Abstract
DHA is important for fetal neurodevelopment. During pregnancy, maternal plasma DHA increases, but the mechanism is not fully understood. Using rats fed a fixed-formula diet (DHA as 0.07% total energy), plasma and liver were collected for fatty acid profiling before pregnancy, at 15 and 20 days of pregnancy, and 7 days postpartum. Phosphatidylethanolamine methyltransferase (PEMT) and enzymes involved in PUFA synthesis were examined in liver. Ad hoc transcriptomic and lipidomic analyses were also performed. With pregnancy, DHA increased in liver and plasma lipids, with a large increase in plasma DHA between day 15 and day 20 that was mainly attributed to an increase in 16:0/DHA phosphatidylcholine (PC) in liver (2.6-fold) and plasma (3.9-fold). Increased protein levels of Δ6 desaturase (FADS2) and PEMT at day 20 and increased Pemt expression and PEMT activity at day 15 suggest that during pregnancy, both DHA synthesis and 16:0/DHA PC synthesis are upregulated. Transcriptomic analysis revealed minor changes in the expression of genes related to phospholipid synthesis, but little insight on DHA metabolism. Hepatic PEMT appears to be the mechanism for increased plasma 16:0/DHA PC, which is supported by increased DHA biosynthesis based on increased FADS2 protein levels.
Keywords: omega-3 fatty acids, phospholipids, fatty acids, nutrition/lipids, tandem mass spectrometry, microarray, liver, blood, prenatal nutritional physiological phenomena, phosphatidylethanolamine methyltransferase, 16:0/docosahexaenoic acid phosphatidylcholine
Maternal intake and blood status of DHA (22:6n-3) can have important effects on the health of infants (1, 2), but blood levels are low in numerous human populations across the globe (3). In a rodent model, the availability of DHA (22:6n-3) during neural development has been shown to be critical for optimal brain function (4). During pregnancy, the amount of DHA in the maternal bloodstream is increased (5–7), especially in the plasma phospholipid fraction (8, 9). While diet is an important predictor in determining blood levels of DHA during pregnancy, it appears that there are also other mechanisms involved with increasing maternal circulating DHA (2, 6, 7, 10, 11).
Increased biosynthesis of DHA from 18:3n-3 through the upregulation of desaturation and elongation enzymes by increased estrogen signaling is one possible mechanism (12). However, in maternal plasma during pregnancy, there appears to be a distinct shift toward increased DHA and decreased EPA (20:5n-3) (6), suggesting additional selectivity for DHA in synthesis and/or mobilization. In addition, partitioning and accretion studies indicate that DHA accumulates in the liver during pregnancy (13, 14), but that DHA in periuterine adipose decreases (14). Therefore, maternal adaptations to meet fetal demand of DHA appear to be a combination of fatty acid biosynthesis and mobilization as well as incorporation and secretion to increase maternal plasma DHA for fetal uptake.
The placenta has the capacity to selectively enrich DHA and arachidonic acid (ARA) (20:4n-6) available for the fetus from the mother (15). While there has been a focus on the maternal-fetal transfer between NEFA pools (2, 7, 11), the placenta has lipase activity (16–20). Therefore, other lipid pools in plasma could be a source of DHA. Plasma lipids include different lipid classes, such as triacylglycerols (TAGs), phospholipids, and cholesteryl esters. The long-chain PUFAs (LC-PUFAs), such as DHA and ARA, tend to concentrate in the phospholipids (21), which in plasma is predominantly composed of phosphatidylcholine (PC) making up the monolayer of lipoproteins (22) and hepatic lipoprotein production is increased during pregnancy (23). PC is synthesized de novo through the Kennedy pathway or by conversion of phosphatidylethanolamine (PE) through methylation by PE methyltransferase (PEMT) (24). Hepatic PE has higher DHA content, as compared with hepatic PC (25–27), and estrogen has been shown to upregulate the PE methylation pathway (28). Additionally, fetal Pemt−/− mice have lower levels of brain DHA (29), plasma levels of DHA in PC have been proposed as a surrogate marker of hepatic PEMT activity in humans (30), and PC derived from PEMT during pregnancy appears to be selectively transferred to the fetus (31).
In the current study, we set out to fully characterize the changes in DHA concentrations in plasma and liver in specific lipid classes before pregnancy, at 15 and 20 days of pregnancy, and 7 days postpartum in Sprague-Dawley rats. We hypothesized that hepatic PEMT would be associated with changes in DHA in plasma PC, therefore, expression and activity were examined. Hepatic enzymes involved in DHA biosynthesis were also examined across all of these time points. Based on the initial findings of a large increase in plasma DHA from 15 to 20 days of pregnancy, additional lipidomic and transcriptomic measurements were completed at these time points. This resulted in the discovery that 16:0/DHA PC was responsible for most of the increases in plasma and liver DHA. PEMT appeared to be involved in DHA mobilization during pregnancy by selectively producing 16:0/DHA PC. We confirmed that increased DHA biosynthesis from n-3 precursors likely occurs during pregnancy based on increased Δ6 desaturase (FADS2) protein levels. However, we cannot rule out that other mechanisms and adaptations could be involved in increasing maternal circulating DHA during pregnancy. Understanding maternal adaptations to increase DHA bioavailability for fetal delivery is necessary to determine precise maternal dietary DHA requirements in order to establish evidence-based recommendations to support pregnancy and fetal development.
MATERIALS AND METHODS
Study design
All animal procedures were approved by the University of Waterloo Animal Care Committee and are in accordance with the guidelines of the Canadian Council on Animal Care. Twenty-four female Sprague-Dawley rats were purchased at 7 weeks of age and mated with 6-month-old proven male breeders (Envigo, Mississauga, Ontario, Canada) at the Central Animal Facility on campus. All rats were fed a commercial fixed-formula rodent diet (8640 Teklad 22/5 Rodent Diet; Envigo) providing 3.0 kcal/g with 17% energy from fat, 29% energy from protein, and 54% energy from carbohydrate throughout the study. The fatty acid composition of the diet was determined by gas chromatography (see details of the methods below). Pregnant rats (n = 6) were euthanized at baseline (nonpregnant rats after 7 days of acclimatization), day 15 and day 20 of pregnancy, and 7 days postpartum by exsanguination following anesthesia using isoflurane after an overnight fast. Exsanguinated blood was collected in the presence of EDTA and plasma was isolated by centrifugation at 1,500 g and the liver was excised, washed in saline (0.9% v/v), and weighed. Samples were flash-frozen in liquid nitrogen and stored at −80°C for various analyses.
The 17β-estradiol concentrations
Plasma 17β-estradiol concentrations were determined by ELISA (Estradiol EIA kit; Cayman Chemical, Ann Arbor, MI). Briefly, 17β-estradiol was extracted from plasma with methylene chloride, and then reconstituted in EIA buffer, loaded to the 96-well plate in duplicate with standards and absorbance at 420 nm wavelength, measured by spectrophotometry, and concentrations determined by comparison to a standard curve.
Gene expression and protein determinations
Gene expression was determined by quantitative real-time PCR and protein levels were determined by immunoblotting according to previous procedures (32). For mRNA expression, liver samples were homogenized in Trizol reagent (Invitrogen, Frederick, MD) and chloroform was used to separate RNA from other cellular components. The integrity of isolated RNA was assessed by measuring intact 18S and 28S rRNA after agarose gel electrophoresis with ethidium bromide. The concentration of the samples was then confirmed using absorbance at 260 nm, while the 260/280 ratios were used to determine sample purity, as determined by a NanoDrop spectrophotometer. Only RNA samples with high purity (260/280 ratio above 1.90) were used for subsequent cDNA synthesis by reverse transcriptase. Primers were designed for Pemt through the Primer-BLAST program on the NCBI website and ordered from Sigma-Aldrich, Oakville, Ontario, Canada (5′-CCCAGCTTTGTGGCGGCTGT-3′). Expression was determined by SYBR Green qPCR master mix and an Applied Biosystems 7500 real-time PCR system (Streetsville, Ontario, Canada) with software version 1.2.3 using 25 μl as the reaction volume with an initial incubation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min. A dissociation curve was obtained by increasing the temperature from 60°C to 95°C at 1°C/min. The threshold cycle number (ΔCt) was determined and normalized to the ΔCt observed for Gapdh, a housekeeping reference gene during pregnancy (33). The expression of target genes was determined using the 2–ΔΔCt method and the values were expressed as the mean expression relative to baseline.
For protein determinations, antibodies used for protein expression analysis were FADS2 [1:1,000 in 5% milk-TBS with 0.5% (v/v) Tween (TBST); Abcam, Cambridge, MA], Δ5 desaturase (FADS1) (1:100 in 5% BSA-TBST; Santa Cruz Biotechnology, Santa Cruz, CA), elongase 2 (ELOVL2) (1:250 in 5% BSA-TBST; Santa Cruz Biotechnology), elongase 5 (ELOVL5) (1:250 in 5% BSA-TBST; Santa Cruz Biotechnology), multifunctional protein-2 (MFP2) (1:200 in 5% milk-TBST; Santa Cruz Biotechnology), and PEMT (kindly donated by Professor Dennis Vance, University of Alberta). Liver samples were homogenized in a buffer containing complete protease inhibitor tablets (0.25 mol/l sucrose, 0.01 mol/l Tris-HCl, 0.01 mol/l MgCl2, and 2.5 mmol/l DTT). Protein quantification was completed using a bicinchoninic acid procedure. Twenty micrograms of the protein were resolved on a 12.5% SDS-PAGE gel and transferred to a polyvinylidene fluoride membrane. Next, 5% milk was used to block the membranes in TBST overnight at 4°C. The membranes were then incubated with primary antibodies (1:1,000 dilution) for 2 h at room temperature. Following incubation, the membranes were washed with TBST, incubated again for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibody (rabbit anti-goat, 1:8,000 dilution; Santa Cruz Biotechnology) and washed again. The proteins were treated with enhanced chemiluminescence Western blotting detection reagents and visualized on a Chemigenius 2 bioimaging system using Genesnap software v 7.07. Finally, the molecular weights of proteins, equal protein loading, and adequate transfer of protein to membrane were confirmed using Ponceau staining and blotting for β actin.
Enzyme activity of PEMT
A liver PEMT activity assay was performed as described by Ridgway and Vance (34). Briefly, to assess activity, we used 50 μg of protein homogenized in Tris-HCl per liter (pH 9.2) and 5 mm DTT buffer per liter (Sigma-Aldrich). Samples were incubated with 200 μmol S-adenosyl-L-methionine per liter containing 0.5 μCi S-adenosyl-L-[methyl-3H]methionine (55.70 Ci/mmol) and 0.4 nmol exogenous phosphatidyl dimethylethanolamine per liter (Avanti Polar Lipids, Alabaster, AL). The reaction was carried out for 60 min at 37°C and then stopped by adding ice-cold CHCL3:methanol:1 N HCL (100:50:1, v/v). Samples were vortexed, washed with 0.1 M KCL in 50% methanol and centrifuged, and the organic phase was collected. Samples were then dried under nitrogen gas and resuspended in chloroform. Samples were then applied to and separated on a Silica Gel GTLC plate (Analtech Inc., Newark, DE) using CHCL3:methanol:acetic acid:water (50:30:5:2, v/v/v/v). The PC band was identified and collected using a reference standard (Sigma-Aldrich) and radioactivity counted by liquid scintillation (Beckman LS6500; Beckman Coulter, Mississauga, Ontario, Canada). PEMT activity was then calculated as PC formed (picomoles per milligram per hour).
Fatty acid composition determinations
The fatty acid composition of total lipids for the rodent diet and total lipids and various lipid classes of plasma and liver were determined. Internal standards used for quantitation of fatty acids were as follows: total lipids [docosatrienoic acid (22:3n-3) ethyl ester; Nu-Check Prep Inc., Elysian, MN], TAG (triheptadecanoate; Nu-Chek Prep Inc.), NEFAs (heptadecanoic acid; Avanti Polar Lipids), cholesteryl esters (cholesteryl heptadecanoate; Avanti Polar Lipids), PC (1,2-diheptadecanoyl-sn-glyercol-3-phosphocholine; Avanti Polar Lipids), PE (1,2-diheptadecanoyl-sn-glyercol-3-phosphoethanolamine; Avanti Polar Lipids), and phosphatidylserine (PS) and phosphatidylinositol (PI) [docosatrienoic acid (22:3n-3) ethyl ester added after separation by thin-layer chromatography]. Fatty acids were extracted by using 2:1 (v/v) chloroform:methanol (35) and 50 μg/ml butyl-hydroxytoluene. The organic phase containing the lipids was isolated by the addition of sodium phosphate buffer followed by centrifugation. Fatty acid methyl esters were then prepared from the total lipid extracts of the rodent diet and tissues. In addition, total lipid extracts of plasma and liver were aliquoted for neutral and polar lipid separation techniques by thin-layer chromatography.
