Abstract
In the last several decades, the genetic ability to taste the bitter compound, 6-n-propyltiouracil (PROP) has attracted considerable attention as a model for understanding individual differences in taste perception, and as an oral marker for food preferences and eating behavior that ultimately impacts nutritional status and health. However, some studies do not support this role. This review describes common factors that can influence the characterization of this phenotype including: (1) changes in taste sensitivity with increasing age; (2) gender differences in taste perception; and (3) effects of smoking and obesity. We suggest that attention to these factors during PROP screening could strengthen the associations between this phenotype and a variety of health outcomes ranging from variation in body composition to oral health and cancer risk.
Keywords: PROP tasting, psychophysical approach, age, gender, weight status
1. Introduction
Taste perception varies from person to person, strongly influencing food preferences and health [1]. In the last several decades, numerous studies have focused on the use of the bitter compound, 6-n-propylthiouracil (PROP) and its chemical relative phenylthiocarbamide (PTC), as genetic markers for oral sensations that have downstream effects on food preferences, eating habits, nutritional status and health [1,2,3]. This approach is based on data indicating that PROP taster status is associated with variations in taste perception and preference for a wide range of oral stimuli including other bitter substances [4,5,6,7,8,9], chemical irritants [10,11], sweet substances [12], sour compounds [13], umami taste [14], fats and high-energy foods [15,16,17], compounds which give rise to astringent sensations [18], and fruits and vegetables [19,20,21]. Some studies suggested that PROP-related sensory differences may be extended to the olfactory system [22,23], and that PROP taster status may influence food perception also via aromas or flavors [24,25]. Relationships between PROP taster status and health indicators such as body mass index (BMI) [26,27], antioxidant status [28], colonic neoplasm risk [29,30,31] and respiratory function [32] have been widely reported.
The validity of PROP tasting as a biomarker depends on the use of robust PROP phenotyping methods. Common screening procedures are based on psychophysical approaches which fall into two general classes: threshold and suprathreshold methods. Threshold measures determine the lowest PROP concentration (or amount) that can be distinguished by an individual. These measures are reliable with a long history of use in the field. They can effectively separate individuals who detect PROP only at high concentrations or not at all (non-tasters), from those who perceive PROP as bitter (tasters). However, thresholds do not distinguish individuals who perceive PROP as extremely bitter (super-tasters) from those who perceive it as moderately bitter (medium tasters) [1,33].
Suprathreshold methods utilize rating scales to assess PROP bitterness across the psychophysical range. Supratheshold scaling can distinguish individuals who are super-tasters from medium tasters, thus classifying individuals as belonging to one of the three PROP taster categories [1,34,35,36,37,38]. Suprathreshold methods are based on ratings of the perceived intensity of PROP following stimulation with multiple samples, a single solution or impregnated filter papers [34,35,36,39].
Bartoshuk and colleagues [34] first pioneered PROP screening methodologies linking PROP intensity ratings with a classification scheme. In the original method, five different concentrations of PROP and five concentrations of sodium chloride were presented to assessors who were classified into groups based on the relative intensity of PROP to NaCl. Those who gave higher ratings to PROP than NaCl were classified as super-tasters. Those who gave similar ratings to both stimuli were classified as medium tasters and those who gave higher ratings to NaCl relative to PROP were classified as non-tasters. Some workers have called into question the validity of NaCl ratings as an independent stimulus [40,41]. This conclusion may be based, in part, on data showing that at high NaCl concentrations (>0.35 M), NaCl ratings are not independent of PROP taster status and diverge in super tasters [41]. Nevertheless, the use of NaCl as a reference standard has been successfully employed by numerous laboratories across the globe [17,18,27,33,34,38,39,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61]. Data described later in this review illustrate this point.
The two predominant scales used in PROP screening are the labeled magnitude scale (LMS) [62], and its variant, the general labeled magnitude scale (gLMS) [63]. Both scales give subjects the freedom to rate the perceived intensity of PROP relative to the ‘strongest imaginable oral sensation’ they had ever experienced (LMS) or the ‘strongest imaginable sensation of any kind’ they had ever experienced, including those evoked by the strongest sound, pain, or light (gLMS). Training in how to use these scales is typically provided to participants in advance.
The need for brief screening methods that could be employed outside of a traditional laboratory setting prompted Tepper and colleagues [35,36] to develop a series of related methods based on the original technique of Bartoshuk et al. [34]. These methods include the 3-solution and 1-solution methods [35] and the paper disk method [36]. The 1-solution and paper disk methods rely on empirically-derived cutoff scores for PROP taster classification. These techniques have been used in numerous investigations in our work [17,18,27,33,38,39,43,45,46,47,48,49,50,51,52,53,54,55,56,57] and the work of others [58,59,60,61]. PROP classifications obtained with this suite of methods is highly correlated with all known TAS2R38 genotypes [27,33,64,65,66,67] and differences in physiological responses such as prefrontal cortex activity [68] and degree of activation of peripheral taste function [69,70].
It has become our practice in recent years to define taster status primarily on the basis of PROP cutoff scores unless a participant gives a ‘borderline’ rating to PROP [18,39,52,54,55,56]. Only in rare instances (~4–6% of the time) is the NaCl rating needed to clarify an individual’s PROP classification. We continue to administer the NaCl disk to reliably classify this small proportion of individuals and to preserve the integrity of the method.
PTC tasting was first described by Fox [71] and was identified as a genetic trait with Mendelian-like inheritance almost 60 years ago [72]. Since then, it has become clear that PROP/PTC tasting is a complex trait, with many factors involved in its determination. First, alleles of the gene that code for the G-protein-linked receptor TAS2R38 explain most of the phenotypic variation in PROP tasting [73,74]. The allelic diversity of this gene, located on chromosome 7 [75], is due to five single-nucleotide polymorphisms (SNPs) [76]. Three of these SNPs, located at base pairs 145 (C/G), 785 (C/T), and 886 (G/A), result in three amino acid substitutions (Pro49Ala, Ala262Val, and Val296Ile) which give rise to 5 distinct haplotypes (PAV, AVI, AAV, AAI, PAI, and PVI which is very rare) [74]. There are two common haplotypes, PAV the dominant (taster) variant and AVI the recessive (non-taster) one. Typically, non-tasters are homozygous for the AVI haplotype, whereas considerable genotypic overlap between the medium and super-taster groups has been reported [11,27,74]. Rare haplotypes (AAV, AAI, and PVI) have been shown to contribute to intermediate sensitivity [67] and are more frequent in African populations [76]. Also, differences in TAS2R38 expression have been strongly associated with PROP bitterness intensity [77]. The TAS2R38 receptor binds synthetic thiourea derivates, such as PROP and PTC, as well as natural ligands, such as glucosinolates of the Brassica family. All of these compounds contain the thiocyanate moiety (N–C=S) which is responsible for their bitter taste [71,78].
Studies also suggest that other modifying genes may be involved in PROP tasting [79,80,81]. For example, a polymorphism in the gene that codes for zinc binding capacity of the salivary protein, gustin (CAVI) [38], has been shown to affect PROP sensitivity by acting as growth factor for taste buds [65]. The discovery of this polymorphism in Gustin provided the first mechanistic explanation for why PROP super-tasters, compared to non-tasters, are more responsive to a wide range of stimuli that are not mediated via the TAS2R38 bitter receptor. Numerous studies have reported that PROP super-tasters have higher papillae densities on the tongue tip than non-tasters [12,34,65,82,83,84]. However, subsequent work by Barbarossa et al. [39] clarified this relationship by showing that fungiform papilla density was more strongly related to variation in the gustin gene than to PROP tasting or TAS2R38 polymorphisms. Together, these findings suggest that there is functional cooperation between these two genes products to influence orosensory perceptions, and these effects are mediated by taste papilla density. It is important to note, however, that other studies failed to find associations between PROP tasting and gustin genotypes [37,85]. Finally, greater PROP sensitivity has also been related to differences in the secretion of other salivary chemical components including proline-rich proteins and other classes of proteins involved in bitter taste and astringency perception [18,45,64,71,86].
Despite the accumulating evidence supporting associations between PROP tasting, nutrition and health, these relationships remain controversial due to the many null reports in the literature [87,88,89,90,91,92]. These inconsistencies may be due, in part, to underlying factors that modify the phenotypic expression of this trait. The purpose of this review is to highlight common factors identified in our research such as age, gender, obesity and smoking that can influence the characterization of the PROP phenotype. Accounting for these factors could lead to more precise phenotyping and more robust relationships between this biomarker, nutrition and health. All of the data presented in this review were collected in individuals under conditions of unobstructed air flow (without nose clips).
