Abstract
Resurrection ecology (RE) is a very powerful approach to address a wide range of question in ecology and evolution. This approach rests on using appropriate model systems, and only few are known to be available. In this study, we show that Artemia has multiple attractive features (short generation time, cyst bank and collections, well‐documented phylogeography, and ecology) for a good RE model. We show in detail with a case study how cysts can be recovered from sediments to document the history and dynamics of a biological invasion. We finally discuss with precise examples the many RE possibilities with this model system: adaptation to climate change, to pollution, to parasites, to invaders and evolution of reproductive systems.
Keywords: biological invasions, cysts, global change, long‐term adaptation, sediment core
1. INTRODUCTION
Many aquatic invertebrate taxa, such as Branchiopoda, Ostracoda, or Copepoda, depend on the production of long‐lived dormant stages, which allow them to persist under unfavorable conditions (drought, high temperatures, extreme salinities, predation, or food scarcity), especially in highly fluctuating environments (Brendonck, 1996; Hairston, 1996; Hand, Denlinger, Podrabsky, & Roy, 2016; Wiggins, Mackay, & Smith, 1980). These dormant stages form egg banks that can be stored in the environment, allowing for an escape strategy in the form of dispersal through time (Venable & Lawlor, 1980). From a practical or applied point of view, because dormant stages can remain viable or at least carry genetic information for decades or even centuries in sediments (Hairston, Ellner, & Kearns, 1996) or in laboratory collections, they can provide researchers with natural archives of recent evolutionary and ecological histories of populations (Brendonck & De Meester, 2003; Kerfoot, Robbins, & Weider, 1999; Weider, Lampert, Wessels, Colbourne, & Limburg, 1997).
With the exception of a handful of long‐term field studies, contemporary evolutionary ecology strongly relies on spatial comparison among populations. These spatial studies are often used to extrapolate about temporal predictions using the so‐called space‐for‐time substitution (Etterson & Shaw, 2001; Pickett, 1989). However, an alternative way to directly capture the temporal component of evolutionary responses of populations without relying on costly long‐term monitoring is by studying ancient populations from propagule banks. This is the approach taken by the emerging field of “resurrection ecology” (Experimental Paleoecology, Kerfoot et al., 1999; Kerfoot & Weider, 2004; thereafter RE). Past populations can be studied by reviving these propagule banks (Etterson et al., 2016; Orsini et al., 2013) or by studying these banks genetically (Angeler, 2007). For example, time‐shift experiments can be used to study temporal adaptation by comparing the fitness of a population under current, past, or future environmental conditions (Blanquart & Gandon, 2013; Gaba & Ebert, 2009). Because of their dormant stages, a number of aquatic animals are ideally suited for “resurrection ecology,” and, as we will see, Artemia is a particularly attractive one, although very limited attempts have been made to exploit its full potential.
Resurrection ecology has been used to study many important questions in ecology and evolution: male–female coevolution (Rode, Charmantier, & Lenormand, 2011), host–parasite coevolution (Decaestecker et al., 2007; Frickel, Sieber, & Becks, 2016; Gaba & Ebert, 2009; Thrall et al., 2012), spatiotemporal variability in community and trophic structure (Anderson & Battarbee, 1994; Vandekerkhove et al., 2005), biological invasions (Kerfoot, Ma, Lorence, & Weider, 2004), and biodiversity dynamics (Gregory‐Eaves & Beisner, 2011). RE can also be particularly useful to study the dynamics of adaptation to changing environments (Bradshaw & Holzapfel, 2001; Frisch et al., 2014; Gaba & Ebert, 2009; Hairston et al., 1999; Pulido & Berthold, 2010) and hence provide better predictions of adaptive responses to global change (Hoffmann & Sgro, 2011). Most RE studies to date have been conducted on diapausing eggs of cladocerans (Angeler, 2007; Decaestecker et al., 2007; Hairston et al., 1999; Miner, De Meester, Pfrender, Lampert, & Hairston, 2012; Orsini, Spanier, & De Meester, 2012; Roy Chowdhury, 2016; Weider et al., 1997), mainly on Daphnia, in freshwater ecosystems. The scarcity of studies in other organisms and environments has been highlighted as an important limitation of RE (Angeler, 2007). Identifying new study systems is therefore essential to fully exploit the potential of RE to address evolutionary and ecological questions.
In this study, we present the hypersaline macrocrustacean brine shrimp Artemia (Crustacea, Branchiopoda, Anostraca) as a valuable model system for RE. We start by reviewing the biological and ecological characteristics that make Artemia an interesting model in RE. We then present how Artemia egg banks can be obtained from sediment cores, with an example of such an approach from our own work in the Aigues‐Mortes saltern in South France. Overall, we discuss for the first time the usefulness and limitations of this model system for RE.
1.1. The biology and ecology of Artemia
Artemia is among the most intensely studied aquatic organisms, due to its importance in aquaculture and its broad use as a model system in ecotoxicology, developmental biology, ecology and evolutionary biology. It is an extreme halophilic organism occurring in hypersaline environments such as salt pans, inland salt lakes, and coastal salt lagoons worldwide (Lenz & Browne, 1991). The genus includes seven sexual species and several parthenogenetic strains of different ploidy (Baxevanis, Kappas, & Abatzopoulos, 2006; Gajardo, Abatzopoulos, Kappas, & Beardmore, 2002). Parthenogenetic populations are widely distributed except in the Americas. Sexual and parthenogenetic Artemia tend to occupy different microhabitats and to be temporally segregated (Agh et al., 2007), although a handful of populations are mixed (Amat, Hontoria, Navarro, Vieira, & Mura, 2007). The latter are particularly interesting to study a diversity of evolutionary and ecological processes.
From an ecological point of view, Artemia is a keystone taxon in hypersaline food webs, where it constitutes the dominant or exclusive macrozooplankton. It is the main prey for aquatic birds (Sánchez, Green, & Castellanos, 2006), the intermediate host for many species of parasites (Georgiev et al., 2005; Rode, Landes, et al., 2013; Rode, Lievens, et al., 2013; Vasileva et al., 2009), and the main consumer of phytoplankton. Because hypersaline ecosystems are simplified, and because of its central role in these ecosystems, Artemia is particularly suited to study cascading effects (Sánchez, Paredes, Lebouvier, & Green, 2016). For instance, it is a good system to explore how genetic changes in one species translate into “cascading” effects throughout the community and ecosystem.
Artemia life history is well studied (Amat et al., 2007; Browne, Davis, & Sallee, 1988; Browne, Sallee, Grosch, Segreti, & Purser, 1984; Browne & Wanigasekera, 2000; Shirdhankar, Thomas, & Barve, 2004). It is characterized by a short generation time, reaching maturity through a series of approximately 15 molts, in less than 20 days (Jensen, 1918), and it has high fecundity, producing up to 250 embryos per brood (Amat et al., 2007). These are key attributes for a good RE model, as it makes it easy to detect evolutionary changes within short periods of time (Hairston et al., 1999; Kerfoot & Weider, 2004; Kerfoot et al., 1999). Artemia females produce free‐living nauplii (ovoviviparity) when environmental conditions are favorable, and produce dormant encysted embryos or diapausing cysts (oviparity) under adverse conditions (i.e., high salinity and temperature, low oxygen levels, and food supply or short photoperiods among others (Criel & Macrae, 2002; Clegg & Trotman, 2002; Nambu, Tanaka, & Nambu, 2004)).
1.2. The Artemia cyst
The cyst is a key feature of Artemia as an important RE model. Cyst production is high in hypersaline ecosystems, reaching hundreds or thousands of metric tons per year (e.g., in the Great Salt Lake, Clegg & Jackson, 1998). Cysts accumulate in sediments as eggs banks (Fig. S1) and can be recorded in sediment cores dated back to 200,000 years (Djamali et al., 2010). Cysts are commercially harvested for aquaculture (Sorgeloos, 1980). They can also be stored in laboratory cyst banks for research purposes (e.g., the cyst bank of the Laboratory of Aquaculture and Artemia Reference Center, ARC). Cysts preserved in airtight drums under cold storage conditions (4°C) can remain viable for long periods and can be hatched following well‐known standardized protocols (Sorgeloos, Persoone, Baeza‐Mesa, Bossuyt, & Bruggeman, 1978). Cysts of Artemia are considered among the most resistant of all animal life history stages to extreme environmental conditions (Clegg & Jackson, 1998; Clegg & Trotman, 2002; Hand & Podrabsky, 2000). They are able to tolerate high levels of UV radiation, prolonged anoxia, extreme temperatures, and repeated cycles of hydration and severe desiccation (Clegg, 2001, 2005; Clegg & Conte, 1980; Liang & MacRae, 1999; Warner, 1989). These are normal conditions that cysts encounter from the moment that they are released from the female into the water, accumulate along the shores, and become covered by sediments. Several features are responsible for such impressive stress resistance of cysts: the disaccharide trehalose, the small heat‐shock protein and molecular chaperone p26, and the RNA‐binding protein Artemin with RNA chaperone activity (Clegg, 1986; Crowe, Clegg, & Crowe, 1998; Warner, Brunet, MacRae, & Clegg, 2004). The cyst shell is also decisive in protecting the embryo from mechanical damage (Clegg, 2005; Clegg & Conte, 1980), UV radiation (Tanguay, Reyes, & Clegg, 2004), desiccation (Clegg, 2005), and resistance to microbial and hydrolytic damage, thus protecting the DNA inside the cyst (Clegg & Conte, 1980).
