derivative of the internal standard (coefficient of variation, <5%). The values of S1P were reported per mole and per gram of HDL protein, as previously described by us (29). To calculate HDL molarity, the following molecular masses of HDL subfractions were used: HDL2b, 404 kDa; HDL2a, 270 kDa; HDL3a, 215 kDa; HDL3b, 199 kDa; and HDL3c, 163 kDa (29).
rHDL preparation and loading with S1P
rHDL was prepared by cholate dialysis from a mixture of human apoA-I, isolated from EDTA plasma, with egg yolk PC at the molar ratio of 1:80 (37). rHDLs were then incubated with or without S1P (40 μmol/l), at a concentration of 100 mg HDL protein per deciliter at 37°C for 2 h, under constant stirring. Subsequently, the rHDLs were extensively dialyzed against PBS (pH 7.4) to remove excessive S1P. S1P concentration was measured in the S1P-enriched rHDL by HPLC with fluorometric detection, as described above.
S1P-loading of albumin
S1P, at a concentration of 2 or 40 μM, was added to a 0.1% w/v solution of human serum albumin, a second major physiologic S1P carrier in human plasma (38), and incubated at 37°C for 2 h. According to previously reported data (15), a maximum of 0.5 μmol of S1P can be loaded per gram of human serum albumin. Because, in our experiments, the S1P/albumin ratios were 0.02 and 0.4 μmol/g, we assumed that all added S1P was bound to albumin at the end of the incubation. Nevertheless, both S1P-enriched and control albumin samples incubated in parallel in the absence of S1P were extensively dialyzed against PBS (pH 7.4) to remove unbound S1P if any.
TG enrichment of HDL
To prepare TG-enriched HDLs, isolated HDL3a and -3b subpopulations (650 μl) were incubated for 3 h at 37°C in the presence of human VLDL (570 μl) and lipoprotein-deficient serum as a source of lipid transfer proteins (80 μl), freshly isolated from three healthy normolipidemic donors by a single-step isopycnic density gradient ultracentrifugation, as described above. The amount of VLDL included in the incubation was sufficient to reach a final TG concentration of 1 mM. TG-enriched HDL was then re-isolated by a single-step isopycnic density gradient ultracentrifugation, as described.
Preparation of oxLDL
LDL (density 1.019–1.063 g/ml) was isolated from human EDTA plasma by sequential flotation ultracentrifugation, as previously described (39). LDL was oxidized by incubating freshly prepared LDL, adjusted to a final concentration of 1.2 g protein per liter in TBS at pH 7.4 with a copper sulfate solution (final concentration, 5 μM) for 24 h at 37°C. At the end of the incubation, oxidation was stopped by adding EDTA to the final concentration of 200 μM and butylated hydroxytoluene to the final concentration of 20 μM. After the 24 h period, oxLDL preparations were dialyzed overnight against Krebs buffer (pH 7.4) and oxLDL was kept at 4°C until use (39).
Ex vivo vasoreactivity of rabbit aortic rings
Vasoreactivity experiments were performed on rabbit aortic rings in accordance with principles of laboratory animal care and the protocol was approved by the local Ethics Committee for animals at the University of Burgundy (Dijon, France). As previously described (7–9), the descending aorta was rapidly removed and transferred into Krebs solution bubbled with 95% O2 plus 5% CO2. The aortas were cut into 3 mm rings and suspended horizontally between two wire hooks in 20 ml jacketed organ baths containing oxygenated Krebs solution (composition: NaCl, 119 mmol/l; KCl, 4.7 mmol/l; KH2PO4, 1.18 mmol/l; MgSO4, 1.17 mmol/l; CaCl2, 2.5 mmol/l; EDTA, 0.027 mmol/l; glucose, 11 mmol/l; and NaHCO3, 25 mmol/l) maintained at 37°C. In brief, aortic rings were initially contracted by 0.3 μmol/l arterenol hydrochloride at a concentration resulting in 75% of the maximal contraction, and acetylcholine in the 1 nmol/l to 0.01 mmol/l concentration range was added cumulatively to relax the rings. After a wash-out step and a further 30 min recovery period, aortic rings were incubated for 2 h with oxLDL alone or with oxLDL in the presence of different HDL subfractions, control rHDL, S1P-enriched rHDL, S1P-enriched albumin, or rHDL supplemented with recombinant human apoJ (0.038 μM) or apoC-III (3 μM) (BioVision Inc.). We used a co-incubation model that more adequately reflected physiological conditions where both HDL and oxLDL are simultaneously present in the same environment. All HDL preparations were compared at a constant concentration of 1 g protein per liter. Subsequently, aortic ring segments were recontracted with arterenol hydrochloride, and then were relaxed again progressively by adding sodium nitroprusside (NO) to verify the exclusive involvement of endothelium-dependent relaxation response.
The maximal relaxation (Emax) evoked by acetylcholine and expressed as a percentage of the contraction to arterenol hydrochloride (0.3 μmol/l) was calculated from experimental data. To reduce variation among experiments resulting from different intrinsic properties of aortic vessels, three different aortic rings were used and the average results were reported (40).
NO production in endothelial cells
Simian vacuolating virus 40 (SV40)-transformed murine endothelial cells (SVECs) (ATCC number: CRL2181) were cultured in DMEM supplemented with 10% FBS to sub-confluency. After overnight starvation, cells were incubated with 4,5-diaminofluorescein diacetate (DAF-2) (1 μM; Cayman Chemical) for 30 min before adding HDL (50 μg protein per milliliter) for 15 min. Triazolofluorescein fluorescence was measured under conditions provided by the manufacturer using excitation/emission wavelengths of 485/520 nm as an indicator of NO production (12).
Antioxidative activity of HDL
Antioxidative activity of HDL3a and -3b subpopulations was evaluated toward oxidation of reference LDL, obtained from a healthy normolipidemic donor, by 2,2′-azobis-(2-amidinopropane) hydrochloride, an azo initiator of lipid peroxidation. Accumulation of conjugated dienes in the samples was measured at 234 nm and average oxidation rate in the propagation phase was calculated, as previously described (36, 41).
Statistical analysis
Between-group differences were analyzed by the nonparametric Wilcoxon’s matched-pair for continuous variables or Chi-square test for discontinuous variables using StatView statistical software (SAS Institute Inc., Berkeley, CA). Spearman’s correlation coefficient was calculated to assess relationships between continuous variables. All results are expressed as mean ± SD except Emax values, which are expressed as mean ± SEM; P < 0.05 was considered statistically significant.