Total lipid extracts from plasma and liver were dried, reconstituted into chloroform, and loaded onto thin-layer chromatography plates using a Hamilton syringe for neutral (Silica Gel G, 20 × 20 cm, 250 μm; Analtech Inc.) and polar lipid separations (Silica Gel G, 20 × 20 cm, 250 μm; Analtech Inc.). Neutral separations were resolved using 60:40:2 heptane:diethyl ether:glacial acetic acid (v/v/v) (36) and polar separations were resolved using 30:9:25:6:18 chloroform:methanol:isopropanol:0.25% potassium chloride in water (w/v):trimethylamine (v/v/v/v) (25). Lipid class bands were visualized under UV light after spraying with 0.1% 2,7-dichlorofluorescin in methanol (w/v) and identified by comparing to external lipid standards. The unesterified or free fatty acids, TAG, and cholesteryl ester bands were collected from the neutral separation, while PC, PE, PS, and PI bands were collected from polar separations. Separated lipid classes were extracted from the scrapings twice with 2:1 chloroform:methanol with butyl-hydroxytoluene.
The fatty acyl groups in total lipid and lipid class extracts were transesterified to fatty acid methyl esters using 14% BF3 in methanol with hexane for 1 h at 95°C. Additional hexane and a water wash were used to collect the fatty acid methyl esters for analysis by gas chromatography with flame ionization detection. Briefly, a fast gas chromatography protocol was employed (37) using a Varian 3900 gas chromatograph equipped with a DB-FFAP 15 m × 0.10 mm injected dose × 0.10 μm film thickness nitroterephthalic acid-modified polyethylene glycol capillary column (J&W Scientific/Agilent Technologies, Mississauga, Ontario, Canada), with hydrogen as the carrier gas and temperature ramping design to maximize peak resolution (38). Individual fatty acid peaks were identified by comparison to a reference mixture of fatty acids (GLC-462; Nu-Chek Prep Inc.), which was also used to calibrate fatty acid response factors on the gas chromatograph. Fatty acid data was expressed as concentrations and relative weight percentages for proper interpretation (3).
Semi-targeted lipidomic analyses
After initial fatty acid composition data was analyzed, ad hoc semi-targeted lipidomic analyses were performed on plasma (PC) and liver (PC and PE) samples for day 15 and day 20 of pregnancy only (n = 3 for each group) at the Analytical Facility for Bioactive Molecules at the Hospital for Sick Children, Toronto, Ontario, Canada. Samples were spiked with 21:0/22:6n-3 PC and 16:0/16:0-d9 PC, and 21:0/22:6n-3 PE as internal standards for PC and PE, respectively. Lipids were then extracted from the samples using a double extraction with 2:1 (v/v) chloroform:methanol. The organic phases were collected, dried under nitrogen gas, and reconstituted in ethanol/formic acid (99.8/0.2, v/v) for analysis by HPLC with MS/MS.
Lipids were separated with a high-performance liquid chromatograph (Agilent 120 series with binary pump; Agilent Technologies, Santa Clara CA) equipped with a Phenomenex Kinetex C18 column with dimensions of 100 × 2.1 mm × 2.6 μm (Phenomenex, Torrance, CA). Mobile phase A consisted of water/acetonitrile/methanol (2/1/1, v/v/v) + 0.05% formic acid and mobile phase B consisted of tetrahydrofuran/acetonitrile/methanol (2/1/1, v/v/v) + 0.05% formic acid using a gradient of 40% to 85% B over the course of 11 min with a flow rate of 0.4 ml/min.
The mass spectrometer (API4000 triple-quadrupole; AB SCIEX, Concord, Ontario, Canada) was operated in positive electrospray ionization mode with multiple reaction monitoring. Analyte-specific precursor to product ion mass transitions were chosen for each lipid. For PC species, a precursor of M+H with a product ion of m/z 184.1 (representing the PC head group) was used. To identify PE species, neutral loss scanning for m/z 141.02 (representing the PE head group) was used. Each PC or PE was then uniquely identified by the precursor to product ion mass transitions and chromatographic retention times. The presence of DHA acyl chains in PC and PE were confirmed in positive MS/MS mode by looking for lysoPC DHA and lysoPE DHA in the product ion spectra. PC data was expressed semi-quantitatively and PE data as relative response ratios.
Transcriptomic microarrays
Similarly, ad hoc transcriptomic analyses were performed on liver samples for day 15 and day 20 of pregnancy only (n = 4 for each group) after initial quantitative (q)PCR data was analyzed. RNA was extracted from livers using Trizol reagent, as described previously (39). Purity of extracted RNA was quantified using the 260/280 ratio on a Nanodrop 2000c prior to shipment to the University of Guelph (Guelph, Ontario, Canada). At Guelph, the integrity of the RNA was confirmed with an Agilent BioAnalyzer (Agilent) prior to hybridization to Affymetrix Rat Gene 2.1 ST array strips (Affymetrix, Fremont, CA), as described previously (40). Briefly, total RNA was used to synthesize cDNA, followed by cRNA synthesis. Second cycle cDNA was then synthesized, fragmented, labeled with biotin, and hybridized to microarrays. Strips were washed, stained, and scanned on the Affymetrix Gene Atlas platform.
Microarray data were corrected for background noise, quantile normalized using RMA, and log transformed (JMP Genomics version 5; SAS, Cary, NC). Principal component analysis was used to assess within and between group variability. ANOVA was used to identify differentially expressed genes with a false discovery rate (FDR) of 0.05 to control for multiple testing. A gene expression list including all genes with P < 0.05 (i.e., not adjusted for multiple testing) was also examined to obtain a more global perspective of the biological pathways effecting DHA metabolism during the late phase of pregnancy. Hierarchical clustering analysis confirmed the two conditions clustered together and did not overlap. FunNet was used to identify biological pathways that were differentially regulated in our gene expression list (41) and characterized into biological themes using pathways established from the Kyoto Encyclopedia of Genes and Genomes (KEGG).
Statistics
The one-way ANOVA test was used to determine differences in mRNA, protein, and fatty acids at different stages of pregnancy in rat models with Tukey post hoc testing to compare individual means after a significant F-value. For lipidomic data, unpaired t-tests were used to compare day 15 and day 20 of pregnancy. Statistical significance was assumed with P < 0.5. Statistical methods used for transcriptomics are described above. Data are presented as mean ± SD.
RESULTS
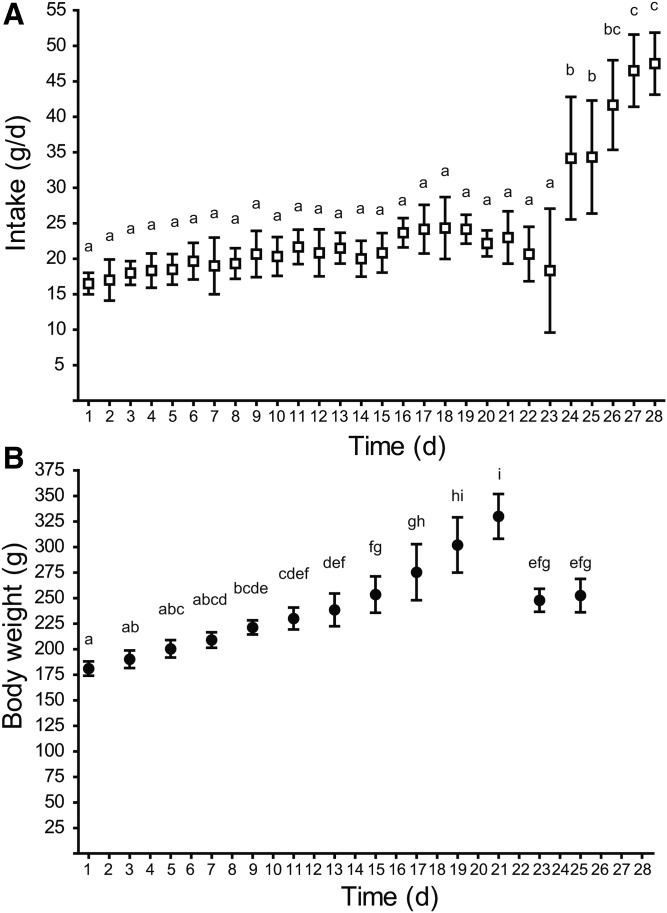
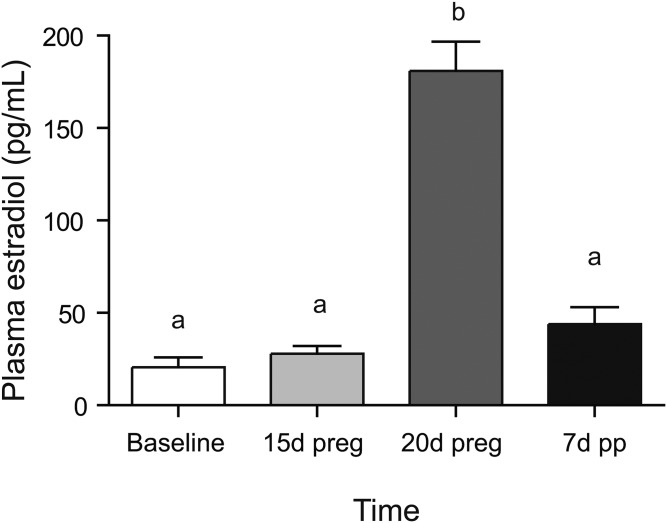
The fatty acid content of the rodent chow was confirmed by analysis by gas chromatography in triplicate. Total fatty acid content was 63.6 ± 1.5 mg fat per gram diet with 18.56 ± 0.13% of the fatty acids being saturates (16:0, 13.24 ± 0.11%; 18:0, 3.68 ± 0.02%), 24.34 ± 0.23% monounsaturates (18:1n-9, 21.82 ± 0.26%), 48.82 ± 0.03% n-6 polyunsaturates (18:2n-6, 48.51 ± 0.05%), and 5.71 ± 0.03% n-3 polyunsaturates (18:3n-3, 4.78 ± 0.01%; 20:5n-3, 0.42 ± 0.01%; 22:6n-3, 0.41 ± 0.02%). DHA intake was therefore 0.07% of total energy. Diet intake was significantly higher in the postpartum starting with 2 days after pup delivery (Fig. 1A), while dam body weight gradually increased during pregnancy and then dropped after pup delivery (Fig. 1B). Plasma estradiol was significantly higher in plasma at 20 days of pregnancy as compared with all other time points (Fig. 2).
Fig. 1.
Effect of pregnancy and postpartum on diet intake (A) and dam body weight (B). Time points (mean ± SD, n = 6 for each point) with different letters are significantly different (P < 0.05) by Bonferroni post hoc after significant F-value by repeated measures ANOVA. d, days.
Fig. 2.
Effect of pregnancy and postpartum on plasma estradiol. Time points (mean ± SD, n = 6 for each point) with different letters are significantly different (P < 0.05) by Bonferroni post hoc after significant F-value by repeated measures ANOVA. d, days; preg, pregnancy; pp, postpartum.
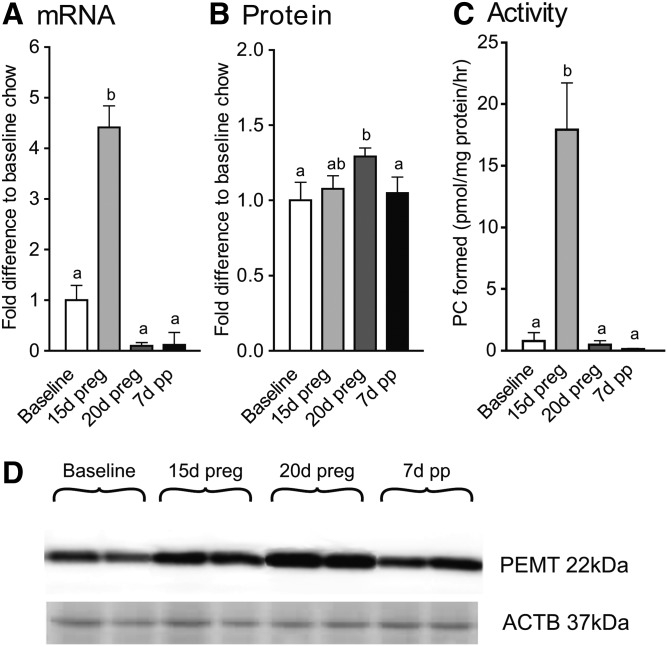
PEMT expression and activity were increased during pregnancy (Fig. 3). For mRNA, expression was increased at 15 days of pregnancy, as compared with all other time points. Differences in protein levels were subtle with measures at 20 days of pregnancy being higher than at baseline and 7 days of postpartum, but similar to levels at 15 days of pregnancy. Activity levels resembled the mRNA response with a significant increase at 15 days of pregnancy only.