Entire cysts have been recovered from sediment cores as old as 27,000 years ago in the Great Salt Lake, Utah, USA (Clegg & Jackson, 1997). The extraordinary stability of encysted embryos has led researchers to consider Artemia as a unique model system to study ancient DNA in paleoecological research (Clegg & Jackson, 1997). For example, Manaffar et al. (2011) analyzed molecularly (by sequencing the exon 7 of the Na/K ATPase gene) the cysts bank of Lake Urmia (Iran) with radiocarbon ages estimated at ~5,000 years. This analysis showed that this ancient population was dominated by A. parthenogenetica (with some A. urmiana). Comparison with the present day niche of those species suggests that the past lake condition was brackish. Similar studies have been made using more recent cysts. Motivated by the intriguing finding of parthenogenetic individuals in a commercial sample from the Great Salt Lake, Utah, USA (Campos‐Ramos et al., 2003), a population usually described as being exclusively made of Artemia franciscana, Endebu et al. (2013) analyzed molecularly, cysts samples collected between 1997 and 2005. They showed that indeed, there has been a period where the two species coexisted.
Additional techniques have been proposed to analyze historical cyst samples. Nielson and Bowen (2010) studied the hydrogen and oxygen isotope ratios of the common structural biopolymer chitin in Artemia (present in cysts and free‐living stages). They suggested that it would be a powerful tool for paleoenvironmental and paleoecological reconstruction, showing that these measures were a good indicator of ecological and biogeochemical changes within lakes. Despite the use of Artemia in paleoecology and paleobiology during the last decade, little attention has so far been paid to its potential use in RE (but see Rode et al., 2011 and below). Cysts from laboratory collections can remain viable for decades (Abatzopoulos, Kappas, Bossier, Sorgeloos, & Beardmore, 2002) making it possible to revive ancient and present populations over significant temporal scales and use them in experiments. However, much less is known about cyst viability and DNA quality in cysts recovered from sediments. We present below a study addressing these questions.
1.3. Origin of cysts for RE studies
Several Artemia cyst collections have been established in different laboratories (e.g., Laboratory of Aquaculture & Artemia Reference Center (ARC) at Ghent University in Belgium and the Institute of Aquaculture of “Torre de la Sal” (CSIC) in Castellón, Spain). Cysts kept in good conditions in these collections have a much higher hatchability than sediment‐collected cysts. Moreover, collections contain Artemia populations from all over the world (the five continents, Sorgeloos, Lavens, Leger, & Tackaert, 1990), thus allowing studies at broad spatial scales, with information about biological parameters of populations and environmental conditions of the collections sites. These collections are readily available for Artemia, are abundant, and span several decades. In comparison, such an approach has been advocated, but only recently initiated, for example, in plants (Etterson et al., 2016). These collections are invaluable and require long‐term maintenance and constant archiving and development (Sorgeloos et al., 1990). The time horizon for cysts hatchability is not entirely clear under these conditions, but even if precise samples may not be enough to obtain complete time series in most populations, these collections offer an excellent starting point for RE studies. These collections can be also exploited from a conservation perspective using them to restore locally extirpated populations (Muñoz et al., 2008), given the dramatic decrease in Artemia biodiversity at a global scale (Amat et al., 2007).
Much less is known about Artemia cysts collected from natural sediment. Very few studies have been conducted in this direction, even though obtaining such samples could extend by far the utility of Artemia as a model system in RE studies. Obtaining and dating such samples can be challenging. We develop in the next section, how this can be achieved, from a case study in the Aigues‐Mortes saltern in South France.
2. SAMPLING AND DATING CYSTS FROM SEDIMENTS
The objective was to document the invasion of A. franciscana (hereafter Af) in the Aigues‐Mortes saltern in southern France, since its introduction in 1970 and to obtain temporal series of cysts of both the local population (A. parthenogenetica, hereafter Ap) and the invading Af species. In contrast to the bottom of lakes, surface sedimentation in commercial salterns can be much more erratic and heavily disturbed by human activity. Yet, these sediments form a very important fraction of Artemia habitats. We chose a favorable sampling site based on prior local historical information. In the saltern, water circulates from the sea to the different ponds by channels. The ponds are connected to the channels through sluice gates. The site we chose (43°31′24.72″N; 4°14′12.67″E) was close to an ancient gate that connected a pond and a channel. It was also located on a shore where cysts tended to accumulate due to the direction of dominant wind. This sluice gate was shut down in the early 1960s (D. Facca, personal communication) and silted up ever since. Cysts accumulated in the sediments, creating a time series. The site is also well connected to the water circuit in the rest of the saltern, such that cysts collected there are representative of the Artemia population in the entire saltern. Four cores were sampled (site map on Fig. S2). The first one (ABB12) was obtained on February 1, 2012, and was used to follow the invasion of Af in the saltern. The other three cores (ABB13_P5 P6 and P7) were obtained on June 26, 2013 using a manual corer and were used to finely study sedimentation patterns in the saltern.
2.1. Cyst quality along the first sediment core
First, the proportion of cysts that could not be molecularly identified in ABB12 core increased from 10% at the surface to 95% at the bottom of the core. This indicates that DNA quality declines with increasing time in the sediments. This does not prevent one from performing molecular analyses, as many cysts are available at each horizon in the cores. However, because of this degradation, methodologies involving long DNA fragments may be difficult to implement. For instance, in the samples taken below 40 cm, DNA fragments were generally smaller than 200 base pairs (bp), which is too small for microsatellite amplification in Artemia (Fig. S3), but sufficient to perform PCR on short diagnostic sequences or for direct sequencing of short fragments.
Among cysts that could be identified molecularly, the proportion of Af increases through time (Figure 1), from 100% in the top horizon to 0% in the last three horizons. This variation reflects the invasion of Af in the saltern since its introduction. Note that although the 20 cysts collected on the surface were Af, Ap is still present in the saltern today (Nougué et al., 2015). Af was intentionally introduced in 1970 (D. Facca, personal communication), which indicates that most of the cores (down to 60 cm depth) were after this date. Hatching success decreased sharply below the surface layer (12.5% at surface, 9.5% and 0.3% in layers 1 and 2, and no success below).
Figure 1.
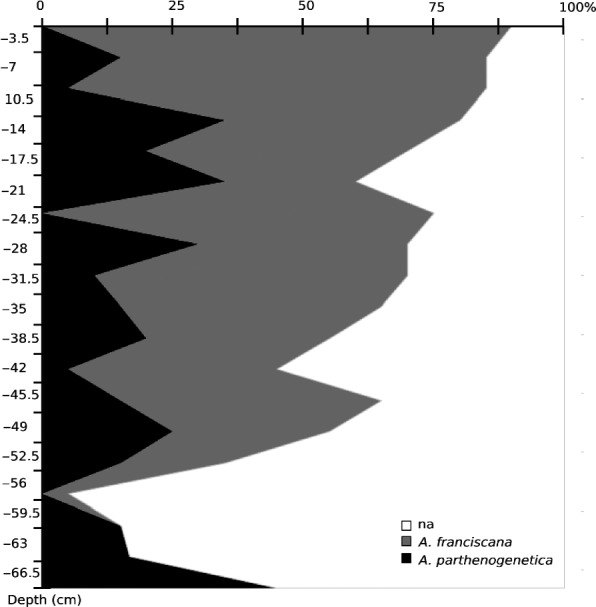
Proportion of Artemia parthenogenetica and Artemia franciscana along the core horizons. na: nonidentifiable cyst; A. franciscana and A. parthenogenetica: cyst identified as sexual and nonsexual species. Data from ABB12 core
2.2. Sediment dating and core synchronization
Visual observation of the cores (Fig. S5) revealed that only ABB13_P6 and ABB13_P7 contained the complete sediment sequence that followed the sluice gate closure. Indeed, in the P6 and P7 cores stirred sediments with no horizons are visible from 81 to 96 cm and 67 to 90 cm, respectively. They correspond to the bottom of the channel that was actively maintained before the gate was definitively closed. No regular patterns of sedimentation (e.g., like annual cycles) were observable in the cores. However, two sandy horizons were visible in the three cores. X‐ray fluorescence observations showed alternation in organic matter containing elements such as sulfur, copper, and bromine and sands containing silicon. The PCA (Fig. S4) confirms this major source of heterogeneity among horizons by exhibiting a first PCA axis (41% of the variance), segregating elements corresponding to organic versus nonorganic matter (clay and sandstone). The second PCA axis (28%) differentiates between elements representing low versus high oxygenated organic matter. As shown in Figure 2, the three cores present alternative horizons with high and low quantities of bromine (element present in organic matter). The two sandy horizons that were visually observable are also observable on the three bromine graphs (yellow zones in Figure 2), as well as the stirred sediments in the bottom of ABB13_P7 (dotted‐shaded zone in Figure 2).
Figure 2.