RESULTS
Compositional characterization of HDL particle subpopulations
As expected, the content of major lipid classes showed a distinct trend to decrease in parallel with increment in HDL total protein and apoA-I [percentage by weight (wt%)] from large light lipid-rich HDL2b to small dense protein-rich HDL3c, with only a minor degree of variation for most lipid classes relative to total lipid (Table 1). In marked contrast, S1P, a minor bioactive lipid, showed significant variation across HDL subpopulations with almost 4-fold enrichment in small dense HDL3 (0.54–0.94 μmol/g protein HDL), as compared with HDL2 particles (0.08–0.24 μmol/g protein HDL), as reported earlier (30). The lowest S1P content was measured in the HDL2a subpopulation (Table 1). In a similar fashion, PON1 activity and apoM were predominantly associated with small dense HDL3 particles, as documented earlier by us and others (32, 36) (ratios of the levels measured in HDL3c relative to HDL2a of 66 and 4.5, respectively).
TABLE 1.
Chemical composition of HDL subpopulations
| HDL2b | HDL2a | HDL3a | HDL3b | HDL3c | |
| Total protein (wt%) | 36.8 ± 4.8 | 36.8 ± 2.8 | 47.5 ± 4.4 | 53.6 ± 4.2 | 60.7 ± 1.8 |
| Phospholipid (wt%) | 29.4 ± 3.9 (43.7) | 29.2 ± 5.2 (46.1) | 25.9 ± 6.3 (46.7) | 22.4 ± 4.7 (46.1) | 18.2 ± 2.3 (44.6) |
| FC (wt%) | 5.7 ± 1.5 (8.4) | 3.7 ± 0.8 (5.9) | 2.9 ± 0.8 (5.1) | 2.2 ± 0.4 (4.5) | 1.8 ± 0.1 (4.4) |
| Cholesteryl ester (wt%) | 25.6 ± 0.8 (38.0) | 23.8 ± 0.2 (37.4) | 21.9 ± 0.8 (39.6) | 20.2 ± 1.8 (41.3) | 17.2 ± 1.8 (42.0) |
| TG (wt%) | 6.7 ± 1.8 (10.0) | 6.7 ± 2.8 (10.6) | 4.7 ± 1.6 (8.6) | 4.0 ± 1.4 (8.2) | 3.7 ± 1.3 (9.0) |
| apoA-I (wt%) | 30.4 ± 4.1 | 28.1 ± 4.6 | 35.1 ± 1.8 | 40.0 ± 3.2 | 45.0 ± 2.6 |
| S1P (mmol/mol HDL) | 19.3 ± 9.6 | 14.4 ± 3.2 | 35.9 ± 3.5 | 65.1 ± 16.4 | 62.7 ± 16.8 |
Values are the means of two measurements performed in preparations of HDL particles obtained from three healthy donors. Lipid composition of HDL expressed as wt% relative to total lipid is shown in parentheses.
Protective effects of HDL particles toward oxLDL-enhanced vasoconstriction in arterenol hydrocholoride-contracted rings
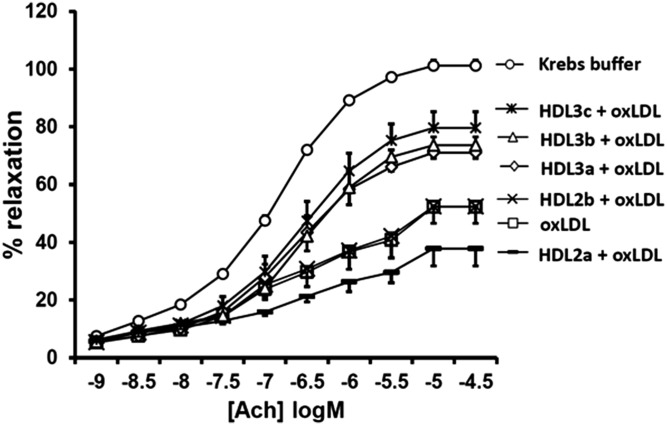
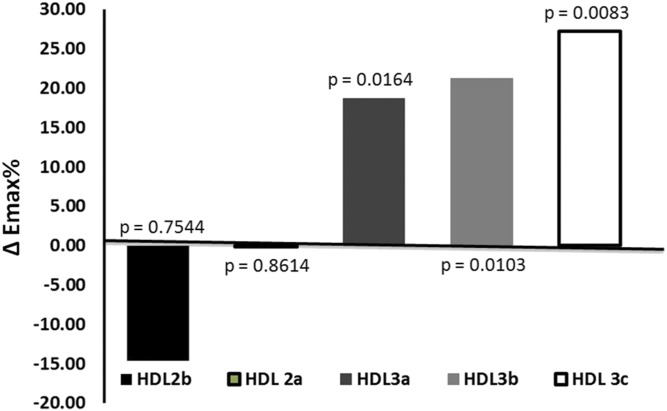
Preincubation of isolated rabbit aortic rings with oxLDL markedly inhibited acetylcholine-induced vasorelaxation, as compared with rings preincubated with a control buffer (Emax= −50%, P < 0.02). We compared the effects of different HDL subpopulations that were matched for their protein content, but differed in S1P content. Large light HDL2b exhibited no effect in counteracting the oxLDL-induced inhibitory effects on the endothelial vasorelaxation response and HDL2a-induced vasoconstriction (Fig. 1). In contrast, HDL3 particle subpopulations potently restored endothelium-dependent vasorelaxation, with the greatest effects observed in the presence of the small dense HDL3c subfraction (Emax= +28%, P < 0.05) followed by HDL3b (Emax= +22%, P < 0.05) and HDL3a (Emax= +20%, P < 0.05) (Fig. 1). As a result, the capacity of HDL particles to protect vessels was proportional to their density (Fig. 2).
Fig. 1.
Influence of HDL subpopulations on oxLDL-induced inhibitory effects toward endothelium-dependent vasorelaxation of rabbit aortic rings. Dose-response curves for acetylcholine (Ach) in aortas contracted with 0.3 μmol/l arterenol hydrochloride and preincubated for 2 h with either Krebs buffer, oxLDL alone, oxLDL and HDL2b, oxLDL and HDL 2a, oxLDL and HDL3a, oxLDL and HDL3b, or oxLDL and HDL3c are shown. All HDL and oxLDL preparations were used at a final concentration of 1 g protein per liter. Values represent the mean ± SEM (n = 10) for 10 experiments performed with three HDL preparations isolated from plasma of three healthy donors.
Fig. 2.