2. Gender and Age Effect
The effects of gender and age on chemosensory perceptions are well known [93,94], but they have been less well studied in relation to PROP sensitivity. Several studies reported a gender difference in PROP perception. Although this difference might be due to hormonal levels, these data showing that women were more sensitive to PROP bitterness than men, were more likely to be tasters [34,48,95], or super-tasters, [34] and had more taste buds and fungiform papillae [34,40]. However, other reports do not substantiate this difference [65,96,97].
Age has also been associated with moderately higher PROP thresholds, accounting for 5–8% of the taste acuity variance [37,65,95,98]. Mennella et al. showed that among TAS2R38 heterozygotes (PAV/AVI), children were more responsive to PROP than adults [99]. These data suggest that the penetrance of this gene (the extent to which environmental factors such as age, influence the phenotypic expression of this trait), varies with age [99]. Further, children have more papillae on the tip of the tongue and they perceive greater intensity from the basic tastes than do adults [100]. These anatomical and functional characteristics could explain why age indirectly affects the PROP genotype-phenotype relationship.
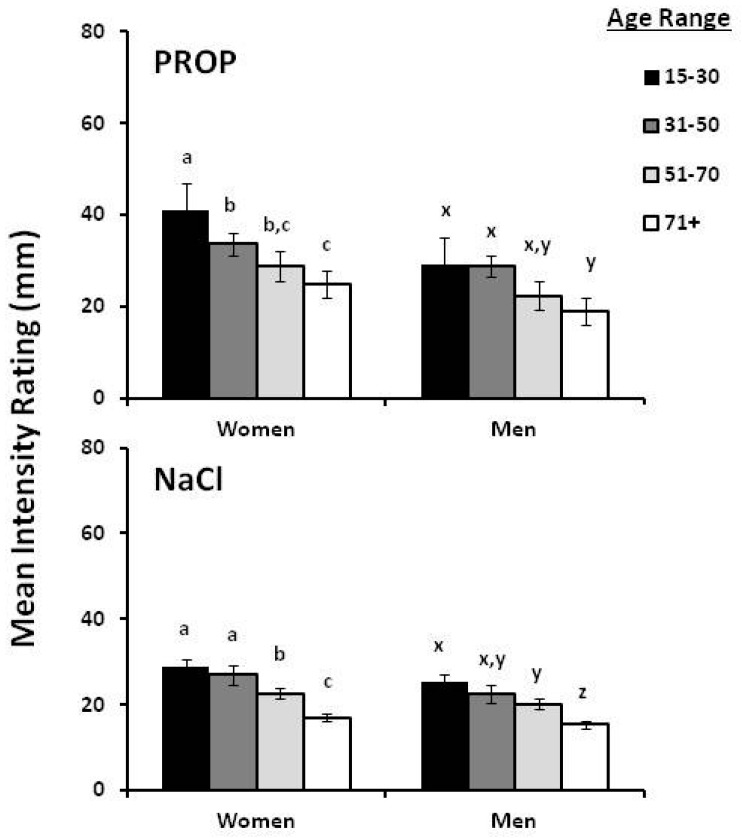
It is well known that population heterogeneity is a common contributor to the problem of non-replication when complex traits, such as PROP phenotype, are under study [101]. Since ethnic homogeneity can reduce noise by diminishing ancestral diversity [101,102,103], we studied gender and age-related changes in the bitterness of PROP and NaCl in 589 adults (18–96 years of age; 343 females; 246 males) residing in the isolated community of Val Borbera located in northwest Italy. All participants were free-living and healthy by self-report, and none were taking medications that might interfere with taste function. Half of the participants were >50 years of age. Participants rated the intensity of PROP and NaCl—impregnated filter papers according to the method of Zhao et al. [36]. Among the youngest participants (15–30-year-old), women gave higher intensity ratings to PROP than men (p < 0.001). PROP ratings declined slowly and consistently with age in both genders (all p-values < 0.001) (Figure 1). These changes presumably reflect well-documented decreases in papillae density, shape, vascularization and function that occur with the aging process [104,105,106] and not a unique effect of TAS2R38 gene on taste function. However, changes in expression of genes related to taste function cannot be excluded as a possibility for decreased PROP perception.
Figure 1.
Mean (±Standard Error, SE) 6-n-propylthiouracil (PROP) (top) and NaCl (bottom) ratings across age groups in both women and men from the isolated community of Val Borbera, Northwest Italy. n = 589; sample size per age group: 59 (15–30 years); 191 (31–50 years); 261 (51–70 years); 78 (71+ years). Ratings by men and women were analyzed separately. For women, mean values within a stimulus type (PROP or NaCl), with different superscripts (a, b and c) are significantly different as a function of age; superscripts x, y and z are used to denote age differences for men. Significant differences, within a stimulus type (PROP or NaCl), are indicated by different letters (a, b and c are used to denote differences for women, while x, y and z for men). For all comparisons, p < 0.001 by Duncan’s Multiple Range test subsequent to ANOVA. Unpublished data from Tepper et al. [107].
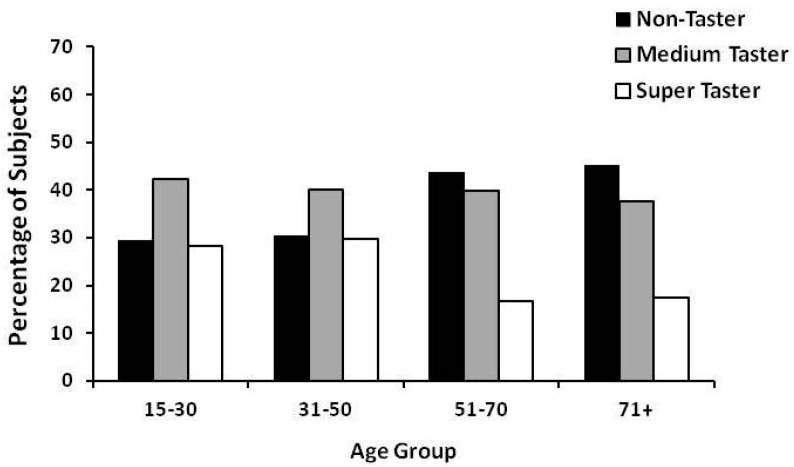
When participants (both men and women) were classified by PROP status (Figure 2) the distribution of taster groups agreed with population norms for western European populations, up to the age of 50 years. After age 50, the percentage of non-tasters increased and the percentage of super-tasters declined by almost 50%. These data suggest that age-related taste loss could have implications for PROP screening and phenotyping, especially in geriatric populations.
Figure 2.
Distribution of PROP taster groups by age in the isolated community of Val Borbera, Northwest Italy. n = 589; sample size per age group: 59 (15–30 years); 191 (31–50 years); 261 (51–70 years); 78 (71+ years). The percentage of non-tasters increased and the percentage of super-tasters decreased in those >50 years of age (by Chi-square analysis; p < 0.001). Unpublished data from Tepper et al. [107].
3. Weight Status Effect
It has often been hypothesized that chemosensory perceptions are blunted in obesity. This supposition is based on evidence showing reductions in oral fat sensation [108,109,110], sweet taste [111], bitter and salty taste [112], umami taste [113] and general taste and smell ability [114] among populations with obesity. However, some studies show no apparent disruptions in the perception of fattiness and sweetness from food products in obese individuals [52], or in the oral detection of fatty acids [115]. Indeed, other studies have come to a different conclusion, showing greater acuity for sweet and salty taste in obese relative to lean individuals [116].
A major focus of our work is on understanding the role of PROP tasting as a biomarker for excess fat/energy intake and increased adiposity [1,3]. Two studies conducted exclusively in women, showed an inverse association between PROP tasting and BMI, i.e., non-taster women were heavier than super-taster women [46,48]. Another mixed-gender study reported this same negative association between PROP tasting and BMI that was specific to women but not men [27]. However, other studies failed to replicate these findings reporting no associations between PROP tasting and weight status in either gender [44,90,91,92,117,118,119,120,121].