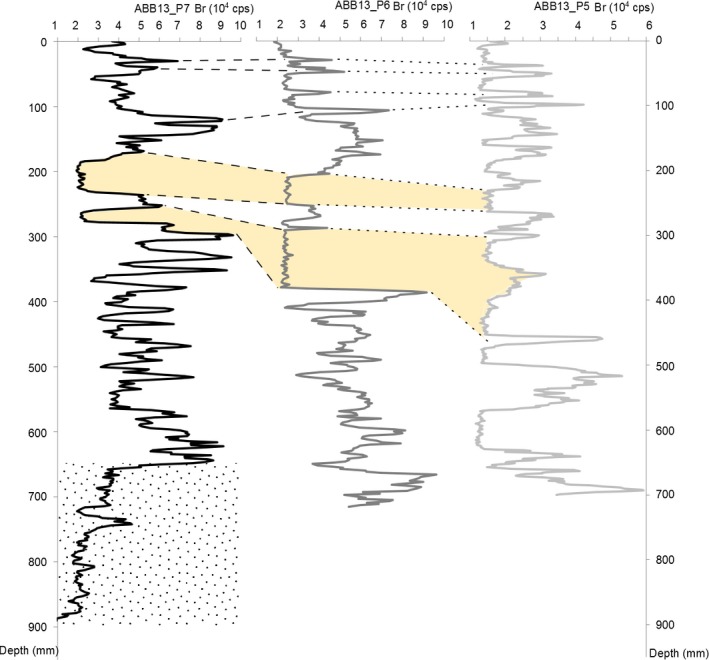
Core correlation using bromine concentration along the cores horizons. Horizontal axis corresponds to the counts per second (cps) recorded at 30 kV for bromine, while vertical axis corresponds to the depth in millimeter for each core. On ABB13_P7 core, the dotted/shaded zone corresponds to the bottom of the core where sediments were stirred. Yellow shaded areas mark the sand area spotted in the cores. Dashed lines are correlations between ABB13_P7 and ABB13_P6, while dotted lines show correspondence between ABB13_P6 and ABB13_P5
To distinguish between recent and older core horizons (Figure 3), we used both continuous time detection (particle diameter and 210Pb) and the detection of event markers (137Cs). The 210Pb signal usually follows a logarithmic decay in sediment. However, 210Pb tends to be washed away from sandy sediments, making this radionuclide difficult to interpret in our cores where different layers exhibit strong heterogeneity in sand content. Particle diameter is negatively correlated with bromine content (Figure 2). The signal for the two major sandstone horizons is visible with high‐diameter particles from 20 to 35 cm deep (labeled Sand 1 and Sand 2 in Figure 3). Another high‐diameter‐particle sandy horizon (labeled Sand 3 hereafter) was detected between 5 and 10 cm (Figure 3), matching with low bromine density in ABB13_P6 (Figure 2). 137Cs was detected in all layers, indicating that the entire time series was probably post‐1954. A maximum of 14.35 mBq/g was detected at 62 cm deep, signaling the fallout maximum of 1963. However, we were not able to detect the Chernobyl peak of 1986. Three possible reasons are as follows: (i) the scale of analysis was too coarse for such a singular event; (ii) the time series stops prior to 1986. In the first case, a finer timescale sampling might be realized especially between 10 and 20 cm depth, where the quantity of 137Cs shows a small increase; (iii) the too low activity of 137Cs in surface soil in this area (Sabatier et al., 2008).
Figure 3.
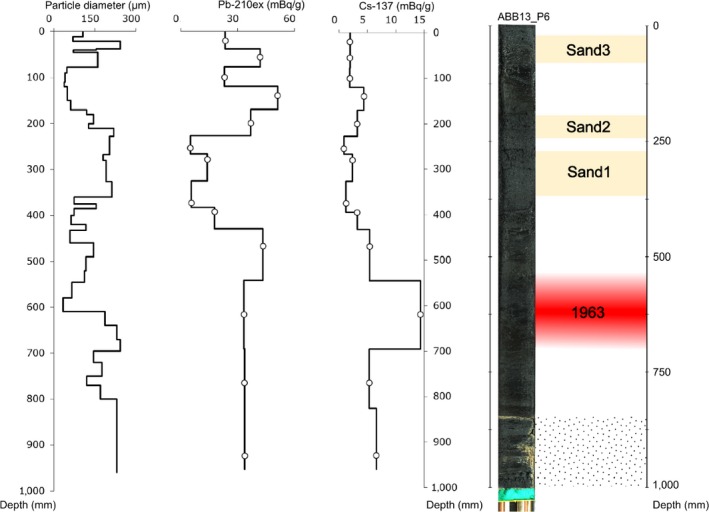
ABB13_P6 core dating. To distinguish between recent and older core horizons, we used both continuous time detection (particle diameter and 210Pb) and the detection of event markers (137Cs)
The three sediment cores were composed of successive layers of sand and organic matter that were more or less oxygenated. Three major sand layers (Sand 1–3 in Figure 3) were useful to correlate the cores. These sandy horizons might correspond to extreme meteorological events (wind storms, flood). Despite very good weather data in the area (Quillé, 2000), there are too many events that could correspond to these layers to be informative. However, the presence of Af cysts around 56 cm (introduced in 1970 in Aigues‐Mortes) shows coherence with the 137Cs peak at 62 cm, which is associated with the maximum radiation fallout of 1963–1964. These data confirmed that the gate closure was prior to this period. We were not able to detect the Chernobyl radiation peak of 1986 so we could not confirm whether the surface sediments were post‐1980s or not. Finally, cyst genotyping showed that most of the cores were post‐1970 (date of Af introduction in the saltern).
2.3. Using Artemia cysts from sediment cores
Our analysis of sediment cores in Aigues‐Mortes saltern and their cyst content revealed important conclusions for the use of Artemia in RE studies. First, it is possible to easily collect and purify cysts from sediments, in relatively large quantities. Second, in salterns, where many large Artemia populations are found worldwide, stratigraphic sampling is possible to provide a good knowledge of the sites. Third, core synchronization is possible, especially using the pattern of thick sandy horizons probably corresponding to extreme events (storms, floods). Fourth, despite a very high variability of the sediment grain size, dating is possible, especially combining an age control from 137Cs and the date of Af introduction in the saltern. Fifth, short DNA sequences can be readily obtained from cysts, even after several decades, which could be directly deep‐sequenced.
There are, however, also important shortcomings. First, hatchability of sedimented cysts is extremely low in sediments a few centimeters below surface [although we obtained some hatching in a similar core at the same site in a previous trial at 40 cm depth, unpublished results], which is a severe limitation. More intensive hatching tests may be required to determine if a small fraction of viable cysts can nevertheless be obtained for older samples, using tailored hatching protocols and perhaps using trials operated immediately after core extraction. Studying patterns of DNA repair in rehydrated cysts would also be particularly insightful to study this more precisely, as in Hespeels et al. (2014). Second, precise sediment dating may be difficult to obtain, due to the lack of a regular sedimentation pattern, as illustrated in our case study.
3. POSSIBLE APPLICATIONS OF ARTEMIA AS RE MODEL
Only a few animal species have been used as models to conduct RE studies (Derry, Arnott, & Boag, 2010; Franks et al., 2008; Hairston et al., 1999; Roy Chowdhury, 2016; Weider et al., 1997). Finding new models from different ecosystems is of key importance to make progress to fully exploit this research strategy. We discuss here how Artemia can be used for RE studies (see overview in Figure 4). First, hypersaline environments where Artemia occur are essential parts of the biosphere (Mohebbi, 2010). They are common in dry regions (one‐third of the earth's land surface) and are distributed worldwide. These extreme environments are diverse (chemical composition, temperature regime) and replicated, allowing for large‐scale studies and comparison of different environmental stresses at different spatial and temporal scales. These systems have a simplified food web and low diversity (compared with, e.g., much more complicated freshwater ecosystems), making them ideal to study ecological and evolutionary dynamics. They are often human‐managed, which implies that there are detailed long‐term records of abiotic (salinity, temperature, oxygen, etc.) and biotic factors (birds, invertebrates, and phytoplankton communities, among others) that can be used to compare environmental change with evolutionary and community changes. They are often exposed to anthropogenic disturbance (Amat et al., 2007; Gajardo & Beardmore, 2012; Muñoz, 2010), therefore providing “natural experiments” that are crucial to understand the dynamics of adaptation to changing environments in natura, and on a long timescale. Hence, the study of Artemia populations offers many opportunities to study ecological and (co)evolutionary change. In the following, we discuss five topics, with specific field examples involving Artemia that could be particularly fruitful, especially with a RE strategy.
Figure 4.