The relationship between the maximal endothelium-dependent relaxation (Emax) of rabbit aortic rings after incubation with different HDL subpopulations and oxLDL. All HDL and oxLDL preparations were used at a final concentration of 1 g protein per liter. The histogram represents differences in Emax values measured in incubations with oxLDL and HDL subpopulations versus values measured in incubations with oxLDL alone; P values for these differences are shown at the bars.
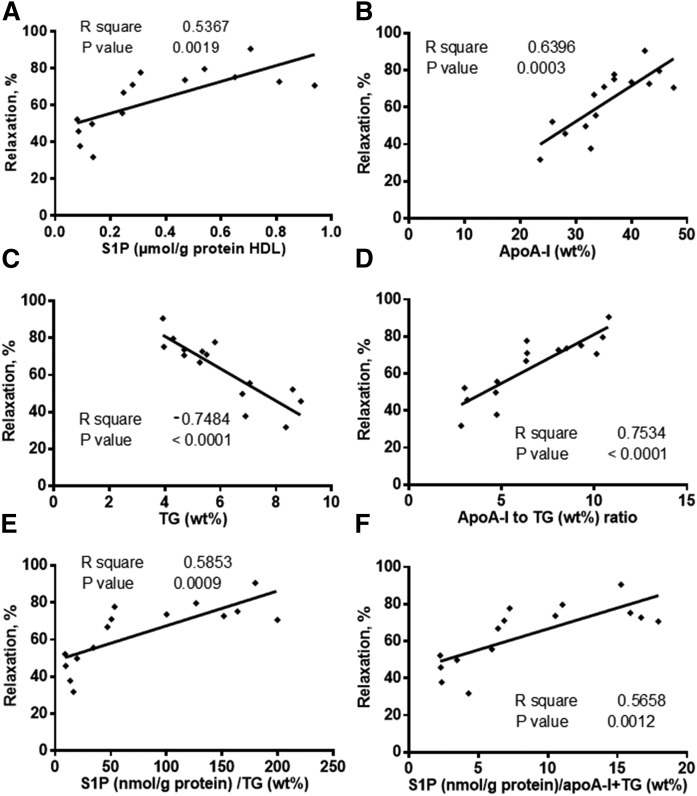
In addition, strong positive correlations were found between the aortic ring vasorelaxation and both S1P (r2 = 0.54, P = 0.0019) and apoA-I (r2 = 0.64, P = 0.0003) content of HDL (Fig. 3A, B), whereas TG content of HDL revealed a negative correlation (r2 = −0.75, P < 0.0001; Fig. 3C). In parallel, S1P and TG contents in HDL subfractions were negatively correlated (r = −0.36, P = 0.02). In order to adjust for the effects of TG and apoA-I, the ratios of S1P/TG and S1P/(apoA-I + TG) were calculated and plotted against the maximal endothelium-dependent relaxation induced by HDL subpopulations. As shown in Fig. 3E, F, these ratios still showed strong positive correlation with the aortic ring vasorelaxation (r2 = 0.59, P = 0.0009 and r2 = 0.57, P = 0.0012, respectively).
Fig. 3.
Correlations between S1P (A), apoA-I (B), and TG (C), apoA-I/TG ratio (D), S1P/TG ratio (E), and S1P/(apoA-I + TG) ratio (F) in HDL subpopulations and the maximal endothelium-dependent relaxation of rabbit aortic rings after incubation with HDL subpopulations and oxLDL. Values are from three HDL preparations isolated from the plasma of three healthy donors.
Next, we found a significant correlation between PON1 activity and the vasodilatory effects of HDL subpopulations (r2 = 0.47, P = 0.03). In addition, a significant relationship between apoM content and vasodilatory activity of HDL particles was obtained (r2 = 0.67, P = 0.0003). Finally, the contents of apoM and S1P were significantly inter-correlated (r2 = 0.26, P = 0.03).
Influence of rHDL on the inhibitory effect of oxLDL on vasorelaxation of rabbit aortic rings
To identify the constituents of HDL particles associated with their vasoprotective effects, the potential role of several candidates displaying vasoprotective properties, such as apoA-I, S1P, and apoJ (clusterin), as well as those associated with HDL dysfunction, such as apoC-III, was assessed.
In the first set of experiments, rHDLs containing only apoA-I and PC, and S1P-enriched rHDL were used to investigate the influence of apoA-I and S1P on aortic ring vasorelaxation.
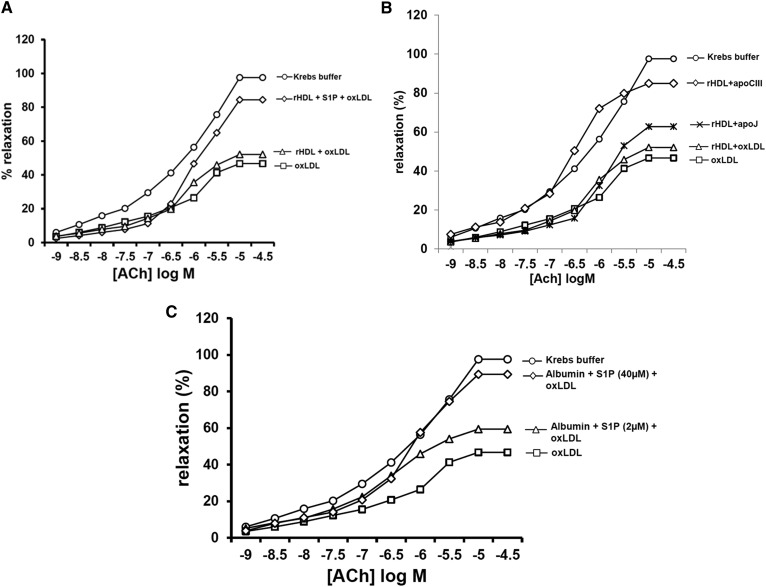
Following incubation with S1P and dialysis, rHDL contained 22.2 ± 1.5 μmol S1P per gram of protein (n = 6) equivalent to 0.7 mol S1P per mole of apoA-I. After 2 h incubation, these particles consistently counteracted oxLDL-induced inhibitory effects toward endothelium-dependent vasorelaxation of rabbit aortic rings (Emax = +84%, P < 0.01; Fig. 4A), while rHDL containing only apoA-I and PC was ineffective (+11%, P > 0.05). Additionally, rHDL incubated with apoJ (0.038 μM) reduced the percentage of oxLDL-induced inhibition of vasorelaxation by 32%, which was not significantly higher than the effect observed for rHDL alone (Fig. 4B). Finally, rHDL incubated with apoC-III (3 μM) reduced the percentage of oxLDL-induced inhibition of vasorelaxation by 75%, which was higher than that observed for rHDL alone and inconsistent with a vasoconstrictive effect of apoC-III.