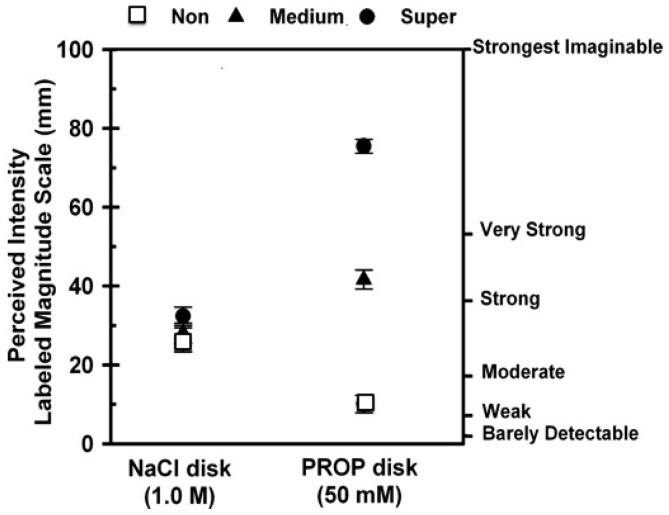
Most studies examining associations between PROP tasting and adiposity have been conducted in mixed subject groups of overweight-obese individuals. Only a few recent studies have been conducted in individuals who meet the criterion for obesity (BMI > 30 kg/m2) [56,120,122]. Presumably, if taste function is altered in obesity, this could impact PROP phenotyping procedures. Two studies described below illustrate this effect. Figure 3 shows typical PROP and NaCl ratings obtained from a group of lean, young women classified by PROP status where NaCl ratings were centered at ~30 mm and did not vary across PROP taster groups.
Figure 3.
Classification of 93 lean women, 18–45 years of age as PROP non-tasters, medium tasters and super-tasters based on intensity ratings (mean ±95% confidence) for paper disks that were impregnated in PROP (50 mM) and NaCl (1.0 M), and then dried. The PROP disks contained 0.28 mg (±2.2% CV) PROP measured by ethanol extraction. Super-tasters were identified with a cutoff score for PROP bitterness >67 mm on the LMS; non-tasters had a cutoff score ≤15 mm. All others were identified as medium tasters. There were no differences in the perception of NaCl across the three subject groups. Data are from Tepper et al. [28] with permission.
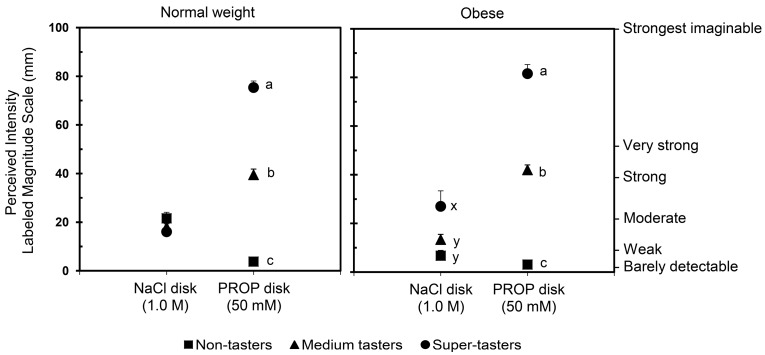
Figure 4 shows the PROP and NaCl ratings from a sample of normal weight (mean BMI = 21.85 ± 0.177 kg/m2) and obese men and women (mean BMI = 35.32 ± 0.61 kg/m2). Results showed that the NaCl ratings of non-tasters and medium tasters with obesity were lower than those of super-tasters with obesity (p = 0.0015 and p = 0.000094, respectively; Duncan’s test subsequent two-way ANOVA). No differences in NaCl ratings were observed across taster groups among normal weight individuals (p > 0.05). Why non-tasters and medium tasters with obesity seem to experience diminished taste responsiveness to NaCl is unknown, and deserves further study. These data suggest that investigators should use caution when interpreting NaCl ratings used as a reference standard in PROP screening among individuals with obesity.
Figure 4.
Mean (±SE) values of perceived intensity NaCl and PROP in an ethnically homogeneous cohort of Sardinia, Italy. n = 134 (48 Males, 86 Females) normal weight subjects; n = 98 (45 Males, 53 Females) obese subjects. Values with different superscripts (a, b, etc.) indicate significant differences (p < 0.034; Duncan’s test subsequent 2-way-ANOVA).
A second issue to consider is that the presence of obesity might alter the relationship between PROP tasting and body weight. Specifically, Carta et al. [120] found that super-tasters who were lean (BMI < 25 kg/m2) had a lower BMI than medium or non-tasters who were lean, consistent with previously reported findings among overweight individuals. However, this relationship was reversed among individuals with obesity (BMI > 30 kg/m2) such that super-tasters with obesity had a higher BMI than medium tasters and non-tasters with obesity.
4. Smoking
Nicotine from cigarettes is both bitter tasting and pungent [123,124]. Aversion to the negative chemosensations of nicotine is thought to reduce the risk of smoking or the severity of nicotine dependence in those who do smoke [125,126]. Several studies have shown that those who are taste blind to PROP (or AVI homozygotes) may be at greater risk for smoking, since non-tasters may be less responsive to the aversive taste of nicotine [125,127,128]. Whether smokers can learn to like the taste of nicotine due to its stimulant properties, is an open question.
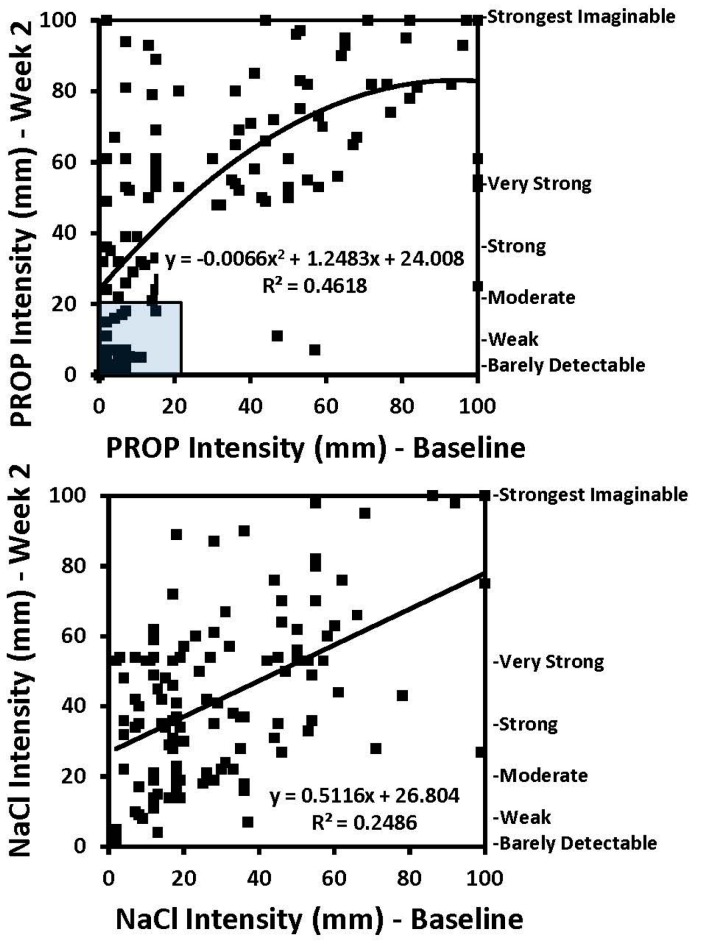
Smoking is also known to diminish both taste and smell sensations, whereas smoking cessation (in the range of 3–6 months duration) partially or fully restores these functions [129,130,131]. Very little work has been done on the acute effects of smoking abstinence, although one study showed partial recovery of taste acuity following 12 h of smoking abstinence [132]. To our knowledge, the study by Ahijevych et al. [57] is the only clinical trial to examine relationships between PROP tasting and smoking cessation in individuals with access to nicotine replacement therapy (NRT). Results showed that PROP non-tasters were more likely to use nicotine lozenges, which is consistent with the notion that oral nicotine exposure is less aversive to non-taster individuals. As a consequence of conducting this trial, we screened 120 smokers (consisting of different races and both genders) for PROP status at baseline and again after two weeks of smoking abstinence (with access to NRT). We regressed the PROP ratings at baseline against the PROP ratings at two weeks to model the changes in PROP taste intensity following the abstinence period; the same analysis was conducted for the NaCl ratings. As shown in Figure 5, most participants gave higher intensity ratings to both PROP and NaCl at week 2 than they did at baseline. Indeed, 30% of participants shifted from PROP non-taster status to PROP taster status by the end of the trial. Only a small group of participants (identified in the shaded box; top panel) were identified as PROP non-tasters both at baseline and after two weeks of abstinence. These data demonstrate that smoking-associated taste loss can impact PROP phenotyping procedures, overestimating the true proportion of PROP non-tasters in smoking trials. Our findings also reveal a rapid recovery of suprathreshold taste intensity after just two weeks of smoking cessation. This finding could have broader implications for understanding the role of inhaled smoke on chemosensory insult and recovery of function.
