Abstract
Background: In recent years, the morbidity of Mycoplasma pneumoniae pneumonia (MPP) has increased significantly in China. A growing number of studies indicate that imbalanced respiratory microbiota is associated with various respiratory diseases. Methods: We enrolled 119 children, including 60 pneumonia patients and 59 healthy children. Nasopharyngeal (NP) and oropharyngeal (OP) sampling was performed for 16S ribosomal RNA (16S rRNA) gene analysis of all children. Sputum and OP swabs were obtained from patients for pathogen detection. Results: Both the NP and OP microbiota of patients differ significantly from that of healthy children. Diseased children harbor lower microbial diversity and a simpler co-occurrence network in NP and OP. In pneumonia patients, NP and OP microbiota showed greater similarities between each other, suggesting transmission of NP microbiota to the OP. Aside from clinically detected pathogens, NP and OP microbiota analysis has also identified possible pathogens in seven cases with unknown infections. Conclusion: NP and OP microbiota in MPP and non-MPP are definitely similar. Respiratory infection generates imbalanced NP microbiota, which has the potential to transmit to OP. Microbiota analysis also promises to compliment the present means of detecting respiratory pathogens.
Keywords: 16S rDNA, microbiota, Mycoplasma pneumoniae, nasopharyngeal, oropharyngeal
1. Introduction
Various pathogens, such as M. pneumoniae, Streptococcus pneumoniae, and respiratory syncytial virus (RSV), are common infectious agents of pneumonia, which is one of the principal causes of childhood morbidity and mortality [1,2,3,4]. Generalized clinical detection of pathogens was primarily based on bronchoalveolar lavage fluid (BALF) sampling, which was confined to pneumonia with severe diseases [5] and easily contaminated by respiratory flora in upper respiratory tracts (URTs) [6]. In addition, culturing and nucleic acid detection of specific pathogens make it difficult to discern infection within the carriage [7,8,9], given the asymptomatically colonized pathogens.
Numerous reports demonstrated an imbalance of microbial flora in pneumonia patients who harbored lower diversity and richness of microbiota in URTs [2,5]. Moreover, this imbalanced respiratory microbiota pattern varied with infectious agents [10]. For instance, the Moraxella lacunata-dominated microbiota structure was identified in viral pneumonia [2], while the S. pneumoniae, Haemophilus influenzae complex or Moraxella catarrhalis dominated in the nasopharyngeal (NP) microbiota of bacterial pneumonia. Furthermore, the clustering of URTs microbiota in patients with pneumonia or bronchiolitis held the potential to predict disease severity, as a previous study demonstrated that the high relative abundance of either lactobacilli, Rothia or S. pneumoniae, was related to a high pneumonia severity index (PSI) [5].
Previous research indicated different NP and oropharyngeal (OP) microbiota between healthy and diseased children [11]. A two-year prospective study suggested that the transmission of imbalanced microbiota between the NP and OP of diseased children held the potential to further trigger lung infection [12]. Over the past few years, M. pneumoniae was a frequent cause of community-acquired pneumonia (CAP) and resulted in a high morbidity ratio in China [4]. In this study, we would like to answer two questions: (1) How does the NP and OP microbiota, and the microbiota transmission in URTs, of Chinese children with pneumonia differ from that of healthy children? (2) Are Mycoplasma pneumoniae pneumonia (MPP) and non-MPP infections associated with different NP and OP microbiota alterations?
2. Materials and Methods
2.1. Ethical Approval
This study was approved by the Ethical Committee of Shenzhen Children’s Hospital with registration number 2016013. All the guardians of recruited children provided their informed consent. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee, as well as the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2.2. Diagnosis, Sampling and Pathogen Examination
Pneumonia was diagnosed through characteristic chest radiographic abnormalities that were consistent with pneumonia (Table 1). Patients were selected based on following criteria: no asthma; no respiratory infection; and no exposure to antibiotics for 1 month before sampling. Age-matched, healthy children were recruited after physical examination in Shenzhen Children’s Hospital according to the following criteria: no asthma or family history of allergy; no history of pneumonia; no wheezing, fever, cough, or other respiratory/allergic symptoms at sampling 1 month prior to the study; no antibiotic exposure for 1 month before the study; no disease symptoms within 1-week after sampling. NP (25-800-A-50, Puritan, Guilford, NC, USA) and OP (155C, COPAN, Murrieta, CA, USA) swabs were collectively frozen at −80 °C within 10 min of sampling. Unused swabs and opened swabs, which were shook in the air for 10 s, were selected as negative controls.
Table 1.