Fig. 4.
Influence of S1P-enriched rHDL (A), S1P-loaded albumin (B), and apoC-III- or apoJ-loaded rHDL (C) on the inhibitory effect of oxLDL toward endothelium-dependent vasorelaxation of rabbit aortic rings. The graph shows mean dose-response curves for acetylcholine (Ach) in aortas contracted with 0.3 μmol/l arterenol hydrochloride and preincubated for 2 h with Krebs buffer, oxLDL alone, oxLDL and S1P-enriched rHDL, and oxLDL and rHDL containing only apoA-I and PC (A); oxLDL and rHDL supplemented with apoJ or apoC-III, and oxLDL and rHDL containing only apoA-I and PC (B); oxLDL and albumin (1g/l) preincubated with S1P (2 and 40 μM) (C). All HDL and oxLDL preparations were used at a final concentration of 1 g protein per liter. Data are presented as the mean of four independent experiments.
Influence of albumin loaded with S1P on the inhibitory effect of oxLDL toward vasorelaxation of rabbit aortic rings
In order to further characterize the distinct role of S1P in the vasorelaxation response induced by HDL, albumin, as a second major plasma carrier of S1P (38), was loaded with S1P and incubated with aortic rings in the presence of oxLDL. As shown in Fig. 4C, such S1P-enriched albumin was able to significantly counteract the inhibitory effect of oxLDL on the vasorelaxation of rabbit aortic rings (up to 1.6-fold, P < 0.01), consistent with the specific capacity of S1P to induce vasorelaxation independent of other HDL components.
Increased S1P1-dependent NO generation in murine aortic endothelial cells (SVECs) treated with small dense HDL3
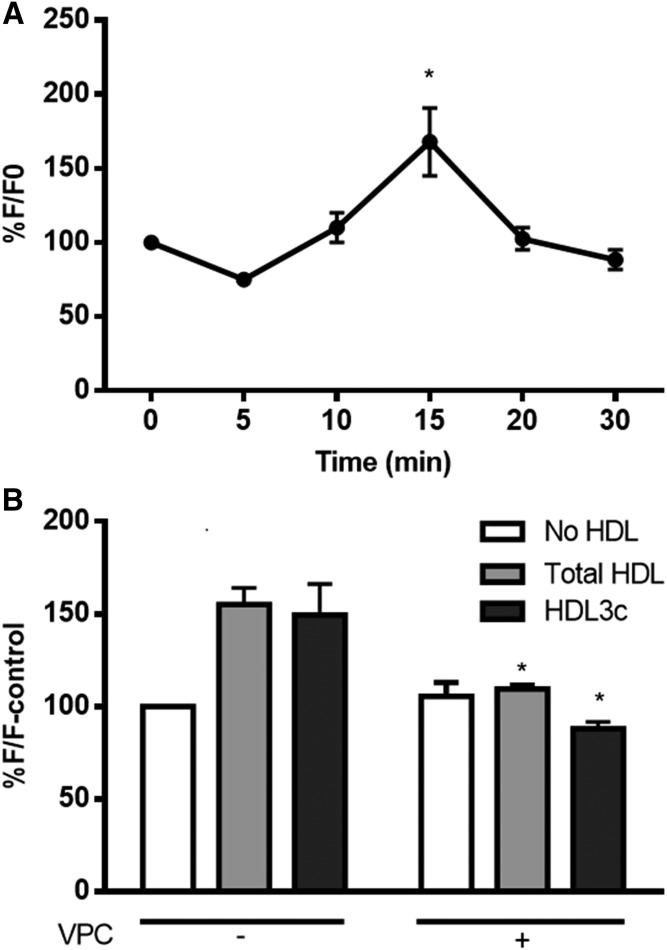
To characterize the potential role of NO in the vasodilatory effects of HDL subpopulations, HDL-dependent NO generation in murine endothelial cells was evaluated by measuring the kinetics of the increase in the fluorescence of DAF-2, a fluorescent probe for NO. Total HDL at a concentration of 50 μg protein per milliliter showed the most reproducible NO increment under our experimental conditions after 15 min incubation time (1.68-fold vs. baseline at time 0; Fig. 5A); this condition was therefore chosen to analyze the effects of individual HDL subpopulations. Small dense HDL3c induced NO production levels in SVECs comparable to those induced by total HDL (1.50-fold vs. 1.55-fold increases, respectively, relative to incubations in the absence of HDL after 15 min; Fig. 5B). The mechanism underlying potent vasodilatory effects of HDL3 was further assessed using the S1P receptor antagonist, VPC 23019 (42). In the presence of VPC 23019, both total HDL and the small, dense HDL3c subpopulation lost their stimulatory effects on endothelial NO production in murine aortic endothelial cells, with a stronger effect on HDL3c (Fig. 5B), indicative of the key role of S1P1/S1P3 receptors in the HDL3c-induced NO production.
Fig. 5.
A: Kinetics of NO formation by murine endothelial cells (SVECs) treated with total HDL (50 μg protein per milliliter). B: NO formation by SVECs treated with total HDL and HDL3c (50 μg protein per milliliter) alone or in combination with the S1P receptor antagonist, VPC 23019 (VPC). CRL2181 cells were preincubated with VPC (10 μM) for 30 min before adding HDL. A fluorescent probe for NO [DAF-2DA (5 μM in DMSO)] was preincubated for 30 min with cells before adding HDL. The fluorescent intensity was recorded at the excitation wavelength of 485 nm and emission wavelength of 520 nm and presented as a percentage of baseline reading obtained at time 0 (F0) (A) or in the absence of HDL (F-control) (B). Data shown are means of four independent experiments. *P < 0.05 versus time 0 (A) or versus corresponding HDL in the absence of VPC (B).
Influence of TG enrichment on antioxidative activity of HDL
To obtain insight into the negative relationship between TG content and vasodilatory activity of HDL, we examined antioxidative activity of TG-enriched HDL particles toward LDL oxidation. Incubation of small dense HDL3a and HDL3b in the presence of isolated VLDL and lipoprotein-deficient serum for 3 h at 37°C markedly (by 57 ± 9%) increased TG content of HDL in three independent experiments performed in three individual HDL samples. Such TG enrichment caused a significant reduction in antioxidative activity of HDL3a and HDL3b particles, evaluated as inhibition of LDL oxidation by 2,2′-azobis-(2-amidinopropane) hydrochloride in the propagation phase (up to −36% and −22%, respectively; P < 0.05).