Fig. 3.
Effect of pregnancy and postpartum on hepatic expression and activity of PEMT. Pemt mRNA (A); PEMT protein (B); PEMT activity (C); representative blots with ACTB as reference (D). Time points (mean ± SD, n = 6 for each point) with different letters are significantly different (P < 0.05) by Bonferroni post hoc after significant F-value by repeated measures ANOVA. d, days; preg, pregnancy; pp, postpartum.
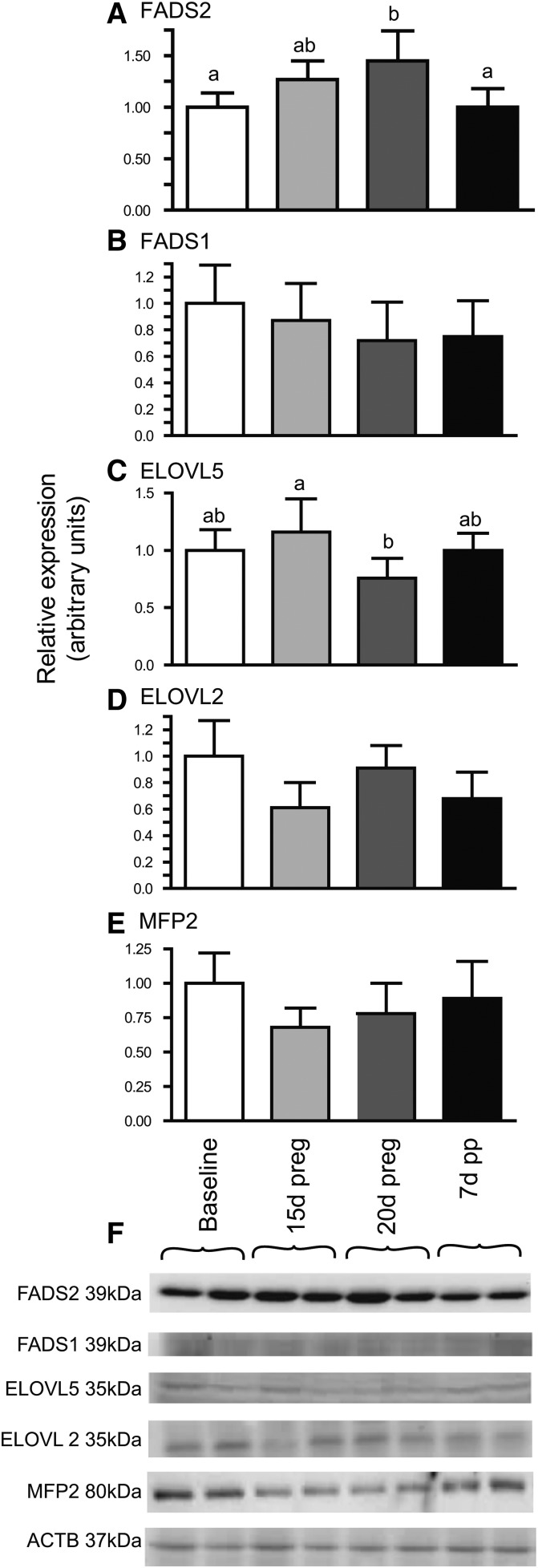
There was also evidence of increased expression of some of the genes involved in PUFA biosynthesis. Protein levels of FADS2 were 45% higher at 20 days of pregnancy, as compared with baseline rats, and returned to baseline levels on 7 days postpartum (Fig. 4A). FADS2 levels at 15 days of pregnancy were intermediate between baseline and 20 days of pregnancy. In contrast, protein levels of ELOVL5 were decreased 30% between post conception days 15 and 20 and returned to baseline levels on day 28 (Fig. 4C). No effects of pregnancy were observed in protein levels of ELOVL2, MFP2, or FADS1.
Fig. 4.
Effect of pregnancy and postpartum on protein levels of hepatic enzymes involved in PUFA synthesis. FADS2 (A); FADS1 (B); ELOVL5 (C); ELOVL2 (D); MFP2 (E); representative blots with ACTB as reference (F). Time points (mean ± SD, n = 6 for each point) with different letters are significantly different (P < 0.05) by Bonferroni post hoc after significant F-value by repeated measures ANOVA. d, days; preg, pregnancy; pp, postpartum.
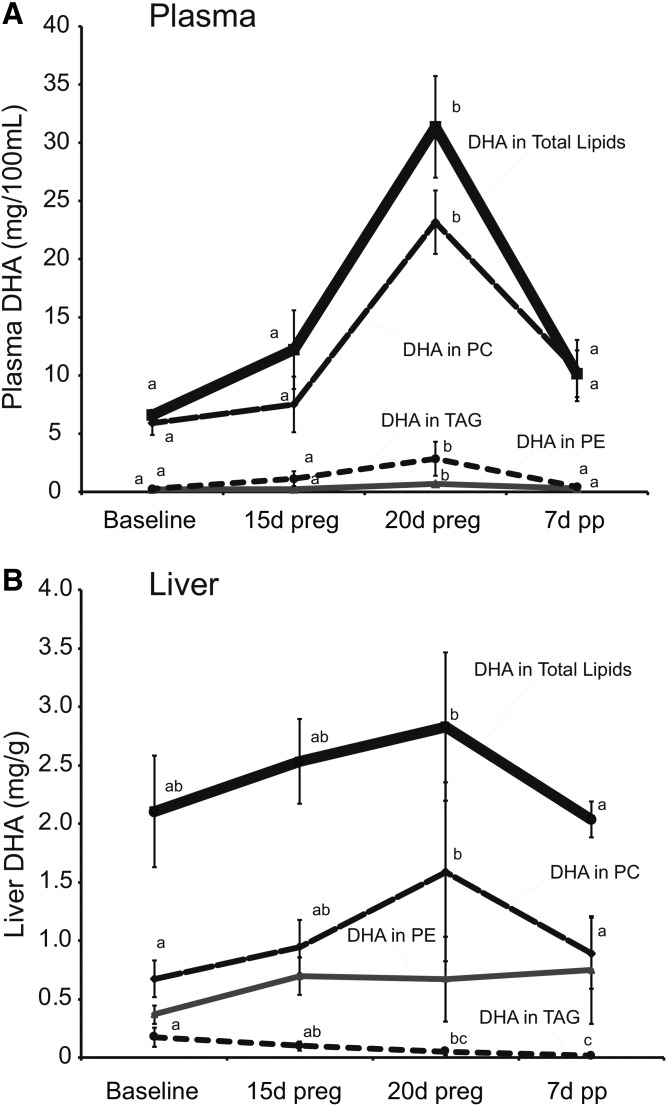
Pregnancy and postpartum had various effects on the fatty acid composition of the various lipid fractions in plasma and liver. In general, plasma total fatty acid concentrations were increased by 140% at 20 days of pregnancy, relative to baseline (P < 0.05 by Tukey’s post hoc after significant F-value by one-way ANOVA), while liver total fatty acids did not differ (data not shown). The increases in plasma total fatty acids at 20 days of pregnancy were also observed in the plasma PC and TAG, but not in the PE fraction (Tables 1–3). DHA concentrations in plasma total lipids increased even more dramatically, particularly at the later stages of pregnancy, with a 1.85-fold increase at 15 days and a 4.75-fold increase at 20 days of pregnancy, relative to baseline (Fig. 5A). While DHA concentrations in plasma TAG and PE also increased at 20 days of pregnancy, their contribution to total DHA in plasma was relatively minor. Therefore, changes in DHA concentrations in the PC fraction, especially during late pregnancy, were largely responsible for the changes observed in the total lipid fraction.
TABLE 1.
Effect of pregnancy and postpartum on the fatty acid composition of plasma PC
| Fatty Acid | Baseline | 15 Days of Pregnancy | 20 Days of Pregnancy | 7 Days Postpartum |
| weight % of total fatty acids | ||||
| 14:0 | 0.41 ± 0.22 | 0.56 ± 0.13 | 0.69 ± 0.09 | 0.74 ± 0.18 |
| 16:0 | 16.86 ± 0.99a | 18.35 ± 1.46a | 26.76 ± 1.73b | 18.32 ± 0.66a |
| 18:0 | 25.46 ± 2.33b | 28.88 ± 2.18bc | 21.55 ± 1.02a | 29.64 ± 1.65c |
| 20:0 | 0.18 ± 0.04 | 0.15 ± 0.07 | 0.13 ± 0.01 | 0.20 ± 0.06 |
| 22:0 | 0.25 ± 0.08b | 0.14 ± 0.05a | 0.10 ± 0.02a | 0.10 ± 0.02a |
| 24:0 | 0.22 ± 0.04 | 0.20 ± 0.08 | 0.24 ± 0.04 | 0.17 ± 0.05 |
| ∑SFAs | 43.92 ± 1.26a | 49.27 ± 2.34b | 51.06 ± 1.73b | 50.47 ± 2.18b |
| 14:1 | 0.05 ± 0.01b | 0.04 ± 0.01b | 0.01 ± 0.01b | 0.03 ± 0.01ab |
| 16:1 | 0.58 ± 0.17 | 0.74 ± 0.17 | 0.78 ± 0.12 | 0.64 ± 0.09 |
| 18:1n-7 | 1.49 ± 0.12b | 1.25 ± 0.11ab | 1.12 ± 0.1a | 1.42 ± 0.17b |
| 18:1n-9 | 9.54 ± 6.09b | 4.30 ± 0.92a | 4.43 ± 0.31ab | 6.54 ± 1.02ab |
| 20:1n-9 | 0.36 ± 0.25 | 0.13 ± 0.05 | 0.10 ± 0.02 | 0.12 ± 0.01 |
| 22:1n-9 | 0.20 ± 0.04ab | 0.27 ± 0.07b | 0.10 ± 0.03a | 0.23 ± 0.03ab |
| 24:1n-9 | 0.24 ± 0.04 | 0.26 ± 0.17 | 0.05 ± 0.03 | 0.10 ± 0.03 |
| ∑MUFAs | 12.49 ± 6.38b | 7.02 ± 0.99ab | 6.59 ± 0.25a | 9.09 ± 1.14ab |
| 18:2n-6 | 15.78 ± 2.34b | 12.56 ± 1.53a | 11.46 ± 1.85a | 14.42 ± 1.93ab |
| 18:3n-6 | 0.74 ± 0.81 | 0.23 ± 0.07 | 0.10 ± 0.02 | 0.15 ± 0.02 |
| 20:2n-6 | 0.26 ± 0.08b | 0.18 ± 0.02a | 0.16 ± 0.02a | 0.24 ± 0.03b |
| 20:3n-6 | 0.41 ± 0.11b | 0.37 ± 0.0ab | 0.22 ± 0.03a | 0.78 ± 0.08c |
| 20:4n-6 | 18.04 ± 3.56b | 19.54 ± 1.23b | 11.61 ± 2.05a | 15.22 ± 1.78ab |
| 22:2n-6 | 0.13 ± 0.09 | 0.12 ± 0.07 | 0.07 ± 0.05 | 0.07 ± 0.02 |
| 22:4n-6 | 0.32 ± 0.08a | 0.23 ± 0.06a | 0.61 ± 0.06b | 0.33 ± 0.07a |
| 22:5n-6 | 0.30 ± 0.04a | 0.33 ± 0.12a | 3.36 ± 0.43b | 0.99 ± 0.32a |
| ∑n-6 PUFAs | 35.98 ± 4.91b | 33.57 ± 1.72b | 27.60 ± 2.68a | 32.20 ± 3.06ab |
| 18:3n-3 | 0.18 ± 0.10 | 0.10 ± 0.02 | 0.12 ± 0.02 | 0.08 ± 0.01 |
| 20:3n-3 | 0.05 ± 0.01 | 0.08 ± 0.05 | 0.04 ± 0.01 | 0.02 ± 0.01 |
| 20:5n-3 | 0.21 ± 0.08b | 0.16 ± 0.07ab | 0.08 ± 0.01a | 0.11 ± 0.02ab |
| 22:5n-3 | 0.84 ± 0.15a | 0.80 ± 0.11a | 1.17 ± 0.12b | 1.36 ± 0.27b |
| 22:6n-3 | 5.69 ± 0.86a | 6.89 ± 1.45a | 12.67 ± 1.13b | 5.69 ± 0.95a |
| ∑n-3 PUFAs | 6.98 ± 1.03a | 8.03 ± 1.45a | 14.09 ± 1.09b | 7.26 ± 1.17a |
| ∑PUFAs | 42.96 ± 5.84 | 41.60 ± 2.44 | 41.69 ± 1.71 | 39.46 ± 2.98 |
| Total fatty acids (mg/100 ml) | 106.81 ± 34.92a | 106.13 ± 27.14a | 184.00 ± 35.52b | 179.90 ± 20.84b |
Values are expressed as mean ± SD, n = 6 for each group. Fatty acids with different superscripts are significantly different by Tukey’s post hoc test (P < 0.05) following significant F-value by one-way ANOVA. SFAs, saturated fatty acids.
