Abstract
Thyroid disorders have emerged as one of the most common immune-related adverse events associated with anti–PD-1 monotherapy or combination anti–PD-1 and anti–CTLA-4 therapy. This study characterizes and compares the evolution of monotherapy and combination therapy-related thyroid disorders. We analyzed the dynamic evolution of thyroid disorders in 45 patients who developed thyroid disorders following treatment with either anti–PD-1 monotherapy or anti–PD-1 and anti–CTLA-4 combination therapy. The patients presented with thyrotoxicosis or hypothyroidism as the initial presentation of their thyroid disorder. Thyrotoxicosis as the initial presentation occurred in the majority of patients (93% and 56% of the patients receiving combination therapy and monotherapy, respectively). The onset pattern of the thyroid disorder was significantly different between the two groups (P = 0.01). Subsequently, 76% and 90% of the patients with thyrotoxicosis evolved to develop hypothyroidism in the combination and monotherapy groups, respectively. In the combination therapy and monotherapy groups, the median times to onset of thyrotoxicosis and hypothyroidism after first treatment were 21 and 63 days, and 31 and 68 days, respectively. The median time for transition from thyrotoxicosis to hypothyroidism was 42 days in both groups. Our study demonstrates that most thyroid disorders induced by either anti–PD-1 or combination anti–PD-1 and anti–CTLA-4 therapy are thyroiditis. The time to onset of thyrotoxicosis after treatment initiation and evolution of thyrotoxicosis to hypothyroidism was short, emphasizing the importance of close monitoring of thyroid function in these patients.
Introduction
Immune checkpoints are cell surface proteins expressed on T lymphocytes and other immune cells (1). Inhibition of immune checkpoints has shown antitumor efficacy across a spectrum of solid and hematologic malignancies in clinical studies (2–5). A common feature of immune checkpoint inhibition is that it may also lead to immune-related adverse events (irAE; refs. 6–8).
Endocrinopathies, such as hypophysitis and thyroid disorders, are among the most common irAEs (9–11). Hypophysitis is more commonly associated with CTLA-4 inhibition, whereas thyroid disorders are more common in patients receiving anti–PD-1 therapy (2, 7, 12, 13). Although the clinical manifestations of immune checkpoint inhibition-related hypophysitis have been well described in several large case series (9–11, 14), few studies have characterized the nature and progression of immune checkpoint inhibition-related thyroid disorders. Clinical trials have found that the incidence of thyroid disorders is high in anti–PD-1 treatment, 5% to 9.5% with pembrolizumab treatment (3, 15) and 8.6% with nivolumab treatment (16). A single institution retrospective review found an incidence of hypophysitis of 8% and of primary hypothyroidism/thyroiditis of 6% in patients receiving ipilimumab (anti–CTLA-4) therapy (11). In the same study, the incidence of thyroid disorders was higher (22%) with combination CTLA-4 and PD-1 blockade, even though the incidence of hypophysitis was 9%, similar to that of anti–CTLA-4 monotherapy (11). Prompt identification and proper management of immune-mediated thyroid disorders will help to avoid severe consequences, such as life-threatening thyroid storm or myxedema coma. Here, we analyzed the dynamic evolution of thyroid disorders associated with anti–PD-1 monotherapy or combination anti–CTLA-4 and anti–PD-1 therapy in 45 patients.
Materials and Methods
Study design
Patients with immune checkpoint inhibition-related thyroid disorders were evaluated clinically in the outpatient endocrinology clinic of Brigham and Women’s Hospital and received continued longitudinal clinical care. This cohort analysis was performed retrospectively by collecting relevant data from chart reviews with removal of individual identifiers. The period for this study was from December 21, 2010 to May 9, 2016. Institutional Review Board approval was obtained for the study. We analyzed the type of checkpoint inhibition therapy, initial presentations of the thyroid disorder, time to onset after treatment initiation, and time for evolution from thyrotoxicosis to hypothyroidism. The patients received either combination therapy or monotherapy. Combination therapy was comprised of ipilimumab (3 mg/kg) and nivolumab (1 mg/kg) every 3 weeks for up to 4 doses followed by nivolumab monotherapy (3 mg/kg) every 2 weeks. Patients on monotherapy received either pembrolizumab (2 mg/kg every 3 weeks, n = 6; 10 mg/kg every 3 weeks, n = 3; 200 mg/infusion every 2–3 weeks, n = 5) or nivolumab (3 mg/kg every 2 weeks, n = 4).
Definition of thyrotoxicosis and hypothyroidism
Thyrotoxicosis was diagnosed based on low thyrotropin (TSH) in conjunction with elevation of at least one of three thyroid hormones: total thyroxine (T4), free T4, or free triiodothyronine (FT3). We use the term thyrotoxicosis rather than hyperthyroidism in this study because hyperthyroidism is defined as overproduction of thyroid hormone by the thyroid gland, as occurs in Grave’s disease, toxic nodular goiter, or TSH-driven hyperthyroidism, whereas thyrotoxicosis is defined as any cause of thyroid hormone excess. Indeed, previous studies have suggested that thyroiditis is the most common type of immune checkpoint blockade-related thyroid disorder (11, 17). Hypothyroidism was diagnosed based on an elevated TSH with a low or normal total or free T4 and/or FT3.