DISCUSSION
The present study, for the first time, demonstrates distinct effects of small dense protein-rich HDL3 in counteracting the inhibitory effects of oxLDL on endothelium-dependent vasorelaxation in rabbit aortic rings. Our data reveal that small dense HDL3 subpopulations enriched in S1P exert potent vasorelaxing activity, thereby suggesting a role of this bioactive constituent for the enhanced vasodilatory activity. In a candidate-based approach, no consistent effect was observed for several other components enriched or depleted in HDL3. Furthermore, small dense HDL3 particles potently induced NO generation in endothelial cells, an effect that was blocked by a S1P receptor antagonist, documenting the involvement of S1P1/S1P3 receptors in the HDL3-mediated vasorelaxation.
Impaired endothelium-dependent vasodilation is a critical event in atherogenesis (43–46). HDL appears to prevent or reverse endothelial dysfunction (2, 10). Several interrelated functions of HDL may contribute to this effect, including cellular cholesterol efflux, protection of LDL against oxidative modification, inhibition of blood cell adhesion to vascular endothelium, protection from endothelial cell death, and vasodilation (27, 30, 47–49). The latter activity of HDL primarily results from the presence of S1P, a bioactive lipid (23, 24, 50–52). Consistent with previous reports (29–31), we observed an asymmetrical distribution of S1P among HDL subpopulations isolated from normolipidemic subjects, featuring almost 4-fold enrichment in small dense HDL3, as compared with large light HDL2.
Due to the high complexity of the lipid and protein composition of HDL, structure-function relationships across HDL subpopulations are complex. Our studies have previously shown the superior atheroprotective functionality of HDL3 particles relative to HDL2 (53). Potential heterogeneity of the vasodilatory properties of HDL was, however, not evaluated in earlier studies. In particular, it remained unclear as to whether S1P enrichment renders superior vasodilatory activity to HDL3. Earlier, HDL-induced endothelial vasorelaxation in aortic segments was shown to be directly related to HDL content of S1P (24). In the present study, small dense protein-rich HDL3 was more potent to prevent oxLDL-induced inhibitory effects on endothelium-dependent vasorelaxation relative to large light HDL2, suggesting beneficial functional consequences of S1P enrichment in HDL3.
Although HDL3 particles are enriched in S1P relative to HDL2, demonstration of a direct link between S1P content and vasorelaxation activity can be complicated by the impact of other components enriched or depleted in HDL3. In a proof-of-concept experiment, S1P-enriched rHDL consistently counteracted oxLDL-induced inhibitory effects toward endothelium-dependent vasorelaxation of rabbit aortic rings, supporting the key role of S1P in the potent vasodilatory properties of HDL3. By contrast, the vasodilatory effect of other HDL components, such as apoJ (10) and apoC-III (11), were either weak or inconsistent with their HDL content (33, 54). Indeed, while apoJ did not significantly enhance vasodilation induced by HDL, the vasodilatory effect of apoC-III was observed, which was inconsistent with its enrichment in large versus small HDL (32, 33).
Further along this line, although PON1 activity was enriched in HDL3 and correlated with the vasodilatory effect of HDL subpopulations, this correlation was weaker, as compared with those calculated for S1P and apoA-I. Importantly, in our study, HDLs were derived from EDTA plasma and as such are well known to display greatly diminished PON1 activity as a result of PON1 inhibition by EDTA (55). We therefore believe that although PON1 activity might, in principle, contribute to the vasodilatory effects of HDL via reducing both oxidative stress and NO inactivation by reactive oxygen species, this pathway is of rather minor importance.
apoM, as the main carrier of S1P, is also enriched in small dense HDL (32, 33). The significant correlation of apoM content with the vasodilatory effect of HDL subpopulations provides an additional argument in support of the major role of S1P in the HDL-induced vasorelaxation. However, the association of HDL S1P and apoM remains incompletely clarified. Despite the fact that apoM was initially proposed as the only interaction partner for S1P in HDL (56), S1P has later been reported to be enriched in HDL independent of apoM (27, 57, 58). Our data obtained using rHDL- and albumin-associated S1P are consistent with a vasodilatory activity of S1P, which can be independent of apoM and HDL binding (59).
Indeed, the magnitude of the vasodilatory effect of S1P against vasoconstriction enhanced by oxLDL in arterenol hydrochloride-contracted rings was preserved following S1P conjugation to albumin instead of HDL, consistent with an S1P-specific mechanism. It is of note that albumin-associated S1P was unlikely to contribute to the vasodilatory properties of HDL in our study, as no albumin was detected by LC/MS/MS in HDL subpopulations isolated by the single-spin isopycnic density gradient ultracentrifugation approach, as previously reported (32).These data imply that albumin-bound S1P may act as a natural substitute of HDL-associated S1P under conditions of HDL and/or apoM deficiency (59).
In addition to S1P, HDL content of apoA-I and TG showed significant positive and negative correlations with endothelium-dependent relaxation activity, respectively, suggesting potential roles of apoA-I and TG in HDL-mediated vasorelaxation. This conclusion is in accordance with our previous studies showing impaired vasodilatory effects of HDL in type 2 diabetes or abdominal obesity in parallel with decreased HDL content of apoA-I and increased content of TG (7, 8). In contrast to S1P, apoA-I content does not considerably differ across HDL subpopulations. Indeed, the concentration of apoA-I was similar across HDL subpopulations when HDL concentration was fixed at 1 g total protein per liter in our aortic ring system. These data suggest that it is conformational status rather than the abundance of apoA-I in small dense HDLs that can favor their enhanced biological functionality (60). Indeed, apoA-I conformation is peculiar in small dense HDL, potentially enhancing its biological activity (22, 60). It is reasonable to consider that such conformational modifications may enhance HDL interactions with receptors involved in vasodilation, such as with scavenger receptor class B type I (SR-BI), which favors NO synthesis (23), or with ABCG1, which promotes NO production by increasing cholesterol efflux (61).
It is important, in this regard, that the negative correlation between the vasodilatory properties of HDL subpopulations and their content of TG can be explained by the role of TG in both altering the conformation of apoA-I (22) and decreasing the antioxidative activity of HDL. Indeed, vascular oxidative stress is well-established to diminish endothelial NO bioavailability (3). HDL antioxidative activity can therefore counteract the inhibitory effect of oxLDL on vasorelaxation. In the present study, we found that TG-enriched small dense HDL displayed significantly reduced antioxidative activity, consistent with the negative correlation between TG content and vasodilatory activity of HDL.