TABLE 3.
Effect of pregnancy and postpartum on the fatty acid composition of plasma TAG
| Fatty Acid | Baseline | 15 Days of Pregnancy | 20 Days of Pregnancy | 7 Days Postpartum |
| weight % of total fatty acids | ||||
| 14:0 | 2.22 ± 1.46b | 1.24 ± 0.63ab | 0.70 ± 0.32a | 1.79 ± 0.38ab |
| 16:0 | 23.40 ± 3.71b | 21.84 ± 0.92ab | 18.55 ± 2.47a | 23.29 ± 2.01b |
| 18:0 | 13.56 ± 1.14c | 8.24 ± 2.80b | 4.78 ± 0.87a | 8.74 ± 2.24b |
| 20:0 | 0.61 ± 0.32b | 0.18 ± 0.16a | 0.15 ± 0.07a | 0.25 ± 0.09a |
| 22:0 | 0.50 ± 0.27b | 0.26 ± 0.20ab | 0.08 ± 0.04a | 0.15 ± 0.15a |
| 24:0 | 0.75 ± 0.44b | 0.11 ± 0.13a | 0.11 ± 0.15a | 0.19 ± 0.05a |
| ∑SFAs | 42.98 ± 6.68c | 33.76 ± 5.72b | 24.75 ± 1.58a | 35.72 ± 4.16b |
| 14:1 | 0.36 ± 0.31 | 0.52 ± 0.44 | 0.15 ± 0.04 | 0.30 ± 0.10 |
| 16:1 | 0.78 ± 0.35 | 0.95 ± 0.22 | 0.73 ± 0.20 | 0.99 ± 0.21 |
| 18:1n-7 | 2.80 ± 1.35 | 1.75 ± 0.20 | 1.71 ± 0.16 | 1.92 ± 0.12 |
| 18:1n-9 | 14.36 ± 3.24a | 18.50 ± 2.26ab | 27.51 ± 12.89b | 24.89 ± 3.53ab |
| 20:1n-9 | 0.37 ± 0.12 | 0.14 ± 0.04 | 0.19 ± 0.05 | 0.32 ± 0.28 |
| 22:1n-9 | 2.04 ± 0.62b | 0.19 ± 0.09a | 0.21 ± 0.22a | 0.63 ± 0.26a |
| 24:1n-9 | 0.27 ± 0.09b | 0.06 ± 0.05a | 0.02 ± 0.01a | 0.06 ± 0.04a |
| ∑MUFAs | 21.37 ± 4.01 | 22.26 ± 2.69 | 30.55 ± 13.05 | 29.21 ± 3.64 |
| 18:2n-6 | 19.54 ± 5.67 | 27.28 ± 5.18 | 24.29 ± 6.18 | 22.78 ± 4.16 |
| 18:3n-6 | 0.89 ± 0.26ab | 0.92 ± 0.29ab | 0.57 ± 0.19a | 1.13 ± 0.19b |
| 20:2n-6 | 0.37 ± 0.20 | 0.41 ± 0.49 | 0.25 ± 0.09 | 0.37 ± 0.35 |
| 20:3n-6 | 0.59 ± 0.31b | 0.26 ± 0.16a | 0.30 ± 0.13ab | 0.23 ± 0.09a |
| 20:4n-6 | 8.53 ± 2.61 | 9.37 ± 2.29 | 11.08 ± 4.08 | 6.91 ± 2.00 |
| 22:2n-6 | 0.26 ± 0.15b | 0.18 ± 0.21ab | 0.03 ± 0.03a | 0.06 ± 0.04ab |
| 22:4n-6 | 0.61 ± 0.29a | 0.45 ± 0.28a | 1.58 ± 0.81b | 0.20 ± 0.12a |
| 22:5n-6 | 0.53 ± 0.33ab | 0.15 ± 0.06a | 0.75 ± 0.33b | 0.33 ± 0.20ab |
| ∑n-6 PUFAs | 31.32 ± 7.66 | 39.02 ± 6.57 | 38.86 ± 10.87 | 31.99 ± 5.19 |
| 18:3n-3 | 0.81 ± 0.31 | 1.22 ± 0.39 | 1.30 ± 0.45 | 0.84 ± 0.22 |
| 20:3n-3 | 0.13 ± 0.03 | 0.09 ± 0.06 | 0.04 ± 0.02 | 0.17 ± 0.15 |
| 20:5n-3 | 1.27 ± 0.47ab | 1.40 ± 0.52b | 0.71 ± 0.22a | 1.03 ± 0.35ab |
| 22:5n-3 | 0.58 ± 0.14a | 0.55 ± 0.25a | 1.52 ± 0.57b | 0.39 ± 0.21a |
| 22:6n-3 | 1.54 ± 0.42b | 1.70 ± 0.56b | 2.26 ± 0.62b | 0.63 ± 0.18a |
| ∑n-3 PUFAs | 4.33 ± 1.17ab | 4.96 ± 1.20b | 5.84 ± 1.45b | 3.07 ± 0.50a |
| ∑PUFAs | 35.65 ± 8.81 | 43.98 ± 7.74 | 44.70 ± 12.22 | 35.06 ± 5.51 |
| Total fatty acids (mg/100 ml) | 11.48 ± 2.74a | 58.66 ± 27.51ab | 116.85 ± 46.66b | 83.17 ± 79.18ab |
Values are expressed as mean ± SD, n = 6 for each group. Fatty acids with different superscripts are significantly different by Tukey’s post hoc test (P < 0.05) following significant F-value by one-way ANOVA. SFAs, saturated fatty acids.
Fig. 5.
Effect of pregnancy and postpartum on plasma (A) and liver (B) concentrations of DHA in total lipids, PC, PE, and TAG fractions. Time points (mean ± SD, n = 6 for each point) with different letters are significantly different within a lipid fraction (P < 0.05) by Bonferroni post hoc after significant F-value by repeated measures ANOVA. d, days; preg, pregnancy; pp, postpartum.
The percentage of DHA in total fatty acids tended to be increased in plasma lipid fractions (Tables 1–3). In plasma PC, the percentages of DHA and 22:5n-6 were increased and the percentages of EPA and ARA were decreased at 20 days of pregnancy (Table 1). This pattern was also evident in plasma PE and TAG, but only the increases in DHA and decreases in EPA were significant in PE (Table 2), and ARA was not decreased in TAG (Table 3). In addition, the percentages of total saturates increased and total monounsaturates decreased in both of the plasma phospholipid fractions at 20 days of pregnancy, but this tended to be reversed in plasma TAG, as the percentage of total saturates was the lowest at 20 days of pregnancy.
TABLE 2.
Effect of pregnancy and postpartum on the fatty acid composition of plasma PE
| Fatty Acid | Baseline | 15 Days of Pregnancy | 20 Days of Pregnancy | 7 Days Postpartum |
| weight % of total fatty acids | ||||
| 14:0 | 2.47 ± 2.48 | 2.14 ± 0.34 | 2.29 ± 0.16 | 2.87 ± 0.53 |
| 16:0 | 15.18 ± 5.29 | 15.11 ± 1.45 | 17.38 ± 1.50 | 18.79 ± 1.02 |
| 18:0 | 23.86 ± 5.51a | 33.44 ± 2.74b | 34.60 ± 2.80b | 38.38 ± 2.37b |
| 20:0 | 0.42 ± 0.09 | 0.40 ± 0.11 | 0.44 ± 0.05 | 0.53 ± 0.04 |
| 22:0 | 0.48 ± 0.15 | 0.38 ± 0.14 | 0.50 ± 0.06 | 0.30 ± 0.03 |
| 24:0 | 0.90 ± 0.46 | 0.66 ± 0.23 | 0.60 ± 0.08 | 0.40 ± 0.04 |
| ∑SFAs | 45.54 ± 7.37a | 55.48 ± 1.14b | 60.62 ± 3.26b | 65.69 ± 1.47b |
| 14:1 | 0.09 ± 0.05 | 0.16 ± 0.06 | 0.01 ± 0.01 | 0.10 ± 0.04 |
| 16:1 | 2.92 ± 1.67 | 3.50 ± 0.40 | 1.94 ± 0.11 | 2.23 ± 0.47 |
| 18:1n-7 | 1.13 ± 1.08 | 0.57 ± 0.11 | 0.59 ± 0.22 | 0.50 ± 0.05 |
| 18:1n-9 | 18.46 ± 8.23b | 7.86 ± 1.15ab | 9.86 ± 2.64ab | 5.91 ± 1.23a |
| 20:1n-9 | 0.36 ± 0.31ab | 0.65 ± 0.18b | 0.27 ± 0.08a | 0.25 ± 0.07ab |
| 22:1n-9 | 0.59 ± 0.21ab | 1.33 ± 0.28b | 0.40 ± 0.14a | 0.92 ± 0.14ab |
| 24:1n-9 | 0.71 ± 0.20 | 0.59 ± 0.26 | 0.35 ± 0.07 | 0.35 ± 0.12 |
| ∑MUFAs | 24.33 ± 7.13b | 14.75 ± 1.28ab | 13.48 ± 2.65a | 10.27 ± 1.34a |
| 18:2n-6 | 4.37 ± 1.36 | 3.58 ± 0.87 | 2.71 ± 0.40 | 2.86 ± 0.57 |
| 18:3n-6 | 2.81 ± 4.07 | 0.48 ± 0.14 | 0.22 ± 0.02 | 0.44 ± 0.07 |
| 20:2n-6 | 0.28 ± 0.13 | 0.24 ± 0.14 | 0.09 ± 0.01 | 0.11 ± 0.02 |
| 20:3n-6 | 0.40 ± 0.22a | 0.50 ± 0.08a | 0.23 ± 0.05 a | 0.92 ± 0.08b |
| 20:4n-6 | 14.27 ± 4.8 | 18.26 ± 2.40 | 15.63 ± 1.47 | 13.83 ± 1.88 |
| 22:2n-6 | 0.34 ± 0.20 | 0.38 ± 0.15 | 0.16 ± 0.06 | 0.20 ± 0.04 |
| 22:4n-6 | 0.50 ± 0.23 | 0.45 ± 0.13 | 0.36 ± 0.08 | 0.44 ± 0.12 |
| 22:5n-6 | 0.76 ± 0.43 | 0.56 ± 0.30 | 0.82 ± 0.20 | 0.36 ± 0.07 |
| ∑n-6 PUFAs | 23.73 ± 5.69 | 24.45 ± 1.73 | 20.22 ± 1.81 | 19.16 ± 1.90 |
| 18:3n-3 | 0.69 ± 0.67 | 0.35 ± 0.13 | 0.26 ± 0.06 | 0.29 ± 0.07 |
| 20:3n-3 | 0.28 ± 0.17 | 0.29 ± 0.17 | 0.24 ± 0.05 | 0.11 ± 0.03 |
| 20:5n-3 | 0.70 ± 0.34b | 0.29 ± 0.08ab | 0.08 ± 0.02a | 0.14 ± 0.05b |
| 22:5n-3 | 0.75 ± 0.35 | 0.66 ± 0.22 | 0.53 ± 0.11 | 0.73 ± 0.16 |
| 22:6n-3 | 1.26 ± 0.22a | 1.84 ± 0.33ab | 3.16 ± 0.67b | 1.53 ± 0.15a |
| ∑n-3 PUFAs | 3.68 ± 0.84 | 3.44 ± 0.65 | 4.27 ± 0.65 | 2.81 ± 0.25 |
| ∑PUFAs | 27.41 ± 6.43 | 27.89 ± 1.80 | 24.49 ± 1.73 | 21.97 ± 2.14 |
| Total fatty acids (mg/100 ml) | 17.68 ± 10.20 | 12.18 ± 1.87 | 21.43 ± 1.17 | 18.97 ± 3.36 |
Values are expressed as mean ± SD, n = 6 for each group. Fatty acids with different superscripts are significantly different by Tukey’s post hoc test (P < 0.05) following significant F-value by one-way ANOVA. SFAs, saturated fatty acids.