Definition of times to onset
Thyroid function tests were performed before each immune checkpoint inhibitor treatment, which was given every 2 to 3 weeks. Time to onset of thyrotoxicosis was defined as the number of days between the administration of the first dose of nivolumab or pembrolizumab in patients receiving monotherapy, or the first dose of ipilimumab and nivolumab in patients receiving combination therapy, and the date of the first biochemical documentation of thyrotoxicosis. Time from the onset of thyrotoxicosis to the onset of hypothyroidism was defined as the number of days between the initial biochemical documentation of thyrotoxicosis and the first biochemical documentation of hypothyroidism. Time to onset of hypothyroidism was defined as the number of days between the administration of the first dose of nivolumab or pembrolizumab in patients receiving monotherapy, or the first dose of ipilimumab and nivolumab in those receiving combination therapy, and the date of the first biochemical documentation of hypothyroidism.
Statistical analysis
Comparisons between monotherapy and combination therapy were analyzed using Fisher exact tests for categorical characteristics or exact Wilcoxon rank-sum tests for characteristics measured on a continuous scale. Presentation of thyroid disorders was divided into three groups: hypothyroidism as initial presentation (hypothyroidism only), thyrotoxicosis alone, or thyrotoxicosis followed by hypothyroidism.
Times to onset of thyrotoxicosis or hypothyroidism were each classified in 21-day increments for patients who developed the respective conditions. The pooled data in each group were analyzed to calculate the median time to onset of thyrotoxicosis, hypothyroidism, as well as the median conversion time from thyrotoxicosis to hypothyroidism. Statistical significance is defined as P < 0.05. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc.) and GraphPad Prism 7 (GraphPad Software).
Results
Demographic data
A total of 45 patients who developed thyroid disorders after either anti–PD-1 monotherapy (pembrolizumab or nivolumab) or combination therapy of anti–CTLA-4 (ipilimumab) and anti–PD-1 (nivolumab) were assessed. Among these 45 patients, 18 patients received anti–PD-1 monotherapy (nivolumab: 4/18; pembrolizumab: 14/18). The other 27 patients received anti–PD-1 (nivolumab) and anti–CTLA-4 (ipilimumab) combination therapy. The demographic data are summarized in Tables 1 and 2. Two-thirds of the patients were female in this cohort study. Although a previous study has shown a higher incidence of anti-PD-1–related thyroid disorders in females (15), we did not make the same statement due to the lack of data for the total number of patients treated with checkpoint inhibitors. The incidence of thyroid disorders was not the focus of this study. Rather, characterization of the nature and time course of the thyroid disorders was the focus. Patients with melanoma comprised 64% of the analyzed patients, followed by patients with breast cancer (11%). Patients with malignancies across the spectrum of solid cancers were also included in the analysis (Tables 1 and 2). Treatment was discontinued in two combination therapy patients due to the development of autoimmune hepatitis and/or colitis.
Table 1.
Demographic data of patients receiving combined anti–PD-1 and anti–CTLA-4 therapy
| Age, y | Gender | Malignancy | Therapy | TSH
|
Free T4
|
Free T3
|
TPO | TSI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Thyrotoxicosis | Hypo | B | Thyrotoxicosis | Hypo | B | Thyrotoxicosis | Hypo | |||||||
| 1 | 58 | F | Bladder ca. | Ipi + Nivo | 1.81 | 0.03 | x | 1.2 | 4.5 | x | 3.2 | 7.4 | x | x | x |
| 2 | 61 | M | Bladder ca. | Ipi + Nivo | 0.58 | 0.02 | x | x | 2.1 | x | x | x | x | x | x |
| 3 | 58 | M | Esophageal ca. | Ipi + Nivo | 2.03 | 0.02 | 32.6 | 1.3 | >5.9 | 0.5 | x | x | x | x | x |
| 4 | 72 | F | Melanoma | Ipi + Nivo | 2.79 | 0.03 | 45.48 | 1.3 | 4.9 | <0.3 | 3.2 | 7.8 | 1.8 | x | x |
| 5 | 49 | F | Melanoma | Ipi + Nivo | 0.84 | 0.2 | 12.46 | 0.9 | 1.5 | 0.6 | 2.5 | 4.1 | 1.9 | x | x |
| 6 | 72 | F | Melanoma | Ipi + Nivo | 1.27 | 0.03 | 45.48 | 1.3 | 4.9 | <0.3 | 2.8 | 7.8 | 1.8 | x | x |
| 7 | 51 | F | Melanoma | Ipi + Nivo | 0.48 | 0.01 | 55.77 | 1.4 | 1.3 | <0.3 | x | x | x | x | x |
| 8 | 36 | F | Melanoma | Ipi + Nivo | 0.7 | 0.02 | x | 1.2 | 2.4 | x | x | x | x | x | x |