Figure 5.
Regression of intensity ratings for 6-n-propylthiouracil (PROP; top panel), and NaCl (bottom panel), at baseline and at two weeks after smoking cessation (controlling for age and gender). Intensity ratings were made using the Labeled Magnitude Scale (0–100 mm). Intensity ratings for both stimuli increased across the trial. PROP intensity ratings have a logarithmic trend line, while NaCl ratings have a linear trend line. Mean intensity ratings for both stimuli rose from baseline to week 2 (for NaCl, 30.2 ± 2.1 vs. 42.3 ± 2.1 and for PROP, 35.9 ± 3.3 vs. 53.2 ± 2.9; p < 0.01 for both). n = 120. The shaded box identifies a small group of participants who were classified as PROP non-tasters at both. Data are from Ahijevych et al. [57] with permission.
5. Conclusions
The data reviewed here suggest that a variety of common characteristics including gender, age, obesity and smoking that are known to influence taste function can also affect PROP phenotyping. These individual effects may be compounded in study populations that include both genders, and participants of variable age, body weight and smoking status. Thus, researchers, clinicians and other practitioners should use caution when phenotyping diverse populations for PROP taster status, and pay close attention to the demographic characteristics of the individuals under study. Statistical procedures to adjust for the effects of these variables should be considered, when feasible.
PROP tasting is a popular tool that has led to important insights into food preferences, eating behavior, obesity and metabolic diseases such as cancer. A possible link between this phenotype or TAS2R38 gene and alcoholism has been claimed [11,133]. More recently, it has been used as a clinical assessment tool in a weight loss intervention [56] and as a biomarker for risk of dental caries [134,135] and upper respiratory infections [32]. Understanding the underlying characteristics of this phenotype is a critical first step for exploiting its utility as a biomarker in health and disease. As phenotypic variation in PROP sensitivity is genetic in origin, we suggest that PROP phenotype is a useful biomarker for nutrition, health and disease because it can be assessed in large populations with scientific rigor, at low cost and non-invasively.
Acknowledgments
Supported in part by NIH DA024765 (K.L.A.), USDA Hatch Act Funds Administered by the NJ Agricultural Experiment Station (B.J.T.), the Rutgers University Office of Research and Economic Development (B.J.T.) and a grant from the University of Cagliari (Fondo Integrativo per la Ricerca, FIR 2016) (I.T.B.).
Author Contributions
B.J.T. and I.T.B. conceived and constructed this manuscript with the assistance of M.M., Y.K., K.L.A. and P.G. contributed to the writing and editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tepper B.J. Nutritional implications of genetic taste variation: The role of PROP sensitivity and other taste phenotypes. Annu. Rev. Nutr. 2008;28:367–388. doi: 10.1146/annurev.nutr.28.061807.155458. [DOI] [PubMed] [Google Scholar]
- 2.Tepper B.J. 6-n-Propylthiouracil: A genetic marker for taste, with implications for food preference and dietary habits. Am. J. Hum. Genet. 1998;63:1271–1276. doi: 10.1086/302124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tepper B.J., Banni S., Melis M., Crnjar R., Barbarossa I.T. Genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) and its association with physiological mechanisms controlling body mass index (BMI) Nutrients. 2014;6:3363–3381. doi: 10.3390/nu6093363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartoshuk L.M., Rifkin B., Marks L.E., Hooper J.E. Bitterness of KCl and benzoate: Related to genetic status for sensitivity to PTC/PROP. Chem. Senses. 1988;13:517–528. doi: 10.1093/chemse/13.4.517. [DOI] [Google Scholar]
- 5.Bartoshuk L.M. The biological basis of food perception and acceptance. Food Qual. Prefer. 1993;4:21–32. doi: 10.1016/0950-3293(93)90310-3. [DOI] [Google Scholar]
- 6.Gent J., Bartoshuk L. Sweetness of sucrose, neohesperidin dihydrochalcone, and saccharin is related to genetic ability to taste the bitter substance 6-n-propylthiouracil. Chem. Senses. 1983;7:265–272. doi: 10.1093/chemse/7.3-4.265. [DOI] [Google Scholar]
- 7.Bartoshuk L., Fast K., Karrer T., Marino S., Price R., Reed D. PROP supertasters and the perception of sweetness and bitterness. Chem. Senses. 1992;17:594. [Google Scholar]
- 8.Bartoshuk L.M. Bitter taste of saccharin related to the genetic ability to taste the bitter substance 6-n-propylthiouracil. Science. 1979;205:934–935. doi: 10.1126/science.472717. [DOI] [PubMed] [Google Scholar]
- 9.Bartoshuk L.M., Rifkin B., Marks L.E., Bars P. Taste and aging. J. Gerontol. 1986;41:51–57. doi: 10.1093/geronj/41.1.51. [DOI] [PubMed] [Google Scholar]
- 10.Prescott J., Swain-Campbell N. Responses to repeated oral irritation by capsaicin, cinnamaldehyde and ethanol in PROP tasters and non-tasters. Chem. Senses. 2000;25:239–246. doi: 10.1093/chemse/25.3.239. [DOI] [PubMed] [Google Scholar]
- 11.Duffy V.B., Davidson A.C., Kidd J.R., Kidd K.K., Speed W.C., Pakstis A.J., Reed D.R., Snyder D.J., Bartoshuk L.M. Bitter Receptor Gene (TAS2R38), 6-n-Propylthiouracil (PROP) Bitterness and Alcohol Intake. Alcohol. Clin. Exp. Res. 2004;28:1629–1637. doi: 10.1097/01.ALC.0000145789.55183.D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeomans M.R., Tepper B.J., Rietzschel J., Prescott J. Human hedonic responses to sweetness: Role of taste genetics and anatomy. Physiol. Behav. 2007;91:264–273. doi: 10.1016/j.physbeh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Prescott J., Soo J., Campbell H., Roberts C. Responses of PROP taster groups to variations in sensory qualities within foods and beverages. Physiol. Behav. 2004;82:459–469. doi: 10.1016/j.physbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Melis M., Tomassini Barbarossa I. Taste Perception of Sweet, Sour, Salty, Bitter, and Umami and Changes Due to l-Arginine Supplementation, as a Function of Genetic Ability to Taste 6-n-Propylthiouracil. Nutrients. 2017;9:541. doi: 10.3390/nu9060541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tepper B.J., Nurse R.J. PROP taster status is related to the perception and preference for fat. Ann. N. Y. Acad. Sci. 1998;855:802–804. doi: 10.1111/j.1749-6632.1998.tb10662.x. [DOI] [PubMed] [Google Scholar]
- 16.Melis M., Sollai G., Muroni P., Crnjar R., Barbarossa I.T. Associations between orosensory perception of oleic acid, the common single nucleotide polymorphisms (rs1761667 and rs1527483) in the CD36 gene, and 6-n-propylthiouracil (PROP) tasting. Nutrients. 2015;7:2068–2084. doi: 10.3390/nu7032068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkmeyer S.V., Tepper B.J. Understanding creaminess perception of dairy products using free-choice profiling and genetic responsivity to 6-n-propylthiouracil. Chem. Senses. 2003;28:527–536. doi: 10.1093/chemse/28.6.527. [DOI] [PubMed] [Google Scholar]
- 18.Melis M., Yousaf N.Y., Mattes M.Z., Cabras T., Messana I., Crnjar R., Tomassini Barbarossa I., Tepper B.J. Sensory perception of and salivary protein response to astringency as a function of the 6-n-propylthioural (PROP) bitter-taste phenotype. Physiol. Behav. 2017;173:163–173. doi: 10.1016/j.physbeh.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Keller K.L., Steinmann L., Nurse R.J., Tepper B.J. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite. 2002;38:3–12. doi: 10.1006/appe.2001.0441. [DOI] [PubMed] [Google Scholar]