The underlying mechanism by which HDL-associated S1P mediates vasorelaxation has been attributed to the activation of eNOS and subsequent NO generation in endothelial cells (24, 62). In our study, the HDL3c subpopulation strongly induced NO production, suggesting the potential role of HDL3-associated S1P in the intracellular signaling pathway activation. Moreover, we showed that a S1P1/S1P3 receptor antagonist effectively blocked NO production in murine aortic endothelial cells incubated with HDL3c, implying that the potent vasodilatory effect of HDL3 is associated with enhanced NO production through the S1P1/S1P3 receptor pathway. Together with the results of aortic ring studies, these findings highlight the importance of the HDL lipid moiety in endothelial vasorelaxation.
Reduced S1P content of HDL in coronary artery disease and myocardial infarction may further reflect the cardioprotective impact of HDL-associated S1P (15, 16, 63, 64). Such a reduction in S1P content of HDL can be linked to oxidative modification of HDL particles (15). Indeed, in vitro oxidation of HDL from healthy subjects resulted in the loss of HDL-S1P with a concomitant reduction in HDL capacity to uptake S1P in vitro (15). Consistent with this observation, oxidation of HDL from healthy subjects reduced its ability to counteract the inhibitory effect of oxLDL on endothelium-dependent vasorelaxation (40).
The protective effect of HDL against cardiovascular disease is well-recognized. However, circulating HDL cholesterol concentration, which is commonly used in clinical practice, does not accurately estimate the cardioprotective functionality of HDL (65). Thus, identification of HDL-related factors involved in, or serving as, biomarkers to cardiovascular disease remains in the focus of research. Available data identify HDL-associated S1P as a potential biomarker of cardiovascular risk. In addition, our findings may have important implications in developing HDL-targeted therapy as novel therapeutic strategies can be envisaged by loading HDL particles with S1P.
In summary, the present study reveals that marked S1P enrichment in small dense HDL3 particles is associated with their potent ability to counteract the inhibitory effects of oxLDL on endothelium-dependent vasorelaxation and to induce NO generation in endothelial cells, suggesting the pivotal role of S1P to the distinct vasodilatory and NO-stimulatory effects of HDL3, which may contribute to HDL-mediated vasoprotection.
Footnotes
Abbreviations:
- DAF-2
- 4,5-diaminofluorescein diacetate
- Emax
- maximal relaxation
- FC
- free cholesterol
- oxLDL
- oxidized LDL
- PC
- phosphatidylcholine
- PON1
- paraoxonase-1
- rHDL
- reconstituted HDL
- S1P
- sphingosine-1-phosphate
- SV40
- simian vacuolating virus 40
- SVEC
- simian vacuolating virus 40-transformed murine aortic endothelial cell
- TC
- total cholesterol
- TG
- triglyceride
- wt%
- percentage by weight
This work was supported by INSERM and ANR (CARINA project) in Paris, France. Additional support was provided by the City of Paris (financial support to M.D.) and National Health and Medical Research Council of Australia Grant APP1037903 (to K.A.R.).
REFERENCES
- 1.Annema W., von Eckardstein A., and Kovanen P. T.. 2015. HDL and atherothrombotic vascular disease. In High Density Lipoproteins: From Biological Understanding to Clinical Exploitation. A. von Eckardstein and D. Kardassis, editors. Springer, Cham Heidelberg New York Dordrecht London. 369–403. [Google Scholar]
- 2.Nofer J-R., Kehrel B., Fobker M., Levkau B., Assmann G., and von Eckardstein A.. 2002. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 161: 1–16. [DOI] [PubMed] [Google Scholar]
- 3.Förstermann U., Xia N., and Li H.. 2017. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 120: 713–735. [DOI] [PubMed] [Google Scholar]
- 4.Cominacini L., Rigoni A., Pasini A. F., Garbin U., Davoli A., Campagnola M., Pastorino A. M., Lo Cascio V., and Sawamura T.. 2001. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J. Biol. Chem. 276: 13750–13755. [DOI] [PubMed] [Google Scholar]
- 5.Riwanto M., and Landmesser U.. 2013. High density lipoproteins and endothelial functions: mechanistic insights and alterations in cardiovascular disease. J. Lipid Res. 54: 3227–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohta T., Takata K., Horiuchi S., Morino Y., and Matsuda I.. 1989. Protective effect of lipoproteins containing apoprotein A-I on Cu2+-catalyzed oxidation of human low density lipoprotein. FEBS Lett. 257: 435–438. [DOI] [PubMed] [Google Scholar]
- 7.Perségol L., Vergès B., Foissac M., Gambert P., and Duvillard L.. 2006. Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia 49: 1380–1386. [DOI] [PubMed]
- 8.Perségol L., Vergès B., Gambert P., and Duvillard L.. 2007. Inability of HDL from abdominally obese subjects to counteract the inhibitory effect of oxidized LDL on vasorelaxation. J. Lipid Res. 48: 1396–1401. [DOI] [PubMed] [Google Scholar]
- 9.Perségol L., Duvillard L., Monier S., Brindisi M-C., Bouillet B., Petit J-M., and Vergès B.. 2014. No improvement of high-density lipoprotein (HDL) vasorelaxant effect despite increase in HDL cholesterol concentration in type 2 diabetic patients treated with glitazones. J. Clin. Endocrinol. Metab. 99: E2015–E2019. [DOI] [PubMed]
- 10.Riwanto M., Rohrer L., Roschitzki B., Besler C., Mocharla P., Mueller M., Perisa D., Heinrich K., Altwegg L., Von Eckardstein A., et al. . 2013. Altered activation of endothelial anti-and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: Role of high-density lipoprotein-proteome remodeling. Circulation. 127: 891–904. [DOI] [PubMed] [Google Scholar]
- 11.Rull A., Martínez-Bujidos M., Pérez-Cuellar M., Pérez A., Ordóñez-Llanos J., and Sánchez-Quesada J. L.. 2015. Increased concentration of clusterin/apolipoprotein J (apoJ) in hyperlipemic serum is paradoxically associated with decreased apoJ content in lipoproteins. Atherosclerosis 241: 463–470. [DOI] [PubMed]