| 9 | 66 | F | Melanoma | Ipi + Nivo | 0.48 | 0.04 | x | 1.2 | 2 | x | 3.1 | 3.6 | x | x | x |
| 10 | 53 | F | Melanoma | Ipi + Nivo | 1.68 | x | 7.28 | 1.1 | x | 0.5 | 3.1 | x | 2.2 | x | x |
| 11 | 64 | F | Melanoma | Ipi + Nivo | 2.39 | 0.03 | 9.23 | 1.2 | 2.8 | 0.7 | 2.7 | 5.9 | x | x | x |
| 12 | 67 | F | Melanoma | Ipi + Nivo | 3.42 | 0.01 | 9.51 | 1 | 4.6 | 0.4 | 2.6 | x | 1.5 | x | x |
| 13 | 66 | F | Melanoma | Ipi + Nivo | x | 0.02 | 8.12 | n/a | 3.5 | 0.6 | x | x | x | x | x |
| 14 | 52 | M | Melanoma | Ipi + Nivo | 3.2 | 0.05 | 9.91 | 1.1 | 1.6 | 0.6 | 3.5 | 4.5 | 3.1 | 10.8 | x |
| 15 | 44 | M | Melanoma | Ipi + Nivo | 1.3 | 0.02 | 5.66 | 1.4 | 5.4 | 0.8 | 3.4 | 8.2 | 2.3 | x | x |
| 16 | 47 | M | Melanoma | Ipi + Nivo | 3.36 | 0.04 | 69.76 | 1 | 2 | <0.3 | 2.8 | 3.7 | 1.5 | x | x |
| 17 | 69 | M | Melanoma | Ipi + Nivo | 2.16 | 0.02 | 18.9 | 1.4 | >5.9 | 4.2 | 2.7 | 15.1 | x | 17.1 | <1 |
| 18 | 25 | M | Melanoma | Ipi + Nivo | 1.14 | 0.02 | x | 1.1 | 2.7 | x | 3.5 | 6.5 | x | x | x |
| 19 | 53 | M | Melanoma | Ipi + Nivo | 4.33 | 0.07 | 9.91 | 1 | 1.6 | 0.6 | 3 | x | 3.1 | 10.8 | <1 |
| 20 | 78 | M | Melanoma | Ipi + Nivo | 3.76 | x | 28.67 | 1.1 | x | <0.3 | x | x | 1.4 | 398 | x |
| 21 | 47 | M | Melanoma | Ipi + Nivo | 3.36 | 0.04 | 69.76 | 1 | 2 | <0.3 | 2.8 | 3.7 | 1.5 | x | x |
| 22 | 64 | M | Melanoma | Ipi + Nivo | 0.89 | 0.02 | 54 | 1.1 | 6.3 | <0.3 | x | x | x | x | x |
| 23 | 58 | M | Melanoma | Ipi + nivo | 2.44 | 0.02 | x | 1.4 | 5.2 | x | x | x | x | x | x |
| 24 | 50 | F | Ovarian ca. | Ipi + Nivo | 3.38 | 0.02 | 20.17 | 1.4 | >7.7 | 0.5 | 2.4 | 3.3 | 1.7 | x | <1 |
| 25 | 33 | F | Ovarian ca. | Ipi + Nivo | 3.63 | 0.09 | 36.76 | 1.4 | >5.9 | 2.9 | 2.4 | 3.3 | 1.7 | 53.9 | <1 |
| 26 | 67 | F | Renal cell ca. | Ipi + Nivo | 4.08 | 0.04 | 55.98 | 1.2 | 1.3 | <0.3 | 2.3 | 2.9 | 1.4 | x | x |
| 27 | 43 | F | Gastric ca. | Ipi + Nivo | 1.1 | 0.01 | x | x | >5.9 | x | x | x | x | 11.1 | <1 |
Abbreviations: M, male; F, female; B, baseline; hypo, hypothyroidism; x, not measured; TPO, thyroid peroxidase; TSI, thyroid-stimulating immunoglobulin; TSH, thyroid-stimulating hormone; free T4, free thyroxine; free T3, free triiodothyronine.
Table 2.
Demographic data of patients receiving anti–PD-1 monotherapy
| Age, y | Gender | Malignancy | Therapy | TSH
|
Free T4
|
Free T3
|
TPO | TSI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Thyrotoxicosis | Hypo | B | Thyrotoxicosis | Hypo | B | Thyrotoxicosis | Hypo | |||||||
| 1 | 59 | M | Anal cancer | Nivolumab | 0.97 | x | 14.86 | 1.1 | x | 0.4 | 3.1 | x | 2 | x | x |
| 2 | 66 | F | Breast ca. | Pembrolizumab | 5.82 | x | 17.38 | 0.9 | x | 0.5 | x | x | x | x | x |
| 3 | 67 | F | Breast ca. | Pembrolizumab | 1.46 | 0.02 | 30.46 | 1.1 | 3.1 | <0.4 | x | x | x | x | x |
| 4 | 62 | F | Breast ca. | Pembrolizumab | 2.62 | 0.13 | 16.7 | 1.3 | 1.7 | 0.6 | x | x | x | x | x |
| 5 | 62 | F | Breast ca. | Pembrolizumab | 0.4 | 0.03 | 20.46 | 1.7 | 2.4 | 0.5 | x | x | x | x | x |
| 6 | 44 | F | Breast ca. | Pembrolizumab | 2.63 | 0.04 | 56.41 | 1 | 1.8 | 0.3 | 3.5 | x | x | x | x |
| 7 | 42 | F | GBM of brain | Nivolumab | 1.55 | 0.09 | 31.62 | 1.1 | 2.3 | <0.3 | 2.8 | 4.7 | <1.4 | x | x |
| 8 | 55 | F | Leiomyosarcoma | Pembrolizumab | 5.54 | x | 54.79 | 1.2 | x | 0.6 | x | x | <1.4 | 73.6 | x |
| 9 | 50 | F | Leiomyosarcoma | Pembrolizumab | 2.57 | x | 58.93 | 1 | x | 0.4 | x | x | x | x | x |
| 10 | 49 | M | Melanoma | Pembrolizumab | 2.56 | x | 187.82 | x | x | x | x | x | x | 20.7 | x |
| 11 | 77 | F | Melanoma | Nivolumab | 1.64 | x | 79.66 | 1 | x | 0.4 | x | x | x | 250.7 | x |
| 12 | 54 | M | Melanoma | Pembrolizumab | 1.15 | 0.01 | x | 1.4 | 2.5 | x | x | x | x | x | <1 |
| 13 | 55 | F | Melanoma | Pembrolizumab | 1.14 | 0.21 | 9.22 | 0.8 | x | 1.1 | x | x | x | x | <1 |
| 14 | 73 | F | Melanoma | Pembrolizumab | 1.09 | 0.01 | 32.64 | x | x | 0.4 | x | x | x | 149 | x |
| 15 | 72 | M | Melanoma | Pembrolizumab | 2.06 | 0.03 | 67.44 | 1 | 1.8 | <0.3 | 2.2 | x | x | 93.8 | <1 |
| 16 | 55 | F | Melanoma | Pembrolizumab | 2.66 | x | 56.78 | x | x | <0.3 | x | x | x | x | x |
| 17 | 41 | F | Melanoma | Pembrolizumab | 0.49 | 0.03 | x | x | 2.7 | x | x | 5.9 | x | x | x |
| 18 | 55 | F | Melanoma | Nivolumab | 1.37 | x | 21.82 | x | x | x | x | x | x | x | x |
Abbreviations: M, male; F, female; B, baseline; hypo, hypothyroidism; GBM, glioblastoma; TSH, thyroid stimulating hormone; free T4, free thyroxine; free T3, free triiodothyronine.