- 20.Bell K.I., Tepper B.J. Short-term vegetable intake by young children classified by 6-n-propylthoiuracil bitter-taste phenotype. Am. J. Clin. Nutr. 2006;84:245–251. doi: 10.1093/ajcn/84.1.245. [DOI] [PubMed] [Google Scholar]
- 21.Dinehart M.E., Hayes J.E., Bartoshuk L.M., Lanier S.L., Duffy V.B. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol. Behav. 2006;87:304–313. doi: 10.1016/j.physbeh.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Yackinous C., Guinard J.X. Relation between PROP taster status and fat perception, touch, and olfaction. Physiol. Behav. 2001;72:427–437. doi: 10.1016/S0031-9384(00)00430-3. [DOI] [PubMed] [Google Scholar]
- 23.Tomassini Barbarossa I., Ozdener M.H., Melis M., Love-Gregory L., Mitreva M., Abumrad N.A., Pepino M.Y. Variant in a common odorant-binding protein gene is associated with bitter sensitivity in people. Behav. Brain Res. 2017;329:200–204. doi: 10.1016/j.bbr.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes J.E., Duffy V.B. Revisiting sugar-fat mixtures: Sweetness and creaminess vary with phenotypic markers of oral sensation. Chem. Senses. 2007;32:225–236. doi: 10.1093/chemse/bjl050. [DOI] [PubMed] [Google Scholar]
- 25.Mattes R.D. 6-n-Propylthiouracil taster status: Dietary modifier, marker or misleader? In: Prescott J., Tepper B.J., editors. Genetic Variation in Taste Sensitivity. Marcel Dekker; New York, NY, USA: 2004. pp. 229–250. [Google Scholar]
- 26.Tepper B.J., Neilland M., Ullrich N.V., Koelliker Y., Belzer L.M. Greater energy intake from a buffet meal in lean, young women is associated with the 6-n-propylthiouracil (PROP) non-taster phenotype. Appetite. 2011;56:104–110. doi: 10.1016/j.appet.2010.11.144. [DOI] [PubMed] [Google Scholar]
- 27.Tepper B.J., Koelliker Y., Zhao L., Ullrich N.V., Lanzara C., d’Adamo P., Ferrara A., Ulivi S., Esposito L., Gasparini P. Variation in the bitter-taste receptor gene TAS2R38, and adiposity in a genetically isolated population in Southern Italy. Obesity. 2008;16:2289–2295. doi: 10.1038/oby.2008.357. [DOI] [PubMed] [Google Scholar]
- 28.Tepper B.J., Williams T.Z., Burgess J.R., Antalis C.J., Mattes R.D. Genetic variation in bitter taste and plasma markers of anti-oxidant status in college women. Int. J. Food Sci. Nutr. 2009;60(Suppl. S2):35–45. doi: 10.1080/09637480802304499. [DOI] [PubMed] [Google Scholar]
- 29.Basson M.D., Bartoshuk L.M., Dichello S.Z., Panzini L., Weiffenbach J.M., Duffy V.B. Association between 6-n-propylthiouracil (PROP) bitterness and colonic neoplasms. Dig. Dis. Sci. 2005;50:483–489. doi: 10.1007/s10620-005-2462-7. [DOI] [PubMed] [Google Scholar]
- 30.Carrai M., Steinke V., Vodicka P., Pardini B., Rahner N., Holinski-Feder E., Morak M., Schackert H.K., Görgens H., Stemmler S., et al. Association between TAS2R38 gene polymorphisms and colorectal cancer risk: A case-control study in two independent populations of Caucasian origin. PLoS ONE. 2011;6:e20464. doi: 10.1371/journal.pone.0020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucock M., Ng X., Boyd L., Skinner V., Wai R., Tang S., Naylor C., Yates Z., Choi J.H., Roach P., et al. TAS2R38 bitter taste genetics, dietary vitamin C, and both natural and synthetic dietary folic acid predict folate status, a key micronutrient in the pathoaetiology of adenomatous polyps. Food Funct. 2011;2:457–465. doi: 10.1039/c1fo10054h. [DOI] [PubMed] [Google Scholar]
- 32.Lee R.J., Xiong G., Kofonow J.M., Chen B., Lysenko A., Jiang P., Abraham V., Doghramji L., Adappa N.D., Palmer J.N., et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calò C., Padiglia A., Zonza A., Corrias L., Contu P., Tepper B.J., Barbarossa I.T. Polymorphisms in TAS2R38 and the taste bud trophic factor, gustin gene co-operate in modulating PROP taste phenotype. Physiol. Behav. 2011;104:1065–1071. doi: 10.1016/j.physbeh.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Bartoshuk L.M., Duffy V.B., Miller I.J. PTC/PROP tasting: Anatomy, psychophysics, and sex effects. Physiol. Behav. 1994;56:1165–1171. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 35.Tepper B.J., Christensen C.M., Cao J. Development of brief methods to classify individuals by PROP taster status. Physiol. Behav. 2001;73:571–577. doi: 10.1016/S0031-9384(01)00500-5. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L., Kirkmeyer S.V., Tepper B.J. A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol. Behav. 2003;78:625–633. doi: 10.1016/S0031-9384(03)00057-X. [DOI] [PubMed] [Google Scholar]
- 37.Genick U.K., Kutalik Z., Ledda M., Destito M.C.S., Souza M.M., Cirillo C.A., Godinot N., Martin N., Morya E., Sameshima K., et al. Sensitivity of Genome-Wide-Association Signals to Phenotyping Strategy: The PROP-TAS2R38 Taste Association as a Benchmark. PLoS ONE. 2011;6:e27745. doi: 10.1371/journal.pone.0027745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padiglia A., Zonza A., Atzori E., Chillotti C., Calò C., Tepper B.J., Barbarossa I.T. Sensitivity to 6-n-propylthiouracil is associated with gustin (carbonic anhydrase VI) gene polymorphism, salivary zinc, and body mass index in humans. Am. J. Clin. Nutr. 2010;92:539–545. doi: 10.3945/ajcn.2010.29418. [DOI] [PubMed] [Google Scholar]
- 39.Barbarossa I.T., Melis M., Mattes M.Z., Calò C., Muroni P., Crnjar R., Tepper B.J. The gustin (CA6) gene polymorphism, rs2274333 (A/G), is associated with fungiform papilla density, whereas PROP bitterness is mostly due to TAS2R38 in an ethnically-mixed population. Physiol. Behav. 2015;138:6–12. doi: 10.1016/j.physbeh.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Prutkin J., Fisher E.M., Etter L., Fast K., Gardner E., Lucchina L.A., Snyder D.J., Tie K., Weiffenbach J., Bartoshuk L.M. Genetic variation and inferences about perceived taste intensity in mice and men. Physiol. Behav. 2000;69:161–173. doi: 10.1016/S0031-9384(00)00199-2. [DOI] [PubMed] [Google Scholar]
- 41.Hayes J.E., Sullivan B.S., Duffy V.B. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol. Behav. 2010;100:369–380. doi: 10.1016/j.physbeh.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drewnowski A., Henderson S.A., Shore A.B. Genetic sensitivity to 6-n-propylthiouracil (PROP) and hedonic responses to bitter and sweet tastes. Chem. Senses. 1997;22:27–37. doi: 10.1093/chemse/22.1.27. [DOI] [PubMed] [Google Scholar]
- 43.Tepper B.J., Nurse R.J. Fat perception is related to PROP taster status. Physiol. Behav. 1997;61:949–954. doi: 10.1016/S0031-9384(96)00608-7. [DOI] [PubMed] [Google Scholar]
- 44.Duffy V.B., Bartoshuk L.M. Food acceptance and genetic variation in taste. J. Am. Diet. Assoc. 2000;100:647–655. doi: 10.1016/S0002-8223(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 45.Melis M., Aragoni M.C., Arca M., Cabras T., Caltagirone C., Castagnola M., Crnjar R., Messana I., Tepper B.J., Barbarossa I.T. Marked increase in PROP taste responsiveness following oral supplementation with selected salivary proteins or their related free amino acids. PLoS ONE. 2013;8:e59810. doi: 10.1371/journal.pone.0059810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tepper B.J., Ullrich N.V. Influence of genetic taste sensitivity to 6-n-propylthiouracil (PROP), dietary restraint and disinhibition on body mass index in middle-aged women. Physiol. Behav. 2002;75:305–312. doi: 10.1016/S0031-9384(01)00664-3. [DOI] [PubMed] [Google Scholar]