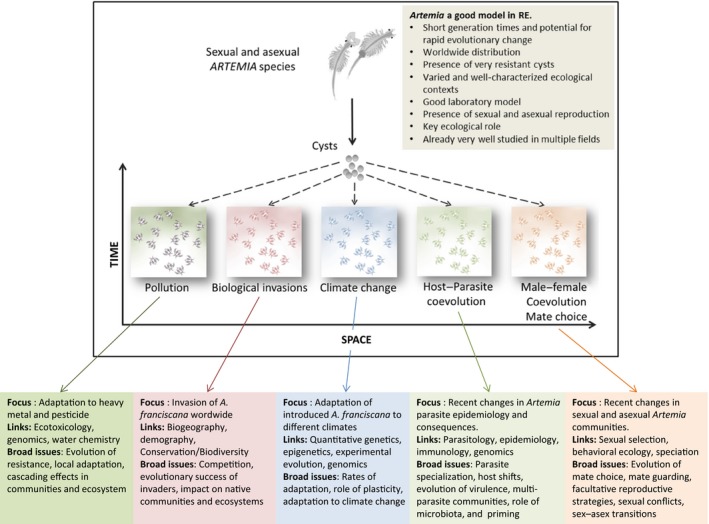
Artemia: a model system for resurrection ecology. Synopsis of key biological features and the main topics where the use of Artemia as a model system can contribute to important breakthroughs and can bridge gaps across fields
3.1. Biological invasions
One of the main components of anthropogenic global change is the introduction of invasive species (Vitousek, Antonio, Loope, & Westbrooks, 1996). Invasion and its multiple steps (Beard & Kulmatiski, 2012) are best studied with a historical perspective. As exemplified in the case of Aigues‐Mortes saltern mentioned above, the RE approach can be used to study these dynamics in detail. Hatching cysts from the past is particularly helpful to document postinvasion evolutionary changes in the invader and the invaded community. Because of their use in aquaculture (Sorgeloos, Dhert, & Candreva, 2001), cysts of the North American Af species have been introduced in many hypersaline systems, rapidly replacing native Artemia species worldwide (Amat et al., 2005, 2007). Af was introduced on to Pacific Islands and Brazil in the 1970s (Van Stappen, 2002) as well as in Europe (see above). It rapidly invaded the entire Mediterranean region (Amat et al., 2005, 2007; Green et al., 2005; Naceur, Jenhani, & Romdhane, 2010) and is now present in the Middle and Far East (Amat et al., 2007) and Australia (Ruebhart, Cock, & Shaw, 2008). This global invasion offers unique opportunities to study microevolutionary changes in Af (the invader) and various invaded communities (of Artemia and their associated parasites), a notoriously difficult issue (Mura et al., 2006; Suarez & Tsutsui, 2008). A RE approach would be particularly useful to measure fitness changes of the invader in different environments, as well as associated phenotypic and genomic changes. This information is not only relevant for fundamental reasons, but also because the understanding of the evolutionary processes that are involved in successful invasions can help to develop management strategies for their prevention or control (Sakai et al., 2001; Suarez & Tsutsui, 2008).
3.2. Global climate change
Artemia have been intentionally introduced in several parts of the world, for various economic reasons. Very often, Artemia were introduced to completely different biotic and abiotic conditions compared to their native habitats (e.g., in terms of temperature, water chemistry, predators, and parasites). Because these factors play a central role in the biology of Artemia (Van Stappen, 2002), they offer natural experiments to study adaptation in the field, especially when cyst time series are available. For example, Af from San Francisco Bay (California, USA) were introduced in new salterns in tropical areas (e.g., in Vietnam in 1982, Quynh & Lam, 1987). The climatic shift in these situations is close to +10°C for annual average temperature (Clegg, Jackson, Van Hoa, & Sorgeloos, 2000; Kappas, Abatzopoulos, Van Hoa, Sorgeloos, & Beardmore, 2004; Sebesvari, Le, & Renaud, 2011), which exceeds the most pessimistic climate change forecast for the 21st century (RCP8.5 Model predicts +6°C, IPCC 2013). These introductions can therefore be used to determine the extent to which species can tolerate and adapt to abrupt increases in temperature on a long‐term basis (hundreds of generations) and in the field. Temperature is a well‐known factor affecting the fitness of Artemia (Amat et al., 2007; Barata, Hontoria, Amat, & Browne, 1996; Browne & Wanigasekera, 2000). Clegg et al. (2000) showed that Af from Vietnam had greater thermal tolerance compared with individuals from San Francisco Bay and that these differences were transmitted to the following generations. These observations could be scaled up to measure rates of adaptation in a time series, as well as associated phenotypic and genotypic changes.
3.3. Global environmental pollution
Most studies in ecotoxicology focus on determining the effect of contaminants on different species, as well as on finding reliable biomarkers for environmental risk assessment. Artemia is extensively used in basic and applied aquatic toxicology (Persoone & Wells, 1987 for review), with a set of robust toxicity protocols (Artemia reference Center, ARC). Studying the evolutionary impact of man‐made change (various pollution, pesticides, heavy metal, etc.) has long been used to provide among the best detailed examples of adaptation (Bijlsma & Loeschcke, 2005; Bishop & Cook, 1981). However, a major limitation to these studies is that they require long‐term monitoring. Further, past or original populations are rarely available and adaptation is studied by comparing populations adapting to different habitats (“local adaptation,” Lenormand, 2002; Räsänen, Laurila, & Merilä, 2003). RE can be an extremely powerful strategy in this context. Several Artemia populations would be particularly interesting in that regard.
Artemia occur in two of the most metal‐polluted ecosystems in Europe, the Ria de Aveiro in Portugal, and the Odiel estuary in South Spain. Ria de Aveiro received a highly contaminated effluent discharged by a mercury cell chlor‐alkali plant from the 1950s until 1994 (Pereira et al., 2009). As a consequence, a total of 33 tons of mercury have accumulated in the wetland, with a significant amount of mercury being stored in sediments (Pereira, Duarte, Millward, Abreu, & Vale, 1998), and released in the saline water of the Ria. In the last decades, mercury discharge has been reduced and the quality of the water improved (Pereira et al., 2009) to reach levels that are today compatible with the EU environmental Directive 82/176/EEC 1982. This historical situation offers an interesting example where a RE approach could be developed to understand adaptation to realistic metal concentrations, as well as relaxation of selection (see also Piscia et al., 2012; for a similar approach). At the physiological and genetic levels, heavy metal‐induced detoxification mechanisms can be studied (Seebaugh & Wallace, 2004), such as those involving metal‐binding proteins (metallothioneins; it is a main metal detoxification strategy in Artemia and other aquatic organisms).
Another kind of pollution, widely occurring in Artemia habitats, is eutrophication. The Great Salt Lake (GSL) ecosystem provides an interesting situation to study biological responses to nutrient input with a RE approach. The lake receives high levels of industrial, urban, mining, and agricultural discharge. The construction of a railroad causeway in 1959 divided the lake into two water bodies, affecting the biogeochemistry and distribution of nutrients (Naftz et al., 2008). Reconstruction of changes in the sediment and water quality of GSL from the early 1700 to 1998 showed that the period from 1979 to 1998 was the most contaminated (Naftz et al., 2008). Comparative studies between the two parts of the lake and as well as during and before the most toxic period can increase our understanding of the evolutionary response of Artemia to nutrient pollution.
3.4. Host–parasite coevolution
Understanding host–parasite coevolutionary dynamics in the field is an important challenge. As we discussed above, many studies have used spatial data to infer patterns of host–parasite interactions, focusing on local adaptation (Greischar & Koskella, 2007). Studying host–parasite coevolution requires long‐term data sets, which is not always feasible. Artemia is the (intermediate) host of a very well‐characterized community of parasites and pathogens, including helminths with complex life cycles through avian final hosts (Georgiev et al., 2005; Vasileva et al., 2009), horizontally transmitted microsporidians (Martinez, Vivares, & Bouix, 1993; Martinez et al., 1992; Rode, Landes, et al., 2013; Rode, Lievens, et al., 2013; Rode, Lievens, Flaven, et al., 2013), yeasts (Codreanu & Codreanu‐Balcescu, 1981), viruses, and bacteria (Crab, Lambert, Defoirdt, Bossier, & Verstraete, 2010; Li, Zhang, Chen, & Yang, 2003; Soto‐Rodriguez, Roque, Lizarraga‐Partida, Guerra‐Flores, & Gomez‐Gil, 2003). Some of these parasites can produce resting stages (e.g., vibrio species which are found in sediments, Williams & LaRock, 1985) which may eventually be recovered from the sediments together with Artemia cysts. Many of these parasites strongly affect host fitness through castration, physiological and behavioral manipulation and increased predation (Amat, Gozalbo, Navarro, Hontoria, & Varó, 1991; Redón, Amat, Sánchez, & Green, 2015; Rode, Lievens, Flaven, et al., 2013; Sánchez, Hortas, Figuerola, & Green, 2009; Sánchez et al., 2012; Sánchez, Thomas, et al., 2009) and are expected to impose strong selective forces potentially leading to rapid evolution of defense mechanisms. So host and parasite populations from different times can be resurrected and used in time‐shift and cross‐infection experiments to provide important insight on long‐term patterns of coevolution in the field (Decaestecker et al., 2007). Furthermore, Artemia immunity (as measured, e.g., by phenoloxidase activity) could be tracked through time in response to the abundance of parasites. The latter usually correlates well to the density of final hosts (Sánchez et al., 2013), which is well documented. For instance, the Mediterranean population of flamingos (Phoenicopterus roseus) has exponentially increased over the last 30 years (Rendón, Green, Aquilera, & Almaraz 2008), and it is expected to be associated with a concomitant increase in the prevalence of the cestode Flamingolepis liguloides in Artemia (the most common avian cestode using Artemia as intermediate host and flamingos as final hosts). The introduction of Af in new regions, which is generally associated with the loss of parasites (Georgiev, Sánchez, Vasileva, Nikolov, & Green, 2007; Sánchez et al., 2012), could provide the opposite scenario with a “relaxed selection” for resistance.
3.5. Male–female coevolution and mate choice
The availability of cysts from different time periods can be used to follow other patterns of coevolution, such as between males and females. The long‐term consequence of sexual conflicts can be studied experimentally in the laboratory on model organisms such as Drosophila (Arnqvist & Rowe, 2005). They are, however, very difficult to study in the field. RE in bisexual Artemia species can be used for this purpose. For instance, Rode et al. (2011) studied male–female coevolution in Af, using a ~160‐generation (c.a. 23 years) time series. They found that females had better survival and longer interbrood intervals when mated with their contemporary males compared to when mated with males from their future or their past, demonstrating fast male–female coevolution in natura. Other evolutionary patterns of mating/reproductive traits could be fruitfully studied using RE in Artemia. In particular, female facultative sex‐ratio adjustment or male mate discrimination could be studied in parthenogenetic native populations recently invaded by Af (Lievens, Henriques, Michalakis, & Lenormand, 2016).