The median age was 58 years in the combination therapy group and 55 years in the monotherapy group. Differences in age between the two groups were not statistically significant (P = 0.69). All patients, except one, had normal baseline thyroid function tests. The patient without normal baseline thyroid function tests received combination therapy and had no history of a thyroid disorder prior to receiving immunotherapy.
Positive TPO (thyroid peroxidase) tests after treatment were found in 2 of the 6 patients tested who received combination therapy and in 4 of the 5 patients tested who received mono-therapy. Thyroid-stimulating immunoglobulins were not detected in the seven patients tested (5 in combination treatment and 2 in monotherapy group). Two patients receiving combination therapy had thyroid uptake tests during the thyrotoxicosis phase. Both had low uptake, but one patient had received iodine treatment for her thyrotoxicosis prior to the uptake test. Both patients developed hypothyroidism within one month.
Conversion of thyrotoxicosis to hypothyroidism
In this study, the patients presented initially with either thyrotoxicosis or hypothyroidism. While thyrotoxicosis was frequently followed by hypothyroidism, no case of hypothyroidism was followed by thyrotoxicosis. We calculated the percentage of patients with hypothyroidism only, thyrotoxicosis only, or thyrotoxicosis followed by hypothyroidism. Only 22% (10/45) of patients presented with hypothyroidism as the initial presentation. The remaining 78% (35/45) of patients manifested initially with thyrotoxicosis (thyrotoxicosis only or thyrotoxicosis followed by hypothyroidism; Fig. 1). Among the patients with thyrotoxicosis, 80% (28/35) subsequently developed hypothyroidism. The percentage of patients with thyrotoxicosis as the initial presentation was significantly higher in the combination therapy group, 93% (25/27), than in the monotherapy group, 56% (10/18; Fig. 1; P = 0.01).
Figure 1.
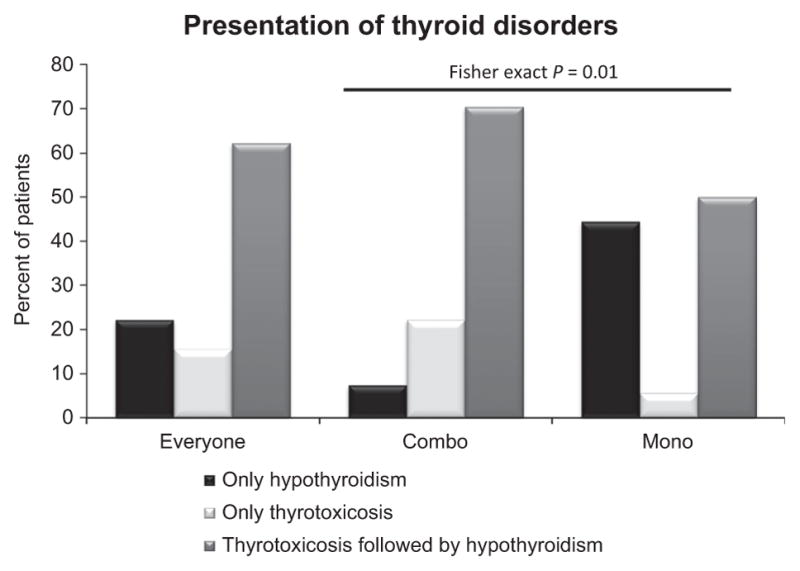
Presentations of thyroid disorders after receiving immune checkpoint inhibition combination therapy or monotherapy. Thyroid disorders were divided into three groups: hypothyroidism only, thyrotoxicosis only, and thyrotoxicosis, followed by hypothyroidism. Percentage of patients with each thyroid disorder, calculated as the percentage of cases presenting with each thyroid disorder out of the total number of patients with thyroid disorders, represented for all patients (left, everyone), combination therapy (middle, combo), or monotherapy (right, mono) groups. Differences in initial presentation between the combination therapy and monotherapy groups were determined using the Fisher test; P = 0.01.
The most common symptoms in patients with thyrotoxicosis were weight loss and tachycardia. Two patients reported neck pain. Among the patients with recorded weight, 90% (20/22) of those receiving combination therapy and 89% (8/9) on monotherapy had weight loss. Among the patients with recorded heart rate, 38% (8/21) receiving combination therapy and 29% (5/17) on monotherapy had tachycardia (heart rate > 90 bpm).