Sample information.
| Healthy Children (n = 59) | Pneumonia Patients (n = 60) | |
|---|---|---|
| Characteristics | ||
| Gender | ||
| Female | 33 | 19 |
| Male | 26 | 41 |
| Age (years) | 2.8 (0.1–9.9) | 2.8 (0.2–12.7) |
| Delivery Mode | ||
| Caesarean section | 20 | 22 |
| Vaginally born | 39 | 38 |
| Feed Pattern | ||
| Breast feed | 18 | 40 |
| Breast feed + Milk feed | 31 | 6 |
| Milk feed | 10 | 14 |
| Family history of allergy | - | 1 |
| History of pneumonia | - | 12 |
| Asthma | - | - |
| Clinical records | ||
| Lung consolidation, atelectasis, infiltration | NA | 60 |
| Hospitalization time (days) | - | 9 (2–37) |
| Fever | - | 26 |
| Cough | - | 57 |
| Wheezing | - | 16 |
| CRP (<0.499 mg/L) | NA | 21 |
| PCT (<0.5 ng/mL) | NA | 60 |
| Eosinophil (0.5–5%) | NA | 33 |
“-” represents no detected; “NA” represents not available; CRP, C-response protein; PCT, Procalcitonin.
Sputum was used for bacterial culturing, which was conducted to detect Acinetobacter baumannii, H. influenzae, Haemophilus parainfluenzae, M. catarrhalis, Staphylococcus aureus, Staphylococcus haemolyticus, and S. pneumoniae [13]. OP swabs were used to conduct nucleic acid testing (NAT). Adenovirus (AdV) and RSV were detected by a D3 Ultra DFA Respiratory Virus Screening & ID Kit (Diagnostic hybirds, Inc., Athens, OH, USA), while Epstein-bar virus (EBV), Cytomegalovirus (CMV), and Influenza A (H1N1) were detected by a EBV PCR Fluorescence quantitative diagnostic Kit, a Diagnostic Kit for Quantification of Human CMV DNA (PCR-fluorescence), and a Diagnostic kit for H1N1 RNA (PCR-fluorescence probing) (DaAnGene, Guangzhou, China, http://daan.joomcn.com/), respectively. M. pneumoniae and Chlamydia pneumoniae were diagnosed through a Diagnostic Kit for M. pneumoniae DNA (PCR-fluorescence probing) (DaAnGene, Guangzhou, China) and Anti C. pneumoniae ELISA (IgM) (EUROIMMUN AG, Lübeck, Germany). The Group A Streptococcus (GAS) detection was performed by Binax NOW® Strep A Card (Alere Scarborough, Inc., Scarborough, ME, USA).
2.3. DNA Extraction, Library Construction, and Sequencing
The genomic DNA of microbiota was extracted from swab samples using the Power Soil DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA, USA). The V3-V4 region of the 16S rRNA gene [14] was amplified using a PCR kit (TransGenAP221-02, Beijing, China) and quantified by Qubit (Thermo Fisher, Singapore). The qualified libraries were then sequenced by Illumina MiSeq sequencing platform (Illumina, San Diego, CA, USA) [15]. All data have been deposited in GenBank under accession number: SRP090593 (healthy children) and SRP109319 (children with pneumonia).
2.4. Bioinformatics Analysis and Visualization
Data filtration, taxonomic classification, and diversity calculation were conducted following our previous study [16]. PERMANOVA was applied to evaluate the confounding factors of microbiota composition and species-level classification of common pathogens referred to as the Live Tree Project (LTP) [17,18]. The inter-group comparison was conducted by the Wilcoxon rank-sum test, and the significance of multiple comparisons was adjusted by FDR. The distance between NP and OP microbiota was calculated by Bray-Curtis dissimilarity. The correlation of components with relative abundance—≥0.1% in microbiota—was assessed by Spearman’s rank correlation coefficient, with parameter coefficient ≥0.6 and p-value ≤0.05. Data visualization was produced by package ‘ggplot2’ of R software (v3.2.3) and Cytoscape (v3.4.0).
3. Results
3.1. Sample Information, Data Output, and Confounder Analysis
In this study, we enrolled 119 children, including 60 pneumonia inpatients and 59 age-matched healthy children (Table 1). According to the clinical detection, 29 cases were infected with M. pneumoniae, while 31 were diagnosed as infected by other or unknown pathogens (Table 1). None of the inpatients were admitted to the pediatric intensive care unit (PICU) or given mechanical ventilation during hospitalization. Most of inpatients remained in the hospital for less than 3 weeks but with one exception: P-33, who suffered the longest hospitalization time (37 days) (Table S1).