- 47.Goldstein G.L., Daun H., Tepper B.J. Adiposity in middle-aged women is associated with genetic taste blindness to 6-n-propylthiouracil. Obes. Res. 2005;13:1017–1023. doi: 10.1038/oby.2005.119. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein G.L., Daun H., Tepper B.J. Influence of PROP taster status and maternal variables on energy intake and body weight of pre-adolescents. Physiol. Behav. 2007;90:809–817. doi: 10.1016/j.physbeh.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Zhao L., Tepper B.J. Perception and acceptance of selected high-intensity sweeteners and blends in model soft drinks by propylthiouracil (PROP) non-tasters and super-tasters. Food Qual. Prefer. 2007;18:531–540. doi: 10.1016/j.foodqual.2006.07.004. [DOI] [Google Scholar]
- 50.Robino A., Mezzavilla M., Pirastu N., Dognini M., Tepper B.J., Gasparini P. A Population-Based Approach to Study the Impact of PROP Perception on Food Liking in Populations along the Silk Road. PLoS ONE. 2014;9:e91716. doi: 10.1371/journal.pone.0091716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robino A., Mezzavilla M., Pirastu N., La Bianca M., Gasparini P., Carlino D., Tepper B.J. Understanding the role of personality and alexithymia in food preferences and PROP taste perception. Physiol. Behav. 2016;157:72–78. doi: 10.1016/j.physbeh.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Burgess B., Rao S.P., Tepper B.J. Changes in liking for sweet and fatty foods following weight loss in women are related to prop phenotype but not to diet. Obesity. 2016;24:1867–1873. doi: 10.1002/oby.21570. [DOI] [PubMed] [Google Scholar]
- 53.Kirkmeyer S.V., Tepper B.J. Consumer reactions to creaminess and genetic sensitivity to 6-n-propylthiouracil: A multidimensional study. Food Qual. Prefer. 2005;16:545–556. doi: 10.1016/j.foodqual.2004.11.003. [DOI] [Google Scholar]
- 54.Shafaie Y., Hoffman D.J., Tepper B.J. Consumption of a high-fat soup preload leads to differences in short-term energy and fat intake between PROP non-taster and super-taster women. Appetite. 2015;89:196–202. doi: 10.1016/j.appet.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 55.Shafaie Y., Koelliker Y., Hoffman D.J., Tepper B.J. Energy intake and diet selection during buffet consumption in women classified by the 6-n-propylthiouracil bitter taste phenotype. Am. J. Clin. Nutr. 2013;98:1583–1591. doi: 10.3945/ajcn.113.058818. [DOI] [PubMed] [Google Scholar]
- 56.Burgess B., Raynor H.A., Tepper B.J. PROP Nontaster Women Lose More Weight Following a Low-Carbohydrate versus a Low-Fat Diet in a Randomized Controlled Trial. Obesity. 2017;25:1682–1690. doi: 10.1002/oby.21951. [DOI] [PubMed] [Google Scholar]
- 57.Ahijevych K., Tepper B.J., Graham M.C., Holloman C., Matcham W.A. Relationships of PROP Taste Phenotype, Taste Receptor Genotype, and Oral Nicotine Replacement Use. Nicotine Tob. Res. 2015;17:1149–1155. doi: 10.1093/ntr/ntu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi S.E. Racial differences between African Americans and Asian Americans in the effect of 6-n-propylthiouracil taste intensity and food liking on body mass index. J. Acad. Nutr. Diet. 2014;114:938–944. doi: 10.1016/j.jand.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 59.Oter B., Ulukapi I., Ulukapi H., Topcuoglu N., Cildir S. The relation between 6-n-propylthiouracil sensitivity and caries activity in schoolchildren. Caries Res. 2011;45:556–560. doi: 10.1159/000332432. [DOI] [PubMed] [Google Scholar]
- 60.Deshaware S., Singhal R. Genetic variation in bitter taste receptor gene TAS2R38, PROP taster status and their association with body mass index and food preferences in Indian population. Gene. 2017;627:363–368. doi: 10.1016/j.gene.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 61.Macht M., Mueller J. Increased negative emotional responses in PROP supertasters. Physiol. Behav. 2007;90:466–472. doi: 10.1016/j.physbeh.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Green B.G., Shaffer G.S., Gilmore M.M. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem. Senses. 1993;18:683–702. doi: 10.1093/chemse/18.6.683. [DOI] [Google Scholar]
- 63.Bartoshuk L.M., Duffy V.B., Green B.G., Hoffman H.J., Ko C.W., Lucchina L.A., Marks L.E., Snyder D.J., Weiffenbach J.M. Valid across-group comparisons with labeled scales: The gLMS versus magnitude matching. Physiol. Behav. 2004;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 64.Melis M., Arca M., Aragoni M.C., Cabras T., Caltagirone C., Castagnola M., Crnjar R., Messana I., Tepper B.J., Tomassini Barbarossa I. Dose-Dependent Effects of l-Arginine on PROP Bitterness Intensity and Latency and Characteristics of the Chemical Interaction between PROP and l-Arginine. PLoS ONE. 2015;10:e0131104. doi: 10.1371/journal.pone.0131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melis M., Atzori E., Cabras S., Zonza A., Calò C., Muroni P., Nieddu M., Padiglia A., Sogos V., Tepper B.J., et al. The gustin (CA6) gene polymorphism, rs2274333 (A/G), as a mechanistic link between PROP tasting and fungiform taste papilla density and maintenance. PLoS ONE. 2013;8:e74151. doi: 10.1371/journal.pone.0074151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oftedal K.N., Tepper B.J. Influence of the PROP bitter taste phenotype and eating attitudes on energy intake and weight status in pre-adolescents: A 6-year follow-up study. Physiol. Behav. 2013;118:103–111. doi: 10.1016/j.physbeh.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boxer E.E., Garneau N.L. Rare haplotypes of the gene TAS2R38 confer bitter taste sensitivity in humans. SpringerPlus. 2015;4:505. doi: 10.1186/s40064-015-1277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bembich S., Lanzara C., Clarici A., Demarini S., Tepper B.J., Gasparini P., Grasso D.L. Individual differences in prefrontal cortex activity during perception of bitter taste using fNIRS methodology. Chem. Senses. 2010;35:801–812. doi: 10.1093/chemse/bjq080. [DOI] [PubMed] [Google Scholar]
- 69.Sollai G., Melis M., Pani D., Cosseddu P., Usai I., Crnjar R., Bonfiglio A., Tomassini Barbarossa I. First objective evaluation of taste sensitivity to 6-n-propylthiouracil (PROP), a paradigm gustatory stimulus in humans. Sci. Rep. 2017;7:40353. doi: 10.1038/srep40353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pani D., Usai I., Cosseddu P., Melis M., Sollai G., Crnjar R., Tomassini Barbarossa I., Raffo L., Bonfiglio A. An automated system for the objective evaluation of human gustatory sensitivity using tongue biopotential recordings. PLoS ONE. 2017;12:e0177246. doi: 10.1371/journal.pone.0177246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fox A.L. The relationship between chemical constitution and taste. Proc. Natl. Acad. Sci. USA. 1932;18:115–120. doi: 10.1073/pnas.18.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalmus H. Improvements in the classification of the taster genotypes. Ann. Hum. Genet. 1958;22:222–230. doi: 10.1111/j.1469-1809.1958.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 73.Kim U.K., Jorgenson E., Coon H., Leppert M., Risch N., Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 74.Bufe B., Breslin P.A., Kuhn C., Reed D.R., Tharp C.D., Slack J.P., Kim U.K., Drayna D., Meyerhof W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drayna D. Human taste genetics. Annu. Rev. Genom. Hum. Genet. 2005;6:217–235. doi: 10.1146/annurev.genom.6.080604.162340. [DOI] [PubMed] [Google Scholar]