The most common symptoms in patients who presented with hypothyroidism were fatigue and weight gain. Among the patients with recorded weight, 89% (16/18) in the combination therapy group and 80% (12/15) in the monotherapy group had weight gain. Rarely, patients may present with severe thyrotoxicosis. Two patients in this study who received combination therapy presented with fever, heart rate > 130 beats per minute, and weakness. Among patients who initially presented with thyrotoxicosis, 76% (19/25) and 90% (9/10) in the combination therapy and monotherapy groups, respectively, subsequently developed hypothyroidism (Fig. 1) during this study period.
Time to onset of thyrotoxicosis is short in both treatment groups
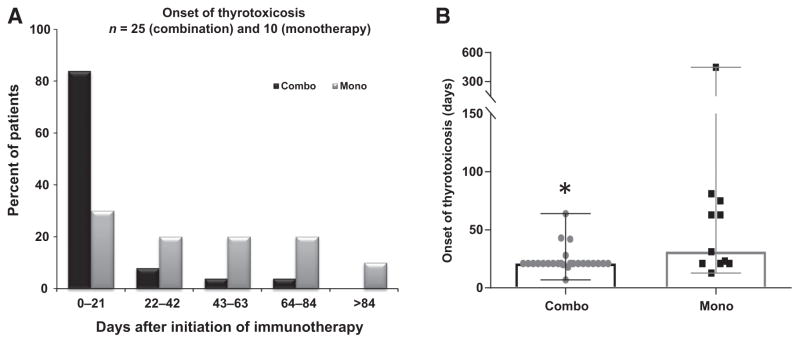
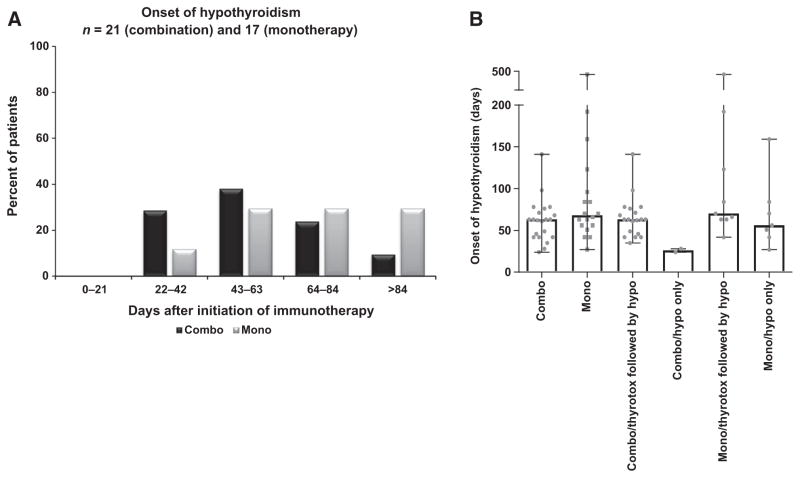
To evaluate the timing of onset in new cases of thyrotoxicosis or hypothyroidism, we classified the time to onset of thyroid disorders by two different methods: (i) time to onset of new cases of thyrotoxicosis (Fig. 2A) and hypothyroidism (Fig. 3A) in 21-day intervals (Fig. 2A and Fig. 3A) and (ii) median time to onset of thyrotoxicosis (Fig. 2B) and hypothyroidism (Fig. 3B) in the combination therapy and monotherapy groups.
Figure 2.
Time to onset of thyrotoxicosis after first treatment with immune checkpoint inhibitors in the combination therapy and monotherapy groups. A, Incidence of onset of thyrotoxicosis in 21-day intervals, calculated by the number of new cases of thyrotoxicosis that occurred in that 21-day interval divided by the total number of thyrotoxicosis cases, expressed as a percentage. Combination therapy: n = 25; monotherapy: n = 10. B, The median onset time (median with range) of thyrotoxicosis after first treatment in combination therapy (combo) and monotherapy (mono) groups. Gray dots, time to onset of thyrotoxicosis in each patient. Significant differences were determined using the Fisher test (A) and exact Wilcoxon rank-sum test (B; *, P < 0.05).
Figure 3.
Time to onset of hypothyroidism after first treatment with immune checkpoint inhibitors in the combination therapy and monotherapy groups. A, Incidence of onset of hypothyroidism in 21-day intervals, calculated by the number of new cases of hypothyroidism that occurred in that 21-day interval divided by the total number of hypothyroidism cases, expressed as a percentage. Combination therapy: n = 21; Monotherapy: n = 17. B, Median onset time (median with range) of hypothyroidism after first treatment. Patients with hypothyroidism were divided into three groups: all patients with hypothyroidism (combo n = 21; mono n = 17); patients with hypothyroidism only (combo n = 2; mono n = 8); and patients who had thyrotoxicosis followed by hypothyroidism (combo n = 19; mono n = 9). Gray dots: time to onset of hypothyroidism in each patient. Combo, combination therapy; mono, monotherapy; thyrotox: thyrotoxicosis; hypo, hypothyroidism. Significant differences were determined using the Fisher test (A) and exact Wilcoxon rank-sum test (B).
In the combination therapy group, 83% of the patients with thyrotoxicosis presented with biochemical evidence of this disorder within 21 days after the first immune checkpoint inhibition treatment (Fig. 2A), whereas the remaining 17% of thyrotoxicosis cases occurred within 84 days (Fig. 2A). Conversely, in the monotherapy group, 30% of patients developed thyrotoxicosis within 21 days, whereas 10% developed thyrotoxicosis more than 84 days after the first treatment (Fig. 2A). For patients with thyrotoxicosis, median onset times were 21 days (range, 7–64 days) and 47 days (range, 14–447 days) in the combination therapy and monotherapy groups, respectively, and were significantly shorter with combination therapy (exact Wilcoxon rank-sum test, P = 0.006).