A total of 9,141,686 high-quality tags were produced, averaging 37,986 (22,208~46,854), 28,669 (15,288~41,626), 42,972 (25,465~47,409), and 43,844 (29,453~47,473) for the NP-health (NP-H), OP-health (OP-H), NP-pneumonia (NP-P), and OP-pneumonia (OP-P) group, respectively. Association analysis indicates that pneumonia onset is the most significant factor (p-value < 0.001) (Table S2) in explaining variations in microbial samples.
3.2. OP and NP Microbiota of Patients Differ Significantly from that of Healthy Children
Generally, bacterial diversity in NP is lower than in that of OP for all children, while the diversity of NP and OP microbiota in patients is also lower than in that of healthy children (Figure 1A). NP and OP microbiota analysis could clearly separate healthy and diseased samples (Figure 1B,C).
Figure 1.
Nasopharyngeal (NP)/ oropharyngeal (OP) microbiota structure in pneumonia patients and healthy children. (A) Shannon index of NP and OP microbiota in patients and healthy infants; (B) principal components analysis (PCA) of NP samples (purple triangles stand for healthy controls, and dark green triangles stand for pneumonia subjects); (C) principal components analysis (PCA) of OP samples (red triangles stand for healthy controls, and yellow triangles stand for pneumonia subjects); (D) comparison of dominated genera between NP/OP microbiota of patients and that of healthy infants. Vertical axis represents genus name, and horizontal axis shows the log10 value of relative abundance. *, ** and *** represent q-value ≤ 0.05, ≤0.01, and ≤0.001, respectively.
Firmicutes predominates, both in OP and NP microbiota of all children, but accumulates significantly in pneumonia patients (OP: 63.55% vs. 47.05% in healthy children, q-value < 0.001; NP: 72.09% vs. 43.06% in healthy children, q-value < 0.001) (Table S3). By contrast, Bacteroidetes are enriched in the OP and NP microbiota of healthy children (OP: 19.64% vs. 5.34%, q-value < 0.001; NP: 7.54% vs. 0.84%, q-value < 0.001) (Table S3). Proteobacteria accounts for a lower proportion of NP and OP microbiota in patients in comparison with healthy children, although this does not have statistical significance.
At genus-level, Mycoplasma, Staphylococcus, Lactobacillus, Ralstonia, Acinetobacter, and Actinomyces amass significantly in both the NP and OP microbiota of inpatients, while Prevotella is particularly enriched in healthy children (Figure 1D, Table S4). Streptococcus prevails in NP-P microbiota (Figure 1D, Table S4), while Moraxella and Dolosigranulum accumulate in NP-H microbiota (Figure 1D, Table S4). Haemophilus accounts for a large proportion of OP-H microbiota, while Corynebacterium similarly enriches OP-P microbiota (Figure 1D, Table S4).
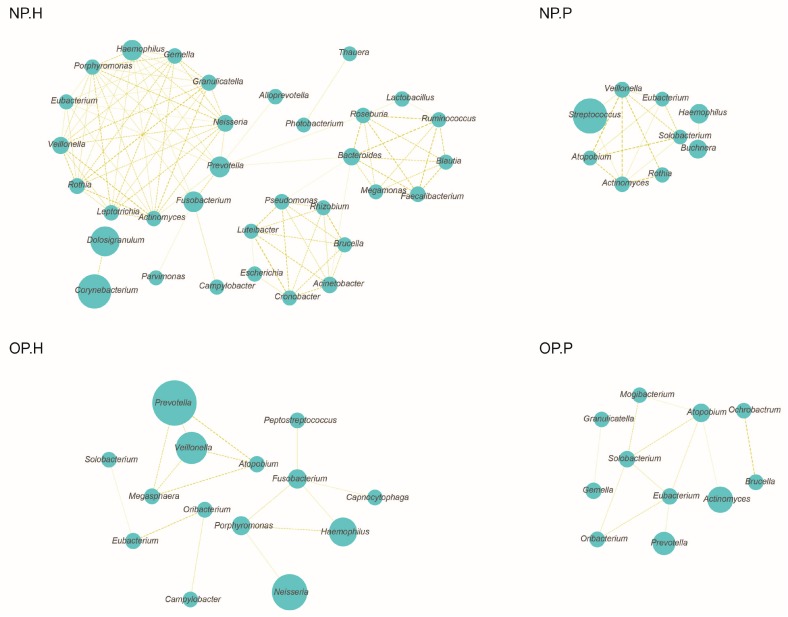
The concurrence bacterial network (Figure 2) of NP-H is comprised of 33 genera featured by three core nodes, namely the Prevotella, Roseburia, and Bacteroides. Nevertheless, only 9 genera are included in bacterial network of the NP-P group. Identical with NP microbiota, the OP microbiota of diseased children also features a simpler concurrent network when compared to healthy children (Figure 2).