4. CONCLUSION
Artemia offers multiple avenues for RE research. New “omics” approaches (genomics, transcriptomics, proteomics, or metabolomics) and the development of Artemia as model system in genomics (De Vos, 2014) would add a new dimension to the use of Artemia in RE, notably to exploit the DNA record that can be obtained in sedimented cysts. Coupled with experimental evolution studies, it would provide resurrection ecologists with stronger insights into a large suite of fundamental questions (local adaptation, host–parasite coevolution, reproductive system evolution), as well as firmer predictions about the effects of global change on organisms, communities, and ecosystems.
DATA ARCHIVING STATEMENT
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.td0g3.
Supporting information
ACKNOWLEDGEMENTS
The authors are grateful to F. Gout (Camargue Pêche) for assistance in the sampling process and to D. Faca from the service des pêches of the Salin du Midi company (Aigues‐Mortes) who gave us major information to find a suitable sampling site. We also thank the Salins du Midi company who allowed us to sample in the Aigues‐Mortes saltern. We thank G. Martin, R. Calatayud who were involved in early core sampling in Aigues‐Mortes. We also wish to thank A.‐L. Develle, C. Pignol, and P. Sabatier from the EDYTEM platform (Université de Savoie, Grenoble, France) for their help in core analysis, and the Centre de Carottage et de Forage National (C2FN, INSU) for help in coring. We are grateful to M. Quillé (Météo Camargue, Grau du Roi) for letting us use the meteorological information he compiled on the Aigues‐Mortes saltern area for the last 70 years. Microsatellite data were partly produced through the technical facilities of the SMGE platform (M.P. Dubois; CEFE‐CNRS, Montpellier) and the PGE platform (F. Cerqueira; UM2, Labex‐CEMEB, Montpellier). We thank L. Weider for many suggestions on the manuscript. We thank Fundación BBVA and the Severo Ochoa Program for Centres of Excellence in R+D+I (SEV‐2012‐0262; Spanish Ministry of Economy and Competitiveness) for funding.
Lenormand T, Nougué O, Jabbour‐Zahab R, et al. Resurrection ecology in Artemia . Evol Appl. 2018;11:76–87. https://doi.org/10.1111/eva.12522
REFERENCES
- Abatzopoulos, T. J. , Kappas, I. , Bossier, P. , Sorgeloos, P. , & Beardmore, J. A. (2002). Genetic characterization of Artemia tibetiana (Crustacea: Anostraca). Biological Journal of the Linnean Society, 75, 333–344. [Google Scholar]
- Agh, N. , Abatzopoulos, T. J. , Kappas, I. , Van Stappen, G. , Razavi Rouhani, S. M. , & Sorgeloos, P. (2007). Coexistence of sexual and parthenogenetic Artemia populations in Lake Urmia and neighbouring lagoons. International Review of Hydrobiology, 92, 48–60. [Google Scholar]
- Amat, F. , Gozalbo, A. , Navarro, J. C. , Hontoria, F. , & Varó, I. (1991). Some aspects of Artemia biology affected by cestode parasitism. Hydrobiologia, 212, 39–44. [Google Scholar]
- Amat, F. , Hontoria, F. , Navarro, J. C. , Vieira, N. , & Mura, G. (2007). Biodiversity loss in the genus Artemia in the Western Mediterranean Region. Limnetica, 26, 387–404. [Google Scholar]
- Amat, F. , Hontoria, F. , Ruiz, O. , Green, A. J. , Sanchez, M. I. , Figuerola, J. , & Hortas, F. (2005). The American brine shrimp as an exotic invasive species in the western Mediterranean. Biological Invasions, 7, 37–47. [Google Scholar]
- Anderson, N. J. , & Battarbee, R. W. (1994). Aquatic community persistence and variability: a palaeolimnological perspective In Giller P. S., Hildrew A. G., & Raffeli D. (Eds.), Aquatic ecology: Scale, pattern and process (pp. 233–259). Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- Angeler, D. G. (2007). Resurrection ecology and global climate change research in freshwater ecosystems. Journal of the North American Benthological Society, 26, 12–22. [Google Scholar]
- Arnqvist, G. , & Rowe, L. (2005). Sexual conflicts (330 p.). Princeton, NJ: Princeton University Press. [Google Scholar]
- Barata, C. , Hontoria, F. , Amat, F. , & Browne, R. (1996). Competition between sexual and parthenogenetic Artemia: Temperature and strain effects. Journal of Experimental Marine Biology and Ecology, 196, 313–328. [Google Scholar]
- Baxevanis, A. D. , Kappas, I. , & Abatzopoulos, T. J. (2006). Molecular phylogenetics and asexuality in the brine shrimp Artemia. Molecular Phylogenetics and Evolution, 40, 724–738. [DOI] [PubMed] [Google Scholar]
- Beard, K. H. , & Kulmatiski, A. (2012). Introduction, establishment, and spread: 50 years of invasion ecology since Elton. Ecology, 2, 437–438. [Google Scholar]
- Bijlsma, R. , & Loeschcke, V. (2005). Environmental stress, adaptation and evolution: An overview. Journal of Evolutionary Biology, 18, 744–749. [DOI] [PubMed] [Google Scholar]
- Bishop, J. A. , & Cook, L. M. (1981). Genetic consequences of man‐made change. London, UK: Academic Press. [Google Scholar]
- Blanquart, F. , & Gandon, S. (2013). Time‐shift experiments and patterns of adaptation across time and space. Ecology Letters, 16, 31–38. [DOI] [PubMed] [Google Scholar]
- Bradshaw, W. , & Holzapfel, C. (2001). Genetic shift in photoperiodic response correlated with global warming. Proceedings Of the National Academy of Sciences, 98, 14509–14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendonck, L. (1996). Diapause, quiescence, hatching requirements: What we can learn from large freshwater branchiopods (Crustacea: Branchiopoda: Anostraca, Notostraca, Conchostraca) In Alekseev V. R. & Fryer G. (Eds.), Diapause in the Crustacea. Developments in hydrobiology 114 (pp. 85–97). Boston, MA: Kluwer Academic Publishers. [Google Scholar]
- Brendonck, L. , & De Meester, L. (2003). Egg banks in freshwater zooplankton: Evolutionary and ecological archives in the sediment. Hydrobiologia, 491, 65–84. [Google Scholar]
- Browne, R. A. , Davis, L. E. , & Sallee, S. E. (1988). Effects of temperature and relative fitness of sexual and asexual brine shrimp Artemia . Journal of Experimental Marine Biology and Ecology, 124, 1–20. [Google Scholar]
- Browne, R. A. , Sallee, S. E. , Grosch, D. S. , Segreti, W. O. , & Purser, S. M. (1984). Partitioning genetic and environmental components of reproduction and lifespan in Artemia . Ecology, 65, 949–960. [Google Scholar]
- Browne, R. A. , & Wanigasekera, G. (2000). Combined effects of salinity and temperature on survival and reproduction of five species of Artemia . Journal of Experimental Marine Biology and Ecology, 244, 29–44. [Google Scholar]
- Campos‐Ramos, R. , Maeda‐Martínez, A. M. , Obregón‐Barboza, H. , Murugan, G. , Guerrero‐Tortolero, D. A. , & Monsalvo‐Spencer, P. (2003). Mixture of parthenogenetic and zygogenetic brine shrimp Artemia (Branchiopoda: Anostraca) in commercial cyst lots from Great Salt Lake, UT, USA. Journal of Experimental Marine Biology and Ecology, 296, 243–251. [Google Scholar]
- Clegg, J. S. (1986). Artemia cysts as a model for the study of water in biological systems. Methods in Enzymology, 127, 230–239. [DOI] [PubMed] [Google Scholar]
- Clegg, J. S. (2001). Cryptobiosis—A peculiar state of biological organization. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 128, 613–624. [DOI] [PubMed] [Google Scholar]
- Clegg, J. S. (2005). Desiccation tolerance in encysted embryos of the animal extremophile, Artemia. Integrative and Comparative Biology, 45, 715–724. [DOI] [PubMed] [Google Scholar]
- Clegg, J. S. , & Conte, F. P. (1980). A review of the cellular and developmental biology of Artemia . The Brine Shrimp Artemia, 2, 11–54. [Google Scholar]
- Clegg, J. S. , & Jackson, S. A. (1997). Significance of cyst fragments of Artemia sp. recovered from a 27,000 year old core taken under the Great Salt Lake, Utah, USA. International Journal of Salt Lake Research, 6, 207–216. [Google Scholar]
- Clegg, J. S. , & Jackson, S. A. (1998). The metabolic status of quiescent and diapause embryos of Artemia franciscana (Kellogg). Ergebnisse der Limnologie, 52, 425–439. [Google Scholar]
- Clegg, J. S. , Jackson, S. A. , Van Hoa, N. , & Sorgeloos, P. (2000). Thermal resistance, developmental rate and heat shock proteins in Artemia franciscana, from San Francisco Bay and southern Vietnam. Journal of Experimental Marine Biology and Ecology, 252, 85–96. [DOI] [PubMed] [Google Scholar]