Hypothyroidism did not develop within 21 days of the first immune checkpoint inhibition treatment in either group of patients. For patients who developed hypothyroidism, the onset times were comparable between combination therapy and monotherapy groups (Fig. 3A). The median time to onset of hypothyroidism was 63 (range, 24–141 days) and 70 (range, 27–475 days) in the combination and monotherapy groups, respectively (exact Wilcoxon rank-sum test, P = 0.09). We analyzed the median time to onset of hypothyroidism in subgroups of the patients. The median times to onset of hypothyroidism in the combination therapy group were 63 days (thyrotoxicosis followed by hypothyroidism; range, 35–141 days) and 26 days (hypothyroidism only; range, 24–28 days), whereas the monotherapy times were 84 days (thyrotoxicosis followed by hypothyroidism; range, 63–475 days) and 56 days (hypothyroidism only; range, 27–159 days; Fig. 3B). Although the data suggest that the median time to onset of hypothyroidism was shorter if hypothyroidism was the initial presentation in both monotherapy and combination therapy groups, the differences in onset times did not reach significance.
Dynamic evolution of thyroid disorders
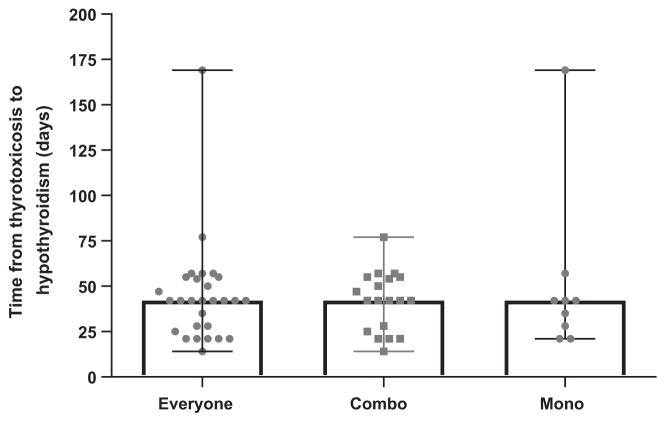
Because most thyrotoxicosis cases progressed to hypothyroidism, we evaluated the conversion time from thyrotoxicosis to hypothyroidism. The median time from onset of thyrotoxicosis to onset of hypothyroidism was 42 days (range, 14–169) and was not significantly different between the combination therapy and monotherapy groups, with the same median time of 42 days in both groups and similar ranges (combination therapy, 14–77 days and monotherapy, 21–169 days; Fig. 4). During our study period, none of the patients had resolution of hypothyroidism, whether they developed hypothyroidism as their primary presentation or following thyrotoxicosis. Seven patients (6 combination therapy and 1 monotherapy) did not develop hypothyroidism after the onset of thyrotoxicosis during follow-up from 1 week to 12 months. One patient presented with thyrotoxicosis followed by euthyroidism for 2 months, with subsequent biochemical evidence of subclinical hyperthyroidism that persisted for more than 8 months until death. No autoantibody testing or thyroid uptake studies were performed in this patient. Therefore, whether the patient developed mild Grave disease or had thyroiditis with a prolonged phase of thyrotoxicosis is unclear. One patient who received monotherapy had preexisting hypothyroidism and was on stable levothyroxine replacement. He developed transient thyrotoxicosis after monotherapy and became euthyroid with continued replacement of levothyroxine. In the combination therapy group, one patient was lost during the follow-up period. Three patients remained euthyroid for 8 to 12 months; 1 patient, as described above, remained hyperthyroid for more than 8 months; and 1 patient had follow-up for less than 1 week.
Figure 4.
Time from the onset of thyrotoxicosis to the onset of hypothyroidism. Median time (median with range) from thyrotoxicosis to hypothyroidism. Gray dots, conversion time from thyrotoxicosis to hypothyroidism in each patient. The times presented, all the patients (left, everyone), combination therapy (middle, combo), and monotherapy (right, mono) groups. Significant differences were determined using the exact Wilcoxon rank-sum test.
Discussion
Thyroid disorders have emerged as one of the most common adverse events associated with anti–PD-1 monotherapy and with combination anti–PD-1 and anti–CTLA-4 therapy (7, 12, 16–18). Although two studies reported 10 to 17 patients with anti-PD-1–related thyroid disorders and described them as painless thyroiditis or inflammatory thyroiditis (17, 19), no studies characterizing the nature and time course of immune checkpoint blockade-related thyroid disorders have been performed.
In this cohort study, we find that the initial presentation of thyroid dysfunction in those receiving combination therapy was predominantly thyrotoxicosis (93%). In contrast, close to half of the patients (44%) receiving anti–PD-1 monotherapy presented with hypothyroidism as their first presentation. The mechanisms underlying the different patterns of presentation of thyroid dysfunction between the two groups remain to be elucidated. With combination therapy, all patients received nivolumab as anti–PD-1 therapy, whereas 14 of 18 patients in the anti–PD-1 monotherapy group received pembrolizumab and 4 of 18 received nivolumab. We evaluated patients receiving nivolumab as anti–PD-1 monotherapy; 50% (2/4) presented with hypothyroidism as initial manifestation, which is similar to patients receiving pembrolizumab. The times to onset of hypothyroidism in these 2 patients were 27 and 84 days, respectively. In the other 2 patients receiving nivolumab monotherapy and manifesting thyrotoxicosis, the time to onset of thyrotoxicosis was 21 and 63 days, respectively.