Figure 2.
Co-occurrence network of NP/OP microbiota in pneumonia patients and healthy children. The circle size represents the relative abundance, and the density of the dashed line represents the Spearman coefficient.
3.3. NP and OP Microbiota Pattern of Inpatients Have an Increased Similarity to Each Other
Compared to healthy children, the dissimilarity of NP and OP microbiota in patients is considerably smaller (Figure 3A). Further analysis indicates that all samples can be clustered into four main groups (Figure 3B). NP and OP samples of diseased children are contained in the same cluster. By contrast, OP samples in healthy children are contained in a cluster, with the exception of H-49. NP samples in healthy children are separated into two clusters, except for the sample H-8, which is assigned to the OP cluster of healthy children.
Figure 3.
Dissimilarity and clustering of NP and OP samples. (A) Divergence between NP and OP microbiota based on Bray-Curtis dissimilarity; (B) the Log10 value of relative abundance was proportional to the color, from blue to red.
Apart from the enrichment of clinically diagnosed pathogens Mycoplasma and Staphylococcus in both the NP-P and OP-P microbiota of patients, we also identified the OP-P microbiota accumulation of Corynebacterium, which primarily dominates in NP-H microbiota.
3.4. Microbiota Analysis Can Be Complementary to Clinical Detection of Respiratory Pathogens
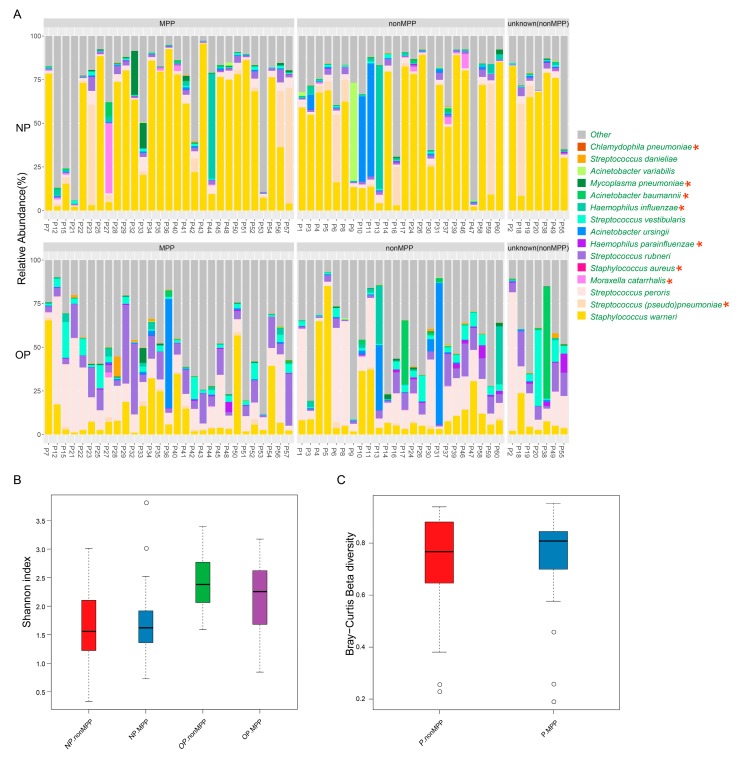
Clinical detection has indicated M. pneumoniae infection in 29 cases (MPP group), non-M. pneumoniae infection in 24 cases (non-MPP group), and an unknown pathogen infection in 7 cases (unknown (non-MPP) group) (Figure 4A, Table S5). The incidence of mixed infection in MPP is 21/29, while it is 8/24 in the non-MPP group. The hospitalization time in MPP, non-MPP, and unknown (non-MPP) averages at 10.4, 9.5, and 7.4 days, respectively. In addition, the MPP group has the highest proportion of fever (72.41%) and abnormal CRP (75.86%).The microbiota diversity of NP/OP differs slightly between MPP and the two other groups (Figure 4B). Moreover, the NP-OP microbiota’s dissimilarity within the MPP group is also similar to that of non-MPP groups (Figure 4C).
Figure 4.