- Clegg, J. S. , & Trotman, C. N. (2002). Physiological and biochemical aspects of Artemia ecology In Abatzopoulos T. J., Beardmore J., Clegg J. S., & Sorgeloos P. (Eds.), Artemia: Basic and applied biology (pp. 129–170). Dordrecht, the Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Codreanu, R. , & Codreanu‐Balcescu, D. (1981). On two Metschnikowia yeast species producing hemocoelic infections in Daphnia magna and Artemia salina (Crustacea, Phyllopoda) from Romania. Journal of Invertebrate Pathology, 37, 22–27. [Google Scholar]
- Crab, R. , Lambert, A. , Defoirdt, T. , Bossier, P. , & Verstraete, W. (2010). The application of bioflocs technology to protect brine shrimp (Artemia franciscana) from pathogenic Vibrio harveyi . Journal of Applied Microbiology, 109, 1643–1649. [DOI] [PubMed] [Google Scholar]
- Criel, G. R. , & Macrae, T. H. (2002). Reproductive biology of Artemia In Abatzopoulos T. J., Beardmore J., Clegg J. S., & Sorgeloos P. (Eds.), Artemia: Basic and applied biology (pp. 39–128). Dordrecht, the Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Crowe, J. H. , Clegg, J. S. , & Crowe, L. E. (1998). Anhydrobiosis: The water replacement hypothesis In Reid D. S. (Ed.), The properties of water in foods ISOPOW 6 (pp. 440–455). New York, NY: Chapman and Hall. [Google Scholar]
- De Vos, S. (2014). Genomic tools and sex determination in the extremophile brine shrimp Artemia franciscana. Doctoral dissertation, Ghent University. [Google Scholar]
- Decaestecker, E. , Gaba, S. , Raeymaekers, J. A. , Stoks, R. , Van Kerckhoven, L. , Ebert, D. , & De Meester, L. (2007). Host–parasite ‘Red Queen’ dynamics archived in pond sediment. Nature, 450, 870–873. [DOI] [PubMed] [Google Scholar]
- Derry, A. M. , Arnott, S. E. , & Boag, P. T. (2010). Evolutionary shifts in copepod acid tolerance in an acid‐recovering lake indicated by resurrected resting eggs. Evolutionary Ecology, 24, 133–145. [Google Scholar]
- Directive 82/176/EEC (1982). Council Directive of 22 March 1982 on limit values and quality objectives for mercury discharges by the chlor‐alkali electrolysis industry. Official Journal of the European Communities, L0176 http://rod.eionet.europa.eu/instruments/619. [Google Scholar]
- Djamali, M. , Ponel, P. , Delille, T. , Thiéry, A. , Asem, A. , Andrieu‐Ponel, V. , … Stevens, L. (2010). A 200,000‐year record of the brine shrimp Artemia (Crustacea: Anostraca) remains in Lake Urmia, NW Iran. International Journal of Aquatic Science, 1, 14–18. [Google Scholar]
- Endebu, M. , Miah, F. , Boon, N. , Catania, F. , Bossier, P. , & Van Stappen, G. (2013). Historic occurrence of parthenogenetic Artemia in Great Salt Lake, USA, as demonstrated by molecular analysis of field samples. Journal of Great Lakes Research, 39, 47–55. [Google Scholar]
- Etterson, J. R. , Franks, S. J. , Mazer, S. J. , Shaw, R. G. , Gorden, N. L. S. , Schneider, H. E. , … Weis, A. E. (2016). Project Baseline: An unprecedented resource to study plant evolution across space and time. American Journal of Botany, 103, 164–173. [DOI] [PubMed] [Google Scholar]
- Etterson, J. R. , & Shaw, R. G. (2001). Constraint to adaptive evolution in response to global warming. Science, 294, 151–154. [DOI] [PubMed] [Google Scholar]
- Franks, S. J. , Avise, J. C. , Bradshaw, W. E. , Conner, J. K. , Etterson, J. R. , Mazer, S. J. , … Weis, A. E. (2008). The resurrection initiative: Storing ancestral genotypes to capture evolution in action. BioScience, 58, 870–873. [Google Scholar]
- Frickel, J. , Sieber, M. , & Becks, L. (2016). Eco‐evolutionary dynamics in a coevolving host–virus system. Ecology Letters, 19, 450–459. [DOI] [PubMed] [Google Scholar]
- Frisch, D. , Morton, P. K. , Chowdhury, P. R. , Culver, B. W. , Colbourne, J. K. , Weider, L. J. , & Jeyasingh, P. D. (2014). A millennial‐scale chronicle of evolutionary responses to cultural eutrophication in Daphnia. Ecology Letters, 17, 360–368. [DOI] [PubMed] [Google Scholar]
- Gaba, S. , & Ebert, D. (2009). Time‐shift experiments as a tool to study antagonistic coevolution. Trends in Ecology and Evolution, 24, 226–232. [DOI] [PubMed] [Google Scholar]
- Gajardo, G. , Abatzopoulos, T. J. , Kappas, I. , & Beardmore, J. A. (2002). Evolution and speciation In Abatzopoulos T. J., Beardmore J. A., Clegg J. S., & Sorgeloos P. (Eds.), Artemia: Basic and applied Biology (pp. 225–250). Dordrecht, the Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Gajardo, G. M. , & Beardmore, J. A. (2012). The brine shrimp Artemia: Adapted to critical life conditions. Frontiers in Physiology, 3, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev, B. B. , Sánchez, M. I. , Green, A. J. , Nikolov, P. N. , Vasileva, G. P. , & Mavrodieva, R. S. (2005). Cestodes from Artemia parthenogenetica (Crustacea, Branchiopoda) in the Odiel Marshes, Spain: A systematic survey. Acta Parasitologica, 50, 105–117. [Google Scholar]
- Georgiev, B. B. , Sánchez, M. I. , Vasileva, G. P. , Nikolov, P. N. , & Green, A. J. (2007). Cestode parasitism in invasive and native brine shrimps (Artemia spp.): Can it explain the rapid invasion of A. franciscana in the Mediterranean region? Parasitology Research, 101, 1647–1655. [DOI] [PubMed] [Google Scholar]
- Green, A. J. , Sanchez, M. I. , Amat, F. , Figuerola, J. , Hontaria, F. , Ruiz, O. , & Hortas, F. (2005). Dispersal of invasive and native brine shrimp Artemia (Anostraca) via waterbirds. Limnology and Oceanography, 50, 737–742. [Google Scholar]
- Gregory‐Eaves, I. , & Beisner, B. E. (2011). Palaeolimnological insights for biodiversity science: An emerging field. Freshwater Biology, 56, 2653–2661. [Google Scholar]
- Greischar, M. A. , & Koskella, B. (2007). A synthesis of experimental work on parasite local adaptation. Ecology Letters, 10, 418–434. [DOI] [PubMed] [Google Scholar]
- Hairston, N. G. (1996). Zooplankton egg banks as biotic reservoirs in changing environments. Limnology and Oceanography, 41, 1087–1092. [Google Scholar]
- Hairston, N. G. Jr , Ellner, S. , & Kearns, C. M. (1996). Overlapping generations: The storage effect and the maintenance of biotic diversity In Rhodes O. E., Jr, Chesser R. K., & Smith M. H. (eds.), Population dynamics in ecological space and time (pp. 109–145). Chicago, IL: University of Chicago Press. [Google Scholar]
- Hairston, N. G. , Lampert, W. , Cáceres, C. E. , Holtmeier, C. L. , Weider, L. J. , Gaedke, U. , … Post, D. M. (1999). Lake ecosystems: Rapid evolution revealed by dormant eggs. Nature, 401, 446. [Google Scholar]
- Hand, S. C. , Denlinger, D. L. , Podrabsky, J. E. , & Roy, R. (2016). Mechanisms of animal diapause: Recent developments from nematodes, crustaceans, insects, and fish. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 310, R1193–R1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand, S. C. , & Podrabsky, J. E. (2000). Bioenergetics of diapause and quiescence in aquatic animals. Thermochimica Acta, 349, 31–42. [Google Scholar]
- Hespeels, B. , Knapen, M. , Hanot‐Mambres, D. , Heuskin, A.‐C. , Pineux, F. , Lucas, S. , … van Doninck, K. (2014). Gateway to genetic exchange? DNA double‐strand breaks in the bdelloid rotifer Adineta vaga submitted to desiccation. Journal of Evolutionary Biology, 27, 1334–1345. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. , & Sgro, C. M. (2011). Climate change and evolutionary adaptation. Nature, 470, 479–485. [DOI] [PubMed] [Google Scholar]
- IPCC (2013). Summary for policymakers In Stocker T. F., Qin D., Plattner G.‐K., Tignor M., Allen S. K., Boschung J., … Midgley P. M. (Eds.), Climate change 2013: The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY: Cambridge University Press. [Google Scholar]
- Jensen, A. C. (1918). Some observations on Artemia gracilis, the brine shrimp of Great Salt Lake. Biological Bulletin, 34, 18–23. [Google Scholar]
- Kappas, I. , Abatzopoulos, T. J. , Van Hoa, N. , Sorgeloos, P. , & Beardmore, J. A. (2004). Genetic and reproductive differentiation of Artemia franciscana in a new environment. Marine Biology, 146, 103–117. [Google Scholar]
- Kerfoot, W. C. , Ma, X. , Lorence, C. S. , & Weider, L. J. (2004). Toward resurrection ecology: Daphnia mendotae and D. retrocurva in the coastal region of Lake Superior, among the first successful outside invaders? Journal of Great Lakes Research, 30, 285–299. [Google Scholar]