In liver, despite the lack of change in liver total fatty acids, DHA concentrations and relative percentages tended to be slightly increased during pregnancy. Again this increase in total DHA concentrations was mainly due to increases in DHA in the PC fraction where concentrations were approximately 50% higher at 20 days of pregnancy as compared with baseline and 7 days postpartum (Fig. 5B), while there was no difference in DHA concentrations in PE and DHA concentrations in liver TAG decreased from baseline through pregnancy and the postpartum (Fig. 5B).
Some of the patterns observed in percentage changes in plasma were also evident in liver, as 22-carbon PUFA tended to be increased and 20-carbon PUFA decreased at 20 days of pregnancy in PC and PE. However, shifts in total saturates and monounsaturates were not consistent across liver lipid fractions and were not consistent with the changes observed in plasma, although a shift toward increased 16:0 and decreased 18:0 at 20 days of pregnancy appeared to be consistent in the phospholipid fractions (Tables 4–6). Interestingly, liver TAG levels of DHA were not increased at 20 days of pregnancy and appeared to be transitioning toward the significantly lower levels observed at 7 days postpartum (Table 6). DHA concentrations in the cholesteryl ester and nonesterified fatty acid fractions in plasma and PS and PI in liver were below levels observed in the PE and TAG fractions in these tissues (data not shown).
TABLE 4.
Effect of pregnancy and postpartum on the fatty acid composition of liver PC
| Fatty Acid | Baseline | 15 Days of Pregnancy | 20 Days of Pregnancy | 7 days Postpartum |
| weight % of total fatty acids | ||||
| 14:0 | 0.45 ± 0.17 | 0.49 ± 0.26 | 0.49 ± 0.13 | 0.4 ± 0.09 |
| 16:0 | 17.59 ± 0.58a | 17.40 ± 0.99a | 26.3 ± 1.22b | 17.19 ± 1.00a |
| 18:0 | 28.95 ± 1.17b | 28.71 ± 1.26b | 19.67 ± 1.42a | 28.06 ± 0.77b |
| 20:0 | 0.08 ± 0.02 | 0.08 ± 0.02 | 0.07 ± 0.02 | 0.06 ± 0.01 |
| 22:0 | 0.09 ± 0.05 | 0.07 ± 0.04 | 0.08 ± 0.04 | 0.05 ± 0.02 |
| 24:0 | 0.08 ± 0.05 | 0.09 ± 0.07 | 0.11 ± 0.02 | 0.10 ± 0.03 |
| ∑SFAs | 48.98 ± 2.39 | 47.37 ± 1.24 | 47.47 ± 0.76 | 46.95 ± 1.36 |
| 14:1 | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.01 ± 0.01 |
| 16:1 | 0.27 ± 0.03 | 0.43 ± 0.13 | 0.36 ± 0.11 | 0.32 ± 0.07 |
| 18:1n-7 | 1.35 ± 0.08ab | 1.27 ± 0.12ab | 1.14 ± 0.09a | 1.49 ± 0.17b |
| 18:1n-9 | 4.86 ± 1.24 | 5.05 ± 0.87 | 6.14 ± 1.05 | 6.72 ± 1.16 |
| 20:1n-9 | 0.10 ± 0.03ab | 0.08 ± 0.03a | 0.07 ± 0.01a | 0.14 ± 0.03b |
| 22:1n-9 | 0.22 ± 0.10b | 0.17 ± 0.10ab | 0.14 ± 0.08ab | 0.09 ± 0.03a |
| 24:1n-9 | 0.13 ± 0.08 | 0.13 ± 0.11 | 0.08 ± 0.03 | 0.06 ± 0.02 |
| ∑MUFAs | 7.33 ± 1.09 | 7.18 ± 1.15 | 7.96 ± 1.18 | 8.87 ± 1.19 |
| 18:2n-6 | 10.78 ± 0.86b | 10.37 ± 0.89ab | 8.39 ± 0.60a | 11.06 ± 1.18ab |
| 18:3n-6 | 0.25 ± 0.03a | 0.35 ± 0.08a | 0.31 ± 0.04a | 0.61 ± 0.12b |
| 20:2n-6 | 0.17 ± 0.04 | 0.17 ± 0.02 | 0.19 ± 0.02 | 0.21 ± 0.02 |
| 20:3n-6 | 0.33 ± 0.05b | 0.29 ± 0.05ab | 0.16 ± 0.01a | 0.61 ± 0.08c |
| 20:4n-6 | 25.34 ± 1.59b | 24.39 ± 1.42b | 16.61 ± 1.06a | 22.24 ± 1.08b |
| 22:2n-6 | 0.05 ± 0.02 | 0.08 ± 0.08 | 0.05 ± 0.01 | 0.04 ± 0.01 |
| 22:4n-6 | 0.20 ± 0.05a | 0.20 ± 0.07a | 0.55 ± 0.04b | 0.23 ± 0.05a |
| 22:5n-6 | 0.17 ± 0.03a | 0.27 ± 0.15a | 3.00 ± 0.40b | 0.89 ± 0.23a |
| ∑n-6 PUFAs | 37.30 ± 1.97b | 36.13 ± 1.69b | 29.26 ± 1.60a | 35.88 ± 1.53b |
| 18:3n-3 | 0.10 ± 0.01 | 0.12 ± 0.04 | 0.14 ± 0.01 | 0.10 ± 0.02 |
| 20:3n-3 | 0.03 ± 0.02 | 0.02 ± 0.02 | 0.05 ± 0.02 | 0.01 ± 0.01 |
| 20:5n-3 | 0.17 ± 0.04 | 0.19 ± 0.04 | 0.15 ± 0.03 | 0.23 ± 0.03 |
| 22:5n-3 | 0.53 ± 0.11a | 0.67 ± 0.11a | 1.06 ± 0.11b | 1.10 ± 0.11b |
| 22:6n-3 | 5.98 ± 10.01a | 7.51 ± 1.23a | 13.31 ± 1.40b | 6.07 ± 0.23a |
| ∑n-3 PUFAs | 6.82 ± 1.04a | 8.52 ± 1.28b | 14.71 ± 1.33c | 7.52 ± 0.23ab |
| ∑PUFAs | 43.11 ± 1.67 | 44.65 ± 2.28 | 43.98 ± 1.54 | 43.39 ± 1.64 |
| Total fatty acids (mg/g) | 11.14 ± 1.84 | 12.46 ± 1.82 | 11.68 ± 4.98 | 14.72 ± 5.54 |
Values are expressed as mean ± SD, n = 6 for each group. Fatty acids with different superscripts are significantly different by Tukey’s post hoc test (P < 0.05) following significant F-value by one-way ANOVA. SFAs, saturated fatty acids.
TABLE 6.
Effect of pregnancy and postpartum on the fatty acid composition of liver TAG
| Fatty Acid | Baseline | 15 Days of Pregnancy | 20 Days of Pregnancy | 7 Days Postpartum |
| weight % of total fatty acids | ||||
| 14:0 | 0.77 ± 0.15a | 0.85 ± 0.17a | 1.14 ± 0.26ab | 1.57 ± 0.70b |
| 16:0 | 23.89 ± 0.75ab | 21.96 ± 1.96a | 26.29 ± 2.85b | 25.89 ± 1.56b |
| 18:0 | 3.48 ± 0.78a | 5.87 ± 1.25ab | 9.10 ± 3.58b | 10.22 ± 3.94b |
| 20:0 | 0.09 ± 0.03a | 0.14 ± 0.04ab | 0.21 ± 0.07ab | 0.46 ± 0.42b |
| 22:0 | 0.03 ± 0.02 | 0.06 ± 0.06 | 0.11 ± 0.10 | 0.14 ± 0.09 |
| 24:0 | 0.01 ± 0.01a | 0.03 ± 0.02ab | 0.07 ± 0.04b | 0.06 ± 0.03b |
| ∑SFAs | 29.19 ± 0.84a | 29.45 ± 3.35a | 37.99 ± 6.15b | 40.13 ± 4.72b |
| 14:1 | 0.09 ± 0.07a | 0.04 ± 0.02a | 0.18 ± 0.06ab | 0.25 ± 0.14b |
| 16:1 | 3.37 ± 2.4 | 2.22 ± 0.35 | 2.05 ± 1.51 | 2.24 ± 1.20 |
| 18:1n-7 | 1.78 ± 0.14 | 1.88 ± 0.12 | 2.01 ± 0.26 | 1.93 ± 0.24 |
| 18:1n-9 | 22.63 ± 3.4 | 21.54 ± 2.20 | 18.60 ± 1.97 | 21.75 ± 2.86 |
| 20:1n-9 | 0.13 ± 0.03a | 0.18 ± 0.06a | 0.29 ± 0.07b | 0.20 ± 0.08ab |
| 22:1n-9 | 0.18 ± 0.06a | 0.38 ± 0.15ab | 0.46 ± 0.09b | 0.40 ± 0.27ab |
| 24:1n-9 | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.07 ± 0.06 | 0.06 ± 0.04 |
| ∑MUFAs | 28.29 ± 5.09 | 26.35 ± 2.51 | 23.79 ± 2.29 | 26.94 ± 2.49 |
| 18:2n-6 | 32.30 ± 3.72b | 30.26 ± 3.90b | 24.06 ± 4.07a | 24.23 ± 2.69a |
| 18:3n-6 | 0.72 ± 0.14ab | 1.04 ± 0.19b | 0.72 ± 0.13a | 0.99 ± 0.28ab |
| 20:2n-6 | 0.20 ± 0.05ab | 0.23 ± 0.08ab | 0.29 ± 0.08b | 0.18 ± 0.04a |
| 20:3n-6 | 0.25 ± 0.07 | 0.32 ± 0.11 | 0.33 ± 0.05 | 0.24 ± 0.05 |
| 20:4n-6 | 3.93 ± 1.13 | 6.52 ± 1.47 | 6.91 ± 2.98 | 4.27 ± 1.37 |
| 22:2n-6 | 0.01 ± 0.01 | 0.04 ± 0.03 | 0.11 ± 0.13 | 0.06 ± 0.05 |
| 22:4n-6 | 0.51 ± 0.20a | 0.74 ± 0.36a | 1.36 ± 0.45b | 0.49 ± 0.12a |
| 22:5n-6 | 0.25 ± 0.09a | 0.26 ± 0.17ab | 0.53 ± 0.25b | 0.32 ± 0.08ab |
| ∑n-6 PUFAs | 38.18 ± 5.12b | 39.42 ± 4.26b | 34.31 ± 3.89ab | 30.78 ± 3.82a |
| 18:3n-3 | 1.76 ± 0.33c | 1.50 ± 0.35bc | 1.20 ± 0.17b | 0.70 ± 0.15a |
| 20:3n-3 | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.03 ± 0.02 |
| 20:5n-3 | 0.65 ± 0.18ab | 0.88 ± 0.33b | 0.46 ± 0.23ab | 0.44 ± 0.25a |
| 22:5n-3 | 0.52 ± 0.16ab | 0.66 ± 0.20ab | 0.96 ± 0.47b | 0.43 ± 0.16a |
| 22:6n-3 | 1.40 ± 0.20b | 1.73 ± 0.32b | 1.25 ± 0.61b | 0.54 ± 0.33a |
| ∑n-3 PUFAs | 4.35 ± 0.54b | 4.79 ± 0.65b | 3.91 ± 1.27b | 2.15 ± 0.81a |
| ∑PUFAs | 42.52 ± 5.51b | 44.20 ± 4.90b | 38.21 ± 4.89ab | 32.93 ± 4.54a |
| Total fatty acids (mg/g) | 12.19 ± 4.10b | 5.95 ± 2.59a | 4.08 ± 0.78a | 2.85 ± 0.63a |
Values are expressed as mean ± SD, n = 6 for each group. Fatty acids with different superscripts are significantly different by Tukey’s post hoc test (P < 0.05) following significant F-value by one-way ANOVA. SFAs, saturated fatty acids.