Microbiota comparison between Mycoplasma pneumoniae pneumonia (MPP) and non-MPP group. (A) Histogram based on species-level of common pathogens in NP/OP. Samples in MPP, non-MPP, and unknown (non-MPP) are ranked by age, respectively, from younger to the older. * stand for pathogens, which were also detected by conventional method; (B) Shannon index of NP/OP microbiota in MPP and non-MPP; (C) distance between NP and OP samples in MPP and non-MPP patients based on Bray-Curtis dissimilarity.
Mycoplasma species show significant accumulation in pneumonia patients, with no statistical difference in NP/OP microbiota between MPP and non-MPP groups (Table S6). In addition to M. pneumoniae, four other Mycoplasma species are also identified (Tables S6 and S7), including Mycoplasma faucium, Mycoplasma salivarium, Mycoplasma sualvi, and Mycoplasma testudinis. M. pneumoniae is the dominant species of Mycoplasma in NP (26/29 in MPP, 19/24 in non-MPP, and 5/6 in the unknown (non-MPP) group) and OP (25/29 in MPP, 19/24 in non-MPP, and 5/6 in unknown (non-MPP) group) of patients (Table S7), followed by M. salivarium. For P-33, who suffered the longest hospitalization period (37 days) (Table S1), a high abundance of M. pneumoniae was identified both in NP and OP microbiota, with proportions of 14.51% and 8.48%, respectively (Figure 4A, Table S5).
Staphylococcus warneri is dominant in the NP microbiota of six cases, where the patient was diagnosed with an unknown pathogen infection. S. (pseudo)pneumoniae accounted for 52.83% of OP microbiota in P-18 (Figure 4A, Table S5), who had no fever and suffered 17 days hospitalization. In OP microbiota, P-38 possessed 63.67% of A. baumannii and P-55 consisted of 10.88% H. parainfluenzae (Figure 4A, Table S5). Streptococcus peroris represented 79.17% of OP microbiota for P-2 (Figure 4A, Table S5), who had a seven day fever during hospitalization. For 24 other patients, NP/OP microbiota analysis results conformed with those of a clinical diagnosis for S. pneumoniae (Figure 4A, Table S5), while M. catarrhalis constituted a low proportion of microbiota, even for patients who were diagnosed with a M. catarrhalis infection.
4. Discussion
Our study shows a simpler NP and OP microbiota structure and co-occurrence network in children with pneumonia who were diagnosed with MPP or non-MPP. The level of respiratory commensals decreased after infection, such as a reduced number of lactic acid-producing bacterium Dolosigranulum [19]. A previous report also indicated decreased levels of Prevotella after a respiratory infection, which antagonized the LPS produced by H. influenzae and inhibited the Toll-like receptor 4 (TLR4) mediated mucosal inflammation [20]. Respiratory pathogens intrude greatly on the respiratory tract through multiple virulence factors [21,22,23,24] and evade the immune response of the host [25,26], which can induce a proliferation of pathogens and may explain the simpler NP and OP microbiota structure of patients [16,27,28].
In addition, we observe a higher similarity between NP and OP microbiota in patients, which can be explained by a robust intrusion of pathogens through the nasal cavity and the transmission from NP to OP and lung [29,30]. Microbes in the air are transmitted to the lung through the nasal cavity and NP [31], which affect the initial infection at NP and subsequent transmission. In parallel, we found OP-P enrichment of Corynebacterium, which was dominant in the NP microbiota of healthy children.
Compared to the non-MPP patients, MPP cases suffered a longer hospitalization time, a higher rate of abnormal CRP values, and fever. This may be related to the robust pathogenicity of M. pneumoniae, such as cytoadherence to respiratory epithelium and cytotoxicity release [32]. Meanwhile, the MPP group has a higher incidence of mixed infection with viruses or bacteria than non-MPP patients. The high ratio of mixed infection in our study is inconsistent with the previous report [33], which implied that the practice for CAP should be country- or region-specific.
Conventional pathogen detection is limited to known typical pathogens [34,35] and emerging reports demonstrating that the 16S/metagenomics method could assist in respiratory pathogen detection [36,37,38]. In a prior report, gram staining and culturing indicated that S. aureus and Streptococcus anginosus gave rise to infections in the lung, yet targeted therapy was not effective [37]. However, microbiota analysis showed that the patient had achieved significant remission from the Streptococcus intermedius infection after 14 days targeted therapy [37]. Several reports indicated the potential of metagenomic sequencing in diagnosing respiratory pathogens in ventilation associated pneumonia (VAP) and hematopoietic cellular transplant (HCT) patients [36,38]. This study shows that microbiota analysis is complementary to the present means of detecting M. pneumoniae, such as enrichment of other Mycoplasma species, for example, M. testudinis. In addition to Mycoplasma, 16S rDNA analysis also provides clues regarding other infectious pathogens affecting seven patients with a clinically unknown infection, including a >50% proportion of A. baumannii and Streptococcus peroris in the OP microbiota of P-38 and P-2, respectively.