Anti–CTLA-4 monotherapy is associated with primary thyroid disorders (4, 20). In our database, 2 of 25 patients with ipilimumab monotherapy-related hypophysitis developed primary thyroid disorders. Both patients presented with thyrotoxicosis followed by a return to a euthyroid state. Another 2 patients in our earlier report (20) receiving combination ipilimumab and bevacizumab, an angiogenesis inhibitor, displayed a similar evolution of the thyroid disorder: thyrotoxicosis followed by euthyroidism. It appears that the nature of thyroid disorders with anti–CTLA-4 monotherapy may be different from those occurring in association with anti–PD-1 monotherapy and those with combination anti–CTLA-4 and anti–PD-1 therapy.
Concomitant endocrine toxicities were not uncommon. More than one third (10/27) of patients with combination therapy-related thyroiditis had synchronous hypophysitis and secondary adrenal insufficiency. In patients with anti-PD-1–related thyroiditis, 22% (4/18) had synchronous hypophysitis and secondary adrenal insufficiency and 1 patient developed metachronous autoimmune diabetes.
Thyroid disorders associated with immune checkpoint inhibitor therapy were described as hyperthyroidism, hypothyroidism, and thyroiditis in previous reports (11, 16, 21). The findings in our study suggest that most immune checkpoint blockade-related thyroid disorders are likely thyroiditis. This conclusion is based on the findings that 80% of the patients who presented initially with thyrotoxicosis subsequently developed hypothyroidism during this study period. The time course of disease progression is consistent with thyroiditis. Hypothyroidism, as an initial presentation in the setting of autoimmunity, is thyroiditis in nature. In this study, measurement of radioiodine thyroid uptake was not routinely performed in patients with thyrotoxicosis. In fact, in our practice, after realizing that most of the thyroid disorders were consistent with thyroiditis, we seldom ordered thyroid uptake testing in order to limit costs. Frequent enhanced CT studies with concurrent iodine-based contrast exposure to monitor the cancer response decreases radioiodine uptake and makes the results of radioiodine thyroid uptake testing unreliable. We recommend thyroid uptake tests in patients with signs suggestive of Grave disease (e.g., goiter, ophthalmopathy, or persistent thyrotoxicosis) and without CT intravenous contrast exposure for at least 1 month.
We analyzed the timing of onset of thyroid disorders in 21-day increments after the first immune checkpoint inhibition treatment. We chose the time interval of 21 days because anti–PD-1 and anti–CTLA-4 therapies were given every 2 to 3 weeks. Calculation of the incidence of thyroid disorders in 21-day intervals provides not only a correlation of the pattern of onset of the thyroid disorder with time but also a correlation between the onset of the thyroid disorder and the number of treatments given. This analysis revealed that 83% and 36% of thyrotoxicosis occurred within 21 days after the first infusion with combination therapy and monotherapy, respectively, and within 84 days after the first treatment, 100% of thyrotoxicosis cases had already occurred in patients receiving combination therapy, whereas 90% of cases of thyrotoxicosis had occurred by this time in the monotherapy group. These findings suggest that close monitoring of thyroid function tests after initiating either combination therapy with anti–PD-1 and CTLA-4 or anti–PD-1 monotherapy is needed. Specifically, TSH and free T4 levels should be checked within 3 weeks after initiating therapy.
No biochemical evidence of hypothyroidism within 21 days after the first immune checkpoint inhibition treatment was observed in either group. Twenty-nine percent and 12% of hypothyroidism cases occurred more than 84 days after treatment initiation in the groups receiving monotherapy and combination therapy, respectively. The checkpoint inhibitors were not discontinued due to the development of thyroid disorders. However, immune checkpoint inhibition therapy was discontinued in 2 patients in combination group because of the development of autoimmune hepatitis and/or colitis. Discontinuation of checkpoint inhibitors did not change the thyroid outcome as no patient recovered from hypothyroidism during our study period.
A previous study of 5 patients who presented with postpartum thyroiditis showed that thyrotoxicosis developed within 1 to 6 months of delivery. In all patients, thyrotoxicosis resolved within four months. Subsequent transient hypothyroidism occurred in four of the five patients, with one developing permanent myxedema (22). Researchers have noted that painless thyroiditis is also self-limited (23). In contrast to postpartum thyroiditis and painless thyroiditis, hypothyroidism induced by anti–PD-1 monotherapy and combination anti–PD-1 and anti–CTLA-4 therapy appears to be permanent or persistent for a long duration. We have not yet seen recovery of thyroid function in any patient who developed hypothyroidism.
The earliest time of onset of thyrotoxicosis was 7 days after the first treatment in a patient receiving combination therapy. In contrast, the latest onset of thyrotoxicosis after the first treatment was 447 days, occurring in a patient receiving anti–PD-1 monotherapy. This patient had continuous exposure to anti–PD-1 treatment before the development of thyrotoxicosis. Nonetheless, as discussed above, most thyrotoxicosis occurred soon after treatment initiation. Because most patients who had an initial presentation of thyrotoxicosis subsequently developed hypothyroidism, continued monitoring of thyroid function is needed due to the differing management strategies for thyrotoxicosis and hypothyroidism.