TABLE 5.
Effect of pregnancy and postpartum on the fatty acid composition of liver PE
| Fatty Acid | Baseline | 15 Days of Pregnancy | 20 Days of Pregnancy | 7 Days Postpartum |
| weight % of total fatty acids | ||||
| 14:0 | 0.75 ± 0.32ab | 0.63 ± 0.20ab | 0.92 ± 0.28b | 0.43 ± 0.13a |
| 16:0 | 15.56 ± 1.62a | 15.67 ± 2.02a | 20.27 ± 0.81b | 15.45 ± 0.41a |
| 18:0 | 29.70 ± 2.31b | 31.22 ± 3.54b | 23.20 ± 1.13a | 27.75 ± 0.77b |
| 20:0 | 0.14 ± 0.04b | 0.10 ± 0.02ab | 0.14 ± 0.03b | 0.05 ± 0.01a |
| 22:0 | 0.12 ± 0.03ab | 0.13 ± 0.08ab | 0.17 ± 0.07b | 0.06 ± 0.02a |
| 24:0 | 0.13 ± 0.05 | 0.13 ± 0.07 | 0.19 ± 0.06 | 0.14 ± 0.03 |
| ∑SFAs | 47.68 ± 0.89b | 48.47 ± 1.31b | 46.77 ± 1.53ab | 45.11 ± 0.45a |
| 14:1 | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.06 ± 0.03 | 0.02 ± 0.01 |
| 16:1 | 0.13 ± 0.02a | 0.46 ± 0.35ab | 0.86 ± 0.49b | 0.29 ± 0.09a |
| 18:1n-7 | 1.02 ± 0.11ab | 0.84 ± 0.11ab | 0.73 ± 0.15a | 1.05 ± 0.14b |
| 18:1n-9 | 6.65 ± 2.58 | 3.94 ± 1.02 | 9.26 ± 3.64 | 6.83 ± 1.55 |
| 20:1n-9 | 0.12 ± 0.05 | 0.07 ± 0.03 | 0.09 ± 0.02 | 0.12 ± 0.02 |
| 22:1n-9 | 0.40 ± 0.10b | 0.34 ± 0.12b | 0.40 ± 0.11b | 0.15 ± 0.05a |
| 24:1n-9 | 0.20 ± 0.09 | 0.17 ± 0.13 | 0.16 ± 0.06 | 0.10 ± 0.02 |
| ∑MUFAs | 8.53 ± 2.69 | 5.89 ± 1.57 | 11.58 ± 3.85 | 8.58 ± 1.68 |
| 18:2n-6 | 5.98 ± 1.07b | 4.48 ± 0.24ab | 2.86 ± 0.42a | 5.64 ± 0.97b |
| 18:3n-6 | 0.16 ± 0.04a | 0.14 ± 0.06a | 0.09 ± 0.03a | 0.28 ± 0.03b |
| 20:2n-6 | 0.12 ± 0.04 | 0.13 ± 0.03 | 0.13 ± 0.03 | 0.16 ± 0.02 |
| 20:3n-6 | 0.29 ± 0.03b | 0.29 ± 0.06b | 0.12 ± 0.03a | 0.58 ± 0.10c |
| 20:4n-6 | 21.57 ± 3.21b | 23.31 ± 3.07b | 13.24 ± 1.20a | 21.27 ± 1.58b |
| 22:2n-6 | 0.10 ± 0.05ab | 0.13 ± 0.07b | 0.09 ± 0.04ab | 0.04 ± 0.01a |
| 22:4n-6 | 0.54 ± 0.09a | 0.56 ± 0.12a | 1.07 ± 0.11b | 0.68 ± 0.08a |
| 22:5n-6 | 0.25 ± 0.06a | 0.44 ± 0.25ab | 3.95 ± 0.71c | 1.42 ± 0.39b |
| ∑n-6 PUFAs | 29.01 ± 3.29b | 29.48 ± 2.81b | 21.54 ± 1.66a | 30.09 ± 2.18b |
| 18:3n-3 | 0.21 ± 0.10 | 0.13 ± 0.03 | 0.16 ± 0.05 | 0.09 ± 0.03 |
| 20:3n-3 | 0.05 ± 0.02ab | 0.07 ± 0.05ab | 0.11 ± 0.04b | 0.02 ± 0.01a |
| 20:5n-3 | 0.24 ± 0.07ab | 0.25 ± 0.05ab | 0.11 ± 0.02a | 0.37 ± 0.09b |
| 22:5n-3 | 1.09 ± 0.26a | 1.27 ± 0.19a | 1.27 ± 0.19a | 2.20 ± 0.23b |
| 22:6n-3 | 11.22 ± 2.16a | 13.77 ± 2.35ab | 17.46 ± 1.82b | 12.47 ± 1.07a |
| ∑n-3 PUFAs | 12.81 ± 1.92a | 15.49 ± 2.44ab | 19.11 ± 1.88b | 15.14 ± 1.33a |
| ∑PUFAs | 41.82 ± 3.33 | 44.97 ± 1.31 | 40.65 ± 3.40 | 45.22 ± 1.99 |
| Total fatty acids (mg/g) | 3.52 ± 0.52 | 5.02 ± 0.91 | 3.74 ± 1.75 | 5.82 ± 3.39 |
Values are expressed as mean ± SD, n = 6 for each group. Fatty acids with different superscripts are significantly different by Tukey’s post hoc test (P < 0.05) following significant F-value by one-way ANOVA. SFAs, saturated fatty acids.
Lipidomics
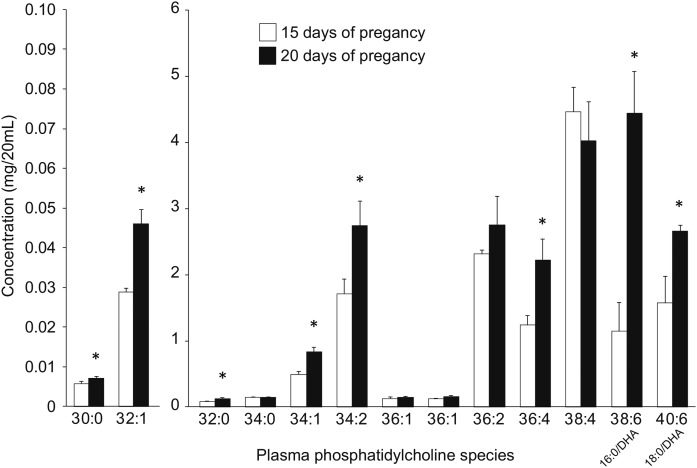
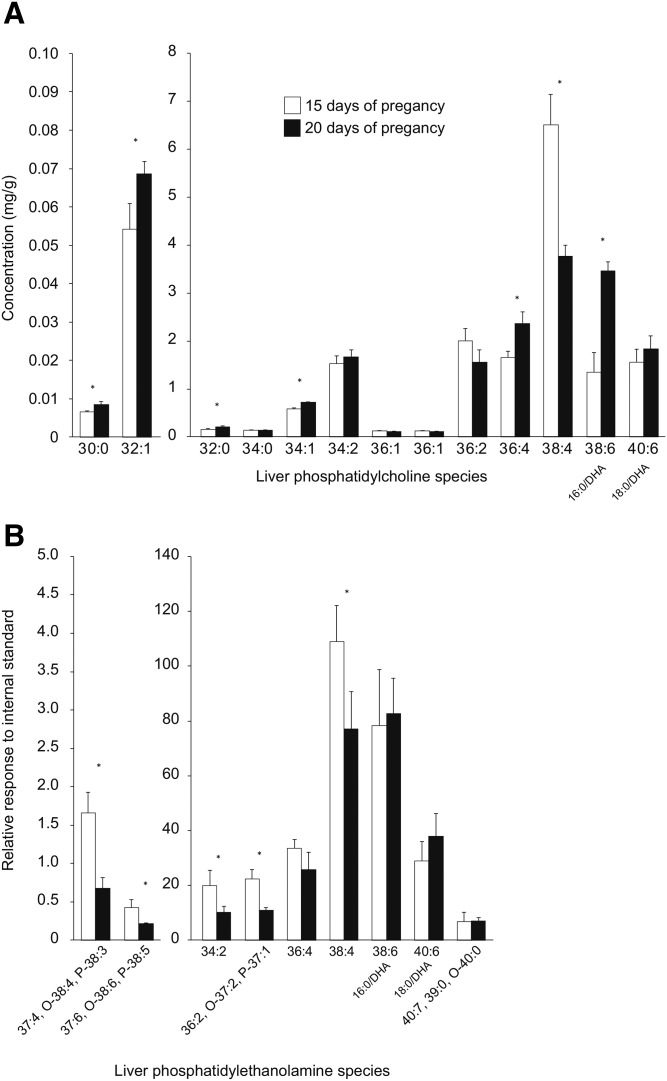
Based on the fatty acid analyses, semi-targeted analyses of plasma and liver PC were completed to determine the acyl species of lipids responsible for the large shift in DHA concentration between 15 days and 20 days of pregnancy. Acyl species of liver PE were also examined at this time, given their role as a potential substrate for PEMT. For PCs, 13 individual species with unique m/z and retention times were identified according to their MS spectrums, of which two contained DHA, as confirmed by MS/MS. In plasma, PC 38:6 (16:0/DHA) was 3.9-fold higher at 20 days relative to 15 days of pregnancy (Fig. 6). PC 40:6 (18:0/DHA) was also higher at 20 days, but only by 1.7-fold. Other PC species that were increased at 20 days were 36:4, 34:2, 34:1, 32:1, 32:0, and 30:0, while no species were decreased. In liver, PC 38:6 (16:0/DHA) was 2.5-fold higher at 20 days relative to 15 days of pregnancy, but PC 40:6 (18:0/DHA) did not change (Fig. 7A). Once again, 36:4, 34:1, 32:1, 32:0, and 30:0 were increased, but 34:2 was not. Interestingly, PC 38:4 in liver decreased from day 15 to day 20 of pregnancy. For PEs, five individual species with unique m/z and retention times were identified according to their MS spectrums. In addition, four peaks were isolated with an m/z with three possible identities (Fig. 7B). In contrast to PCs, the PE species generally decreased from 15 days to 20 days of pregnancy, but the DHA-containing species, PE 38:6 (16:0/DHA) and PE 40:6 (18:0/DHA), did not change.
Fig. 6.
Changes in plasma PC species between day 15 and day 20 of pregnancy. *Significantly different from day 15 by independent t-test (P < 0.05, mean ± SD, n = 3 for each bar).
Fig. 7.
Changes in liver PC (A) and PE (B) species between day 15 and day 20 of pregnancy. *Significantly different from day 15 by independent t-test (P < 0.05, mean ± SD, n = 3 for each bar). O-, alkyl ether; P-, alkenyl ether.
Transcriptomics
Using a FDR of 5%, 76 transcripts were differentially expressed, of which 57 had unique Gene IDs (supplemental Table S1). KEGG pathway analysis indicated that at 20 days of pregnancy, C21-steroid, cysteine and phenylalanine metabolism, and phenylalanine, tyrosine, tryptophan, and alkaloid biosynthesis pathways were upregulated. Downregulated pathways at 20 days of pregnancy included biosynthesis of steroids, metabolism of cytochrome P450, butanoate, pyruvate, propanoate, and linoleic acid, synthesis and degradation of ketone bodies, and degradation of valine, leucine, isoleucine, lysine γ-hexachlorocyclohexane, and benzoate. When expanding the list of differentially expressed genes by relaxing our statistical criteria to P < 0.01 without a FDR, 712 transcripts having unique Gene IDs were identified. A total of 413 genes were upregulated, which included choline phosphotransferase 1 (Chpt1) and diacylglycerol O-acyltransferase 1 (Dgat1), and 219 genes were downregulated that included phosphate cytidylyltransferase 2 ethanolamine (Pcyt2), and acyl-CoA synthetase long-chain family member 1 (Acsl1).