Several limitations affecting this study should be noted. The first is the small number of recruited cases and lack of microbiota analysis in sputum/lung samples, which made it difficult to clearly comprehend the microbial transmission mode, as well as the significance of NP/OP microbiota analysis. Although the pneumonia samples were divided into MPP and non-MPP groups, we could not discover group-specific patterns to deepen our understanding of infection-related dysbiosis. The 16S rDNA analysis cannot identify species-level pathogens definitively, nor explain functional dysbiosis in infection-caused microbiota imbalance.
5. Conclusions
This study provides insight into imbalanced URTs microbiota in Chinese MPP and non-MPP children. Profiling possible pathogens through microbiota analysis also holds the potential to complement present means of pathogen detection.
Acknowledgments
This study was supported by the Key Medical Disciplines Building Project of Shenzhen (201506053), Key Medical Disciplines Building Project of Shenzhen (SZXJ2017005), Guangdong Medical Research Fund (A2016501), Sanming Project of Medicine in Shenzhen (2016029), Shenzhen public service platform for clinical drug trials (20151964), and Shenzhen Science and Technology Project (JCYJ20170303155012371).
Supplementary Materials
The following are available online at www.mdpi.com/2073-4425/8/12/380/s1. Table S1: Sample information of pneumonia patients and healthy children. Table S2: Evaluation of factors’ contribution by PERMANOVA. Table S3: Wilcoxon rank-sum test result of NP and OP at phylum level between healthy children and pneumonia patients. Table S4: Wilcoxon rank-sum test result of NP and OP at genus level between healthy children and pneumonia patients. Table S5: Species-level detection between conventional and sequencing method. Table S6: Comparison of Mycoplasma species in NP/OP among MPP, non-MPP, and healthy group. Table S7: Distribution of Mycoplasma species in NP and OP of patients and healthy children.
Author Contributions
Y.Z. and S.L. managed the project. Z.L. and H.W. performed the sampling for all children and for the detection of common pathogens. Yan.L. and D.L. performed the bioinformatics analysis. Z.L. and W.D. interpreted the analysis results and wrote the paper. G.X. and Q.Z. prepared the DNA and optimized the graphs. Z.Y. and Yin.L. polished the article. All authors reviewed this manuscript.
Conflicts of Interest
The authors declare that no other organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript, had any relevant affiliation or financial involvement, apart from those disclosed.
References
- 1.Rudan I., O’Brien K.L., Nair H., Liu L., Theodoratou E., Qazi S., Luksic I., Fischer Walker C.L., Black R.E., Campbell H., et al. Epidemiology and etiology of childhood pneumonia in 2010: Estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J. Glob. Health. 2013;3:10401. doi: 10.7189/jogh.03.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakwinska O., Bastic Schmid V., Berger B., Bruttin A., Keitel K., Lepage M., Moine D., Ngom Bru C., Brussow H., Gervaix A. Nasopharyngeal microbiota in healthy children and pneumonia patients. J. Clin. Microbiol. 2014;52:1590–1594. doi: 10.1128/JCM.03280-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Garcia M.L., Calvo C., Pozo F., Villadangos P.A., Perez-Brena P., Casas I. Spectrum of respiratory viruses in children with community-acquired pneumonia. Pediatr. Infect. Dis. J. 2012;31:808–813. doi: 10.1097/INF.0b013e3182568c67. [DOI] [PubMed] [Google Scholar]
- 4.Qin Q., Xu B., Liu X., Shen K. Status of Mycoplasma Pneumoniae pneumonia in chinese children: A systematic review. Adv. Microbiol. 2014;4:704–711. doi: 10.4236/aim.2014.411076. [DOI] [Google Scholar]
- 5.De Steenhuijsen Piters W.A., Huijskens E.G., Wyllie A.L., Biesbroek G., van den Bergh M.R., Veenhoven R.H., Wang X., Trzcinski K., Bonten M.J., Rossen J.W., et al. Dysbiosis of upper respiratory tract microbiota in elderly pneumonia patients. ISME J. 2016;10:97–108. doi: 10.1038/ismej.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen Y., Xiao F., Wang C., Wang Z. The impact of different methods of DNA extraction on microbial community measures of balf samples based on metagenomic data. Am. J. Transl. Res. 2016;8:1412–1425. [PMC free article] [PubMed] [Google Scholar]