- 76.Campbell M.C., Ranciaro A., Froment A., Hirbo J., Omar S., Bodo J.M., Nyambo T., Lema G., Zinshteyn D., Drayna D., et al. Evolution of functionally diverse alleles associated with PTC bitter taste sensitivity in Africa. Mol. Biol. Evol. 2012;29:1141–1153. doi: 10.1093/molbev/msr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lipchock S.V., Mennella J.A., Spielman A.I., Reed D.R. Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. Am. J. Clin. Nutr. 2013;98:1136–1143. doi: 10.3945/ajcn.113.066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fahey J.W., Zalcmann A.T., Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/S0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 79.Olson J.M., Boehnke M., Neiswanger K., Roche A.F., Siervogel R.M. Alternative genetic models for the inheritance of the phenylthiocarbamide taste deficiency. Genet. Epidemiol. 1989;6:423–434. doi: 10.1002/gepi.1370060305. [DOI] [PubMed] [Google Scholar]
- 80.Reed D.R., Nanthakumar E., North M., Bell C., Bartoshuk L.M., Price R.A. Localization of a gene for bitter-taste perception to human chromosome 5p15. Am. J. Hum. Genet. 1999;64:1478–1480. doi: 10.1086/302367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drayna D., Coon H., Kim U.K., Elsner T., Cromer K., Otterud B., Baird L., Peiffer A.P., Leppert M. Genetic analysis of a complex trait in the Utah Genetic Reference Project: A major locus for PTC taste ability on chromosome 7q and a secondary locus on chromosome 16p. Hum. Genet. 2003;112:567–572. doi: 10.1007/s00439-003-0911-y. [DOI] [PubMed] [Google Scholar]
- 82.Bajec M.R., Pickering G.J. Thermal taste, PROP responsiveness, and perception of oral sensations. Physiol. Behav. 2008;95:581–590. doi: 10.1016/j.physbeh.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 83.Essick G., Chopra A., Guest S., McGlone F. Lingual tactile acuity, taste perception, and the density and diameter of fungiform papillae in female subjects. Physiol. Behav. 2003;80:289–302. doi: 10.1016/j.physbeh.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 84.Shahbake M., Hutchinson I., Laing D.G., Jinks A.L. Rapid quantitative assessment of fungiform papillae density in the human tongue. Brain Res. 2005;1052:196–201. doi: 10.1016/j.brainres.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 85.Feeney E.L., Hayes J.E. Exploring associations between taste perception, oral anatomy and polymorphisms in the carbonic anhydrase (gustin) gene CA6. Physiol. Behav. 2014;128:148–154. doi: 10.1016/j.physbeh.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cabras T., Melis M., Castagnola M., Padiglia A., Tepper B.J., Messana I., Tomassini Barbarossa I. Responsiveness to 6-n-propylthiouracil (PROP) is associated with salivary levels of two specific basic proline-rich proteins in humans. PLoS ONE. 2012;7:e30962. doi: 10.1371/journal.pone.0030962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaminski L.C., Henderson S.A., Drewnowski A. Young women’s food preferences and taste responsiveness to 6-n-propylthiouracil (PROP) Physiol. Behav. 2000;68:691–697. doi: 10.1016/S0031-9384(99)00240-1. [DOI] [PubMed] [Google Scholar]
- 88.Timpson N.J., Christensen M., Lawlor D.A., Gaunt T.R., Day I.N., Ebrahim S., Davey Smith G. TAS2R38 (phenylthiocarbamide) haplotypes, coronary heart disease traits, and eating behavior in the British Women’s Heart and Health Study. Am. J. Clin. Nutr. 2005;81:1005–1011. doi: 10.1093/ajcn/81.5.1005. [DOI] [PubMed] [Google Scholar]
- 89.Yackinous C.A., Guinard J.X. Relation between PROP (6-n-propylthiouracil) taster status, taste anatomy and dietary intake measures for young men and women. Appetite. 2002;38:201–209. doi: 10.1006/appe.2001.0481. [DOI] [PubMed] [Google Scholar]
- 90.Drewnowski A., Henderson S.A., Cockroft J.E. Genetic Sensitivity to 6-n-Propylthiouracil Has No Influence on Dietary Patterns, Body Mass Indexes, or Plasma Lipid Profiles of Women. J. Am. Diet. Assoc. 2007;107:1340–1348. doi: 10.1016/j.jada.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 91.Gorovic N., Afzal S., Tjonneland A., Overvad K., Vogel U., Albrechtsen C., Poulsen H.E. Genetic variation in the hTAS2R38 taste receptor and brassica vegetable intake. Scand. J. Clin. Lab. Investig. 2011;71:274–279. doi: 10.3109/00365513.2011.559553. [DOI] [PubMed] [Google Scholar]
- 92.O’Brien S.A., Feeney E.L., Scannell A.G., Markey A., Gibney E.R. Bitter taste perception and dietary intake patterns in Irish children. J. Nutrigenet. Nutrigenomics. 2013;6:43–58. doi: 10.1159/000348442. [DOI] [PubMed] [Google Scholar]
- 93.Doty R.L., Kamath V. The influences of age on olfaction: A review. Front. Psychol. 2014;5:20. doi: 10.3389/fpsyg.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Methven L., Allen V.J., Withers C.A., Gosney M.A. Ageing and taste. Proc. Nutr. Soc. 2012;71:556–565. doi: 10.1017/S0029665112000742. [DOI] [PubMed] [Google Scholar]
- 95.Whissell-Buechy D., Wills C. Male and female correlations for taster (P.T.C.) phenotypes and rate of adolescent development. Ann. Hum. Biol. 1989;16:131–146. doi: 10.1080/03014468700006982. [DOI] [PubMed] [Google Scholar]
- 96.Zuniga J.R., Davis S.H., Englehardt R.A., Miller I.J., Schiffrman S.S., Phillips C. Taste performance on the anterior human tongue varles with fungiform taste bud density. Chem. Senses. 1993;18:449–460. doi: 10.1093/chemse/18.5.449. [DOI] [Google Scholar]
- 97.Correa M., Hutchinson I., Laing D.G., Jinks A.L. Changes in Fungiform Papillae Density during Development in Humans. Chem. Senses. 2013;38:519–527. doi: 10.1093/chemse/bjt022. [DOI] [PubMed] [Google Scholar]
- 98.Whissell-Buechy D. Effects of age and sex on taste sensitivity to phenylthiocarbamide (PTC) in the Berkeley Guidance sample. Chem. Senses. 1990;15:39–57. doi: 10.1093/chemse/15.1.39. [DOI] [Google Scholar]
- 99.Mennella J., Pepino M.Y., Duke F., Reed D. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2010;11:60. doi: 10.1186/1471-2156-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Segovia C., Hutchinson I., Laing D.G., Jinks A.L. A quantitative study of fungiform papillae and taste pore density in adults and children. Dev. Brain Res. 2002;138:135–146. doi: 10.1016/S0165-3806(02)00463-7. [DOI] [PubMed] [Google Scholar]
- 101.Sillanpaa M.J., Auranen K. Replication in genetic studies of complex traits. Ann. Hum. Genet. 2004;68:646–657. doi: 10.1046/j.1529-8817.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- 102.Tian C., Gregersen P.K., Seldin M.F. Accounting for ancestry: Population substructure and genome-wide association studies. Hum. Mol. Genet. 2008;17:R143–R150. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thornton-Wells T.A., Moore J.H., Haines J.L. Genetics, statistics and human disease: Analytical retooling for complexity. Trends Genet. 2004;20:640–647. doi: 10.1016/j.tig.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 104.Fischer M.E., Cruickshanks K.J., Schubert C.R., Pinto A., Klein R., Pankratz N., Pankow J.S., Huang G.H. Factors related to fungiform papillae density: The beaver dam offspring study. Chem. Senses. 2013;38:669–677. doi: 10.1093/chemse/bjt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pavlidis P., Gouveris H., Anogeianaki A., Koutsonikolas D., Anogianakis G., Kekes G. Age-related changes in electrogustometry thresholds, tongue tip vascularization, density, and form of the fungiform papillae in humans. Chem. Senses. 2013;38:35–43. doi: 10.1093/chemse/bjs076. [DOI] [PubMed] [Google Scholar]
- 106.Mojet J., Heidema J., Christ-Hazelhof E. Taste perception with age: Generic or specific losses in supra-threshold intensities of five taste qualities? Chem. Senses. 2003;28:397–413. doi: 10.1093/chemse/28.5.397. [DOI] [PubMed] [Google Scholar]
- 107.Tepper B.J., Koelliker Y., Lanzara C., Pirastu N., Gasparini P., Sala C., Toniolo D. Age-related changes in the bitterness of 6-n-propylthiouracil (PROP) and food preferences in an isolated population in Northwest Italy. Abstracts from the Thirty-Third Annual Meeting of the Association for Chemoreception Sciences. Chem. Senses. 2011;36:A118–A119. [Google Scholar]