DISCUSSION
In the present study, changes in maternal plasma and liver DHA status in various lipid classes throughout pregnancy were examined in a rat model using fatty acid and lipidomic analyses. This is the most comprehensive report of the fatty acid composition of the lipid classes of plasma and liver throughout pregnancy to date and allowed us to perform more targeted lipidomic assessment to determine that 16:0/DHA PC is the specific lipid responsible for most of the DHA increases in liver and plasma during pregnancy. Based on a preliminary hypothesis that PEMT was responsible for the mobilization of hepatic DHA into the PC of lipoproteins, mRNA, protein, and enzyme activity were assessed. Dietary intake was also monitored and targeted protein assays were used to examine pathways related to DHA biosynthesis. In addition, transcriptomic screening was also used to identify potential gene products that could be involved in the large increase in DHA that was observed in circulating plasma phospholipids. We demonstrated that hepatic PEMT expression and activity is increased and appears to be involved in supporting the mobilization of maternal DHA to meet fetal demand by selectively increasing plasma 16:0/DHA PC between 15 and 20 days of pregnancy. We also report increased protein concentrations of FADS2 during pregnancy for the first time, which confirms a previous observation by mRNA (42). This observation and shifts in the n-3 PUFA pool in the liver and plasma toward higher amounts of DHA and fewer n-3 PUFA precursors suggest that increased DHA biosynthesis during pregnancy through upregulated FADS2 supports the production of 16:0/DHA PC.
In our model, we showed that DHA increases in maternal plasma during pregnancy, which has been observed previously in humans (5–8) and rats (14, 42). In rats, the increase in plasma DHA occurs primarily in late pregnancy, as we and others (42) found an increase in DHA in plasma at 20 days of pregnancy, but not earlier (12–15 days of pregnancy). While DHA increased in various lipid classes in plasma, the increase in DHA in plasma phospholipids accounted for 82% of the increase in DHA in all plasma lipids from 15 to 20 days of pregnancy. Ad hoc lipidomic analyses identified two DHA-containing PCs, with 18:0/DHA PC increasing by 69% and 16:0/DHA PC increasing by 288%. While an increase in DHA in plasma phospholipids has been documented (8, 11), most of the interest in the supply of DHA for maternal-fetal transfer of DHA has been focused on plasma NEFA and TAG (7, 11). This appears to be based on placental transfer studies and documented maternal lipidemia (2, 7, 11); however, the importance of DHA accumulation in plasma phospholipids has been documented (43). The focus on NEFA and TAG has also led to a focus on maternal adipose stores as a mobilizable source of DHA during pregnancy (2, 14), despite the fact that these lipid pools tend to have relatively low concentrations of DHA (42, 44, 45). Our acyl species analysis indicating the specific increase in plasma 16:0/DHA PC suggests considerable hepatic involvement of DHA mobilization and a mechanism where DHA enrichment of PC in the lipoprotein monolayer may be a delivery mechanism. The placenta can bind lipoproteins (46) and placental lipases can release NEFA from the TAG of lipoproteins by lipoprotein lipase (16, 19). However, the placenta is known to have considerable endothelial lipase activity, which has a considerable phospholipase activity that would generate NEFA and lysoPC (17, 18, 20). It has also been proposed that docosahexaenoyl lysoPC could be a carrier for placental transport of DHA (47) based on studies of brain uptake of DHA (48, 49), although it has also been proposed that DHA NEFA is the main source for brain uptake (50). Regardless, 16:0/DHA PC has the potential to serve as a source of DHA for placental transport.
Hepatic involvement has been proposed previously, with the liver either repackaging circulating NEFA into TAG (7, 11) and/or biosynthesis of DHA from precursor omega-3 PUFA released from adipose, such as EPA and 18:3n-3 (42). In the present study, it appears that hepatic TAG DHA could be getting mobilized to support DHA incorporation into PC. It has been demonstrated that estrogen is linked to increased DHA biosynthesis (12), and that there is a shift in human plasma toward 22-carbon PUFA at the expense of 20-carbon PUFA during pregnancy (6). These patterns were observed presently at day 20 of pregnancy, as FADS2 protein levels were increased and the percentages of DHA and 22:5n-6 were increased and the percentages of EPA and ARA were decreased in plasma PC. We did not observe changes in the protein concentrations of FADS1, ELOVL2, and MFP2, while ELOVL5 concentrations were actually lower at 20 days, as compared with 15 days of pregnancy. These observations are consistent with previous research in this area examining the effect of sex hormones on these enzymes, with differences in mRNA expression occurring in several enzymes, but protein concentrations being restricted to an increase in FADS2 and a slight decrease in ELOVL5 (25, 32).
The lack of a FADS1 response seems to agree with the observations that EPA was decreased at day 20, while 18:3n-3 was relatively unchanged during pregnancy in various plasma and liver lipid pools. The increase in the FADS2 protein and higher DHA and 22:5n-6 levels suggest an adaptation during pregnancy to increase the ability of FADS2 to engage in what is considered a second round of desaturation. This is generally considered a Δ6 desaturation of a 24-carbon PUFA that is then peroxisomally β-oxidized back to a 22-carbon PUFA; however, it is possible that the upregulated FADS2 could be catalyzing a Δ4 desaturation reaction (51). This latter possibility could explain the lack of upregulation of ELOVL2 and ELOVL5 that should produce 24-carbon PUFA. However, a consistent shift away from 20-carbon PUFA toward 22-carbon PUFA was observed without support from increased ELOVL2 and ELOVL5 expression. This confirms previous work indicating that gene expression of ELOVL2 and ELOVL5 may not reflect PUFA synthesis. Interestingly, ELOVL6 is consistently upregulated at the mRNA and protein level in response to female sex hormones. Further examination of PUFA elongation during pregnancy should consider a comprehensive assessment of the elongase isoforms and examine substrate specificity using activity assays.
Interestingly, the increase in DHA was much greater in circulating plasma as compared with the increase in DHA in the liver, while relatively modest changes in ARA in plasma appeared to be accompanied by decreased liver ARA. This was also reflected in the lipidomic analysis, as the 288% increase in 16:0/DHA PC and 69% increase in 18:0/DHA PC in plasma were accompanied by a 156% increase in 16:0/DHA PC and no changes in 18:0/DHA PC or DHA species in PE in liver. The shifts in ARA-containing phospholipid species were interesting to contrast, as 38:4 PC did not change in plasma, but both 38:4 PC and 38:4 PE decreased in liver. These results appear to suggest that both ARA and DHA are being mobilized from liver stores to the plasma, but additional mechanisms, such as a shift in PUFA biosynthesis toward 22-carbon, as described above, are occurring to provide additional DHA, while large hepatic ARA stores are mobilized instead. The responses of other PC species in plasma and liver and PE in liver provided support of hepatic mobilization of PE toward PE to support increased PC plasma content with pregnancy-induced hyperlipidemia.
The observation of a specific increase in 16:0/DHA PC in plasma during pregnancy supports the hypothesis of an active role of PEMT in mobilizing DHA. PEMT is increased during pregnancy (31) and estrogen influences PEMT expression and activity (52). Potential estrogen response element motifs have been identified on the PEMT gene promoter region and treating hepatic cell cultures with estrogen at doses mimicking concentrations in humans (0–100 nmol/l) significantly upregulates mRNA expression and enzyme activity of PEMT (28). While the increased PEMT activity during pregnancy appears to be an evolutionary mechanism to meet the high demand for choline to support anabolic pathways (31), plasma PC content of DHA is also directly correlated with PEMT activity (30). Conversion of PE to PC may serve as a mechanism to mobilize hepatic DHA for fetal transport. PE has a higher content of DHA, which resides predominantly on the inner bilayer of membranes, as compared with PC (25–27). PEMT can then convert DHA-rich PE to PC that can then be transferred to plasma as the principal phospholipid component of lipoproteins. PC derived from PEMT has been shown, in humans, to partition toward the fetus in a tracer study using choline labeled with deuterium (31). It has also been shown that PEMT has a high selectivity for converting 16:0/DHA PE to 16:0/DHA PC, but that the fatty acyls on the newly synthesized PC are remodeled to lower DHA content within 6–12 h (53). This remodeling could result in increased DHA hepatic pools of NEFAs and/or acyl-CoAs and be available for incorporation into other lipid classes. However, in our study, DHA enrichment was the largest in 16:0/DHA, suggesting that DHA remains or is selectively reincorporated in this lipid during pregnancy. Unfortunately, comparing the hepatic transcriptomes between 15 and 20 days of pregnancy was not successful in identifying changes in the transcriptome that could directly explain the increase in plasma 16:0/DHA PC. This is likely due to the fact that numerous metabolic changes occur during pregnancy, which may consequently mask subtler changes in the transcriptome. In addition, our analysis was limited to a comparison between 15 days and 20 days of pregnancy. We cannot exclude that changes in mRNA expression related to 16:0/DHA PC synthesis may have occurred in the period between baseline and 15 days of pregnancy. When a less conservative statistical approach was used (P < 0.01 without FDR), there was some evidence supporting increased PC synthesis (choline phosphotransferase 1 was upregulated) and decreased PE synthesis (phosphate cytidylyltransferase 2 ethanolamine was downregulated).
We have provided evidence that the increased DHA in plasma that is observed during pregnancy is largely driven by a specific increase in 16:0/DHA PC. It also appears that the plasma increase in 16:0/DHA PC is at least partially mediated by the conversion of DHA-rich PE to PC through increased expression and activity of PEMT in the liver. Other metabolic pathways, such as DHA biosynthesis, also appear to support the increase in DHA in plasma, but hepatic transcriptomics did not identify changes in genes coding for enzymes involved in fatty acid transport and incorporation into complex lipids. This may be due to our decision to compare 15 days of pregnancy with 20 days of pregnancy rather than comparisons to baseline, as changes in mRNA expression may occur before the biochemical phenomenon. Further analyses at additional time points between baseline and 15 days of pregnancy are warranted to investigate changes in the expression of genes related to hepatic fatty acid and lipid metabolism. Given the large increase in 16:0/DHA PC in plasma, it also appears that future studies examining maternal hepatic adaptations during pregnancy should consider the role of other maternal tissues as a potential source of DHA. In addition, manipulations of dietary levels of DHA and other n-3 PUFAs during pregnancy could reveal additional maternal adaptations. A comprehensive understanding of maternal adaptations is required to understand the resiliency in maternal-fetal DHA transport in order to establish dietary DHA requirements during pregnancy.
Supplementary Material
Footnotes
Abbreviations:
- ARA
- arachidonic acid
- ELOVL2
- elongase 2
- ELOVL5
- elongase 5
- FADS1
- Δ5 desaturase
- FADS2
- Δ6 desaturase
- FDR
- false discovery rate
- KEGG
- Kyoto Encyclopedia of Genes and Genomes
- LC-PUFA
- long-chain PUFA
- MFP2
- multifunctional protein-2
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PEMT
- phosphatidylethanolamine methyltransferase
- PI
- phosphatidylinositol
- PS
- phosphatidylserine
- TAG
- triacylglycerol
- TBST
- TBS with 0.5% (v/v) Tween
This work was supported by an operating grant from the Natural Sciences and Engineering Research Council of Canada (327149-2013) to K.D.S.; personnel awards from the Natural Sciences and Engineering Research Council of Canada to A.P.K. and J.J.A.H. (Graduate Scholarships) and J.L.E. and D.M.E.L-K. (Undergraduate Research Awards); an Ontario Graduate Scholarship to A.C.; and an Ontario Women’s Health Scholarship to K.A.M. K.D.S. also receives salary support from the Canada Research Chairs program as a Chair in Nutritional Lipidomics (950-228125).