- 7.Murdoch D.R., O’Brien K.L., Scott J.A., Karron R.A., Bhat N., Driscoll A.J., Knoll M.D., Levine O.S. Breathing new life into pneumonia diagnostics. J. Clin. Microbiol. 2009;47:3405–3408. doi: 10.1128/JCM.01685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vissing N.H., Chawes B.L., Bisgaard H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am. J. Respir. Crit. Care Med. 2013;188:1246–1252. doi: 10.1164/rccm.201302-0215OC. [DOI] [PubMed] [Google Scholar]
- 9.Shaikh N., Leonard E., Martin J.M. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: A meta-analysis. Pediatrics. 2010;126:e557–e564. doi: 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- 10.Bousbia S., Papazian L., Saux P., Forel J.M., Auffray J.P., Martin C., Raoult D., La Scola B. Repertoire of intensive care unit pneumonia microbiota. PLoS ONE. 2012;7:e32486. doi: 10.1371/journal.pone.0032486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Steenhuijsen Piters W.A., Heinonen S., Hasrat R., Bunsow E., Smith B., Suarez-Arrabal M.C., Chaussabel D., Cohen D.M., Sanders E.A., Ramilo O., et al. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am. J. Respir. Crit. Care Med. 2016;194:1104–1115. doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosch A.A., de Steenhuijsen Piters W.A., van Houten M.A., Chu M., Biesbroek G., Kool J., Pernet P., de Groot P.C.M., Eijkemans M.J.C., Keijser B.J.F., et al. Maturation of the infant respiratory microbiota, environmental drivers and health consequences: A prospective cohort study. Am. J. Respir. Crit. Care Med. 2017 doi: 10.1164/rccm.201703-0554OC. [DOI] [PubMed] [Google Scholar]
- 13.Pettigrew M.M., Gent J.F., Kong Y., Wade M., Gansebom S., Bramley A.M., Jain S., Arnold S.L., McCullers J.A. Association of sputum microbiota profiles with severity of community-acquired pneumonia in children. BMC Infect. Dis. 2016;16:317. doi: 10.1186/s12879-016-1670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teo S.M., Mok D., Pham K., Kusel M., Serralha M., Troy N., Holt B.J., Hales B.J., Walker M.L., Hollams E., et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozich J.J., Westcott S.L., Baxter N.T., Highlander S.K., Schloss P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl. Environ. Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Dai W., Qiu C., Li S., Wang W., Xu J., Li Z., Wang H., Li Y., Yang Z., et al. Mycoplasma Pneumoniae and streptococcus pneumoniae caused different microbial structure and correlation network in lung microbiota. J. Thorac. Dis. 2016;8:1316–1322. doi: 10.21037/jtd.2016.04.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly B.J., Imai I., Bittinger K., Laughlin A., Fuchs B.D., Bushman F.D., Collman R.G. Composition and dynamics of the respiratory tract microbiome in intubated patients. Microbiome. 2016;4:7. doi: 10.1186/s40168-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yilmaz P., Parfrey L.W., Yarza P., Gerken J., Pruesse E., Quast C., Schweer T., Peplies J., Ludwig W., Glockner F.O. The silva and “all-species living tree project (ltp)” taxonomic frameworks. Nucleic Acids Res. 2014;42:D643–D648. doi: 10.1093/nar/gkt1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biesbroek G., Bosch A.A., Wang X., Keijser B.J., Veenhoven R.H., Sanders E.A., Bogaert D. The impact of breastfeeding on nasopharyngeal microbial communities in infants. Am. J. Respir. Crit. Care Med. 2014;190:298–308. doi: 10.1164/rccm.201401-0073OC. [DOI] [PubMed] [Google Scholar]
- 20.Miller S.I., Ernst R.K., Bader M.W. Lps, tlr4 and infectious disease diversity. Nat. Rev. Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 21.Lother S.A., Demczuk W., Martin I., Mulvey M., Dufault B., Lagace-Wiens P., Keynan Y. Clonal clusters and virulence factors of group c and g streptococcus causing severe infections, Manitoba, Canada, 2012–2014. Emerg. Infect. Dis. 2017;23:1079–1088. doi: 10.3201/eid2307.161259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadioglu A., Weiser J.N., Paton J.C., Andrew P.W. The role of streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 23.Bayer-Santos E., Durkin C.H., Rigano L.A., Kupz A., Alix E., Cerny O., Jennings E., Liu M., Ryan A.S., Lapaque N., et al. The salmonella effector sted mediates march8-dependent ubiquitination of mhc ii molecules and inhibits t cell activation. Cell Host Microbe. 2016;20:584–595. doi: 10.1016/j.chom.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira S.G., Rosa A.C., Ferreira A.S., Moreira L.M., Proenca D.N., Morais P.V., Cardoso O. Virulence factors and infection ability of pseudomonas aeruginosa isolates from a hydropathic facility and respiratory infections. J. Appl. Microbiol. 2014;116:1359–1368. doi: 10.1111/jam.12463. [DOI] [PubMed] [Google Scholar]