- 108.Chevrot M., Passilly-Degrace P., Ancel D., Bernard A., Enderli G., Gomes M., Robin I., Issanchou S., Verges B., Nicklaus S., et al. Obesity interferes with the orosensory detection of long-chain fatty acids in humans. Am. J. Clin. Nutr. 2014;99:975–983. doi: 10.3945/ajcn.113.077198. [DOI] [PubMed] [Google Scholar]
- 109.Sayed A., Sery O., Plesnik J., Daoudi H., Rouabah A., Rouabah L., Khan N.A. CD36 AA genotype is associated with decreased lipid taste perception in young obese, but not lean, children. Int. J. Obes. 2015;39:920–924. doi: 10.1038/ijo.2015.20. [DOI] [PubMed] [Google Scholar]
- 110.Stewart J.E., Newman L.P., Keast R.S. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin. Nutr. 2011;30:838–844. doi: 10.1016/j.clnu.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 111.Feeney E.L., O’Brien S.A., Scannell A.G., Markey A., Gibney E.R. Suprathreshold measures of taste perception in children—Association with dietary quality and body weight. Appetite. 2017;113:116–123. doi: 10.1016/j.appet.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 112.Rawal S., Huedo-Medina T.B., Hoffman H.J., Swede H., Duffy V.B. Structural equation modeling of associations among taste-related risk factors, taste functioning, and adiposity. Obesity. 2017;25:781–787. doi: 10.1002/oby.21785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Donaldson L.F., Bennett L., Baic S., Melichar J.K. Taste and weight: Is there a link? Am. J. Clin. Nutr. 2009;90:800S–803S. doi: 10.3945/ajcn.2009.27462Q. [DOI] [PubMed] [Google Scholar]
- 114.Fernandez-Garcia J.C., Alcaide J., Santiago-Fernandez C., Roca-Rodriguez M.M., Aguera Z., Banos R., Botella C., de la Torre R., Fernandez-Real J.M., Fruhbeck G., et al. An increase in visceral fat is associated with a decrease in the taste and olfactory capacity. PLoS ONE. 2017;12:e0171204. doi: 10.1371/journal.pone.0171204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tucker R.M., Kaiser K.A., Parman M.A., George B.J., Allison D.B., Mattes R.D. Comparisons of Fatty Acid Taste Detection Thresholds in People Who Are Lean vs. Overweight or Obese: A Systematic Review and Meta-Analysis. PLoS ONE. 2017;12:e0169583. doi: 10.1371/journal.pone.0169583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hardikar S., Hochenberger R., Villringer A., Ohla K. Higher sensitivity to sweet and salty taste in obese compared to lean individuals. Appetite. 2017;111:158–165. doi: 10.1016/j.appet.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 117.Feeney E., O’Brien S., Scannell A., Markey A., Gibney E.R. Genetic variation in taste perception: Does it have a role in healthy eating? Proc. Nutr. Soc. 2011;70:135–143. doi: 10.1017/S0029665110003976. [DOI] [PubMed] [Google Scholar]
- 118.Baranowski T., Baranowski J.C., Watson K.B., Jago R., Islam N., Beltran A., Martin S.J., Nguyen N., Tepper B.J. 6-n-propylthiouracil taster status not related to reported cruciferous vegetable intake among ethnically diverse children. Nutr. Res. 2011;31:594–600. doi: 10.1016/j.nutres.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mennella J.A., Pepino M.Y., Reed D.R. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115:e216–e222. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carta G., Melis M., Pintus S., Pintus P., Piras C.A., Muredda L., Demurtas D., Di Marzo V., Banni S., Barbarossa I.T. Participants with Normal Weight or with Obesity Show Different Relationships of 6-n-Propylthiouracil (PROP) Taster Status with BMI and Plasma Endocannabinoids. Sci. Rep. 2017;7:1361. doi: 10.1038/s41598-017-01562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomassini Barbarossa I., Carta G., Murru E., Melis M., Zonza A., Vacca C., Muroni P., Di Marzo V., Banni S. Taste sensitivity to 6-n-propylthiouracil is associated with endocannabinoid plasma levels in normal-weight individuals. Nutrition. 2013;29:531–536. doi: 10.1016/j.nut.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 122.Karmous I., Plesnik J., Khan A.S., Sery O., Abid A., Mankai A., Aouidet A., Khan N.A. Orosensory detection of bitter in fat-taster healthy and obese participants: Genetic polymorphism of CD36 and TAS2R38. Clin. Nutr. 2017 doi: 10.1016/j.clnu.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 123.Hummel T., Hummel C., Pauli E., Kobal G. Olfactory discrimination of nicotine-enantiomers by smokers and non-smokers. Chem. Senses. 1992;17:13–21. doi: 10.1093/chemse/17.1.13. [DOI] [Google Scholar]
- 124.Scott T.R., Giza B.K., Yan J. Electrophysiological responses to bitter stimuli in primate cortex. Ann. N. Y. Acad. Sci. 1998;855:498–501. doi: 10.1111/j.1749-6632.1998.tb10613.x. [DOI] [PubMed] [Google Scholar]
- 125.Enoch M.A., Harris C.R., Goldman D. Does a reduced sensitivity to bitter taste increase the risk of becoming nicotine addicted? Addict. Behav. 2001;26:399–404. doi: 10.1016/S0306-4603(00)00117-9. [DOI] [PubMed] [Google Scholar]
- 126.Snedecor S.M., Pomerleau C.S., Mehringer A.M., Ninowski R., Pomerleau O.F. Differences in smoking-related variables based on phenylthiocarbamide “taster” status. Addict. Behav. 2006;31:2309–2312. doi: 10.1016/j.addbeh.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 127.Mangold J.E., Payne T.J., Ma J.Z., Chen G., Li M.D. Bitter taste receptor gene polymorphisms are an important factor in the development of nicotine dependence in African Americans. J. Med. Genet. 2008;45:578–582. doi: 10.1136/jmg.2008.057844. [DOI] [PubMed] [Google Scholar]
- 128.Risso D.S., Kozlitina J., Sainz E., Gutierrez J., Wooding S., Getachew B., Luiselli D., Berg C.J., Drayna D. Genetic Variation in the TAS2R38 Bitter Taste Receptor and Smoking Behaviors. PLoS ONE. 2016;11:e0164157. doi: 10.1371/journal.pone.0164157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ajmani G.S., Suh H.H., Wroblewski K.E., Pinto J.M. Smoking and olfactory dysfunction: A systematic literature review and meta-analysis. Laryngoscope. 2017;127:1753–1761. doi: 10.1002/lary.26558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cheruel F., Jarlier M., Sancho-Garnier H. Effect of cigarette smoke on gustatory sensitivity, evaluation of the deficit and of the recovery time-course after smoking cessation. Tob. Induc. Dis. 2017;15:15. doi: 10.1186/s12971-017-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pavlidis P., Gouveris H., Kekes G. Electrogustometry Thresholds, Tongue Tip Vascularization, Density, and Form of the Fungiform Papillae Following Smoking Cessation. Chem. Senses. 2017;42:419–423. doi: 10.1093/chemse/bjx009. [DOI] [PubMed] [Google Scholar]
- 132.Mullings E.L., Donaldson L.F., Melichar J.K., Munafo M.R. Effects of acute abstinence and nicotine administration on taste perception in cigarette smokers. J. Psychopharmacol. 2010;24:1709–1715. doi: 10.1177/0269881109105395. [DOI] [PubMed] [Google Scholar]
- 133.Pelchat M.L., Danowski S. A possible genetic association between PROP-tasting and alcoholism. Physiol. Behav. 1992;51:1261–1266. doi: 10.1016/0031-9384(92)90318-V. [DOI] [PubMed] [Google Scholar]
- 134.Alanzi A., Minah G., Romberg E., Catalanotto F., Bartoshuk L., Tinanoff N. Mothers’ taste perceptions and their preschool children’s dental caries experiences. Pediatr. Dent. 2013;35:510–514. [PubMed] [Google Scholar]
- 135.Yildiz G., Ermis R.B., Calapoglu N.S., Celik E.U., Turel G.Y. Gene-environment Interactions in the Etiology of Dental Caries. J. Dent. Res. 2016;95:74–79. doi: 10.1177/0022034515605281. [DOI] [PubMed] [Google Scholar]