Timely monitoring and proper management of immune checkpoint inhibition-induced thyroid disorders will help to prevent life-threatening consequences, such as thyroid storm and myxedema coma, and improve the quality of life. We recommend measuring baseline TSH and free T4 prior to initiation of immune checkpoint inhibition therapy and repeat before each infusion for at least 5 cycles. If patients develop thyrotoxicosis, they can be treated with a beta-blocker, such as propranolol (10–30 mg t.i.d. PRN for pulse > 100 bpm), for symptomatic control if they are at low risk for cardiovascular events. In patients with severe thyrotoxicosis or with high risk for cardiovascular events, such as a previous history of coronary artery disease, cardiac arrhythmia, or heart failure, treatment with high-dose corticosteroids (prednisone 1–2 mg/kg daily for 2–3 weeks) can be considered. Thyroid function should be monitored every 2 to 3 weeks, as a transition from thyrotoxicosis to hypothyroidism may occur. If thyrotoxicosis resolves and hypothyroidism develops, the beta-blocker should be discontinued and levothyroxine treatment initiated. We recommend initiating treatment with levothyroxine at 0.8 mcg/kg daily in young patients without a history of cardiovascular disease. We recommend repeating TSH and free T4 measurements one month after levothyroxine initiation for further levothyroxine dose titration. In elderly patients or patients with a history of cardiovascular disease, we recommend initiating levothyroxine treatment with a lower dose (usually 25 mcg daily) and increasing the dose slowly, as tolerated. Based on our current study and previous studies (17, 19), measurement of TPO and TSI may not be helpful in terms of clinical management. However, if a patient with thyrotoxicosis has signs of Graves’ disease (i.e., a large goiter or Graves ophthalmopathy), we recommend testing TSI and performing a thyroid uptake test (if the patient has had no recent intravenous radiographic contrast administration).
In summary, immune checkpoint inhibitors display effective and durable anticancer effects across a broad spectrum of advanced malignancies. With the increasing use of this type of therapy, clinicians should be aware of irAEs including thyroid disorders in these patients. Most cases of thyroid dysfunction occurred within 12 weeks after the first treatment with either combination therapy or monotherapy, and most thyrotoxicosis converted to hypothyroidism in a relatively short period, underscoring the importance of close monitoring of thyroid function after initiation of immunotherapy. Our study provides guidance for appropriate, timely screening with thyroid function tests in this group of the patients.
Acknowledgments
This research was supported by NICHD/NIH K08 HD070957 (to L. Min) and NIH R01 HD019938 and HD082314 (to U.B. Kaiser).
Footnotes
Disclosure of Potential Conflicts of Interest
F.S. Hodi reports receiving a commercial research grant from Bristol-Myers Squibb to an institution and is a consultant/advisory board member for Bristol-Myers Squibb, Merck, Genentech, Novartis, Amgen, and EMD Serono. P.A. Ott is a consultant/advisory board member for Bristol-Myers Squibb, Genentech, Alexion, Novartis, Pfizer, Merck, Celldex, and CytomX. R. Haq reports receiving a commercial research grant from Bristol-Myers Squibb. S. Tolaney is a consultant/advisory board member for Merck, AstraZeneca, Eli Lilly, Novartis, Eisai, Pfizer, and NanoString. L. Min reports receiving a commercial research grant from Bristol-Myers Squibb. No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions
Conception and design: H. Lee, R.S. Carroll, U.B. Kaiser, L. Min
Development of methodology: H. Lee, L. Min
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): H. Lee, F.S. Hodi, P.A. Ott, E.I. Buchbinder, R. Haq, S. Tolaney, K. Zhang, H. Donahue, M. Davis, K.M. Kelley, L. Min
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): H. Lee, F.S. Hodi, A. Giobbie-Hurder, R. Barroso-Sousa, K. Zhang, U.B. Kaiser, L. Min
Writing, review, and/or revision of the manuscript: H. Lee, F.S. Hodi, P.A. Ott, E.I. Buchbinder, R. Haq, S. Tolaney, R. Barroso-Sousa, M.E. Gargano, K.M. Kelley, U.B. Kaiser, L. Min
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): H. Lee, K. Zhang, L. Min
Study supervision: R.S. Carroll, L. Min
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–18. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33:3193–8. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min L, Hodi FS, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2015;21:749–55. doi: 10.1158/1078-0432.CCR-14-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary. 2016;19:82–92. doi: 10.1007/s11102-015-0671-4. [DOI] [PubMed] [Google Scholar]
- 11.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–81. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Velasco G, Je Y, Bosse D, Awad MM, Ott PA, Moreira RB, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5:312–8. doi: 10.1158/2326-6066.CIR-16-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99:4078–85. doi: 10.1210/jc.2014-2306. [DOI] [PubMed] [Google Scholar]
- 15.Robert C, Joshua AM, Kefford R, Joseph RW, Wolchok JD, Hodi FS, et al. Association of immune-related thyroid disorders with pembrolizumab (pembro, MK-3475) in patients (pts) with advanced melanoma treated in KEYNOTE-001. J Clin Oncol. 2015;33(Suppl):9050. [Google Scholar]
- 16.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab. 2015;100:1738–41. doi: 10.1210/jc.2014-4560. [DOI] [PubMed] [Google Scholar]
- 18.Larkin J, Lao CD, Urba WJ, McDermott DF, Horak C, Jiang J, et al. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1:433–40. doi: 10.1001/jamaoncol.2015.1184. [DOI] [PubMed] [Google Scholar]
- 19.de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, et al. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab. 2016;101:4431–9. doi: 10.1210/jc.2016-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2010;164:303–7. doi: 10.1530/EJE-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 22.Ginsberg J, Walfish PG. Post-partum transient thyrotoxicosis with painless thyroiditis. Lancet. 1977;1:1125–8. doi: 10.1016/s0140-6736(77)92384-4. [DOI] [PubMed] [Google Scholar]
- 23.Woolf PD. Transient painless thyroiditis with hyperthyroidism: a variant of lymphocytic thyroiditis? Endocr Rev. 1980;1:411–20. doi: 10.1210/edrv-1-4-411. [DOI] [PubMed] [Google Scholar]