- 12.Besler C., Heinrich K., Rohrer L., Doerries C., Riwanto M., Shih D. M., Chroni A., Yonekawa K., Stein S., Schaefer N., et al. . 2011. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Invest. 121: 2693–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomaraschi M., Ossoli A., Favari E., Adorni M. P., Sinagra G., Cattin L., Veglia F., Bernini F., Franceschini G., and Calabresi L.. 2013. Inflammation impairs eNOS activation by HDL in patients with acute coronary syndrome. Cardiovasc. Res. 100: 36–43. [DOI] [PubMed] [Google Scholar]
- 14.Sattler K., Lehmann I., Gräler M., Bröcker-Preuss M., Erbel R., Heusch G., and Levkau B.. 2014. HDL-bound sphingosine 1-phosphate (S1P) predicts the severity of coronary artery atherosclerosis. Cell. Physiol. Biochem. 34: 172–184. [DOI] [PubMed] [Google Scholar]
- 15.Sattler K., Gräler M., Keul P., Weske S., Reimann C-M., Jindrová H., Kleinbongard P., Sabbadini R., Bröcker-Preuss M., Erbel R., et al. . 2015. Defects of high-density lipoproteins in coronary artery disease caused by low sphingosine-1-phosphate content: correction by sphingosine-1-phosphate-loading. J. Am. Coll. Cardiol. 66: 1470–1485. [DOI] [PubMed] [Google Scholar]
- 16.Sattler K. J. E., Elbasan Ş., Keul P., Elter-Schulz M., Bode C., Gräler M. H., Bröcker-Preuss M., Budde T., Erbel R., Heusch G., et al. . 2010. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res. Cardiol. 105: 821–832. [DOI] [PubMed] [Google Scholar]
- 17.Navab M., Hama S. Y., Anantharamaiah G. M., Hassan K., Hough G. P., Watson A. D., Reddy S. T., Sevanian A., Fonarow G. C., and Fogelman A. M.. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J. Lipid Res. 41: 1495–1508. [PubMed] [Google Scholar]
- 18.Kontush A., Chantepie S., and Chapman M. J.. 2003. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 23: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 19.Watson, A. D., J. A. Berliner, S. Y. Hama, B. N. La Du, K. F. Faull, A. M. Fogelman, and M. Navab. 1995. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J. Clin. Invest. 96: 2882–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou J., Wang J., Xu H., Ou Z., Sorci-Thomas M. G., Jones D. W., Signorino P., Densmore J. C., Kaul S., Oldham K. T., et al. . 2005. Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ. Res. 97: 1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holzer M., Wolf P., Curcic S., Birner-Gruenberger R., Weger W., Inzinger M., El-Gamal D., Wadsack C., Heinemann A., and Marsche G.. 2012. Psoriasis alters HDL composition and cholesterol efflux capacity. J. Lipid Res. 53: 1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtiss L. K., Bonnet D. J., and Rye K. A.. 2000. The conformation of apolipoprotein A-I in high-density lipoproteins is influenced by core lipid composition and particle size: a surface plasmon resonance study. Biochemistry 39: 5712–5721. [DOI] [PubMed]
- 23.Mineo C., Yuhanna I. S., Quon M. J., and Shaul P. W.. 2003. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J. Biol. Chem. 278: 9142–9149. [DOI] [PubMed] [Google Scholar]
- 24.Nofer J. R., Van Der Giet M., Tölle M., Wolinska I., Von Wnuck Lipinski K., Baba H. A., Tietge U. J., Gödecke A., Ishii I., Kleuser B., et al. . 2004. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Invest. 113: 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuvin J. T., Rämet M. E., Patel A. R., Pandian N. G., Mendelsohn M. E., and Karas R. H.. 2002. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am. Heart J. 144: 165–172. [DOI] [PubMed] [Google Scholar]
- 26.Rämet M. E., Rämet M., Lu Q., Nickerson M., Savolainen M. J., Malzone A., and Karas R. H.. 2003. High-density lipoprotein increases the abundance of eNOS protein in human vascular endothelial cells by increasing its half-life. J. Am. Coll. Cardiol. 41: 2288–2297. [DOI] [PubMed] [Google Scholar]
- 27.Levkau B. 2015. HDL-S1P: Cardiovascular functions, disease-associated alterations, and therapeutic applications. Front. Pharmacol. 6: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okajima F. 2002. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim. Biophys. Acta 1582: 132–137. [DOI] [PubMed]
- 29.de Souza J. A., Vindis C., Nègre-Salvayre A., Rye K. A., Couturier M., Therond P., Chantepie S., Salvayre R., Chapman M. J., and Kontush A.. 2010. Small, dense HDL 3 particles attenuate apoptosis in endothelial cells: Pivotal role of apolipoprotein A-I. J. Cell. Mol. Med. 14: 608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kontush A., Therond P., Zerrad A., Couturier M., Négre-Salvayre A., de Souza J. A., Chantepie S., and Chapman M. J.. 2007. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler. Thromb. Vasc. Biol. 27: 1843–1849. [DOI] [PubMed] [Google Scholar]
- 31.Kontush A., Lhomme M., and Chapman M. J.. 2013. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 54: 2950–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson W. S., Silva R. A. G. D., Chantepie S., Lagor W. R., Chapman M. J., and Kontush A.. 2009. Proteomic analysis of defined hdl subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 29: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah A. S., Tan L., Long J. L., and Davidson W. S.. 2013. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 54: 2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guérin M., Le Goff W., Lassel T. S., Van Tol A., Steiner G., and Chapman M. J.. 2001. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes: impact of the degree of triglyceridemia. Arterioscler. Thromb. Vasc. Biol. 21: 282–288. [DOI] [PubMed]
- 35.Chapman M. J., Goldstein S., Lagrange D., and Laplaud P. M.. 1981. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J. Lipid Res. 22: 339–358. [PubMed] [Google Scholar]
- 36.Hansel B., Giral P., Nobecourt E., Chantepie S., Bruckert E., Chapman M. J., and Kontush A.. 2004. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J. Clin. Endocrinol. Metab. 89: 4963–4971. [DOI] [PubMed] [Google Scholar]
- 37.Matz C. E., and Jonas A.. 1982. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J. Biol. Chem. 257: 4535–4540. [PubMed] [Google Scholar]
- 38.Murata N., Sato K., Kon J., Tomura H., Yanagita M., Kuwabara A., Ui M., and Okajima F.. 2000. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 352 Pt 3: 809–815. [PMC free article] [PubMed]