- 25.Norris S.J. Antigenic variation with a twist—The borrelia story. Mol. Microbiol. 2006;60:1319–1322. doi: 10.1111/j.1365-2958.2006.05204.x. [DOI] [PubMed] [Google Scholar]
- 26.Reddick L.E., Alto N.M. Bacteria fighting back: How pathogens target and subvert the host innate immune system. Mol. Cell. 2014;54:321–328. doi: 10.1016/j.molcel.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Q., Dai W., Zhou Q., Fu D., Zheng Y., Wang W., Liu Y., Yang Q., Dai D., Liu S., et al. Dynamic oropharyngeal and faecal microbiota during treatment in infants hospitalized for bronchiolitis compared with age-matched healthy subjects. Sci. Rep. 2017;7:11266. doi: 10.1038/s41598-017-11311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Man W.H., de Steenhuijsen Piters W.A., Bogaert D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017;15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassis C.M., Erb-Downward J.R., Dickson R.P., Freeman C.M., Schmidt T.M., Young V.B., Beck J.M., Curtis J.L., Huffnagle G.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio. 2015;6:e00037-15. doi: 10.1128/mBio.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charlson E.S., Bittinger K., Haas A.R., Fitzgerald A.S., Frank I., Yadav A., Bushman F.D., Collman R.G. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am. J. Respir. Crit. Care Med. 2011;184:957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahin-Yilmaz A., Naclerio R.M. Anatomy and physiology of the upper airway. Proc. Am. Thorac. Soc. 2011;8:31–39. doi: 10.1513/pats.201007-050RN. [DOI] [PubMed] [Google Scholar]
- 32.Al Masalma M., Armougom F., Scheld W.M., Dufour H., Roche P.H., Drancourt M., Raoult D. The expansion of the microbiological spectrum of brain abscesses with use of multiple 16s ribosomal DNA sequencing. Clin. Infect. Dis. 2009;48:1169–1178. doi: 10.1086/597578. [DOI] [PubMed] [Google Scholar]
- 33.Uehara S., Sunakawa K., Eguchi H., Ouchi K., Okada K., Kurosaki T., Suzuki H., Tsutsumi H., Haruta T., Mitsuda T., et al. Japanese guidelines for the management of respiratory infectious diseases in children 2007 with focus on pneumonia. Pediatr. Int. 2011;53:264–276. doi: 10.1111/j.1442-200X.2010.03316.x. [DOI] [PubMed] [Google Scholar]
- 34.Musher D.M., Thorner A.R. Community-acquired pneumonia. N. Engl. J. Med. 2014;371:1619–1628. doi: 10.1056/NEJMra1312885. [DOI] [PubMed] [Google Scholar]
- 35.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M., Grijalva C.G., Anderson E.J., Courtney D.M., Chappell J.D., et al. Community-acquired pneumonia requiring ospitalization among U.S. Adults. N. Engl. J. Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langelier C., Zinter M.S., Kalantar K., Yanik G.A., Christenson S., O’Donovan B., White C., Wilson M., Sapru A., Dvorak C.C., et al. Metagenomic sequencing detects respiratory pathogens in hematopoietic cellular transplant patients. Am. J. Respir. Crit. Care Med. 2017 doi: 10.1164/rccm.201706-1097LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noguchi S., Yatera K., Kawanami T., Yamasaki K., Fukuda K., Naito K., Akata K., Nagata S., Ishimoto H., Taniguchi H., et al. Pneumonia and empyema caused by streptococcus intermedius that shows the diagnostic importance of evaluating the microbiota in the lower respiratory tract. Intern. Med. 2014;53:47–50. doi: 10.2169/internalmedicine.53.0971. [DOI] [PubMed] [Google Scholar]
- 38.Pendleton K.M., Erb-Downward J.R., Bao Y., Branton W.R., Falkowski N.R., Newton D.W., Huffnagle G.B., Dickson R.P. Rapid pathogen identification in bacterial pneumonia using real-time metagenomics. Am. J. Respir. Crit. Care Med. 2017 doi: 10.1164/rccm.201703-0537LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.