- Kerfoot, W. C. , Robbins, J. A. , & Weider, L. J. (1999). A new approach to historical reconstruction: Combining descriptive and experimental paleolimnology. Limnology and Oceanography, 44, 1232–1247. [Google Scholar]
- Kerfoot, W. C. , & Weider, L. J. (2004). Experimental paleoecology (resurrection ecology): Chasing Van Valen's Red Queen hypothesis. Limnology and Oceanography, 49(4 Part 2), 1300–1316. [Google Scholar]
- Lenormand, T. (2002). Gene flow and the limits to natural selection. Trends in Ecology and Evolution, 17, 183–189. [Google Scholar]
- Lenz, P. H. , & Browne, R. A. (1991). Ecology of Artemia In Browne R. A., Sorgeloos P., & Trotman C. N. A. (Eds.), Artemia biology (10, pp. 237–253). Boston, MA, CRC Press. [Google Scholar]
- Li, Q. , Zhang, J. , Chen, Y. , & Yang, F. (2003). White spot syndrome virus (WSSV) infectivity for Artemia at different developmental stages. Diseases of Aquatic Organisms, 57, 261–264. [DOI] [PubMed] [Google Scholar]
- Liang, P. , & MacRae, T. H. (1999). The synthesis of a small heat shock/α‐crystallin protein in Artemia and its relationship to stress tolerance during development. Developmental Biology, 207, 445–456. [DOI] [PubMed] [Google Scholar]
- Lievens, E. J. , Henriques, G. J. , Michalakis, Y. , & Lenormand, T. (2016). Maladaptive sex ratio adjustment in the invasive brine shrimp Artemia franciscana . Current Biology, 26, 1463–1467. [DOI] [PubMed] [Google Scholar]
- Manaffar, R. , Zare, S. , Agh, N. , Siyabgodsi, A. , Soltanian, S. , Mees, F. , … Van Stappen, G. (2011). Sediment cores from Lake Urmia (Iran) suggest the inhabitation by parthenogenetic Artemia around 5,000 years ago. Hydrobiologia, 671, 65–74. [Google Scholar]
- Martinez, M. A. , Vivares, C. P. , & Bouix, G. (1993). Ultrastructural study of Endoreticulatus durforti n. sp., a new microsporidian parasite of the intestinal epithelium of Artemia (Crustacea, Anostraca). Journal of Eukaryotic Microbiology, 40, 677–687. [Google Scholar]
- Martinez, M. A. , Vivares, C. P. , de Medeiros Rocha, R. , Fonseca, A. C. , Andral, B. , & Bouix, G. (1992). Microsporidiosis on Artemia (Crustacea, Anostraca): Light and electron microscopy of Vavraia anostraca sp. nov. (Microsporidia, Pleistophoridae) in the Brazilian solar salterns. Aquaculture, 107, 229–237. [Google Scholar]
- Miner, B. E. , De Meester, L. , Pfrender, M. E. , Lampert, W. , & Hairston, N. G. (2012). Linking genes to communities and ecosystems: Daphnia as an ecogenomic model. Proceedings of the Royal Society of London B: Biological Sciences, 279, 1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebbi, F. (2010). The Brine Shrimp Artemia and hypersaline environments microalgal composition: A mutual interaction. International Journal of Aquatic Science, 1, 19–27. [Google Scholar]
- Muñoz, J. (2010). Diversity and distribution of diapausing aquatic invertebrates in inland wetlands: An ecosystem conservation viewpoint. Journal for Nature Conservation, 18, 55–62. [Google Scholar]
- Muñoz, J. , Gómez, A. , Green, A. J. , Figuerola, J. , Amat, F. , & Rico, C. (2008). Phylogeography and local endemism of the native Mediterranean brine shrimp Artemia salina (Branchiopoda: Anostraca). Molecular Ecology, 17, 3160–3177. [DOI] [PubMed] [Google Scholar]
- Mura, G. , Kappas, I. , Baxevanis, A. D. , Moscatello, S. , D'Amico, Q. , Lopez, G. M. , … Abatzopoulos, T. J. (2006). Morphological and molecular data reveal the presence of the invasive Artemia franciscana in Margherita di Savoia salterns (Italy). International Review of Hydrobiology, 91, 539–554. [Google Scholar]
- Naceur, H. B. , Jenhani, A. B. R. , & Romdhane, M. S. (2010). Biological characterization of the new invasive brine shrimp Artemia franciscana in Tunisia: Sabkhet Halk El‐Menzel. World Academy of Science, Engineering and Technology, International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering, 4, 107–113. [Google Scholar]
- Naftz, D. , Angeroth, C. , Kenney, T. , Waddell, B. , Darnall, N. , Silva, S. , … Whitehead, J. (2008). Anthropogenic influences on the input and biogeochemical cycling of nutrients and mercury in Great Salt Lake, Utah, USA. Applied Geochemistry, 23, 1731–1744. [Google Scholar]
- Nambu, Z. , Tanaka, S. , & Nambu, F. (2004). Influence of photoperiod and temperature on reproductive mode in the brine shrimp, Artemia franciscana . Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 301, 542–546. [DOI] [PubMed] [Google Scholar]
- Nielson, K. E. , & Bowen, G. J. (2010). Hydrogen and oxygen in brine shrimp chitin reflect environmental water and dietary isotopic composition. Geochimica et Cosmochimica Acta, 74, 1812–1822. [Google Scholar]
- Nougué, O. , Rode, N. O. , Jabbour‐Zahab, R. , Ségard, A. , Chevin, L.‐M. , Haag, C. R. , & Lenormand, T. (2015). Automixis in Artemia: Solving a century‐old controversy. Journal of Evolutionary Biology, 28, 2337–2348. [DOI] [PubMed] [Google Scholar]
- Orsini, L. , Schwenk, K. , De Meester, L. , Colbourne, J. K. , Pfrender, M. E. , & Weider, L. J. (2013). The evolutionary time machine: Using dormant propagules to forecast how populations can adapt to changing environments. Trends in Ecology & Evolution, 28, 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini, L. , Spanier, K. I. , & De Meester, L. U. C. (2012). Genomic signature of natural and anthropogenic stress in wild populations of the waterflea Daphnia magna: Validation in space, time and experimental evolution. Molecular Ecology, 21, 2160–2175. [DOI] [PubMed] [Google Scholar]
- Pereira, M. E. , Duarte, A. C. , Millward, G. E. , Abreu, S. N. , & Vale, C. (1998). An estimation of industrial mercury stored in sediments of a confined area of the Lagoon of Aveiro (Portugal). Water Science and Technology, 37, 125–130. [Google Scholar]
- Pereira, M. E. , Lillebø, A. I. , Pato, P. , Válega, M. , Coelho, J. P. , Lopes, C. B. , … Duarte, A. C. (2009). Mercury pollution in Ria de Aveiro (Portugal): A review of the system assessment. Environmental Monitoring and Assessment, 155, 39–49. [DOI] [PubMed] [Google Scholar]
- Persoone, G. , & Wells, P. G. (1987). Artemia in aquatic toxicology: A review. Artemia Research and its Applications, 1, 259–275. [Google Scholar]
- Pickett, S. T. A. (1989). Space‐for‐time substitution as an alternative to long‐term studies In Likens G. (Ed.), Long‐term studies in ecology (pp. 110–135). New York, NY: Springer. [Google Scholar]
- Piscia, R. , Guilizzoni, P. , Fontaneto, D. , Vignati, D. A. L. , Appleby, P. G. , & Manca, M. (2012). Dynamics of rotifer and cladoceran resting stages during copper pollution and recovery in a subalpine lake. International Journal of Limnology, 48, 151–160. [Google Scholar]
- Pulido, F. , & Berthold, P. (2010). Current selection for lower migratory activity will drive the evolution of residency in a migratory bird population. Proceeding of the National Academy of Sciences of the United States of America, 107, 7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillé, M. (2000). Météo Camargue. Retrieved from www.meteo-camargue.sup.fr
- Quynh, V. D. , & Lam, N. N. (1987). Inoculation of Artemia in experimental ponds in central Vietnam: An ecological approach and a comparison of three geographical strains. Artemia Research and its Applications, 3, 253–269. [Google Scholar]
- Räsänen, K. , Laurila, A. , & Merilä, J. (2003). Geographic variation in acid stress tolerance of the moor frog, Rana arvalis. I. Local adaptation. Evolution, 57, 352–362. [PubMed] [Google Scholar]
- Redón, S. , Amat, F. , Sánchez, M. I. , & Green, A. J. (2015). Comparing cestode infections and their consequences for host fitness in two sexual branchiopods: Alien Artemia franciscana and native A. salina from syntopic‐populations. PeerJ, 3, e1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendón, M. A. , Green, A. J. , Aquilera, E. , & Almaraz, P. (2008). Status, distribution and long‐term changes in the waterbird community wintering in Doñana, South‐West Spain. Biological Conservation, 141, 1371–1388. [Google Scholar]
- Rode, N. O. , Charmantier, A. , & Lenormand, T. (2011). Male–female coevolution in the wild: Evidence from a time series in Artemia franciscana . Evolution, 65, 2881–2892. [DOI] [PubMed] [Google Scholar]