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Bisgaard H., Stokholm J., Chawes B. L., Vissing N. H., Bjarnadottir E., Schoos A. M., Wolsk H. M., Pedersen T. M., Vinding R. K., Thorsteinsdottir S., et al. 2016. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N. Engl. J. Med. 375: 2530–2539. [DOI] [PubMed] [Google Scholar]
- 2.Lauritzen L., and Carlson S. E.. 2011. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern. Child Nutr. 7(Suppl 2): 41–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stark K. D., Van Elswyk M. E., Higgins M. R., Weatherford C. A., and Salem N. Jr. 2016. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 63: 132–152. [DOI] [PubMed] [Google Scholar]
- 4.Lim S. Y., Hoshiba J., and Salem N. Jr. 2005. An extraordinary degree of structural specificity is required in neural phospholipids for optimal brain function: n-6 docosapentaenoic acid substitution for docosahexaenoic acid leads to a loss in spatial task performance. J. Neurochem. 95: 848–857. [DOI] [PubMed] [Google Scholar]
- 5.Al M. D., van Houwelingen A. C., Kester A. D., Hasaart T. H., de Jong A. E., and Hornstra G.. 1995. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br. J. Nutr. 74: 55–68. [DOI] [PubMed] [Google Scholar]
- 6.Stark K. D., Beblo S., Murthy M., Buda-Abela M., Janisse J., Rockett H., Whitty J. E., Martier S. S., Sokol R. J., Hannigan J. H., et al. 2005. Comparison of bloodstream fatty acid composition from African-American women at gestation, delivery, and postpartum. J. Lipid Res. 46: 516–525. [DOI] [PubMed] [Google Scholar]
- 7.Haggarty P. 2010. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 30: 237–255. [DOI] [PubMed] [Google Scholar]
- 8.Otto S. J., van Houwelingen A. C., Badart-Smook A., and Hornstra G.. 2001. Changes in the maternal essential fatty acid profile during early pregnancy and the relation of the profile to diet. Am. J. Clin. Nutr. 73: 302–307. [DOI] [PubMed] [Google Scholar]
- 9.Childs C. E., Romijn T., Enke U., Hoile S., and Calder P. C.. 2010. Maternal diet during pregnancy has tissue-specific effects upon fetal fatty acid composition and alters fetal immune parameters. Prostaglandins Leukot. Essent. Fatty Acids. 83: 179–184. [DOI] [PubMed] [Google Scholar]
- 10.Stark K. D., Beblo S., Murthy M., Whitty J. E., Buda-Abela M., Janisse J., Rockett H., Martier S. S., Sokol R. J., Hannigan J. H., et al. 2005. Alcohol consumption in pregnant, black women is associated with decreased plasma and erythrocyte docosahexaenoic acid. Alcohol. Clin. Exp. Res. 29: 130–140. [DOI] [PubMed] [Google Scholar]
- 11.Haggarty P. 2004. Effect of placental function on fatty acid requirements during pregnancy. Eur. J. Clin. Nutr. 58: 1559–1570. [DOI] [PubMed] [Google Scholar]
- 12.Kitson A. P., Stroud C. K., and Stark K. D.. 2010. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids. 45: 209–224. [DOI] [PubMed] [Google Scholar]
- 13.Yang J., Chen Z. Y., and Cunnane S. C.. 1994. Application of the balance method to determining accumulation, metabolism, and apparent oxidation of linoleic and alpha-linolenic acids in the pregnant rat. Metabolism. 43: 940–944. [DOI] [PubMed] [Google Scholar]
- 14.Metherel A. H., Kitson A. P., Domenichiello A. F., Lacombe R. J. S., Hopperton K. E., Trepanier M. O., Alashmali S. M., Lin L., and Bazinet R. P.. 2017. Maternal liver docosahexaenoic acid (DHA) stores are increased via higher serum unesterified DHA uptake in pregnant long Evans rats. J. Nutr. Biochem. 46: 143–150. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree J. T., Gordon M. J., Campbell F. M., and Dutta-Roy A. K.. 1998. Differential distribution and metabolism of arachidonic acid and docosahexaenoic acid by human placental choriocarcinoma (BeWo) cells. Mol. Cell. Biochem. 185: 191–198. [DOI] [PubMed] [Google Scholar]
- 16.Duttaroy A. K. 2009. Transport of fatty acids across the human placenta: a review. Prog. Lipid Res. 48: 52–61. [DOI] [PubMed] [Google Scholar]
- 17.Gauster M., Hiden U., Blaschitz A., Frank S., Lang U., Alvino G., Cetin I., Desoye G., and Wadsack C.. 2007. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. J. Clin. Endocrinol. Metab. 92: 2256–2263. [DOI] [PubMed] [Google Scholar]
- 18.Gauster M., Rechberger G., Sovic A., Horl G., Steyrer E., Sattler W., and Frank S.. 2005. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J. Lipid Res. 46: 1517–1525. [DOI] [PubMed] [Google Scholar]
- 19.Hanebutt F. L., Demmelmair H., Schiessl B., Larque E., and Koletzko B.. 2008. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin. Nutr. 27: 685–693. [DOI] [PubMed] [Google Scholar]
- 20.McCoy M. G., Sun G. S., Marchadier D., Maugeais C., Glick J. M., and Rader D. J.. 2002. Characterization of the lipolytic activity of endothelial lipase. J. Lipid Res. 43: 921–929. [PubMed] [Google Scholar]
- 21.Stark K. 2008. Analytical implications of routine clinical testing for omega-3 fatty acid biomarkers. Lipid Technol. 20: 177–179. [Google Scholar]
- 22.Christie W. W. 1985. Rapid separation and quantification of lipid classes by high performance liquid chromatography and mass (light-scattering) detection. J. Lipid Res. 26: 507–512. [PubMed] [Google Scholar]
- 23.Herrera E. 2002. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development–a review. Placemta. 23 (Suppl. A): S9–S19. [DOI] [PubMed] [Google Scholar]
- 24.Ridgway N. D., and Vance D. E.. 1988. Kinetic mechanism of phosphatidylethanolamine N-methyltransferase. J. Biol. Chem. 263: 16864–16871. [PubMed] [Google Scholar]
- 25.Kitson A. P., Marks K. A., Shaw B., Mutch D. M., and Stark K. D.. 2013. Treatment of ovariectomized rats with 17beta-estradiol increases hepatic delta-6 desaturase enzyme expression and docosahexaenoic acid levels in hepatic and plasma phospholipids. Prostaglandins Leukot. Essent. Fatty Acids. 89: 81–88. [DOI] [PubMed] [Google Scholar]
- 26.Kramer J. K. 1973. Changes in liver lipid composition of male rats fed rapeseed oil diets. Lipids. 8: 641–648. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto A., Isozaki M., Hirayama K., and Sakai Y.. 1965. Influence of dietary fatty acids on phospholipid fatty acid composition in subcellular particles of rat liver. J. Lipid Res. 6: 295–300. [PubMed] [Google Scholar]
- 28.Resseguie M., Song J., Niculescu M. D., da Costa K. A., Randall T. A., and Zeisel S. H.. 2007. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 21: 2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Costa K. A., Rai K. S., Craciunescu C. N., Parikh K., Mehedint M. G., Sanders L. M., McLean-Pottinger A., and Zeisel S. H.. 2010. Dietary docosahexaenoic acid supplementation modulates hippocampal development in the Pemt−/− mouse. J. Biol. Chem. 285: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Costa K. A., Sanders L. M., Fischer L. M., and Zeisel S. H.. 2011. Docosahexaenoic acid in plasma phosphatidylcholine may be a potential marker for in vivo phosphatidylethanolamine N-methyltransferase activity in humans. Am. J. Clin. Nutr. 93: 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan J., Jiang X., West A. A., Perry C. A., Malysheva O. V., Brenna J. T., Stabler S. P., Allen R. H., Gregory J. F. 3rd, and Caudill M. A.. 2013. Pregnancy alters choline dynamics: results of a randomized trial using stable isotope methodology in pregnant and nonpregnant women. Am. J. Clin. Nutr. 98: 1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitson A. P., Smith T. L., Marks K. A., and Stark K. D.. 2012. Tissue-specific sex differences in docosahexaenoic acid and Delta6-desaturase in rats fed a standard chow diet. Appl. Physiol. Nutr. Metab. 37: 1200–1211. [DOI] [PubMed] [Google Scholar]
- 33.Rekawiecki R., Rutkowska J., and Kotwica J.. 2012. Identification of optimal housekeeping genes for examination of gene expression in bovine corpus luteum. Reprod. Biol. 12: 362–367. [DOI] [PubMed] [Google Scholar]
- 34.Ridgway N. D., and Vance D. E.. 1992. Phosphatidylethanolamine N-methyltransferase from rat liver. Methods Enzymol. 209: 366–374. [DOI] [PubMed] [Google Scholar]
- 35.Folch J., Lees M., and Sloane Stanley G. H. S.. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 36.Armstrong J. M., Metherel A. H., and Stark K. D.. 2008. Direct microwave transesterification of fingertip prick blood samples for fatty acid determinations. Lipids. 43: 187–196. [DOI] [PubMed] [Google Scholar]
- 37.Stark K. D., and Salem N. Jr. 2005. Fast gas chromatography for the identification of fatty acid methyl esters from mammalian samples. Lipid Technol. 17: 181–185. [Google Scholar]
- 38.Metherel A. H., Buzikievich L. M., Charkhzarin P., Patterson A. C., Peel A. C., Howorth A. M., Kishi D. M., and Stark K. D.. 2012. Omega-3 polyunsaturated fatty acid profiling using fingertip-prick whole blood does not require overnight fasting before blood collection. Nutr. Res. 32: 547–556. [DOI] [PubMed] [Google Scholar]
- 39.Marks K. A., Kitson A. P., and Stark K. D.. 2013. Hepatic and plasma sex differences in saturated and monounsaturated fatty acids are associated with differences in expression of elongase 6, but not stearoyl-CoA desaturase in Sprague-Dawley rats. Genes Nutr. 8: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marks K. A., Kitson A. P., Shaw B., Mutch D. M., and Stark K. D.. 2013. Stearoyl-CoA desaturase 1, elongase 6 and their fatty acid products and precursors are altered in ovariectomized rats with 17beta-estradiol and progesterone treatment. Prostaglandins Leukot. Essent. Fatty Acids. 89: 89–96. [DOI] [PubMed] [Google Scholar]
- 41.Prifti E., Zucker J. D., Clement K., and Henegar C.. 2008. FunNet: an integrative tool for exploring transcriptional interactions. Bioinformatics. 24: 2636–2638. [DOI] [PubMed] [Google Scholar]
- 42.Childs C. E., Hoile S. P., Burdge G. C., and Calder P. C.. 2012. Changes in rat n-3 and n-6 fatty acid composition during pregnancy are associated with progesterone concentrations and hepatic FADS2 expression. Prostaglandins Leukot. Essent. Fatty Acids. 86: 141–147. [DOI] [PubMed] [Google Scholar]
- 43.Larqué E., Demmelmair H., Gil-Sánchez A., Prieto-Sánchez M. T., Blanco J. E., Pagán A., Faber F. L., Zamora S., Parrilla J. J., and Koletzko B.. 2011. Placental transfer of fatty acids and fetal implications. Am. J. Clin. Nutr. 94: 1908S–1913S. [DOI] [PubMed] [Google Scholar]
- 44.Stark K. D., Lim S. Y., and Salem N. Jr. 2007. Artificial rearing with docosahexaenoic acid and n-6 docosapentaenoic acid alters rat tissue fatty acid composition. J. Lipid Res. 48: 2471–2477. [DOI] [PubMed] [Google Scholar]
- 45.Lin Y. H., and Salem N. Jr. 2007. Whole body distribution of deuterated linoleic and alpha-linolenic acids and their metabolites in the rat. J. Lipid Res. 48: 2709–2724. [DOI] [PubMed] [Google Scholar]
- 46.Naoum H. G., De Chazal R. C., Eaton B. M., and Contractor S. F.. 1987. Characterization and specificity of lipoprotein binding to term human placental membranes. Biochim. Biophys. Acta. 902: 193–199. [DOI] [PubMed] [Google Scholar]
- 47.Gil-Sánchez A., Demmelmair H., Parrilla J. J., Koletzko B., and Larqué E.. 2011. Mechanisms involved in the selective transfer of long chain polyunsaturated Fatty acids to the fetus. Front. Genet. 2: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lagarde M., Bernoud N., Brossard N., Lemaitre-Delaunay D., Thies F., Croset M., and Lecerf J.. 2001. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 16: 201–204, discussion 215–221. [DOI] [PubMed] [Google Scholar]
- 49.Thiés F., Delachambre M. C., Bentejac M., Lagarde M., and Lecerf J.. 1992. Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J. Neurochem. 59: 1110–1116. [DOI] [PubMed] [Google Scholar]
- 50.Chen C. T., Kitson A. P., Hopperton K. E., Domenichiello A. F., Trepanier M. O., Lin L. E., Ermini L., Post M., Thies F., and Bazinet R. P.. 2015. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci. Rep. 5: 15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park H. G., Park W. J., Kothapalli K. S., and Brenna J. T.. 2015. The fatty acid desaturase 2 (FADS2) gene product catalyzes Delta4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J. 29: 3911–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vance D. E. 2014. Phospholipid methylation in mammals: from biochemistry to physiological function. Biochim. Biophys. Acta. 1838: 1477–1487. [DOI] [PubMed] [Google Scholar]
- 53.Ridgway N. D., and Vance D. E.. 1988. Specificity of rat hepatic phosphatidylethanolamine N-methyltransferase for molecular species of diacyl phosphatidylethanolamine. J. Biol. Chem. 263: 16856–16863. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.