- 39.De Mello W. C. 1996. Impaired regulation of cell communication by β-adrenergic receptor activation in the failing heart. Hypertension. 27: 265–268. [DOI] [PubMed] [Google Scholar]
- 40.Perségol L., Brindisi M-C., Rageot D., Pais de Barros J-P., Monier S., Vergès B., and Duvillard L.. 2015. Oxidation-induced loss of the ability of HDL to counteract the inhibitory effect of oxidized LDL on vasorelaxation. Heart Vessels 30: 845–849. [DOI] [PubMed]
- 41.Nobécourt E., Jacqueminet S., Hansel B., Chantepie S., Grimaldi A., Chapman M. J., and Kontush A.. 2005. Defective antioxidative activity of small dense HDL3 particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycaemia. Diabetologia 48: 529–538. [DOI] [PubMed]
- 42.Mulders A. C., Hendriks-Balk M. C., Mathy M-J., Michel M. C., Alewijnse A. E., and Peters S. L.. 2006. Sphingosine kinase-dependent activation of endothelial nitric oxide synthase by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 26: 2043–2048. [DOI] [PubMed]
- 43.Kitchens R. L., Thompson P. A., Viriyakosol S., O’Keefe G. E., and Munford R. S.. 2001. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J. Clin. Invest. 108: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitamoto S., and Egashira K.. 2004. Endothelial dysfunction and coronary atherosclerosis. Curr. Drug Targets Cardiovasc. Haematol. Disord. 4: 13–22. [DOI] [PubMed]
- 45.Endemann D. H., and Schiffrin E. L.. 2004. Endothelial dysfunction. J. Am. Soc. Nephrol 15: 1983–1992. [DOI] [PubMed]
- 46.Davignon J., and Ganz P.. 2004. Role of endothelial dysfunction in atherosclerosis. Circulation 109: III27–III32. [DOI] [PubMed]
- 47.Riwanto M., Rohrer L., Roschitzki B., Besler C., Mocharla P., Mueller M., Perisa D., Heinrich K., Altwegg L., von Eckardstein A., et al. . 2013. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation 127: 891–904. [DOI] [PubMed]
- 48.Calabresi L., Canavesi M., Bernini F., and Franceschini G.. 1999. Cell cholesterol efflux to reconstituted high-density lipoproteins containing the apolipoprotein A-I(Milano) dimer. Biochemistry. 38: 16307–16314. [DOI] [PubMed] [Google Scholar]
- 49.Kontush A., and Chapman M. J.. 2012. High-Density Lipoproteins: Structure, Metabolism, Function and Therapeutics. John Wiley & Sons, Inc., New York. [Google Scholar]
- 50.Yuhanna I. S., Zhu Y., Cox B. E., Hahner L. D., Osborne-Lawrence S., Lu P., Marcel Y. L., Anderson R. G., Mendelsohn M. E., Hobbs H. H., et al. . 2001. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 7: 853–857. [DOI] [PubMed] [Google Scholar]
- 51.Theilmeier G., Schmidt C., Herrmann J., Keul P., Schäfers M., Herrgott I., Mersmann J., Larmann J., Hermann S., Stypmann J., et al. . 2006. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 114: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 52.Prüfer N., Kleuser B., and van der Giet M.. 2015. The role of serum amyloid A and sphingosine-1-phosphate on high-density lipoprotein functionality. Biol. Chem. 396: 573–583. [DOI] [PubMed]
- 53.Camont L., Lhomme M., Rached F., Le Goff W., Nègre-Salvayre A., Salvayre R., Calzada C., Lagarde M., Chapman M. J., and Kontush A.. 2013. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler. Thromb. Vasc. Biol. 33: 2715–2723. [DOI] [PubMed] [Google Scholar]
- 54.HDL Proteome Watch. Accessed December 8, 2017, at http://homepages.uc.edu/~davidswm/HDLproteome.html.
- 55.Mackness B., Durrington P. N., and Mackness M. I.. 1998. Human serum paraoxonase. Gen. Pharmacol. 31: 329–336. [DOI] [PubMed]
- 56.Christoffersen C., Obinata H., Kumaraswamy S. B., Galvani S., Ahnström J., Sevvana M., and Egerer-Sieber C.. Y. A. Muller, T. Hla, L. B. Nielsen, et al. 2011. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA. 108: 9613–9618. [DOI] [PMC free article] [PubMed]
- 57.Arkensteijn B. W. C., Berbée J. F. P., Rensen P. C. N., Nielsen L. B., and Christoffersen C.. 2013. The apolipoprotein M-sphingosine-1-phosphate axis: Biological relevance in lipoprotein metabolism, lipid disorders and atherosclerosis. Int. J. Mol. Sci. 14: 4419–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karuna R., Park R., Othman A., Holleboom A. G., Motazacker M. M., Sutter I., Kuivenhoven J. A., Rohrer L., Matile H., Hornemann T., et al. . 2011. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis 219: 855–863. [DOI] [PubMed] [Google Scholar]
- 59.Wilkerson B. A., Grass G. D., Wing S. B., Argraves W. S., and Argraves K. M.. 2012. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier. J. Biol. Chem. 287: 44645–44653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du X. M., Kim M. J., Hou L., Le Goff W., Chapman M. J., Van Eck M., Curtiss L. K., Burnett J. R., Cartland S. P., Quinn C. M., et al. . 2015. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ. Res. 116: 1133–1142. [DOI] [PubMed] [Google Scholar]
- 61.Terasaka N., Westerterp M., Koetsveld J., Fernandez-Hernando C., Yvan-Charvet L., Wang N., Sessa W. C., and Tall A. R.. 2010. ATP-binding cassette transporter G1 and high-density lipoprotein promote endothelial NO synthesis through a decrease in the interaction of caveolin-1 and endothelial NO synthase. Arterioscler. Thromb. Vasc. Biol. 30: 2219–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura T., Sato K., Malchinkhuu E., Tomura H., Tamama K., Kuwabara A., Murakami M., and Okajima F.. 2003. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler. Thromb. Vasc. Biol 23: 1283–1288. [DOI] [PubMed]
- 63.Argraves K. M., Sethi A. A., Gazzolo P. J., Wilkerson B. A., Remaley A. T., Tybjaerg-Hansen A., Nordestgaard B. G., Yeatts S. D., Nicholas K. S., Barth J. L., et al. . 2011. S1P, dihydro-S1P and C24:1-ceramide levels in the HDL-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis. 10: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knapp M., Baranowski M., Czarnowski D., Lisowska A., Zabielski P., Górski J., and Musiał W.. 2009. Plasma sphingosine-1-phosphate concentration is reduced in patients with myocardial infarction. Med. Sci. Monit. 15: CR490–CR493. [PubMed] [Google Scholar]
- 65.Rader D. J., and Tall A. R.. 2012. The not-so-simple HDL story: is it time to revise the HDL cholesterol hypothesis? Nat. Med 18: 1344–1346. [DOI] [PubMed] [Google Scholar]