- Rode, N. O. , Landes, J. , Lievens, E. J. , Flaven, E. , Segard, A. , Jabbour‐Zahab, R. , … Lenormand, T. (2013). Cytological, molecular and life cycle characterization of Anostracospora rigaudi ng, n. sp. and Enterocytospora artemiae ng, n. sp., two new microsporidian parasites infecting gut tissues of the brine shrimp Artemia . Parasitology, 140, 1168. [DOI] [PubMed] [Google Scholar]
- Rode, N. O. , Lievens, E. J. , Elodie, F. , Segard, A. , Jabbour‐Zahab, R. , & Lenormand, T. (2013). Cryptic microsporidian parasites differentially affect invasive and native Artemia spp. International Journal for Parasitology, 43, 795–803. [DOI] [PubMed] [Google Scholar]
- Rode, N. O. , Lievens, E. J. P. , Flaven, E. , Segard, A. , Jabbour‐Zahab, R. , Sanchez, M. I. , & Lenormand, T. (2013). Why join groups? Lessons from parasite‐manipulated Artemia . Ecology Letters, 16, 493–501. [DOI] [PubMed] [Google Scholar]
- Roy Chowdhury, P. (2016). Differences in phosphorus use between ancient and extant Daphnia genotypes alters algal stoichiometry and abundance. Inland Waters, 6, 165–172. [Google Scholar]
- Ruebhart, D. R. , Cock, I. E. , & Shaw, G. R. (2008). Invasive character of the brine shrimp Artemia franciscana Kellogg 1906 (Branchiopoda: Anostraca) and its potential impact on Australian inland hypersaline waters. Marine and Freshwater Research, 59, 587–595. [Google Scholar]
- Sabatier, P. , Dezileau, L. , Condomines, M. , Briqueu, L. , Colin, C. , Bouchette, F. , … Blanchemanche, P. (2008). Reconstruction of paleostorm events in a coastal lagoon (Herault, South of France). Marine Geology, 251, 224–232. [Google Scholar]
- Sakai, A. K. , Allendorf, F. W. , Holt, J. S. , Lodge, D. M. , Molofsky, J. , With, K. A. , … Weller, S. G. (2001). The population biology of invasive species. Annual Review of Ecology and Systematics, 32, 305–332. [Google Scholar]
- Sánchez, M. I. , Green, A. J. , & Castellanos, E. M. (2006). Temporal and spatial variation of an aquatic invertebrate community subjected to avian predation at the Odiel salt pans (SW Spain). Archive für Hydrobiologie, 166, 199–223. [Google Scholar]
- Sánchez, M. I. , Hortas, F. , Figuerola, J. , & Green, A. J. (2009). Sandpipers select red brine shrimps rich in carotenoids but also parasites. Ethology, 115, 196–200. [Google Scholar]
- Sánchez, M. I. , Nikolov, P. N. , Georgieva, D. D. , Georgiev, B. B. , Vasileva, G. , Pankov, P. , … Green, A. J. (2013). High prevalence of cestode parasites throughout the annual cycle of Artemia salina and A. parthenogenetica in coastal Spain: Relationship with abundance of avian final hosts. Parasitology Research, 112, 1913–1923. [DOI] [PubMed] [Google Scholar]
- Sánchez, M. I. , Paredes, I. , Lebouvier, M. , & Green, A. J. (2016). Functional role of native and invasive filter‐feeders, and the effect of parasites: Learning from hypersaline ecosystems. PLoS ONE, 11, e0161478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez, M. I. , Rode, N. O. , Flaven, E. , Redón, S. , Amat, F. , Vasileva, G. P. , & Lenormand, T. (2012). Differential susceptibility to parasites of invasive and native species of Artemia living in sympatry: Consequences for the invasion of A. franciscana in the Mediterranean Region. Biological Invasions, 14, 1819–1829. [Google Scholar]
- Sánchez, M. I. , Thomas, F. , Perrot‐Minnot, M. J. , Biron, D. G. , Bertrand‐Michel, J. , & Missé, D. (2009). Neurological and physiological disorders in Artemia harboring manipulative cestodes. Journal of Parasitology, 95, 20–24. [DOI] [PubMed] [Google Scholar]
- Sebesvari, Z. , Le, T. T. H. , & Renaud, F. G. (2011). Climate change adaptation and agrochemicals in the Mekong Delta, Vietnam In Stewart M. A. & Coclanis P. A. (Eds.), Environmental change and agricultural sustainability in the Mekong Delta (pp. 219–239). Dordrecht, the Netherlands: Springer. [Google Scholar]
- Seebaugh, D. R. , & Wallace, W. G. (2004). Importance of metal‐binding proteins in the partitioning of Cd and Zn as trophically available metal (TAM) in the brine shrimp Artemia franciscana . Marine Ecology Progress Series, 272, 215–230. [Google Scholar]
- Shirdhankar, M. M. , Thomas, P. C. , & Barve, S. K. (2004). Phenotypic estimates and heritability values of Artemia franciscana . Aquaculture Research, 35, 35–39. [Google Scholar]
- Sorgeloos, P. (1980). The use of the brine shrimp Artemia in aquaculture. The Brine Shrimp Artemia, 3, 25–46. [Google Scholar]
- Sorgeloos, P. , Dhert, P. , & Candreva, P. (2001). Use of the brine shrimp, Artemia spp., in marine fish larviculture. Aquaculture, 200, 147–159. [Google Scholar]
- Sorgeloos, P. , Lavens, P. , Leger, P. , & Tackaert, W. (1990). Activities of the laboratory of aquaculture & artemia reference center. Biologish Jaarboek Dodonaea, 58, 35–38. [Google Scholar]
- Sorgeloos, P. , Persoone, G. , Baeza‐Mesa, M. , Bossuyt, E. , & Bruggeman, E. (1978). The use of Artemia cysts in aquaculture: The concept of “hatching efficiency” and description of a new method for cyst processing. Journal of the World Aquaculture Society, 9, 715–721. [Google Scholar]
- Soto‐Rodriguez, S. A. , Roque, A. , Lizarraga‐Partida, M. L. , Guerra‐Flores, A. L. , & Gomez‐Gil, B. (2003). Virulence of luminous vibrios to Artemia franciscana nauplii. Diseases of Aquatic Organisms, 53, 231–240. [DOI] [PubMed] [Google Scholar]
- Suarez, A. V. , & Tsutsui, N. D. (2008). The evolutionary consequences of biological invasions. Molecular Ecology, 17, 351–360. [DOI] [PubMed] [Google Scholar]
- Tanguay, J. A. , Reyes, R. C. , & Clegg, J. S. (2004). Habitat diversity and adaptation to environmental stress in encysted embryos of the crustacean Artemia. Journal of Biosciences, 29, 489–501. [DOI] [PubMed] [Google Scholar]
- Thrall, P. , Laine, A. , Ravensdale, M. , Nemri, A. , Dodds, P. , Barrett, L. , & Burdon, J. J. (2012). Rapid genetic change underpins antagonistic coevolution in a natural host‐pathogen metapopulation. Ecology Letters, 15, 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stappen, G. (2002). Zoogeography In Abatzopoulos T. J., Beardmore J. A., Cleeg J. S., & Sorgeloos P. (Eds.), Artemia basic and applied biology (pp. 171–215). Dordrecht, the Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Vandekerkhove, J. , Declerck, S. , Brendonck, L. , Conde‐Porcuna, J. , Jeppesen, E. , & Meester, L. D. (2005). Hatching of cladoceran resting eggs: Temperature and photoperiod. Freshwater Biology, 50, 96–104. [Google Scholar]
- Vasileva, G. P. , Redón, S. , Amat, F. , Nikolov, P. N. , Sánchez, M. I. , Lenormand, T. , & Georgiev, B. B. (2009). Records of cysticercoids of Fimbriarioides tadornae (Maksimova, 1976) and Branchiopodataenia gvozdevi (Maksimova, 1988) (Cyclophyllidea: Hymenolepididae) from brine shrimps at the Mediterranean coasts of Spain and France, with a key to cestodes from Artemia spp. from the Western Mediterranean. Acta Parasitologica, 54, 143–150. [Google Scholar]
- Venable, D. L. , & Lawlor, L. (1980). Delayed germination and dispersal in desert annuals: Escape in space and time. Oecologia, 46, 272–282. [DOI] [PubMed] [Google Scholar]
- Vitousek, P. M. , Antonio, C. M. , Loope, L. L. , & Westbrooks, R. (1996). Biological invasions as global environmental change. American Scientist, 84, 468–478. [Google Scholar]
- Warner A. (Ed.). (1989). Cell and molecular biology of Artemia development Nato Science Series A: Book 174. New York: Springer. 453 pp. [Google Scholar]
- Warner, A. H. , Brunet, R. T. , MacRae, T. H. , & Clegg, J. S. (2004). Artemin is an RNA‐binding protein with high thermal stability and potential RNA chaperone activity. Archives of Biochemistry and Biophysics, 424, 189–200. [DOI] [PubMed] [Google Scholar]
- Weider, L. J. , Lampert, W. , Wessels, M. , Colbourne, J. K. , & Limburg, P. (1997). Long‐term genetic shifts in a microcrustacean egg bank associated with anthropogenic changes in the Lake Constance ecosystem. Proceedings of the Royal Society B, 264, 1613–1618. [Google Scholar]
- Wiggins, G. B. , Mackay, R. J. , & Smith, I. M. (1980). Evolutionary and ecological strategies of animals in annual temporary pools. Archiv für Hydrobiologie Supplement, 58, 97–206. [Google Scholar]
- Williams, L. A. , & LaRock, P. A. (1985). Temporal occurrence of Vibrio species and Aeromonas hydrophila in estuarine sediments. Applied and Environmental Microbiology, 50, 1490–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


